Abstract
Background
The Centers for Medicare and Medicaid Services stipulate shared decision-making (SDM) counseling as a prerequisite to lung cancer screening (LCS) reimbursement, despite well-known challenges implementing SDM in practice.
Research Question
How have health-care organizations implemented SDM for LCS?
Study Design and Methods
For this qualitative study, we used data from in-depth, semistructured interviews with key informants directly involved in implementing SDM for LCS, managing SDM for LCS, or both. We identified respondents using a snowball sampling technique and used template analysis to identify and analyze responses thematically.
Results
We interviewed 30 informants representing 23 health-care organizations located in 12 states and 4 Census regions. Respondents described two types of SDM for LCS programs: centralized models (n = 7), in which front-end practitioners (eg, primary care providers) referred patients to an LCS clinic where trained staff (eg, advanced practice nurses) delivered SDM at the time of screening, or decentralized models (n = 10), in which front-end practitioners delivered SDM before referring patients for screening. Some organizations used both models simultaneously (n = 6). Respondents discussed tradeoffs between SDM quality and access. They perceived centralized models as enhancing SDM quality, but limiting patient access to care, and vice versa. Respondents reported ongoing challenges with limited resources and budgetary constraints, ambiguity regarding what constitutes SDM, and an absence of benchmarks for evaluating SDM for LCS quality.
Interpretation
Those responsible for developing and managing SDM for LCS programs voiced concerns regarding both patient access and SDM quality, regardless of organizational context, or the SDM for LCS model implemented. The challenge facing these organizations, and those wanting to help patients and clinicians balance the tradeoffs inherent with LCS, is how to move beyond a check-box documentation requirement to a process that enables LCS to be offered to all high-risk patients, but used only by those who are informed and for whom screening represents a value-concordant service.
Key Words: implementation, informed decision-making, lung cancer, lung cancer screening, shared decision-making
Abbreviations: CMS, Centers for Medicare and Medicaid Services; IQ, illustrative quotation; LCS, lung cancer screening; SDM, shared decision-making
FOR EDITORIAL COMMENT, SEE PAGE 23
Since 2015, the Centers for Medicare and Medicaid Services (CMS) has stipulated a counseling and shared decision-making (SDM) visit as a reimbursable prerequisite for lung cancer screening (LCS). The CMS-required components of counseling and SDM for LCS include assessment of screening eligibility, SDM with the use of at least one decision aid, and counseling on the importance of annual screening, the impact of comorbidities, and the willingness or ability to undergo diagnostic evaluation and treatment along with smoking cessation counseling.1 As such, LCS is the first (and so far only) cancer screening service with financial implications if SDM is not delivered before a patient is screened.1 Although CMS provides general guidance regarding expected activities, most of the decisions regarding how to implement SDM are left to providers and their organizations.
Although screening with low-dose CT scanning among high-risk patients can reduce lung cancer mortality,2,3 it also introduces risks of incidental findings, false-positive results, overdiagnosis, and harm resulting from cumulative radiation exposure.4, 5, 6 Because of these tradeoffs, LCS decisions are complex, requiring patients to consider the balance of expected benefits and harms in light of their own personal values and preferences and in the context of evolving empirical evidence.2,7, 8, 9, 10
Given that CMS has expanded its policies to require SDM within other clinical contexts11 and the consistent evidence that SDM has proven challenging to implement in practice,12, 13, 14, 15 it is informative to understand how SDM for LCS has been implemented. The objectives of this study were to describe how organizations have implemented SDM for LCS and to identify the factors considered as these programs were developed and implemented as well as the challenges faced.
Methods
Study Design
We conducted a qualitative study using in-depth, semistructured interviews with key informants involved in implementing SDM for LCS programs, or managing SDM programs for LCS, or both. We conducted interviews between November 2018 and April 2019.
Selection of Study Respondents
We used a snowball sampling approach16 to identify key informants who were involved directly with implementing the SDM for LCS program at their institution. When the individual directly involved with program implementation had left the organization, we interviewed the person currently responsible for SDM for LCS management. To initiate the sampling process, we sent a study introductory e-mail to 26 people. Twenty-one of these individuals were known by a study team member and were thought to have knowledge of their organizations’ LCS programs. An additional five people were identified from the LCS literature (ie, authored an article describing SDM counseling in the context of LCS). Among those contacted, seven people met the study inclusion criteria and agreed to participate in the study, eight either did not respond or indicated they were not involved with SDM for LCS within their institution, and 11 provided referrals to other people whom they considered to be appropriate for study participation and who were employed either by their organization or elsewhere. This resulted in 18 new people, of whom 14 agreed to participate in the study. From these 21 initial study participants, we received an additional 9 referrals, all of whom agreed to study participation, resulting in a total of 30 study participants representing 23 organizations. We recruited respondents until data saturation was achieved.17
Data Collection
We used domains and constructs within the Consolidated Framework for Implementation Research to develop an interview guide.18 We selected four domains (ie, intervention characteristics, inner setting, outer setting, and process), and 10 constructs (relative advantage, adaptability, cost, external policies and incentives, structural characteristics, available resources, access to knowledge and information, planning, opinion leaders, and reflecting and evaluating) that most aligned with our research aims. The guide also contained probing questions to allow respondents to expand on and clarify responses as well as an initial question asking the respondent to describe how SDM for LCS was carried out in their organization (e-Appendix 1, e-Table 1), Probes for the latter included questions regarding types of workflows used, program staffing, and program monitoring activities.
We conducted two pilot interviews to ensure clarity and to minimize interview length and repetitiveness. Each interview took between 25 and 50 minutes. Before initiating an interview, we obtained permission to audio-record and use the individual’s responses. The University of North Carolina at Chapel Hill Institutional Review Board approved this study (Identifier: 18-2382). All interviews were transcribed verbatim before analyses.
Data Analysis
We used a template analysis approach19 to identify and analyze interview data thematically. Two coders (A. A. T. and K. T.) read half of the transcripts independently to become familiar with the data. Using five purposefully selected transcripts that represented a diversity of organizations, the two coders developed a preliminary codebook and themes20 using a template (https://cfirguide.org/). They also developed additional codes as new themes emerged. Within each interview, they coded passages into the related theme(s) and selected illustrative quotations (IQs) explanatory of each theme. Data from interviews were considered holistically, not in response to specific questions. The coders discussed and came to a consensus regarding any discrepancies in coding. We organized, labeled, and reported themes, using Dedoose version 8.1.8 software (SocioCultural Research Consultants).
Results
Study Respondents
Respondents (N = 30) represented 23 organizations (including academic medical centers, community hospitals, and Veteran Administration hospitals) located in 12 states that collectively represented the four United States Census regions (Table 1). Respondents included one surgeon, seven nurse practitioners, 17 pulmonologists, two primary care physicians, and three nonclinician researchers.
Table 1.
Type of Shared Decision-making Model and Geographic Location of Organizations (n = 23) and Respondents (N = 30) Represented by the Sample
| SDM for LCS Model | Geographic Region |
Total | |||
|---|---|---|---|---|---|
| Northeast | Midwest | South | West | ||
| Organizations | |||||
| Centralized model | 1 | 3 | 1 | 1 | 7 |
| Decentralized model | 2 | 4 | 2 | 2 | 10 |
| Both models simultaneouslya | 2 | 2 | 2 | 1 | 6 |
| Total | 5 | 9 | 5 | 4 | 23 |
| Respondents | |||||
| Centralized model | 2 | 4 | 1 | 1 | 8 |
| Decentralized model | 2 | 4 | 4 | 2 | 12 |
| Both models simultaneouslya | 2 | 3 | 4 | 1 | 10 |
| Total | 6 | 11 | 9 | 4 | 30 |
LCS = lung cancer screening; SDM = shared decision-making.
Within these organizations, each type of model was provided independently.
Types of SDM for LCS Models
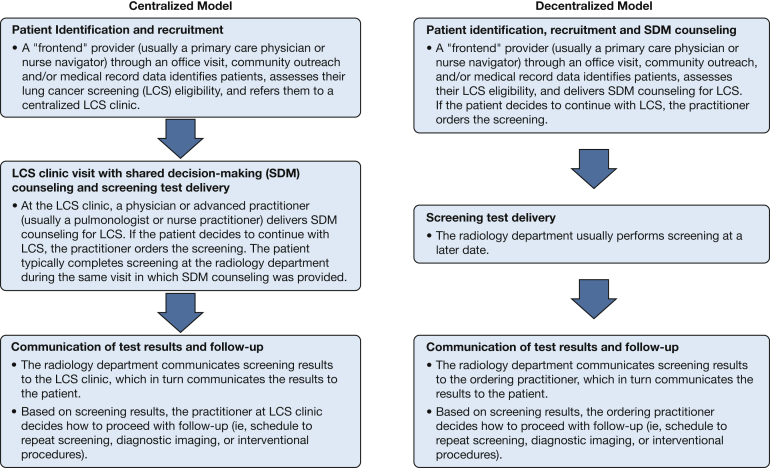
Figure 1 provides an overview of the two main types of programs that respondents described. In decentralized models, front-end practitioners (eg, primary care physicians) were responsible for identifying screening-eligible patients, providing SDM counseling, ordering the screening test, and providing follow-up after test results became available. Within decentralized models, the SDM counseling was conducted during the office visit at which screening eligibility was determined and before a referral for testing (IQ 1) (Table 2). In centralized models, although a front-end practitioner determined patient screening eligibility, these practitioners did not provide SDM counseling. Instead, the front-end practitioner referred patients to a centralized LCS clinic. Within the LCS clinic, a second practitioner (typically a nurse practitioner or pulmonologist) reviewed the patient’s screening eligibility, provided SDM, ordered the screening test, and provided follow-up. Within centralized models, the patient typically completed screening during the same visit in which SDM counseling was provided (IQ 2) (Table 2). Some organizations reported simultaneously using each type of model independently. In such organizations, front-end practitioners had the liberty either to refer patients to a centralized LCS clinic for SDM and follow-up (ie, centralized model) or to provide SDM counseling and follow-up themselves (ie, decentralized model; IQ 3) (Table 2).
Figure 1.
Diagram summarizing shared decision-making for lung cancer screening program models.
Table 2.
Illustrative Quotations Regarding Characteristics of SDM Models Implemented for LCS
| SDM Model Characteristic | Quotation No. | Illustrative Quotation (Study Respondent Letter) |
|---|---|---|
| SDM for LCS process | 1 | “Our decentralized program will usually start with primary care . . . after identifying eligible patients we’ve discussed the risks and benefits of screening, we include briefly what the risks are, you know, radiation exposure, overdiagnosis, false positive, unnecessary procedures, and the patient agrees that they are willing and able to undergo the CT and any recommended follow-up. Um, and then from that point the patient, when they place the order, the patient will be contacted to schedule the CT scan, um, and again the CT scan could happen at a variety of imaging centers around our area.” (19A) |
| 2 | “It is centralized. I conduct all of the consults. Um, the patient is referred from their primary care provider to our program and then once a patient’s referred, they are contacted by a medical assistant who will call the patient, confirm eligibility, or just confirm it according to what’s, in the referral, and then, get the patient in for the consult. So schedule the patient for the consult and then the patient is able to walk down the hall to radiology to have the imaging, after the consult.” (6A) | |
| 3 | “We have hybrid program either they can order it on their own or they can send it to our centralized program. When they decide to do it on their own it’s like, oh well, I can do the shared decision-making, um, just as much as, as you can.” (7B) | |
| SDM for LCS evolution | 4 | “I just think you know as we’ve evolved through this process over the last couple years. We’ve understood how complex that it is, in terms of really running a quality program, making sure that smoking cessation is the center of our program and that patients have the resources for that . . . . We are in the process of somewhat decentralizing, um, as we’ve recently acquired some satellite locations and it becomes more widespread and understood and so we are educating our primary doctors, on doing shared decision-making with the patients, then they will order, the primary care doctor will perform the shared decision-making and order the scan and all abnormal screens will come to our clinic.” (18A) |
| 5 | “What drove the change was, um, they were realizing that there were patients being referred into the program who were not necessarily, even eligible for lung cancer screening or had active symptoms of lung cancer. There was some misunderstanding among referring providers about screening vs diagnostic CTs, they felt like they wanted a little bit more control over the process and decided to shift that responsibility to the, lung cancer screening nurse coordinator.” (14A) | |
| Implementation leaders | 6 | “We have a group of providers from the chest radiology, PCP, and also pulmonary, so we are the core people. We work with, uh, multidiscipline oncology group but pretty much we just report it back to them what we decide just to make sure they know. Um, so the decision we made ‘this is the way we want to do’ is basically, based on what was available. So, we cannot do the centralized system because we don’t have the budget for nurse navigator.” (4A) |
| 7 | “We had a steering committee that consisted of, uh, thoracic surgery, radiation oncology, the chief of radiology, the chief for primary care. All of us got together before we ever started to implement, enroll out and so shared decision-making was one of the components of implementing lung screening.” (7B) | |
| Access-quality tradeoff | 8 | “There is a tension between sort of the centralization of the process that is everyone comes to a central place to get their shared decision-making conversation, to get their tobacco cessation counseling, and to get their screening. I’m absolutely certain that is the highest-quality way to provide a lung cancer screening program. The problem I think is the reach for that is limited and there’s an unquantifiable number of people that just will never get screened who may benefit from screening because they don’t have the wherewithal or the desire to travel an hour, two hours, three or even sometimes four hours to get to that centralized clinic.” (13A) |
| 9 | “We felt like, funneling the patients through a single care provider or office would limit the growth and access to lung cancer screenings so we wanted to leave it in the hands of the ordering provider.” (2A) | |
| 10 | “So we do a large, uh, community outreach portion and our community outreach is done by our patient navigators who bring in hard-to-reach patients and through that they help to facilitate that conversation so they help find out what the patients’ needs are in terms of lung cancer screening, smoking cessation, and from there then they can communicate to providers to help fill the provider in with what that patient needs to help facilitate that conversation. . . . I think one of the things we do really well is including that community engagement piece into our clinical health programs, our implementation science program, so really being able to reach marginalized communities, is an important component of this.” (16A) | |
| 11 | “One of our sites is doing this by [a proprietary online meeting service] so the patient comes in to the clinic locally where they have a video monitor and connects with the more central site by video monitor. There has to be a nurse in the room with the patient for that to be a billed event, but that way we are doing some remote, shared decision-making as well.” (17A) | |
| 12 | “Because it’s a centralized program, you know, we had input from the primary care providers, very shortly, and after implementation they were thrilled that they did not have to do these conversations. One, they didn’t have the time in a busy clinic practice to do shared decision-making, two, they didn’t understand the nuisances of risks and benefits for screening. They’d rather just leave it for the trained experts.” (7B) | |
| 13 | “We have discovered that not all providers answer the questions. And they would just skip over it. In April we have, um, an upgrade to [proprietary EHR vendor]. During that, um [proprietary EHR vendor], upgrade the, the questions regarding decision-making and smoking cessation will be hard stop questions and a provider must order the, or answer them, in order to move forward. So, it will be mandatory to be answered.” (9A) | |
| 14 | “CMS has certain requirements for what needs to be documented, so we can monitor for that. So, if you were to argue that you know appropriately documenting the things CMS requires to be documented as quality, that we can monitor, but what really is happening in terms of, you know, quality, no.” (20A) | |
| 15 | “Although when you look at the documentation there’s a checked box indicated the right CMS verbiage for billing, but whether or not they’re actually conducting a shared decision-making visit is hard for us to know.” (7B) | |
| 16 | “[T]he con of centralized, or you could say the benefit of doing it decentralized, physician recommendation really matters to patients like it’s out in the literature. It’s like, you know when your doctor tells you to do some kind of cancer screening at least in the other cancer screenings you know, it’s, it’s highly predictive of the patient doing it . . . . But, when we put a team on things and that team is not, you know, speaking with the voice of the physician I think patients ignore it.” (20A) |
CMS = Centers for Medicare and Medicaid Services; EHR = electronic health record; PCP = primary care physician. See Table 1 legend for expansion of other abbreviations.
Some respondents mentioned that the SDM for LCS model their organization used had evolved over time. In some organizations, these evolutions reflected a change from one model to the other (IQs 4-5) (Table 2). Regardless of the model used by the organization, respondents reported that a multidisciplinary clinical team (that included stakeholders from pulmonology, thoracic oncology and surgery, and primary care) developed SDM for LCS programs and adapted these over time based on organizational structural changes or feedback received from providers or patients (IQs 6-7) (Table 2).
Access-Quality Tradeoff
Respondents discussed a tradeoff between quality and access in centralized vs decentralized models. Respondents generally perceived centralized models as enhancing SDM counseling quality, but limiting patient access (IQ 8) (Table 2). For example, because centralized models required patients to travel to LCS clinics often located in different facilities from where individuals typically received care and that tended to offer services on limited days, times, or both, respondents perceived them as less accessible than those offered via decentralized models (IQ 9) (Table 2). Some centralized models used patient navigators who were responsible for identifying eligible patients through community outreach and educational programs, used remote SDM sessions through online meeting services, or both in attempts to improve access (IQs 10-11) (Table 2).
However, respondents’ perceptions were that centralized models had a higher level of SDM quality because practitioners who specifically were trained in SDM delivered the SDM counseling and did not face the same time constraints that primary care providers faced (IQ 12) (Table 2). Some decentralized programs reported modifying the electronic health record to require physicians to respond to specific questions regarding the SDM components as stipulated by CMS policy in an attempt to address CMS quality measures (IQ 13) (Table 2). However, respondents expressed concern that providers may be documenting SDM (to comply with CMS requirements), regardless of the counseling content provided (IQ 14-15) (Table 2).
Respondents also discussed the importance of patient-provider trust when engaging in SDM, acknowledging how established patient-provider relationships could be an advantage in decentralized models and that a trusting relationship may enable patients to understand and apply their personal values to the tradeoffs inherent in LCS (IQ 16) (Table 2).
Determinants of SDM for LCS Implementation
Organizational Characteristics
Respondents reported that their organization’s size and structure (such as the number and geographic distance among affiliated facilities) were key determinants to implementing SDM for LCS programs. Notably, some respondents mentioned how the size and complexity of their organization (ie, multiple clinics in remote areas) precluded a centralized SDM for LCS model (IQ 1) (Table 3), whereas others perceived centralized models to be better suited to large organizations, given the difficulty associated with training a large number of providers on SDM requirements (IQ 2) (Table 3). The existence of an in-house smoking cessation program also was a consideration, because some respondents reported using resources (eg, nurse counselors) associated with their organization’s smoking cessation program to provide SDM (IQ 3) (Table 3). However, the extent to which organizations integrated their SDM for LCS and in-house smoking cessation program varied.
Table 3.
Illustrative Quotations Regarding Determinants of SDM for LCS Implementation
| Determinants of SDM for LCS Implementation | Quotation No. | Illustrative Quotation (Study Respondent Letter) |
|---|---|---|
| Health system characteristics | 1 | “Our program is a decentralized program. Our university, hospital, and clinic are part of a larger health system in the metropolitan area, which, uh, has several, I think four or five hospitals over a probably 100-mile range and many, many clinics. . . . So because our system is decentralized we never really felt like it was feasible to have a screening clinic and that was never really considered an option.” (19A) |
| 2 | “Our program was developed of using mid-level providers, and we have a very large organization so training all the physicians of all the requirements of the SDMs did not seem to be a feasible task.” (1B) | |
| 3 | “The [name of state] grant opportunity which has been going on for two previous years and now we just got funding, to help integrate the smoking cessation and the lung cancer screening. And that has allowed us to have a programmer, uh myself, another MPH person, to really track the data feedback information to, the lung cancer screening clinic.” (1A) | |
| Available resources and budgetary considerations | 4 | “We looked at the cost of our staffing. We looked at the cost of IT stuff, you know, they would need laptops, and how to present the PowerPoint. We looked at the cost of the brochures and everything we need to hand out to the patients. We looked at the cost of how much we’re gonna get reimbursed per CT, how much you’re gonna be reimbursed per SDM, and then, we looked at the benefits to the patients and we looked at the downstream that could come from all this, and we decided as a health system hiring nurse practitioners that do shared decision-making visits wasn’t cost effective. Instead what would be the best use of everyone to the highest level they’re licensed.” (2A) |
| 5 | “The other sites literally don’t have the manpower or they didn’t have the financial buy-in from administration to do the shared decision making visit . . . . I’m in a position where I can see the difference between how we as an academic center try to do it vs how the community hospitals who are affiliated with our health system do it.” (12A) | |
| 6 | “We do have some grant funding from a local grant fund[er]. I think we would love to increase our federal funding for lung cancer screening. Just to make sure we could increase our capacity. We also need funding that could help us partner with other clinics and hospitals to increase capacity to at-risk populations, a lot of whom don’t interact with health care systems with a regular basis, so really if we really want to impact health disparities, funding that helps us extend our community outreach to reach people.” (16A) | |
| 7 | “We did allocate our thoracic oncology navigators to be part of the lung cancer screening program and they are the ones who kind of keep track. We did also allocate some time for our cancer registrar to maintain the registry.” (6B) | |
| 8 | “We hired a nurse practitioner, who’s excellent, and also expensive. The challenge is keeping her employed because, while she generates a lot of revenue for the health system through surgeries and scans etcetera, she’s actually housed in the Department of Pulmonology, which in the cancer center that doesn’t really directly see that revenue. So, we have to keep advocating and reminding people that she is making money for the health system in general, and she may not be putting money in the cancer center specifically, but she’s generating and causing, several life-saving cancer surgeries.” (7A) | |
| 9 | “I’ve spent a lot of time with nurses from various, outlying clinics to teach them that. The problem is there’s enough turnover in that position that every few years I have to go out and teach it all over again.” (13A) | |
| External policy incentives | 10 | “We work with compliance to make sure that we’re meeting the Medicare, um, like our internal compliance officer to make sure we were meeting Medicare’s rules, and were covered of um, you know, reimbursements and uh, violating all of those CMS policies, um, by having all those attestations statements on the statements that the ordering provider has to sign.” (2A) |
| 11 | “Um, so we were moving towards a closed program, uh, with visits before CMS’s mandate but, CMS’s mandate helped to accelerate that and, uh, allowed us to justify it with our, you know, with our health system and the primary care community.” (21A) | |
| 12 | “While the Medicare patients have to have it done in order to reimburse[], we feel that it is important that all patients to have one, and we sort of consider that our standard of care.” (5A) | |
| 13 | “CMS doesn’t pay for patients who are in the upper USPSTS-recommended age range so we’re gonna put a note on it, just, you know, make sure folks are aware that if they order it for those older patients CMS won’t cover the payment for the, for the test.” (8B) | |
| 14 | “I think just in the general sense that CMS criteria and USPSTS criteria are different. That just adds confusion for people as well.” (17A) |
Available Resources and Budgetary Considerations
Respondents mentioned that available resources and budgetary considerations were key determinants to implementing SDM for LCS programs. Respondents described costly infrastructure changes, such as hiring new staff to track patients and deliver SDM counseling, which required financial resources that only some organizations could afford (IQs 4-5) (Table 3). Several respondents reported that external grant funding helped their organization to implement and maintain their SDM for LCS program (IQ 6) (Table 3). Lacking such external resources, some organizations educated existing nurse practitioners to provide SDM or reassigned available staff (IQ 7) (Table 3). Respondents discussed challenges faced when trying to secure staffing and other resources for SDM counseling. Many reported challenges stemming from their organization’s cost accounting system within which the costs associated with patient identification and SDM counseling were borne in one department, while the downstream procedural revenues from LCS accrued to another (IQ 8) (Table 3). In addition, respondents mentioned challenges with staff turnover and the need continually to retrain SDM counseling staff (IQ 9) (Table 3).
External Policies and Incentives
Respondents described how CMS requirements influenced their SDM for LCS program. For example, the CMS requirement influenced who provided SDM counseling and what information was discussed during counseling (IQ 10) (Table 3). Moreover, some respondents described how their organization was already leaning toward providing SDM for LCS and how the CMS requirement provided an opportunity to move forward (IQ 11) (Table 3). Respondents noted how discrepancies between CMS screening eligibility criteria and those of other organizations (eg, United States Preventive Services Task Force) created confusion regarding health insurance coverage for LCS. For example, based on CMS criteria, patients are eligible for LCS if they are 55 to 77 years of age, whereas the upper limit of United States Preventive Services Task Force guideline recommendations is 80 years of age. Resulting organizational policies varied in their age eligibility. All respondents noted their organization decided to offer SDM to all patients, regardless of the patient’s insurance carrier (IQ 12) (Table 3). However, to address differences in eligibility, respondents indicated their organizations provided practitioner alerts regarding insurance coverage of LCS (IQs 13-14) (Table 3).
Challenges of SDM Implementation
Time Constraints
One of the key challenges reported by respondents, particularly those from organizations with decentralized models, was the lack of protected time for primary care physicians to provide SDM. Respondents explained how primary care providers’ schedules were constrained as compared with those of specialty providers (IQ 1) (Table 4). To address the lack of time, some organizations used posters and brochures in the waiting area or referred patients to watch educational videos in an effort to reduce time demands (IQ 2) (Table 4).
Table 4.
Illustrative Quotations Regarding Challenges of SDM for LCS Implementation
| Challenges of SDM for LCS Implementation | Quotation No. | Illustrative Quotation (Study Respondent Letter) |
|---|---|---|
| Time constraints | 1 | “So they’re supposed to be identifying patients who need the flu shot, they’re supposed to be identifying patients who need the shingles shot, so there’s a lot of competing agendas, and they don’t have any protected time for do this work.” (20A) |
| 2 | “It’s, I guess, certainly a daunting task there. If you do well it probably takes several minutes, and it could kind of be a hard sell getting an already busy clinician who’s, um, dealing with three or four chronic medical problems in a 15-minute time slot to commit to actually have the discussion . . . . We made a video and a couple of nurse practitioner[s] and I, uh, who worked on this basically had a shared decision-making visit into a camera with the thought being that, you know, someone could be put in the room for primary care visit. This video could, you know, then be played while they’re waiting on the provider to come in and to start the visit.” (5A) | |
| Knowledge and beliefs about SDM for LCS | 3 | “I think initially this is a very new concept, uh, the concept of a shared decision-making visit before a cancer screening test. We don’t do that for colon cancer screening, don’t really do it for breast cancer screening, anything, or anything else.” (5A) |
| 4 | “I don’t think there was a lot of information, to be honest in general, regarding shared decision-making. It was just one more element. There was more emphasis in, having a program to report to CMS the data.” (3A) | |
| 5 | “Primary care providers in particular, but really all referring clinicians, were feeling like they didn’t necessarily have the in-depth knowledge about lung cancer screening and what the tradeoff[s] of that were to be able to conduct the full shared decision-making process.” (14A) | |
| 6 | “We try to educate, we do grand rounds, we encourage the primary care providers to refer to our program. We did have paper decision aids that were made available to the primary care providers should they want to engage in that conversation. Um, with time we had some computer-based, uh, web-based decision aids, available through [the] VA, one is, uh, is, lung decision precision.com, which includes the risks and benefits of lung cancer screening as well as an individualized risk for developing lung cancer based on the bock model.” (7B) | |
| Reflecting and evaluating | 7 | “I think we need something like that, but as of now there’s no system in place to monitor the quality of shared decision-making.” (14A) |
| 8 | “I think what we need to do is define the bare minimum, content of a discussion between a patient and a physician that constitutes shared decision-making . . . . I think if we want to audit ourselves as providers we need to be able to go back and see what we did and how we did it and how well we did it, and then see does that to really, does that, you know, connected with patient-centered outcomes, right? . . . . And you know I’ve had conversations with other experts about, how do you even measure the quality of a shared decision-making conversation? Is there some metric that we can come up with? And I think people even struggle with that which is, how do you know whether it’s happened and how do you know whether it’s happened with quality?” (13A) | |
| 9 | “We did surveys of our patients before the visit about their knowledge, um, and then immediately after the visit surveys about their knowledge and whether they felt the visit helped them make an informed decision . . . . Those results had showed, uh, that knowledge at the end of the visit was much better, particularly about the harms of screening. Um, and the patients felt more comfortable with their decisions.” (21A) | |
| 10 | “We have implemented in our EHR that the, uh, a little box comes up if a patient reaches, uh, is correct age, if their smoking history is correct, to remind the physician that this could be a patient that could be eligible for lung cancer screening. When providers order low-dose CT, providers [are] asked to go through the checklist for the CMS, you know, documentation purpose: make sure patients eligible, make sure patient does not have a fever, or come up with any symptom of the cancer, discussed the benefit and risk, um, including false positive, blah, blah, blah. That’s CMS documentation part.” (4A) |
Practitioner Knowledge and Beliefs About SDM
Respondents acknowledged the novelty of requiring and a general lack of practitioner knowledge regarding SDM (IQs 3-4) (Table 4) undermined the ability to provide high-quality SDM for LCS (IQ 5) (Table 3). To address these concerns, organizations offered educational opportunities for practitioners via grand rounds, educational brochures, or educational resources such as web-based decision aids (eg, LCS for providers; IQ 6) (Table 4).
Reflecting and Evaluating
Respondents noted that CMS has not provided a set of benchmarks by which to measure the quality or consistency of SDM for LCS (IQ 7) (Table 4). As a result, respondents reported not knowing how to monitor the quality of SDM counseling (IQ 8) (Table 4). A few respondents described developing internal mechanisms to monitor the quality of the SDM counseling process, such as checking in with patients to see what information they retained from the SDM counseling visit, conducting observations of staff performing SDM counseling, or conducting surveys to assess patient satisfaction (IQ 9) (Table 4). Some respondents reported their organization modified the electronic health record to require practitioners to document SDM before being able to order LCS. Such so-called hard stops often required the provider to respond to specific questions that parallel CMS SDM counseling requirements (IQ 10) (Table 4).
Discussion
We studied how health-care organizations implement SDM for LCS. Resultant programs fell into one of two basic models: a centralized model in which front-end practitioners referred patients to an LCS clinic at which trained practitioners delivered SDM at the time of testing or a decentralized model in which front-end practitioners delivered SDM before referring patients for testing. Some organizations used the combination of both models and offered both a centralized and decentralized SDM for LCS model separately, albeit simultaneously. Importantly, although the term hybrid21 has been used to describe such programs, no respondent in our sample incorporated components of each model into an integrated program.
Consistent with prior findings, respondents raised concerns regarding time constraints providers face for conducting SDM,13,22, 23, 24 a lack of SDM knowledge,25, 26, 27, 28 and the inability to standardize counseling and SDM processes. Such concerns seem well grounded given that a study found that LCS discussions between patients and primary care physicians or pulmonologists, on average, lasted less than 1 min and none met established criteria for SDM.29 Respondents perceived providers in centralized programs as being better trained in SDM and as having relatively more time for discussions with patients. Such sentiments are consistent with those identified previously30 and with studies that have found centralized programs led to increased patient knowledge and more informed decision-making.31
Nonetheless, also consistent with others’ opinions,29,32 respondents voiced support for conducting SDM in the context of an established and trusting patient-physician relationship.15,33 In addition to the relationship context afforded with decentralized SDM models, decentralized models further ensure that SDM counseling occurs early in the screening process, when patients have time to consider the information discussed before having to commit to traveling to a screening clinic.34 This is important because although the quality of the counseling in a centralized model is considered relatively higher than that in decentralized models, the reach and quality of the overall decision process may not be. For example, a recent study35 found that all patients receiving SDM counseling for LCS within a centralized model went on to complete LCS. Other studies likewise have found that substantially fewer patients opt out of screening after SDM counseling in centralized as compared with decentralized models.31,36,37 It maybe because patients lower their benefit-to-risk threshold for LCS after counseling receipt in centralized models as a way of reducing cognitive dissonance with the decision they already made to be screened. Therefore, it is important to ensure that patients do not equate an LCS clinic referral with the need to complete screening. Such competing advantages (and disadvantages) of centralized and decentralized SDM models merely illustrate the ongoing challenges of implementing SDM in practice.38 The use of telehealth for providing SDM may help centralized, decentralized, and hybrid screening programs to address some of these problems. Providing SDM sessions through telehealth (eg, video- or phone-based decision-making counseling sessions),39 allows SDM counselling to occur outside of time-limited office visits, increased access to SDM counselling, and could help patients make informed decisions while maintaining their existing patient-physician relationship.
Service access, particularly among traditionally underserved populations,40, 41, 42 remains an important consideration, especially given that LCS is significantly underused relative to other recommended cancer screening tests.5,12 Decentralized models were reported as offering wider access and being able to screen more people relative to centralized models, a consideration particularly important to individuals of lower socioeconomic status who may face multiple barriers to receiving services. Testing ineligible patients (eg, those with severe comorbidities or older than 80 years) for screening likely would increase the harms and decrease the benefits of LCS, highlighting the need for decentralized programs to establish rigorous eligibility checks at either the time of ordering the screening or through quality assurance feedback to ordering practitioners. Regardless of the SDM for LCS model, both patient and provider engagement and education are paramount to identifying high-risk individuals eligible for screening and thus to maximizing the benefit-to-risk ratio of LCS.
Current CMS policies and organizational monitoring seem insufficient to enable either an understanding of the extent to which patients who are eligible for LCS screening are being offered testing or the extent to which those who undergo screening are knowledgeable of the tradeoffs inherent with LCS. Although arguably no gold standard exists by which to measure SDM quality, a range of related outcomes, such as patient knowledge and perceived decision-making participation, are available and could be used as proxy measures or with adaptation.15,43, 44, 45 In the absence of standardized metrics and quality oversight, the CMS policy regarding SDM for LCS counseling may have little impact on actual clinical practice. Our findings suggest that the policy may be a “parchment guarantee” in that providers can check a box within the electronic health record to indicate that SDM counseling occurred, but because of their lack of understanding regarding what SDM is or other factors, the policy may not be ensuring that patients offered LCS are receiving appropriate counseling.
Consistent with findings from prior studies reporting physician’s perceptions of SDM for LCS,27,46 the results indicate that implementing SDM for LCS is challenging because of a lack of available resources. Also as reported elsewhere,47 respondents reported relying on external grant funding, struggling to maintain the resources needed, or both to create and sustain an LCS clinic and the staffing to deliver SDM. Such challenges likely are compounded by the relatively small reimbursement amount for LCS counseling and SDM in comparison with either the costs of providing such services or the reimbursement offered for other procedures as well as the organizational accounting disconnect between departments that provide counseling and those that are reimbursed for it. Whether organizations will begin to develop internal accounting systems that more appropriately align with the overall impact of LCS on the organization’s fiscal health remains to be seen. The larger challenge facing organizations likely derives from the relatively modest reimbursement for SDM. Without additional innovation in service delivery, it is difficult to envision current SDM for LCS models being sustainable for the delivery of high-quality SDM.
Our study is not without limitations. First, although interviews enabled an in-depth understanding of how the organizations in our sample have implemented SDM for LCS programs, the responses of a selected group of individuals from a limited number of organizations may not represent all programs. We similarly were not able to characterize the challenges and successes encountered by all organizations that are implementing LCS. Second, our interviews targeted the individual responsible for overseeing the development of the SDM for LCS program, in some instances the program’s ongoing management, or both, and it is possible that the perception of such program leaders differs from that of others or that other unknown bias is present. However, we were able to provide an in-depth exploration of how SDM for LCS has been developed and implemented in a select group of organizations, something that has not been done previously. In addition, we interviewed representatives from diverse organizations located in multiple states, and continued until we reached data saturation.
Conclusions
Despite limited guidance or evidence of best practices, organizations have implemented SDM for LCS to enable Medicare reimbursement. Respondents not only reported facing ongoing resource-related challenges but also lamented the SDM access-quality tradeoff. Those responsible for developing and managing these programs voiced concerns regarding program access and SDM quality, regardless of organizational context or the SDM for LCS model implemented. Despite such challenges, all programs devised ways to enable Medicare reimbursement for LCS. The challenge facing these organizations, and thus those wanting to help patients and clinicians balance the tradeoffs inherent with LCS, is how to move beyond a check-box documentation requirement to a process that enables LCS to be offered to all high-risk patients, who are informed and for whom screening represents a value-concordant service.
Acknowledgments
Author contributions: J. E. L., C. N. D., and A. A. T. conceived and designed the study. J. E. L. supervised the conduct of the study and data collection. A. A. T. and J. E. L. undertook recruitment of participating key informants and managed the data, including quality control. A. A. T. conducted interviews. A. A. T. and K. T. performed the coding and data analysis. M. P. R., K. T., D. S. R., and C. N. D. provided critical revision of the manuscript for important intellectual content. J. E. L. provided administrative, technical, or logistic support. A. A. T. and J. E. L. drafted the manuscript, and all authors contributed substantially to its revision. J. E. L. and A. A. T. take responsibility for the article as a whole.
Financial/nonfinancial disclosures: None declared.
Role of the sponsors: The sponsors did not participate in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Additional information: The e-Appendix and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the Lineberger Comprehensive Cancer Center. Dr Elston Lafata is supported by the Division of Cancer Prevention, National Cancer Institute [Grant number R01CA197205].
Supplementary Data
References
- 1.Centers for Medicare and Medicaid Services Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) 2015 https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 [Google Scholar]
- 2.De Koning H., Van Der Aalst C., Ten Haaf K., Oudkerk M. Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population based trial. J Thorac Oncol. 2018;13(10) [Google Scholar]
- 3.Humphrey L.L., Deffebach M., Pappas M. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159(6):411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor G.T., Hatabu H. Lung cancer screening, radiation, risks, benefits, and uncertainty. JAMA. 2012;307(22):2434–2435. doi: 10.1001/jama.2012.6096. [DOI] [PubMed] [Google Scholar]
- 5.Bach P.B., Mirkin J.N., Oliver T.K. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patz E.F., Jr., Pinsky P., Gatsonis C. NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa E.J.M., Jr. Lung cancer screening overdiagnosis: reports of overdiagnosis in screening for lung cancer are grossly exaggerated. Acad Radiol. 2015;22(8):976–982. doi: 10.1016/j.acra.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Woolf S.H., Harris R.P., Campos-Outcalt D. Low-dose computed tomography screening for lung cancer: how strong is the evidence? JAMA Intern Med. 2014;174(12):2019–2022. doi: 10.1001/jamainternmed.2014.5626. [DOI] [PubMed] [Google Scholar]
- 9.Gill R.R., Jaklitsch M.T., Jacobson F.L. Controversies in lung cancer screening. J Am Coll Radiol. 2013;10(12):931–936. doi: 10.1016/j.jacr.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Wiener R.S. Balancing the benefits and harms of low-dose computed tomography screening for lung cancer: Medicare’s options for coverage. Ann Intern Med. 2014;161(6):445–446. doi: 10.7326/M14-1352. [DOI] [PubMed] [Google Scholar]
- 11.Merchant F.M., Dickert N.W., Howard D.H. Mandatory shared decision making by the Centers for Medicare & Medicaid Services for cardiovascular procedures and other tests. JAMA. 2018;320(7):641–642. doi: 10.1001/jama.2018.6617. [DOI] [PubMed] [Google Scholar]
- 12.Joseph-Williams N., Elwyn G., Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. doi: 10.1016/j.pec.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Légaré F., Ratté S., Gravel K., Graham I.D. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–535. doi: 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Gravel K., Légaré F., Graham I.D. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1(1):16. doi: 10.1186/1748-5908-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shay L.A., Lafata J.E. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–131. doi: 10.1177/0272989X14551638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palinkas L.A., Horwitz S.M., Green C.A. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533–544. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guest G., Bunce A., Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82. [Google Scholar]
- 18.Damschroder L.J., Aron D.C., Keith R.E., Kirsh S.R., Alexander J.A., Lowery J.C. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):1–5. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King N. Template analysis. In: Symon G., Cassel C., editors. Qualitative Data Analysis in Organisational Research: A Practical Guide. Sage; London: 1998. pp. 118–134. [Google Scholar]
- 20.Denzin N.K., Lincoln Y.S. Sage; London: 2011. The Sage Handbook of Qualitative Research; pp. 1–28. [Google Scholar]
- 21.Tanner N.T., Silvestri G.A. Shared decision-making and lung cancer screening: let’s get the conversation started. Chest. 2019;155(1):21–24. doi: 10.1016/j.chest.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Henderson L.M., Jones L.M., Marsh M.W. Opinions, practice patterns, and perceived barriers to lung cancer screening among attending and resident primary care physicians. Risk Manag Healthc Policy. 2017;10:189. doi: 10.2147/RMHP.S143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanodra N.M., Pope C., Halbert C.H., Silvestri G.A., Rice L.J., Tanner N.T. Primary care provider and patient perspectives on lung cancer screening. A qualitative study. Ann Am Thorac Soc. 2016;13(11):1977–1982. doi: 10.1513/AnnalsATS.201604-286OC. [DOI] [PubMed] [Google Scholar]
- 24.Yarnall K.S., Pollak K.I., Østbye T., Krause K.M., Michener J.L. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raz D.J., Wu G.X., Consunji M. The effect of primary care physician knowledge of lung cancer screening guidelines on perceptions and utilization of low-dose computed tomography. Clin Lung Cancer. 2018;19(1):51–57. doi: 10.1016/j.cllc.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elston Lafata J., Brown R.F., Pignone M.P., Ratliff S., Shay L.A. Primary care physicians’ support of shared decision making for different cancer screening decisions. Med Decis Making. 2017;37(1):70–78. doi: 10.1177/0272989X16660547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iaccarino J.M., Clark J., Bolton R. A national survey of pulmonologists’ views on low-dose computed tomography screening for lung cancer. Ann Am Thorac Soc. 2015;12(11):1667–1675. doi: 10.1513/AnnalsATS.201507-467OC. [DOI] [PubMed] [Google Scholar]
- 28.Klabunde C.N., Marcus P.M., Han P.K. Lung cancer screening practices of primary care physicians: results from a national survey. Ann Fam Med. 2012;10(2):102–110. doi: 10.1370/afm.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner A.T., Malo T.L., Margolis M. Evaluating shared decision making for lung cancer screening. JAMA Intern Med. 2018;178(10):1311–1316. doi: 10.1001/jamainternmed.2018.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiener R.S., Koppelman E., Bolton R. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018 doi: 10.1007/s11606-018-4350-9. ;33(7):1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzone P.J., Tenenbaum A., Seeley M. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151(3):572–578. doi: 10.1016/j.chest.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Cohen E.E., LaMonte S.J., Erb N.L. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203–239. doi: 10.3322/caac.21343. [DOI] [PubMed] [Google Scholar]
- 33.Lafata J.E., Morris H.L., Dobie E., Heisler M., Werner R.M., Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013;92(1):94–99. doi: 10.1016/j.pec.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobler C.C., Midthun D.E., Montori V.M. Quality of shared decision making in lung cancer screening: the right process, with the right partners, at the right time and place. Mayo Clin Proc. 2017;92(11):1612–1616. doi: 10.1016/j.mayocp.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Han P.K., Lary C., Black A. Effects of personalized risk information on patients referred for lung cancer screening with low-dose CT. Med Decis Making. 2019;39(8):950–961. doi: 10.1177/0272989X19875966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinsinger L.S., Anderson C., Kim J. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 37.Reuland D.S., Cubillos L., Brenner A.T., Harris R.P., Minish B., Pignone M.P. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak. 2018;18(1):5. doi: 10.1186/s12911-018-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elwyn G., Scholl I., Tietbohl C. “Many miles to go . . .”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(2):S14. doi: 10.1186/1472-6947-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagan H.B., Fournakis N.A., Jurkovitz C. Telephone-based shared decision-making for lung cancer screening in primary care. J Cancer Educ. 2020;35(4):766–773. doi: 10.1007/s13187-019-01528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin A.N., Hassinger T.E., Kozower B.D., Camacho F., Anderson R.T., Yao N. Disparities in lung cancer screening availability: lessons from Southwest Virginia. Ann Thorac Surg. 2019;108(2):412–416. doi: 10.1016/j.athoracsur.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henley S.J., Anderson R.N., Thomas C.C., Massetti G.M., Peaker B., Richardson L.C. Invasive cancer incidence, 2004-2013, and deaths, 2006-2015, in nonmetropolitan and metropolitan counties—United States. MMWR Surveill Summ. 2017;66(14):1. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao N., Alcalá H.E., Anderson R., Balkrishnan R. Cancer disparities in rural Appalachia: incidence, early detection, and survivorship. J Rural Health. 2017;33(4):375–381. doi: 10.1111/jrh.12213. [DOI] [PubMed] [Google Scholar]
- 43.Joosten E.A., DeFuentes-Merillas L., De Weert G.H., Sensky T., Van Der Staak C.P.F., de Jong C.A. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 44.Agency for Healthcare Research and Quality Supplemental items for the CAHPS Cancer Care Survey: shared decision making. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/cahps/surveys-guidance/item-sets/cancer/suppl-shared-decision-making-items.html
- 45.Rimer B.K., Briss P.A., Zeller P.K., Chan E.C., Woolf S.H. Informed decision making: what is its role in cancer screening? Cancer Interdiscip Int J Am Cancer Soc. 2004;101(S5):1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman R.M., Sussman A.L., Getrich C.M. Attitudes and beliefs of primary care providers in New Mexico about lung cancer screening using low-dose computed tomography. Prev Chronic Dis. 2015;12:E108. doi: 10.5888/pcd12.150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J., Chung S., Wei E.K., Luft H.S. New recommendation and coverage of low-dose computed tomography for lung cancer screening: uptake has increased but is still low. BMC Health Serv Res. 2018;18(1):525. doi: 10.1186/s12913-018-3338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.