Abstract
Comprehensive reviews and large population-based cohort studies have played an important role in the diagnosis and treatment of pancreatitis and its sequelae. The incidence and mortality of pancreatitis have been reduced significantly due to substantial advancements in the pathophysiological mechanisms and clinically effective treatments. The study of extracellular vesicles (EVs) has the potential to identify cell-to-cell communication in diseases such as pancreatitis. Exosomes are a subset of EVs with an average diameter of 50~150 nm. Their diverse and unique constituents include nucleic acids, proteins, and lipids, which can be transferred to trigger phenotypic changes of recipient cells. In recent years, many reports have indicated the role of EVs in pancreatitis, including acute pancreatitis, chronic pancreatitis and autoimmune pancreatitis, suggesting their potential influence on the development and progression of pancreatitis. Plasma exosomes of acute pancreatitis can effectively reach the alveolar cavity and activate alveolar macrophages to cause acute lung injury. Furthermore, upregulated exosomal miRNAs can be used as biomarkers for acute pancreatitis. Here, we summarized the current understanding of EVs in pancreatitis with an emphasis on their biological roles and their potential use as diagnostic biomarkers and therapeutic agents for this disease.
Keywords: pancreatitis, extracellular vesicles, exosomes, biomarkers
Introduction
Pancreatitis refers to an inflammatory disorder of the pancreas, in which pancreatic enzymes damage pancreatic tissue, leading to acinar cell death, as well as local and systemic inflammation 1. Previous studies have shown that acute pancreatitis, recurrent acute pancreatitis, and chronic pancreatitis represent a continuum of disease progression. Per 100,000 people in the general population, the global incidence of acute pancreatitis is 33.74 cases per year and that of chronic pancreatitis is 9.62 cases per year 2. Similar to acute pancreatitis, chronic pancreatitis is most prevalent in middle-aged and older patients 3,4. However, the incidence of chronic pancreatitis was higher among men than women, although there was no significant difference between sexes for acute pancreatitis. The global transition rate data indicated that the transition from the first episode of acute pancreatitis to recurrent acute pancreatitis occurs in approximately 21% of cases and that from recurrent acute pancreatitis to chronic pancreatitis occurs in approximately 36% of cases 5. The global mortality rates of acute pancreatitis and chronic pancreatitis were 1.60 and 0.09 per 100,000 persons per year, respectively 2. Recently, clinical and experimental data have shed light on the pathophysiology of pancreatitis, indicating that premature intrapancreatic activation of digestive proteases is critical in the pathogenesis of pancreatitis 6. Furthermore, the progression and severity of pancreatitis may be influenced by dysregulated autophagy, which promotes the inflammatory response in the pancreas, leading to local and systemic inflammatory responses and multiorgan failure 7. Unfortunately, the sequelae and mortality of pancreatitis remain substantial. Concerted efforts by not only surgeons but also researchers should strive to reduce the incidence of pancreatitis and effectively improve the treatment of its sequelae 8-10.
EVs are cell-derived membranous structures that are present in biological fluids and are involved in physiological and pathological processes of inflammatory disease or cancer 11-15. EVs were initially regarded as membrane debris with no biological function 16. However, in 2007, exosomes were shown to transfer mRNAs and microRNAs to recipient cells, remained functional and changed the behavior of target cells 17. EVs exert their effects on fundamental biological processes by directly merging with the recipient cell plasma membrane and delivering their contents, including transcription factors, oncogenes, microRNAs and mRNAs, into recipient cells 18-20. In this manner, EVs participate in the pathophysiological process of disease, for example, stem cell therapy 21, tissue repair 22, immune surveillance 23, and tumor progression and metastasis 24,25. In addition, several studies have reported the potential applications of EVs in the diagnosis and treatment of disease based on their own characteristics.
Here, we report a comprehensive overview of the relationship between EVs and pancreatitis, with a special focus on their roles in pathogenesis and their potential clinical application as diagnostic biomarkers and therapeutic targets in pancreatitis. We also discuss the advantages and limitations among current studies and the need for further research. Finally, we discuss the prospects and applications of EVs in pancreatitis.
EVs: clinical applications
EVs are regarded as a mechanism for intercellular communication, transferring proteins, lipids and genetic material between cells 26. Based on the current knowledge of their biogenesis by transmission electron microscopy, NanoSight analysis and other biochemical means, EVs can be broadly divided into two main categories: exosomes and microvesicles (MVs) 27,28. In addition, the pathophysiological roles of EVs are applied in the diagnosis of diseases including cancer and inflammatory diseases, especially in their potential treatments for therapeutic intervention 29,30.
Diagnostic potential of EVs
The biomedical applications of EVs take advantage of their contents in the diagnosis and treatment of disease. The characteristic properties of EVs involve delivering functional cargos to diseased cells or EVs derived from diseased cells can affect normal cells; furthermore, EVs remain ill-defined in terms of their biological characteristics and functions 31. EVs contain a large number of extracellular and intracellular molecular components, which can be used as minimally invasive liquid biopsies for comprehensive, multiparameter disease diagnosis. EVs are diagnostic biomarkers for diseases include stroke 32, Alzheimer's disease 33, cardiovascular diseases 34 and cancer 35. Exosomal miRNAs are the most widely used diagnostic biomarkers, especially in cancer 36. Specific exosomal miRNAs may be diagnostic or prognostic markers in cancer. Furthermore, highly expressed oncogenic and tumor-suppressor miRNAs in exosomes may provide high diagnostic value due to their differential expression between cancer cells and normal cells, especially in the early diagnosis of diseases 37. Similarly, exosomal proteins also have diagnostic potential for diseases. Several studies have reported the utility of glypican-1 (GPC1)-positive exosomes in the diagnosis of pancreatic cancer 38-40. GPC1 is specifically enriched in pancreatic patient serum-derived exosomes, distinguishing chronic patients and healthy people from patients with early- or late-stage pancreatic cancer. Thus, the multicomponent and combinatorial nature of exosomal proteins and miRNAs could potentially enhance the specificity and sensitivity of cancer diagnosis and prognostic evaluation. Therefore, EVs can be used as biomarkers for disease diagnosis because disease-generating exosomes can reflect disease-specific changes.
Therapeutic potential of EVs
According to the characteristics of EVs that can contain DNA, RNA and proteins, exosomes by themselves or as vehicles for drug delivery have therapeutic potential in diseases 41-43. Exosomes, as natural endogenous drugs, have obvious advantages in delivering functional cargo to cells. Compared with liposomes, exosomes are widely distributed in body fluids with low immunogenicity and minimal immune clearance. Furthermore, their phospholipid bilayer effectively protects the loaded drugs, making them stable in the blood 44. Several studies reported that exosomes from mesenchymal stem cells (MSCs) or dendritic cells inhibit disease progression by transporting siRNAs 45, miRNAs 46,47, and chemotherapy drugs 48-50. In addition, ligand-modified exosomes may be used to enhance their targeting ability to specific cell types 51,52. For example, previous studies reported that the integrin-specific recognition peptide RGD was applied for exosome membrane modification to enhance the exosome targeting capability 53. Tian et al. reported that exosomes derived from immature dendritic cells deliver doxorubicin to human breast cancer cells, inhibiting tumor progression without obvious toxicity 54. Together, these clinical and experimental data contribute to the development of exosomes as therapeutic vesicles.
EVs and AP
EVs in the pathogenesis of AP
Previous studies have reported that the pathogenesis of acute pancreatitis includes calcium signaling 55, premature trypsinogen activation 56, autophagy 57, endoplasmic reticulum stress, the unfolded protein response 58, intraductal fluid stasis 59, immune system 60, genetic mutations 61, unsaturated fatty acids 62 and mesenteric lymph 63, which mainly lead to trypsinogen activation and injury of acinar cells. The most common and earliest organ dysfunction of AP-associated complications is acute lung injury (ALI), accounting for approximately 10-25% of the incidence and 60% of the mortality 63-65. Underlying mechanisms of AP-associated ALI are complex and poorly understood, although recent perspectives have indicated that pancreatic phospholipase A2, proinflammatory cytokines, neutrophil sequestration and bacterial translocation are involved in the mechanisms of AP and ALI 66-68.
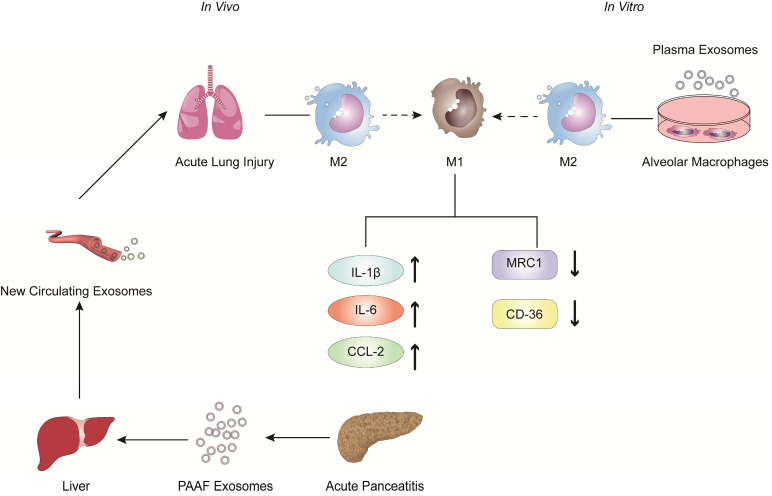
In recent years, the role of exosomes has been gradually clarified in the pathogenesis and treatment of inflammatory diseases, especially in AP 69-75. Bonjoch et al. illustrated that the increased plasma exosomes of acute pancreatitis effectively reach the alveolar cavity and activate alveolar macrophages in an experimental rat model of taurocholate-induced acute pancreatitis 76. Moreover, in vitro experiments showed that plasma exosomes activate alveolar macrophages from the M2 phenotype to a proinflammatory M1 phenotype, concurrent with significantly increased expression of the M1 marker cytokines IL-1β and IL-6 and the chemokine CCL-2 and decreased expression of the M2 markers MRC1 and CD36. In addition, mass spectrometry-driven proteomic analysis of plasma exosomes indicated that the 33 significantly differentially expressed proteins were mainly derived from liver and immune cells; however, the expression of protein derived from the pancreas was downregulated. Thus, proteomic analysis suggested that the most likely origin of plasma exosomes could be the liver instead of the pancreas. Tracking analysis and histological analysis revealed that the liver retains almost 75% of exosomes from pancreatitis-associated ascitic fluid (PAAF). Furthermore, exosomes filtered by the liver changed not only in number but also in protein content. These results indicated that the liver could be generating and releasing new exosomes during AP, which could reach the alveoli and activate alveolar macrophages to a proinflammatory phenotype (Figure 1).
Figure 1.
The role of extracellular vesicles in the mechanism of AP-related alveolar macrophage activation. The figure shows that in vitro experiments (left) revealed that part of the PAAF exosomes released from the pancreas during AP entered the liver directly through the portal system, and most of them were retained in liver tissue. During AP, the formation of new circulating exosomes from the liver reached the alveoli and activated the alveolar macrophages from the M2 phenotype to the pro-inflammatory M1 phenotype, leading to significantly increased expression of cytokines IL-1, IL-6 and chemokine CCL2, while the expression of MRC1 and CD36 was decreased. In vivo experiments (right) showed that plasma exosomes from AP promoted the activation of alveolar macrophages from the M2 phenotype to the pro-inflammatory M1 phenotype, and resulted in significantly increased expression of the inflammatory cytokines IL-1 and the chemokine CCL2 in alveolar macrophages.
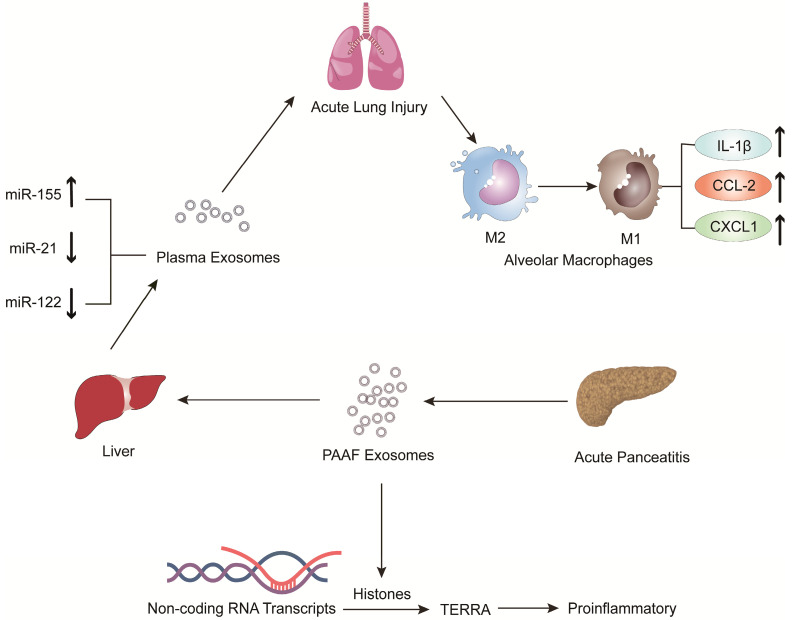
Jiménez-Alesanco et al. performed further experiments to show that the liver could be the source of plasma exosomes that activate the inflammatory response in the lung, rather than the pancreas, during AP 77. These researchers provided evidence that plasma exosomes and PAAF exosomes differ in microRNA (miRNA) content, protein, distribution and physiological effects. Exosomal miRNA analysis revealed that plasma exosomes contained high expression of miR-155 and low expression of miR-122 and miR-21; however, the expression of these miRNAs in PAAF exosomes was similar to that in the control group. Previous studies have shown that miR-155 has a proinflammatory role that can promote M1 polarization of macrophages 78. In contrast, miR-122, which is mainly produced by the liver, plays an anti-inflammatory role 79,80. Therefore, the results suggested that proinflammatory miR-155 expression was significantly upregulated, concurrent with a significant decrease in anti-inflammatory miR-21 and miR-122 expression in plasma exosomes, which could play a proinflammatory response by activating macrophages and promoting the release of inflammatory cytokines. Moreover, proteomic analysis revealed that the proteins of plasma exosomes were mainly from the liver; however, only two specific pancreatic proteins were detected. PAAF exosomes contained high levels of pancreatic enzymes, which confirmed their pancreatic origin. However, histones and ribosomal proteins were more enriched in PAAF exosomes but not in plasma exosomes. Furthermore, histone proteins produce telomeric repeat-containing RNA (TERRA) by regulating noncoding RNA transcripts, which are carried by exosomes from damaged cells to induce an inflammatory response 81,82. They also evaluated the different effects of plasma exosomes and PAAF exosomes on alveolar macrophages. The results showed that plasma exosomes significantly increased the expression of the inflammatory cytokine IL-1β and chemokines CCL2 and CXCL1 in alveolar macrophages; however, the increase in inflammatory factors was not statistically significant in PAAF exosomes compared with that in the control group. The results indicated that these highly expressed miRNAs and proteins could be targeted for designed therapeutic drugs for the treatment of AP (Figure 2).
Figure 2.
The expression of pro-inflammatory miR-155 in plasma exosomes produced during AP was significantly increased, while the expressions of anti-inflammatory miR-122 and miR-21 were decreased. The arrival of plasma exosomes to the alveoli and by activating alveolar macrophages leads to increased expression of the inflammatory cytokines IL-1 and the chemokines CCL2 and CXCL1, thus exacerbating AP-related lung injury. In addition, PAAF exosomes produced during AP contain more histones, which induce inflammation by regulating the transcription of non-coding RNA to produce TERRA.
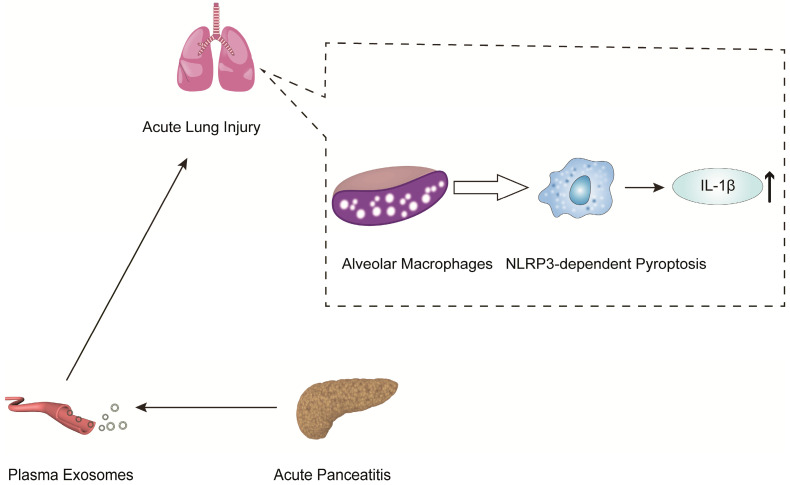
In another mechanistic study of pancreatitis-associated ALI, Wu et al. indicated that plasma exosomes triggered NOD-like receptor protein 3 (NLRP3)-dependent pyroptosis in alveolar macrophages, which induced AP-associated ALI 83 (Figure 3). The present work revealed that plasma exosomes stimulated alveolar macrophages to activate the NLRP3 inflammasome, released IL-1β and induced pyroptosis, suggesting that the plasma exosome-mediated NLRP3 pathway is a potential therapeutic target for the treatment of ALI during AP (Figure 3).
Figure 3.
AP-generated plasma exosomes activate NLRP3 inflammasomes in alveolar macrophages and induce NLRP3-dependent pyroptosis, leading to apoptosis in alveolar macrophages and increased expression of the inflammatory cytokine IL-1.
EVs in the diagnosis of AP
There are many studies on exosomal miRNAs as a diagnostic marker of inflammatory disease, including alcoholic hepatitis 84, inflammatory liver diseases 85, diabetes mellitus 86, liver disease 87, and Parkinson's disease 88. However, there are only a few studies on exosomal miRNAs as diagnostic biomarkers for AP 89. Zhao et al. indicated that 115 differentially expressed exosomal miRNAs of the pancreatic acinar cell line AR42J were identified by a miRNA microarray. Among the differentially expressed miRNAs, 30 were upregulated and 85 were downregulated. Therefore, these 30 upregulated miRNAs may be used as biomarkers for AP. However, the results of this study are only derived from in vitro experiments and have not been verified by in vivo experiments and human samples. It is not yet known whether there are any types of interference, such as differential expression and exosome rupture. Furthermore, target genes of the identified miRNAs were predicted using TargetScan and analyzed by KEGG pathway analysis. The pathways included cell adhesion molecules (CAMs), glycerophospholipid metabolism, the Wnt signaling pathway, the MAPK signaling pathway and the Hedgehog signaling pathway. After further analysis and verification, the target genes regulated macrophage and NFκB activation through the TRAF6-TAB2-TAK1-NIK/IKK-NFκB pathway, which is one of the MAPK signaling pathways. Therefore, the present study provides new ways to alleviate pancreatitis-associated macrophage activation and potential diagnostic exosomal biomarkers for AP.
EVs in the treatment of AP
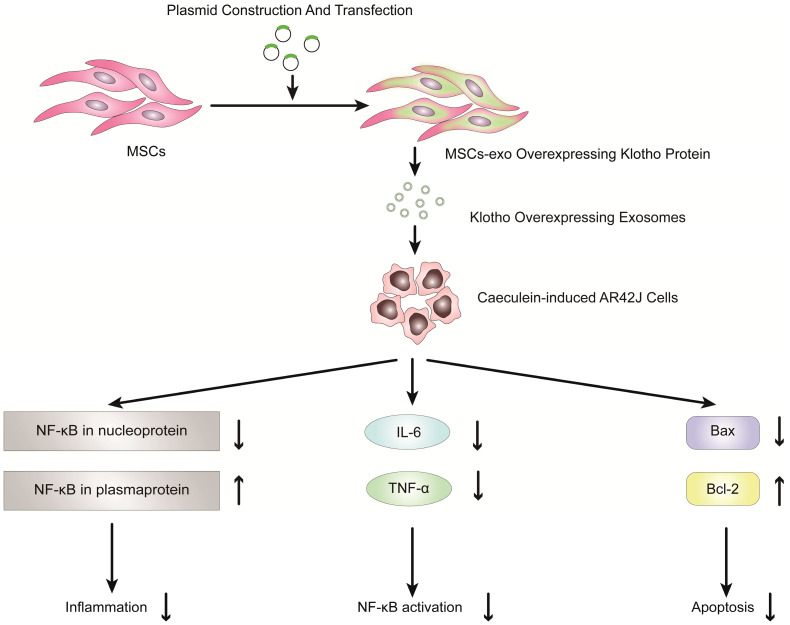
Previous studies have shown that exosomes derived from mesenchymal stem cells (MSCs) can be used to reduce inflammatory responses and treat inflammatory diseases 90-100. Therefore, MSC-derived exosomes have potential clinical value in the treatment of inflammatory diseases, especially pancreatitis. Wang et al. reported that exosomes derived from mesenchymal stem cells that overexpress Klotho attenuated the severity of pancreatic inflammation in caerulein-stimulated AR42J cells 101. Klotho, which is expressed in pancreases, is essential for digestive enzyme secretion from pancreatic acinar cells 102. In this study, exosomes derived from MSCs that overexpressed Klotho (MSCs-exo Klotho) decreased the expression of IL-6 and TNF-α compared to that of the control group. Furthermore, the expression of Bax and NF-kB in nucleoproteins was significantly downregulated in the MSC-exo Klotho group, concurrent with a significant increase in the expression of Bcl-2 and NF-kB in plasma proteins. In conclusion, these results showed that MSC-exo Klotho alleviated inflammation and apoptosis in AP and that Klotho could be a potential targeted therapy for clinical treatment in AP (Figure 4).
Figure 4.
Overexpression of Klotho protein in exosomes from genetically engineered mesenchymal stem cells can reduce the inflammatory response of pancreatic acinar cells (AR42J cells) in the model of acute pancreatitis induced by caerulein.
EVs and CP
EVs in the pathogenesis of CP
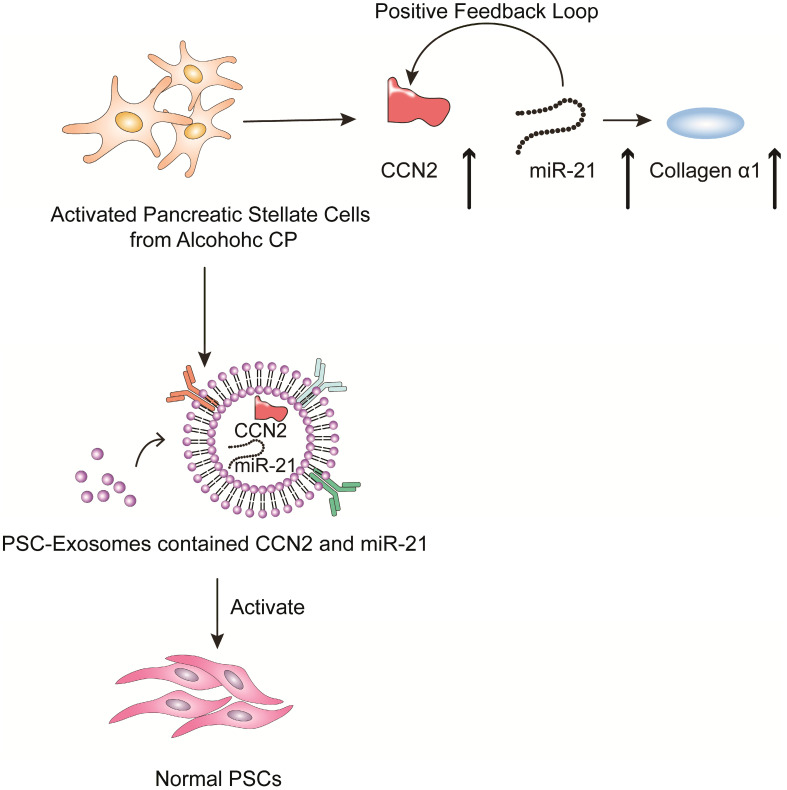
Chronic pancreatitis is a chronic inflammatory disease that is characterized by fibrosis and inflammation of the pancreas, with genetic, environmental, and other risk factors 103-107. The pathophysiological processes of CP mainly involve acinar cell injury 108,109, inflammation 110 and fibrosis by activated pancreatic stellate cells 111. Studies have shown that activated pancreatic stellate cells (PSCs) are considered a promoter of pancreatic fibrosis, which is a crucial hallmark of CP 112-115. Activated PSCs are the main producers of connective tissue growth factor (CCN2), which plays an important role in driving fibrogenic pathways to stimulate extracellular matrix collagen production. Charrier et al. found that the expression of CCN2 and miR-21 is upregulated in PSCs 116. CCN2 not only drives collagen expression but also stimulates the expression of miR-21, which can itself increase CCN2 expression. Thus, upregulated CCN2 and miR-21 are components of a positive feedback loop that may be a mechanism for enhanced collagen production in CP. Additionally; the study indicated that the exosomes derived from activated PSCs contain CCN2 and miR-21, which can be shuttled to activate normal PSCs. Therefore, inhibiting exosome secretion and the expression of CNN2 and miR-21 can reduce the inflammatory response caused by PSC activation in the development of CP (Figure 5).
Figure 5.
Role of extracellular vesicles in CP - associated pancreatic fibrosis. The figure shows that in activated PSC in alcoholic chronic pancreatitis, up-regulated CCN2 and miR-21 constitute a positive feedback pathway that promotes collagen α1 production. In addition, exosomes produced by activated PSCs contained CCN2 and miR-21, and these exosomes could activate more PSCs and produce more exosomes and collagen α1.
EVs in the diagnosis of CP
In the diagnosis of chronic pancreatitis, effective diagnostic biomarkers are still lacking.
At present, no study has indicated that exosomes can be used as diagnostic biomarkers to distinguish chronic pancreatitis from normal conditions. Pancreatic ductal adenocarcinoma (PDAC) is sometimes difficult to distinguish from chronic pancreatitis in the early clinical diagnosis. Furthermore, CP may be misdiagnosed as PDAC, leading to unnecessary pancreatic resection. Therefore, accurate early diagnosis and clear differentiation between PDAC and CP are crucial for patients 104,106.
In addition, several studies have shown that exosomal miRNAs can distinguish patients with chronic pancreatitis from those with PDAC. Lai et al. 117 found that high expression of exosomal miR-10b, miR-20a, miR-21, miR-30c, miR-106b and miR-181a and low expression of exosomal miR-let7a can effectively differentiate patients with PDAC from those with CP. Moreover, after resection, the high expression of exosomal miR-10b, miR-20a, miR-21, miR-30c, and miR-106b decreased to normal values. Nakamura et al. 118 reported that they used exosomal miRNAs from pancreatic juice to distinguish patients with PDAC from those with CP. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) showed that the expression levels of exosomal miR-21 and miR-155 were significantly higher in the PDAC patients than in the CP patients. However, there were no significant differences observed in the expression levels of free miR-21 and free miR-155 in PDAC and CP patients. Furthermore, the AUC values of exosomal miR-21 and miR-155 levels were significantly higher than those for the serum CA19-9 levels. Therefore, exosomal miRNAs may be useful and stable biomarkers for distinguishing patients with chronic pancreatitis from those with PDAC.
Reese et al. used qRT-PCR to show that the expression of miR-200b and miR-200c was significantly downregulated in serum exosomes of PDAC patients compared to healthy controls (HCs) and patients with CP. Moreover, the expression of exosomal miR-125b was significantly upregulated in patients with CP compared to those with HC, and the expression of exosomal miR-148a was significantly upregulated in patients with CP compared to PDAC patients 119. Therefore, exosomal miR-125b and miR-148a can be used as specific diagnostic biomarkers to distinguish CP patients from patients with HC and PDAC, respectively. Similarly, other studies have shown that exosomal miR-10b and miR‑23b‑3p can also distinguish CP from PDAC 120,121.
Furthermore, several studies have shown that exosomal DNA can distinguish patients with chronic pancreatitis from those with PDAC. Yang et al. 122 indicated that circulating exosomal double stranded genomic DNA derived from PDAC patients enabled the detection of prevalent KRAS and TP53 mutations. Digital PCR of exosomal DNA identified KRAS mutations in 29 of 48 (39.6%) cases and TP53 mutations in 2 of 48 (4.2%) cases in PDAC patients. Moreover, they found that 3 of 7 (42.8%) IPMN patients harbored the KRAS mutation, and one of these patients also coharbored the TP53 mutation. For CP patients, the KRAS mutation was found in 5 of 9 (55.6%) cases; however, none had the TP53 mutation. In addition, 5 of 12 (41.7%) patients with other diseases, such as autoimmune pancreatitis, common bile duct cancer, pancreatic cystadenoma, and pancreatic neuroendocrine tumor, harbored the KRAS mutation, and only 1 had the TP53 mutation. In healthy subjects, the KRAS mutation was observed in 3 of 114 (2.6%) cases, and none had the TP53 mutation. Therefore, this study demonstrates that circulating exosomal KRAS and TP53 mutations can be used to distinguish healthy subjects from those with PDAC, PC and other diseases.
Several studies have applied exosomal miRNAs and DNA as diagnostic biomarkers of disease; however, whether the concentrations and diameter of EVs could discriminate malignant and benign disease has not been determined. Severino et al. 123 used the concentrations of EVs to discriminate malignant from nonmalignant CBD stenoses. They collected EVs derived from bile and blood samples and assessed them by nanoparticle tracking analyses (NTA). In bile samples, the concentration of EVs ranged between 1.78×1012 and 1.31×1016 nanoparticles/L, with an overall median value of 6.66×1014 nanoparticles/L. In the PDAC group vs the biliary stones group, the median concentration of EVs was 2.41×1015 vs 1.60×1014 nanoparticles/L. Furthermore, a threshold of 9.46×1014 nanoparticles/L in bile samples distinguished patients with PDAC from those with biliary stones. In the PDAC group vs the CP group, the median concentration of EVs was 4.00×1015 vs 1.26×1014 nanoparticles/L. The threshold of 9.46×1014 nanoparticles/L discriminated PDAC from CP with 100% accuracy. In serum samples, the concentration of EVs was significantly lower than that in bile samples, with an overall median of 2.67×1013 nanoparticles/L. The median concentration of EVs in the PDAC group vs the biliary stone group was 3.55×1014 vs 1.74×1013 nanoparticles/L. In the PDAC group vs the CP group, the median concentration of EVs was 4.64×1013 vs 7.58×1012 nanoparticles/L. In addition, the average diameter of EVs in the PDAC group was 277.8 nm; however, the average diameter of EVs in the CP group was 169.9 nm. Furthermore, EVs derived from bile have larger sizes and contain more proteins in the malignant vs nonmalignant group. Thus, the concentrations and diameters of EVs could be used to discriminate malignant from benign disease with optimal accuracy.
EVs and AIP
Autoimmune pancreatitis (AIP) is a special form of CP that has a pivotal role in inducing fibroinflammatory disorders of the pancreas 124,125. The diagnosis and treatment of AIP have not achieved satisfactory clinical effects. Nakamaru et al. reported that the expression of miR-21 was significantly upregulated in extracellular vesicles derived from the serum of patients with type 1 autoimmune pancreatitis 126. This study included 27 patients with type 1 AIP, 23 patients with chronic pancreatitis and 23 healthy controls (HCs). Microarray analysis showed 165 differentially expressed miRNAs in patients with type 1 AIP. Furthermore, 132 miRNAs were upregulated and 33 were downregulated in type 1 AIP patients compared with HCs. Among these results, the expression levels of miR-659-3p, miR-27a-3p, miR-99a-5p, miR-21-5p, miR-205-5p, miR-100-5p, miR-29c-3p, and miR-126b-1-3p were significantly upregulated, concurrent with a significant decrease in miR-4252 and miR-5004-1-5p expression relative to that of the HCs. Quantitative evaluation of EV miRNA expression levels by RT-PCR showed that only the expression level of miR-21-5p was significantly higher in type 1 AIP patients than in HCs. Furthermore, the results of in situ hybridization (ISH) of resected specimens of type 1 AIP patients showed that the expression of miR-21 in pancreatic duct epithelium was similar between type 1 AIP patients and HCs. However, miR-21 was highly expressed in pancreatic acinar cells in type 1 AIP patients compared to HCs. Therefore, this study demonstrated that miR-21 in EVs derived from AIP patients' serum could be used as a diagnostic marker to distinguish AIP from healthy people.
Shortcoming and perspectives
The study of EVs has the potential to identify cellular and molecular communication and value in the diagnosis and treatment of diseases 26,31. Due to their diverse and unique contents, such as nucleic acids, proteins, lipids, and metabolites, EVs not only can reflect their origin cells but can also be used as a diagnostic marker for disease. In addition, EVs protect their contents through their stable membrane structure and serve as an effective carrier for drug delivery in the therapeutics of cancer and inflammatory diseases 15,36,127.
In recent years, EV research has focused on the classification of EVs, isolation methods, and their functions in disease diagnosis, progression and therapy 30,128-130. Despite the increase in different isolation methods of EVs, there are still no uniform and standardized methods available for the purification and isolation of EVs 131,132. Therefore, it remains unclear whether different isolation methods of EVs may lead to different results 26. Currently, there is a need to establish standardized methods of sample collection, storage, and application to minimize the influence of the complexity and heterogeneity of EVs 133. In addition, the conventional isolation methods of exosomes in blood, such as ultracentrifugation, cannot completely remove lipoprotein, which is similar in size and density to EVs 134. However, the volume and time of blood collection, handling, storage condition and application of anticoagulants all impact the isolation of EVs 135. For the isolation of EVs from cultured cells, it is recommended to use serum-free medium or EV-free serum in cell culture medium 136. For clinical blood sample collection, it is important to minimize the influence of the activation and release of platelet and red blood cell-derived EVs and the contamination of cell debris. A number of studies have shown that -80°C and minimized freeze-thaw cycles are the optimum conditions for the storage of EVs based on size, composition, and functionality. An unfavorable temperature and increased freeze-thaw cycles can cause EV aggregation and lysis, leading to an increase in size, a reduction in counting and a loss of content. The current methods of EV isolation mainly include ultracentrifugation, size-exclusion chromatography, filtration, commercial reagents, microfluidics, asymmetric flow field-flow fractionation, and nanoflow cytometry 133. Diverse methods have their advantages and disadvantages. Moreover, high-efficiency isolation of EVs is needed to eliminate protein contamination and increase purity for clinical application of EVs.
In the diagnosis of AP, there are only a few studies on exosomal biomarkers for AP. Zhao et al. indicated that 30 exosomal miRNAs were upregulated in pancreatic acinar AR42J cells and could be used as biomarkers for AP. However, the results of this study are only derived from in vitro experiments and have not been verified by in vivo experiments and human samples 89. Therefore, for an EV diagnostic biomarker of AP, large samples and multicenter clinical studies are needed. In the diagnosis of CP, no literature has indicated that EVs can be used as diagnostic biomarkers to distinguish CP patients from healthy people. However, PDAC is sometimes difficult to distinguish from CP in the early clinical diagnosis, leading to unnecessary pancreatic resection 104,106. Therefore, Lai et al. 117 found that high expression of exosomal miR-10b, miR-20a, miR-21, miR-30c, miR-106b and miR-181a can effectively differentiate patients with PDAC from those with CP. After resection, the high expression of these miRNAs decreased to normal values. Moreover, Nakamura et al. 118 reported that the expression of exosomal miR-21 and miR-155 from pancreatic juice was significantly higher in PDAC patients than in CP patients. Compared with those of serum CA19-9 levels, the AUC values of exosomal miR-21 and miR-155 levels were significantly higher. Therefore, exosomal miRNAs may be useful and stable biomarkers for distinguishing patients with CP from those with PDAC. Furthermore, several studies have shown that exosomal DNA can also distinguish patients with CP from those with PDAC and healthy subjects. Yang et al. 122 indicated that exosomal DNA identified KRAS mutations in 29 of 48 (39.6%) cases and TP53 mutations in 2 of 48 (4.2%) cases in PDAC patients. For CP patients, the KRAS mutation was found in 5 of 9 (55.6%) cases; however, none had the TP53 mutation. In healthy subjects, the KRAS mutation was observed in 3 of 114 (2.6%) individuals, and none had the TP53 mutation. Therefore, the study indicates that circulating exosomal KRAS and TP53 mutations can be used to distinguish patients with CP from those with PDAC and healthy subjects.
In recent years, compared with exosomal miRNAs and DNA as diagnostic biomarkers of disease, the concentrations and diameters of EVs could discriminate PDAC and CP patients 123. In bile samples, the median concentration of EVs was 4.00×1015 vs 1.26×1014 nanoparticles/L in the PDAC group vs the CP group. In serum samples, the median concentration of EVs was 4.64×1013 vs 7.58×1012 nanoparticles/L in the PDAC group vs the CP group. In addition, the average diameter of EVs in the PDAC group was 277.8 nm; however, the average diameter of EVs in the CP group was 169.9 nm. Thus, EVs derived from bile have larger sizes and contain more proteins in the PDAC vs CP group.
In the diagnosis of AIP, Nakamaru et al. reported that the expression of miR-21 was significantly upregulated in extracellular vesicles derived from serum from patients with type 1 autoimmune pancreatitis 126. This study included 27 patients with type 1 AIP, 23 patients with chronic pancreatitis and 23 healthy controls (HCs). Microarray analysis and RT-PCR showed that the expression level of miR-21-5p was significantly higher in type 1 AIP patients than in HCs. Therefore, miR-21 of EVs derived from AIP patients' serum could be used as a diagnostic marker to distinguish AIP patients from healthy people. At present, there are no reports on potential therapeutic application of EVs in the treatment of AIP. However, it has been reported that EVs have potential therapeutic effect in other autoimmune diseases, such as type 1 diabetes mellitus, multiple sclerosis, systemic lupus erythematosus 134. Research has been suggested that mesenchymal stem cells-derived exosomes might protect the pancreatic islets of patients with Type 1 diabetes by immunomodulatory effect to slow disease progression 135. Similarly, Multiple sclerosis (MS) is a T cell-mediated autoimmune disease, which underlying mechanisms are unclear. Kimura have showed that MS derived exosomal let-7i regulates MS pathogenesis by blocking the insulin like growth factor 1 receptor and transforming growth factor beta receptor 1 pathway 136. Therefore, more studies are needed to further investigate the mechanism and treatment of EVs in AIP.
In the treatment of AP, previous studies have shown that exosomes derived from MSCs can be used to reduce inflammatory responses and treat inflammatory diseases 90-100. Therefore, Wang et al. reported that exosomes derived from mesenchymal stem cells that overexpress Klotho attenuated the severity of pancreatic inflammation in caerulein-stimulated AR42J cells 101. In this study, exosomes derived from MSCs that overexpressed Klotho decreased the expression of IL-6 and TNF-α compared to the control group. In conclusion, these results showed that MSC-exo Klotho alleviated inflammation and apoptosis in AP and that Klotho could be a potential targeted therapy for clinical treatment in AP. However, EVs have not been applied for drug delivery in the treatment of AP. The application of drug-loaded EVs can effectively improve the targeting ability of drugs. In addition, compared with liposomes, EVs have an advantage in the application of drug delivery for targeted treatment.
Conclusion
In this review, we report a comprehensive overview of the relationship between EVs and pancreatitis, with a special focus on their roles in pathogenesis and their potential clinical applications as diagnostic biomarkers and therapeutic targets in pancreatitis. We have discussed EV isolation and application of clinical-grade EVs and the advantages and limitations of current studies, as well as the need for further research. EVs are regarded as a mechanism for intercellular communication, transferring proteins, lipids and genetic material in pancreatitis.
EVs are involved in the pathogenesis of pancreatitis and are used as diagnostic markers for pancreatitis and have clear potential as treatment targets in pancreatitis. However, there are still no uniform and standardized methods available for the purification and isolation of EVs. Therefore, it remains unclear whether different isolation methods of EVs may lead to different results. Currently, there is a need to establish standardized methods of sample collection, storage, and application to minimize the influence of the complexity and heterogeneity of EVs. EVs have not been applied drug delivery in the treatment of AP. Therefore, the clinical application of EVs in the diagnosis and treatment of pancreatitis is promising, and additional extensive research is required before clinical application.
Table 1.
The role of EVs in pancreatitis
| Pancreatitis | Category | Source of EVs | Related molecules | Effects | References |
|---|---|---|---|---|---|
| AP | Pathogenesis | Mice plasma | IL-1β, IL-6, CCL-2, MRC1, CD36 | The increased plasma exosomes of acute pancreatitis effectively reach the alveolar cavity and activate alveolar macrophages in AP. | 76 |
| Mice plasma, Mice PAAF | miR-155, miR-122, miR-21, TERRA, IL-1β, CCL2, CXCL1 | The liver could be the source of plasma exosomes that activate the inflammatory response in the lung, rather than the pancreas, during AP. | 77 | ||
| Mice plasma | NLRP3,IL-1β | Plasma exosomes triggered NOD-like receptor protein 3 (NLRP3)-dependent pyroptosis in alveolar macrophages, which induced AP-associated ALI. | 83 | ||
| Diagnosis | AR42J cell | miRNAs | Upregulated extracellular vesicle miRNAs in TRAF6-TAB2-TAK1-NIK/IKK-NFκB pathway may be used as biomarkers for AP. | 89 | |
| Treatment | MSCs | Klotho, IL-6, TNF-α,Bax, Bcl-2 | Exosomes derived from mesenchymal stem cells that overexpress Klotho attenuated the severity of pancreatic inflammation in caerulein-stimulated AR42J cells. | 101 | |
| Mice plasma | NLRP3,IL-1β | Exosome-mediated NLRP3 pathway is a potential therapeutic target for the treatment of ALI during AP. | 83 | ||
| CP | Pathogenesis | PSC | CCN2,miR-21 | CCN2 up-regulation in PSC is associated with increased expression of miR-21 which, in turn, is able to stimulate CCN2 expression further via a positive feedback loop. Additionally miR-21 and CCN2 were identified in PSC-derived exosomes which effected their delivery to other PSC. | 116 |
| Diagnosis | Human plasma | miR-10b, miR-20a, miR-21, miR-30c, miR-106b, miR-181a, miR-let7a | Clear differentiation between PDAC and CP. | 117 | |
| Human Pancreatic juice | miR-21, miR-155 | Clear differentiation between PDAC and CP. | 118 | ||
| Human serum | miR-125b, miR-148a | Clear differentiation between PDAC and CP. | 119 | ||
| Human serum | miR-10b,miR‑23b‑3p | Clear differentiation between PC and CP. | 120,121 | ||
| Human serum | DNA | Circulating exosomal KRAS and TP53 mutations can be used to distinguish healthy subjects from those with PDAC and PC. | 122 | ||
| Human bile, Human serum | EVs' concentrations | Discriminate malignant from nonmalignant CBD stenoses. | 123 | ||
| Treatment | PSC | CCN2, miR-21 | Inhibiting exosome secretion and the expression of CNN2 and miR-21 may reduce the inflammatory response caused by PSC activation in the development of CP. | 116 | |
| AIP | Pathogenesis | Human serum | miR-21 | Diagnostic marker to distinguish AIP from healthy people. | 126 |
Abbreviations
- EVs
Extracellular vesicles
- AP
Acute pancreatitis
- CP
Chronic pancreatitis
- AIP
Autoimmune pancreatitis
- MVs
Exosomes and microvesicles
- GPC1
Glypican-1
- MSCs
Mesenchymal stem cells
- ALI
Acute lung injury
- PAAF
pancreatitis-associated ascitic fluid
- miRNAs
microRNAs
- TERRA
Telomeric repeat-containing RNA
- NLRP3
NOD-like receptor protein 3
- CAMs
Cell adhesion molecules
- MSCs
Mesenchymal stem cells
- MSCs-exo klotho
Exosomes derived from MSCs which overexpressed klotho
- PSCs
Pancreatic stellate cells
- CCN2
Connective tissue growth factor
- PDAC
Pancreatic ductal adenocarcinoma
- qRT-PCR
Quantitative real-time reverse transcription polymerase chain reaction
- HC
Healthy control
- IPMN
Intraductal papillary mucinous neoplasm
- NTA
Nanoparticle tracking analyses
- HCs
Healthy controls
- ISH
In situ hybridization
References
- 1.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 3.Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: A population-based study. Dig Liver Dis. 2017;49(5):540–544. doi: 10.1016/j.dld.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Pendharkar SA, Mathew J, Zhao J, Windsor JA, Exeter DJ, Petrov MS. Ethnic and geographic variations in the incidence of pancreatitis and post-pancreatitis diabetes mellitus in New Zealand: a nationwide population-based study. N Z Med J. 2017;130(1450):55–68. [PubMed] [Google Scholar]
- 5.Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. 2015;149(6):1490–1500. doi: 10.1053/j.gastro.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 6.Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156(7):1951–1968.e1. doi: 10.1053/j.gastro.2018.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gukovskaya AS, Gukovsky I, Algül H, Habtezion A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology. 2017;153(5):1212–1226. doi: 10.1053/j.gastro.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–496. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 9.Bakker OJ, Issa Y, van Santvoort HC, Besselink MG, Schepers NJ, Bruno MJ. et al. Treatment options for acute pancreatitis. Nature Review. 2014;11(8):462–9. doi: 10.1038/nrgastro.2014.39. [DOI] [PubMed] [Google Scholar]
- 10.Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. doi: 10.1136/bmj.l6227. [DOI] [PubMed] [Google Scholar]
- 11.Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 12.Lindenbergh MFS, Stoorvogel W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu Rev Immunol. 2018;36:435–459. doi: 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 13.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30(6):836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L. et al. Exosomes and Nanoengineering: A Match Made for Precision Therapeutics. Adv Mater Weinheim. 2020;32(18):e1904040. doi: 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262(19):9412–20. [PubMed] [Google Scholar]
- 17.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 18.Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Syn NL, Wang L, Chow EK, Lim CT, Goh BC. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017;35(7):665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 20.LeBleu VS, Kalluri R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer. 2020;6(9):767–774. doi: 10.1016/j.trecan.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant. 2018;27(3):349–363. doi: 10.1177/0963689717723636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu H, Liu S, Wu K, Zhao R, Cao L, Wang H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J Cosmet Dermatol. 2020;19(3):574–581. doi: 10.1111/jocd.13215. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE. Exosomes and immune surveillance of neoplastic lesions: a review. Biotech Histochem. 2012;87(3):161–8. doi: 10.3109/10520291003659042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbichler TB, Dudás J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–181. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45:1–12. doi: 10.1016/j.drup.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros T, Myette RL, Almeida JR, Silva AA, Burger D. Extracellular Vesicles: Cell-Derived Biomarkers of Glomerular and Tubular Injury. Cell Physiol Biochem. 2020;54(1):88–109. doi: 10.33594/000000207. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Feng Q, Sun J. Lipid Nanovesicles by Microfluidics: Manipulation, Synthesis, and Drug Delivery. Adv Mater Weinheim. 2019;31(45):e1804788. doi: 10.1002/adma.201804788. [DOI] [PubMed] [Google Scholar]
- 29.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2020;10:3137. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 30.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2020;9(4):1044. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R, LeBleu VS. The biology function and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otero-Ortega L, Laso-García F, Gómez-de Frutos M, Fuentes B, Diekhorst L, Díez-Tejedor E. et al. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl Stroke Res. 2019;10(3):241–249. doi: 10.1007/s12975-018-0654-7. [DOI] [PubMed] [Google Scholar]
- 33.Hamlett ED, Goetzl EJ, Ledreux A, Vasilevko V, Boger HA, LaRosa A. et al. Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimers Dement. 2017;13(5):541–549. doi: 10.1016/j.jalz.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen F, Li Q. Exosomes as Diagnostic Biomarkers in Cardiovascular Diseases. Adv Exp Med Biol. 2017;998:61–70. doi: 10.1007/978-981-10-4397-0_4. [DOI] [PubMed] [Google Scholar]
- 35.Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V. et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer. 2017;70:122–132. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019;30(4):656–673. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: Challenges and opportunities. J Cell Physiol. 2018;233(9):6370–6380. doi: 10.1002/jcp.26481. [DOI] [PubMed] [Google Scholar]
- 38.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–82. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86–93. doi: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu H, Niu F, Liu F, Gao J, Sun Y, Zhao X. et al. Elevated glypican-1 expression is associated with an unfavorable prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6(6):1181–1191. doi: 10.1002/cam4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 42.Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D. et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65(3):342–7. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery-a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–51. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Stremersch S, Vandenbroucke RE, Van Wonterghem E, Hendrix A, De Smedt SC, Raemdonck K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release. 2016;232:51–61. doi: 10.1016/j.jconrel.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 46.Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y. et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351–361. doi: 10.1016/j.canlet.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 47.Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C. et al. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol Ther. 2016;24(7):1290–301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiao L, Hu S, Huang K, Su T, Li Z, Vandergriff A. et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10(8):3474–3487. doi: 10.7150/thno.39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL. et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–70. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 51.Gilligan KE, Dwyer RM. Engineering Exosomes for Cancer Therapy. Int J Mol. Sci. 2017;18(6):1122. doi: 10.3390/ijms18061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J. et al. Engineered Exosomes with Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J Am Heart Assoc. 2018;7(15):e008737. doi: 10.1161/JAHA.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Li W, Lu Z, Zhang L, Hu Y, Li Q. et al. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale. 2017;9(40):15598–15605. doi: 10.1039/c7nr04425a. [DOI] [PubMed] [Google Scholar]
- 54.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 55.Maléth J, Hegyi P. Ca2+ toxicity and mitochondrial damage in acute pancreatitis: translational overview. Philos. Trans. R. Soc. Lond, B, Biol. Sci. 2016;371(1700):20150425. doi: 10.1098/rstb.2015.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saluja A, Dudeja V, Dawra R, Sah RP. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology. 2019;156(7):1979–1993. doi: 10.1053/j.gastro.2019.01.268. [DOI] [PubMed] [Google Scholar]
- 57.Antonucci L, Fagman JB, Kim JY, Todoric J, Gukovsky I, Mackey M. et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein. synthesis and prevents ER stress. Proc. Natl. Acad. Sci. U.S.A. 2015;112(45):E6166–74. doi: 10.1073/pnas.1519384112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 59.Hegyi P, Wilschanski M, Muallem S, Lukacs GL, Sahin-Tóth M, Uc A. et al. CFTR: a new horizon in the pathomechanism and treatment of pancreatitis. Rev Physiol Biochem. Pharmacol. 2016;170:37–66. doi: 10.1007/112_2015_5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10(2):283–298. doi: 10.1038/mi.2016.101. [DOI] [PubMed] [Google Scholar]
- 61.Hasan A, Moscoso DI, Kastrinos F. The role of genetics in pancreatitis. Gastrointest. Endosc. Clin. N. Am. 2018;28(4):587–603. doi: 10.1016/j.giec.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Khatua B, El-Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33(5):374–382. doi: 10.1097/MOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Landahl P, Ansari D, Andersson R. Severe acute pancreatitis: gut barrier failure, systemic inflammatory response, acute lung injury, and the role of the mesenteric lymph. Surg Infect (Larchmt) 2015;16(6):651–6. doi: 10.1089/sur.2015.034. [DOI] [PubMed] [Google Scholar]
- 64.Akbarshahi H, Rosendahl AH, Westergren-Thorsson G, Andersson R. Acute lung injury in acute pancreatitis-awaiting the big leap. Respir Med. 2012;106(9):1199–210. doi: 10.1016/j.rmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Zhou MT, Chen CS, Chen BC, Zhang QY, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094–9. doi: 10.3748/wjg.v16.i17.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vrolyk V, Singh B. Animal models to study the role of pulmonary intravascular macrophages in spontaneous and induced acute pancreatitis. Cell Tissue Res. 2020;380(2):207–222. doi: 10.1007/s00441-020-03211-y. [DOI] [PubMed] [Google Scholar]
- 67.Elder AS, Saccone GT, Dixon DL. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology. 2012;12(1):49–56. doi: 10.1016/j.pan.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Browne GW, Pitchumoni CS. Pathophysiology of pulmonary complications of acute pancreatitis. World J. Gastroenterol. 2006;12(44):7087–96. doi: 10.3748/wjg.v12.i44.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan BD, Wong WY, Lee MM, Cho WC, Yee BK, Kwan YW. et al. Exosomes in Inflammation and Inflammatory Disease. Proteomics. 2019;19(8):e1800149. doi: 10.1002/pmic.201800149. [DOI] [PubMed] [Google Scholar]
- 70.Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M, Hashemi SM. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 2019;234(7):9910–9926. doi: 10.1002/jcp.27934. [DOI] [PubMed] [Google Scholar]
- 71.Tran TH, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160(1):46–58. doi: 10.1016/j.clim.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 72.Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N. et al. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Adv Exp Med Biol. 2018;1089:47–57. doi: 10.1007/5584_2018_219. [DOI] [PubMed] [Google Scholar]
- 73.Hough KP, Deshane JS. Exosomes in Allergic Airway Diseases. Curr Allergy Asthma Rep. 2019;19(5):26. doi: 10.1007/s11882-019-0857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hough KP, Chanda D, Duncan SR, Thannickal VJ, Deshane JS. Exosomes in immunoregulation of chronic lung diseases. Allergy. 2017;72(4):534–544. doi: 10.1111/all.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–171. doi: 10.1016/j.cca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Bonjoch L, Casas V, Carrascal M, Closa D. Involvement of exosomes in lung inflammation associated with experimental acute pancreatitis. J Pathol. 2016;240(2):235–45. doi: 10.1002/path.4771. [DOI] [PubMed] [Google Scholar]
- 77.Jiménez-Alesanco A, Marcuello M, Pastor-Jiménez M, López-Puerto L, Bonjoch L, Gironella M. et al. Acute pancreatitis promotes the generation of two different exosome populations. Sci Rep. 2019;9(1):19887. doi: 10.1038/s41598-019-56220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bala S, Csak T, Saha B, Zatsiorsky J, Kodys K, Catalano D. et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J. Hepatol. 2016 Jun;64(6):1378–87. doi: 10.1016/j.jhep.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR-122-a key factor and therapeutic target in liver disease. J. Hepatol. 2015;62(2):448–57. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H. et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Invest. 2012;122(8):2871–83. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Lieberman PM. The crosstalk of telomere dysfunction and inflammation through cell-free TERRA containing exosomes. RNA Biol. 2016;13(8):690–5. doi: 10.1080/15476286.2016.1203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Deng Z, Dahmane N, Tsai K, Wang P, Williams DR. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc Natl Acad Sci U.S.A. 2015;112(46):E6293–300. doi: 10.1073/pnas.1505962112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu XB, Sun HY, Luo ZL, Cheng L, Duan XM, Ren JD. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5):165685. doi: 10.1016/j.bbadis.2020.165685. [DOI] [PubMed] [Google Scholar]
- 84.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J. et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56(5):1946–57. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang W, Wang J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells. 2019;8(8):853. doi: 10.3390/cells8080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S, Wang JQ, Lv XW. Exosomal miRNAs as biomarkers in the diagnosis of liver disease. Biomark Med. 2017;11(6):491–501. doi: 10.2217/bmm-2017-0011. [DOI] [PubMed] [Google Scholar]
- 88.Harischandra DS, Ghaisas S, Rokad D, Zamanian M, Jin H, Anantharam V, Kimber M. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson's disease: Relevance to α-synuclein misfolding in metal neurotoxicity. Neurotoxicology. 2018;64:267–277. doi: 10.1016/j.neuro.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y, Wang H, Lu M, Qiao X, Sun B, Zhang W. et al. Pancreatic Acinar Cells Employ miRNAs as Mediators of Intercellular. Communication to Participate in the Regulation of Pancreatitis-Associated Macrophage Activation. Mediators Inflamm. 2018;64:267–277. doi: 10.1155/2016/6340457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C. et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than. microparticles in inflammatory arthritis. Theranostics. 2018;8(5):1399–1410. doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J, Xue H, Li T, Chu X, Xin D, Xiong Y. et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–572. doi: 10.1016/j.bbrc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10(1):98. doi: 10.1186/s13287-019-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB. et al. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma. 2017;34(24):3388–3396. doi: 10.1089/neu.2017.5063. [DOI] [PubMed] [Google Scholar]
- 94.Jafarzadeh N, Safari Z, Pornour M, Amirizadeh N, Forouzandeh Moghadam M. et al. Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes. J Cell Physiol. 2019;234(4):3697–3710. doi: 10.1002/jcp.27142. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Jin D, Xie W, Wen L, Chen W, Xu J. et al. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr Stem Cell Res Ther. 2018;13(5):362–368. doi: 10.2174/1574888X13666180403163456. [DOI] [PubMed] [Google Scholar]
- 96.Liu J, Chen T, Lei P, Tang X, Huang P. Exosomes Released by Bone Marrow Mesenchymal Stem Cells Attenuate Lung Injury Induced by Intestinal Ischemia Reperfusion via the TLR4/NF-κB Pathway. Int J Med Sci. 2019;16(9):1238–1244. doi: 10.7150/ijms.35369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y. et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed Res Int. 2017;2017:5356760. doi: 10.1155/2017/5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S, Jiang L, Hu H, Wang H, Wang X, Jiang J. et al. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020;246:117401. doi: 10.1016/j.lfs.2020.117401. [DOI] [PubMed] [Google Scholar]
- 99.Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N. et al. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Adv Exp Med Biol. 2018;1089:47–57. doi: 10.1007/5584_2018_219. [DOI] [PubMed] [Google Scholar]
- 100.Ren J, Liu N, Sun N, Zhang K, Yu L. Mesenchymal Stem Cells and their Exosomes: Promising Therapeutics for Chronic Pain. Curr Stem Cell Res Ther. 2019;14(8):644–653. doi: 10.2174/1574888X14666190912162504. [DOI] [PubMed] [Google Scholar]
- 101.Wang N, Ma J, Ren Y, Xiang S, Jia R. Secreted klotho from exosomes alleviates inflammation and apoptosis in acute pancreatitis. Am J Transl Res. 2019;11(6):3375–3383. [PMC free article] [PubMed] [Google Scholar]
- 102.Coate KC, Hernandez G, Thorne CA, Sun S, Le TDV, Vale K. et al. FGF21 Is an Exocrine Pancreas Secretagogue. Cell Metab. 2017;25(2):472–480. doi: 10.1016/j.cmet.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA. 2019;322(24):2422–2434. doi: 10.1001/jama.2019.19411. [DOI] [PubMed] [Google Scholar]
- 104.Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T. et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. doi: 10.1038/nrdp.2017.60. [DOI] [PubMed] [Google Scholar]
- 105.Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387(10031):1957–66. doi: 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- 106.Issa Y, Bruno MJ, Bakker OJ. et al. Treatment options for chronic pancreatitis. Nat Rev Gastroenterol Hepatol. 2014;11(9):556–64. doi: 10.1038/nrgastro.2014.74. [DOI] [PubMed] [Google Scholar]
- 107.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic. Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112(9):1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 108.Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol. 2010;25(12):1816–1826. doi: 10.1111/j.1440-1746.2010.06445.x. [DOI] [PubMed] [Google Scholar]
- 109.Lee AT, Xu Z, Pothula SP, Patel MB, Pirola RC, Wilson JS, Apte MV. Alcohol and cigarette smoke components activate human pancreatic stellate cells: implications for the progression of chronic pancreatitis. Alcohol Clin Exp Res. 2015;39(11):2123–2133. doi: 10.1111/acer.12882. [DOI] [PubMed] [Google Scholar]
- 110.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ. et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.ApteM PirolaR, WilsonJ. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid Redox Signal. 2011;15(10):2711–2722. doi: 10.1089/ars.2011.4079. [DOI] [PubMed] [Google Scholar]
- 112.Ramakrishnan P, Loh WM, Gopinath SCB, Bonam SR, Fareez IM, Mac Guad R. et al. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm Sin B. 2020;10(3):399–413. doi: 10.1016/j.apsb.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J. et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50(4):535–41. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bynigeri RR, Jakkampudi A, Jangala R, Subramanyam C, Sasikala M, Rao GV. et al. Pancreatic stellate cell: Pandora's box for pancreatic disease biology. World J Gastroenterol. 2017;23(3):382–405. doi: 10.3748/wjg.v23.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L, Wang G, Hu JS, Zhang GQ, Chen HZ, Yuan Y. et al. RB1CC1-enhanced autophagy facilitates PSCs activation and pancreatic fibrogenesis in chronic pancreatitis. Cell Death Dis. 2018;9(10):952. doi: 10.1038/s41419-018-0980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M. et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis. J Cell Commun Signal. 2014;8(2):147–56. doi: 10.1007/s12079-014-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86–93. doi: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakamura S, Sadakari Y, Ohtsuka T, Okayama T, Nakashima Y, Gotoh Y. et al. Pancreatic Juice Exosomal MicroRNAs as Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2019;26(7):2104–2111. doi: 10.1245/s10434-019-07269-z. [DOI] [PubMed] [Google Scholar]
- 119.Reese M, Flammang I, Yang Z, Dhayat SA. Potential of Exosomal microRNA-200b as Liquid Biopsy Marker in Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2020;12(1):197. doi: 10.3390/cancers12010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pang Y, Wang C, Lu L, Wang C, Sun Z, Xiao R. Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens Bioelectron. 2019;130:204–213. doi: 10.1016/j.bios.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 121.Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y. et al. Upregulated exosomic miR-23b-3p plays regulatory roles in the progression of pancreatic cancer. Oncol Rep. 2017;38(4):2182–2188. doi: 10.3892/or.2017.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P. et al. Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer Biol Ther. 2017;18(3):158–165. doi: 10.1080/15384047.2017.1281499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Severino V, Dumonceau JM, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X. et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology. 2017;153(2):495–504. doi: 10.1053/j.gastro.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 124.Watanabe T, Minaga K, Kamata K. et al. Mechanistic Insights into Autoimmune Pancreatitis and IgG4-Related Disease. Trends Immunol. 2018;39(11):874–889. doi: 10.1016/j.it.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Kamisawa T, Takuma K, Egawa N, Tsuruta K, Sasaki T. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7(7):401–9. doi: 10.1038/nrgastro.2010.81. [DOI] [PubMed] [Google Scholar]
- 126.Nakamaru K, Tomiyama T, Kobayashi S, Ikemune M, Tsukuda S, Ito T. et al. Extracellular vesicles microRNA analysis in type 1 autoimmune pancreatitis: Increased expression of microRNA-21. Pancreatology. 2020;20(3):318–324. doi: 10.1016/j.pan.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 127.Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 128.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–15. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18(1):52. doi: 10.1186/s12943-019-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gholizadeh S, Shehata Draz M, Zarghooni M, Sanati-Nezhad A, Ghavami S, Shafiee H. et al. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens Bioelectron. 2017;91:588–605. doi: 10.1016/j.bios.2016.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126(4):1152–62. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L. et al. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020;38(10):1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu H, Jia S, Xu H. Potential therapeutic applications of exosomes in different autoimmune diseases. Clin Immunol. 2019;205:116–124. doi: 10.1016/j.clim.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Garcia-Contreras M, Brooks RW, Boccuzzi L. et al. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21(12):2940–2956. [PubMed] [Google Scholar]
- 136.Kimura K, Hohjoh H, Fukuoka M. et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9(1):17. doi: 10.1038/s41467-017-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]