Abstract
Purpose
The objectives of this study are to (a) identify speech-language pathologists' (SLPs') familiarity with transcranial direct current stimulation (tDCS), (b) quantify what SLPs consider necessary tDCS-related improvement in aphasia severity (i.e., tDCS enhancement; desired improvement above and beyond traditional behavioral therapy) to implement this adjuvant therapy for the clinical management of aphasia, and (c) identify concerns that could potentially hinder the clinical adoption of tDCS.
Method
A brief (14-question) survey was disseminated via e-mail and social media outlets targeting SLPs working with individuals with aphasia.
Results
Two hundred twenty-one individuals responded, and 155 valid surveys were analyzed. Seventy-one percent of participants reported familiarity with tDCS prior to taking the survey. Clinicians reported a desired mean enhancement of 22.9% additional points on the Western Aphasia Battery–Revised Aphasia Quotient. Importantly, 94.2% of SLPs reported concerns regarding the implementation of tDCS in clinical settings (i.e., safety, cost, administrative approval, reimbursement and training).
Conclusions
This is the first study to identify SLPs' perspectives regarding the clinical adoption of tDCS. Results suggest the majority of queried SLPs were familiar with tDCS prior to taking the survey. Although SLPs report a desired improvement of approximately 23% additional points on the Western Aphasia Battery–Revised Aphasia Quotient to consider adopting tDCS into practice, many SLPs reported concerns regarding clinical adoption. Responses from the current survey offer important preliminary evidence to begin bridging the research-to-practice gap as it relates to the clinical implementation of tDCS. Relatedly, these results will inform future clinical trials.
In the field of speech-language pathology, clinicians are encouraged and expected to implement evidence-based practice. However, there are significant research-to-practice gaps in the field, and it is concerning that it takes, on average, 17 years for 14% of research findings to be adopted in clinical practice (Balas & Boren, 2000; Green et al., 2009). Moreover, successful execution of evidence-based practice bears many challenges, such as time and cost constraints, utility restrictions, and misunderstood professional roles (Harold, 2019). When it comes to the management of aphasia, it is clear that speech-language pathologists (SLPs) employ a variety of approaches, delivery methods, and interventions that often are not rooted in evidence-based practice (Brady et al., 2016). Researchers and clinicians should be collaborators in research implementation; however, applied research may not always reflect what clinicians consider to be important for clinical translation (Schmittdiel et al., 2010; Westfall et al., 2009). One way to ameliorate this situation is to involve stakeholders, such as clinicians, before the start of costly clinical trials to increase the chance that the expected outcomes will be embraced in practice. Accordingly, the current study surveyed practicing SLPs about their opinions regarding the use of transcranial direct current stimulation (tDCS) as an adjunct to aphasia treatment in clinical practice. The motivation behind this survey was to begin bridging the research-to-practice gap for this potential treatment approach. The following sections outline the importance of this work for aphasia therapy, as well as preliminary evidence that motivates the clinical implementation of tDCS in practice.
Following a stroke, 21%–38% of survivors suffer from aphasia (Engelter et al., 2006; Laska et al., 2001), a disorder resulting from damage to the neural networks that support language processing. For 40%–60% of individuals with aphasia (IWA), the impairment continues into the chronic stages of recovery (Pederson et al., 2004). A recent report by Simmons-Mackie (2018) suggests there are over 2 million individuals in North America living with aphasia. Poststroke survival rates are on the rise (Fisher et al., 2014), yielding increased demands on health care systems (Demaerschalk et al., 2010), which include longer hospital stays and increased use of rehabilitation services (Dickey et al., 2010; Pederson et al., 2004). Current aphasia rehabilitation approaches rely on behavioral speech-language therapy, with documented gains including improved communication abilities and quality of life (Barnes & Nickels, 2017; Basso & Macis, 2011; Breitenstein et al., 2017; Fridriksson, Hubbard, et al., 2012; Moss & Nicholas, 2006; Pulvermüller et al., 2001; Smania et al., 2010).
Traditionally, aphasia recovery was thought to be limited to a 3- to 6-month window following stroke (Demeurisse et al., 1980). Recent randomized controlled trials (Breitenstein et al., 2017; Nouwens et al., 2015; Pulvermüller & Berthier, 2008), however, support the notion that therapy-induced improvements are possible in the chronic stage (i.e., beyond 6 months poststroke). Although studies on aphasia rehabilitation reveal promising results related to recovery potential, many are limited in terms of the potential to inform translational research as they include small sample sizes, they include treatment schedules that do not often match what is possible in many rehabilitation settings, and long-term outcomes are seldom reported (Brady et al., 2016). In contrast to most aphasia therapy trials, Breitenstein et al. (2017) conducted a large-scale, Class I randomized controlled trial (N = 158) to evaluate the effectiveness of language therapy for chronic aphasia. Their findings demonstrated that 3 weeks of intensive speech and language therapy improved verbal communication and quality of life in chronic aphasia. Accordingly, studies such as this and others (Basso & Macis, 2011; Fridriksson, Richardson, et al., 2012; Moss & Nicholas, 2006; Pulvermüller et al., 2001; Smania et al., 2010) strongly suggest that aphasia rehabilitation can yield positive outcomes.
Despite these documented benefits, the actual outcomes are often modest (Hope et al., 2017). In order to advance best practice and maximize recovery, there is a crucial need to improve the effect sizes associated with aphasia therapy. Although behavioral therapy remains the mainstay in clinical settings, recent studies suggest noninvasive brain stimulation may boost outcomes (Fridriksson et al., 2011; Turkeltaub, 2015). Since the seminal study of Nitsche and Paulus (2000) that found tDCS can modulate cortical activity, there has been a steady rise in the study of tDCS as an adjuvant for aphasia rehabilitation (Fridriksson et al., 2011; Marangolo et al., 2011; Meinzer et al., 2016). Compared to other noninvasive brain stimulation methods such as transcranial magnetic stimulation, tDCS is less expensive, is more portable, and demonstrates a more reliable sham condition (Bolognini et al., 2009). While the underlying neural mechanism is not completely understood (Weiss & Bikson, 2014), tDCS provides noninvasive, nonpainful electrical brain stimulation that modulates cortical excitability through the application of weak electrical current. Anodal tDCS (A-tDCS) is thought to induce neuronal excitability by bringing membrane potentials closer to thresholds for action potential generation, whereas cathodal stimulation induces inhibition and has the opposite effect (Nitsche et al., 2008; Paulus & Nitsche, 2001).
Our group conducted the first two studies suggesting A-tDCS may boost the effect of language therapy in aphasia (Baker et al., 2010; Fridriksson et al., 2011). Results from these studies revealed that 1 mA of A-tDCS is safe when dispensed in daily, 20-min sessions. In the last decade, several other groups have similarly capitalized on this method, showing that tDCS paired with traditional speech and language therapy in poststroke aphasia may improve naming (Kang et al., 2011; Marangolo et al., 2011; Meinzer et al., 2016; Wu et al., 2015), as well as the recovery of articulatory (Marangolo, Fiori, Calpagnano, et al., 2013; Marangolo et al., 2011) and conversational discourse abilities (Marangolo, Fiori, Campana, et al., 2014; Marangolo, Fiori, Cipollari, et al., 2013; Marangolo, Fiori, Gelfo, et al., 2014). These effects are documented for trained (Fridriksson et al., 2011; Marangolo, Fiori, Calpagnano, et al., 2013; Marangolo et al., 2011; Wu et al., 2015), untrained (Kang et al., 2011), and both trained and untrained items (Marangolo, Fiori, Campana, et al., 2014; Marangolo, Fiori, Gelfo, et al., 2014; Meinzer et al., 2016). While many studies support the use of A-tDCS as an adjuvant to traditional behavioral therapy and suggest that further clinical trials of tDCS are worth investigating, the overall effectiveness and potential for clinical application are not conclusive due to variable study characteristics, such as stimulation parameters, participant inclusion criteria, and characteristics of the behavioral treatments (de Aguiar et al., 2015).
Recently, our group has tested a futility hypothesis in a Phase II, double-blinded, prospective randomized clinical trial (Fridriksson et al., 2018). The primary aim was to examine the effects of A-tDCS as an adjunct intervention to speech therapy in chronic stroke (> 6 months postonset). Seventy-four participants received either A-tDCS or sham (placebo) stimulation paired with 3 weeks of behavioral language training that targeted lexical-semantic processing (15 sessions, 45 min each). A-tDCS was significantly better than sham stimulation in improving trained and untrained naming ability, suggesting that A-tDCS application to the left hemisphere has the potential to improve rehabilitation outcome in IWA secondary to stroke (Fridriksson et al., 2019, 2018). This recent Phase II clinical trial informs future, definitive trials to investigate A-tDCS as an option for aphasia rehabilitation.
Although naming is commonly included as the primary end point in aphasia therapy studies, including those that incorporate tDCS, it is not a measure of overall aphasia severity. Given the recent Research Outcome Measurement in Aphasia consensus statement, which recommends the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2007) as the preferred measure of language processing outcomes for clinical trials (Wallace et al., 2019), along with evidence of its widespread use in both research and clinical settings (Kiran et al., 2018), we use improvement on the WAB-R as the outcome measure in the current study. Specifically, we surveyed SLPs regarding their opinions about tDCS-related language improvement as it relates to performance on the WAB-R Aphasia Quotient (AQ). The WAB-R AQ is a composite score based on subtests of speech fluency, auditory comprehension, speech repetition, and naming. It is measured on a scale of 0–100, where a score below 93.8 indicates aphasia (0–25 typically indicates a very severe aphasia, 26–50 is severe aphasia, 51–75 is moderate aphasia, and ≥ 76 suggests mild aphasia; Kertesz, 2007).
Consistent with the current state of affairs in the field, there is a research-to-practice gap in our understanding of clinicians' perspectives of tDCS. Although tDCS is not yet approved for clinical practice, it is possible that positive Phase III trials will pave the way for its adoption as part of the SLP's clinical arsenal. To better understand what SLPs consider the minimum tDCS-related improvement (tDCS enhancement) in aphasia therapy outcome, the current study surveyed the opinions of practicing SLPs with varying experiences in aphasia management. The goal of this study aligns closely with existing implementation science literature. According to the Promoting Action on Research Implementation in Health Services (PARiHS) framework (Kitson et al., 1998, 2008; Rycroft-Malone, 2004), the implementation of research to practice is dependent on supporting evidence, the context of adoption (e.g., the clinic), and factors that promote the facilitation of the new practice (e.g., support from key personnel). This survey directly addresses one aspect of the PARiHS framework, that is, SLPs’ perceptions, and discusses the role of external factors that may be influential in the clinical adoption of tDCS for aphasia therapy. The long-term goals of this research are to inform potential future clinical trials on tDCS as adjuvant to aphasia therapy and to improve the chances of clinical translation.
Method
Study data were collected and managed using Research Electronic Data Capture (Harris et al., 2009) software hosted at the University of South Carolina. Research Electronic Data Capture is a secure, web-based application designed to support data capture for research studies. The survey link was posted on various social media outlets (e.g., Twitter, Facebook) and distributed via e-mail (e.g., departmental listservs, state speech-language-hearing association listservs). Per the survey invitation letter, medical SLPs who worked with IWA were invited to participate. Survey responses were anonymous; no personally identifiable information was collected. A copy of the survey is presented in the Appendix. Study procedures were reviewed by the University of South Carolina Institutional Review Board and determined exempt. Participants consented to survey participation by clicking the survey link provided at the bottom of a participant invitation letter.
Participants
The target population for this survey was SLPs who work clinically with IWA. A survey was returned from 221 participants. Responses were reviewed for completion, and incomplete surveys (n = 55) were removed from the final sample. Surveys were considered incomplete if any of the following criteria were met: (a) respondent did not answer Question 8 (i.e., did not report work setting; n = 49), (b) respondent did not answer Questions 11 and/or 12 pertaining to WAB-R administration and/or their WAB-R use (n = 51), and/or (c) respondent did not answer the main question pertaining to tDCS enhancement (n = 37; Question 2). In addition, individuals who reported that they did not work directly with IWA (responded “0” to Question 9) were excluded from the final sample (n = 11). We excluded respondents who did not report working with IWA because these individuals do not reflect our target population and including these clinicians could introduce unnecessary error in the data set. It is important to note that the aforementioned groups are not mutually exclusive and, therefore, may be represented twice in the specifications of “invalid surveys.”
After the initial review, surveys from 155 individuals (70%) were determined to be valid, and these data were included in the final analysis. On average, respondents had a median of 9.5 (interquartile range: 16; Q1 = 4, Q3 = 20) years of experience working as SLPs and reported that 40.4% (SD = 27, range: 1%–100%) of their caseload was composed of IWA. Eighty-seven percent of respondents held a Certificate of Clinical Competence (CCC) from the American Speech-Language-Hearing Association. It should be noted that some respondents were clinical fellows (i.e., working toward their CCC; n = 8) and some worked in other countries where American Speech-Language-Hearing Association CCCs are not necessary for practice (Australia, n = 6; Canada, n = 4; Iceland, n = 3; United Kingdom, n = 2; India, n = 2; Pakistan, n = 1; China, n = 1). Participant demographics are represented in Table 1.
Table 1.
Demographics of survey respondents.
| Professional characteristics | M (SD), range |
|---|---|
| Years of professional experience | 12.6 (10.5), > 1–42 |
| Percentage of caseload composed of IWA | 40.4 (27), 1–100 |
|
Work setting |
% (n) |
| Acute/subacute care | 27 (42) |
| Outpatient/SNF/LTC/HH | 27 (42) |
| Academic/university | 12.3 (19) |
| Multiple settings | 33.6 (52) |
|
Country of practice |
% (n) |
| United States | 87.7 (136) |
| Canada | 2.6 (4) |
| United Kingdom | 1.3 (2) |
| Australia | 3.9 (6) |
| Other | 4.5 (7) b |
Note. IWA = individuals with aphasia; SNF = skilled nursing facility; LTC = long-term care; HH = home health.
Other countries reported as follows: India, n = 2; Pakistan, n = 1; Iceland, n = 3; Hong Kong, n = 1.
Outcome Measure
As previously stated, we framed our survey in the context of the WAB-R. For this reason, Survey Questions 11 and 12 inquire about clinical use of the WAB-R to determine if respondents have ever used the WAB-R in clinical practice and, if so, how often they use this battery for the purpose of aphasia diagnostics. Of the clinicians we queried, 92.90% (n = 144) report having administered the WAB (WAB, WAB-R, or WAB Bedside) at some point in their clinical practice. Of these respondents, 6.45% (n = 9) report never using the WAB for aphasia diagnostics, 25.16% (n = 36) report using it “rarely,” 18.06% (n = 26) report using it “sometimes,” 29.68% (n = 43) report using it “often,” and 20.65% (n = 30) report using it “always.”
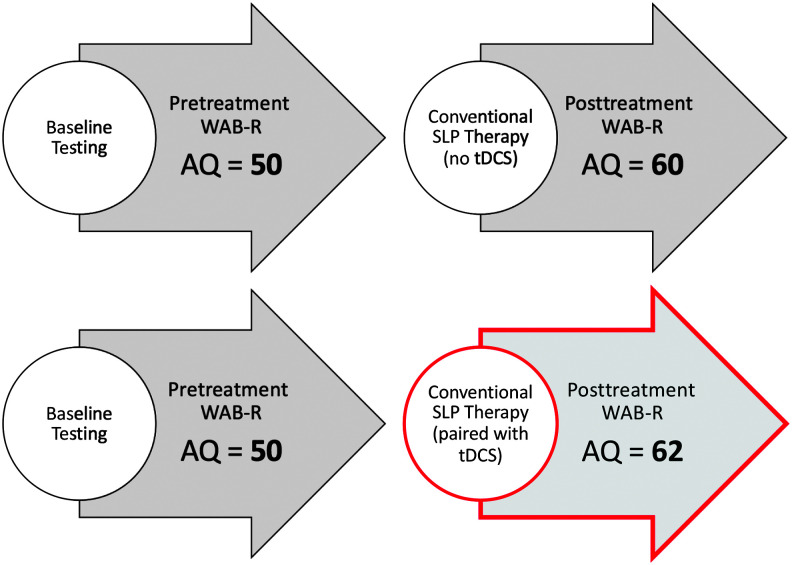
Importantly, to address our second objective, we consider the desired tDCS enhancement SLPs would need to see to implement tDCS clinically. To better clarify this concept, we use a hypothetical example, as follows: Consider a patient with aphasia who, when tested at baseline, obtains a WAB-R AQ of 50 points. After behavioral therapy alone, the patient improves by 10 points on the WAB-R AQ, yielding a new WAB-R AQ of 60. Considering an alternative rehabilitative approach (i.e., tDCS, as presented here), we focus on the gains above and beyond this 10-point improvement that SLPs would need to see to use tDCS in practice (tDCS enhancement). This concept is illustrated, in the context of our results, in Figure 1. This example is hypothetical and simply describes how we calculated the described tDCS enhancement in the context of the current study.
Figure 1.
Example of approximately 23% transcranial direct current stimulation (tDCS) enhancement reported by speech-language pathologists (SLPs) to implement tDCS in practice. For a patient who improves by 10 Western Aphasia Battery–Revised Aphasia Quotient (WAB-R AQ) points following aphasia therapy, the mean reported tDCS enhancement would correspond to a 2-point increase above and beyond conventional SLP therapy. In this hypothetical example, this means clinicians desire a total of 12 points of improvement for conventional SLP therapy paired with tDCS.
Statistical Analyses
Our analyses focused on three main objectives. First, we determined SLPs' familiarity with tDCS. Second, we analyzed what SLPs considered as the necessary tDCS enhancement in overall improvement on the WAB-R AQ needed to consider incorporating tDCS into practice. To better understand the outcome, we considered factors such as work setting, years of experience, and proportion of caseload consisting of IWA. Note that the variables “tDCS enhancement” and “years of experience” were not normally distributed (Shapiro–Wilk test, p < .05); therefore, nonparametric tests (Kruskal–Wallis, Mann–Whitney U, and Spearman correlations) were used for subsequent analyses that incorporated the “tDCS enhancement” and “years of experience” variables. Of note, work settings were grouped as follows: (a) acute/subacute settings (n = 42); (b) post—acute settings (n = 43), including outpatient setting, skilled nursing facilities (SNFs), long-term care (LTC), and home health; (c) academic settings (n = 19); and (d) multiple settings (n = 51). SNFs were grouped with LTC on the survey (Question 8) and intended to represent post—acute settings (see the Appendix). Group stratifications are consistent with previous studies surveying SLPs (Riley, 2017; Rose et al., 2018). Outpatient clinics, SNFs, LTC, and home health were grouped together as they were considered to be post—acute medical settings, whereas academic settings were considered to be associated with a university clinic. Academic settings were categorized independently as they typically do not have the same demands as other clinical settings, for example, productivity standards and effects of the research environment. In addition, those in university clinics may be more regularly exposed to research in that environment, so their opinions regarding more experimental therapeutic strategies may differ from those working in other settings.
To compare groups by years of work experience, we grouped participants according to experience, based on percentile ranks. Participants were grouped as follows: those with less than 4 years of experience (n = 37), those with 4–9 years of experience (n = 41), those with 10–19 years of experience (n = 38), and those with greater than 20 years of experience (n = 39).
Finally, for the third objective, we considered concerns SLPs may have for incorporating tDCS into practice. We evaluated whether the number of concerns reported influenced responses to the tDCS enhancement question. Themes for clinician concerns were identified by a consensus discussion between authors L. K., A. B., and J. F.
Results
tDCS Familiarity and Desired Enhancement
Seventy-one percent (n = 110) of participants reported familiarity with tDCS prior to taking the survey. Although not specified on the survey, “familiar” was intended to reflect if SLPs had, at the very least, heard of tDCS prior to taking the survey.
On average, SLPs reported a mean 22.9% (SD = 20, range: 0–100) desired tDCS enhancement (see Figure 1) of WAB-R AQ. Thirteen participants (8.4%) did not provide a numeric response for this question; rather, they provided qualitative responses. More specifically, of these 13, one participant stated, “Any consistent improvement would be sufficient,” 10 responded with uncertainty (e.g., “I'm not sure” or “I don't know”), and two reported they would look for functional language gains as opposed to changes on a standardized battery (e.g., “I'm less concerned with changes to the scores on any aphasia battery and far more concerned with real-world effects reported by clients”). Although these responses are not numeric, survey data for these individuals were included because a response to this question was indeed provided. These responses offer valuable insight to SLP opinions regarding the clinical adoption of tDCS. We expand upon this point in the Discussion section.
Response by Work Setting
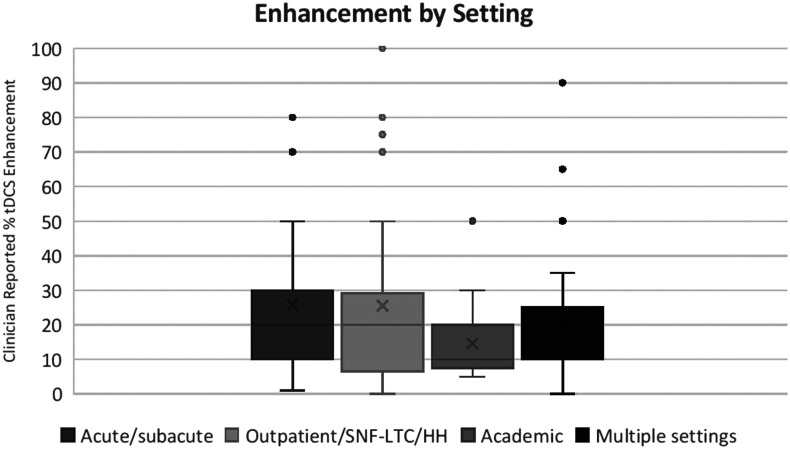
Kruskal–Wallis analysis of variance for tDCS enhancement by work setting was not statistically significant, χ2(3) = 5.8, p = .12. As seen in Figure 2, each group was highly variable in its response (mean tDCS enhancement response for acute/subacute settings = 26% [SD = 20.93], post—acute settings = 25% [SD = 24.87], multiple settings = 22% [SD = 17.02]); however, those in academic settings tended to report numerically less tDCS enhancement (M = 14.7%, SD = 11.1) compared to the other three groups.
Figure 2.
Box plot of transcranial direct current stimulation (tDCS) enhancement response by work setting. Kruskal–Wallis analysis of variance was not significant, χ2(3) = 5.8, p = .12; however, those in academic settings generally tended to respond with a smaller tDCS enhancement to adopt compared to the other three groups. SNF-LTC = skilled nursing facility–long-term care; HH = home health.
To investigate this trend further, we compared the tDCS enhancement response between SLPs who reported working exclusively in academic settings (n = 19) and those who worked in an academic setting and at least one other setting (n = 16). The mean tDCS enhancement response for those who work in academic settings and other settings was an average of 19.6% (SD = 20.6) tDCS enhancement. A Mann–Whitney U test revealed that there was no difference in desired tDCS enhancement between these two groups (Mann–Whitney U = 129, z = −0.79, p = .43). We suggest that this may indicate that those in academic settings generally report lower tDCS enhancement, whether or not they work in multiple settings. In short, there were no significant differences in tDCS enhancement by setting.
Response by Years of Experience
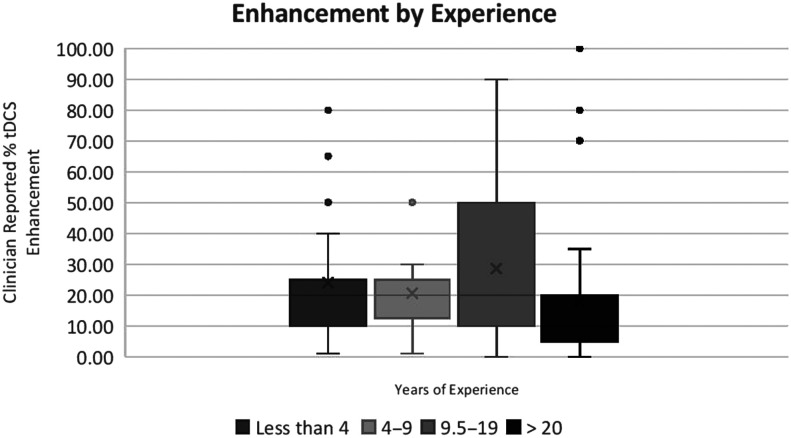
There was a nonsignificant trend toward a correlation between years of experience and tDCS enhancement, rs = −.16, p = .059. Kruskal–Wallis analysis of variance revealed a significant main effect of years of experience for tDCS enhancement, χ2(3) = 9.3, p = .025 (see Figure 3). Pairwise comparison revealed that those with over 20 years of experience reported a smaller necessary tDCS enhancement when compared to those with < 1–4 years of experience (Mann–Whitney U = 442.5, z = −2.5, p = .013), those with 4–9 years of experience (Mann–Whitney U = 352, z = −2.6, p = .009), and those with 9.5–19 years of experience (Mann–Whitney U = 468.5, z = −2.4, p = .019). The relationship between years of experience and desired tDCS enhancement did not appear to be driven by a correlation between tDCS familiarity and experience, rs = .002, p = .979.
Figure 3.
Box plot of transcranial direct current stimulation (tDCS) enhancement by years of experience. Kruskal–Wallis analysis of variance reveals a significant main effect of years of experience for tDCS enhancement, χ2(3) = 9.3, p = .025.
Response by Caseload
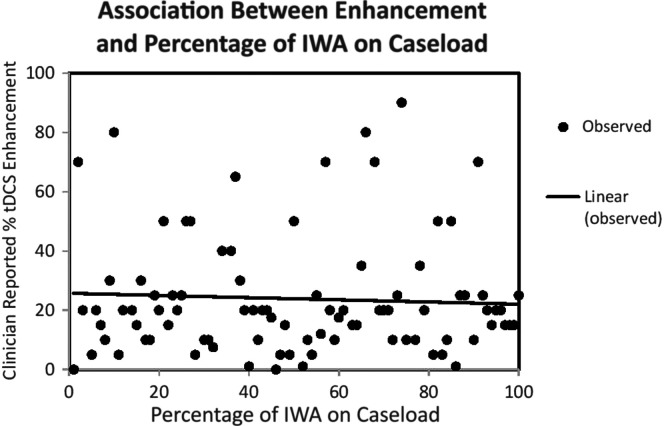
Across all years of experience, there was a significant negative relationship between desired tDCS enhancement and percentage of caseload consisting of IWA, rs = −.17, p = .043 (see Figure 4). This suggests that clinicians with a greater number of IWA on their caseload reported less of the tDCS enhancement required to implement tDCS in practice. Importantly, there was a positive association between the use of the WAB-R and the number of IWA on caseload, rs = .17, p < .033.
Figure 4.
Transcranial direct current stimulation (tDCS) enhancement response by proportion of individuals with aphasia (IWA) caseload. This scatter plot illustrates the negative correlation between tDCS enhancement and percentage of IWA on caseload, rs = −.17, p = .043.
Concerns for Adopting tDCS Into Practice
Only 5.8% of respondents (n = 9) reported no concerns regarding tDCS adoption into clinical practice. When provided with five broad categories (safety, cost, administrative approval, reimbursement concerns, and tDCS training and education/continuing education; see survey in the Appendix) of concerns and the option to select more than one concern, the majority of concerns (68.4%, n = 99) were related to clinician training and continuing education (frequency of concerns is presented in Table 2). The second highest concern was related to administrative support (50.3%, n = 78), followed by cost (47.1%, n = 73), safety (45.8%, n = 67), and reimbursement (41.9%, n = 61). Of note, “cost” pertains to the cost of the tDCS equipment, whereas “reimbursement” refers to insurance coverage for actual administration of tDCS and consideration of stimulation as a reimbursable therapy. Approximately one third (30.3%) of the respondents (n = 47) reported at least three concerns. When considering those who reported at least three concerns, compared to those who reported less than three (n = 108), there was no significant difference in desired tDCS enhancement of the WAB-R AQ (Mann–Whitney U test, z = 1.65, p = .1).
Table 2.
Concerns for adopting transcranial direct current stimulation.
| Concern | % Reported |
|---|---|
| Training/continuing education | 68.4 |
| Administrative approval | 50.3 |
| Cost | 47.1 |
| Safety | 45.8 |
| Reimbursement | 41.9 |
| No concern | 5.8 |
Note. The concerns listed here represent those provided in the survey. Additional “free response” concerns are listed in Table 3.
Thirty-six of the 155 survey participants (approximately 23%) used the “free response” box to expand upon their concerns or report additional concerns that were not listed within the five broad categories included on the survey instrument. SLPs voiced concerns related to tDCS treatment protocols (“What factors would inform an individualized tDCS electrode placement montage?”), inclusion criteria (“protocols for client selection,” “severity of aphasia prior to tDCS,” “presence of underlying cognitive deficits”), patient and family perceptions (“patients who are worried about brain stimulation,” “patient would not be open to it because they believe it is unsafe…too far ‘out there,’” “overblown expectations of patients and family”), and facility resources (“productivity impact,” “having a supervising neurologist,” “available detailed MRI information”). Other respondents were skeptical about the adoption of tDCS given that there are no Phase III clinical trials for aphasia or Food and Drug Administration approval. Several respondents expanded upon issues related to administration, training, cost (prices associated with tDCS equipment and associated setup/maintenance), and reimbursement (payments from third-party payors to reimburse speech-language pathology therapies that incorporate tDCS). These themes and example responses are presented in Table 3.
Table 3.
Themes related to concerns for adopting transcranial direct current stimulation (tDCS) and qualitative responses.
| Concern | Comment |
|---|---|
| Training/continuing education | “No formal training for clinicians that I am aware of for clinical implementation.” |
| Administrative approval | “Hospital administration may not approve…due to safety concerns and reimbursement issues….” |
| Cost | “The cost listed above is not likely to be the cost paid by patients because someone must be paid to set up and break down the equipment and apply it properly. If the SLP does this there will be less time for therapy so someone else must be employed as a tDCS tech and the cost of that person's salary will be borne by the patients.” |
| Safety | “Strong evidence of ‘no harm’ would be the first paper I would look for.” |
| Reimbursement | “…patients do not have finances (either through insurance or as private payers) to afford prolonged treatment. Most insurance plans do not cover either extended periods of time for or number of sessions.” |
| Efficacy | “No phase 3 trials have shown efficacy. The current evidence for tDCS in aphasia is similar to the evidence for rTMS for motor recovery a few years ago. Then the pivotal phase 3 trial of rTMS for motor recovery was stopped early for lack of efficacy. A multicenter trial is needed before charging patients for tDCS.” |
| tDCS treatment protocols | “What factors would inform an individualized tDCS electrode placement montage?” |
| Inclusion criteria | “…protocols for client selection,” “severity of aphasia prior to tDCS,” “…presence of underlying cognitive deficits” |
| Patient/family perceptions | “I'm concerned that patients would not be open to it because they believe it is unsafe, or because they feel it is too far ‘out there’” |
| Facility resources | “Maintenance of equipment considering my rural location.” |
Note. Concerns included here are from free responses and describe additional concerns from Table 2. rTMS = repetitive transcranial magnetic stimulation.
Discussion
The current study polled SLPs about their familiarity with tDCS, determined the threshold of language improvement on the WAB-R needed to adopt tDCS (tDCS enhancement), and identified specific concerns related to the clinical adoption of tDCS into practice. Survey results are important not only to inform future clinical trials related to this adjuvant therapy but also to bridge the translational gap between clinical research and clinical practice, which exists in the field. Seventy-one percent of the SLPs (110 clinicians) reported familiarity with tDCS prior to the survey, suggesting research related to tDCS is largely accessible to practicing clinicians. Notably, the survey places SLPs' reported tDCS-related enhancement in the context of WAB-R outcomes. The selection of the WAB-R, as the reference measure, shaped our study and the reported outcome, which we expand upon below.
The mean desired increase in WAB-R AQ to incorporate tDCS into practice was 22.9% additional points on the WAB-R AQ. This reported percentage of tDCS enhancement or gain above and beyond behavioral therapy alone can be applied to any amount of behavioral improvement on the WAB-R. Using our example of a 10-point increase in WAB-R AQ (see Figure 1), the reported 22.9% enhancement is equivalent to approximately a 2-point increase in WAB-R AQ. To illustrate this, consider the same clinical scenario as presented above (see Outcome Measure section). For this hypothetical patient who improves by 10 WAB-R AQ points following behavioral aphasia therapy, SLPs would adopt tDCS as part of their therapy program if the patient were to improve by 2 additional AQ points (22.9% additional improvement calculated as follows: 10 × 1.229 ≅ 12 points). Although the majority of respondents provided a numeric response to the question regarding desired tDCS enhancement of outcome, 13 SLPs provided an alternative, qualitative response instead. These responses indicate that SLPs may value other gains, for example, functional outcomes, rather than those that can be measured directly on the WAB-R.
We recognize that a 2-point WAB-R AQ increase is not clinically significant (Holland et al., 2017). This increase may, however, reflect an improvement in relevant or comprehensive speech output. Importantly, we also recognize that this 2-point difference is less than the standard error of the mean for the WAB-R (2.5 points; Kertesz, 2007). However, considering this 2-point change in the context of our results, a 1-point increase on either the Information Content rating of the Spontaneous Speech subtest (maximum points = 10, as awarded by responding correctly to a personally relevant question or providing an extra detail in response to the Picture Description task) or the Fluency portion (maximum points = 10, scored for fluency, presence/absence of paraphasias, and grammatical competence) results in a 2-point increase in AQ. For patients with limited speech output, even this considerably small change may enable them to verbalize wants and needs and to become more independent communicators. It is important to emphasize that the 2-point increase on the WAB-R reported here should not be taken as an indication of necessary minimum significant improvement as an end point for a Phase III trial on tDCS to enhance aphasia therapy outcome. Rather, the mean for the SLPs' desired improvement (22.9%) could be used as a reference point for interpreting the outcome of a clinical trial in the context of likely clinical adoption.
A smaller desired tDCS enhancement for implementation was reported by those with more experience in the field, those with a higher percentage of IWA on their caseload, and those in academic settings. A negative correlation between years of experience and tDCS enhancement suggests that clinicians with more experience in the field reported less of the required tDCS enhancement to implement tDCS in practice, which may reflect that clinicians with more experience may expect smaller gains to be made in individuals with chronic aphasia. This indicates that these clinician characteristics are influential in the decision to adopt tDCS in a clinical setting. Notably, those in academic settings also had more years of experience than clinicians in other settings, and therefore, this result is consistent with the significant main effect of years of experience for tDCS enhancement. Clinicians with less clinical experience and fewer IWA on their caseload may be less familiar with the administration and scoring of the WAB-R and research related specifically to aphasia therapies. Accordingly, these SLPs may wish for a greater tDCS enhancement to incorporate this modality into practice. Furthermore, experience working with aphasia (in terms of both years of practice and percentage of IWA on caseload) might influence clinician responses due to familiarity with the WAB-R and the persistent nature of chronic aphasia. Clinicians with more experience may also recognize the relatively small benefits that are typically seen with behavioral speech and language therapy for this population, which is a perception that may be reflected in their lower desired tDCS enhancement.
When considering the percentage of IWA on a clinician's caseload, there was a significant negative correlation between tDCS enhancement and percentage of caseload consisting of IWA. Although the correlation was small, this is comparable to the trend with greater experience yielding a lower tDCS enhancement needed for implementation. It may be that those with a greater number of IWA on their caseload are satisfied with less of an enhancement to incorporate tDCS into practice. These clinicians also have a greater familiarity or comfort level working with IWA, and this familiarity or “expertise” influenced responses. As demonstrated by our results, clinicians with a higher number of IWA on their caseload use the WAB-R more often than their counterparts with fewer IWA on their caseload, rs = .17, p < .033.
Despite the fact that the survey included select references highlighting the efficacy and safety of tDCS (see the Appendix), 94.2% of clinicians (n = 146 SLPs) reported concerns related to the implementation of tDCS (see Table 3). Of those, a majority were related to clinician training and continuing education. The second-highest concern was related to administrative support, followed by cost, safety, and reimbursement. In addition to the categories of concerns listed in Survey Question 3, clinicians expressed hesitation in adopting this method of brain stimulation based on efficacy, stimulation protocols, inclusion criteria for selecting appropriate patients, clinician training, patient/family perceptions, and facility resources (see Table 3). Many concerns emphasize the practicality of implementation in a clinical setting, especially related to administrative approval, productivity, and billing. Clinicians also expressed concerns related to having an available neurologist or neuroimaging to correctly identify the best tDCS stimulation site. These concerns bring to light many of the logistical and fiscal obstacles SLPs might face upon adopting tDCS in the clinical setting and are important considerations for the development of future clinical trials focused on tDCS in aphasia and related disorders. While clinician concerns are not necessarily surprising, these responses can help direct future research to consider needs relevant to clinicians and the settings in which they practice. Crucially, research that aligns well with clinical reality is necessary for evidence-based practice (Harold, 2019).
Limitations
The first and primary limitation of this study is the survey-based method of data collection, which is subject to biases, namely, that the data collected are that of a convenience sample. Therefore, the sample itself may not be representative of the average clinician working with IWA. The social media outreach for survey distribution (i.e., Twitter and Facebook) likely reached a variety of SLPs who are active in clinical and/or research capacities. Similarly, clinicians who responded to the survey through state association listservs may similarly misrepresent the whole of clinicians who work with IWA as these state association e-mails are more likely to be read by SLPs with an active interest in current affairs in the field. It is recognized that this is a limitation that may affect survey results; however, for this purpose of the current study, this is important preliminary information to begin bridging that gap between research and practice and begin identifying clinician perspectives regarding important technical research for aphasia recovery.
Second, the reported years of experience reflect clinicians' time working in the field as an SLP and are not specific to the amount of time SLPs spent in practice with a caseload that includes IWA. This is an important consideration for future work, as experience with the clinical management of aphasia may influence SLPs' perceptions of realistic treatment outcomes, which may not necessarily be related to other clinical experiences (i.e., time spent working with other patient populations). Future studies should include more specific questions targeting the nature of SLPs' work with IWA, as these factors may influence SLP opinions pertaining to the feasibility of adopting tDCS.
Third, future studies should consider how factors such as organizational structure, reimbursement factors, and level of care may influence SLPs' opinions regarding tDCS. As we discussed above, we grouped SLPs by work setting to largely reflect the types of patients seen at each level of care. Although we did not find differences across settings for desired tDCS enhancement, future work could consider other measures designed to quantify elements of the PARiHS framework (Kitson et al., 1998, 2008; Rycroft-Malone, 2004), with considerations of leadership and cultural aspects within an organization.
Finally, another limitation of this study is that we have considered SLP reports of tDCS enhancement in the context of a single outcome measure, namely, the WAB-R. Although recently recommended as a measure of language in clinical trials (Wallace et al., 2019) and used by the majority (92.9%) of queried SLPs, it may be the case that this singular outcome measure does not capture the use of other instruments used after the initial diagnosis, which may be used to measure improvement. Furthermore, we acknowledge that this test better represents communication than confrontation naming tests, but it does not necessarily capture functional communicative abilities as a whole.
Future Directions
As a whole, this investigation offers important preliminary evidence related to SLPs' perspectives on tDCS. This corresponds to one of the three aspects of translation of evidence to practice by the PARiHS framework: engagement of clinicians to understand clinical perceptions of evidence. The current results represent a very broad understanding of this topic but are a reasonable starting point that can be expanded upon in future studies. For example, future work may consider the addition of a clinical or patient advisory group for project planning. Specifically, an in-depth exploration of clinical perspectives as related to clinical settings will be important, moving forward. In other implementation models, examining clinical settings based on reimbursement, productivity policies, or organizational structures may better inform how tDCS can be incorporated into a health care system.
Conclusions
This study is the first to identify clinician familiarity with tDCS and to quantify a desired tDCS-related enhancement that SLPs deem necessary for clinical adoption for poststroke aphasia in the rehabilitation setting. Our group and others have reported improved naming associated with A-tDCS coupled with behavioral aphasia therapy (Baker et al., 2010; Fiori et al., 2011; Fridriksson, Richardson, et al., 2012; Kang et al., 2011; Meinzer et al., 2016; Wu et al., 2015). For example, our recent Phase II trial showed that such effects in expressive naming lasted up to 6 months post-therapy (Fridriksson et al., 2019). While these results reveal that further investigation into the implementation of tDCS is necessary, there is a paucity of evidence related to translational research, including the potential for clinical implementation of tDCS. Responses from the current survey emphasize the need for improved dissemination and access to new and relevant research in this area. Furthermore, there are probably several stigmatizing beliefs associated with brain stimulation (Cabrera & Reiner, 2015), which may surface as doubts, indecision, or declination regarding tDCS as part of the rehabilitation model. On the basis of the current study, SLPs report that, on average, a 22.9% tDCS enhancement of a behavioral language outcome would encourage them to consider incorporating tDCS as part of aphasia therapy. Given the results of the Phase II clinical trial from our group, it is straightforward to propose that a positive Phase III trial has the potential to encourage clinical adoption of A-tDCS as a standard of care for aphasia rehabilitation. This modality of brain stimulation has the potential to lead to considerable improvement in overall therapy outcomes and improved quality of life for stroke survivors living with aphasia. SLPs' perspectives regarding tDCS as part of the aphasia therapy regimen may inform future clinical trials and, as importantly, should be considered an integral factor for estimating potential for clinical translation.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grants U01 DC011739 (PI: Julius Fridriksson) and NIH T32 DC014435 (coauthor: Alexandra Basilakos, trainee). The authors gratefully acknowledge participants who completed the survey.
Appendix
Survey
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grants U01 DC011739 (PI: Julius Fridriksson) and NIH T32 DC014435 (coauthor: Alexandra Basilakos, trainee).
References
- Baker, J. M. , Rorden, C. , & Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke, 41, 1229–1236. https://doi.org/10.1161/STROKEAHA.109.576785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas, E. A. , & Boren, S. (2000). Managing clinical knowledge for health care improvement. Yearbook of Medical Information, 9(1), 65–70. https://doi.org/10.1055/s-0038-1637943 [PubMed] [Google Scholar]
- Barnes, S. , & Nickels, L. (2017). Interaction-focussed therapy for aphasia: Effects on communication and quality of life. International Journal of Speech-Language Pathology, 20(5), 528–540. https://doi.org/10.1080/17549507.2017.1329851 [DOI] [PubMed] [Google Scholar]
- Basso, A. , & Macis, M. (2011). Therapy efficacy in chronic aphasia. Behavioural Neurology, 24(4), 317–325. https://doi.org/10.3233/BEN-2011-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini, N. , Pascual-Leone, A. , & Fregni, F. (2009). Using non-invasive brain stimulation to augment motor training-induced plasticity. Journal of NeuroEngineering and Rehabilitation, 6(8), 1–13. https://doi.org/10.1186/1743-0003-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, M. C. , Kelly, H. , Godwin, J. , Enderby, P. , & Campbell, P. (2016). Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews, (6) Aricle CD000425. https://doi.org/10.1002/14651858.CD000425.pub4.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein, C. , Grewe, T. , Flöel, A. , Ziegler, W. , Springer, L. , Martus, P. , Huber, W. , Willmes, K. , Ringelstein, E. B. , Haeusler, K. G. , Abel, S. , Glindemann, R. , Domahs, F. , Regenbrecht, F. , Schlenck, K.-J. , Thomas, M. , Obrig, H. , de Langen, E. , Rocker, R. , … Baumgaertner, A. (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. The Lancet, 389(10078), 1528–1538. https://doi.org/10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- Cabrera, L. Y. , & Reiner, P. B. (2015). Understanding public (mis)understanding of tDCS for enhancement. Frontiers in Integrative Neuroscience, 9(30), 1–5. https://doi.org/10.3389/fnint.2015.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar, V. , Paolazzi, C. L. , & Miceli, G. (2015). tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex, 63, 296–316. https://doi.org/10.1016/j.cortex.2014.08.015 [DOI] [PubMed] [Google Scholar]
- Demaerschalk, B. M. , Hwang, H. M. , & Leung, G. (2010). US cost burden of ischemic stroke: A systematic literature review. The American Journal of Managed Care, 16(17), 525–533. [PubMed] [Google Scholar]
- Demeurisse, G. , Demol, O. , de Beuckelaer, R. , Coekaerts, M. J. , & Capon, A. (1980). Quantitative study of the rate of recovery from aphasia due to ischemic stroke. Stroke, 11(5), 455–458. https://doi.org/10.1161/01.STR.11.5.455 [DOI] [PubMed] [Google Scholar]
- Dickey, L. , Kagan, A. , Lindsay, M. P. , Fang, J. , Rowland, A. , & Black, S. (2010). Incidence and profile of inpatient stroke-induced aphasia in Ontario, Canada. Archives of Physical Medicine and Rehabilitation, 91(2), 196–202. https://doi.org/10.1016/j.apmr.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Engelter, S. T. , Gostynski, M. , Papa, S. , Frei, M. , Born, C. , Ajdacic-Gross, V. , Gutzwiller, F. , & Lyrer, P. A. (2006). Epidemiology of aphasia attributable to first ischemic stroke. Stroke, 37(6), 1379–1384. https://doi.org/10.1161/01.STR.0000221815.64093.8c [DOI] [PubMed] [Google Scholar]
- Fiori, V. , Coccia, M. , Marinelli, C. V. , Vecchi, V. , Bonifazi, S. , Ceravolo, M. G. , Provinciali, L. , Tomaiuolo, F. , & Marangolo, P. (2011). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience, 23(9), 2309–2323. https://doi.org/10.1162/jocn.2010.21579 [DOI] [PubMed] [Google Scholar]
- Fisher, A. , Martin, J. , Srikusalanukul, W. , & Davis, M. (2014). Trends in stroke survival incidence rates in older Australians in the new millennium and forecasts into the future. Journal of Stroke & Cerebrovascular Diseases, 23(4), 759–770. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.06.035 [DOI] [PubMed] [Google Scholar]
- Fridriksson, J. , Basilakos, A. , Stark, B. C. , Rorden, C. , Elm, J. , Gottfried, M. , George, M. S. , Sen, S. , & Bonilha, L. (2019). Transcranial direct current stimulation to treat aphasia: Longitudinal analysis of a randomized controlled trial. Brain Stimulation, 12(1), 190–191. https://doi.org/10.1016/j.brs.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Hubbard, H. I. , Hudspeth, S. G. , Holland, A. L. , Bonilha, L. , Fromm, D. , & Rorden, C. (2012). Speech entrainment enables patients with Broca's aphasia to produce fluent speech. Brain: A Journal of Neurology, 135(Pt. 12), 3815–3829. https://doi.org/10.1093/brain/aws301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Richardson, J. D. , Baker, J. M. , & Rorden, C. (2011). Transcranial direct current stimulation improves naming reaction time in fluent aphasia. Stroke, 42(3), 819–821. https://doi.org/10.1161/STROKEAHA.110.600288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Richardson, J. D. , Fillmore, P. , & Cai, B. (2012). Left hemisphere plasticity and aphasia recovery. NeuroImage, 60(2), 854–863. https://doi.org/10.1016/j.neuroimage.2011.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Rorden, C. , Elm, J. , Sen, S. , George, M. S. , & Bonilha, L. (2018). Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: A randomized clinical trial. JAMA Neurology, 75(12), 1470–1476. https://doi.org/10.1001/jamaneurol.2018.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, L. W. , Ottoson, J. M. , García, C. , & Hiatt, R. A. (2009). Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annual Review of Public Health, 30(1), 151–174. https://doi.org/10.1146/annurev.publhealth.031308.100049 [DOI] [PubMed] [Google Scholar]
- Harold, M. (2019). The research translation problem: A modest proposal. The ASHA Leader, 24(7), 52–61. https://doi.org/10.1044/leader.FTR2.24072019.52 [Google Scholar]
- Harris, P. A. , Taylor, R. , Thielke, R. , Payne, J. , Gonzalez, N. , & Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Information, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, A. , Fromm, D. , Forbes, M. , & MacWhinney, B. (2017). Long-term recovery in stroke accompanied by aphasia: A reconsideration. Aphasiology, 31(2), 152–165. https://doi.org/10.1080/02687038.2016.1184221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope, T. M. H. , Leff, A. P. , Prejawa, S. , Bruce, R. , Haigh, Z. , Lim, L. , Ramsden, S. , Oberhuber, M. , Ludersdorfer, P. , Crinion, J. , Seghier, M. L. , & Price, C. J. (2017). Right hemisphere structural adaptation and changing language skills years after left hemisphere stroke. Brain: A Journal of Neurology, 140(6), 1718–1728. https://doi.org/10.1093/brain/awx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, E. K. , Kim, Y. K. , Sohn, H. M. , Cohen, L. G. , & Paik, N.-J. (2011). Improved picture naming in aphaisa patients treated with cathodal tDCS to inhibit the right Broca's homologue area. Restorative Neurology and Neuroscience, 29(3), 141–152. https://doi.org/10.3233/RNN-2011-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz, A. (2007). Western Aphasia Battery–Revised. The Psychological Corporation. [Google Scholar]
- Kiran, S. , Cherney, L. R. , Kagan, A. , Haley, K. L. , Antonucci, S. M. , Schwartz, M. , Holland, A. L. , & Simmons-Mackie, N. (2018). Aphasia assessments: A survey of clinical and research settings. Aphasiology, 32(Suppl. 1), 47–49. https://doi.org/10.1080/02687038.2018.1487923 [Google Scholar]
- Kitson, A. L. , Harvey, G. , & McCormack, B. (1998). Enabling the implementation of evidence based practice: A conceptual framework. BMJ Quality & Safety, 7(3), 149–158. https://doi.org/10.1136/qshc.7.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson, A. L. , Rycroft-Malone, J. , Harvey, G. , McCormack, B. , Seers, K. , & Titchen, A. (2008). Evaluating the successful implementation of evidence into practice using the PARiHS framework: Theoretical and practical challenges. Implementation Science, 3(1), 1–12. https://doi.org/10.1186/1748-5908-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska, A. C. , Hellblom, A. , Murray, V. , Kahan, T. , & Von Arbin, M. (2001). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249(5), 413–422. https://doi.org/10.1046/j.1365-2796.2001.00812.x [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Fiori, V. , Calpagnano, M. A. , Campana, S. , Razzano, C. , Caltagirone, C. , & Marini, A. (2013). tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience, 7, 1–10. https://doi.org/10.3389/fnhum.2013.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo, P. , Fiori, V. , Campana, S. , Antonietta, M. , Razzano, C. , Caltagirone, C. , & Marini, A. (2014). Neuropsychologia something to talk about: Enhancement of linguistic cohesion through tdCS in chronic non fluent aphasia. Neuropsychologia, 53, 246–256. https://doi.org/10.1016/j.neuropsychologia.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Fiori, V. , Cipollari, S. , Campana, S. , Razzano, C. , Di Paola, M. , Koch, G. , & Caltagirone, C. (2013). Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. European Journal of Neuroscience, 38(9), 3370–3377. https://doi.org/10.1111/ejn.12332 [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Fiori, V. , Gelfo, F. , Shofany, J. , & Razzano, C. (2014). Bihemispheric tDCS enhances language recovery but does not alter BDNF levels in chronic aphasic patients. Restorative Neurology and Neuroscience, 32(2), 367–379. https://doi.org/10.3233/RNN-130323 [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Marinelli, C. V. , Bonifazi, S. , Fiori, V. , Ceravolo, M. G. , Provinciali, L. , & Tomaiuolo, F. (2011). Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behavioural Brain Research, 225(2), 498–504. https://doi.org/10.1016/j.bbr.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Meinzer, M. , Darkow, R. , Lindenberg, R. , & Flöel, A. (2016). Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain: A Journal of Neurology, 139(4), 1152–1163. https://doi.org/10.1093/brain/aww002 [DOI] [PubMed] [Google Scholar]
- Moss, A. , & Nicholas, M. (2006). Language rehabilitation in chronic aphasia and time postonset: A review of single-subject data. Stroke, 37(12), 3043–3051. https://doi.org/10.1161/01.STR.0000249427.74970.15 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Cohen, L. G. , Wassermann, E. M. , Priori, A. , Lang, N. , Antal, A. , Paulus, W. , Hummel, F. , Boggio, P. S. , Fregni, F. , & Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3), 206–233. https://doi.org/10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–639. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwens, F. , Visch-Brink, E. G. , Van de Sandt-Koenderman, M. M. , Dippel, D. W. , Koudstaal, P. J. , & de Lau, L. M. (2015). Optimal timing of speech and language therapy for aphasia after stroke: More evidence needed. Expert Review of Neurotherapeutics, 15(8), 885–893. https://doi.org/10.1586/14737175.2015.1058161 [DOI] [PubMed] [Google Scholar]
- Paulus, W. , & Nitsche, M. A. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901. https://doi.org/10.1212/WNL.57.10.1899 [DOI] [PubMed] [Google Scholar]
- Pederson, P. , Vinter, K. , & Olsen, T. S. (2004). Aphasia after stroke: Type, severity and prognosis. Cerebrovascular Diseases, 17, 35–43. https://doi.org/10.1159/000073896 [DOI] [PubMed] [Google Scholar]
- Pulvermüller, F. , & Berthier, M. L. (2008). Aphasia therapy on a neuroscience basis. Aphasiology, 22(6), 563–599. https://doi.org/10.1080/02687030701612213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller, F. , Neininger, B. , Elbert, T. , Mohr, B. , Rockstroh, B. , Koebbel, P. , & Taub, E. (2001). Aphasia after stroke. Stroke, 32(7), 2–7. https://doi.org/10.1161/01.STR.32.7.1621 [DOI] [PubMed] [Google Scholar]
- Riley, E. A. (2017). Patient fatigue during aphasia treatment: A survey of speech-language pathologists. Communication Disorders Quarterly, 38(3), 143–153. https://doi.org/10.1177/1525740116656330 [Google Scholar]
- Rose, T. A. , Balse, A. , Osmond, S. , Poon, A. , Simons, N. , & Wallace, S. J. (2018). Aphasia education: Speech-language pathologists' perspectives regarding current and optimal practice. Aphasiology, 32(8), 967–988. https://doi.org/10.1080/02687038.2018.1472366 [Google Scholar]
- Rycroft-Malone, J. (2004). The PARIHS framework—A framework for guiding the implementation of evidence-based practice. Journal of Nursing Care Quality, 19(4), 297–304. https://doi.org/10.1097/00001786-200410000-00002 [DOI] [PubMed] [Google Scholar]
- Schmittdiel, J. A. , Grumbach, K. , & Selby, J. V. (2010). System-based participatory research in health care: An approach for sustainable translational research and quality improvement. Annals of Family Medicine, 8(3), 256–259. https://doi.org/10.1370/afm.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons-Mackie, N. (2018). Aphasia in North America. Aphasia Access. [Google Scholar]
- Smania, N. , Gandolfi, M. , Aglioti, S. M. , Girardi, P. , Fiaschi, A. , & Girardi, F. (2010). How long is the recovery of global aphasia? Twenty-five years of follow-up in a patient with left hemisphere stroke. Neurorehabilitation and Neural Repair, 24(9), 871–875. https://doi.org/10.1177/1545968310368962 [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. (2015). Brain stimulation and the role of the right hemisphere in aphasia recovery. Current Neurology and Neuroscience Reports, 15(11), Article 72. https://doi.org/10.1007/s11910-015-0593-6 [DOI] [PubMed] [Google Scholar]
- Wallace, S. , Worrall, L. , Rose, T. , LeDorze, G. , Breitenstein, C. , Hilari, K. , Babbitt, E. , Bose, A. , Brady, M. , Cherney, L. R. , Copland, D. , Cruice, M. , Enderby, P. , Hersh, D. , Howe, T. , Kelly, H. , Kiran, S. , Laska, A.-C. , Marshall, J. , … Webster, J. (2019). A core outcome set for aphasia treatment research: The ROMA consensus statement. International Journal of Stroke, 14(2), 180–185. https://doi.org/10.1177/1747493018806200 [DOI] [PubMed] [Google Scholar]
- Weiss, S. , & Bikson, M. (2014). Open questions on the mechanisms of neuromodulation with applied and endogenous electric fields. Frontiers in Human Neuroscience, 8, 227 https://doi.org/10.3389/fnhum.2014.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall, J. M. , Fagnan, L. J. , Handley, M. , Salsberg, J. , McGinnis, P. , Zittleman, L. K. , & Macaulay, A. C. (2009). Practice-based research is community engagement. The Journal of the American Board of Family Medicine, 22(4), 423–427. https://doi.org/10.3122/jabfm.2009.04.090105 [DOI] [PubMed] [Google Scholar]
- Wu, D. , Wang, J. , & Yuan, Y. (2015). Effects of transcranial direct current stimulation on naming and cortical excitability in stroke patients with aphasia. Neuroscience Letters, 589, 115–120. https://doi.org/10.1016/j.neulet.2015.01.045 [DOI] [PubMed] [Google Scholar]