Abstract
F1FO ATP synthase is responsible for the production of >95% of all ATP synthesis within the cell. Dysregulation of its expression, activity or localization is linked to various human diseases including cancer, diabetes, and Alzheimer’s and Parkinson’s disease. In addition, ATP synthase is a novel and viable drug target for the development of antimicrobials as evidenced by bedaquiline, which was approved in 2012 for the treatment of tuberculosis. Historically, natural products have been a rich source of ATP synthase inhibitors that help unravel the role of F1FO ATP synthase in cellular bioenergetics. During the last decade, new modulators of ATP synthase have been discovered through the isolation of novel natural products as well as through a ligand-based drug design process. In addition, new data has been obtained with regards to the structure and function of ATP synthase under physiological and pathological conditions. Crystal structure studies have provided a significant insight into the rotary function of the enzyme and may provide additional opportunities to design a new generation of inhibitors. This review provides an update on recently discovered ATP synthase modulators as well as an update on existing scaffolds
Keywords: F1FO, ATP synthase, ATPase, inhibitors, oligomycin, anticancer
Graphical Abstract
1. Introduction
F1FO ATP synthase (F1FO-ATPase) is a well-documented and highly studied enzyme due to its critical role in the synthesis of ATP, the primary currency for energy used in life, but also for its role in various diseases including cancer, neurodegenerative disorders, and mitochondrial disease resulting from genetic mutations. Since isolation of the F1 portion of ATP synthase by Racker in 1961, followed by identification of the chemiosmotic mechanism of ATP synthesis in the same year by Mitchell, significant efforts have been invested to improve our understanding of F1FO-ATPase’s structure, its function, and its role in disease.[1] In addition, extensive research has been pursued to develop compounds that modulate F1FO-ATPase activity. In 2008, a review was published by Hong and Pedersen, which identified over 250 compounds that target this molecular machine.[2] The purpose of this review is to provide a more recent update that outlines F1FO-ATPase research that has primarily occurred since 2008.
2. F1FO ATP Synthase
F1FO-ATPase is a membrane-bound multiprotein complex that is responsible for producing the majority of ATP synthesis within the cell via exploitation of the proton motive force that can drive the phosphorylation of ADP to ATP under aerobic conditions (oxidative phosphorylation). Although predominantly located within the mitochondria in the inner mitochondrial membrane (IMM), F1FO-ATPase is also expressed on the surface of various cell types (ectopic F1FO-ATPase) and can play various roles to modulate lipid metabolism, cell differentiation, survival and proliferation, angiogenesis and cancer, as well as intracellular pH.[3]
3. F1FO ATP Synthase Structure
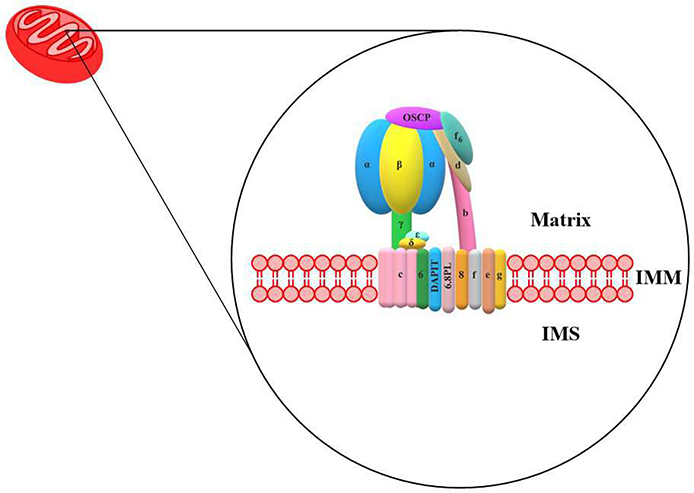
F1FO-ATPase is a heteroprotein complex that is composed of 29 subunits, with each subunit comprised of a single protein.[4,5] The DNA code for 27 of the proteins are nuclear genes, which are synthesized by cytosolic ribosomes and then transported into the mitochondria for assembly into the heteroprotein complex. The other 2 proteins (subunits 6 and 8) are encoded by mitochondrial DNA and are synthesized by ribosomes within the mitochondria. The genes associated with each subunit are provided in Table 1.
Table 1.
Mitochondrial F1FO-ATPase subunits and associated genes
| Groups | Subunit | Ratio | HGNC Symbol |
|---|---|---|---|
| F1 Catalytic Head | α | 3 | ATP5F1A |
| β | 3 | ATP5F1B | |
| F1 Central Stalk | γ | 1 | ATP5F1C |
| δ | 1 | ATP5F1D | |
| ε | 1 | ATP5F1E | |
| FO Rotor | 6 (ATP6) | 1 | MT-ATP6 |
| 8 (ATP8) | 1 | MT-ATP8 | |
| c | 8 | ATP5MC1 | |
| ATP5MC2 | |||
| ATP5MC3 | |||
| FO Peripheral Stalk | b | 1 | ATP5PB |
| d | 1 | ATP5PD | |
| f6 | 1 | ATP5PF | |
| OSCP | 1 | ATP5PO | |
| FO Supernumerary Subunits | e | 1 | ATP5ME |
| f | 1 | ATP5MF | |
| g | 1 | ATP5MG | |
| ATP5MGL | |||
| 6.8PL | 1 | ATP5MPL | |
| DAPIT | 1 | ATP5MD | |
| Inhibitory Factor 1 | IF1 | 1 | ATP5IF1 |
F1FO-ATPase is comprised of two major regions, which consists of the F1 and FO domains (Fig. 1). The F1 domain is derived from the name “Fraction 1”. The FO domain is named with the subscript “O”, due to the binding of oligomycin to this region. The F1 domain can be subdivided into two additional subunits, the F1 catalytic head and the F1 central stalk. The F1 catalytic head is composed of three alternating α and β subunits and the F1 central stalk contains one γ, δ, and ε subunits. The FO domain is subdivided into three groups: the FO rotor, the FO peripheral stalk, and the FO supernumerary subunits. The FO rotor consists of the subunits 6 (‘a’ or ATP6) and 8 (ATP8) and the c-ring (c8-ring) composed of eight identical c subunits. Inhibitory Factor 1 (IF1) is a inhibitory protein that can also regulate the activity of F1FO ATP synthase.
Fig. 1.
Human mitochondria F1FO-ATPase.
4. F1FO ATP Synthase Function
F1FO-ATPase is an ATP synthase and is solely dependent upon a proton gradient that is generated by the electron transport chain (ETC). The catalytic phosphorylation of ADP begins via the movement of a proton into F1FO-ATPase and ends with the release of ATP into the mitochondrial matrix. A proton from the IMS binds to an influx channel that exists between the c-ring and subunit 6, which ultimately leads to protonation of a hydrophilic residue. This change in hydrophobicity results in a counterclockwise rotation (as seen from the matrix) of the c-ring. Each proton that binds to subunit c will elicit a full rotation of the c-ring before it reaches the efflux channel. When the protonated c subunit reaches the efflux channel, it is readily deprotonated, and the proton is released into the matrix. This rotation of the c-ring is very rapid and occurs at a rate of approximately 130 revolutions per second.[6]
Rotation of the c-ring imparts a torque on the F1 central stalk that results in its rotation within the F1 catalytic head and is facilitated by the δ and ε subunits to provide additional support for the c-ring.[7] The F1 catalytic head is anchored by the FO peripheral stalk and held in a stationary position relative to the rotating F1 central stalk. The hexameric F1 catalytic head (α3β3) is the site of ATP catalysis, specifically within the β subunits.
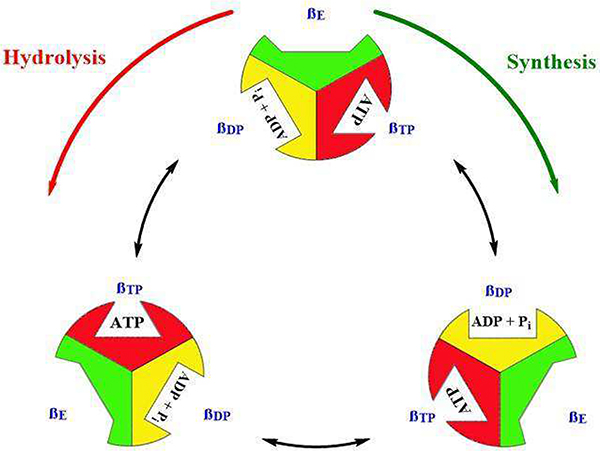
Rotation of the F1 central stalk results in perturbations of the F1 catalytic head that subsequently result in small conformational changes (Fig. 2). The catalytic sites exist in three unique binding states; βE (open or empty), βDP (loose or ADP bound) and βTP (tight or ATP bound).[8] When the catalytic site is in an open state, ADP and phosphate (Pi) can occupy the site. During the next 120° rotation, the catalytic site changes from an open state to a loose state wherein bound ADP + Pi are bound more closely. The next 120° rotation results in transformation of the catalytic site into a tight state wherein the synthesis of ATP occurs. During the final 120° rotation, the catalytic site returns to an open state and newly synthesized ATP is released. Hydrolysis of ATP occurs in a similar manner, but in which rotation is reversed.[9]
Fig. 2.
F1FO-ATPase catalytic states. Open states are shown in green, loose states in yellow, and tight states in red.
The FO subunits are not directly involved in the synthesis of ATP, but perform supporting roles for the function of ATP synthase, which includes structural support (particularly the peripheral stalk), dimerization, and oligomerization. Cristae formation within the mitochondria has been shown to be influenced by dimerization and oligomerization of F1FO-ATPase to increase the overall surface area of IMM for the respiratory chain.[10] Moreover, knockdown studies of DAPIT and the 6.8PL subunits have demonstrated a decrease in both the population of ATP synthase and ATP production, demonstrating their importance in the biogenesis and proper functioning of the ATP synthase.[4,11]
5. Implications for Disease
Given the complexity of the molecular machinery of F1FO-ATPase in terms of interconnectivity and interactions among subunits, minor disruptions to the structure or conformation inhibit or impair ATP synthesis and/or hydrolysis. Mitochondrial diseases which often result from genetic mutations, lead to disruption of F1FO-ATPase biogenesis and an underdeveloped cristae, insufficient F1FO-ATPase populations and ATP synthesis, and poor prognosis for those affected. The therapeutic role of F1FO-ATPase spans various areas of human health including cancer, neurodegenerative disorders (Alzheimer’s and Parkinson’s), cardiovascular and other metabolic diseases as diabetes.[2,12] The aberrant ectopic expression of the β subunit has been demonstrated in various cancers, including non-small cell lung cancer, acute myeloid leukemia and prostate cancer.[13–15] Inhibition of the ectopic β subunit by a monoclonal antibody (Mc178-Ab) led to apoptosis via the MAPK and Akt pathways.[16] Downregulation of the mitochondrial β subunit in tumors such as breast, lung, colon and squamous lung carcinomas was affected at the translation step, which may be regulated by the AMPK and/or GCN2–ATF4 pathway.[17,18] It was also shown that mitochondrial F1FO-ATPase translocates to cell surface in hepatocytes and has high activity in tumor-like acidic and hypoxic environments.[19] In addition, overexpression of the ε subunit was shown to promote metastasis in colorectal cancer.[20] Endogenous p53 has also been shown to interact with a subunit OSCP and may play an important role in tumor suppression via the regulation of F1FO-ATPase activity.[21] In neurodegeneration, the α subunit of the ATP-synthase is targeted for oxidative damage in the very early stages (Braak stages I/II) of AD pathology.[22] Moreover, Aβ in Alzheimer’s and α synuclein in Parkinson disease, interact with the F1FO-ATPase to open the mitochondrial permeability transition pore (mPTP).[23,24] F1FO-ATPase-Cyclophilin D (CyPD) interactions were also shown to increase during diabetic encephalopathy.[25]
6. Compounds Targeting F1FO ATP Synthase
6.1. Polyketides Inhibitors
Polyketides are a group of secondary metabolites produced by various microorganisms. Many polyketides have shown antibacterial, antifungal, and/or immunosuppressive activities. Some of these polyketides such as oligomycin, aurovertin, apoptolidin, mandelalide and cruentaren have been shown to manifest inhibitory activity against F1FO ATP synthase, and thus, exhibiting anti-cancer activity (Fig. 3–6).
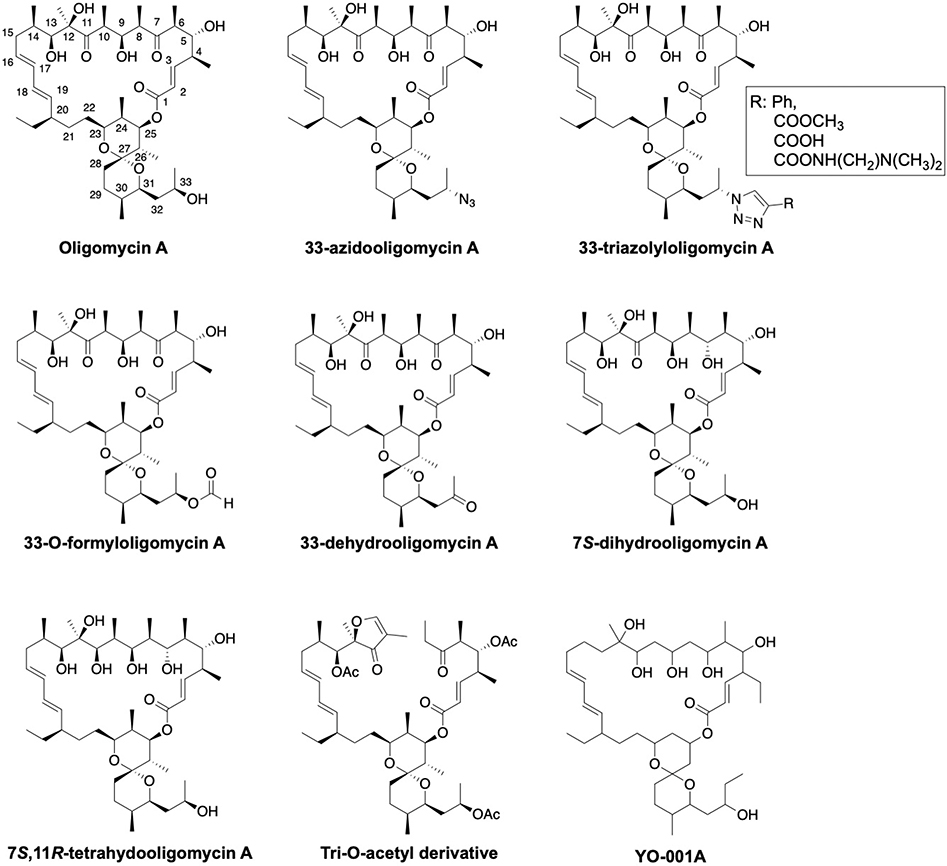
Fig. 3:
Structures of oligomycin A and derivatives
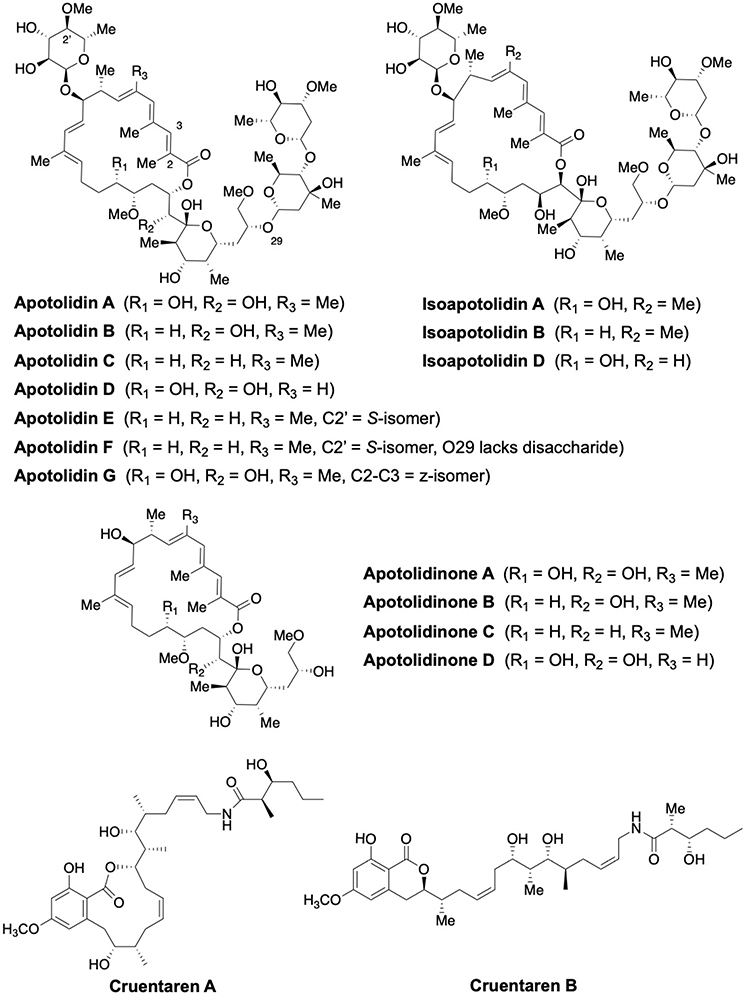
Fig. 6:
Structures of apoptolidins A-G, Isoapoptolidin A, B and D, Apoptolidinone A-D and Cruentaren A-B
Oligomycins, which are produced by Streptomyces sp. constitute a class of 26-membered macrolactones that contain a spiroketal. Oligomycin targets the c subunit, which blocks ATP synthesis and inhibits oxidative phosphorylation.[26] There is a strong interest to develop oligomycin as an anticancer therapeutic due to its selective cytotoxic activity against tumor cells, however, high toxicity and low water solubility hinder its clinical application and development. In addition, oligomycin also inhibits P-glycoprotein (P-gp), and prevents oncogenic K-Ras activation by disrupting its localization to the plasma membrane, further complicating the development of this class of molecules.[27,28]
Limited structure-activity relationship (SAR) studies for oligomycin A have been performed in an effort to overcome these shortcomings (Fig. 3). Side-chain substitutions (azide, triazole and formyl) onto the spiroketal ring resulted in compounds that retained activity against cancer cell lines (human leukemia cell line K562 and colon carcinoma cell line HCT116; IC50 = 0.1–1 μM, excepting a COOH derivative) similar to that of oligomycin A.[29,30] 33-Dehydrooligomycin A was ~3.7 times more potent against K-562 cells and ~1.7 times less potent against HCT116 cells when compared to oligomycin A.[31] Enantioselective reduction of the C7 and C11 ketones at provided (7S)-dihydrooligomycin A and (7S,11R)-tetrahydrooligomycin A derivatives that also maintained inhibitory activity against K562 and the doxorubicin-resistant subline, K562/4, but were slightly less active against HCT116 (~3.5-fold) and its doxorubicin-resistant subline HCT116(−/−) (~1.8-fold).[32] The IC50 values of a tri-O-acetyl derivative of oligomycin A were 3.1 μM (HCT116 cell line) and 0.9 μM (K562 cell line), while oligomycin A exhibited IC50 values of 1 μM (HCT116 cell line) and 0.2 μM (K562 cell line).[33]
YO-001A is a recently identified antifungal macrolide from the oligomycin family that was isolated via a soil sample collected at Toyama Prefecture in Japan (Fig. 3). Compared to oligomycin A, YO-001A does not contain functionalities at C13, C14 and C24. However, YO-001A was shown to inhibit F1FO-ATPase with an IC50 of 1 μM against isolated bovine heart mitochondria, and demonstrated modest cytotoxicity in the HeLa (IC50 = 8.2 μM) and HL-60 (IC50 = 5.8 μM) cancer cell lines.[34]
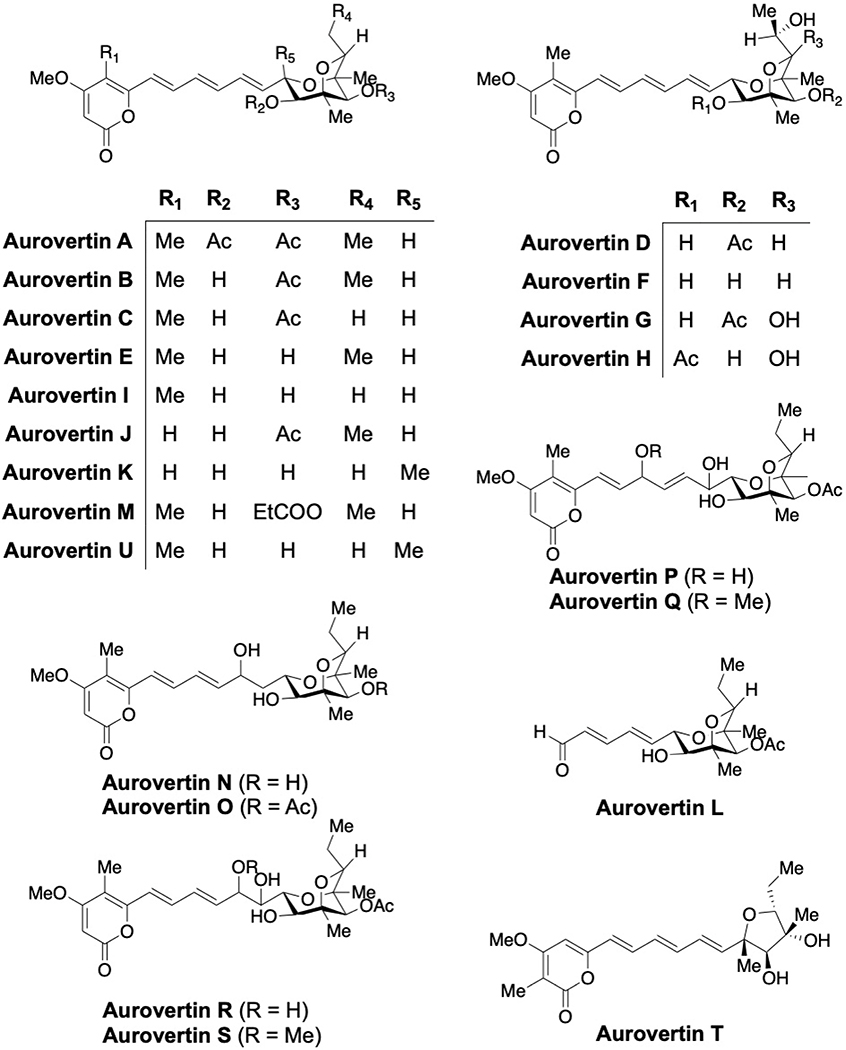
Aurovertins A-E are secondary metabolites that are produced by the fungus Calcarisporium arbuscular and contain a polyketide structure, a 2,6-dioxabicyclo[3.2.1]octane ring system and an α-pyrone moiety.[35–39] Aurovertins F-U have recently been isolated from other fungi (Fig. 4).[40–43] Reports of the synthesis and production of aurovertins and structurally related compounds were recently reviewed.[44]
Fig. 4:
Structures of aurovertins A-U
Aurovertin B binds to the β subunits wherein β(Arg412) and β(Tyr458) are responsible for making important interactions that result in the inhibition of F1FO-ATP synthase.[45,46] Aurovertin is a mixed, noncompetitive inhibitor of F1FO-ATPase. In fact, it inhibits ATP synthesis more strongly than ATP hydrolysis. Interestingly, inhibition of ATP hydrolysis by aurovertin is never complete, even under saturating conditions. A mechanistic study attempted to address the basis for the inhibitory behavior of aurovertin using F1FO-ATPase complex from bovine heart mitochondria and E. coli membrane.[47] Stoichiometric experiments suggested that two molecules of aurovertin are required to bind the F1 domain at low substrate concentration, while cooperativity decreases to one at higher concentrations. This data may explain the differential activity observed for ATP hydrolysis (Ki(ES) = 120 nM) vs synthesis (Ki(ES) = 25 nM), illustrating the fact that aurovertin exhibits a higher affinity for the F1 binding site during ATP synthesis. In contrast, loosely bound aurovertin appears to hinder catalytic interactions during ATP hydrolysis, and thus, prolonging ADP-release, which is the rate-limiting step.
Aurovertin B targets the β subunit of ectopic F1FO-ATPase in the breast cancer cell lines T-47D, MDA-MB-231 and MCF-7, which also inhibits cellular proliferation with IC50 values of 0.89, 5.52, and 0.09 μM, respectively, while remaining relatively non-toxic to the normal MCF-10A human breast cell line at 10 μM.[2,48] In addition, aurovertin B was able to sensitize colorectal cancer cells to recognition and lysis by natural killer cells.[49] Aurovertin P displayed moderate cytotoxicity against various tumor cell lines (SMMC-7721; IC50 = 18.3 μM and SW480; IC50 = 14.4 μM) whereas aurovertin U inhibited the growth of MDA-MB-231 cells with an IC50 of 5.43 μM.[42,43] Interestingly, aurovertin T was ineffective in a cytotoxicity assay, which illustrates the importance of the 2,6-dioxabicyclo[3.2.1]octane system for anti-cancer activity.[43]
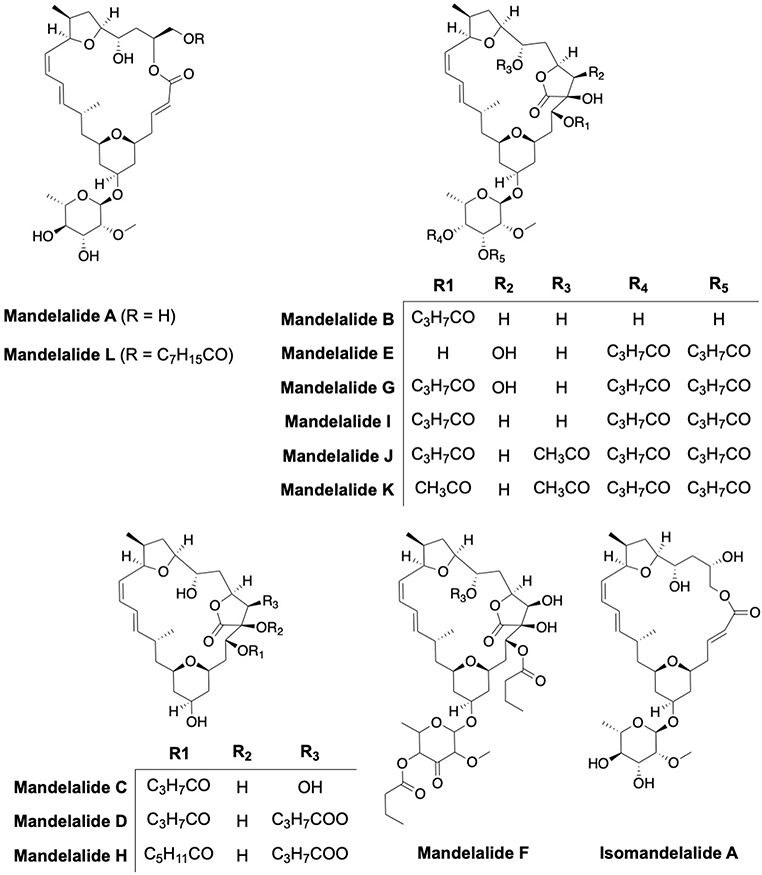
Mandelalides are marine macrocyclic polyketides produced by Lissoclinum sp; a rare ascidian or sea squirt species (Fig. 5). Mandelalides A-L manifest cytotoxicity against the HeLa cervical and NCI-H460 lung cancer cells with mandelalides A, B, F and L manifesting EC50s at the nanomolar concentration range (2.8 nM to 270 nM).[50–52] Mandelalides A and B, which are similar to oligomycin and apoptolidin, were found to be selective inhibitors of the ATP synthase. The IC50 values for inhibition of F1FO-ATPase by mandelalides A and C were 2.2 nM and 3.4 μM, respectively.47 A synthetic ring-expanded isomandelalide A analog exhibited an EC50 value of 17 nM and 30 nM against HeLa and NCI-H460 cells, respectively.[53]
Fig. 5:
Structures of mandelalides A-L and isomandelalide A
Apoptolidin A, a 20-membered macrolide was first isolated by the Seto group in 1993 from the culture broth of Nocardiopsis sp.[54,55] Later, Wender’s group extracted compounds with a similar structure, which they referred to as Apoptolidins B-D.[56,57] Isolation of apoptolidin E-H followed (Fig. 6) and subsequent studies determined that mitochondrial F1FO-ATPase was the molecular target of apoptolidin A.[58–61] Semisynthetic analogs of apoptolidin have also been prepared and evaluated with hopes that compounds exhibiting superior stability and pharmacokinetic properties could be identified.[62–65]
Growth inhibition of human lung carcinoma H292 cells by apoptolidins has been reported to occur at concentrations between 7–150 nM, when the glycoside is present.[56,57] Less active compounds 2′-O-succinyl-apoptolidin A and 3′-O-succinyl-apoptolidin A, were also isolated from Amycolatopsis sp. Serrill and co-workers confirmed that apoptolidin A and C activate AMPK, which triggers autophagy in cells containing the sensitive metabolic phenotype.[66,67] Isoapoptolidin A is 10-fold less potent at the inhibition of F1FO-ATP synthase as compared to apoptolidin A.[68] The synthetic aglycones, apoptolidinones A and D, were inactive against the H292 cell line highlighting the need for the sugar appendages.[69]
Cruentaren A and B are members of the benzolactone class of macrolides, which were isolated from Myxobacterium Byssovorax cruenta and exhibit both antifungal and anticancer activities (Fig. 6). Cruentaren A manifested high cytotoxicity (IC50 = 1.2 ng/mL) towards the L929 mouse fibroblast cell line, while congener B was inactive (1 μg/mL).[70] Cruentaren A showed growth inhibition against multiple human cancer cells including cervix carcinoma KB-3–1, multi-drug resistant KB-V1, chronic myelogenous leukemia K-562 and kidney carcinoma A498. Cruentaren A was shown to bind the F1 domain and to selectively inhibit ATP synthesis with an IC50 of 15–20 nM, but without inhibition of Na+/K+ ATPase or V-ATPase up to 1 μM concentration.[71] It was reported that F1FO ATP synthase acts as an ancillary protein during the Hsp90-mediated protein folding process, which is a promising target for anti-cancer therapy.[72,73] A number of groups have reported the total synthesis and biological evaluation of cruentaren A and a few analogs, which led to the identification of a few key functional groups that are important for inhibitory activity.[74–77]
6.2. Polyphenols
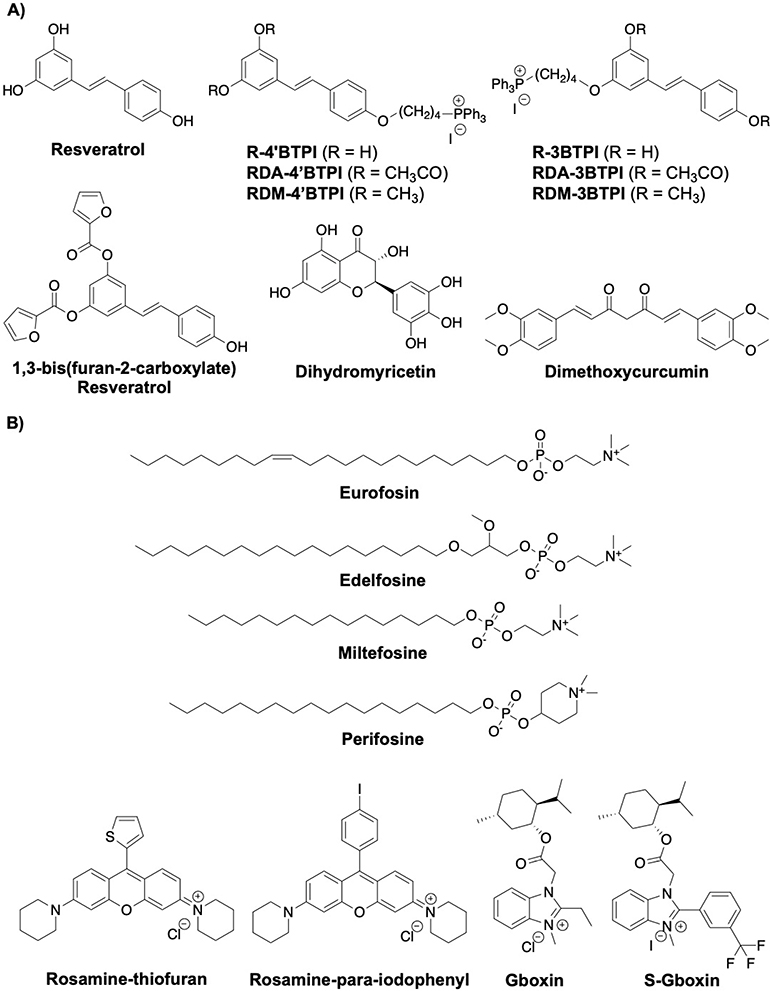
Polyphenols represent a class of natural products found in plants that appear to play a vital role in human health. The protective effects of polyphenols have been widely demonstrated in various diseases such as obesity, neurodegenerative disease, type 2 diabetes, and cardiovascular disease.[78] Polyphenols such as resveratrol, dihydromyricetin and curcumin can affect multiple targets while continuing to inhibit F1FO ATP synthase (Fig. 7A).
Fig. 7:
Structures of polyphenols and cationic compounds
Resveratrol was shown to interact with a hydrophobic pocket in the F1 domain that is created between the γ subunit and the βTP subunits. Upon binding, resveratrol blocks rotation of the γ subunit, thereby inhibiting the catalytic activity of the F1FO complex.[79] The acetyl and methyl capped analogs (RDA compounds) were also shown to inhibit ATPase at 50 μM, while R-3BTPI and R-4′BTPI did not affect the hydrolytic activity.[80] Resveratrol derivatives with heterocyclic extensions were also synthesized and evaluated for anticancer activity against MCF-7 cells. A synthetic derivative that contains a furan (IC50 = 42.7 μM) was 2-fold more potent than resveratrol and appears to inhibit F1FO-ATPase.[81]
Dihydromyricetin is a bioactive component that can be isolated from the medicinal plant species, Ampelopsis, and exhibits a variety of pharmacological effects such as antioxidant, antibacterial, anticancer, antiobesity and neuroprotective activity. Dihydromyricetin (DHM), a natural flavonoid, inhibits F1FO-ATPase activity and increases the AMP/ATP ratio which activates AMPK. The inhibitory activity manifested by DHM induces autophagy and prevents insulin-resistant related metabolic diseases.[82]
Curcumin, found in the rhizome of Curcuma sp. has also been reported to inhibit F1FO-ATP synthase.[2,83] A synthetic analog, dimethoxycurcumin exhibits cytotoxic activity against MCF-7 cells via induction of reactive oxygen species (ROS), DNA damage, mitochondrial dysfunction, and disruption of the p53/p21/CDK4 pathway. It also decreases cellular ATP levels by suppressing the α, β, γ and ε subunits of the ATPase complex.[84]
6.3. Cationic Inhibitors
Cationic compounds have been shown to accumulate selectively in the mitochondria due to the negative potential that exists across the IMM (Fig. 7B). Therefore, compounds that contain triphenylphosphine moiety or a choline ester that is positively charged are often used to aid uptake into the mitochondria. Some cationic compounds have also been shown to possess favorable pharmacokinetic properties, enhancing their potential for clinical activity.[85]
The alkylphosphocholine or alkylphospholipid (APL) analogs constitute a class of anticancer agents that manifest various medicinal properties and include promising lead compounds/drugs, such as miltefosine, edelfosine, erufosine and perifosine. Miltefosine, sold as Impavido, is the first orally-active treatment for visceral leishmaniasis.[86] Miltefosine is also an ingredient of Miltex, which is used topically for the treatment of skin metastases resulting from breast cancer.[87]
Erucylphosphohomocholine (ErPC3, Erufosine) was shown to target the mitochondria by activating an apoptotic pathway via the 18 kDa Translocator Protein (TSPO).[88] Effects on ROS generation and mitochondrial membrane potential ( Δψm) have also been observed with Erufosine. Concomitant treatment of cells with respiratory chain inhibitors and F1FO complex inhibitors support a role for the FO subunit in this apoptotic pathway.[89] Erufosin induced selective apoptosis of a highly apoptosis-resistant malignant glioma cell line, but not normal cells.[90]
The induction of apoptosis by edelfosine was shown to involve the mitochondria and cholesterol-rich lipid rafts. Edelfosine was the most active APL, followed by perifosine, miltefosine and erufosine. These compounds exhibited promising anti-cancer activity against several human cancer cell lines and include leukemia (HL-60, Jurkat and CEM-C7H2), multiple myeloma (MM144), and cervical cancers (HeLa). Genetic deletion in S. cerevisiae and other experiments indicate F1FO-ATPase plays a critical role in the generation of ROS. Proteomic analysis demonstrated a translocation of the F1FO-ATPase β subunit to the lipid raft in drug-treated Jurkat cells. The exact mechanism has not been established, however it appears that edelfosine may perturb membrane integrity, which alters the proton gradient and thus, stimulates the ATP hydrolase activity and cell death.[91]
Fluorone dyes like rhodamine 123 have been reported to inhibit F1FO-ATP synthase by binding the F1 domain.[2,92] Rosamines are a structurally related class of molecules that lack a carboxylic acid at the meso-position on the phenyl ring. Lim and co-workers synthesized 16 rosamine derivatives, two of which contained a thiofuran or a para-iodophenyl substitution at the meso position, which were found to inhibit human leukemia HL-60 at an IC50 of ~0.1 μM.[93] These compounds showed potent anti-proliferative activity against several cancers such as colon, breast, oral and nasopharynx. It was proposed that the rosamine derivatives localize specifically to the mitochondria to affect both complex II and the ATP synthase. The thiofuran and para-iodophenyl derivatives inhibited ATP synthase activity with IC50 values of 3 and 3.9 μM, respectively. Unfortunately, the thiofuran compounds showed very modest activity in 4T1 murine breast cancer-bearing female BALB/c mice model in terms of tumor growth delay.[94]
A high-throughput screen with primary glioblastoma cells identified the imidazole-based small molecule, Gboxin as an F1FO-ATPase inhibitor. It was determined that gboxin binds ATP synthase through various biochemical and pull-down assays. Gboxin also selectively inhibited the growth of cancer cells (IC50 = 150 nM against high-throughput GBM sphere cells), while sparring normal embryonic fibroblasts and neonatal astrocytes. Structure-activity relationship studies led to the discovery of S-Gboxin (IC50 = 470 nM), which contains a 3-trifluoromethylphenyl group. S-Gboxin decreased the tumor volume in in vivo studies, but failed to ablate the tumor.[95]
6.4. Natural Peptides and Endogenous Ligands
A variety of natural peptides and endogenous ligands have shown diverse activities against F1FO ATP synthase on the cell surface, which stimulates downstream effects that ultimately, induce anticancer activity (Fig. 8 and 9).
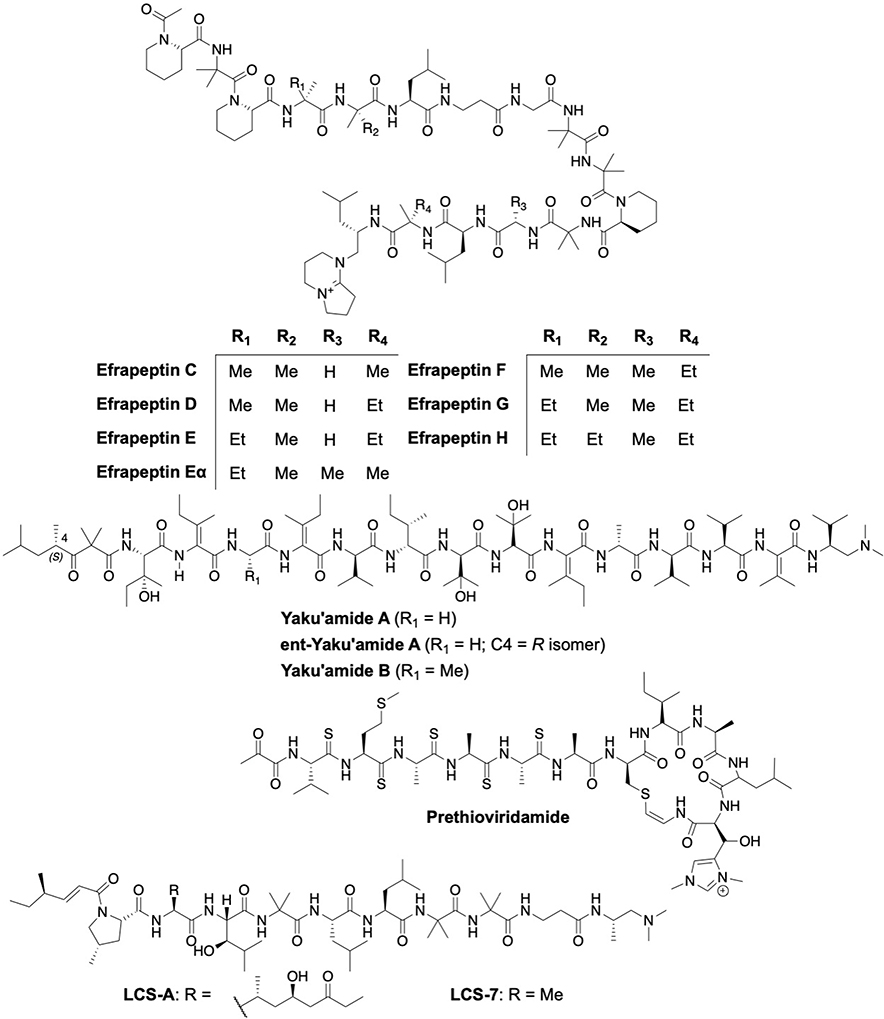
Fig. 8:
Structures of natural peptides
Fig. 9:
Structures of endogenous ligands
Efrapeptins are peptaibiotics that contain a 15-mer linear peptide rich in Cα-dialkyl amino acids that are produced non-ribosomally by the fungi, Tolypocladium. Structurally, these metabolites consist of non-proteinogenic amino acids such as α-aminoisobutyric acid, isovaline, β-alanine or pipecolic acid, and have an acetylated N-terminus and an unusual cationic C-terminal cap. The co-crystal structure determined efrapeptin to bind the F1 domain. Efrapeptin inhibits F1FO catalytic activity by preventing the βE subunit to assemble into a conformation that is amenable for nucleotide binding.[96] Efrapeptin displayed potent cytotoxic activity against a variety of organisms via inhibition of the 20S proteasome, F1FO synthase and/or other ATPases.[97,98] Efrapeptin F displays preferential cytotoxicity (IC50 = 52 nM) to pancreatic cancer cells (PANC-1) under nutrient-deprived conditions in vitro and in vivo.[99] Efrapeptin was also shown to selectively eliminate liver cancer cells that express high levels of SALL4.[100] In addition, interactions between F1FO-ATPase and Hsp90 are perturbed by efrapeptins, which resulted in the degradation of select Hsp90 clients as well as decreased levels of Hsp70, Hsp90, and Hsp27.[101]
Yaku’amide A and B are anticancer tetradecapeptides produced by the rare deep-sea sponge, Ceratopsion, and contain four β,β-dialkylated α,β-unsaturated amino acid residues.[102] Analysis with synthetic chemical probes of Yaku’amide B revealed it to predominantly localize in the mitochondria and to interact with the F1FO-ATP synthase complex, resulting in the growth arrest of MCF-7 (IC50 = 10 nM) and P388 mouse leukemia cell lines (IC50 = 0.51 nM). Moreover, an unnatural enantiomer of Yaku’amide B was synthesized that inhibited MCF-7 cell growth at 3-fold lower potency as compared to the natural peptide. Yaku’amide B acts via two distinct modes of action; inhibition of ATP synthesis and increased ATP hydrolysis. Yaku’amide B inhibits ATP synthesis 9-fold more effectively than its enantiomer. In contrast, Yaku’amide B enhances the hydrolytic activity of the ATPase complex by 300%, whereas the enantiomer is completely inactive.[103] In addition, 14 stereoisomers of Yaku’amide B were synthesized and evaluated, the results from which indicate non-specific hydrophobic interactions contribute to their inhibitory activity.[104]
Amycolatopsis alba and Streptomyces sp. produce ribosomally processed and post-translationally modified peptides (RiPPs), which include thioviridamide, prethioviridamide, JBIR-140, neothioviridamide (thiostreptamide S87 or thioholgamide A), thioalbamide, thiostreptamide S4, and thioholgamide B. All of these natural products manifest cytotoxicity against various human cancer cell lines and serve as starting points for modern drug discovery efforts.[105–112] These unique peptides contain an unusual thioamide bond. It was determined that prethioviridamide induced the integrated stress response (ISR) via the GCN2-eIF2α-ATF4 pathway by inhibition of the F1FO-ATP synthase complex. Thioviridamide and prethioviridamide both exhibit >25-fold selective cytotoxicity towards E1A-3Y1 (transfected with adenovirus oncogene E1A) cells over 3Y1 (rat fibroblasts) cells and were also active against the HeLa S3 human cervical carcinoma cell line.[106,113]
Leucinostatin (LCS)-A, a nanopeptide from the fungi, Paecilomyces lilacinus, and its synthetic derivative LCS-7 were shown to inhibit ATP synthase activity (IC50 = 2.8 μg/mL). Inhibition of mitochondria ATP synthase activity suppressed the growth of prostate cancer DU-145 cells in vitro and in vivo via reduction of insulin-like growth factor-1 secretion by prostate stromal cells. This activity resulted in strong antiproliferative effects by LCS-A and LCS-7 on DU-145 (androgen-unresponsive) and LNCaP (androgen-responsive) cells in cultures and included prostate stromal cells (PrSCs).[114,115]
PEDF is a multifunctional 50 kDa protein that belongs to the serpin superfamily and is secreted by most cell types. PEDF was shown to bind the β-subunit of ectopic F1FO-ATPase on endothelial cells.[116] Structure-activity relationship studies demonstrated that a PEDF-derived 34-mer peptide composed of amino acids 44–77 performed similar to the full-length PEDF peptide as a measurement of ATP synthase inhibiton and anti-tumor activity.[117] The anti-tumor activity against neuroblastoma was first reported by Crawford and coworkers wherein the ganglionic and Schwann cells produced PEDF that inhibited angiogenesis while promoting the growth of Schwann cells.[118] PEDF exhibits promising anti-cancer activity against multiple cancer types such as glioma, breast, lung, prostate, pancreatic carcinomas, ovarian, melanoma, and osteosarcoma.[119] PEDF also modulates interactions between macrophages and prostate cancer cells to induce tumor cell apoptosis and phagocytosis.[120] Moreover, PEDF acts as a tumor-suppressor in fibroblasts as well as in nasopharyngeal carcinoma during epithelial– mesenchymal transition (EMT) and metastasis.[121,122]
Human alpha-lactalbumin made lethal to tumor cells (HAMLET) is a proteolipid complex that manifests anti-tumor activity in in vitro and in vivo studies.[123] HAMLET reduced cellular ATP levels in A549 lung carcinoma cells and was found to localize the α and β subunits of F1FO-ATPase. Upon binding to the F1 domain with a KD value of 20.5 μM, HAMLET led to reduced rotation rates of the molecular motor.[124]
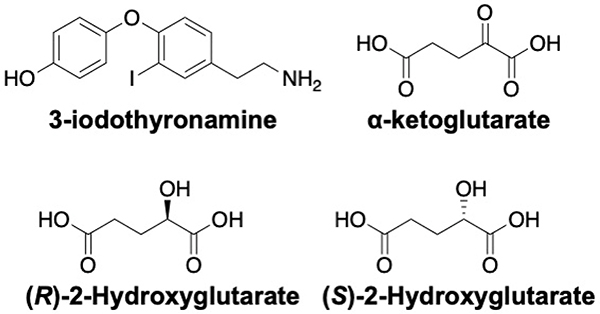
3-iodothyronamine (T1AM) is an endogenously produced signaling molecule that manifests various physiological properties by the modulation of various extra- and intra-cellular targets (Fig. 9). T1AM was found to be a non-competitive inhibitor of F1FO-ATP synthase and binds two different sites; one within the IF1 binding site in the F1 domain and the other at the aurovertin binding site. The binding of T1AM was mutually exclusive with IF1 and aurovertin B. T1AM increases mitochondrial respiration in H9c2 cardiomyocytes at low nanomolar concentrations by displacing IF1 (high affinity site) at micromolar concentrations, it also binds to the aurovertin site to inhibit enzymatic activity.[125]
α-ketoglutarate (α-KG), an important intermediate in the Krebs cycle was shown to inhibit ATP synthase in bovine heart mitochondria and to extend the lifespan of C. elegans. In a similar manner, both 2-Hydroxyglutarate (2-HG) enantiomers, which are produced by mutant IDH1/2, were also demonstrated to act on F1FO-ATPase in U87 human glioblastoma cells. Accordingly, inhibition of F1FO-ATPase by α-KG or 2-HG was sufficient for antiproliferative and cytotoxic activity in glioblastoma cells under low glucose conditions.[126,127]
6.5. Natural and Synthetic Miscellaneous Modulators
Small molecules from natural as well as synthetic origins have been successfully developed to act on diverse biological targets, including F1FO-ATPase (Fig. 10 and 11). In addition, the repurposing of approved drugs has gained momentum to reduce the cost and time needed for lengthy clinical trials. Alternatively, RNA oligonucleotides have been viewed as a potential therapeutic approach due to their high affinity and specificity (Fig. 11).
Fig. 10:
Structures of natural small molecules
Fig. 11:
Structures of synthetic modulators
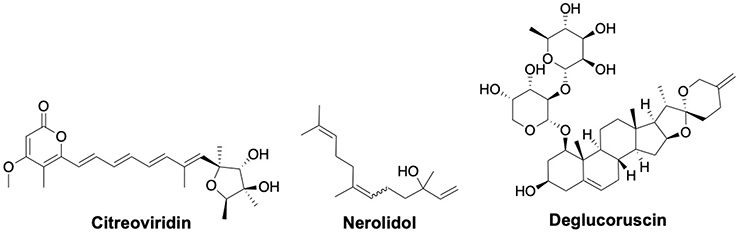
Fungi such as Penicillium and Aspergillus produce citreoviridin which has been shown to inhibit F1FO-ATPase activity by binding to the β subunit at a site different than that of aurovertin.[128,129] Further, it was determined that it acts as an uncompetitive inhibitor of ATP hydrolysis and as a noncompetitive inhibitor of ATP synthesis.[130] Ectopic F1FO-ATPase and ETC on lung adenocarcinoma and breast cancer cells has been shown to contribute to tumor growth. Citreoviridin, which inhibits ectopic F1FO-ATPase activity, increased the unfolded protein response (UPR) and ROS to selectively block cancer cell proliferation.[131,132] Proteomic analysis of citreoviridin administered in a lung cancer xenograft model suggested that gluconeogenesis is activated by upregulating the expression of gluconeogenic enzymes, namely UDP-glucose pyrophosphorylase, inositol-3-phosphate synthase 1 and aldose reductase. Reduced glycolytic intermediates that are required for macromolecule synthesis resulted in the inhibition of cell proliferation.[133]
Nerolidol or peruviol is a sesquiterpene alcohol present in the essential oils from various plants. Nerolidol is approved by the FDA for use as a flavoring agent in food. Nerolidol also manifests various biological activities including antitumor, antioxidant, anti-inflammatory, antiulcer, and antimicrobial.[134] Nerolidol may affect the activity of F1FO-ATPase to decrease ATP/ADP levels in a concentration-dependent manner, as it was shown that nerolidol inhibits F1FO ATPase activity at low micromolar concentrations.[135]
A multi-disciplinary study based on chemical proteomics, molecular modelling and bio-organic assays concluded that deglucoruscin, a spirostanol component isolated from an extract of Ruscus aculeatus, interacts with F1FO-ATP synthase with a Ki value of 10 μM.[136,137] Docking studies suggested that it may bind similar to 8-chloroadenosine and resveratrol and serve as a competitive inhibitor to trap the enzyme in an inactive conformation.[137]
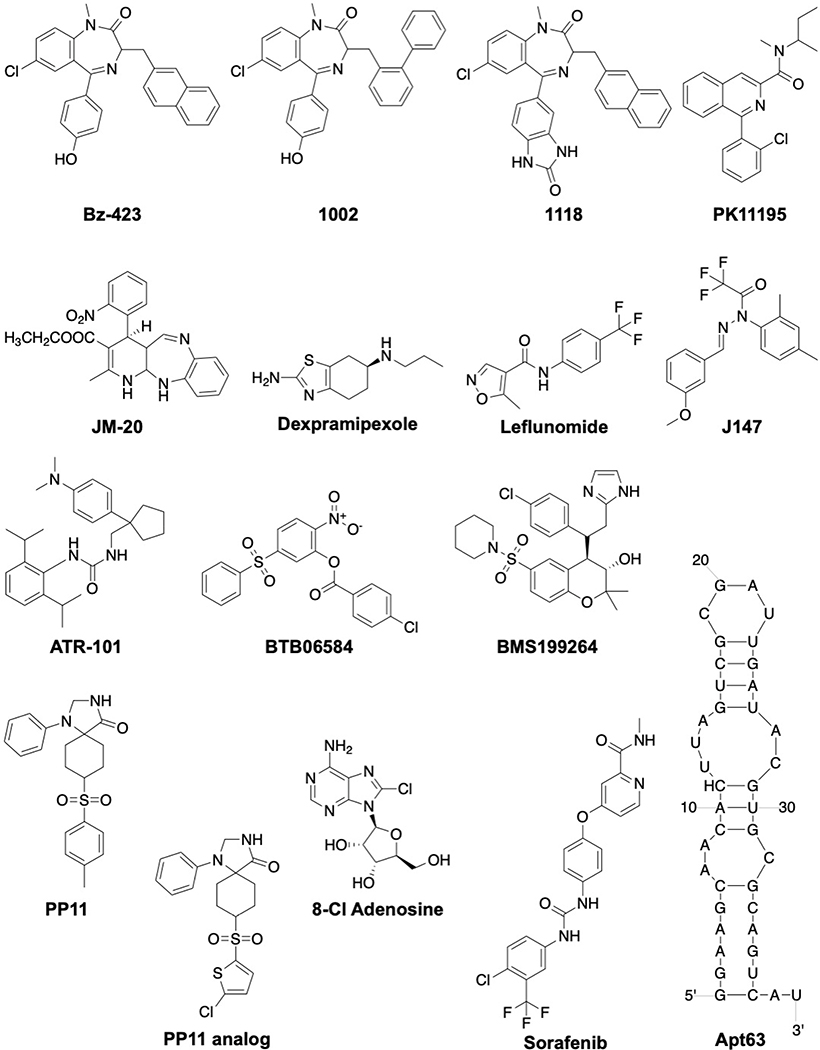
Benzodiazepine (Bz)-423 has been shown to selectively induce apoptosis in CD4+ T cells in an animal model of lupus.[138] The binding site for Bz-423 was localized to a shoulder region of OSCP via NMR spectroscopy and exhibit an allosteric mode of inhibition.[139] This binding site, which is also shared by cyclophilin D, was recently confirmed as the mechanism by which Bz-423 sensitizes the mPTP to Ca2+.[140–142] Bz-423 reduces the rate of both ATP synthesis and hydrolysis and promotes superoxide formation, which leads to inactivation of anti-apoptotic PI3K-Akt signaling and selective upregulation of Noxa and Bak pro-apoptotic proteins in pathogenic lymphocytes.[143] Bz-423 was also shown to selectively kill alloreactive T cells manifesting graft-versus-host disease in bone marrow transplant models without affecting hematopoietic engraftment or lymphocyte reconstitution both in vitro and in vivo.[144] Two analogs (1002, 1118) that differ by substitutions on the diazepinone ring were reported to manifest comparable activity to Bz-423.[145]
PK11195 inhibits both ATP synthesis (EC50 = 33 μM) and hydrolysis (EC50 = 230 μM) by presumably by binding the OSCP subunit.[146,147] As a consequence, PK11195 alters the mitochondrial integrity and selectively targets Bcl-2 knockdowned in HeLa cells and chronic lymphocytic leukemia (CLL) cells.[148,149] In comparison, JM-20 inhibits only the hydrolytic activity of F1FO-ATPase and increases cellular ATP levels, and thus, manifests cytoprotective activity. This was confirmed by a JM-20 mediated rescue of PC-12 cells at low nanomolar concentrations.[150]
Dexpramipexole is the R-enantiomer of the antiparkinsonian drug, pramipexole, which binds to subunits b and OSCP to increase the rate of ATP synthesis and improve mitochondrial bioenergetics. It was also hypothesized that dexpramipexole binding to the FO domain could inhibit opening of the mPTP pore.[151] Unfortunately, the cytoprotective effects of dexpramipexole did not yield positive outcomes in a Phase III clinical trial for the treatment of amyotrophic lateral sclerosis (ALS).[152]
Leflunomide is an anti-inflammatory drug that is used for the treatment of rheumatoid arthritis. During an investigation of its off-target effects, it was found that leflunomide and its active metabolite, A771726, inhibited the ATP synthase with IC50 values of 35.0 μM and 63.7 μM respectively. The ensuing depletion of ATP levels led to cytotoxic response in HepG2 cells, and provides rationale for the adverse effects manifested by leflunomide on the liver.[153]
A phenotypic screening assay based on age-associated pathologies identified a small molecule (J147) that showed neuroprotective activity in vitro and in vivo.[154,155] J147 was found to localize in the mitochondria and to partially inhibit the ATP synthase (EC50 = 20 nM) via modulation of the α subunit. In vivo, J147 prevented age-associated drift in mice and extended the lifespan of drosophila.[155] Based on the structure of J147, a pharmacophore model was built to identify additional compounds (ZINC04549531, ZINC70656000 and ZINC70656005) that exhibited similar biological profiles.[154–157]
ATR-101 (Nevanimibe or PD132301–02) is a urea derivative and a potent acetyl-CoA acetyltransferase (ACAT1) inhibitor. It exhibits anti-tumor activity against the adrenocortical carcinoma (ACC)-derived H295R xenograft model in vivo. Nevanimibe was also found to inhibit F1FO-ATP synthase, resulting in mitochondrial hyperpolarization.[158] Evaluation of nevanimibe in a Phase I study concluded that the requisite drug concentrations needed to induce apoptosis was not achieved and thus, resulting in its discontinuation for the treatment of ACC.[159]
BTB06584 was discovered in a chemoinformatic screen based on the structure of BMS199264, which was previously shown to exhibit cardioprotective effects.[160,161] BTB06584 inhibited F1FO-ATPase hydrolytic activity while having no effect on ATP synthesis as illustrated by ΔΨm and O2 consumption. The activity manifested by BTB06584 was dependent upon the expression of IF1. Docking experiments suggested interactions between BTB06584 and the F1 portion of F1FO-ATPase.[162] Recently, it was shown that BTB06584 increased the sensitivity of non-small cell lung cancer cells to radiotherapy via the inhibition of ATP hydrolysis.[163]
The 1,3,8-triazaspiro[4.5]decane-scaffold represents a first-in-class small molecule mPTP inhibitor that was designed to target subunit c of F1FO-ATPase. The triazaspiro core was identified via a high-throughput screen based on a similar region of oligomycin, a well-known inhibitor of mPTP opening.26 Proximity ligation assay (PLA)-based studies indicated the spiro compounds (PP11 and analog) to stabilize ATP synthase dimers and desensitize mPTP for protection against cell death in a cardiovascular model of disease.[164]
8-chloroadenosine (8-Cl-Ado) is a ribonucleoside analog that integrates into RNA and inhibits polyadenylation to impede transcription.[165,166] In addition, it decreases cellular ATP levels suggesting an interference with ATP synthase. Molecular docking and functional assays indicated that 8-Cl-ADP and 8-Cl-ATP may act as a substrate and inhibitor of F1FO ATP synthase, respectively.[167] This hypothesis is supported by both basal mitochondrial respiration and glycolysis measurements following 8-Cl-Ado treatment.[166] Depletion of ATP levels by 8-Cl-Ado was shown to induce AMPK phosphorylation and inhibit mTOR activity in sensitive clear cell renal cell carcinoma cells.[168] 8-Cl-Ado has entered Phase I/II clinical trial as a single agent for the treatment of relapsed or refractory acute myeloid leukemia (AML) (ClinicalTrials.gov identifier: NCT02509546). Unfortunately, adverse effects of 8-chloroadenosine were recently reported on human coronary artery endothelial cells and macrophages, which may affect clinical advancement.[169,170]
Sorafenib (Nexavar) is a marketed multikinase inhibitor used for the treatment of advanced renal-cell carcinoma and liver cancer.[171] Sorafenib was found to activate ubiquitin E3 ligase Parkin to result in mitochondrial damage. The PINK1-Parkin pathway is activated as a result of dual inhibition of complex II/III (~10 μM) and ATP synthase (~2.5 μM).[172]
Apt63 was identified via a differential Cell-SELEX (Systematic Evolution of Ligands by EXponential enrichment) RNA aptamer that distinguishes ligands based on their selective targeting of the metastatic cancer cells (LN3) in preference to normal and non-metastatic cells (LNCaP and Pro5). The Apt63 aptamer was shown to target ectopic F1FO-ATPase at the β-subunit, which led to the translocation of endonuclease G from the mitochondria and into the nucleus, DNA fragmentation, and apoptosis in tumor cells (IC50 = 1.030 nM, LN3 cells). Apt63 induced no signs of toxicity to non-transformed epithelial cells in vitro and adjacent normal tissue in vivo.[173]
7. Summary and Outlook
F1FO-ATPase is a promising target for the treatment of human disease, as it plays a central role in cellular bioenergetics. During the last decade, new structural and functional information has emerged to help understand the role played by F1FO-ATPase during cell growth and survival. While post-translational modifications help to regulate the ATP synthase activity under normal and pathological conditions, additional studies are needed to further deconvolute their effects. For example, Cys244 and Cys294 in the α subunit are S-sulfhydrated by endogenous H2S to maintain ATP synthase activity under physiological condition.[174] In obese individuals, the phosphorylation of Thr213 and Tyr361 in the β subunit results in an impaired insulin-stimulated glucose disposal, clearly highlighting the opposing roles of post-translational modifications on F1FO-ATPase function.[175]
The involvement of F1FO-ATPase in mPTP formation is an area of debate. Giorgio and co-workers reported that Ca2+ binding to the β subunit can trigger opening of mPTP, which appears to be formed by dimers of the ATP synthase.[142,176] In addition, it was reported that the c subunit is required for mPTP formation along with an uncoupling channel within the c-subunit ring that acts as mPTP.[177–179] In contrast, Walker and coworkers reported persistence of a mitochondrial permeability transition (mPT) in the absence of FO subunits.[180–182] Bonora et al suggested dissociation of the ATP synthase dimer during mPT.[183]
There is a great deal of published research regarding various small molecule modulators of F1FO ATP synthase (Table 2).[2] In fact, ATP synthase modulators can be classified into various classes, such as polyketide, polyphenols, cationic, peptides, endogenous ligands, natural small molecules and synthetic modulators. Endogenous ligands such as IF1 and PEDF play a crucial role in regulating cell survival and tumor growth. IF1 has been shown to exhibit both antiapoptotic and tumorigenic function whereas the silencing of IF1 inhibits bladder cancer growth.[184,185] The overexpression of IF1 inhibits ATP synthase activity in neurons and promotes metabolic reprogramming, highlighting its role in neuroprotection.[186] Inhibition of ectopic ATP synthase leads to intracellular acidification and contributes to the inhibition of cell proliferation. Coupled with the inhibition of ATP synthesis, ectopic ATP synthase inhibitors can induce apoptosis.[187] Ligands such as aurovertin, cruentaren A, resveratrol, efrapeptin, prethioviridamide, PEDF, HAMLET, α-KG, citreoviridin, J147, and Apt63 bind the F1 domain to modulate ATP synthase activity, whereas oligomycin A, leucinostatins, Bz-423 and dexpramipexole target the FO subunit.
Table 2:
Summary of biological activities of F1FO ATP synthase modulators
| Modulators | Mechanism of actiona | Biological activity |
|---|---|---|
| Polyketide | ||
| Oligomycin A | Targets subunit c to inhibit ATP synthesis and hydrolysis[26] | IC50 values: 0.2 μM (K-562), 0.9 μM (HCT116)[31] |
| YO-001A | Inhibits ATP synthase from isolated bovine heart mitochondria with an IC50 of 1 μM[34] | IC50 values: 8.2 μM (HeLa), 5.8 μM (HL-60)[34] |
| Aurovertin B | IC50 values: 0.89 μM (T-47D), 5.52 μM (MDA-MB-231), 0.09 μM (MCF-7), 14.7 μM (HL-60), 10.8 μM (SMMC-7721), 14.7 μM (A-549), 18.8 μM (MCF-7), 22.4 μM (SW480)[42,43,48] | |
| Aurovertin D | Aurovertin B and D binds βTP and βE subunits; Aurovertin B inhibits ATP synthesis (Ki = 25 nM), slows rate of ATP hydrolysis (Ki = 120 nM)[46] | IC50 values: 0.08 μM (MDA-MB-231)[43] |
| Aurovertin E | IC50 values: 8.79 μM (MDA-MB-231)[43] | |
| Aurovertins J-S (except P) | IC50 values: >40 μM (HL-60, SMMC-7721, A-549, MCF-7, SW480)[42] | |
| Aurovertin P | IC50 values: >40 μM (HL-60), 18.2 μM (SMMC-7721), >40 μM (A-549), >40 μM (MCF-7), 14.4 μM (SW480)[42] | |
| Aurovertin T | IC50 values: >50 μM (MDA-MB-231)[43] | |
| Aurovertin U | IC50 values: 5.43 μM (MDA-MB-231)[43] | |
| Mandelalide A | IC50 values: 9.9 nM (HeLa), 12 nM (NCI-H460), 44 nM (Neuro-2A)[50,52] | |
| Mandelalide B | IC50 values: 23 nM (HeLa), 44 nM (NCI-H460), 84 nM (Neuro-2A), 61 nM (U87-Mg), 54 nM (HCT116)[50–52] | |
| Mandelalide C | IC50 values: >3000 nM (HeLa, NCI-H460)[51,52] | |
| Mandelalide D | IC50 values: 660 nM (HeLa), 1700 nM (NCI-H460)[52] | |
| Mandelalide E | Mandelalides A and C inhibits ATP synthase with an IC50 of 2.2 nM and 3.4 μM respectively[52] | IC50 values: 1900 nM (HeLa), 2000 nM (NCI-H460), >3000 nM (U87-MG), >3000 nM (HCT116)[51,52] |
| Mandelalide F | IC50 values: 50 nM (HeLa), 270 nM (NCI-H460)[52] | |
| Mandelalide G | IC50 values: >3000 nM (HeLa, NCI-H460)[52] | |
| Mandelalide H | IC50 values: 330 nM (HeLa), 2600 nM (NCI-H460)[52] | |
| Mandelalide I | IC50 values: 2000 nM (HeLa), 910 nM (NCI-H460)[52] | |
| Mandelalide J | IC50 values: >3000 nM (HeLa), 790 nM (NCI-H460)[52] | |
| Mandelalide K | IC50 values: >3000 nM (HeLa), 2400 nM (NCI-H460)[52] | |
| Mandelalide L | IC50 values: 2.8 nM (HeLa), 9.8 nM (NCI-H460)[52] | |
| Apoptolidin A | IC50 values: 32 nM (H292)[56] | |
| Apoptolidin B | IC50 values: 7 nM (H292)[56] | |
| Apoptolidin C | IC50 values: 24 nM (H292)[56] | |
| Apoptolidin D | IC50 values: 110 nM (H292)[57] | |
| Apoptolidin E | IC50 values: <100 nM (H292)[58] | |
| Apoptolidin F | Apoptolidin A inhibits mitochondrial ATP synthase with IC50 value of 0.7 μM in mitochondria[62] | IC50 values: >1000 nM (H292)[58] |
| Apoptolidin G | IC50 values: 150 nM (H292)[59] | |
| Isoapoptolidin | 17 μM (Ki against isolated yeast mitochondria)[68] | |
| 2′-O-succinyl-apoptolidin A | IC50 values: 91 nM (H292), 240 nM (HeLa)[66] | |
| 3′-O-succinyl-apoptolidin A | IC50 values: 82 nM (H292), 260 nM (HeLa)[66] | |
| Cruentaren A | Cruentaren A binds F1 domain inhibiting beef and yeast ATP synthase with IC50 values of 15–30 nM[71] | IC50 values: 0.3 ng/mL (KB-3-1), 0.6 ng/mL (KB-V1), 0.6 ng/mL (K-562), 0.1 ng/mL (U-937), 0.4 ng/mL (A-549), 1.0 ng/mL (SK-V-3), 0.4 ng/mL (A-498), 1.2 ng/mL (L929)[70,71] |
| Cruentaren B | IC50 >1000 ng/mL (L929)[70] | |
| Polyphenols | ||
| Resveratrol | IC50 = 80 μM (MCF-7)[81] | |
| Resveratrol derivatives | Resveratrol binds F1 and inhibits ATP synthase activity[79] | RDM and RDA analogs inhibit >40% ATPase activity at 50 μM |
| Furan-resveratrol | IC50 = 42.7 μM (MCF-7)[81] | |
| Dihydromyricetin | Inhibits F1FO ATP synthase activity to induce autophagy[82] | Improved skeletal muscle insulin resistance in rat skeletal muscle L6 myoblast cells[82] |
| Dimethoxycurcumin | Suppressed levels of the α, β, γ and ε subunits[84] | Cytotoxic to MCF-7 cells at 5–50 μM[84] |
| Cationics | ||
| Alkylphosphocholines | Translocates β subunit to lipid raft[91] | Induced apoptosis-like cytotoxicity in HL-60, Jurkat, CEM-C7H2, MM144, HeLa cells[91] |
| Rosamines | Inhibits ATP synthase activity with IC50 of 3–4 μM[94] | IC50 = 0.1 μM (HL-60)[93] |
| Gboxin | Inhibits ATP synthase activity[95] | Gboxin: IC50 = 150 nM and S-Gboxin: IC50 = 470 nM (high-throughput GBM sphere cells)[95] |
| Natural peptides | ||
| Efrapeptin D | Binds F1 preventing βE to assume nucleotide binding conformation and disrupts interactions between F1FO-ATPase and Hsp90[96,101] | IC50 = 15 nM (SNU-398 with SALL4hi)[100] |
| Efrapeptin Eα | IC50 = 5 nM (SNU-398 with SALL4hi)[100] | |
| Efrapeptin G | IC50 = 9 nM (SNU-398 with SALL4hi)[100] | |
| Efrapeptin H | IC50 = 6 nM (SNU-398 with SALL4hi)[100] | |
| Efrapeptin F | IC50 = 52 nM (PANC-1)[96,99] | |
| Yaku’amide A | Yaku’amide B inhibits ATP synthesis and increases rate of ATP hydrolysis[103] | IC50 = 0.88 nM (P388)[102] |
| Yaku’amide B | IC50 = 0.51 nM (P388), IC50 = 10 nM (MCF-7 and P388)[102,103] | |
| Thioviridamide | IC50 values: 3.9 ng/mL (Ad12-3Y1), 32 ng/mL (E1A-3Y1), 630 ng/mL (SR-3Y1), 460 ng/mL (SV-3Y1), 200 ng/mL (HR-3Y1), 38 μM (SKOV-3), 27.8 μM (Meso-1), 12.5 μM (Jurkat) [105,107] | |
| Thioalbamide | Prethioviridamide binds β subunit in the F1 domain[113] | IC50 values: 48 nM (A549), 59 nM (MCF7), 72 nM (MDA-MB-231), 50 nM (HeLa), 65 nM (PA-TU-8988T)[110] |
| Neothioviridamide/Thioholgamide A/thiostreptamide S87 | IC50 values: 2.1 μM (SKOV-3), 0.7 μM (Meso-1), 0.4 μM (Jurkat), 0.176 μM (HCT116), 0.141 μM (Huh7), 0.48 μM (MCF7), 1.16 μM (A549), 0.157 μM (RIL175)[108,111,112] | |
| JBIR-140 | IC50 values: 10.8 μM (SKOV-3), 14.3 μM (Meso-1), 5.4 μM (Jurkat)[107] | |
| Prethioviridamide | IC50 values: 0.36 μM (HeLa S3), 16 nM (Ad12-3Y1), 21 nM (E1A-3Y1)[106,113] | |
| Thioholgamide B | IC50 values: 1.47 μM (HL60), 0.83 μM (MCF-7), 5.02 μM (H1299), 1.18 μM (LOVO), 20.89 μM (SKOV-3), 5.28 μM (Jurkat), 0.51 μM (HCT116), 4.49 μM (KB-3-1), 12.17 (SW480), 16.94 μM (U937)[109,111] | |
| Thiostreptamide S4 | IC50 values: 0.61 μM (HL60), 4.98 μM (MCF-7), 5.08 μM (H1299), 1.83 μM (LOVO)[109] | |
| Leucinostatins (LCS-A and LCS-7) | Binds the FO domain of ATP synthase; IC50 = 2.8 μg/mL[114] | DU-145 cells cocultured with PrSCs vs DU-145 cells monoculture (LCS-A: coculture IC50 = 0.045 μg/ml, monoculture IC50 > 1 μg/ml; LCS-7: coculture IC50 = 0.21 μg/ml, monoculture IC50 > 1 μg/ml); Maximum tolerated dose in mice: LCS-A (2.5 mg/kg) and LCS-7 (12.5 mg/kg)[114,115] |
| Endogenous ligands | ||
| Pigment epithelium-derived factor (PEDF) | Binds β subunit on the ectopic ATP synthase[116] | Showed anticancer activity in vitro and in vivo against lung, breast, prostatic, ovarian and pancreatic carcinomas, melanoma, glioma, as well as osteosarcoma[119] |
| HAMLET | Binds F1 domain with KD value of 20.5 μM[124] | A peroral agent for colon cancer, with APC mutation, prevention and treatment[123] |
| 3-iodothyronamine | Non-competitive inhibition of ATP synthase (IC50 = 27.5 μM), binds at two different sites (IF1 and aurovertin binding sites) | Increased ATP synthesis at low concentration (50 nM) in H9c2 cells by displacing IF1[125] |
| α-KG and 2-HG | Binds the β subunit of ATP synthase | Extends the lifespan of adult Caenorhabditis elegans[126,127] |
| Natural small molecules | ||
| Citreoviridin | Binds β subunit and inhibits ecto-F1FO-ATPase; uncompetitive inhibitor of ATP hydrolysis and as a noncompetitive inhibitor of ATP synthesis[128,130] | IC50 values: 1.5 μM (A549), 4.65 μM (CL1-0); cytotoxic to MCF7, T47D, and MDA-MB-231 cells[131–133] |
| Nerolidol | Inhibits ATP synthesis at 1.2 and 2.4 μM | Inhibited cell proliferation of HepG2 cells[135] |
| Deglucoruscin | Inhibits F1FO-ATPase with Ki = 10 μM[137] | Reduced thrombin-induced hyperpermeability of endothelial cells (HMEC-1) by 41.9%[137,189] |
| Synthetic modulators | ||
| Keratinocyte EC50 | ||
| Bz-423 | Binds OSCP disrupting OSCP-F1 interactions[139] | Bz-423: 3.1 μM |
| 1002: 2.2 μM | ||
| 1118: 2.1 μM[145] | ||
| PK11195 | Inhibits ATP synthesis (EC50 = 33 μM) and hydrolysis (EC50 = 230 μM)[146] | IC50 values: 5.4 nM (MCF-7) 6 nM (T47D)[190] |
| JM-20 | Inhibits 67% ATP hydrolytic activity at 5 μM concentration | Rescued PC-12 cells from glutamate and KCN induced damage with IC50 of 29 nM and 8 nM respectively[150] |
| Dexpramipexole | Binds subunits b and OSCP[151] | Increased mitochondrial ATP production and resistance to in vitro ischemia of primary cultures of neurons or glia[152] |
| Leflunomide | Inhibits ATP synthase activity, IC50 = 35 μM | IC50 = 109.5 μM (HepG2)[153] |
| J147 | Targets α subunit; EC50 = 20 nM[155] | Rescued primary embryonic cortical cells with an EC50 of 25 nM; Blocked extracellular amyloid toxicity using rat hippocampal neurons with an EC50 of about 200 nM[154] |
| ATR-101 | Depletes ATP levels by inhibiting ATP synthase | Inhibited proliferation of H295R cells in vitro and in vivo[158] |
| BTB06584 | Selectively inhibits ATP hydrolysis[162] | Enhanced radiosensitivity in non-small lung cancer cell[163] |
| 1,3,8-Triazaspiro[4.5]decane | May bind subunit c to affect mPTP | Inhibited mPTP opening and protected against cell death in cardiovascular model[164] |
| 8-Cl adenosine | 8-Cl ADP may act as a substrate and 8-Cl ATP as an inhibitor of ATP synthase[167] | IC50 = 2 μM (CAKI-1), tumoricidal to MCF-7 and active in in vivo BT-474 tumor[166,168] |
| Sorafenib | Dual inhibition of complex II/III and ATP synthase (IC50 = 2.5 μM) | Broad spectrum marketed anti-tumor drug for treatment of renal-cell carcinoma and liver cancer[171,172] |
| Apt63 | Targets β subunit on ectopic ATP synthase | IC50 = 1.030 nM (LN3)[173] |
For certain modulator classes, mechanism of action was tested only for one or two members. It is assumed that compounds from the same class act via similar mechanism of action.
Combination therapy with ATP synthase inhibitors have also been suggested. In fact, it was shown that citreoviridin along with the 26S proteasome inhibitor, bortezomib, caused higher endoplasmic reticulum (ER) stress and resulted in effective anticancer activity.[132] Similar results were obtained with a combination of efrapeptins and the glycolysis inhibitor, 2-deoxyglucose. However, they acted as an antagonist in vivo, most likely due to the downregulation of F1FO-ATPase.[98] Moreover, it was found that acquired resistance to HER2-targeted therapies may render cancer vulnerable to ATP synthase inhibition, suggesting another potential combination strategy.[188]
This new data underscores the role played by ATP synthase in various diseases and highlights new opportunities for drug discovery. In fact, the current work provides a solid foundation for the development of new ATP synthase modulators for the treatment of diseases ranging from cancer to neurodegeneration.
Highlights.
F1FO ATP synthase, being the generator of cell energy currency (ATP), plays a pivotal role in cell survival and growth in disease states.
No current drug has been approved that specifically targets mitochondrial F1FO ATP synthase.
Natural and synthetic modulators of F1FO ATP synthase are covered in this review.
The biological data for small molecule modulators of F1FO ATP synthase is presented.
9. Acknowledgements
This work was supported by the National Institutes of Health [grant number CA216919].
Footnotes
8. Declaration of competing interest
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- [1].Mitchell P, Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism, Nature. 191 (1961) 144–148. [DOI] [PubMed] [Google Scholar]
- [2].Hong S, Pedersen PL, ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas, Microbiol. Mol. Biol. Rev. 72 (2008) 590–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taurino F, Gnoni A, Systematic review of plasma-membrane ecto-ATP synthase: A new player in health and disease, Exp. Mol. Pathol. 104 (2018) 59–70. [DOI] [PubMed] [Google Scholar]
- [4].He J, Ford HC, Carroll J, Douglas C, Gonzales E, Ding S, Fearnley IM, Walker JE, Assembly of the membrane domain of ATP synthase in human mitochondria, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) 2988–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Song J, Pfanner N, Becker T, Assembling the mitochondrial ATP synthase, Proc. Natl. Acad. Sci. U. S. A. 115 (2018) 2850–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yasuda R, Noji H, Yoshida M, Kinosita K, Itoh H, Resolution of distinct rotational substeps by submillisecond kinetic analysis of F 1-ATPase, Nature. 410 (2001) 898–904. [DOI] [PubMed] [Google Scholar]
- [7].Kulish O, Wright AD, Terentjev EM, F1 rotary motor of ATP synthase is driven by the torsionally-asymmetric drive shaft, Sci. Rep. 6 (2016) 28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abrahams JP, Leslie AG, Lutter R, Walker JE, Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria, Nature. 370 (1994) 621–628. [DOI] [PubMed] [Google Scholar]
- [9].Nakamoto RK, Scanlon JAB, Al-Shawi MK, The rotary mechanism of the ATP synthase, Arch. Biochem. Biophys. 476 (2008) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arselin G, Vaillier J, Salin B, Schaeffer J, Giraud M, Dautant A, Brèthes D, Velours J, The modulation in subunits e and g amounts of yeast ATP synthase modifies mitochondrial cristae morphology, J. Biol. Chem. 279 (2004) 40392–40399. [DOI] [PubMed] [Google Scholar]
- [11].Ohsakaya S, Fujikawa M, Hisabori T, Yoshida M, Knockdown of DAPIT (diabetes-associated protein in insulin-sensitive tissue) results in loss of ATP synthase in mitochondria, J. Biol. Chem. 286 (2011) 20292–20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nesci S, Trombetti F, Algieri C, Pagliarani A, A Therapeutic Role for the F1FO-ATP Synthase, SLAS Discov. 24 (2019) 893–903. [DOI] [PubMed] [Google Scholar]
- [13].Lu ZJ, Song QF, Jiang SS, Song Q, Wang W, Zhang GH, Kan B, Chen LJ, Yang JL, Luo F, Qian ZY, Wei YQ, Gou LT, Identification of ATP synthase beta subunit (ATPB) on the cell surface as a non-small cell lung cancer (NSCLC) associated antigen, BMC Cancer. 9 (2009) 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wen-Li Z, Jian W, Yan-Fang T, Xing F, Yan-Hong L, Xue-Ming Z, Min Z, Jian N, Jian P, Inhibition of the ecto-beta subunit of F1F0-ATPase inhibits proliferation and induces apoptosis in acute myeloid leukemia cell lines, J. Exp. Clin. Cancer Res. 31 (2012) 92–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li W, Li Y, Li G, Zhou Z, Chang X, Xia Y, Dong X, Liu Z, Ren B, Liu W, Li Y, Ectopic expression of the ATP synthase beta subunit on the membrane of PC-3M cells supports its potential role in prostate cancer metastasis, Int. J. Oncol. 50 (2017) 1312–1320. [DOI] [PubMed] [Google Scholar]
- [16].Wang WJ, Ma Z, Liu YW, He YQ, Wang YZ, Yang CX, Du Y, Zhou MQ, Gao F, A monoclonal antibody (Mc178-Ab) targeted to the ecto-ATP synthase beta-subunit-induced cell apoptosis via a mechanism involving the MAPKase and Akt pathways, Clin. Exp. Med. 12 (2012) 3–12. [DOI] [PubMed] [Google Scholar]
- [17].Willers IM, Isidoro A, Ortega AD, Fernandez PL, Cuezva JM, Selective inhibition of beta-F1-ATPase mRNA translation in human tumours, Biochem. J. 426 (2010) 319–326. [DOI] [PubMed] [Google Scholar]
- [18].Martinez-Reyes I, Sanchez-Arago M, Cuezva JM, AMPK and GCN2-ATF4 signal the repression of mitochondria in colon cancer cells, Biochem. J. 444 (2012) 249–259. [DOI] [PubMed] [Google Scholar]
- [19].Ma Z, Cao M, Liu Y, He Y, Wang Y, Yang C, Wang W, Du Y, Zhou M, Gao F, Mitochondrial F1Fo-ATP synthase translocates to cell surface in hepatocytes and has high activity in tumor-like acidic and hypoxic environment, Acta Biochim. Biophys. Sin. (Shanghai). 42 (2010) 530–537. [DOI] [PubMed] [Google Scholar]
- [20].Huang Y, Jan Y, Chang Y, Tsai H, Wu ATH, Chen C, Hsiao M, ATP synthase subunit epsilon overexpression promotes metastasis by modulating AMPK signaling to induce epithelial-to-mesenchymal transition and is a poor prognostic marker in colorectal cancer patients, J. Clin. Med. 8 (2019) 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bergeaud M, Mathieu L, Guillaume A, Moll UM, Mignotte B, Le Floch N, Vayssiere JL, Rincheval V, Mitochondrial p53 mediates a transcription-independent regulation of cell respiration and interacts with the mitochondrial F1F0-ATP synthase, Cell Cycle. 12 (2013) 2781–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I, Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology, Brain Pathol. 20 (2010) 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beck SJ, Guo L, Phensy A, Tian J, Wang L, Tandon N, Gauba E, Lu L, Pascual JM, Kroener S, Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease, Nat. commun. 7 (2016) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ludtmann MHR, Angelova PR, Horrocks MH, Choi ML, Rodrigues M, Baev AY, Berezhnov AV, Yao Z, Little D, Banushi B, Al-Menhali A, Ranasinghe RT, Whiten DR, Yapom R, Dolt KS, Devine MJ, Gissen P, Kunath T, Jaganjac M, Pavlov EV, Klenerman D, Abramov AY, Gandhi S, alpha-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease, Nat. Commun. 9 (2018) 2293–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yan S, Du F, Wu L, Zhang Z, Zhong C, Yu Q, Wang Y, Lue L, Walker DG, Douglas JT, F1F0 ATP synthase–cyclophilin D interaction contributes to diabetes-induced synaptic dysfunction and cognitive decline, Diabetes. 65 (2016) 3482–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Symersky J, Osowski D, Walters DE, Mueller DM, Oligomycin frames a common drug-binding site in the ATP synthase, Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 13961–13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li YC, Fung KP, Kwok TT, Lee CY, Suen YK, Kong SK, Mitochondria-targeting drug oligomycin blocked P-glycoprotein activity and triggered apoptosis in doxorubicin-resistant HepG2 cells, Chemotherapy. 50 (2004) 55–62. [DOI] [PubMed] [Google Scholar]
- [28].Salim AA, Tan L, Huang X, Cho K, Lacey E, Hancock JF, Capon RJ, Oligomycins as inhibitors of K-Ras plasma membrane localisation, Org. Biomol. Chem. 14 (2016) 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Omelchuk OA, Belov NM, Tsvetkov VB, Korolev AM, Dezhenkova LG, Grammatikova NE, Lysenkova LN, Bekker OB, Danilenko VN, Shchekotikhin AE, Synthesis and biological activity of 16, 33-O, O-diformyl-16, 17-dihydro-16 (S), 17 (R)-dihydroxyoligomycin A and 33-O-formyloligomycin A, Macroheterocycles. 11 (2018) 181–192. [Google Scholar]
- [30].Lysenkova LN, Turchin KF, Korolev AM, Dezhenkova LG, Bekker OB, Shtil AA, Danilenko VN, Preobrazhenskaya MN, Synthesis and cytotoxicity of oligomycin A derivatives modified in the side chain, Bioorg. Med. Chem. 21 (2013) 2918–2924. [DOI] [PubMed] [Google Scholar]
- [31].Lysenkova LN, Saveljev OY, Grammatikova NE, Tsvetkov VB, Bekker OB, Danilenko VN, Dezhenkova LG, Bykov EE, Omelchuk OA, Korolev AM, Shchekotikhin AE, Verification of oligomycin A structure: synthesis and biological evaluation of 33-dehydrooligomycin A, J. Antibiot. 70 (2017) 871–877. [DOI] [PubMed] [Google Scholar]
- [32].Omelchuk OA, Lysenkova LN, Belov NM, Korolev AM, Dezhenkova LG, Grammatikova NE, Bekker OB, Danilenko VN, Shchekotikhin AE, Synthesis and biological activity of 7(7,11)-hydroderivatives of oligomycin A, Makrogeterotsikly. 11 (2018) 322–328. [Google Scholar]
- [33].Lysenkova LN, Turchin KF, Korolev AM, Bykov EE, Danilenko VN, Bekker OB, Trenin AS, Elizarov SM, Dezhenkova LG, Shtil AA, Preobrazhenskaya MN, A novel acyclic oligomycin A derivative formed via retro-aldol rearrangement of oligomycin A, J. Antibiot. (Tokyo). 65 (2012) 405–411. [DOI] [PubMed] [Google Scholar]
- [34].Yamamoto K, Futamura Y, Uson-Lopez RA, Aono H, Shimizu T, Osada H, YO-001A, a new antifungal agent produced by Streptomyces sp. YO15-A001, J. Antibiot. (Tokyo). 72 (2019) 986–990. [DOI] [PubMed] [Google Scholar]
- [35].Baldwin CL, Biological and chemical properties of aurovertin, a metabolic product of Calcarisporium abuscula, Lloydia. 27 (1964) 88–95. [Google Scholar]
- [36].Steyn PS, Vleggaar R, Wessels PL, Biosynthesis of the aurovertins B and D. The role of methionine and propionate in the simultaneous operation of two independent biosynthetic pathways, J. Chem. Soc., Perkin Trans. 1. (1981) 1298–1308. [Google Scholar]
- [37].Mulheirn LJ, Beechey RB, Leworthy DP, Osselton MD, Aurovertin B, a metabolite of Calcarisporium arbuscula, J. Chem. Soc., Chem. Commun. (1974) 874–876. [Google Scholar]
- [38].Nishiyama S, Toshima H, Kanai H, Yamamura S, Total synthesis and the absolute configuration of aurovertin B, Tetrahedron Lett. 27 (1986) 3643–3646. [Google Scholar]
- [39].Mao X, Zhan Z, Grayson MN, Tang M, Xu W, Li Y, Yin W, Lin H, Chooi Y, Houk KN, Efficient biosynthesis of fungal polyketides containing the dioxabicyclo-octane ring system, J. Am. Chem. Soc. 137 (2015) 11904–11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Azumi M, Ishidoh K, Kinoshita H, Nihira T, Ihara F, Fujita T, Igarashi Y, Aurovertins F–H from the entomopathogenic fungus Metarhizium anisopliae, J. Nat. Prod. 71 (2008) 278–280. [DOI] [PubMed] [Google Scholar]
- [41].Niu X, Wang Y, Chu Y, Xue H, Li N, Wei L, Mo M, Zhang K, Nematodetoxic aurovertin-type metabolites from a root-knot nematode parasitic fungus Pochonia chlamydosporia, J. Agric. Food Chem. 58 (2010) 828–834. [DOI] [PubMed] [Google Scholar]
- [42].Guo H, Feng T, Li Z, Liu J, Ten new aurovertins from cultures of the basidiomycete Albatrellus confluens, Nat. Products Bioprospect. 3 (2013) 8–13. [Google Scholar]
- [43].Zhao H, Wu R, Ma L, Wo L, Hu Y, Chen C, Zhan Z, Aurovertin-Type Polyketides from Calcarisporium arbuscula with Potent Cytotoxic Activities against Triple-Negative Breast Cancer, Helv. Chim. Acta. 99 (2016) 543–546. [Google Scholar]
- [44].Li W, Ma Z, Chen L, Yin W, Synthesis and production of the antitumor polyketide aurovertins and structurally related compounds, Appl. Microbiol. Biotechnol. 102 (2018) 6373–6381. [DOI] [PubMed] [Google Scholar]
- [45].Van Raaij MJ, Abrahams JP, Leslie AG, Walker JE, The structure of bovine F1-ATPase complexed with the antibiotic inhibitor aurovertin B, Proc. Natl. Acad. Sci. U. S. A. 93 (1996) 6913–6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Verschoor GJ, Slater EC, The binding of aurovertin to isolated beta subunit of F1 (mitochondrial ATPase). Stoicheiometry of beta subunit in F1. Biochim. Biophys. Acta. 462 (1977) 438–449. [DOI] [PubMed] [Google Scholar]
- [47].Johnson KM, Swenson L, Opipari AW Jr., Reuter R, Zarrabi N, Fierke CA, Boersch M, Glick GD, Mechanistic basis for differential inhibition of the F1F0-ATPase by aurovertin, Biopolymers. 91 (2009) 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang T, Chang H, Hsu C, Kuo W, Chang K, Juan H, Targeting therapy for breast carcinoma by ATP synthase inhibitor aurovertin B, J. Proteome Res. 7 (2008) 1433–1444. [DOI] [PubMed] [Google Scholar]
- [49].Zhu H, Wang F, Ju X, Kong L, An T, Zhao Z, Liu J, Li Y, Aurovertin B sensitizes colorectal cancer cells to NK cell recognition and lysis, Biochem. Biophys. Res. Commun. 503 (2018) 3057–3063. [DOI] [PubMed] [Google Scholar]
- [50].Sikorska J, Hau AM, Anklin C, Parker-Nance S, Davies-Coleman MT, Ishmael JE, McPhail KL, Mandelalides A-D, cytotoxic macrolides from a new Lissoclinum species of South African tunicate, J. Org. Chem. 77 (2012) 6066–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nazari M, Serrill JD, Sikorska J, Ye T, Ishmael JE, McPhail KL, Discovery of mandelalide E and determinants of cytotoxicity for the mandelalide series, Org. Lett. 18 (2016) 1374–1377. [DOI] [PubMed] [Google Scholar]
- [52].Nazari M, Serrill JD, Wan X, Nguyen MH, Anklin C, Gallegos DA, Smith3 AB, Ishmael JE, McPhail KL, New Mandelalides Expand a Macrolide Series of Mitochondrial Inhibitors, J. Med. Chem. 60 (2017) 7850–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Veerasamy N, Ghosh A, Li J, Watanabe K, Serrill JD, Ishmael JE, McPhail KL, Carter RG, Enantioselective total synthesis of mandelalide A and isomandelalide A: discovery of a cytotoxic ring-expanded isomer, J. Am. Chem. Soc. 138 (2016) 770–773. [DOI] [PubMed] [Google Scholar]
- [54].Kim JW, Adachi H, Shin-Ya K, Hayakawa Y, Seto H, Apoptolidin, a new apoptosis inducer in transformed cells from Nocardiopsis sp. J. Antibiot. 50 (1997) 628–630. [DOI] [PubMed] [Google Scholar]
- [55].Hayakawa Y, Kim JW, Adachi H, Shin-ya K, Fujita K, Seto H, Structure of apoptolidin, a specific apoptosis inducer in transformed cells, J. Am. Chem. Soc. 120 (1998) 3524–3525. [Google Scholar]
- [56].Wender PA, Sukopp M, Longcore K, Apoptolidins B and C: Isolation, structure determination, and biological activity, Org. Lett. 7 (2005) 3025–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wender PA, Longcore KE, Isolation, structure determination, and anti-cancer activity of apoptolidin D, Org. Lett. 9 (2007) 691–694. [DOI] [PubMed] [Google Scholar]
- [58].Wender PA, Longcore KE, Apoptolidins E and F, new glycosylated macrolactones isolated from Nocardiopsis sp. Org. Lett. 11 (2009) 5474–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bachmann BO, McNees R, Melancon BJ, Ghidu VP, Clark R, Crews BC, DeGuire SM, Marnett LJ, Sulikowski GA, Light-Induced Isomerization of Apoptolidin A leads to Inversion of C2–C3 Double Bond Geometry, Org. Lett. 12 (2010) 2944–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].DeGuire SM, Earl DC, Du Y, Crews BA, Jacobs AT, Ustione A, Daniel C, Chong KM, Marnett LJ, Piston DW, Fluorescent probes of the apoptolidins and their utility in cellular localization studies, Angew Chem Int. Ed. Engl. 127 (2015) 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Salomon AR, Voehringer DW, Herzenberg LA, Khosla C, Apoptolidin, a selective cytotoxic agent, is an inhibitor of F0F1-ATPase, Chem. Biol. 8 (2001) 71–80. [DOI] [PubMed] [Google Scholar]
- [62].Wender PA, Jankowski OD, Tabet EA, Seto H, Toward a Structure-Activity Relationship for Apoptolidin: Selective Functionalization of the Hydroxyl Group Array, Org. Lett. 5 (2003) 487–490. [DOI] [PubMed] [Google Scholar]
- [63].Wender PA, Jankowski OD, Tabet EA, Seto H, Facile synthetic access to and biological evaluation of the macrocyclic core of apoptolidin, Org. Lett. 5 (2003) 2299–2302. [DOI] [PubMed] [Google Scholar]
- [64].Wender PA, Jankowski OD, Longcore K, Tabet EA, Seto H, Tomikawa T, Correlation of F0F1-ATPase inhibition and antiproliferative activity of apoptolidin analogues, Org. Lett. 8 (2006) 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lewis CA, Longcore KE, Miller SJ, Wender PA, An approach to the site-selective diversification of apoptolidin A with peptide-based catalysts, J. Nat. Prod. 72 (2009) 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sheng Y, Fotso S, Serrill JD, Shahab S, Santosa DA, Ishmael JE, Proteau PJ, Zabriskie TM, Mahmud T, Succinylated apoptolidins from Amycolatopsis sp. ICBB 8242, Org. Lett. 17 (2015) 2526–2529. [DOI] [PubMed] [Google Scholar]
- [67].Serrill JD, Tan M, Fotso S, Sikorska J, Kasanah N, Hau AM, McPhail KL, Santosa DA, Zabriskie TM, Mahmud T, Viollet B, Proteau PJ, Ishmael JE, Apoptolidins A and C activate AMPK in metabolically sensitive cell types and are mechanistically distinct from oligomycin A, Biochem. Pharmacol. 93 (2015) 251–265. [DOI] [PubMed] [Google Scholar]
- [68].Wender PA, Gulledge AV, Jankowski OD, Seto H, Isoapoptolidin: Structure and activity of the ring-expanded isomer of apoptolidin, Org. Lett. 4 (2002) 3819–3822. [DOI] [PubMed] [Google Scholar]
- [69].Ghidu VP, Wang J, Wu B, Liu Q, Jacobs A, Marnett LJ, Sulikowski GA, Synthesis and evaluation of the cytotoxicity of apoptolidinones A and D, J. Org. Chem. 73 (2008) 4949–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kunze B, Steinmetz H, Höfle G, Huss M, Wieczorek H, Reichenbach H, Cruentaren, a new antifungal salicylate-type macrolide from Byssovorax cruenta (Myxobacteria) with inhibitory effect on mitochondrial ATPase activity, J. Antibiot. 59 (2006) 664–668. [DOI] [PubMed] [Google Scholar]
- [71].Kunze B, Sasse F, Wieczorek H, Huss M, Cruentaren A, a highly cytotoxic benzolactone from Myxobacteria is a novel selective inhibitor of mitochondrial F1-ATPases, FEBS Lett. 581 (2007) 3523–3527. [DOI] [PubMed] [Google Scholar]
- [72].Papathanassiu AE, MacDonald NJ, Bencsura A, Vu HA, F1F0-ATP synthase functions as a co-chaperone of Hsp90–substrate protein complexes, Biochem. Biophys. Res. Commun. 345 (2006) 419–429. [DOI] [PubMed] [Google Scholar]
- [73].Hall JA, Kusuma BR, Brandt GE, Blagg BS, Cruentaren A binds F1F0 ATP synthase to modulate the Hsp90 protein folding machinery, ACS Chem. Biol. 9 (2014) 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vintonyak VV, Calà M, Lay F, Kunze B, Sasse F, Maier ME, Synthesis and biological evaluation of cruentaren A analogues, Chem. Eur. J. 14 (2008) 3709–3720. [DOI] [PubMed] [Google Scholar]
- [75].Bindl M, Jean L, Herrmann J, Muller R, Furstner A, Preparation, modification, and evaluation of cruentaren A and analogues, Chem. Eur. J. 2. 15 (2009) 12310–12319. [DOI] [PubMed] [Google Scholar]
- [76].FoucheÌ M, Rooney L, Barrett AG, Biomimetic Total Synthesis of Cruentaren A via Aromatization of Diketodioxinones, J. Org. Chem. 77 (2012) 3060–3070. [DOI] [PubMed] [Google Scholar]
- [77].Kusuma BR, Brandt GE, Blagg BS, Synthesis of cruentaren A, Org. Lett. 14 (2012) 6242–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cory H, Passarelli S, Szeto J, Tamez M, Mattei J, The role of polyphenols in human health and food systems: a mini-review, Front. Nutr. 5 (2018) 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gledhill JR, Montgomery MG, Leslie AG, Walker JE, Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols, Proc. Natl. Acad. Sci. U. S. A.,. 104 (2007) 13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sassi N, Mattarei A, Azzolini M, Szabo I’, Paradisi C, Zoratti M, Biasutto L, Cytotoxicity of mitochondria-targeted resveratrol derivatives: Interactions with respiratory chain complexes and ATP synthase, Biochim. Biophys. Acta, Bioenerg. 1837 (2014) 1781–1789. [DOI] [PubMed] [Google Scholar]
- [81].Du C, Dong M, Ren Y, Jin L, Xu C, Design, synthesis and antibreast cancer MCF-7 cells biological evaluation of heterocyclic analogs of resveratrol, J. Asian Nat. Prod. Res. 19 (2017) 890–902. [DOI] [PubMed] [Google Scholar]
- [82].Shi L, Zhang T, Liang X, Hu Q, Huang J, Zhou Y, Chen M, Zhang Q, Zhu J, Mi M, Dihydromyricetin improves skeletal muscle insulin resistance by inducing autophagy via the AMPK signaling pathway, Mol. Cell. Endocrinol. 409 (2015) 92–102. [DOI] [PubMed] [Google Scholar]
- [83].Zheng J, Ramirez VD, Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals, Br. J. Pharmacol. 130 (2000) 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kunwar A, Jayakumar S, Srivastava AK, Priyadarsini KI, Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis, Arch. Toxicol. 86 (2012) 603–614. [DOI] [PubMed] [Google Scholar]
- [85].Murphy MP, Smith RA, Targeting antioxidants to mitochondria by conjugation to lipophilic cations, Annu. Rev. Pharmacol. Toxicol. 47 (2007) 629–656. [DOI] [PubMed] [Google Scholar]
- [86].Mollinedo F, Antitumour ether lipids: proapoptotic agents with multiple therapeutic indications, Expert Opin. Ther. Pat. 17 (2007) 385–405. [Google Scholar]
- [87].Leonard R, Hardy J, Van Tienhoven G, Houston S, Simmonds P, David M, Mansi J, Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer, J. Clin. Oncol. 19 (2001) 4150–4159. [DOI] [PubMed] [Google Scholar]
- [88].Kugler W, Veenman L, Shandalov Y, Leschiner S, Spanier I, Lakomek M, Gavish M, Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine, Anal. Cell Pathol. 30 (2008) 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Veenman L, Alten J, Linnemannstons K, Shandalov Y, Zeno S, Lakomek M, Gavish M, Kugler W, Potential involvement of F0F1-ATP(synth)ase and reactive oxygen species in apoptosis induction by the antineoplastic agent erucylphosphohomocholine in glioblastoma cell lines : a mechanism for induction of apoptosis via the 18 kDa mitochondrial translocator protein, Apoptosis. 15 (2010) 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jendrossek V, Kugler W, Erdlenbruch B, Eibl H, Lang F, Lakomek M, Erucylphosphocholine-induced apoptosis in chemoresistant glioblastoma cell lines: involvement of caspase activation and mitochondrial alterations. Anticancer Res. 21 (2001) 3389–3396. [PubMed] [Google Scholar]
- [91].Villa-Pulgarin J, Gajate C, Botet J, Jimenez A, Justies N, Varela-M R, Cuesta-Marban A, Muller I, Modolell M, Revuelta JL, Mollinedo F, Mitochondria and lipid raft-located FOF1-ATP synthase as major therapeutic targets in the antileishmanial and anticancer activities of ether lipid edelfosine, PLoS Negl. Trop. Dis. 11 (2017) e0005805/1–e0005805/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wieker H, Kuschmitz D, Hess B, Inhibition of yeast mitochondrial F1-ATPase, F0F1-ATPase and submitochondrial particles by rhodamines and ethidium bromide, Biochim. Biophys. Acta. 892 (1987) 108–117. [DOI] [PubMed] [Google Scholar]
- [93].Lim SH, Wu L, Burgess K, Lee HB, New cytotoxic rosamine derivatives selectively accumulate in the mitochondria of cancer cells, Anticancer Drugs. 20 (2009) 461–468. [DOI] [PubMed] [Google Scholar]
- [94].Lim SH, Wu L, Kiew LV, Chung LY, Burgess K, Lee HB, Rosamines targeting the cancer oxidative phosphorylation pathway, PLoS One. 9 (2014) e82934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shi Y, Lim SK, Liang Q, Iyer SV, Wang HY, Wang Z, Xie X, Sun D, Chen YJ, Tabar V, Gutin P, Williams N, De Brabander JK, Parada LF, Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma, Nature. 567 (2019) 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Abrahams JP, Buchanan SK, Van Raaij MJ, Fearnley IM, Leslie AG, Walker JE, The structure of bovine F1-ATPase complexed with the peptide antibiotic efrapeptin, Proc. Natl. Acad. Sci. U. S. A.,. 93 (1996) 9420–9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Weigelt S, Huber T, Hofmann F, Jost M, Ritzefeld M, Luy B, Freudenberger C, Majer Z, Vass E, Greie J, Synthesis and conformational analysis of efrapeptins, Chem. Eur. J. 18 (2012) 478–487. [DOI] [PubMed] [Google Scholar]
- [98].Papathanassiu AE, MacDonald NJ, Emlet DR, Vu HA, Antitumor activity of efrapeptins, alone or in combination with 2-deoxyglucose, in breast cancer in vitro and in vivo, Cell Stress Chaperones. 16 (2011) 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Momose I, Ohba S, Tatsuda D, Kawada M, Masuda T, Tsujiuchi G, Yamori T, Esumi H, Ikeda D, Mitochondrial inhibitors show preferential cytotoxicity to human pancreatic cancer PANC-1 cells under glucose-deprived conditions, Biochem. Biophys. Res. Commun. 392 (2010) 460–466. [DOI] [PubMed] [Google Scholar]
- [100].Tan JL, Li F, Yeo JZ, Yong KJ, Bassal MA, Ng GH, Lee MY, Leong CY, Tan HK, Wu C, New high-throughput screening identifies compounds that reduce viability specifically in liver cancer cells that express high levels of SALL4 by inhibiting oxidative phosphorylation, Gastroenterology. 157 (2019) 1615–1629. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Papathanassiu AE, MacDonald NJ, Bencsura A, Vu HA, F1F0-ATP synthase functions as a co-chaperone of Hsp90–substrate protein complexes, Biochem. Biophys. Res. Commun. 345 (2006) 419–429. [DOI] [PubMed] [Google Scholar]
- [102].Ueoka R, Ise Y, Ohtsuka S, Okada S, Yamori T, Matsunaga S, Yaku’amides A and B, Cytotoxic Linear Peptides Rich in Dehydroamino Acids from the Marine Sponge Ceratopsion sp. J. Am. Chem. Soc. 132 (2010) 17692–17694. [DOI] [PubMed] [Google Scholar]
- [103].Kitamura K, Itoh H, Sakurai K, Dan S, Inoue M, Target Identification of Yaku’amide B and Its Two Distinct Activities against Mitochondrial FoF1-ATP Synthase, J. Am. Chem. Soc. 140 (2018) 12189–12199. [DOI] [PubMed] [Google Scholar]
- [104].Mutoh H, Sesoko Y, Kuranaga T, Itoh H, Inoue M, The total synthesis and functional evaluation of fourteen stereoisomers of yaku’amide B. The importance of stereochemistry for hydrophobicity and cytotoxicity, Org. Biomol. Chem. 14 (2016) 4199–4204. [DOI] [PubMed] [Google Scholar]
- [105].Hayakawa Y, Sasaki K, Adachi H, Furihata K, Nagai K, Shin-Ya K, Thioviridamide, a novel apoptosis inducer in transformed cells from Streptomyces olivoviridis, J. Antibiot. 59 (2006) 1–5. [DOI] [PubMed] [Google Scholar]
- [106].Izawa M, Nagamine S, Aoki H, Hayakawa Y, Identification of essential biosynthetic genes and a true biosynthetic product for thioviridamide, J. Gen. Appl. Microbiol. 64 (2018) 50–53. [DOI] [PubMed] [Google Scholar]
- [107].Izumikawa M, Kozone I, Hashimoto J, Kagaya N, Takagi M, Koiwai H, Komatsu M, Fujie M, Satoh N, Ikeda H, Novel thioviridamide derivative—JBIR-140: heterologous expression of the gene cluster for thioviridamide biosynthesis, J. Antibiot. 68 (2015) 533–536. [DOI] [PubMed] [Google Scholar]
- [108].Kawahara T, Izumikawa M, Kozone I, Hashimoto J, Kagaya N, Koiwai H, Komatsu M, Fujie M, Sato N, Ikeda H, Neothioviridamide, a polythioamide compound produced by heterologous expression of a Streptomyces sp. cryptic RiPP biosynthetic gene cluster, J. Nat. Prod. 81 (2018) 264–269. [DOI] [PubMed] [Google Scholar]
- [109].Li Y, Liu J, Tang H, Qiu Y, Chen D, Liu W, Discovery of New Thioviridamide- Like Compounds with Antitumor Activities, Chin. J. Chem. 37 (2019) 1015–1020. [Google Scholar]
- [110].Frattaruolo L, Lacret R, Cappello AR, Truman AW, A genomics-based approach identifies a thioviridamide-like compound with selective anticancer activity, ACS Chem. Biol. 12 (2017) 2815–2822. [DOI] [PubMed] [Google Scholar]
- [111].Tang J, Lu J, Luo Q, Wang H, Discovery and biosynthesis of thioviridamide-like compounds, Chin. Chem. Lett. 29 (2018) 1022–1028. [Google Scholar]
- [112].Dahlem C, Siow WX, Lopatniuk M, Tse WK, Kessler SM, Kirsch SH, Hoppstädter J, Vollmar AM, Müller R, Luzhetskyy A, Thioholgamide A, a New Anti-Proliferative Anti-Tumor Agent, Modulates Macrophage Polarization and Metabolism, Cancers. 12 (2020) 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Takase S, Kurokawa R, Kondoh Y, Honda K, Suzuki T, Kawahara T, Ikeda H, Dohmae N, Osada H, Shin-ya K, Kushiro T, Yoshida M, Matsumoto K, Mechanism of Action of Prethioviridamide, an Anticancer Ribosomally Synthesized and Post-Translationally Modified Peptide with a Polythioamide Structure, ACS Chem. Biol. 14 (2019) 1819–1828. [DOI] [PubMed] [Google Scholar]
- [114].Ohishi T, Abe H, Sakashita C, Saqib U, Baig MS, Ohba S, Inoue H, Watanabe T, Shibasaki M, Kawada M, Inhibition of mitochondria ATP synthase suppresses prostate cancer growth through reduced insulin†like growth factor†1 secretion by prostate stromal cells, Int. J. Cancer. 146 (2020) 3474–3484. [DOI] [PubMed] [Google Scholar]
- [115].Abe H, Kawada M, Sakashita C, Watanabe T, Shibasaki M, Structure-activity relationship study of leucinostatin A, a modulator of tumor–stroma interaction, Tetrahedron. 74 (2018) 5129–5137. [Google Scholar]
- [116].Notari L, Arakaki N, Mueller D, Meier S, Amaral J, Becerra SP, Pigment epithelium-derived factor binds to cell-surface F1-ATP synthase, FEBS J. 277 (2010) 2192–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Deshpande M, Notari L, Subramanian P, Notario V, Becerra SP, Inhibition of tumor cell surface ATP synthesis by pigment epithelium-derived factor: implications for antitumor activity, Int. J. Oncol. 41 (2012) 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Crawford SE, Stellmach V, Ranalli M, Huang X, Huang L, Volpert O, De Vries GH, Abramson LP, Bouck N, Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity, J. Cell. Sci. 114 (2001) 4421–4428. [DOI] [PubMed] [Google Scholar]
- [119].Broadhead ML, Dass CR, Choong PF, In vitro and in vivo biological activity of PEDF against a range of tumors, Expert Opin. Ther. Targets . 13 (2009) 1429–1438. [DOI] [PubMed] [Google Scholar]
- [120].Martinez-Marin D, Jarvis C, Nelius T, De Riese W, Volpert OV, Filleur S, PEDF increases the tumoricidal activity of macrophages towards prostate cancer cells in vitro, PloS one. 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang T, Yin P, Zhang Z, Xu B, Che D, Dai Z, Dong C, Jiang P, Hong H, Yang Z, Deficiency of pigment epithelium-derived factor in nasopharyngeal carcinoma cells triggers the epithelial–mesenchymal transition and metastasis, Cell death dis. 8 (2017) e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Nwani NG, Deguiz ML, Jimenez B, Vinokour E, Dubrovskyi O, Ugolkov A, Mazar AP, Volpert OV, Melanoma cells block PEDF production in fibroblasts to induce the tumor-promoting phenotype of cancer-associated fibroblasts, Cancer Res. 76 (2016) 2265–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Puthia M, Storm P, Nadeem A, Hsiung S, Svanborg C, Prevention and treatment of colon cancer by peroral administration of HAMLET (human α-lactalbumin made lethal to tumour cells), Gut. 63 (2014) 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ho J, Sielaff H, Nadeem A, Svanborg C, Gruber G, The molecular motor F-ATP synthase is targeted by the tumoricidal protein HAMLET, J. Mol. Biol. 427 (2015) 1866–1874. [DOI] [PubMed] [Google Scholar]
- [125].Cumero S, Fogolari F, Domenis R, Zucchi R, Mavelli I, Contessi S, Mitochondrial F0F1-ATP synthase is a molecular target of 3-iodothyronamine, an endogenous metabolite of thyroid hormone, Br. J. Pharmacol. 166 (2012) 2331–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR, Nature. 510 (2014) 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, Pai MY, Li S, Ta L, Fazlollahi F, Chen C, Prins RM, Teitell MA, Nathanson DA, Lai A, Faull KF, Jiang M, Clarke SG, Cloughesy TF, Graeber TG, Braas D, Christofk HR, Jung ME, Reue K, Huang J, 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling, Cell. Metab. 22 (2015) 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gause EM, Buck MA, Douglas MG, Binding of citreoviridin to the beta subunit of the yeast F1-ATPase. J. Biol. Chem. 256 (1981) 557–559. [PubMed] [Google Scholar]
- [129].Satre M, Bof M, Vignais PV, Interaction of Escherichia coli adenosine triphosphatase with aurovertin and citreoviridin: inhibition and fluorescence studies. J. Bacteriol. 142 (1980) 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sayood SF, Suh H, Wilcox CS, Schuster SM, Effect of citreoviridin and isocitreoviridin on beef heart mitochondrial ATPase, Arch. Biochem. Biophys. 270 (1989) 714–721. [DOI] [PubMed] [Google Scholar]
- [131].Chang H, Huang H, Huang T, Yang P, Wang Y, Juan H, Ectopic ATP Synthase Blockade Suppresses Lung Adenocarcinoma Growth by Activating the Unfolded Protein Response, Cancer Res. 72 (2012) 4696–4706. [DOI] [PubMed] [Google Scholar]
- [132].Chang HY, Huang TC, Chen NN, Huang HC, Juan HF, Combination therapy targeting ectopic ATP synthase and 26S proteasome induces ER stress in breast cancer cells, Cell death dis. 5 (2014) e1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wu Y, Hu C, Chien C, Chen Y, Huang H, Juan H, Quantitative proteomic analysis of human lung tumor xenografts treated with the ectopic ATP synthase inhibitor citreoviridin, PloS one. 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chan W, Tan LT, Chan K, Lee L, Goh B, Nerolidol: a sesquiterpene alcohol with multi-faceted pharmacological and biological activities, Molecules. 21 (2016) 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ferreira FM, Palmeira CM, Oliveira MM, Santos D, Simoes AM, Rocha SM, Coimbra MA, Peixoto F, Nerolidol effects on mitochondrial and cellular energetics, Toxicol. In. Vitro. 26 (2012) 189–196. [DOI] [PubMed] [Google Scholar]
- [136].Dunouau C, Belle R, Oulad-Ali A, Anton R, David B, Triterpenes and sterols from Ruscus aculeatus, Planta Med. 62 (1996) 189–190. [DOI] [PubMed] [Google Scholar]
- [137].Del Gaudio F, Festa C, Mozzicafreddo M, Vasaturo M, Casapullo A, De Marino S, Riccio R, Monti MC, Biomolecular proteomics discloses ATP synthase as the main target of the natural glycoside deglucoruscin, Mol. Biosyst. 12 (2016) 3132–3138. [DOI] [PubMed] [Google Scholar]
- [138].Bednarski JJ, Warner RE, Rao T, Leonetti F, Yung R, Richardson BC, Johnson KJ, Ellman JA, Opipari AW Jr, Glick GD, Attenuation of autoimmune disease in fas-deficient mice by treatment with a cytotoxic benzodiazepine, Arthritis Rheum. 48 (2003) 757–766. [DOI] [PubMed] [Google Scholar]
- [139].Stelzer AC, Frazee RW, Van Huis C, Cleary J, Opipari AW Jr, Glick GD, Al-Hashimi HM, NMR studies of an immunomodulatory benzodiazepine binding to its molecular target on the mitochondrial F1F0-ATPase, Biopolymers. 93 (2010) 85–92. [DOI] [PubMed] [Google Scholar]
- [140].Starke I, Johnson KM, Petersen J, Graeber P, Opipar AW Jr., G.D. Glick, M. Boersch, Binding of the immunomodulatory drug Bz-423 to mitochondrial FoF1-ATP synthase in living cells by FRET acceptor photobleaching, Proc. SPIE 9712 (2016) 97120P. [Google Scholar]
- [141].Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW Jr, Glick GD, Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423, Chem. Biol. 12 (2005) 485–496. [DOI] [PubMed] [Google Scholar]
- [142].Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P, Dimers of mitochondrial ATP synthase form the permeability transition pore, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 5887–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sundberg TB, Swenson L, Wahl DR, Opipari AW Jr, Glick GD, Apoptotic signaling activated by modulation of the F0F1-ATPase: implications for selective killing of autoimmune lymphocytes, J. Pharmacol. Exp. Ther. 331 (2009) 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Glick GD, Ferrara JL, Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease, Sci. Transl. Med. 3 (2011) 67ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Bhagavathula N, Nerusu KC, Hanosh A, Aslam MN, Sundberg TB, Opipari AW, Johnson K, Kang S, Glick GD, Varani J, 7-chloro-5-(4-hydroxyphenyl)-1-methyl-3-(naphthalen-2-ylmethyl)-4, 5-dihydro-1H-benzo [b][1, 4] diazepin-2 (3H)-one (Bz-423), a benzodiazepine, suppresses keratinocyte proliferation and has antipsoriatic activity in the human skin-severe, combined immunodeficient mouse transplant model, J. Pharmacol. Exp. Ther. 324 (2008) 938–947. [DOI] [PubMed] [Google Scholar]
- [146].Cleary J, Johnson KM, Opipari AW Jr, Glick GD, Inhibition of the mitochondrial F1F0-ATPase by ligands of the peripheral benzodiazepine receptor, Bioorg. Med. Chem. Lett. 17 (2007) 1667–1670. [DOI] [PubMed] [Google Scholar]
- [147].Krestinina OV, Grachev DE, Odinokova IV, Reiser G, Evtodienko YV, Azarashvili TS, Effect of peripheral benzodiazepine receptor (PBR/TSPO) ligands on opening of Ca2+ -induced pore and phosphorylation of 3.5-kDa polypeptide in rat brain mitochondria, Biochem. (Mosc). 74 (2009) 421–429. [DOI] [PubMed] [Google Scholar]
- [148].Seneviratne MSD, Faccenda D, De Biase V, Campanella M, PK11195 inhibits mitophagy targeting the F1Fo-ATPsynthase in Bcl-2 knock-down cells, Curr. Mol. Med. 12 (2012) 476–482. [DOI] [PubMed] [Google Scholar]
- [149].Jitschin R, Hofmann AD, Bruns H, Giessl A, Bricks J, Berger J, Saul D, Eckart MJ, Mackensen A, Mougiakakos D, Mitochondrial metabolism contributes to oxidative stress and reveals therapeutic targets in chronic lymphocytic leukemia, Blood. 123 (2014) 2663–2672. [DOI] [PubMed] [Google Scholar]
- [150].Nunez-Figueredo Y, Ramirez-Sanchez J, Delgado-Hernandez R, Porto-Verdecia M, Ochoa-Rodriguez E, Verdecia-Reyes Y, Marin-Prida J, Gonzalez-Durruthy M, Uyemura SA, Rodrigues FP, Curti C, Souza DO, Pardo-Andreu G, JM-20, a novel benzodiazepine-dihydropyridine hybrid molecule, protects mitochondria and prevents ischemic insult-mediated neural cell death in vitro, Eur. J. Pharmacol. 726 (2014) 57–65. [DOI] [PubMed] [Google Scholar]
- [151].Alavian KN, Dworetzky SI, Bonanni L, Zhang P, Sacchetti S, Li H, Signore AP, Smith PJ, Gribkoff VK, Jonas EA, The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole, Mol. Pharmacol. 87 (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Muzzi M, Gerace E, Buonvicino D, Coppi E, Resta F, Formentini L, Zecchi R, Tigli L, Guasti D, Ferri M, Camaioni E, Masi A, Pellegrini-Giampietro D, Mannaioni G, Bani D, Pugliese AM, Chiarugi A, Dexpramipexole improves bioenergetics and outcome in experimental stroke, Br. J. Pharmacol. 175 (2018) 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Xuan J, Ren Z, Qing T, Couch L, Shi L, Tolleson WH, Guo L, Mitochondrial dysfunction induced by leflunomide and its active metabolite, Toxicology. 396–397 (2018) 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease, PloS one. 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Molto P, Cuezva JM, Maher P, Petrascheck M, Schubert D, The mitochondrial ATP synthase is a shared drug target for aging and dementia, Aging Cell. 17 (2018) e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Emmanuel IA, Olotu F, Agoni C, Soliman MES, Broadening the horizon: Integrative pharmacophore-based and cheminformatics screening of novel chemical modulators of mitochondria ATP synthase towards interventive Alzheimer’s disease therapy, Med. Hypotheses. 130 (2019) 109277. [DOI] [PubMed] [Google Scholar]
- [157].Emmanuel IA, Olotu FA, Agoni C, Soliman MES, Deciphering the ‘Elixir of Life’: Dynamic Perspectives into the Allosteric Modulation of Mitochondrial ATP Synthase by J147, a Novel Drug in the Treatment of Alzheimer’s Disease, Chem. Biodivers. 16 (2019) e1900085. [DOI] [PubMed] [Google Scholar]
- [158].Cheng Y, Kerppola RE, Kerppola TK, ATR-101 disrupts mitochondrial functions in adrenocortical carcinoma cells and in vivo, Endocr. Relat. Cancer. 23 (2016) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Smith DC, Kroiss M, Kebebew E, Habra MA, Chugh R, Schneider BJ, Fassnacht M, Jafarinasabian P, Ijzerman MM, Lin VH, A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma, Invest. New Drugs. (2020) 1–9. [DOI] [PubMed] [Google Scholar]
- [160].Atwal KS, Wang P, Rogers WL, Sleph P, Monshizadegan H, Ferrara FN, Traeger S, Green DW, Grover GJ, Small molecule mitochondrial F1F0 ATPase hydrolase inhibitors as cardioprotective agents. Identification of 4-(N-arylimidazole)-substituted benzopyran derivatives as selective hydrolase inhibitors, J. Med. Chem. 47 (2004) 1081–1084. [DOI] [PubMed] [Google Scholar]
- [161].Grover GJ, Malm J, Pharmacological profile of the selective mitochondrial F1F0 ATP hydrolase inhibitor BMS-199264 in myocardial ischemia. Cardiovasc. ther. 26 (2008) 287–296. [DOI] [PubMed] [Google Scholar]
- [162].Ivanes F, Faccenda D, Gatliff J, Ahmed AA, Cocco S, Cheng CHK, Allan E, Russell C, Duchen MR, Campanella M, The compound BTB06584 is an IF1-dependent selective inhibitor of the mitochondrial F1Fo-ATPase, Br. J. Pharmacol. 171 (2014) 4193–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wang Y, Hou Q, Xiao G, Yang S, Di C, Si J, Zhou R, Ye Y, Zhang Y, Zhang H, Selective ATP hydrolysis inhibition in F1Fo ATP synthase enhances radiosensitivity in non-small-cell lung cancer cells (A549), Oncotarget. 8 (2017) 53602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Morciano G, Morciano G, Pedriali G, Rizzo P, Ferrari R, Campo G, Pinton P, Preti D, Aquila G, Missiroli S, Fantinati A, Caroccia N, Pacifico S, Bonora M, Talarico A, Morganti C, Giorgi C, Trapella C, Ferrari R, Campo G, Wieckowski MR, Discovery of Novel 1,3,8-Triazaspiro[4.5]decane Derivatives That Target the c Subunit of F1/FO-Adenosine Triphosphate (ATP) Synthase for the Treatment of Reperfusion Damage in Myocardial Infarction, J. Med. Chem. 61 (2018) 7131–7143. [DOI] [PubMed] [Google Scholar]
- [165].Stellrecht CM, Rodriguez CO, Ayres M, Gandhi V, RNA-directed actions of 8-chloro-adenosine in multiple myeloma cells, Cancer Res. 63 (2003) 7968–7974. [PubMed] [Google Scholar]
- [166].Stellrecht CM, Vangapandu HV, Le X, Mao W, Shentu S, ATP directed agent, 8-chloro-adenosine, induces AMP activated protein kinase activity, leading to autophagic cell death in breast cancer cells, J. hematol. oncol. 7 (2014) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chen LS, Nowak BJ, Ayres ML, Krett NL, Rosen ST, Zhang S, Gandhi V, Inhibition of ATP synthase by chlorinated adenosine analogue, Biochem. Pharmacol. 78 (2009) 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kearney AY, Fan Y, Giri U, Saigal B, Gandhi V, Heymach JV, Zurita AJ, 8-chloroadenosine sensitivity in renal cell carcinoma is associated with AMPK activation and mTOR pathway inhibition, PloS one. 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Macer-Wright JL, Sileikaite I, Rayner BS, Hawkins CL, 8-Chloroadenosine Alters the Metabolic Profile and Downregulates Antioxidant and DNA Damage Repair Pathways in Macrophages, Chem. Res. Toxicol. (2019). [DOI] [PubMed] [Google Scholar]
- [170].Tang V, Fu S, Rayner BS, Hawkins CL, 8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response, Redox biol. 26 (2019) 101274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis, Cancer Res. 64 (2004) 7099–7109. [DOI] [PubMed] [Google Scholar]
- [172].Zhang C, Liu Z, Bunker E, Ramirez A, Lee S, Peng Y, Tan AC, Eckhardt SG, Chapnick DA, Liu X, Sorafenib targets the mitochondrial electron transport chain complexes and ATP synthase to activate the PINK1-Parkin pathway and modulate cellular drug response, J. Biol. Chem. 292 (2017) 15105–15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Speransky S, Serafini P, Caroli J, Bicciato S, Lippman ME, Bishopric NH, A novel RNA aptamer identifies plasma membrane ATP synthase beta subunit as an early marker and therapeutic target in aggressive cancer, Breast Cancer Res. Treat. 176 (2019) 271–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Modis K, Ju Y, Ahmad A, Untereiner AA, Altaany Z, Wu L, Szabo C, Wang R, S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics, Pharmacol. Res. 113 (2016) 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hojlund K, Yi Z, Lefort N, Langlais P, Bowen B, Levin K, Beck-Nielsen H, Mandarino LJ, Human ATP synthase beta is phosphorylated at multiple sites and shows abnormal phosphorylation at specific sites in insulin-resistant muscle, Diabetologia. 53 (2010) 541–551. [DOI] [PubMed] [Google Scholar]
- [176].Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, Argenton F, Forte M, Tosatto S, Lippe G, Bernardi P, Ca(2+) binding to F-ATP synthase beta subunit triggers the mitochondrial permeability transition, EMBO Rep. 18 (2017) 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P, Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition, Cell. Cycle. 12 (2013) 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA, An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 10580–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Neginskaya MA, Solesio ME, Berezhnaya EV, Amodeo GF, Mnatsakanyan N, Jonas EA, Pavlov EV, ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel, but Lack the Permeability Transition Pore, Cell. Rep. 26 (2019) 11–17.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE, Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase, Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 3409–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].He J, Carroll J, Ding S, Fearnley IM, Walker JE, Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase, Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Carroll J, He J, Ding S, Fearnley IM, Walker JE, Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 12816–12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, Kroemer G, Wieckowski MR, Galluzzi L, Pinton P, Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation, EMBO Rep. 18 (2017) 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Faccenda D, Tan CH, Seraphim A, Duchen MR, Campanella M, IF1 limits the apoptotic-signalling cascade by preventing mitochondrial remodelling, Cell Death Differ. 20 (2013) 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Wei S, Fukuhara H, Kawada C, Kurabayashi A, Furihata M, Ogura S, Inoue K, Shuin T, Silencing of ATPase Inhibitory Factor 1 Inhibits Cell Growth via Cell Cycle Arrest in Bladder Cancer, Pathobiology. 82 (2015) 224–232. [DOI] [PubMed] [Google Scholar]
- [186].Formentini L, Pereira MP, Sanchez-Cenizo L, Santacatterina F, Lucas JJ, Navarro C, Martinez-Serrano A, Cuezva JM, In vivo inhibition of the mitochondrial H+-ATP synthase in neurons promotes metabolic preconditioning, EMBO J. 33 (2014) 762–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Wang WJ, Shi XX, Liu YW, He YQ, Wang YZ, Yang CX, Gao F, The mechanism underlying the effects of the cell surface ATP synthase on the regulation of intracellular acidification during acidosis, J. Cell. Biochem. 114 (2013) 1695–1703. [DOI] [PubMed] [Google Scholar]
- [188].Gale M, Li Y, Cao J, Liu ZZ, Holmbeck MA, Zhang M, Lang SM, Wu L, Carmo MD, Gupta S, Aoshima K, DiGiovanna MP, Stern DF, Rimm DL, Shadel GS, Chen X, Yan Q, Acquired resistance to HER2-targeted therapies creates vulnerability to ATP synthase inhibition, Cancer Res. 80 (2020) 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Barbic M, Willer EA, Rothenhöfer M, Heilmann J, Fürst R, Jürgenliemk G, Spirostanol saponins and esculin from Rusci rhizoma reduce the thrombin-induced hyperpermeability of endothelial cells, Phytochemistry. 90 (2013) 106–113. [DOI] [PubMed] [Google Scholar]
- [190].Xu J, Shen D, Mao W, Lin Q, Lin F, Lu C, The effects of PK11195 on the MCF-7 and T47D were associated with the allopregnanolone biosynthesis, which was mediated by Translocator Protein 18 KDa, Cancer Biomark. 17 (2016) 11–16. [DOI] [PubMed] [Google Scholar]