Abstract
Background:
Central sensitization (CS) is a condition characterized by a disproportionate response to pain stimuli. We sought to investigate the prevalence of CS in patients with inflammatory arthritides and its association with measures of disease activity and functional disability.
Methods:
We conducted an observational retrospective study in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) patients. We administered to all the subjects in the study the CS inventory (CSI), a questionnaire that has been used for the diagnosis of CS. Demographic and clinical characteristics were collected as well as measures or disease activity [i.e. Simple Disease Activity Index, Disease Activity Score in PsA (DAPSA)] and functional disability [Health Assessment Questionnaire Disability Index (HAQ-DI)]. Patients with fibromyalgia were excluded from the analyses. The primary outcome measure was the presence of functional disability as assessed by HAQ-DI >1.
Results:
We enrolled 150 patients with inflammatory arthritides (78 PsA and 72 RA). Prevalence of CS was observed in 35.3% of the overall sample (29% in RA, 42.9% in PsA). Binary logistic regressions showed a strong, independent and linear association between functional disability and CS in both PsA and RA patients. The strength of this association was greater in PsA than in RA.
Conclusion:
CS is an important determinant of functional disability in patients with chronic inflammatory arthritides. PsA appeared to be more vulnerable to CS. In addition, in the presence of CS, DAPSA did not adequately capture the occurrence of functional disability. Therefore, special attention should be paid to PsA patients, in whom the concomitant diagnosis of CS should be routinely ruled out.
Keywords: biopsychosocial, central sensitization, disability, pain, psoriasis, psoriatic arthritis, rheumatoid arthritis
Introduction
In patients with rheumatic diseases, pain is mixed; nociceptive and neuropathic mechanisms are involved at both the peripheral and central levels. Chronic inflammation in conditions as rheumatoid arthritis (RA), psoriatic arthritis (PsA) and spondyloarthritis may trigger both peripheral and central sensitization (CS) via central modifications of the pain pathways.1–4
Furthermore, pain and tenderness are present not only in joints directly affected but also in apparently normal tissues. In a proportion of patients, the magnitude of symptoms may not necessarily correlate with the severity of the underlying disease, and symptoms may persist even when disease exacerbations have apparently settled.5 Pain in chronic arthritides is primarily due to inflammatory mechanisms; nonetheless, the neuropathic pain component commonly occurs in such conditions. However, the large part of the pain previously labelled as neuropathic is, rather, nociplastic owing to a central sensitization mechanism.6
Nociplastic pain is commonly defined as an altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors. The International Association for the Study of Pain defines CS as a type of nociplastic pain that presents as an “increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input”.7 Pain amplification in CS is probably secondary to an increased excitability of neurons’ membrane as well as to a reduced synaptic inhibition, which facilitates hyperexcitability and elicits pain hypersensitivity.8 These mechanisms explain part of the chronic pain seen in various diseases such as musculoskeletal disorders, degenerative and inflammatory articular diseases and fibromyalgia.6 Nevertheless, fibromyalgia is a disorder characterized by a varying degree of generalized pain and CS contributes to only a portion of the perceived pain, possibly through top-down or bottom-up processes.9
In 2012 the CS inventory (CSI) was developed for the diagnosis of CS.10 The CSI consists of 25 Likert-type questions with a score ranging from 0 to 100;10 the CSI showed to have good clinimetric properties in different populations with chronic pain11–13 and a cut-off of 40 points allowed a correct identification of approximately eight out of 10 patients with CS.12
There are data documenting that 15–40% of patients with inflammatory rheumatic diseases may have concomitant CS.4 When CS is concomitantly present with PsA, disease activity measures which include patient-reported outcomes (PROs) are nearly twice as severe when compared with PsA subjects without CS.4 PsA patients with CS are less likely to achieve targets of treatment such as minimal disease activity.4 Furthermore, lack of association between local tenderness at enthesial signs and evidence of enthesitis at ultrasound imaging (US) has also been documented in such populations.14,15
The prevalence of fibromyalgia (FM) is estimated to be around 21% in RA16 and 16–22% in PsA.14 Even if it is likely that CS and FM constructs overlap to a certain extent, they are not just two different terms to identify the same condition. Currently, there are very few data on the prevalence of CS in arthritic patients, especially in individuals with PsA. Furthermore, it is currently unknown to what extent CS interferes with the assessment of clinical disease activity or affects the functional ability of such individuals. Therefore, we conducted a cross-sectional study to investigate the prevalence of CS in a cohort of patients affected by either RA or PsA. In addition, we analysed the relationship between CS, its severity, clinical disease activity and measures of functional disability.
Materials and methods
We enrolled patients affected by RA classified according to the ACR/EULAR criteria17 or patients affected by PsA classified according to the CASPAR criteria,18 who consecutively attended our arthritides outpatient clinic of the University of Verona. Patients were seen in their usual appointment scheduling and were asked to participate in the study.
We excluded patients with: (a) established diagnosis of major depressive disorders, (b) patients with an established diagnosis of fibromyalgia (c) patients receiving treatment with antidepressant or anticonvulsants, (d) patients not able to autonomously complete the questionnaires, (e) presence or history of axial involvement (as assessed by magnetic resonance imaging (MRI) or X-rays).
For each patient, data on C-reactive protein, Simple Disease Activity Index (SDAI), Disease Activity Score in PsA (DAPSA), Health Assessment Questionnaire Disability Index (HAQ-DI), CSI score, intake of concomitant glucocorticoids (GCs), conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs) and biologic disease modifying anti-rheumatic drug (bDMARD) were collected.
Patients were classified as being in remission or having low disease activity, moderate disease activity or high disease activity according to the established SDAI cut-offs for subjects affected by RA and DAPSA cut-offs for subjects affected by PsA.19–22
The conventional threshold of HAQ-DI >123 was adopted for the definition of functional disability, and a CSI score >40 was the considered cut-off for the definition of CS.12 CSI questionnaire was analysed either as a continuous variable or as positive/negative (threshold 40 points) or divided into four categories, that is, subclinical (⩽29), mild (30–39), moderate (40–49), severe/extreme (⩾50).24 The CSI has been cross-culturally adapted and tested in Italian.11
Data concerning disease duration, time from presentations of symptoms and the start of the first csDMARD or bDMARD, presence of erosions at hands X-rays, the positivity of rheumatoid factor and anti-citrullinated peptides antibodies, history of dactylitis and/or enthesitis, axial involvement (defined as fulfilling the radiological criteria, either at X-rays or MRI, established by ASAS25) and psoriasis (current or anamnestic) were collected for each patient as well.
Whenever possible, grey scale and power Doppler ultrasound examination was performed (General Electric ultrasound machine with a 18 MHz linear probe) and Ultrasound 7 Joints Score26 was obtained.
The study was conducted within the protocol 1483CESC approved by our local Ethics Committee, in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants included.
Statistical analysis
Data are presented as medians [inter-quartile-ranges (IQRs)] for non-normally distributed variables, mean ± standard deviation (SD) for normally distributed ones and percentages for proportions. Normality for all variables was tested by Shapiro–Wilk test. Differences in main clinical characteristics among groups were tested by Student’s t-test for normally distributed variables and the Mann–Whitney U test for independent samples for non-normally distributed variables. Differences in the proportion of the reported features (i.e. functional disability, CS, ongoing treatment with csDMARD, bDMARD or GCs) among groups were tested by the Fisher’s exact test. Correlations were calculated by Spearman’s rho. In order to estimate the independent contribution of the different items on the risk for being classified as having functional disability, binary logistic regressions were run from CSI score, disease duration, age, body mass index (BMI) and grade of disease activity. CSI was either considered as a continuous variable (model 1) or as a categorical variable divided into four categories (model 2). Grade of disease activity was considered as a categorical variable when RA and PsA groups were pooled together. In the disease specific sub-analyses SDAI and DAPSA were considered continuous variables. Two-sided p values of 0.05 or less were considered statistically significant. Data were analysed using SPSS software, Version 22 (SPSS, Inc., Chicago, IL, USA).
Results
A total of 150 patients (72 RA and 78 PsA) were enrolled in this study. The clinical characteristics of the overall sample and of the RA and PsA subgroups are reported in Table 1.
Table 1.
Clinical characteristics of the samples. Data reported as median (IQR) for non-normally distributed variables or percentages for proportions. Differences in the clinical features between RA and PsA groups were tested by Mann–Whitney U test for independent samples.
| Overall | Rheumatoid arthritis | Psoriatic arthritis | p value | |
|---|---|---|---|---|
| Gender, n (female) | 150 (98) | 72 (56) | 78 (42) | <0.01 |
| Age, years (IQR) | 57.5 (48.2–66.6) | 58.0 (50.8–68.8) | 56.1 (46.5–65.3) | NS |
| BMI, kg/m2 (IQR) | 25.4 (23.4–29.0) | 25.0 (22.9–29.2) | 26.1 (24.2–28.7) | NS |
| Disease duration, years (IQR) | 7 (3.7–11) | 7 (4–11) | 7 (3–10) | NS |
| ACPA positive, % | 28.7 | 64.2 | 0 | NA |
| RF positive, % | 26.7 | 61.5 | 0 | NA |
| Erosions, X-rays, % | 54.0 | 62.5 | 46.2 | 0.051 |
| History or current dactylitis/enthesitis, % | NA | NA | 67.9 | NA |
| Current psoriasis, % | NA | 0 | 19.6 | NA |
| CRP, mg/L (IQR) | 1.9 (1.0–5.0) | 1.2 (1.0–6.0) | 1.9 (1.0–4.0) | NS |
| pGA (IQR) | 4 (1–7) | 4 (2–7) | 4.5 (1–7) | NS |
| DAPSA (IQR) | NA | NA | 12.0 (3.8–21.2) | NA |
| SDAI (IQR) | NA | 10 (4.1–19.4) | NA | NA |
| Disease activity* | NS | |||
| Remission | 23.5% | 20.8% | 25.6% | |
| Low disease activity | 31.5% | 30.6% | 32.1% | |
| Moderate disease activity | 32.2% | 31.9% | 32.1% | |
| High disease activity | 12.8% | 15.3% | 10.3% | |
| CSI score ± SD | 35.6 ± 16.5 | 34.18 ± 15.1 | 37 ± 17.6 | NS |
| Central sensitization** | 35.3% | 29% | 42.9% | 0.08 |
| HAQ-DI (IQR) | 0.375 (0–0.125) | 0.250 (0–1.25) | 0.375 (0–1.25) | NS |
| Disability | 29.3% | 27.8% | 30.8% | NS |
| csDMARD | 76.3% | 90.3% | 63.8% | <0.01 |
| bDMARD | 64.5% | 56.7% | 71.6% | NS |
| Treatment with GCs | 24.7% | 44.9% | 6.4% | <0.01 |
| Median GC dose, mg (IQR) | 5.0 (2.5–6.0) | 5.0 (2.5–6.25) | 3.5 (1.5–6.8) | NS |
| 7US total score (IQR) | 4 (1–6) | 4 (2–7) | 3 (0–6) | NS |
| 7US grey scale score (IQR) | 2 (1–4) | 2 (1–4) | 2 (0–4) | NS |
| 7US PDUS score (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | NS |
| 7US erosion score (IQR) | 0 (0–1) | 1 (0–3) | 0 (0–1) | <0.01 |
7US, joints ultrasound score; ACPA, anti-citrullinated proteins antibodies; bDMARD, biologic disease modifying anti-rheumatic drug; BMI, body mass index; CRP, C-reactive protein; csDMARD, conventional synthetic disease modifying anti-rheumatic drug; CSI, central sensitization index; DAPSA, Disease Activity in Psoriatic Arthritis; GC, glucocorticoid prednisone equivalent; HAQ-DI, Health Assessment Questionnaire Disability Index; IQR, interquartile range; NA, not applicable; NS, not significant; PDUS, power Doppler ultrasound; pGA, patient global assessment; RF, rheumatoid factor; SDAI, Simple Disease Activity Index.
DAPSA and SDAI pooled at the respective thresholds.
Central sensitization defined as CSI >40.
Notably, the CSI values of the overall sample were normally distributed [mean 35.6, 95% confidence interval (CI) 33–38.2], as they were for the RA (mean 34.2 95% CI 30.7–37.7) and PsA (mean 37, 95% CI 33.3–40.7) samples.
When all patients were considered, a significant strong correlation was found between HAQ-DI and CSI scores (p < 0.01, rs = 0.600). Significant moderate correlations were also found between HAQ-DI and the grade of disease activity (p < 0.01, rs = 0.487) and between CSI score and the grade of disease activity (p < 0.01, rs = 0.525). A very weak correlation was found between BMI and HAQ-DI (p = 0.01, rs = 0.227). No difference was found in the prevalence of CS in steroid users and non-users (χ2 2.464, p NS).
In the univariate analysis, the strength of the correlation between HAQ-DI and CSI score was the highest in the PsA group (p < 0.01, rs = 0.706). In PsA patients, HAQ-DI and the grade of disease activity assessed by DAPSA (p < 0.01, rs = 0.633) and CSI score with disease activity assessed by DAPSA (p < 0.01, rs = 0.695) were strongly associated. A weak correlation was found between HAQ-DI and BMI (p = 0.01, 0.320). No difference was found in terms of the prevalence of CS in PsA patients using steroids and non-using steroids (data not shown). Similar findings, though with slightly weaker rs were observed with SDAI in the RA group (data not shown) except for BMI. No correlation between BMI and HAQ-DI was found in this cohort. The analysis on the correlation between HAQ-DI and CSI score ranks is reported in Figure 1 and in Figure 2, divided according to the different diagnosis. When the overall sample was considered, the model 1 analysis (CSI considered as a continuous variable) showed that CSI score was significantly associated with higher odds of being functionally disabled [adjusted odds ratio (aOR) 1.071, 95% CI 1.034–1.109, p < 0.0001]. We found that higher scores of CSI were associated with increased risk of functional disability in model 2. Patients with CSI score ⩾40 had an increased risk of being disabled (aOR 7.241, 95% CI 1.538–34.081 and aOR 17.108, 95% CI 3.711–78.876 for moderate sensibilization and severe sensibilization respectively). Both the models were statistically significant, p < 0.01. Model 1 explained 48% (Nagelkerke R2) of the variance in the presence of functional disability and correctly classified 83.9% of cases. Model 2 explained 52% (Nagelkerke R2) of the variance in the presence of functional disability and correctly classified 86.6% of cases. In RA patients, SDAI was independently associated with the presence of functional disability, while CSI was not. Indeed, when the RA sample was considered the models remained statistically significant, p < 0.01. The models explained 47% and 59% (Nagelkerke R2) of the variance in the presence of functional disability and correctly classified 84.4% and 89.1% of cases respectively. When the PsA sample was considered the models remained statistically significant, p < 0.01. The models explained 52% and 53% (Nagelkerke R2) of the variance in the presence of functional disability and correctly classified 85% and 85% of cases. The results of the analysis are reported in Table 2.
Figure 1.
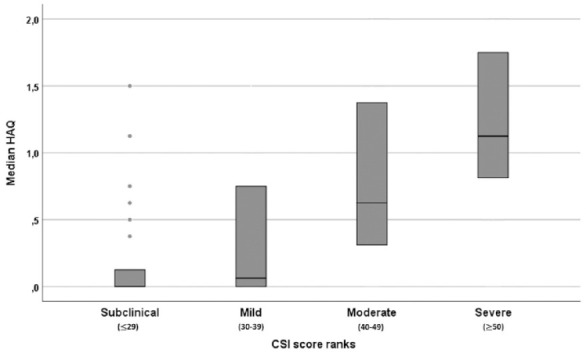
Distribution of the HAQ-DI score according to the different CSI score ranks of the overall cohort. Bars show median and interquartile range.
CSI, central sensitization inventory; HAQ-DI, Health Assessment Questionnaire Disability Index.
Figure 2.
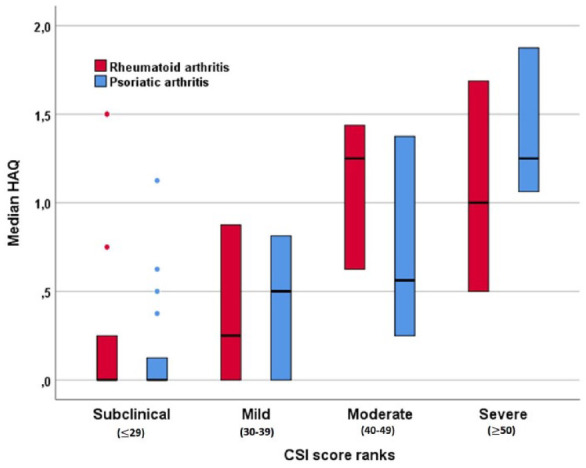
Distribution of the HAQ-DI score according to the different CSI score ranks for the rheumatoid arthritis and psoriatic arthritis subgroups. Bars show median and interquartile range.
CSI, central sensitization inventory; HAQ-DI, Health Assessment Questionnaire Disability Index.
Table 2.
Binary logistic regression for the risk for functional disability (defined as HAQ-DI >1).
| Variables | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Overall sample – Model 1 | |||
| CSI score | 1.079 | 1.039–0.119 | <0.01 |
| Disease duration | 1.020 | 0.956–1.088 | NS |
| Age | 1.060 | 1.012–1.111 | 0.01 |
| BMI | 1.006 | 0.896–1.129 | NS |
| Remission* | Ref. | ||
| Low disease activity | 2.950 | 0.437–19.936 | NS |
| Moderate disease activity | 10.879 | 1.796–65.905 | <0.01 |
| High disease activity | 37.039 | 4.876–281.370 | <0.01 |
| Overall sample – Model 2 | |||
| CSI score ⩽29 | Ref. | ||
| CSI score 30–39 | 1.370 | 0.213–8.822 | NS |
| CSI score 40–49 | 7.241 | 1.538–34.081 | 0.01 |
| CSI score ⩾50 | 17.108 | 3.711–78.876 | <0.01 |
| Disease duration | 1.018 | 0.954–1.086 | NS |
| Age | 1.055 | 1.006–1.108 | 0.02 |
| BMI | 1.001 | 0.887–1.128 | NS |
| Remission* | Ref. | ||
| Low disease activity | 2.673 | 0.384–18.602 | NS |
| Moderate disease activity | 10.796 | 1.784–66.664 | 0.01 |
| High disease activity | 40.965 | 5.135–326.783 | <0.01 |
| RA – Model 1 | |||
| CSI score | 1.049 | 0.989–1.114 | NS |
| Disease duration | 1.050 | 0.952–1.159 | NS |
| Age | 1.101 | 1.015–1.194 | 0.02 |
| BMI | 1.055 | 0.901–1.235 | NS |
| SDAI | 1.124 | 1.035–1.221 | <0.01 |
| RA – Model 2 | |||
| CSI score ⩽29 | Ref. | ||
| CSI score 30–39 | 2.554 | 0.181–36.002 | NS |
| CSI score 40–49 | 37.264 | 2.663–521.389 | <0.01 |
| CSI score ⩾50 | 5.272 | 0.344–80.827 | NS |
| Disease duration | 1.078 | 0.962–1.209 | NS |
| Age | 1.127 | 1.018–1.247 | 0.02 |
| BMI | 1.092 | 0.917–1.301 | NS |
| SDAI | 1.150 | 1.039–1.273 | <0.01 |
| PsA – Model 1 | |||
| CSI score | 1.102 | 1.037–1.171 | <0.01 |
| Disease duration | 1.010 | 0.867–1.176 | NS |
| Age | 1.020 | 0.958–1.085 | NS |
| BMI | 0.977 | 0.810–1.177 | NS |
| DAPSA | 1.058 | 0.982–1.141 | NS |
| PsA – Model 2 | |||
| CSI score ⩽29 | Ref. | ||
| CSI score 30–39 | 1.674 | 0.072–38.790 | NS |
| CSI score 40–49 | 4.078 | 0.326–51.071 | NS |
| CSI score ⩾50 | 31.173 | 2.352–413.133 | <0.01 |
| Disease duration | 0.998 | 0.857–1.164 | NS |
| Age | 1.023 | 0.962–1.089 | NS |
| BMI | 0.963 | 0.791–1.173 | NS |
| DAPSA | 1.067 | 0.977–1.165 | NS |
SDAI and DAPSA were pooled together according to the respective cut-offs.
BMI, body mass index; CI, confidence interval; CSI, central sensitization index; DAPSA, Disease Activity in Psoriatic Arthritis; HAQ-DI, Health Assessment Questionnaire Disability Index; NS, not significant; PsA, psoriatic arthritis; RA, rheumatoid arthritis; Ref., reference; SDAI, Simple Disease Activity Index.
The 7 joints ultrasound score (US7 score) US7 score was performed in 69 patients (46% of the overall sample). No significant correlation was found between the grey scale US7 score and the CSI score (data not shown).
Discussion
In our study we investigated the associations between clinical and ultrasonographic parameters and CS to pain in patients with inflammatory arthritides. CS was common, with approximately one-third of the patients having CSI score above 40. We found that CS was independently associated with functional disability in PsA patients, in whom DAPSA was not. On the contrary, in RA patients, SDAI was independently associated with the presence of functional disability, while CSI was not. We also found a significant correlation between disability and the grade of disease activity and between CSI score and the degree of disease activity.
To estimate the degree the severity of CS we chose the CSI questionnaire, a validated tool that explores several aspects of pain.12 The CSI proved to be reliable in identifying patients with CS syndrome with good sensitivity and specificity and with good test–retest reliability.10,13,27 We selected the CSI among other questionnaires that had been used to assess CS. The painDETECT questionnaire, for example, had been administered in RA and PsA patients, obtaining acceptable intraclass correlation coefficients and classification consistency.28 Very recently, a study on PsA patients observed characteristics of neuropathic pain in 25.4% of the sample, with a significant association with functional disability also after exclusion of subjects with comorbid FM.29 However, the painDETECT questionnaire is an instrument originally developed for neuropathic pain and some controversy regarding its appropriateness in central pain sensitization still exits.30
The prevalence of CS in our cohort was roughly 30%. Our findings are in line with previous studies that explored CSI using the CSI questionnaire. In RA patients, the prevalence of CS ranged from 20% in the study by Chiarotto et al. to 41%, as reported by Guler and colleagues.11,31 Data on the prevalence of CS in PsA patients are lacking and, to our knowledge, this is the first study assessing CS using the CSI questionnaire in such patients.
The high prevalence of CS in inflammatory arthritides is coherent with the pathophysiology of these conditions. Pro-inflammatory cytokines and vasoactive peptides produced by immune cells act directly on nociceptive neurons of the dorsal horn of the spinal cord and they contribute to peripheral sensitization and CS.32–34 Studies on animal models had shown that several cytokines are involved in the pathogenesis of CS to pain. Tumour necrosis factor-alpha, interleukin-1beta, interleukin-6 and interleukin-17 receptors have been identified on nociceptive and sensory neurons of mice and their activation led to an increased C-fibre action potentials frequency.32–35 Also, this pathophysiologic process might well explain the correlation between CSI score and disease activity that we found. However, a simpler explanation could be true as well; disease activity scores might be influenced by CS and not the opposite. SDAI and DAPSA are calculated upon the collection of both physician- and patient-reported components, including, for example, patient global assessment of disease activity (pGA). In this scenario, the pGA, persistently high as a result of the CS syndrome, may drive the elevation of SDAI score, eventually resulting in the untruthful association between this score and the CSI score. Nevertheless, the CSI score, in both the unadjusted and adjusted analyses, was significantly associated with HAQ-DI, a measure of the physical burden of the disease. Moreover, an exposure–response relationship between CSI ranks and HAQ-DI was observed among our study population. Indeed, we found higher odds of functional disability in patients with moderate CS (CSI 40–49, aOR 7.241) or severe CS (CSI ⩾50, aOR 17.108) when compared with patients without CS (CSI <40). Therefore, we can speculate that the association between CSI score and the grade of disease activity was primarily mediated by the persistent inflammation and was not the consequence of flawed patietnt global assessment (pGA) and/or visual analogue scale (VAS) pain. In our opinion, these findings support the hypothesis of a mechanistic association between CS and functional disability.
Steroid use was more common in the RA group. Interestingly, the mean CSI score was higher, even though non-significant, in the PsA group as compared with the RA group and this difference might be partially attributable to steroid use. However, manifestations seen only in PsA patients which usually do not require steroid treatment (e.g. enthesitis) might have accounted for the different prevalence of CS that we found in the two populations as well, leading to a false apparent negative association between steroid use and CSI. In addition, we found no difference in the prevalence of CS in steroid users and non-users.
Notably, in PsA patients, disease activity assessed with DAPSA was not independently associated with the presence of functional disability. There are a few explanations for this seemingly unexpected result. First, PsA is a heterogeneous disease, with variable involvement of several disease “domains”.36 We excluded patients with axial involvement but our cohort, as expected, still presented a considerable proportion of subjects with enthesitis/dactylitis. These manifestations of the disease might have interfered with measures of functional disability though while “flying under the DAPSA radar”. Second, DAPSA has been demonstrated to be not wholly representative of the functional status in patients with mono-oligo articular involvement.36 Third, the DAPSA algorithm includes both pGA and pain VAS, while SDAI includes pGA and Evaluator Global Assessment. This difference might account for a somewhat increased “vulnerability” of DAPSA by the influence of CS on PROs. This hypothesis is also in line with the higher strength of the correlation between DAPSA and CSI scores in the PsA cohort. In this scenario, the DAPSA, which in many patients could depend on subjective parameters such as pGA and pain VAS, might have been influenced by concomitant CS, which prominent effect might have overcome the outcome of the DAPSA on the risk of functional disability in the logistic regression analysis.
Interestingly, the presence of enthesitis has been associated with worse quality of life, greater work impairment and concurrent fibromyalgia.37,38 Our data show that the DAPSA score might be significantly influenced by CS and, therefore, this domain should not be overlooked when evaluating disease activity. Moreover, we excluded patients who received a diagnosis of fibromyalgia and still the association was evident, thus further highlighting the DAPSA limitations in patients with CS.
Our study has strengths and limitations. We enrolled a reasonably large and homogeneous sample of RA and PsA patients. However, our results are not easily generalizable to all patients affected by these conditions. Indeed, cultural and social factors might play a crucial role in the development of CS and these data need to be replicated in different regions of the world and need to involve other ethnic groups as the strength of these relationships may vary accordingly. Since we excluded patients with a diagnosis of fibromyalgia and/or major depressive disorder, our findings cannot be generalized to the overall arthritic population. Indeed, the prevalence of CS could have been possibly underestimated when compared with the general RA and PsA population. Nevertheless, albeit excluding patients with a diagnosis of fibromyalgia and/or major depressive disorder could have decreased the generalizability of our study, it might have improved the specificity of our results. Moreover, pooling the disease activity thresholds for DAPSA and SDAI has not been validated, possibly limiting the validity of the overall sample analysis. In addition, sub-group analyses might have been limited by the smaller sample size. In addition, given the retrospective and exploratory nature of the study, we cannot infer on the nature of the association between disease activity and CS.
In conclusion, we used the CSI questionnaire to investigate the relationship between CS, disease activity and functional disability in a cohort of patients affected either by RA or by PsA. For the first time, we demonstrated a striking correlation between these descriptors of different aspects of the disease burden. In particular, we found a remarkable contribution of CS on measures of functional disability. Interestingly, while in the RA subgroup the assessment of disease activity (SDAI) and not the CSI score was found to be a significant determinant of functional disability, in the PsA subgroup the CSI score played a major role, while the DAPSA contribution was non-significant. In our opinion, the interference of CS on the quality of life of patients with inflammatory arthritis needs to be investigated further. Particular attention should be paid in PsA, in which subjects with concomitant CS are less likely or even unable to achieve goals such as minimal disease activity.4
To date, there is no evidence on the efficacy of any csDMARD, bDMARD, or thosa DMARD (tsDMARD) on CS outcomes. For this reason, especially in PsA patients, given a high risk of interference of CS on DAPSA, adjusting for CSI severity when assessing disease activity might represent an effective strategy to avoid unnecessary treatment escalations or repeated drugs switching.
Footnotes
Availability of data and materials: The dataset used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflict of interest statement: Angelo Fassio reports personal fees from Abiogen, Novartis, Neopharmed, outside the submitted work.
Luca Idolazzi reports personal fees from Eli-Lilly, Merck Sharp & Dohme, Novartis, Sanofi, Celgene, UCB outside the submitted work.
Maurizio Rossini reports personal fees from AbbVie, Abiogen, Eli-Lilly, Merck Sharp & Dohme, Novartis, Sanofi, UCB, outside the submitted work.
Davide Gatti has received advisory board honoraria, consultancy fees and/or speaker fees from Abiogen, Celgene, Eli-Lilly, Neopharmed-Gentili, Pfizer, UCB.
All other authors do not have anything to disclose.
Ethics approval and consent to participate: The study was conducted within the protocol 1483CESC approved by our local Ethics Committee, in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants included.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Giovanni Adami  https://orcid.org/0000-0002-8915-0755
https://orcid.org/0000-0002-8915-0755
Angelo Fassio  https://orcid.org/0000-0001-9187-232X
https://orcid.org/0000-0001-9187-232X
Contributor Information
Giovanni Adami, Rheumatology Unit, University of Verona, Verona, Italy.
Elisabetta Gerratana, Rheumatology Unit, University of Messina, Messina, Italy.
Fabiola Atzeni, Rheumatology Unit, University of Messina, Messina, Italy.
Camilla Benini, Rheumatology Unit, University of Verona, Verona, Italy.
Elisabetta Vantaggiato, Rheumatology Unit, University of Verona, Verona, Italy.
Denise Rotta, Rheumatology Unit, University of Verona, Verona, Italy.
Luca Idolazzi, Rheumatology Unit, University of Verona, Verona, Italy.
Maurizio Rossini, Rheumatology Unit, University of Verona, Verona, Italy.
Davide Gatti, Rheumatology Unit, University of Verona, Verona, Italy.
Angelo Fassio, Rheumatology Unit, University of Verona, Policlinico GB Rossi, Piazzale A. Scuro, 37134 Verona, Italy.
References
- 1. Bas DB, Su J, Wigerblad G, et al. Pain in rheumatoid arthritis: models and mechanisms. Pain Manag 2016; 6: 265–284. [DOI] [PubMed] [Google Scholar]
- 2. Sarzi-Puttini P, Salaffi F, Di Franco M, et al. Pain in rheumatoid arthritis: a critical review. Reumatismo 2014; 66: 18–27. [DOI] [PubMed] [Google Scholar]
- 3. Bidad K, Gracey E, Hemington KS, et al. Pain in ankylosing spondylitis: a neuro-immune collaboration. Nat Rev Rheumatol 2017; 13: 410–420. [DOI] [PubMed] [Google Scholar]
- 4. Winthrop KL, Weinblatt ME, Bathon J, et al. Unmet need in rheumatology: reports from the targeted therapies meeting 2019. Ann Rheum Dis 2020; 79: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris VH, Cruwys SC, Kidd BL. Characterisation of capsaicin-induced mechanical hyperalgesia as a marker for altered nociceptive processing in patients with rheumatoid arthritis. Pain 1997; 71: 179–186. [DOI] [PubMed] [Google Scholar]
- 6. Bailly F, Cantagrel A, Bertin P, et al. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open 2020; 6: e001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raja SN, Carr DB, Cohen M, et al. The revised International association for the study of pain definition of pain: concepts, challenges, and compromises. Pain 2020; 161: 1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hazra S, Venkataraman S, Handa G, et al. A cross-sectional study on central sensitization and autonomic changes in fibromyalgia. Front Neurosci 2020; 14: 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract 2012; 12: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiarotto A, Viti C, Sulli A, et al. Cross-cultural adaptation and validity of the Italian version of the central sensitization inventory. Musculoskelet Sci Pract 2018; 37: 20–28. [DOI] [PubMed] [Google Scholar]
- 12. Neblett R, Cohen H, Choi Y, et al. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 2013; 14: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuesta-Vargas AI, Neblett R, Chiarotto A, et al. Dimensionality and reliability of the central sensitization inventory in a pooled multicountry sample. J Pain 2018; 19: 317–329. [DOI] [PubMed] [Google Scholar]
- 14. Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol 2017; 29: 304–310. [DOI] [PubMed] [Google Scholar]
- 15. Højgaard P, Ellegaard K, Nielsen SM, et al. Pain mechanisms and ultrasonic inflammatory activity as prognostic factors in patients with psoriatic arthritis: a prospective cohort study. Arthritis Care Res (Hoboken) 2019; 71: 798–810. [DOI] [PubMed] [Google Scholar]
- 16. Duffield SJ, Miller N, Zhao S, et al. Concomitant fibromyalgia complicating chronic inflammatory arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2018; 57: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muratore F, Salvarani C, Macchioni P. Contribution of the new 2012 EULAR/ACR classification criteria for the diagnosis of polymyalgia rheumatica. Reumatismo 2018; 70: 18–22. https [DOI] [PubMed] [Google Scholar]
- 18. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 19. Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003; 42: 244–257. [DOI] [PubMed] [Google Scholar]
- 20. Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis 2008; 67: 1360–1364. [DOI] [PubMed] [Google Scholar]
- 21. Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. nn Rheum Dis 2010; 69: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 22. Schoels MM, Aletaha D, Alasti F, et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. Epub ahead of print 12 August 2015. DOI: 10.1136/annrheumdis-2015-207507. [DOI] [PubMed] [Google Scholar]
- 23. Krishnan E, Tugwell P, Fries JF. Percentile benchmarks in patients with rheumatoid arthritis: health assessment questionnaire as a Quality Indicator (QI). Arthritis Res Ther 2004; 6: R505–R513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neblett R, Hartzell MM, Mayer TG, et al. Establishing clinically relevant severity levels for the central sensitization inventory. Pain Pract 2017; 17: 166–175. [DOI] [PubMed] [Google Scholar]
- 25. Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis International Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009; 68(Suppl. 2): ii1–ii44. [DOI] [PubMed] [Google Scholar]
- 26. Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 2009; 61: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 27. Neblett R, Hartzell MM, Cohen H, et al. Ability of the central sensitization inventory to identify central sensitivity syndromes in an outpatient chronic pain sample. Clin J Pain 2015; 31: 323–332. [DOI] [PubMed] [Google Scholar]
- 28. Rifbjerg-Madsen S, Wæhrens EE, Danneskiold-Samsøe B, et al. Psychometric properties of the painDETECT questionnaire in rheumatoid arthritis, psoriatic arthritis and spondyloarthritis: Rasch analysis and test–retest reliability. Health Qual Life Outcomes 2017; 15: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Carlo M, Muto P, Benfaremo D, et al. The neuropathic pain features in psoriatic arthritis: a cross-sectional evaluation of prevalence and associated factors. J Rheumatol 2020; 47: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 30. Zhang A, Lee YC. Mechanisms for joint pain in Rheumatoid Arthritis (RA): from cytokines to central sensitization. Curr Osteoporos Rep 2018; 16: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guler MA, Celik OF, Ayhan FF. The important role of central sensitization in chronic musculoskeletal pain seen in different rheumatic diseases. Clin Rheumatol 2020; 39: 269–274. [DOI] [PubMed] [Google Scholar]
- 32. Brenn D, Richter F, Schaible H-G. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum 2007; 56: 351–359. [DOI] [PubMed] [Google Scholar]
- 33. Copray JC, Mantingh I, Brouwer N, et al. Expression of interleukin-1 beta in rat dorsal root ganglia. J Neuroimmunol 2001; 118: 203–211. [DOI] [PubMed] [Google Scholar]
- 34. Schaible H-G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther 2014; 16: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 2017; 38: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tucker LJ, Coates LC, Helliwell PS. Assessing disease activity in psoriatic arthritis: a literature review. Rheumatol Ther 2019; 6: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sunar I, Ataman S, Nas K, et al. Enthesitis and its relationship with disease activity, functional status, and quality of life in psoriatic arthritis: a multi-center study. Rheumatol Int 2020; 40: 283–294. [DOI] [PubMed] [Google Scholar]
- 38. Mease PJ, Liu M, Rebello S, et al. Characterization of patients with axial spondyloarthritis by enthesitis presence: data from the corrona psoriatic arthritis/spondyloarthritis registry. ACR Open Rheumatol 2020; 2: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]


