Abstract
Problem:
Previous studies identified circulating CD14+HLA-DRlo/-monocytic cells as an immune suppressive subset in solid malignancies, such as prostate, renal cell carcinoma, and pancreatic cancer. Such monocytic cells have been implicated not only in tumor progression but also as a potential barrier for immunotherapy. This study examined the relationship between the frequency of circulating monocytic cells and epithelial ovarian cancer (EOC) progression pre- and post-frontline chemotherapy, defined by disease stage, which is a leading prognostic factor for this malignancy.
Method of study:
Incident cases of 236 women with EOC were recruited and comprehensive flow cytometry was utilized to assess the frequency of peripheral blood CD33+CD11b+HLA-DR−/lowCD14+CD15− monocytic cells, henceforth termed CD14+HLA-DRlo/- monocytic cells, prior to and after completion of frontline chemotherapy. Multivariable odds ratios (OR) were used to estimate the association between CD14+HLA-DRlo/- monocytic cell percentages and disease stage. Wilcoxon signed-rank tests evaluated changes in these monocytic cell levels pre- and post-chemotherapy in a patient subset (n=70).
Results:
Patients with elevated frequencies of circulating CD14+HLA-DRlo/- monocytic cells at diagnosis were at 3.33-fold greater odds of having advanced stage (III/IV) EOC (CI: 1.04–10.64), with a significant trend in increasing CD14+HLA-DRlo/- monocytic cell levels (p=0.04). There was a 2.02% median decrease of these monocytic cells post-chemotherapy among a subset of patients with advanced stage disease (p<0.0001).
Conclusion:
These findings support the potential clinical relevance of CD14+HLA-DRlo/- monocytic cells in EOC for prognosis and may indicate a non-invasive biomarker to measure disease progression.
Keywords: Monocytes, MDSC, ovarian cancer, biomarker, monocytic cells
Introduction
Ovarian cancer has a low rate of incidence, but a high rate of mortality [1]. Most women diagnosed with this disease have distant metastasis at initial presentation, with a five-year relative survival of approximately 30% [1–3]. Immunosuppression in ovarian cancer may represent an important mechanism for poorer survival; therefore, considerable interest is devoted to the design of immune-based therapeutic interventions that convert a pro-tumor- to an antitumor microenvironment to prolong survival [4].
Various types of immune cells within both the adaptive and innate immune systems have been shown to either inhibit or promote the growth of epithelial ovarian cancer (EOC) [5]. On the one hand, tumor-infiltrating CD8+ T cells of the adaptive immune system have been associated with improved prognostic outcomes among women with EOC. For example, among women with high-grade serous EOC, increasing levels of tumor-infiltrating lymphocytes are associated with improved survival [6]. Furthermore, CD4+ T cells of the adaptive immune system have been associated with improved EOC prognosis, largely related to their role in expanding the antitumor CD8+ T cell response [5]. On the other hand, it has been well documented that interactions with other immune cells, such as those of the innate immune system, namely the myeloid compartment, can inhibit T cell activity and aid solid tumor progression, including metastasis [5, 7–10].
Such myeloid cells can be further divided into specific populations derived from either the granulocytic or the monocytic lineage. The granulocytic lineage of myeloid cells includes populations of neutrophils and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) associated with the progression of solid tumors, including EOC [8, 9, 11, 12]. The monocytic lineage of myeloid cells consists of macrophages, monocytes, dendritic cells, and monocytic MDSCs (M-MDSCs) [5, 8, 13]. The work presented in this study focuses on monocytic cells in EOC progression, which are of particular interest given their capacity to promote immune suppression, tumor angiogenesis and limit immunotherapy efficacy [8, 14–16]. Given that immunotherapeutic approaches in women with EOC have been largely unsuccessful, identifying potential barriers for such therapies, such as immune suppressive myeloid cells, may guide future approaches to enhance treatment success [17].
Monocytic cells that contribute to tumor growth and metastasis have been identified in the ovarian tumor microenvironment, and continue to be of interest to study with regard to EOC prognosis [18]. An immunosuppressive subset of monocytic cells defined as CD14+HLA-DRlo/-, which may contain monocytes and M-MDSCs, have been observed across diverse solid malignancies [14, 16, 19–22]. Such cells act to inhibit T cell responses and, importantly, a high lymphocyte-to-monocyte ratio has been found to be beneficial for EOC prognosis [7, 8, 23, 24]. However, the impact of the association between these CD14+HLA-DRlo/- monocytic cells and EOC disease progression or prognosis has not been rigorously studied with respect to the consistency of cell surface markers needed to identify this cell population, as well as recognition of clinical variables to address potential confounding, and small sample sizes across existing studies [25–30]. Therefore, we examined the relevance of this cell population as a potential non-invasive biomarker in the peripheral blood of EOC patients for its association with progression.
Methods
Data collection
Pre- and post-treatment peripheral blood samples and clinical data were collected on patients with incident ovarian cancer seen at Hillman Cancer Center (HCC, n=133), Roswell Park Comprehensive Cancer Center (n=88), and Mayo Clinic-Rochester (Mayo, n=15) between 2016 and 2019. Clinical and demographic data collected from medical records and self-administered questionnaires included: age at diagnosis, presence of ascites (yes/no), tumor grade (well/moderately differentiated or poorly differentiated), FIGO stage (I/II or III/IV), tumor histotype (serous or non-serous), body mass index at diagnosis (BMI), age at menopause (<50 years or ≥50 years), smoking history including pack-years, and history of autoimmune disease (any vs none).
Sample processing
Peripheral blood was collected by trained hospital phlebotomists in an EDTA-containing lavender top tube, prior to any treatment; additionally, a second sample was collected on patients from HCC and Roswell Park when possible (n=70), after completion of first-line treatment. Whole-blood samples collected from patients at HCC and Mayo Clinic were shipped ambient to Roswell Park overnight, where they were processed along with samples from Roswell Park patients. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of patients using lymphocyte separation medium (LSM) (Mediatech, 25–072-CV) and 50 mL SepMate tubes (Stemcell Technologies, 85450) following manufacturer’s protocol, followed by centrifugation in a SepMate at 1200g for 10 minutes. Cells in interphase were collected and centrifuged with Dulbecco’s Phosphate-Buffered Solution (DPBS) at 1200g for 10 minutes. The cell pellet was cryopreserved in freezing medium (70% RPMI, 20% FBS, 10% DMSO), aliquoted into 1.5 mL cryovials, which were placed into containers with alcohol for controlled rate freezing, placed in a −80 freezer for 2–24 hours, then for long-term storage in liquid nitrogen. Samples underwent controlled cell thawing prior to flow cytometry.
Flow cytometry
Cells for immunophenotyping were pre-blocked using 200μg of mouse IgG (Invitrogen) at room temperature for 30 minutes. Up to 1×106 cells per evaluation were incubated with a reactive monoclonal antibody mixture (Table 1), while in a dark environment for 30 minutes. Cells were fixed with 0.5% paraformaldehyde in PBS. Acquisition was performed using a FACSCanto (BD BioSciences, San Jose, CA) flow cytometer equipped with 405 nm, 488 nm, and 640 nm lasers. Minimum target event count was set at 150,000. The raw flow cytometry data were then analyzed using WinList 3.0 (Verity Software House). Statistical analysis examined percent of CD45+ mononuclear cells using a panel of surface markers for monocytic cells of interest (Lymphoid lineage negative, CD33+CD11b+HLA-DRlo/-CD14+CD15−). Gating strategy for flow cytometry is demonstrated in Figure 1.
Table 1.
Antibodies used in flow cytometry analysis
| Fluorophore | Immune marker | Supplier details |
|---|---|---|
| FITC | CD11b | BC Bear1 |
| PerCP | CD45 | BD clone 2D1 |
| PECy7 | CD33 | BC clone D3HL60.251 |
| APC | HLA-DR | Thermo Fisher clone TU36 |
| APCH7 | CD14 | BD clone MφP9 |
| V450 | CD15 | BD clone MMA |
| BV510 | DUMP (CD3, CD19, CD56) | CD3: BD HIT3a; CD19: BioLegend HIB19 CD56: BD NCAM16.2 |
Beckman Coulter (BC), BD Bioscience (BD)
Figure 1.
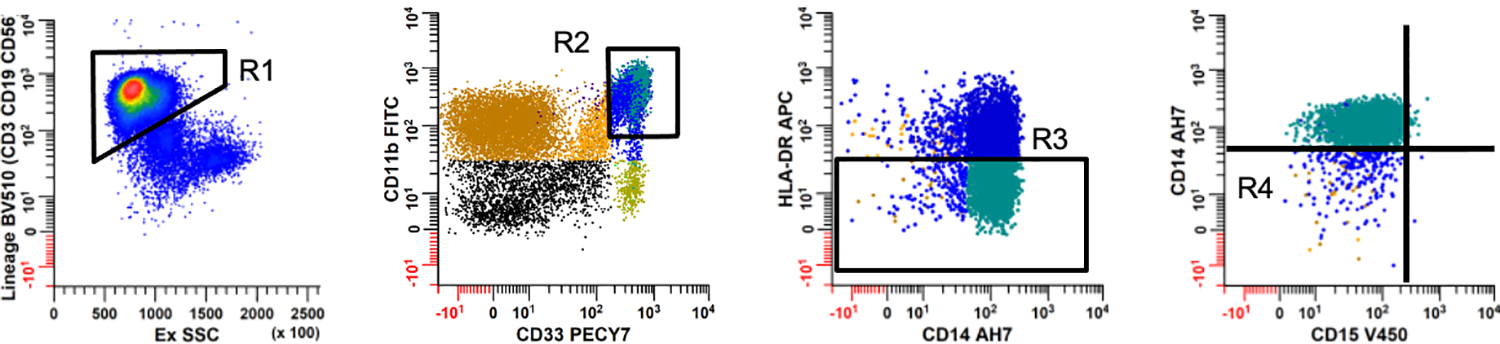
Representative strategy used to identify CD14+HLA-DRlo/- monocytic cell subsets in women with epithelial ovarian cancer. Mononuclear cells were analyzed using a series of sequential regions to exclude debris, apoptotic events, aggregates and CD45 negative events (not shown). Panel 1: A series of regions were created to exclude lineage positive (CD3, CD19, and CD56) cells (R1); Panel 2: CD33 and CD11b dual positive cells (R2); and Panel 3: HLADR negative to dim cells (R3). Panel 4: The CD15 vs. CD14 dotplot gated on R2, R3, and excluding cells in R1 was used to define CD14+HLA-DRlo/- monocytic cells (excludes cells in R4).
Statistical analysis
CD14+HLA-DRlo/- monocytic cells were categorized into levels by tertile cut-off measurements (low: <3.6%, intermediate: 3.6–9.2%, high: ≥9.3%). Age at diagnosis, BMI at diagnosis, and lifetime smoking pack-years were analyzed in relation to monocytic cell tertiles by ANOVA testing. All other categorical variables were evaluated using chi-square tests to identify any significant differences in clinical and demographic variables among monocytic cell tertiles of patients. To estimate the association between monocytic cell levels and tumor stage, multivariable odds ratios (OR) with corresponding 95% confidence intervals (CIs) were calculated, adjusting for shipment, tumor histotype and tumor grade. The decision to not include presence of ascites in the multivariable model was made a priori, given the strong collinearity of stage and ascites, and that ascites is thought to be a direct consequence of stage, therefore, is not a confounder [31]. Stratified analysis for site (Roswell Park, HCC) were performed using multivariable logistic regression to examine the association between CD14+HLA-DRlo/- monocytic cells and disease stage to compare ORs among patients from different clinic sites. We were unable to look at site specific ORs for Mayo Clinic, due to small sample size. To examine how tumor histology may play a role in CD14+HLA-DRlo/- monocytic cells and the relationship with disease stage, multivariable ORs were performed, stratifying by histology (serous/non-serous). We calculated differences in pre- and post-treatment monocytic cell measurements (continuous % gated) among women with available data (n=70), using Wilcoxon signed-rank tests to examine stage-group differences in the median. For all analyses, p-values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.4.
Results
Patients with the highest tertile of CD14+HLA-DRlo/- monocytic cells (≥9.3% of gated CD45+ cells) were more likely to have advanced stage disease at diagnosis, and present with ascites (Table 2). Those with intermediate or high levels of CD14+HLA-DRlo/- monocytic cells were more likely to have serous histotype (as compared to non-serous consisting of mucinous, endometroid, clear cell, and unspecified epithelial). CD14+HLA-DRlo/- monocytic cell distributions also differed by clinical site, suggesting potential effects from shipment of samples, which was subsequently included in the multivariable model. There were no significant differences in race, BMI, age at menopause, smoking history, history of autoimmune disease, or tumor grade across monocytic cell tertiles. However, with a small sample size of patients identified as a race/ethnicity that is not White, and a small sample size of patients with a documented history of autoimmune disease, these variables may require further analyses in future studies where these populations are larger, and therefore these results described here are limited.
Table 2.
Distribution of clinical and epidemiologic characteristics among patients with ovarian cancer, by level of CD14+HLA-DRlo/- monocytic cells†.
| Characteristics | Low | Intermediate | High | P-Value |
|---|---|---|---|---|
| N | 78 | 80 | 78 | |
| Clinic site: | ||||
| Roswell Park | 23 (29.5%) | 21 (26.3%) | 44 (56.4%) | |
| HCC | 54 (69.2%) | 54 (67.5%) | 25 (32.1%) | |
| Mayo | 1 (1.3%) | 5 (6.3%) | 9 (11.5%) | <.0001 |
| Age at diagnosis: | ||||
| Mean (SD) | 63.1 (11.9) | 63.7 (13.1) | 62.6 (11.2) | 0.83 |
| Race: | ||||
| White | 73 (93.6%) | 72 (90%) | 71 (91%) | |
| Other | 4 (5.1%) | 5 (6.3%) | 7 (9%) | |
| Unknown | 1 (1.3%) | 3 (3.8%) | 0 | 0.36 |
| Age at menopause: | ||||
| <50 years | 23 (29.5%) | 18 (22.5%) | 22 (28.2%) | |
| ≥50 years | 22 (28.2%) | 18 (22.5%) | 17 (21.8%) | |
| Unknown | 33 (42.3%) | 44 (55%) | 39 (50%) | 0.57 |
| BMI: | ||||
| Mean (SD) | 30.6 (7.9) | 30.4 (6.4) | 28.1 (5.6) | 0.08 |
| Smoking history: | ||||
| Never | 34 (43.6%) | 44 (55%) | 36 (46.2%) | |
| Former | (28.2%) | 19 (13.8%) | 19 (24.4%) | |
| Current | 14 (18%) | 15 (18.8%) | 7 (9%) | |
| Unknown | 8 (10.3%) | 10 (12.5%) | 16 (20.5%) | 0.08 |
| Smoking pack years: | ||||
| Mean (SD) | 25.4 (18.8) | 25.3 (17.7) | 20.6 (20.2) | 0.64 |
| Autoimmune disease history‡: | ||||
| Any | 3 (3.9%) | 4 (5%) | 6 (7.8%) | |
| None | 75 (96.1%) | 76 (95%) | 72 (92.3%) | 0.56 |
| Evidence of malignant ascites: | ||||
| Yes | 37 (47.4%) | 46 (57.5%) | 48 (61.5%) | |
| No | 33 (42.3%) | 23 (28.8%) | 14 (18%) | |
| Unknown | 8 (10.3%) | 11 (13.8%) | 16 (20.5%) | 0.02 |
| Tumor grade§: | ||||
| Low | 15 (19.2%) | 11 (13.8%) | 8 (10.3%) | |
| High | 33 (42.3%) | 48 (60%) | 49 (62.8%) | |
| Unknown | 30 (38.5%) | 21 (26.3%) | 21 (26.9%) | 0.09 |
| Tumor histotype¶: | ||||
| Serous | 49 (62.8%) | 66 (82.5%) | 63 (80.8%) | |
| Non-serous | 29 (37.2%) | 14 (17.5%) | 15 (19.2%) | 0.007 |
| FIGO stage: | ||||
| I/II | 36 (46.2%) | 22 (27.5%) | 16 (20.5%) | |
| III/IV | 38 (48.7%) | 48 (60%) | 59 (75.6%) | |
| Unknown | 4 (5.1%) | 10 (12.5%) | 3 (3.9%) | 0.001 |
CD14+HLA-DRlo/- monocytic cells: Low: <3.6%, Intermediate: 3.6–9.2%, High: ≥9.3%
Includes rheumatoid arthritis, systemic lupus erythematosus, Graves’ disease, and Hashimoto’s disease
Low: well or moderately differentiated tumor, High: poorly differentiated tumor
Non-serous histotype includes mucinous, clear cell, endometrioid, and mixed cell type
There was a 3.33-fold greater probability of being diagnosed with advanced stage disease (OR: 3.33, 95% CI: 1.04–10.64) among patients in the highest tertile of CD14+HLA-DRlo/- monocytic cells when compared to the lowest tertile (Table 3a). There was a significant trend in increasing levels of monocytic cells and odds of advanced stage (p=0.04). When stratifying to compare by clinic site in sub-analysis, we did not find significant increased odds of advanced stage disease among women with elevated frequencies of CD14+HLA-DRlo/- monocytic cells diagnosed with ovarian cancer at Roswell Park (OR: 5.35, 95% CI: 0.90–31.69, Supplementary Table 1a) and at HCC (OR: 3.32, CI: 0.46–24.17, Supplementary Table 1b). Though these associations by clinic site were not statistically significant, this is likely a result of decreased sample sizes in the stratified analysis.
Table 3a.
Association between CD14+HLA-DRlo/- monocytic cells percent gated and advanced ovarian cancer stage.
| Monocytic cells | Crude OR (95% CI) | †Adj. OR (95% CI) |
|---|---|---|
| Low (<3.6%) | 1.00 (ref.) | 1.00 (ref.) |
| Intermediate (3.6–9.2%) | 2.07 (1.05–4.08) | 1.51 (0.54–4.18) |
| High (≥9.3%) | 3.49 (1.71–7.15) | 3.33 (1.04–10.64) |
| P for trend | 0.0005 | 0.04 |
Multivariable logistic regression adjusted for shipment, grade and tumor histotype
As previously noted, patients with serous tumors had elevated levels of CD14+HLA-DRlo/- monocytic cells (Table 2). When stratifying by tumor histology, there remained a significant association between increasing CD14+HLA-DRlo/- monocytic cell levels and disease stage among patients with serous ovarian tumor histology (Supplementary Table 2). Patients with serous ovarian tumors presenting with highest levels of CD14+HLA-DRlo/- monocytic cell levels had greater odds of disease progression (OR: 8.81, CI: 1.88–41.27). There was a significant trend demonstrating that increasing CD14+HLA-DRlo/- monocytic cell levels were associated with greater odds of disease progression among patients with serous disease (p=0.005). However, there was no significant association observed among patients with non-serous ovarian tumors, but this may be limited by small sample size of patients with non-serous ovarian cancer.
First-line chemotherapy treatment primarily included platinum and taxane-based regimens, either alone or together, in patients with available post-chemotherapy monocytic cell measurements (n=70), regardless of disease stage. Among these patients with blood samples available for both time points, there was a significant decrease in median CD14+HLA-DRlo/- monocytic cell levels, comparing pre- to post-chemotherapy, among serous ovarian cancer patients (n=49) with advanced stage disease (Table 3b, Figure 2). Median measurements were 8.45% gated pre-treatment, and 4.88% gated post-treatment, and a 2.02% median decrease in monocytic cells (p<0.0001). Furthermore, there was a decrease in these cells post-treatment among patients (n=21) with early stage disease, but it was not statistically significant, likely due to small sample size (1.18% reduction, p=0.19).
Table 3b.
CD14+HLA-DRlo/- monocytic cells levels among serous ovarian cancer patients pre- and post-treatment, by disease stage.‡
| Median CD14+HLA-DRlo/- monocytic cells % gated | ||||
|---|---|---|---|---|
| Disease stage | Pre-treatment | Post-treatment | Median Decrease | P-value |
| Stage I/II (n=21) | 4.93 | 3.57 | 1.18 | 0.19 |
| Stage III/IV (n=49) | 8.45 | 4.88 | 2.02 | <0.0001 |
Among patients with available post-treatment CD14+HLA-DRlo/- monocytic cells levels
Figure 2.
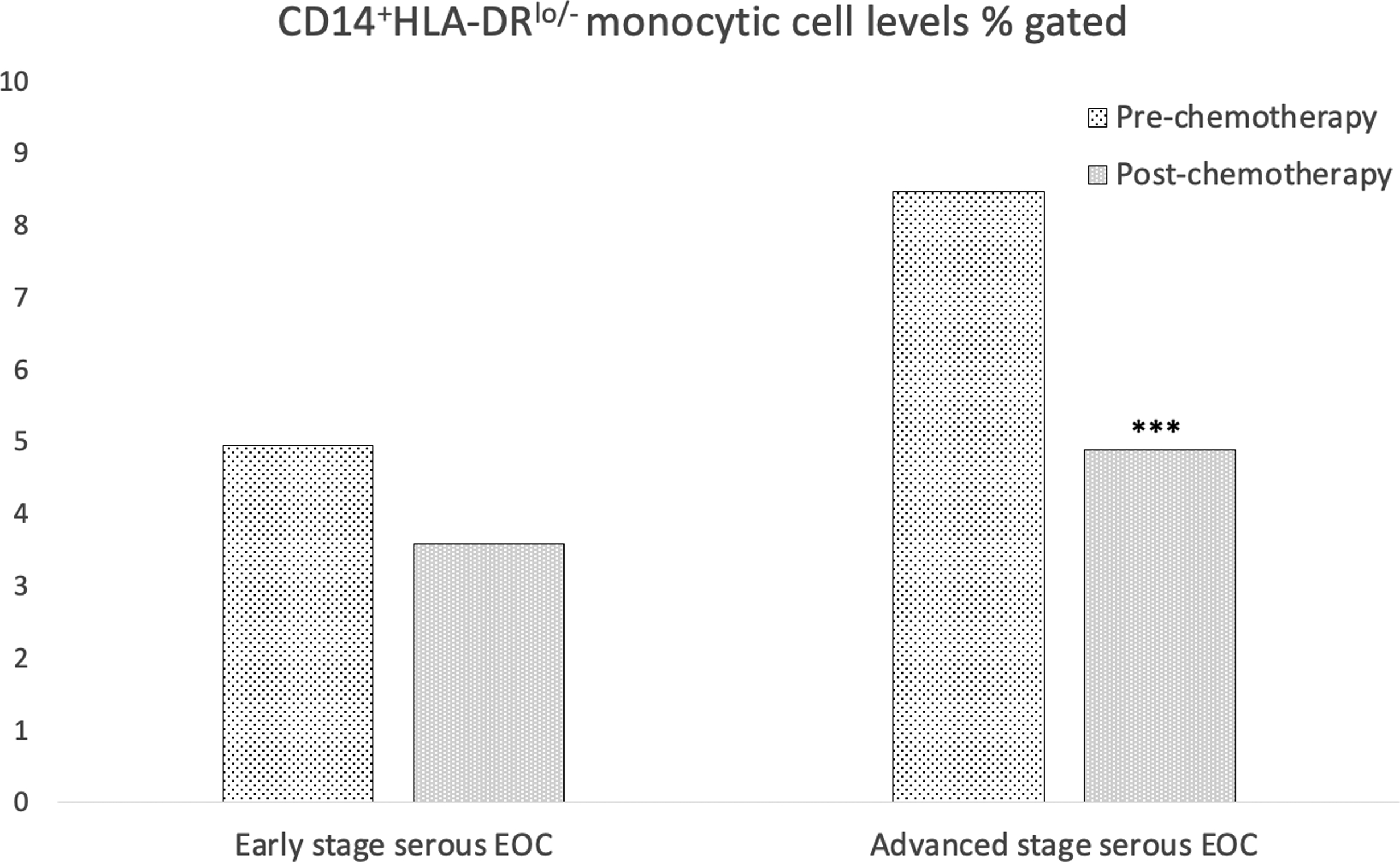
CD14+HLA‐DRlo/− monocytic cell levels % gated pre‐ and post‐chemotherapy intervention. Among women with early stage serous EOC, CD14+HLA‐DRlo/− monocytic cell levels decreased from a median of 4.93% cells gated pre‐chemotherapy to 3.57% cells gated post‐chemotherapy (P = .19). Among women with advanced stage serous EOC, CD14+HLA‐DRlo/− monocytic cell levels were found to decrease from a median of 8.45% cells gated pre‐chemotherapy to 4.88% cells gated post‐chemotherapy (P < .0001)
Discussion
The findings from this study identified a significant association between an increasing frequency of circulating CD14+HLA-DRlo/- monocytic cells and advancing stage of EOC patients at diagnosis. This is an important finding given the strong role disease stage plays in ovarian cancer survival [1]. Furthermore, these monocytic cells decreased following chemotherapeutic treatment. Our panel of surface markers was specific for monocytic cells of the myeloid lineage that were negative or expressed low levels of HLA-DR. The panel of surface markers used in this study also likely captured M-MDSCs, which includes those expressing a CD14+HLA-DRlo/- phenotype [14–16, 19–21].
Monocytes observed in different malignancies, including EOC, have demonstrated the potential to exhibit immune suppressive effects in response to exposure to tumor-derived factors (TDFs) [5, 14, 15, 32, 33]. Additionally, it’s been described that TDFs in EOC promote the differentiation of peripheral blood monocytes into M2-like polarized macrophages, also known to promote immune suppression and angiogenesis [18, 34]. Furthermore, it has been suggested that CD14+HLA-DRlo/- monocytes may be indicative of disease unresponsive to immunotherapy [15]. Lack of HLA-DR expression in monocytes across malignant and non-malignant conditions has been associated with monocytic cell dysfunction and immune suppression.
As previously mentioned, our monocytic population also captured CD14+HLA-DRlo/- M-MDSCs. MDSCs are predominantly immature, dysfunctional cells of the myeloid lineage [35–38]. These cells have the ability to suppress CD8+ T cell activity, increase tumor-supportive M2-like macrophage and regulatory T cell responses, and release inflammatory cytokines and mediators, which are all implicated in pro-metastatic behavior [39, 40]. Two primary subsets of MDSCs are present during malignant progression (M-MDSCs and PMN-MDSCs). However, M-MDSCs have been described as the more immunosuppressive subset, and therefore may be a key barrier in EOC immunity [40].
This clinical study, through a multi-center collaboration, had a larger sample size of patients compared to previous work exploring CD14+HLA-DRlo/- monocytic cell populations in EOC [19, 26–29]. We not only approached our study with a larger sample size, but we included clinical variables that are critical to consider in relation to ovarian cancer, which may guide future prognostic studies. Our study builds on existing literature, producing new information regarding populations of CD14+HLA-DRlo/- monocytic cells in EOC disease progression, and the impact of standard-of-care chemotherapeutic intervention on these cells.
Though our study adds to the understanding of CD14+HLA-DRlo/- monocytic cells in EOC, there are also limitations that should be noted. Our study measured immunophenotypically defined monocytic cell levels solely in the peripheral blood and not in the tumor microenvironment. Future studies exploring this cell population collected from other environments, such as those in the ascites fluid, may add to the growing literature and knowledge on this topic. However, developing a prognostic biomarker in the blood will expand its potential merit in a broader patient population, as well as potentially offer non-invasive methods for predicting disease progression. Our cell populations were defined phenotypically through flow cytometry and did not include functional studies to examine immunosuppressive cell behavior. Future work should examine additional features of these populations, particularly their ability to inhibit T cell function and how that may change in relation to disease status or response to cancer treatment. Finally, there was some heterogeneity observed in cell levels across clinic sites. However, it will be important to continue collaborative efforts to obtain larger patient sample sizes, and future studies should examine strategies to preserve cell populations.
Our study demonstrated a significant association between CD14+HLA-DRlo/- monocytic cells and advanced stage disease in EOC patients at diagnosis. Continuing to examine these monocytic cells in EOC may identify a biomarker for monitoring disease progression and prognostic outcomes. Our results demonstrated a reduction of CD14+HLA-DRlo/- monocytic cells with chemotherapy intervention, and it is plausible that further depletion of this population through additional therapies may enhance an antitumor immune response. Further research using large patient cohorts, with the inclusion of prognostic clinical variables, will be necessary for identifying and strengthening potential prognostic associations of the frequency of these monocytic cells with response to therapy. Such studies may identify patients who would be selected for clinical trials aimed at depletion or modulation of their pro-tumorigenic behavior.
Supplementary Material
Acknowledgements:
The authors would like to thank the Roswell Flow Cytometry and Data Bank and Biorepository shared resource, the HCC Gynecologic Cancer Biorepository and Data Bank, and the Mayo Clinic tumor registry program for support for this research.
Funding: Research included in this article was supported by funding from the National Cancer Institute’s Specialized Programs of Research Excellence (SPOREs) program, under award number: P50CA159981 and P50CA136393. This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by the Hillman Cancer Center Grant award P30CA047904. Additional funds were provided by the Dean’s Faculty Advancement Fund (FM).
Footnotes
Conflicts of Interest: There are no conflicts of interests to declare.
Ethics approval and consent to participate: IRB approval and oversight was obtained, and all patients were consented prior to the initiation of any study procedures. All research was performed in accordance with the Declaration of Helsinki.
Data availability: Upon reasonable request, the data utilized for this study may be available from the corresponding author.
References
- 1.Durot C, Dohan A, Boudiaf M et al. Cancer of the Anal Canal: Diagnosis, Staging and Follow-Up with MRI. Korean journal of radiology 2017; 18: 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham JGJ, Allegra CJ. The Bethesda Handbook of Clinical Oncology. In 4 Edition Wolters Kluwer; 2014. [Google Scholar]
- 3.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017; 14: 9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chodon T, Lugade AA, Battaglia S, Odunsi K. Emerging Role and Future Directions of Immunotherapy in Advanced Ovarian Cancer. Hematol Oncol Clin North Am 2018; 32: 1025–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drakes ML, Stiff PJ. Regulation of Ovarian Cancer Prognosis by Immune Cells in the Tumor Microenvironment. Cancers 2018; 10: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goode EL, Block MS, Kalli KR et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol 2017; 3: e173290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colligan SH, Tzetzo SL, Abrams SI. Myeloid-driven mechanisms as barriers to antitumor CD8(+) T cell activity. Mol Immunol 2020; 118: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer immunology research 2017; 5: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singel KL, Emmons TR, Khan ANH et al. Mature neutrophils suppress T cell immunity in ovarian cancer microenvironment. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenzel AE, Abrams SI, Moysich KB. A Call for Epidemiological Research on Myeloid-Derived Suppressor Cells in Ovarian Cancer: A Review of the Existing Immunological Evidence and Suggestions for Moving Forward. Frontiers in Immunology 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature Reviews Cancer 2016; 16: 431–446. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Seminars in Immunology 2018; 35: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nature Reviews Cancer 2016; 16: 447–462. [DOI] [PubMed] [Google Scholar]
- 14.Javeed N, Gustafson MP, Dutta SK et al. Immunosuppressive CD14+HLA-DRlo/neg monocytes are elevated in pancreatic cancer and “primed” by tumor-derived exosomes. OncoImmunology 2017; 6: e1252013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengos AE, Gastineau DA, Gustafson MP. The CD14+HLA-DRlo/neg Monocyte: An Immunosuppressive Phenotype That Restrains Responses to Cancer Immunotherapy. Frontiers in Immunology 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuk-Pavlović S, Bulur PA, Lin Y et al. Immunosuppressive CD14+HLA-DRlow/-monocytes in prostate cancer. The Prostate 2010; 70: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odunsi K Immunotherapy in ovarian cancer. Annals of Oncology 2017; 28: viii1–viii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen JM, Coleman RL, Sood AK. Targeting the tumour microenvironment in ovarian cancer. European Journal of Cancer 2016; 56: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loercher AE, Nash MA, Kavanagh JJ et al. Identification of an IL-10-Producing HLA-DR-Negative Monocyte Subset in the Malignant Ascites of Patients with Ovarian Carcinoma That Inhibits Cytokine Protein Expression and Proliferation of Autologous T Cells. The Journal of Immunology 1999; 163: 6251. [PubMed] [Google Scholar]
- 20.Bronte V, Brandau S, Chen SH et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandruzzato S, Brandau S, Britten CM et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother 2016; 65: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafson MP, Lin Y, Bleeker JS et al. Intratumoral CD14+ Cells and Circulating CD14+HLA-DRlo/neg Monocytes Correlate with Decreased Survival in Patients with Clear Cell Renal Cell Carcinoma. Clinical Cancer Research 2015; 21: 4224. [DOI] [PubMed] [Google Scholar]
- 23.Eo WK, Chang HJ, Kwon SH et al. The Lymphocyte-Monocyte Ratio Predicts Patient Survival and Aggressiveness of Ovarian Cancer. Journal of Cancer 2016; 7: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J-Y, Liu C-C, Wang L et al. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. Journal of Cancer 2017; 8: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horikawa N, Abiko K, Matsumura N et al. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells. Clin Cancer Res 2017; 23: 587–599. [DOI] [PubMed] [Google Scholar]
- 26.Obermajer N, Muthuswamy R, Odunsi K et al. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res 2011; 71: 7463–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santegoets S, de Groot AF, Dijkgraaf EM et al. The blood mMDSC to DC ratio is a sensitive and easy to assess independent predictive factor for epithelial ovarian cancer survival. Oncoimmunology 2018; 7: e1465166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Deng Z, Peng Y et al. Ascites-derived IL-6 and IL-10 synergistically expand CD14(+)HLA-DR(-/low) myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget 2017; 8: 76843–76856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okla K, Czerwonka A, Wawruszak A et al. Clinical Relevance and Immunosuppressive Pattern of Circulating and Infiltrating Subsets of Myeloid-Derived Suppressor Cells (MDSCs) in Epithelial Ovarian Cancer. Front Immunol 2019; 10: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JM, Botesteanu DA, Tomita Y et al. Patients with BRCA mutated ovarian cancer may have fewer circulating MDSC and more peripheral CD8(+) T cells compared with women with BRCA wild-type disease during the early disease course. Oncol Lett 2019; 18: 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kipps E, Tan DSP, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nature reviews. Cancer 2013; 13: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. Journal of Autoimmunity 2017; 85: 117–125. [DOI] [PubMed] [Google Scholar]
- 33.Caronni N, Savino B, Bonecchi R. Myeloid cells in cancer-related inflammation. Immunobiology 2015; 220: 249–253. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Zhao X, Wang K et al. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Science 2013; 104: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messmer MN, Netherby CS, Banik D, Abrams SI. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer immunology, immunotherapy: CII 2015; 64: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182: 4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenzel AE, Abrams SI, Moysich KB. A Call for Epidemiological Research on Myeloid-Derived Suppressor Cells in Ovarian Cancer: A Review of the Existing Immunological Evidence and Suggestions for Moving Forward. Front Immunol 2019; 10: 1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology 2012; 12: 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


