Abstract
Substance use disorder (SUD) is a behavioral disorder characterized by volitional drug consumption. Mouse models of SUD allow for the use of molecular, genetic, and circuit-level tools, providing enormous potential for defining the underlying mechanisms of this disorder. However, the relevance of results depends on the validity of the mouse models used. Self-administration models have long been the preferred preclinical model for SUD as they allow for volitional drug consumption, thus providing strong face validity. While previous work has defined the parameters that influence intravenous cocaine self-administration in other species—such as rats and primates—many of these parameters have not been explicitly assessed in mice. In a series of experiments, we showed that commonly used mouse models of self-administration, where behavior is maintained on a fixed-ratio schedule of reinforcement, show similar levels of responding in the presence and absence of drug delivery—demonstrating that it is impossible to determine when drug consumption is and is not volitional. To address these issues, we have developed a novel mouse self-administration procedure where animals do not need to be pretrained on sucrose and behavior is maintained on a variable-ratio schedule of reinforcement. This procedure increases rates of reinforcement behavior, increases levels of drug intake, and results in clearer delineation between drug-reinforced and saline conditions. Together, these data highlight a major issue with fixed-ratio models in mice that complicates subsequent analysis and provide a simple approach to minimize these confounds with variable-ratio schedules of reinforcement.
Keywords: cocaine, reinforcement, self-administration, sex differences, substance use disorder
A sizable effort has focused on creating mouse models of substance use disorders (SUDs) to define the molecular and circuit-based mechanisms that underlie addictive behaviors. Intravenous self-administration is the preferred preclinical model of SUD because it allows for the complex assessment of multiple components of volitional drug intake and provides an animal model for changes observed in humans throughout the transition to SUD (Belin-Rauscent, Fouyssac, Bonci, & Belin, 2016; Caine, Stevens Negus, & Mello, 1999; Calipari et al., 2014; Contet, Whisler, Jarrell, Kenny, & Markou, 2010; Ferris, Calipari, Yorgason, & Jones, 2013; Fowler & Kenny, 2011; Lesscher & Vanderschuren, 2012; Markou et al., 1993; Schuster & Thompson, 1969; Simon & Moghaddam, 2017; Thomsen & Caine, 2005, 2007). The development of mouse self-administration models along with novel molecular, circuit, and genetic tools has allowed for the expansion of our understanding of the underpinnings of these behaviors with unprecedented resolution (Carpenter et al., 2020; Chandra et al., 2017; Fowler, Lu, Johnson, Marks, & Kenny, 2011; Ozburn, Larson, Self, & McClung, 2012; Pascoli et al., 2014; Wang et al., 2016; Yap & Miczek, 2007). However, in many cases, behaviors that have been validated in other model systems have been applied to mice without considering the different factors that influence these behavioral read-outs across species (Roberts, Morgan, & Liu, 2007). Here, we conducted a series of studies to empirically evaluate interpretations from existing mouse self-administration models that are standard in the field. We then used these evaluations to establish a mouse self-administration procedure to produce consistent drug intake, produce more consistent responding, and more clearly delineate saline from drug-reinforced conditions.
Volitional drug consumption is a core feature that makes self-administration models more translationally relevant than experimenter-delivered injections (Chen et al., 2008; Collins, Weeks, Cooper, Good, & Russell, 1983; Epstein, Preston, Stewart, & Shaham, 2006; Fowler et al., 2011; Kawa, Allain, Robinson, & Samaha, 2019; Liu, Roberts, & Morgan, 2005; Lynch, Nicholson, Dance, Morgan, & Foley, 2010; Markou et al., 1993; Shaham, Shalev, Lu, de Wit, & Stewart, 2003). However, generating robust operant self-administration models in mice has been challenging. Currently, mouse models commonly rely on food pretraining where responses on the active operanda results in the delivery of food with a conditioned cue (Anderson et al., 2018; Caine et al., 1999; Carpenter et al., 2020; Fowler et al., 2011; Thomsen & Caine, 2007; Thomsen et al., 2005). In subsequent sessions, the reinforcer is switched to drug, representing a contingency switch where the mouse now learns that the same response results in a different outcome. Typically, if responding continues on the active operanda after the contingency is switched to drug, mice are said to have learned/acquired the self-administration task and are included in the study. This approach has been used regularly with mouse self-administration protocols in the neuroscience field, especially as it relates to understanding how repeated volitional drug use changes the brain on the genetic, molecular, and circuit level (Engeln et al., 2020; Lotfipour et al., 2013; Rocha et al., 1998; Yap & Miczek, 2007).
However, while it is assumed that previously food-trained mice are now deliberately self-administering cocaine, confounding this interpretation is that the previous food training may produce cue-food associations, and these conditioned cues may be capable of supporting robust responding on their own (Chaudhri et al., 2006; Olausson, Jentsch, & Taylor, 2004; R. R. Miller, Barnet, & Grahame, 1992). Further, neutral cues alone can support reinforcement, even on high-effort schedules in mice (Olsen, Childs, Stanwood, & Winder, 2010; Olsen & Winder, 2009, 2010). Thus, responding on the active operanda could be maintained by the cues, conditioned reinforcement, or lack of food response extinction—rather than being maintained by the delivery of drug. This has the potential for false positives by including animals that meet self-administration criteria but exhibit behavior driven by nondrug-associated factors, as others have previously shown (Thomsen & Caine, 2011). These factors make it difficult to ascertain if drug consumption is truly volitional, and without clear controls to interpret these behaviors, setting empirically derived acquisition/inclusion criteria is impossible.
Here, we sought to identify the core factors that underlie operant behavior in mice and to develop a mouse training model that does not require food training, results in higher intake with stable rates of behavior, and clarifies the delineation between drug-maintained behavior and saline controls. First, we found that traditional training criteria are particularly problematic in mice where they can meet these criteria in the absence of a reinforcer. Next, we ran a series of studies to identify some of the factors that contribute to the high rates of behavior seen in the absence of a traditional reinforcer in mice. Finally, we developed a simple procedure that relies on variable-ratio schedules of reinforcement, which eliminates the need for food pretraining as well as the previously observed behavioral bias seen in the unreinforced condition. Moving forward, this standardized model will be effective in further characterizing the neural mechanisms underlying drug-induced plasticity.
Method
Animals
Eight-week-old male and female C57BL/6J mice were purchased from the Jackson Laboratory and maintained in a 12-hr 6:00/6:00 reverse dark/light cycle, with food and water provided ad libitum. During self-administration, animals were food restricted to ~95% body weight, with water provided ad libitum. All behavioral experiments were conducted during the animal’s dark cycle. Experiments were approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center. All experiments were conducted according to the National Institutes of Health guidelines for animal care and use.
Jugular Catheter Implantation
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally and implanted with chronic indwelling jugular catheters, as previously described (Calipari et al., 2014; Walker et al., 2018). Catheters were custom made and consisted of a back-mounted pedestal with silicone tubing (Access Technologies #BC-2S; 0.3mmID, 0.6mmOD), and a silicone bead was placed 1 cm from the end of the tubing as an anchor to suture the catheter into the vein once implanted. Ampicillin (0.5 mg/kg)/heparin (10U/mL) in 0.9% saline was administered intravenously daily. Mice recovered > 3 days before commencing training.
Drugs
Cocaine (National Institute on Drug Abuse Drug Supply Program) was dissolved in 0.9% sterile saline on the day of the experiment and administered intravenously. Ketamine and xylazine (Patterson Veterinary) were mixed fresh in 0.9% sterile saline on the day of the surgery and administered intraperitoneally.
Self-Administration
Mice were trained in standard operant chambers (Med Associates, St. Albans, VT) equipped with two illuminated nose pokes and a white noise generator with speaker. During each daily session (2 hr), the initiation of white noise signaled the beginning of, and remained on throughout, the session. For each task, one nose poke was designated as the “active poke” that would result in the delivery of the reinforcer, and the other nose poke was the “inactive poke” and had no delivered reinforcer, but program consequences depended on each experiment, as outlined next.
Sucrose Self-Administration and Conditioned Reinforcement (Figure 1)
Figure 1.
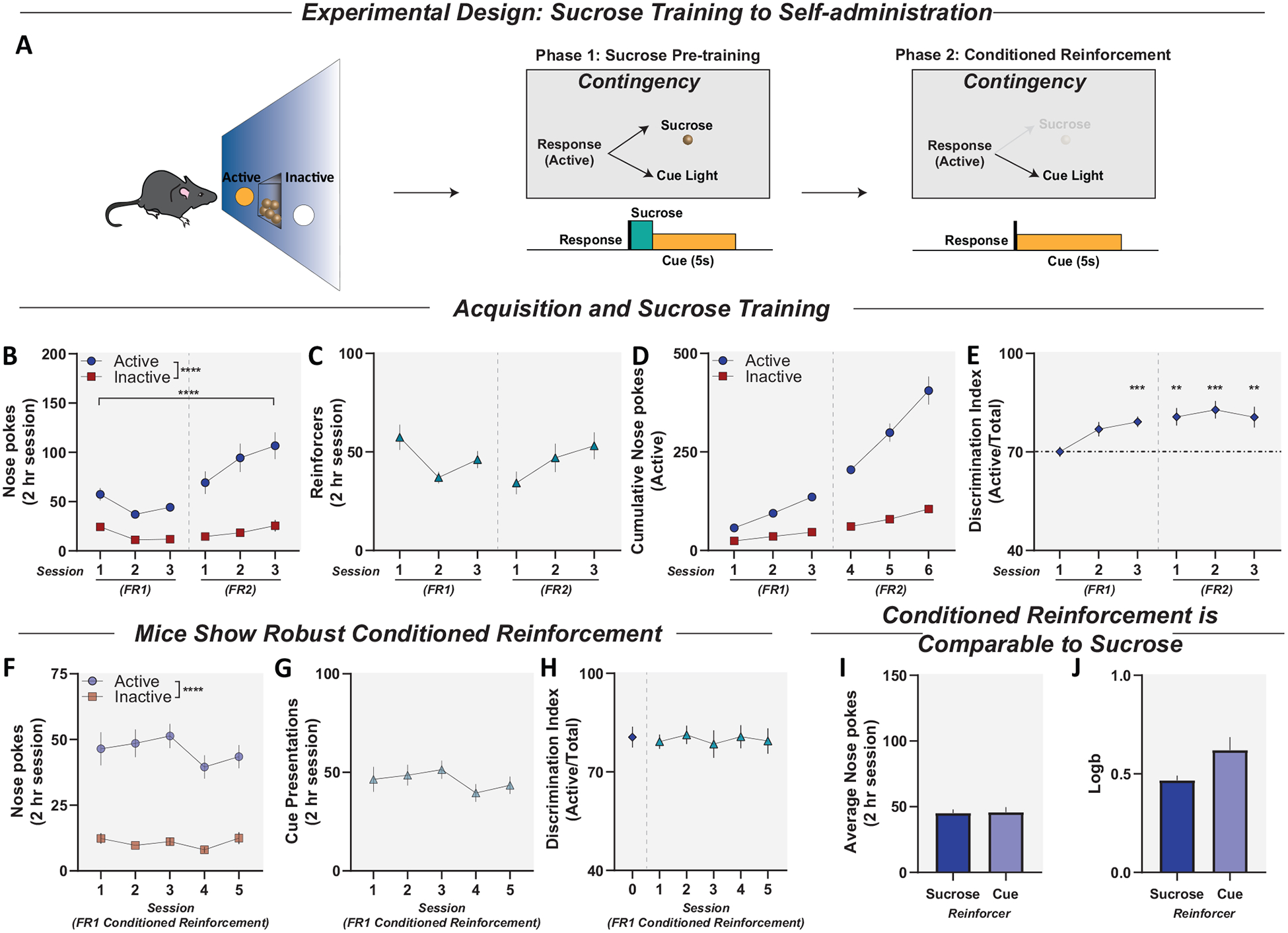
Typical pretraining approaches confound drug self-administration experiments in mice. Previous sucrose-paired cues support conditioned reinforcement. Panel A: Illustration of operant training procedures. Phase 1, operant training: Active nose pokes delivered a sucrose pellet concurrent with a 5-s cue light on a fixed ratio (FR) 1 and FR2 schedule of reinforcement. Phase 2, conditioned reinforcement: Mice were switched from sucrose responding to an FR1 schedule—like would be seen in cocaine self-administration training— however, active pokes resulted in only a 5-s cue light presentation, with no reinforcer. In all phases, inactive nose pokes were recorded but had no programmed consequence. Panel B: Active and inactive nose pokes during FR1 and FR2 sucrose training. Mice showed higher responding on the active nose poke compared to inactive and increased active responding during FR2. Panel C: Sucrose pellets earned across training. Animals earned reinforcers at a stable rate across FR1 and FR2 schedules of reinforcement. Panel D: Cumulative record of active and inactive responses across training sessions. Panel E: Discrimination index across sucrose training plotted as active/total responses. Panel F: Active and inactive nose pokes during FR1 during conditioned reinforcement. Mice maintained active responding for the presentation of the cue alone for 5 consecutive days. Panel G: Cue presentations earned across sessions. Animals earned stable cue presentations over 5 consecutive days. Panel H: Discrimination index across cue testing (Days 1–6) showing that mice still met discrimination criteria with no reinforcer present and maintained discrimination indices comparable to final day for sucrose training (Day 0). Panel I: Number of active nose pokes for sucrose or for cues alone (with no reinforcer present) are indistinguishable under an FR1 schedule. Panel J: Mice showed similar bias (measured by Logb) for active nose poke when reinforced by sucrose or cue light presentations alone. Data reported as mean ± standard error of the mean. ** p < .01. *** p < .001. **** p < .0001. Multiple comparisons were corrected for using Bonferroni correction of alpha threshold.
Male (n = 6) and female (n = 6) mice were trained to respond for sucrose pellets. Active nose pokes resulted in the delivery of a single 45-mg sucrose pellet (Dustless Precision Pellets, Bio-Serv F0025, chocolate flavor) paired with a nose poke light illumination (5 s) on a fixed-ratio (FR) 1 schedule. Inactive nose pokes had no programmed consequences. Following 3 consecutive days of FR1 training, mice were trained for 3 days under an FR2 schedule, as described previously (Caine et al., 1999). Next, mice were switched from sucrose self-administration to conditioned reinforcement where mice responded for just the delivery of the 5-s nose poke light, in the absence of the sucrose reinforcer.
Single-Cue Self-Administration in Mice (Figure 2)
Figure 2.
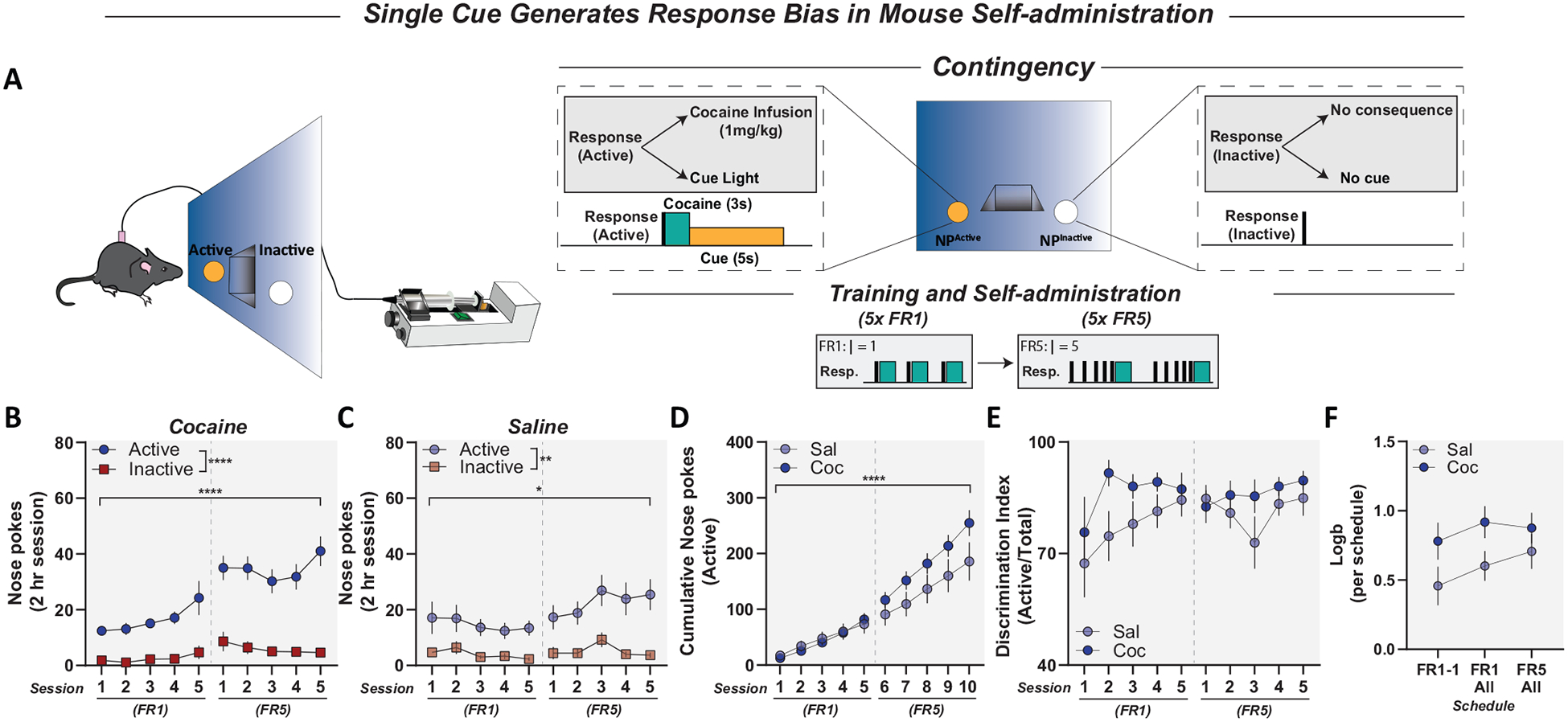
Mice showed biased responding in single-cue paradigms in both cocaine and saline conditions. Panel A: Schematic for mouse intravenous cocaine self-administration under fixed-ratio (FR) schedules of reinforcement with a single cue. Panel B: Active and inactive responses for cocaine (1 mg/kg/inj) self-administration in mice under FR1 and FR5 schedules of reinforcement when a consequent stimulus was presented following active, but not inactive, responses. Cocaine animals showed preference for the active nose poke and increased responding from FR3 to FR5. Panel C: Active and inactive responses for saline in mice under FR1 and FR5 schedules of reinforcement. Mice in the saline group showed preference for active nose poke and increased responding from FR1 to FR5. Panel D: Cumulative active responses for saline and cocaine in mice under FR1 and FR5 single-cue schedules of reinforcement. There were no significant differences in cumulative responding between saline and cocaine mice. Panel E: Discrimination indices for saline and cocaine animals; both saline and cocaine animals maintained suprathreshold discrimination indices. Panel F: Logb for saline and cocaine animals across self-administration under FR1 and FR5 single-cue schedules of reinforcement; there was no difference in response biases between saline and cocaine animals. Data reported as mean ± standard error of the mean. * p < .05. ** p < .01. **** p < .0001.
Male and female mice underwent intravenous cocaine self-administration where active nose pokes resulted in a single infusion of cocaine (1 mg/kg/infusion; 0.035 ml, 3 s) or sterile saline (0.9% NaCl; 0.035 ml, 3 s) and a concurrent 5-s illumination of the active nose poke light. Reinforced responses on the active nose poke initiated a 5-s timeout (concurrent with reinforcer + cue delivery). Inactive nose pokes resulted in no programmed consequences. Thus, only responses on the active poke resulted in cue delivery. This procedure was used under multiple schedules of reinforcement outlined next.
FR reinforcement.
Mice were trained to self-administer cocaine (n = 10; or saline as control [n = 10]), as described above, under FR1. Following acquisition, defined as 2 consecutive days of > 70%, or 3 consecutive days of > 60% discrimination on the active nose poke (the percentage of active vs. total nose pokes), mice self-administered for 5 consecutive days and were then switched to an FR5 schedule for the next 5 consecutive days.
Variable-ratio (VR) reinforcement.
Mice were trained to self-administer saline (n = 8) or cocaine (n = 11) under an FR1 schedule until they reached a minimum of six infusions per session. These criteria were purely intake based and thus independent of responses on active pokes relative to inactive. Mice were subsequently trained on a series of VR schedules. VR schedules result in reinforcement following an unpredictable number of responses around an average number of responses per reinforcer delivery and result in steady and high rates of responding that exceed those of other FR or interval schedules (Field, Tonneau, Ahearn, & Hineline, 1996; Spealman & Goldberg, 1978; Wanchisen, Tatham, & Mooney, 1989). Mice were switched to VR2 (range: 1, 2, 3) for 5 consecutive days or until criteria were met. Criteria were defined as 2 consecutive days of > 70% or 3 consecutive days of > 60% discrimination (defined as the percentage of active vs. total nose pokes). Following acquisition, animals were moved to self-administer under a VR3 (range: 1, 2, 3, 4, 5) schedule for 5 days and then to VR5 (range: 1, 3, 5, 7, 9) for an additional 5 days, equaling 10 days of self-administration total. During VR3/5 self-administration, animals were excluded if they did not maintain > 50% baseline infusions (defined as the average infusions during the first 3 days of VR3 training) for 70% of self-administration sessions.
Dual-Cue VR Self-Administration Model in Mice (Figures 3, 4, 5)
Figure 3.
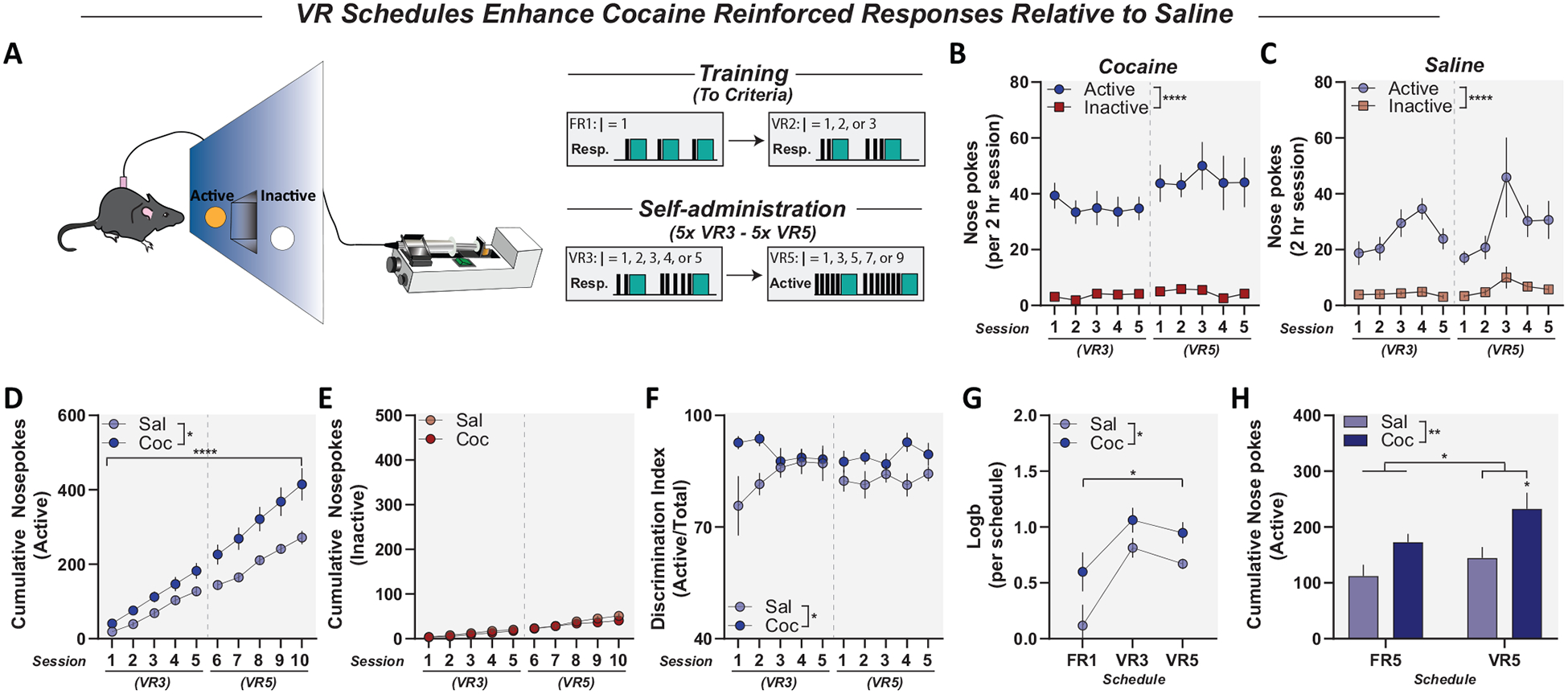
Variable-ratio (VR) schedules increased response rates for cocaine reinforcement in mice. Panel A: Schematic and self-administration schedule under VR schedules of reinforcement with a single consequent stimulus (cue) paired with responses on the active nose poke. Panel B: Active and inactive responses in mice under VR3 and VR5 schedules of reinforcement. Mice self-administering cocaine showed preference for active nose poke under VR3 and VR5 schedules. Panel C: Active and inactive responses for saline in mice under VR3 and VR5. Saline animals also showed preference for active nose poke under VR3 and VR5. Panel D: Cumulative active responses for saline and cocaine in mice under VR3 and VR5 single-cue schedules of reinforcement. Cocaine animals showed increased cumulative active responding compared to saline controls. Panel E: Cumulative inactive responses for saline and cocaine in mice under VR3 and VR5 single-cue schedules of reinforcement did not differ. Panel F: Discrimination indices for saline and cocaine animals. Cocaine self-administering mice maintained higher discrimination indices than saline controls. Panel G: Logb for saline and cocaine animals across self-administration under VR3 and VR5. Cocaine animals maintained higher Logb than saline controls. Panel H: Total active responding under fixed ratio (FR) 5 compared to VR5 single-cue schedules in saline and cocaine animals. Cocaine animals under VR5 had increased cumulative responding compared to saline controls, which was not seen with cocaine animals under FR5. Data reported as mean ± standard error of the mean. * p < .05. ** p < .01. **** p < .0001.
Figure 4.
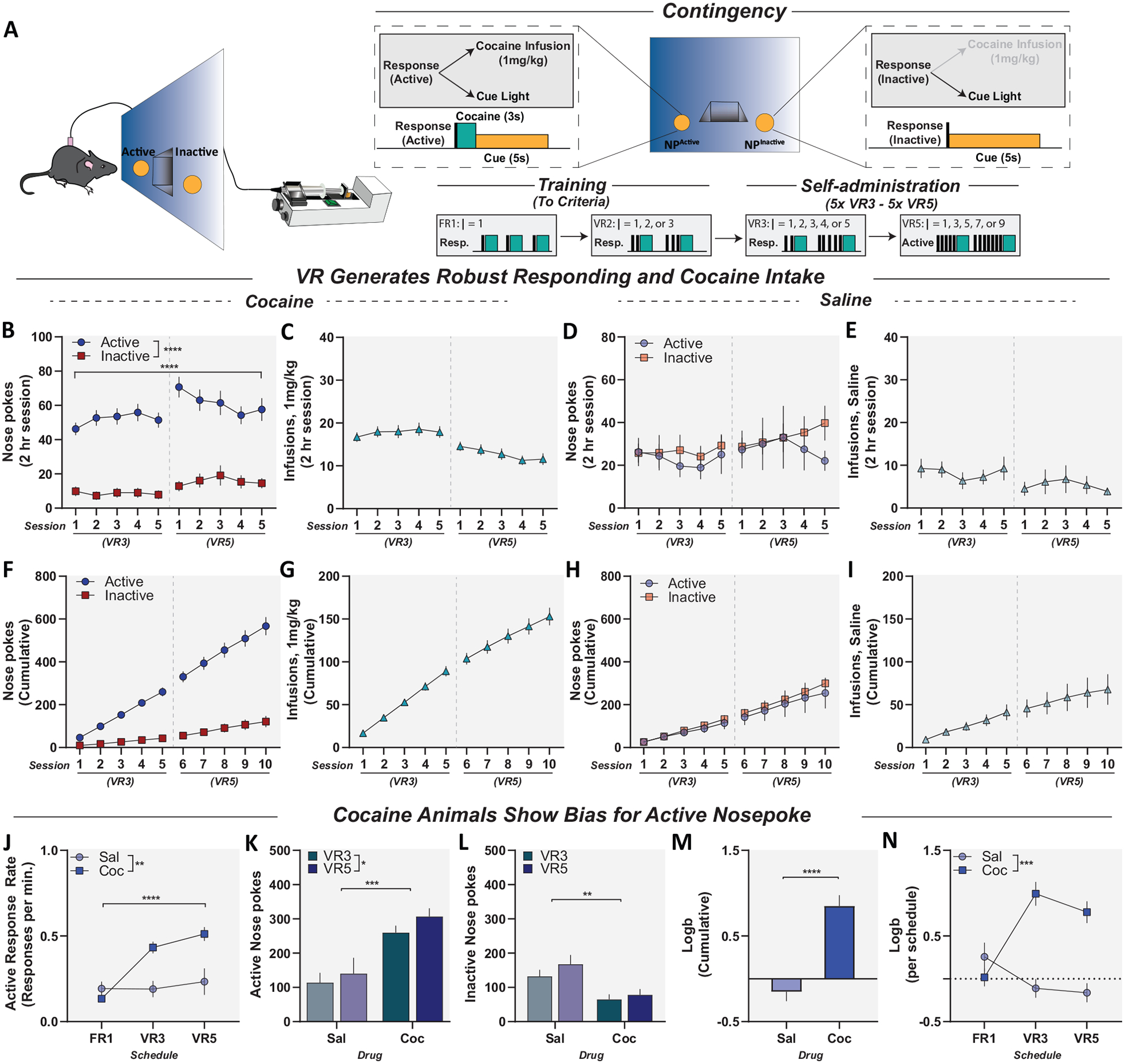
New procedure for drug self-administration in mice that requires no food/sucrose pretraining, shows robust self-administration, and increases drug intake. Panel A, left: Schematic for mouse intravenous cocaine self-administration; right: training and self-administration schedule. Panel B: Active and inactive responses for cocaine (1 mg/kg/inj) self-administration in mice on variable-ratio (VR) 3 and VR5 schedules of reinforcement. Animals showed a preference for active nose poke compared to inactive that is schedule dependent. Panel C: Total cocaine infusions per session. Panel D: Active and inactive responses for saline in mice on VR3 and VR5 schedules of reinforcement. Mice did not show a preference for either the active or inactive nose poke. Panel E: Total saline infusions per session. Panel F: Cumulative record of active and inactive responses across cocaine self-administration. Panel G: Cumulative record of cocaine infusions across cocaine self-administration. Panel H: Cumulative record of active and inactive responses across saline self-administration. Panel I: Cumulative record of saline infusions across saline self-administration. Panel J: Response rates on the active poke throughout training and self-administration. Cocaine animals demonstrated increased response rates on the active nose poke throughout cocaine self-administration compared to saline controls. Panel K: Total active responses for saline and cocaine animals during VR3 and VR5. Cocaine animals responded more on active poke and increased responding under VR5 compared to saline controls. Panel L: Total inactive responses for saline and cocaine animals during VR3 and VR5. Cocaine animals responded less on the inactive poke compared to saline controls. Panel M: Cocaine animals demonstrated increased bias for active nose poke during VR self-administration compared to saline controls. Panel N: Cocaine animals showed increased bias during VR self-administration compared to saline controls. Data reported as mean ± standard error of the mean. FR = fixed ratio. * p < .05. ** p < .01. *** p < .001. **** p < .0001.
Figure 5.
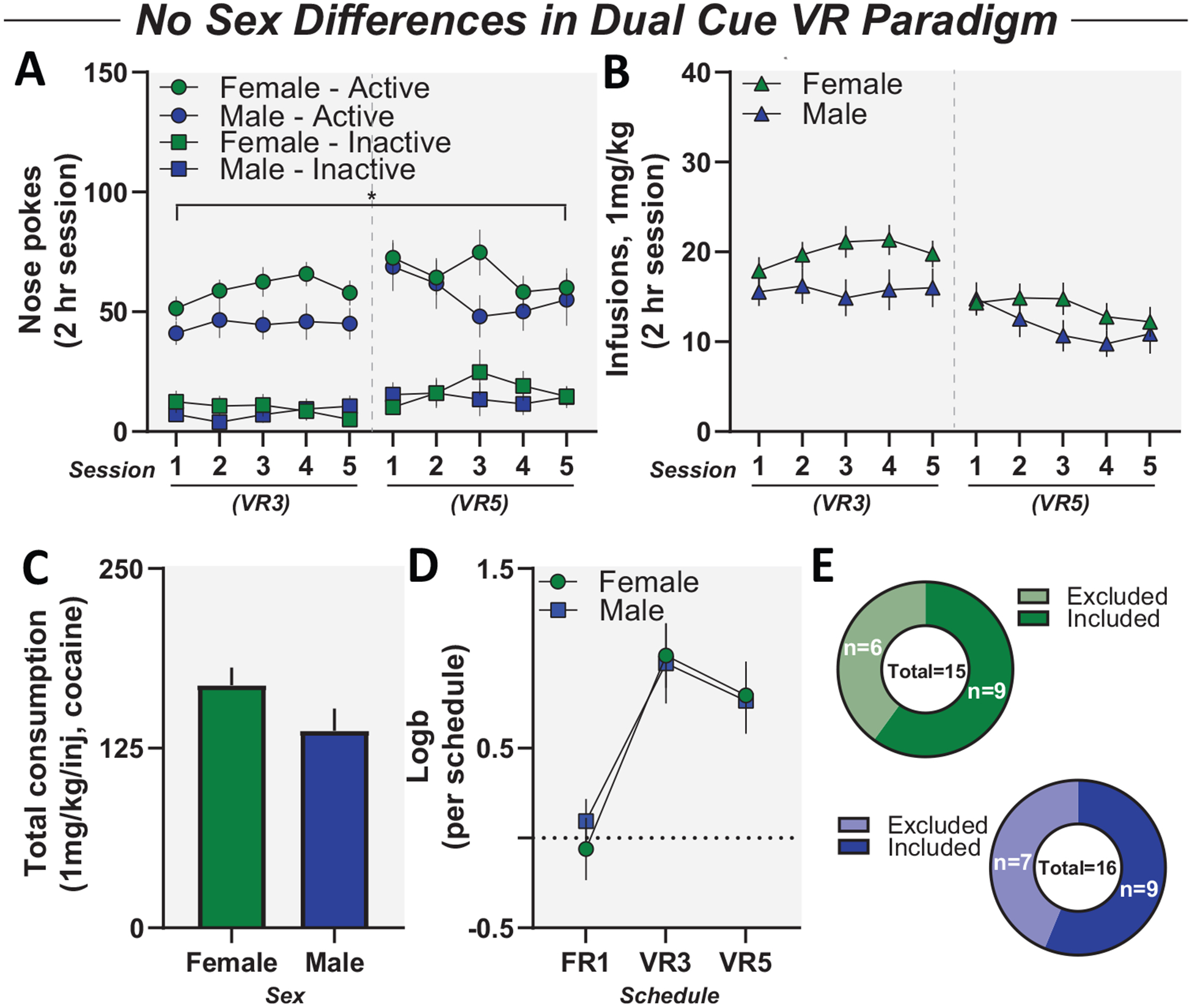
No sex differences in variable-ratio (VR)-based self-administration in mice. Panel A: Active and inactive responses for female and male mice during cocaine self-administration. Male and female mice showed similar preference for active nose poke compared to inactive. Panel B: Cocaine infusions for female and male mice during cocaine self-administration. Female and male mice self-administered similar levels of cocaine. Panel C: Total cocaine consumption in self-administration (VR3 and VR5). Female and male mice consumed similar levels of cocaine. Panel D: Female and male mice acquired bias for active nose poke at similar rates during cocaine self-administration. Panel E: Female and male animals excluded based on VR criteria. Data reported as mean ± standard error of the mean. FR = fixed ratio.
Male and female mice (saline, n = 8; cocaine, n = 31) underwent intravenous cocaine self-administration where active nose pokes initiated a 5-s timeout (concurrent with cocaine [or saline] + cue delivery). Inactive nose pokes resulted in inactive nose poke light delivery with the same parameters as the active nose poke with no concurrent infusion.
Animals were first trained on an FR1 schedule of reinforcement as described above and then switched to VR2 (range: 1, 2, 3) for 5 consecutive days or until criteria (defined above) were met. Animals that failed to meet acquisition in 5 days were moved to VR3 (range: 1, 2, 3, 4, 5) self-administration, and animals that failed to increase VR3 active responding by > 30% were excluded from analyses. Following acquisition, animals were moved to self-administer under a VR3 schedule for 5 days and then to VR5 (range: 1, 3, 5, 7, 9) for an additional 5 days, equaling 10 days of self-administration total. During VR3/5 self-administration, animals were excluded if they did not maintain > 50% baseline cocaine intake for 70% of cocaine self-administration sessions (baseline intake is defined as the average infusions during the first 3 days of VR3 training).
Analysis Parameters
For within-subject comparisons, a repeated-measures analysis of variance (ANOVA) was used. For comparisons between groups, a two-tailed t test was used. Each statistical test is denoted with the appropriate statistics in the results section. We also used a computational analysis to determine the parameters of response bias (Logb), as described previously (Davison & Tustin, 1978; Johnstone & Alsop, 1999; Kutlu et al., 2020). Briefly, Logb was computed as the measure for behavioral bias using a logarithmic scale for the multiplication of the ratio between correct and incorrect responses: Log b = 0.5 × log[(Active + 0.5)2/(Inactive + 0.5)2]. Type I error rate (alpha) was set to 0.05 for all statistical tests, unless otherwise noted. Data are represented as the mean ± standard error of the mean in figures. Data were analyzed and graphed using Graphpad Prism 8.2 (La Jolla, CA).
Results
Current Approaches for Cocaine Self-Administration Do Not Meet the Criteria for Clearly Defined Volitional Drug Consumption
Drug self-administration training paradigms frequently begin with food/sucrose pretraining to enhance acquisition and reduce attrition rates (Fowler & Kenny, 2011; Fowler et al., 2011; Roberts et al., 2007; Thomsen & Caine, 2005). To determine if current approaches for self-administration were adequate, mice were first trained briefly to self-administer sucrose with a single cue pairing under the control of a single active nose poke (Figure 1A). Mice showed higher responding on the active nose poke compared to inactive when sucrose was delivered on an FR1 schedule, and active responding increased further when response requirements were raised to an FR2 schedule (Figure 1B); main effect of nose poke, F(1, 20) = 67.17, p < .0001; main effect of session, F(5, 99) = 11.83, p < .0001; interaction, F(5, 99) = 6.532, p < .0001 (Figure 1D). Mice showed stable intake of sucrose pellets across sessions and schedules of reinforcement (Figure 1C). Last, mice exhibited discrimination indices above threshold criteria typically used to determine acquisition (Figure 1E); one-sample t test (theoretical M = 70), FR11: t(10) = 0.04012, p = .9688; FR12: t(10) = 3.133, p = .0106; FR13: t(9) = 5.788, p = .0003; FR21: t(10) = 3.994, p = .0025; FR22: t(10) = 4.797, p = .0007; FR23: t(10) = 3.319, p = .0078; Bonferroni postcorrection of multiple comparisons α = .008.
Next, we aimed to test if responding for the previously sucrose-paired cue—that is, conditioned reinforcement—was sufficient to maintain responding. Here, active responses resulted in only the presentation of the previously paired cue. The animals maintained high levels of active responding for the conditioned reinforcer alone (Figure 1F); main effect of nose poke, F(1, 20) = 82.6, p < .0001. Mice also showed no difference in cue deliveries over sessions (Figure 1G) and maintained discrimination indices comparable to those when responding is reinforced by sucrose (Figure 1H); repeated-measures one-way ANOVA, no effect of session, F(3.5, 35.0) = 0.1532, p = .9464. Active responding for the cue alone was not different from active responding for sucrose during the FR1 sessions (Figure 1I, Figure 1J). Thus, animals that undergo sucrose pretraining continue to meet inclusion criteria for drug self-administration, even in the absence of positive reinforcers (such as food, sucrose or drug). These results indicate that the standard food/sucrose pretraining and acquisition criteria that are used in most drug self-administration studies in mice will not differentiate animals that are responding for conditioned reinforcement and those that are responding for drug.
FR1 Self-Administration in the Absence of Sucrose Training Still Engendered Robust Response Biases in Control Animals
The data presented above clearly show that conditioned reinforcement for sucrose-associated cues is capable of confounding behavioral data. However, in addition to conditioned reinforcement, previous studies have demonstrated that mice will self-administer sensory stimuli, including lights and auditory stimuli in the absence of previous experience (Olsen et al., 2010; Olsen & Winder, 2009, 2010). Thus, eliminating food/sucrose pretraining may not be sufficient to eliminate difficulty in interpretation. To test this hypothesis, we conducted a series of studies to determine how a single conditioned stimulus (cue light) on the active operanda influences self-administration in mice.
Male and female mice were trained to self-administer cocaine (or saline) under either FR (Figure 2A) or VR (Figure 3A) schedules of reinforcement. In these studies, active responses resulted in cocaine/saline delivery concurrent with a conditioned stimulus, while inactive responses had no programmed consequence. Mice responding for cocaine showed a bias for the active nose poke under both FR1 and FR5 schedules; two-way ANOVA, main effect of nose poke, F(1, 18) = 70.31, p < .0001; main effect of session, F(3.404, 60.51) = 11.37, p < .0001; significant interaction, F(9, 160) = 5.437, p < .0001 (Figure 2B). Moreover, mice self-administering cocaine showed an increase in active responding during FR5 (post hoc analysis indicated significant increase in responding during FR5–1, p = .0039; FR5–2, p = .0003; FR5–3, p = .0167; FR5–4, p = .0089; FR5–5, p = .0014; Figure 2B). However, mice responding for saline also demonstrated a bias for the active nose poke under FR1 and FR5 schedules; two-way ANOVA, main effect of nose poke, F(1, 18) = 15.16, p = .0011 (Figure 2C). In addition, saline animals increased responding from FR1 to FR5 schedules; two-way ANOVA, main effect of session, F(2.309, 40.80) = 3.342, p = .0390 (Figure 2C). There was also no difference in cumulative active responding between saline and cocaine animals; two-way ANOVA, no main effect of drug, F(1, 18) = 1.042, p = .3208 (Figure 2D). Although there was a significant interaction, F(9, 162) = 4.749, p < .0001, post hoc analysis showed no significant difference in cumulative responses between saline and cocaine groups during any session. Moreover, both saline and cocaine groups maintained discrimination indices above 70% and were not different from each other; two-way ANOVA, no effect of drug, F(1, 18) = 2.761, p = .1139 (Figure 2E). Last, saline and cocaine animals acquired and maintained preference for active nose poke (measured by Logb), with a modest, but not significant, increase in cocaine animals; two-way ANOVA, no effect of drug, F(1, 18) = 4.104, p = .0579 (Figure 2F).
VR Reinforcement Schedules Engendered High Rates of Responding and Cocaine Intake
VR schedules engender higher and more consistent response rates than FR schedules; thus, we sought to determine if VR schedules would affect response biases seen with the single-cue tasks. We trained mice to self-administer cocaine or saline under VR3 and VR5 schedules (Figure 2G). Cocaine self-administering animals showed increased active responding compared to inactive; two-way ANOVA, main effect of nose poke, F(1, 20) = 72.47, p < .0001 (Figure 3B). Saline animals also showed increased active responding as compared to inactive; two-way ANOVA, main effect of nose poke, F(1, 14) = 134.5, p < .0001 (Figure 3C). However, under VR3 and VR5, cocaine animals demonstrated increased cumulative active responses compared to saline mice; two-way ANOVA, main effect of drug, F(1, 17) = 7.637, p = .0133; main effect of session, F(1.296, 22.03) = 124.8, p < .0001; significant interaction, F(9, 153) = 5.032, p < .0001 (Figure 3D), with no difference in inactive responding; two-way ANOVA, no effect of drug, F(1, 17) = 0.4248, p = .5233 (Figure 3E). In addition, cocaine animals showed increased discrimination indices compared to saline; two-way ANOVA, main effect of drug, F(1, 17) = 6.893, p = .0177 (Figure 3F). While both cocaine and saline animals acquired and maintained a bias for the active nose poke (measured by Logb), cocaine self-administering mice maintained significantly higher Logb values across reinforcement schedules compared to saline controls; two-way ANOVA, main effect of drug, F(1, 17) = 8.285, p = .0104; main effect of session, F(11.332, 22.65) = 11.81, p = .0011 (Figure 3G). Last, although in both FR5 and VR5 schedules of reinforcement, saline animals had a significant preference for the active nose poke, under VR5, cocaine animals increased active responding compared to FR5; two-way ANOVA, main effect of drug, F(1, 35) = 10.86, p = .0023; main effect of schedule, F(1, 35) = 4.141, p = .0495; post hoc analysis showed increased cocaine VR5 versus saline, t(35) = 2.707, p = .0207 (Figure 3H). Thus, although saline animals still maintain a biased response toward active operandi under VR schedules, cocaine self-administering mice show increased levels of responding, allowing for a more effective dissociation from control animals in cocaine self-administration.
Adding Control Conditioned Stimuli Following Inactive Nose Poke Responses Eliminated Response Biases Seen in Control Animals
In previous work, response biases were generated by the inclusion of consequent stimuli (i.e., a light cue) only on the active operanda. Indeed, we have shown that use of cues in this manner paired with sucrose pretraining in operant tasks can produce high discrimination indices in the absence of a primary reinforcer (see Figure 1F–J). Moreover, we demonstrated that VR schedules are able to partially mitigate this concern by increasing responding in cocaine-reinforced animals. Nevertheless, the use of VR schedules alone was unable to eliminate the response bias seen in control animals. Therefore, to circumvent these drawbacks, we used an operant schedule where active and inactive responses both generate programmed consequences (Figure 4A, left). Active responses resulted in delivery of both a positive reinforcer (i.e., a single cocaine infusion) and an active nose-poke-specific cue light, whereas inactive responses resulted only in the presentation of an inactive nose-poke-specific cue light (Figure 4A, top right). Further, we developed a novel operant training procedure that does not include food/sucrose pretraining. Mice were trained to self-administer cocaine (1 mg/kg/inj) under FR1 and VR2 reinforcement schedules until an adapted response criterion was met (Figure 4A, bottom right; see method section for criteria). Mice then self-administered cocaine under VR3 for 5 consecutive days and under VR5 for 5 consecutive days. Following training, mice responding for cocaine showed a preference for active nose poke compared to inactive that is schedule dependent (Figure 4B); main effect of nose poke, F(1, 34) = 80.05, p < .001; main effect of session, F(9, 306) = 5.062, p < .0001 (see Figure 4F). Mice responding for cocaine also maintained steady levels of cocaine intake, although they showed a slight decrease in intake under VR5 (Figure 4C); F(4.763, 80.97) = 11.81, p < .0001 (see Figure 4G). Mice in the saline group showed no preference for the active nose poke and did not increase active responding from VR3 to VR5 (Figure 4D, H). Saline infusions did not change over schedules of reinforcement; however, saline animals received fewer infusions than cocaine (Figure 4E); main effect of cocaine, F(1, 24) = 19.18, p = .0002. Mice responding for cocaine demonstrated increased response rates on the active nose poke throughout cocaine self-administration compared to saline controls (Figure 4J); main effect of schedule, F(1.522, 35.77) = 22.61, p < .0001; main effect of cocaine, F(1, 24) = 9.619, p = .0049; interaction, F(2, 47) = 16.75, p < .0001. Mice self-administering cocaine also demonstrated higher active responding and showed an increase in active responding under VR5, while generating less inactive responses compared to saline controls (Figure 4K); active responding, main effect of cocaine, F(1, 24) = 15.51, p = .0006; main effect of schedule, F(1, 24) = 6.416, p = .0183 (Figure 4L); inactive responding, main effect of cocaine, F(1, 24) = 9.643, p = .0048. Lastly, mice self-administering cocaine acquire an increased bias for the active nose poke during VR self-administration compared to saline controls (Figure 4M); two-tailed t test, t(24) = 4.897, p < .0001 (Figure 4N); two-way ANOVA, main effect of cocaine, F(1, 24) = 15.47, p = .0006; interaction, F(2, 48) = 18.41, p < .0001. Our novel dual-cue VR training model allows for rapid acquisition of cocaine self-administration. Critically, saline animals did not demonstrate active response bias in the absence of a positive reinforcer, as seen with other operant models (see Figure 1).
Dual-Cue VR Schedules Did Not Show Sex Differences in Behavior
Sex as a biological variable has become an increasingly important component in neurobiological mechanisms underlying SUD. As such, we assessed sex-specific effects in our dual-cue VR training paradigm. During cocaine self-administration, there was no difference in active responses between female and male mice and no difference in inactive responses between female and male mice (Figure 5A). Female and male mice received similar levels of cocaine infusions and comparable levels of total cocaine intake (Figure 5B, C). In addition, female and male mice acquired bias for active responding at equal rates (Figure 5D); main effect of schedule, F(1.306, 20.90) = 24.71, p < .0001; no effect of sex, F(1, 16) = 0.02026, p = .8886. Last, we found no sex-specific effects on our adapted exclusion criteria as female and male mice were equally excluded (Figure 5E).
Evaluating Behavioral Profiles of Mice That Were Included and Excluded Based on Criteria in Dual-Cue VR Paradigm
In evaluating our VR inclusion criteria, it was critical to assess the behavioral profile of not only cocaine-included animals (Figure 4) but also animals that failed to meet inclusion criteria (cocaine-excluded). Here, we found that the behavioral profile of cocaine-excluded animals was more comparable to saline controls. Cocaine-excluded mice showed no difference in active or inactive responding (Figures 6A, B). Cocaine-excluded animals did not take more infusions than saline controls (Figures 6C, D). Cocaine-excluded animals demonstrated no difference in total active or inactive responding throughout self-administration (Figures 6E, F, G). With regard to reinforcers, cocaine-excluded animals showed no increase in total infusions compared to saline controls (Figures 6H, I). Last, cocaine-excluded animals did not show response bias throughout self-administration and did not acquire response bias over self-administration sessions (Figures 6J, K). These data demonstrate that mice that fail to meet inclusion criteria (cocaine-excluded) perform similarly to saline controls.
Figure 6.
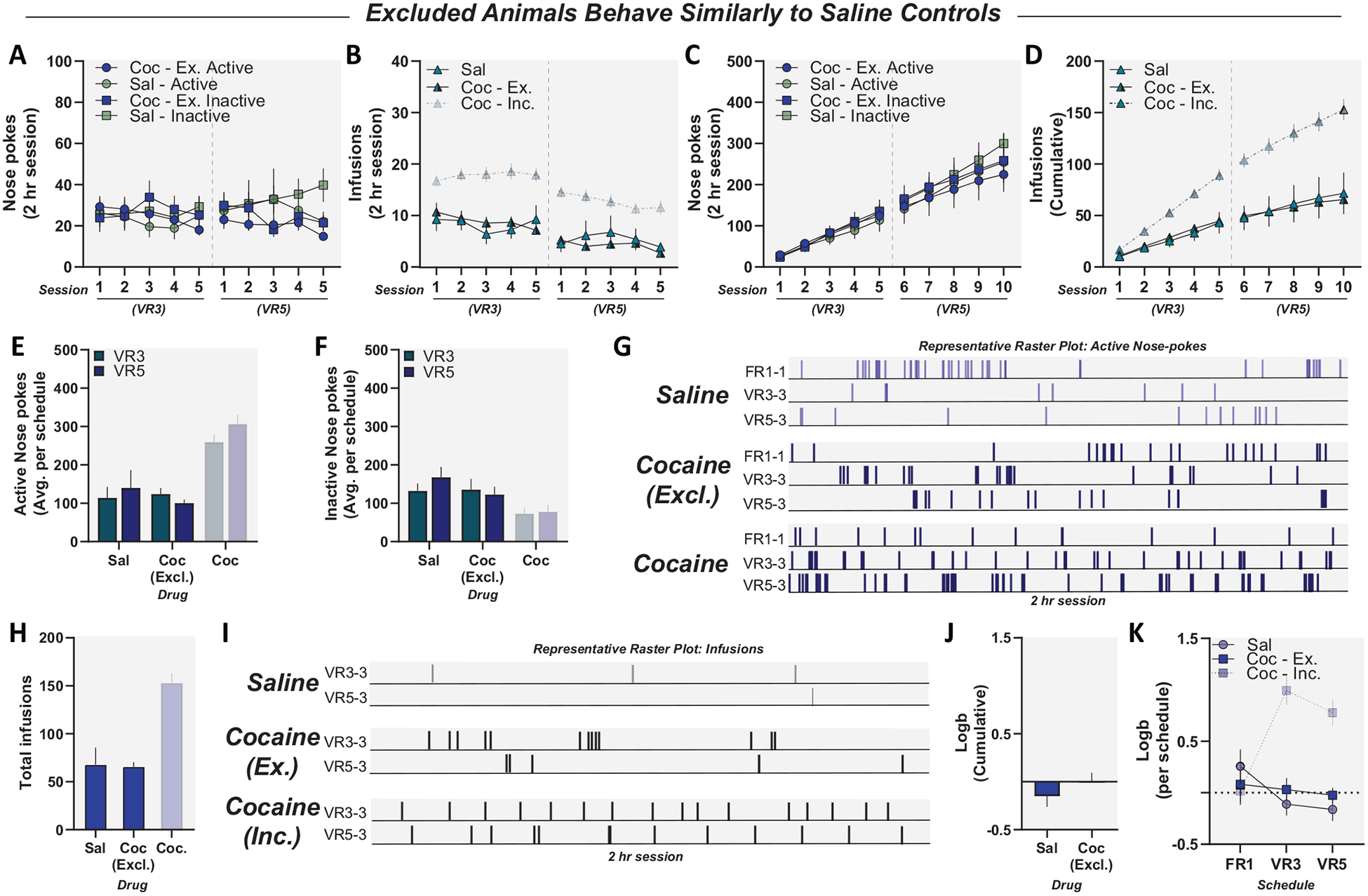
Mice excluded based on acquisition and performance criteria were not significantly different than saline controls (grayscale: cocaine-included animals, excluded from subsequent analysis). Panel A: Active and inactive responses for cocaine-excluded and saline animals during self-administration. Cocaine-excluded animals showed comparable active and inactive responses compared to saline controls. Panel B: Infusions of cocaine or saline during self-administration. Cocaine-excluded animals did not take more infusions than saline controls. Panel C: Cumulative active and inactive responses of cocaine-excluded animals during self-administration. Panel D: Cumulative infusions of cocaine-excluded and saline animals during self-administration. Cocaine-excluded animals earned similar infusions to saline controls. Panel E: Total active responses for cocaine-excluded animals and saline controls during variable ratio (VR) 3 and VR5. Cocaine-excluded animals showed no difference in active responding compared to saline controls. Panel F: Total inactive responses for cocaine-excluded animals and saline controls during VR3 and VR5. Cocaine-excluded animals showed no difference in inactive responding compared to saline controls. Panel G: Active response raster plots for representative (top) saline control, (middle) cocaine-excluded, and (bottom) cocaine-included animals. Panel H: Total infusions during cocaine self-administration, with no difference between saline controls and cocaine-excluded animals. Panel I: Infusion raster plots for representative (top) saline control, (middle) cocaine-excluded, and (bottom) cocaine-included animals. Panel J: Saline and cocaine-excluded animals showed no difference in response bias during self-administration. Panel K: Saline and cocaine-excluded animals did not acquire response bias for active nose pokes throughout self-administration. Data reported as mean ± standard error of the mean. FR = fixed ratio.
Discussion
Together, we show that traditional mouse models of self-administration result in high levels of operant responding in the absence of a reinforcer—with equivalent response rates between reinforced and nonreinforced sessions—demonstrating that with this form of pretraining, it is impossible to determine when drug consumption is volitional and goal oriented or if mice are responding for the food-conditioned reinforcer and have not associated this action with the delivery of cocaine. Given that the core assumption of self-administration models is that drug intake is goal directed and voluntary, this poses a particularly large problem for studies outlining the mechanisms of contingent drug consumption. Here, we present a novel training procedure for operant cocaine self-administration that allows for rapid acquisition of self-administration without the need for food pretraining (Figures 4–6). The addition of a programmed consequence of inactive responding (e.g., schedule-dependent delivery of a cue light) prevents the generation of a response bias in the absence of a reinforcer (e.g., saline delivery). Further, our paradigm shows robust responding in both males and females, allowing for the simultaneous inclusion of male and female subjects in studies using this task (Figure 5E). This procedure is simple to implement in mice, eliminates the confounds of previous training, and provides several independent measures that can be combined with novel circuit-and molecular-dissecting approaches. Moreover, this training procedure maintains attrition and throughput rates comparable to, if not above, those reported in previous studies (Bock et al., 2013; Caine et al., 2007), while decreasing the inclusion of false positive mice, as seen with commonly used inclusion criteria (Thomsen & Caine, 2011). Moving forward, this will be a powerful tool to rigorously assess various drug-induced adaptations in molecular and circuit functions.
As the use of transgenic mouse models increases, the field has translated several behavioral paradigms optimized in rats to assess drug taking in mice. However, due to practical limitations of these designs in mice (such as functional life of mouse jugular catheters), it is common to pretrain mice to respond for sucrose or food under various FR schedules of reinforcement prior to catheterization and drug self-administration. Here, we demonstrate that following sucrose self-administration, mice demonstrate robust conditioned reinforcement, where they continue to respond on the active operanda in the absence of any reinforcer (see Figure 1). Behaviorally, this is relatively straightforward to resolve as conditioned reinforcement can be parsed from the acquisition of drug self-administration via extinction of sucrose responding prior to training for intravenous drug infusions (Thomsen & Caine, 2007). However, in experiments that have end goals to assess the molecular and circuit adaptations induced by drug seeking and drug taking, a history of extinction training introduces a confound for subsequent drug-induced adaptations and altered circuit function (Lalumiere, Niehoff, & Kalivas, 2010; Lalumiere, Smith, & Kalivas, 2012). The self-administration procedures we outline provide an effective method for conducting mouse self-administration studies where volitional and nonvolitional drug consumption can be easily parsed without behavioral or pharmacological intervention. As such, this method can be employed easily in studies for the assessment of circuit and molecular adaptations.
Commonly used drug self-administration procedures employ a cued active operanda to indicate reward delivery, while the inactive operanda has no programmed consequences. However, previous work has demonstrated that mice perform operant tasks for visual stimuli alone and will do so in a manner that meets various operant inclusion criteria, including active operanda discrimination, schedule-dependent changes in responding, and resistance to extinction (Olsen et al., 2010; Olsen & Winder, 2009, 2010). As such, operant tasks programmed to deliver cues only to active responses can generate active-operanda bias independent of drug or food/sucrose reinforcer delivery. This is particularly problematic in drug self-administration as bias for the active operanda is often used as the read-out for not only volitional drug consumption but also catheter patency. In animals trained in single-cue paradigms, this active bias can be a product of the cue delivery and not drug seeking. Indeed, we demonstrate that in saline controls, there is a reliable bias for the active nose poke (see Figures 2–3) that is sensitive to changes in delivery schedule. We subsequently demonstrate that the use of VR schedules combined with the addition of a programmed control cue for the inactive operanda blunts this baseline bias seen in the unreinforced condition (see Figures 2–4).
A major component of addiction research has been characterizing the behavioral strategies and adaptations in drug-seeking and drug-taking paradigms (Ahmed & Koob, 1998; Bale et al., 2019; Belin, Mar, Dalley, Robbins, & Everitt, 2008; Edwards & Koob, 2013; Fouyssac, Everitt, & Belin, 2017; Liu et al., 2005; Murray et al., 2015). A plurality of studies, however, have focused only on the male phenotype as female subjects are either underpowered or excluded altogether (Becker & Koob, 2016). As a result, there has been a recent emphasis in accurately and reliably assessing sex as a biological variable with regard to preclinical models of SUD (Johnson et al., 2019; Kiraly, Walker, & Calipari, 2018; L. R. Miller et al., 2017; Roth, Cosgrove, & Carroll, 2004; Shansky, 2018; Zachry, Johnson, & Calipari, 2019). Here, we assessed the sex-specific effects of our cocaine self-administration procedure. Previous work has shown sex differences in baseline and drug-induced motor activity (Caldarone, King, & Picciotto, 2008; Cosgrove, Hunter, & Carroll, 2002; Van Haaren & Meyer, 1991; Võikar, Kõks, Vasar, & Rauvala, 2001), two factors in mice that can further interact with the above parameters to alter the behavioral read-out of self-administration. As such, we demonstrate that our training procedure does not induce sexually dimorphic behaviors and generates comparable cocaine intake across male and female mice (see Figure 5A–E). This will be a powerful tool for studying molecular and circuit-based sex differences as they can be studied using this procedure without confounds of sex differences in intake or reinforcement rate.
Our results highlight several problems with currently employed operant training procedures in mice. The procedure presented here relies on VR schedules of reinforcement to minimize these confounds. VR schedules result in reinforcement following an unpredictable number of responses around an average number of responses per reinforcer delivery. VR schedules are particularly powerful as they create steady and high rates of responding that exceed those of other FR or interval schedules (Field et al., 1996; Spealman & Goldberg, 1978; Wanchisen et al., 1989; see Figure 3H). Here, we show that VR schedules minimize inactive responses and generate high rates of active responses, allowing for a clear delineation between animals that have acquired the task and those that have not (see Figures 3–6). Further, the training schedule does not require food pretraining, allowing for drug reinforcement that is not influenced by other factors and will be critical in the study to dissociate drug-reinforcer-induced from natural-reinforcer-induced changes. Some previous studies have employed drug self-administration paradigms in mice without food pretraining (Deroche-Gamonet et al., 2003; Fiancette, Balado, Piazza, & Deroche-Gamonet, 2010; van der Veen et al., 2008). However, many of these studies have focused on behavioral pharmacology where doses are changed, or pharmacological agents are given, to show that mice operant behavior is in fact being reinforced. This is more difficult to do for genetic or ex vivo studies as pharmacological or behavioral interventions that ascertain whether reinforcement is occurring will confound subsequent analysis of how consistent volitional drug intake alters physiology or gene regulation. Further, these studies still rely on single-cue operant programs that make parsing volitional drug intake difficult as previous studies have shown that mice will respond at high rates for the presentation of cues alone, even on progressive ratio schedules of reinforcement (Olsen & Winder, 2009, 2010). Here, we provide a procedure that makes it easier to define volitional drug consumption and allows for many independent measures that will be able to be used to correlate with the neuronal measures in molecular and circuit-based studies.
It is important to note that there is no “one size fits all” behavioral paradigm for studying drug effects on the brain and behavior. Studies investigating the molecular and circuit response to drugs of abuse often employ self-administration as a model for stable, repeated drug consumption. The goal of this study was to develop a reliable schedule of reinforcement in mice to ensure relatively stable, volitional cocaine consumption in mice for subsequent ex vivo analysis. Along these lines, we limited our studies to a single dose of cocaine (1 mg/kg/inj) to demonstrate that stable drug consumption can be achieved over many consecutive days. Moreover, we demonstrate in our dual-cue VR paradigm that this single dose of cocaine (1 mg/kg/inj) can be effectively used to study both males and females as there were no observed sex differences in either acquisition or total consumption. Nevertheless, several studies have demonstrated that pharmacology and dosing of cocaine can have a significant impact on behavioral outputs with regard to intravenous self-administration, including total consumption and motivation (Mantsch, Yuferov, Mathieu-Kia, Ho, & Kreek, 2004; Oleson & Roberts, 2009; Roberts, Gabriele, & Zimmer, 2013). As such, future studies should carefully consider the effects of various doses of cocaine in mouse self-administration paradigms prior to ex vivo analyses.
The goal of preclinical SUD work is to understand drug-taking behavior on a mechanistic level that would allow for the development of novel pharmacotherapeutic targets for treatment in clinical populations (Czoty, Stoops, & Rush, 2016; Sweis, Thomas, & Redish, 2018; Volkow & Morales, 2015). SUD is a complex neuropsychiatric disorder characterized by aberrant learning regarding drugs of abuse and drug-associated cues (Barrett & Wood, 2008; Campbell & Wood, 2019; López, Siciliano, & Calipari, 2019; Mews & Calipari, 2017; Sultan & Day, 2011). It is important to note that the complexity of SUD makes it difficult, if not impossible, to create a single behavioral model that can encapsulate all components of SUD. Rather, here we identify factors in some currently employed behavioral models of SUD that are likely to confound subsequent study of the underlying cellular mechanisms driving drug intake and volitional consumption. In recent years, the field has focused on identifying the various molecular and circuity adaptations induced by drugs of abuse and underlying drug-associated behaviors (Calipari et al., 2016; Ebner, Larson, Hearing, Ingebretson, & Thomas, 2018; Fennell, Pitts, Sexton, & Ferris, 2020; Heller et al., 2016; López, Hemstedt, et al., 2019, López, Jia, et al., 2019; Malvaez et al., 2018; Savell et al., 2019; White et al., 2016). However, our ability to effectively characterize the components driving drug-seeking and addictive phenotypes depend entirely on having translational and rigorous mouse models of volitional drug consumption. Here, we identify the problems with the current models and create a new optimized procedure that minimizes these issues. Together, this approach will allow for more rigorous studies that result in a more complete and definitive understanding of the behavior at the core of these translational models.
Public Health Significance.
While recent advances in molecular, circuit-based, and genetic techniques have become optimized for in vivo use in mouse models, intravenous drug self-administration has not. For these approaches to effectively provide insight into the adaptations that drive drug-taking behavior, there is a need for reliable mouse models of volitional drug consumption. Here, we demonstrated significant limitations with standard mouse self-administration protocols and present an optimized self-administration model for the study of the molecular and circuit adaptations in mice.
Acknowledgments
The authors have no conflicts of interest to disclose. This work was supported by the National Institutes of Health (DA042111 and DA048931 to Erin S. Calipari, DA041838 to Alberto J. López, DA047777 to Amy R. Johnson, T32GM007347 to Ansley J. Kunnath, DA045103 to Cody A. Siciliano, GM07628 to Jennifer E. Zachry, T32MH064913 to Kimberly C. Thibeault) as well as funds from the Brain and Behavior Research Foundation in the form of NARSAD Young Investigator Grants (to Munir G. Kutlu, Erin S. Calipari, and Cody A. Siciliano), the Whitehall Foundation (to Erin S. Calipari), and the Edward Mallinckrodt Jr. Foundation (to Erin S. Calipari). We thank the National Institute on Drug Abuse Drug Supply Program for providing cocaine HCl used within this study. An earlier version of this article was posted on bioRxiv in preprint form and can be found here: https://doi.org/10.1101/786616.
References
- Ahmed SH, & Koob GF (1998). Transition from moderate to excessive drug intake: Change in hedonic set point. Science, 282, 298–300. 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- Anderson EM, Larson EB, Guzman D, Wissman AM, Neve RL, Nestler EJ, & Self DW (2018). Overexpression of the histone dimethyltransferase G9a in nucleus accumbens shell increases cocaine self-administration, stress-induced reinstatement, and anxiety. The Journal of Neuroscience, 38, 803–813. 10.1523/JNEUROSCI.1657-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Abel T, Akil H, Carlezon WA Jr., Moghaddam B, Nestler EJ, … Thompson SM (2019). The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology, 44, 1349–1353. 10.1038/s41386-019-0405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RM, & Wood MA (2008). Beyond transcription factors: The role of chromatin modifying enzymes in regulating transcription required for memory. Learning & Memory, 15, 460–467. 10.1101/lm.917508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex differences in animal models: Focus on addiction. Pharmacological Reviews, 68, 242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, & Everitt BJ (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science, 320, 1352–1355. 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A, & Belin D (2016). How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biological Psychiatry, 79, 39–46. 10.1016/j.biopsych.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, … Alvarez VA (2013). Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature Neuroscience, 16, 632–638. 10.1038/nn.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Stevens Negus S, & Mello NK (1999). Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology, 147, 22–24. 10.1007/s002130051134 [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, … Xu M (2007). Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. The Journal of Neuroscience, 27, 13140–13150. 10.1523/JNEUROSCI.2284-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, & Picciotto MR (2008). Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neuroscience Letters, 439, 187–191. 10.1016/j.neulet.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, … Nestler EJ (2016). In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proceedings of the National Academy of Sciences of the United States of America, 113, 2726–2731. 10.1073/pnas.1521238113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DCS, & Jones SR (2014). Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addiction Biology, 19, 145–155. 10.1111/j.1369-1600.2012.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RR, & Wood MA (2019). How the epigenome integrates information and reshapes the synapse. Nature Reviews Neuroscience, 20, 133–147. 10.1038/s41583-019-0121-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MD, Hu Q, Bond AM, Lombroso SI, Czarnecki KS, Lim CJ, … Heller EA (2020). Nr4a1 suppresses cocaine-induced behavior via epigenetic regulation of homeostatic target genes. Nature Communications, 11, 504. 10.1038/s41467-020-14331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R, Engeln M, Schiefer C, Patton MH, Martin JA, Werner CT, … Lobo MK (2017). Drp1 mitochondrial fission in D1 neurons mediates behavioral and cellular plasticity during early cocaine abstinence. Neuron, 96, 1327–1341. 10.1016/j.neuron.2017.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, … Sved AF (2006). Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology, 189, 27–36. 10.1007/s00213-006-0522-0 [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, … Bonci A (2008). Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron, 59, 288–297. 10.1016/j.neuron.2008.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, & Russell RR (1983). Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology, 82(1–2), 6–13. 10.1007/BF00426372 [DOI] [PubMed] [Google Scholar]
- Contet C, Whisler KN, Jarrell H, Kenny PJ, & Markou A (2010). Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacologia, 212, 283–299. 10.1007/s00213-010-1950-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, & Carroll ME (2002). Wheel-running attenuates intravenous cocaine self-administration in rats: Sex differences. Pharmacology, Biochemistry and Behavior, 73, 663–671. 10.1016/S0091-3057(02)00853-5 [DOI] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, & Rush CR (2016). Evaluation of the “pipeline” for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacological Reviews, 68, 533–562. 10.1124/pr.115.011668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison MC, & Tustin RD (1978). The relation between the generalized matching law and signal-detection theory. Journal of the Experimental Analysis of Behavior, 29, 331–336. 10.1901/jeab.1978.29-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, … Piazza PV (2003). The glucocorticoid receptor as a potential target to reduce cocaine abuse. The Journal of Neuroscience, 23, 4785–4790. 10.1523/JNEUROSCI.23-11-04785.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Larson EB, Hearing MC, Ingebretson AE, & Thomas MJ (2018). Extinction and reinstatement of cocaine-seeking in self-administering mice is associated with bidirectional AMPAR-mediated plasticity in the nucleus accumbens shell. Neuroscience, 384, 340–349. 10.1016/j.neuroscience.2018.05.043 [DOI] [PubMed] [Google Scholar]
- Edwards S, & Koob GF (2013). Escalation of drug self-administration as a hallmark of persistent addiction liability. Behavioural Pharmacology, 24, 356–362. 10.1097/FBP.0b013e3283644d15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeln M, Mitra S, Chandra R, Gyawali U, Fox ME, Dietz DM, & Lobo MK (2020). Sex-specific role for Egr3 in nucleus accumbens D2-medium spiny neurons following long-term abstinence from cocaine self-administration. Biological Psychiatry, 87, 992–1000. 10.1016/j.biopsych.2019.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, & Shaham Y (2006). Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology, 189, 1–16. 10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell AM, Pitts EG, Sexton LL, & Ferris MJ (2020). Phasic dopamine release magnitude tracks individual differences in sensitization of locomotor response following a history of nicotine exposure. Scientific Reports, 10, 173. 10.1038/s41598-019-56884-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, & Jones SR (2013). Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chemical Neuroscience, 4, 693–703. 10.1021/cn400026v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiancette JF, Balado E, Piazza PV, & Deroche-Gamonet V (2010). Mifepristone and spironolactone differently alter cocaine intravenous self-administration and cocaine-induced locomotion in C57BL/6J mice. Addiction Biology, 15, 81–87. 10.1111/j.1369-1600.2009.00178.x [DOI] [PubMed] [Google Scholar]
- Field DP, Tonneau F, Ahearn W, & Hineline PN (1996). Preference between variable-ratio and fixed-ratio schedules: Local and extended relations. Journal of the Experimental Analysis of Behavior, 66, 283–295. 10.1901/jeab.1996.66-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouyssac M, Everitt BJ, & Belin D (2017). Cellular basis of the intrastriatal functional shifts that underlie the development of habits: Relevance for drug addiction. Current Opinion in Behavioral Sciences, 13, 144–151. 10.1016/j.cobeha.2016.11.018 [DOI] [Google Scholar]
- Fowler CD, & Kenny PJ (2011). Intravenous nicotine self-administration and cue-induced reinstatement in mice: Effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology, 61, 687–698. 10.1016/j.neuropharm.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, & Kenny PJ (2011). Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature, 471, 597–601. 10.1038/nature09797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Peña CJ, Neve RL, & Nestler EJ (2016). Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. The Journal of Neuroscience, 36, 4690–4697. 10.1523/JNEUROSCI.0013-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Thibeault KC, Ĺopez AJ, Peck EG, Sands LP, Sanders CM, … Calipari ES (2019). Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology, 44, 1189–1197. 10.1038/s41386-019-0320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone V, & Alsop B (1999). Stimulus presentation ratios and the outcomes for correct responses in signal-detection procedures. Journal of the Experimental Analysis of Behavior, 72, 1–20. 10.1901/jeab.1999.72-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Allain F, Robinson TE, & Samaha AN (2019). The transition to cocaine addiction: The importance of pharmacokinetics for preclinical models. Psychopharmacology, 236, 1145–1157. 10.1007/s00213-019-5164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, & Calipari ES (2018). Modeling drug addiction in females: How internal state and environmental context facilitate vulnerability. Current Opinion in Behavioral Sciences, 23, 27–35. 10.1016/j.cobeha.2018.02.003 [DOI] [Google Scholar]
- Kutlu MG, Zachry JE, Brady LJ, Melugin PR, Kelly SJ, Sanders C, … Calipari ES (2020). A novel multidimensional reinforcement task in mice elucidates sex-specific behavioral strategies. Neuropsychopharmacology, 53, 690–750. 10.1038/s41386-020-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, & Kalivas PW (2010). The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learning & Memory, 17, 168–175. 10.1101/lm.1576810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, & Kalivas PW (2012). Neural circuit competition in cocaine-seeking: Roles of the infralimbic cortex and nucleus accumbens shell. European Journal of Neuroscience, 35, 614–622. 10.1111/j.1460-9568.2012.07991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HMB, & Vanderschuren LJMJ (2012). Compulsive drug use and its neural substrates. Reviews in the Neurosciences, 23(5–6), 731–745. 10.1515/revneuro-2012-0066 [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DCS, & Morgan D (2005). Sensitization of the reinforcing effects of self-administered cocaine in rats: Effects of dose and intravenous injection speed. European Journal of Neuroscience, 22, 195–200. 10.1111/j.1460-9568.2005.04195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- López AJ, Hemstedt TJ, Jia Y, Hwang PH, Campbell RR, Kwapis JL, … Wood MA (2019). Epigenetic regulation of immediate-early gene Nr4a2/Nurr1 in the medial habenula during reinstatement of cocaine-associated behavior. Neuropharmacology, 153, 13–19. 10.1016/j.neuropharm.2019.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López AJ, Jia Y, White AO, Kwapis JL, Espinoza M, Hwang P, … Wood MA (2019). Medial habenula cholinergic signaling regulates cocaine-associated relapse-like behavior. Addiction Biology, 24, 403–413. 10.1111/adb.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López AJ, Siciliano CA, & Calipari ES (2019). Activity-dependent epigenetic remodeling in cocaine use disorder. In Hurd Y & Nader M (Eds.), Handbook of experimental pharmacology (pp. 1–33). 10.1007/164_2019_257 [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Byun JS, Leach P, Fowler CD, Murphy NP, Kenny PJ, … Boulter J (2013). Targeted deletion of the mouse α2 nicotinic acetylcholine receptor subunit gene (Chrna2) potentiates nicotine-modulated behaviors. The Journal of Neuroscience, 33, 7728–7741. 10.1523/JNEUROSCI.4731-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, & Foley PL (2010). Animal models of substance abuse and addiction: Implications for science, animal welfare, and society. Comparative Medicine, 60, 177–188. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2890392/ [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Greenfield VY, Matheos DP, Angelillis NA, Murphy MD, Kennedy PJ, … Wassum KM (2018). Habits are negatively regulated by histone deacetylase 3 in the dorsal striatum. Biological Psychiatry, 84, 383–392. 10.1016/j.biopsych.2018.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, & Kreek MJ (2004). Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology, 175, 26–36. 10.1007/s00213-004-1778-x [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, & Koob GF (1993). Animal models of drug craving. Psychopharmacology, 112(2–3), 163–182. 10.1007/BF02244907 [DOI] [PubMed] [Google Scholar]
- Mews P, & Calipari ES (2017). Cross-talk between the epigenome and neural circuits in drug addiction. In Calvey T & Daniels WMU (Eds.), Progress in brain research (Vol. 235, 1st ed., pp. 19–63). 10.1016/bs.pbr.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, … Clayton JA (2017). Considering sex as a biological variable in preclinical research. The FASEB Journal, 31, 29–34. 10.1096/fj.201600781r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Barnet RC, & Grahame NJ (1992). Responding to a conditioned stimulus depends on the current associative status of other cues present during training of that specific stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 18, 251–264. 10.1037/0097-7403.18.3.251 [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin-Rauscent A, Simon M, Giuliano C, Benoit-Marand M, Everitt BJ, & Belin D (2015). Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nature Communications, 6, 10088. 10.1038/ncomms10088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, & Taylor JR (2004). Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology, 173(1–2), 98–104. 10.1007/s00213-003-1702-9 [DOI] [PubMed] [Google Scholar]
- Oleson EB, & Roberts DCS (2009). Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology, 34, 796–804. 10.1038/npp.2008.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Childs DS, Stanwood GD, & Winder DG (2010). Operant sensation seeking requires metabotropic glutamate receptor 5 (mGluR5). PLoS ONE, 5, e15085. 10.1371/journal.pone.0015085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, & Winder DG (2009). Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology, 34, 1685–1694. 10.1038/npp.2008.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, & Winder DG (2010). Operant sensation seeking in the mouse. Journal of Visualized Experiments, 45, 4–7. 10.3791/2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Larson EB, Self DW, & McClung CA (2012). Cocaine self-administration behaviors in ClockΔ19 mice. Psychopharmacology, 223, 169–177. 10.1007/s00213-012-2704-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, & Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature, 509, 459–464. 10.1038/nature13257 [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Gabriele A, & Zimmer BA (2013). Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: Methodological and interpretational considerations. Neuroscience and Biobehavioral Reviews, 37, 2026–2036. 10.1016/j.neubiorev.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Morgan D, & Liu Y (2007). How to make a rat addicted to cocaine. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31, 1614–1624. 10.1016/j.pnpbp.2007.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, … Caron MG (1998). Cocaine self-administration in dopamine-transporter knockout mice. Nature Neuroscience, 1, 132–137. 10.1038/381 [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, & Carroll ME (2004). Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neuroscience and Biobehavioral Reviews, 28, 533–546. 10.1016/j.neubiorev.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Savell KE, Bach SV, Zipperly ME, Revanna JS, Goska NA, Tuscher JJ, … Day JJ (2019). A neuron-optimized CRISPR/dCas9 activation system for robust and specific gene regulation. ENeuro, 6, 1–17. 10.1523/ENEURO.0495-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, & Thompson T (1969). Self administration of and behavioral dependence on drugs. Annual Review of Pharmacology, 9, 483–502. 10.1146/annurev.pa.09.040169.002411 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, & Stewart J (2003). The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology, 168(1–2), 3–20. 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Shansky RM (2018). Sex differences in behavioral strategies: Avoiding interpretational pitfalls. Current Opinion in Neurobiology, 49, 95–98. 10.1016/j.conb.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Simon NW, & Moghaddam B (2017). Methylphenidate has nonlinear dose effects on cued response inhibition in adults but not adolescents. Brain Research, 1654, 171–176. 10.1016/j.brainres.2016.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, & Goldberg SR (1978). Drug self-administration by laboratory animals: Control by schedules of reinforcement. Annual Review of Pharmacology and Toxicology, 18, 313–339. 10.1146/annurev.pa.18.040178.001525 [DOI] [PubMed] [Google Scholar]
- Sultan FA, & Day JJ (2011). Epigenetic mechanisms in memory and synaptic function. Epigenomics, 3, 157–181. 10.2217/epi.11.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweis BM, Thomas MJ, & Redish AD (2018). Beyond simple tests of value: Measuring addiction as a heterogeneous disease of computation-specific valuation processes. Learning & Memory, 25, 501–512. 10.1101/lm.047795.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2005). Chronic intravenous drug self-administration in rats and mice. Current Protocols in Neuroscience, 32, 1–40. 10.1002/0471142301.ns0920s32 [DOI] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2007). Intravenous drug self-administration in mice: Practical considerations. Behavior Genetics, 37, 101–118. 10.1007/s10519-006-9097-0 [DOI] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2011). False positive in the intravenous drug self-administration test in C57BL/6J mice. Behavioural Pharmacology, 22, 239–247. 10.1097/FBP.0b013e328345f8f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DPD, Wörtwein G, Fink-Jensen A, Wess J, & Caine SB (2005). Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. The Journal of Neuroscience, 25, 8141–8149. 10.1523/JNEUROSCI.2077-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, & Deroche-Gamonet V (2008). Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS ONE, 3(5), e2245. 10.1371/journal.pone.0002245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haaren F, & Meyer ME (1991). Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacology, Biochemistry and Behavior, 39, 923–927. 10.1016/0091-3057(91)90054-6 [DOI] [PubMed] [Google Scholar]
- Võikar V, Kõks S, Vasar E, & Rauvala H (2001). Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiology & Behavior, 72(1–2), 271–281. 10.1016/S0031-9384(00)00405-4 [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Morales M (2015). The brain on drugs: From reward to addiction. Cell, 162, 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, … Nestler EJ (2018). Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry. Biological Psychiatry, 84, 867–880. 10.1016/j.biopsych.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchisen BA, Tatham TA, & Mooney SE (1989). Variable-ratio conditioning history produces high- and low-rate fixed-interval performance in rats. Journal of the Experimental Analysis of Behavior, 52, 167–179. 10.1901/jeab.1989.52-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Martin JA, Mueller LE, Caccamise A, Werner CT, Neve RL, … Dietz DM (2016). BRG1 in the nucleus accumbens regulates cocaine-seeking behavior. Biological Psychiatry, 80, 652–660. 10.1016/j.biopsych.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AO, Kramár EA, López AJ, Kwapis JL, Doan J, Saldana D, … Wood MA (2016). BDNF rescues BAF53b-dependent synaptic plasticity and cocaine-associated memory in the nucleus accumbens. Nature Communications, 7, 11725. 10.1038/ncomms11725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap JJ, & Miczek KA (2007). Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology, 192, 261–273. 10.1007/s00213-007-0712-4 [DOI] [PubMed] [Google Scholar]
- Zachry JE, Johnson AR, & Calipari ES (2019). Sex differences in value-based decision making underlie substance use disorders in females. Alcohol and Alcoholism, 54, 339–341. 10.1093/alcalc/agz052 [DOI] [PMC free article] [PubMed] [Google Scholar]


