Abstract
Metal-based carbon monoxide (CO)-releasing molecules have been shown to exert anti-inflammatory and anti-oxidative properties maintaining gastric mucosal integrity. We are interested in further development of metal-free CO-based therapeutics for oral administration. Thus, we examine the protective effect of representative CO prodrug, BW-CO-111, in rat models of gastric damage induced by necrotic ethanol or aspirin, a representative non-steroidal anti-inflammatory drug. Treatment effectiveness was assessed by measuring the microscopic/macroscopic gastric damage area and gastric blood flow by laser flowmetry. Gastric mucosal mRNA and/or protein expressions of HMOX1, HMOX2, nuclear factor erythroid 2-related factor 2, COX1, COX2, iNos, Anxa1 and serum contents of TGFB1, TGFB2, IL1B, IL2, IL4, IL5, IL6, IL10, IL12, tumor necrosis factor α, interferon γ, and GM-CSF were determined. CO content in gastric mucosa was assessed by gas chromatography. Pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) increased gastric mucosal content of CO and reduced gastric lesions area in both models followed by increased GBF. These protective effects of the CO prodrug were supported by changes in expressions of molecular biomarkers. However, because the pathomechanisms of gastric damage differ between topical administration of ethanol and aspirin, the possible protective and anti-inflammatory mechanisms of BW-CO-111 may be somewhat different in these models.
KEY WORDS: Gastroprotection, Carbon monoxide, Prodrug, Anti-inflammation, NSAID, Ethanol, Gastric mucosal damage
Graphical abstract
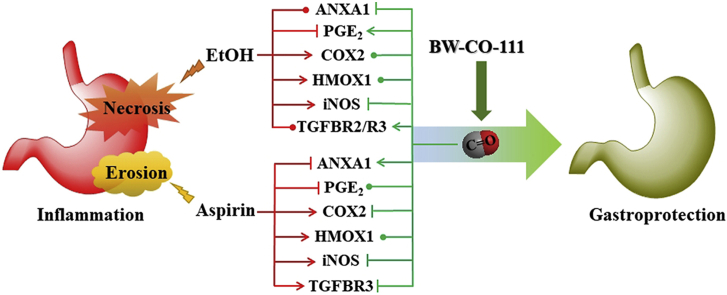
Carbon monoxide (CO) is an endogenous molecule exerting anti-inflammatory activity. BW-CO-111 is an organic CO prodrug. BW-CO-111 exerts gastroprotective effect against ethanol- and aspirin-induced damage. The specific mechanism of the beneficial activity of CO released from BW-CO-111 depends on the type of gastric tissue injury.
1. Introduction
Carbon monoxide (CO) is an endogenous signaling molecule produced by heme oxygenase (HMOX)-mediated heme degradation and has been shown to exhibit cytoprotective effects among others1. Taking into account its molecular activity and ability to modulate a variety of intracellular pathways under physiological and pathological conditions, CO is a sibling gasotransmitter to endogenously produced hydrogen sulfide (H2S) and nitric oxide (NO)2. CO has been shown to modulate synaptic plasticity and tissue regeneration within central nervous system and also to affect gastric mucosal integrity and to modulate duodenal secretion within gastrointestinal (GI) tract2, 3, 4, 5. Morevoer, CO producing HMOX1 is known to be induced by oxidation and inflammation and is considered as a part of self-defense feedback mechanism activated within particular tissues in response to the exposure to various stressors6, 7, 8. Importantly, CO has a relatively high toxicity threshold reaching up to 10% of carboxyhemoglobin (COHb) concentration in blood9. Therefore, the last two decades have seen much effort in developing CO as a therapeutic agent10, 11, 12, 13 for treating inflammatory conditions and in offering cytoprotection in various organs including kidney14, 15, 16, 17, 18, heart19, 20, 21, liver22, 23, 24, brain25, 26, 27 and GI2,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 among others10,11. Along this line, the issue of route of administration is a critical pharmaceutical factor. Earlier work was largely focused on using inhaled CO gas as the choice of administration. However, for a broad range of potential applications, it is desirable to develop non-inhalation forms of delivery. CO is known to have high affinity for a range of transition metals; thus metal-immobilized carbonyls attracted much attention for the experimental delivery of CO. As early as in 1891, McKendrick and Snodgrass39 experimented nickel tetracarbonyl and found it to be an antipyretic. However, nickel tetracarbonyl is highly toxic. Motterlini and others11,12,40, 41, 42, 43 developed alternatives as metal-based CO-releasing molecules (CO-RMs) using metals such as ruthenium, manganese, molybdenum and iron, which are sufficiently benign to allow for biological and pharmacological studies at the cellular level in vitro and in animal models. Aiming at improving delivery properties and minimizing metal-related toxicity, modified CO-RMs have been reported including enzyme-controlled release44,45 and encapsulated metal-based CO-RMs46, 47, 48, 49, 50, as well as photosensitive organic (metal-free) CO-RMs51, 52, 53, 54, 55, 56 and oral forms of CO in a solution57. Most recently, we have been working on developing metal-free organic CO prodrugs belonging to different structural classes10,58, 59, 60 with tunable CO release rates58,60, 61, 62, 63. Some of these prodrugs are capable of triggered release (pH-62, esterase-61,64,65, and ROS-sensitive66), mitochondrion-targeting22, employing dual-triggers60,64, and delivering more than one payload using a single prodrug60,61,64,67.
Previously, ruthenium-based CORM-2 and CO have been studied for their gastroprotective and ulcer healing effects30,31,33. However, recent studies of ruthenium-based CO-RMS have led some to ask for “a major reappraisal of the biological effects of CORM-2 and related CORMs”68 because of ruthenium's ability to undergo chemical reactions with thiols68, 69, 70 and other molecules or proteins71, 72, 73, 74. Recently, organic CO prodrugs have been shown to be effective in treating systemic inflammation, experimental colitis, chemically induced liver injury, and ischemia reperfusion kidney injury17,22,64,75. It is worth mentioning that endogenously produced CO due to activity of HMOX/nuclear factor erythroid 2-related factor 2 (NRF2) pathway has been shown to play beneficial roles in the self-defensive response to noxious agents2,35,37,76,77. Herein, we describe our effort of studying for the first time an organic CO prodrug for its gastroprotection of injuries induced by aspirin, a classic NSAID or a chemical agent known to evoke necrotizing injury to the stomach such as ethanol.
Thus, in this study we aimed to investigate for the first time if pretreatment with a novel CO prodrug, BW-CO-11162, prevents gastric mucosa against aspirin- or necrotic, ethanol-induced gastric damage on the micro- and macroscopic levels and if this effect is accompanied by the alterations in gastric blood flow (GBF). We focused on possible effects of BW-CO-111 on modulation of inflammation by screening serum concentrations of 11 inflammatory markers and transforming growth factor β (TGFB). Possible modulation of HMOX1/HMOX2 and NRF2 pathways by this CO prodrug has also been investigated. Alterations in gastric mucosal mRNA and/or protein expression for proinflammatory inducible nitric oxide synthase (iNos) and anti-inflammatory annexin-A1 (Anxa1), TGFB1 receptors (Tgfbr1, Tgfbr2, and Tgfbr3), and physiological gastroprotective barrier components, prostaglandin (PG)E2-producing enzymes, cyclooxygenase (COX1 and COX2) were also assessed.
2. Materials and methods
2.1. Experimental design
Fifty male Wistar rats with average weight of 220–300 g were used in the study. Animals were fasted for 24 h with free access to tap water before each experiment. All procedures were approved by the I Local Ethical Committee for Care and Use of Experimental Animals, held by Faculty of Pharmacy, Jagiellonian University Medical College in Cracow (Decision No.: 311/2019; Date: 17 July 2019). Experiments were run with implications for replacement, refinement or reduction (the 3Rs) principle. Animal studies are reported in compliance with the ARRIVE guidelines.
Rats were randomly assigned to the appropriate experimental groups (5 rats each) and were pretreated i.g. by orogastric tube with 1 mL of 1) dimethyl sulfoxide (DMSO)/H2O (1:9) as vehicle, 2) CO prodrug BW-CO-111 (0.02–5 mg/kg) or 3) BW-CP-111, the product after CO release from BW-CO-111, applied at a dose of 0.1 mg/kg, which is the equivalent of the effective dose of BW-CO-111 capable of reducing ethanol-induced injury area by more than 50%62. After 30 min, animals were administered i.g. with 1 mL of 75% ethanol or 1.5 mL of ASA (125 mg/kg dissolved in 0.2 mol/L HCl), based on previously implemented and described experimental models of drugs-induced or necrotic gastric mucosal injuries35,78. BW-CO-111 was selected due to its half-live for CO release62. In a separate series of experiments, animals were pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg) and 30 min later gastric mucosal biopsies were collected for determination of gastric mucosal CO content as described below.
All compounds and chemicals were purchased from Sigma–Aldrich (Schnelldorf, Germany) unless otherwise stated. BW-CO-111 and BW-CP-111 were synthesized following procedures described previously62.
2.2. GBF determination, macro- and microscopic gastric damage assessment and sample collection
One hour after administration of ethanol or aspirin (ASA), under isoflurane anesthesia, the abdomen was opened for the GBF measurement by laser flowmetry, as described previously38. Briefly, the GBF was determined in the oxyntic part of the gastric mucosa not involving ethanol- or aspirin-induced mucosal damage using laser flowmeter (Laserflo, model BPM 403A, Blood Perfusion Monitor, Vasamedics, St. Paul, MN, USA). Average values of three measurements were expressed in mL/min per 100 g of gastric tissue. Blood samples were collected from the vena cava and separated serum was stored at −80 °C until further analysis38,79. Next, the stomach was excised, opened along the greater curvature and the area of gastric damage was determined planimetrically and expressed in mm2 35. Gastric mucosal samples were scraped off on ice, snap-frozen in liquid nitrogen and stored at −80 °C until further analysis35. Gastric mucosal biopsies were collected as described previously for determination of CO content80.
For microscopic analysis, the gastric tissue sections were excised and fixed in 10% buffered formalin, pH = 7.4. Samples were dehydrated by passing them through a series of alcohols with incremental concentrations, equilibrated in xylene for 10–15 min and embedded in paraffin; paraffin blocks were cut into about 4 μm sections using a microtome. The prepared specimens were stained with haematoxylin/eosin (H&E). Tissue slides were evaluated using a light microscope (AxioVert A1, Carl Zeiss, Oberkochen, Germany)80. Digital documentation of histological slides was obtained using above mentioned microscope and ZEN Pro 2.3 software (Carl Zeiss, Oberkochen, Germany)80.
2.3. Determination of gastric mucosal mRNA fold changes by real-time PCR
Gastric mucosal mRNA expression fold change for Hmox1, Hmox2, iNos, Cox1, Cox2, Anxa1 and Tgfb1 was assessed by real time PCR, as described previously80. Briefly, total RNA was isolated using commercially available kit with spin-columns (GeneMATRIXUniversal RNA Purification Kit, EURx, Gdansk, Poland) according to manufacturer's protocols. Reversed transcription (RT) was performed using PrimeScript™RTMasterMix (Perfect Real Time Takara Bio Inc., Kyoto, Japan). RNA concentration was measured using Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). For each RT reaction, total RNA concentration was adjusted to (1 μg) per sample. Samples from healthy (intact) gastric mucosa were further used as reference control during calculations. Expression of mRNA for Hmox1, Hmox2, iNos, Cox1, Cox2, Anxa1, Il1b and succinate dehydrogenase complex, subunit A (Sdha) and β-actin (Actb) as reference genes was determined using specific primers35,80. To determine Tgfbr1, 5′-ACTCCCAACTACAGAAAAGCA-3′ forward and 5′-AAGGGCGATCTAGTGAGGGA-3′ reverse primers were used. To determine Tgfbr2, 5′-CCCCCGTTTGGTTCCAGAGT-3′ forward and 5′-CGGTCTCTCAGCACGTTGTC-3′ reverse primers were used. To determine Tgfbr3, 5′-GCTCCCAACAGTATCGGCTT-3′ forward and 5′-GCCTGTAGCCATTGTCCAGT-3′ reverse primers were used.
PCR reaction was run using thermal cycler Quant Studio 3 (Thermo Fisher Scientific) and SYBR Green I dye including kit [SG qPCR Master Mix (2 ×), EURx]. To maintain the same PCR reaction efficiency in all analyzed samples, the same amount of cDNA per each well was used. After reaction, melting curve for each sample, its technical replicates and for appropriate negative control were analyzed to exclude the data derived from potentially unintended products. Results were analyzed using the −ΔΔCt method81.
2.4. Determination of proteins expression in gastric mucosa by Western blot
Protein expression for HMOX1, HMOX2, NRF2, COX1 and COX2 in gastric mucosa was determined using Western blot as described previously79. Rabbit monoclonal anti-HMOX1 (ab68477, Abcam, Cambridge, UK) in dilution of 1:1000, rabbit polyclonal anti-COX1 (13393-1-AP, Proteintech, Manchester, UK) in dilution of 1:1000, rabbit polyclonal anti-NRF2 (163936-1-AP, Proteintech) in dilution of 1:500, rabbit polyclonal anti-COX-2 (ab 15191, Abcam) in dilution of 1:1000, rabbit polyclonal anti-HMOX-2 (14817-1AP, Proteintech) in dilution of 1:1000 and rabbit monoclonal anti-GAPDH, (2118, Cell Signaling Technology, Danvers, MA, USA) in dilution of 1:2000 were used as primary antibodies. Protein expression was visualized using horseradish peroxidase-linked secondary anti-rabbit IgG antibody (7074, Cell Signaling Technology) or anti-mouse IgG antibody (7076, Cell Signaling Technology) in dilution of 1:2000 where appropriate. All primary and secondary antibodies were diluted in 5% non-fat milk, except anti-NRF2 antibody, which was diluted in 5% BSA.
Chemiluminescence was developed using WesternSure® ECL Substrate (LI-COR, Lincoln, NE, USA) or WesternBright Quantum (Advansta, Menlo Park, CA, USA) and was measured using C-DiGit® Blot Scanner (LI-COR). The intensity of bands was determined and analyzed using Image Studio 4.0 software (LI-COR). The expression of each protein of interest was determined using 5 samples per experimental group and obtained values were normalized to the expression of ACTB or GAPDH as loading controls78,79.
2.5. Luminex microbeads fluorescent assays
Determination of serum concentrations of interleukin IL1B, IL2, IL4, IL5, IL6, IL10, IL12, IL13, interferon γ (IFNG), tumor necrosis factor α (TNF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) was performed using Luminex microbeads fluorescent assay (Bio-Rad, Hercules, CA, USA) and Luminex MAGPIX system (Luminex Corp., Austin, TX, USA). Results were calculated from calibration curves and expressed in pg/mL73.
2.6. Determination of CO content in gastric mucosa by gas chromatography (GC)
CO concentration in the gastric mucosa biopsies was determined as reported previously but using modified GC-based method described in detail elsewhere and briefly below 80.
Sample preparation: 10 mL of water were added to the tissue fragments (about 400–600 mg) and homogenized by sonication (Sonoplus, BANDELIN Electronic GmbH & Co. KG, Berlin, Germany). The volume of 2.5 mL of homogenate was pipetted into two 10 mL headspace vials (2 test samples). To obtain calibration samples, about 5 mL of the remaining volume of the homogenate was saturated with CO for 20 min (100% saturation CO). The CO used to saturate the calibration samples was obtained by reacting concentrated sulfuric acid with 80% formic acid. Unbound CO was removed by flushing with nitrogen for 3 min. Calibration solutions with CO saturation 1.25%, 2.5%, 5% and 10% were prepared from 100% saturated homogenates. 2.5 mL of each calibration solution was pipetted into headspace vials (4 calibration samples). The vials were then sealed with an aluminum cap and silicon Teflon septum. Each vial was gently flushed with helium for 30 s and then 1.5 mL of 20% potassium hexacyanoferrate was added with a syringe.
GC/O-FID (flame ionization detector)-headspace analysis: for the GC/O-FID-headspace analysis, a Thermo Trace GC Ultra (Thermo Electron Corp., Waltham, MA, USA) equipped with O-FID detector (FID with jet nickel microcatalytic methanizer) was used. The jet nickel microcatalyzer converts CO to methane at 330 °C, which increases the sensitivity of CO detection. The system was equipped with a Thermo TriPlus HS autosampler (Thermo Electron Corp.). The prepared samples were mixed and incubated at 70 °C for 8 min in autosampler agitator to the achieve complete CO liberation. 200 μL of gas-phase of each sample were injected with an autosampler gas-tight syringe (heated at 72 °C). Split/splitess injector (200 °C) with closed split was used. GC separation was performed with HP-Molesieve column (Agilent Technologies, Santa Clara, CA, USA; 30 m/0.53 mm ID/0.25 μm) at constant flow 15 mL/min of helium as a carrier gas. The temperature program consisted of the following steps: 60 °C for 2 min followed by 120 °C for 2 min achieved by a heating rate 60 °C/min.
2.7. Determination of CO release from BW-CO-111 in vitro by GC
GC was performed on an Agilent 7820 GC (Agilent Technologies) equipped with a thermal conductivity detector (TCD) and a packed molecular sieve column Carboxen 1000, 15 ft × 2.1 mm (RESTEK, Bellefonte, PA, USA); helium was used as the carrier gas. Oven temperature was programed as follows: 35 °C in 5 min, then to 225 °C at a rate of 20 °C/min, and then hold for 5.5 min. TCD detector was set at 125 °C. Calibration curve was generated by injecting a series of varying volumes of pure CO gas taken from a gas sampling bag into a 6-mL head-space vial pre-sealed with 2.8 mL phosphate buffered saline (PBS)/DMSO (1:6, v:v) medium, which was the same volume used in the CO release experiment from the prodrug. After incubation at 37 °C overnight, 250 μL of the head-space gas was injected to the GC. The peak area of CO was normalized with the peak area of oxygen as the internal standard (from the air in the head space). The ratio between CO and O2 was plotted against the injected pure CO molar quantity to give the calibration curve.
2.8. Determination of CO release yield and profile of BW-CO-111 in PBS and simulated gastric fluid (SGF)
Phosphate buffered saline (PBS) was purchased from Corning (Corning, NY, USA) and SGF was made by dissolving NaCl (0.2%, w/v; Sigma–Aldrich, St. Louis, MO, USA) in pH 1.2 hydrochloric acid (HCl, Sigma–Aldrich) solution. DMSO was purchased form Sigma–Aldrich. About 2 mg of BW-CO-111 was used by dissolving in a mixed solvent with PBS (or SGF)/DMSO (1:6, v:v) to give a solution of about 1 mmol/L. Specifically, approximately 2 mg of BW-CO-111 was weighed into a 6-mL headspace vial (total volume 5.8 mL). After dissolving in 2.4 mL DMSO, 0.4 mL of PBS or SGF was added and the cap was sealed instantly. For the release yield determination, the vial was incubated at 37 °C for 6 h and 250 μL of headspace gas was injected into GC. For the release profile determination, 250 μL of headspace gas was taken from the vial at different time points (10–180 min) and injected into GC. After each sampling, 250 μL of air was injected into the vial to balance the pressure. The ratio between the calculated CO amount and the molar quantity of BW-CO-111 was used to determine the release yield and the release profile. The experiments were conducted in triplicate and the results are reported as mean ± SD.
2.9. Statistical analysis
Results were analyzed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Results are presented as mean ± SEM. Statistical analysis was conducted using Student's t-test or ANOVA with Dunnett's multiple comparison post hoc test if more than two experimental groups were compared. The group size for each experimental group was of n = 5 and P < 0.05 was considered as statistically significant.
3. Results
3.1. CO release from BW-CO-111 in vitro and CO content in gastric mucosa after pretreatment with this prodrug
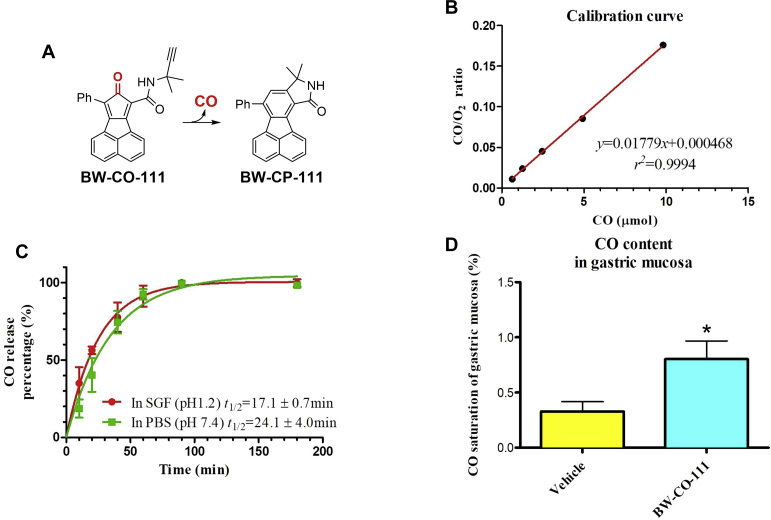
Fig. 1A shows the reaction of CO release from BW-CO-111 to BW-CP-11162. In vitro CO release profile was tested with gas chromatography. CO calibration curve and the regression formula is shown in Fig. 1B and the goodness of fitting is r2 = 0.9994. All data points of CO/O2 ratio for the tested samples fell within the range of the calibration levels (0.625–9.821 μmol). As shown in Table 1 and Fig. 1C, BW-CO-111 released stoichiometric amount of CO under both acidic and neutral conditions. The release kinetics gave a half-life of 24.1 ± 4.0 min in the PBS/DMSO mixture and 17.1 ± 0.7 min in the SGF/DMSO mixture. The difference in release half-life likely reflects the solvent-sensitive nature of the controlling Diels–Alder reaction58. Fig. 1D shows that CO content in gastric mucosa was significantly increased 45 min after i.g. administration of BW-CO-111 (0.1 mg/kg) as compared with vehicle treated group (P < 0.05).
Figure 1.
Chemical structures of CO prodrug BW-CO-111 and BW-CP-111 (A). Calibration curve for CO release from BW-CO-111 in vitro (B). Percentage of CO release in vitro and half-life at pH 7.4 and 1.2 for BW-CO-111 (C). Results are reported as mean ± SD. Gastric mucosal CO contents in gastric mucosa with or without pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) 45 min prior to sampling (D). Results are mean ± SEM of 5 rats per group. ∗P < 0.05 compared with intact.
Table 1.
CO release yield of BW-CO-111.
| Solution | Release yield (%) |
|---|---|
| SGF (pH 1.2; HCl:DMSO = 1:6) | 103.0 ± 1.9 |
| PBS (pH 7.4; buffer:DMSO = 1:6) | 100.7 ± 1.7 |
Results are reported as mean ± SD.
3.2. Gastroprotective effect of pretreatment with BW-CO-111 against ethanol- or aspirin-induced damage and possible modulation of gastric microcirculation
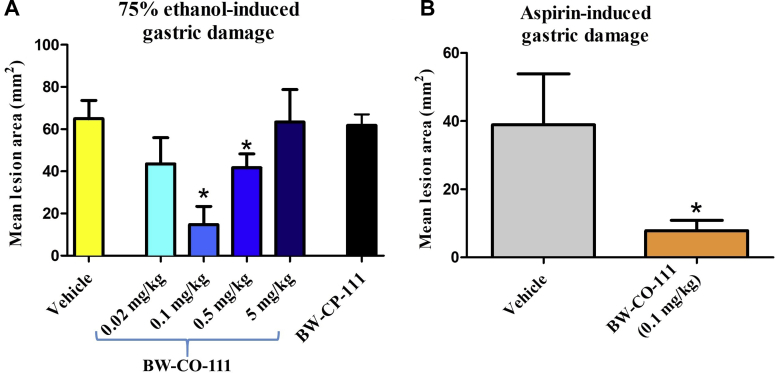
Fig. 2A shows that pretreatment with BW-CO-111 applied i.g. at a dose of 0.1 and 0.5 mg/kg, but not at a dose 0.02 or 5 mg/kg, significantly decreased ethanol-induced gastric damage area (P < 0.05). However, the dose of 0.1 mg/kg i.g. decreased gastric lesion area by more than 50% (Fig. 2A). The negative control BW-CP-111 (0.1 mg/kg, i.g.) did not significantly affect ethanol-induced gastric lesions area (Fig. 2A). Fig. 2B shows that pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) significantly decreased aspirin-induced gastric lesion area (P < 0.05).
Figure 2.
Ethanol- (A) and aspirin-induced (B) gastric lesion areas in rats pretreated i.g. with vehicle, CO prodrug BW-CO-111 (0.02–5 mg/kg) or BW-CP-111 (0.1 mg/kg). Results are mean ± SEM of 5 rats per group. ∗P < 0.05 compared with the vehicle-control group.
In rats administered with ethanol or aspirin, the GBF was significantly decreased as compared with intact gastric mucosa (P < 0.05, Table 2). Pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) but not with BW-CP-111 (0.1 mg/kg, i.g.) significantly elevated GBF in gastric mucosa administered with ethanol and aspirin as compared with rats pretreated with vehicle (P < 0.05, Table 2).
Table 2.
Gastric blood flow (GBF) levels in gastric mucosa of rats pretreated i.g. with vehicle, CO prodrug BW-CO-111 (0.1 mg/kg) or BW-CP-111 (0.1 mg/kg) 45 min prior to treatment with 75% ethanol (EtOH) or aspirin (ASA, 125 mg/kg).
| Experimental group | GBF (mL/min per 100 g of gastric tissue) |
|---|---|
| Intact | 41.80 ± 0.8602 |
| Vehicle + EtOH | 27.40 ± 1.631∗ |
| BW-CO-111 + EtOH | 34.00 ± 1.140∗∗ |
| BW-CP-111 + EtOH | 29.20 ± 0.6633 |
| Vehicle + ASA | 26.60 ± 1.503∗ |
| BW-CO-111 + ASA | 32.40 ± 1.536# |
Intact refers to the values obtained in healthy gastric mucosa without ethanol or aspirin-induced gastric damage. Results are mean ± SEM of 5 rats per group. ∗P < 0.05 compared with the respective values in intact gastric mucosa. ∗∗P < 0.05 compared with the respective values in vehicle-control group administered with EtOH. #P < 0.05 compared with the respective values in vehicle-control group administered with ASA.
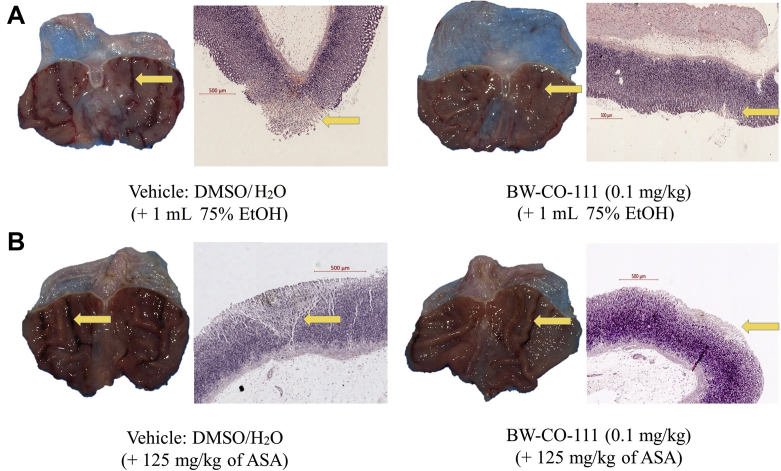
Fig. 3A and B shows macroscopic and microscopic appearance of gastric injury in rats administered with 75% ethanol (A) or aspirin (B) and pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg). In rats pretreated with vehicle, i.g. administration of 75% ethanol or aspirin (125 mg/kg) resulted in macroscopic hemorrhagic erosions and microscopic necrotic damage penetrating into gastric mucosa with notable leukocytes infiltration into submucosal layer with accompanying desquamation and necrosis of epithelium surface (Fig. 3A and B). Pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) reduced the depth and surface area of the necrotic layer (Fig. 3A and B).
Figure 3.
Macroscopic and microscopic appearance of randomly selected representative gastric mucosa of rats pretreated with vehicle or BW-CO-111 (0.1 mg/kg) and exposed to 75% ethanol (EtOH) (A) or aspirin (ASA) (B). Histological slides were stained with H/E. Yellow arrows indicate the macroscopic and microscopic gastric lesions.
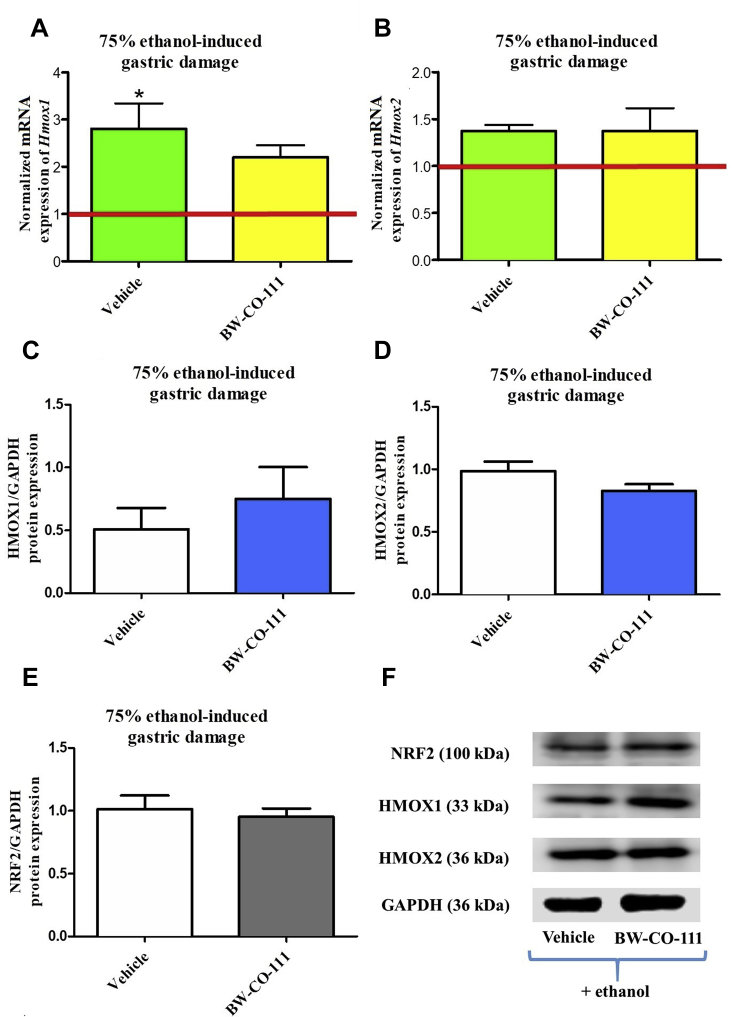
3.3. Alterations in Hmox1/HMOX1 and Hmox2/HMOX2 mRNA/protein expression in gastric mucosa compromised by EtOH after pretreatment with BW-CO-111
Administration of 75% ethanol significantly increased Hmox1 but not Hmox2mRNA expression in gastric mucosa as compared with rats without any treatments (Fig. 4A and B, P < 0.05). Fig. 4A and B shows that gastric mucosal mRNA expression for Hmox1 and Hmox2 was not significantly affected in rats pretreated with BW-CO-111 followed by treatment with 75% ethanol 30 min later. Gastric mucosal protein expression for HMOX1, HMOX2 and NRF2 were not significantly affected in rats pretreated i.g. with BW-CO-111 (0.1 mg/kg) as compared with vehicle (Fig. 4C–E).
Figure 4.
Hmox1 (A), Hmox2 (B) mRNA and HMOX1 (C, F), HMOX2 (D, F), NRF2 (E, F) proteins expression in gastric mucosa of rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg) followed by 75% ethanol administration 30 min later. Results are mean ± SEM of 5 rats per experimental group. Red line indicates baseline value of mRNA expression in healthy gastric mucosa without any treatments. ∗P < 0.05 compared with healthy gastric mucosa.
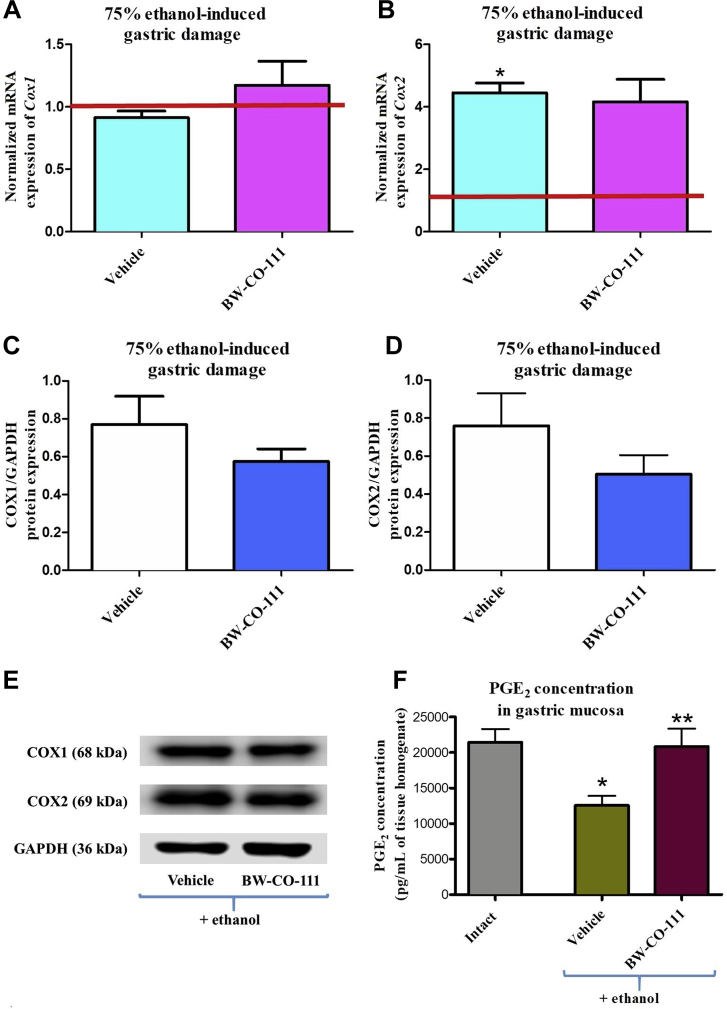
3.4. Modulation of gastroprotective PGE2 content and Cox1/COX1 and Cox2/COX2 mRNA/protein expression in gastric mucosa compromised by EtOH after pretreatment with BW-CO-111
Fig. 5A and B shows that gastric mucosal mRNA expression for Cox2 but not Cox1 was significantly upregulated in rats administered with 75% ethanol as compared with rats without any treatments (P < 0.05). In rats pretreated with BW-CO-111 followed by ethanol administration 30 min later, the gastric mucosal Cox1 and Cox2 mRNA expression was not significantly affected as compared with vehicle-treated animals (Fig. 5A and B). The gastric mucosal protein expression for COX1 and COX2 was not significantly affected in rats pretreated i.g. with BW-CO-111 (0.1 mg/kg) as compared with vehicle (Fig. 5C–E). Fig. 5F shows that PGE2 concentration in gastric mucosa was significantly decreased after exposure to ethanol as compared with intact (P < 0.05). Pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) significantly increased PGE2 content as compared with vehicle in animals exposed to ethanol (P < 0.05).
Figure 5.
Cox1 (A), Cox2 (B) mRNA, COX1 (C, E), COX2 (D, E) proteins expression and prostaglandin E2 content (PGE2, F) in gastric mucosa of rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg) and administered 30 min later with 75% ethanol. Results are mean ± SEM of 5 rats per experimental group. Red line indicates baseline value of mRNA expression in healthy gastric mucosa without any treatments (Intact). ∗P < 0.05 compared with healthy gastric mucosa; ∗∗P < 0.05 compared with vehicle.
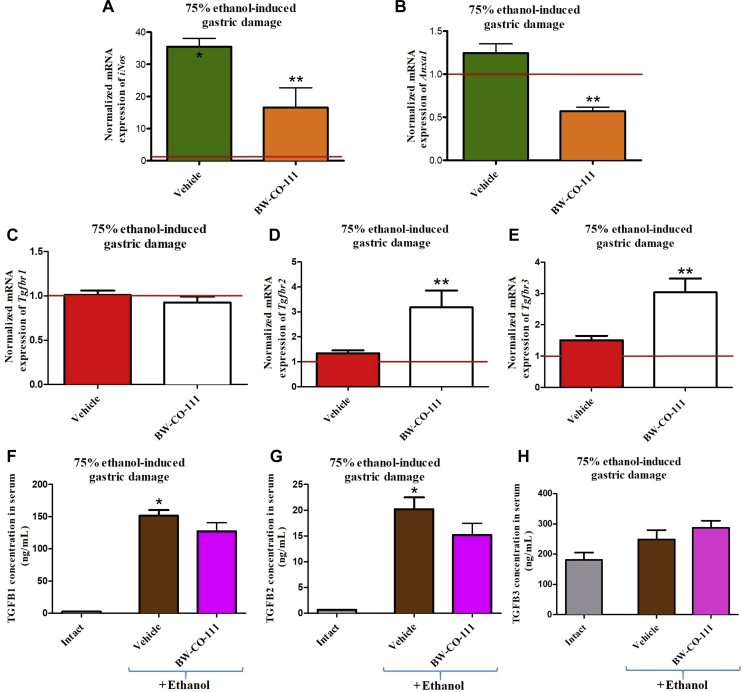
3.5. Involvement of anti-inflammatory Anxa1 and TGFB pathway in the gastroprotective effect of pretreatment with BW-CO-111 in gastric mucosa compromised by EtOH
Fig. 6A shows that gastric mucosal mRNA expression for iNos was significantly upregulated in rats treated with 75% ethanol after vehicle-pretreatment 30 min earlier as compared with animals without any treatments (P < 0.05). BW-CO-111 (0.1 mg/kg, i.g.) significantly decreased iNos mRNA expression fold changes as compared with vehicle-control group (P < 0.05, Fig. 6A). Fig. 6B shows that gastric mucosal mRNA expression of Anxa1 was significantly downregulated in rats administered i.g. with 75% ethanol and pretreated i.g. with BW-CO-111 (0.1 mg/kg) as compared with vehicle (P < 0.05). Fig. 6C–E shows that gastric mucosal mRNA expressions for Tgfbr1, Tgfbr2 and Tgfbr3 were not significantly altered in rats administered i.g. with 75% ethanol and pretreated with vehicle as compared with healthy gastric mucosa (P < 0.05). Pretreatment with BW-CO-111 upregulated Tgfbr2 and Tgfbr3 but not Tgfbr1 mRNA expression in gastric mucosa as compared with vehicle (P < 0.05, Fig. 6C–E). Fig. 6F and G shows respectively that TGFB1 and TGFB2 serum concentrations in rats pretreated with vehicle and administered with ethanol were significantly increased as compared with intact animals (P < 0.05). BW-CO-111 (0.1 mg/kg, i.g.) did not affect TGFB1 and TGFB2 serum concentration in rats administered with ethanol as compared with vehicle-pretreated group (P < 0.05, Fig. 6F and G). TGFB3 serum concentration was not significantly changed after administration of ethanol in rats with or without pretreatment with BW-CO-111 (Fig. 6H).
Figure 6.
Expression of iNos (A), Anxa1 (B) and Tgfb receptor 1 (Tgfbr1) (C), Tgfbr2 (D), Tgfbr3 (E) mRNA in gastric mucosa and alterations in serum concentration of TGFB1 (F), TGFB2 (G), TGFB3 (H) in rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg, i.g.) 30 min before administration of 75% ethanol. Intact refers to healthy rats which were not treated with ethanol. Results are mean ± SEM of 5 samples per each experimental group with statistical significance marked only if 2-fold up- or downregulation was reached. Results are expressed as fold change of normalized gastric mucosal iNos, Anxa1, Tgfbr1, Tgfbr2 and Tgfbr3 mRNA expression. Red line indicates baseline value of mRNA expression in healthy gastric mucosa without any treatments. ∗P < 0.05 compared with healthy gastric mucosa; ∗∗P < 0.05 compared with respective values obtained in vehicle-pretreated group.
Gastric mucosal mRNA expression of anti-inflammatory Anxa1 and pro-inflammatory iNos and Il1b was not significantly downregulated in rats pretreated with BW-CP-111 (0.1 mg/kg, i.g.) as compared with vehicle-pretreatment (Supporting Information Fig. S1A–S1C). Gastric mucosal mRNA expression of iNos and Il1b but not of Anxa1 in 75% ethanol treated rats was significantly upregulated as compared with intact gastric mucosa (P < 0.05, Fig. S1A–S1C).
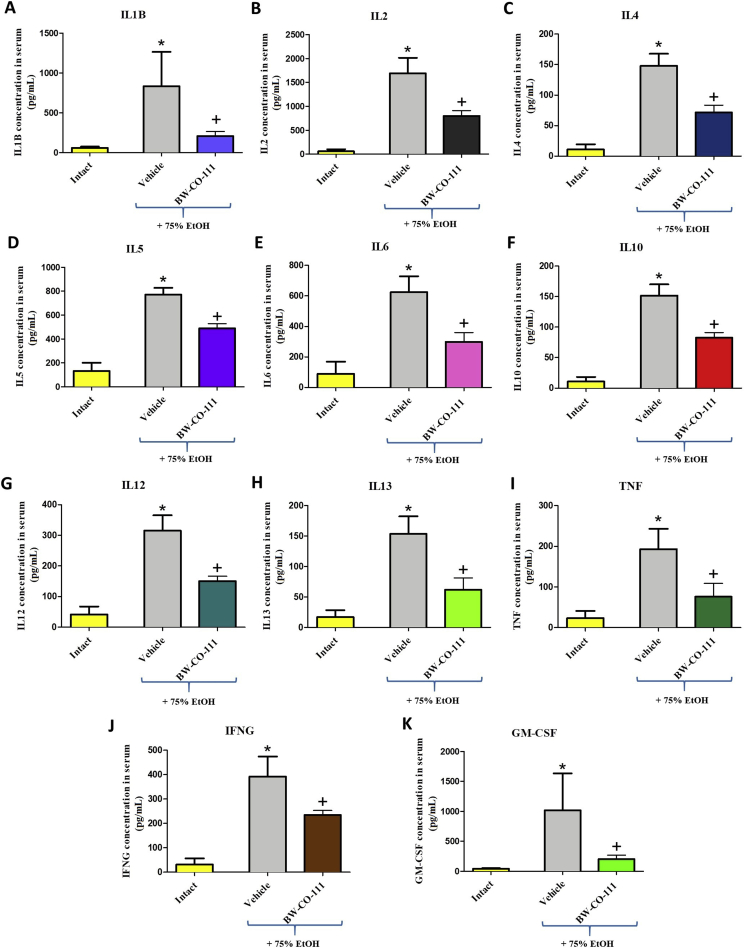
3.6. The effect of pretreatment with BW-CO-111 on systemic inflammatory response in rats administered with EtOH
Fig. 7 shows that administration of 75% ethanol significantly increased serum concentration of IL1B (A), IL2 (B), IL4 (C), IL5 (D), IL6 (E), IL10 (F), IL12 (G), IL13 (H), TNF (I), IFNG (J), and GM-CSF (K) as compared with intact rats (P < 0.05). Fig. 7 shows that pretreatment with BW-CO-111 before administration of 75% ethanol significantly decreased serum concentration of IL1B (A), IL2 (B), IL4 (C), IL5 (D), IL6 (E), IL10 (F), IL12 (G), IL13 (H), TNF (I), IFNG (J), and GM-CSF (K) as compared with vehicle-control group (P < 0.05).
Figure 7.
Changes in serum concentration of interleukin IL1B (A), IL2 (B), IL4 (C), IL5 (D), IL6 (E), IL10 (F), IL12 (G), IL13 (H), tumor necrosis factor TNF (I), interferon IFNG (J), and granulocyte-macrophage colony-stimulating factor GM-CSF (K) in rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg) and administered with 75% ethanol (EtOH). Intact refers to serum concentration of cytokines in rats without any treatments. Results are mean ± SEM of 5 samples per each experimental group. ∗P < 0.05 compared with respective values obtained in intact rats; +P < 0.05 compared with respective values obtained in vehicle-control group.
3.7. Alterations in Hmox1/HMOX1 and Hmox2/HMOX2 mRNA/protein expression in gastric mucosa compromised by aspirin after pretreatment with BW-CO-111
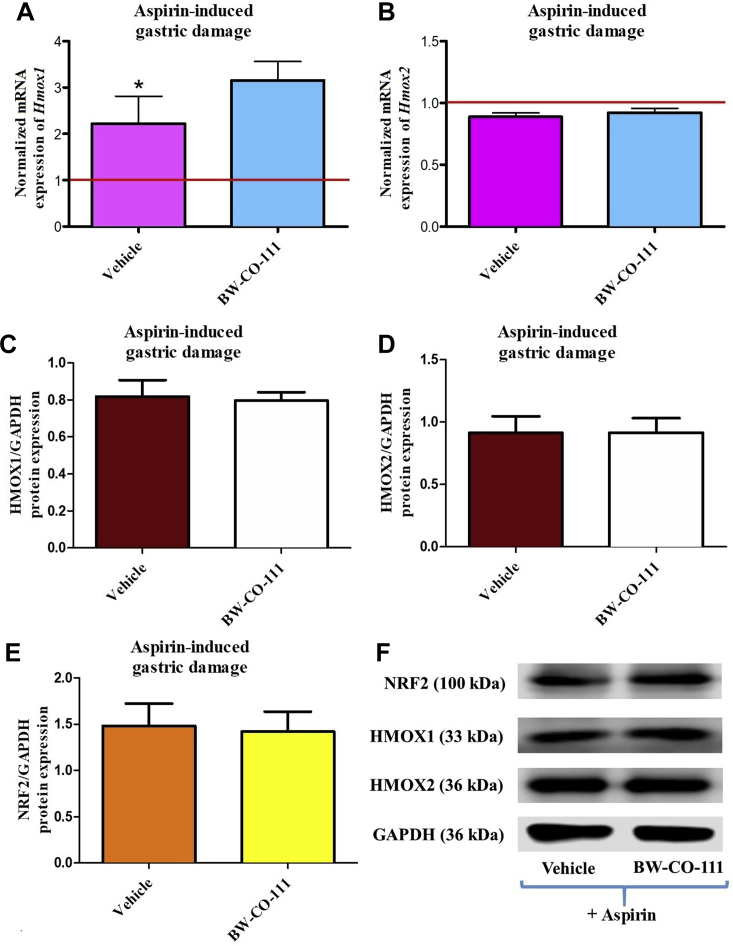
Fig. 8A shows that mRNA expression for Hmox1 in gastric mucosa but not that for Hmox2, was significantly increased as compared with expressions of Hmox1 and Hmox2 mRNA recorded in healthy animals without any treatments (P < 0.05). Fig. 8A and B shows that gastric mucosal mRNA expression for Hmox1 and Hmox2 was not significantly affected in rats pretreated with BW-CO-111 as compared with vehicle in rats administered 30 min later with aspirin (125 mg/kg, i.g.). Gastric mucosal protein expression for HMOX1, HMOX2 and NRF2 were not significantly affected in rats pretreated i.g. with BW-CO-111 (0.1 mg/kg) as compared with vehicle (Fig. 8C–E).
Figure 8.
Hmox1 (A), Hmox2 (B) mRNA and HMOX1 (C, F), HMOX2 (D, F), NRF2 (E, F) proteins expression in gastric mucosa of rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg) and administered 30 min later with aspirin (125 mg/kg, i.g.). Results are mean ± SEM of 5 rats per experimental group. Red line indicates baseline value of mRNA expression in healthy gastric mucosa without any treatments. ∗P < 0.05 compared with healthy gastric mucosa.
3.8. Modulation of gastroprotective PGE2 content and Cox1/COX1 and Cox2/COX2 mRNA/protein expression in gastric mucosa compromised by aspirin after pretreatment with BW-CO-111
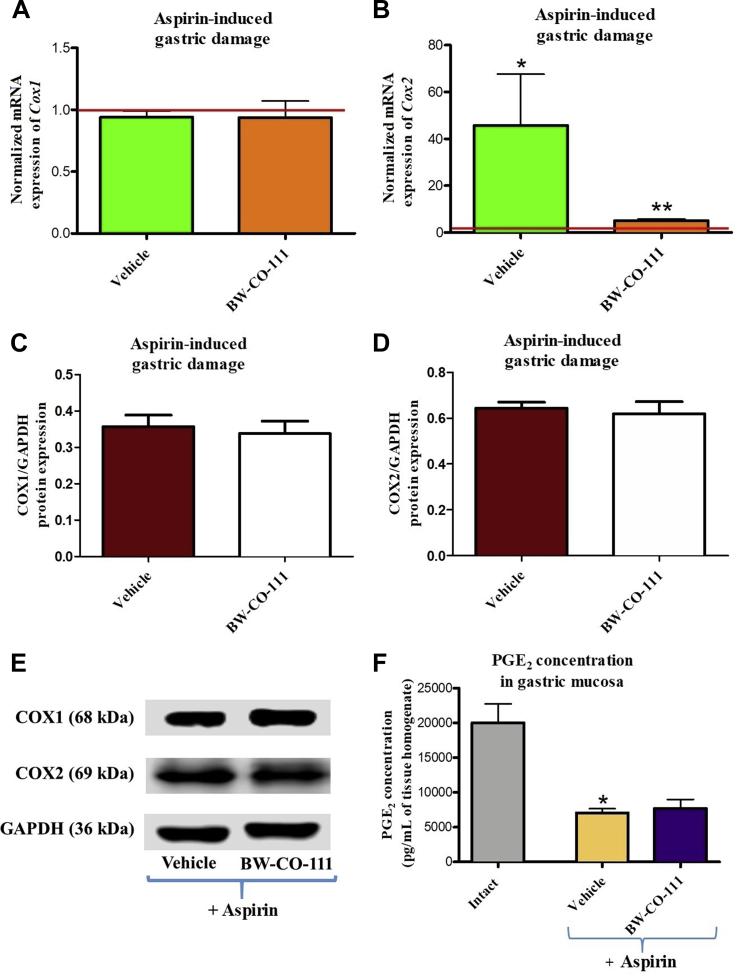
Fig. 9A and B shows that gastric mucosal Cox2 but not Cox1 mRNA expression was significantly increased by aspirin treatment as compared with animals without any treatments (P < 0.05). Fig. 9A and B shows that gastric mucosal mRNA expression for Cox2 but not mRNA expression of Cox1 was significantly downregulated in rats pretreated with BW-CO-111 and administered 30 min later with aspirin (P < 0.05). Gastric mucosal protein expression for COX1 and COX2 was not significantly affected in rats pretreated i.g. with BW-CO-111 (0.1 mg/kg) as compared with vehicle (Fig. 9C and D). PGE2 concentration in gastric mucosa was significantly decreased after administration of aspirin as compared with the respective values observed in intact gastric mucosa (P < 0.05, Fig. 9F). BW-CO-111 (0.1 mg/kg, i.g.) did not significantly affect PGE2 concentration in gastric mucosa administered with aspirin as compared with vehicle pretreatment (Fig. 9F).
Figure 9.
Effect of pretreatment with vehicle or BW-CO-111 (0.1 mg/kg) administered 30 min prior aspirin (125 mg/kg, i.g.) on alterations in Cox1 (A), Cox2 (B) mRNA, COX1 (C, E), COX2 (D, E) proteins expression and the mucosal concentrations of prostaglandin E2 (PGE2, F). Results are mean ± SEM of 5 rats per experimental group. Red line indicates baseline value of mRNA expression in healthy gastric mucosa without any treatments (Intact). ∗P < 0.05 compared with healthy gastric mucosa; ∗∗P < 0.05 compared with respective values obtained in vehicle-pretreated group.
3.9. Involvement of anti-inflammatory Anxa1 and TGFB pathway in the protective effect of pretreatment with BW-CO-111 in gastric mucosa compromised by aspirin
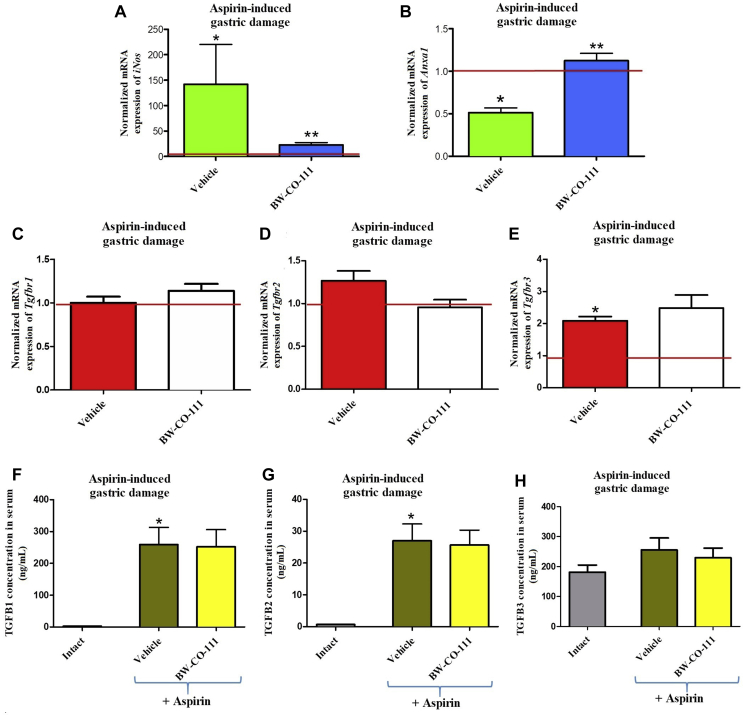
Fig. 10A shows that gastric mucosal mRNA expression for iNos was significantly upregulated in vehicle-pretreated rats administered 30 min later with aspirin (125 mg/kg, i.g., P < 0.05). BW-CO-111 (0.1 mg/kg, i.g.) significantly decreased iNos mRNA expression fold changes as compared with vehicle-control group (P < 0.05, Fig. 10A). In rats administered with aspirin and pretreated i.g. with BW-CO-111 (0.1 mg/kg), the gastric mucosal mRNA expression for Anxa1 was significantly upregulated as compared with vehicle (P < 0.05). Fig. 10C–E shows that mRNA expression for Tgfbr3 but not Tgfbr1 or Tgfbr2 mRNA expression was significantly upregulated in gastric mucosa exposed to aspirin (P < 0.05). Pretreatment with BW-CO-111 before aspirin administration, did not affect mRNA expression for Tgfbr1, Tgfbr2 or Tgfbr3 in gastric mucosa as compared with vehicle (P < 0.05, Fig. 10C–E). As presented in Fig. 10F–H, TGFB1 and TGFB2 but not TGFB3 serum concentrations is significantly increased in rats pretreated with vehicle and administered with aspirin as compared with intact animals (P < 0.05). BW-CO-111 (0.1 mg/kg, i.g.) did not affect TGFB1, TGFB2 and TGFB3 serum concentrations in rats administered with aspirin as compared with vehicle-pretreated group (P < 0.05, Fig. 10F–H).
Figure 10.
Expression of iNos (A), Anxa1 (B) and Tgfb receptor 1 (Tgfbr1) (C), Tgfbr2 (D), Tgfbr3 (E) mRNA in gastric mucosa and the changes in TGFB1 (F), TGFB2 (G), TGFB3 (H) serum concentrations of rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg, i.g.) 30 min before administration of aspirin (125 mg/kg, i.g.). Intact refers to healthy rats which were not treated with aspirin. Results are mean ± SEM of 5 samples per each experimental group with statistical significance marked only if 2-fold up- or downregulation was reached. Results are expressed as fold change of normalized gastric mucosal iNos, Anxa1 and Tgfbr1, Tgfbr2, Tgfbr3 mRNA expression. Red line indicates baseline value of mRNA expression in healthy gastric mucosa without any treatments (Intact). ∗P < 0.05 compared with intact rats; ∗∗P < 0.05 compared with respective values obtained in vehicle-pretreated group.
3.10. The effect of pretreatment with BW-CO-111 on systemic inflammatory response in rats administered with aspirin
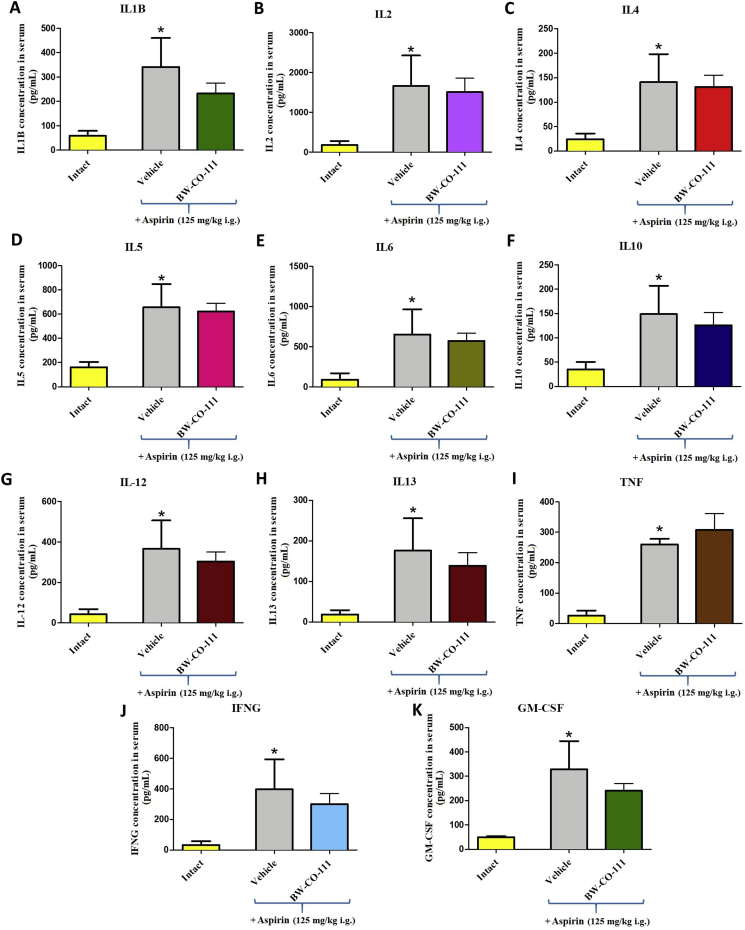
The administration of aspirin (125 mg/kg, i.g.) significantly increased serum concentration of IL1B (A), IL2 (B), IL4 (C), IL5 (D), IL6 (E), IL10 (F), IL12 (G), IL13 (H), TNF (I), IFNG (J), GM-CSF (K) as compared with the values of these cytokines measured in intact rats (P < 0.05) (Fig. 11). Pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) before administration of aspirin did not significantly affect serum concentration of IL1B (A), IL2 (B), IL4 (C), IL5 (D), IL6 (E), IL10 (F), IL12 (G), IL13 (H), TNF (I), IFNG (J), GM-CSF (K) as compared with vehicle-control group (Fig. 11).
Figure 11.
Alterations in serum concentration of interleukin IL1B (A), IL2 (B), IL4 (C), IL5 (D), IL6 (E), IL10 (F), IL12 (G), IL13 (H), tumor necrosis factor TNF (I), interferon IFNG (J), and granulocyte-macrophage colony-stimulating factor GM-CSF (K) in rats pretreated i.g. with vehicle or BW-CO-111 (0.1 mg/kg) and administered with aspirin (125 mg/kg, i.g.). Intact refers to serum of healthy rats without any treatments. Results are mean ± SEM of 5 samples per each experimental group. ∗P < 0.05 compared with respective values obtained in intact rats.
4. Discussion
Gastric mucosa is permanently exposed to exogenous noxious factors derived orally, very often with food, such as chemical necrotizing irritants including ethanol or drugs82, 83, 84, 85. Clinical use of NSAIDs such as aspirin is very often associated with serious side effects including induction of the hemorrhagic gastric erosions and microbleedings86,87. In spite of that, NSAIDs are still widely used because of its effectiveness in modulation of inflammation, fever and pain intensity86,87. Proton pomp inhibitors (PPI) are commonly used in clinics to prevent NSAIDs-induced GI complications. However, it has been observed that the prolonged treatment with PPI resulting in achlorhydria may lead to small intestinal bacterial overgrowth88.
On the other hand, endogenous gaseous mediators, such as hydrogen sulfide (H2S), CO or nitric oxide (NO) have been shown to be involved in the maintenance of physiological GI tract integrity and mucosal defense89. Moreover, H2S-releasing pharmacological tools can prevent ethanol- or drugs-induced gastric damage and were effective in acceleration of gastric ulcer healing36,79,90, 91, 92. Interestingly, novel gaseous mediators-releasing hybrids of NSAIDs, such as H2S-releasing derivative of naproxen, ATB-346 passed successfully the phase 2 of clinical trial93. Similarly to H2S, ruthenium containing CO-releasing CORM-2 is capable to prevent gastric mucosa against oxidative- or necrotic-gastric damage78,80. However, the utility of metal-complexes with attached CO-ligands in CO signaling research is debatable94.
In this study, we have demonstrated for the first time that novel organic and metal-free CO prodrug BW-CO-111 applied i.g. at a dose of 0.1 mg/kg protected the gastric mucosa against necrotic, ethanol- and aspirin-induced mucosal damage reducing by more than 50% the area of gastric lesions at the micro- and macroscopic levels as observed using well-known animal models35,78. This acute aspirin-induced GI injury has been previously reported to reflect topical and systemic actions of acidified form of this NSAID, including the reduced production of cytoprotective PGE2 and the accompanying fall in gastric microcirculation95,96. It has been reported previously that CO can exert vasodilatory action possibly mediated by soluble guanylyl cyclase activity35,97,98. In our study, the pretreatment with BW-CO-111 counteracted the decrease in GBF in gastric mucosa compromised by ethanol or aspirin. In contrast, the pretreatment with BW-CP-111, a release product control of BW-CO-111, without ability to release CO, was not effective in gastric mucosal protection and GBF modulation. Moreover, prior administration of BW-CP-111 did not decrease pro-inflammatory markers mRNA expression upregulated in chemically damaged gastric mucosa exposed to 75% ethanol. These observations confirm that beneficial effects of BW-CO-111 could be due to its ability to release CO.
The chemical characterization and CO release kinetics for BW-CO-111 has been described in detail previously62. CO release is stoichiometric with a t1/2 of approximately 17 min at pH 1.2 and 24 min at pH 7.4 as we have demonstrated in this study. BW-CO-111 applied i.g. at a dose of 0.1 mg/kg increased CO content in gastric mucosa as we observed by direct measurement of CO concentration in gastric tissue. This amount of CO was adequate to prevent gastric mucosa against ethanol- or aspirin-induced damage. Interestingly, ruthenium containing CORM-2 was effective at a dose of 5 mg/kg i.g 35,78. Thus, because of the difference in t1/2 for CO release, the novel CO-donating compound BW-CO-111 appears to act as a promising and safe pharmacological alternative for further studies related to gastric disorders. This notion is supported by our present finding that intragastric treatment with BW-CO-111 exerted gastroprotection at a dose of 0.1 and 0.5 mg/kg and BW-CP-111 did not increase cytotoxic effect of aspirin or ethanol within gastric epithelium. Additionally, BW-CP-111 did not further elevate gastric mucosal mRNA expression for pro-inflammatory iNos or Il1b upregulated by ethanol. These observations not only provide the evidence that the beneficial effects of BW-CO-111 is due to its ability to release CO but also indicate that this compound is not cytotoxic, at least in this experimental model. Thus, this aspect seems to require further toxicological studies to be fully confirmed. Interestingly, BW-CO-111 applied at a dose of 5 mg/kg was not observed to prevent gastric mucosa against ethanol-induced damage. This is in pair with previously published data showing that CO-releasing CORM-2 was also not effective or even cytotoxic when applied at higher doses78,99, 100, 101. Such results are consistent with the biphasic nature of the dose–response curves for compounds with pleotropic effects, especially gasotransmitters such as hydrogen sulfide. In practice, such results also set a window for effective doses for future considerations.
We have observed in our present study that BW-CO-111 maintained upregulated gastric mucosal mRNA expression of endogenous CO producing Hmox1 in rats administered with aspirin or ethanol. Indeed, it has been previously observed that mRNA and protein expression for HMOX1 was increased and decreased, respectively in gastric mucosa administered with ethanol or aspirin35,78. Such results suggest that in contrast to CORM-2, BW-CO-111 can attenuate gastric mucosal necrotic lesions and drug-induced gastrotoxicity without additional synergistic stimulation of endogenous CO production. The observed earlier CORM-2-mediated upregulation of Hmox1 mRNA in gastric mucosa exposed to ethanol or aspirin could be due to the presence of Ruthenium in the structure of CORM-2, which in fact, did not interfere with the potential gastroprotective activity of this CO donor35,78. It needs to be noted that recent studies have found that ruthenium-based CO-RMs such as CORM-2 can participate in many reactions under physiological conditions68, 69, 70, 71, 72, 73, 74. Thus, it is reasonable to conclude that the interpretation of the protective gastroprotective effects of CORM-2 can be attributed to yet unrecognized factors and mechanisms besides the release of CO.
Interestingly, BW-CO-111 (0.1 mg/kg, i.g.) downregulated gastric mucosal mRNA expression for anti-inflammatory Anxa1, upregulated mucosal mRNA expression for Tgfbr2 and r3 and maintained elevated TGFB1 and TGFB2 serum concentration in rats administered with ethanol. It has been previously reported that CORM-2 can antagonize TGFB activity through internalization of TGFB receptor 1 (ALK5) inhibiting profibrotic effect of this inflammatory factor102. However, we observed that Anxa1 mRNA expression was decreased in rats administered with high dose of aspirin but pretreatment with BW-CO-111 increased the gastric mucosal mRNA expression for this protein and similarly to rats administered with ethanol, this CO-prodrug maintained elevated TGFB1 and TGFB2 serum contents and maintained upregulated mucosal mRNA expression for Tgfbr3 by aspirin. It is worth highlighting that ANXA1 is assumed to exert its anti-inflammatory activity due to inhibition of prostaglandins (PGs) biosynthesis103,104. However, PGE2 produced by the activity of COXs is a crucial component of gastric mucosal barrier105. Interestingly, it has been reported that ANXA1 is involved in gastroprotection of dexamethasone against indomethacin-induced gastric damage under experimental conditions similar to those with aspirin in our study, in which the PG generation is also suppressed106. This could explain the discrepancies in Anxa1 mRNA expression between gastric mucosa pretreated with BW-CO-111 and compromised by ethanol and aspirin. In chronic ulcer study, CO-releasing CORM-2 accelerated gastric ulcer healing increasing Anxa1 mRNA expression at ulcer margin after 3 days of treatment and maintaining TGFB2 serum concentration after 6 days of treatments107. Additionally, it has been reported that CO-releasing CORM-A1 increased TGFB production by pancreatic lymph node cells resulting in enhancement of beta cells regeneration in experimental mice model of diabetes108.
CO donors were reported to modulate inflammatory response in various experimental models including digestive system pathologies6,109, 110, 111. In our study, we have observed that pretreatment with BW-CO-111 (0.1 mg/kg, i.g.) attenuated the systemic inflammatory response induced by 75% ethanol application as reflected in the decreased serum concentrations of eleven inflammatory biomarkers including IL1B, IL2, IL4, IL5, IL6, IL10, IL12, IL13, TNF, IFNG, and GM-CSF. Moreover, BW-CO-111 downregulated gastric mucosal mRNA expression of pro-inflammatory iNos and Cox2 elevated in gastric mucosa exposed to aspirin. Similarly, it has been reported previously that CORM-2 (5 mg/kg, i.g.) prevented ischemia/reperfusion-induced gastric damage in rats by mechanism involving the decrease in the systemic levels of these cytokines80.
Finally, BW-CO-111 (0.1 mg/kg, i.g.) maintained upregulated gastric mucosal protein expression for COX2 in rats as a result of treatment with aspirin or ethanol. However, BW-CO-111 increased cytoprotective PGE2 mucosal content in gastric mucosa compromised by ethanol but not by aspirin. In the case of aspirin treatment, BW-CO-111 was able to alleviate the damaging effect without directly altering decreased PGE2 gastric mucosal production caused by this drug. Since the expression of Cox2 mRNA but not that of Cox1 was elevated in gastric mucosa of ethanol treated rats with or without BW-CO-111 pretreatment comparing with healthy gastric mucosa, one would assume that COX2 derived PG are predominantly responsible for this CO donor-induced protection and the increase in GBF. Thus, we assume that this CO donor enhanced the activity of PGE2 system being involved in the physiological defensive mechanism against necrotic effects of ethanol.
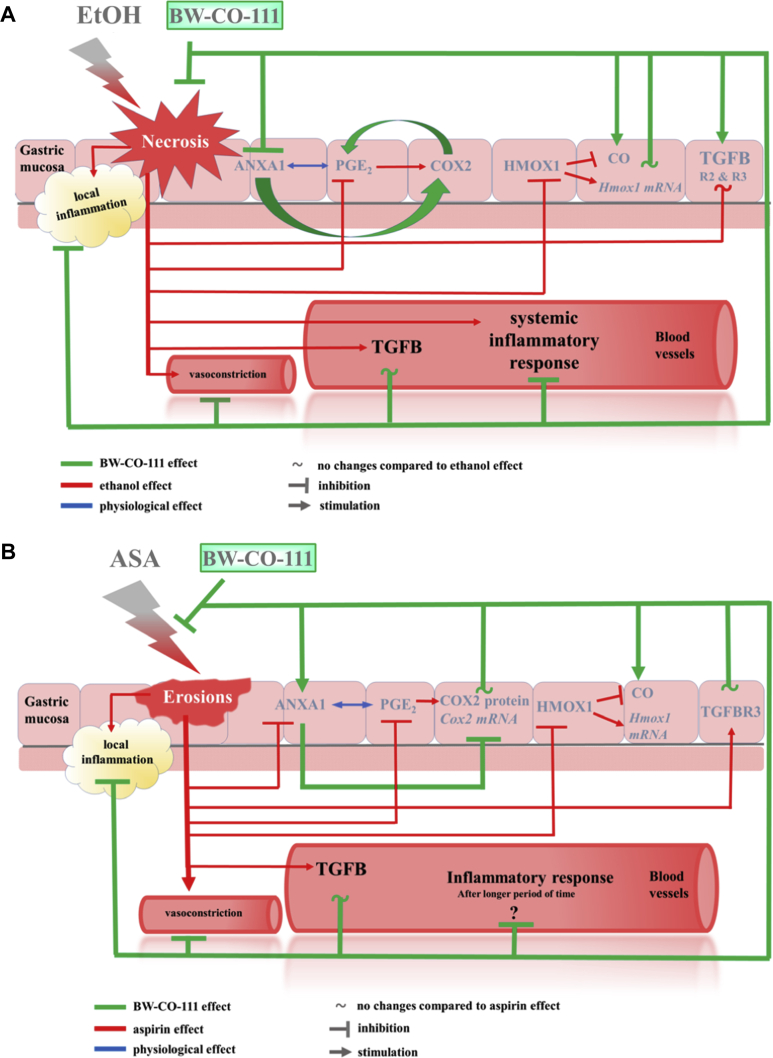
Taken together, we conclude that BW-CO-111 (0.1 mg/kg) applied i.g. protected gastric mucosa against necrotic ethanol-induced and aspirin-induced damage due to its ability to release CO possibly responsible for the increase in gastric microcirculation observed in our present study. However, because the pathophysiology of gastric damage differs between topical administration of ethanol and aspirin, the possible mechanisms of BW-CO-111-mediated gastroprotection seems to be somewhat different in these two models. Its gastroprotective activity was also associated with the similar significant attenuation of systemic and gastric mucosal inflammatory response possibly mediated by the anti-inflammatory ANXA1, TGFB and its receptors activity. Based on our present evidence, the NRF2 triggering effect of HMOX pathway by BW-CO-111 is questionable. Moreover, BW-CO-111 could affect COX2/PGE2 resulting in gastroprotection against ethanol-induced gastric damage and this CO donor could counteract the alterations in the COX2/PGE2 pathway in gastric mucosa induced by aspirin (Fig. 12A and B, respectively).
Figure 12.
Possible mechanisms of BW-CO-111-mediated gastroprotection against ethanol- (A) and aspirin-induced (B) gastric damage.
Acknowledgments
This study was supported by statutory grant for Marcin Magierowski received from Jagiellonian University Medical College (N41/DBS/000106, Poland). Part of molecular and biochemical assessments in Marcin Magierowski's laboratory were supported by a grant from National Science Centre Poland (UMO-2019/33/B/NZ4/00616). Marcin Magierowski received financial support from the Foundation for Polish Science (START 62.2018, Poland). We would like to acknowledge Anna Chmura, MSc for her input related to the technical preparation of histological slides. Binghe Wang acknowledges the financial support from the Georgia Research Alliance Eminent Scholar Fund, and internal resources at Georgia State University (USA) including its Department of Chemistry and the Molecular Basis of Disease program.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.08.005.
Contributor Information
Binghe Wang, Email: wang@gsu.edu.
Marcin Magierowski, Email: m.magierowski@uj.edu.pl.
Author contributions
Conceptualization: Dominik Bakalarz and Marcin Magierowski. Supervision: Binghe Wang and Marcin Magierowski. Funding acquisition: Binghe Wang and Marcin Magierowski. Investigation: Dominik Bakalarz, Marcin Surmiak, Xiaoxiao Yang, Dagmara Wójcik, Edyta Korbut, Zbigniew Śliwowski, Grzegorz Ginter, Grzegorz Buszewicz, Tomasz Brzozowski, Jakub Cieszkowski, Urszula Głowacka, Katarzyna Magierowska, Zhixiang Pan, and Marcin Magierowski. Methodology: Dominik Bakalarz, Marcin Surmiak, Xiaoxiao Yang, Edyta Korbut, and Grzegorz Buszewicz. Visualization: Dominik Bakalarz, Xiaoxiao Yang, Katarzyna Magierowska, and Marcin Magierowski. Writing-original draft: Dominik Bakalarz and Marcin Magierowski. Writing-review and editing: Dominik Bakalarz, Edyta Korbut, Tomasz Brzozowski, Xiaoxiao Yang, Binghe Wang and Marcin Magierowski.
Conflicts of interest
The authors have nothing to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wu L., Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 2.Magierowska K., Brzozowski T., Magierowski M. Emerging role of carbon monoxide in regulation of cellular pathways and in the maintenance of gastric mucosal integrity. Pharmacol Res. 2018;129:56–64. doi: 10.1016/j.phrs.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi K., Aihara E., Kimura M., Dogishi K., Hara T., Hayashi S. Gas mediators involved in modulating duodenal HCO3− secretion. Curr Med Chem. 2012;19:43–54. doi: 10.2174/092986712803413962. [DOI] [PubMed] [Google Scholar]
- 4.Shefa U., Kim D., Kim M.S., Jeong N.Y., Jung J. Roles of gasotransmitters in synaptic plasticity and neuropsychiatric conditions. Neural Plast. 2018;2018:1824713. doi: 10.1155/2018/1824713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H., Choi Y.K. Regenerative effects of heme oxygenase metabolites on neuroinflammatory diseases. Int J Mol Sci. 2018;20:78. doi: 10.3390/ijms20010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryter S.W. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch Biochem Biophys. 2019;678:108186. doi: 10.1016/j.abb.2019.108186. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo-Pereira C., Dias-Pedroso D., Soares N.L., Vieira H.L.A. CO-mediated cytoprotection is dependent on cell metabolism modulation. Redox Biol. 2020;32:101470. doi: 10.1016/j.redox.2020.101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin X., Lv J., Ge D., Bai H., Yang Y., Wu J. Heme oxygenase-1 alleviates eosinophilic inflammation by inhibiting STAT3-SOCS3 signaling. Pediatr Pulmonol. 2020;55:1440–1447. doi: 10.1002/ppul.24759. [DOI] [PubMed] [Google Scholar]
- 9.Faizan M., Muhammad N., Niazi K.U.K., Hu Y., Wang Y., Wu Y. CO-releasing materials: an emphasis on therapeutic implications, as release and subsequent cytotoxicity are the part of therapy. Materials. 2019;12:1643. doi: 10.3390/ma12101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji X., Damera K., Zheng Y., Yu B., Otterbein L.E., Wang B. Toward carbon monoxide-based therapeutics: critical drug delivery and developability issues. J Pharmaceut Sci. 2016;105:406–416. doi: 10.1016/j.xphs.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motterlini R., Otterbein L.E. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 12.Adach W., Olas B. Carbon monoxide and its donors—heir implications for medicine. Future Med Chem. 2019;11:61–73. doi: 10.4155/fmc-2018-0215. [DOI] [PubMed] [Google Scholar]
- 13.Stojak M., Kaczara P., Motterlini R., Chlopicki S. Modulation of cellular bioenergetics by CO-releasing molecules and NO-donors inhibits the interaction of cancer cells with human lung microvascular endothelial cells. Pharmacol Res. 2018;136:160–171. doi: 10.1016/j.phrs.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Uddin M.J., Pak E.S., Ha H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J Physiol Pharmacol. 2018;22:567–575. doi: 10.4196/kjpp.2018.22.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y., Rong J. Therapeutic potential of heme oxygenase-1/carbon monoxide system against ischemia−reperfusion injury. Curr Pharmaceut Des. 2017;23:3884–3898. doi: 10.2174/1381612823666170413122439. [DOI] [PubMed] [Google Scholar]
- 16.Abe T., Yazawa K., Fujino M., Imamura R., Hatayama N., Kakuta Y. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest. 2017;97:468–477. doi: 10.1038/labinvest.2016.157. [DOI] [PubMed] [Google Scholar]
- 17.Correa-Costa M., Gallo D., Csizmadia E., Gomperts E., Lieberum J.L., Hauser C.J. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A. 2018;115:E2302–E2310. doi: 10.1073/pnas.1716747115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vera T., Henegar J.R., Drummond H.A., Rimoldi J.M., Stec D.E. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 19.Suliman H.B., Carraway M.S., Ali A.S., Reynolds C.M., Welty-Wolf K.E., Piantadosi C.A. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H.H., Choi S. Therapeutic aspects of carbon monoxide in cardiovascular disease. Int J Mol Sci. 2018;19:2381. doi: 10.3390/ijms19082381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y.Q., Ji X.Y., Yu B.C., Ji K.L., Gallo D., Csizmadia E. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat Chem. 2018;10:787–794. doi: 10.1038/s41557-018-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J., Guo E., Yang J., Yang Y., Liu S., Hu J. Carbon monoxide ameliorates hepatic ischemia/reperfusion injury via sirtuin 1-mediated deacetylation of high-mobility group box 1 in rats. Liver Transplant. 2017;23:510–526. doi: 10.1002/lt.24733. [DOI] [PubMed] [Google Scholar]
- 24.Li S., Fujino M., Takahara T., Li X.K. Protective role of heme oxygenase-1 in fatty liver ischemia−reperfusion injury. Med Mol Morphol. 2019;52:61–72. doi: 10.1007/s00795-018-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Che X., Fang Y., Si X., Wang J., Hu X., Reis C. The role of gaseous molecules in traumatic brain injury: an updated review. Front Neurosci. 2018;12:392. doi: 10.3389/fnins.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi Y.K., Maki T., Mandeville E.T., Koh S.H., Hayakawa K., Arai K. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med. 2016;22:1335–1341. doi: 10.1038/nm.4188. [DOI] [PubMed] [Google Scholar]
- 27.Parfenova H., Pourcyrous M., Fedinec A.L., Liu J., Basuroy S., Leffler C.W. Astrocyte-produced carbon monoxide and the carbon monoxide donor CORM-A1 protect against cerebrovascular dysfunction caused by prolonged neonatal asphyxia. Am J Physiol Heart Circ Physiol. 2018;315:H978–H988. doi: 10.1152/ajpheart.00140.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P., Yao L., Zhou L.L., Liu Y.S., Chen M.D., Wu H.D. Carbon monoxide improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. Int J Biol Sci. 2016;12:1000–1009. doi: 10.7150/ijbs.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegazi R.A.F., Rao K.N., Mayle A., Sepulveda A.R., Otterbein L.E., Plevy S.E. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh S.Z., Hegazi R.A., Kobayashi T., Onyiah J.C., Russo S.M., Matsuoka K.M. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186:5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiger C., Uchiyama K., Takagi T., Mizushima K., Higashimura Y., Gutmann M. Prevention of colitis by controlled oral drug delivery of carbon monoxide. J Control Release. 2016;239:128–136. doi: 10.1016/j.jconrel.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Takagi T., Naito Y., Mizushima K., Akagiri S., Suzuki, Hirata I. Inhalation of carbon monoxide ameliorates TNBS-induced colitis in mice through the inhibition of TNF-α expression. Dig Dis Sci. 2010;55:2797–2804. doi: 10.1007/s10620-009-1112-x. [DOI] [PubMed] [Google Scholar]
- 33.Takagi T., Naito Y., Uchiyama K., Suzuki T., Hirata I., Mizushima K. Carbon monoxide liberated from carbon monoxide-releasing molecule exerts an anti-inflammatory effect on dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2011;56:1663–1671. doi: 10.1007/s10620-010-1484-y. [DOI] [PubMed] [Google Scholar]
- 34.Uddin M.J., Jeong S.O., Zheng M., Chen Y., Cho G.J., Chung H.T. Carbon monoxide attenuates dextran sulfate sodium-induced colitis via inhibition of GSK-3beta signaling. Oxid Med Cell Longev. 2013;2013:210563. doi: 10.1155/2013/210563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magierowski M., Magierowska K., Hubalewska-Mazgaj M., Adamski J., Bakalarz D., Sliwowski Z. Interaction between endogenous carbon monoxide and hydrogen sulfide in the mechanism of gastroprotection against acute aspirin-induced gastric damage. Pharmacol Res. 2016;114:235–250. doi: 10.1016/j.phrs.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Magierowski M., Magierowska K., Szmyd J., Surmiak M., Sliwowski Z., Kwiecien S. Hydrogen sulfide and carbon monoxide protect gastric mucosa compromised by mild stress against alendronate injury. Dig Dis Sci. 2016;61:3176–3189. doi: 10.1007/s10620-016-4280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasnos K., Magierowski M., Kwiecien S., Brzozowski T. Carbon monoxide in human physiology—its role in the gastrointestinal tract. Postepy Hig Med Dosw. 2014;68:101–109. doi: 10.5604/17322693.1087527. [DOI] [PubMed] [Google Scholar]
- 38.Magierowski M., Magierowska K., Hubalewska-Mazgaj M., Sliwowski Z., Ginter G., Pajdo R. Carbon monoxide released from its pharmacological donor, tricarbonyldichlororuthenium (II) dimer, accelerates the healing of pre-existing gastric ulcers. Br J Pharmacol. 2017;174:3654–3668. doi: 10.1111/bph.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKendrick J.G., Snodgrass W. On the physiological action of carbon monoxide of nickel. Br Med J. 1891;1:1215. doi: 10.1136/bmj.1.1588.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motterlini R., Foresti R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am J Physiol Cell Physiol. 2017;312:C302–C313. doi: 10.1152/ajpcell.00360.2016. [DOI] [PubMed] [Google Scholar]
- 41.Vummaleti S.V., Branduardi D., Masetti M., de Vivo M., Motterlini R., Cavalli A. Theoretical insights into the mechanism of carbon monoxide (CO) release from CO-releasing molecules. Chemistry. 2012;18:9267–9275. doi: 10.1002/chem.201103617. [DOI] [PubMed] [Google Scholar]
- 42.Motterlini R., Clark J.E., Foresti R., Sarathchandra P., Mann B.E., Green C.J. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 43.Jeremias H.F., Lousa D., Hollmann A., Coelho A.C., Baltazar C.S., Seixas J.D. Study of the interactions of bovine serum albumin with a molybdenum(II) carbonyl complex by spectroscopic and molecular simulation methods. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romanski S., Stamellou E., Jaraba J.T., Storz D., Kramer B.K., Hafner M. Enzyme-triggered CO-releasing molecules (ET-CORMs): evaluation of biological activity in relation to their structure. Free Radic Biol Med. 2013;65:78–88. doi: 10.1016/j.freeradbiomed.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Stamellou E., Storz D., Botov S., Ntasis E., Wedel J., Sollazzo S. Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation. Redox Biol. 2014;2:739–748. doi: 10.1016/j.redox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin H., Fang J., Liao L., Nakamura H., Maeda H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J Control Release. 2014;187:14–21. doi: 10.1016/j.jconrel.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Hasegawa U., van der Vlies A.J., Simeoni E., Wandrey C., Hubbell J.A. Carbon monoxide-releasing micelles for immunotherapy. J Am Chem Soc. 2010;132:18273–18280. doi: 10.1021/ja1075025. [DOI] [PubMed] [Google Scholar]
- 48.Pierri A.E., Huang P.J., Garcia J.V., Stanfill J.G., Chui M., Wu G. A photoCORM nanocarrier for CO release using NIR light. Chem Commun. 2015;51:2072–2075. doi: 10.1039/c4cc06766e. [DOI] [PubMed] [Google Scholar]
- 49.Dordelmann G., Pfeiffer H., Birkner A., Schatzschneider U. Silicium dioxide nanoparticles as carriers for photoactivatable CO-releasing molecules (PhotoCORMs) Inorg Chem. 2011;50:4362–4367. doi: 10.1021/ic1024197. [DOI] [PubMed] [Google Scholar]
- 50.Matson J.B., Webber M.J., Tamboli V.K., Weber B., Stupp S.I. A peptide-based material for therapeutic carbon monoxide delivery. Soft Matter. 2012;8:2689–2692. doi: 10.1039/C2SM25785H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antony L.A., Slanina T., Sebej P., Solomek T., Klan P. Fluorescein analogue xanthene-9-carboxylic acid: a transition-metal-free CO releasing molecule activated by green light. Org Lett. 2013;15:4552–4555. doi: 10.1021/ol4021089. [DOI] [PubMed] [Google Scholar]
- 52.Palao E., Slanina T., Muchova L., Solomek T., Vitek L., Klan P. Transition-metal-free CO-releasing BODIPY derivatives activatable by visible to NIR light as promising bioactive molecules. J Am Chem Soc. 2016;138:126–133. doi: 10.1021/jacs.5b10800. [DOI] [PubMed] [Google Scholar]
- 53.Peng P., Wang C., Shi Z., Johns V.K., Ma L., Oyer J. Visible-light activatable organic CO-releasing molecules (PhotoCORMs) that simultaneously generate fluorophores. Org Biomol Chem. 2013;11:6671–6674. doi: 10.1039/c3ob41385c. [DOI] [PubMed] [Google Scholar]
- 54.Anderson S.N., Richards J.M., Esquer H.J., Benninghoff A.D., Arif A.M., Berreau L.M. A structurally-tunable 3-hydroxyflavone motif for visible light-induced carbon monoxide-releasing molecules (CORMs) ChemistryOpen. 2015;4:590–594. doi: 10.1002/open.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popova M., Soboleva T., Ayad S., Benninghoff A.D., Berreau L.M. Visible-light-activated quinolone carbon-monoxide-releasing molecule: prodrug and albumin-assisted delivery enables anticancer and potent anti-inflammatory effects. J Am Chem Soc. 2018;140:9721–9729. doi: 10.1021/jacs.8b06011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schatzschneider U. Novel lead structures and activation mechanisms for CO-releasing molecules (CORMs) Br J Pharmacol. 2015;172:1638–1650. doi: 10.1111/bph.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belcher J.D., Gomperts E., Nguyen J., Chen C., Abdulla F., Kiser Z.M. Oral carbon monoxide therapy in murine sickle cell disease: beneficial effects on vaso-occlusion, inflammation and anemia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji X., Wang B. Strategies toward organic carbon monoxide prodrugs. Acc Chem Res. 2018;51:1377–1385. doi: 10.1021/acs.accounts.8b00019. [DOI] [PubMed] [Google Scholar]
- 59.Ji X., Wang B. Toward carbon monoxide based therapeutics: carbon monoxide in a pill. Pharm Pat Anal. 2017;6:171–177. doi: 10.4155/ppa-2017-0013. [DOI] [PubMed] [Google Scholar]
- 60.Ji X., Aghoghovbia R.E., De La Cruz L.K.C., Pan Z., Yang X., Yu B. Click and release: a high-content bioorthogonal prodrug with multiple outputs. Org Lett. 2019;21:3649–3652. doi: 10.1021/acs.orglett.9b01086. [DOI] [PubMed] [Google Scholar]
- 61.Ji X., Ji K., Chittavong V., Aghoghovbia R.E., Zhu M., Wang B. Click and fluoresce: a bioorthogonally activated smart probe for wash free fluorescent labeling of biomolecules. J Org Chem. 2017;82:1471–1476. doi: 10.1021/acs.joc.6b02654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Z., Ji X., Chittavong V., Li W., Ji K., Zhu M. Organic CO-prodrugs: structure CO-release rate relationship studies. Chem Eur J. 2017;23:9838–9845. doi: 10.1002/chem.201700936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang D., Viennois E., Ji K., Damera K., Draganov A., Zheng Y. A click-and-release approach to CO prodrugs. Chem Commun. 2014;50:15890–15893. doi: 10.1039/c4cc07748b. [DOI] [PubMed] [Google Scholar]
- 64.Ji X., Pan Z., Li C., Kang T., De La Cruz L.K.C., Yang L. Esterase-sensitive and pH-controlled carbon monoxide prodrugs for treating systemic inflammation. J Med Chem. 2019;62:3163–3168. doi: 10.1021/acs.jmedchem.9b00073. [DOI] [PubMed] [Google Scholar]
- 65.Ji X., Ji K., Chittavong V., Yu B., Pan Z., Wang B. An esterase-activated click and release approach to metal-free CO-prodrugs. Chem Commun. 2017;53:8296–8299. doi: 10.1039/c7cc03832a. [DOI] [PubMed] [Google Scholar]
- 66.Pan Z., Zhang J., Chittavong V., Ji X., Wang B. Organic CO prodrugs activated by endogenous ROS. Org Lett. 2018;20:8–11. doi: 10.1021/acs.orglett.7b02775. [DOI] [PubMed] [Google Scholar]
- 67.De La Cruz K., Benoit S.L., Pan Z., Maier R., Ji X., Wang B. Click, release, and fluoresce: a chemical strategy for a cascade prodrug system for codelivery of carbon monoxide, a drug payload, and a fluorescent reporter. Org Lett. 2018;20:897–900. doi: 10.1021/acs.orglett.7b03348. [DOI] [PubMed] [Google Scholar]
- 68.Southam H.M., Smith T.W., Lyon R.L., Liao C., Trevitt C.R., Middlemiss L.A. A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3. Redox Biol. 2018;18:114–123. doi: 10.1016/j.redox.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seixas J.D., Chaves-Ferreira M., Montes-Grajales D., Goncalves A.M., Marques A.R., Saraiva L.M. An N-acetyl cysteine ruthenium tricarbonyl conjugate enables simultaneous release of CO and ablation of reactive oxygen species. Chemistry. 2015;21:14708–14712. doi: 10.1002/chem.201502474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos-Silva T., Mukhopadhyay A., Seixas J.D., Bernardes G.J., Romao C.C., Romao M.J. CORM-3 reactivity toward proteins: the crystal structure of a Ru(II) dicarbonyl-lysozyme complex. J Am Chem Soc. 2011;133:1192–1195. doi: 10.1021/ja108820s. [DOI] [PubMed] [Google Scholar]
- 71.McLean S., Mann B.E., Poole R.K. Sulfite species enhance carbon monoxide release from CO-releasing molecules: implications for the deoxymyoglobin assay of activity. Anal Biochem. 2012;427:36–40. doi: 10.1016/j.ab.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 72.Nobre L.S., Jeremias H., Romao C.C., Saraiva L.M. Examining the antimicrobial activity and toxicity to animal cells of different types of CO-releasing molecules. Dalton Trans. 2016;45:1455–1466. doi: 10.1039/c5dt02238j. [DOI] [PubMed] [Google Scholar]
- 73.Santos-Silva T., Mukhopadhyay A., Seixas J.D., Bernardes G.J., Romao C.C., Romao M.J. Towards improved therapeutic CORMs: understanding the reactivity of CORM-3 with proteins. Curr Med Chem. 2011;18:3361–3366. doi: 10.2174/092986711796504583. [DOI] [PubMed] [Google Scholar]
- 74.Yuan Z., Yang X., De La Cruz L.K., Wang B. Nitro reduction-based fluorescent probes for carbon monoxide require reactivity involving a ruthenium carbonyl moiety. Chem Commun. 2020;56:2190–2193. doi: 10.1039/c9cc08296d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji X., Zhou C., Ji K., Aghoghovbia R., Pan Z., Chittavong V. Click and release: a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels–Alder reaction. Angew Chem Int Ed Engl. 2016;55:15846–15851. doi: 10.1002/anie.201608732. [DOI] [PubMed] [Google Scholar]
- 76.Lee J.S., An J.M., Kang E.A., Han Y.M., Kim Y.S., Lee H.J. Host nuclear factor erythroid 2-related factor-2 defense system determines the outcome of dextran sulfate sodium-induced colitis in mice. J Physiol Pharmacol. 2018;69:755–767. doi: 10.26402/jpp.2018.5.10. [DOI] [PubMed] [Google Scholar]
- 77.Kim K.J., Park J.M., Lee J.S., Kim Y.S., Kangwan N., Han Y.M. Oligonol prevented the relapse of dextran sulfate sodium-ulcerative colitis through enhancing NRF2-mediated antioxidative defense mechanism. J Physiol Pharmacol. 2018;69:359–371. doi: 10.26402/jpp.2018.3.03. [DOI] [PubMed] [Google Scholar]
- 78.Magierowska K., Magierowski M., Hubalewska-Mazgaj M., Adamski J., Surmiak M., Sliwowski Z. Carbon monoxide (CO) released from tricarbonyldichlororuthenium (II) dimer (CORM-2) in gastroprotection against experimental ethanol-induced gastric damage. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magierowski M., Magierowska K., Hubalewska-Mazgaj M., Surmiak M., Sliwowski Z., Wierdak M. Cross-talk between hydrogen sulfide and carbon monoxide in the mechanism of experimental gastric ulcers healing, regulation of gastric blood flow and accompanying inflammation. Biochem Pharmacol. 2018;149:131–142. doi: 10.1016/j.bcp.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 80.Magierowska K., Korbut E., Hubalewska-Mazgaj M., Surmiak M., Chmura A., Bakalarz D. Oxidative gastric mucosal damage induced by ischemia/reperfusion and the mechanisms of its prevention by carbon monoxide-releasing tricarbonyldichlororuthenium (II) dimer. Free Radic Biol Med. 2019;145:198–208. doi: 10.1016/j.freeradbiomed.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 81.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 82.Wallace J.L. Recent advances in gastric ulcer therapeutics. Curr Opin Pharmacol. 2005;5:573–577. doi: 10.1016/j.coph.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Ham M., Kaunitz J.D. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2008;24:665–673. doi: 10.1097/MOG.0b013e328311cd93. [DOI] [PubMed] [Google Scholar]
- 84.Tarnawski A.S., Ahluwalia A. Increased susceptibility of aging gastric mucosa to injury and delayed healing: clinical implications. World J Gastroenterol. 2018;24:4721–4727. doi: 10.3748/wjg.v24.i42.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krzysiek-Maczka G., Wrobel T., Targosz A., Szczyrk U., Strzalka M., Ptak-Belowska A. Helicobacter pylori-activated gastric fibroblasts induce epithelial-mesenchymal transition of gastric epithelial cells in vitro in a TGF-beta-dependent manner. Helicobacter. 2019;24 doi: 10.1111/hel.12653. [DOI] [PubMed] [Google Scholar]
- 86.Kauffman G. Aspirin-induced gastric mucosal injury: lessons learned from animal models. Gastroenterol. 1989;96:606–614. doi: 10.1016/s0016-5085(89)80056-3. [DOI] [PubMed] [Google Scholar]
- 87.Bjarnason I., Scarpignato C., Takeuchi K., Rainsford K.D. Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment Pharmacol Ther. 2007;26:95–106. doi: 10.1111/j.1365-2036.2007.03348.x. [DOI] [PubMed] [Google Scholar]
- 88.Compare D., Pica L., Rocco A., De Giorgi F., Cuomo R., Sarnelli G. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur J Clin Invest. 2011;41:380–386. doi: 10.1111/j.1365-2362.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 89.Wallace J.L., Ianaro A., de Nucci G. Gaseous mediators in gastrointestinal mucosal defense and injury. Dig Dis Sci. 2017;62:2223–2230. doi: 10.1007/s10620-017-4681-0. [DOI] [PubMed] [Google Scholar]
- 90.Wallace J.L., Dicay M., McKnight W., Martin G.R. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 91.Nicolau L.A., Silva R.O., Damasceno S.R., Carvalho N.S., Costa N.R., Aragao K.S. The hydrogen sulfide donor, Lawesson's reagent, prevents alendronate-induced gastric damage in rats. Braz J Med Biol Res. 2013;46:708–714. doi: 10.1590/1414-431X20133030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magierowski M., Magierowska K., Surmiak M., Hubalewska-Mazgaj M., Kwiecien S., Wallace J.L. The effect of hydrogen sulfide-releasing naproxen (ATB-346) versus naproxen on formation of stress-induced gastric lesions, the regulation of systemic inflammation, hypoxia and alterations in gastric microcirculation. J Physiol Pharmacol. 2017;68:749–756. [PubMed] [Google Scholar]
- 93.Wallace J.L., Nagy P., Feener T.D., Allain T., Ditroi T., Vaughan D.J. A proof-of-concept, phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br J Pharmacol. 2020;177:769–777. doi: 10.1111/bph.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stucki D., Krahl H., Walter M., Steinhausen J., Hommel K., Brenneisen P. Effects of frequently applied carbon monoxide releasing molecules (CORMs) in typical CO-sensitive model systems—a comparative in vitro study. Arch Biochem Biophys. 2020;687:108383. doi: 10.1016/j.abb.2020.108383. [DOI] [PubMed] [Google Scholar]
- 95.Fiorucci S., Antonelli E., Distrutti E., Rizzo G., Mencarelli A., Orlandi S. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 96.Konturek S.J., Brzozowski T., Piastucki I., Radecki T. Prevention of ethanol and aspirin-induced gastric mucosal lesions by paracetamol and salicylate in rats: role of endogenous prostaglandins. Gut. 1982;23:536–540. doi: 10.1136/gut.23.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kocer G., Nasircilar Ulker S., Senturk U.K. The contribution of carbon monoxide to vascular tonus. Microcirculation. 2018;25 doi: 10.1111/micc.12495. [DOI] [PubMed] [Google Scholar]
- 98.de Backer O., Elinck E., Sips P., Buys E., Brouckaert P., Lefebvre R.A. Role of the soluble guanylyl cyclase alpha1/alpha2 subunits in the relaxant effect of CO and CORM-2 in murine gastric fundus. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008;378:493–502. doi: 10.1007/s00210-008-0315-6. [DOI] [PubMed] [Google Scholar]
- 99.Moon H., Jang J.H., Jang T.C., Park G.H. Carbon monoxide ameliorates 6-hydroxydopamine-induced cell death in C6 glioma cells. Biomol Ther (Seoul) 2018;26:175–181. doi: 10.4062/biomolther.2018.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Magierowska K., Magierowski M., Surmiak M., Adamski J., Mazur-Bialy A.I., Pajdo R. The protective role of carbon monoxide (CO) produced by heme oxygenases and derived from the CO-releasing molecule CORM-2 in the pathogenesis of stress-induced gastric lesions: evidence for non-involvement of nitric oxide (NO) Int J Mol Sci. 2016;17:442. doi: 10.3390/ijms17040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Magierowska K., Bakalarz D., Wójcik D., Korbut E., Danielak A., Głowacka U. Evidence for cytoprotective effect of carbon monoxide donor in the development of acute esophagitis leading to acute esophageal epithelium lesions. Cells. 2020;9:1203. doi: 10.3390/cells9051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hovater M.B., Ying W.Z., Agarwal A., Sanders P.W. Nitric oxide and carbon monoxide antagonize TGF-beta through ligand-independent internalization of TbetaR1/ALK5. Am J Physiol Ren Physiol. 2014;307:F727–F735. doi: 10.1152/ajprenal.00353.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perretti M., D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 104.Goppelt-Struebe M., Wolter D., Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989;98:1287–1295. doi: 10.1111/j.1476-5381.1989.tb12676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarnawski A., Ahluwalia A., Jones M.K. Gastric cytoprotection beyond prostaglandins: cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr Pharmaceut Des. 2013;19:126–132. doi: 10.2174/13816128130117. [DOI] [PubMed] [Google Scholar]
- 106.Zanardo R.C., Perretti M., Wallace J.L. Annexin-1 is an endogenous gastroprotective factor against indomethacin-induced damage. Am J Physiol Gastrointest Liver Physiol. 2005;288:G481–G486. doi: 10.1152/ajpgi.00299.2004. [DOI] [PubMed] [Google Scholar]
- 107.Magierowska K., Bakalarz D., Wojcik D., Chmura A., Hubalewska-Mazgaj M., Licholai S. Time-dependent course of gastric ulcer healing and molecular markers profile modulated by increased gastric mucosal content of carbon monoxide released from its pharmacological donor. Biochem Pharmacol. 2019;163:71–83. doi: 10.1016/j.bcp.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Nikolic I., Saksida T., Vujicic M., Stojanovic I., Stosic-Grujicic S. Anti-diabetic actions of carbon monoxide-releasing molecule (CORM)-A1: immunomodulation and regeneration of islet beta cells. Immunol Lett. 2015;165:39–46. doi: 10.1016/j.imlet.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 109.Lin C.C., Hsiao L.D., Cho R.L., Yang C.M. CO-releasing molecule-2 induces Nrf2/ARE-dependent heme oxygenase-1 expression suppressing TNF-alpha-induced pulmonary inflammation. J Clin Med. 2019;8:436. doi: 10.3390/jcm8040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takagi T., Naito Y., Tanaka M., Mizushima K., Ushiroda C., Toyokawa Y. Carbon monoxide ameliorates murine T-cell-dependent colitis through the inhibition of Th17 differentiation. Free Radic Res. 2018;52:1328–1335. doi: 10.1080/10715762.2018.1470327. [DOI] [PubMed] [Google Scholar]
- 111.Babu D., Motterlini R., Lefebvre R.A. CO and CO-releasing molecules (CO-RMs) in acute gastrointestinal inflammation. Br J Pharmacol. 2015;172:1557–1573. doi: 10.1111/bph.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.