Abstract
Purpose
Functional brain imaging is playing an increasingly important role in the diagnosis and treatment of communication disorders, yet many populations and settings are incompatible with functional magnetic resonance imaging and other commonly used techniques. We conducted a systematic review of neuroimaging studies using functional near-infrared spectroscopy (fNIRS) with individuals with speech or language impairment across the life span. We aimed to answer the following question: To what extent has fNIRS been used to investigate the neural correlates of speech-language impairment?
Method
This systematic review was preregistered with PROSPERO, the international prospective register of systematic reviews (CRD42019136464). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol for preferred reporting items for systematic reviews. The database searches were conducted between February and March of 2019 with the following search terms: (a) fNIRS or functional near-infrared spectroscopy or NIRS or near-infrared spectroscopy, (b) speech or language, and (c) disorder or impairment or delay.
Results
We found 34 fNIRS studies that involved individuals with speech or language impairment across nine categories: (a) autism spectrum disorders; (b) developmental speech and language disorders; (c) cochlear implantation and deafness; (d) dementia, dementia of the Alzheimer's type, and mild cognitive impairment; (e) locked-in syndrome; (f) neurologic speech disorders/dysarthria; (g) stroke/aphasia; (h) stuttering; and (i) traumatic brain injury.
Conclusions
Though it is not without inherent challenges, fNIRS may have advantages over other neuroimaging techniques in the areas of speech and language impairment. fNIRS has clinical applications that may lead to improved early and differential diagnosis, increase our understanding of response to treatment, improve neuroprosthetic functioning, and advance neurofeedback.
The past few decades have seen an impressive increase in research on the neural mechanisms that underlie speech and language impairment. In his book (Ingham, 2008) on neuroimaging studies of communication disorders, Roger Ingham reported a fivefold increase in neuroimaging studies between 1994 and 2004. He then explained that “[t]he explosive growth in neuroimaging since the early 1990s is truly astounding.” In a review for The ASHA Leader on the ways in which neuroimaging has contributed to our understanding of the link between the brain and language, Beeson (2010) observes that “approaches to the study of the neural substrates of language have advanced dramatically during the last three decades” and “[o]ur discipline contributes substantially to the theoretical motivation.”
A better understanding of the neural underpinnings of speech and language impairment is fundamental to advancing diagnosis and treatment at all points of the life span. Neuroimaging may improve the early identification of brain-based risk factors, which may lead to an earlier diagnosis of a range of developmental and neurogenic communication disorders, from mild cognitive impairment (MCI) in aging adults to autism spectrum disorder (ASD) in infants. Similarly, neuroimaging may provide biomarkers that can improve the differential diagnosis of communication disorders, such as for the aphasias (Kiran & Thompson, 2019) and dementias (Oh & LaPointe, 2017). Also, neuroimaging is a fundamental next step in identifying the source of profound language deficits, for example, in people with ASD (Tager-Flusberg, 2016; Tager-Flusberg & Kasari, 2013). In the treatment of communication disorders, a better understanding of the neural correlates of response to treatment and recovery has the potential to advance the field. Perhaps one of the most salient examples of the transformative role of neuroimaging in treatment is the principles of neuroplasticity in aphasia treatment (Kiran & Thompson, 2019; Kleim & Jones, 2008).
Not all neuroimaging techniques, however, are highly compatible with people with speech and language disorders. Some neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), require a participant to lie supine and motionless in an enclosed, noisy space. This method proves difficult with young children and people with sensory sensitivities such as are common in people with ASD, unless under sedation. In populations that may be compatible, imaging the brain while an individual is supine or sedated does not allow for highly ecologically valid investigations. Most of the time and in clinical practice, individuals are upright and interacting with their environment.
Functional near-infrared spectroscopy (fNIRS) is an emerging neuroimaging technique that may offer some key advantages for research and clinical practice with individuals with speech and language impairment due to its use in more ecologically valid situations and with infants and children (among other reasons discussed in depth below). In 2014, Vanderwert and Nelson observed that the use of fNIRS as a neuroimaging technique has grown exponentially over the past decade. In fact, the number of published fNIRS articles has doubled every 3.5 years for 20 years with over 200 publications using fNIRS for brain imaging in 2012 (Boas et al., 2014). In addition, several researcher groups have highlighted the clinical applications of fNIRS (e.g., Arenth et al., 2007; Irani et al., 2007; Kober et al., 2014; Ludlow, 2012; Obrig, 2014). The goal of this systematic review is to identify to what extent fNIRS has been used with people with speech and language disorders. We also aim to discuss the potential applications of fNIRS to the assessment and treatment of speech and language impairment. First, we briefly review current major neuroimaging techniques and provide a background on fNIRS methodology.
Current Brain Imaging Techniques
Current brain imaging techniques include two broad approaches: (a) electrophysiological indicators of cognitive activity and (b) localized changes in cerebral blood flow as indicators of neuronal activity. Instruments for measuring electrophysiological indicators of cognitive activity include magnetoencephalography (MEG) and electroencephalography (EEG). MEG measures rapid changes in magnetic fields in the brain. EEG records electrical signals from the scalp using high-density arrays (head caps with numerous electrodes). Both MEG and EEG are noninvasive and offer the best temporal resolution of all current brain imaging techniques, so they can detect electrophysiological changes in response to rapid stimuli (Baars & Gage, 2013).
Indicators of neuronal activity that measure localized changes in cerebral blood flow include positron emission tomography (PET), fMRI, and fNIRS. PET is an invasive technique since it involves the injection of a radioactive agent into the bloodstream. As the radioactive substance reaches the brain, brain cells consume more of it with greater activity, which can be detected and reconstructed as a three-dimensional (3D) image. fMRI detects neural activity based on changes in blood oxygenation by using magnetic fields to manipulate the nuclei of hydrogen atoms, which are slightly magnetized. These signals can then be transformed into images. fMRI provides a high-resolution image with good contrast between different tissues. Since it was discovered that fMRI can be sensitive to brain activity and not just anatomy, it became the most common functional imaging technique in language research (see Chouinard et al., 2016, for a tutorial on fMRI in clinical populations). Though fMRI has excellent spatial resolution, it has a number of inherent features that are not highly compatible with individuals with speech-language impairment—it is noisy and not tolerant of motion. Listening in a noisy environment and the inherent motion involved in producing speech present confounds for fMRI studies.
fNIRS Background
fNIRS is a noninvasive method of imaging the brain by using near-infrared spectrum light that was first described by Jöbsis (1977). Neuroimaging research with fNIRS has increased exponentially over the past two decades because it is low cost, safe and noninvasive, portable, and more tolerant of motion than other brain imaging methods (Hoshi, 2003; Izzetoglu et al., 2004; Obrig & Villringer, 2003; Strangeman et al., 2002; Villringer & Chance, 1997; Zabel & Chute, 2002). Because fNIRS is quiet, safe, noninvasive, and more tolerant of motion, its use as a neuroimaging tool has increased exponentially over the past few decades (Boas et al., 2014), particularly in studies with infants/children (Wilcox & Biondi, 2015) and atypical/clinical populations (Obrig, 2014; Vanderwert & Nelson, 2014). These features of fNIRS make it a useful technique for studying the brain basis of speech and language impairment, which we discuss in more depth below.
Detecting Localized Changes in Cerebral Blood Flow With Near-Infrared Light
Near-infrared light in the range of 650–900 nm is readily absorbed by oxygenated hemoglobin (O2Hb; also abbreviated as oxy-Hb or HbO) and deoxygenated hemoglobin (HHb; also abbreviated as deoxy-Hb or HbR) in the human cortex. At the same time, it is relatively transparent to human tissues. Like fMRI, fNIRS relies on the physiological principle of neurovascular coupling, which describes the relationship between neuronal activity and localized changes in cerebral blood flow (known as the hemodynamic response or the blood oxygenation level–dependent response; Friston et al., 1995; Raichle & Mintun, 2006). This means that an increase in O2Hb is taken as an indicator of neuronal activity (just as in fMRI). More specifically, in fNIRS, a canonical hemodynamic response involves an increase in O2Hb accompanied by a decrease in HHb (see more below). For fNIRS-fMRI cross-validation studies, the reader can see Chance et al. (1998), Cui et al. (2011), Kleinschmidt et al. (1996), and Strangeman et al. (2002). Continuous wave fNIRS systems were the most commonly used in this review, but other approaches exist (frequency-resolved and time-resolved; see Scholkmann et al., 2014, for an overview).
Data and Analyses
In an fNIRS system, light is emitted by laser or LED diodes (sources), detected by photodiodes (detectors), and transmitted through fiberoptic bundles to the instrument (see Figure 1). The sources and detectors are typically placed on a headband or cap, which is secured to the head of a participant (see Figure 2). These sources and detectors are normally placed 3 cm apart, a distance at which tissue oxygenation can be probed to a depth of about 1–2 cm (see Figure 3). In addition, detectors placed at a short distance from a source (around 1 cm) can be used to detect and control for more superficial scalp hemodynamics. A source–detector pair makes up a channel, and researchers most often report the number of channels used. Many fNIRS researchers use either data processing tools developed within their labs or a toolbox designed for use with MATLAB (MathWorks) called HomER (Huppert et al., 2009), the most recent installation being HomER3 (https://github.com/BUNPC/Homer3). Basic processing of fNIRS data involves three steps: (a) converting intensity to optical density and then to hemoglobin concentration by the modified Beer–Lambert law (Ferrari & Quaresima, 2012), (b) filtering, and (c) block-averaging and more advanced processing (Selb, 2018). Advanced processing then involves (a) short separation regression, (b) general linear regression, and (c) anatomical rendering (Cooper, 2018; Yücel, 2018).
Figure 1.

Continuous wave functional near-infrared spectroscopy system.
Figure 2.
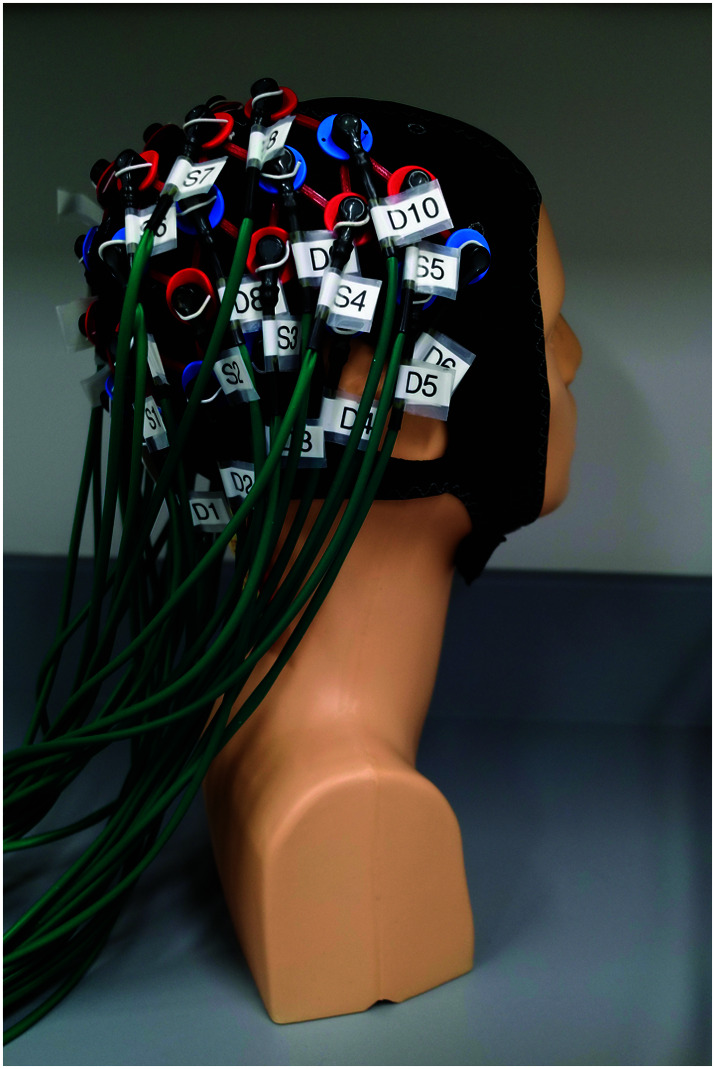
Functional near-infrared spectroscopy cap with sources (red) and detectors (blue).
Figure 3.
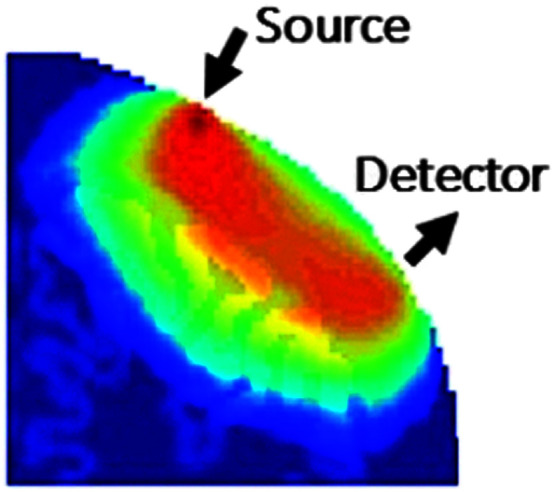
Spatial sensitivity of a source–detector pair (image reproduced with permission from the Boston University Neurophotonics Center).
In the advanced processing stage, short separation regression (Gagnon et al., 2011) can be used to detect and control for the signal from the scalp and skull (see also the double short separation technique of Gagnon et al., 2014). Other physiological variables (e.g., blood pressure, respiration) are then controlled for to reduce noise in the signal. After preprocessing, the hemodynamic response function can be predefined using the general linear model (GLM). Then, β values resulting from the GLM are taken to reflect how heavily weighted that response is in each channel (Cooper, 2018; see Figure 4). An anatomical representation of the hemodynamic response in the brain can be generated using AtlasViewer (Aasted et al., 2015; see Figure 5), a function in the HomER3 toolbox (Yücel, 2018). Though these methods are perhaps the most common, they are not the only approaches. Some fNIRS studies reviewed here used functional data analysis or other approaches. We discuss this more below.
Figure 4.
Oxygenated (red) and deoxygenated (blue) and total (green) hemoglobin concentration changes by channel.
Figure 5.
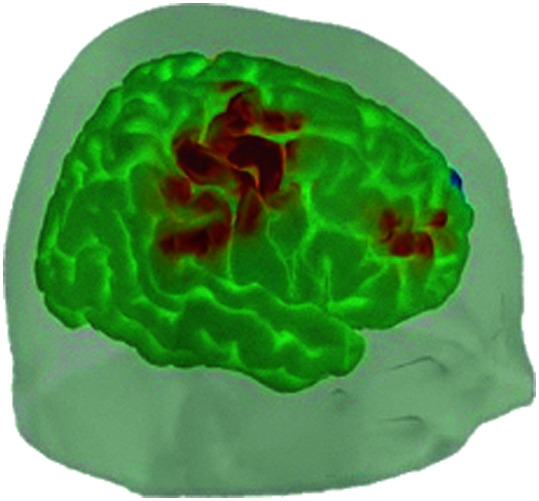
Anatomical rendering of the hemodynamic response (image reproduced with permission from the Boston University Neurophotonics Center).
Some Challenges of fNIRS
Though fNIRS offers many advantages in the study of the brain bases of speech and language impairment, it is not without inherent challenges. Three primary challenges pertain to the use of fNIRS with people with speech and language disorders and more broadly: (a) methods and analyses, (b) motion artifacts, and (c) localization and interpretation.
Methods and Analyses
Since fNIRS is an emerging neuroimaging technique, it is to be expected that there is variation in the methods and analyses as evidence of the best approaches is being established. Most studies reviewed here used a GLM-based analysis, but other studies used functional data analysis to avoid violating the assumptions of the GLM when the data were not normally distributed. Some of the early exploratory fNIRS studies that we reviewed relied on a more descriptive than analytical approach to fNIRS data. A number of researchers are working toward standardizing methods and streamlining the analysis of fNIRS data. In fact, the journal Neurophotonics published by the Society for functional Near-Infrared Spectroscopy was established in 2014 as a venue for researchers to publish peer-reviewed research on “advances in optical technology applicable to study of the brain and their impact on the basic and clinical neuroscience applications” (https://www.spiedigitallibrary.org/journals/neurophotonics). Current techniques are quickly improving with the increase in fNIRS research across disciplines (e.g., see Tak & Ye, 2014, for a discussion of the history and current trends in the statistical analysis of fNIRS data).
Motion Artifacts
Motion from the participant while fNIRS measurements are being taken represents a potential source of noise in the signal (Huppert, 2016). This is of particular concern when an fNIRS participant is doing a language production task since speaking necessarily involves movement in the head, jaw, and neck. Techniques for reducing the effects of motion-induced noise in the fNIRS data involve discarding trials with excessive movement and using mathematical correction techniques (see, e.g., Brigadoi et al., 2014; Chiarelli et al., 2015; Cooper et al., 2012; Scholkmann et al., 2010; Tsuzuki et al., 2012; Yücel et al., 2014). While new techniques are being developed and evaluated to improve the removal of motion artifacts and facilitate the preprocessing of fNIRS data (e.g., Delgado Reyes et al., 2018; Jahani et al., 2018), motion remains a potential source of noise in the signal. Motion artifacts may be a special concern for individuals with speech or language disorders who may have comorbid motor, sensory, or behavioral challenges.
Localization and Interpretation
While fNIRS has better spatial resolution than EEG and better temporal resolution than fMRI, its spatial resolution is not as sensitive as fMRI. The hemodynamic response obtained from fNIRS is restricted to the surface cortical areas of the brain (about 1–2 cm below the surface of the skull; Ferrari & Quaresima, 2012). This means that fNIRS is not well suited to investigate questions relating to white matter areas of the brain. Also, the fNIRS measurements are taken without direct anatomical information about the brain. Techniques exist to localize the position of the probes on the scalp to internal structures of the brain, such as using the international 10–20 system (as used in EEG research), anatomical–functional coregistration (Wilcox & Biondi, 2015), and 3D digitization of optode locations. Nevertheless, careful controls are required to accurately localize the brain areas that contribute to the observed signal. Also, the hemodynamic response may be modulated to some degree by experimental design, age, and cortical region (Issard & Gervain, 2018). As an example, to improve localization and interpretation of the fNIRS signal, Saliba et al. (2016) reported that their lab had recently transitioned from a four-channel system to a 140-channel system. Increasing the density of the channels, among other things, as in whole-head recording, will result in finer sampling of the cortex (Minagawa-Kawai et al., 2008). However, as the distance between the source and detector decreases (to accommodate more channels), the penetration depth also decreases (Patil et al., 2011).
Some Advantages of fNIRS
Despite inherent challenges in the use of fNIRS for the study of the brain basis of speech and language impairment, it may offer distinct advantages. Two primary advantages of fNIRS for imaging studies with individuals with speech or language impairment are (a) increasing ecological validity and (b) imaging with populations incompatible with other techniques.
Increasing Ecological Validity
fNIRS offers a distinct advantage over brain imaging techniques that require the participant to be supine and nearly immobile in that it can be used in naturalistic settings, increasing the ecological validity of brain imaging studies. fNIRS can be used to study speech and language production processes, which inherently involve motion of the face and neck. fNIRS can be used as a patient interacts with a clinician at a table or in a clinical treatment room. This means that fNIRS could offer a way to image functional brain changes as a result of or even during treatment. In a similar vein, wearable fNIRS systems have been used for long-term monitoring of neural responses in acute patients with stroke and epilepsy (Kassab et al., 2018) and could be adapted to investigate neural processes in naturalistic and clinical settings.
Imaging With Populations Incompatible With Other Techniques
fNIRS may offer major advantages in the field of communication disorders in that we can use it with populations who are not compatible with fMRI and other neuroimaging techniques. As discussed previously, people who use cochlear implants (CIs) are incompatible with fMRI due to the electrical and magnetic components in the implant (Bandettini, 2012; Bisconti et al., 2016; Quaresima et al., 2012; Sevy et al., 2010). The electrical signals inherent in CI functioning create data artifacts with EEG (Gilley et al., 2006; Timm et al., 2014). PET is possible, but it involves ionizing radiation that is potentially harmful to human health (Talavage et al., 2014). For brain imaging studies with people who use CIs, fNIRS offers major advantages.
For infants, young children, and people with sensory sensitivities, fNIRS may offer advantages as well. While fMRI involves noise, fNIRS is virtually silent so fNIRS offers advantages for brain imaging studies in these populations (Aslin et al., 2015; Gervain, 2014; Gervain et al., 2011; Kovelman et al., 2012; Lloyd-Fox et al., 2010; Wilcox & Biondi, 2015) and people with ASD (Liu et al., 2019; Mazzoni et al., 2018; Zhang & Roeyers, 2019). These populations may be sensitive not only to the noise inherent in fMRI studies but also to the enclosed space of the magnet and large, unfamiliar equipment setup.
Goals of This Study
The goal of this study was to conduct a systematic review of empirical fNIRS studies of speech and language disorders. We aimed to answer the following questions:
To what extent has fNIRS been used to investigate the neural correlates of speech-language impairment?
a. What is the range of populations with whom fNIRS has been used (age, etiology)?
b. What are the tasks and brain regions of interest (ROIs) that have been investigated using fNIRS?
Next, we outline the methods used in this systematic review followed by a discussion of the results.
Methods
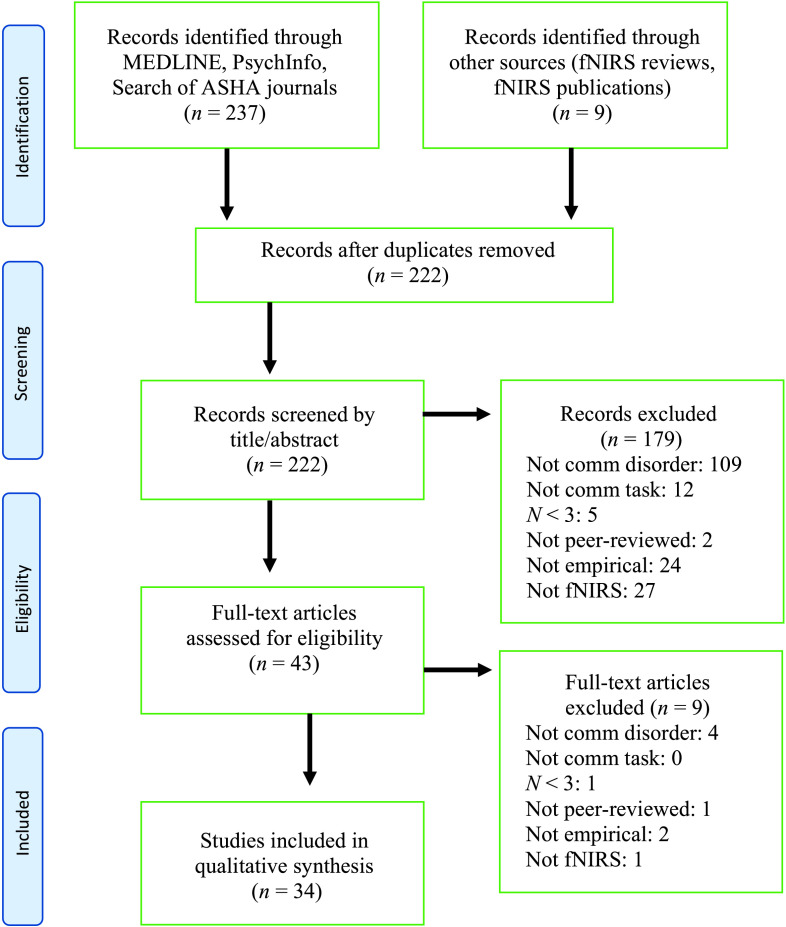
We conducted a systematic search for empirical studies that used fNIRS to investigate the neural basis of speech and language disorders. This systematic review protocol has been preregistered with PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=136464), the international prospective register of systematic reviews (CRD42019136464). We followed the PRISMA protocol for preferred reporting items for systematic reviews (Moher et al., 2009). The search procedures are described below (and see the flow diagram in Figure 6). Our goal was to include as many fNIRS studies as possible since fNIRS is a relatively new brain imaging technology. At the same time, we aimed to keep the results as relevant as possible to researchers and clinicians who work with individuals with speech and language disorders.
Figure 6.
PRISMA Moher et al., (2009) flow diagram of search methods. fNIRS = functional near-infrared spectroscopy.
Search Procedures
The database searches were conducted between February and March of 2019. The following databases were searched: MEDLINE via the PubMed interface, PsycINFO, and Google Scholar. The following search terms were used: (a) fNIRS or functional near-infrared spectroscopy or NIRS or near-infrared spectroscopy, (b) speech or language, and (c) disorder or impairment or delay. In addition, the following individual e-journals were searched with the search term “fNIRS or functional near-infrared spectroscopy or NIRS or near-infrared spectroscopy”: Journal of Speech-Language-Hearing Research; American Journal of Speech-Language Pathology; Language, Speech, and Hearing Services in Schools; and Journal of Communication Disorders. We also searched the works cited within these results for additional studies that met the criteria.
The first author compiled a list of the search results and removed duplicates. The first author then screened those results by title and abstract for those that met the inclusion/exclusion criteria (outlined below). Then, the first and second authors further assessed the abstracts and full-text articles for eligibility. Any disagreements or questions about eligibility were discussed openly during lab meetings with the second author's lab members, who are not authors on this review article but who conduct research on language and language impairment. Decisions were made based on the majority opinion.
Inclusion Criteria
The year of publication was not restricted. We included only empirical studies published in peer-reviewed journals (not a conference abstract/paper) in English. We included only studies with individuals with speech or language disorders. We included studies of infants at high risk for developing ASD due to having an older sibling with the diagnosis. Because of the increased risk of developing ASD, studying high-risk infants has led to important discoveries in the early identification of ASD and associated language impairment. Similarly, we included studies of MCI due to its role in the early identification of dementia. We included only studies that used tasks of speaking or listening. We included only studies with three or more participants due to a large number of single-subject studies of fNIRS-based brain–computer interfaces for individuals with locked-in syndrome (LIS) and single-case medical reports of fNIRS used for bedside monitoring, both of which fell outside the scope of this review.
Exclusion Criteria
Studies were excluded if they did not involve a task of speaking or listening, so we excluded studies with nonverbal working memory, motor, visual, executive function, and attention tasks that did not have a communicative component. We excluded studies of typical adults with autistic traits (one study) since they did not have a diagnosis of ASD or social communication disorder. Though we aimed to include as many studies as possible, some etiologies fell outside the scope of this review even though they may fall within the American Speech-Language-Hearing Association's (2016) scope of practice policy, which itself is not a comprehensive list (these were attention-deficit/hyperactivity disorder, bipolar disorder, depressive disorder, public speaking anxiety, social anxiety disorder, and schizophrenia). After removing duplicate records and applying the inclusion/exclusion criteria, we found 34 studies that met criteria (see Figure 6).
Quality Analysis
In order to provide a critical appraisal of the quality of these studies, we outlined nine major methodological features of each of the 34 studies, shown in Table 10. The major methodological features include (a) number of participants, (b) inclusion of a control group, (c) ROI–probe match, (d) oxygenation measure, (e) the removal or correction of motion artifacts from the data, (f) the removal of systemic physiology from the signal, (g) the use of short separation detectors to control for the signal arising from scalp hemodynamics, (h) the sampling rate in hertz, and (i) whether the study included 3D digitization of the optode locations in order to improve localization and anatomical accuracy of the results. We consulted with members of the Neurophotonics Center at Boston University in order to select the features on which to rate the methodological quality of fNIRS studies. Then, we rated each study on these features by awarding each study a quality rating in each category as follows:
number of participants (N): high-quality rating given for N of 20 or greater (mean N for all studies was 19.2);
control group: high-quality rating given for the inclusion of a control group;
ROI–probe match: high-quality rating given for studies in which the fNIRS probe covered the intended brain ROI;
type of oxygenation measure: high-quality rating given for analyses that included O2Hb and HHb measures rather than just O2Hb;
motion artifact removal or correction: high-quality rating given for any method of motion artifact removal or correction;
systemic physiology removed: high-quality rating given for any method of removing noise from the signal induced by systemic physiology (e.g., blood pressure, respiration);
short separation detectors: high-quality rating given for using one or more short separation detectors to remove scalp hemodynamics (applied to adult and adolescent participants only);
sampling rate (Hz): high-quality rating given for a sampling rate of 5 Hz or higher since sampling at 5 Hz or higher is necessary to control for cardiac oscillations; and
3D digitization: high-quality rating given for the 3D digitization of optode locations to improve localization of underlying brain regions (not applied to participants with CIs).
For the ROI–probe match rating, we extracted the research question, ROI, and probe design. We compared these three aspects to judge that the brain ROI matched the research question and that the probe design matched the brain ROI. There were two reasons that studies did not receive a high-quality rating in this category: (a) the number of measurement channels was too small to cover the ROI and (b) the brain region(s) covered by the probe did not match the research question (e.g., a language task that examined prefrontal cortex only or auditory cortex only).
For studies that involved infants and children under the age of 12 years, the short separation category was not included in the quality analysis rating. Due to infants and children having thinner skulls, it is not clear that short separation detectors would be necessary for this population. For studies that involved individuals with CIs, the 3D digitization category was not included in the quality analysis rating. 3D digitization may not be appropriate with this population because the magnetic field may interfere with the CI. The quality ratings are shown in Table 10 and summarized below.
Data Extraction and Synthesis
Our systematic review led to a total of 34 empirical studies that we further analyzed in terms of characteristics of the study populations, tasks/brain ROIs, techniques, and findings. All three authors discussed the major features of the studies and agreed on the list of features that could most effectively contrast fNIRS studies across categories of speech-language disorder. More specifically, we outline the following major features of all 34 studies in Tables 1 through 9: age, population, number of participants, task, measurement of hemoglobin, number of channels, cortical ROI, results, and source/reference.
Results
The thirty-four fNIRS studies that we reviewed involved a total of 665 individuals with speech or language disorders. The study with the smallest number of participants had four participants, and the study with the largest number of participants had 55. We grouped the studies into the following categories: (a) ASD; (b) developmental speech and language disorders; (c) cochlear implantation and deafness; (d) dementia, dementia of the Alzheimer's type (DAT), and MCI; (e) LIS; (f) neurologic speech disorders/dysarthria; (g) stroke/aphasia; (h) stuttering; and (i) traumatic brain injury (TBI).
ASD
Three recent systematic reviews discussed fNIRS studies of a wide range of brain functions (functional connectivity, social functioning/perception/interaction, face/emotional processing, auditory and language processing, working memory and inhibition, executive function, joint attention) in with people with ASD (Liu et al., 2019; Mazzoni et al., 2018; Zhang & Roeyers, 2019). These reviews suggest that fNIRS is a promising tool for studying brain function in people with ASD.
Our systematic review revealed nine empirical fNIRS studies focused on speech or language processes in children and adults with ASD (see Table 1). The populations that were the focus of these studies can be categorized into three main groups: (a) infants at high risk of developing ASD due to having an older sibling with the diagnosis (two studies), (b) adults with intact language and intellectual ability (Asperger's, pervasive developmental disorder–not otherwise specified; four studies), and (c) children with ASD with varying levels of intellectual ability (nonverbal IQ above and below 70; one study). These studies investigated a total of 152 individuals with ASD, but only four of these have intellectual disability, a group that is understudied in autism research (Tager-Flusberg & Kasari, 2013). The tasks used in these fNIRS studies fall into two broad categories: speech perception (repeating vs. nonrepeating syllables, forward vs. backward speech, words with phonemic vs. prosodic contrasts) and word production (letter and category fluency tasks).
Table 1.
Functional near-infrared spectroscopy studies in populations with autism spectrum disorder (ASD).
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 11- to 18-year-old adolescents | ASD | 22 | Letter fluency, category fluency | O2Hb | 16 | Prefrontal cortex | Typical adolescents showed activation in lateral frontal regions, adolescents with ASD showed activation across lateral and medial frontal regions, associated with poorer word retrieval performance | Yeung et al. (2019) |
| 3-month-old infants | High risk, sibling with ASD | 21 high-risk ASD, 17 low-risk controls | Repeating or nonrepeating trisyllabic sequences | O2Hb, HHb | 24 | Left and right temporal regions | Neural activation in female low-risk infants decreased over exposure to repetition; no changes in neural activity in female high-risk infants with exposure | Edwards et al. (2017) |
| 4- to 6-month-old infants | High risk, sibling with ASD | 5 (16 low-risk controls) | Social videos vs. nonsocial images, human vocalizations vs. nonvocal sounds | O2Hb, HHb, total Hb | 26 | Inferior frontal gyrus, posterior temporal, left-lateralized temporal | Reduced activation to social auditory and visual stimuli compared to nonsocial vs. controls | Lloyd-Fox et al. (2017) |
| 3-, 6-, 9-, and 12-month-old infants | High risk, sibling with ASD | 27 high risk, 3 low risk | Repeating or nonrepeating trisyllabic sequences | O2Hb, HHb | 24 | 3-month high risk: bilateral posterior and left anterior temporal, 12-month low risk: left anterior and bilateral posterior temporal | High-risk infants showed increased connectivity at 3 months, no differences at 6 and 9 months, and decreased connectivity by 12 months compared to low-risk infants | Keehn et al. (2013) |
| 10- to 22-year-old adolescents and 20- to 32-year-old adults | ASD | 11, 12 controls | Listen to or ignore forward speech (story) and backward speech | O2Hb, HHb | 32 | Auditory cortex, prefrontal cortex | Increased O2Hb in response to intentional listening vs. ignoring in ASD and controls, decreased laterality in ASD compared with controls when asked to ignore | Funabiki et al. (2012) |
| 18- to 35-year-old adults | ASD | 20, 18 controls | Category and letter fluency | O2Hb | 52 | Prefrontal cortex | People with Asperger's significantly lower activation than controls in letter fluency, no difference in category fluency | Iwanami et al. (2011) |
| 9- to 16-year-old children and adults | ASD (HFA, PDD-NOS) | 27 HFA, 27 nonaffected sibs, 27 unrelated controls | Letter fluency test | O2Hb, HHb | 8 | Bilateral prefrontal cortex | For adults, the O2Hb increases were significantly smaller in ASD vs. controls (but similar task performance), change was intermediate in adult siblings | Kawakubo et al. (2009) |
| 6- to 11-year-old children | ASD | 9, 9 controls | Real words with phonemic vs. prosodic contrasts | O2Hb | 8 | Auditory cortex | Increased left hemisphere dominance for phonemic cues and increased right hemisphere dominance for prosodic cues (controls), less localization to the left hemisphere (ASD) | Minagawa-Kawai et al. (2009) |
| 18- to 37-year-old adults | ASD (PDD) | 10, 10 controls | Letter fluency task | O2Hb, HHb, total Hb | 16 | Prefrontal cortex | Bilateral reduction in O2Hb in PDD vs. controls (no difference in task performance) | Kuwabara et al. (2006) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin; HFA = high-functioning autism; PDD = pervasive developmental disorder; NOS = not otherwise specified.
Edwards et al. (2017) used fNIRS to investigate neural activation in the left and right temporal regions in 3-month-old infants at high risk of developing ASD in response to repeating versus nonrepeating syllables. They found that neural activation in low-risk infant girls decreased over exposure to repeated syllables. On the other hand, they found no changes in neural activity in high-risk infant girls with exposure to repeated syllables. In a similar fNIRS study with high-risk infants presenting repeating versus nonrepeating trisyllabic sequences, Keehn et al. (2013) investigated neural activation in a longitudinal study of infants at the ages of 3, 6, 9, and 12 months. They found high-risk infants at the age of 3 months showed increased overall functional connectivity compared to low-risk infants. No significant differences were found between high-risk and low-risk infants at the ages of 6 and 9 months, but at 12 months, high-risk infants showed decreased connectivity compared to low-risk infants. Lloyd-Fox et al. (2017) also used fNIRS with 4- to 6-month-old high-risk infants. They showed social versus nonsocial videos and played recordings of human voices versus nonhuman sounds. They reported that the high-risk infants showed reduced activation to social stimuli, both auditory and visual, compared to low-risk controls. Because fNIRS is suitable for research with infants, these studies have identified differences in neural patterns in very young infants associated with a higher risk of developing ASD.
Minagawa-Kawai et al. (2009) used fNIRS with Japanese-speaking children with ASD to investigate neural activation in auditory cortex for Japanese words with phonemic versus prosodic contrasts. They found increased left hemisphere activity for phonemic cues and increased right hemisphere activity for prosodic cues in controls. In children with ASD, they found less localization in the left hemisphere compared to controls.
Funabiki et al. (2012) used fNIRS with adolescents and adults with ASD in a task in which the participants were asked to ignore forward and backward speech stimuli. Focusing on the prefrontal cortex, they found decreased laterality compared with controls when participants with ASD were asked to ignore auditory stimuli. In auditory cortex, no differences were detected between the two groups when they were asked to pay attention to auditory stimuli.
Five fNIRS studies examined the neural correlates of verbal fluency in adults with ASD. Verbal fluency tasks ask participants to name as many words as they can in a category in a 60-s time frame. A typical category fluency task is to name as many animals as possible in 60 s. A typical letter fluency task is to name as many words that start with “s” as possible in 60 s. The particular categories and letters that are used may vary.
Iwanami et al. (2011) found that people with Asperger's syndrome showed significantly lower activation in prefrontal cortex compared to controls in letter fluency but no difference in category fluency. Kawakubo et al. (2009) found that, for adults doing a letter fluency test, the O2Hb increases were significantly smaller in ASD versus control groups (though the groups showed similar task performance). They found that the neural activation in unaffected adults who had siblings with ASD was intermediate between ASD and control groups. Kuwabara et al. (2006) found a bilateral reduction in O2Hb in prefrontal cortex in adults with PDD versus controls in a letter fluency task (but no group differences in task performance between the groups). Yeung et al. (2019) found that typical adolescents showed activation in lateral frontal regions, while adolescents with ASD showed activation across lateral and medial frontal regions. They found that this pattern of activation in individuals with ASD was associated with poorer word retrieval performance.
These fNIRS-based studies of speech perception and production in ASD demonstrate the feasibility of using fNIRS to study brain activation with very young infants at high risk of developing ASD. One of the fNIRS studies successfully used fNIRS with children with ASD with intellectual disability. The studies with adults and children with ASD but no intellectual disability showed that, despite no differences between individuals with ASD and controls in the behavioral task (e.g., verbal fluency), fNIRS revealed differences in brain activation and atypical lateralization of response to linguistic stimuli.
Developmental Speech and Language Disorders
There is a small but growing body of neuroimaging research on developmental speech and language disorders (Mayes et al., 2015; Morgan et al., 2016). Mayes et al. (2015) conducted a systematic review of neuroimaging studies of child language disorders. They found 18 studies, including both structural and functional imaging studies. Unlike this review, they included tasks that were not necessarily restricted to speech or language processes and included all neuroimaging techniques. Our systematic review revealed four empirical studies that used fNIRS to investigate the neural correlates of language processes in children with language impairment. This included one study of children with specific language impairment (SLI) and two studies of children and young adults with dyslexia (see Table 2).
Table 2.
Functional near-infrared spectroscopy studies in developmental speech and language disorders.
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| Children, M age = 12 years | Dyslexia | 15 (15 controls) | Phoneme deletion, phonological short-term memory, rapid automatized naming, amplitude rise tasks | O2Hb, HHb, total Hb | 44 | Left superior temporal gyrus and left angular gyrus, right supramarginal gyrus and right angular gyrus | Asymmetric bilateral pattern of regions more active in children with dyslexia than controls for slow vs. fast stimuli | Cutini et al. (2016) |
| 8- to 12-year-old children | SLI | 15 (15 controls) | Sentence comprehension | O2Hb, HHb, total Hb | 44 | Bilateral perisylvian areas | Significant differences between SLI and controls in O2Hb mean trends in bilateral inferior frontal and left inferior posterior parietal regions; significant differences in HHb mean trends in right inferior posterior parietal cortex and left temporal parietal junction | Fu et al. (2016) |
| Young adults (M = 25.65 years, SD = 2.67) | Dyslexia | 17 (17 controls, 17 control 12-year-olds) | Lexical decision | O2Hb, HHb | 16 | Upper left frontal lobe | Readers with dyslexia showed lower activity under pseudoword condition vs. real words | Sela et al. (2014) |
| Children, Grades 3–5 | Dyslexia | 20 (20 controls) | Consonant–vowel task | O2Hb, HHb, total Hb | 16 | Left middle frontal gyrus | Children with dyslexia had decreased amounts of O2Hb and total Hb in the left dorsolateral prefrontal cortex | Song et al. (2014) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin; SLI = specific language impairment.
Fu et al. (2016) studied neural patterns in children with SLI with fNIRS using a nonparametric statistical approach rather than the typical GLM-based approach to avoid the a priori assumption that the data would show a Gaussian distribution. Using functional data analysis, they reported significant differences in O2Hb trends in bilateral inferior frontal gyrus (IFG) and left inferior posterior parietal regions. They also reported differences in HHb trends in the right inferior posterior parietal cortex and left temporal parietal junction in 8- to 12-year-old children with SLI (developmental language disorder or DLD) compared to controls.
Cutini et al. (2016) used fNIRS to examine the neural correlates of phoneme deletion, phonological short-term memory, rapid automatized naming, and amplitude rise tasks at fast and slow speeds in children with developmental dyslexia and typical controls. They found higher activation in the right supramarginal gyrus for fast versus slow linguistic stimuli in children with developmental dyslexia compared to controls. Sela et al. (2014) reported that young adults with dyslexia had lower activation compared to controls in the left frontal brain region in response to pseudowords versus real words. Using fNIRS, Song et al. (2014) found lower O2Hb in the left dorsolateral prefrontal cortex (DLPFC) in a consonant–vowel task in children with dyslexia compared to controls.
These studies demonstrate the feasibility of using fNIRS to investigate the neural correlates of speech and language processing in children with developmental speech and language disorders. They all found differences in neural activation in response to linguistic stimuli for children and young adults with SLI and dyslexia compared to typical controls. These results are similar to what has been found with fMRI and other neuroimaging methods for children with speech and language disorders (Mayes et al., 2015; Morgan et al., 2016) and dyslexia (D'Mello & Gabrieli, 2018).
Deafness/CI
fNIRS is particularly well suited to study neural activation in recipients of CIs because they cannot undergo fMRI or EEG due to the ferromagnetic and electrical components of the implant (Bisconti et al., 2016; Sevy et al., 2010; see Saliba et al., 2016, for a more in-depth review of fNIRS with CI recipients). Our systematic review revealed six empirical studies of communication in recipients of CIs using fNIRS (see Table 3).
Table 3.
Functional near-infrared spectroscopy studies in populations with deafness and cochlear implant (CI).
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 36- to 78-year-old adults | Deafness, cochlear implant |
15 adults pre- and post-CI (17 controls) | Visual speech recognition (lipreading) | O2Hb, HHb | 24 | Bilateral superior temporal cortex | Nine CI users showed a decrease in cross-modal activation, six showed an increase | Anderson et al. (2017) |
| 21- to 74-year-old adults | Cochlear implant | 14 CI recipients, 13 controls | Phonological awareness (rhyme judgment), passage comprehension | O2Hb, HHb | 32 | Frontal, temporal cortices spanning bilateral inferior and middle frontal, superior and middle temporal regions, and parietal cortex | CI and controls activation in left temporal and right frontal regions in rhyme vs. tone judgment, left frontal region less activated in CI group, controls and CI groups bilateral activation over frontal and temporal cortices during passage comprehension | Bisconti et al. (2016) |
| 23- to 86-year-old adult CI users | Cochlear implant | 32 CI recipients, 35 controls | Normal, channelized, and scrambled speech | O2Hb, HHb | 31 | Lateral temporal cortex, superior temporal gyrus | CI users with good speech perception and control showed reduced activation as speech becomes less intelligible; CI users with poor speech perception showed increased activation for all speech conditions | Olds et al. (2016) |
| 55- to 59-year-old adult CI users | Cochlear implant | 5 (33 controls, 18–62 years old) | Children's stories read aloud | O2Hb, HHb | 4 | Temporal cortex | Auditory, visual, and audiovisual speech stimuli evoked concentration changes in both cohorts, auditory larger than visual | van de Rijt et al. (2016) |
| 18- to 60-year-old adults | Profoundly deaf | 30 (30 controls) | Responses to auditory, visual, somatosensory stimulation | O2Hb, HHb | 24 | Left and right auditory cortices (Heschl's gyrus and the superiotemporal gyrus) | Visual stimuli evoked significantly larger right ROI responses in profoundly deaf individuals, nonsignificant trend for auditory responses to be larger in controls vs. profoundly deaf individuals | Dewey & Hartley (2015) |
| 3- to 12-year-old children, 22- to 28-year-old adults | Cochlear implant | 36 early-implanted children, 9 newly implanted children (11 control children, 11 control adults) | Passive listening, stories | O2Hb, HHb | 4 | Temporal lobe and superior temporal gyrus | Speech-evoked cortical activity observed in 100% of normal-hearing adults, 82% of normal-hearing children, 78% of early-implanted deaf children, and 78% of newly implanted children | Sevy et al. (2010) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin.
Anderson et al. (2017) used fNIRS to investigate the differences in cross-modal activation of auditory brain regions in a visual speech task (lipreading) in deaf adults before and 6 months following cochlear implantation. Nine CI users showed a decrease in activation from pre- to postimplantation, and six CI users showed an increase. Participants who had become deaf more recently showed an increase in cross-modal activation from pre- to postimplantation. On the other hand, participants with a longer duration of deafness showed a decrease in cross-modal activation. The authors suggest that their results run counter to the generalization that visual linguistic abilities are maladaptive for hearing restoration after cochlear implantation. Rather, this may depend on duration of deafness before implantation as well as other individual differences.
Bisconti et al. (2016) used fNIRS with adult CI recipients during a phonological awareness (rhyme vs. tone judgment) task and a passage comprehension task. In the rhyme versus judgment task, they found that CI recipients showed less activation than controls in left frontal regions. In the passage comprehension task, however, they found no differences in activation between CI recipients and controls in bilateral frontal and temporal cortices.
Olds et al. (2016) used fNIRS to investigate neural activation in response to more and less intelligible speech in adult CI users. They found the CI users with good speech perception abilities and controls showed reduced activation as speech became less intelligible. On the other hand, they found that CI users with poor speech perception showed increased activation for all speech conditions regardless of intelligibility. van de Rijt et al. (2016) used fNIRS with adult CI recipients while they listened to children's stories read aloud. They found no differences between CI recipients and controls in activation in auditory, visual, and audiovisual brain regions (temporal cortex), with greater activation in auditory compared to visual brain regions. Sevy et al. (2010) used fNIRS with children who were new CI recipients (newly implanted) and children who were CI recipients at an early age (early implanted) in a passive listening task in which children listened to stories read aloud. They found speech-evoked cortical activity in the temporal lobe and superior temporal gyrus in 78% of newly implanted and early-implanted children (compared to 82% in children who hear normally and 100% in adults who hear normally).
Dewey and Hartley (2015) used fNIRS with adults who are profoundly deaf (but not CI recipients) to examine responses to audio, visual, and somatosensory stimuli. They found that visual stimuli evoked significantly greater neural responses in the right auditory cortex in individuals who are profoundly deaf compared to controls.
For neuroimaging studies with CI recipients, fNIRS is the primary safe and noninvasive technique since CI users cannot undergo fMRI or EEG. The studies reviewed here represent an important step in demonstrating the feasibility of fNIRS with CI recipients. They have also begun to identify differences in neural activation and address important theoretical questions with applications to rehabilitation.
Dementia/Alzheimer's/MCI
Neuroimaging plays a central role in basic and translational science for MCI, dementia, and DAT. Narayanan and Murray (2016) suggest that research on neuroimaging is fundamental to improving the early detection, diagnosis, and monitoring of the progression of dementia and related impairments. Along the same lines, Oh and LaPointe (2017) suggest that neuroimaging could potentially improve the differential diagnosis of the various types of dementias. Our systematic review resulted in six studies that used fNIRS to investigate the neural correlates of verbal fluency in people with dementia, DAT, and MCI (see Table 4).
Table 4.
Functional near-infrared spectroscopy studies in populations with dementia, Alzheimer's dementia, and mild cognitive impairment (MCI).
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 70- to 77-year-old adults | MCI | 55 (55 controls) | Verbal fluency | O2Hb, HHb | 52 | Inferior frontotemporal regions | Decreased hemodynamic response in the inferior frontotemporal cortex in MCI | Katzorke et al. (2018) |
| 60- to 91-year-old adults | MCI | 26 (26 controls) | Category fluency | O2Hb, HHb | 16 | Left-lateralized frontal | Controls showed left lateralization of frontal activations, MCI group did not | Yeung et al. (2016) |
| 50- to 90-year-old adults | Probable Alzheimer's dementia (AD) | 16 | Verbal fluency (3 time points: premedication, target dose, 8 weeks post target dose) | O2Hb, HHb | 22 | Left and right prefrontal and temporal cortices | Cholinesterase inhibitors resulted in increase in O2Hb in speech-related brain areas from pre to post (with improvements in behavioral performance) | Metzger et al. (2015) |
| MCI M
age = 63.0 years (SD = 6.4) AD M age = 59.2 years (SD = 3.9) |
MCI/AD | 15 MCI, 15 AD, 32 controls | Verbal fluency | O2Hb | 24 | Frontal, bilateral parietal, occipital | Significantly lower amplitude changes in the frontal and bilateral parietal areas in AD; MCI was significantly lower only in the right parietal area | Arai et al. (2006) |
| M age = 67.3 years, SD = 10.6 | Dementia of the Alzheimer's type (DAT) | 10 (10 controls) | Letter fluency, category fluency | O2Hb, HHb | 2 | Left and right hemispheric prefrontal areas | Patients with DAT showed less lateralization of the relative concentration of oxyhemoglobin in frontal brain tissue | Fallgatter et al. (1997) |
| M age = 71 years, SD = 10 | AD | 19 (19 controls) | Verbal fluency | O2Hb HHb, total Hb | 4 | Frontal and parietal cortex | Controls showed increases in concentrations of O2Hb and total Hb over left superior parietal cortex, patients with AD showed significant decreases in O2Hb and total Hb | Hock et al. (1997) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin.
Katzorke et al. (2018) used fNIRS to examine the neural correlates of verbal fluency in 70- to 77-year-old adults with MCI compared to age-matched controls. They found a decreased hemodynamic response in the inferior frontotemporal cortex in people with MCI compared to controls. Yeung et al. (2016) used fNIRS to examine neural responses during a category fluency task with 60- to 91-year-old adults with MCI and healthy age-matched controls. They found that, while controls showed a left-lateralized hemodynamic response in frontal brain regions, the MCI group did not. Metzger et al. (2015) used fNIRS to investigate the changes in neural activation in response to a pharmaceutical intervention with cholinesterase inhibitors in individuals with probable DAT. They measured neural activation during a verbal fluency task before participants were administered the medication at target dose and 8 weeks after the target dose. They reported that the cholinesterase inhibitors resulted in an increase of O2Hb in speech-related brain areas from pre- to postintervention (with improvements in behavioral performance).
Arai et al. (2006) used fNIRS to investigate hemodynamic responses during a verbal fluency task in adults with MCI, adults with DAT, and healthy age-matched controls. They found a significantly lower response in the frontal and bilateral parietal areas in people with DAT compared to controls. The MCI group showed significantly lower activation only in the right parietal areas compared to controls. Fallgatter et al. (1997) used fNIRS in letter and category fluency tasks in people with DAT and age-matched controls. They reported that patients with DAT showed a less lateralized hemodynamic response in the frontal cortex compared to controls. Hock et al. (1997) used fNIRS in a verbal fluency task with people with DAT and age-matched controls. They reported that patients with DAT showed a significantly lower hemodynamic response in the left superior parietal cortex compared to controls.
In summary, several research groups have used fNIRS to investigate hemodynamic responses during verbal fluency tasks with individuals with dementia, DAT, and MCI. These studies demonstrate the feasibility of fNIRS and could be used to detect early changes in brain activation associated with MCI, DAT, and dementia. One study compared fNIRS measurements at three time points in response to a pharmaceutical intervention. fNIRS may help improve the diagnosis and treatment of MCI, DAT, and dementia as these studies have suggested.
LIS
fNIRS has been explored (often in conjunction with EEG) as a brain–computer interface and a potential mode of communication for individuals with LIS. Naseer and Hong (2015) presented a review of fNIRS-based brain–computer interface studies. Two studies using fNIRS met the inclusion/exclusion criteria and were included in our systematic review. These are summarized in Table 5 and discussed below.
Table 5.
Functional near-infrared spectroscopy (fNIRS) studies in populations with locked-in syndrome.
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 24-, 61-, 68-, 76-year-old adults | Locked-in (ALS) | 4 | Think answers to known yes/no questions | O2Hb, HHb | 8 | Frontocentral region | Online fNIRS classification using linear support vector machine resulted in above-chance-level correct response rate over 70% | Chaudhary et al. (2017) |
| 22- to 80-year-old adults | Locked-in ALS and not locked-in ALS | 23 ALS, 17 ALS locked-in | Think answers to known yes/no questions | O2Hb | 1 | Frontal lobe | Average rate of correct detection of answer was almost 80% (applicable to only 40% of locked-in patients) | Naito et al. (2007) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; ALS = amyotrophic lateral sclerosis; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin.
Chaudhary et al. (2017) used fNIRS with four patients with amyotrophic lateral sclerosis (ALS) and LIS to investigate the hemodynamic response in frontocentral regions (designed to cover language regions) while patients mentally responded to known yes/no questions. Based on an analysis of the neural response patterns using a linear support vector machine, they classified correct answers to yes/no questions at a rate of 70%. Naito et al. (2007) also used fNIRS with 23 patients with ALS and LIS and 17 controls with ALS and without LIS. An analysis of the hemodynamic response was able to determine the correct answer to known yes/no questions at a rate of 80% for patients with ALS and without LIS and at a rate of 40% for patients with ALS and LIS. These studies demonstrate the feasibility of fNIRS with individuals with ALS/LIS and are important steps in the development of brain–computer interfaces, which could improve access to communication for individuals in a locked-in state.
Neurologic Speech Disorders/Dysarthria
Our systematic search resulted in only one study that focused on neurologic speech disorders (see Table 6). Caliandro et al. (2013) used fNIRS to investigate the neural correlates of phonemic verbal fluency in the prefrontal cortex in 29 adults with myotonic dystrophy, Type 1, and 30 healthy controls. They found no significant difference in the hemodynamic response of patients with myotonic dystrophy and controls, but this study demonstrates the initial feasibility of this method with individuals with neurologic speech disorders.
Table 6.
Functional near-infrared spectroscopy studies in populations with neurologic speech disorders/dysarthria.
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| Adults, M age = 44.46 years, SD = 14.23 | Myotonic dystrophy Type 1 (DM1) | 29 (30 controls) | Phonemic verbal fluency | O2Hb, HHb | 2 | Prefrontal cortex | Control group O2Hb increased and HHb significantly decreased, no difference found for DM1 patients | Caliandro et al. (2013) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin.
Stroke/Aphasia
In a systematic review, Yang et al. (2019) reviewed 66 published studies of fNIRS and stroke across a wide range of categories (though none focused on communicative processes). Though Yang et al.'s systematic review primarily resulted in studies of acute stroke risk, monitoring, and motor recovery, their conclusion that fNIRS has a wide range of research and clinical applications in the evaluation and treatment of stroke is relevant. Our review of fNIRS studies of speech or language processes in people with poststroke aphasia resulted in two studies (see Table 7). Despite a paucity of fNIRS-based research on speech or language processes in poststroke patients with aphasia, Yang et al. show that fNIRS has been used successfully and extensively with this population and has a range of clinical applications (discussed in more depth below).
Table 7.
Functional near-infrared spectroscopy studies in populations with stroke/aphasia.
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 42- to 75-year-old adults | Aphasia, receiving low- or high-frequency TMS | 8 | Word repetition | O2Hb | 48 | Inferior frontal gyrus, superior temporal gyrus | Low-frequency TMS group showed reduced left and right activation, stronger activation in ipsilateral hemisphere; high-frequency TMS group showed stronger activation in contralateral vs. ipsilateral hemisphere pre- and postintervention | Hara et al. (2017) |
| 48- to 59-year-old adults | Poststroke nonaphasic, nonfluent aphasic, controls | 10 nonfluent aphasia (6 poststroke nonaphasia, 13 healthy controls) | Naming, counting, narrative task | O2Hb, HHb, total Hb | 1 | Left prefrontal cortex | 50% of aphasics increase in HHb and O2Hb during language task, 23.1% of controls and 16.7% of nonaphasic CVD patients | Sakatani et al. (1998) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; TMS = transcranial magnetic stimulation; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin; CVD = cerebrovascular disease.
Hara et al. (2017) used fNIRS in a word repetition task with eight adults with aphasia receiving low- or high-frequency transcranial magnetic stimulation (TMS) pre- and posttreatment. The ROIs included the IFG and superior temporal gyrus. They found that the low-frequency TMS group showed reduced left and right hemisphere activation and stronger activation in the hemisphere ipsilateral to the lesion. In the high-frequency group, they found stronger activation following high-frequency TMS and stronger activation in the contralateral hemisphere versus ipsilateral from pre- to postintervention. Sakatani et al. (1998) used fNIRS to compare naming, counting, and narrative production tasks in the left prefrontal cortex in 10 patients with nonfluent aphasia, six patients poststroke without aphasia, and 13 healthy controls. They reported no differences between patients with aphasia, patients without aphasia, and healthy controls in O2Hb or total hemoglobin. They found a significant difference in HHb in patients with aphasia compared to nonaphasia patients and healthy controls.
In summary, in poststroke patients, fNIRS has been used to investigate brain activation in response to TMS and in language production tasks. Given that Yang et al. (2019) found that fNIRS had been successfully used with patients who have had stroke, fNIRS studies of speech or language processing in individuals with stroke/aphasia could lead to a better understanding of the neural correlates of recovery. Since fNIRS is easier and less expensive than fMRI and well tolerated for repeated measurements in close time intervals (Dieler et al., 2012; Keenan et al., 2002), it would be particularly useful for monitoring changes in brain activation session by session or even during therapy sessions.
Stuttering
In a recent systematic literature review of neuroimaging research on stuttering, Etchell et al. (2018) found far fewer studies that had included children who stutter despite the recognized importance of studying children who stutter (Chang, 2014). Etchell et al. cite methodological difficulties with imaging studies that include children, such as the need to remain still for an extended time and anxiety about the scanning environment. Since fNIRS is safe and noninvasive, it is well suited for research with infants and children. Based on our systematic search, we found that fNIRS has been used in three studies to examine the neural correlates of auditory processing and language production in adults, children who stutter, and children who had recovered from stuttering (see Table 8).
Table 8.
Functional near-infrared spectroscopy studies in people who stutter.
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 8- to 16-year-old children | Children who recovered from stuttering | 46: stuttering n = 16; control n = 16; recovered from stuttering n = 14 | Picture description | O2Hb, HHb | 18 | Inferior frontal gyrus (IFG), superior temporal gyrus (STG), precentral gyrus, premotor cortex (PMC) | Significantly reduced NAUS in left IFG, increased Hjorth mobility parameters, denoting increased variability in left IFG In children who stutter, neural correlate of 71.43% of children who recovered classified as controls rather than stuttering |
Hosseini et al. (2018) |
| 7- to 11-year-old children | Stuttering | 16 children who stutter, 16 controls | Picture description | O2Hb, HHb | 18 | Ventral and dorsal IFG, STG, precentral gyrus/PMC | Activation over left dorsal IFG and left PMC in controls, deactivation over these left hemisphere regions in children who stutter | Walsh et al. (2017) |
| 3- to 12-year-old children, 18- to 44-year-old adults | Stuttering | 10 adults who stutter, 13 children who stutter | Auditory processing of phonemic and prosodic contrasts | Total Hb | 12 | Bilateral temporal areas | Preschool children who stutter: right-lateralized response for all stimuli, school-age children who stutter: no lateralization, controls: left-lateralized response to phonemic and right-lateralized response to prosodic stimuli | Sato et al. (2011) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin; NAUS = normalized area under the signal.
Hosseini et al. (2018) used fNIRS to examine hemodynamic responses with a picture description task in children who stutter and children who had recovered from stuttering. In children who stutter, they found significantly reduced activation in left IFG channels compared to controls. They reported that the neural patterns of 71% of children who recovered from stuttering could be classified as controls rather than children who stutter. Walsh et al. (2017) used fNIRS to investigate hemodynamic responses in a picture description task in children who stutter. They found significantly less activation in the left dorsal IFG and the left premotor cortex with children who stutter compared to controls. Sato et al. (2011) used fNIRS to examine the neural correlates of auditory processing of phonemic and prosodic contrasts in Japanese-speaking children and adults who stutter. Preschool-age children who stutter showed a more right-lateralized response for all stimuli, while controls showed a left-lateralized response to phonemic stimuli and a right-lateralized response to prosodic stimuli.
These fNIRS studies of adults and children who stutter demonstrate the feasibility of the method and have identified differences in neural activation for people who stutter compared to typical controls. Going beyond feasibility, these researchers have begun to use fNIRS to identify neural patterns that correlate with recovery from stuttering.
TBI
Table 9 summarizes the one fNIRS study with people with TBI that met the inclusion criteria. Rodriguez Merzagora et al. (2014) used fNIRS to investigate the neural correlates of a verbal n-back working memory task in the left and right DLPFC in six adults with TBI and 11 healthy controls. They found significantly higher activation in the left DLPFC in people with TBI compared to controls. This study demonstrates the feasibility of fNIRS to detect differences in cerebral blood oxygenation in the DLPFC during a verbal n-back task in adults with TBI and healthy controls.
Table 9.
Functional near-infrared spectroscopy studies in populations with traumatic brain injury (TBI).
| Age | Population | N | Task | Meas | Ch | ROI | Results | Source |
|---|---|---|---|---|---|---|---|---|
| 32- to 52-year-old adults | TBI | 6 TBI, 11 controls | Verbal n-back | O2Hb, HHb, total Hb, metabolic ratio between HHb and O2Hb | 16 | Left and right dorsolateral prefrontal cortex | Activation for TBI group higher than controls at left DLPFC | Rodriguez Merzagora et al. (2014) |
Note. Meas = oxygenation measure; Ch = number of measurement channels; ROI = region of interest; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin; DLPFC = dorsolateral prefrontal cortex.
The results of this systematic review suggest that fNIRS is feasible with a range of individuals with speech or language impairment across the life span. fNIRS is highly suitable for use with infants, children, and recipients of CIs. Some of the studies reviewed have used fNIRS for repeated measurements, across three to eight sessions. Most studies found differences in neural patterns between people with speech or language impairment and controls, but these patterns varied. Some studies found decreased neural activation compared to controls. Other studies found atypical connectivity or more diffuse rather than localized patterns of neural activation. Yet other studies found atypical lateralization of activation, with less left-lateralized responses. Some of these studies uncovered atypical neural patterns in the absence of atypical behavioral responses. In the next section, we present a quality analysis of the fNIRS studies included in this systematic review.
Results of the Quality Analysis
We critically appraised the quality of the fNIRS studies of speech-language impairment on the following nine properties: (a) number of participants, (b) inclusion of a control group, (c) ROI–probe match, (d) oxygenation/deoxygenation measurement, (e) correction of motion artifacts, (f) removal of systemic physiology from the signal, (g) use of short separation detectors to remove the signal arising from scalp hemodynamics, (h) the sampling rate in hertz, and (i) whether the study included 3D digitization of the optode locations. Table 10 shows these nine methodological characteristics and the quality ratings for the 34 studies that were included in this review.
Table 10.
Quality analysis of functional near-infrared spectroscopy studies.
| Category | Reference | N | Control group | ROI–probe match | Oxy measure | Motion removal correc | Syst phys removed | Short sep | Sampling rate | 3D dig | Quality criteria met |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults, adolescents | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 123456789 | |
| ASD | Yeung et al. (2019) | 22 | Yes | No | O2Hb | Yes | No | No | 12.21 | No | 1258 |
| ASD | Funabiki et al. (2012) | 11 | Yes | Yes | O2Hb, HHb | No | No | No | 7.69 | No | 2348 |
| ASD | Iwanami et al. (2011) | 20 | Yes | Yes | O2Hb | Yes | No | No | 10 | No | 12358 |
| ASD | Kuwabara et al. (2006) | 10 | Yes | Yes | O2Hb, HHb | Yes | No | No | 10 | No | 23458 |
| DLD/D | Cutini et al. (2016) | 15 | Yes | Yes | O2Hb, HHb | Yes | Yes | No | n/r | No | 23456 |
| DLD/D | Sela et al. (2014) | 17 | Yes | No | O2Hb | Yes | Yes | No | 1 | No | 256 |
| D/CI | Dewey & Hartley (2015) | 30 | Yes | Yes | O2Hb, HHb | Yes | Yes | No | 10 | No | 1234568 |
| DemAlz | Katzorke et al. (2018) | 55 | Yes | Yes | O2Hb, HHb | Yes | Yes | No | 10 | No | 1234568 |
| DemAlz | Yeung et al. (2016) | 26 | Yes | No | O2Hb, HHb | Yes | Yes | No | 12.21 | No | 124568 |
| DemAlz | Metzger et al. (2015) | 16 | No | Yes | O2Hb, HHb | Yes | Yes | No | 10 | No | 34568 |
| DemAlz | Arai et al. (2006) | 30 | Yes | Yes | O2Hb | Yes | Yes | No | n/r | No | 12356 |
| DemAlz | Fallgatter et al. (1997) | 10 | Yes | No | O2Hb, HHb | No | Yes | No | 1 | No | 246 |
| DemAlz | Hock et al. (1997) | 19 | Yes | No | O2Hb, HHb | No | No | No | 0.5 | No | 24 |
| LIS | Chaudhary et al. (2017) | 4 | No | Yes | O2Hb, HHb | No | Yes | No | 6.25 | No | 3468 |
| LIS | Naito et al. (2007) | 17 | Yes | No | O2Hb | No | Yes | No | 5 or 10 | No | 268 |
| Neurologic speech | Caliandro et al. (2013) | 29 | Yes | No | O2Hb, HHb | No | No | No | 2 | No | 124 |
| Stroke/aphasia | Hara et al. (2017) | 8 | No | Yes | O2Hb | No | No | No | n/r | No | 3 |
| Stroke/aphasia | Sakatani et al. (1998) | 10 | Yes | No | O2Hb, HHb | No | No | No | n/r | No | 24 |
| TBI | Rodriguez Merzagora et al. (2014) | 6 | Yes | No | O2Hb, HHb | No | No | No | 2 | No | 24 |
| Adult CI users | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | n/a | 12345678 | |
| D/CI | Anderson et al. (2017) | 15 | Yes | Yes | O2Hb, HHb | Yes | Yes | No | 10 | n/a | 234568 |
| D/CI | Bisconti et al. (2016) | 14 | Yes | Yes | O2Hb, HHb | Yes | Yes | No | 20 | n/a | 234568 |
| D/CI | Olds et al. (2016) | 32 | Yes | Yes | O2Hb, HHb | Yes | Yes | No | 6.25 | n/a | 1234568 |
| D/CI | van de Rijt et al. (2016) | 5 | Yes | No | O2Hb, HHb | Yes | Yes | No | 10 | n/a | 24568 |
| Infants, children | 1 | 2 | 3 | 4 | 5 | 6 | n/a | 8 | 9 | 12345689 | |
| ASD | Edwards et al. (2017) | 21 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | 10 | No | 1234568 |
| ASD | Lloyd-Fox et al. (2017) | 5 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | n/r | No | 23456 |
| ASD | Keehn et al. (2013) | 27 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | 10 | No | 1234568 |
| ASD | Kawakubo et al. (2009) | 27 | Yes | No | O2Hb, HHb | No | No | n/a | 2 | No | 124 |
| ASD | Minagawa-Kawai et al. (2009) | 9 | Yes | No | O2Hb | Yes | No | n/a | 10 | No | 258 |
| DLD/D | Fu et al. (2016) | 15 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | n/r | Yes | 234569 |
| DLD/D | Song et al. (2014) | 20 | Yes | No | O2Hb, HHb | No | No | n/a | 1 | No | 124 |
| Stuttering | Hosseini et al. (2018) | 30 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | 25 | Yes | 12345689 |
| Stuttering | Walsh et al. (2017) | 16 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | 25 | Yes | 2345689 |
| Stuttering | Sato et al. (2011) | 23 | No | No | HbTotal | Yes | No | n/a | n/r | Yes | 159 |
| Child CI users | 1 | 2 | 3 | 4 | 5 | 6 | n/a | 8 | n/a | 1234568 | |
| D/CI | Sevy et al. (2010) | 40 | Yes | Yes | O2Hb, HHb | Yes | Yes | n/a | n/r | n/a | 123456 |
Note. ROI = region of interest; Oxy = oxygenation; Motion removal correc = removal or correction of motion artifacts from the data; Syst phys removed = removal of systemic physiology from the signal; Short sep = use of short separation detectors; 3D dig = 3D digitization; ASD = autism spectrum disorder; O2Hb = oxygenated hemoglobin; HHb = deoxygenated hemoglobin; DLD/D = developmental language disorder/dyslexia; D/CI = deafness/cochlear implant; DemAlz = dementia/Alzheimer's (including mild cognitive impairment [MCI]); LIS = locked-in syndrome; TBI = traumatic brain injury; CI = cochlear implant; n/a = not applicable, n/r = not reported.
Strengths
In three of the nine properties, overall study quality was relatively strong.
Control Group
All but three of the 34 studies reviewed included a control group, which was an overall strength of the studies reviewed.
Oxygenation Measure
Twenty-five out of 34 studies reported measurements of both O2Hb and HHb. This has been shown to be the best correlate of neural activity, and in fact, the signal can be misinterpreted (due to spontaneous hemodynamic changes) if both chromophores are not taken into consideration, particularly for anterior temporal regions important for speech and language (Zimeo Morais et al., 2017). Though many of the studies reviewed reported both chromophores, all fNIRS studies can improve quality by reporting and analyzing O2Hb and HHb concentrations.
Motion Correction
Twenty-three of the 34 studies used some type of motion correction, which is an important step to avoid misinterpretation, as rises in the signal may be due to head motion and not to hemodynamic changes. Though the use of motion correction improves quality, not all motion correction techniques are equal. Many techniques exist, and many recent advances have been made. At the current time, a new hybrid method based on spline interpolation and Savitzky–Golay filtering (Jahani et al., 2018) is the gold standard, but it depends on the nature of the motion. For example, for speech tasks, the motion is correlated with the stimulus (not due to spontaneous head movements), and hybrid approaches may be appropriate (cf. Novi et al., 2020).
Weaknesses
In six of the nine properties, overall study quality was relatively weak, and future studies can address these areas to advance fNIRS research and address replicability.
Number of Participants
Only 12 of the 34 studies reviewed had more than 20 participants, and some had as few as four or five. Given that fNIRS is safe, noninvasive, low cost, and portable, studies with larger sample sizes should be easier in these regards than with other neuroimaging techniques (though increasing sample size may be more challenging for some categories of speech-language impairment than others).
ROI–Probe Match
Nineteen of the 34 studies demonstrated a careful consideration for the design of the fNIRS probe and how well it covered the brain ROIs. Of the remaining 15 studies, many did not discuss the probe design in sufficient detail to determine how well the probe matched the intended ROIs. For other studies, the probe design was too small to cover the ROIs relevant to the task. In future studies, the probe should be carefully designed to cover the brain ROIs relevant to the task, and the reporting of the results should provide sufficient detail to assess the ROI–probe match. The ROI–probe match information is also important so that other researchers can replicate these studies.
Systemic Physiology
Only 21 of the 34 studies reviewed applied filters to remove noise in the signal arising from systemic physiology (e.g., blood pressure, respiration). Systemic physiology in the signal can lead to misinterpretation of the results if not filtered. Bandpass filtering to remove this source of noise should be a standard step of preprocessing NIRS data to reduce noise and improve quality.
Short Separation Detectors
Short separation detectors are essential to reduce the significant signal contamination that comes from the hemodynamics of the superficial layers of the scalp and skull (Gagnon et al., 2011). Though the technique was established around 2009–2011, none of the studies reviewed used short separation detectors. This is an area in which future studies can greatly improve the methodological quality and reduce the risk of misinterpretation of the data.
Sampling Rate
The sampling rate should be 5 Hz or higher. This is necessary to control for cardiac oscillations, which will occur at a rate lower than 5 Hz and can then be filtered out of the signal. Eight studies did not report the sampling rate. Seven studies sampled at a rate lower than 5 Hz. Nineteen studies had sampling rates of 5 Hz or higher, ranging from 5 to 25 Hz. Sampling higher than 5 Hz and reporting the sampling rate can improve the quality and reliability of fNIRS studies.
3D Digitization
By digitizing the locations of the optodes after the cap is placed on the participant's head, we can verify how well the probe covers the intended brain regions for each individual participant. Individual participants will vary in terms of head size and shape, which affects the placement of the cap and probe. With digital information about the placement of the probe for each participant, researchers can assure that the probe covered the intended brain ROI. Researchers can also use this information to have a systematic and objective way to exclude data from participants whose caps were not placed with fidelity. 3D digitization also allows researchers to render an anatomical representation (brain image) of the results of the study (Aasted et al., 2015). These are important factors in ensuring the quality of future fNIRS studies.
Discussion
The results of this systematic review suggest that fNIRS is feasible with individuals with speech and language impairment across a wide range of ages and categories of impairment. In this section, we discuss the potential clinical applications of fNIRS.
What Are the Clinical Applications of fNIRS in Assessing and Treating Speech-Language Impairment?
The clinical translation of neuroimaging studies currently faces two major challenges: high costs (which have not come down over time) and low ecological validity. Shuster (2018) notes that the cost of a brain magnetic resonance imaging may range between $1,000 and $5,000 according to CostHelper Health (http://health.costhelper.com). While the cost of a magnetic resonance imaging machine is in the range of millions of dollars, fNIRS systems range in cost from $25,000 to $250,000, which could translate to lower cost at a consumer level and greater potential for clinical utility. Despite its growing use in research, fNIRS is not yet widely applied in clinical settings (Bortfeld et al., 2007; Lloyd-Fox et al., 2014; Mahmoudzadeh et al., 2013; Olds et al., 2016; Villringer et al., 1993; Zaramella et al., 2001). Though fNIRS is in its infancy as a neuroimaging technique, it may be a clinically useful tool for the identification of biomarkers associated with patterns of deficits, outcomes, or response to treatment. Like any new technique, however, it is important to establish the sensitivity and specificity, which includes replication studies. Through our systematic review, four main areas stood out in which fNIRS may have important clinical applications: (a) early and differential diagnosis, (b) response to treatment and patterns of recovery, (c) neuroprosthetic functioning, and (d) neurofeedback.
Early and Differential Diagnosis
Early detection and accurate diagnosis of speech and language disorders is fundamental to treatment and recovery outcomes. Biomarkers of speech and language impairment based on patterns of brain activation may improve early and differential diagnosis of speech and language impairment. fNIRS has been used to reveal patterns of neural activation that could serve as a potential biomarker of early MCI (Katzorke et al., 2018) and ASD in very young infants (Lloyd-Fox et al., 2017). Though fNIRS research with infants at high risk for ASD is emerging, fNIRS (and EEG) studies have begun to converge on potential biomarkers, such as atypical functional connectivity (Keehn et al., 2013) that could serve as a reliable diagnostic tool in very young children (under the age of 3 years) with ASD (Zwaigenbaum et al., 2016). The early detection of ASD significantly improves prognosis (Reiersen, 2017), but it is complicated by phenotypic and genetic overlap between neurodevelopmental disorders (Lichtenstein et al., 2010; Pettersson et al., 2013).
Response to Treatment
A fundamental question in speech-language pathology is who will respond to treatment. A related question is which regions of the brain support the changes brought about by interventions when brain regions that typically support speech and language are impaired. For example, in an fMRI study of response to naming treatment in aphasia, researchers found that more highly preserved global and left temporal lobe structural brain networks were associated with better treatment outcomes (Bonilha et al., 2016; see Kiran & Thompson, 2019, for an overview of neuroplasticity of language networks in aphasia for additional examples).
Questions of response to treatment can uniquely be addressed by longitudinal neuroimaging studies. Longitudinal monitoring of brain changes in response to therapy is highly compatible with fNIRS because repeated measurements in close time intervals are affordable and well tolerated (Dieler et al., 2012; Keenan et al., 2002). fNIRS has a range of clinical applications including providing neurobiological information that could improve our understanding of response to treatment.
Neuroprosthetic Functioning
Neuroprosthetic devices, such as CIs, require initial and repeated calibration to support proper functioning. fNIRS may be a promising clinical tool to guide postimplant programming that could improve the speech and language outcomes of CI recipients (Basura et al., 2018; Saliba et al., 2016). These authors note that, for a child who is a CI recipient, fNIRS could help indicate that the language areas of the brain are appropriately activated, which could then improve the child's chances for learning speech and language. Similarly, it could aid in early detection of child CI recipients who may be on a suboptimal language development trajectory. fNIRS could also improve neuroprosthetic functioning for young infants who are CI recipients and not yet able to provide a verbal or behavioral response, which is required for current calibration techniques. In this way, fNIRS could provide an objective measurement of brain activation in speech-related areas to monitor and predict language development in very young CI users (Saliba et al., 2016). Given that fNIRS is safe, noninvasive, low cost, and portable, it is compatible with repeated measurements that would be required of CI calibration and ongoing recalibration. Saliba et al. (2016) discuss fNIRS as an “exciting possibility” in current clinical practice of CI programming and speech and language therapy for CI recipients.
Neurofeedback
A person's ability to make use of real-time visual neurofeedback to exercise voluntary control over their own brain activation would have useful therapeutic applications in the future given stronger evidence of the effectiveness in clinical practice. Because of increased accessibility, increased portability, and lower cost of fNIRS compared to fMRI, there is growing interest in fNIRS-based neurofeedback. Mihara et al. (2012) used fNIRS in a neurofeedback application with participants who got real or sham feedback about changes in blood oxygenation during a motor imagery task. They found that participants who got real feedback showed significantly greater activation compared to participants who got sham feedback. Kober et al. (2014) replicated the Mihara et al. study to investigate the efficacy of fNIRS-based neurofeedback training over eight training sessions. They found that participants who received real neurofeedback showed more specific (vs. diffuse) brain activation, which increased in specificity over the eight training sessions compared to participants who got sham feedback over eight sessions. Though fNIRS is very new as a therapeutic tool for neurofeedback, so far, the findings suggest that it may be a promising tool for increasing voluntary control over specific brain regions. The clinical applications of fNIRS for neurofeedback could increase the effectiveness of treatment, but more evidence is needed to evaluate the feasibility and effectiveness.
Limitations of This Study
Because fNIRS is an emerging neuroimaging technique, many of the studies included in this review are feasibility studies that have a low number of participants, may lack a control group, and use descriptive rather than analytic or predictive data analysis approaches. fNIRS methods and data analysis techniques are quickly advancing and have changed markedly over the past 5–10 years (Tak & Ye, 2014). This posed particular challenges to carrying out the quality analysis, so our quality analysis appraises the methodological quality rather than directly assessing the evidence, but we think it is an important first step. Though the authors have expertise across different etiologies and different ends of the life span, a more complete discussion of the clinical applications of fNIRS across the broad range of speech and language impairments and levels of severity is beyond the scope of this review. In addition, some etiologies that are within the scope of practice of speech-language pathology fell out of the scope of this review. Finally, a more objective approach to deciding which studies to include in the first phase of this systematic review could have been adopted.
Conclusion
In this systematic review, we analyzed 34 empirical studies that examined the neural correlates of speech and language impairment using fNIRS. These 34 studies span nine different categories of speech or language impairment across the life span. Despite inherent challenges in adapting and using a new technique such as fNIRS and specific challenges for using it with an individual with speech or language impairment, the results of this systematic review suggest that it is feasible with a range of ages across diverse categories of speech-language impairment. Our quality analysis showed that there is a lot of variation in the methodological quality of fNIRS studies, which may affect the interpretation of the results. We outlined ways in which future fNIRS studies can improve in quality. Despite challenges, fNIRS has the advantages of affordability and suitability for repeated measurements in close intervals. It can be used in ecologically valid contexts and with populations that are incompatible with other neuroimaging techniques. fNIRS may have a range of clinical applications including providing neurophysiological evidence that could improve early detection, response to treatment, neuroprosthetic functioning, and neurofeedback, but much more research is needed to evaluate the evidence in these areas.
Acknowledgments
L. K. B. was supported by an NIH-NIDCD grant (T32 DC013017 (PI Christopher A. Moore). This work was supported by NIH-NIDCD Grant 1P50 DC012283 to SK. HTF was supported by NIH-NIDCD Grants P50 DC13027 (PI), P50 18006 (PI), and R01 DC10290 (PI). We thank Meryem A. Yücel and members of the Boston University Neurophotonics Center and the Aphasia Research Lab for discussion of this article. We thank the Neurophotonics Center for permission to reproduce images.
Funding Statement
L. K. B. was supported by an NIH-NIDCD grant (T32 DC013017 (PI Christopher A. Moore). This work was supported by NIH-NIDCD Grant 1P50 DC012283 to SK. HTF was supported by NIH-NIDCD Grants P50 DC13027 (PI), P50 18006 (PI), and R01 DC10290 (PI).
References
- Aasted, C. M. , Yücel, M. A. , Cooper, R. J. , Dubb, J. , Tsuzuki, D. , Becerra, L. , Petkov, M. P. , Borsook, D. , Dan, I. , & Boas, D. A. (2015). Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics, 2(2), Article 020801. https://doi.org/10.1117/1.NPh.2.2.020801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association. (2016). Scope of practice in speech-language pathology [Scope of practice]. http://www.asha.org/policy
- Anderson, C. A. , Wiggins, I. M. , Kitterick, P. T. , & Hartley, D. E. H. (2017). Adaptive benefit of cross-modal plasticity following cochlear implantation in deaf adults. Proceedings of the National Academy of Sciences of the United States of America, 114(38), 10256–10261. https://doi.org/10.1073/pnas.1704785114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, H. , Takano, M. , Miyakawa, K. , Ota, T. , Takahashi, T. , Asaka, H. , & Kawaguchi, T. (2006). A quantitative near-infrared spectroscopy study: A decrease in cerebral hemoglobin oxygenation in Alzheimer's disease and mild cognitive impairment. Brain and Cognition, 61(2), 189–194. https://doi.org/10.1016/j.bandc.2005.12.012 [DOI] [PubMed] [Google Scholar]
- Arenth, P. M. , Ricker, J. H. , & Schultheis, M. T. (2007). Applications of functional near-infrared spectroscopy (fNIRS) to neurorehabilitation of cognitive disabilities. The Clinical Neuropsychologist, 21(1), 38–57. https://doi.org/10.1080/13854040600878785 [DOI] [PubMed] [Google Scholar]
- Aslin, R. N. , Shukla, M. , & Emberson, L. L. (2015). Hemodynamic correlates of cognition in human infants. Annual Review of Psychology, 66, 349–379. https://doi.org/10.1146/annurev-psych-010213-115108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars, B. J. , & Gage, N. M. (2013). Fundamentals of cognitive neuroscience: A beginner's guide. Academic Press. [Google Scholar]
- Bandettini, P. A. (2012). Twenty years of functional MRI: The science and the stories. NeuroImage, 62(2), 575–588. https://doi.org/10.1016/j.neuroimage.2012.04.026 [DOI] [PubMed] [Google Scholar]
- Basura, G. J. , Hu, X.-S. , San Juan, J. , Tessier, A.-M. , & Kovelman, I. (2018). Human central auditory plasticity: A review of functional near-infrared spectroscopy (fNIRS) to measure cochlear implant performance and tinnitus perception. Laryngoscope Investigative Otolaryngology, 3(6), 463–472. https://doi.org/10.1002/lio2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, P. M. (2010). Neuroimaging research: Brain–behavior relationships and language. The ASHA Leader, 15(8), 18–21. https://doi.org/10.1044/leader.FTR3.15082010.18 [Google Scholar]
- Bisconti, S. , Shulkin, M. , Hu, X. , Basura, G. J. , Kileny, P. R. , & Kovelman, I. (2016). Functional near-infrared spectroscopy brain imaging investigation of phonological awareness and passage comprehension abilities in adult recipients of cochlear implants. Journal of Speech, Language, and Hearing Research, 59(2), 239–253. https://doi.org/10.1044/2015_JSLHR-L-14-0278 [DOI] [PubMed] [Google Scholar]
- Boas, D. A. , Elwell, C. E. , Ferrari, M. , & Taga, G. (2014). Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. NeuroImage, 85, 1–5. https://doi.org/10.1016/j.neuroimage.2013.11.033 [DOI] [PubMed] [Google Scholar]
- Bonilha, L. , Gleichgerrcht, E. , Nesland, T. , Rorden, C. , & Fridriksson, J. (2016). Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabilitation and Neural Repair, 30(3), 266–279. https://doi.org/10.1177/1545968315593808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld, H. , Wruck, E. , & Boas, D. A. (2007). Assessing infants' cortical response to speech using near-infrared spectroscopy. NeuroImage, 34(1), 407–415. https://doi.org/10.1016/j.neuroimage.2006.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadoi, S. , Ceccherini, L. , Cutini, S. , Scarpa, F. , Scatturin, P. , Selb, J. , Gagnon, L. , Boas, D. A. , & Cooper, R. J. (2014). Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. NeuroImage, 85, 181–191. https://doi.org/10.1016/j.neuroimage.2013.04.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliandro, P. , Silvestri, G. , Padua, L. , Bianchi, M. L. E. , Simbolotti, C. , Russo, G. , Masciullo, M. , & Rossini, P. M. (2013). fNIRS evaluation during a phonemic verbal task reveals prefrontal hypometabolism in patients affected by myotonic dystrophy type 1. Clinical Neurophysiology, 124(11), 2269–2276. https://doi.org/10.1016/j.clinph.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Chance, B. , Anday, E. , Nioka, S. , Zhou, S. , Hong, L. , Worden, K. , Li, C. , Murray, T. , Ovetsky, Y. , Pidikiti, D. , & Thomas, R. (1998). A novel method for fast imaging of brain function, non-invasively, with light. Optics Express, 2(10), 411–423. https://doi.org/10.1364/OE.2.000411 [DOI] [PubMed] [Google Scholar]
- Chang, S.-E. (2014). Research updates in neuroimaging studies of children who stutter. Seminars in Speech and Language, 35(2), 067–079. https://doi.org/10.1055/s-0034-1382151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, U. , Xia, B. , Silvoni, S. , Cohen, L. G. , & Birbaumer, N. (2017). Brain–computer interface-based communication in the completely locked-in state. PLOS Biology, 15(1), Article e1002593. https://doi.org/10.1371/journal.pbio.1002593 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chiarelli, A. M. , Maclin, E. L. , Fabiani, M. , & Gratton, G. (2015). A kurtosis-based wavelet algorithm for motion artifact correction of fNIRS data. NeuroImage, 112, 112–137. https://doi.org/10.1016/j.neuroimage.2015.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard, B. , Boliek, C. , & Cummine, J. (2016). How to interpret and critique neuroimaging research: A tutorial on use of functional magnetic resonance imaging in clinical populations. American Journal of Speech-Language Pathology, 25(3), 269–289. https://doi.org/10.1044/2016_AJSLP-15-0013 [DOI] [PubMed] [Google Scholar]
- Cooper, R. J. (2018, November). Physiological interference & short-separation regression [Technical report]. Boston University Neurophotonics Center. [Google Scholar]
- Cooper, R. J. , Selb, J. , Gagnon, L. , Phillip, D. , Schytz, H. W. , Iversen, H. K. , Ashina, M. , & Boas, D. A. (2012). A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Frontiers in Neuroscience, 6, 147 https://doi.org/10.3389/fnins.2012.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, X. , Bray, S. , Bryant, D. M. , Glover, G. H. , & Reiss, A. L. (2011). A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage, 54(4), 2808–2821. https://doi.org/10.1016/j.neuroimage.2010.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutini, S. , Szücs, D. , Mead, N. , Huss, M. , & Goswami, U. (2016). Atypical right hemisphere response to slow temporal modulations in children with developmental dyslexia. NeuroImage, 143, 40–49. https://doi.org/10.1016/j.neuroimage.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado Reyes, L. M. , Bohache, K. , Wijeakumar, S. , & Spencer, J. P. (2018). Evaluating motion processing algorithms for use with functional near-infrared spectroscopy data from young children. Neurophotonics, 5(2), Article 025008. https://doi.org/10.1117/1.NPh.5.2.025008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, R. S. , & Hartley, D. E. H. (2015). Cortical cross-modal plasticity following deafness measured using functional near-infrared spectroscopy. Hearing Research, 325, 55–63. https://doi.org/10.1016/j.heares.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Dieler, A. C. , Tupak, S. V. , & Fallgatter, A. J. (2012). Functional near-infrared spectroscopy for the assessment of speech related tasks. Brain and Language, 121, 90–109. https://doi.org/10.1016/j.bandl.2011.03.005 [DOI] [PubMed] [Google Scholar]
- D'Mello, A. M. , & Gabrieli, J. D. E. (2018). Cognitive neuroscience of dyslexia. Language, Speech, and Hearing Services in Schools, 49(4), 798–809. https://doi.org/10.1044/2018_LSHSS-DYSLC-18-0020 [DOI] [PubMed] [Google Scholar]
- Edwards, L. A. , Wagner, J. B. , Tager-Flusberg, H. , & Nelson, C. A. (2017). Differences in neural correlates of speech perception in 3 month olds at high and low risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 47, 3125–3138. https://doi.org/10.1007/s10803-017-3222-1 [DOI] [PubMed] [Google Scholar]
- Etchell, A. C. , Civier, O. , Ballard, K. J. , & Sowman, P. F. (2018). A systematic literature review of neuroimaging research on developmental stuttering between 1995 and 2016. Journal of Fluency Disorders, 55, 6–45. https://doi.org/10.1016/j.jfludis.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Fallgatter, A. J. , Sitzmann, M. R. L. , Heidrich, A. , Mueller, T. J. , & Strik, W. K. (1997). Loss of functional hemispheric asymmetry in Alzheimer's dementia assessed with near-infrared spectroscopy. Cognitive Brain Research, 6(1), 67–72. https://doi.org/10.1016/S0926-6410(97)00016-5 [DOI] [PubMed] [Google Scholar]
- Ferrari, M. , & Quaresima, V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage, 63, 921–935. https://doi.org/10.1016/j.neuroimage.2012.03.049 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Frith, C. D. , Turner, R. , & Frackowiak, R. S. J. (1995). Characterizing evoked hemodynamics with fMRI. NeuroImage, 2(2), 157–165. https://doi.org/10.1006/nimg.1995.1018 [DOI] [PubMed] [Google Scholar]
- Fu, G. , Wan, N. J. A. , Baker, J. M. , Montgomery, J. W. , Evans, J. L. , & Gillam, R. B. (2016). A proof of concept study of function-based statistical analysis of fNIRS data: Syntax comprehension in children with specific language impairment compared to typically-developing controls. Frontiers in Behavioral Neuroscience, 10(108). https://doi.org/10.3389/fnbeh.2016.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki, Y. , Murai, T. , & Toichi, M. (2012). Cortical activation during attention to sound in autism spectrum disorders. Research in Developmental Disabilities, 33(2), 518–524. https://doi.org/10.1016/j.ridd.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Gagnon, L. , Perdue, K. L. , Greve, D. N. , Goldenholz, D. , Kaskhedikar, G. , & Boas, D. A. (2011). Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. NeuroImage, 56(3), 1362–1371. https://doi.org/10.1016/j.neuroimage.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, L. , Yücel, M. A. , Boas, D. A. , & Cooper, R. J. (2014). Further improvement in reducing superficial contamination in NIRS using double short separation measurements. NeuroImage, 85(1), 127–135. https://doi.org/10.1016/j.neuroimage.2013.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain, J. (2014). Near-infrared spectroscopy: Recent advances in infant speech perception and language acquisition research. Frontiers in Psychology, 5(1–2), 916 https://doi.org/10.3389/fpsyg.2014.00916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain, J. , Mehler, J. , Werker, J. F. , Nelson, C. A. , Csibra, G. , Lloyd-Fox, S. , Shukla, M. , & Aslin, R. N. (2011). Near-infrared spectroscopy: A report from the McDonnell infant methodology consortium. Developmental Cognitive Neuroscience, 1(1), 22–46. https://doi.org/10.1016/j.dcn.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley, P. M. , Sharma, A. , Dorman, M. , Finley, C. C. , Panch, A. S. , & Martin, K. (2006). Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clinical Neurophysiology, 117(8), 1772–1782. https://doi.org/10.1016/j.clinph.2006.04.018 [DOI] [PubMed] [Google Scholar]
- Hara, T. , Abo, M. , Kakita, K. , Mori, Y. , Yoshida, M. , & Sasaki, N. (2017). The effect of selective transcranial magnetic stimulation with functional near-infrared spectroscopy and intensive speech therapy on individuals with post-stroke aphasia. European Neurology, 77(3–4), 186–194. https://doi.org/10.1159/000457901 [DOI] [PubMed] [Google Scholar]
- Hock, C. , Villringer, K. , Müller-Spahn, F. , Wenzel, R. , Heekeren, H. , Schuh-Hofer, S. , Hofmann, M. , Minoshima, S. , Schwaiger, M. , Dirnagl, U. , & Villringer, A. (1997). Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer's disease monitored by means of near-infrared spectroscopy (NIRS)—Correlation with simultaneous rCBF-PET measurements. Brain Research, 755(2), 293–303. https://doi.org/10.1016/S0006-8993(97)00122-4 [DOI] [PubMed] [Google Scholar]
- Hoshi, Y. (2003). Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology, 40(4), 511–520. https://doi.org/10.1111/1469-8986.00053 [DOI] [PubMed] [Google Scholar]
- Hosseini, R. , Walsh, B. , Tian, F. , & Wang, S. (2018). An fNIRS-based feature learning and classification framework to distinguish hemodynamic patterns in children who stutter. IEEE Transactions on Neural Systems Rehabilitation Engineering, 26(6), 1254–1263. https://doi.org/10.1109/TNSRE.2018.2829083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, T. J. (2016). Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics, 3(1), Article 010401. https://doi.org/10.1117/1.NPh.3.1.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, T. J. , Diamond, S. G. , Franceschini, M. A. , & Boas, D. A. (2009). HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics, 48(10), D280–D298. https://doi.org/10.1364/AO.48.00D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham, R. J. (Ed.). (2008). Neuroimaging in communication sciences and disorders. Plural. [Google Scholar]
- Irani, F. , Platek, S. M. , Bunce, S. , Ruocco, A. C. , & Chute, D. (2007). Functional near-infrared spectroscopy (fNIRS): An emerging neuroimaging technology with important applications for the study of brain disorders. The Clinical Neuropsychologist, 21(1), 9–37. https://doi.org/10.1080/13854040600910018 [DOI] [PubMed] [Google Scholar]
- Issard, C. , & Gervain, J. (2018). Variability of the hemodynamic response in infants: Influence of experimental design and stimulus complexity. Developmental Cognitive Neuroscience, 33, 182–193. https://doi.org/10.1016/j.dcn.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami, A. , Okajima, Y. , Ota, H. , Tani, M. , Yamada, T. , Hashimoro, R. , Kanai, C. , Watanabe, H. , Yamasue, H. , Kawakubo, Y. , & Kato, N. (2011). Task dependent prefrontal dysfunction in persons with Asperger's disorder investigated with multi-channel near-infrared spectroscopy. Research in Autism Spectrum Disorders, 5(3), 1187–1193. https://doi.org/10.1016/j.rasd.2011.01.005 [Google Scholar]
- Izzetoglu, K. , Bunce, S. , Onaral, B. , Pourrezaei, K. , & Chance, B. (2004). Functional optical brain imaging using near-infrared during cognitive tasks. International Journal of Human–Computer Interaction, 17(2), 211–227. https://doi.org/10.1207/s15327590ijhc1702_6 [Google Scholar]
- Jahani, S. , Setarehdan, S. K. , Boas, D. A. , & Yücel, M. A. (2018). Motion artifact detection and correction in functional near-infrared spectroscopy: A new hybrid method based on spline interpolation method and Savitzky–Golay filtering. Neurophotonics, 5(1), Article 015003. https://doi.org/10.1117/1.NPh.5.1.015003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis, F. F. (1977). Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science, 198, 1264–1267. https://doi.org/10.1126/science.929199 [DOI] [PubMed] [Google Scholar]
- Kassab, A. , Le Lan, J. , Tremblay, J. , Vannasing, P. , Dehbozorgi, M. , Pouliot, P. , Gallagher, A. , Lesage, F. , Sawan, M. , & Nguyen, D. K. (2018). Multichannel wearable fNIRS-EEG system for long-term clinical monitoring. Human Brain Mapping, 39(1), 7–23. https://doi.org/10.1002/hbm.23849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzorke, A. , Zeller, J. B. M. , Müller, L. D. , Lauer, M. , Polak, T. , Deckert, J. , & Herrmann, M. J. (2018). Decreased hemodynamic response in inferior frontotemporal regions in elderly with mild cognitive impairment. Psychiatry Research: Neuroimaging, 274, 11–18. https://doi.org/10.1016/j.pscychresns.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Kawakubo, Y. , Kuwabara, H. , Watanabe, K. , Minowa, M. , Someya, T. , Minowa, I. , Kono, T. , Nishida, H. , Sugiyama, T. , Kato, N. , & Kasai, K. (2009). Impaired prefrontal hemodynamic maturation in autism and unaffected siblings. PLOS ONE, 4, 6–13. https://doi.org/10.1371/journal.pone.0006881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn, B. , Wagner, J. B. , Tager-Flusberg, H. , & Nelson, C. A. (2013). Functional connectivity in the first year of life in infants at-risk for autism: A preliminary near-infrared spectroscopy study. Frontiers in Human Neuroscience, 7, 444 https://doi.org/10.3389/fnhum.2013.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, R. P. , Kim, D. , Maki, A. , Koizumi, H. , & Constable, R. T. (2002). Non-invasive assessment of language lateralization by transcranial near infrared optical topography and functional MRI. Human Brain Mapping, 16(3), 183–189. https://doi.org/10.1002/hbm.10039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran, S. , & Thompson, C. K. (2019). Neuroplasticity of language networks in aphasia: Advances, updates, and future challenges. Frontiers in Neurology, 10, 295 https://doi.org/10.3389/fneur.2019.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim, J. A. , & Jones, T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research, 51(1), S225–S239. https://doi.org/10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- Kleinschmidt, A. , Obrig, H. , Requardt, M. , Merboldt, K.-D. , Dirnagl, U. , Villrunger, A. , & Frahm, J. (1996). Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. Journal of Cerebral Blood Flow & Metabolism, 16(5), 817–826. https://doi.org/10.1097/00004647-199609000-00006 [DOI] [PubMed] [Google Scholar]
- Kober, S. E. , Wood, G. , Kurzmann, J. , Friedrich, E. V. C. , Stangl, M. , Wippel, T. , Väljamäe, A. , & Neuper, C. (2014). Near-infrared spectroscopy based neurofeedback training increases specific motor imagery related cortical activation compared to sham feedback. Biological Psychology, 95, 21–30. https://doi.org/10.1016/j.biopsycho.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Kovelman, I. , Mascho, K. , Millott, L. , Mastic, A. , Moiseff, B. H. , & Shalinsky, M. (2012). At the rhythm of language: Brain bases of language-related frequency perception in children. NeuroImage, 60(1), 673–682. https://doi.org/10.1016/j.neuroimage.2011.12.066 [DOI] [PubMed] [Google Scholar]
- Kuwabara, H. , Kasai, K. , Takizawa, R. , Kawakubo, Y. , Yamasue, H. , Rogers, M. A. , Ishijima, M. , Watanabe, K. , & Kato, N. (2006). Decreased prefrontal activation during letter fluency task in adults with pervasive developmental disorders: A near-infrared spectroscopy study. Behavioral Brain Research, 172(2), 272–277. https://doi.org/10.1016/j.bbr.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Lichtenstein, P. , Carlström, E. , Råstam, M. , Gillberg, C. , & Anckarsäter, H. (2010). The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. American Journal of Psychiatry, 167(11), 1357–1363. https://doi.org/10.1176/appi.ajp.2010.10020223 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Liu, X. , Yi, L. , Zhu, C. , Markey, P. S. , & Pelowski, M. (2019). Assessing autism at its social and developmental roots: A review of autism spectrum disorder studies using functional near-infrared spectroscopy. NeuroImage, 185, 955–967. https://doi.org/10.1016/j.neuroimage.2017.09.044 [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox, S. , Blasi, A. , & Elwell, C. E. (2010). Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neuroscience & Biobehavioral Reviews, 34(3), 269–284. https://doi.org/10.1016/j.neubiorev.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox, S. , Blasi, A. , Pasco, G. , Gliga, T. , Jones, E. J. H. , Murphy, D. G. M. , Elwell, C. E. , Charman, T. , & Johnson, M. H. (2017). Cortical responses before 6 months of life associate with later autism. European Journal of Neuroscience, 47(6), 736–749. https://doi.org/10.1111/ejn.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox, S. , Papademetriou, M. , Darboe, M. K. , Everdell, N. L. , Wegmuller, R. , Prentice, A. M. , Moore, S. E. , & Elwell, C. E. (2014). Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Scientific Reports, 4, Article 4740. https://doi.org/10.1038/srep04740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow, C. L. (2012). Using neuroimaging and neuromodulation to study changes in brain functioning with therapy. Seminars in Speech and Language, 33(3), 175–187. https://doi.org/10.1055/s-0032-1320038 [DOI] [PubMed] [Google Scholar]
- Mahmoudzadeh, M. , Dehaene-Lambertz, G. , Fournier, M. , Kongolo, G. , Goudjil, S. , Dubois, J. , Grebe, R. , & Wallois, F. (2013). Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proceedings of the National Academy of Sciences of the United States of America, 110(12), 4846–4851. https://doi.org/10.1073/pnas.1212220110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes, A. K. , Reilly, S. , & Morgan, A. T. (2015). Neural correlates of childhood language disorder: A systematic review. Developmental Medicine & Child Neurology, 57(8), 706–717. https://doi.org/10.1111/dmcn.12714 [DOI] [PubMed] [Google Scholar]
- Mazzoni, A. , Grove, R. , Eapen, V. , Lenroot, R. K. , & Bruggemann, J. (2018). The promise of functional near-infrared spectroscopy in autism research: What do we know and where do we go? Social Neuroscience, 14(5), 505–518. https://doi.org/10.1080/17470919.2018.1497701 [DOI] [PubMed] [Google Scholar]
- Metzger, F. G. , Ehlis, A.-C. , Haeussinger, F. B. , Fallgatter, A. J. , & Hagen, K. (2015). Effects of cholinesterase inhibitor on brain activation in Alzheimer's patients measured with functional near-infrared spectroscopy. Psychopharmacology, 232(23), 4383–4391. https://doi.org/10.1007/s00213-015-4066-z [DOI] [PubMed] [Google Scholar]
- Mihara, M. , Miyai, I. , Hattori, N. , Hatakenaka, M. , Yagura, H. , Kawano, T. , Okibayashi, M. , Danjo, N. , Ishikawa, A. , Inoue, Y. , & Kubota, K. (2012). Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLOS ONE, 7(3), Article e32234. https://doi.org/10.1371/journal.pone.0032234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai, Y. , Mori, K. , Hebden, J. C. , & Dupoux, E. (2008). Optical imaging of infants' neurocognitive development: Recent advances and perspectives. Developmental Neurobiology, 68(6), 712–728. https://doi.org/10.1002/dneu.20618 [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai, Y. , Naoi, N. , Kikuchi, N. , Yamamoto, J. , Nakamura, K. , & Kojima, S. (2009). Cerebral laterality for phonemic and prosodic cue decoding in children with autism. NeuroReport, 20(13), 1219–1224. https://doi.org/10.1097/WNR.0b013e32832fa65f [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , & Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLOS Medicine, 6(7), Article e1000097. https://doi.org/10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, A. , Bonthrone, A. , & Liégeois, F. J. (2016). Brain basis of childhood speech and language disorders: Are we closer to clinically meaningful MRI markers? Current Opinion in Pediatrics, 28(6), 725–730. https://doi.org/10.1097/MOP.0000000000000420 [DOI] [PubMed] [Google Scholar]
- Naito, M. , Michioka, Y. , Ozawa, K. , Ito, Y. , Kiguchi, M. , & Kanazawa, T. (2007). A communication means for totally locked-in ALS patients based on changes in cerebral blood volume measured with near-infrared light. IEICE Transactions on Information and Systems, E90(7), 1028–1037. https://doi.org/10.1093/ietisy/e90-d.7.1028 [Google Scholar]
- Narayanan, L. , & Murray, A. D. (2016). What can imaging tell us about cognitive impairment and dementia? World Journal of Radiology, 8(3), 240–254. https://doi.org/10.4329/wjr.v8.i3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseer, N. , & Hong, K. (2015). fNIRS-based brain–computer interfaces: A review. Frontiers in Human Neuroscience, 9(3). https://doi.org/10.3389/fnhum.2015.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novi, S. L. , Roberts, E. , Spagnuolo, D. , Spilsbury, B. M. , Price, D. C. , Imbalzano, C. A. , Forero, E. , Yodh, A. G. , Tellis, G. M. , Tellis, C. M. , & Mesquita, R. C. (2020). Functional near-infrared spectroscopy for speech protocols: Characterization of motion artifacts and guidelines for improving data analysis. Neurophotonics, 7(1), Article 015001. https://doi.org/10.1117/1.NPh.7.1.015001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig, H. (2014). NIRS in clinical neurology—A “promising” tool? NeuroImage, 85, 535–546. https://doi.org/10.1016/j.neuroimage.2013.03.045 [DOI] [PubMed] [Google Scholar]
- Obrig, H. , & Villringer, A. (2003). Beyond the visible—Imaging the human brain with light. Journal of Cerebral Blood Flow & Metabolism, 23(1), 1–18. https://doi.org/10.1097/01.WCB.0000043472.45775.29 [DOI] [PubMed] [Google Scholar]
- Oh, C. , & LaPointe, L. (2017). “Where is dementia?” A systematic literature review exploring neuroanatomical aspects of dementia. Perspectives of the ASHA Special Interest Groups, 2(15), 9–23. https://doi.org/10.1044/persp2.SIG15.9 [Google Scholar]
- Olds, C. , Pollonini, L. , Abaya, H. , Larky, J. , Loy, M. , Bortfeld, H. , Beauchamp, M. S. , & Oghalai, J. S. (2016). Cortical activation patterns correlate with speech understanding after cochlear implantation. Ear and Hearing, 37(3), e160–e172. https://doi.org/10.1097/AUD.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, A. V. , Safaie, J. , Moghaddam, H. A. , Wallois, F. , & Grebe, R. (2011). Experimental investigations of NIRS spatial sensitivity. Biomedical Optics Express, 2(6), 1478–1493. https://doi.org/10.1364/BOE.2.001478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson, E. , Anckarsäter, H. , Gillberg, C. , & Lichtenstein, P. (2013). Different neurodevelopmental symptoms have a common genetic etiology. The Journal of Child Psychology and Psychiatry, 54(12), 1356–1365. https://doi.org/10.1111/jcpp.12113 [DOI] [PubMed] [Google Scholar]
- Quaresima, V. , Bisconti, S. , & Ferrari, M. (2012). A brief review of the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain and Language, 121(2), 79–89. https://doi.org/10.1016/j.bandl.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. , & Mintun, M. A. (2006). Brain work and brain imaging. Annual Review of Neuroscience, 29, 449–476. https://doi.org/10.1146/annurev.neuro.29.051605.112819 [DOI] [PubMed] [Google Scholar]
- Reiersen, A. M. (2017). Early identification of autism spectrum disorder: Is diagnosis by age 3 a reasonable goal? Journal of the American Academy of Child & Adolescent Psychiatry, 56(4), 284–285. https://doi.org/10.1016/j.jaac.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Rodriguez Merzagora, A. C. , Izzetoglu, M. , Onaral, B. , & Schultheis, M. T. (2014). Verbal working memory impairments following traumatic brain injury: An fNIRS investigation. Brain Imaging and Behavior, 8, 446–459. https://doi.org/10.1007/s11682-013-9258-8 [DOI] [PubMed] [Google Scholar]
- Sakatani, K. , Xie, Y. , Lichty, W. , Li, S. , & Zuo, H. (1998). Language-activated cerebral blood oxygenation and hemodynamic changes of the left prefrontal cortex in poststroke aphasic patients: A near-infrared spectroscopy study. Stroke, 29(7), 1299–1304. https://doi.org/10.1161/01.STR.29.7.1299 [DOI] [PubMed] [Google Scholar]
- Saliba, J. , Bortfeld, H. , Levitin, D. J. , & Oghalai, J. S. (2016). Functional near-infrared spectroscopy for neuroimaging in cochlear implant recipients. Hearing Research, 338, 64–75. https://doi.org/10.1016/j.heares.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y. , Mori, K. , Koizumi, T. , Minagawa-Kawai, Y. , Tanaka, A. , Ozawa, E. , Wakaba, Y. , & Mazuka, R. (2011). Functional lateralization of speech processing in adults and children who stutter. Frontiers in Psychology, 2(70). https://doi.org/10.3389/fpsyg.2011.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann, F. , Kleiser, S. , Metz, A. J. , Zimmermann, R. , Mata Pavia, J. , Wolf, U. , & Wolf, M. (2014). A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage, 85(1), 6–27. https://doi.org/10.1016/j.neuroimage.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Scholkmann, F. , Spichtig, S. , Muehlemann, T. , & Wolf, M. (2010). How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiological Measurement, 31(5), 649–662. https://doi.org/10.1088/0967-3334/31/5/004 [DOI] [PubMed] [Google Scholar]
- Sela, I. , Izzetoglu, M. , Izzetoglu, K. , & Onaral, B. (2014). A functional near-infrared spectroscopy study of lexical decision task supports the dual route model and the phonological deficit theory of dyslexia. Journal of Learning Disabilities, 47(3), 279–288. https://doi.org/10.1177/0022219412451998 [DOI] [PubMed] [Google Scholar]
- Selb, J. (2018, November). Basic fNIRS data analysis with Homer [Technical report]. Boston University Neurophotonics Center.
- Sevy, A. B. , Bortfeld, H. , Huppert, T. J. , Beauchamo, M. S. , Tonini, R. E. , & Oghalai, J. S. (2010). Neuroimaging with near-infrared spectroscopy demonstrates speech-evoked activity in the auditory cortex of deaf children following cochlear implantation. Hearing Research, 270(1–2), 39–47. https://doi.org/10.1016/j.heares.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster, L. I. (2018). Considerations for the use of neuroimaging technologies for predicting recovery of speech and language in aphasia. American Journal of Speech-Language Pathology, 27(1S), 291–305. https://doi.org/10.1044/2018_AJSLP-16-0180 [DOI] [PubMed] [Google Scholar]
- Song, R. , Zhang, J. , Wang, B. , Zhang, H. , & Wu, H. (2014). A near-infrared brain function study of Chinese dyslexic children. Neurocase, 19(4), 382–389. https://doi.org/10.1080/13554794.2012.690422 [DOI] [PubMed] [Google Scholar]
- Strangeman, G. , Culver, J. P. , Thompson, J. H. , & Boas, D. A. (2002). A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage, 17(2), 719–731. https://doi.org/10.1006/nimg.2002.1227 [PubMed] [Google Scholar]
- Tager-Flusberg, H. (2016). Risk factors associated with language in autism spectrum disorder: Clues to underlying mechanisms. Journal of Speech, Language, and Hearing Research, 59(1), 143–154. https://doi.org/10.1044/2015_JSLHR-L-15-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg, H. , & Kasari, C. (2013). Minimally verbal school-aged children with autism spectrum disorders: The neglected end of the spectrum. Autism Research, 6(6), 468–478. https://doi.org/10.1002/aur.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak, S. , & Ye, J. C. (2014). Statistical analysis of fNIRS data: A comprehensive review. NeuroImage, 85(1), 72–91. https://doi.org/10.1016/j.neuroimage.2013.06.016 [DOI] [PubMed] [Google Scholar]
- Talavage, T. M. , Gonzalez-Castillo, J. , & Scott, S. K. (2014). Auditory neuroimaging with fMRI and PET. Hearing Research, 307, 4–15. https://doi.org/10.1016/j.heares.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm, L. , Vuust, P. , Brattico, E. , Agrawal, D. , Debener, S. , Büchner, A. , Dengler, R. , & Wittfoth, M. (2014). Residual neural processing of musical sound features in adult cochlear implant users. Frontiers in Human Neuroscience, 8, 181 https://doi.org/10.3389/fnhum.2014.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki, D. , Cai, D. , Dan, H. , Kyutoku, Y. , Fujita, A. , Watanabe, E. , & Dan, I. (2012). Stable and convenient spatial registration of stand-alone NIRS data through anchor-based probabilistic registration. Neuroscience Research, 72(2), 163–171. https://doi.org/10.1016/j.neures.2011.10.008 [DOI] [PubMed] [Google Scholar]
- van de Rijt, L. P. H. , van Opstal, A. J. , Mylanus, E. A. M. , Straatman, L. V. , Hu, H. Y. , Snik, A. F. M. , & van Wanrooij, M. M. (2016). Temporal cortex activation to audiovisual speech in normal-hearing and cochlear implant users measured with functional near-infrared spectroscopy. Frontiers in Human Neuroscience, 10(48). https://doi.org/10.3389/fnhum.2016.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert, R. E. , & Nelson, C. A. (2014). The use of near-infrared spectroscopy in the study of typical and atypical development. NeuroImage, 85, 264–271. https://doi.org/10.1016/j.neuroimage.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer, A. , & Chance, B. (1997). Non-invasive optical spectroscopy and imaging of human brain function. Trends in Neuroscience, 20(10), 435–442. https://doi.org/10.1016/S0166-2236(97)01132-6 [DOI] [PubMed] [Google Scholar]
- Villringer, A. , Planck, J. , Hock, C. , Schleinkofer, L. , & Dirnagl, U. (1993). Near infrared spectroscopy (NIRS): A new tool to study hemodynamic changes during activation of brain function in human adults. Neuroscience Letters, 154(1–2), 101–104. https://doi.org/10.1016/0304-3940(93)90181-J [DOI] [PubMed] [Google Scholar]
- Walsh, B. , Tian, F. , Tourville, J. A. , Yücel, M. A. , Kuczek, T. , & Bostian, A. J. (2017). Hemodynamics of speech production: An fNIRS investigation of children who stutter. Scientific Reports, 7, Article 4034. https://doi.org/10.1038/s41598-017-04357-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox, T. , & Biondi, M. (2015). fNIRS in the developmental sciences. WIREs Cognitive Science, 6(3), 263–283. https://doi.org/10.1002/wcs.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Yang, Z. , Yuan, T. , Feng, W. , & Wang, P. (2019). A systemic review of functional near-infrared spectroscopy for stroke: Current application and future directions. Frontiers in Neurology, 10(58). https://doi.org/10.3389/fneur.2019.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, M. K. , Lee, T. L. , & Chan, A. S. (2019). Frontal lobe dysfunction underlies the differential word retrieval impairment in adolescents with high-functioning autism. Autism Research, 12(4), 600–613. https://doi.org/10.1002/aur.2082 [DOI] [PubMed] [Google Scholar]
- Yeung, M. K. , Sze, S. L. , Woo, J. , Kwok, T. , Shum, D. H. K. , Yu, R. , & Chan, A. S. (2016). Altered frontal lateralization underlies the category fluency deficits in older adults with mild cognitive impairment: A near-infrared spectroscopy study. Frontiers in Aging Neuroscience, 8(59). https://doi.org/10.3389/fnagi.2016.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel, M. A. (2018, November). Importing true subject anatomy using AtlasViewer [Technical report]. Boston University Neurophotonics Center.
- Yücel, M. A. , Delb, J. , Cooper, R. J. , & Boas, D. A. (2014). Targeted principle component analysis: A new motion artifact correction approach for near-infrared spectroscopy. Journal of Innovative Optical Health Sciences, 7(2). https://doi.org/10.1142/S1793545813500661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel, T. A. , & Chute, D. L. (2002). Educational neuroimaging: A proposed neuropsychological application of near-infrared spectroscopy. The Journal of Head Trauma Rehabilitation, 17(5), 477–488. https://doi.org/10.1097/00001199-200210000-00008 [DOI] [PubMed] [Google Scholar]
- Zaramella, P. , Freato, F. , Amigoni, A. , Salvadori, S. , Marangoni, P. , Suppjei, A. , Schiavo, B. , & Chiandetti, L. (2001). Brain auditory activation measured by near-infrared spectroscopy (NIRS) in neonates. Pediatric Research, 49, 213–219. https://doi.org/10.1203/00006450-200102000-00014 [DOI] [PubMed] [Google Scholar]
- Zhang, F. , & Roeyers, H. (2019). Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies. International Journal of Psychophysiology, 137, 41–53. https://doi.org/10.1016/j.ijpsycho.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Zimeo Morais, G. A. , Scholkmann, F. , Balardin, J. B. , Furucho, R. A. , Viera de Paula, R. C. , Biazoli, C. E. , & Sato, J. R. (2017). Non-neuronal evoked and spontaneous hemodynamic changes in the anterior temporal region of the human head may lead to misinterpretations of functional near-infrared spectroscopy signals. Neurophotonics, 5(1), Article 011002. https://doi.org/10.1117/1.NPh.5.1.011002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum, L. , Bryson, S. E. , Brian, J. , Smith, I. M. , Roberts, W. , Szatmari, P. , Roncadin, C. , Garon, N. , & Vaillancourt, T. (2016). Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Research, 9(7), 790–800. https://doi.org/10.1002/aur.1585 [DOI] [PubMed] [Google Scholar]