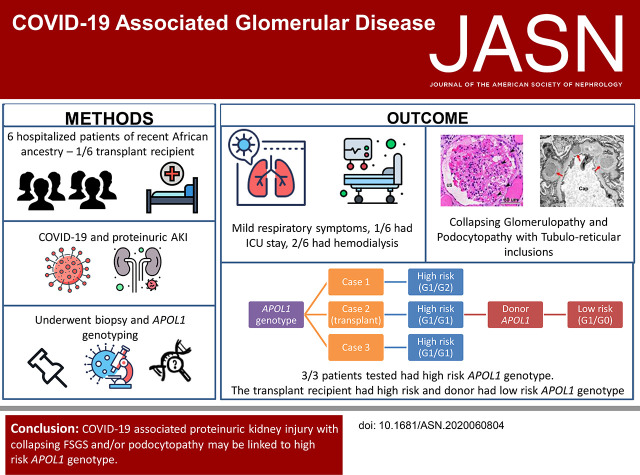
Significance Statement
Studies have found AKI with high-grade proteinuria in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In this patient series, the authors describe six patients of recent African ancestry who developed COVID-19–associated AKI with podocytopathy, collapsing glomerulopathy, or both. Respiratory symptoms among these patients were generally mild, and none required ventilator support. Previous research has demonstrated an association between high-risk gene variants in the APOL1 gene, which encodes the APOL1 protein, and collapsing glomerulopathy in patients with another viral infection, HIV. Genetic testing in three of the patients in this study confirmed that they had high-risk APOL1 genotypes. In one of these patients, collapsing glomerulopathy occurred in the engrafted kidney, which was transplanted from a donor who carried an APOL1 low-risk genotype, a finding inconsistent with current models of APOL1-mediated kidney injury.
Keywords: COVID-19, collapsing glomerulopathy, APOL1
Visual Abstract
Abstract
Background
Studies have documented AKI with high-grade proteinuria in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In some patients, biopsies have revealed collapsing glomerulopathy, a distinct form of glomerular injury that has been associated with other viruses, including HIV. Previous patient reports have described patients of African ancestry who developed nephrotic-range proteinuria and AKI early in the course of disease.
Methods
In this patient series, we identified six patients with coronavirus disease 2019 (COVID-19), AKI, and nephrotic-range proteinuria. COVID-19 was diagnosed by a positive nasopharyngeal swab RT-PCR for SARS-CoV-2 infection. We examined biopsy specimens from one transplanted kidney and five native kidneys. Three of the six patients underwent genetic analysis of APOL1, the gene encoding the APOL1 protein, from DNA extracted from peripheral blood. In addition, we purified genomic DNA from paraffin-embedded tissue and performed APOL1 genotype analysis of one of the native biopsies and the donor kidney graft.
Results
All six patients were of recent African ancestry. They developed COVID-19–associated AKI with podocytopathy, collapsing glomerulopathy, or both. Patients exhibited generally mild respiratory symptoms, and no patient required ventilator support. Genetic testing performed in three patients confirmed high-risk APOL1 genotypes. One APOL1 high-risk patient developed collapsing glomerulopathy in the engrafted kidney, which was transplanted from a donor who carried a low-risk APOL1 genotype; this contradicts current models of APOL1-mediated kidney injury, and suggests that intrinsic renal expression of APOL1 may not be the driver of nephrotoxicity and specifically, of podocyte injury.
Conclusions
Glomerular disease presenting as proteinuria with or without AKI is an important presentation of COVID-19 infection and may be associated with a high-risk APOL1 genotype.
AKI is reported to occur in 20% of all hospitalized patients and in 30% of critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In addition to kidney failure due to acute tubular necrosis and injury secondary to hypotension, sepsis, and/or cytokine storm that occur in the later phase of SARS-CoV-2 infection, a substantial number of patients have been reported to exhibit proteinuria and hematuria.1,2 Previous patient reports describe patients of African ancestry who developed nephrotic-range proteinuria and AKI, early in the course of disease.3–10 Biopsies performed in some of these patients showed collapsing GN, a distinct form of glomerular injury that has been associated with other viruses, including HIV and parvovirus.11 Importantly, kidney disease did not correlate with the severity of respiratory symptoms, and there was little evidence of cytokine storm. Below, we report six patients who presented to our hospital within a 3-week period with coronavirus disease 2019 (COVID-19), AKI, nephrotic-range proteinuria, and severe podocytopathy.
Methods
Six patients with COVID-19 with AKI and nephrotic-range proteinuria were identified within a 3-week period at a single center in Cook County, Illinois (Supplemental Figures 1–5). COVID-19 was diagnosed by positive nasopharyngeal swab RT-PCR for SARS-CoV-2 infection. One transplant kidney and five native kidney biopsy specimens from patients were examined. Three of the six patients underwent APOL1 genetic analysis using DNA extracted from peripheral blood (OHSU Knight Genetics laboratory). In addition, genomic DNA was purified from paraffin-embedded tissue, and APOL1 genotype analysis of one of the native biopsies and the donor kidney graft was performed as described5 (institutional review board no. STU00104548-MOD0030; approved by NU institutional review board for use on or after March 13, 2020).
Patient 1
A 64-year-old man of recent African ancestry, with no known past medical history (PMH), presented to an outside hospital with fever but no other symptoms. His physical examination was unremarkable, and he was not hypoxemic. Nasopharyngeal swab RT-PCR was positive for SARS-CoV-2 infection. Computed tomography chest demonstrated bilateral patchy peripheral ground-glass opacities. His initial serum creatinine (SCr) was 1.2 mg/dl. He was discharged after 3 days and then readmitted to our hospital the next day for recurrent fever. At the time of readmission, his SCr was 1.9 mg/dl, and urinalysis showed proteinuria and hematuria, consistent with AKI. A 24-hour urine collection contained 7.6 g protein. Kidneys were normal sized with increased echogenicity on ultrasound. Workup for autoimmune, monoclonal, and other infectious etiologies of glomerular disease was negative. A renal biopsy (Figure 1) showed collapsing glomerulopathy on light microscopy. Electron microscopy showed podocytopathy with shrinkage of the capillary tuft and expansion of the urinary space. Many protein absorption droplets were observed in the podocytes and proximal tubules, which also showed vacuolar degeneration. Tubuloreticular inclusions were present. APOL1 genotyping of DNA from peripheral blood and biopsy tissue revealed high-risk genotype of G1/G2. The patient received supportive care but no SARS-CoV-2–specific therapy. On day 5, he required noninvasive oxygen supplementation and spent 48 hours in the intensive care unit. AKI continued to worsen, with peak SCr of 7.2 mg/dl on day 8 of admission followed by gradual improvement in renal function without need for RRT. He was discharged on day 16. On follow-up of 47 days after initial presentation, his SCr and proteinuria had partially improved to 2.5 mg/dl and 1.4 g/24 h, respectively.
Figure 1.
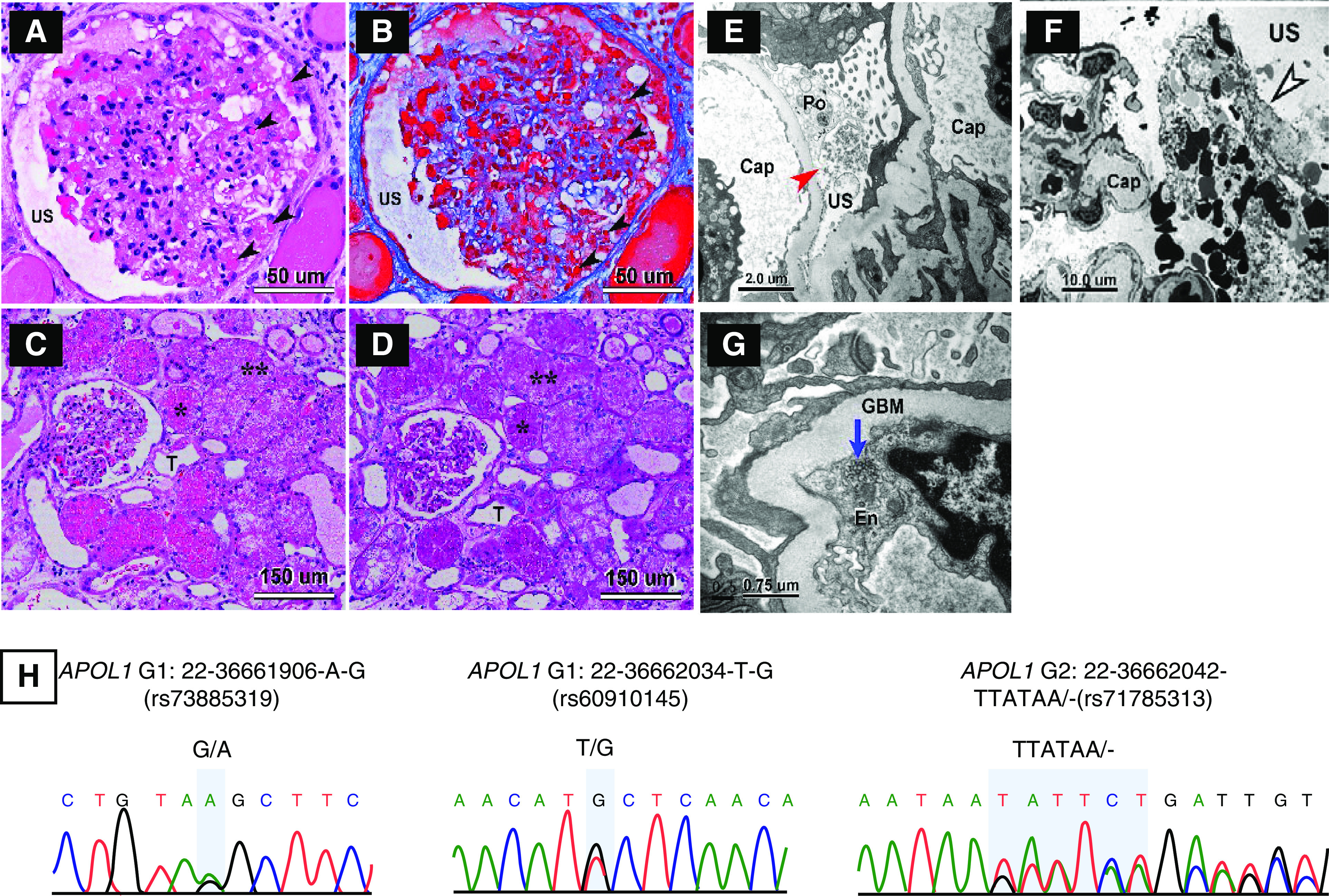
Biopsy findings showing CGN and APOL1 genotyping showing high risk genotype for patient 1. (A–D) Light micrographs of glomerular and tubular morphologic features in patient’s renal biopsy. (A and B) Mild to moderate focal glomerular hypercellularity with shrinkage of the capillary tuft is seen. The lumina of many capillary loops are effaced. The urinary space (US) is expanded. Many protein absorption lysosomal droplets are seen in the glomerular podocytes and parietal epithelial cells (black arrowheads). (A and C) Hematoxylin and eosin stain. (B) Trichrome stain. (D) Periodic acid–Schiff (PAS) stain. T, tubule. *In (C and D), numerous protein absorption lysosomal PAS-positive droplets are seen in the tubular epithelia. **Many tubules have undergone severe vacuolar degeneration. (E–G) Electron micrographs of glomerular and tubular morphologic features in patient’s renal biopsy. (E and F) Podocyte effacement (Po) and dilation of USs (red arrow) with cellular debris are present in the US (black and white arrowhead), which is likely made up of deformed red blood cells and fibrin tactoids. (G) shows tubular reticular inclusions (TRIs) in the rough endoplasmic reticulum of glomerular endothelium (En; blue arrow). GBM, glomerular basement membrane. (H) Sequencing for APOL1 was performed on DNA obtained from blood and native kidney biopsy. Genotypes were concordant and revealed a high–renal risk APOL1 G1/G2 genotype.
Patient 2
A 50-year-old woman of recent African ancestry presented to the emergency department with fever, diarrhea, fatigue, and dyspnea on exertion. Her PMH included hypertension and ESKD status after two kidney transplants in 2003 and 2009, the latter from a deceased Hispanic donor. Her post-transplant course was complicated by Banff 1B acute cellular rejection and CKD stage 4 with baseline creatinine 2.5 mg/dl and baseline urine protein-creatinine ratio of 1.4 g/g. She was not hypoxemic and had an unremarkable physical examination. Nasopharyngeal swab RT-PCR was positive for SARS-CoV-2 infection. Initial blood and urine testing showed SCr of 4.93 mg/dl, proteinuria, and hematuria consistent with AKI. A 24-hour urine protein collection revealed 6.11 g. Workup for autoimmune, monoclonal, and other infectious etiologies of glomerular disease was negative. A transplant renal biopsy (Figure 2) showed collapsing glomerulopathy with mesangial expansion. Electron microscopy showed podocytopathy characterized by effacement and numerous tubuloreticular inclusions. There was also evidence of subepithelial, intramembranous, and a few mesangial electron dense deposits. APOL1 genotyping of DNA from peripheral blood revealed that the patient carried a high-risk G1/G1 genotype. Interestingly, the transplanted kidney carried a low-risk G1/G0 APOL1 genotype. She received supportive care but no COVID-19–specific therapies and did not require intensive care unit admission. Immunosuppressive medications included mycophenolate mofetil, which was reduced to one-third of the original dose, and tacrolimus. Kidney function declined with peak SCr 5.99 mg/dl on day 7. She was discharged from the hospital after 13 days. On follow-up of 93 days after initial presentation, her SCr and proteinuria had partially improved to 4.81 mg/dl and 3.3 g/24 h, respectively.
Figure 2.

Biopsy findings showing CGN and APOL1 genotyping showing discordance between donor and recipient for patient 2. (A–D) Light micrographs of glomerular and tubular morphologic features in renal biopsy. (A–C) Moderate diffuse glomerular hypercellularity with compression of the capillary tuft is seen. The lumina of some capillary loops (Cap) are not readily discernible. (C) A portion of the glomerular tuft with intact Cap is seen only with silver stain sections. There is marked proliferation of visceral epithelial cells/podocytes (Po). Many protein absorption lysosomal (Ly) droplets are seen in the glomerular Po (black arrowheads in A and B). (D) Numerous protein absorption Ly periodic acid–Schiff (PAS)-positive droplets are seen in the tubular epithelia (T). Some tubules have undergone vacuolar degeneration, as seen in lower right corner of (D). (A) Hematoxylin and eosin stain. (B) Trichrome stain. (C) Silver stain. (D) PAS stain. (E–H) Electron micrographs showing glomerular morphologic features in renal biopsy. (E) shows a Po containing numerous Ly droplets and focal effacement of the foot processes. Electron-dense immune complex deposits are seen in the subepithelial (red arrows) and paramesangial (black and green arrow) regions of the glomerulus. (F) shows disrupted Po with dilated urinary space (US; red arrowheads) flanked by glomerular basement membrane (GBM) of two Cap. Tubuloreticular inclusions (black and white arrowhead) are present in the glomerular endothelium (En). (G) shows the presence of subepithelial and intramembranous electron-dense deposits (red arrows). (H) shows an intramembranous electron-dense deposit (red arrow) flanked by striated collagen fibers (black and green arrowheads). (I) Sequencing of APOL1 from DNA obtained from renal allograft demonstrates a G1/G0 genotype, whereas (J) analysis of DNA obtained from peripheral blood shows a G1/G1 genotype, which was discordant from the renal allograft.
Patient 3
A 64-year-old man of recent African ancestry, with a PMH of hypertension, diabetes mellitus, and prostate cancer, presented with fever, diarrhea, fatigue, and productive cough for 2 weeks. On admission, he was not hypoxic and had an unremarkable physical examination. A chest x-ray demonstrated diffuse bilateral lung opacities. A nasopharyngeal SARS-CoV-2 RT-PCR test was positive. Initial laboratory values were significant for AKI with SCr 10.82 mg/dl, increased from a baseline of 1 mg/dl, and a urinalysis showing proteinuria and hematuria. A urine protein-creatinine ratio was 8.3. The patient rapidly became anuric, precluding a 24-hour urine collection for protein quantification. Workup for autoimmune, monoclonal, and other infectious etiologies of glomerular disease was negative. The kidney biopsy demonstrated mild mesangial hypercellularity, diffuse podocytopathy, and focal increase of phagolysosomal activity. APOL1 genotyping of DNA from peripheral blood revealed that the patient had the high-risk genotype of G1/G1. The subsequent hospital course was marked by persistent hyperkalemia and volume overload in the setting of oligoanuric AKI, requiring intermittent hemodialysis. The patient did not receive COVID-19–specific therapies. He was discharged after 10 days of hospital admission, but he was still oligoanuric and on intermittent hemodialysis three times a week. However, his renal function subsequently improved, and 3 weeks later, hemodialysis was discontinued. On follow-up of 42 days after initial presentation, off hemodialysis, his SCr and proteinuria had almost completely recovered to 1.2 g/dl and 0.4 g/24 h, respectively.
Patient 4
A 48-year-old woman of recent African ancestry, with a PMH of hypertension and CKD, presented with 12 days of diarrhea, dry cough, myalgias, and anosmia. Three days prior to her presentation to our ED, she had been tested for COVID-19 by nasopharyngeal SARS-CoV-2 PCR, which was positive. Of note, the patient works in a nursing home. On admission, she was febrile to 39.2 °C, tachycardic, normotensive, and not hypoxic. Her physical examination was otherwise unremarkable. A chest x-ray demonstrated bilateral patchy airspace opacities. Initial laboratory work revealed AKI with a SCr of 7.63 mg/dl, increased from a baseline of 1.3 mg/dl, and urinalysis showing proteinuria and hematuria. A 24-hour urine protein quantification revealed 12 g. Workup for autoimmune, monoclonal, and other infectious etiologies of glomerular disease was negative. The kidney biopsy showed mild mesangial hypercellularity, diffuse podocytopathy, and mild focal increase of phagolysosomal activity. The patient declined APOL1 genotyping. The patient received supportive care during her hospital course. No specific therapy for COVID-19 was administered. She did not require RRT. During her hospital stay, her AKI continued to worsen, despite adequate fluid intake and resolution of diarrhea, with SCr peaking at 10.5 mg/dl on hospital day 11 followed by slow improvement to an SCr of 6.7 mg/dl at discharge on hospital day 18. On follow-up of 90 days after initial presentation, her SCr and proteinuria had partially improved to 1.8 g/dl and 0.8 g/24 h, respectively.
Patient 5
A 54-year-old woman of recent African ancestry, with PMH of hypertension, presented with neck swelling and sore throat. She was not hypoxic, and examination was remarkable for a malar rash, mild neck swelling, and bilateral lower extremity edema. Initial nasopharyngeal SARS-CoV-2 RT-PCR test was negative. A computed tomography scan of her neck showed a retropharyngeal effusion anterior to the cervical spine that was thought to be sterile. Initial laboratory values were significant for a creatinine of 2.3 mg/dl, increased from a baseline of 1.7 mg/dl, as well as proteinuria and hematuria. A 24-hour urine protein collection showed 11.7 g of protein, and her serum albumin was 1.8 g/dl. Workup for autoimmune, monoclonal, and other infectious etiologies of glomerular disease was negative. Renal biopsy showed focal collapsing glomerulopathy with podocytopathy and increased phagolysosomal activity, associated with an unremarkable immunofluorescence. She declined APOL1 genotyping. Because of the high suspicion for COVID-19–associated glomerulopathy, the patient was retested for COVID-19, and the repeat nasopharyngeal SARS-CoV-2 RT-PCR was positive. She was managed with supportive care, with no COVID-19–specific therapies, and she was discharged after a 5-day hospital stay, at which point her renal function had not shown any improvement. On follow-up of 47 days after initial presentation, SCr and proteinuria were 2.3 g/dl and 17 g/24 h, respectively. The patient was initiated on high-dose prednisone and RAS blockade for treatment of CGN with persistent nephrotic-range proteinuria.
Patient 6
A 56-year-old man of recent African ancestry, with a PMH significant for hypertension, diabetes mellitus, stage 4 CKD, and monoclonal gammopathy of undetermined significance, presented with a 10-day history of fever, diarrhea, cough, and nasal congestion. On admission, he was normotensive and not hypoxic, with physical examination being unremarkable. Chest x-ray demonstrated clear lung fields bilaterally. Initial laboratory studies revealed a SCr of 7.87 mg/dl, increased from a baseline of 4.0 mg/dl, as well as proteinuria without hematuria. Of note, during a recent nephrology evaluation 3 months prior, he was found to have nephrotic-range proteinuria of 7.5 g/24 h and stage 4 CKD, attributed to diabetic nephropathy given the long-standing history of poorly controlled diabetes and diabetic retinopathy. Workup for autoimmune, monoclonal, and other infectious etiologies of glomerular disease was negative. The kidney biopsy showed marked focal and global glomerulosclerosis and hyalinosis, moderate mesangial hypercellularity, and diffuse podocytopathy with focal increase in phagolysosomal activity. He declined APOL1 genotyping. The hospital course was notable for worsening oligoanuric AKI with SCr peaking at 8.82 mg/dl, requiring intermittent hemodialysis for 6 days. On hospital day 9, the urine output started to improve, and hemodialysis was held. No COVID-19–specific therapies were administered, and he was discharged after a 24-day hospital stay. On follow-up of 65 days after initial presentation, his SCr and proteinuria were 4.53 g/dl and 2.7 g, respectively, which are near his baseline.
Discussion
This report highlights features of an emerging and common COVID-19–related kidney disease that is characterized by podocytopathy and tubular injury. In this small patient series, we observed disproportionately high prevalence of APOL1 renal risk genotypes (100% of tested patients versus 15% expected12,13) and show that this serious glomerular kidney disease can occur in the setting of mild or moderate respiratory symptoms.
All six reported patients with COVID-19–related kidney disease shared several common pathologic features representing a continuum of podocyte injury. Four of the six patients had no history of preexisting kidney disease. In all six patients, significant podocyte injury with effacement and protein overload tubulopathy were observed together with the presence of tubuloreticular inclusions in endothelial cells, characteristic of IFN-mediated viral infection. Three of the patients showed florid collapsing glomerulopathy, similar to lesions observed in HIV-associated nephropathy.14 In all cases, podocytopathy is a central feature of the disease, suggesting a possible tropism of the virus for podocytes, which is supported by autopsy reports (Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al.: Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection. medRxiv 2020.03.04.20031120, 2020).15,16 Podocytes have been reported to express all components required for SARS-CoV-2 entry into a cell, including the ACE2 receptor, the TrpMS22 protease, and furin,17,18 although it is clear that ACE2 is expressed at much higher levels in the brush border of proximal tubular cells.16 Although some studies have shown evidence of SARS-CoV-2 viral RNA in kidney tissue,7,16,19 many others have failed to demonstrate presence of SARS-CoV-2 viral particles in kidney tissue of patients with glomerular disease.9,10 Additional research is required to determine whether direct intrarenal viral infection or systemic cytokine release triggered by extrarenal viral infection is responsible for kidney and podocyte injury.
Three patients underwent genetic analysis and were found to carry high-risk APOL1 genotypes (Table 1). Although still small numbers, there are growing numbers of patient reports linking APOL1 high-risk genotype to COVID-19–related CGN. The risk of HIV-related CGN (HIVAN) is 29-fold higher in patients carrying high-risk APOL1 genotypes versus low-risk genotypes (G1/G0, G0/G0, or G2/G0), supporting a common viral-mediated trigger for renal injury in both diseases. APOL1 encodes for APOL1, which is an apolipoprotein involved in innate immunity,20 and can be secreted into the bloodstream where it circulates in complex with HDL3. The high-risk APOL1 allele G1 carries one or two missense mutations, and the G2 variant carries a six–base pair deletion.21 Both high-risk alleles provide protection from trypanosomiasis, which is thought to explain their high prevalence (15% in the United States) in individuals of recent African ancestry.21,22 It is now well established that high-risk genotypes (G1/G1, G2/G2, or G1/G2) are associated with a high risk of ESKD and CKD related to hypertension, primary glomerular disease, lupus, sickle cell disease, and HIV.22 Several mechanisms have been proposed to explain increased risk of kidney disease in individuals with a high-risk APOL1 genotype, although the pathogenesis is not yet fully understood. All of the proposed mechanisms are believed to cause direct cellular toxicity and death of podocytes, a specialized cell of the renal glomerulus.23–28 In cell-based studies, IFN and toll-like receptor agonists lead to enhanced APOL1 expression,29 suggesting that viral infection may lead to higher levels of endogenous APOL1 in patients, which may enhance toxicity.
Table 1.
Demographics and clinical features of the six patients
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age, yr/race/sex | 64/AA/M | 50/AA/W | 64/AA/M | 49/AA/W | 54/AA/W | 56/AA/M |
| PMH | None | ESKD s/p DDRT, HTN, DM, CKD | HTN, DM, prostate cancer | HTN, HLD, CKD | HTN, CKD | DM, HTN, MGUS, CKD |
| Presenting symptoms | Fever | Fever, fatigue, DOE, diarrhea | Fever, fatigue, diarrhea, cough | Diarrhea, cough myalgias | Sore throat, neck swelling | Fever, vomiting, sore throat, cough |
| Symptoms onset to COVID-19 Dx, d | 2 | 28 | 14 | 2 | 4 | 10 |
| Proteinuria, g | 7.6 | 6.11 | 8.3a | 12.06 | 11 | 0.6b |
| Oliguria | No | No | Yes | No | No | Yes |
| Baseline SCr, mg/dl | 1.2 | 2.5 | 1 | 1.5 | 1.7 | 4 |
| Peak SCr, mg/dl | 7.2 | 5.99 | 13.52 | 10.46 | 2.35 | 8.82 |
| Latest SCr, mg/dl | 2.5 | 4.81 | 1.2 | 1.8 | 2.34 | 4.53 |
| Required hemodialysis | No | No | Yes | No | No | Yes |
| Duration of hospital stay/duration of follow-up, d | 16/47 | 13/93 | 10/42 | 18/90 | 5/47 | 24/65 |
| Duration of ICU stay, d | 2 | 0 | 0 | 0 | 0 | 0 |
| Ventilator dependent | No | No | No | No | No | No |
| COVID-19–specific medications | No | No | No | No | No | No |
| APOL1 test on blood | G1/G2 | G1/G1 | G1/G1 | Declined | Declined | Declined |
| APOL1 test on kidney biopsy tissue | G1/G2 | G1/G0c | Not done | Declined | Declined | Declined |
| Type of kidney biopsy | Native | Transplant | Native | Native | Native | Native |
| Biopsy findings | Collapsing GN, podocytopathy, TRI protein overload tubulopathy | Collapsing GN, podocytopathy, TRI, protein overload tubulopathy | Podocytopathy, TRI, protein overload tubulopathy | Podocytopathy, TRI, protein overload tubulopathy | Collapsing GN, podocytopathy, protein overload tubulopathy | Podocytopathy, protein overload tubulopathy |
AA, recent African ancestry; M, men; W, women; s/p, status post; DDRT, deceased donor renal transplant; HTN, hypertension; DM, diabetes mellitus; HLD, hyperlipidemia; MGUS, monoclonal gammopathy of unknown significance; DOE, dyspnea on exertion; Dx, diagnosis; ICU, intensive care unit; TRI, tubuloreticular inclusion.
Spot urine protein creatinine ratio (UPCR).
Oliguria.
Donor kidney.
Current models of APOL1-induced nephrotoxicity suggest that intrinsic renal expression is important for nephrotoxicity and specifically, for podocyte injury.27,30,31 Patient 2 showed discordance between recipient and transplant kidney APOL1 genotype. The patient (recipient) carried the high-risk APOL1 genotype, whereas the donor graft carried low-risk genotype G1/G0. We also sequenced all exons of APOL1 from DNA derived from the renal allograft. Consistent with prior studies of HIVAN and FSGS, we did not identify additional rare coding variants that could explain increased risk of CGN in the setting of heterozygosity for APOL1 risk alleles.32,33 This finding raises the important possibility that systemic APOL1 expression in extrarenal organs or cells might also be important in pathogenesis of kidney disease. APOL1 is highly expressed by liver, lung, and placenta, and it is found in macrophages, where it has been shown to play a role in limiting HIV infection.34 Of note, inflammatory cells including lymphocyte and macrophage infiltration have been reported in kidneys of patients infected with SARS-CoV-2 (Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al.: Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection. medRxiv 2020.03.04.20031120, 2020). Alternatively, this finding may support the notion that presence of a single APOL1 risk allele might confer increased risk of glomerular disease in COVID-19, as has been suggested in HIVAN.35,36
Given that COVID-19 infections will continue, there is an urgency to identify specific therapies, like targeted antiviral or anti-inflammatory therapies, to protect the kidney in patients at risk. Remdesivir improves respiratory outcomes in patients with COVID-19, but patients with eGFR<30 ml/min are currently excluded from receiving it due to limited safety data.37 Steroids have been used in collapsing GN and other podocytopathies without strong evidence-based guidelines38; however, the risk of nosocomial infection and potential harm makes it difficult to endorse their use. The benefit of other COVID-19–specific therapies, such as IL-6 receptor antagonists, is not clear, and the six patients reported here did not develop evidence of cytokine storm, when IL-6 antagonists are clinically recommended.
The development of severe proteinuric kidney disease without severe respiratory or systemic symptoms should be recognized in COVID-19. In a period of 3 weeks at a single center, we observed six patients with severe acute kidney disease and podocytopathy. It is likely that only the patients with the most severe cases were referred to the nephrology service. Further studies to determine the incidence of proteinuria and/or decline in kidney function in larger cohorts of patients with COVID-19 should be performed.
Disclosures
A. Gharavi receives consulting fees from the AstraZeneca Center for Genomics Research and Goldfinch Bio. S. Quaggin has applied for patents related to therapeutic targeting of the Angiopoietin-Tie2/Tek (ANGPT-TEK) pathway in ocular hypertension and glaucoma and receives research support, owns stock in, and is a director of Mannin Research. S. Quaggin also receives consulting fees from AstraZeneca, Janssen, the Lowy Medical Research Foundation, and Roche/Genentech; is Chair of the External Scientific Advisory Board for AstraZeneca; and is a scientific advisor or member of AstraZeneca, Genentech/Roche, JCI, the Karolinska CVRM Institute, the Lowy Medical Research Institute, Mannin, and Novartis. A. Shetty consults for Veloxis Pharmaceuticals, receives investigator support from Angion Biomedica Corp., receives honoraria from Veloxis Pharmaceuticals, and receives speaker fees from Veloxis Pharmaceuticals. All remaining authors have nothing to disclose.
Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants NIH P30DK114857 (to S. Quaggin) and NIH DK60635 (to Y. Kanwar), and Feinberg School of Medicine Northwestern University Clinical and Translational Sciences Institute (NUCATS) COVID fund (to S. Quaggin).
Supplementary Material
Acknowledgments
We acknowledge Jung Soo Kim, Dr. Rafael Gras-Pena, and Dr. Hila Rasouly for technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “COVID-19 and APOL1: Understanding Disease Mechanisms through Clinical Observation,” on pages 1–2.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060804/-/DCSupplemental.
Supplemental Figure 1. Light and electron micrograph findings for patient 3.
Supplemental Figure 2. Light and electron micrograph findings for patient 4.
Supplemental Figure 3. Light and electron micrograph findings for patient 5.
Supplemental Figure 4. Light micrograph findings for patient 6.
Supplemental Figure 5. Chromatograms of APOL1 exons from renal allograft from patient 2.
References
- 1.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al.: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al.: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kissling S, Rotman S, Fakhouri F: The authors reply. Kidney Int 98: 232, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA: Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 5: P935–P939, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, et al.: Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 5: 940–945, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2 [published correction appears in J Am Soc Nephrol 31: 2494, 2020 10.1681/ASN.2020081117]. J Am Soc Nephrol 31: 1683–1687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. : Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 31: 1959–1968, 2020. 10.1681/ASN.2020060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, et al.: COVID-19-related glomerulopathy: A report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med 2: 488–492, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR: COVID-19-associated collapsing focal segmental glomerulosclerosis: A report of 2 cases. Kidney Med 2: 493–497, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, et al.: AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 31: 1688–1695, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyatt CM, Rosenstiel PE, Klotman PE: HIV-associated nephropathy. Contrib Nephrol 159: 151–161, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Pollak MR, Genovese G, Friedman DJ: APOL1 and kidney disease. Curr Opin Nephrol Hypertens 21: 179–182, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Limou S, Nelson GW, Kopp JB, Winkler CA: APOL1 kidney risk alleles: Population genetics and disease associations. Adv Chronic Kidney Dis 21: 426–433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al.: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al.: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Boucher E, Mayer G, Londono I, Bendayan M: Expression and localization of MT1-MMP and furin in the glomerular wall of short- and long-term diabetic rats. Kidney Int 69: 1570–1577, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Westhoff TH, Seibert FS, Bauer F, Stervbo U, Anft M, Doevelaar AAN, et al.: Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient [published online ahead of print July 26, 2020]. Am J Transplant 10.1111/ajt.16223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdi J, Schaub C, Thomson R, Raper J: All you ever wanted to know about APOL1 and TLFs and did not dare ask. Methods Mol Biol 2116: 463–483, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, et al.: A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 78: 698–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese G, Friedman DJ, Pollak MR: APOL1 variants and kidney disease in people of recent African ancestry. Nat Rev Nephrol 9: 240–244, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Shah SS, Lannon H, Dias L, Zhang JY, Alper SL, Pollak MR, et al.: APOL1 kidney risk variants induce cell death via mitochondrial translocation and opening of the mitochondrial permeability transition pore. J Am Soc Nephrol 30: 2355–2368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovinazzo JA, Thomson RP, Khalizova N, Zager PJ, Malani N, Rodriguez-Boulan E, et al.: Apolipoprotein L-1 renal risk variants form active channels at the plasma membrane driving cytotoxicity. Elife 9: e51185, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd, Heneghan JF, et al.: APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JY, Wang M, Tian L, Genovese G, Yan P, Wilson JG, et al.: UBD modifies APOL1-induced kidney disease risk. Proc Natl Acad Sci U S A 115: 3446–3451, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, et al.: Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 23: 429–438, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uzureau S, Lecordier L, Uzureau P, Hennig D, Graversen JH, Homblé F, et al. : APOL1 C-terminal variants may trigger kidney disease through interference with APOL3 control of actomyosin. Cell Rep 30: 3821–3836.e13, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, et al.: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman BI, Moxey-Mims MM, Alexander AA, Astor BC, Birdwell KA, Bowden DW, et al.: APOL1 long-term kidney transplantation outcomes network (APOLLO): Design and rationale. Kidney Int Rep 5: 278–288, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoriello D, Husain SA, De Serres SA, Bomback AS, Crew RJ, Vasilescu ER, et al.: Donor APOL1 high-risk genotypes are associated with increased risk and inferior prognosis of de novo collapsing glomerulopathy in renal allografts. Kidney Int 94: 1189–1198, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limou S, Nelson GW, Lecordier L, An P, O’hUigin CS, David VA, et al.: Sequencing rare and common APOL1 coding variants to determine kidney disease risk. Kidney Int 88: 754–763, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papeta N, Kiryluk K, Patel A, Sterken R, Kacak N, Snyder HJ, et al.: APOL1 variants increase risk for FSGS and HIVAN but not IgA nephropathy. J Am Soc Nephrol 22: 1991–1996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor HE, Khatua AK, Popik W: The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol 88: 592–603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. : Association of trypanolytic apoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, et al. : APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol 26: 2882–2890, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigel JH, Tomashek KM, Dodd LE: Remdesivir for the treatment of Covid-19—preliminary report. Reply. N Engl J Med 383: 994, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Laurin LP, Gasim AM, Derebail VK, McGregor JG, Kidd JM, Hogan SL, et al.: Renal survival in patients with collapsing compared with not otherwise specified FSGS. Clin J Am Soc Nephrol 11: 1752–1759, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.