Abstract
Background
Acute kidney injury is associated with high mortality, and the optimal time to start renal replacement therapy for acute kidney injury is unknown despite several randomised controlled trials on the subject. We performed a systematic review and meta-analysis to assess the effect of earlier initiation of renal replacement therapy for acute kidney injury on mortality and reported secondary outcomes.
Methods
All literature in databases EMBASE, MEDLINE and CENTRAL was searched from January 1970 to March 2019 using terms related to renal replacement therapy, timing and randomised controlled trials. All randomised controlled trials with 25 or more adult participants suffering from acute kidney injury comparing timing of renal replacement therapy were included. The results of the selected studies were pooled and expressed in terms of risk ratios (RR) and 95% confidence intervals (95% CI) using a random effects model.
Results
A total of 7008 records were identified; 94 were selected for full text review of which 10 were included in the final meta-analysis. The 10 studies comprised 1956 participants (989 ‘early’ group; 967 ‘late’ group) with 918 total deaths; the analysis demonstrated no significant differences between the ‘early’ and ‘late’ renal replacement therapy groups (RR = 0.98 (95% CI = 0.84, 1.15)) for mortality. No significant differences between groups were evident for period-wise mortality; dialysis dependence; recovery of renal function; length of intensive care unit or hospital stay; or number of renal replacement therapies, mechanical ventilation and vasopressor-free days.
Conclusions
Current evidence does not support the use of early renal replacement therapy for patients with acute kidney injury. Data from ongoing and future randomised controlled trials are required to strengthen the evidence base in the area.
Keywords: Acute kidney injury, renal replacement therapy, timing, meta-analysis
Introduction
Acute kidney injury (AKI) is common within the critically ill and hospitalised patients. AKI, an evolution of the term acute renal failure (ARF), has been subjected to several classifications,1–3 making the reported incidence of AKI in patients admitted to intensive care units (ICUs) vary significantly (35%–67%).4–7 Owing to the high incidence within the critically ill, an increase in the severity of AKI is associated with increasing all-cause mortality of up to 57%.6–8
Renal replacement therapy (RRT) is a key strategy in the treatment of severe AKI with life-threatening complications, such as refractory hyperkalaemia, metabolic acidosis and volume overload unresponsive to medical therapy. While RRT is accepted as an impactful treatment, its implementation remains a matter of debate. Studies have compared differences between modalities of RRT, such as intermittent haemodialysis versus continuous RRT,9,10 haemofiltration versus haemodialysis11 or dose.12
Further, the timing to initiate RRT for AKI remains a challenge. Many randomised controlled trials (RCTs) have been executed to determine whether ‘early’ compared to ‘delayed’ initiation is of benefit; two studies13,14 reported evidence on the subject in 2016, followed by several meta-analyses.15–17 However, a disparity between conclusions persists, with reports that no difference is evident between groups,16,17 while others conclude that earlier initiation of RRT conveys a decrease in mortality.18,19 Three subsequent RCTs published in 2018 added further data.20–22 A Cochrane Review was also published but excluded studies of patients not admitted to ICU.23
Our objective was to conduct a systematic review and meta-analysis on all patients suffering from AKI who required RRT. Analysis would be carried out on studies comparing timing of the initiation of RRT in two groups of patients: the first group classified as ‘early’ and the second group classified as ‘late’, ‘delayed’ or ‘standard treatment’. The studies must report on all-cause mortality to be included in the analysis. We specifically add the three RCTs published in 2018 to update previous meta-analyses and assess what these new data contribute to this area of study.
Methods
Registration
The review is registered with PROSPERO’s Register of Systematic Reviews, ID Number: CRD42019145074.
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019145074
Eligibility criteria
The inclusion criteria followed agreed RCT guidelines on reporting differences in the timing of initiating RRT (early vs. late, standard vs. early, early vs. delayed). Non-RCTs, the paediatric population and patient population without AKI, were excluded. No guidelines as to defining RRT timing exist; therefore, the definition of ‘early’ and ‘late’ is according to the individual studies’ interpretation unless the definition of ‘late’ was outwith that considered a ‘standard’ RRT initiation which has resulted in two ‘early’ group classifications. Studies that defined the ‘late’ group as initiation within 12 h of diagnosis with any stage AKI were also excluded.
Search strategy
Three databases (EMBASE, MEDLINE and Cochrane Central Register of Controlled Trials (CENTRAL)) were interrogated for the period January 1974 to March 2019. The search strategy was as broad as possible to capture all RCTs conducted on the subject; the only filter applied was to restrict results to English language. MEDLINE and CENTRAL searches used MeSH terms (supplementary Figure s1.1). A near identical search interrogated EMBASE, but with certain terms altered to match Emtree headings (Figure s1.2). In order to identify ongoing or not published completed trials, the International Trials Registry (https://www.who.int/ictrp/en/) and the National Institutes of Health’s registry (https://www.clinicaltrials.gov) were searched.
Study selection
Two authors (MA, RS) independently compiled a list of citations gathered from the three sources. Obvious duplicate citations were removed by databases when merging; however, if any two citations had discrepancies, they were both retained for title review. Both authors reviewed the titles independently and selected eligible studies for abstract review; a thorough abstract review was then conducted to select studies eligible for full text review. A concluding, full text review was then executed and any differences between the two reviewers were referred to a third reviewer (KP) to make a final decision on eligibility.
Data extraction
The papers were each initially assessed for time-period mortality reported on and then the data were recorded independently using a pre-defined form. Two independent reviewers (MA, RS) extracted key data including the number of patients recruited, definition of ‘early’ and ‘late’ RRT groups and measured outcomes. After consolidation, data on the number of events and the total for both ‘early’ and ‘late’ groups were collected and outcomes in terms of mean, median, mode and interquartile ranges were extracted as reported.
Outcome measures
The following outcomes were extracted:
Primary outcomes
Overall mortality rate, in-ICU, in-hospital, 28-, 60- and 90-day mortality rates.
Secondary outcomes
Dialysis dependence at 28, 60 and 90 days, recovery of renal function (return to baseline) at 90 days, adverse events, length of ICU stay, length of hospital stay, number of RRT days, number of RRT-free days, number of mechanical ventilation-free days and number of vasopressor-free days.
Risk of bias
Each study was assessed independently by two authors (MA, RS) for potential risk of bias using the seven domains cited in the Cochrane Collaboration’s tool24; a funnel plot categorised the risk of publication bias across the studies. The quality of evidence for the primary outcomes was assessed independently using the GRADE tool.25
Data synthesis
The results were expressed in terms of risk ratio (RR) and 95% confidence intervals (95% CI) for mortality and secondary outcomes. Heterogeneity between studies was determined through the I2 statistic; a value of >40% was interpreted as a significant degree of heterogeneity. RR for each outcome was estimated using both fixed and random effects to identify high degrees of heterogeneity between studies. Statistical comparison was captured as a P-value for each analysis; a value of <0.05 was considered statistically significant. Any outcome reported in terms of continuous data was expressed in terms of pooled raw differences between the two groups medians (a negative difference favouring early RRT) and 95% CIs. This has been previously described as comparing favourably to methods which transform medians and IQRs to mean and standard deviation.26 All data were analysed using the software R (R version 3.5.1, The R Foundation).
The following pre-defined sub-groups were analysed for overall mortality to assess possible sources of heterogeneity including risk of bias, RRT modality, severity of illness and patient population.
Low risk versus high or unclear risk of bias
Intermittent haemodialysis versus continuous RRT versus mixed
ICU-only population versus mixed population
Medical versus surgical versus mixed patients
Results
Selected studies
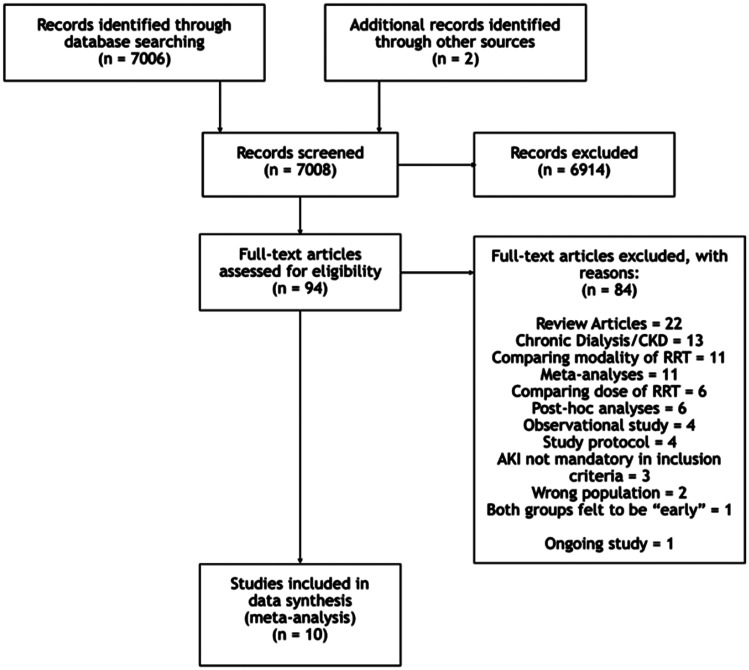
The literature search returned a total 7008 references after duplicate removal (Figure 1). The features of the 10 studies selected for inclusion in the review are detailed in Table 1; studies varied in size from 28 patients29 to 488 patients.22 Of the 10, 8 included ICU patients only.
Figure 1.
PRISMA flow diagram.
AKI: acute kidney injury; CKD: chronic kidney disease; RRT: renal replacement therapy.
Table 1.
Study characteristics.
| Study | Setting/patient group | Inclusion criteria | Numbers | RRT modality | Early definition for RRT | Late definition for RRT | Outcomes |
|---|---|---|---|---|---|---|---|
| Pursnani et al.27 | Single centre; all inpatients; medical only | Diagnosis of acute tubular necrosis with serum creatinine <7 mg% and blood urea <120 mg% | Total = 35 Early = 18 Late = 17 | IHD | Early hemodialysis (as soon as met eligibility criteria) | Conservative management | Overall mortality Length of hospital stay Adverse events |
| Bouman et al.28,a | Two centres, single country; ICU only; mixed patients | Urine output <30 ml/h for >6 h and creatinine clearance <20 ml/min | Total = 106 Early = 70 Late = 36 | CRRT | RRT started within 12 h of inclusion | Plasma Urea level >40 mmol/l, potassium >6.5 mmol/l or severe pulmonary oedema | ICU, hospital and 28-day mortality Recovery of renal function at 90 days Duration of ICU and hospital stay Adverse events |
| Sugahara and Suzuki29 | Single centre; ICU only; surgical only | Post-CABG patients. Hourly urine output <30 ml/h and serum creatinine increased at rate of 0.5 mg/dl/day or more | Total = 28 Early = 14 Late = 14 | CRRT | Urine output <30 ml/h for three consecutive hours (or daily urinary output 750 ml or less) | Urine output <20 ml/h for two consecutive hours (or daily urinary output 500 ml or less) | 14-day mortality Changes in BP, urine output and creatinine |
| Jamale et al.30 | Single centre; all inpatients; medical only | Severe AKI with increasing serum urea and creatinine levels | Total = 208 Early = 102 Late = 106 | IHD | Serum urea >70 mg/dl and/or creatinine level >7 mg/dl | Treatment refractory hyperkalaemia, volume overload, acidosis. Uremic nausea and anorexia with inability to maintain oral intake | In hospital mortality Dialysis dependence at 90 days Number of RRT days Adverse events |
| Wald et al.31 | Multiple centres, single country; ICU only; mixed patients | Volume repletes severe AKI with two criteria from three: creatinine doubled from baseline, urine output <6 ml/kg in last 12 h or whole blood NGAL >400 ng/ml. Absence of urgent indications for RRT. | Total = 100 Early = 48 Late = 52 | Mixed | RRT started within 12 h of fulfilling eligibility criteria | Potassium >6.0 mmol/l, serum bicarbonate <10 mmol/l, PaO2/FiO2 <200 with infiltrates on chest radiograph suggestive of pulmonary oedema | ICU, hospital and 90-day mortality Dialysis dependence at 90 days Length of ICU and hospital stay Adverse events |
| Gaudry et al.13 | Multiple centres, single country; ICU only; mixed patients | KDIGO3 stage 3 AKI compatible with a diagnosis of ischaemic or toxic acute tubular necrosis and receiving mechanical ventilation and/or catecholamine infusion. | Total = 619 Early = 311 Late = 308 | Mixed | RRT commenced within 6 h after documentation of KDIGO3 stage 3 AKI | Urea >40 mmol/l, potassium >6 mmol/l (or >5.5 mmol/l despite medical treatment), pH <7.15, pulmonary oedema due to fluid overload requiring oxygen >5 l/ or FiO2 >50%, oliguria or anuria >72 h | 28- and 60-day mortality Dialysis dependence at 28 and 60 days Length of ICU and hospital stay Number of RRT, mechanical ventilation and vasopressor free days |
| Zarbock et al.14 | Single centre; ICU only; mixed patients | KDIGO stage 2 AKI (baseline creatinine doubled or urinary output <0.5 ml/kg/h for >12 h) despite optimal resuscitation, NGAL >150 ng/ml and one of: severe sepsis, use of vasopressors, refractory fluid overload and progression of non-renal organ dysfunction | Total = 231 Early = 112 Late = 119 | Mixed | RRT started within 8 h of diagnosis of KDIGO stage 2 AKI | Commenced within 12 h of diagnosis of stage 3 AKI, or if urea >100 mg/dL, potassium >6.0 mmol/l and or ECG changes, urine output <200 ml in 12 h or organ oedema resistant to diuretic treatment | 28-, 60- and 90-day mortality Dialysis dependence at 28, 60 and 90 days Length of ICU and hospital stay Length of mechanical ventilation and RRT Adverse events |
| Srisawat et al.20,b | Single centre; ICU only; mixed patients | Patients aged 18 or older diagnosed with AKI by RIFLE criteria1 | Total = 40 Early = 20 Late = 20 | CRRT | RRT started within 12 h of randomization | Severe refractory acidosis (pH < 7.2 or HCO3 <15), severe peripheral oedema, pulmonary oedema, no response to diuretics, refractory hyperkalaemia (K > 6.2 or ECG changes), anuria or oliguria or high BUN (>60) | 28-day mortality Dialysis dependence at 28 days Mechanical ventilation free days ICU-free days Renal recovery at 28 days Balance of input and output fluid |
| Lumlertgul et al.21,c | Multiple centres, single country; ICU only; mixed patients | AKI with diagnosis of Acute Tubular Necrosis, clinically resuscitated and euvolaemic, no urgent indication or contraindications for RRT. | Total = 118 Early = 58 Late = 60 | CRRT | RRT was started in the early group within 6 h of randomisation | Urea >100 mg/dl, potassium >6 mmol/l, serum bicarbonate <12 mmol/l, pH <7.15, PaO2/FiO2 ratio <200 or chest radiographs compatible with pulmonary oedema | 28-day mortality Dialysis dependence and renal recovery at 28 days Length of ICU and hospital stay Number of RRT and mechanical ventilation-free days |
| Barbar et al.22 | Multiple centres, single country; ICU only; mixed patients | Early phase of septic shock (within 48 h of start of vasopressor therapy) developing AKI with at least one criterion of the failure stage of the RIFLE classification system1 | Total = 488 Early = 246 Late = 242 | Mixed | RRT commenced within 12 h of documentation of ‘failure’ stage AKI1 | RRT commenced 48 h after diagnosis of AKI or if prior to this: serum potassium >6.5 mmol/l, pH <7.15 or fluid overload with pulmonary oedema | 28, 90 and 180 day mortality Dialysis dependence at 28 and 90 days Length of ICU and hospital stay RRT, mechanical ventilation and vasopressor-free days Adverse events |
AKI: acute kidney injury; BUN: blood urea nitrogen; CABG: coronary artery bypass graft; CRRT: continuous renal replacement therapy; ECG: electrocardiogram; ICU: intensive care unit; IHD: ischaemic heart disease; KDIGO: kidney disease improving global outcomes; NGAL: neutrophil gelatinase-associated lipocalin; RIFLE: isk, injury, failure, loss, end-stage kidney disease; RRT: renal replacement therapy.
Patients in early group split into low-volume (n = 35) and high-volume (n = 35) hemofiltration.
Patients were tested for plasma neutrophil gelatinase associated lipocalin (pNGAL) levels after recruitment. Patients with pNGAL level greater than or equal to 400ng/ml were randomized into early or late groups.
Patients underwent a furosemide stress test first. If they were non-responsive they were randomised into early or late groups.
Overall mortality
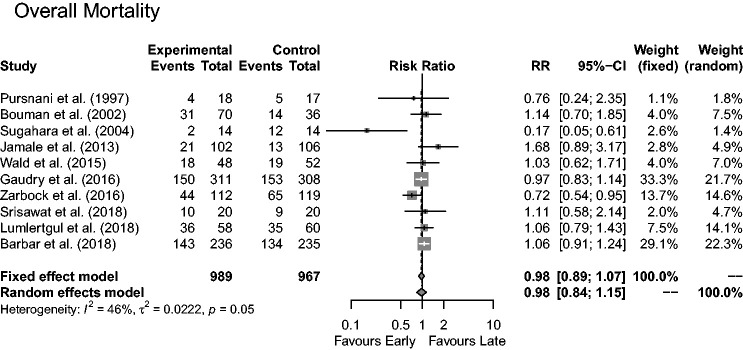
The 10 studies comprising 1956 patients reported on overall all-cause mortality at varying times: 989 into the ‘early’ and 967 into the ‘late’ groups. A total of 918 deaths were reported; 459 in the ‘early’ and 459 in the ‘late’ group, corresponding to a mortality rate of 46.4% for patients receiving early and 47.5% for those receiving conventional/late RRT.
Figure 2 illustrates results from the 10 studies depicting no significant difference between ‘early’ or ‘late’ initiation of RRT for mortality rates: RR = 0.98 (95% CI = 0.84,1.15 (random effects modelling)). A marked heterogeneity between studies was evident with an I2 of 46% (P = 0.05). Pre-defined subgroup analyses were carried out to further explore the possible cause.
Figure 2.
Forest plot of the effect of early versus late RRT on overall mortality.
Impact on mortality after accounting for risk of bias
Two studies were assessed to have either a high or unclear risk of bias (supplementary Table s1.1), and their pooled results suggested a mortality benefit for ‘early’ RRT (Figure s1.3); RR = 0.37 (95% CI = 0.08,1.65). The remaining eight studies were assessed as low risk of bias, with pooled results showing no statistically significant difference between groups; RR = 1.00 (95% CI = 0.89,1.13). The heterogeneity in the low risk of bias group decreased to I2 of 23% (from the overall analysis value of 46%).
Impact on mortality after accounting for RRT modality
The RRT modality used to deliver the intervention and its impact on mortality is presented in supplementary Figure s1.4; two studies used intermittent haemodialysis with no significant difference between the ‘early’ and ‘late’ arms: RR = 1.30 (95% CI = 0.63,2.70); four used only continuous RRT with no significant difference between groups: RR = 0.91 (95% CI = 0.57,1.46) and the remaining four studies utilised a mixture of these two modalities and also found no significant difference between groups: RR = 0.95 (95% CI = 0.81,1.11).
Impact on mortality after consideration of critical illness
Two studies included all inpatients (supplementary Figure s1.5). The difference between the ‘early’ and ‘late’ groups was not statistically significant; RR = 1.30 (95% CI = 0.63,2.70). The remaining eight studies included ICU patients only with no observable difference between the two groups: RR = 0.95 (95% CI = 0.80,1.12).
Impact on mortality by admission type: Medical versus surgical versus mixed population
Two studies only involved patients from a medical cohort (supplementary Figure s1.6); no differences in mortality between the two RRT groups were observed: RR = 1.30 (95% CI = 0.63,2.70). Only one study used participants from the surgical cohort, the result indicating a mortality benefit in the early RRT group: RR = 0.17 (95% CI = 0.05,0.61). The remaining seven studies contained a mixed population of patients and no statistical difference existed between groups: RR = 0.98 (95% CI = 0.88,1.10).
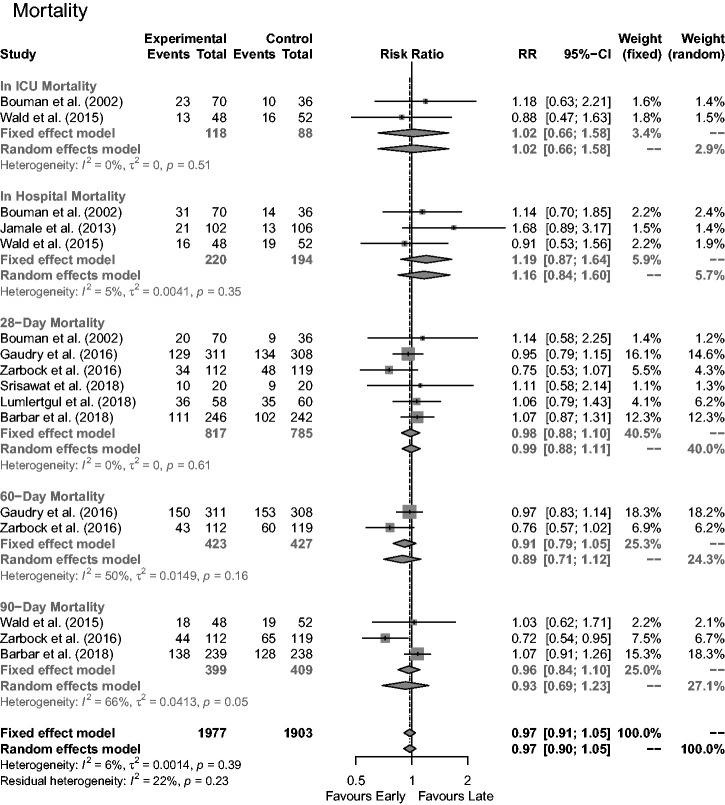
Time-based mortality
All studies reported mortality numbers over differing time periods, sub-categorised into in-ICU, in-hospital, 28, 60 and 90 days (Figure 3). ICU mortality was reported by two studies; no statistical difference was evident between the two RRT treatment groups: RR = 1.02 (95% CI = 0.66,1.58). In-hospital mortality was reported by three studies with no significant difference between groups: RR = 1.16 (95% CI = 0.84,1.60). Six reported 28-day mortality with no significant difference found: RR = 0.99 (95% CI = 0.88,1.11). Two reported 60-day mortality with no significant difference observed: RR = 0.89, 95% CI = 0.71,1.12). Three reported 90-day mortality with no statically significant difference between early and late groups: RR = 0.93 (95% CI = 0.69,1.23).
Figure 3.
Impact of early versus late RRT on mortality rates at various time periods.
ICU: intensive care unit.
Secondary outcomes
Dialysis dependence was reported at 28, 60 and 90 days. Four studies reported on rates of dialysis dependence in surviving patients after 90 days (supplementary Figure s1.7). The pooled data demonstrated no significant differences between ‘early’ and ‘late’ groups: 16/279 versus 18/289 patients (RR = 0.87). Four studies reported on rates of dialysis dependence after 28 days with no statistically significant difference (supplementary Figure s1.8): 65/423 versus 76/425 patients (RR = 0.84). Dialysis dependence at day 60 was reported by two studies with a benefit suggested in the early RRT group (supplementary Figure s1.5): 14/226 versus 22/214 patients (RR = 0.59).
No statistically significant differences between the ‘early’ and ‘late’ groups was observable for all adverse events except catheter-related complications; the results of these can be found in Table 2 with forest plots presented in supplementary Figures s1.9–s1.17. Analysis of the six studies reporting catheter-related complications (supplementary Figure s1.18) suggested an increase in complications within the ‘early’ group: RR = 1.85 (95% CI = 1.18,2.88).
Table 2.
Summary of secondary outcomes related to adverse events and recovery of renal function at 90 days.
| Outcome | Number of participants (studies) | Risk ratio | 95% Confidence intervals |
|---|---|---|---|
| Recovery of renal function to baseline at 90 days | 181 (2 studies) | 1.00 | 0.94–1.06 |
| Bleeding events | 1905 (8 studies) | 0.80 | 0.56–1.15 |
| Arrhythmias | 1591 (6 studies) | 1.11 | 0.84–1.45 |
| Dialysis-related hypotension | 1080 (6 studies) | 1.14 | 0.82–1.57 |
| Hypokalaemia | 737 (2 studies) | 1.04 | 0.77–1.40 |
| Thrombocytopenia | 725 (2 studies) | 1.03 | 0.89–1.19 |
| Hypocalcaemia | 449 (3 studies) | 1.12 | 0.92–1.36 |
| Hypophosphatemia | 737 (2 studies) | 2.68 | 0.62–11.58 |
| Hyperkalaemia | 1107 (2 studies) | 0.27 | 0.01–5.85 |
Two studies13,28 reported median and interquartile values as two separate classes for early RRT. In Bouman et al.,28 the ‘early’ group was segmented into high- and low-volume haemofiltration; in Gaudry et al.,13 values were given for survivors/non-survivors in both ‘early’ and ‘late’ groups; these two studies were excluded from the analysis since no composite values were reported. In the remaining four studies,14,21,22,31 medians and interquartile ranges were pooled, showing no statistically significant difference between the ‘early’ and ‘late’ groups for either length of ICU stay (estimated difference in length of stay = 0.34 days (95% CI = −1.60,2.28, P = 0.73)), or length of hospital stay (estimated difference in length of stay = −1.75 days (95% CI −5.84,2.34, P = 0.40)).
Three studies reported on the impact of the number of RRT days. One study30 reported in terms of mean, ±SD and therefore was excluded; the other two reporting in terms of median and interquartile ranges.14,22 Although a large estimated difference in medians was evident, they were considered statistically insignificant; estimated difference = −5.99 (95% CI = −23.52,11.53, P = 0.50); this was also the case for number of mechanical ventilation-free days (estimated difference in length of stay = 6.94 days (95% CI = −4.59,18.48, P = 0.24)). No clear difference between groups in terms of the number of RRT-free days (estimated difference in length of stay = −1.33 days (95% CI = −3.66,1.01, P = 0.27)), or vasopressor-free days (estimated difference in length of stay = −0.45 days (95% CI = −3.22,2.32, P = 0.75)) was observable.
Risk of bias across studies
The risk of bias was estimated through a funnel plot using the overall mortality as an outcome. The inverted standard error against the RR is shown in supplementary Figure s1.19, where the ‘dotted’ lines signify the expected distribution of the studies. One study29 is a significant outlier; otherwise distributions corroborate a reduced risk of bias across the selected studies.
Discussion
The systematic literature review identified a total of 10 studies that describe the impact of early versus conventional/late-RRT on mortality. While the time period for follow-up varied throughout, the analysis showed no statistically significant difference in terms of overall, in-ICU, in-hospital, 28-, 60- and 90-day mortality. Further, subgroup analyses detected no significant differences between modality of RRT, or general hospital inpatients versus ICU patients only.
On removal of studies with a high or unclear risk of bias, the heterogeneity reduced (I2 from 46% to 23%), but with no impact on the difference in mortality between groups, suggesting these studies are likely to have influenced the consistency of the overall analysis.
The only subgroup that identified a difference in outcome as a function of RRT initiation was the surgical only population where ‘early’ RRT resulted in an improvement in mortality. However, it must be noted that the conclusion was based on a single, small study,29 which reported vastly different mortality rates between the ‘early’ and ‘late’ groups (14.29% vs. 85.71%). The study was the smallest included in the present meta-analysis (n = 28) and owing to its limited extent, the impact of a few additional patients will markedly alter the statistical significance between groups. In addition, the study was also assessed to have an overall unclear risk of bias as well as high risk of reporting incomplete outcome data; therefore, it is likely that the study with a purely surgical population has skewed results significantly. However, it should be noted that while limited conclusions can be drawn, this may indeed represent a difference based on patient population and that further studies may provide better understanding.
The meta-analysis did not identify any association between timing of RRT for AKI and dialysis dependence at 28 or 90 days, but it should be noted that absolute numbers were small. Although results from two studies13,14 investigating dependence at day 60 suggested a benefit in the early group, fewer studies reported day 60 compared to days 28 and 90. In both studies, the absolute numbers of dialysis-dependent patients at 60 days were relatively small which potentially skew the conclusions drawn. Further, Zarbock et al.14 also reported on dialysis dependence at day 90 with no significant difference between the groups. Other reported secondary outcomes such as renal recovery, length of ICU stay, length of hospital stay, number of RRT days, RRT-free days, mechanical ventilation-free days and vasopressor-free days also showed no statistically significant differences between groups.
The pooled results of the majority of adverse events showed no significant difference between groups with the exception of one: higher rates of catheter related complications were seen in the ‘early’ group which is likely due to the increased number of catheters inserted compared to the ‘late’ group.
The variability in the classification of ‘early’ and ‘late’ contribute to increasing the difficulty in pooling data for direct comparisons. Recent studies for the early group13,14,20,21,22,31 have adopted a time frame from eligibility while others utilised physiological variables to determine the initiation of RRT. Timeframes ranged from commencement within 6- to 12-h window from meeting eligibility criteria, whereas physiological criteria ranged from varying urine outputs to serum creatinine or urea levels. In addition to the difference between timing versus physiological factors, studies utilising international guidelines for either inclusion or to determine commencement of early RRT used varying classifications.1–3 While a known factor prior to devising the search strategy, it was nevertheless deemed that a systematic comparison of differing strategies would be informative despite the paucity of available data.
The value of initiating RRT earlier has been subjected to extensive debate, and while theoretical benefits have been postulated such as limiting fluid overload and organ dysfunction as well as removal of inflammatory mediators,32 the hypothesis has not been supported through an assessment of measured patient outcomes. Initiation of RRT at an earlier stage will also result in a higher proportion of patients receiving RRT which may, in turn, result in higher rates of complications as well as significant increases to cost.
Previous meta-analyses have reached differing conclusions; two conducted prior to the RCTs from 2013, suggested that ‘early’ RRT may convey a mortality benefit.18,19 In contrast, more recent RCTs concluded that there was no difference in mortality between groups.16,17 In 2018, three further RCTs20–22 concluded no difference of note in mortality; the large IDEAL-ICU trial22 was stopped early due to futility.
Evidence drawn from the pooling of studies tends to indicate little significant differences exist between early and late initiation of RRT for AKI. In addition, with the exception of 28-day mortality which was found to be of moderate quality, all pooled primary outcomes assessed using the GRADE tool were found to be of low quality (supplementary Table s1.2). The currently ongoing STARRT-AKI trial33 will add valuable data in an area where there is still a paucity of contextualised data which will, in turn, fuel significant debate.
Conclusions
The systematic review and meta-analysis revealed no significant difference between early and late initiation of RRT for AKI with regard to the primary outcome of overall mortality and multiple secondary outcomes such as length of ICU and hospital stay and dialysis dependence at 90 days. This agrees with recent previous meta-analyses that current evidence does not support the use of early RRT for patients with AKI. Additional data from ongoing and future RCTs are necessary to strengthen the evidence base.
Supplemental Material
Supplemental material, INC901688 Supplemental Material1 for Timing of renal replacement therapy for patients with acute kidney injury: A systematic review and meta-analysis by Mark Andonovic, Richard Shemilt, Malcolm Sim, Jamie P Traynor, Martin Shaw, Patrick B Mark and Kathryn A Puxty in Journal of the Intensive Care Society
Supplemental material, INC901688 Supplemental Material2 for Timing of renal replacement therapy for patients with acute kidney injury: A systematic review and meta-analysis by Mark Andonovic, Richard Shemilt, Malcolm Sim, Jamie P Traynor, Martin Shaw, Patrick B Mark and Kathryn A Puxty in Journal of the Intensive Care Society
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Mark Andonovic https://orcid.org/0000-0002-5290-4680
Jamie P Traynor https://orcid.org/0000-0002-4339-0366
References
- 1.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta R, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khwaja A. KIDGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: 179–184. [DOI] [PubMed] [Google Scholar]
- 4.Hoste E, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423. [DOI] [PubMed] [Google Scholar]
- 5.Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit Care Med 2010; 38: 261–275. [DOI] [PubMed] [Google Scholar]
- 6.Ostermann M, Chang R. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007; 35: 1837–1843. [DOI] [PubMed] [Google Scholar]
- 7.Hoste E, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10: R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchino S, Bellomo R, Goldsmith D, et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006; 34: 1913–1917. [DOI] [PubMed] [Google Scholar]
- 9.Schwenger V, Weigand MA, Hoffmann O, et al. Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury – a randomized interventional trial: the REnal Replacement Therapy Study in Intensive Care Unit PatiEnts. Crit Care 2012; 16: R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant 2009; 24: 512–518. [DOI] [PubMed] [Google Scholar]
- 11.Wald R, Friedrich JO, Bagshaw SM, et al. The optimal mode of renal replacement therapy in acute kidney injury (OMAKI): a pilot randomized controlled trial of CVVH vs. CVVHD. J Am Soc Nephrol 2011; 22: 10B. [Google Scholar]
- 12.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000; 356: 26–30. [DOI] [PubMed] [Google Scholar]
- 13.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375: 122–133. [DOI] [PubMed] [Google Scholar]
- 14.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016; 315(20): 2190–2199. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Gao J, Zheng X, Zhong B, Na Y, Wei J. Timing of initiation of renal replacement therapy for acute kidney injury: a systematic review and meta-analysis of randomized-controlled trials. Clin Exp Nephrol 2017; 21: 552–562. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt GC, Das RR. Early versus late initiation of renal replacement therapy in patients with acute kidney injury-a systematic review & meta-analysis of randomized controlled trials. BMC Nephrol 2017; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierstra BT, Kadri S, Alomar S, et al. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care 2016; 20: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 2011; 15: R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seabra VF, Balk EM, Liangos O, et al. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis 2008; 52: 272–284. [DOI] [PubMed] [Google Scholar]
- 20.Srisawat N, Laoveeravat P, Limphunudom P, et al. The effect of early renal replacement therapy guided by plasma neutrophil gelatinase associated lipocalin on outcome of acute kidney injury: A feasibility study. J Crit Care 2018; 43: 36–41. [DOI] [PubMed] [Google Scholar]
- 21.Lumlertgul N, Peerapornratana S, Trakarnvanich T, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care 2018; 22: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018; 379: 1431–1442. [DOI] [PubMed] [Google Scholar]
- 23.Ali F, Buamscha DG, Ciapponi A. Timing of renal replacement therapy initiation for acute kidney injury. Cochrane Database Syst Rev 2018; 12: CD010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath S, Sohn H, Steele R, et al. Meta-analysis of the difference of medians. Biom J 2019; 38(6): 969–984. [DOI] [PubMed] [Google Scholar]
- 27.Pursnani ML, Hazra DK, Singh B, et al. Early haemodialysis in acute tubular necrosis. J Assoc Physicians India 1997; 45: 850–852. [PubMed] [Google Scholar]
- 28.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, et al. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 2002; 30: 2205–2211. [DOI] [PubMed] [Google Scholar]
- 29.Sugahara S, Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodial Int 2004; 8: 320–325. [DOI] [PubMed] [Google Scholar]
- 30.Jamale TE, Hase NK, Kulkarni M, et al. Earlier-start versus usual-start dialysis in patients with community-acquired acute kidney injury: a randomized controlled trial. Am J Kidney Dis 2013; 62: 1116–1121. [DOI] [PubMed] [Google Scholar]
- 31.Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int 2015; 88: 897–904. [DOI] [PubMed] [Google Scholar]
- 32.Lameire N, Vanmassenhove J. Timing of dialysis in sepsis and acute respiratory distress syndrome. Am J Respir Crit Care Med 2018; 198: 4–5. [DOI] [PubMed] [Google Scholar]
- 33.Smith OM, Wald R, Adhikari NK, et al. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials 2013; 14: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, INC901688 Supplemental Material1 for Timing of renal replacement therapy for patients with acute kidney injury: A systematic review and meta-analysis by Mark Andonovic, Richard Shemilt, Malcolm Sim, Jamie P Traynor, Martin Shaw, Patrick B Mark and Kathryn A Puxty in Journal of the Intensive Care Society
Supplemental material, INC901688 Supplemental Material2 for Timing of renal replacement therapy for patients with acute kidney injury: A systematic review and meta-analysis by Mark Andonovic, Richard Shemilt, Malcolm Sim, Jamie P Traynor, Martin Shaw, Patrick B Mark and Kathryn A Puxty in Journal of the Intensive Care Society