Abstract
Aim
To assess a total population of school‐age children with cerebral palsy (CP) for autism and attention‐deficit/hyperactivity disorder (ADHD) with a view to determining their prevalence and to relate findings to motor function, intellectual disability, and other associated impairments.
Method
Of 264 children, born between 1999 and 2006, from the CP register of western Sweden, 200 children (109 males, 91 females, median age at assessment 14y, range 7–18y) completed comprehensive screening and further neuropsychiatric clinical assessments.
Results
Ninety children (45%) were diagnosed with autism, ADHD, or both, 59 (30%) were diagnosed with autism, and 60 (30%) were diagnosed with ADHD. Intellectual disability was present in 51%. Two‐thirds had autism, ADHD, and/or intellectual disability. In regression models, autism was mainly predicted by intellectual disability (odds ratio [OR]=4.1) and ADHD (OR=3.2), and ADHD was predicted by intellectual disability (OR=2.3) and autism (OR=3.0). Autism was more common in children born preterm (OR=2.0). Gross motor function was not associated with autism. ADHD prevalence was low in children with severe motor impairment, possibly due to diagnostic limitations.
Interpretation
Autism and ADHD were common in this population of children with CP and were mainlyindependent of motor severity and CP type. The strongest predictor of autism/ADHD was intellectual disability. Assessment for autism and ADHD is warranted as part of the evaluation in CP.
What this paper adds
Forty‐five percent of the children with cerebral palsy also had autism, attention‐deficit/hyperactivity disorder (ADHD), or both.
Autism and ADHD were predicted mainly by intellectual disability.
Established diagnostic instruments worked well for all but the most disabled group of children.
What this paper adds
Forty‐five percent of the children with cerebral palsy also had autism, attention‐deficit/hyperactivity disorder (ADHD), or both.
Autism and ADHD were predicted mainly by intellectual disability.
Established diagnostic instruments worked well for all but the most disabled group of children.
This article is commented on by Gecz and Berry on pages 247–248 of this issue.
Cerebral palsy (CP) is a group of motor disorders caused by early disturbances in the developing brain. The motor disorders of CP are often accompanied by other impairments and neurodevelopmental disorders. 1 Neuropsychiatric disorders, for example autism spectrum disorder (autism) and attention‐deficit/hyperactivity disorder (ADHD), are more common in children with CP than in typically developing children. 2 , 3 , 4 , 5 , 6 A recent systematic review found the occurrence of autism to be between 3% and 16% in studies of cohorts with CP. 7 ADHD is also common in children with CP, but results vary due to different diagnostic approaches and few if any studies are population‐based. To the best of our knowledge, no previous study has actively assessed a total population of children with CP for these neuropsychiatric disorders.
Children with CP have a varying, and sometimes complex, profile of impairments, with motor disability often not being the most restricting. There is a need to identify associated impairments to provide the right support at the right time. Early diagnosis and adequate interventions may give a better prognosis in terms of function and participation for children with autism 8 and ADHD, 9 in children with and without CP.
The present study is the third in a project aiming to establish accurate prevalence rates of autism and ADHD in children with CP. The original study population of 264 children, derived from the CP register for western Sweden, comprised 8 birth year cohorts of children and adolescents with CP born between 1999 and 2006 in the county of Västra Götaland, a region with 1.6 million inhabitants. 10 , 11 The first study described the occurrence of autism (18%) and ADHD (21%), and other associated impairments, for children aged between 10 and 17 years, based on retrospective medical and habilitation records. 12 In the second study, the same 264 children were screened for autism and ADHD through comprehensive parent‐completed questionnaires. 13 Of the 232 responses obtained, 213 were possible to evaluate and showed high screen positivity for both autism (35%) and ADHD (50%), strongly indicating the need to assess all children with CP (Fig. S1, online supporting information).
The aims of this study were: (1) to actively estimate the prevalence of autism and ADHD in a population of school‐age children with CP, (2) to describe autism and ADHD in relation to CP type, gross motor function, sex, gestational age, intellectual disability, and other associated impairments, and (3) to establish how well screening predicted the diagnoses of autism and ADHD.
Method
Participants
The population of the present study originated from the 213 children who had participated in the screening study. 13 Their screening results were compared with the diagnoses of autism and ADHD already identified and reported in the first published paper of this project 12 (Fig. S1). The 28 children whose positive results from the screening were fully concordant with the diagnoses of autism, ADHD, or both (group 1 in Fig. S1) were not assessed further since all diagnoses had been made by clinically experienced teams. The 82 children with negative screening and no diagnoses of autism or ADHD (group 5 in Fig. S1) were also not assessed further. They had been evaluated repeatedly by multidisciplinary habilitation teams throughout childhood and several school years without an identified need for neuropsychiatric assessment. It was concluded that no further assessment would be needed for these 110 children.
The remaining 103 children and their families were approached for clinical assessment. Twelve children with a positive screen declined further participation in the study and one child had died. The remaining 90 children (groups 2, 3, and 4 in Fig. S1) participated in the neuropsychiatric examinations.
Assessments
The children were assessed at a tertiary centre for children with impairments by professionals experienced in the field, either on a single day by a neuropaediatrician (MP), neuropsychologist, and speech and language pathologist, or as part of a comprehensive, clinically requested examination by a multidisciplinary team over 1 week. Some complementing parental interviews were also made by telephone (n=10).
The instruments used to diagnose autism were the Diagnostic Interview for Social and Communication disorders (DISCO), 14 the Childhood Autism Rating Scale (CARS), 15 and when applicable the Autism Diagnostic Observation Schedule, Second Edition (ADOS‐2). 16 The Swanson, Nolan and Pelham Scale (SNAP‐IV) 17 was used by parents, and teachers if further clarification was needed, to diagnose ADHD. Adaptive behaviour was assessed with Vineland Adaptive Behavior Scales, Second Edition. 18 Unless not done in the last year, intellectual level was tested with the appropriate Wechsler intelligence scale. The assessments took place from 2013 to 2019, mainly during 2017 and 2018. Children were 7 years 3 months to 17 years 11 months at assessment (median age 14y 5mo).
Intellectual level was established on the basis of clinical examination, individualized standardized cognitive testing, and assessment of adaptive functioning, according the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) criteria. 19 Children classified at an intellectual level of less than 1 year were not considered further to diagnose autism and children at an intellectual level of less than 3 years were not considered further to diagnose ADHD.
Clinical diagnoses of autism and ADHD were made on the basis of the relevant DSM‐5 criteria 19 by the multidisciplinary assessment team; clinical diagnoses were reached by consensus. The diagnoses were based on developmental and medical history, results of the instruments administered, 14 , 15 , 16 , 17 , 18 and clinical observations and examinations. Children were evaluated with special consideration to intellectual level and other impairments. If there was any uncertainty (n=25), children were evaluated further with the final decision made by consensus by the three authors in a case conference, that is, two neuropaediatricians and a very experienced child and adolescent psychiatrist (CG).
The results from the 90 children newly assessed were added to the results from the 110 children screened and assessed previously (groups 1 and 5 in Fig. S1), resulting in a total study population of 200 children (109 males, 91 females).
Definitions
CP types were classified according to the Surveillance of Cerebral Palsy in Europe (SCPE) into unilateral spastic, bilateral spastic, dyskinetic, and ataxic CP. 20 Gross motor function was classified according to the Gross Motor Function Classification System (GMFCS). 21
Gestational age groups were classified as children born extremely preterm (birth occurring at less than 28 completed gestational weeks), very preterm (28–31wks), moderately preterm (32–36wks), and term (37 completed weeks or more).
Intellectual level was defined as normal if IQ was greater than or equal to 85 and borderline intellectual functioning if IQ was 70 to 84. Intellectual disability was divided into mild (IQ=50–69), moderate (IQ=35–49), severe (IQ=20–34), and profound (IQ<20).
Visual impairment was defined as an acuity of no more than 0.3 in the best eye with correction. Speech was classified according to the four levels of the Viking Speech Scale from I (speech not affected by motor disorder) to IV (no understandable speech). 22 Epilepsy was defined as epilepsy under treatment according to the medical records.
Ethical statement
The study was approved by the Regional Ethical Review Board in Gothenburg (refs 145‐07 and 398‐12). Written informed consent for research and publication was obtained from the parents of all assessed participating children.
Statistical analysis
Descriptive statistics were used to compare groups. For the association between categorical variables, the χ 2 test for independence was used; if more than two categories were present within an ordinal scale, the Cochran–Armitage χ 2 test for trend was used. Spearman's rank correlation (ρ) was used to analyse the relationship between classification scales. A p‐value of less than 0.05 was regarded as statistically significant.
An outcome of autism and ADHD respectively was analysed using a multiple regression model including dichotomized variables: sex; born preterm/at term; mild (GMFCS levels I and II) or moderate‐to‐severe (GMFCS levels III–V) impaired gross motor function; and associated impairments as no or some impairment. In order to select variables to include in the various models, we used the process described in Bursac et al. 23 The first step was a bivariate analysis retaining variables with a p‐value of less than 0.25. The second step was a multiple regression model using the remaining variables, where we removed variables with a p‐value greater than 0.10, but not confounding variables; that is, variables changing the estimates by more than 15%. In the third and final step, we refitted the multiple regression model, adding stepwise the variables excluded previously, keeping those with a p‐value less than 0.10. Odds ratios (ORs) with 95% confidence intervals (CIs) were then calculated for the remaining variables associated with autism or ADHD in the models.
The diagnoses of autism and ADHD in the 200 children were compared with the screening results for the same children. 13 Screening sensitivity and specificity were then calculated.
Analyses were conducted in R v3.6.2. (R Foundation for Statistical Computing, Vienna, Austria); Figure 1 was produced in RStudio (RStudio, Boston, MA, USA; https://rstudio.com/) using the R package eulerr v.6.0.0 (https://cran.r‐project.org/web/packages/eulerr/index.html).
Figure 1.
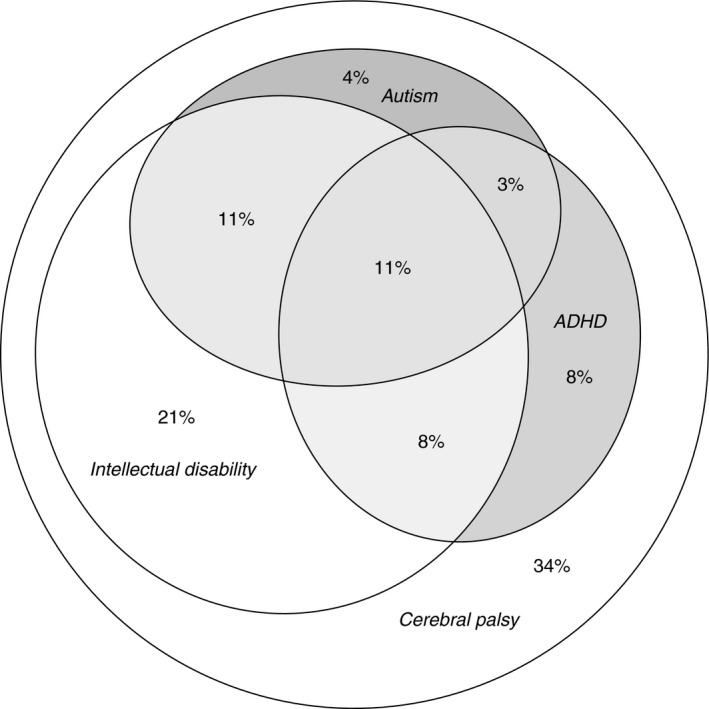
Autism, attention‐deficit/hyperactivity disorder (ADHD), and intellectual disability diagnosed in a population‐based group of 200 school‐age children with cerebral palsy, illustrated proportionally to area in an Euler diagram.
Results
First, the results from the children who were newly assessed (n=90) are presented; then, these are merged with the existing results from the other 110 children, resulting in a total population of 200 children.
Assessments
Seven of the 90 children performed at too low an intellectual level to be able to further assess autism or ADHD: four below an intellectual level of 1 year had no diagnosis of autism and three at an intellectual level between 1 and 3 years had already been diagnosed with autism but not ADHD.
Of the remaining 83 children, 40 had previously been diagnosed with autism, ADHD, or both. All these diagnoses (23 autism and 21 ADHD) were evaluated and met the current DSM‐5 criteria. Additional diagnoses were made in nine children, five with autism and four with ADHD. Of the 43 children with no previous diagnoses of autism and ADHD, 19 met the diagnostic criteria: 10 presented with autism, seven with ADHD, and two with both autism and ADHD.
In total, 17 new diagnoses of autism and 13 new diagnoses of ADHD were made. Another nine children presented with obvious autistic traits that did not fully meet the diagnostic criteria for autism, while several children with attention deficits did not meet the full criteria for an ADHD diagnosis.
The 28 children with new diagnoses were more often classified in GMFCS levels III to IV (10 out of 17 children diagnosed with autism and 6 out of 13 diagnosed with ADHD) and more often had mild‐to‐severe intellectual disability (12 out of 17 with autism and 9 out of 13 with ADHD). Children with dyskinetic CP were overrepresented in the group newly diagnosed with autism (6 out of 17).
Nineteen of the 90 children assessed performed at another intellectual level than previously described, that is, at a lower level in all but one child.
Total study population
The total study population consisted of 200 children (109 males and 91 females), making up 76% of the original population of 264 children; and 94% of the 213 children screened for autism and ADHD. The 13 children who did not participate did not differ significantly from the 200 who did regarding sex, gestational age, CP type, gross motor function, or associated impairments.
Of the 200 children, 90 (45%) were diagnosed with autism, ADHD, or both. Fifty‐nine children (30%) had autism and 60 children (30%) had ADHD; diagnoses of autism and ADHD overlapped in 29 children, who had both diagnoses (Fig. 1). Associations with sex, gestational age, CP type, gross motor function, intellectual disability, and other associated impairments are presented in Table 1.
Table 1.
Diagnoses of autism and attention‐deficit/hyperactivity disorder (ADHD) in a total population of school‐age children with cerebral palsy (CP)
| Total, n | Autism only, n (%) | Autism+ADHD, n (%) | ADHD only, n (%) | None, n (%) | Autism total, n (%) | ADHD total, n (%) | |
|---|---|---|---|---|---|---|---|
| Population size | 200 | 30 (15) | 29 (14) | 31 (16) | 110 (55) | 59 (30) | 60 (30) |
| Sex | |||||||
| Males | 109 | 20 (18) | 17 (16) | 16 (15) | 56 (51) | 37 (34) | 33 (30) |
| Females | 91 | 10 (11) | 12 (13) | 15 (17) | 54 (59) | 22 (24) | 27 (30) |
| Gestational age, wks | |||||||
| 23–27 | 20 | 4 (20) | 7 (35) | 4 (20) | 5 (25) | 11 (55) | 11 (55) |
| 28–31 | 22 | 5 (23) | 2 (9) | 2 (9) | 13 (59) | 7 (32) | 4 (18) |
| 32–36 | 35 | 6 (17) | 4 (11) | 3 (9) | 22 (63) | 10 (29) | 7 (20) |
| 37–42 | 123 | 15 (12) | 16 (13) | 22 (18) | 70 (57) | 31 (25) | 38 (31) |
| CP type | |||||||
| Unilateral spastic CP | 82 | 7 (9) | 11 (13) | 17 (21) | 47 (57) | 18 (22) | 28 (34) |
| Bilateral spastic CP | 73 | 16 (22) | 9 (12) | 7 (10) | 41 (56) | 25 (34) | 16 (22) |
| Dyskinetic CP | 31 | 6 (19) | 3 (10) | 5 (16) | 17 (55) | 9 (29) | 8 (26) |
| Ataxic CP | 14 | 1 (7) | 6 (43) | 2 (14) | 5 (36) | 7 (50) | 8 (57) |
| GMFCS level | |||||||
| I | 101 | 9 (9) | 11 (11) | 20 (20) | 61 (60) | 20 (20) | 31 (31) |
| II | 33 | 5 (15) | 10 (30) | 3 (9) | 15 (46) | 15 (45) | 13 (39) |
| III | 18 | 3 (17) | 3 (17) | 4 (22) | 8 (44) | 6 (33) | 7 (39) |
| IV | 29 | 8 (28) | 4 (14) | 3 (10) | 14 (48) | 12 (41) | 7 (24) |
| V | 19 | 5 (27) | 1 (5) | 1 (5) | 12 (63) | 6 (32) | 2 (11) |
| Visual impairment | |||||||
| No | 170 | 20 (12) | 26 (15) | 31 (18) | 93 (55) | 46 (27) | 57 (34) |
| Yes | 30 | 10 (33) | 3 (10) | 0 (0) | 17 (57) | 13 (43) | 3 (10) |
| Intellectual level | |||||||
| Normal | 79 | 6 (8) | 3 (4) | 13 (16) | 57 (72) | 9 (11) | 16 (20) |
| Borderline | 20 | 2 (10) | 4 (20) | 3 (15) | 11 (55) | 6 (30) | 7 (35) |
| Mild intellectual disability | 45 | 5 (11) | 12 (27) | 11 (24) | 17 (38) | 17 (38) | 23 (51) |
| Moderate intellectual disability | 20 | 4 (20) | 6 (30) | 4 (20) | 6 (30) | 10 (50) | 10 (50) |
| Severe intellectual disability | 20 | 7 (35) | 4 (20) | 0 (0) | 9 (45) | 11 (55) | 4 (20) |
| Profound intellectual disability | 16 | 6 (37) | 0 (0) | 0 (0) | 10 (63) | 6 (38) | 0 (0) |
| Viking Speech Scale level | |||||||
| I | 97 | 10 (10) | 12 (12) | 16 (17) | 59 (61) | 22 (23) | 28 (29) |
| II | 49 | 8 (16) | 9 (19) | 8 (16) | 24 (49) | 17 (35) | 17 (35) |
| III | 16 | 0 (0) | 2 (12) | 6 (38) | 8 (50) | 2 (13) | 8 (50) |
| IV | 38 | 12 (31) | 6 (16) | 1 (3) | 19 (50) | 18 (47) | 7 (18) |
| Epilepsy | |||||||
| No | 125 | 14 (11) | 14 (11) | 20 (16) | 77 (62) | 28 (22) | 34 (27) |
| Active | 75 | 16 (21) | 15 (20) | 11 (15) | 33 (44) | 31 (41) | 26 (35) |
The results are shown in relation to sex, gestational age, CP type, gross motor function, and associated impairments. The percentages describe the proportion of the subgroups. GMFCS, Gross Motor Function Classification System.
Autism and ADHD were found in all CP types (Fig. 2). Children with ataxic CP often presented with a combination of autism and ADHD. The overrepresentation of autism was not significant (χ2=3.04; p=0.081), whereas it was significant for ADHD (χ2=5.28; p=0.022). There was a non‐significant trend for spastic CP, with autism more common in children with bilateral spastic CP and ADHD more common in children with unilateral spastic CP (p=0.088 and p=0.092 respectively).
Figure 2.
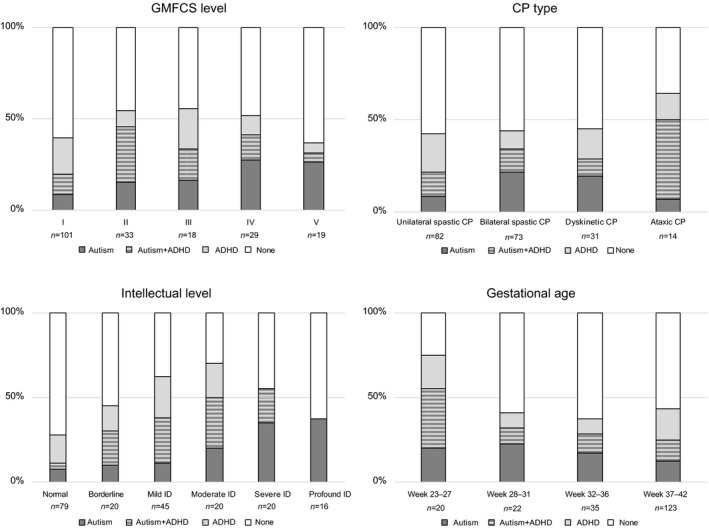
Diagnoses of autism and attention‐deficit/hyperactivity disorder (ADHD) or both in a population‐based group of 200 school‐age children with cerebral palsy (CP) after screening and clinical assessment. Results are presented in relation to gross motor function, CP type, intellectual level, and gestational age. GMFCS, Gross Motor Function Classification System.
Autism was less prevalent in GMFCS level I than GMFCS levels II to V (χ2=15.03; p<0.001), while ADHD prevalence was low in GMFCS levels IV and V (Fig. 2).
There was no significant difference in sex distribution for autism or ADHD. Children born extremely preterm had autism (χ2=6.95; p=0.008) and ADHD (χ2=6.61; p=0.010) more often than children born after 27 weeks of gestation (Fig. 2).
Intellectual disability was found in 101 of the 200 children (51%). The prevalence of autism increased with lower intellectual level (=18.84; p<0.001). ADHD was also more prevalent in children with lower intellectual level (=3.86; p=0.049), profound intellectual disability was excluded due to no ADHD diagnoses per definition at this intellectual level (Fig. 2).
Speech ability classified according to the Viking Speech Scale was associated with GMFCS (ρ=0.75; p<0.001) and intellectual level (ρ=0.67; p<0.001); however, speech impairment was not significantly associated with either autism or ADHD. Autism was more prevalent in children with epilepsy (χ2=8.08; p=0.004), but epilepsy was not associated with ADHD.
Autism was predicted by three variables: intellectual disability, ADHD, and preterm birth. CP type, gross motor function, sex, speech ability, and epilepsy did not provide any additional information. The ORs for an outcome of autism were 4.1 for intellectual disability (95% CI 2.1–8.6), 3.2 for ADHD (95% CI 1.6–6.5), and 2.0 for preterm birth (95% CI 1.0–3.9).
ADHD was also predicted by three variables: intellectual disability, autism, and mild gross motor impairment. CP type, sex, preterm birth, speech ability, and epilepsy did not provide any additional information. The ORs for an outcome of ADHD were 2.3 for intellectual disability (95% CI 1.0–4.9), 3.0 for autism (95% CI 1.5–6.1), and 2.8 for mild gross motor impairment (95% CI 1.3–6.3).
The final diagnoses of autism and ADHD were compared with the screening performed in the 200 children. The screening for autism showed a sensitivity of 83% and a specificity of 87%, while the screening for ADHD showed a sensitivity of 87% and a specificity of 69%.
The clinical characteristics of children with autism were intellectual disability, epilepsy, speech impairment, more severe gross motor impairment, and preterm birth (especially being born extremely preterm). The clinical characteristics of children with ADHD were mild‐to‐severe intellectual disability, less severe gross motor impairment, and extremely preterm birth. Half of the children with autism had ADHD and half of the children with ADHD had autism (Fig. 1).
There were few children with CP without associated impairments. Sixty‐eight (of 200) children (34%) had CP without autism, ADHD, or intellectual disability. Forty‐eight (of 200) children (24%) had CP without any of the associated impairments studied, that is, autism, ADHD, intellectual disability, speech/language disorders, visual/hearing impairments, or epilepsy.
Discussion
Autism and ADHD are common in children with CP. 2 , 3 , 4 , 5 , 6 , 7 In this in‐depth, comprehensive study of a total population of children with CP, prevalence rates were higher than previously reported from population‐based studies; 45% had autism, ADHD, or both. This prevalence rate is similar to those for intellectual disability and epilepsy respectively.
Previous population‐based studies of autism, including our first study in the present series, 12 mainly reported already identified diagnoses. Delobel‐Ayoub et al. 6 reported varying prevalence rates (4.0–16.7%) from four areas in Europe, probably reflecting differences in both methodology and awareness of autism in CP. In a larger study from four locations in the USA, the reported frequency of autism co‐occurring with CP was 6.9% of children with CP. 5 Other studies focused on certain subpopulations. Kilincaslan et al. 3 reported autism in 15% of children with CP within a specialized hospital setting, while Goodman and Graham 2 assessed children with hemiplegia and found autism in 3%. In addition, the number of autism diagnoses in the general population has also increased during the last decades. 24
Population‐based studies of ADHD in CP are scarce. ADHD may be hard to diagnose in children with speech impairment and severe motor impairment. Some studies have reported attention deficits but not diagnoses, while some have used a screening procedure for ADHD symptoms rather than diagnoses. 7 , 13 By interviewing parents, Bjorgaas et al. 4 identified symptoms of ADHD in 50% of children in GMFCS levels I to IV.
Studies based on records and registers most likely underestimate the prevalence of autism and ADHD and screening usually includes more children than those actually meeting the diagnostic criteria. The present study in the current series may contribute substantially to the lack of knowledge pointed out by Craig et al. 7 in a systematic review.
Most associated impairments increase with more severe GMFCS levels. 25 , 26 In our register study, we speculated that autism and ADHD were underdiagnosed especially in children in GMFCS levels III and IV. 12 Indeed, in the present study, we found a high proportion of new diagnoses of autism and ADHD in these GMFCS levels. However, autism did not increase with GMFCS level in the same way as other associated impairments; in fact, ADHD prevalence even decreased in children in the most severe GMFCS levels. It is most likely that ADHD, but also autism, were underdiagnosed due to diagnostic limitations in children with a complex clinical picture or at too low an intellectual level. 27 With this in mind, predicting mild motor impairment for an ADHD outcome may be incorrect.
There were no clear differences between CP types, except for the small group of children with ataxic CP where ADHD was significantly more common. This group may need further studying, as suggested by Åhsgren et al., 28 who found high rates of autism and hyperactivity disorders in children with ataxia.
The multiple regression models showed covariation between autism, ADHD, and intellectual disability in children with CP. Associations with other factors, like epilepsy, were explained by their correlation with intellectual disability. Intellectual disability was a predictor of both autism and ADHD, and autism and ADHD often co‐occurred. The concept of Early Symptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations (ESSENCE) 29 underscores this; having one neurodevelopmental impairment is a strong risk factor for having other impairments.
The screening showed high sensitivity and specificity for autism and high sensitivity but lower specificity for ADHD, possibly reflecting that too many children were included in the assessment. There is a need to adjust the cut‐offs to be more accurate regarding ADHD. Screening tools with fewer items than in our original questionnaire are also needed. 7 , 13
The diagnostic procedure with established instruments we used worked well for the vast majority of children with CP but was not suitable for the most disabled children. Neither screening nor assessment instruments could be applied to this smaller group. Nonetheless, autism and ADHD may be important parts of their overall impairment profile. We need more knowledge about autism and ADHD in these children and which assessment methods are adequate to use for clinical validation. 4 , 7 , 19 , 27
Study strengths and limitations
A strength of this study was the coverage provided by a total well‐defined population of children with CP. More than 75% of children completed the process of screening and assessment. A second strength is that all diagnoses were made by clinical assessment and validation. This argues in favour of the results being generalizable, at least in other high‐income countries that are likely to have a similar panorama of CP.
One limitation was that not all children were personally examined in the present study; thus, some of the data presented are retrospective in nature. The existing retrospective data used included assessments made by different multidisciplinary teams, albeit in a very similar and almost systematic fashion. Our own team had made one‐third of these assessments.
Another limitation was that children with several and severe associated impairments were difficult to assess in a standardized way. The methods and instruments used could not be applied without adaptations for the most disabled group.
Starting out with a register of 264 children, followed by screening for autism and ADHD and in‐depth assessment if needed, a total of 200 children were examined and evaluated. The 200 participants and the 64 children who did not participate were similar in terms of CP type, sex, gestational age, and intellectual disability. There was a higher proportion of the most severely disabled children among non‐participants. In summary, in spite of its limitations, we regard the study results as sufficiently representative of the total population of children studied. By actively screening and assessing this population‐based group of children with CP, we have come as close as possible to establishing a more accurate prevalence of autism and ADHD in children with CP.
Conclusion
Autism and ADHD were very common in this study of a total population of children with CP; 30% had autism and 30% had ADHD. Half of all children had an intellectual disability and two‐thirds had autism, ADHD, and/or an intellectual disability. Among other well‐known associated impairments, autism and ADHD are common in children with CP. However, autism and ADHD are not correlated with the severity of motor impairment but are mainly predicted by intellectual disability. The high prevalence of autism and ADHD in this population emphasizes the need to screen, and if indicated, assess all children with CP for these important neuropsychiatric impairments.
Supporting information
Figure S1: Flow chart of the study recruitment process.
Acknowledgements
We thank all participating children and their parents. This study was supported by grants from the Gothenburg Society of Medicine, the Linnea and Josef Carlsson Foundation, the Queen Silvia Children’s Hospital Research Foundations, the RBU Research Foundation, the Sahlgrenska University Hospital Foundation, and the Swedish State under the agreement between the Swedish Government and country councils, namely ALF agreement no. ALFGBG‐726001.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy. Dev Med Child Neurol Suppl 2007; 109: 8–14. [PubMed] [Google Scholar]
- 2. Goodman R, Graham P. Psychiatric problems in children with hemiplegia: cross sectional epidemiological survey. BMJ 1996; 312: 1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kilincaslan A, Mukaddes NM. Pervasive developmental disorders in individuals with cerebral palsy. Dev Med Child Neurol 2009; 51: 289–94. [DOI] [PubMed] [Google Scholar]
- 4. Bjorgaas HM, Hysing M, Elgen I. Psychiatric disorders among children with cerebral palsy at school starting age. Res Dev Disabil 2012; 33: 1287–93. [DOI] [PubMed] [Google Scholar]
- 5. Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co‐occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 2014; 56: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delobel‐Ayoub M, Klapouszczak D, van Bakel MME, et al. Prevalence and characteristics of autism spectrum disorders in children with cerebral palsy. Dev Med Child Neurol 2017; 59: 738–42. [DOI] [PubMed] [Google Scholar]
- 7. Craig F, Savino R, Trabacca A. A systematic review of comorbidity between cerebral palsy, autism spectrum disorders and Attention Deficit Hyperactivity Disorder. Eur J Paediatr Neurol 2019; 23: 31–42. [DOI] [PubMed] [Google Scholar]
- 8. Nygren G, Cederlund M, Sandberg E, et al. The prevalence of autism spectrum disorders in toddlers: a population study of 2‐year‐old Swedish children. J Autism Dev Disord 2012; 42: 1491–7. [DOI] [PubMed] [Google Scholar]
- 9. Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention‐deficit/hyperactivity disorder outcomes for children treated in community‐based pediatric settings. Arch Pediatr Adolesc Med 2010; 164: 160–5. [DOI] [PubMed] [Google Scholar]
- 10. Himmelmann K, Hagberg G, Uvebrant P. The changing panorama of cerebral palsy in Sweden. X. Prevalence and origin in the birth‐year period 1999–2002. Acta Paediatr 2010; 99: 1337–43. [DOI] [PubMed] [Google Scholar]
- 11. Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden. XI. Changing patterns in the birth‐year period 2003–2006. Acta Paediatr 2014; 103: 618–24. [DOI] [PubMed] [Google Scholar]
- 12. Påhlman M, Gillberg C, Himmelmann K. One third of school‐aged children with cerebral palsy have neuropsychiatric impairments in a population‐based study. Acta Paediatr 2019; 108: 2048–55. [DOI] [PubMed] [Google Scholar]
- 13. Påhlman M, Gillberg C, Wentz E, Himmelmann K. Autism spectrum disorder and attention‐deficit/hyperactivity disorder in children with cerebral palsy: results from screening in a population‐based group. Eur Child Adolesc Psychiatry 2020; 29(11): 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wing L, Leekam SR, Libby SJ, Gould J, Larcombe M. The Diagnostic Interview for Social and Communication Disorders: background, inter‐rater reliability and clinical use. J Child Psychol Psychiatry 2002; 43: 307–25. [DOI] [PubMed] [Google Scholar]
- 15. Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord 1980; 10: 91–103. [DOI] [PubMed] [Google Scholar]
- 16. Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord 2007; 37: 613–27. [DOI] [PubMed] [Google Scholar]
- 17. Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry 2001; 40: 168–79. [DOI] [PubMed] [Google Scholar]
- 18. Sparrow SS, Cicchetti DV, Balla DA, Doll EA. Vineland‐II: Vineland Adaptive Behavior Scales. 2nd edn Circle Pines, MN: American Guidance Service, 2005. [Google Scholar]
- 19. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th edn Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 20. Surveillance of Cerebral Palsy in Europe . Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol 2000; 42: 816–24. [DOI] [PubMed] [Google Scholar]
- 21. Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol 2008; 50: 744–50. [DOI] [PubMed] [Google Scholar]
- 22. Pennington L, Virella D, Mjøen T, et al. Development of The Viking Speech Scale to classify the speech of children with cerebral palsy. Res Dev Disabil 2013; 34: 3202–10. [DOI] [PubMed] [Google Scholar]
- 23. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundström S, Reichenberg A, Anckarsäter H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 2015; 350: h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Himmelmann K, Beckung E, Hagberg G, Uvebrant P. Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol 2006; 48: 417–23. [DOI] [PubMed] [Google Scholar]
- 26. Sigurdardóttir S, Thórkelsson T, Halldórsdóttir M, Thorarensen O, Vik T. Trends in prevalence and characteristics of cerebral palsy among Icelandic children born 1990 to 2003. Dev Med Child Neurol 2009; 51: 356–63. [DOI] [PubMed] [Google Scholar]
- 27. Thurm A, Farmer C, Salzman E, Lord C, Bishop S. State of the field: differentiating intellectual disability from autism spectrum disorder. Front Psychiatry 2019; 10: 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Åhsgren I, Baldwin I, Goetzinger‐Falk C, Eriksson A, Flodmark O, Gillberg C. Ataxia, autism, and the cerebellum: a clinical study of 32 individuals with congenital ataxia. Dev Med Child Neurol 2005; 47: 193–8. [DOI] [PubMed] [Google Scholar]
- 29. Gillberg C. The ESSENCE in child psychiatry: Early Symptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations. Res Dev Disabil 2010; 31: 1543–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flow chart of the study recruitment process.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


