Abstract
Introduction: Cannabidiol (CBD), the nonintoxicating constituent of cannabis, is largely employed for pharmaceutical and cosmetic purposes. CBD can be extracted from the plant or chemically synthesized. Impurities of psychotropic cannabinoids Δ9-tetrahydrocannabinol (Δ9-THC) and Δ8-THC have been found in extracted CBD, thus hypothesizing a possible contamination from the plant.
Materials and Methods: In this study, synthetic and extracted CBD samples were analyzed by ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry and the parameters that can be responsible of the conversion of CBD into THC were evaluated by an accelerated stability test.
Results: In synthetic and extracted CBD no trace of THC species was detected. In contrast, CBD samples stored in the dark at room temperature on the benchtop for 3 months showed the presence of such impurities. Experiments carried out under inert atmosphere in the absence of humidity or carbon dioxide led to no trace of THC over time even at high temperature.
Conclusions: The results suggested that the copresence of carbon dioxide and water from the air could be the key for creating the acidic environment responsible for the cyclization of CBD. These findings suggest that it might be appropriate to review the storage conditions indicated on the label of commercially available CBD.
Keywords: Δ9-tetrahydrocannabinol, cannabidiol, impurity, liquid chromatography–mass spectrometry
Introduction
Cannabis sativa L. is a plant that has always attracted great attention due to the plethora of applications in numerous fields from medical to nutraceutical, manufacturing and food industry, and so on. Its main peculiarity is the biosynthesis of a unique class of bioactive organic molecules called phytocannabinoids. Among these, cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC) are the most investigated due to their numerous biological activities.
Unlike Δ9-THC, which is the major psychoactive component that confers the well-known euphoriant properties of cannabis, CBD showed no effect linkable to such activity, but rather proved to exert other different pharmacological effects.1 Moreover, CBD is void of abuse risks or potential dependence. In the past 20 years, several studies showed that CBD is a promising therapeutic agent for many diseases according to its numerous proprieties, including antiseizure, anxiolytic, antipsychotic, antiparkinsonian, antioxidative, neuroprotective, anti-inflammatory, and analgesic effects.1,2
In cannabis plants, CBD as well as THC is present as acid form, cannabidiolic acid (CBDA) and tetrahydrocannabinolic acid (THCA), respectively, and only as a (−)-trans isomer. It is converted into the corresponding neutral form by a heat-mediated decarboxylation reaction.3 Therefore, the neutral form can be extracted from leaves and flowers of previously decarboxylated Cannabis sativa or it can be obtained from the extraction and subsequent decarboxylation of its acid precursor.
The extraction is generally carried out with an organic solvent or supercritical carbon dioxide. Then, the waxes are removed from the extract through a “winterization” step, which involves the freezing of the extract at temperatures below −20°C for 2 days followed by filtration. Finally, CBD is purified by chromatography or crystallization from pentane or hexane.
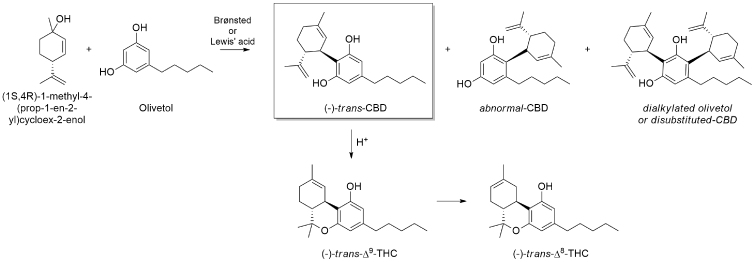
Alternatively, CBD can be produced through several stereoselective synthetic approaches.4 The most followed and easily accessible synthetic procedure requires the single-step Friedel–Craft allylation of commercially available olivetol with (1S,4R)-1-methyl-4-(prop-1-en-2-yl)cycloex-2-enol according to Petrzilka et al. and Baek et al.,5,6 in the presence of Bronsted or Lewis acid catalysts such as p-toluenesulfonic acid,7–13 BF3.Et2O,6,14 zinc triflate,15,16 or ZnCl2.17,18 However, this synthetic approach always leads to the formation of two main by-products, namely “abnormal CBD” and “dialkylated olivetol” (Fig. 1).6,10–13,17,19,20
FIG. 1.
Friedel–Craft allylation of olivetol with (1S,4R)-1-methyl-4-(prop-1-en-2-yl)cycloex-2-enol for the synthesis of CBD according to Petrzilka et al. procedure. The main by-products formed with this procedure and the further conversion of CBD to Δ9-THC and Δ8-THC are reported. CBD, cannabidiol; THC, tetrahydrocannabinol.
In addition, from our practical experience and as reported in literature and patents, traces of Δ9-THC and Δ8-THC may be present since the acidic conditions of the medium allow the reaction to proceed, during time, with the cyclization of CBD to Δ9-THC, first, and then to the more thermodynamically stable isomer Δ8-THC (Fig. 1).10–13,21 Hence, cumbersome chromatographic purifications are necessary to provide CBD with a pharmaceutical grade purity.
At present, CBD is not subject to international control. However, CBD as an extract of cannabis is in Schedule I of the 1961 Single Convention.22 Notwithstanding it is reported only one CAS (Chemical Abstract Service) number for both extracted and synthetic CBD, which are, therefore, chemically identical, it has been suggested that extracted CBD can retain traces of the psychotropic Δ9-THC after extraction from cannabis.
Conversely, synthetic CBD should be void of such contamination. Based on this premise, the European Commission has undertaken the policy of distinction between the “two forms” of CBD and excluded extracted CBD from the CosIng database, which is an exhaustive database of allowed cosmetic ingredients.23,24 Therefore, the origin of CBD makes the difference for its trade and use, notwithstanding it is reported only one CAS number for both extracted and synthetic CBD, since they are the same chemical entity.
Unfortunately, according to the aforementioned standard procedure, also the synthesis of CBD can lead to a product with traces of Δ9-THC, requiring the same or more complex purification procedures to remove the contaminant by-products.
Our recent study regarding the analysis of impurities of commercial CBD samples extracted from hemp highlighted that both Δ8-THC and Δ9-THC were far below the limit of detection (LOD) of the analytical method.10 In contrast, the major impurities detected were cannabidivarin (CBDV) and cannabidibutol (CBDB), the latter characterized for the first time by our research team.10,11 These findings indicate that not all CBD samples extracted from hemp contain THC.
As a continuation of this study, herein we analyzed the impurities of synthetic CBD to assess the presence of THC. As expected, the latter was not found in synthetic CBD samples even in traces. However, after 3 months of continuous use and storage on the laboratory bench-top at room temperature but in the dark, as specified on the label, the routine analysis of synthetic CBD samples revealed the presence of several peaks in the chromatogram obtained by liquid chromatography coupled to a UV detector.
To confirm the nature of such impurities, we employed an ultrahigh-performance liquid chromatography-based method coupled to high-resolution Orbitrap mass spectrometry detection (UHPLC-HRMS), which allows to provide extremely accurate qualitative responses with high sensitivity. Two of these peaks were found to be Δ9-THC and Δ8-THC by match of their HRMS features to those of authentic standards. Given that the labels of both commercially available synthetic and extracted CBD recommend storage at room temperature in the dark, this study investigates the parameters of temperature and humidity, which could affect the formation of such impurities in pure synthetic CBD samples.
Since synthetic and extracted CBD are currently discriminated for the presence of the psychotropic Δ9-THC in the latter, the ultimate goal of this study is to shed light on the still confusing difference between synthetic and extracted CBD.
Methods
Materials and instrumentation
Acetonitrile (ACN), water, and formic acid were all LC–MS grade and purchased from Carlo Erba. The analytical standard of CBDV, CBDA, CBD, cannabinol (CBN), Δ9-THC, Δ8-THC, THCA, and THC-d3 were bought from Cerilliant Corporation (Sigma Aldrich, Milan, Italy). CBDB was available from in-house synthesis.10 Extracted CBD was kindly provided by the CBDepot company (Teplice, Czech republic), whereas synthetic CBD was provided by the Farmabios company (Gropello Cairoli, Pavia, Italy).
Controlled stability was evaluated using a test cabinet TK 120 (Nüve, Akyurt/Ankara, Turkey).
Ultrahigh-performance liquid chromatography analyses were carried out on a Thermo Fisher Scientific Ultimate 3000 equipped with a vacuum degasser, a binary pump, a thermostated autosampler, a thermostated column compartment, and a Q-Exactive Orbitrap high-resolution mass spectrometer with a heated electrospray ionization source (UHPLC-HRMS).
Stability
The stability of synthetic pure CBD was tested at two temperatures (50°C and 60°C), two relative humidity (RH) percentages (75% and 55%), and under nitrogen (N2) or carbon dioxide (CO2) atmosphere.
Forty CBD samples were prepared by weighing 2 mg of powder and divided as follows: (1) 10 samples placed in open vials in oven set at 50°C and 75% of RH, (2) 10 samples placed in open vials at 60°C and 25% RH, (3) 10 samples placed in sealed vials under N2 atmosphere, and (4) 10 samples placed in sealed vials under CO2 atmosphere. All samples were stored for 35 days and two samples were analyzed by UHPLC-HRMS at each selected time points at 7-day intervals.
UHPLC-HRMS analyses and sample preparation
Chromatographic separation was performed using a column Poroshell 120 EC-C18 (3.0×100 mm, 2.7 μm; Agilent, Milan, Italy) and eluting 0.1% aqueous formic acid (A) and 0.1% formic acid in ACN (B) as mobile phase. An isocratic elution at 70% B was set for 15 min, followed by another isocratic elution at 95% B for 13 min, concluding with a drastic re-equilibration to the initial conditions (70% B), to give a total run time of 30 min. The flow rate was maintained constant at 0.5 mL/min and the injection volume was 10 μL.
The HESI parameters were capillary temperature, 320°C; vaporizer temperature, 280°C; electrospray voltage, 4.2 kV (positive mode) and 3.8 kV (negative mode); sheath gas, 55 arbitrary units; auxiliary gas, 30 arbitrary units; and S lens RF level, 45. The analyses were acquired in full scan data-dependent acquisition (FS-dd-MS2) in positive (ESI+) and negative (ESI−) mode at a resolving power of 70,000 FWHM at m/z 200. The software used to acquire the analyses is the Xcalibur 3.0 software (Thermo Fisher Scientific, San Jose, CA). The mass analyzer parameters were scan range, m/z 250–400; AGC, 3e6; injection time, 100 ms; isolation window for the filtration of the precursor ions, m/z 0.7. Fragmentation of precursors ions was performed at a collision energy of 20 eV. Detection was based on calculated [M+H]+ and [M-H]- molecular ions with an accuracy of 5 ppm, retention time and MS/MS spectrum match with pure analytical standards.
A stock solution of internal standard (IS, Δ9-THC-d3 100 ng/mL) was prepared diluting (1:1000) a solution of THC-d3 (100 μg/mL) in ACN. Samples were prepared by diluting a CBD stock solution at a concentration of 10 mg/mL with IS to a final concentration of 100 μg/mL.
To provide a semiquantitative analysis of the main impurities, we performed a calibration curve for each analyte under investigation. A standard stock solution of CBDV, CBDB, CBDA, CBN, Δ9-THC, Δ8-THC, and THCA at the concentration of 10 μg/mL was properly diluted with IS to obtain seven calibration standards (1, 5, 10, 50, 100, 500, and 1000 ng/mL). The LOD and the limit of quantification (LOQ) were established at 3 and 10 times the signal-to-noise ratio, respectively. The LOD was 1.5 ng/mL and the LOQ was 5 ng/mL for all analytes.
All samples were analyzed in triplicate; therefore, the result at each time point is the mean of six analyses and the values are expressed as mean±standard deviation of micrograms of substance per gram of CBD.
Results
Impurity profile of extracted and synthetic CBD
It is possible to distinguish extracted CBD from chemically synthesized CBD by analyzing its impurity profile. Previous studies concerning the impurities of CBD samples derived from industrial hemp extraction have highlighted the presence of considerable amounts of two major impurities corresponding to CBDV (0.07–0.41%, w/w) and CBDB (0.08–0.19%, w/w).10 To analyze the impurity profile of synthetic CBD samples, we developed a simple and fast UHPLC-HRMS method with an isocratic elution of ACN/water 70:30 (v/v) with 0.1% of formic acid that allowed for the detection of the main cannabinoids in the following order: CBDV, CBDB, CBDA, CBD, CBN, Δ9-THC, Δ8-THC, and THCA.
Since CBD samples were injected at an unusually high concentration for such a sensitive instrument (100 μg/mL), after the elution of the last compound, which occurred within 15 min, a washing step with ACN was set for 13 min and a re-equilibration of the column with the starting conditions allowed to remove any contamination that could affect the subsequent analyses. The impurity profile was evaluated for both extracted and synthetic CBD.
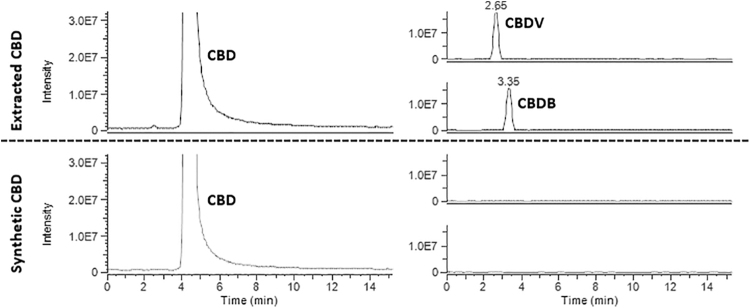
As expected, the analysis of extracted CBD showed the presence of the two major impurities with the extraction of the [M+H]+ molecular ions at m/z 287.2006 and 301.2162 from the total ion current for CBDV and CBDB, respectively. The extraction of the m/z 315.2319 corresponding to the CBD, Δ9-THC and Δ8-THC revealed only the presence of the main component of the sample, CBD.
In contrast, UHPLC-HRMS analysis of freshly opened bottle of synthetic CBD showed the complete absence of CBDV and CBDB when the corresponding [M+H]+ molecular ions were extracted from the total ion current. Freshly opened synthetic CBD, similar to freshly opened extracted CBD, showed no trace of either Δ9-THC or Δ8-THC. The impurity profile of extracted and synthetic CBD is reported in Figure 2.
FIG. 2.
Comparison of UHPLC-HRMS chromatograms of extracted CBD and synthetic CBD. The extraction of the molecular ion [M+H]+ at m/z 315.2319 showed only the presence of the main component (CBD) and no trace of Δ9-THC or Δ8-THC. The extraction of the molecular ions [M+H]+ at m/z 287.2006 and 301.2162 showed the presence of the peaks corresponding to CBDV and CBDB, respectively, only in extracted CBD, whereas they were absent in synthetic CBD. CBDB, cannabidibutol; CBDV, cannabidivarin; UHPLC-HRMS, ultrahigh-performance liquid chromatography-based method coupled to high-resolution Orbitrap mass spectrometry detection.
Identification of decomposition products of synthetic CBD
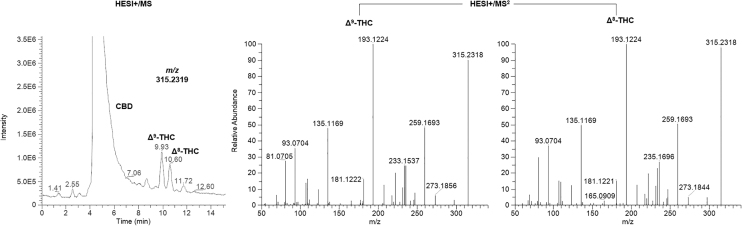
Although freshly opened synthetic CBD seemed void of any kind of impurities, it was surprising to find several peaks, not corresponding to the retention time of either CBDV or CBDB, in synthetic CBD samples analyzed after a 3-month storage on the bench-top in the dark. It is important to mention that, although complying with the provisions of storage reported on the label, these samples were continuously used for routine analysis, such as identification and calibration purposes, which unavoidably expose the material to air/moisture. We, therefore, investigated the nature of those impurities by analyzing their HRMS fragmentation spectra. Figure 3 shows the extracted [M+H]+ molecular ion with the corresponding HRMS spectrum of each peak.
FIG. 3.
Chromatograms of the impurities extracted from the total ion current in positive ionization mode (HESI+/MS) of synthetic CBD samples left on the bench-top for 3 months. HRMS spectra are shown on the same line as the corresponding chromatogram (HESI+/MS2). A fragmentation scheme is also proposed.
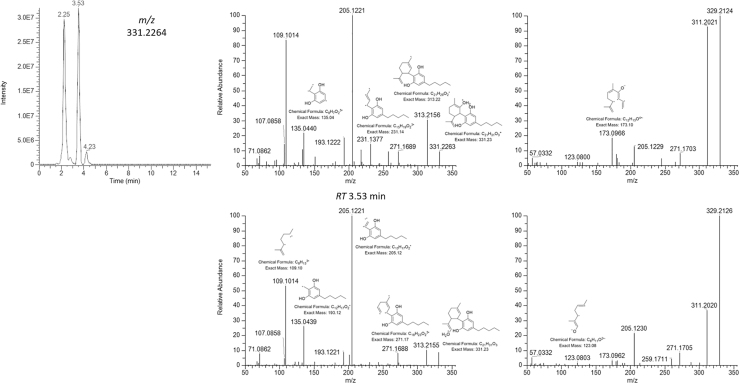
The first extracted molecular ion had m/z at 331.2264, which showed two peaks eluting at 2.25 and 3.53 min. The analysis of the fragments differing only for their relative abundance suggested a molecular formula C21H31O3 (Δppm=−1.64), which could likely correspond to two isomers of a hydroxylated CBD. Moreover, its chromatographic retention time is indicative of a molecule more polar than CBD. Unfortunately, the sole HRMS spectrum is not diagnostic of the exact position of the hydroxyl group. However, the base peak at m/z 205.1221, the fragment at m/z 135.0440, and the highly intense fragment at m/z 109.1014 suggested that the -OH should be on the terpenyl moiety rather than on the resorcinyl group or on the side chain.
The exact mass of the fragments is diagnostic of the chemical formula and, consequently, of the structure. In particular, the base peak corresponded to the olivetolic group with two oxygen atoms and two carbon atoms belonging to the terpenyl moiety; thus, no C-OH bond was broken on either the resorcinyl moiety or the side chain. The fragment at m/z 135.0440 was completely different from the one usually present in either CBD or THC spectrum (m/z 135.1167) as the exact mass suggested the chemical formula C8H7O2, which indicated the resorcinyl core rather than the terpene group.
Finally, the fragment at m/z 109 was unusually high compared with the one found in either CBD or THC spectrum, which is generally very low, most likely because the presence of the hydroxyl group on the terpene moiety favors that particular fragmentation. It was not possible though to establish whether the hydroxyl group was on the methyl group of the cyclohexene or on the propene moiety. It is reasonable to hypothesize that the two isomers differ exactly for the position of that hydroxyl group. In Figure 3, we tried to hypothesize the possible fragmentation scheme of the two isomers based on the suggested elemental composition of the fragments. HRMS spectrum obtained in negative ionization mode supported our hypothesis as shown by the hypothesized fragments structure.
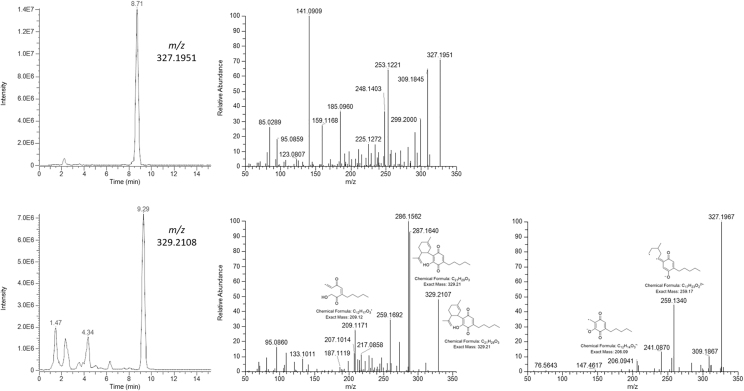
Another impurity appeared at 8.71 min with m/z at 327.1951 with a likely molecular formula C21H27O3 (Δppm=−0.21). The fragmentation patterns showed a high degree of oxidation/decomposition, thus very difficult to interpret. It is noteworthy though that no fragmentation was obtained in negative ionization mode, probably due to the lack of ionizable functional groups such as -OH.
Another peak was detected at 9.29 min with m/z at 329.2108, which corresponded to the [M+H]+ ion of HU-331, the well-known oxidation product of CBD with chemical formula C21H27O3 (Δppm=−1.30). Indeed, its HRMS spectrum in both positive and negative ionization mode matched the data found in the literature and on available spectral libraries.25
Surprisingly, the extraction of the [M+H]+ molecular ion at m/z 315.2319 showed the presence of two additional peaks besides the major component CBD. The two impurities eluted at 9.93 and 10.60 min. The investigation of their HRMS spectra suggested the formation of THC-like species. By comparing their exact mass (Δppm <0.1) and HRMS fragmentation spectra with those of pure standards of Δ9-THC and Δ8-THC, we were able to confirm their identity. Figure 4 shows the peaks of the two THC species extracted from the total ion current of synthetic CBD samples analyzed in positive ionization mode with the corresponding well-known HRMS fragmentation spectra.12,13,25–28
FIG. 4.
Representative chromatogram of the extracted [M+H]+ molecular ion at m/z 315.2318. The HRMS fragmentation spectra of the peaks eluting at 9.93 min (Δ9-THC) and 10.60 min (Δ8-THC) are also reported.
CBD-accelerated stability test
To understand how CBD decomposes over the time and to effectively establish that it can convert into Δ9-THC and Δ8-THC during storage, its stability was evaluated under controlled temperature and humidity.29 In particular, a stress test was performed to evaluate the likely degradation products during storage in accelerated conditions across a 5-week time. Temperature and RH were evaluated to assess their influence on CBD degradation. At different conditions, we registered the formation of decomposition products at 7-day intervals.
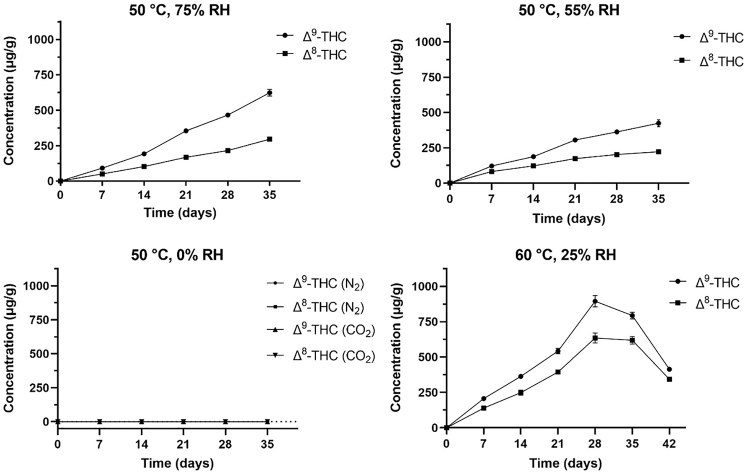
In particular, as expected, we observed the formation of two new peaks corresponding to the retention time and exact mass of Δ9-THC and Δ8-THC when CBD was kept at 50°C and 75% RH. By using the authentic standards of these two molecules, we were able to confirm that THC species were formed upon CBD decomposition. Their concentration increased over the time and the highest value registered was 623.5±22.9 and 295.2±10.6 μg/g for Δ9-THC and Δ8-THC, respectively, after 35 days.
With the aim to understand which is in particular the parameter responsible for the cyclization of CBD into THC, we kept the temperature at 50°C and lowered the RH to 55%. The THC-type cannabinoids were detected with the same trend but at lower concentrations reaching the highest value of 423.5±35.3 and 221.9±8.7 μg/g for Δ9-THC and Δ8-THC, respectively, after 35 days. This data supported the hypothesis that the presence of water (humidity) plays a key role in the conversion of CBD into THC.
Therefore, humidity was completely removed and the samples were stored in sealed vials insufflated either with an inert atmosphere of nitrogen (N2) or in the presence of carbon dioxide (CO2). In both cases, no formation of either Δ9-THC or Δ8-THC was observed, confirming that water is the necessary but not sufficient condition for this reaction to occur.
The next step consisted in increasing the temperature to 60°C setting the RH at 25%. In this case, the peak was reached after 28 days for both Δ9-THC and Δ8-THC (895.6±40.3 and 634.6±35.6 μg/g, respectively), then the concentrations decreased significantly for Δ9-THC and slightly for Δ8-THC at 35 days. To better describe this trend, which was not observed in the other conditions, a further time point at 42 days was added. Indeed, we observed that the concentration of the THC species abruptly decreased to 414.0±5.1 and 343.4±9.9 μg/g for Δ9-THC and Δ8-THC, respectively. This could be probably due to the oxidation/decomposition of the molecule.
To support our observations, we noticed that some of the impurities found in synthetic CBD samples left on the bench-top were also present in the synthetic CBD samples stored at 60°C and 25% RH for 42 days. However, the formation of the new products had completely different kinetics from those of CBD and THC, thus difficult to calculate at this stage given the number of species present in the chromatogram and mostly with unknown identity. Figure 5 shows the trend of Δ9-THC and Δ8-THC under different storage conditions tested for CBD-accelerated stability.
FIG. 5.
Concentrations of Δ9-THC and Δ8-THC over time under different storage conditions. Values are expressed in micrograms of THC species per gram of CBD powder as mean±SD. SD, standard deviation.
Calibration data on Δ9-THC and Δ8-THC for quantitative determination and purity assessment of CBD samples are reported in Supplementary Figures S1 and S2 and Supplementary Tables S1 and S2.
Discussion
CBD has been for long time, and until a few years ago, considered a minor less interesting cannabinoid compared with its isomer Δ9-THC, probably due to the lack of psychotropic activity typical of the latter. However, today CBD represents one of the most studied molecules for its multiple pharmacological activities, as evidenced by the numerous scientific articles published with an almost daily frequency. A hot debate about CBD regards its conversion through isomerization into Δ9-THC.
The occasional observation of altered cognitive states similar to those caused by THC after the administration of high doses of pure CBD to epileptic children30 led researchers to hypothesize a possible conversion of the latter in vivo into THC. In particular, Merrick and coworkers observed that CBD underwent a remarkable conversion into Δ9-THC and Δ8-THC, and other unknown cannabinoids in simulated gastric fluids.31 This finding prompts researchers to hypothesize a conversion in the stomach when CBD was administered to humans. This hypothesis though was not confirmed by in vivo experiments since no trace of THC species, including its metabolites, were detected when high doses of CBD were administered to either humans or animals.32–34
To explain the adverse effects observed after administration of high doses of CBD, a second hypothesis led to searching for a possible THC impurity in CBD starting material. Actually, such impurity had been already found in CBD extracted from hemp, even with varieties low in THC.35 Therefore, it was reasonable to think that the presence of THC, along with CBD, in the hemp plant could pollute the extracted CBD. The percentage of THC detected in CBD samples, though, was generally <0.1%, which represents the limit for an impurity to be identified according to ICH guidelines.10,36 Thus, the high doses of CBD required for the treatment of severe epilepsy could explain, according to some authors, the altered states in some children after administration of high doses of CBD.37
The presence of THC in CBD samples is thought to be due to its copresence in the hemp plant from which CBD is extracted. Actually, our experience with CBD synthesis indicated that CBD is converted into Δ9-THC and Δ8-THC in the presence of an acidic environment. The analysis of the impurity profile of synthetic CBD suggested that THC does not form if the samples are stored in the dark and at a temperature of 4–8°C (fridge). In contrast, bad storage conditions, such as room temperature and exposition to air and light, led to the formation of Δ9-THC and Δ8-THC. This also should apply to extracted CBD, which is also sensitive to air, light, and temperature.
Interestingly, our experiments on accelerated stability of synthetic CBD under controlled temperature and humidity allowed us to make new hypotheses on the formation of these impurities. Specifically, we initially thought that a residue of the reagents used for the synthesis of CBD was responsible for the cyclization of CBD into THC.
However, the experiments performed with lower RH (55% instead of 75%) suggested that this parameter should be somehow important for the reaction. Indeed, our initial hypothesis failed when nitrogen gas was insufflated at the same temperature as our previous experiments into CBD containing sealed vials to remove any external contamination and no trace of THC species was detected. We then hypothesized that water was the element necessary to trigger the reaction. Therefore, we carried out the same experiment in the absence of moisture (water) but in the presence of CO2. Again, THC was not detected, suggesting that water alone is not able to trigger the reaction, but instead needs CO2 to form carbonic acid (H2CO3), which then acts as catalyst for the cyclization into THC.
As a result, these findings, along with the related interpretation of the reaction rationale, can realistically apply to extracted CBD. In particular, this study points out the possibility of finding THC impurities in both forms of CBD, synthetic and extracted, since there is no inherent component in either one or the other that can alone cause the conversion. The only elements that could be responsible for the formation of THC from pure CBD are water and CO2 present in the air.
This suggests that incorrect storage conditions that do not protect against air and moisture may lead to the same results on both synthetic and extracted CBD. At the same time, the results obtained suggest that such conversion should not occur for CBD-based pharmaceutical products if the specified storage conditions are strictly respected. For example, product information leaflet of Epidiolex® (GW Pharmaceuticals, UK) clearly states that the quality of the content is guaranteed if stored between 20°C and 25°C for 12 weeks at most with the cap tightly closed.
To summarize, this study has elucidated the chemical conditions necessary for the conversion of CBD into THC under inappropriate storage conditions, highlighting that humidity and temperature can lead over time to the formation of oxidized products, along with the psychotropic Δ9-THC and Δ8-THC. Finally, this study supported previous findings on the actual difference between synthetic and extracted CBD, consisting of the presence of CBDV and CBDB only in the latter without exposing it to air, light, or high temperature.
In contrast, THC impurities can be formed in both extracted and synthetic CBD under particular storage conditions. Considering the current distinction between synthetic and extracted CBD and the different treatment reserved by the scientific community and the institutions to the two forms, this study may be helpful to shed some light in this regard, suggesting to take into account that they are, instead, the same chemical entity.
Supplementary Material
Acknowledgment
The authors thank the “Fondazione Cassa di Risparmio di Modena” for funding the UHPLC-ESI-Q Exactive system at the “Centro Interdipartimentale Grandi Strumenti” (CIGS).
Abbreviations Used
- ACN
acetonitrile
- CBD
cannabidiol
- CBDA
cannabidiolic acid
- CBDB
cannabidibutol
- CBDV
cannabidivarin
- CBN
cannabinol
- IS
internal standard
- LOD
limit of detection
- LOQ
limit of quantification
- RH
relative humidity
- SD
standard deviation
- THC
tetrahydrocannabinol
- THCA
tetrahydrocannabinolic acid
- UHPLC-HRMS
ultrahigh-performance liquid chromatography-based method coupled to high-resolution Orbitrap mass spectrometry detection
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by UNIHEMP research project “Use of iNdustrIal Hemp biomass for Energy and new biocheMicals Production” (ARS01_00668) funded by Fondo Europeo di Sviluppo Regionale (FESR) (within the PON R&I 2017-2020—Axis 2—Action II—OS 1.b). Grant decree UNIHEMP prot. no. 2016 of 27/07/2018; CUP B76C18000520005.
Supplementary Material
Cite this article as: Citti C, Russo F, Linciano P, Strallhofer SS, Tolomeo F, Forni F, Vandelli MA, Gigli G, Cannazza G (2021) Origin of Δ9-tetrahydrocannabinol impurity in synthetic cannabidiol, Cannabis and Cannabinoid Research 6:1, 28–39, DOI: 10.1089/can.2020.0021.
References
- 1. Pisanti S, Malfitano AM, Ciaglia E, et al. . Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150 [DOI] [PubMed] [Google Scholar]
- 2. Crippa JA, Guimarães FS, Campos AC, et al. . Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimura M, Okamoto K. Distribution of tetrahydrocannabinolic acid in fresh wild cannabis. Experientia. 1970;26:819–820 [DOI] [PubMed] [Google Scholar]
- 4. Jung B, Lee JK, Kim J, et al. . Synthetic strategies for (−)-cannabidiol and its structural analogs. Chem Asian J. 2019;14:3749–3762 [DOI] [PubMed] [Google Scholar]
- 5. Petrzilka T, Haefliger W, Sikemeier C, et al. . [Synthesis and optical rotation of the (−)-cannabidiols]. Helv Chim Acta. 1967;50:719–723 [DOI] [PubMed] [Google Scholar]
- 6. Baek S-H, Srebnik M, Mechoulam R. Boron triflouride etherate on alimina—a modified Lewis acid reagent.: an improved synthesis of cannabidiol. Tetrahedron Lett. 1985;26:1083–1086 [Google Scholar]
- 7. Kinney WA, McDonnell ME, Zhong HM, et al. . Discovery of KLS-13019, a cannabidiol-derived neuroprotective agent, with improved potency, safety, and permeability. ACS Med Chem Lett. 2016;7:424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Audra Lynn Stinchcomb MJG, Dana Carmel Hammell, Jeffrey Lynn Howard, et al (2009) Prodrugs of cannabidiol, compositions comprising prodrugs of cannabidiol and methods of using the same. US Patent US8293786B2, February 5, 2009 [Google Scholar]
- 9. Papahatjis D, Nikas S, Andreou T, et al. . Novel 1′,1′-chain substituted Δ8-tetrahydrocannabinols. Bioorg Med Chem Lett. 2002;12:3583–3586 [DOI] [PubMed] [Google Scholar]
- 10. Citti C, Linciano P, Forni F, et al. . Analysis of impurities of cannabidiol from hemp. Isolation, characterization and synthesis of cannabidibutol, the novel cannabidiol butyl analog. J Pharm Biomed Anal. 2019;175:112752. [DOI] [PubMed] [Google Scholar]
- 11. Citti C, Linciano P, Forni F, et al. . Chemical and spectroscopic characterization data of “cannabidibutol,” a novel cannabidiol butyl analog. Data Brief. 2019;26:104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linciano P, Citti C, Luongo L, et al. . Isolation of a high affinity cannabinoid for human CB1 receptor from a medicinal cannabis variety: D9-tetrahydrocannabutol, the butyl homologue of D9-tetrahydrocannabinol. J Nat Prod. 2020;83:88–98 [DOI] [PubMed] [Google Scholar]
- 13. Citti C, Linciano P, Russo F, et al. . A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci Rep. 2019;9:20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniel Dickman DL. Crystalline Form of Cannabidiol. US Patent, 20170349518, May 31, 2017 [Google Scholar]
- 15. Marc Bencivenga MFH, Paul Jass, Surendra Singh, et al. . Synthetic cannabidiol compositions and methods of making the same. WO2019046806A1, August 31, 2018 [Google Scholar]
- 16. Lukas Dialer DP, Ulrich Weigl. Process for preparation of (−)-Δ9-tetrahydrocannabinol and derivatives thereof. WO2017011210A1, January 19, 2017 [Google Scholar]
- 17. Kupper RJ. Cannabinoid active pharmaceutical ingredient for improved dosage forms. WO2006133941A2, December 21, 2006 [Google Scholar]
- 18. Gutman AL, Nisnevich GA, Rukhman I, et al. Methods for purifying trans-(−)-Δ9-tetrahydrocannabinol and trans-(+)-Δ9-tetrahydrocannabinol, US Patent US 8383842B2, February 26, 2013 [Google Scholar]
- 19. David C. Burdick SJC, Frédéric Jos, et al. Process for production of delta-9-tetrahydrocannabinol. US Patent US7674922B2, April 12, 2007 [Google Scholar]
- 20. Makriyannis A, Nikas SP, Alapafuja SO. Angiogenic resorcinol derivatives as activators of non-CB1 and non-CB2 cannabinoid receptors. WO/2011/006099, January 13, 2011 [Google Scholar]
- 21. Crombie L, Crombie WML, Jamieson SV, et al. . Acid-catalysed terpenylations of olivetol in the synthesis of cannabinoids. J Chem Soc Perkin Trans 1988;1:1243–1250 [Google Scholar]
- 22. Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol (revised edition 2013). New York: United Nations, November 2013 [Google Scholar]
- 23. Citti C, Linciano P, Cannazza G. Is cannabidiol a scheduled controlled substance? Origin makes the difference. Drug Discov Today. 2020;25:628–632 [DOI] [PubMed] [Google Scholar]
- 24. Cosmetic Ingredient Database (CosIng): Cannabidiol (2019) European Commission. https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.results. Accessed November7, 2019
- 25. Citti C, Battisti UM, Braghiroli D, et al. . A Metabolomic approach applied to a liquid chromatography coupled to high-resolution tandem mass spectrometry method (HPLC-ESI-HRMS/MS): towards the comprehensive evaluation of the chemical composition of cannabis medicinal extracts. Phytochem Anal. 2018;29:144–155 [DOI] [PubMed] [Google Scholar]
- 26. Citti C, Linciano P, Panseri S, et al. . Cannabinoid profiling of hemp seed oil by liquid chromatography coupled to high-resolution mass spectrometry. Front Plant Sci. 2019;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Citti C, Ciccarella G, Braghiroli D, et al. . Medicinal cannabis: principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J Pharm Biomed Anal. 2016;128:201–209 [DOI] [PubMed] [Google Scholar]
- 28. Pavlovic R, Panseri S, Giupponi L, et al. . Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Front Plant Sci. 2019;10:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. ICH Topic Q1A (R2): Stability Testing of new Drug Substances and Products (CPMP/ICH/2736/99). European Medicines Agency: London, 2003 [Google Scholar]
- 30. Press CA, Knupp KG, Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015;45:49–52 [DOI] [PubMed] [Google Scholar]
- 31. Merrick J, Lane B, Sebree T, et al. . Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis Cannabinoid Res. 2016;1:102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palazzoli F, Citti C, Licata M, et al. . Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal. 2018;150:25–32 [DOI] [PubMed] [Google Scholar]
- 33. Citti C, Palazzoli F, Licata M, et al. . Untargeted rat brain metabolomics after oral administration of a single high dose of cannabidiol. J Pharm Biomed Anal. 2018;161:1–11 [DOI] [PubMed] [Google Scholar]
- 34. Gerhard Nahler FG, Antonio Waldo Zuardi, José A.S. Crippa. A conversion of oral cannabidiol to delta9-tetrahydrocannabinol seems not to occur in humans. Cannabis Cannabinoid Res. 2017;2:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guy G, Wright S, Mead A, et al. Use of cannabinoids in the treatment of epilepsy. US Patent US Patent WO2016059403A1, 2016
- 36. ICH Q3A (R2) Impurities in new drug substances (CPMP/ICH/2737/99). UK: European Medicines Agency, 2006
- 37. Lachenmeier DW, Habel S, Fischer B, et al. . Are side effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination? F1000Research 2020;8:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.