Abstract
The association of obesity with cardiovascular disease is well established. However, the interplay of obesity and vascular dysfunction in peripheral tissues such as skeletal muscle, which plays a key in role metabolic homeostasis, requires further study. In particular, there is a paucity of data with regard to sex-differences. Therefore, using a murine model (C57BL/6) of high-fat diet-induced obesity and insulin resistance, we investigated changes in vascular function in gluteus maximus muscle of female and male mice. Diet-induced obesity resulted in alterations in microvascular function. Obese male mice displayed impaired vasoconstriction in second order arterioles compared to lean, male mice, whereas arterioles of obese, female mice displayed significant impairments of both vasodilation and vasoconstrictor responses compared to lean, female mice. Overall, this study identifies distinct differences in how obesity impacts the female and male murine response to skeletal muscle vascular function. This work advances our understanding of sex-specific risk of metabolic complications of obesity and indicates the need for expansion of this study as well as detailed investigation of sex-specific differences in obesity pathology in the future.
Keywords: Diet-induced obesity, Insulin resistance, Microvascular function, Sex-differences
Diet-induced obesity, Insulin resistance, Microvascular function, Sex-differences.
1. Introduction
The epidemic increase in obesity continues to contribute to the rise in cardiovascular disease globally [1, 2]. Obesity is the number one risk factor for diabetes, which itself confers cardiovascular risk [3]. Furthermore, obesity has been associated with cardiovascular disease including microvascular disease, coronary artery disease, and valvular heart disease [4, 5, 6].
The link between obesity and cardiovascular disease is highlighted by factors such as marked increases in blood pressure and systemic vascular resistance, vascular inflammation, and hyperinsulinemia [7]. During positive energy balance, white adipose tissue (WAT) undergoes hypertrophy or hyperplasia [8] to accommodate excess energy intake. While this response of the WAT is an adaptive response to maintain homeostasis, chronic activation of these pathways results in changes in adipokine profiles that become dysregulated in the obese adipose tissue, leading to induction of inflammation and oxidative stress pathways [9]. Moreover, adipokines and cytokines produced in WAT activate intracellular pathways that initiate and maintain inflammation as well as promote insulin resistance in peripheral tissues, such as skeletal muscle, and dampen insulin signaling in the vasculature and metabolic organs [10].
Functionally, the negative interplay of obesity and the vascular physiology has been demonstrated [11]. During the development of obesity-induced insulin resistance using isolated aortas and saphenous arterioles of male rats, Zhao et al. (2015) showed that the microvasculature of skeletal muscle became insulin resistant after only 3 days of high fat feeding and microvascular vasodilation was impaired by four weeks [12]. Several other studies have also assessed microvascular function in models of diet-induced metabolic syndrome or diabetes, showing impaired microvascular function and endothelial dysfunction in the late stages of metabolic disease [13, 14]. Moreover, it was shown that changes in vascular function were more pronounced in pre-diabetic females in comparison to males in isolated mesenteric vasculature [15], suggesting sex-specific differences in the impact of obesity on microvascular health.
Despite these studies, our overall understanding of the effect of obesity on microvascular function in both sexes is lacking and requires further investigation to identify new targets to prevent cardiovascular damage in the face of obesity. In particular, understanding the impact of obesity on non-cardiovascular vascular beds, such as skeletal muscle, is limited with studies restricted to males. This is of particular interest given the pivotal role of skeletal muscle in metabolic homeostasis and thereby obesity. Therefore, to further our understanding of how microvascular function is impaired in obesity and impacted by sex, we used in vivo intravital microscopy (IVM) to assess vascular function in a murine model of diet-induced obesity and insulin resistance in gluteus maximus, a non-sex specific skeletal muscle. These investigations provide insight into the link between obesity and the microcirculation to identify potential therapeutic targets to mitigate obesity-induced vascular dysfunction and identify sexual dimorphism in pathology.
2. Materials & methods
2.1. Animal Care
Animal use was approved by the Animal Care and Use Committee of the University of Northern British Columbia and performed in accordance with the Canadian Council of Animal Care (CCAC) guidelines for the use of Laboratory Animals. Mice (C57BL/6, Charles River Laboratories, Wilmington, MA, USA) were housed at 21 °C with a 12-hour light/dark cycle and were fed standard rodent chow (Rodent LabDiet, 5001, Leduc AB, Canada) ad libitum unless stated. Mice acclimatized for one week prior to beginning experimental procedures. Experiments were conducted in both male and female mice and using two cohorts: vascular function was evaluated by IVM (a terminal procedure) in cohort 1 in the fed state and in cohort 2 in the fasted state.
2.2. Diet-induced obesity
2.2.1. Mouse model
Starting at seven weeks of age, mice were fed standard rodent chow diet (13% kcal as fat, Rodent LabDiet, 5001, Leduc AB, Canada) or high fat diet containing 45% kcal as fat (D12451, Research Diets, New Brunswick NJ, USA) ad libitum for 14–16 weeks. Body weight was measured weekly. Blood was sampled from the saphenous vein of mice in a fasted (16 h fast) and random fed state between 09:00 and 11:00. Blood glucose was measured biweekly using a hand held glucose monitor (Lifescan, Burnaby BC, Canada). Body composition (fat and lean mass) was determined by time domain nuclear magnetic resonance [16] (TD-NMR, minispec LF50, Bruker, Billerica, MA, USA) every four weeks. Fasted and fed plasma insulin (heparinized blood collection tubes, Ultrasensitive Insulin ELISA, ALPCO, Salem NH, USA), glucose tolerance (oral glucose tolerance test, 2 g/kg D-glucose, described below) and insulin sensitivity (insulin tolerance test, 1.5U insulin/kg, described below) were assessed after 12 weeks of feeding.
2.2.2. Insulin tolerance test
Fasted mice (4 h) were given an intraperitoneal (ip) injection of insulin (1.5U/kg, Humalog, Eli Lilly, Indianapolis, IN) and blood was sampled (1–2 drops) from the saphenous vein and used to test blood glucose; blood glucose (mmol/L) was measured at 0, 10, 20, 30, 60, and 120 minutes post insulin injection using a handheld glucometer (Lifescan, Burnaby BC, Canada).
2.2.3. Oral glucose tolerance test
Fasted mice (16 h fast) were given 2 g/kg d-glucose in water by oral gavage and blood glucose (mmol/L) was measured at 0, 10, 30, 60, 120 and 180 minutes post glucose load.
2.3. In vivo assessment of microvascular function by intravital microscopy
2.3.1. General animal preparation
Vascular function was assessed by IVM, which allows for real-time functional assessment of the microcirculation in vivo (adapted from Bearden et al. 2004) [17], in male and female mice after 14–16 weeks of high fat or chow feeding in the fed or fasted state. Mice were anesthetized with sodium pentobarbital (initial dose of 60 mg/kg ip with top-up doses given subcutaneously at 15 mg/kg/h) or isoflurane with an induction rate of 2–3% and supplemented as needed with a maintenance rate 0.5–1% to maintain a surgical plane. All chemicals were purchased from Sigma Aldrich unless otherwise noted (Oakville, ON, Canada).
2.3.2. Preparation of gluteus maximus muscle
Once anesthetized, mice were placed under a stereomicroscope. The left gluteus maximus area of the mouse was shaved and cleaned to remove the fur. The gluteus maximus muscle was chosen over the cremaster muscle, as used in other experiments such as Payne et al. (2003) [18], because the cremaster is only found in males and we performed these experiments in both males and females. The skin covering the muscle area was cut away and the proximal edge of the gluteus maximus muscle cut parallel to the spine and reflected away from the mouse ensuring preservation of the vascular supply to the tissue. The muscle was then positioned to lay flat and the proximal edge pinned down on a Sylgard (Dow Corning, Midland, MI, USA) board. Physiological saline solution (PSS) made of bicarbonate (18.0 mM) and basic salt solution (131.9mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2•2H2O, 1.17 mM MgSO4•7H2O) at pH 7.4 was superfused over the muscle tissue to mimic physiological conditions. The solution was kept at a temperature of 37 °C using a heated perfusion reservoir (Radnoti, USA).
2.3.3. Intravital microscopy
The prepared mouse was moved onto the intravital microscope (modified model 20T, Zeiss) and the muscle preparation was allowed to equilibrate under PSS flow. Brightfield illumination (ACH/APL condenser; numerical aperture 0.32) was used to observe arterioles through a Zeiss UD40 objective (numerical aperture 0.41) coupled to a video camera (C2400; Hamamatsu, Japan), with a final magnification on the video monitor (model PVM-132; Sony, Japan) of 950X. The vessel diameter was measured as the distance between luminal edges by using a video caliper (modified model 321; Colorado Video Inc, Boulder, CO, US) with spatial resolution of at least 2 μm. Data were collected at 40 Hz using PowerLab coupled to a computer (AD Instruments, Australia).
2.3.4. Baseline measurements (dose-response curves)
As described previously [17], second order arterioles (one per mouse) were studied as they are ideally positioned for the control of blood flow within the muscle; serial dilutions (10−9 M to 10−5 M) of acetylcholine (ACh) and phenylephrine (PE) were used to verify vasodilatory and vasoconstrictor vasomotor response respectively. Once equilibrated for 45–60 min following surgery, resting diameter was measured. Vascular activity was then recorded while superfusing increasing concentrations of ACh or PE over the tissue. At each concentration, arteriolar diameter was allowed to stabilize for two minutes, after which the diameter was recorded. Following the response to 10−5 M concentration, the tissue was superfused with PSS for 15 min to washout the chemicals, allowing resting diameter to be restored prior to testing additional chemicals. At the conclusion of each experiment, 10−2 M sodium nitroprusside (SNP) was used to measure maximal diameter of the arteriole.
2.4. Data analysis
Body mass (g), blood glucose (mM), insulin tolerance (area under the curve following an insulin tolerance test), and arteriole diameter (μm) are expressed as mean ± standard error of the mean. Analyses were performed using students t-tests or 2-way ANOVAs followed by Bonferroni or Šidák post hoc comparisons using GraphPad Prism 9.0 software (La Jolla CA, USA). Significance was declared if ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3. Results
3.1. Induction of obesity and insulin resistance with 12 weeks of high fat feeding
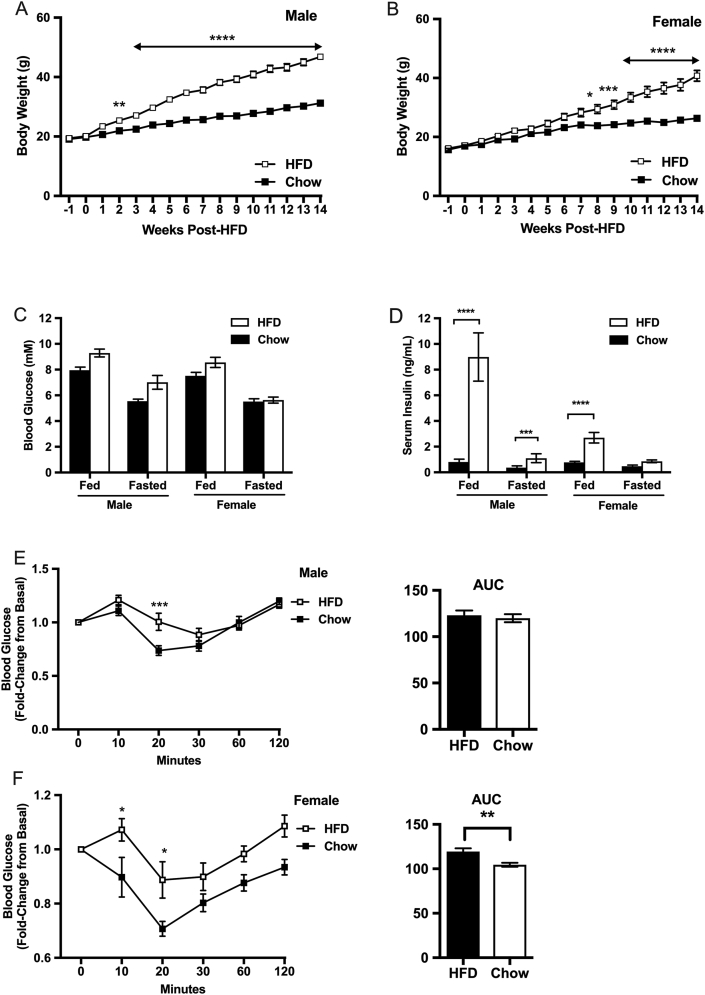
Twelve weeks of high fat feeding induced obesity and insulin resistance (Figure 1); body weight increased significantly in the high fat diet (HFD)-fed mice compared to chow-fed mice in both male and female cohorts (Figure 1A, B). Male and female HFD mice had significantly higher serum insulin levels compared to chow-fed control mice in the fed state for comparable glucose levels. Notably, males had a more severe phenotype than females (Fig. 1C, D). Insulin tolerance tests revealed that both male and female HFD mice were insulin insensitive compared to chow-fed mice (Figure 1E, F). For example, male and female HFD mice had significantly impaired reduction in blood glucose levels 20 min post insulin challenge compared to chow-fed controls (Fig. 1E, F).
Figure 1.
Body weight and carbohydrate metabolism in male and female C57BL6 mice fed chow and high fat diet for 12 weeks (n = 12 per group). Body weight measured over 14 weeks of chow or high fat feeding, in male (A) and female (B) mice. Blood glucose (mmol/L) levels (C) and plasma insulin (ng/mL) (D) measured after 12 weeks of chow or high fat feeding in male and female mice, in the fed (random fed) and fasted (16hr fast) state. Plasma insulin measured in the fed and fasted state (D). Blood glucose (mmol/L) levels expressed over time and as area under the curve (AUC) in response to an insulin tolerance test (ip 1.5U/kg) in male (E) and female (F) mice fed chow or high fat diet for 12 weeks. ∗p < 0.05, ∗∗P < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
3.2. Microvascular function is impaired in male and female obese, insulin resistant mice
3.2.1. Normal (fed) state
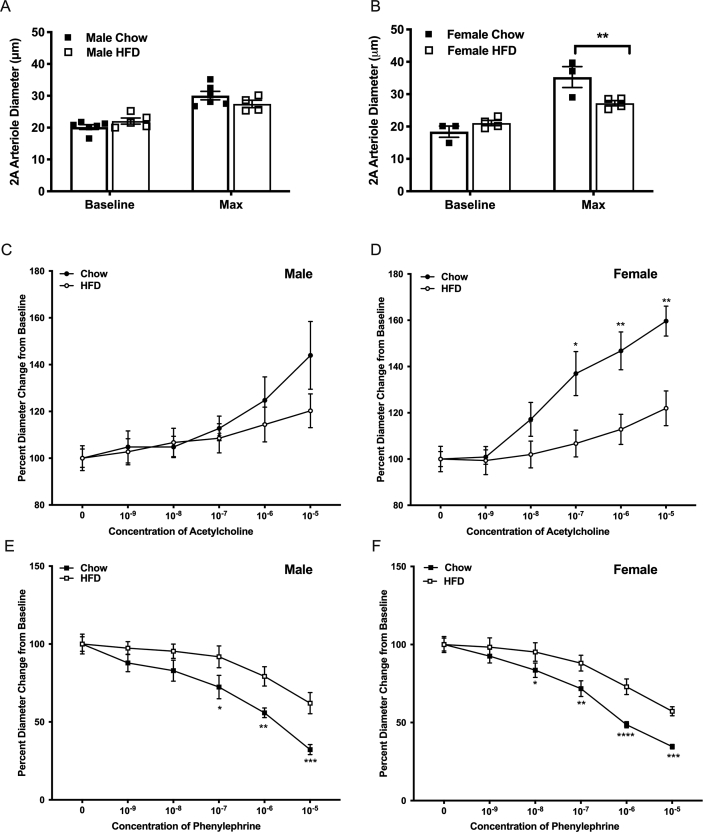
We observed no difference in baseline and maximal vessel diameter of second-order arterioles in male mice fed HFD compared to regular chow (Figure 2A). In contrast however, maximal vessel diameter was significantly smaller in females mice fed HFD compared to regular chow-fed controls (Figure 2B).
Figure 2.
Intravital microscopy conducted on male and female C57BL/6 mice fed a chow (male n = 6, female n = 5) or high-fat (male n = 4, female n = 5) diet for 14–16 weeks. Intravital microscopy was performed in mice who were in a fed state prior to the procedure (cohort 1). Vessel diameter of 2A arteriole after 45-minute equilibrium period (baseline) and at maximal dilation with addition of SNP 10−2 in male (A) and female (B) mice. Acetylcholine (C, D) and phenylephrine (E, F) dose response curves illustrating percent diameter change from baseline arteriole diameter in male (C, E) and female (D, F) mice. ∗p < 0.05, ∗∗P < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Mice fed a HFD exhibited alterations in microvascular function. Male HFD mice exhibited an impairment of the vasoconstrictor response in second order arterioles exposed to phenylephrine (p = 0.0278) with significant differences at concentrations from 10−7 through 10−5 M compared to chow-fed male mice (Figure 2E) but there was no significant differences in vasodilatory response to ACh (p = 0.8604) (Figure 2C), although there was a notably large variation in response to 10−5 M phenylephrine in chow fed males. Interestingly, female HFD mice displayed significant impairments (p < 0.05) of both the vasodilatory and vasoconstrictor responses; when exposed to ACh (2 way ANOVA across all concentrations, p = 0.129, with significant differences at 10−7 through 10−5 M) and PE (2 way ANOVA across all concentrations, p = 0.0080, with significant differences at 10−8 through 10−5 M) HFD-fed females differed compared to chow-fed female mice (Figure 2D, F).
3.3. Fasted state
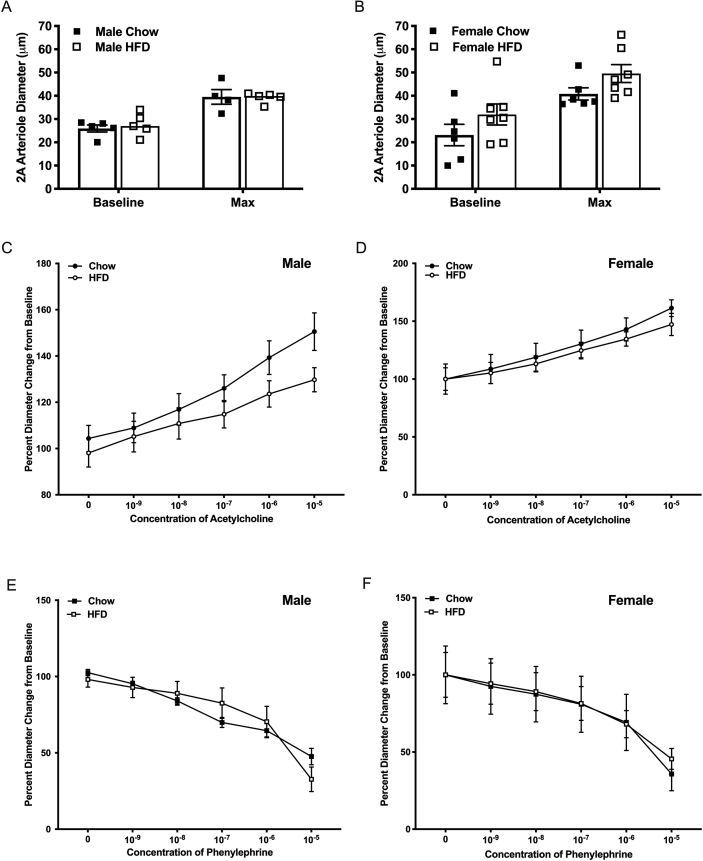
To assess if fasting had any impact on microvascular reactivity, cohorts of fasted male and female mice were assessed identically to the experiments outlined for the non-fasted mice. No differences in either baseline or maximal diameter, regardless of diet consumed, were observed (Figure 3A, B). Additionally, the vasoconstrictor or vasodilatory responses in second order arterioles were unaffected by diet in both male (ACh, p = 0.2481, PE, p = 0.9722) and female fasted mice (ACh, p = 0.6443, PE, p = 0.9165) (Figure 3C-F).
Figure 3.
Intravital microscopy conducted on male and female C57BL6 mice fed a chow (male n = 5, female n = 6) or high fat (male n = 5, female n = 6) diet for 14–16 weeks and that were in a fasted state (15–19 h) prior to the procedure (cohort 2). Vessel diameter of 2A arteriole after 45-minute equilibrium period (baseline) and at maximal dilation with addition of SNP 10−2 in male (A) and female (B) mice. Acetylcholine (C, D) and phenylephrine (E, F) dose response curves illustrating percent diameter change from baseline arteriole diameter in male (C, E) and female (D, F) mice.
4. Discussion
The link between obesity, insulin and adipose signaling, and the microvasculature is at the nexus of understanding adverse vascular events in obese and/or diabetic patients. Using an animal model of metabolic disease, our study adds to our understanding of this link by evaluating differences in female and male microvascular function. Specifically, using a rodent model of diet-induced obesity and insulin resistance, we demonstrated altered microvascular reactivity in both male and female C57BL/6 mice fed a high-fat diet compared to chow-fed control mice. The effects of diet-induced obesity and insulin resistance on microvascular reactivity were more substantial in obese female mice compared to obese male mice, thus adding vital insights into sex differences in microvascular function in the context of metabolic disease. While further study of the molecular mechanisms driving this difference is needed, this is an important novel finding which draws attention to such sex differences. While there are a number of potential pathways towards impaired insulin sensitivity and subsequent dysregulation of glucose and lipid metabolism in diabetes, it is notable that the inflammatory and endocrine decompensation that develop in obesity is thought to contribute to microvascular dysfunction, particularly at the level of the endothelium [19].
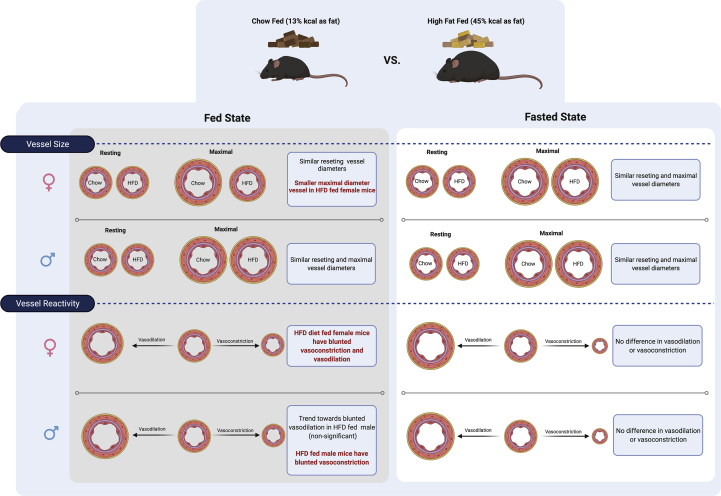
After 12 weeks of high fat feeding both male and female animals were both obese (Figure 1A, B) and insulin resistant, as determined by a significantly dampened response to an insulin tolerance test (Figure 1E, F) and significantly elevated circulating insulin levels in the fed state in females and in the fed and fasted state in males (Figure 1D), thus representing an obese, insulin resistant, pre-diabetic state. Obese male mice had no defined changes in either the basal or maximal vessel diameter (Figure 2A), however, they did exhibit an impaired vasodilatory response to acetylcholine with a trend of blunting beginning starting at 10−7 M and continuing through 10−5 M (Figure 2C). Although this did not reach statistical significance, this may be due to the large standard deviation at 10−5 M. These results suggest potential impairment in muscarinic receptor signaling [20] which could be due to a loss of the number of receptors or uncoupling of the signaling mechanisms, which need to be determined by future studies. In obese female mice, no differences were observed in basal vessel diameter while stimulation of maximal vessel diameter with sodium nitroprusside, a nitric oxide donor, was significantly impaired in obese compared to lean mice (Figure 2B). Significantly impaired vasodilation was also observed in obese female mice in an Acetylcholine dose-dependent manner (Figure 2D). An additional measure of vascular reactivity, vasoconstriction, was assessed by measuring changes in 2A arteriole diameter in response to PE. This revealed significantly reduced vasoconstriction in obese mice compared to lean controls in a dose-dependent manner in both sexes (Figure 2E, F). Taken together these results show an overall dampening of vasoreactivity in 2A arterioles of obese mice compared to lean controls and highlight a substantial effect of obesity and insulin resistance facilitating vascular dysfunction in female mice. We have summarized our findings in terms of vessel size and vasoconstriction and dilation capacity in Figure 4. Previous work by Sivitz et al. (2007) using multivariate analysis demonstrated sex and obesity are associated with changes in ACh and SNP responses [21]. These data provide insight into cardiac disease in women who are much more likely to have coronary microvascular disease associated with obesity [22]. This is evidenced in our results by the pronounced differences in vasoreactivity in female vs male mice (Figure 2C, D). Since the majority of studies assessing metabolic complications of obesity and vascular reactivity in rodent models are performed in male mice, these results highlight important differences in male and female physiology that warrant further investigations with respect to the underlying mechanism.
Figure 4.
Summary of findings for A2 arteriole size and vasodilation and vasoconstriction response in female and males mice, fed high fat diet or chow diet in a fed or fasted state. This figure was created using Biorender.com.
Previous work using intravital microscopy (IVM) to assess vessel function in metabolic disease but has not addressed the integral component of sex differences. For example, Costa et al. (2011) performed IVM experiments on a lesser used model, the male Syrian golden hamster where vasodilatory responses of peripheral vasculature within the cheek pouch or the male-specific cremaster muscle were assessed [23]. Similar to our results they showed diminished vasodilation in pre-diabetic mice in the fed state. Given the interesting sex specific changes we have observed, the advantage of our model is the assessment of the microcirculation in the gluteus maximus muscle, a functional muscle in both males and females.
Perhaps the most significant findings of our paper are the more substantial effects of obesity and insulin resistance on vascular reactivity of obese female mice compared to obese male mice. It is well established that laboratory mice exhibit sexually dimorphic characteristics in fat mass gain and fat distribution (a phenomenon also seen in humans [24]) and the metabolic complications of obesity with respect to glucose and lipid metabolism tend to be more significant in male vs female mice [25, 26]. The anatomical distribution of WAT is different in mice than humans, however, adipose tissue depots affect metabolism similarly [8]. In humans, females have more subcutaneous fat in the gluteal-femoral and peripheral regions, whereas males have more visceral fat in the abdominal region [24, 27]. In experimental mice fed both standard and high fat diets, male mice have significantly more visceral than subcutaneous fat, while in females visceral and subcutaneous depots expand in a similar way [24], thus contributing to the more significant effect on metabolic decompensation. High fat diet feeding results in males gaining more fat mass than their female counterparts [24], as we observed (Figure 1A, B). It is known that adipogenesis and mature adipocyte function differs between the sexes due to influences from sex hormones; Estrogen has been shown to play a protective factor in females, helping to prevent fat accumulation, improve insulin action, as well as reducing inflammatory signaling [24]. Androgens however, negatively affect metabolic health in females, with high levels leading to increased risk of cardiovascular and metabolic diseases [24]. In contrast, in males, androgens improve adipogenesis by heightening insulin sensitivity and reducing the rate of insulin resistance [24].
Therefore, this study suggests the need to assess the progression of microvascular dysfunction early in the development of obesity and metabolic disease as this may identify novel therapeutic targets for the treatment or prevention of metabolic co-morbidities.
5. Limitations
This initial study highlights an evaluation changes in the microcirculation of skeletal muscle in both male and female mice in response to high fat diet. The sex component is extremely relevant given differences in male and female mice in the development of diabetes. We recognize there is future evaluation needed in repetition of these experiments by other groups and expansion beyond the initial study including potential remodeling changes of vasculature, hormonal changes across the sexes and extensive analyses of inflammatory changes in adipose tissue linked to microcirculation. This will be explored in subsequent experiments beyond this initial assessment.
Declarations
Author contribution statement
Danielle A. Sidsworth: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Stephanie L. Sellers: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jennifer P. Reutens-Hernandez: Performed the experiments.
Elizabeth A. Dunn: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sarah L. Gray: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Geoffrey W. Payne: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Sarah L. Gray was supported by operating funding from a Canada Research Chair in Integrative Physiology of Diabetes. Geoffrey W. Payne was supported by University of Northern British Columbia (seed grant). Stephanie L. Sellers was supported by fellowships from the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the staff of the NHSRF Animal Care Facility at UNBC, Sianne Vautour, Natalie DeBruyn and Lydia Troc for their dedication to caring for the mice used in this study.
References
- 1.Ortega F.B., Lavie C.J., Blair S.N. Obesity and cardiovascular disease. Circ. Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 2.Scherer P.E., Hill J.A. Obesity, diabetes, and cardiovascular diseases: a compendium. Circ. Res. 2016;118(11):1703–1705. doi: 10.1161/CIRCRESAHA.116.308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman J.A., Creager M.A. Vascular complications of diabetes. Circ. Res. 2016;118(11):1771–1785. doi: 10.1161/CIRCRESAHA.115.306884. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj N.S., Osborne M.T., Gupta A., Tavakkoli A., Bravo P.E., Vita T., Bibbo C.F., Hainer J., Dorbala S., Blankstein R., Bhatt D.L., Di Carli M.F., Taqueti V.R. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J. Am. Coll. Cardiol. 2018;72(7):707–717. doi: 10.1016/j.jacc.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson S.C., Wolk A., Hakansson N., Back M. Overall and abdominal obesity and incident aortic valve stenosis: two prospective cohort studies. Eur. Heart J. 2017;38(28):2192–2197. doi: 10.1093/eurheartj/ehx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logue J., Murray H.M., Welsh P., Shepherd J., Packard C., Macfarlane P., Cobbe S., Ford I., Sattar N. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart. 2011;97(7):564–568. doi: 10.1136/hrt.2010.211201. [DOI] [PubMed] [Google Scholar]
- 7.Koliaki C., Liatis S., Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. doi: 10.1016/j.metabol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery E., Wing A., Holtrup B., Sebo Z., Kaplan J.L., Saavedra-Pena R., Church C.D., Colman L., Berry R., Rodeheffer M.S. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metabol. 2016;24(1):142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 10.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuster J.J., Ouchi N., Gokce N., Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016;118(11):1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L., Fu Z., Wu J., Aylor K.W., Barrett E.J., Cao W., Liu Z. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin. Sci. (Lond.) 2015;129(12):1025–1036. doi: 10.1042/CS20150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Candela J., Wang R., White C. Microvascular endothelial dysfunction in obesity is driven by macrophage-dependent hydrogen sulfide depletion. Arterioscler. Thromb. Vasc. Biol. 2017;37(5):889–899. doi: 10.1161/ATVBAHA.117.309138. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento A.R., Machado M., de Jesus N., Gomes F., Lessa M.A., Bonomo I.T., Tibirica E. Structural and functional microvascular alterations in a rat model of metabolic syndrome induced by a high-fat diet. Obesity. 2013;21(10):2046–2054. doi: 10.1002/oby.20358. [DOI] [PubMed] [Google Scholar]
- 15.Shaligram S., Sanguesa G., Akther F., Alegret M., Laguna J.C., Rahimian R. Differential effects of high consumption of fructose or glucose on mesenteric arterial function in female rats. J. Nutr. Biochem. 2018;57:136–144. doi: 10.1016/j.jnutbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halldorsdottir S., Carmody J., Boozer C.N., Leduc C.A., Leibel R.L. Reproducibility and accuracy of body composition assessments in mice by dual energy x-ray absorptiometry and time domain nuclear magnetic resonance. Int. J. Body Compos. Res. 2009;7(4):147–154. [PMC free article] [PubMed] [Google Scholar]
- 17.Bearden S.E., Payne G.W., Chisty A., Segal S.S. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J. Physiol. 2004;561(Pt 2):535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne G.W., Madri J.A., Sessa W.C., Segal S.S. Abolition of arteriolar dilation but not constriction to histamine in cremaster muscle of eNOS-/- mice. Am. J. Physiol. Heart Circ. Physiol. 2003;285(2):H493–H498. doi: 10.1152/ajpheart.00071.2003. [DOI] [PubMed] [Google Scholar]
- 19.El Husseny M.W., Mamdouh M., Shaban S., Ibrahim Abushouk A., Zaki M.M., Ahmed O.M., Abdel-Daim M.M. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J. Diabetes Res. 2017;2017:8095926. doi: 10.1155/2017/8095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagher P., Segal S.S. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol. 2011;202(3):271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivitz W.I., Wayson S.M., Bayless M.L., Sinkey C.A., Haynes W.G. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J. Diabet. Complicat. 2007;21(3):149–157. doi: 10.1016/j.jdiacomp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Bagi Z., Broskova Z., Feher A. Obesity and coronary microvascular disease - implications for adipose tissue-mediated remote inflammatory response. Curr. Vasc. Pharmacol. 2014;12(3):453–461. doi: 10.2174/1570161112666140423221843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa R.R., Villela N.R., Souza M., Boa B.C., Cyrino F.Z., Silva S.V., Lisboa P.C., Moura E.G., Barja-Fidalgo T.C., Bouskela E. High fat diet induces central obesity, insulin resistance and microvascular dysfunction in hamsters. Microvasc. Res. 2011;82(3):416–422. doi: 10.1016/j.mvr.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald S.J., Janorkar A.V., Barnes A., Maranon R.O. A new approach to study the sex differences in adipose tissue. J. Biomed. Sci. 2018;25(1):89. doi: 10.1186/s12929-018-0488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link J.C., Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu. Rev. Nutr. 2017;37:225–245. doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma G., Prossnitz E.R. G-Protein-Coupled estrogen receptor (GPER) and sex-specific metabolic homeostasis. Adv. Exp. Med. Biol. 2017;1043:427–453. doi: 10.1007/978-3-319-70178-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloor I.D., Symonds M.E. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm. Behav. 2014;66(1):95–103. doi: 10.1016/j.yhbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.