Abstract
K-ras mutant lung adenocarcinoma (LUAD) is the most common type of lung cancer, displays abysmal prognosis and is tightly linked to tumor-promoting inflammation, which is increasingly recognized as a target for therapeutic intervention. We have recently shown a gender-specific role for epithelial Stat3 signaling in the pathogenesis of K-ras mutant LUAD. The absence of epithelial Stat3 in male K-ras mutant mice (LR/Stat3Δ/Δ mice) promoted tumorigenesis and induced a nuclear factor-kappaB (NF-κB)-driven pro-tumor immune response while reducing tumorigenesis and enhancing anti-tumor immunity in female counterparts. In the present study, we manipulated estrogen and NF-κB signaling to study the mechanisms underlying this intriguing gender-disparity. In LR/Stat3Δ/Δ females, estrogen deprivation by bilateral oophorectomy resulted in higher tumor burden, an induction of NF-κB-driven immunosuppressive response, and reduced anti-tumor cytotoxicity, whereas estrogen replacement reversed these changes. On the other hand, exogenous estrogen in males successfully inhibited tumorigenesis, attenuated NF-κB-driven immunosuppression and boosted anti-tumor immunity. Mechanistically, genetic targeting of epithelial NF-κB activity resulted in reduced tumorigenesis and enhanced the anti-tumor immune response in LR/Stat3Δ/Δ males, but not females. Our data suggest that estrogen exerts a context-specific anti-tumor effect through inhibiting NF-κB-driven tumor-promoting inflammation and provide insights into developing novel personalized therapeutic strategies for K-ras mutant LUAD.
Through lost- and gain-of-function studies, our data suggest that estrogen exerts a context-specific anti-tumor effect through inhibiting NF-κB-driven tumor-promoting inflammation and provide insights into developing novel personalized therapeutic strategies for K-ras mutant LUAD.
Introduction
Lung cancer is the second most common cancer in both men and women and the most common cause of cancer death worldwide (1). Non-small cell lung cancer accounts for 80% of lung cancer cases, and lung adenocarcinoma (LUAD) is the most common histological subtype of non-small cell lung cancer (2). About 30% of LUAD patients harbor activating mutations of K-ras, which are more common in lifetime smokers (3,4). K-ras mutant LUAD is resistant to most forms of systemic and targeted therapeutic strategies and displays abysmal outcome (4), clearly indicating the urgent need for alternative strategies that target the downstream effectors or cooperative pathways of K-ras.
Tumor-promoting inflammation is a major hallmark of cancer (5,6). Our group and others have found that K-ras mutant LUAD is associated with airway inflammation and release of cytokines that reprogram the tumor microenvironment (TME) and promote carcinogenesis and tumor progression (7–13). In our previous studies using a lung epithelial-specific K-ras mutant LUAD mouse model mimicking the K-ras mutation and lung cancer development and progression found in K-ras mutant LUAD patients, we found that K-ras mutations activate nuclear factor-kappaB (NF-κB) pathway, thus increasing interleukin (IL)-6 that signal through Stat3 pathway to generate a pro-tumor immune response characterized by increased infiltration of tumor-associated macrophages, neutrophils and myeloid-derived suppressor cells (MDSCs) (7). Targeting IL-6 resulted in reduced tumorigenesis and Stat3 activation, along with reduced tumor-associated macrophage and MDSC infiltration and increased cytotoxic T-cell response (7,14). These findings indicate that inflammation is a vulnerability factor for K-ras mutant LUAD and suggest therapeutically targeting the TME may combat this currently ‘undruggable’ disease.
To further study the role of IL-6/Stat3 pathway in K-ras mutant LUAD, we generated lung epithelial-specific K-ras mutant and Stat3 knockout (LR/Stat3Δ/Δ) mice and observed an intriguing disparity regarding tumor burden and lung TME between female and male mice (15). We specifically found that deletion of Stat3 in K-ras mutant lung epithelia significantly reduced tumor burden in female mice, which was associated with an enhanced anti-tumor immune response, but surprisingly caused a dramatic enhancement of tumorigenesis and induction of an NF-κB-driven pro-tumor immune response characterized by increased IL-6, CXCL1 and neutrophil infiltration in male mice (15). We also found a significant positive correlation between pSTAT3 and estrogen receptor beta (ERβ) expression in female but not in male patients with LUAD.
Our findings reveal a sex-specific role for the IL-6/Stat3 pathway in K-ras mutant LUAD suggesting that lung cancer cells have different immune and molecular mechanisms for survival in males and females. Lung cancer risk and outcome for men and women smokers are distinct (16). It seems that women have an increased risk of lung cancer for a given level of smoke exposure (16–19). However, their survival, prognosis and response to treatment remain better than men (20–25). Studies of human lung cancer tissues suggest different immune responses and signatures between sexes that could be related to different clinical outcomes (26). Despite data illustrating profound differences between the sexes in immune function, mechanisms of sex differences in the immunopathogenesis of lung cancer are vastly overlooked. Accordingly, we studied the inflammatory mechanisms mediating/affecting K-ras induced lung tumorigenesis in a sex-specific manner, especially the dynamic interplay between immunomodulatory pathways (Stat3/NF-κB) and sex-specific molecular signals (estrogen/ER signaling) in the tumor and how this relationship impinges on the development and promotion of K-ras mutant lung cancer. In our previous study (15), we observed elevated DNA binding activity of NF-κB in LR/Stat3Δ/Δ male mice but not females, suggesting a pro-tumorigenic role for NF-κB activity in male LR/Stat3Δ/Δ mice. However, these effects were suppressed in female LR/Stat3Δ/Δ mice. Estrogen has been reported to inhibit NF-κB activity in various ways, including increasing IκBα levels (27) and inhibiting DNA binding activity (28–30); thus, we hypothesized that estrogen might be the protective factor from K-ras mutant lung tumorigenesis in the absence of epithelial Stat3 signaling by suppressing NF-κB activity and its roles in increasing IL-6, CXCL1 and subsequent neutrophil recruitment.
To test our hypothesis, we performed loss- and gain-of-function studies to manipulate estrogen as well as NF-κB signaling in both female and male LR/Stat3Δ/Δ mice to study modulating roles of estrogen in K-ras mutant lung tumorigenesis and its interplay with Stat3/NF-κB-mediated cytokine network.
Materials and methods
Animal housing and experiments
CCSPCre/LSL-K-rasG12D (CC-LR) and LR/Stat3Δ/Δ mice were generated as we previously described (15). Briefly, by crossing mice harboring LSL-K-rasG12D allele with mice containing Cre recombinase inserted into the Club Cell Specific Protein locus, we generated CC-LR mice, which has K-ras mutant tumor initiation specifically in lung epithelium. Then CC-LR mice were crossed with Stat3fl/fl mice to generate LR/Stat3Δ/Δ mice, which have Stat3-deficient K-ras mutant lung cancer. LR/Stat3Δ/Δ/IKKβ Δ/Δ mice, with loss of lung epithelial Stat3 and NF-κB activity, were generated by crossing LR/Stat3Δ/Δ mice with IKKβ fl/fl mice (31) kindly provided by Dr Michael Karin (University of California, San Diego, CA). All mice were housed under specific pathogen-free conditions and handled in accordance with the institutional animal care and use committee of the University of Texas MD Anderson Cancer Center. Mice were monitored daily for evidence of disease or death.
Oophorectomy and estrogen pellet implantation
As shown in Supplementary Figure 1 (available at Carcinogenesis online), 6-week-old CC-LR and LR/Stat3Δ/Δ female mice were anesthetized using isoflurane, and bilateral ovaries were resected through a midline incision in the abdomen. Six-week-old CC-LR, as well as male and oophorectomized female LR/Stat3Δ/Δ mice, were implanted with estrogen pellet (17-β estradiol, 0.18 mg/pellet, Innovative Research of America, Sarasota, FL) under the back skin. All mice were monitored daily after surgery and were euthanized at 14 weeks of age. Five mice in each group were randomly chosen to measure estrogen levels in plasma samples using Estradiol ELISA kit (Item No. 582251, Cayman Chemical, Ann Arbor, MI).
Estrogen receptor blockade
Six-week old CC-LR and LR/Stat3Δ/Δ female mice (n = 6) were injected intraperitoneally with 3 mg fulvestrant (FASLODEX, AstraZeneca) for 2 days per week until 14 weeks.
NF-κB inhibition
Six-week-old CC-LR and LR/Stat3Δ/Δ male mice were injected intraperitoneally with 25 mg/kg dosage of IKKβ inhibitor IMD-0354 (MedChemExpress, Monmouth Junction, NJ) daily for 8 weeks (n = 6). IMD-0354 was resuspended in 1% carboxymethylcellulose sodium (Sigma, St. Louis, MO).
Assessment of lung tumor burden and inflammation
Fourteen-week-old mice were injected with Avertin (0.8 ml, 0.25 mg/ml) (Sigma), and their tracheas were cannulated and sutured into place. Surface tumor numbers were counted if visible. Subsequently, the lungs were perfused with phosphate-buffered saline through the right ventricle. For half of the mice, lungs were inflated with 10% buffered formalin (Sigma) for 10 min and collected for histological studies. Tumor/lung area percentages were assessed as we had previously done (15). For the other half of the mice, bronchoalveolar lavage fluid (BALF) was collected by sequentially instilling and collecting two aliquots of phosphate-buffered saline through a tracheostomy cannula. Lungs were snap-frozen and stored for RNA analysis. Total WBC count in BALFs was determined using a hematocytometer, and differential cell populations were determined by cytocentrifugation of BALFs and subsequent Wright–Giemsa (Sigma) staining. WBCs in BALFs were pelleted by centrifuging and stored in TRIzol (Invitrogen, Carlsbad, CA) for future RNA analysis.
Histochemistry/immunostaining
Hematoxylin and eosin, and immunohistochemical staining for proliferation marker Ki-67 (1:200; ab16667; Abcam, MA) and angiogenesis marker ERG (1:200; ab92513; Abcam) were done as described previously (7). The percentages of labeled positive cells were analyzed using Nuclear v9 in ImageScope 12.3.3 (Leica, Nussloch, Germany) as fractions of positive cells in total tumor cells per 20× field.
Quantitative RT-PCR analysis
Total RNA from the whole lung was extracted using E.Z.N.A total RNA kit (Omega, GA). RNA from WBCs in BALF was extracted using Direct-zol RNA Microprep kit (Zymo Research, Irvine, CA). Reverse transcription PCR was performed using the qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). qPCR was performed using SYBR Green FastMix (Quanta Bioscience) on CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Data are presented as fold changes between test groups and controls as indicated in figure legends. Primers are listed in Supplementary Table S5 (available at Carcinogenesis online).
Cytokines/chemokine ELISA measurement
Total lung protein from the whole lung tissue was extracted with Cell Lysis Buffer 2 (R&D System, Minneapolis, MN). Two hundred micrograms of protein was used for each sample. Mouse Magnetic Luminex Assay (R&D System) for CXCL1 and IL-6 was used, and the whole procedure was performed according to instructions of the manufacturer. Standard curves were generated using a five-parameter logistic curve fitting equation (StarStation V 2.0; Applied Cytometry Systems). Each sample reading was interpolated from the appropriate standard curve. Medium Fluorescent Intensity was measured using Luminex MAGPIX (R&D System).
Western blot
Lung tissues were homogenized, and then cytoplasmic and nuclear proteins were isolated using NE-PER™ Nuclear and Cytoplasmic Extraction Reagent (Thermo Scientific, USA). Cytoplasmic protein concentrations were determined using the BCA assay (Thermo Scientific, USA). Western blot for IκBα (1:1000, ab32518-100, Abcam) and β-actin (1:1000, 4970S, Cell Signaling Technology) was performed as previously described (10).
Measurement of NF-κB activity in lung tissues
Lung tissues were homogenized, then cytoplasmic and nuclear proteins were isolated using NE-PER™ Nuclear and Cytoplasmic Extraction Reagent (Thermo Scientific, USA). Nuclear protein concentrations were determined using the BCA assay (Thermo Scientific, USA). NF-κB (p65) binding activity was determined using NF-κB (p65) Transcription Factor Assay Kit (Cayman Chem, Ann Arbor, MI) according to the manufacturer’s instructions with 20 μg of nuclear extract in duplicates. Optic density (OD) was determined with a microplate reader set to 450 nm and represented the activity of p65 binding ability. Fold change of p65 binding activity is determined by normalizing the OD value of each group to control.
Human studies
ESR2A and ESR2B (both encode ERβ), CD3, CD8 and CD4 expression levels were determined by array analysis (Illumina v3) of surgically resected lung adenocarcinomas from 150 patients who did not receive neoadjuvant therapy. This cohort was obtained from the Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT) study, developed in 2006 at M.D. Anderson Cancer Center (32), with known clinical characteristics as we previously described (7). Expression values were log (base 2) transformed. Correlations between genes, separately in male and female LUAD, were statistically evaluated using Pearson’s correlation coefficients and summarized plotted as correlation matrices. All analysis was performed in the R statistical language and environment (R-project.org; version 3.5.1).
Statistics
Data are presented as mean ± SEM. SPSS Statistics 21 (IBM, Armonk, NY) was used for statistical analysis, and Student’s t-test was used for comparison between two groups. Differences were considered significant for P < 0.05.
Study approval
All animal experiments were performed in accordance with guidelines approved by the University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee.
Results
Oophorectomy in female LR/Stat3Δ/Δ mice increased tumor burden, and estrogen replacement rescued this effect
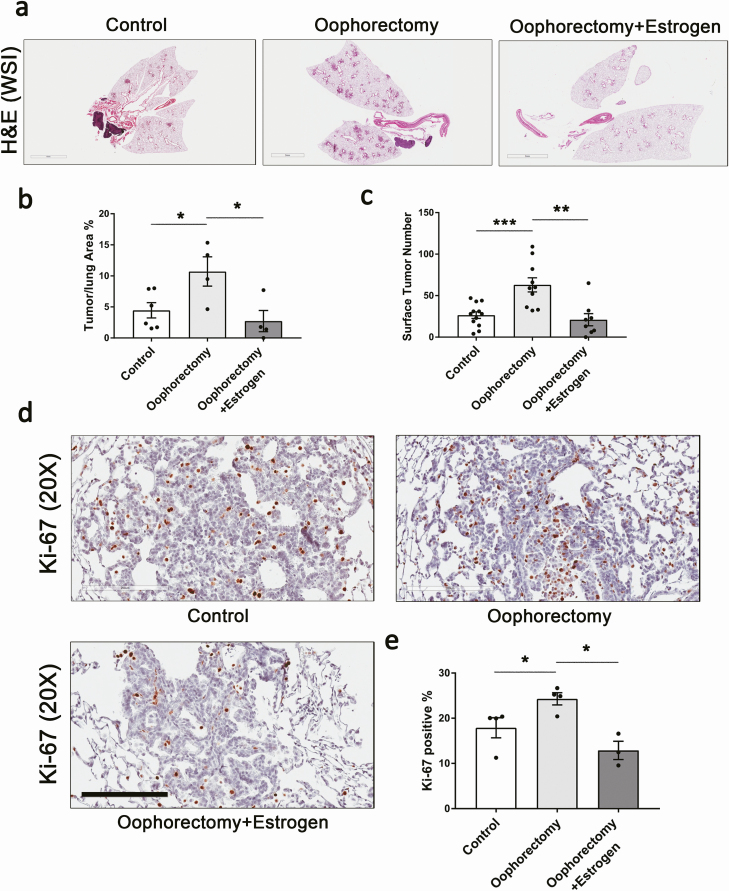
To dissect the mechanism of Stat3-dependent sex disparity in the pathogenesis of K-ras mutant lung cancer, we performed loss- and gain-of-function studies to manipulate estrogen signaling. Female LR/Stat3Δ/Δ mice underwent either oophorectomy or oophorectomy plus estrogen pellet implantation (Supplementary Table S1, available at Carcinogenesis online). Depletion of estrogen by bilateral oophorectomy significantly increased tumor burden in female LR/Stat3Δ/Δ mice evidenced by increased lung surface tumor numbers of 2.4-fold and increased tumor/lung area percentage of 2.4-fold (Figure 1a–c). There were increased proliferative lesions in oophorectomized females as presented by higher Ki-67-positive staining (Figure 1d and e). However, the angiogenesis marker ERG did not change significantly among the groups, and oophorectomized mice even had slightly lower levels of ERG staining (Supplementary Figure S2, available at Carcinogenesis online). This might be due to the known angiogenic effect of estrogen. Furthermore, when oophorectomized LR/Stat3Δ/Δ female mice were implanted with estrogen pellets, the phenotype was reversed, as presented by reduced surface tumor numbers to 33.3% and tumor/lung area percentage to 25% of oophorectomized group (Figure 1a–c). Ki-67-positive cells were reduced by estrogen replacement (Figure 1d and e), but ERG expression did not show a significant difference (Supplementary Figure S2, available at Carcinogenesis online). Then we blocked ERs in 6-week-old LR/Stat3Δ/Δ female mice using a selective ER antagonist, fulvestrant (3 mg/day), 2 days per week for a total of 8 weeks. Treatment with fulvestrant resulted in increased tumor/lung area percentage by 2.5-fold (Supplementary Figure S3a and b, available at Carcinogenesis online), increased tumor proliferation rate (Supplementary Figure S3c and d, available at Carcinogenesis online) and increased angiogenesis (Supplementary Figure S3e and f, available at Carcinogenesis online). To check whether estrogen has the same effect on K-ras mutant lung tumorigenesis in mice with intact epithelial Stat3, we performed oophorectomy or oophorectomy plus estrogen pellet implantation in female CC-LR mice (Supplementary Table S2, available at Carcinogenesis online). However, oophorectomy and estrogen replacement in CC-LR females did not change surface tumor number nor tumor/lung area significantly, although there was slightly increased tumor burden with oophorectomy, and this trend was reversed by estrogen supplement (Supplementary Figure S4, available at Carcinogenesis online). These data suggest that estrogen protects against tumorigenesis in LR/Stat3Δ/Δ female mice, as blockade of ER signaling changed the tumorigenesis phenotype of LR/Stat3Δ/Δ female to resemble the increased tumorigenesis observed in LR/Stat3Δ/Δ males.
Figure 1.
Oophorectomy in female LR/Stat3Δ/Δ mice increased tumor burden, and estrogen replacement rescued this effect. (a) Representative photomicrographs of the whole slide images of lung gematoxylin and eosin (H&E) stained sections (scale bar = 3 mm) and (b) Tumor/lung area percentage (n = 4–6). (c) Lung surface tumor number (n = 8–12). (d) Representative photomicrographs of Ki-67 stained sections (n = 3–4) (scale bar = 200 μm), and (e) proliferative rate presented as the percentage of Ki-67-positive cells. Data represent mean ± SEM; ***P < 0.001, **P < 0.01, *P < 0.05, experimental replicate #3.
Oophorectomy in LR/Stat3Δ/Δ female mice reprogrammed the TME to a pro-tumor phenotype, and estrogen replacement reversed these changes
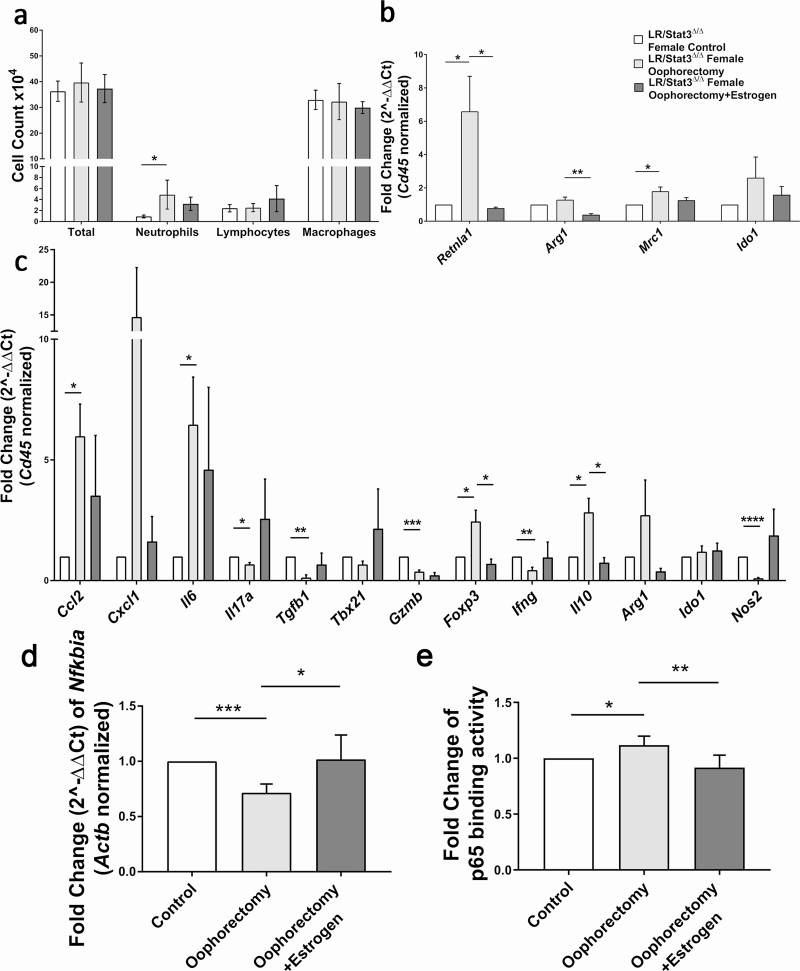
Our previous study showed that the TME in LR/Stat3Δ/Δ females was marked by enhanced anti-tumor and attenuated immunosuppressive characteristics (15). In the present study, we sought to determine whether oophorectomy could reprogram the TME in LR/Stat3Δ/Δ females. First, we looked at immune cell composition in BALF. Oophorectomy did not change the total WBC, however, the neutrophil count was significantly increased in oophorectomized mice (Figure 2a). Estrogen replacement reduced BALF neutrophils slightly in oophorectomized mice (Figure 2a). We next looked at the expression level of M1 and M2 macrophage-associated genes in BALF using quantitative real-time PCR. After normalizing to CD45, a pan-hematopoietic marker, we found oophorectomy increased M2 macrophage markers, including resistin-like alpha (Retnla1) and mannose receptor 1 (Mrc1), and slightly increased 2,3-dioxygenase (Ido1) (Figure 2b). Estrogen replacement led to reduced Retnla1 and arginase 1 (Arg1) compared with oophorectomized mice (Figure 2b). These data indicate that estrogen deprivation can lead to an immunosuppressive phenotype with pro-tumor function in TME. Although BALF immune cell profile does not completely represent the lung immune profile, however, it well reflects the quantitative and phenotypic changes in lung immune profile respective to tumor development and promotion or in response to a specific intervention. To further support our findings in BALF, we next measured the levels of pro-inflammatory genes in the whole lung. We found increased expression of interleukin 6 (Il6), chemoattractant chemokine ligand 2 (Ccl2) and C-X-C motif ligand 1 (Cxcl1) in oophorectomized mice, which most likely explains the significant increase in neutrophils (Figures 2a and c). Estrogen replacement appeared to suppress the expression of these genes, although this did not reach a statistical significance (Figure 2c). The changes in IL-6 and CXCL1 protein expression followed the same pattern as their mRNA expression (Supplementary Figure S5a and c, available at Carcinogenesis online). Oophorectomy also significantly increased expression of forkhead box P3 (Foxp3) and interleukin 10 (Il10), which indicated enhanced T regulatory (Treg) cell differentiation and activity, and their expression significantly decreased with estrogen replacement (Figure 2c). We also observed reduced expression of interleukin 17 (Il17a) and transforming growth factor beta (Tgfb1), which indicates Treg and T helper 17 cell (Th17) function, but we postulate that the effect of their reduction was surpassed by increased Foxp3 and Il10. Moreover, granzyme B (Gzmb) and interferon gamma (Ifng) were significantly decreased with oophorectomy (Figure 2c), indicating attenuated anti-tumor cytotoxic activity. Oophorectomy also significantly decreased the expression of nitric oxide synthase 2 (Nos2) (Figure 2c), an M1 macrophage marker, which corresponds well with the M1 to M2 polarization we observed in BALF cells. The elevated expression of Ccl2, Il6, Cxcl1 and increased neutrophil infiltration in oophorectomized females were very similar to the phenotype of LR/Stat3Δ/Δ male mice, which had increased NF-κB activity according to our previous studies (15), so we evaluated NF-κB activity in oophorectomized LR/Stat3Δ/Δ females. qPCR of the whole lung showed a decreased level of IκBα, an NF-κB inhibitor, in oophorectomized mice, and its expression was restored with estrogen replacement (Figure 2d). We then measured the DNA binding ability of p65 in nuclear extract of the whole lung from oophorectomized mice which showed stronger binding activity than the control group, and estrogen replacement reversed this change (Figure 2e). These data showed that blockade of estrogen signaling unleashed NF-κB activation in LR/Stat3Δ/Δ female mice and reprogrammed the TME into a pro-tumor inflammatory phenotype, which was reversed by estrogen replacement.
Figure 2.
Oophorectomy in LR/Stat3Δ/Δ female mice reprogrammed lung tumor microenvironment to a pro-tumor phenotype, and estrogen replacement reversed these changes. (a) Total inflammatory cell and lineage-specific leukocyte numbers from BALF (n = 5–9). (b) Relative mRNA expression of Retnla1, Arg1, Mrc1 and Ido1 mRNA in BALF inflammatory cells, normalized by Cd45 expression (n = 4). (c) Relative mRNA expression of Ccl2, Cxcl1, Il6, Il17a, Tgfb1, Tbx21, Gzmb, Foxp3, Ifng, Il10, Arg1, Ido1 and Nos2 in the whole lung, normalized by Cd45 expression. (d) Relative mRNA expression of IκBα in the whole lung normalized to β-actin. (e) Fold change of p65 binding activity represented by fold change of OD value. Data represent mean ± SEM; ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, experimental replicate #3.
Estrogen treatment in male LR/Stat3Δ/Δ mice reduced tumor burden
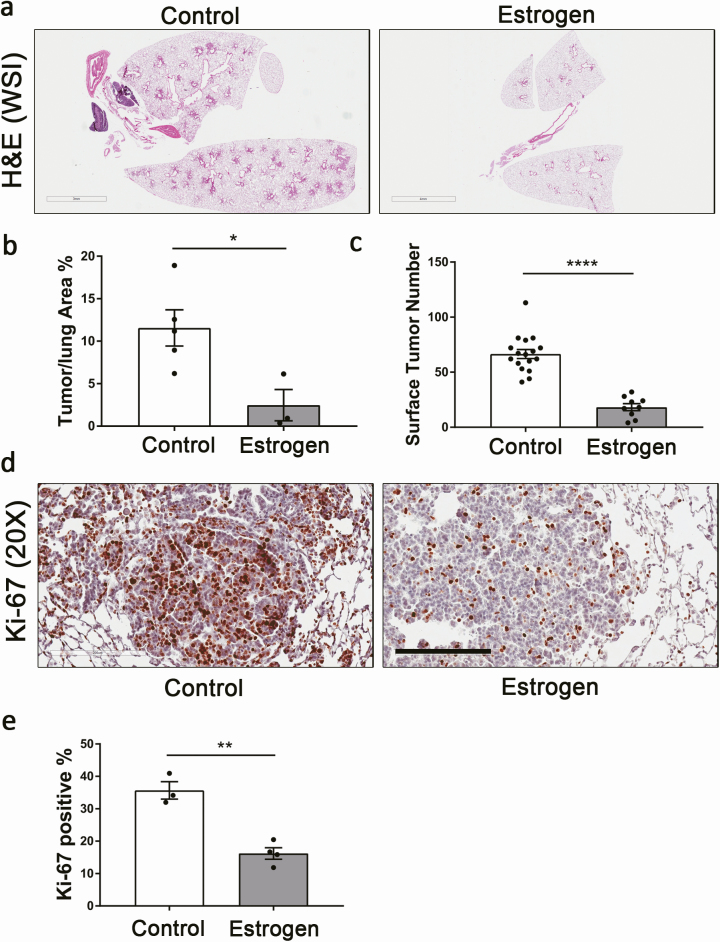
We further studied the effect of estrogen in rescuing the tumorigenic phenotype of male LR/Stat3Δ/Δ mice, and we found that 8 weeks of estrogen replacement therapy (Supplementary Table S3 and Supplementary Figure S1, available at Carcinogenesis online) reduced the lung surface tumor count to 27% and tumor/lung area percentage to 20.8% of the control group (Figure 3a–c). Tumor proliferation was inhibited as Ki-67 immunoreactivity was reduced compared with the control group (Figure 3d and e). The angiogenesis marker ERG was reduced slightly, but not significantly in the estrogen-treated group (Supplementary Figure S6, available at Carcinogenesis online). To determine whether estrogen has a protective effect in K-ras mutant LUAD in general, we also implanted CC-LR mice with estrogen pellets (Supplementary Table S4, available at Carcinogenesis online). We observed a significant reduction in tumor surface numbers (40%); however, it was not as robust as those of Stat3-deficient mice (Supplementary Figure S7a, available at Carcinogenesis online). Notably, changes in tumor/lung area percentage were not significant (Supplementary Figure S7b and c, available at Carcinogenesis online). These data suggest that estrogen might play a protective role in K-ras mutant LUAD with normal Stat3 activity but not as strong as its role in the absence of epithelial Stat3. The reduction in tumor count in CC-LR males might be caused by the inhibitory effect of estrogen on the baseline increase in the activation of NF-κB in K-ras mutant LUAD. It also suggests that estrogen may reduce tumor initiation but may not affect the progression of developing tumors. These findings further confirmed that estrogen prevents K-ras mutant LUAD progression in the absence of epithelial Stat3 signaling.
Figure 3.
Estrogen treatment in male LR/Stat3Δ/Δ mice reduced tumor burden. (a) Representative photomicrographs of the whole slide images of lung H&E stained sections (scale bar = 3 mm) and (b) tumor/lung area percentage (n = 3–4). (c) Lung surface tumor number (n = 9–17). (d) Representative photomicrographs of Ki-67 stained sections (n = 3–4) (scale bar = 200 μm), and (e) proliferative rate presented as percentage of Ki-67-positive cells. Data represent mean ± SEM; ****P < 0.0001, **P < 0.01, *P < 0.05, experimental replicate #3.
Estrogen treatment in LR/Stat3Δ/Δ male mice reprogrammed the TME to an anti-tumor phenotype through suppressing NF-κB activity
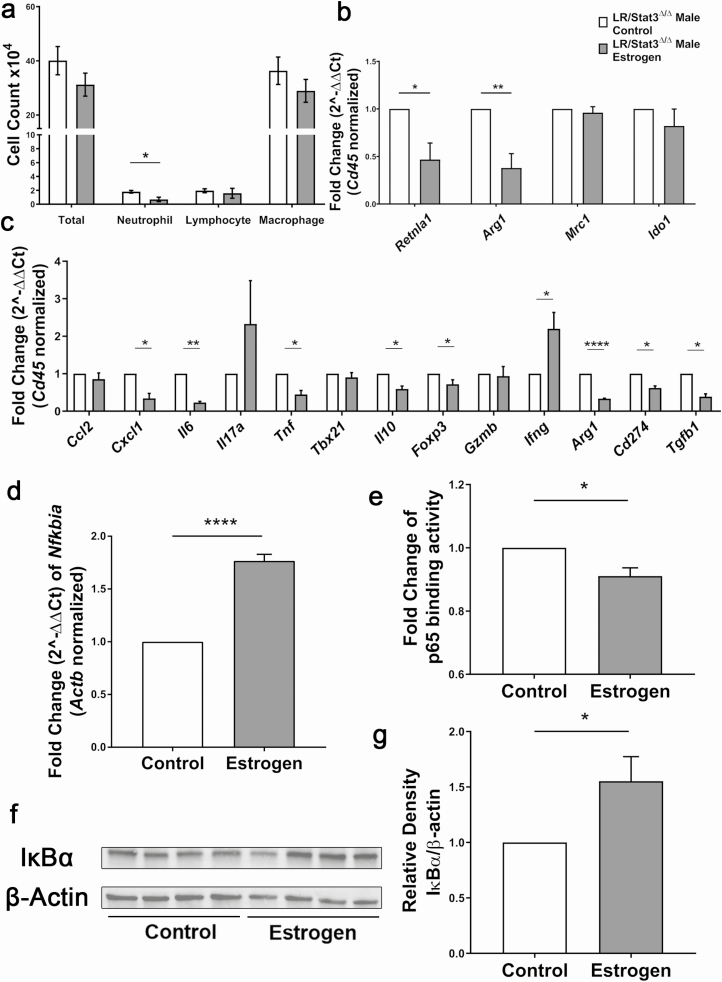
In our previous studies, we found an NF-κB-driven pro-tumor immune response in LR/Stat3Δ/Δ male mice (15). In the present study, we evaluated whether exogenous estrogen could suppress the NF-κB induced changes in the TME. We first looked at changes in WBC differentiation in BALFs, and we found decreased neutrophil infiltration in males treated with estrogen, but no changes in total WBC, lymphocytes or macrophages (Figure 4a). qPCR analysis of RNA extracted from BALF cells showed decreased expression of M2 markers Retnla and Arg1, which indicates a shift from M2 to M1 phenotype, however, expression of Mrc1 and Ido1 were not changed (Figure 4b). We next measured the expression of a panel of inflammatory genes in the whole lung using qPCR. Estrogen treatment resulted in reduced expression of Il6 and Cxcl1 (Figure 4c), which may explain the reduced neutrophil infiltration and indicates suppressed NF-κB activity. However, these changes were not reflected at the protein level (Supplementary Figure S5b and d, available at Carcinogenesis online). Expression of Arg1, PD-L1 (Cd274), Il10 and Foxp3 was also reduced in estrogen-treated males (Figure 4c), which suggests attenuated immunosuppression on cytotoxic T cells and decreased Treg differentiation and activity. These were associated with Ifng upregulation (Figure 4c), suggesting stronger cytotoxic anti-tumor activities; however, no significant changes in Gzmb were found. Estrogen treatment also led to reduced expression of tumor necrosis factor alpha (Tnf) and Tgfb1 (Figure 4c). These data showed that exogenous estrogen in male mice enhanced cytotoxic T-cell activity and diminished the immunosuppressive pro-tumor response. Because we previously reported stronger NF-κB activity in LR/Stat3Δ/Δ males and our qPCR results showed reduced expression of NF-κB downstream genes after estrogen treatment, we next sought to measure NF-κB activity in estrogen-treated males. mRNA and protein levels of IκBα were both higher in estrogen-treated males, which suggested stronger inhibition of NF-κB nuclear translocation (Figure 4d, f and g). DNA binding assay of p65 showed decreased binding activity in estrogen-treated males (Figure 4e). These changes together indicate that exogenous estrogen in LR/Stat3Δ/Δ males attenuates the pro-TME through the downregulation of NF-κB activity.
Figure 4.
Estrogen treatment in male LR/Stat3Δ/Δ mice reprogrammed lung tumor microenvironment to an anti-tumor phenotype through suppressing NF-κB activity. (a) Total inflammatory cell and lineage-specific leukocyte numbers from BALF (n = 6, 7). (b) Relative mRNA expression of Retnla1, Arg1, Mrc1 and Ido1 mRNA in BALF inflammatory cells, normalized by Cd45 expression. (c) Relative mRNA expression of Ccl2, Cxcl1, Il6, Il17a, Tnf, Tbx21, Il10, Foxp3, Gzmb, Ifng, Arg1, Cd274 and Tgfb1 in the whole lung, normalized by Cd45 expression. (d) Relative mRNA expression of IκBα in the whole lung normalized to β-actin. (e) Fold change of p65 binding activity represented by fold change of OD value. (f) Western blot analysis of IκBα and β-actin protein levels in whole lung tissue. (g) Quantification of (f) relative density of IκBα/β-actin. Data represent mean ± SEM; ****P < 0.0001, **P < 0.01, *P < 0.05, experimental replicate #3.
Interestingly, using an available mRNA expression dataset extracted from a cohort of tumor tissues surgically resected from patients with LUAD that did not receive neoadjuvant therapy, we found a significant positive correlation between expression levels of ERβ and T cell-specific markers including CD3, CD8 and CD4 in both females and males, which was stronger in females (Supplementary Figure S8, available at Carcinogenesis online). This further supports the observed changes in cytotoxic T-cell activities in response to estrogen deprivation in female mice and estrogen treatment in male mice and suggests an important role for ER signaling in attenuation of pro-tumor immunosuppressive response which we assume to be stronger in females due to higher levels of estrogen and ER signaling.
Inhibition of NF-κB activity suppressed lung tumorigenesis in LR/Stat3Δ/Δ males
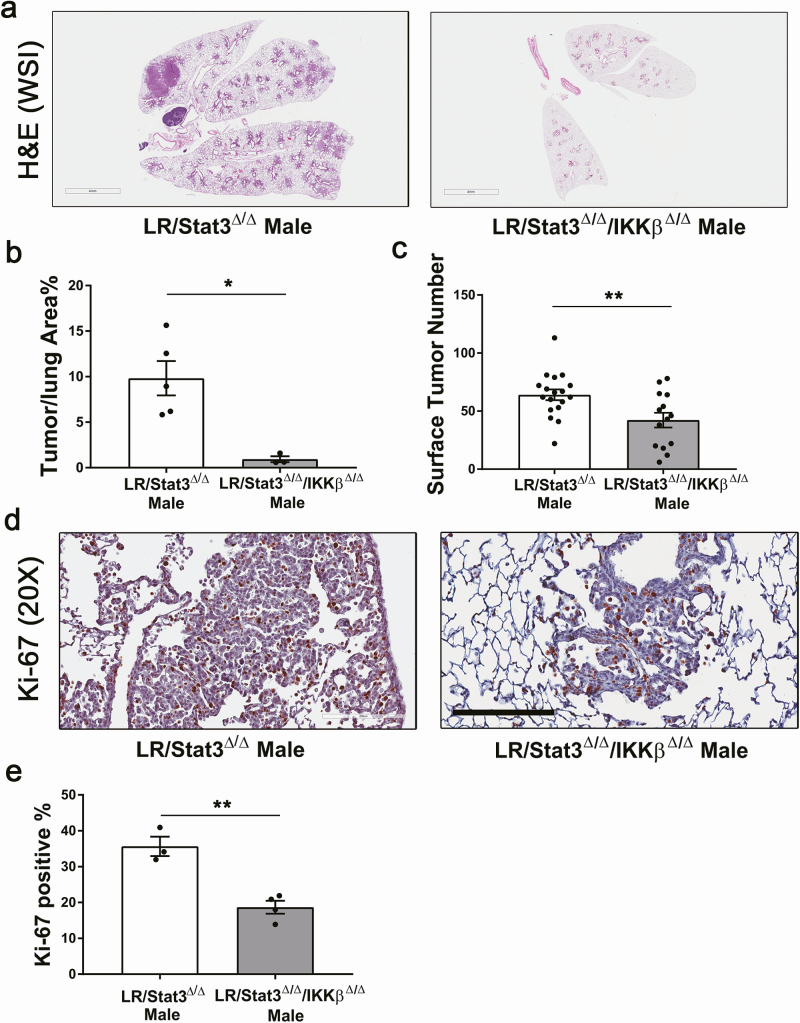
Because LR/Stat3Δ/Δ males have significantly elevated NF-κB activity but females do not, we sought to determine whether the inhibition of IKKβ, which plays a critical role in NF-κB translocation and activation, affects tumorigenesis. We crossed LR/Stat3Δ/Δ mice with IKKβ fl/fl mice (31) to generate double knockout LR/Stat3Δ/Δ/IKKβ Δ/Δ mice. At 14 weeks of age, we found that the surface tumor count and tumor/lung area percentage reduced to 66.7% and 9.1%, respectively, in LR/Stat3Δ/Δ/IKKβ Δ/Δ male mice (Figure 5a–c), but no difference in tumor burden was observed between the two female groups (Supplementary Figure S9a and b, available at Carcinogenesis online). Tumors from LR/Stat3Δ/Δ/IKKβ Δ/Δ males also had significantly reduced proliferation and angiogenesis as assessed by Ki-67 (Figure 5d and e) and ERG staining (Supplementary Figure S10, available at Carcinogenesis online). We next inhibited NF-κB signaling in LR/Stat3Δ/Δ male mice using intraperitoneally injection of a selective IKKβ inhibitor, IMD-0354, daily for 8 weeks. IMD-0354 treatment resulted in a 66.7% reduction in tumor/lung area percentage compared with 0.5% carboxymethylcellulose vehicle-treated group (Supplementary Figure 11Sa and b, available at Carcinogenesis online); however, no differences in Ki-67 or ERG staining were observed (Supplementary Figure 11Sc–f, available at Carcinogenesis online). Surprisingly, the IMD-0354-treated group had slightly stronger staining for Ki-67, probably because most of the lesions in the IMD-0354-treated group were early proliferative hyperplasia, whereas adenomas and adenocarcinomas were mostly present in the vehicle group. These data further confirmed that the increased tumor burden in LR/Stat3Δ/Δ males is caused by increased NF-κB activity and that inhibition of IKKβ can reverse the effect. Because LR/Stat3Δ/Δ females already have endogenous estrogen that inhibits NF-κB activity, inhibition of IKKβ does not have such a dramatic effect in suppressing tumorigenesis in females. However, we knowledge that inclusion of a complementary experiment showing the effect of IMD-0354 treatment in oophorectomized LR/Stat3Δ/Δ female mice could further support this conclusion.
Figure 5.
Lung epithelial-specific deletion of IKKβ suppressed tumorigenesis in LR/Stat3Δ/Δ males. (a) Representative photomicrographs of the whole slide images of lung H&E stained sections (scale bar = 4 mm) and (b) tumor/lung area percentage (n = 3–5). (c) Lung surface tumor number (n = 14–18). (d) Representative photomicrographs of Ki-67 stained sections (n = 3–4) (scale bar = 200 μm), and (e) proliferative rate presented as the percentage of Ki-67-positive cells. Data represent mean ± SEM; **P < 0.01, *P < 0.05, experimental replicate #3.
Targeting of NF-κB activity in lung epithelium reprogrammed the TME in LR/Stat3Δ/Δ male mice
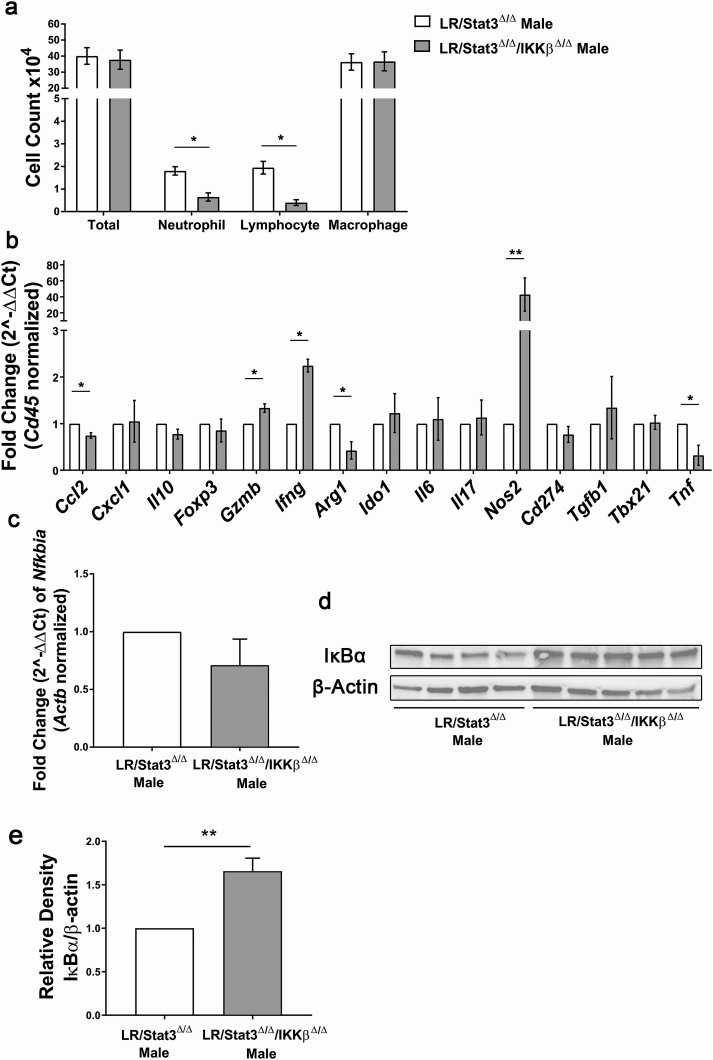
As described above, double knockout of Stat3 and IKKβ in lung epithelium led to lower tumor burden compared with LR/Stat3Δ/Δ males. We next sought to determine whether the inhibition of IKKβ affected TME characteristics. In BALFs, there was a significant reduction in neutrophils and lymphocytes in LR/Stat3Δ/Δ/IKKβ Δ/Δ male mice, but no significant changes in total WBC and macrophages were seen (Figure 6a). However, we did not observe changes in the different WBC populations in female LR/Stat3Δ/Δ/IKKβ Δ/Δ mice (Supplementary Figure S9c, available at Carcinogenesis online). qPCR analysis of RNA extracted from the whole lung showed reduced Ccl2, Arg1 and Tnf expression (Figure 6b) in LR/Stat3Δ/Δ/IKKβ Δ/Δ males, which indicated decreased immunosuppressive function. There was a sharp increase in Nos2 (Figure 6b), an M1 macrophage marker, which suggested a polarization to M1 phenotype. Notably, even though lymphocyte number was decreased in LR/Stat3Δ/Δ/IKKβ Δ/Δ males, there were still significant increases in Gzmb and Ifng (Figure 6b), which suggests a shift toward an anti-tumor cytotoxic response. We then studied NF-κB activity by measuring the level of IκBα. In LR/Stat3Δ/Δ/IKKβ Δ/Δ males, IκBα protein levels were significantly higher than LR/Stat3Δ/Δ counterparts, although mRNA levels did not show significant changes (Figure 6c–e), which suggested inhibited NF-κB activation in LR/Stat3Δ/Δ/IKKβ Δ/Δ males. Changes in TME characteristics and NF-κB activity in LR/Stat3Δ/Δ/IKKβ Δ/Δ males were similar to estrogen-treated LR/Stat3Δ/Δ males, further confirming our hypothesis that estrogen acts as a protective factor in K-ras mutant lung tumorigenesis in the absence of epithelial Stat3 signaling by suppressing NF-κB activity.
Figure 6.
Lung epithelial-specific deletion of IKKβ reprogrammed lung tumor microenvironment in LR/Stat3Δ/Δ male mice through suppressing NF-κB activity. (a) Total inflammatory cell and lineage-specific leukocyte numbers from BALF (n = 6, 7). (b) Relative mRNA expression of Ccl2, Cxcl1, Il10, Foxp3, Gzmb, Ifng, Arg1, Ido1, Il6, Il17, Nos2, Cd 274, Tgfb1, Tbx21 and Tnf in the whole lung normalized by Cd45 expression. (c) Relative mRNA expression of IκBα in the whole lung normalized to β-actin. (d) Western blot analysis of IκBα and β-actin protein levels in whole lung tissue. (e) Quantification of (d) relative density of IκBα/β-actin. Data represent mean ± SEM; **P < 0.01, *P < 0.05, experimental replicate #3.
Discussion
To test whether estrogen might be the protective factor in K-ras mutant lung tumorigenesis by suppressing NF-κB activity, we manipulated either estrogen or NF-κB signaling in LR/Stat3Δ/Δ mice. Oophorectomy in LR/Stat3Δ/Δ females increased tumor burden and immunosuppression while decreasing anti-tumor cytotoxic activity. These changes were similar to the phenotype of LR/Stat3Δ/Δ males and were reversed by estrogen replacement therapy. In LR/Stat3Δ/Δ males, estrogen treatment led to reduced tumorigenesis and immunosuppression along with increased anti-tumor immunity, which are the characteristics of LR/Stat3Δ/Δ females. Furthermore, we found increased NF-κB activity in oophorectomized females, which was decreased in males receiving estrogen. In LR/Stat3Δ/Δ males, both genetic and pharmacologic inhibition of NF-κB activity resulted in reduced tumor burden, increased anti-tumor immune response and attenuated immunosuppression. Overall, our findings suggest that estrogen plays a protective role against K-ras mutant lung cancer by suppressing NF-κB activity in the absence of epithelial Stat3 activity.
Sex disparity is a complex and controversial topic in lung cancer. Women are more likely to develop adenocarcinoma than men (33), and women smokers are more susceptible to lung cancer (34); however, women with lung cancers have better clinical outcomes regardless of stages and histological subtypes (35). Interestingly, most lung cancers are seen in older people with smoking history except for non-smoker females with non-K-ras targetable mutations (36,37). The effect of hormone replacement therapy (HRT) in lung cancer patients also remains controversial. Several studies suggest that HRT increases lung cancer incidence and shortens median survival time (38), whereas some find that women under HRT show less incidence of lung cancer (39) and decreased mortality rate (40,41), and some studies found neutral results (42–44). ERs are members of the nuclear steroid receptor superfamily that respond to estrogen. Normally, ERβ is highly expressed in lung epithelia and is required for maintaining the extracellular matrix of the lung (45,46). ERβ is also the predominant form of ER in lung cancer (46), and studies find that nuclear ERβ favors a good prognosis; however, cytoplasmic ERβ is associated with poor prognosis (47,48). Roles of estrogen signaling in K-ras-driven malignancies are controversial, too. In breast cancer, estrogen withdrawal or a low estrogen state predicts aggressive tumor biology in women with K-ras mutant tumors (49). In K-rasV12 hepatocellular carcinoma, estrogen inhibits tumor growth (50). In lung cancer, women who smoke are more likely to develop tumors harboring K-ras mutations (16,51); however, our studies have found that epithelial Stat3-deleted female mice with K-ras mutant LUAD had reduced tumorigenesis whereas their male counterparts had increased tumor burden (15). In this study, we found that inhibiting ER signaling by either oophorectomy or fulvestrant in LR/Stat3Δ/Δ females significantly increased tumor growth and estrogen replacement therapy reversed these changes. Moreover, estrogen treatment in LR/Stat3Δ/Δ males inhibited tumorigenesis. Our findings confirmed our previous report that estrogen prevents K-ras mutant lung tumorigenesis in the absence of epithelial Stat3 signaling.
K-ras mutant lung tumorigenesis is characterized by activation of NF-κB and Stat3 signaling pathways, increased inflammatory mediators IL-6, CXCL1, CCL2, IL-17, and subsequent infiltration of tumor-promoting neutrophils, M2 macrophages, Th-17 cells and MDSCs (7–10,52). Estrogen plays an important role in regulating inflammation. Although ERβ is the major ER expressed in lung tissues, inflammatory cells infiltrating the lungs express both ERα and ERβ (53). It has long been recognized that steroid hormones regulate the immune response to infection or tissue damage and modulate all levels of the innate and adaptive immune systems (54). Estrogen restricts recruitment of neutrophils through downregulating production of neutrophil chemoattractants CXCL1, CXCL2 and CXCL3 in rodent models of intestinal (55,56) and lung injuries (56,57), and limiting adhesion of neutrophils to endothelial cells (58). Estrogen inhibits CD206+ M2 macrophages and prevents tumor growth in hepatocellular carcinoma (59). Estrogen also attenuates the release of pro-inflammatory mediators TNFα, IL-1β and IL-6 from neutrophils and macrophages (60). In our oophorectomized LR/Stat3Δ/Δ females, we found increased neutrophil infiltration, increased M2 macrophage polarization and increased NF-κB-driven pro-inflammatory cytokines (i.e. IL-6 and CCL2), and estrogen treatment in LR/Stat3Δ/Δ males resulted in reduced neutrophil infiltration and M1 macrophage polarization and lowered IL-6, CXCL1, TNFα, Arg1 and TGFβ expression. These findings suggest that estrogen in epithelial Stat3-deleted K-ras LUAD dampens immunosuppressive characteristics of TME by inhibiting neutrophil infiltration, reducing M2 macrophage polarization and suppressing MDSCs. Estrogen also regulates immune function through affecting adaptive immunity. Low levels of estrogen promote Th1-type response and increase IFNγ produced by T cells, whereas high levels of estrogen inhibit the production of IL-17 by Th17 cells (61). In the present study, oophorectomized LR/Stat3Δ/Δ females had lower cytotoxic anti-tumor immunity with reduced expression of GzmB and IFNγ, whereas their male counterparts treated with estrogen had increased IFNγ. These data suggest that estrogen may inhibit Stat3 deficient K-ras mutant LUAD growth through activating cytotoxic anti-tumor immunity. Estrogen also mediates immunosuppression through activating Treg cells, such as in pregnancy, to maintain fetal-maternal tolerance (62). In our model, loss of estrogen boosted Treg activity through upregulating Foxp3 and IL-10, and suppressed anti-tumor immunity. This contrasting effect of estrogen on Tregs suggests that estrogen might play different roles under specific pathological conditions.
As discussed above, K-ras mutant LUAD is associated with increased NF-κB activation (7,10), and estrogen has been widely reported to suppress NF-κB activity (27–30,63). Estrogen suppresses NF-κB induced inflammation by increasing IκBα levels (27) and decreasing phosphorylated IκBα levels (63). ERβ inhibits DNA binding ability of NF-κB p50, c-Rel and p65/p50 dimers, and both ERs prevent NF-κB from binding to IL-6 promoter (28–30). In K-ras mutant LUAD, CXCL1, CCL2, IL-6 are important inflammatory cytokines and chemokines that are all regulated by NF-κB activity. In our previous study, we found increased NF-κB activity in LR/Stat3Δ/Δ males, but not females (15). In the present study, we found increased NF-κB activity in oophorectomized LR/Stat3Δ/Δ females, which were rescued by estrogen replacement, and LR/Stat3Δ/Δ males treated with exogenous estrogen had reduced NF-κB activity. Moreover, both genetic and pharmacologic inhibition of IKKβ resulted in reduced tumor burden, attenuated immunosuppression and enhanced anti-tumor immunity only in LR/Stat3Δ/Δ males, but not females. Our findings align well with our previous findings and suggest that estrogen inhibits K-ras mutant lung tumorigenesis in the absence of epithelial Stat3 through inhibiting NF-κB activity.
In summary, we found that in K-ras mutant lung tumors without epithelial Stat3 expression, NF-κB gets further activated (unleashed) to compensate for the loss of Stat3 signaling. In females, with the presence of strong estrogen signaling, NF-κB is suppressed, which results in reduced tumorigenesis, reduced pro-tumor immunosuppression and enhanced anti-tumor cytotoxicity. However, in males, with lower estrogen signaling, NF-κB activity becomes dominant, promotes tumorigenesis and induces a pro-tumor TME. These findings are in line with our previous findings in LUAD patients showing a significant positive correlation between pSTAT3 and ERβ expression in female but not in male LUAD patients (15), which here we further corroborated by demonstrating a positive correlation between expression levels of ERβ and T-cell-specific markers CD3, CD8 and CD4. Overall, our study underscores the interplay between the Stat3/NF-κB cytokine network and estrogen signaling and their gender-specific functions in K-ras mutant tumorigenesis. This suggests an important role for in-depth immuno-genomic phenotyping of each patient with lung tumor prior to decision making. It provides novel cues in developing personalized treatment and preventive strategies against this currently ‘undruggable‘ malignancy based on each patient’s sex-specific immuno-genomic phenotype. Furthermore, our data showing the mechanisms of interaction between the TME and cancer cells in a sex-specific fashion is crucial in the context of immunotherapy [e.g. immune checkpoint blockade (ICB)]. Although ICB has shown promising results against lung cancer, most patients do not sustain durable responses to current ICB regimens. Interestingly, the response rate to ICB could be influenced by patient sex due to differences in TME contexture between sexes (64–69). Our study also prompts the use of complementary treatment approaches using IL-6/Stat3 inhibitors in combination with ICB or HRT. It will also help to better understand sex-specific predictive and prognostic biomarkers and explore mechanisms of susceptibility or resistance to therapy.
Supplementary Material
Glossary
Abbreviations
- BALF
bronchoalveolar lavage fluid
- ERβ
estrogen receptor beta
- HRT
hormone replacement therapy
- ICB
immune checkpoint blockade
- IL
interleukin
- LUAD
lung adenocarcinoma
- MDSCs
myeloid-derived suppressor cells
- NF-κB
nuclear factor-kappaB
- OD
optic density
- TME
tumor microenvironment
- Treg
T regulatory
Funding
This study was supported in part by lung cancer discovery award from the American Lung Association (LCD-503769) and National Cancer Institute (NCI) grant R01CA225977 (both to S.J.M.), as well as University of Texas Lung Specialized Programs of Research Excellence grant (P50CA70907) to I.I.W., and M. D. Anderson Institutional Tissue Bank award (2P30CA016672) from the National Institute of Health, National Cancer Institute. S.D. was partly supported by the China Scholarship Council.
Author contributions
S.J.M. conceived, supervised and conceptualized the study; S.J.M. and M.S.C. designed research; S.D., M.R., W.V.V., B.A.G, M.J.C., O.N., M.Z., E.J.O., L.A., Y.D., S.Y., C.B., H.K. and S.J.M. performed research; S.D., H.K. and S.J.M. analyzed data; I.I.W., L.P.S., H.K. and S.S.W. reviewed the paper; S.D. and S.J.M. wrote the paper.
Conflict of Interest Statement: None declared.
References
- 1. Siegel, R.L. et al. (2019) Cancer statistics, 2019. CA. Cancer J. Clin., 69, 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw, P. et al. (2011) Non-small-cell lung cancer. Lancet, 378, 1727–1740. [DOI] [PubMed] [Google Scholar]
- 3. Network CGAR. (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature., 511, 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cully, M (2016) Cancer: closing the door on KRAS-mutant lung cancer. Nat. Rev. Drug Discov., 15, 747. [DOI] [PubMed] [Google Scholar]
- 5. Hanahan, D. et al. (2011) Hallmarks of cancer: the next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 6. Hanahan, D. et al. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21, 309–322. [DOI] [PubMed] [Google Scholar]
- 7. Caetano, M.S. et al. (2016) IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras-mutant lung cancer. Cancer Res., 76, 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang, S.H. et al. (2014) T helper 17 cells play a critical pathogenic role in lung cancer. Proc. Natl Acad. Sci. USA, 111, 5664–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gong, L. et al. (2013) Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol. Cancer, 12, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong, L. et al. (2016) Tumor necrosis factor links chronic obstructive pulmonary disease and K-ras mutant lung cancer through induction of an immunosuppressive pro-tumor microenvironment. Oncoimmunology, 5, e1229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moghaddam, S.J. et al. (2009) Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am. J. Respir. Cell Mol. Biol., 40, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Busch, S.E. et al. (2016) Lung cancer subtypes generate unique immune responses. J. Immunol., 197, 4493–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji, H. et al. (2006) K-ras activation generates an inflammatory response in lung tumors. Oncogene, 25, 2105–2112. [DOI] [PubMed] [Google Scholar]
- 14. Ochoa, C.E. et al. (2011) Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev. Res. (Phila.), 4, 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caetano, M.S. et al. (2018) Sex specific function of epithelial STAT3 signaling in pathogenesis of K-ras mutant lung cancer. Nat. Commun., 9, 4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kiyohara, C. et al. (2010) Sex differences in lung cancer susceptibility: a review. Gend. Med., 7, 381–401. [DOI] [PubMed] [Google Scholar]
- 17. Kligerman, S. et al. (2011) Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR Am. J. Roentgenol., 196, 287–295. [DOI] [PubMed] [Google Scholar]
- 18. Risch, H.A. et al. (1993) Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am. J. Epidemiol., 138, 281–293. [DOI] [PubMed] [Google Scholar]
- 19. Zang, E.A. et al. (1996) Differences in lung cancer risk between men and women: examination of the evidence. J. Natl Cancer Inst., 88, 183–192. [DOI] [PubMed] [Google Scholar]
- 20. North, C.M. et al. (2013) Women and lung cancer: what is new? Semin. Thorac. Cardiovasc. Surg., 25, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egleston, B.L. et al. (2009) Population-based trends in lung cancer incidence in women. Semin. Oncol., 36, 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Perrot, M. et al. (2000) Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J. Thorac. Cardiovasc. Surg., 119, 21–26. [DOI] [PubMed] [Google Scholar]
- 23. Radzikowska, E. et al. (2002) Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann. Oncol., 13, 1087–1093. [DOI] [PubMed] [Google Scholar]
- 24. Visbal, A.L. et al. (2004) Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann. Thorac. Surg.., 78, 209–215; discussion 15. [DOI] [PubMed] [Google Scholar]
- 25. Siddiqui, F. et al. (2010) The influence of gender, race, and marital status on survival in lung cancer patients: analysis of Radiation Therapy Oncology Group trials. J. Thorac. Oncol., 5, 631–639. [DOI] [PubMed] [Google Scholar]
- 26. Araujo, J.M. et al. (2016) Repeated observation of immune gene sets enrichment in women with non-small cell lung cancer. Oncotarget, 7, 20282–20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xing, D. et al. (2012) Estrogen modulates NFκB signaling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-β. PLoS One, 7, e36890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pelzer, T. et al. (2001) Estrogen effects in the myocardium: inhibition of NF-kappaB DNA binding by estrogen receptor-alpha and -beta. Biochem. Biophys. Res. Commun., 286, 1153–1157. [DOI] [PubMed] [Google Scholar]
- 29. Stein, B. et al. (1995) Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol., 15, 4971–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galien, R. et al. (1997) Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res., 25, 2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li, Z.-W. et al. (2003) IKKβ is required for peripheral B cell survival and proliferation. J. Immunol., 170, 4630–4637. [DOI] [PubMed] [Google Scholar]
- 32. Cardnell, R.J. et al. (2015) An integrated molecular analysis of lung adenocarcinomas identifies potential therapeutic targets among TTF1-negative tumors, including DNA repair proteins and Nrf2. Clin. Cancer Res., 21, 3480–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donington, J.S et al. (2011) Sex and gender differences in non-small cell lung cancer. Semin. Thorac. Cardiovasc. Surg., 23, 137–145. [DOI] [PubMed] [Google Scholar]
- 34. Wakelee, H.A. et al. (2007) Lung cancer incidence in never-smokers. J. Clin. Oncol., 25, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas, L. et al. (2005) Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest, 128, 370–381. [DOI] [PubMed] [Google Scholar]
- 36. Smolle, E. et al. (2019) Non-smoking-associated lung cancer: a distinct entity in terms of tumor biology, patient characteristics and impact of hereditary cancer predisposition. Cancers (Basel), 11, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudin, C.M. et al. (2009) Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin. Cancer Res., 15, 5646–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ganti, A.K. et al. (2006) Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol., 24, 59–63. [DOI] [PubMed] [Google Scholar]
- 39. Schabath, M.B. et al. (2004) Hormone replacement therapy and lung cancer risk: a case–control analysis. Clin. Cancer Res., 10(1 Pt 1), 113–123. [DOI] [PubMed] [Google Scholar]
- 40. Clague, J. et al. (2014) Menopausal hormone therapy and lung cancer-specific mortality following diagnosis: the California Teachers Study. PLoS One, 9, e103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ettinger, B. et al. (1996) Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet. Gynecol., 87, 6–12. [DOI] [PubMed] [Google Scholar]
- 42. Chlebowski, R.T. et al. (2010) Lung cancer among postmenopausal women treated with estrogen alone in the women’s health initiative randomized trial. J. Natl Cancer Inst., 102, 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang, B. et al. (2009) Hormone replacement therapy and survival in lung cancer in postmenopausal women in a rural population. Cancer, 115, 4167–4175. [DOI] [PubMed] [Google Scholar]
- 44. Ayeni, O. et al. (2009) Hormone replacement therapy and outcomes for women with non-small-cell lung cancer: can an association be confirmed? Curr. Oncol., 16, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsu, L.-H. et al. (2017) Estrogen, estrogen receptor and lung cancer. Int J Mol Sci., 18, 1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carey, M.A. et al. (2007) The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am. J. Physiol. Lung Cell. Mol. Physiol., 293, L272–L278. [DOI] [PubMed] [Google Scholar]
- 47. Baik, C.S. et al. (2012) Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel), 4, 969–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawai, H (2014) Estrogen receptors as the novel therapeutic biomarker in non-small cell lung cancer. World J. Clin. Oncol., 5, 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McVeigh, T.P. et al. (2015) Estrogen withdrawal, increased breast cancer risk and the KRAS-variant. Cell Cycle, 14, 2091–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li, Y. et al. (2017) Males develop faster and more severe hepatocellular carcinoma than females in krasV12 transgenic zebrafish. Sci. Rep., 7, 41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gasperino, J (2011) Gender is a risk factor for lung cancer. Med. Hypotheses, 76, 328–331. [DOI] [PubMed] [Google Scholar]
- 52. Kitajima, S. et al. (2016) Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. Semin. Cell Dev. Biol., 58, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siegfried, J.M. et al. (2014) Estrongenic steroid hormones in lung cancer. Semin. Oncol., 41, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nadkarni, S. et al. (2013) Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr. Opin. Pharmacol., 13, 576–581. [DOI] [PubMed] [Google Scholar]
- 55. Sheh, A. et al. (2011) 17β-Estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in helicobacter pylori–infected INS-GAS male mice. Cancer Prev. Res., 4, 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doucet, D. et al. (2010) Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS One, 5, e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang, S.J. et al. (2011) Akt pathway is required for oestrogen-mediated attenuation of lung injury in a rodent model of cerulein-induced acute pancreatitis. Injury, 42, 638–642. [DOI] [PubMed] [Google Scholar]
- 58. Nadkarni, S. et al. (2011) Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes. Arterioscler. Thromb. Vasc. Biol., 31, 2749–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang, W. et al. (2012) Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs). J. Biol. Chem., 287, 40140–40149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toyoda, Y. et al. (2011) Estradiol and progesterone modulate halothane-induced liver injury in mice. Toxicol. Lett., 204, 17–24. [DOI] [PubMed] [Google Scholar]
- 61. Klein, S.L. et al. (2016) Sex differences in immune responses. Nat. Rev. Immunol., 16, 626–638. [DOI] [PubMed] [Google Scholar]
- 62. Aluvihare, V.R. et al. (2004) Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol., 5, 266–271. [DOI] [PubMed] [Google Scholar]
- 63. Murphy, A.J. et al. (2010) Estradiol suppresses NF-κB activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J. Immunol., 184, 5029–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pinto, J.A. et al. (2018) Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open, 3, e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Conforti, F. et al. (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet. Oncol., 19, 737–746. [DOI] [PubMed] [Google Scholar]
- 66. Conforti, F. et al. (2019) Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J. Natl Cancer Inst., 111, 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang, S. et al. (2019) Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules, 24, 3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang, S. et al. (2019) The predictive power of tumor mutational burden in lung cancer immunotherapy response is influenced by patients’ sex. Int. J. Cancer, 145, 2840–2849. [DOI] [PubMed] [Google Scholar]
- 69. Capone, I. et al. (2018) Sexual dimorphism of immune responses: a new perspective in cancer immunotherapy. Front. Immunol., 9, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.