Abstract
Injury of articular cartilage can cause osteoarthritis and seriously affect the physical and mental health of patients. Unfortunately, current surgical treatment techniques that are commonly used in the clinic cannot regenerate articular cartilage. Regenerative medicine involving stem cells has entered a new stage and is considered the most promising way to regenerate articular cartilage. In terms of theories on the mechanism, it was thought that stem cell-mediated articular cartilage regeneration was achieved through the directional differentiation of stem cells into chondrocytes. However, recent evidence has shown that the stem cell secretome plays an important role in biological processes such as the immune response, inflammation regulation, and drug delivery. At the same time, the stem cell secretome can effectively mediate the process of tissue regeneration. This new theory has attributed the therapeutic effect of stem cells to their paracrine effects. The application of stem cells is not limited to exogenous stem cell transplantation. Endogenous stem cell homing and in situ regeneration strategies have received extensive attention. The application of stem cell derivatives, such as conditioned media, extracellular vesicles, and extracellular matrix, is an extension of stem cell paracrine theory. On the other hand, stem cell pretreatment strategies have also shown promising therapeutic effects. This article will systematically review the latest developments in these areas, summarize challenges in articular cartilage regeneration strategies involving stem cells, and describe prospects for future development.
1. Introduction
Articular cartilage is an important weight-bearing tissue of synovial joints. Due to the lack of blood vessels, nerves, and lymphatic vessels and the restriction of the dense extracellular matrix (ECM) on cartilage cells, the self-healing ability of articular cartilage after injury is very limited. If left untreated, damage to articular cartilage can lead to osteoarthritis (OA) [1]. OA has a high incidence and disability rate, affecting 250 million patients worldwide [2]. Unfortunately, none of the cartilage repair techniques currently in clinical use can completely regenerate hyaline cartilage [3].
Stem cells are an important milestone in the field of tissue engineering and regenerative medicine. Stem cell therapy is considered to be a promising method to solve the regeneration of articular cartilage [4, 5]. A large number of preclinical and clinical studies have shown that compared with traditional repair techniques such as microfractures, stem cell therapy can form more typical hyaline cartilage and can better control symptoms [6–8]. On the other hand, compared with autologous chondrocytes, stem cells have a wider source and stronger ability to expand in vitro, which makes tissue-engineered cartilage involving stem cells more advantageous than tissue-engineered cartilage involving autologous chondrocytes. Tissue engineering strategies involving stem cells involve the implantation of exogenous stem cells and homing of endogenous stem cells to achieve cartilage regeneration in situ. The basis of the exogenous stem cell implantation strategy is finding suitable types of stem cells. Mesenchymal stem cells (MSCs) derived from various tissues are currently the most studied tissue engineering articular cartilage seed cell type [9]. Embryonic stem cells (ESCs) have the potential to differentiate into any cell type, but due to ethical disputes, ESCs are in only the preclinical experimental stage. Induced pluripotent stem cells (iPSCs) can theoretically be obtained by reprogramming any type of terminally differentiated cell, removing limitations of the cell source and reducing ethical disputes, thus becoming a new type of seed cell that is gradually emerging. However, stem cell transplantation also poses the risk of tumorigenesis, immune rejection, disease transmission, and the functional heterogeneity of cells from different individuals [10–13].
In this review, we first introduced the two main theories of stem cell-mediated articular cartilage regeneration and then reviewed the application of exogenous stem cell implantation strategies and endogenous stem cell homing and in situ cartilage regeneration strategies. Second, we reviewed the research progress of stem cell pretreatment strategies, derivatives, and delivery scaffolds. Finally, we summarized problems in stem cell research related to articular cartilage regeneration and looked toward the future directions of this field.
2. Theories on Cartilage Regeneration Involving Stem Cells
As immature tissue precursor cells, stem cells can self-renew and have the ability to form clonal cell populations and differentiate into multiple cell lineages [14]. These special properties are particularly attractive for restoring the functions of a variety of organs. At present, stem cells can be divided into three general categories: (1) ESCs derived from early embryos, (2) iPSCs, and (3) adult stem cells, including hematopoietic stem cells, neural stem cells, and MSCs. A large number of studies have confirmed the beneficial role of stem cells in the regeneration of articular cartilage, and their potential mechanisms are mainly divided into two theories (Figure 1): the first is the “differentiation theory,” which states that stem cells directly differentiate into chondrocytes and repair damaged cartilage by adding or replacing chondrocytes [15]. The other is the “paracrine theory,” in which stem cells secrete bioactive factors, extracellular vesicles (EVs), and ECM [16], changing the biological behavior of receptor cells (including endogenous stem cells, chondrocytes, and macrophages), such as proliferation, differentiation, migration, polarization, metabolism, and apoptosis, and regulating the local microenvironment to repair and regenerate articular cartilage. Early studies focused on the direct differentiation and replacement of stem cells. In recent years, there has been an increasing amount of evidence that the therapeutic benefits of stem cells may be attributed to their paracrine effects.
Figure 1.
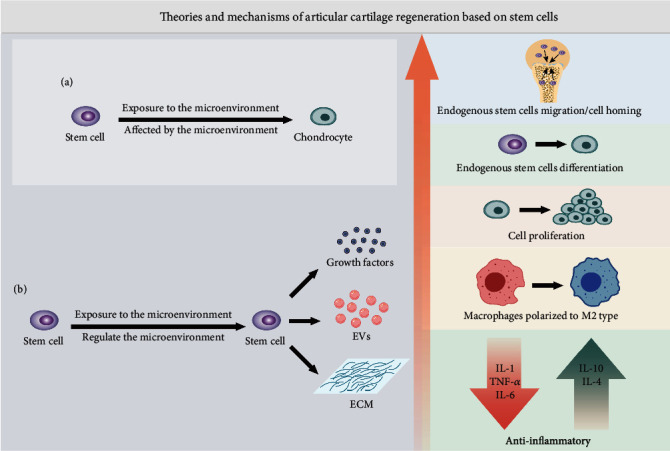
Two theories of articular cartilage regeneration involving stem cells. (a) Stem cell differentiation theory. Stem cells are affected by the microenvironment and directly differentiate into chondrocytes. (b) Paracrine theory of stem cells. Stem cells are affected by the microenvironment and secrete various derivatives, including growth factors, EVs, and ECM. These derivatives have been proven to induce homing of endogenous stem cells, promote the differentiation of endogenous stem cells into chondrocytes, promote the proliferation of chondrocytes, induce macrophages to polarize to the M2 type, and regulate the level of inflammatory factors to exert anti-inflammatory effects. EVs: extracellular vesicles; ECM: extracellular matrix.
2.1. Differentiation Theory
From the perspective of chondrogenesis, cartilage formation begins with mesenchymal condensation, which causes MSCs to differentiate into cartilage. Then, a dense matrix forms, which serves as a template for the subsequent formation of subchondral bone and cartilage [17]. In addition, a large number of studies have indicated that MSCs maintain pluripotency after repeated proliferation cycles in vitro and can differentiate into matrix-producing chondrocytes [18, 19]. Based on these findings, most previous studies attributed the role of stem cells in regenerating articular cartilage to their ability to differentiate into multiple lineages [20, 21]. A large number of studies focused on the development of materials and methods to induce stem cells to differentiate into cells with a chondrocyte phenotype [22]. Abir and colleagues demonstrated that autologous MSCs that were intra-articularly injected differentiated into mature chondrocyte-like cells [23]. This conclusion strongly supports this theory. Researchers suspended donkey autologous bone marrow-derived MSCs (BMSCs) labeled with green fluorescent protein (GFP) in hyaluronic acid (HA) for intra-articular injections in an attempt to treat wrist OA induced by amphotericin B. The results of up to 6 months of follow-up showed that the intra-articular injection of autologous BMSCs combined with HA resulted in an improved therapeutic effect compared with that of HA injections alone. GFP-labeled MSCs were detected in all the examined articular cartilage. Some cells showed a chondrocyte-like phenotype (round and surrounded by cavities), which proved that the injected MSCs differentiated into chondrocytes. To further verify this conclusion, similar work in a dog knee cartilage defect model also proved that injected MSCs differentiated into mature chondrocytes [24]. The same results were obtained in a study by Kotaka et al., who found that human iPSCs can repair knee cartilage defects in nude mice. The immunofluorescence of antihuman mitochondrial antibodies was found in newborn chondrocytes, which suggested that implanted iPSCs differentiated into chondrocytes [25]. In a recent study in a rat KOA model, researchers injected fluorescein-labeled human adipose-derived MSCs (ADSCs) into the articular cavity and found that the injected cells had a good therapeutic effect on OA. The existence of human cells in the rat meniscus and cartilage was confirmed by immunohistochemistry with antihuman mitochondria and antihuman Ki67 antibodies, and some of the cells were in the proliferative phase [26]. Although this study did not explore whether injected cells differentiated into mature chondrocytes, the fluorescence signal in OA rats lasted for approximately 10 weeks, which at least indicated that the implanted stem cells could be retained in the articular cavity for a long time. The above studies provide strong evidence for the “differentiation theory” of stem cells.
2.2. Paracrine Theory
Researchers have long known that the conditioned medium (CM) of stem cells can promote cell proliferation and differentiation in vitro and can promote tissue repair and regeneration in vivo [27]. It has been shown that stem cells secrete many cytokines and proteins. The synergistic effect of small molecules secreted by MSCs can reduce cell damage and improve the repair ability of tissue [28]. Second, the immunomodulatory effect of stem cells has been increasingly reported. Stem cells can regulate the immune microenvironment during the process of tissue repair and provide a good environment for tissue regeneration [29]. MSCs in the immune microenvironment can promote chondrogenesis through immune regulation [30]. At the same time, a large number of studies on the coculture of MSCs and chondrocytes in vitro have proven that paracrine signaling is an important feature of MSCs [31–33]. The nutritional function of MSCs has led researchers to increasingly regard them as therapeutic delivery agents, and it has been recommended to rename them “medicinal signaling cells” [34]. Their paracrine signaling drives the endogenous response [35]. On the other hand, some in vitro studies [36–38] found that the differentiation of MSCs is not as strong as originally thought, and it is difficult to achieve stable and effective differentiation. Especially in the case of differentiation into chondrocytes, the progression of stem cells to terminal hypertrophy is a frustrating problem [39]. Early in vivo follow-up studies showed that few cells can survive for more than a few weeks after implantation [40, 41]. A recent clinical study described the ultimate results of stem cell implantation. Tommy et al. implanted allogeneic MSCs into full-thickness femoral cartilage defects. After a 12-month repair period, histological samples were examined, and no allogeneic MSC DNA was detected in the repaired tissue. This indicated that implanted MSCs provided the initial stimulation but then died and were cleared from the tissue [42]. The above studies suggest that the function of stem cells in tissue repair and regeneration is mediated by active components secreted by stem cells rather than by their direct differentiation into target cells.
At present, the mechanism of stem cell-mediated cartilage regeneration is still unclear, and the above theory provides some insights. The complete regeneration process may be coordinated by multiple mechanisms, and stem cells may play different roles in different stages of the actual repair process. The precise control of the changing roles of stem cells may be an effective way to achieve the desired regeneration effect.
3. Cartilage Regeneration Strategies Involving Stem Cells
Stem cells used for tissue engineering and cell therapy are usually obtained from four basic sources: (1) embryonic tissue; (2) fetal tissue, such as fetus, amniotic fluid, and umbilical cord (Wharton jelly, blood); (3) a specific location in adult organisms (such as fat, bone marrow, and synovium); and (4) somatic cells after genetic reprogramming, i.e., iPSC [43, 44]. Among the sources of stem cells, adipose tissue seems to be the most promising choice. It have many unparalleled advantages. Specifically, adipose tissue is available in relatively high quantity in many patients and can be collected by “waste tissue” produced by surgical procedures (such as liposuction or abdominal plastic surgery), which can effectively solve problems with local morbidity, safety, and ethical issues. Moreover, compared with other tissues, adipose tissue produces a large number of living stem cells. Studies have shown that ADSCs in lipoaspiration account for 2% of nuclear cells, and the output per gram of adipose tissue is approximately 5000 fibroblast colony forming units (CFU-F). In contrast, the production of bone marrow MSCs (BMSCs) is only 100–1000 CFU-F/ml bone marrow [45]. Due to the tissue diversity and individual differences of MSC sources, the MSC population has obvious heterogeneity. Adult MSCs have obvious differences in their cartilage differentiation ability due to their different inherent tissue sources. Studies have compared adult MSCs derived from different tissues, and the results show that MSCs derived from joint synovium (SMSCs) have the strongest cartilage differentiation ability, which may be determined by their inherent cell characteristics and growth characteristics [46]. Researchers found high expression of proline arginine-rich end leucine-rich repeat protein (PRELP) in SMSCs, which is a glycoprotein rich in cartilage, but little or no content in stem cells outside the joints [47]. In addition, SMSCs remained multidirectional in 10 generations in vitro, and cell senescence was limited [48]. However, the acquisition of synovium is accompanied by invasive operation of the joint cavity, and the source of synovium is limited, which greatly limits the application of SMSCs. Compared with cells isolated from adult tissues, embryonic or neonatal-derived stem cells are characterized by faster proliferation and more passages in vitro before aging [49]. There is no study to compare the chondrogenic differentiation ability of neonatal/ESCs and adult stem cells, but studies have shown that single-cell-derived colonies of marrow stromal cells contained three morphologically distinct cell types: spindle-shaped cells, large flat cells, and very small round cells, and the small cells had a greater potential for multipotential differentiation [50].
With the development of high-throughput analysis technology, the heterogeneity of stem cells has become more obvious at the genetic molecular level [51]. Cell surface molecules that may be markers of stem cell pluripotency have been identified including but not limited to CD34 [52], CD146 [53], and CD49f [54]. Animal experiments show that CD146+ ADSCs can inhibit the inflammation of the joint cavity and promote the regeneration of articular cartilage [55]. Although no studies have confirmed the special role of CD34 and CD49f-specific stem cells in cartilage regeneration, the beneficial effects of CD34+ stem cells on cardiac repair and regeneration have been confirmed [56]. Studies have also found that the CM of CD34+ stem cells contains 32 soluble factors related to cell proliferation, survival, tissue repair, and wound healing, which can promote liver repair and regeneration in vivo [57]. MSCs with high expression of CD49f play an important role in the maintenance of hair follicle epithelial cells [58]. Directly implanting exogenous stem cells into joint cavities or articular cartilage defects seems to be the most direct stem cell application strategy. However, the strategy of stem cell homing and in situ regeneration was the first to be applied. Its history can even be traced back to 1959. Pridie [59] reported for the first time the subchondral bone drilling method used to treat cartilage injury. The bone marrow (containing BMSCs) was drained to the cartilage defect to form a blood clot, and then, cartilage tissue formed. However, there was no concept of “homing” stem cells at that time. This chapter will discuss these two strategies in detail.
3.1. Exogenous Stem Cell Implantation Strategy
We searched for studies applying exogenous stem cell implantation strategies to treat articular cartilage defects or OA on PubMed from the past 4 years (2017-2020) and summarized the representative studies in Table 1 (animal experiments) and Table 2 (clinical research). According to the search results, most studies showed good therapeutic effects. Most of the animal models used in animal experiments involved rats, rabbits, pigs, sheep, and horses. The pathological process of OA in these quadrupeds may be quite different from that in humans. One study used a model of OA in primates (rhesus monkeys) [60]. Encouragingly, the results of this study showed that both xenogenic ESC-derived MSCs (EMSCs) and allogeneic BMSC transplantation had therapeutic effects on knee joint OA in rhesus monkeys, and the results were better than those in the control group. There are relatively few clinical studies, and there are only 2 clinical studies with a large sample (more than 100 cases) [61, 62]. In terms of the follow-up time, the evaluation time for animal experiments ranged from 3 weeks to 64 weeks. The shortest follow-up time for a clinical study was 6 months, and the longest follow-up time was more than 36 months. Because articular cartilage is in an ischemic and hypoxic environment that relies on only synovial fluid to supply nutrients, the regeneration of articular cartilage often takes a long time [63]. Therefore, long-term follow-up has more reference value. In terms of the stem cell dose, the single dose used in most studies was 106-107 cells. Although a higher number of cells would theoretically increase the number of successful stem cell transplants, there may be a plateau, beyond which the results will not continue to improve. For example, a study by Wu et al. confirmed that the intravenous injection of 1 × 106 MSCs improved the neurological function of rats with brain injury, but increasing the dose to 3 × 106 cells did not lead to a greater improvement in function [64]. In addition, some studies have shown that the repeated delivery of stem cells can have a better therapeutic effect [65], and no serious adverse events, such as tumorigenesis, were found during the 2-year follow-up. However, increased treatment costs, tedious cell culture and expansion procedures, and potential infection risks are problems that cannot be ignored. The delivery mode of stem cells determines the success rate of stem cell transplantation to some extent. We summarize the commonly used delivery methods in Figure 2. Because of the lack of blood vessels in articular cartilage, it is difficult to deliver drugs through the intravenous or arterial system. Most studies directly inject stem cells into the articular cavity, usually using normal saline, phosphate-buffered saline (PBS), or HA as cell carriers. After the direct injection of stem cells into the articular cavity, it is impossible to accurately target the area of cartilage injury. Although studies by Xia et al. [66] and others have shown that superparamagnetic iron oxide-labeled BMSCs gather at the location of cartilage defects after injection into the articular cavity, the practicability of the technique needs to be further verified. Magnetic targeted delivery and cell-scaffold constructs may solve this problem, but magnetic targeted delivery is still in the preclinical research stage [25], and the long-term effect of magnetic iron particles on cell and tissue regeneration is unclear. The cell-scaffold construct strategy has been used in the clinic. According to the search results, three commercial scaffold products have been used [62, 67, 68]. This may be due to the incomplete supervision and management policies of various countries on cell products, especially stem cell products, which restricts the translation of related products into clinical practice. Although there are still few commercial products of stem cell-scaffold constructs at present, commercial products of autologous chondrocyte-scaffold constructs have been widely used, and their therapeutic effects are ideal [69]. We have reason to believe that stem cells with stronger proliferation and differentiation ability have better application prospects.
Table 1.
| Cell types | References | Animal model | Carrier/scaffold material/delivery method | Groups | Cell source | Single dose | Transplant number | Time point (W) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| BMSCs | Lang Li [70] | Canines (n = 24) Full-thickness cartilage defects |
HA Intra-articular injection |
(1) HA (2) BMSCs+HA (3) Control: saline |
Allogeneic | 1 × 107 | 1 | 28 | BMSCs+HA can regenerate articular cartilage better than HA alone. |
| Wu et al. [71] | Rabbits (n = 24) Osteochondral defect |
PBS Intra-articular injection |
(1) BMSCs (2) PRFr (3) BMSCs+PRFr (4) Control: untreated |
Autologous | 3 × 106 | 2 | 12 | The BMSCs+PRFr group had better results in histological evaluation and GAG production. | |
| Barrachina et al. [72] | Equine (n = 18) Chemically induced OA |
LRS Intra-articular injection |
(1) MSCs-primed+LRS (2) MSCs-naïve+LRS (3) Control: LRS |
Allogeneic | 1 × 107 | 2 | 8 and 24 | MSCs-primed+LRS improved clinical symptoms and reduced synovial inflammation, but there was no significant difference from the control group. | |
| Vayas et al. [73] | Rabbits (n = 36) Full-thickness cartilage defects |
PLGA microspheres dispersed in a pluronic F-127 solution Intra-articular injection |
(1) MF (2) MSCs (3) BMP (3) (4) MF-BMP (3) (5) MSCs-BMP (3) (6) BMP (12) (7) MF-BMP (12) (8) MSCs-BMP (12) (9) Control: untreated |
Allogeneic | 2.5 × 105 | 1 | 12 and 24 | Compared with MF, BMP-2 and MSCs repaired articular cartilage defects better and were less invasive. | |
| Xia et al. [66] | Pigs (n = 6) Bilateral medial meniscectomy-induced OA |
SPIO nanoparticles Intra-articular injection |
(1) SPIO-BMSCs (2) Control: untreated |
Allogenic | 1 × 107 | 4 | 11 | The treatment effect of the MSC group was not significantly different from that of the control group. | |
| Jiang et al. [74] | Rats (n = 60) Cartilage defect |
PCL-PTHF Cell-scaffold construct implantation |
(1) PCL-PTHF with rat tail-derived collagen nanofibers+BMSCs (2) PCL-PTHF with chondroitin sulfate nanofibers+BMSCs (3) Control: untreated |
Allogenic | 3.14 × 105 | 1 | 4 and 8 | The PCL-PTHF with rat tail-derived collagen nanofiber group showed better chondrogenesis potential in vitro and in vivo. | |
| ADSCs | Kohli et al. [75] | Athymic nude rats (n = 15) Osteochondral defect |
Alpha Chondro Shield Cell-scaffold construct implantation |
(1) PA MSCs+Alpha Chondro Shield (2) CD271+ MSCs+Alpha Chondro Shield (3) Control: Alpha Chondro Shield |
Xenogeneic (human) | 5 × 104 | 1 | 3 | CD271+ADSCs had a stronger ability to promote cartilage regeneration in vivo. |
| Li et al. [55] | Rabbits (n = 60) Cartilage defect |
AECM scaffold Cell-scaffold construct implantation |
(1) ADSCs+scaffold (2) CD146+ ADSCs+scaffold (3) Scaffold (4) Positive control: sham-operated (5) Negative control: untreated |
Xenogeneic (human) | 5 × 105 | 1 | 12 and 24 | CD146+ ADSCs promoted better cartilage regeneration than that in the control group. | |
| Mei et al. [76] | Rats (n = 60) ACLT-induced OA |
PBS Intra-articular injection |
(1) ADSCs+PBS (2) Control: PBS |
Allogenic | 1 × 106 | 1 | 8 and 12 | ADSCs had anti-inflammatory effects and inhibited articular cartilage degeneration. | |
| Critchley et al. [77] | Caprine (n = 14) Osteochondral defect |
3D-printed PCL alginate hydrogel biphasic scaffold Cell-scaffold construct implantation |
(1) The biphasic constructs (2) Maioregen scaffolds (Finceramica) |
Allogenic | 3 × 106 ADSCss+1 × 106 chondrocytes | 1 | 24 | The biphasic constructs regenerated hyaline cartilage in vivo. | |
| SMSCs/SFMSCs | Yan et al. [78] | Mice (n = 20) Bovine type II collagen-induced OA |
PBS Intra-articular injection |
(1) SMSCs+PBS (2) Control: PBS |
Xenogeneic (human) | 1 × 106 | 3 | 10 | SMSCs prevented arthritis development and suppressed immune responses. |
| Kondo et al. [79] | Pigs (n = 13) Full-thickness osteochondral defects |
No carrier or scaffold MSC aggregate implantation |
(1) MSC aggregates (2) Control: untreated |
Autologous | 4 × 106 | 1 | 4 and 12 | Autologous synovial MSC aggregates promoted articular cartilage regeneration in vivo. | |
| Neybecker et al. [80] | Nude rats (n = 48) ACLT-induced OA |
Saline Intra-articular injection |
(1) SFMSCs+ saline (2) Saline (3) Control: sham + saline |
Xenogeneic (human) | 1 × 106 | 2 | 4 and 8 | Xenogenic SFMSCs exerted neither chondroprotection nor inflammation in ACLT-induced OA. | |
| Li et al. [81] | Rats (n = 30) Full-thickness cartilage defects |
Hyperbranched poly-PEGDA/HA hydrogel Injected into the cartilage defect site |
(1) AFF-MSCs/hydrogel (2) Hydrogel (3) Control: PBS |
Xenogeneic (human) | 1 × 106 | 1 | 4 and 8 | The composite material significantly repaired articular cartilage defects. | |
| UCBMSCs/WJMSCs | Zhang et al. [6] | Goats (n = 6) Full-thickness cartilage defects |
AECM scaffold Cell-scaffold construct implantation |
(1) MSCs+ scaffold (2) MF |
Xenogeneic (human) | 1 × 106 | 1 | 24 and 36 | The cell-scaffold constructs maintained the integrity of subchondral bone and regenerated hyaline cartilage. |
| Liu et al. [82] | Rabbits (n = NS) Full-thickness cartilage defects |
ECM scaffold Cell-scaffold construct implantation |
(1) hWJMSCs-scaffold (2) hWJMSCs-C-scaffold (3) Scaffold (4) Control: untreated |
Xenogeneic (human) | NS | 1 | 12, 24, 28, and 64 | WJMSC composite ECM scaffold regenerated hyaline cartilage in vivo. Undifferentiated WJMSCs had a better repair effect. | |
| Xing et al. [83] | Rats (n = 18) ACLT and medial meniscectomy-induced OA |
HA Intra-articular injection |
(1) HA+MSCs (2) HA (3) Control: saline |
Xenogeneic (human) | 1 × 106 | 1 | 6 and 12 | At 6 weeks, the therapeutic effect of the HA+MSCs group was significantly better than that of other groups, but there was no significant difference between the groups at 12 weeks. | |
| iPSCs | Rim et al. [84] | Rats (n = NS) Full-thickness cartilage defects |
hiChondroPellet group: no carrier or scaffold Transplant directly to the defect site hiChondrocytes group: PBS Injected into the cartilage defect site |
(1) hiChondroPellet (2) hiChondrocytes+PBS (3) Defect control: untreated (4) Normal control |
Xenogeneic (human) | hiChondroPellets or 1 × 106 hiChondrocytes | 1 | 8 | Both the chondropellets and the chondrocytes derived from iPSCs had therapeutic effects on osteochondral defects. |
| Kotaka et al. [25] | Nude rats (n = 54) Full-thickness cartilage defects |
Atelocollagen Transplant directly to the defect site by an external magnetic field |
(1) Magnetic force+iPS+ atelocollagen (2) iPS+ atelocollagen (3) Control: atelocollagen |
Xenogeneic (human) | 1 × 105 | 1 | 4, 6, and 8 | The histological score of the treatment group was significantly better than that of the control group. | |
| ESCs/EMSCs | Jiang et al. [60] | Rhesus macaques (n = 8) Spontaneous OA |
Saline Intra-articular injection |
(1) EMSCs (2) BMSCs (3) Control: saline |
EMSC group: xenogeneic (human) BMSC group: allogeneic |
5 × 106 | 3 | 4, 8, 12, 16, 24, and 36 | The degrees of joint swelling and imaging examination results in the EMSC group and BMSC group were significantly improved. |
| Gibson et al. [85] | Nude rats (n = 15) Cartilage defects |
No carrier or scaffold Transplant MSC pellets directly to the defect site |
(1) MSC pellets (untreated) (2) MSC pellets (pretreated with BMP-2 and Wnt5a) (3) Control: empty defects |
Xenogeneic (human) | 2.5 × 105 | 1 | 4 and 8 | Cartilage progenitor cell particles derived from hESCs pretreated with BMP-2 and Wnt5a induced hyaline cartilage regeneration in vivo. |
Table 2.
| Cell types | References | Cell source | Single dose | Transplant number | K-L grade | Age | Sample size | Carrier/scaffold material | Follow-up (M) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| BMSCs/BMACs | Chahal et al. [86] | Autologous | 1 × 106 1 × 107 5 × 107 |
1 | III-IV | 40-65 | 12 | Excipient | 12 | The clinical symptoms significantly improved in the 5 × 107 cell group. |
| Shapiro et al. [87] | Autologous | 5 mL BMAC (1.7 × 105 cells) | 1 | I-III | 42-68 | 25 | Platelet-poor bone marrow plasma | 12 | BMACs relieved pain caused by OA. However, at 12 months, BMACs had no significant advantage compared with saline. | |
| Emadedin et al. [88] | Autologous | 4 × 107 | 1 | II-IV | 18-65 | 43 | Saline +2% human serum albumin | 6 | BMSCs significantly relieved the pain of patients with OA. | |
| Shadmanfar et al. [89] | Autologous | 4 × 107 | 1 | II-IV | 18-65 | 30 | Saline | 12 | BMSCs alleviated clinical symptoms, but their efficacy at 12 months was not significantly different from that of the placebo. | |
| ADSCs | Kim and Koh [61] | Autologous | 4.26 × 106 | 1 | III-IV | 53-65 | 100 | NS | At least 36 | HTO combined with ADSCs improved IKDC and Lysholm scores in patients with OA. |
| Song et al. [65] | Autologous | 1 × 107 2 × 107 5 × 107 |
3 | ≥II | 40-70 | 14 | NS | 24 | Autologous ADSCs were safe and significantly improved the symptoms of OA. The effect of repeated injections of high-dose cells was more obvious. | |
| Kim et al. [67] | Autologous | 4.7 × 106 | 1 | III-IV | 42-68 | 70 | Allogenic cartilage (MegaCartilage) or fibrin glue (Greenplast kit®) | 27.6 | HTO+autologous MSCs+allogeneic cartilage implantation more effectively treated OA. | |
| UCBMSCs/WJMSCs | Sadlik et al. [68] | Allogenic | NS | 1 | NS | NS | 5 | Porcine type I/II collagen matrix scaffold (ChondrO-Gide) | 12 | WJMSCs can be used to induce articular cartilage regeneration. |
| Matas et al. [90] | Allogenic | 2 × 107 | 2 | I-III | 40-65 | 26 | Saline with 5% AB plasma | 13 | Repeated injection of WJMSCs was safe and significantly improved the clinical symptoms of OA. | |
| Song et al. [62] | Allogenic | 2.5 × 106 cells/cm2 | 1 | I-III | >40 | 128 | 4% HA (CARTISTEM®) | 24 | Allogeneic UCBMSCs significantly reduced the pain of OA joints and improved joint function. | |
| ESCs or PLMSCs | Khalifeh Soltani et al. [91] | Allogenic | 5 − 6 × 107 | 1 | II-IV | 35-75 | 20 | NS | 6 | Allogeneic PLMSCs relieved the symptoms of OA joints. |
BMAC: bone marrow aspiration and concentration; SMSCs/SFMSCs: synovial-derived mesenchymal stem cells/synovial fluid-derived mesenchymal stem cells; UCBMSCs/WJMSCs: umbilical cord blood-derived mesenchymal stem cells/umbilical cord Wharton's jelly derived mesenchymal stem cells; iPSCs: induced pluripotent stem cells; ESCs/EMSCs: embryonic stem cells/ESC-derived mesenchymal stem cells; LRS: lactate's ringer solution; MF: microfracture; SPIO: superparamagnetic iron oxide; PAMSCs: plastic adherent MSCs; AECM: articular cartilage extracellular matrix; HTO: high tibial osteotomy; RA: rheumatoid arthritis; WOMAC: the Western Ontario and McMaster Universities Arthritis Index; VAS: visual analog scale; PLMSCs: placenta-derived MSCs; PCL-PTHF: electrospun nanofibers composed of cartilage matrix components (collagen or chondroitin sulfate) and poly(-caprolactone)-polytetrahydrofuran; NS: not specified; PRFr: platelet-rich fibrin releasate; K-L grade: Kellgrene-Lawrence grade.
Figure 2.
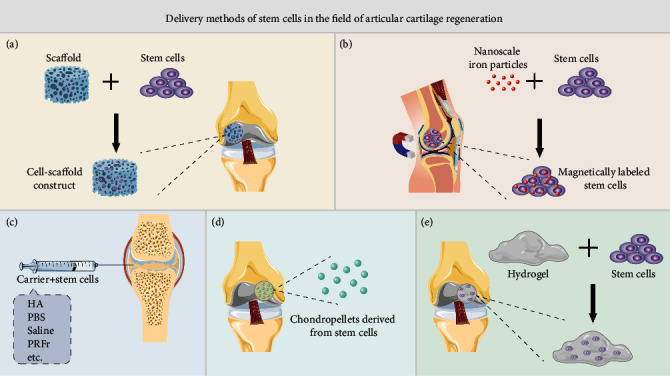
Stem cell delivery for repairing articular cartilage defects or treating OA. (a) Cell-scaffold construct. Stem cells are planted on a tissue engineering scaffold, cultured in vitro until the cells adhere to the scaffold, and then, the cell-scaffold construct is implanted into the cartilage defect. (b) Magnetic targeting. Place a magnet on the back of the cartilage defect (popliteal fossa), use nanoiron particles to label stem cells, and then implant the stem cells into the cartilage defect. Under the attraction of the magnet, the stem cells are tightly fixed to the bottom of the cartilage defect. (c) Intra-articular injection. The stem cells are resuspended in hyaluronic acid (HA), phosphate-buffered saline (PBS), physiological saline or platelet-rich fibrin releasate (PRFr), and other carriers and then injected into the joint cavity. (d) Chondrocyte pellets. The stem cells are cultured and differentiated in vitro to form cartilage pellets, and then, the cartilage pellets are implanted into the cartilage defect. (e) Cell-hydrogel construct. The stem cells are mixed into the injectable hydrogel material, and then, the cell-hydrogel construct is injected into the cartilage defect.
A large amount of clinical follow-up evidence has proven that MF, cartilage transplantation, ACI, etc., can regenerate fibrocartilage, but the long-term treatment effects are not good. An increasing number of scholars have attempted to combine stem cell transplantation with these traditional repair methods. Song et al. [62] combined human umbilical cord blood-derived MSC transplantation with MF, and Kim et al. [67] combined autologous ADSC transplantation with allogeneic cartilage transplantation (MegaCartilage, particulate allogenic cartilage, L&CBio, Seoul, KR), which significantly improved the clinical symptoms of OA patients. These results provide a reference for the combined use of stem cells and traditional cartilage repair techniques.
However, the limitations of exogenous stem cell implantation strategies cannot be ignored, such as the risk of tumorigenesis, the risk of disease transmission, the risk of immune rejection, and the restrictions on stem cell regulatory policies in different countries.
3.2. Stem Cell Homing and In Situ Regeneration Strategy
The term “homing” was first proposed by Gallatin et al. [92] in 1983. It was first used to describe the phenomenon in which lymphocytes in circulating blood tend to migrate to the sites that they were originally derived from, such as lymph nodes, which is referred to as “lymphocyte homing,” and then was gradually extended to stem cells. The term has recently been used to emphasize the ability of stem cells to respond to extracellular signals, such as migration stimuli and guidance cues, for targeted transport and migration [93]. Most tissues initiate the recruitment of stem cells to a certain extent when they are injured or inflamed, which promotes the homing of stem cells to the damaged area and exerts the potential for a variety of repair types, including ECM reconstruction and microenvironment regulation [94, 95]. Recruited stem cells can come directly from the stem cell pool of the tissue around the injury or be recruited from the circulatory system. As endogenous stem cells/progenitor cells do not need to be cultured and expanded in vitro and there is no risk of immunogenicity and disease transmission, researchers have focused on in situ cartilage regeneration by triggering endogenous stem cells/progenitor cells to undergo “homing” [96].
To enhance the homing behavior of stem cells, researchers tested the following strategies.
3.2.1. Artificially Increasing the Concentration of Chemokines in the Injured Site
For example, the stromal cell-derived factor (SDF-1)/CXCR4 signaling pathway has been shown to play a key role in endogenous stem cell homing [97, 98]. Zhang et al. successfully repaired part of a thickness cartilage defect in a rabbit knee joint with a type I collagen scaffold containing SDF-1 and confirmed that increasing the concentration of chemokines at the injured site promoted the homing of endogenous stem cells and mediated cartilage regeneration [99]. In another recent study, researchers embedded transforming growth factor β1 (TGF-β1) in photocrosslinked glycidyl methacrylate (GM-HPCH) to repair articular cartilage defects in rats. The results showed that compared with GM-HPCH alone, GM-HPCH+TGF-β1 could repair cartilage defects more effectively through its ability to recruit stem cells [100]. In similar studies, increases in interleukin 8 (IL-8) and macrophage inflammatory protein 3α (MIP-3α) were shown to promote stem cell homing to articular cartilage injury sites and mediate articular cartilage regeneration [101].
3.2.2. Increasing the Number of Stem Cells in the Damaged Local Microenvironment
For example, MF can stimulate and release BMSCs. Min and others demonstrated that the MF channel caused by the hollow cone is more unobstructed than that caused by a traditional blunt cone and can mobilize more BMSCs to the location of a cartilage defect [102]. Baboolal et al. stirred joint synovium with a special stem cell mobilization device (StemDevice) for 1 minute and collected joint cavity lavage fluid for cell culture. Compared with ordinary cytological brushes, this stem cell mobilization device greatly increased the number of synovial stem cells in the lavage fluid [103]. Encouragingly, both of these techniques have been applied in the clinic, and both are arthroscopic-assisted operations with the advantages of being minimally invasive.
3.2.3. Construct Scaffolds Conducive to Stem Cell Homing, Adhesion, Proliferation, and Differentiation
For example, Sun et al. combined self-assembled peptide nanofiber hydrogels (RAD/SKP) with acellular cartilage matrix (DCM) scaffolds. It was confirmed in animal experiments that the DCM-RAD/SKP functional scaffold system significantly promoted the recruitment of endogenous stem cells and regenerated hyaline cartilage [104].
It is worth noting that at present, many studies are not limited to the application of one of these strategies, but a variety of strategies can be combined to improve the repair effect. In a recent study, researchers first used 3D-bioprinting technology to construct a silk fibroin-gelatin composite scaffold (SFG), which had a porous structure suitable for cell adhesion and good mechanical strength. The scaffold was then combined with a BMSC-specific affinity peptide (E7), which was shown to have the ability to recruit BMSCs. In the rabbit knee articular cartilage defect model, the SFG-E7 composite scaffold was combined with MF. After 24 weeks, the cartilage defect was completely filled, and the new tissue had obvious characteristics of hyaline cartilage [105]. The research team modified the acellular porcine peritoneal matrix (APM) scaffold with the E7 polypeptide, which had good biocompatibility and a surface suitable for cell growth. The combined application of the APM-E7 scaffold and MF greatly enhanced the recruitment of endogenous stem cells and regenerated rabbit knee cartilage [106].
Endogenous stem cell recruitment and in situ regeneration strategies also face many limitations. The biologically active ingredients used to recruit stem cells often require high synthesis techniques and conditions. At the same time, in order to exert a sustained recruitment effect, the delivery materials need to have a slow-release function.
4. Stem Cell Pretreatment Strategy
The microenvironment of damaged articular cartilage is adverse, with inflammation, hypoxia, and insufficient blood supply. In addition, most stem cells used in clinical applications come from adults, and the functions of these cells are compromised. The above factors lead to a very low survival rate of transplanted cells [107], and the use of stem cells for cartilage regeneration has not yet achieved the desired effect. Studies have shown that pretreatment is an effective way to enhance the ability of stem cells to resist adverse microenvironments. Stem cell pretreatment can improve cell survival and differentiation potential, regulate the immune response, inhibit fibrosis, and enhance cell secretion of anti-inflammatory factors. These effects promote the regeneration and functional recovery of organs and tissues after cell implantation [108, 109]. Stem cell pretreatment strategies reported in the field of cartilage regeneration mainly include the following aspects:
4.1. Hypoxia
In natural cartilage, cells are exposed to very low oxygen pressure: approximately 7% (53 mmHg) in the superficial area and only 1% (5-8 mmHg) in the deep area [110]. Hypoxic pretreatment not only enhances the survival and migration ability of stem cells after implantation but also promotes the proliferation and differentiation of stem cells [111]. Under the same conditions for cartilage-induced differentiation, compared with MSCs without hypoxia pretreatment, MSCs with hypoxia pretreatment have been shown to enhance matrix deposition and reduce the expression of hypertrophy markers such as type X collagen [112]. Additionally, hypoxic pretreatment can also upregulate genes related to growth, cell signaling, metabolism, and cellular stress response pathways [113]. In a rabbit knee joint trauma and focal early OA model, hypoxia-pretreated MSC+HA hydrogel caused a significant improvement in the cartilage repair score [114]. The mechanism through which hypoxia affects cells is mainly regulated by HIF-1. The latest evidence shows that HIF-1α promotes cartilage matrix gene expression and upregulation and that HIF-3α can help stabilize the cartilage phenotype. In contrast, HIF-2α upregulates hypertrophy genes and matrix-degrading enzymes [112]. Some studies have explored the specific mechanism of hypoxia that regulates HIF. Studies have shown that hypoxia can induce an increase in phosphorylated AKT and p38 MAPK to stabilize HIF-1α [115], resulting in the upregulation of the glucose-6-phosphate transporter and an increase in the MSC survival rate [116].
4.2. Pharmacological or Chemical Agents
The use of pharmacological or chemical reagents to protect stem cells and improve the effect of stem cells on cartilage regeneration is another pretreatment strategy. For example, vitamin E pretreatment can make MSCs resistant to H2O2-induced oxidative stress, upregulate the expression of proliferation markers and transforming growth factor-β (TGF-β), and downregulate the expression of apoptosis-related genes. After the above pretreatment, MSCs increased the content of proteoglycan in the cartilage matrix in a surgically induced OA rat model, upregulated a chondrogenesis marker, and promoted the differentiation of MSCs into cartilage [117]. Kartogenin (KGN) has been proven to be a chondrogenesis and cartilage protective agent that is more effective in inducing cartilage regeneration than growth factors [118]. Jing and colleagues found that KGN pretreatment may improve the chondrogenesis and differentiation of human WJMSCs by promoting human WJMSCs to enter the prechondral phase, enhancing JNK phosphorylation and inhibiting dicatenin [119]. A recent study found that EVs derived from human WJMSCs pretreated with KGN contain a unique miRNA, miR-381-3p. Researchers found that miR-381-3p directly inhibited TAOK1 by targeting the 3′ untranslated region of TAOK1, thus inhibiting the Hippo signaling pathway and mediating cartilage formation [120].
4.3. Trophic Factors and Cytokines
The interaction between specific nutritional factors and their receptors can activate downstream signal transduction and promote cell survival and differentiation. Therefore, the pretreatment of stem cells with nutritional factors and cytokines is a promising strategy for improving the therapeutic effect of stem cells. Stem cells pretreated with FGF-2 have been shown to have an enhanced proliferation ability and to retain the potential to differentiate into cartilage after 30 population doublings, while stem cells that were not pretreated lost their ability to differentiate into cartilage after approximately 20 doublings [121]. The pretreatment of stem cells with specific growth factors can promote their chondrogenic differentiation potential and their ability to repair cartilage defects in vivo [111]. For example, pretreatment with an appropriate concentration of IL-1β can not only promote proliferation but also enhance the chondrogenic potential of synovial MSCs. However, high concentrations of IL-1β adversely affected synovial MSCs by reducing their adhesion and pluripotency [122]. BMSCs pretreated with soluble IL-6R effectively repaired articular cartilage defects in vivo [123].
4.4. Physical Factors
Articular cartilage is a load-bearing tissue, so mechanical stimulation is very important for the development and maintenance of articular cartilage. A 3D culture model can mimic the natural growth state of cells in vivo, provide enough space for stem cell proliferation, and produce more biochemical and biomechanical clues by providing intensive cell-to-cell interactions. Therefore, with these advantages, a variety of physical factors can be applied to the 3D microenvironment in vitro or in vivo to improve the performance of stem cells [124]. For example, Zhang et al. found that radial shock waves not only significantly improved the proliferation and self-renewal ability of MSCs in vitro but also safely promoted the repair cartilage defects by MSCs in vivo [125]. The articular cartilage matrix contains a large amount of collagen type II (COLII), and the expression of the SOX9 gene is positively correlated with COLII. Continuous low-intensity ultrasound pretreatment upregulated SOX9 gene expression and enhanced the nuclear localization of SOX9 protein in MSCs compared with control stimulation by discontinuous low-intensity ultrasound [126]. In addition, a new method involves combining cells with carriers/scaffolds before physical stimulation. To date, researchers have designed different types of cell carriers with appropriate physical and chemical properties for cell transplantation, such as injectable hydrogels, large scaffolds, microcarriers, and microspheres [127]. Cheng et al. loaded BMSCs onto an autologous platelet-rich fibrin (PRF) membrane scaffold and applied hydrostatic pressure to the cell-scaffold construct before transplantation. The results showed that the cell scaffold pretreated by hydrostatic pressure significantly increased the formation rate and matrix content of new cartilage and enhanced its mechanical properties [128].
4.5. Genetic Modification
A large number of studies have genetically engineered stem cells to reduce their tendency to differentiate into a hypertrophic phenotype or to induce the overexpression of transcription factors and growth factors to promote the formation of new cartilage in vivo [129, 130]. The overexpression of specific growth factors before implantation is a controllable and effective way to improve the efficacy of stem cell therapy. Genes for specific factors can be introduced into cells by nonviral or viral techniques. For example, compared with simple cellular or acellular scaffolds, BMSCs overexpressing TGF-β1 can be implanted into polylactic acid (PLA) scaffolds to achieve good cartilage tissue repair in a rabbit knee osteochondral defect model [131]. With regard to the specific progress of gene modification in cartilage repair, please refer to relevant reviews [129, 130].
There are still few in vivo animal experiments on stem cell pretreatment strategies, and there is currently a lack of standard protocols. The optimal length and dosage of stem cells need to be explored in depth. At the same time, it is necessary to clarify the molecular mechanism of physical, chemical, and genetic processing methods to promote cartilage regeneration.
5. Composition and Characteristics of Stem Cell Derivatives for Cartilage Regeneration
Stem cell derivatives are an extension of the paracrine theory of stem cells (Figure 1), in which the secretome is considered to be the mechanism through which stem cells exert their tissue repair and regeneration effects [132]. The secretome is a general term for bioactive factors and EVs secreted from the cell to the extracellular space. The secretome of cells is specific but changes in physiological or pathological conditions that directly affect it [133]. Bioactive factors include growth factors, cytokines, chemokines, and enzymes [134]. EVs are considered an important component of the therapeutic efficacy of MSCs. According to the size, composition, and origin of EVs, they can be divided into three types: apoptotic bodies, microvesicles, and exosomes [135, 136]. There are relatively few studies on apoptotic bodies, which are generated only during apoptosis, have a diameter of 50-5000 nm, and carry nuclear fragments and organelles. Microvesicles are small vesicles with a diameter of 50-1000 nm released by cells in the form of budding, which can be obtained by 10,000-20,000 g centrifugation. Exosomes are formed by the multivesicular endosomal pathway and are usually a complex of proteins, nucleic acids, and lipids with a diameter of 50-200 nm that can be obtained from very high-speed centrifugation at or above 100,000 g. Although stem cells have become powerful tools for clinical applications, they still have limitations in terms of delivery, safety, and the heterogeneity of therapeutic responses. The secretome composed of cytokines, chemokines, growth factors, proteins, and EVs may represent an effective alternative [16]. Notably, MSC-derived EVs (MSC-EVs) have been demonstrated to have a similar effect to MSCs and may have advantages over parent cells because of their specific miRNA load [135]. The focus of current research has shifted from stem cells to their secretome while attempting to overcome the limitations of cell-based therapies.
In addition, stem cell-derived ECM can be obtained by decellularizing stem cells cultured in vitro, and the ECM is a noncellular component that contains macromolecules secreted by various cells. The ECM may vary among cell type sources, but it is mainly composed of proteoglycans, such as growth factors, glycosaminoglycan (GAG), and matrix proteins, as well as collagen, fibronectin, elastin, vitronectin, and laminin [137]. After removing cellular components, such as DNA and cellular components that trigger an immune response, the ECM retains natural biochemical and biophysical signals [138]. Recent studies have shown that the ECM can promote cell proliferation and chondrogenic potential and is a potential biomaterial for tissue-engineered articular cartilage [139, 140].
The following sections will discuss in detail three aspects of the application of stem cell derivatives in cartilage regeneration and OA treatment: stem cell-derived CM, purified EVs (microvesicles and exosomes), and stem cell-derived ECM.
5.1. Stem Cell-Derived CM
Compared with stem cells, CM can be stored in a low-temperature environment, which is convenient for transportation, and does not have the risk of tumorigenesis. Compared with EVs and certain growth factors, CM components are more complex, including all components of the cell secretome, and do not need to be isolated and extracted, making it is convenient to use. Recently, Islam et al. studied the secretome of stromal cells obtained from the Hoffa fat pad (HFPSCs), synovial (SMSCs), umbilical cord (UCSCs), and cartilage (ACs) by quantitative liquid chromatography-mass spectrometry (LC-MS/MS) proteomics [141]. They identified more than 1000 proteins in each type of cell-derived CM. The secretome contained a large number of growth factors and cytokines. More importantly, compared with stromal cells from adult tissues, UCSCs had stronger anti-inflammatory and immunosuppressive properties. Recent studies reported that stem cell-derived CM plays a role in anti-inflammation and immune regulation and increases the synthesis of cartilage matrix in arthritis and osteochondral defect models. Ishikawa et al. intravenously injected CM derived from human dental pulp stem cells into the joint cavity of rheumatoid arthritis mice and found that CM relieved joint symptoms and synovial inflammation. The histological scores of bone erosion and cartilage damage in the CM group were significantly better than those in the control group, and the gene expression levels of proinflammatory cytokines were significantly reduced [142]. Alasdair found that the intra-articular injection of MSC-CM reduced cartilage damage and inhibited the immune response by reducing the cleavage of aggrecan, enhancing Treg function, and regulating the ratio of Treg : Th17 [143]. In addition, the application of BMSC-CM in a rat model of antigen-induced arthritis significantly reduced edema and thermal hyperalgesia as well as serum levels of TNF-α [144]. The anti-inflammatory effect of CM is related to its various immunomodulatory factors, including TGF-β, thrombospondin 1 (TSP1), and prostaglandin E2 (PGE2) [134]. Moreover, MSC-CM can also inhibit the progression of OA by balancing the ratio of MMP-13 to TIMP-1 in cartilage, inhibiting chondrocyte apoptosis and enhancing autophagy [145]. In a rabbit osteochondral defect repair experiment, the application of BMSC-CM led to only fibrocartilage regeneration [146]. Widhiyanto et al. composited ADSC-CM into porous scaffolds to repair rabbit trochlear cartilage defects, and new hyaline cartilage was observed at 12 weeks [147]. Interestingly, contradictory results were reported in a rabbit ear cartilage regeneration study. Researchers subcutaneously injected ADSCs, ADSC-CM, and PBS and found that there was no significant difference between the ADSC-CM and PBS groups at 4 or 8 weeks [148]. The above studies preliminarily proved that stem cell-derived CM repaired articular cartilage defects and relieved OA. The differences in experimental results in vivo may be related to the application method. When using scaffolds as carriers, CM can be retained in the defect area and gradually released. Stem cell-derived CM contains the whole secretome, and different stem cells and pretreatments can significantly affect the composition of CM. Researchers need to find more effective CM collection conditions to promote cartilage regeneration and to ensure that there are effective concentrations of effector substances at the target location to achieve better cartilage regeneration. Researchers also need to determine the exact biological mechanism of CM in vivo.
5.2. Stem Cell-Derived EVs
Unlike the direct use of stem cell-derived CM, EVs need to be separated and purified. The current methods used to obtain EVs include but are not limited to ultrafiltration and size-exclusion chromatography [149, 150], ultracentrifugation [151], and immunoaffinity [152]. Recently, an increasing number of reports have indicated that exosomes are the main therapeutic agents secreted by MSCs that enhance the regeneration and immunomodulatory ability of MSCs during tissue repair [135]. It has been reported that stem cell-derived EVs can promote cartilage regeneration and prevent cartilage degeneration induced by OA [153–156]. In a rat model of osteochondral defects, the weekly injection of human ESC-derived exosomes into the joint cavity induced cartilage and subchondral bone tissue regeneration within 2 weeks, and the orderly regeneration of the two tissues was observed at 12 weeks [153]. Compared with MSC injection, a single intra-articular injection of exosomes or microvesicles derived from mouse BMSCs had similar effects in preventing the development of collagenase-induced OA in mice [154]. Exosomes derived from human ESCs also showed cartilage protective effects in a mouse OA model [155]. Another study compared the therapeutic effects of iPSC-derived exosomes and synovial-derived exosomes in a collagenase-induced mouse OA model. The results showed that iPSC-derived exosomes could more effectively delay the progression of OA [157]. The biodistribution of EVs after intra-articular injection is not clear. Encapsulating EVs in a suitable biomaterial can prevent the rapid clearance of EVs and achieve a sustained release effect. Liu et al. encapsulated hiPSC-MSC-derived exosomes in a photocrosslinked hydrogel, which resulted in the retention of exosomes in vitro and achieved cartilage regeneration and repair in a rabbit femoral condylar cartilage defect model [63]. Chen et al. used desktop stereolithography to fabricate 3D-printed cartilage ECM/methacrylic acid gelatin (GelMA)/exosome scaffolds with radial channels. In vivo experiments showed that the 3D-printed scaffolds significantly promoted cartilage regeneration [158]. In vitro mechanistic studies showed that EVs derived from MSCs mediate cartilage repair by enhancing proliferation, reducing cell apoptosis, and regulating the immune response [159].
With the in-depth study of the therapeutic mechanism of EVs, the anti-inflammatory effects of EVs have been reviewed in detail [160, 161]. A growing number of scholars believe that the therapeutic efficacy of EVs can be attributed to their nucleic acid composition [162]. An increasing number of studies have described a complex picture of how miRNA regulates or influences OA [163]. Wu et al. reported that ADSC-derived exosomes from the human subpatellar fat pad protected articular cartilage from damage and improved gait abnormalities in OA mice by maintaining cartilage homeostasis, which may have been related to the inhibition of the mTOR autophagy pathway regulated by miR100-5p [156]. Another study proved that exosomes derived from SMSCs with high miR-140 expression promoted articular cartilage regeneration in rats [164]. In addition, early molecular mechanism studies showed that miR-92a regulates the PI3K/AKT/mTOR signaling pathway by targeting noggin3, thus upregulating chondrocyte proliferation and matrix synthesis [165, 166]. Exosome miR-23b induced human MSCs to differentiate into chondrocytes by inhibiting the protein kinase A (PKA) signaling pathway [167]. On the other hand, miR-125b and miR-320 reduced ECM damage by downregulating the expression of aggrecanase-1 (ADAMTS-4) and MMP-13, while these two ECM proteases were significantly upregulated in human OA chondrocytes [168]. Recently, Enrico et al. conducted high-throughput screening of the human adipose-derived MSC secretome and identified 60 kinds of miRNAs that can protect cartilage and induce macrophages to polarize to an M2 phenotype through bioinformatics analysis [169]. Increasing evidence indicates that stem cell-derived EVs may promote cartilage regeneration and treat OA by regulating a complex miRNA network [163]. Finally, the application of stem cell-derived EVs in treatment may have more advantages than using stem cells alone, mainly for the following reasons: (1) they cannot proliferate and are easy to preserve and transport [170]; (2) EVs are nontoxic, have no risk of tumorigenesis, low immunogenicity, and higher safety [171]; and (3) compared with the regulatory and ethical restrictions on stem cell products, the application of EVs is less restricted. However, in the field of cartilage repair, there are still many questions about the therapeutic effect, biodynamics, biodistribution, and delivery methods of stem cell-derived EVs that need to be answered in large animal experiments.
5.3. Stem Cell-Derived ECM
Stem cell-derived ECM is a natural biomaterial with strong biological activity and good biocompatibility. A large number of studies have shown that stem cell-derived ECM can enhance cell proliferation, prevent chondrocyte dedifferentiation, and maintain the stemness of stem cells [172, 173]. Stem cell-derived ECM provides a better platform for the expansion of chondrocytes/stem cells in vitro. Many studies have shown that compared with tissue culture polystyrene (TCPS), stem cell-derived ECM can significantly improve the proliferation of chondrogenic cells. At the same time, chondrogenic cells expanded on stem cell-derived ECM have stronger chondrogenic potential [174, 175]. Pei et al. showed that compared with cell culture plates, porcine synovial stem cell-derived ECM increased the proliferation of chondrocytes and delayed the dedifferentiation of porcine chondrocytes [174]. At the same time, stem cell-derived ECM can be used as a substrate for stem cell culture in vitro, which can restore the lineage differentiation ability of stem cells in aging mice [176]. Research by Yang et al. showed that compared with chondrocytes grown on TCP, chondrocytes inoculated on human BMSC-ECM showed a significantly increased proliferation rate and maintained a better cartilage phenotype. After expanding to the same number of cells and placing them in high-density micromass culture, chondrocytes from the BMSC-ECM group showed better cartilage differentiation characteristics than those from the TCP group [175]. Interestingly, the age of host that cells were derived from and different cell sources seem to be important factors affecting the ECM. Chee et al. found that fetal BMSC-ECM was superior to adult BMSC-ECM or human neonatal dermal fibroblasts in promoting the proliferation and pluripotency of adult BMSCs [177]. In addition to promoting cell proliferation and lineage-specific differentiation, recent studies have shown that SMSC-ECM enhanced the anti-inflammatory properties of rabbit articular chondrocytes through the SIRT1 pathway [178].
In addition to being used as a cell culture substrate, stem cell ECM can also be used alone or in combination with polymer materials to make 3D scaffolds to promote cartilage formation in vivo/in vitro. Tang et al. evaluated the cartilage repair ability of autologous BMSC-derived ECM scaffolds in two kinds of cartilage defect animal models. Six months after surgery, the histological characteristics and biochemical content of the bone marrow stimulation + ECM group were similar to those of normal hyaline cartilage [179]. Makiko et al. inoculated human amniotic MSCs on PLGA, successfully prepared an ECM-PLGA scaffold by removing cellular components, and implanted the scaffold into an osteochondral defect in the rat femoral trochlea. The results showed that ECM-PLGA induced gradual tissue regeneration and resulted in hyaline cartilage repair that was superior to that in the empty control group [180].
Current research shows that various stem cell derivatives play beneficial roles in cartilage regeneration and OA treatment. However, compared with the direct application of stem cells, the most substantial problem faced by stem cell derivatives is the cumbersome collection process, which undoubtedly increases the cost of treatment. In addition, how to increase the yield of exosomes and other derivatives and ensure the unity between batches is an urgent problem to be solved.
6. Stem Cell Delivery Biomaterials and Scaffolds
The key factor determining the effectiveness of stem cell therapy is the survival rate of stem cells during and after transplantation. Biomaterials used for cartilage repair not only provide mechanical support for cartilage defects but also provide support matrix for stem cells to induce cell growth, diffusion, and differentiation [181]. Biomaterial-based cell delivery systems can be extracted from naturally occurring materials, such as HA [182], gelatin [183], alginate [184], collagen, and decellularized matrix [185, 186] or based on synthetic materials, such as poly(ethylene glycol) (PEG) [187], poly(N-isopropylacrylamide) (PNIPAM) [188], poly(lactic-co-glycolic acid) (PLGA) [189], and polycaprolactone (PCL) [190]. The material is usually made into a porous structure to facilitate cell inoculation or hydrated polymeric networks, hydrogels for cell encapsulation [191]. Natural materials have better biological effects such as promoting cell adhesion, proliferation, and cartilage differentiation [192]. However, the mechanical properties and degradation rate of synthetic materials are more adjustable, and it is easier to customize according to cartilage or bone cartilage [193]. By combining biomaterials or natural ECM components with synthetic polymers, it is beneficial to highlight their respective advantages while limiting their disadvantages [194–197].
Early researchers used the material as a stem cell delivery platform to ensure the survival rate of stem cell transplantation to the defect and enhance the cell retention and therapeutic effect at the local administration site. Vahedi et al. inoculated adipose-derived stem cells into PCL scaffolds, and the ASC-PCL construct treated with low-intensity ultrasound achieved effective cartilage regeneration in a sheep model of a femoral condylar cartilage defect [198]. Collagen exists widely in a variety of biological tissues, has good biocompatibility and biodegradability, and has good plasticity [199]. Shi et al. successfully fabricated silk-fibroin-gelatin composite scaffolds using 3D printing technology and introduced BMSC-specific-affinity peptide [105]. This composite scaffold not only provides a suitable three-dimensional structure for stem cell proliferation, differentiation, and extracellular matrix synthesis but also achieves articular cartilage regeneration by recruiting endogenous BMSCs. In cartilage tissue engineering, the use of decellularized ECM is a relatively new concept. Our study group has proven that decellularized cartilage ECM porous scaffolds can promote stem cell adhesion, proliferation, and cartilage differentiation. At the same time, preclinical studies have proven that decellularized cartilage scaffolds have an excellent cartilage repair effect [200–203].
Although collagen type II and HA are key components of cartilage ECM, mainly type I collagen and HA have been developed as hydrogels for experimental and clinical cartilage repair [204]. To develop injectable scaffolds for the treatment of cartilage, the effects of HA hydrogel on chondrogenic differentiation and cartilage repair of hMSCs have been evaluated in vitro and in vivo. Result showed that the hydrogels reduce the fast leakage of the encapsulated growth factors, leading to the enhanced chondrogenesis of hMSCs and neocartilage formation [205]. Hydrogel can also achieve better cartilage repair by encapsulating functional biological small molecules. Xu et al. demonstrated that hydrogel encapsulation resulted in more sustained release of kartogenin and TGF-β1, which led to enhanced chondrogenesis of encapsulated human bone marrow MSCs in vitro and in vivo [206]. For the treatment of cartilage and osteochondral defects, the exact size and shape can be determined only after debridement. Therefore, methods such as in situ 3D bioprinting or hydrogel application are the most appropriate procedures for providing personalized treatment. The customizability of traditional solid scaffolds is weak, while the limitations of hydrogels include poor mechanical integrity and rapid degradation when exposed to inflammatory environment [207]. With the deepening of the intersection of biology and material manufacturing disciplines, any strategy aimed at imitating the composition and regional organization of articular cartilage will be more likely to reconstruct engineering tissue with the potential for successful clinical application [208].
The current biomaterials and scaffolds used for the delivery of stem cells still have many problems to be solved. For example, the biocompatibility of polymer materials is poor, and their degradation products may cause changes in the pH of the microenvironment. The mechanical properties and degradation rate of natural biomaterials are difficult to control, and its activity and functionality in the body are still to be clarified.
7. Conclusions and Future Perspectives
In the field of articular cartilage regeneration and OA treatment, research involving stem cells has moved from the laboratory to the clinic [209, 210]. However, several problems remain that restrict the application of tissue-engineered cartilage involving stem cells.
First, the functional heterogeneity of stem cells is a substantial obstacle to their clinical transformation [211]. Therefore, before using stem cells, it is necessary to screen out specific subgroups to more accurately explore the molecular mechanism of cartilage regeneration. Second, the problem of premature differentiation of stem cells in vitro expansion has not been resolved. Finding specific targets that regulate stem cell differentiation may solve this problem. For example, methyltransferase inhibitors can inhibit Setd7 protein, prevent cell differentiation, and maintain cell division. Researchers used stem cells containing methyltransferase inhibitors to treat muscle atrophy mice, and the results showed that the strength of regenerating muscle was significantly improved [212]. Finally, standard animal models of articular cartilage defects and OA have not yet been established [213]. Rodents such as mice and rats maintain open endochondral ossification throughout their lives, and the healing of cartilage defects may be greatly affected by spontaneous internal healing [214]. Using large animals such as pigs and horses often limits the number of samples due to high prices. Therefore, finding a balance between effectiveness and economic benefits is necessary in the choice of animal models.
The use of stem cell derivatives to regenerate articular cartilage is a promising development direction [133]. miRNA is considered to be the main component that mediates the biological effects of EVs. However, the main problem currently encountered is that it is technically challenging to produce a sufficient number of EVs, and the amount of nucleic acid packages for EVs is too low [215, 216]. Cell nanoporation biochips can not only increase the production of exosomes but also realize the encapsulation of specific miRNAs [217]. This new technology may help translate the EV-based cartilage regeneration strategy into clinical practice.
The treatment of articular cartilage defects has gone through several stages of development: drug treatment can only relieve symptoms (Figures 3(a) and 3(b)). MF and other techniques often use fibrocartilage to temporarily fill cartilage defects (Figures 3(b) and 3(c)) [218]. Artificial joint replacement surgery temporarily restores the smooth joint surface, but the artificial material has a limited life span (Figures 3(b) and 3(d)). The use of hyaline cartilage to restore the joint surface (Figure 3(e)) is the consummate appeal [219]. A recent study suggested that we might not consider hyaline cartilage as a “final” goal, but as an intermediate stage (Figures 3(e) and 3(f)), and try to stay at this stage. The cells go through the hyaline cartilage stage before forming bone tissue [220]. Researchers used bone morphogenetic protein 2 (BMP2) to initiate the bone formation process after MF and then used an antagonist (VEGFR1) to block the vascular endothelial growth factor, thereby stopping the bone formation process and leaving the new tissue in the hyaline cartilage stage [221].
Figure 3.
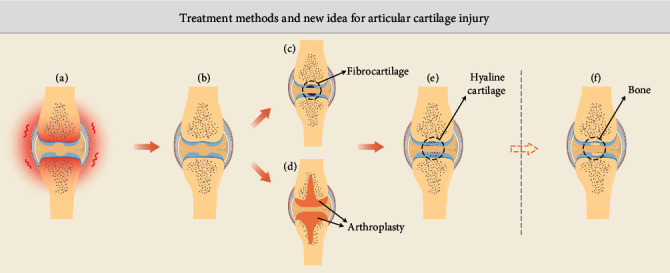
Treatment methods and new idea for articular cartilage injury. (a) Joint cartilage defects cause joint inflammation. (b) Medication can relieve symptoms. (c) Traditional repair techniques such as MF form fibrocartilage. (d) Artificial joint replacement surgery reconstructs the articular surface. (e) Ideal form of cartilage regeneration. (f) New ideas for cartilage regeneration.
In summary, the articular cartilage regeneration strategy involving stem cells has achieved encouraging results. The joint cooperation of practitioners from multiple disciplines and fields will help overcome current challenges, and the change in thinking style may open up new strategies for articular cartilage regeneration.
Acknowledgments
This work was supported by the National Key R&D Program of China (2019 YFA 0110600), the National Natural Science Foundation of China (81772319), and the China Postdoctoral Science Foundation Grant (2019TQ0379, 2019M663262).
Contributor Information
Shuyun Liu, Email: clear_ann@163.com.
Weimin Guo, Email: guowm5@mail.sysu.edu.cn.
Quanyi Guo, Email: doctorguo_301@163.com.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Shuangpeng Jiang and Guangzhao Tian contributed equally to this work.
References
- 1.Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis and rheumatism. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett R. Osteoarthritis. Lancet. 2018;391(10134):1985–2078. doi: 10.1016/S0140-6736(18)31064-X. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S., Guo W., Tian G., et al. Clinical application status of articular cartilage regeneration techniques: tissue-engineered cartilage brings new hope. Stem Cells International. 2020;2020:16. doi: 10.1155/2020/5690252.5690252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam Y., Rim Y. A., Lee J., Ju J. H. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem cells international. 2018;2018:20. doi: 10.1155/2018/8490489.8490489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medvedeva E. V., Grebenik E. A., Gornostaeva S. N., et al. Repair of damaged articular cartilage: current approaches and future directions. International journal of molecular sciences. 2018;19(8) doi: 10.3390/ijms19082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Liu S., Guo W., et al. Human umbilical cord Wharton's jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthritis Cartilage. 2018;26(7):954–965. doi: 10.1016/j.joca.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Koh Y. G., Kwon O. R., Kim Y. S., Choi Y. J., Tak D. H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized Trial. Arthroscopy. 2016;32(1):97–109. doi: 10.1016/j.arthro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Wang A. T., Feng Y., Jia H. H., Zhao M., Yu H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: a concise review. World journal of stem cells. 2019;11(4):222–235. doi: 10.4252/wjsc.v11.i4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrell C. R., Markovic B. S., Fellabaum C., Arsenijevic A., Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomedicine & pharmacotherapy. 2019;109:2318–2326. doi: 10.1016/j.biopha.2018.11.099. [DOI] [PubMed] [Google Scholar]
- 10.Houghton J., Stoicov C., Nomura S., et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306(5701):1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 11.Večerić-Haler Ž., Cerar A., Perše M. (Mesenchymal) stem cell-based therapy in cisplatin-induced acute kidney injury animal model: risk of immunogenicity and tumorigenicity. Stem Cells International. 2017;2017:17. doi: 10.1155/2017/7304643.7304643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of ageing and development. 2008;129(3):163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Murphy J. M., Dixon K., Beck S., Fabian D., Feldman A., Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis & Rheumatism. 2002;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 14.Kolios G., Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 15.Daneshmandi L., Shah S., Jafari T., et al. Emergence of the stem cell secretome in regenerative engineering. Trends in Biotechnology. 2020;38(12):1373–1384. doi: 10.1016/j.tibtech.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L P. K., Kandoi S., Misra R., S V., K R., Verma R. S. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine & growth factor reviews. 2019;46(1-9):1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Bernhard J. C., Vunjak-Novakovic G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem cell research & therapy. 2016;7(1) doi: 10.1186/s13287-016-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou S., Chen S., Jiang Q., Pei M. Determinants of stem cell lineage differentiation toward chondrogenesis versus adipogenesis. Cellular and Molecular Life Sciences. 2019;76(9):1653–1680. doi: 10.1007/s00018-019-03017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T., Nimkingratana P., Smith C. A., Cheng A., Hardingham T. E., Kimber S. J. Enhanced chondrogenesis from human embryonic stem cells. Stem cell research. 2019;39:p. 101497. doi: 10.1016/j.scr.2019.101497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo J. U., Barthel T. S., Nishimura K., et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. The Journal of Bone and Joint Surgery. 1998;80(12):1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kang H., Lu S., Peng J., et al. In vivo construction of tissue-engineered cartilage using adipose-derived stem cells and bioreactor technology. Cell Tissue Bank. 2015;16(1):123–133. doi: 10.1007/s10561-014-9448-7. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell K. L., Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthritis and cartilage. 2015;23(3):351–362. doi: 10.1016/j.joca.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokbel A. N., El Tookhy O. S., Shamaa A. A., Rashed L. A., Sabry D., El Sayed A. M. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. 2011;12(1) doi: 10.1186/1471-2474-12-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokbel A., El-Tookhy O., Shamaa A. A., Sabry D., Rashed L., Mostafa A. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clinical and Experimental Rheumatology-Incl Supplements. 2011;29(2):275–284. [PubMed] [Google Scholar]
- 25.Kotaka S., Wakitani S., Shimamoto A., et al. Magnetic targeted delivery of induced pluripotent stem cells promotes articular cartilage repair. Stem cells international. 2017;2017:7. doi: 10.1155/2017/9514719.9514719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., Luo X., Lv X., et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem cell research & therapy. 2016;7(1) doi: 10.1186/s13287-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawitan J. A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed research international. 2014;2014:14. doi: 10.1155/2014/965849.965849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplan A. I., Dennis J. E. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 29.Li H. J., Shen S., Fu H. T., et al. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells International. 2019;2019:18. doi: 10.1155/2019/9671206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding J., Chen B., Lv T., et al. Bone Marrow Mesenchymal Stem Cell‐Based Engineered Cartilage Ameliorates Polyglycolic Acid/Polylactic Acid Scaffold‐Induced Inflammation Through M2 Polarization of Macrophages in a Pig Model. STEM CELLS Translational Medicine. 2016;5(8):1079–1089. doi: 10.5966/sctm.2015-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L., Leijten J. C., Georgi N., Post J. N., van Blitterswijk C., Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9-10):1425–1436. doi: 10.1089/ten.tea.2010.0517. [DOI] [PubMed] [Google Scholar]
- 32.Acharya C., Adesida A., Zajac P., et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. Cellular Physiology. 2012;227(1):88–97. doi: 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Guo W., Wang M., et al. Co-culture systems-based strategies for articular cartilage tissue engineering. Journal of Cellular Physiology. 2018;233(3):1940–1951. doi: 10.1002/jcp.26020. [DOI] [PubMed] [Google Scholar]
- 34.Caplan A. I. Mesenchymal Stem Cells: Time to Change the Name! STEM CELLS Translational Medicine. 2017;6(6):1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoddart M. J., Bara J., Alini M. Cells and secretome--towards endogenous cell re-activation for cartilage repair. Advanced Drug Delivery Reviews. 2015;84:135–145. doi: 10.1016/j.addr.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q., Wang J., Chen Y., et al. Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomaterialia. 2018;76:29–38. doi: 10.1016/j.actbio.2018.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelttari K., Winter A., Steck E., et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 38.Mueller M. B., Tuan R. S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58(5):1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Studer D., Millan C., Öztürk E., Maniura-Weber K., Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. European Cells and Materials. 2012;24:118–135. doi: 10.22203/eCM.v024a09. [DOI] [PubMed] [Google Scholar]
- 40.Emans P. J., Pieper J., Hulsbosch M. M., et al. Differential cell viability of chondrocytes and progenitor cells in tissue-engineered constructs following implantation into osteochondral defects. Tissue Engineering. 2006;12(6):1699–1709. doi: 10.1089/ten.2006.12.1699. [DOI] [PubMed] [Google Scholar]
- 41.Quintavalla J., Uziel-Fusi S., Yin J., et al. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23(1):109–119. doi: 10.1016/S0142-9612(01)00086-2. [DOI] [PubMed] [Google Scholar]
- 42.de Windt T. S., Vonk L. A., Slaper-Cortenbach I. C., et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1):256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 43.Andrzejewska A., Lukomska B., Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37(7):855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacakova L., Zarubova J., Travnickova M., et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnology Advances. 2018;36(4):1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Bajek A., Gurtowska N., Olkowska J., Kazmierski L., Maj M., Drewa T. Adipose-derived stem cells as a tool in cell-based therapies. Archivum Immunologiae et Therapiae Experimentalis. 2016;64(6):443–454. doi: 10.1007/s00005-016-0394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzute T., Lynch K., Pei M. Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Reviews and Reports. 2015;11(1):119–132. doi: 10.1007/s12015-014-9546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segawa Y., Muneta T., Makino H., et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. Journal of Orthopaedic Research. 2009;27(4):435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 48.De Bari C., Dell'Accio F., Tylzanowski P., Luyten F. P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 49.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell communication and signaling: CCS. 2011;9(1):12–12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colter D. C., Sekiya I., Prockop D. J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proceedings of the National Academy of Sciences. 2001;98(14):7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen L., Tang F. Single-cell sequencing in stem cell biology. Genome Biology. 2016;17(1) doi: 10.1186/s13059-016-0941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sidney L. E., Branch M. J., Dunphy S. E., Dua H. S., Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell K. C., Phinney D. G., Lacey M. R., Barrilleaux B. L., Meyertholen K. E., O'Connor K. C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28(4):788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 54.Krebsbach P. H., Villa-Diaz L. G. The Role of Integrin α6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Cells and Development. 2017;26(15):1090–1099. doi: 10.1089/scd.2016.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X., Guo W., Zha K., et al. Enrichment of CD146+Adipose-Derived Stem Cells in Combination with Articular Cartilage Extracellular Matrix Scaffold Promotes Cartilage Regeneration. Theranostics. 2019;9(17):5105–5121. doi: 10.7150/thno.33904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marvasti T. B., Alibhai F. J., Weisel R. D., Li R. K. CD34(+) stem cells: promising roles in cardiac repair and regeneration. Canadian Journal of Cardiology. 2019;35(10):1311–1321. doi: 10.1016/j.cjca.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 57.Mintz P. J., Huang K. W., Reebye V., et al. Exploiting human CD34+ stem cell-conditioned medium for tissue repair. Molecular Therapy. 2014;22(1):149–159. doi: 10.1038/mt.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z., Ma S., Cao R., et al. CD49fhigh Defines a Distinct Skin Mesenchymal Stem Cell Population Capable of Hair Follicle Epithelial Cell Maintenance. ournal of Investigative Dermatology. 2020;140(3):544–555.e9. doi: 10.1016/j.jid.2019.08.442. [DOI] [PubMed] [Google Scholar]
- 59.Pridie K. A method of resurfacing osteoarthritis knee joints. Journal of Bone and Joint Surgery. 1959;41 [Google Scholar]
- 60.Jiang B., Fu X., Yan L., et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics. 2019;9(22):6587–6600. doi: 10.7150/thno.35391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y. S., Koh Y. G. Comparative Matched-Pair Analysis of Open-Wedge High Tibial Osteotomy With Versus Without an Injection of Adipose-Derived Mesenchymal Stem Cells for Varus Knee Osteoarthritis: Clinical and Second-Look Arthroscopic Results. The American Journal of Sports Medicine. 2018;46(11):2669–2677. doi: 10.1177/0363546518785973. [DOI] [PubMed] [Google Scholar]
- 62.Song J. S., Hong K. T., Kim N. M., et al. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regenerative Therapy. 2020;14:32–39. doi: 10.1016/j.reth.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X., Yang Y., Li Y., et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–4438. doi: 10.1039/C7NR00352H. [DOI] [PubMed] [Google Scholar]
- 64.Wu J., Sun Z., Sun H. S., et al. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transplant. 2008;16(10):993–1005. [PubMed] [Google Scholar]
- 65.Song Y., Du H., Dai C., et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regenerative Medicine. 2018;13(3):295–307. doi: 10.2217/rme-2017-0152. [DOI] [PubMed] [Google Scholar]
- 66.Xia T., Yu F., Zhang K., et al. The effectiveness of allogeneic mesenchymal stem cells therapy for knee osteoarthritis in pigs. Annals of Translational Medicine. 2018;6(20):p. 404. doi: 10.21037/atm.2018.09.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y. S., Chung P. K., Suh D. S., Heo D. B., Tak D. H., Koh Y. G. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2020;28(2):544–554. doi: 10.1007/s00167-019-05729-3. [DOI] [PubMed] [Google Scholar]
- 68.Sadlik B., Jaroslawski G., Gladysz D., et al. Knee Cartilage Regeneration with Umbilical Cord Mesenchymal Stem Cells Embedded in Collagen Scaffold Using Dry Arthroscopy Technique. Clinical Research and Practice. 2017;1020:113–122. doi: 10.1007/5584_2017_9. [DOI] [PubMed] [Google Scholar]
- 69.Huang B. J., Hu J. C., Athanasiou K. A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L., Duan X., Fan Z., et al. Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Scientific Reports. 2018;8(1):p. 9900. doi: 10.1038/s41598-018-27737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu C. C., Sheu S. Y., Hsu L. H., Yang K. C., Tseng C. C., Kuo T. F. Intra-articular Injection of platelet-rich fibrin releasates in combination with bone marrow-derived mesenchymal stem cells in the treatment of articular cartilage defects: Anin vivostudy in rabbits. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2017;105(6):1536–1543. doi: 10.1002/jbm.b.33688. [DOI] [PubMed] [Google Scholar]
- 72.Barrachina L., Remacha A. R., Romero A., et al. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Veterinary Research. 2018;14(1):p. 241. doi: 10.1186/s12917-018-1556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vayas R., Reyes R., Arnau M. R., Évora C., Delgado A. Injectable Scaffold for Bone Marrow Stem Cells and Bone Morphogenetic Protein-2 to Repair Cartilage. Cartilage. 2019:p. 194760351984168. doi: 10.1177/1947603519841682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang T., Heng S., Huang X., et al. Biomimetic poly(poly(ε-caprolactone)-polytetrahydrofuran urethane) based nanofibers enhanced chondrogenic differentiation and cartilage regeneration. Journal of Biomedical Nanotechnology. 2019;15(5):1005–1017. doi: 10.1166/jbn.2019.2748. [DOI] [PubMed] [Google Scholar]
- 75.Kohli N., Al-Delfi I. R. T., Snow M., et al. CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells. Scientific Reports. 2019;9(1):p. 3194. doi: 10.1038/s41598-019-39715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mei L., Shen B., Ling P., et al. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS One. 2017;12(4):p. e0176107. doi: 10.1371/journal.pone.0176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Critchley S., Sheehy E. J., Cunniffe G., et al. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020;113:130–143. doi: 10.1016/j.actbio.2020.05.040. [DOI] [PubMed] [Google Scholar]
- 78.Yan M., Liu X., Dang Q., Huang H., Yang F., Li Y. Intra-articular injection of human synovial membrane-derived mesenchymal stem cells in murine collagen-induced arthritis: assessment of immunomodulatory capacity in vivo. Stem Cells International. 2017;2017:12. doi: 10.1155/2017/9198328.9198328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kondo S., Nakagawa Y., Mizuno M., et al. Transplantation of aggregates of autologous synovial mesenchymal stem cells for treatment of cartilage defects in the femoral condyle and the femoral groove in microminipigs. The American Journal of Sports Medicine. 2019;47(10):2338–2347. doi: 10.1177/0363546519859855. [DOI] [PubMed] [Google Scholar]
- 80.Neybecker P., Henrionnet C., Pape E., et al. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res Ther. 2018;9(1):p. 329. doi: 10.1186/s13287-018-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J., Huang Y., Song J., et al. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018;79:202–215. doi: 10.1016/j.actbio.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S., Jia Y., Yuan M., et al. Repair of osteochondral defects using human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in a rabbit model. BioMed Research International. 2017;2017 doi: 10.1155/2017/8760383.8760383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xing D., Wu J., Wang B., et al. Intra-articular delivery of umbilical cord-derived mesenchymal stem cells temporarily retard the progression of osteoarthritis in a rat model. Int J Rheum Dis. 2020;23(6):778–787. doi: 10.1111/1756-185X.13834. [DOI] [PubMed] [Google Scholar]
- 84.Rim Y. A., Nam Y., Park N., Lee J., Park S. H., Ju J. H. Repair potential of nonsurgically delivered induced pluripotent stem cell-derived chondrocytes in a rat osteochondral defect model. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(8):1843–1855. doi: 10.1002/term.2705. [DOI] [PubMed] [Google Scholar]
- 85.Gibson J. D., O'Sullivan M. B., Alaee F., et al. Regeneration of Articular Cartilage by Human ESC-Derived Mesenchymal Progenitors Treated Sequentially with BMP-2 and Wnt5a. STEM CELLS Translational Medicine. 2017;6(1):40–50. doi: 10.5966/sctm.2016-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chahal J., Gómez-Aristizábal A., Shestopaloff K., et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. STEM CELLS Translational Medicine. 2019;8(8):746–757. doi: 10.1002/sctm.18-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapiro S. A., Arthurs J. R., Heckman M. G., et al. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage. 2018;10(4):432–443. doi: 10.1177/1947603518796142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emadedin M., Labibzadeh N., Liastani M. G., et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(10):1238–1246. doi: 10.1016/j.jcyt.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Shadmanfar S., Labibzadeh N., Emadedin M., et al. Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(4):499–506. doi: 10.1016/j.jcyt.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Matas J., Orrego M., Amenabar D., et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. STEM CELLS Translational Medicine. 2019;8(3):215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khalifeh Soltani S., Forogh B., Ahmadbeigi N., et al. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: a pilot study. Cytotherapy. 2019;21(1):54–63. doi: 10.1016/j.jcyt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 93.Yin Y., Li X., He X. T., Wu R. X., Sun H. H., Chen F. M. Leveraging stem cell homing for therapeutic regeneration. Journal of Dental Research. 2017;96(6):601–609. doi: 10.1177/0022034517706070. [DOI] [PubMed] [Google Scholar]
- 94.Zhang S., Hu B., Liu W., et al. Articular cartilage regeneration: The role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin Arthritis Rheum. 2020;50(2):198–208. doi: 10.1016/j.semarthrit.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Karp J. M., Leng Teo G. S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 96.Li X., He X. T., Yin Y., Wu R. X., Tian B. M., Chen F. M. Administration of signalling molecules dictates stem cell homing forin situregeneration. Journal of Cellular and Molecular Medicine. 2017;21(12):3162–3177. doi: 10.1111/jcmm.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li M., Sun X., Ma L., et al. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Scientific Reports. 2017;7(1):p. 40161. doi: 10.1038/srep40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peyvandi A. A., Roozbahany N. A., Peyvandi H., et al. Critical role of SDF-1/CXCR4 signaling pathway in stem cell homing in the deafened rat cochlea after acoustic trauma. Neural Regeneration Research. 2018;13(1):154–160. doi: 10.4103/1673-5374.224382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W., Chen J., Tao J., et al. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34(3):713–723. doi: 10.1016/j.biomaterials.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 100.Ji X., Lei Z., Yuan M., et al. Cartilage repair mediated by thermosensitive photocrosslinkable TGFβ1-loaded GM-HPCH via immunomodulating macrophages, recruiting MSCs and promoting chondrogenesis. Theranostics. 2020;10(6):2872–2887. doi: 10.7150/thno.41622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park M. S., Kim Y. H., Jung Y., et al. In Situ Recruitment of Human Bone Marrow-Derived Mesenchymal Stem Cells Using Chemokines for Articular Cartilage Regeneration. Cell Transplant. 2015;24(6):1067–1083. doi: 10.3727/096368914X681018. [DOI] [PubMed] [Google Scholar]
- 102.Min B. H., Truong M. D., Song H. K., et al. Development and Efficacy Testing of a "Hollow Awl" That Leads to Patent Bone Marrow Channels and Greater Mesenchymal Stem Cell Mobilization During Bone Marrow Stimulation Cartilage Repair Surgery. Arthroscopy. 2017;33(11):2045–2051. doi: 10.1016/j.arthro.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 103.Baboolal T. G., Khalil-Khan A., Theodorides A. A., Wall O., Jones E., McGonagle D. A Novel Arthroscopic Technique for Intraoperative Mobilization of Synovial Mesenchymal Stem Cells. The American Journal of Sports Medicine. 2018;46(14):3532–3540. doi: 10.1177/0363546518803757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun X., Yin H., Wang Y., et al. In situ articular cartilage regeneration through endogenous reparative cell homing using a functional bone marrow-specific scaffolding system. ACS Applied Materials & Interfaces. 2018;10(45):38715–38728. doi: 10.1021/acsami.8b11687. [DOI] [PubMed] [Google Scholar]
- 105.Shi W., Sun M., Hu X., et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Advanced Materials. 2017;29(29) doi: 10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- 106.Meng Q., Hu X., Huang H., et al. Microfracture combined with functional pig peritoneum-derived acellular matrix for cartilage repair in rabbit models. Acta Biomater. 2017;53:279–292. doi: 10.1016/j.actbio.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 107.Kalamegam G., Memic A., Budd E., Abbas M., Mobasheri A. A Comprehensive Review of Stem Cells for Cartilage Regeneration in Osteoarthritis. Advances in Experimental Medicine and Biolog. 2018;1089:23–36. doi: 10.1007/5584_2018_205. [DOI] [PubMed] [Google Scholar]
- 108.Liu S., Zhou J., Zhang X., et al. Strategies to optimize adult stem cell therapy for tissue regeneration. International Journal of Molecular Sciences. 2016;17(6) doi: 10.3390/ijms17060982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu S. P., Wei Z., Wei L. Preconditioning strategy in stem cell transplantation therapy. Translational Stroke Research. 2013;4(1):76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silver I. A. Measurement of pH and ionic composition of pericellular sites. Philosophical Transactions of the Royal Society of London. B, Biological Sciences. 1975;271(912):261–272. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 111.Pei M. Environmental preconditioning rejuvenates adult stem cells' proliferation and chondrogenic potential. Biomaterials. 2017;117:10–23. doi: 10.1016/j.biomaterials.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pattappa G., Johnstone B., Zellner J., Docheva D., Angele P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. International Journal of Molecular Sciences. 2019;20(3):p. 484. doi: 10.3390/ijms20030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peck S. H., Bendigo J. R., Tobias J. W., et al. Hypoxic Preconditioning Enhances Bone Marrow–Derived Mesenchymal Stem Cell Survival in a Low Oxygen and Nutrient-Limited 3D Microenvironment. Cartilage. 2019:p. 194760351984167. doi: 10.1177/1947603519841675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pattappa G., Krueckel J., Schewior R., et al. Physioxia expanded bone marrow derived mesenchymal stem cells have improved cartilage repair in an early osteoarthritic focal defect model. Biology. 2020;9(8):p. 230. doi: 10.3390/biology9080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kanichai M., Ferguson D., Prendergast P. J., Campbell V. A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. Journal of Cellular Physiology. 2008;216(3):708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 116.Lord-Dufour S., Copland I. B., Levros L. C., Jr., et al. Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1α: Targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells. Stem Cells. 2009;27(3):489–497. doi: 10.1634/stemcells.2008-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhatti F. U., Mehmood A., Latief N., et al. Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide- induced oxidative stress _in vitro_ and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthritis Cartilage. 2017;25(2):321–331. doi: 10.1016/j.joca.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 118.Cai G., Liu W., He Y., et al. Recent advances in kartogenin for cartilage regeneration. J Drug Target. 2019;27(1):28–32. doi: 10.1080/1061186X.2018.1464011. [DOI] [PubMed] [Google Scholar]
- 119.Jing H., Zhang X., Gao M., et al. Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating JNK and β-catenin-related pathways. The FASEB Journal. 2019;33(4):5641–5653. doi: 10.1096/fj.201802137RRR. [DOI] [PubMed] [Google Scholar]
- 120.Jing H., Zhang X., Luo K., et al. miR-381-abundant small extracellular vesicles derived from kartogenin- preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020;231:p. 119682. doi: 10.1016/j.biomaterials.2019.119682. [DOI] [PubMed] [Google Scholar]
- 121.Solchaga L. A., Penick K., Goldberg V. M., Caplan A. I., Welter J. F. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16(3):1009–1019. doi: 10.1089/ten.tea.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsumura E., Tsuji K., Komori K., Koga H., Sekiya I., Muneta T. Pretreatment with IL-1β enhances proliferation and chondrogenic potential of synovium-derived mesenchymal stem cells. Cytotherapy. 2017;19(2):181–193. doi: 10.1016/j.jcyt.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 123.Yamagata K., Nakayamada S., Zhang T., Zhang X., Tanaka Y. Soluble IL-6R promotes chondrogenic differentiation of mesenchymal stem cells to enhance the repair of articular cartilage defects using a rat model for rheumatoid arthritis. Clin Exp Rheumatol. 2020;38(4):670–679. [PubMed] [Google Scholar]
- 124.Sart S., Agathos S. N., Li Y., Ma T. Regulation of mesenchymal stem cell 3D microenvironment: From macro to microfluidic bioreactors. Biotechnol J. 2016;11(1):43–57. doi: 10.1002/biot.201500191. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H., Li Z. L., Yang F., et al. Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing. Stem Cell Research & Therapy. 2018;9(1) doi: 10.1186/s13287-018-0805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sahu N., Budhiraja G., Subramanian A. Preconditioning of mesenchymal stromal cells with low-intensity ultrasound: influence on chondrogenesis and directed SOX9 signaling pathways. Stem Cell Research & Therapy. 2020;11(1):p. 6. doi: 10.1186/s13287-019-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Armiento A. R., Stoddart M. J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 128.Cheng B., Tu T., Shi X., et al. A novel construct with biomechanical flexibility for articular cartilage regeneration. Stem Cell Res Ther. 2019;10(1):p. 298. doi: 10.1186/s13287-019-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan X., Chen Y. R., Song Y. F., et al. Scaffold-based gene therapeutics for osteochondral tissue engineering. Frontiers in Pharmacology. 2020;10(1534) doi: 10.3389/fphar.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Graceffa V., Vinatier C., Guicheux J., et al. State of art and limitations in genetic engineering to induce stable chondrogenic phenotype. Biotechnology Advances. 2018;36(7):1855–1869. doi: 10.1016/j.biotechadv.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Guo X., Zheng Q., Yang S., et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006;1(4):206–215. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 132.Vizoso F. J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences. 2017;18(9):p. 1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.González-González A., García-Sánchez D., Dotta M., Rodríguez-Rey J. C., Pérez-Campo F. M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World Journal of Stem Cells. 2020;12(12):1529–1552. doi: 10.4252/wjsc.v12.i12.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harrell C. R., Fellabaum C., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8(5):p. 467. doi: 10.3390/cells8050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wiklander O. P. B., Brennan M., Lötvall J., Breakefield X. O., El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Science Translational Medicine. 2019;11(492) doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doyle L. M., Wang M. Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7):p. 727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Järveläinen H., Annele Sainio A., Koulu M., Wight T. N., Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacological Reviews. 2009;6(2):198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng C. W., Solorio L. D., Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnology Advances. 2014;32(2):462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pérez-Castrillo S., González-Fernández M. L., López-González M. E., Villar-Suárez V. Effect of ascorbic and chondrogenic derived decellularized extracellular matrix from mesenchymal stem cells on their proliferation, viability and differentiation. Ann Anat. 2018;220:60–69. doi: 10.1016/j.aanat.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 140.Tang C., Jin C., Xu Y., Wei B., Wang L. Chondrogenic Differentiation Could Be Induced by Autologous Bone Marrow Mesenchymal Stem Cell–Derived Extracellular Matrix Scaffolds Without Exogenous Growth Factor. Tissue Eng Part A. 2016;22(3-4):222–232. doi: 10.1089/ten.tea.2014.0491. [DOI] [PubMed] [Google Scholar]
- 141.Islam A., Urbarova I., Bruun J. A., Martinez-Zubiaurre I. Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells. European Cells and Materials. 2019;37:153–174. doi: 10.22203/ecm.v037a10. [DOI] [PubMed] [Google Scholar]
- 142.Ishikawa J., Takahashi N., Matsumoto T., et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone. 2016;83:210–219. doi: 10.1016/j.bone.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 143.Kay A. G., Long G., Tyler G., et al. Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Scientific Reports. 2017;7(1):p. 18019. doi: 10.1038/s41598-017-18144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nazemian V., Manaheji H., Sharifi A. M., Zaringhalam J. Long term treatment by mesenchymal stem cells conditioned medium modulates cellular, molecular and behavioral aspects of adjuvant-induced arthritis. Cell Mol Biol (Noisy-le-grand) 2018;64(1):19–26. doi: 10.14715/cmb/2018.64.2.5. [DOI] [PubMed] [Google Scholar]
- 145.Chen W., Sun Y., Gu X., et al. Conditioned medium of mesenchymal stem cells delays osteoarthritis progression in a rat model by protecting subchondral bone, maintaining matrix homeostasis, and enhancing autophagy. Journal of Tissue Engineering and Regenerative Medicine. 2019;13(9):1618–1628. doi: 10.1002/term.2916. [DOI] [PubMed] [Google Scholar]
- 146.Veronesi F., Desando G., Fini M., et al. Bone marrow concentrate and expanded mesenchymal stromal cell surnatants as cell-free approaches for the treatment of osteochondral defects in a preclinical animal model. International Orthopaedics. 2019;43(1):25–34. doi: 10.1007/s00264-018-4202-6. [DOI] [PubMed] [Google Scholar]
- 147.Widhiyanto L., Utomo D. N., Perbowo A. P., Hernugrahanto K. D., Purwati Macroscopic and histologic evaluation of cartilage regeneration treated using xenogenic biodegradable porous sponge cartilage scaffold composite supplemented with allogenic adipose derived mesenchymal stem cells (ASCs) and secretome: An in vivo experimental study. Journal of Biomaterials Applications. 2020;35(3):422–429. doi: 10.1177/0885328220934938. [DOI] [PubMed] [Google Scholar]
- 148.Oh S. J., Choi K. U., Choi S. W., et al. Comparative analysis of adipose-derived stromal cells and their secretome for auricular cartilage regeneration. Stem Cells Internationa. 2020;2020, article 8595940 doi: 10.1155/2020/8595940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Monguió-Tortajada M., Morón-Font M., Gámez-Valero A., Carreras-Planella L., Borràs F. E., Franquesa M. Extracellular-vesicle isolation from different biological fluids by size-exclusion chromatography. Curr Protoc Stem Cell Biol. 2019;49(1):p. e82. doi: 10.1002/cpsc.82. [DOI] [PubMed] [Google Scholar]
- 150.Guerreiro E. M., Vestad B., Steffensen L. A., et al. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS One. 2018;13(9):p. e0204276. doi: 10.1371/journal.pone.0204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim S. Y., Phan T. H., Limantoro C., Kalionis B., Chrzanowski W. Isolation and characterization of extracellular vesicles from mesenchymal stromal cells. Methods in Molecular Biology. 2019;2029:15–23. doi: 10.1007/978-1-4939-9631-5_2. [DOI] [PubMed] [Google Scholar]
- 152.Brett S. I., Lucien F., Guo C., et al. Immunoaffinity based methods are superior to kits for purification of prostate derived extracellular vesicles from plasma samples. Prostate. 2017;77(13):1335–1343. doi: 10.1002/pros.23393. [DOI] [PubMed] [Google Scholar]
- 153.Zhang S., Chuah S. J., Lai R. C., Hui J. H. P., Lim S. K., Toh W. S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 154.Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Scientific Reports. 2017;7(1):p. 16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y., Yu D., Liu Z., et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Research & Therapy. 2017;8(1):p. 189. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu J., Kuang L., Chen C., et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 157.Zhu Y., Wang Y., Zhao B., et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research & Therapy. 2017;8(1):p. 64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen P., Zheng L., Wang Y., et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim K. H., Jo J. H., Cho H. J., Park T. S., Kim T. M. Therapeutic potential of stem cell-derived extracellular vesicles in osteoarthritis: preclinical study findings. Laboratory animal research. 2020;36(1) doi: 10.1186/s42826-020-00043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim Y. G., Choi J., Kim K. Mesenchymal Stem Cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnology Journal. 2020;15(12):p. 2000082. doi: 10.1002/biot.202000082. [DOI] [PubMed] [Google Scholar]
- 161.Mianehsaz E., Mirzaei H. R., Mahjoubin-Tehran M., et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1) doi: 10.1186/s13287-019-1445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eleuteri S., Fierabracci A. Insights into the secretome of mesenchymal stem cells and its potential applications. International journal of molecular sciences. 2019;20(18):p. 4597. doi: 10.3390/ijms20184597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Toh W. S., Lai R. C., Hui J. H. P., Lim S. K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 164.Tao S.-C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ning G., Liu X., Dai M., Meng A., Wang Q. _MicroRNA-92a_ Upholds Bmp Signaling by Targeting _noggin3_ during Pharyngeal Cartilage Formation. Dev Cell. 2013;24(3):283–295. doi: 10.1016/j.devcel.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 166.Hou C., Zhang Z., Zhang Z., et al. Presence and function of microRNA-92a in chondrogenic ATDC5 and adipose-derived mesenchymal stem cells. Molecular medicine reports. 2015;12(4):4877–4886. doi: 10.3892/mmr.2015.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ham O., Song B. W., Lee S. Y., et al. The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling. Biomaterials. 2012;33(18):4500–4507. doi: 10.1016/j.biomaterials.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 168.Meng F., Zhang Z., Chen W., et al. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1β-induced chondrocyte responses. Osteoarthritis Cartilage. 2016;24(5):932–941. doi: 10.1016/j.joca.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 169.Ragni E., Perucca Orfei C., De Luca P., et al. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: the example of joint disease. Stem cell research & therapy. 2020;11(1):165–165. doi: 10.1186/s13287-020-01677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mardpour S., Hamidieh A. A., Taleahmad S., Sharifzad F., Taghikhani A., Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. Journal of Cellular Physiology. 2018;234(6):8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 171.Saleh A. F., Lázaro-Ibáñez E., Forsgard M. A., et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11(14):6990–7001. doi: 10.1039/C8NR08720B. [DOI] [PubMed] [Google Scholar]
- 172.Chun S. Y., Lim J. O., Lee E. H., et al. Preparation and characterization of human adipose tissue-derived extracellular matrix, growth factors, and stem cells: a concise review. Tissue Engineering and Regenerative Medicine. 2019;16(4):385–393. doi: 10.1007/s13770-019-00199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi J. S., Kim B. S., Kim J. D., Choi Y. C., Lee H. Y., Cho Y. W. In vitro cartilage tissue engineering using adipose-derived extracellular matrix scaffolds seeded with adipose-derived stem cells. Tissue Engineering Part A. 2012;18(1-2):80–92. doi: 10.1089/ten.tea.2011.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pei M., He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. Journal of Cellular Physiology. 2012;227(5):2163–2174. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang Y., Lin H., Shen H., Wang B., Lei G., Tuan R. S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes _in vitro_ and _in vivo_. Acta Biomater. 2018;69:71–82. doi: 10.1016/j.actbio.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li J., Hansen K. C., Zhang Y., et al. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35(2):642–653. doi: 10.1016/j.biomaterials.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 177.Ng C. P., Sharif A. R., Heath D. E., et al. Enhanced _ex vivo_ expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35(13):4046–4057. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 178.Yan J., Chen X., Pu C., et al. Synovium stem cell-derived matrix enhances anti-inflammatory properties of rabbit articular chondrocytes via the SIRT1 pathway. Materials Science and Engineering: C. 2020;106:p. 110286. doi: 10.1016/j.msec.2019.110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tang C., Jin C., Li X., et al. Evaluation of an autologous bone mesenchymal stem cell-derived extracellular matrix scaffold in a rabbit and minipig model of cartilage repair. Medical Science Monitor. 2019;25:7342–7350. doi: 10.12659/MSM.916481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nogami M., Kimura T., Seki S., et al. A human amnion-derived extracellular matrix-coated cell-free scaffold for cartilage repair: in vitro and in vivo studies. Tissue Engineering Part A. 2016;22(7-8):680–688. doi: 10.1089/ten.tea.2015.0285. [DOI] [PubMed] [Google Scholar]
- 181.Ashammakhi N., Ahadian S., Darabi M. A., et al. Minimally invasive and regenerative therapeutics. Advanced Materials. 2019;31(1):p. e1804041. doi: 10.1002/adma.201804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Prestwich G. D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. Journal of Controlled Release. 2011;155(2):193–199. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Klotz B. J., Gawlitta D., Rosenberg A., Malda J., Melchels F. P. W. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34(5):394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rastogi P., Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4):p. 042001. doi: 10.1088/1758-5090/ab331e. [DOI] [PubMed] [Google Scholar]
- 185.Yang X., Lu Z., Wu H., Li W., Zheng L., Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Materials Science and Engineering: C. 2018;83:195–201. doi: 10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 186.Xia C., Mei S., Gu C., et al. Decellularized cartilage as a prospective scaffold for cartilage repair. Materials Science and Engineering: C. 2019;101:588–595. doi: 10.1016/j.msec.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 187.Musumeci G., Loreto C., Castorina S., Imbesi R., Leonardi R., Castrogiovanni P. New perspectives in the treatment of cartilage damage. Poly(ethylene glycol) diacrylate (PEGDA) scaffold. A review. Italian Journal of Anatomy and Embryology. 2013;118(2):204–210. [PubMed] [Google Scholar]
- 188.Suntornnond R., An J., Chua C. K. Bioprinting of thermoresponsive hydrogels for next generation tissue engineering: a review. Macromolecular Materials and Engineering. 2017;302(1):p. 1600266. doi: 10.1002/mame.201600266. [DOI] [Google Scholar]
- 189.Pan Z., Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2(3):366–377. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mondal D., Griffith M., Venkatraman S. S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. International Journal of Polymeric Materials and Polymeric Biomaterials. 2016;65(5):255–265. doi: 10.1080/00914037.2015.1103241. [DOI] [Google Scholar]
- 191.Cucchiarini M., Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nature Reviews Rheumatology. 2019;15(1):18–29. doi: 10.1038/s41584-018-0125-2. [DOI] [PubMed] [Google Scholar]
- 192.Toh W. S., Spector M., Lee E. H., Cao T. Biomaterial-mediated delivery of microenvironmental cues for repair and regeneration of articular cartilage. Molecular Pharmaceutics. 2011;8(4):994–1001. doi: 10.1021/mp100437a. [DOI] [PubMed] [Google Scholar]
- 193.Rezwan K., Chen Q. Z., Blaker J. J., Boccaccini A. R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 194.Cui Y. L., Qi A. D., Liu W. G., et al. Biomimetic surface modification of poly(L-lactic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials. 2003;24(21):3859–3868. doi: 10.1016/S0142-9612(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 195.Chen G., Kawazoe N. Porous scaffolds for regeneration of cartilage, bone and osteochondral tissue. Advances in Experimental Medicine and Biology. 2018;1058:171–191. doi: 10.1007/978-3-319-76711-6_8. [DOI] [PubMed] [Google Scholar]
- 196.Chen G., Sato T., Ushida T., Ochiai N., Tateishi T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Engineering. 2004;10(3-4):323–330. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 197.Liao J., Qu Y., Chu B., Zhang X., Qian Z. Biodegradable CSMA/PECA/graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 2015;5(1) doi: 10.1038/srep09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vahedi P., Roshangar L., Jarolmasjed S., Shafaei H., Samadi N., Soleimanirad J. Effect of low-intensity pulsed ultrasound on regenerative potential of transplanted ASCs -PCL construct in articular cartilage defects in sheep. Indian Journal of Animal Sciences. 2016;86(10):1111–1114. [Google Scholar]
- 199.Fu N., Dong T., Meng A., Meng Z., Zhu B., Lin Y. Research progress of the types and preparation techniques of scaffold materials in cartilage tissue engineering. Current Stem Cell Research & Therapy. 2018;13(7):583–590. doi: 10.2174/1574888X12666170718152611. [DOI] [PubMed] [Google Scholar]
- 200.Yang Q., Peng J., Guo Q., et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29(15):2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 201.Zheng X., Yang F., Wang S., et al. Fabrication and cell affinity of biomimetic structured PLGA/articular cartilage ECM composite scaffold. Journal of Materials Science: Materials in Medicine. 2011;22(3):693–704. doi: 10.1007/s10856-011-4248-0. [DOI] [PubMed] [Google Scholar]
- 202.Zheng X.-F., Lu S.-B., Zhang W.-G., Liu S.-Y., Huang J.-X., Guo Q.-Y. Mesenchymal stem cells on a decellularized cartilage matrix for cartilage tissue engineering. Biotechnology and Bioprocess Engineering. 2011;16(3):593–602. doi: 10.1007/s12257-010-0348-9. [DOI] [Google Scholar]
- 203.Kang H., Peng J., Lu S., et al. In vivocartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. Journal of Tissue Engineering and Regenerative Medicine. 2014;8(6):442–453. doi: 10.1002/term.1538. [DOI] [PubMed] [Google Scholar]
- 204.Vega S. L., Kwon M. Y., Burdick J. A. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater. 2017;33:59–75. doi: 10.22203/eCM.v033a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei K., Zhu M., Sun Y., et al. Robust biopolymeric supramolecular “Host−Guest Macromer” hydrogels reinforced byin SituFormed multivalent nanoclusters for cartilage regeneration. Macromolecules. 2016;49(3):866–875. doi: 10.1021/acs.macromol.5b02527. [DOI] [Google Scholar]
- 206.Xu J., Feng Q., Lin S., et al. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials. 2019;210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 207.Jha A. K., Tharp K. M., Browne S., et al. Matrix metalloproteinase-13 mediated degradation of hyaluronic acid-based matrices orchestrates stem cell engraftment through vascular integration. Biomaterials. 2016;89:136–147. doi: 10.1016/j.biomaterials.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tatman P. D., Gerull W., Sweeney-Easter S., Davis J. I., Gee A. O., Kim D. H. Multiscale biofabrication of articular cartilage: bioinspired and biomimetic approaches. Tissue Engineering Part B: Reviews. 2015;21(6):543–559. doi: 10.1089/ten.teb.2015.0142. [DOI] [PubMed] [Google Scholar]
- 209.McIntyre J. A., Jones I. A., Han B., Vangsness C. T., Jr. Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. The American Journal of Sports Medicine. 2018;46(14):3550–3563. doi: 10.1177/0363546517735844. [DOI] [PubMed] [Google Scholar]
- 210.Vega A., Martín-Ferrero M. A., Del Canto F., et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 211.Costa L. A., Eiro N., Fraile M., et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cellular and Molecular Life Sciences. 2020 doi: 10.1007/s00018-020-03600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Judson R. N., Quarta M., Oudhoff M. J., et al. Inhibition of Methyltransferase Setd7 Allows the _In Vitro_ Expansion of Myogenic Stem Cells with Improved Therapeutic Potential. Cell Stem Cell. 2018;22(2):177–190.e7. doi: 10.1016/j.stem.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tessaro I., Nguyen V. T., Di Giancamillo A., et al. Animal models for cartilage repair. Journal of Biological Regulators & Homeostatic Agents. 2018;32(6) Supplemet. 1:105–116. [PubMed] [Google Scholar]
- 214.Dawson A. B. The age order of epiphyseal union in the long bones of the albino rat. The Anatomical Record. 1925;31(1):1–17. doi: 10.1002/ar.1090310102. [DOI] [Google Scholar]
- 215.Kamerkar S., LeBleu V. S., Sugimoto H., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.el Andaloussi S., Mäger I., Breakefield X. O., Wood M. J. A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 217.Yang Z., Shi J., Xie J., et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nature Biomedical Engineering. 2020;4(1):69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Knutsen G., Engebretsen L., Ludvigsen T. C., et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. The Journal of Bone & Joint Surgery. 2004;86(3):455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 219.Armiento A. R., Alini M., Stoddart M. J. Articular fibrocartilage - why does hyaline cartilage fail to repair? Adv Drug Deliv Rev. 2019;146:289–305. doi: 10.1016/j.addr.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 220.Berendsen A. D., Olsen B. R. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Murphy M. P., Koepke L. S., Lopez M. T., et al. Articular cartilage regeneration by activated skeletal stem cells. Nature Medicine. 2020;26(10):1583–1592. doi: 10.1038/s41591-020-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


