Abstract
OBJECTIVE
We evaluated the associations between changes in plant-based diets and subsequent risk of type 2 diabetes.
RESEARCH DESIGN AND METHODS
We prospectively followed 76,530 women in the Nurses’ Health Study (NHS) (1986–2012), 81,569 women in NHS II (1991–2017), and 34,468 men in the Health Professionals Follow-up Study (1986–2016). Adherence to plant-based diets was assessed every 4 years with the overall plant-based diet index (PDI), healthful PDI (hPDI), and unhealthful PDI (uPDI). We used multivariable Cox proportional hazards models to estimate hazard ratios (HRs). We pooled results of the three cohorts using meta-analysis.
RESULTS
We documented 12,627 cases of type 2 diabetes during 2,955,350 person-years of follow-up. After adjustment for initial BMI and initial and 4-year changes in alcohol intake, smoking, physical activity, and other factors, compared with participants whose indices remained relatively stable (±3%), participants with the largest decrease (>10%) in PDI and hPDI over 4 years had a 12–23% higher diabetes risk in the subsequent 4 years (pooled HR, PDI 1.12 [95% CI 1.05, 1.20], hPDI 1.23 [1.16, 1.31]). Each 10% increment in PDI and hPDI over 4 years was associated with a 7–9% lower risk (PDI 0.93 [0.91, 0.95], hPDI 0.91 [0.87, 0.95]). Changes in uPDI were not associated with diabetes risk. Weight changes accounted for 6.0–35.6% of the associations between changes in PDI and hPDI and diabetes risk.
CONCLUSIONS
Improving adherence to overall and healthful plant-based diets was associated with a lower risk of type 2 diabetes, whereas decreased adherence to such diets was associated with a higher risk.
Introduction
Type 2 diabetes remains an important public health problem. Currently, more than 450 million people live with type 2 diabetes worldwide, and the number is projected to reach 700 million by 2045 (1). The high prevalence of type 2 diabetes places an enormous economic and clinical burden on patients and health care systems. Diet is an important modifiable lifestyle factor in the prevention of type 2 diabetes (2). Recently, plant-based diets, characterized by higher consumption of plant foods and lower or no intake of animal foods, have gained significant attention for their health benefits. National dietary guidelines and public health agencies recommend consuming a plant-based diet, particularly rich in high-quality plant foods (e.g., whole grains, vegetables, fruits, and nuts) for chronic disease prevention (3). Emerging evidence from observational studies has demonstrated that adherence to an overall plant-based diet emphasizing all plant foods, and a healthful plant-based diet emphasizing high-quality plant foods, has consistently been associated with a lower risk of type 2 diabetes (4). In a recent meta-analysis of nine observational studies, representing 307,099 participants, greater adherence to overall and healthful plant-based diets was associated with a 23–30% lower type 2 diabetes risk (4). However, most previous studies measured adherence to plant-based diets only at baseline (5–8). In real life, a person’s eating behavior is likely to change over time (9), and a single measurement of diet at baseline would not adequately capture these dynamic changes during follow-up. Therefore, it is critical to examine how changes in adherence to plant-based diets over time (i.e., how gradually increasing consumption of plant foods while progressively decreasing animal food intake over time or vice versa) is associated with subsequent risk of type 2 diabetes. This would provide a more complete understanding of the associations between plant-based diets and type 2 diabetes risk. Furthermore, evaluating the associations of changes in adherence to plant-based diets over different time periods (e.g., 4-year changes and 8-year changes) with subsequent risk of type 2 diabetes will also help determine how quickly such dietary changes may impact diabetes risk. However, to our knowledge, no study has examined the associations between changes in adherence to plant-based diets and subsequent risk of type 2 diabetes.
In the current study, we aimed to examine associations between changes in adherence to plant-based diets and subsequent risk of type 2 diabetes in three large ongoing prospective cohort studies: the Nurses’ Health Study (NHS), the NHS II, and the Health Professionals Follow-up Study (HPFS). In these three cohorts, dietary data were collected every 4 years; information on lifestyle (e.g., smoking status, physical activity), health conditions (e.g., BMI), and chronic diseases (e.g., type 2 diabetes) was collected every 2 years for >30 years of follow-up. The availability of these repeated measures and the long duration of follow-up allowed us to evaluate the associations between 4-year changes in adherence to plant-based diets and risk of type 2 diabetes in the next 4 years. In sensitivity analyses, we also examined longer-term (8-year) changes in plant-based diets in relation to type 2 diabetes risk in the subsequent 8-year period.
Research Design and Methods
Study Population
The NHS started in 1976 with 121,701 female nurses aged 30–55 years, and NHS II began in 1989 with 116,430 female nurses aged 25–42 years (10). The HPFS, the male counterpart of the NHS, started in 1986 with 51,529 male health professionals aged 40–75 years (11). In all three cohorts, self-administered questionnaires collected information on lifestyle and medical history every 2 years, with a response rate of ∼90% per cycle. Starting in 1986 for NHS and the HPFS, and 1991 for NHS II, diet was assessed every 4 years. The years 1990 in NHS and HPFS and 1995 in NHS II were used as baseline for the current analysis because our primary exposure was 4-year changes in adherence to plant-based diets. Specifically, we used changes in adherence to plant-based diets that were updated every 4 years as a time-varying exposure to examine associations with type 2 diabetes risk in the subsequent 4 years. For instance, changes in plant-based diets between 1986 and 1990 were used to examine associations with the risk of diabetes between 1990 and 1994, changes in plant-based diets between 1990 and 1994 were used to examine associations with the risk between 1994 and 1998, and so on. In the current analysis, follow-up ended on 30 June 2012 for NHS, 30 June 2017 for NHS II, and 31 January 2016 for HPFS.
We excluded participants with diabetes (type 1, type 2, gestational diabetes mellitus), cancer, or cardiovascular disease and those who died before baseline (1990/1995: the baseline time point of this current analysis). We also excluded participants whose last returned questionnaire was at baseline, those who did not complete two consecutive food-frequency questionnaires (FFQs) (e.g., in 1990 and 1994), and those who reported implausible calorie intakes (<500 or >3,500 kcal/day for women or <800 or >4,200 kcal/day for men). However, these participants reentered the analysis when data from two consecutive FFQs were available and plausible (12). After exclusions, we included 76,530 women in NHS, 81,569 women in NHS II, and 34,468 men in HPFS in the current analysis (Supplementary Fig. 1). The study protocol was approved by the Institutional Review Board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard T.H. Chan School of Public Health.
Dietary Assessment
Dietary data were collected with use of previously validated semiquantitative FFQs of ∼131 items every 4 years, starting in 1986 for NHS and HPFS and 1989 for NHS II (13). These FFQs were largely the same for each of the cohorts, but changes were made to the FFQs to accommodate introduction of new foods to the food system. For each food item, participants were asked to report their usual intake of a standard portion of each food item from the previous year. The daily nutrient intake was assessed based on the Harvard Food Composition Database, which is derived from the U.S. Department of Agriculture nutrient data (14). The reproducibility and validity of nutrient, food, and dietary pattern measurements in the NHS and HPFS have previously been described in detail (15–18).
Considering that quality and health effects of plant foods may differ widely, we previously developed and validated three distinct plant-based diet quality indices that capture adherence to different dimensions of a plant-based diet: the overall plant-based diet index (PDI), the healthful PDI (hPDI), and the unhealthful PDI (uPDI) (19). The PDI captures overall plant-based diet, which emphasizes higher intake of all plant foods and lower intake of all animal foods. The hPDI emphasizes higher intake of healthy plant foods (e.g., whole grains, vegetables, fruits) and lower intakes of unhealthy plant foods (e.g., refined grains, potatoes, sugary beverages) and all animal foods. The uPDI emphasizes consumption of unhealthy plant foods and lower intake of healthy plant foods and all animal foods. In the current analysis, for each participant at each cycle, we calculated a PDI, an hPDI, and a uPDI to assess the degree of adherence to overall, healthful, and unhealthful plant-based diets, respectively, using methods described previously (19). Briefly, we created 18 food groups based on nutrient and culinary similarities to calculate the PDIs (Supplementary Table 1). Of the 18 food groups, 7 were healthy plant food groups (including whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea/coffee), 5 were unhealthy plant food groups (fruit juices, sugar-sweetened beverages, refined grains, potatoes, and sweets/desserts), and 6 were animal food groups (animal fats, dairy, eggs, fish/seafood, meat [poultry and red meat], and miscellaneous animal-based foods [e.g., pizza]). The 18 food groups were ranked into quintiles, and each quintile was assigned a score ranging from 1 to 5. For creating the PDI, for overall plant-based diet, higher intakes of healthy and unhealthy plant food groups were given higher scores, while animal food groups were given reverse scores. For the hPDI, healthy plant food groups were given positive scores and unhealthy plant food groups and animal food groups were given reverse scores. For the uPDI, unhealthy plant food groups received positive scores, while healthy plant food groups and animal food groups received reverse scores. The quintile scores of the 18 food groups were summed to obtain the PDIs. The theoretical range of the indices was, therefore, from 18 to 90. Higher scores of all indices reflected lower animal food intake (19). Changes in adherence to overall, healthful, and unhealthful plant-based diets were assessed by calculation of changes in PDI, hPDI, and uPDI between FFQs, respectively. Alcohol beverages were not included in the indices, as alcohol beverages were not clearly associated in one direction with several health outcomes (19). Due to changes in the fatty acid composition of margarine over time from high trans fat to high unsaturated fat, we also excluded margarine from the indices (19). However, we adjusted for initial and change in alcohol intake (g/day, quintiles) and initial and changes in margarine intake (serving/day, quintiles) in the analyses.
Assessment of Type 2 Diabetes
Incident type 2 diabetes was the primary outcome of the current analysis. Participants who first self-reported type 2 diabetes on the main biennial questionnaire were sent a supplementary questionnaire on the symptoms, diagnostic tests, and treatment of diabetes. Cases before 1998 were confirmed in accordance with National Diabetes Data Group criteria (20), which included at least one of the following criteria: 1) one or more classic symptoms (excessive thirst, polyuria, weight loss, hunger) and fasting glucose concentrations ≥7.8 mmol/L or random glucose concentrations ≥11.1 mmol/L, 2) two or more elevated glucose concentrations on different occasions (fasting concentrations ≥7.8 mmol/L, random glucose concentrations ≥11.1 mmol/L, and/or concentrations of ≥11.1 mmol/L after ≥2 h shown by oral glucose tolerance testing) in the absence of symptoms, or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). After 1998, cases were identified with use of the American Diabetes Association criteria, which lowered the threshold for fasting glucose for the diagnosis of diabetes to 7.0 mmol/L, instead of 7.8 mmol/L (21). The validity of the supplementary questionnaire for diagnosis of type 2 diabetes was previously demonstrated (22–24). Only incident diabetes cases confirmed with the supplementary questionnaire (using National Diabetes Data Group criteria before 1998 and the American Diabetes Association criteria after 1998) were considered for the current study.
Assessment of Covariates
Updated information on height, body weight, cigarette smoking, physical activity, family history of diabetes, and history of hypertension and hypercholesterolemia was collected using the biennial follow-up questionnaires. Among women, information on menopausal status, postmenopausal hormone use (NHS and NHS II), and oral contraceptive use (NHS II only) was also assessed. Alcohol intake was assessed every 4 years with the FFQs.
Statistical Analysis
For minimization of the influence of outliers, changes in PDIs <0.5th and >99.5th percentiles were recoded into the value of the 0.5th and the 99.5th percentiles, respectively. We divided participants into five categories of changes in plant-based diet indices: no change or relatively stable indices (±3%, reference group), small-to-moderate increase (3–10%) or decrease, and large increase (>10%) or decrease. We calculated person-time for each participant from the date of return of the baseline questionnaire (1990 in NHS and HPFS and 1995 in NHS II) to date of diagnosis of type 2 diabetes or return of the last questionnaire before death, loss to follow-up, or the end of the follow-up (30 June 2012 for NHS, 30 June 2017 for NHS II, and 31 January 2016 for the HPFS)—whichever came first.
We used time-dependent Cox proportional hazards regression models to calculate hazard ratios (HRs) for type 2 diabetes in relation to 4-year changes in the indices. In model 1, we adjusted for baseline age and initial corresponding PDI score (PDI, hPDI, or uPDI, quintiles) with stratification by calendar year in 4-year intervals. In model 2, we additionally adjusted for ethnicity (White, non-White), family history of diabetes (yes/no), initial and 4-year change in total energy (kcal/day, quintiles), initial and 4-year change in alcohol intake (g/day, quintiles), initial and 4-year change in margarine intake (serving/day, quintiles), initial and 4-year change in physical activity (MET h/week, quintiles), 4-year change in smoking status (never to never, never to current, past to past, past to current, current to past, current to current, or missing indicator), initial BMI (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, ≥32.0 kg/m2), updated history of hypertension (yes/no, updated every 4 years), updated history of hypercholesterolemia (yes/no, updated every 4 years), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator) (NHS and NHS II), and oral contraceptive use (never, current, past, missing indicator; NHS II only). We tested for linear trend across categories of changes in plant-based diet indices by treating the median value of each category of change as a continuous variable in the models. We also additionally examined associations between each 10% incremental increase in the indices over 4 years with risk of type 2 diabetes in the subsequent 4 years.
To test the robustness of our findings, we conducted several sensitivity analyses based on our fully adjusted model (model 2). First, because change in body weight may be a potential mediator of the association between changes in plant-based diets and risk of type 2 diabetes, we further adjusted for concurrent 4-year changes in body weight (quintiles) (25,26). We also estimated the percentage of the association between changes in plant-based diets and diabetes risk that was mediated by concurrent changes in body weight. For this, we used the SAS macro %mediate (publicly available from https://www.hsph.harvard.edu/donna-spiegelman/software/mediate/) and the formula of 1 − (βmediator model / βbase model) ∗ 100 to evaluate the mediation effect of concurrent 4-year body weight change on the association between changes in plant-based diets and subsequent diabetes risk (27). Second, we conducted a 4-year lagged analysis to minimize the impact of recent dietary changes made among individuals at higher risk. In this analysis, changes in plant-based diet indices from 1986 to 1990 were used to evaluate the risk of type 2 diabetes between 1994 and 1998, and so on. Third, as aforementioned, in addition to 4-year changes, we also evaluated the associations between longer-term changes (8 years) in adherence to plant-based diets and risk of type 2 diabetes in the subsequent 8 years. For example, changes in plant-based diet indices from 1986 to 1994 were used to evaluate the risk of type 2 diabetes between 1994 and 2002. Fourth, because individuals at higher risk are likely to be screened for diabetes and diagnosed more rapidly, leading to potential surveillance bias, we evaluated the association of 4-year changes in plant-based diets and risk of symptomatic diabetes (21), ascertained by the report of at least one symptom of diabetes (e.g., excessive thirst) in the supplementary questionnaire. Fifth, we conducted stratified analyses according to potential effect modifiers of the association between changes in plant-based diets and risk of type 2 diabetes. These include analyses stratified by baseline age, sex, initial plant-based diet indices, smoking status, 4-year change in physical activity level, initial BMI, 4-year concurrent weight change, family history of diabetes, history of hypertension, and history of hypercholesterolemia. We tested interactions using likelihood ratio tests by including cross product terms of each stratum and change in plant-based diets in the multivariable models. Given the potential for multiple testing, we set the statistical level for significance for these interactions at 0.005 (0.05/10 comparisons). Finally, as changes in PDIs were driven by changes in healthy and unhealthy plant foods and animal foods, to further examine the individual contributions of 4-year changes in intakes of healthy and unhealthy plant foods and animal foods to subsequent risk of diabetes, we included variables for 4-year changes in all three food types (healthy plant foods, unhealthy plant foods, and animal foods) simultaneously in the model in place of PDIs.
Analyses were conducted separately in each cohort, and results were pooled with use of an inverse variance–weighted, fixed-effects meta-analysis. If significant heterogeneity across cohorts was found (P < 0.10 assessed by Cochran Q test), we used an inverse variance–weighted, random-effects meta-analysis. All other statistical significance (except those for interaction tests) was considered at P < 0.05 (two-sided), and analyses were performed using SAS software, version 9.4.
Results
During a total of 2,955,350 person-years of follow-up, we documented 12,627 incident cases of type 2 diabetes (5,993 [7.8% of analyzed participants] in NHS, 4,190 [5.1%] in NHS II, and 2,444 [7.1%] in HPFS). Table 1 presents the age-adjusted characteristics of participants according to baseline 4-year changes in PDI. In all three cohorts, compared with those who had relatively stable PDI (±3% [±2 points]), participants who had the largest decrease in PDI (>10% [>7 points]) had a higher initial PDI score, a higher initial energy intake, and a greater decrease in energy intake in the first 4-year period, whereas participants who made the largest increase (>10% [>7 points]) had a lower initial PDI score, a lower initial energy intake, and a greater increase in energy intake in the first 4-year period. Supplementary Table 2 presents the age-adjusted characteristics of participants across baseline 4-year changes in hPDI and uPDI. Participants with the largest decrease in hPDI had a higher initial hPDI score, a lower initial energy intake, and a greater increase in energy intake in the first 4-year period, while participants with the largest hPDI increase had a lower initial hPDI score, a higher initial energy intake, and a larger decrease in energy intake. Similar characteristics were observed for changes in uPDI in the first 4-year period.
Table 1.
Age-adjusted characteristics of participants according to baseline 4-year changes in overall plant-based diet
| Changes in overall plant-based diet (% score change) | |||||
|---|---|---|---|---|---|
| Decrease | No change or relatively stable (± <3%) | Increase | |||
| Large (>10%) | Small to moderate (3–10%) | Small to moderate (3–10%) | Large (>10%) | ||
| NHS (n = 76,530) | |||||
| Participants, n | 8,268 | 17,330 | 24,466 | 17,594 | 8,872 |
| Age, years† | 57.7 (8.1) | 57.6 (7.9) | 58.1 (7.9) | 58.4 (7.8) | 58.9 (7.6) |
| Overall PDI | |||||
| Initial | 59.6 (5.7) | 56.9 (5.8) | 54.6 (5.8) | 52.3 (5.9) | 49.3 (5.9) |
| Change | −10.6 (2.4) | −4.7 (1.4) | 0.0 (1.4) | 4.7 (1.4) | 10.6 (2.5) |
| Energy intake, kcal/day | |||||
| Initial | 1,872 (526) | 1,813 (533) | 1,755 (529) | 1,707 (522) | 1,646 (499) |
| Change | −252 (472) | −120 (440) | −12 (426) | 83 (441) | 204 (463) |
| Alcohol intake, g/day | |||||
| Initial | 5.9 (10.2) | 6.0 (10.3) | 6.0 (10.3) | 5.9 (10.2) | 6.0 (10.6) |
| Change | −0.87 (6.9) | −0.78 (6.9) | −0.72 (7.1) | −0.65 (7.2) | −0.66 (8.1) |
| Initial BMI, kg/m2 | 25.6 (4.8) | 25.4 (4.7) | 25.3 (4.7) | 25.3 (4.8) | 25.4 (4.7) |
| Weight change, kg | 1.2 (5.5) | 1.2 (5.1) | 1.2 (5.14) | 1.2 (5.3) | 1.0 (5.9) |
| Physical activity, MET h/week | |||||
| Initial | 15.3 (20.5) | 15.3 (22.9) | 15.3 (21.4) | 15.2 (20.3) | 15.3 (21.5) |
| Change | 0.71 (21.7) | 0.92 (24.0) | 1.2 (22.3) | 1.7 (21.2) | 2.1 (22.3) |
| White ethnicity, % | 97.3 | 97.6 | 97.7 | 97.7 | 97.4 |
| Current smoker, % | 18.2 | 18.4 | 18.6 | 18.8 | 18.7 |
| Hypertension, % | 32.0 | 31.5 | 31.8 | 31.9 | 32.3 |
| High cholesterol, % | 38.8 | 38.4 | 40.3 | 42.3 | 46.4 |
| Family history of diabetes, % | 27.7 | 27.5 | 27.3 | 27.5 | 28.0 |
| NHS II (n = 81,569) | |||||
| Participants, n | 10,198 | 19,258 | 25,274 | 17,904 | 8,935 |
| Age, years† | 40.9 (5.1) | 41.1 (5.3) | 41.1 (5.4) | 41.3 (5.6) | 41.3 (5.7) |
| Overall PDI | |||||
| Initial | 59.8 (5.7) | 57.0 (5.9) | 54.5 (5.9) | 52.3 (5.8) | 49.4 (5.8) |
| Change | −10.7 (2.6) | −4.8 (1.4) | −0.01 (1.4) | 4.7 (1.4) | 10.7 (2.6) |
| Energy intake, kcal/day | |||||
| Initial | 1,927 (548) | 1,851 (548) | 1,782 (549) | 1,717 (530) | 1,639 (511) |
| Change | −239 (501) | −89 (470) | 38 (464) | 141 (470) | 285 (508) |
| Alcohol intake, g/day | |||||
| Initial | 3.4 (6.4) | 3.3 (6.2) | 3.3 (6.5) | 3.3 (6.4) | 3.3 (6.5) |
| Change | 0.37 (5.6) | 0.37 (5.2) | 0.38 (5.3) | 0.45 (5.5) | 0.49 (5.6) |
| Initial BMI, kg/m2 | 24.8 (5.3) | 24.5 (5.2) | 24.5 (5.2) | 24.7 (5.3) | 24.7 (5.3) |
| Weight change, kg | 3.2 (6.7) | 3.0 (6.3) | 3.1 (6.3) | 3.0 (6.5) | 2.8 (6.7) |
| Physical activity, MET h/week | |||||
| Initial | 24.4 (33.6) | 23.8 (35.5) | 23.8 (35.3) | 23.6 (33.7) | 24.5 (33.6) |
| Change | −2.9 (32.1) | −2.7 (33.4) | −3.2 (32.9) | −2.8 (32.1) | −3.4 (31.9) |
| White ethnicity, % | 96.5 | 96.8 | 96.9 | 96.8 | 96.2 |
| Current smoker, % | 12.4 | 11.5 | 11.0 | 11.2 | 11.1 |
| Hypertension, % | 9.8 | 8.7 | 8.5 | 8.6 | 8.9 |
| High cholesterol, % | 21.2 | 20.4 | 19.9 | 20.0 | 20.5 |
| Family history of diabetes, % | 34.6 | 34.8 | 34.5 | 34.3 | 34.3 |
| HPFS (n = 34,468) | |||||
| Participants, n | 3,229 | 7,752 | 11,174 | 8,241 | 4,072 |
| Age, years† | 57.4 (9.7) | 57.5 (9.8) | 57.4 (9.6) | 57.7 (9.7) | 57.7 (9.5) |
| Overall PDI | |||||
| Initial | 59.8 (5.8) | 57.0 (5.9) | 54.9 (6.1) | 52.8 (6.0) | 50.2 (5.9) |
| Change | −10.5 (2.4) | −4.7 (1.4) | 0.01 (1.4) | 4.7 (1.4) | 10.7 (2.5) |
| Energy intake, kcal/day | |||||
| Initial | 2,140 (628) | 2,061 (627) | 2,013 (631) | 1,950 (614) | 1,868 (574) |
| Change | −361 (549) | −189 (484) | −68 (485) | 38 (491) | 169 (531) |
| Alcohol intake, g/day | |||||
| Initial | 11.2 (15.4) | 11.5 (15.0) | 11.3 (15.0) | 11.8 (15.6) | 11.7 (15.3) |
| Change | −1.1 (11.2) | −1.2 (10.0) | −0.87 (9.8) | −1.0 (10.7) | −1.1 (11.3) |
| Initial BMI, kg/m2 | 25.6 (3.2) | 25.5 (3.1) | 25.4 (3.2) | 25.4 (3.1) | 25.4 (3.2) |
| Weight change, kg | 0.85 (4.3) | 0.8 (4.1) | 0.70 (4.1) | 0.55 (4.1) | 0.21 (4.5) |
| Physical activity, MET h/week | |||||
| Initial | 20.4 (26.8) | 20.1 (26.4) | 19.8 (27.4) | 19.8 (25.4) | 19.7 (25.9) |
| Change | 0.59 (24.9) | 0.92 (25.3) | 2.0 (25.9) | 1.9 (23.5) | 3.1 (24.7) |
| White ethnicity, % | 94.9 | 95.8 | 95.9 | 95.5 | 95.6 |
| Current smoker, % | 8.1 | 9.3 | 8.0 | 8.4 | 7.9 |
| Hypertension, % | 26.1 | 24.7 | 24.8 | 25.4 | 26.0 |
| High cholesterol, % | 28.1 | 28.8 | 29.5 | 32.1 | 39.3 |
| Family history of diabetes, % | 26.1 | 26.0 | 25.8 | 25.7 | 25.3 |
Data are means (SD) unless otherwise indicated and are standardized to the age distribution of the study population.
Value is not age adjusted.
Additionally, we observed that the main driver of changes in the PDIs over 4 years was the change in healthy plant food intake (Supplementary Table 3). Changes in the intake of unhealthy plant foods and of animal foods had a smaller impact on changes in PDIs.
Table 2 shows pooled HRs for incident type 2 diabetes in the subsequent 4 years according to updated 4-year changes in adherence to plant-based diet indices. In pooled multivariable analysis (model 2), compared with participants who maintained a relatively stable PDI (±3%), individuals with the largest decreases in PDI (>10%) over a 4-year period had a 12% (95% CI 5, 20) higher risk of type 2 diabetes in the subsequent 4 years, whereas individuals with the largest 4-year increases in PDI (>10%) had a 9% (3, 14) lower risk of type 2 diabetes in the subsequent 4 years. Each 10% incremental increase in the PDI score was associated with a 7% (5, 9) lower risk of type 2 diabetes. Likewise, compared with participants with stable hPDI scores, those with the largest 4-year decrease in hPDI (>10%) scores had a 23% (16, 31) higher risk of type 2 diabetes in the subsequent 4-year period. Each 10% incremental increase in the hPDI score was associated with a 9% (5, 13) lower risk of type 2 diabetes. Changes in uPDI were not associated with risk of type 2 diabetes. Results were roughly similar across each cohort (Supplementary Table 4).
Table 2.
Pooled HRs for type 2 diabetes according to updated 4-year changes in plant-based diets
| Decrease | No change or relatively stable (± <3%) | Increase | Ptrend* | Per 10% score increase | |||
|---|---|---|---|---|---|---|---|
| Large (>10%) | Small to moderate (3–10%) | Small to moderate (3–10%) | Large (>10%) | ||||
| Overall PDI score, pooled | |||||||
| Model 1 | 1.26 (1.11, 1.43)† | 1.10 (1.04, 1.15) | 1.00 | 0.92 (0.88, 0.96) | 0.91 (0.85, 0.96) | <0.0001 | 0.89 (0.87, 0.91) |
| Model 2 | 1.12 (1.05, 1.20) | 1.05 (1.00, 1.11) | 1.00 | 0.93 (0.89, 0.98) | 0.91 (0.86, 0.97) | <0.0001 | 0.93 (0.91, 0.95) |
| hPDI score, pooled | |||||||
| Model 1 | 1.47 (1.34, 1.62)† | 1.17 (1.12, 1.23) | 1.00 | 0.95 (0.90, 0.99) | 0.98 (0.84, 1.14)† | 0.0002† | 0.86 (0.80, 0.92)† |
| Model 2 | 1.23 (1.16, 1.31) | 1.11 (1.05, 1.16) | 1.00 | 0.97 (0.92, 1.02) | 0.97 (0.85, 1.10)† | 0.002† | 0.91 (0.87, 0.95)† |
| uPDI score, pooled | |||||||
| Model 1 | 1.07 (0.93, 1.23)† | 1.06 (0.98, 1.15)† | 1.00 | 1.05 (0.99, 1.10) | 1.19 (1.12, 1.27) | 0.77† | 1.01 (0.94, 1.09)† |
| Model 2 | 1.03 (0.92, 1.17)† | 1.07 (0.98, 1.16)† | 1.00 | 1.02 (0.97, 1.07) | 1.06 (0.99, 1.13) | 0.78† | 0.99 (0.94, 1.05)† |
Data are HR (95% CI). Model 1: adjustment for age and initial corresponding plant-based diet score (overall, healthful, or unhealthful, quintiles) and stratification by calendar year in 4-year intervals. Model 2: additional adjustment for ethnicity (White, non-White), family history of diabetes (yes/no), initial and change in total energy (kcal/day, quintiles), initial and change in alcohol intake (g/day, quintiles), initial and change in margarine intake (serving/day, quintiles), initial and change in physical activity (MET h/week, quintiles), change in smoking status (never to never, never to current, past to past, past to current, current to past, current to current, or missing indicator), initial BMI (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, ≥32.0 kg/m2), medical history of hypertension (yes/no, updated every 4 years), medical history of hypercholesterolemia (yes/no, updated every 4 years), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator) (NHS and NHS II), and oral contraceptive use (never, current, past, missing indicator) (NHS II).
The P value when we assigned the median value to each category and entered this as a continuous variable in the model. We calculated pooled results using an inverse variance–weighted, fixed-effects meta-analysis, unless specified otherwise.
We calculated pooled results using a random-effects meta-analysis because of statistically significant heterogeneity across studies assessed by Cochran Q test, with P < 0.1.
Our findings remained consistent in several sensitivity analyses. When we further adjusted our final multivariable model for concurrent weight change, results remained similar (Supplementary Table 5). We also estimated that concurrent weight change statistically accounted for 6.0% (95% CI 2.9, 12.0) of the association between changes in overall plant-based diet (per 10% increase) with subsequent diabetes risk and 35.6% (27.3, 45.0) of the associations between changes in healthful plant-based diets (per 10% increase) and subsequent diabetes risk. Our results remained unchanged when we performed a 4-year lagged analysis and an 8-year changes analysis and when we restricted the cases to symptomatic diabetes (cases, n = 5,385) (Supplementary Table 6). The significant associations between changes in PDIs and subsequent type 2 diabetes persisted when we stratified by potential effect modifiers and none of the tested interaction terms was statistically significant (Fig. 1 and Supplementary Table 7). For example, the associations remained similar across low, medium, and high baseline PDIs (P values for interactions of baseline PDI, hPDI, and uPDI were 0.74, 0.47, and 0.54, respectively). In addition, compared with participants whose healthy plant food intake remained relatively stable, those with the greatest decrease in intake of healthy plant foods over a 4-year period had a 10% (95% CI 4, 17) higher risk in a subsequent 4-year period (Supplementary Table 8). Participants with the greatest increase in animal foods had a 10% (4, 17) higher risk of type 2 diabetes. In line with this, each 1-serving incremental increase of animal foods was associated with 2% higher risk of type 2 diabetes (HR 1.02 [95% CI 1.01, 1.03]) (Supplementary Table 8). We found no associations between changes in intake of unhealthy plant foods and diabetes risk.
Figure 1.
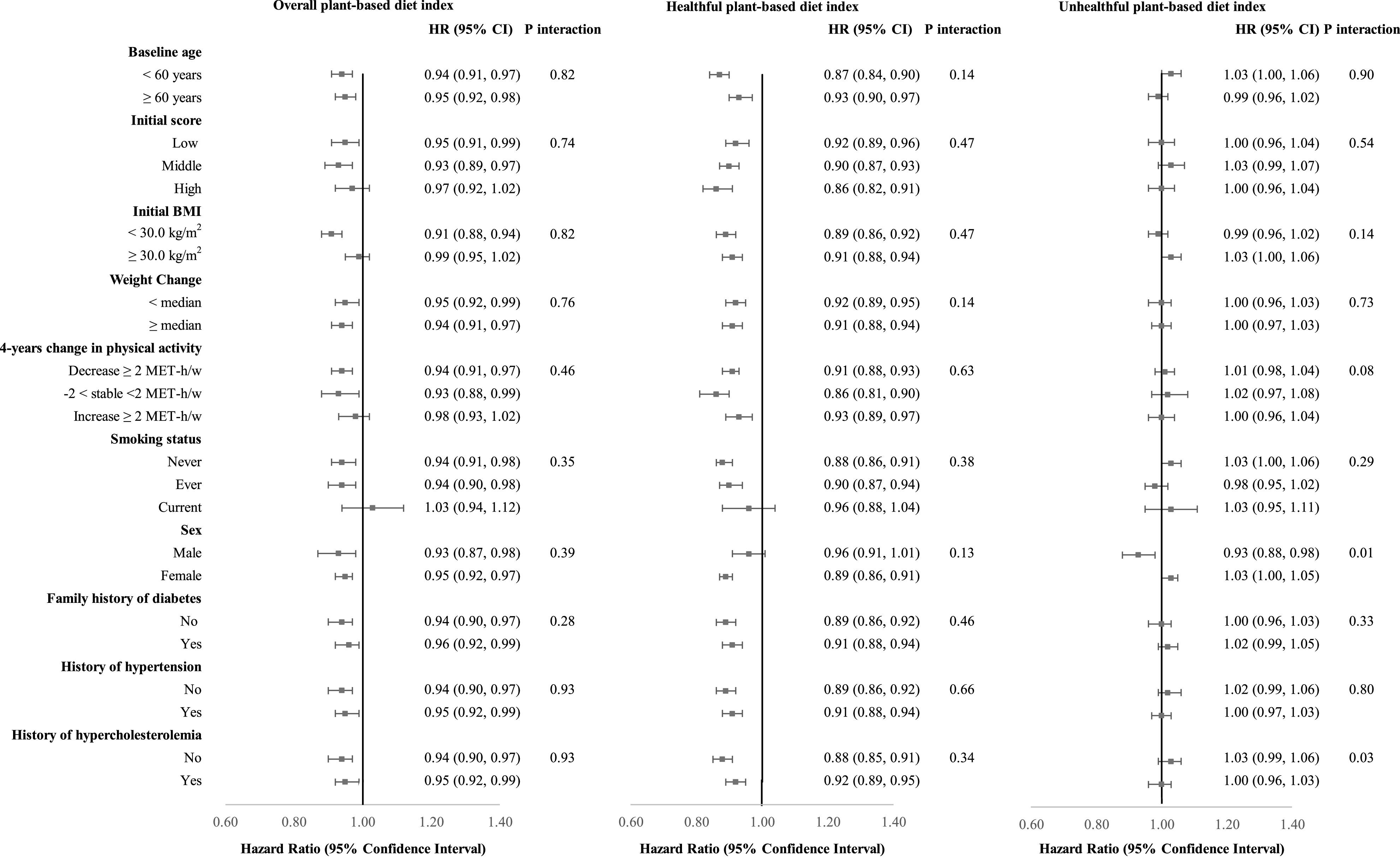
Subgroup analyses between changes in plant-based diets and subsequent type 2 diabetes risk per 10% score increase in each index score. Values are reported as HR (95% CI). Results were adjusted for age, adjusted for initial corresponding plant-based diet score (overall, healthful, or unhealthful, quintiles), stratified by calendar year in 4-year intervals, and adjusted for ethnicity (White, non-White), family history of diabetes (yes/no), initial and change in total energy (kcal/day, quintiles), initial and change in alcohol intake (g/day, quintiles), initial and change in margarine intake (serving/day, quintiles), initial and change in physical activity (MET h/week [w], quintiles), change in smoking status (never to never, never to current, past to past, past to current, current to past, current to current, or missing indicator), initial BMI (<21.0, 21.0–24.9, 25.0–29.9, 30.0–31.9, ≥32.0 kg/m2), medical history of hypertension (yes/no, updated every 4 years), medical history of hypercholesterolemia (yes/no, updated every 4 years), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal + current use, postmenopausal + past use, postmenopausal + never use, missing indicator) (NHS and NHS II), and oral contraceptive use (never, current, past, missing indicator) (NHS II) (based on model 2 as defined in Table 2). P values for interaction were calculated with use of the likelihood ratio test. Given the potential for multiple testing, the statistical level for significance was set at 0.005 (0.05/10 comparisons).
Conclusions
In these three large cohort studies of U.S. women and men, we observed that improved adherence to overall and healthful plant-based diets over 4 years was associated with a lower risk of type 2 diabetes in the subsequent 4 years. On the contrary, decreased adherence to overall and healthful plant-based diets over 4 years was associated with a 12–23% higher subsequent risk of type 2 diabetes. Concurrent body weight changes partially explained these associations.
A recent meta-analysis documented inverse associations between overall and healthful plant-based diets with type 2 diabetes risk, with estimates ranging from 23 to 30% lower type 2 diabetes risk for greater adherence to overall and healthful plant-based diets (4). Studies included in this meta-analysis evaluated the relation between either a baseline or an updated measure of adherence to plant-based diets and diabetes risk. However, this approach does not capture changes that individuals make to their dietary habits. Understanding how changes in adherence to plant-based diets are associated with subsequent diabetes risk allows us to mimic an intervention study where individuals make real-life changes to their adherence to plant-based diets. The findings of the current study not only confirm previous reports but also demonstrate that both 4-year and longer-term (8-year) improvements in adherence to overall and healthful plant-based diets are associated with lower diabetes risk.
An important issue in examining changes in plant-based diets and subsequent risk of type 2 diabetes is to adequately control for initial and concomitant changes in other behaviors (such as changes in physical activity and smoking), dietary factors (such as changes in energy intake and alcohol intake), and time-varying measures of subclinical risk factors of diabetes (such as hypertension and hypercholesterolemia). For example, in our study, participants with the greatest increase in PDI scores had lower initial PDI scores and higher initial total energy intake. Therefore, we adjusted not only for initial and changes in covariates but also for initial PDI, as participants with lower initial PDI scores tended to have increases in their PDIs scores. Furthermore, to consider the ceiling effect of the initial diet quality, we stratified our analysis by the initial PDI and observed similar results. The robustness of our findings in sensitivity analyses suggests that improving adherence to overall and healthful plant-based diets may lower type 2 diabetes risk, irrespective of baseline diet quality.
Our present results on hPDI are consistent with those of recent meta-analyses showing higher diet quality scores that focus on plant foods such as the Alternate Healthy Eating Index (AHEI), Alternate Mediterranean Diet, Dietary Approaches to Stop Hypertension, and the Healthy Eating Index-2010 (28,29). However, studies included in these meta-analyses did not examine short- or long-term changes in diet. Recently, Ley et al. (30) examined associations between 4-year changes in AHEI and subsequent risk of type 2 diabetes. In line with our findings, Ley et al. (30) observed that each 10% incremental increase in AHEI score over a 4-year period was associated with an 11% lower risk of type 2 diabetes in the subsequent 4 years. However, while the AHEI focuses on overall diet quality, our plant-based diet indices focus on the quality of plant foods and score all animal foods negatively, irrespective of their health benefits. Our additional analyses also showed that increases in consumption of healthy plant foods over a 4-year period were associated with lower diabetes risk in the subsequent 4 years, while increases in consumption of animal foods were associated with higher risk of diabetes. Our results extend the previous findings to suggest that shifting consumption of animal foods away toward more consumption of healthy plant foods over time is followed by a lower diabetes risk.
The mechanisms through which plant-based diets improve diabetes risk can be multifactorial. In the current study, the largest contributors to improvement in both PDI and hPDI were healthy plant food groups such as whole grains, fruits, vegetables, tea, and coffee. Higher intakes of these healthy plant food groups have been associated with lower risk of type 2 diabetes through various biological pathways, such as weight control, anti-inflammation, antioxidant, and improved gut microbiome, due to higher intake of dietary fiber and polyphenols and lower intake of saturated fat in these healthy plant food groups (31–34). On the other hand, decreased intakes of animal foods also contributed to the inverse associations of improved PDI and hPDI with type 2 diabetes risk. The associations may be partially explained by some components of animal foods, such as heme iron, animal protein, and saturated fat, found mainly in animal foods, such as red and processed meat and poultry (35–37). These components have been associated with higher risk of diabetes in several prospective cohort studies through various pathways, such as oxidative stress, proinflammation, and weight gain (36,38,39). Indeed, in our present study, we observed a potential mediation effect of weight change in the associations of overall and healthful plant-based diets and subsequent diabetes risk, and the potential mediation effect was stronger for hPDI. In a previous analysis, using the same cohorts, Satija et al. (25) observed that a 1-SD increase in hPDI was associated with 0.68 kg less weight gain over 4-year periods, while a 1-SD increase in PDI was associated with 0.04 kg less weight gain over 4-year periods. Additionally, in the current study, we observed no associations between changes in unhealthful plant-based diet and subsequent risk of type 2 diabetes. Changes in uPDI were driven by changes in all three foods groups: unhealthy plant foods, healthy plant foods, and animal foods. In line with previous analyses focusing on cumulative intakes of unhealthy plant foods (19), we observed no associations between changes in unhealthy plant foods and subsequent diabetes risk. Moreover, for changes in uPDI, a harmful association of decreased intakes of healthy plant foods could be compensated by a beneficial association of decreased intakes of animal foods, potentially resulting in a null net association.
Strengths and Limitations
The strengths of the current study include a large sample size with high follow-up rates. The presence of repeated assessments of dietary and lifestyle variables provided us with the unique ability to investigate the associations of dynamic changes in plant-based diets, independent of initial intakes, with type 2 diabetes risk. While randomized controlled trials are ideal to address the true causal associations between changes in plant-based diets and type 2 diabetes risk, such studies are difficult to conduct, given the long follow-up time needed for the development of type 2 diabetes, high costs, uncertainty regarding the ideal intervention period, and poor adherence to longer-term dietary interventions. In our present study we, can, to some extent, address these methodological difficulties by taking advantage of the long follow-up period and the availability of repeated measures of diet and lifestyles in our cohorts.
The current study has several limitations. First, measurement errors in dietary intake using FFQs are inevitable, and random error in the setting of a prospective cohort study may have led to an underestimation of associations. Second, our study participants were health professionals of primarily European ancestry, which could limit the generalizability of the findings to other populations of different nationalities and races. Furthermore, the observed associations may also not be generalizable to other populations with different sociodemographic and lifestyle characteristics. However, their occupations are a distinct advantage, as this allows us to collect high-quality data using self-reported questionnaires and enhance the internal validity of the study by reducing confounding by socioeconomic status. Furthermore, the health benefits of plant-based diets assessed by baseline PDIs have been observed in other populations (7,8). Finally, due to the observational nature of the study design, we cannot exclude the presence of residual confounding, such as changes in sleep habits and stress, which may be caused by the fact that changes in diet are usually accompanied by changes in other lifestyle behaviors related to diabetes (40).
In conclusion, improving adherence to overall and healthful plant-based diets over time was associated with a lower risk of developing type 2 diabetes in the subsequent years in prospective cohorts of U.S. adults, whereas decreasing adherence to such plant-based diets was associated with a higher risk of the development of type 2 diabetes in the subsequent years.
Article Information
Funding. This study was funded by the National Institutes of Health with research grants UM1 CA186107, P01 CA87969, R01 CA49449, R01 DK112940, R01 HL60712, P30 DK46200, R01 HL034594, R01 HL088521, U01 CA176726, R01 CA67262, and U01 CA167552. Z.C. was partially supported by a fellowship from the Nutricia Research Foundation (2019-T2). J.-P.D.-C. was supported by a Banting Postdoctoral Fellowship from the Canadian Institutes of Health Research (BPF-156628). M.Y.B. was supported by a fellowship from the Manpei Suzuki Diabetes Foundation.
The funders had no role to play in the design of the study or in the interpretation of the results.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Z.C. developed the hypotheses, conducted the analyses, interpreted the data, and drafted, reviewed, and edited the manuscript. J.-P.D.-C. contributed to the development of the hypotheses and the analysis and reviewed and edited the manuscript. Y.L. contributed to the development of the hypotheses and reviewed and edited the manuscript. M.Y.B. contributed to the analysis and reviewed and edited the manuscript. J.E.M., W.C.W., and T.V. reviewed and edited the manuscript. F.B.H. contributed to the development of the hypothesis, interpreted the data, and reviewed and edited the manuscript. S.N.B. contributed to the development of the hypotheses and the analysis, interpreted the data, and reviewed and edited the manuscript. Z.C. and S.N.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13312577.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.International Diabetes Federation . International Diabetes Federation Diabetes Atlas, 9th edition. Brussels, Belgium, International Diabetes Federation. Accessed 20 March 2020. Available from https://diabetesatlas.org/en/resources/ [Google Scholar]
- 2.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millen BE, Abrams S, Adams-Campbell L, et al. The 2015 Dietary Guidelines Advisory Committee scientific report: development and major conclusions. Adv Nutr 2016;7:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med 2019;179:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis 2013;23:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koloverou E, Panagiotakos DB, Georgousopoulou EN, et al.; ATTICA Study Group . Dietary patterns and 10-year (2002-2012) incidence of type 2 diabetes: results from the ATTICA cohort study. Rev Diabet Stud 2016;13:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Zuurmond MG, van der Schaft N, et al. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam Study. Eur J Epidemiol 2018;33:883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G-C, Koh W-P, Neelakantan N, Yuan J-M, Qin L-Q, van Dam RM. Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese Health Study. Am J Epidemiol 2018;187:2651–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian DJJ. Dietary intake among US adults, 1999-2012. JAMA 2016;315:2542–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health 2016;106:1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468 [DOI] [PubMed] [Google Scholar]
- 12.Drouin-Chartier J-P, Li Y, Ardisson Korat AV, et al. Changes in dairy product consumption and risk of type 2 diabetes: results from 3 large prospective cohorts of US men and women. Am J Clin Nutr 2019;110:1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 14.Agricultural Research Service, U.S. Department of Agriculture . Welcome to the USDA National Nutrient Database for Standard Reference, 2015. Accessed 4 June 2015. Available from https://fdc.nal.usda.gov/data-documentation.html
- 15.Mullie P, Clarys P, Hulens M, Vansant G. Reproducibility and validity of a semiquantitative food frequency questionnaire among military men. Mil Med 2009;174:852–856 [DOI] [PubMed] [Google Scholar]
- 16.Willett W, Lenart EJ. Reproducibility and validity of food-frequency questionnaires. In Nutritional Epidemiology. Larent E, Willett W, Eds. Oxford, U.K., Oxford University Press, 2013, pp. 96–117 [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–249 [DOI] [PubMed] [Google Scholar]
- 19.Satija A, Bhupathiraju SN, Rimm EB, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 21.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Rimm EB, Stampfer M, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 24.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581–1586 [DOI] [PubMed] [Google Scholar]
- 25.Satija A, Malik V, Rimm EB, Sacks F, Willett W, Hu FB. Changes in intake of plant-based diets and weight change: results from 3 prospective cohort studies. Am J Clin Nutr 2019;110:574–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Schoufour JD, Rivadeneira F, et al. Plant-based diet and adiposity over time in a middle-aged and elderly population: the Rotterdam Study. Epidemiology 2019;30:303–310 [DOI] [PubMed] [Google Scholar]
- 27.Lin D, Fleming T, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–1527 [DOI] [PubMed] [Google Scholar]
- 28.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2018;118:74–100.e11 [DOI] [PubMed] [Google Scholar]
- 29.Schwingshackl L, Missbach B, König J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr 2015;18:1292–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley SH, Pan A, Li Y, et al. Changes in overall diet quality and subsequent type 2 diabetes risk: three U.S. prospective cohorts. Diabetes Care 2016;39:2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Hébert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition 2008;24:941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H, Xia M, Zou T, Ling W, Zhong R, Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem 2012;23:349–360 [DOI] [PubMed] [Google Scholar]
- 33.Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H, Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr 2015;102:172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holscher HD, Caporaso JG, Hooda S, Brulc JM, Fahey GC Jr., Swanson KS. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr 2015;101:55–64 [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira Otto MC, Alonso A, Lee D-H, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr 2012;142:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol 2016;183:715–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–424 [DOI] [PubMed] [Google Scholar]
- 38.Van Woudenbergh GJ, Kuijsten A, Tigcheler B, et al. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: the Rotterdam Study. Diabetes Care 2012;35:1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med 2018;28:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.St-Onge M-P, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr 2016;7:938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]


