Abstract
Circadian disruptions, along with altered affective and reward states, are commonly associated with psychiatric disorders. In addition to genetics, the enduring influence of environmental factors in programming neural networks is of increased interest in assessing the underpinnings of mental health. The duration of daylight or photoperiod is known to impact both the serotonin and dopamine systems, which are implicated in mood and reward-based disorders. This review first examines the effects of circadian disruption and photoperiod in the serotonin system in both human and preclinical studies. We next highlight how brain regions crucial for the serotoninergic system (i.e., dorsal raphe nucleus; DRN), and dopaminergic (i.e., nucleus accumbens; NAc and ventral tegmental area; VTA) system are intertwined in overlapping circuitry, and play influential roles in the pathology of mood and reward-based disorders. We then focus on human and animal studies that demonstrate the impact of circadian factors on the dopaminergic system. Lastly, we discuss how environmental factors such as circadian photoperiod can impact the neural circuits that are responsible for regulating affective and reward states, offering novel insights into the biological mechanisms underlying the pathophysiology, systems, and therapeutic treatments necessary for mood and reward-based disorders.
Keywords: photoperiod, circadian, serotonin, dopamine, affect, reward
Introduction
One key question in neuroscience research is: how do environmental factors induce enduring changes to the function of neural circuits resulting in increased risk for neuropsychiatric disorders? A pervasive environmental factor that appears to play a critical role in this line of questioning is the duration of daylight, or circadian photoperiod. Circadian influences have long been associated with psychiatric disorders (McClung, 2013); however, the neural substrates are still not fully understood. The circadian system is known to impact both mood and reward-based circuitry (Logan et al., 2014; Ketchesin et al., 2018), which are critical components of mental health. Especially relevant, photoperiod exposure results in plasticity and developmental programming of the circadian system itself (Ciarleglio et al., 2011b), along with downstream enduring effects in both the serotonin and dopamine systems (Green et al., 2015; Young et al., 2018), key nodes in the mood and reward systems. We argue that changes to circuits underlying mood and reward, driven through circadian encoding of photoperiod, can play an important role in the risk for psychiatric disorders. We propose that circadian photoperiod influences aspects of both of these overlapping circuits, and modulates risk for mood and reward-based disorders, which may be of great relevance for future diagnosis and treatment. In this review, we will discuss how circadian influences and environmental factors, such as photoperiod, play an integral role in modulating the underlying mechanisms and circuits necessary for mood and reward-based pathophysiology.
Influences on mood by the circadian and the serotonergic systems
Mood disorders have a significant health impact. Globally it is estimated that over 300 million individuals suffer from depression and 16 million Americans have reported experiencing at least one depressive episode ((NIMH), 2019; (WHO), 2019). This results in about 7% of the US population being affected by depression with an estimated economic cost of over $200 billion ((CDC), 2016; (NIMH), 2019). Anxiety disorders are estimated to impact 19% of individuals in the US with approximately 31% of adults experiencing an anxiety disorder during their lifetime ((NIMH), 2017).
Seasonal affective disorder or “winter depression” results in individuals presenting atypical depressive symptoms (Magnusson and Boivin, 2003) arising most frequently during the fall and winter seasons and typically regressing during the spring and summer seasons, providing a clear link between circadian alterations and mood (Zauderer and Ganzer, 2015). In addition, seasonal affective disorder is estimated to impact approximately 5% of the US population (Kurlansik and Ibay, 2012) and is commonly comorbid with other mood disorders such as major depression and anxiety (Winthorst et al., 2017). Lastly, treatments for seasonal affective disorder and major depression normally target the serotonin system with the use of antidepressants, however, light therapy has also been shown to be an effective treatment option for both of these disorders as well (Tuunainen et al., 2004; Terman and Terman, 2005; Even et al., 2008). Thus, affective disorders impact the global population, present a great economic cost, and due to high comorbidity suggest common underlying mechanisms and circuits, which may be specifically sensitive to circadian influences.
Clinical observations have consistently linked circadian disruption with several psychiatric disorders including major depression, anxiety, bipolar disorder, addiction, stress, and seasonal affective disorder (Benedetti et al., 2003; Magnusson and Boivin, 2003; Nievergelt et al., 2006; McClung, 2007a; Glickman, 2010; Sipilä et al., 2010; Landgraf et al., 2014a; Parekh et al., 2015; Geoffray et al., 2016; Ketchesin et al., 2020). In addition, these neuropsychiatric disorders are significantly associated with the serotonin system (Cook and Leventhal, 1996; Lesch et al., 1996; Mahmood and Silverstone, 2001; Magnusson and Boivin, 2003; Eley et al., 2004; Willeit et al., 2008; Daut and Fonken, 2019). The circadian and serotonin systems share underlying neural connections, with the central pace-maker in the brain, the suprachiasmatic nucleus (SCN), being upstream of and projecting to the dorsal raphe nucleus (DRN) (Ciarleglio et al., 2011a), the main hub for serotonin (5-HT) signaling in the brain (Gaspar et al., 2003) (Figure 1). Affective disorders, in particular, are classically associated with atypical signaling and circuitry in the serotonin system, are highly prevalent worldwide, and are thus a main focus of this review.
Figure 1.
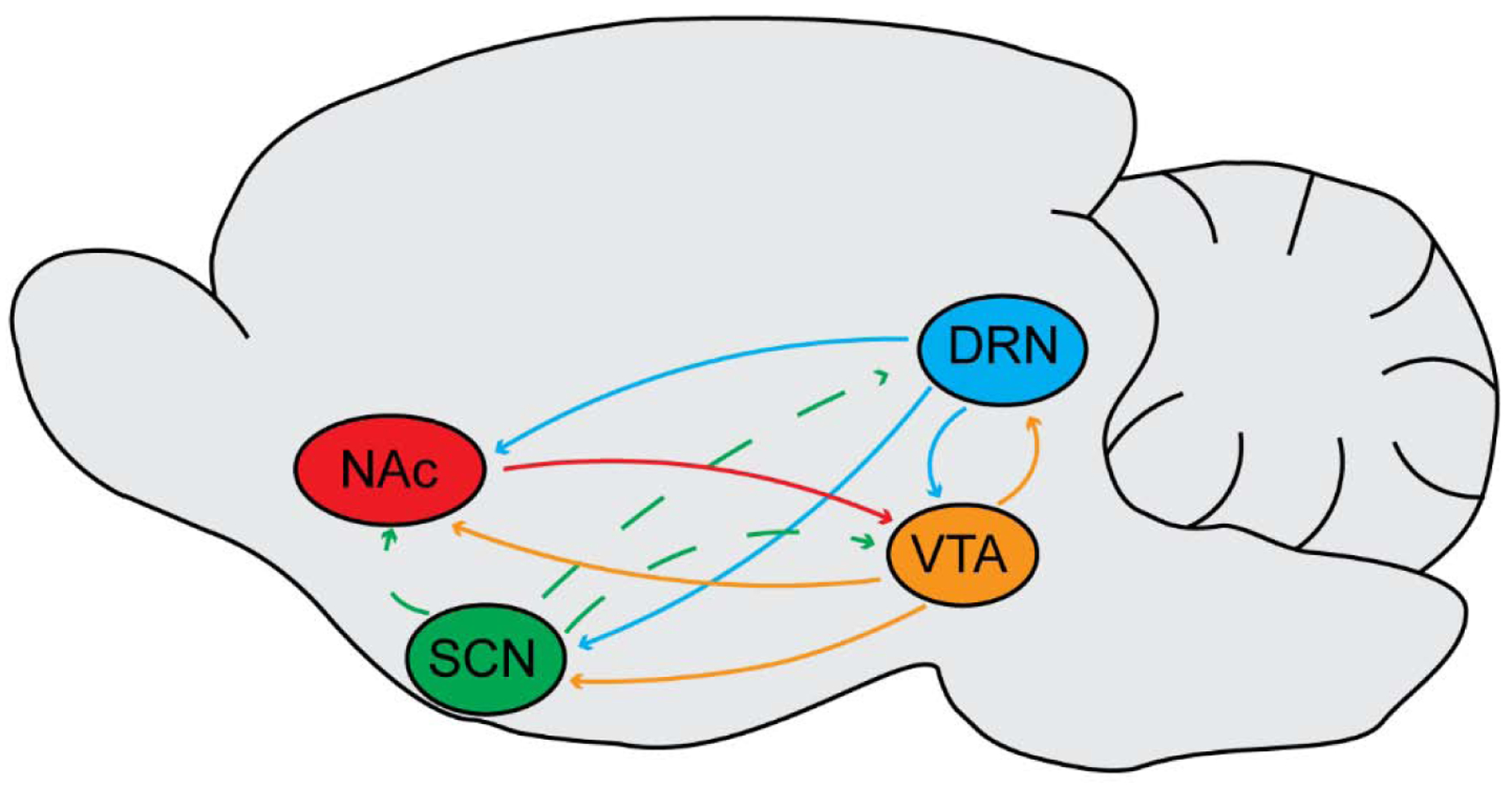
Connecting the circadian (SCN), mood (DRN), and reward (VTA, NAc) systems. Note the solid lines represent direct connections, and the dashed lines represent indirect connections. SCN = suprachiasmatic nucleus, DRN = dorsal raphe nucleus, VTA = ventral tegmental area, and NAc = nucleus accumbens. Listed are the following direct connections: DRN to SCN (Kiyasova et al., 2011; Paulus and Mintz, 2012), DRN to VTA (Vertes and Linley, 2007), DRN to NAc (Vertes and Linley, 2007), VTA to SCN (Grippo et al., 2017), VTA to DRN (Kirouac et al., 2004), VTA to NAc (Russo and Nestler, 2013), and NAc to VTA (Russo and Nestler, 2013). Listed are the following indirect connections: SCN to DRN (Deurveilher and Semba, 2008) and SCN to VTA (Luo and Aston-Jones, 2009).
Studies in humans have identified genetic associations within both the circadian and serotonin systems with affective disorders (McClung, 2007a). For example, genes that form the core of the molecular circadian clockwork (i.e., clock genes), such as Clock Locomotor Output Cycles Kaput (CLOCK), Brain and Muscle ARNT-Like 1 (BMAL1), Period (Per), and Cryptochrome (Cry) have been associated with depression, anxiety, and bipolar disorder (Benedetti et al., 2003; Nievergelt et al., 2006; Benedetti et al., 2008; Sipilä et al., 2010; Partonen, 2012; Bunney et al., 2015; Buoli et al., 2018). Polymorphisms in key serotonin signaling genes such as the rate-limiting enzyme for serotonin synthesis, tryptophan hydroxylase (TPH), the serotonin 2A receptor (5-HT 2A), and specifically the serotonin transporter (SERT), are implicated in affective disorders as well (Lesch et al., 1996; Caspi et al., 2003; Eley et al., 2004; Zill et al., 2004; Canli and Lesch, 2007; Uher and McGuffin, 2008). Importantly, studies have also found associations between clock genes such as Per2, ARNTL, NPAS2, and risk for seasonal affective disorder or winter depression (Johansson et al., 2003; Partonen et al., 2007; Westrin and Lam, 2007). In addition, patients with seasonal affective disorder have shown alterations in the 5-HT 2A receptor gene along with SERT function and binding (Rosenthal et al., 1998; Enoch et al., 1999; Arias et al., 2001; Praschak-Rieder et al., 2008; Willeit et al., 2008; Kalbitzer et al., 2010; Tyrer et al., 2016). Serotonin depletion in these individuals can result in significant depressive symptoms during remission (Lam et al., 1996; Neumeister et al., 1997; Neumeister et al., 1998; Lam et al., 2000), and both selective serotonin reuptake inhibitors and light therapy are known to be beneficial treatment options (Terman et al., 1989; Swedo et al., 1997; Terman and Terman, 2005; Lam et al., 2006; Kurlansik and Ibay, 2012). Lastly, altered diurnal patterns of both mood and serotonin levels (Pietraszek et al., 1992; Peeters et al., 2006), shifted and decreased circadian gene expression patterns in brain regions implicated in depression (Li et al., 2013), and disrupted circadian activity patterns which correlates with decreased affect, have all been observed in individuals with major depression (Lyall et al., 2018). This underscores the fact that disruptions to and the interactions between the circadian and serotonin systems, in humans, may be integral in the pathophysiology of affective disorders (Daut and Fonken, 2019).
Preclinical animal model research has demonstrated reciprocal interactions and closely intertwined circuitry between the circadian and serotonin systems (Cagampang and Inouye, 1994; Recio et al., 1996; Cuesta et al., 2008; Ciarleglio et al., 2011a; Paulus and Mintz, 2012; Landgraf et al., 2016a). Studies have shown that activating the serotonin system by electrically stimulating the DRN or with pharmacology targeting 5-HT1A/7 receptors can result in release of 5-HT in the SCN, altered clock gene expression levels, and phase resetting of circadian behavior (Glass et al., 2000; Cuesta et al., 2008). Also, genetically knocking out Pet-1, a transcriptional factor needed for proper 5-HT neuron development, results in period lengthening of the circadian rhythms in the SCN and increased circadian behavior activity levels (Paulus and Mintz, 2013; Ciarleglio et al., 2014). Conversely, manipulating the circadian day length can increase DRN 5-HT content, shift the diurnal peak of serotonin content, and knocking out clock genes in animal models results in significant increases in mania and depressive-like behaviors (Cagampang et al., 1993; Roybal et al., 2007; Barnard and Nolan, 2008; Landgraf et al., 2014b). In addition to genetic disruptions, studies have investigated the effects of environmental factors such as jet lag or shift work on mental health (Vogel et al., 2012; Foster et al., 2013).
Rapid travel across multiple time zones (i.e., jet lag) or exposure to light during atypical work hours relative to the light/dark cycle (i.e., shift work) can result in significant disruptions to the SCN and circadian system (Navara and Nelson, 2007; Choy and Salbu, 2011). In addition, jet lag and shift work has been shown to impact the serotonin system and is associated with decreased mood and a higher incidence of affective disorders (Montange et al., 1981; Versteeg et al., 2015; Lee et al., 2017; Daut and Fonken, 2019). For example, altered circadian light cycles and light presented at night, either chronically or even acutely, can produce deleterious effects such as the desynchronization of circadian clock neurons, altered circadian rhythms and clock gene expression levels, learning and memory deficits, and elevated anxiety and depressive-like behaviors (Ohta et al., 2005; Ohta et al., 2006; Fonken et al., 2009; Bedrosian et al., 2011; Fonken and Nelson, 2011; LeGates et al., 2012; Bedrosian and Nelson, 2013; Fonken and Nelson, 2013; Bedrosian and Nelson, 2017; Walker et al., 2020a; Walker et al., 2020b). Interestingly, it was found that chronic selective serotonin reuptake inhibitor (SSRI) treatment could reverse some of these affective behavioral deficits due to circadian disruptions (Bedrosian et al., 2012). These studies demonstrate that manipulation of the circadian system via genetic alterations or environmental factors directly impacts affective behavior, and regulates key components of the serotonin system (Ciarleglio et al., 2011a).
Photoperiodic programming of the serotonin system
An important environmental factor, the duration of daylight or photoperiod has been implicated as a risk factor for numerous psychiatric disorders (Modai et al., 1994; Torrey et al., 1997; Castrogiovanni et al., 1998; Foster and Roenneberg, 2008; Lee et al., 2008; Disanto et al., 2012; Tonetti et al., 2012). Monoamine turnover of serotonin and dopamine is lower during the winter and fall seasons (Chotai and Adolfsson, 2002; Lambert et al., 2002), SERT binding can fluctuate across the seasons, (Praschak-Rieder et al., 2008; Kalbitzer et al., 2010; Tyrer et al., 2016), and an interaction between candidate genes for affective disorders and births in winter seasons has been identified, demonstrating a gene × environment risk for these disorders (Chotai et al., 2003). These findings are important because they highlight how photoperiod can modulate aspects of the serotonin and dopamine systems, underscoring the impact that photoperiod may have on the development and underlying mechanisms associated with affective disorders. Exposure to early life adversity and environmental factors such as stress and trauma during key neurodevelopmental time points including gestation and postnatal development have been associated with increased risk for psychiatric disorders later in life (Nestler et al., 2002; Andersen, 2015). Recently, human epidemiological work has demonstrated that high magnitude photoperiodic changes during the second trimester of gestation can result in decreased risk for depression in the offspring later in life (Devore et al., 2018). Thus, the day length or photoperiod is a critical environmental factor associated with psychiatric disorders, and importantly may play an underappreciated role in the development and pathophysiology of affective disorders (Figure 2).
Figure 2.
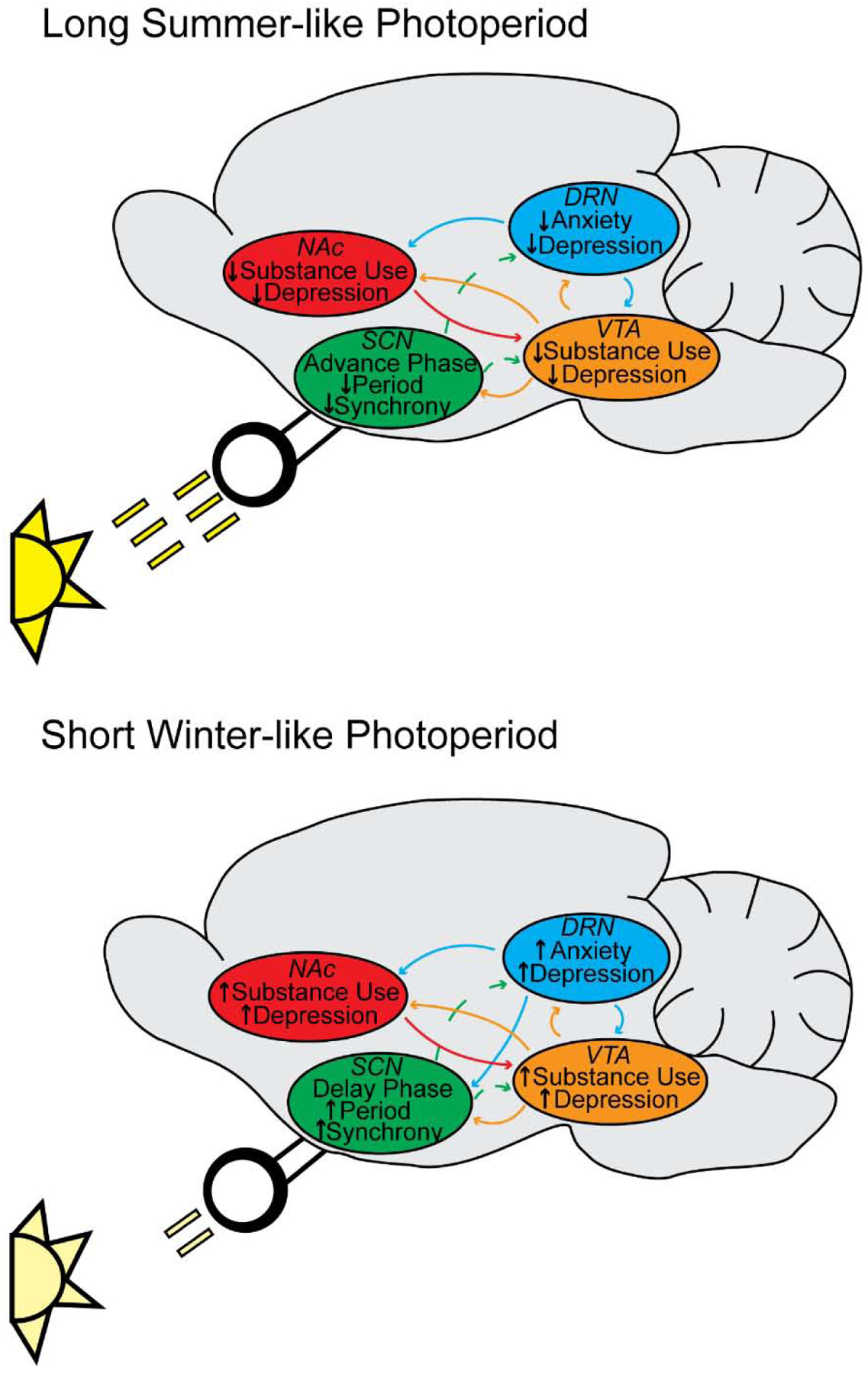
Hypothetical model connecting photoperiodic input with circadian (SCN), mood (DRN), and reward (VTA, NAc) systems and the associated psychiatric disorders. A) We hypothesize that that the SCN acts by mediating circadian entrainment under Long summer-like photoperiods (Ciarleglio et al., 2011b) and impacting the DRN, VTA, and NAc resulting in a potential decreased risk for anxiety, depression, or substance use disorders. B) We hypothesize that that the SCN acts by mediating circadian entrainment under Short winter-like photoperiods (Ciarleglio et al., 2011b) and impacting the DRN, VTA, and NAc resulting in a potential increased risk for anxiety, depression, or substance use disorders.
The serotonergic system is impacted by the duration of daylight and has been consistently implicated in affective disorders (Uher and McGuffin, 2008; Ciarleglio et al., 2011a; Spindelegger et al., 2012). Animal studies have shown that aberrant serotonin signaling during prenatal and perinatal development can result in dramatic and lasting molecular, systems level and behavioral changes in adulthood (Bennett et al., 2002; Gaspar et al., 2003; Bonnin et al., 2011; Bonnin and Levitt, 2011). Knockout rodent models targeting various aspects of the serotonergic system exhibit atypical serotonin (5-HT) neuronal development and viability, (Azmitia, 2001; Hendricks et al., 2003; Persico et al., 2003) altered circuit formation and monoamine content (Bennett-Clarke et al., 1993; Cases et al., 1995; Cases et al., 1996; Upton et al., 1999; Richardson-Jones et al., 2011; Migliarini et al., 2013) along with increased anxiety and depressive-like behaviors (Gross et al., 2002; Holmes et al., 2002; Holmes et al., 2003; Kalueff et al., 2007).
Preclinical rodent studies have shown that, depending on the daylight duration, photoperiodic exposure can significantly increase DRN serotonin neuronal firing rate (Green et al., 2015; Giannoni-Guzman, 2020), elevate monoamine signaling (Otsuka et al., 2014; Goda et al., 2015; Siemann, 2020), and reduce anxiety and depressive-like behaviors (Prendergast and Nelson, 2005; Einat et al., 2006; Pyter and Nelson, 2006; Krivisky et al., 2011; Xu et al., 2016; Takai et al., 2018). For example, prior work has shown that animals developed under Long (summer-like) photoperiods demonstrate enduringly increased firing rates in DRN serotonin neurons, elevated 5-HT and norepinephrine (NE) concentrations in the midbrain, along with decreased time spent immobile in the forced swim task and reduced time spent in the closed arms in the elevated zero maze task compared to mice developed under either Equinox or Short (winter-like) photoperiods (Green et al., 2015). Green et al also demonstrated that these photoperiodic effects were melatonin 1 receptor dependent, and the increased firing rate in DRN serotonin neurons is driven by developmental (i.e., prior to adolescence) rather than proximal photoperiodic effects and lasted up to 5 months after initial photoperiod exposure (Green et al., 2015). These were the first findings to show the enduring impact of photoperiod on 5-HT neurophysiology, monoamine signaling, and the associated affective behaviors. In addition, these results suggested that developmental periods may exist, which could be sensitive to photoperiodic programming, and further indicated a potential role in the pathology of affective disorders.
As a result of these novel findings, we have recently investigated the effects of photoperiodic programming during sensitive periods of prenatal and postnatal development. We found that prenatal Long photoperiodic exposure, in mice, results in enduring changes increasing DRN 5-HT neuronal firing rate, when evaluated in adolescence and adulthood (Siemann et al., 2019). In addition, there are critical temporal windows within postnatal development that are programmed by Long photoperiod resulting in lasting changes to gene expression, monoamine signaling, and affective behaviors (Siemann et al., 2019; Siemann, 2020). Specifically, Long (summer-like) photoperiod exposure during gestation consistently impacts the serotonin system by elevating DRN 5-HT neuronal firing rate, increases midbrain monoamine concentrations, and decreases affective behavior later in life (Siemann et al., 2019). We hypothesize that photoperiodic programming may occur in sequential steps. First, with lasting changes to DRN neuronal firing rate occurring prenatally, which may then “set the serotonin system”, followed by programming of monoamine signaling and behavior during sensitive periods of postnatal development. Importantly, there is emerging clinical and preclinical evidence implicating photoperiod in the development of mood disorders (Devore et al., 2018; Siemann et al., 2019; Siemann, 2020). While these effects are intriguing and warrant further investigation; independent of development, circadian photoperiod clearly impacts the cellular, signaling, and behavioral components of the serotonin system. This begs the question – are the outputs and targets from this system, specifically from the DRN, similarly impacted by circadian influences and photoperiod, resulting in lasting changes to underlying circuits critical for affective disorders?
Connecting mood & reward
There is overwhelming evidence demonstrating the role of the serotonin and dopamine systems in mood and reward (Nestler et al., 2002; Anguelova et al., 2003; Nestler and Carlezon Jr, 2006; Ishikawa et al., 2013; Russo and Nestler, 2013). Studies have shown genetic associations specifically between SERT and depression (Lesch et al., 1996; Jönsson et al., 1998; Canli and Lesch, 2007), the role of environmental factors such as stress on serotonergic and dopaminergic genes, demonstrating gene × environment associations (Pani et al., 2000; Caspi et al., 2003; Eley et al., 2004; Martens and van Loo, 2007; Uher and McGuffin, 2008; Heim and Binder, 2012), and most commonly prescribed antidepressants target the serotonin and dopamine systems (Pirraglia et al., 2003; Dailly et al., 2004). Serotonin is consistently implicated in affective disorders (Anguelova et al., 2003); however, studies have more recently investigated the role of this system in disorders of reward, including addiction (Kirby et al., 2011; Müller and Homberg, 2015; Heifets et al., 2019).
Classic hallmarks of reward-based disorders include dopamine gene variants, atypical dopaminergic signaling, and overall alterations to the dopamine system (Nestler and Carlezon Jr, 2006; Volkow et al., 2011). In addition, clinical observations have shown associations between affective disorders and the dopaminergic system, specifically in depression (Nestler et al., 2002; Dunlop and Nemeroff, 2007; Krishnan and Nestler, 2008; Fox and Lobo, 2019). Interestingly, comorbidities between substance abuse and mood disorders have been established (Volkow, 2004; Quello et al., 2005; Destoop et al., 2019), and alterations in the serotonin and dopamine systems have also been found in individuals with seasonal affective disorder (Neumeister et al., 1998; Rosenthal et al., 1998; Enoch et al., 1999; Lam et al., 2000; Praschak-Rieder and Willeit, 2011). These findings highlight that disruptions to the serotonin and dopamine systems are commonly found in affective and reward-based disorders, strongly suggesting shared and overlapping circuitry, and demonstrate that both of these systems are impacted by environmental factors, including circadian photoperiod.
Underlying circuitry connecting the serotonergic & dopaminergic systems
Major targets of the DRN include the ventral tegmental area (VTA) and nucleus accumbens (NAc), which are critical to the dopamine system and reward-based circuitry involved in mood disorders (Nestler et al., 2002; Nestler and Carlezon Jr, 2006; Russo and Nestler, 2013). The mesolimbic dopamine (DA) system has been extensively implicated in depression in both preclinical and clinical studies (Treadway and Zald, 2011; Friedman et al., 2014; Addy et al., 2015; Small et al., 2016). Both the NAc and VTA integrate information in the form of glutamatergic inputs from limbic, cortical, and thalamic regions and neuromodulatory inputs from key structures including serotonergic inputs from the dorsal raphe (Soghomonian et al., 1989; Brown and Molliver, 2000; Turner et al., 2017) (Figure 1). For example, electrical stimulation of the DRN can result in increased dopamine release in the NAc (De Deurwaerdère and Spampinato, 1999), along with modulation of DA neuronal firing rate and response properties in the VTA (Gervais and Rouillard, 2000).
The DRN, VTA, and NAc are key components of serotonergic and dopaminergic circuitry that have been associated with affective and reward-based disorders (Nestler and Carlezon Jr, 2006; Cohen et al., 2012; Cohen et al., 2015; Wong-Lin et al., 2017). To this point, two recent human imaging studies have demonstrated that patients with major depression who are either medication free or being treated with antidepressants show altered fMRI resting state activity and functional connectivity in the DRN and VTA (Wagner et al., 2017; Wohlschläger et al., 2018). In addition, preclinical model work has shown that ethanol consumption or self-administration results in altered 5-HT levels in the DRN and DA content in the VTA and NAc (McBride et al., 1993), and chronic cocaine administration increases both 5-HT and DA levels in the DRN and NAc (Parsons and Justice Jr, 1993). Studies have also begun to identify the neuronal populations and connections between these brain regions that are necessary for producing these behavioral effects. For example, inhibition of VTA DA neurons can result in depressive-like behavior and phasic activation of these neurons can reverse these behavioral deficits (Tye et al., 2013). While investigating the underlying mechanisms of depressive states, studies have observed that differences in VTA neuronal activity can result in susceptible or resilient behavioral populations (Friedman et al., 2014), the excitation or inhibition of the connections between the VTA and NAc can also produce susceptible or resilient behavioral responses to social-defeat stress (Chaudhury et al., 2013), and lesioning VTA neurons results in elevated depressive-like behavior, which can be reversed with the administration of an SSRI (Winter et al., 2007).
Recent studies have attempted to connect mood and reward-based pathways utilizing optogenetics to investigate the underlying circuitry between the serotonin and dopamine systems (Nakamura, 2013; Liu et al., 2014; Fonseca et al., 2015; Hayashi et al., 2015; Li et al., 2016; Browne et al., 2019; Li et al., 2019; Wang et al., 2019; Nagai et al., 2020). Studies have shown that nonserotonergic DRN neurons provide glutamatergic excitation on VTA DA neurons and are responsible for reinforcing behavior (McDevitt et al., 2014), DRN 5-HT neurons fire tonically for reward and phasically for reward acquisition, and DRN GABAergic neurons are inhibited for reward stimuli presentations (Li et al., 2016). In addition, DRN 5-HT neuronal activity over short compared to long time scales produce rewarding vs. aversive responses (Nakamura, 2013; Hayashi et al., 2015), and direct stimulation of DRN 5-HT terminals projecting to the VTA can impact reward-like behavior (Browne et al., 2019).
Prior work utilizing Pet-1 Cre-driven expression of channelrhodopsin to target optogenetic stimulation of DRN 5-HT neurons, found this results in the release of both 5-HT and glutamate leading to increased self-administration and reward-based behaviors (Liu et al., 2014). In addition, DRN 5-HT-vGlut3 terminals synapse onto DA VTA neurons, which activates the 5-HT terminals in the VTA causing the excitation of VTA DA cells (Wang et al., 2019). Excitation of these 5-HT terminals in the VTA contribute to increasing reward-like behavior via cocaine conditioned place preference (CPP) and downstream release of DA in the NAc (Wang et al., 2019). Also, using a Tph2 promoter, either stimulation or inhibition of DRN 5-HT neurons projecting to the VTA can result in significant increases and decreases in reward-based behavior, respectively (Nagai et al., 2020). Lastly, DRN 5-HT neuronal projections to the NAc have been associated with reward-based disorders (Chang et al., 2011; You et al., 2016). 5-HT neuronal projections and specifically 5-HT1A receptors are critical for DRN-NAc circuits involved in reducing reward behavior, via CPP, and producing anti-depressive-like behaviors (You et al., 2016). In addition, it has been found that 5-HT1B receptors in the NAc are necessary for significantly decreasing social reward behavior (Dölen et al., 2013). These elegant circuit-based studies demonstrate the complexity between the serotonin and dopamine systems, provide novel insights into the underlying mechanisms necessary for mood and reward, and present novel opportunities to investigate circadian-driven DRN 5-HT effects in the VTA and NAc.
There are clear connections and direct interactions between the key components of the serotonin (i.e., DRN) and dopamine (i.e., VTA and NAc) systems with studies identifying the underlying neural populations and circuits needed to produce affective and reward-based behaviors. Circadian influences and specifically photoperiodic exposure can result in dramatic and lasting changes to various components of the serotonin system. Alterations in the dopaminergic system are classic hallmarks of mood and in particular reward-based disorders. As the serotonergic and dopaminergic systems clearly share overlapping circuitry, it is then logical to ask 1) if circadian changes and specifically photoperiodic exposures produce enduring effects to dopamine rich nuclei, and 2) does this result in potential circadian-dependent changes in circuitry critical for mood and reward?
Bidirectional regulation of reward by the circadian and dopaminergic systems
In the US it is estimated that 20 million adults suffer from addiction or a substance abuse disorder ((NIMH), 2016), resulting in an estimated $740 billion economic cost annually ((NIDA), 2020), and approximately 8 million individuals present comorbidities with additional psychiatric disorders ((NIMH), 2016). In addition, there are known comorbidities between substance abuse and affective disorders (Quello et al., 2005) further highlighting the shared underlying mechanisms between mood and reward. Psychiatric disorders such as addiction, stress, major depression, bipolar, and substance abuse disorder are associated with the dopamine system (Pani et al., 2000; Nestler and Carlezon Jr, 2006; Moriam and Sobhani, 2013; Russo and Nestler, 2013; Belujon and Grace, 2015), with alterations in the circadian system being consistently identified as well (Benedetti et al., 2003; McClung, 2007a; 2013; Landgraf et al., 2014a; Logan et al., 2014). Substance abuse disorders are highly prevalent, present a significant economic cost, and based on the direct regulation by circadian influences, warrant further investigation into these underlying relationships.
Human genetic associations have been found between the circadian system and substance abuse disorders (McClung, 2007b; Falcón and McClung, 2009; Parekh et al., 2015; Partonen, 2015). For example, polymorphisms in Clock and Per genes have been implicated in alcohol, cocaine use, and addiction (Spanagel et al., 2005; Kovanen et al., 2010), and have been found in individuals displaying comorbid mood disorders as well (Sjöholm et al., 2010). Animal model studies have demonstrated that circadian disruptions via clock gene knockouts and knockdowns can disrupt dopamine rich nuclei, such as the VTA and NAc, and produce a variety of reward and addiction-like behaviors (Andretic et al., 1999; Falcón and McClung, 2009; Logan et al., 2014; Parekh et al., 2015). For example, knockout animal models or mutations to Per genes can result in altered alcohol consumption and cocaine reward-based behavior, however differential findings are observed when evaluating either Per1 or Per2 genes (Abarca et al., 2002; Spanagel et al., 2005; Zghoul et al., 2007; Perreau-Lenz et al., 2009). In addition, disruptions to Clock can result in elevated dopaminergic neuronal activity and tyrosine hydroxylase expression levels in the VTA along with increased reward and self-administrative behaviors (McClung et al., 2005; Coque et al., 2011). These Clock mutant mouse models also present manic-like behavior, such as hyperactivity, decreased sleep, and reduced anxiety and depressive-like behaviors, which can be reversed with lithium treatment along with viral administration of a functional Clock into the VTA (Roybal et al., 2007). Intriguingly, genetic knockdown of Clock specifically in the VTA results in altered affective and reward-based behaviors, and disrupts circadian rhythms in the SCN along with circadian behavior (Mukherjee et al., 2010). Lastly, Clock mutant animals and knockdown of VTA Clock expression levels produces altered glutamatergic tone, disruptions in clock gene expression levels in the VTA, and elevated ethanol consumption behavior (Ozburn et al., 2013).
In addition, environmental factors such as jet lag, shift work, and the time of day can modulate aspects of the dopaminergic system as well (Webb, 2017). Jet lag and shift work have been associated with increased risk for substance abuse disorders (Trinkoff and Storr, 1998; Bildt and Michélsen, 2002; Rogers and Reilly, 2002; Dorrian and Skinner, 2012), and preclinical work in rodents has shown that shifts to the circadian light/dark cycle can modulate alcohol consumption (Gauvin et al., 1997; Clark et al., 2007; Rosenwasser et al., 2010). In addition, the circadian and dopamine systems can regulate each other, demonstrating reciprocal interactions. For example, dopamine D1 receptors in the SCN are necessary for synchronizing circadian rhythms (Grippo et al., 2017), VTA dopaminergic neurons project to the SCN (Grippo and Güler, 2019), and dopamine and dopamine receptors demonstrate circadian diurnal expression levels in the VTA and NAc (Schade et al., 1995; Shieh et al., 1997; Weber et al., 2004). Lastly, the time of day can directly impact cocaine self-administration behavior in rodents (Baird and Gauvin, 2000; Sleipness et al., 2005), VTA dopamine neuronal firing rate along with tyrosine hydroxylase activity demonstrate diurnal rhythms, and this dopaminergic rhythmic activity throughout the day is responsible for manic-like behaviors (Sidor et al., 2015). Thus, there is evidence of not only genetic, but environmental circadian influences that can modulate various aspects of dopaminergic neurobiology and reward behaviors.
One clear link connecting serotoninergic and dopaminergic circuits is through the circadian system (Figure 1). Genetic manipulations of key clock genes in the VTA and NAc results in altered neuronal firing rate and anxiety and depressive-like behaviors (Mukherjee et al., 2010; Coque et al., 2011; Spencer et al., 2013; Landgraf et al., 2016b). Intriguing findings indicate that the VTA and NAc contain molecular circadian clockworks similar to the SCN central clock (Logan et al., 2014; Landgraf et al., 2016b; Porcu et al., 2020). Targeted knockdowns of Clock in the VTA results in dopaminergic neurophysiological and reward-based behavioral deficits similar to those observed utilizing global Clock mutations, and reintroducing a functional Clock into the VTA can reverse these effects (McClung et al., 2005; Roybal et al., 2007; Mukherjee et al., 2010; Coque et al., 2011; Ozburn et al., 2013). These findings critically demonstrate a direct relationship between circadian influences and dopaminergic nuclei such as the VTA, and these interactions appear to be critical for addiction and reward processing (Parekh et al., 2015).
Interestingly, disruption of NAc circadian molecular rhythms is associated with vulnerable rather than resilient populations in a learned helplessness model, indicating a link between NAc clock function and depressive-like behaviors (Landgraf et al., 2016b). Furthermore, specific genetic elements of the NAc clockworks regulate dopamine signaling in dopamine 1 receptor medium spiny neurons (D1-MSNs), and genetic disruption of the NAc circadian clock is associated with reduced helpless behavior (Porcu et al., 2020). Prior work has also demonstrated that developmental photoperiod drives enduring changes in the molecular waveform of the central SCN circadian clock, along with long-term changes in how the SCN responds to photoperiods later in life (Ciarleglio et al., 2011b). Clearly circadian genetic and environmental influences impact the dopaminergic system directly resulting in changes to reward and mood. This begs the question – can circadian photoperiod drive similar enduring changes in the molecular clockworks of the VTA and NAc, which impact affective and reward behaviors?
Mechanisms of photoperiodic programming on dopaminergic circuitry
Both human and animal model studies have shown that photoperiodic exposure can result in lasting changes to numerous aspects of the dopamine system, including neurophysiology (Meng et al., 2018), monoamine levels (Dulcis et al., 2013; Aumann et al., 2016; Itzhacki et al., 2018), and reward/addiction-like behaviors (Sorg et al., 2011; Young et al., 2018). Circadian variation in the dopamine transporter (DAT) function has been shown to underlie daily rhythms in dopaminergic tone in the NAc core (Ferris et al., 2014), seasonal changes can impact SERT function, and both SERT and DAT have been associated with seasonal affective disorder (Rosenthal et al., 1998; Neumeister et al., 2001; Praschak-Rieder et al., 2008; Kalbitzer et al., 2010; Praschak-Rieder and Willeit, 2011; Tyrer et al., 2016) suggesting that these transporters may be potential key points of regulation by circadian photoperiod. To this point, prior work has shown that circadian photoperiod can impact reward-based behavior differentially in DAT KO animals, highlighting a direct role of the effects of photoperiod on DAT for reward-like behaviors (Young et al., 2018). These findings demonstrate that circadian photoperiod can impact dopamine rich nuclei, however, future studies will need to investigate the neural components under differing photoperiodic conditions to more fully investigate these brain-behavior based relationships.
Long term functional changes in NAc neurons along with VTA dopaminergic and GABAergic neuron synapses are key features of mood disorders (Russo and Nestler, 2013; Fox and Lobo, 2019). The NAc and VTA receive convergent glutamatergic inputs from multiple regions, each thought to encode specific information, which is integrated and relayed through the reward system (Lüscher and Malenka, 2011; Grueter et al., 2012; Turner et al., 2017). Activity-dependent changes in synaptic strength at glutamatergic synapses are considered to be the fundamental mechanism in information processing and storage in the brain, and contribute to the development of neural circuit and experience-dependent behavioral plasticity. Consistently, the molecular and cellular bases of depressive-like behavior in animal models include changes in glutamatergic and GABAergic synapses, and membrane excitability in VTA and NAc neurons (Russo and Nestler, 2013). The NAc is comprised of parallel output loops (D1 and D2 dopamine receptor expressing medium spiny neurons; MSNs) whose function is differentially linked to behavioral outcomes (Lobo et al., 2010; Bock et al., 2013; Pascoli et al., 2015). The functional output of the NAc is gated by the strength of glutamatergic synapses onto D1 and D2 dopamine (DA) receptor-expressing GABAergic MSNs (Turner et al., 2017; Baimel et al., 2019). Adding to the complexity of the circuitry, the expression of a depressive-like behavioral state correlates with differential reorganization of excitatory synapses onto D1, but not D2 MSNs (Lim et al., 2012; Francis et al., 2017; Francis et al., 2019), and synaptic and membrane property adaptations at both DAergic and GABAergic neurons in the VTA have been reported as well (O’Dell and Parsons, 2004; Wang et al., 2019). Often overlooked, but functionally impactful are interneurons within the NAc (Schall et al., 2020). These microcircuits are necessary for behaviors associated with psychiatric disorders (Wang et al., 2018; Pisansky et al., 2019), and receive similar excitatory inputs that also express synaptic plasticity (Manz et al., 2020). However, there is a paucity of information regarding consequences of photoperiod on reward circuit synapses leading to the question: does photoperiod drive similar enduring synaptic plasticity of the NAc that then impact affective and reward-based behaviors?
Importantly, studies have evaluated the effects of clock gene disruption on dopaminergic synaptic plasticity in the NAc and the direct impact this has on the resultant reward-like behaviors (Parekh et al., 2018; Parekh et al., 2019). Clock mutant animals demonstrate reduced glutamate receptor (GLUA1) expression levels in the NAc, decreased synaptic excitatory strength and transmission along with lower intrinsic excitability in dopaminergic MSNs, and overexpressing GLUA1 directly in the NAc can rescue manic-like and reward-based behavioral deficits (Parekh et al., 2018). In addition, recent work has shown that knockdown of a key circadian gene, NPAS2, in the NAc, results in altered synaptic strength in specifically D1-MSNs, and NPAS2 expression in D1-MSNs, not D2-MSNs, is necessary for cocaine-induced behavior (Parekh et al., 2019). Based on these prior findings, we hypothesize that circadian photoperiodic exposure may differentially scale excitatory gain in the VTA and NAc, which may be responsible for the observed enduring changes in affective and reward-like behaviors. These studies highlight the cell-type specificity needed for circadian influences on reward-dependent circuitry and behavior, and allow for novel opportunities to investigate the effects of photoperiod on synaptic and neural drive in the VTA and NAc.
Enduring effects of circadian photoperiod on mood and reward
Studies have shown that photoperiod can program aspects of the circadian system including the neural firing rate in the master pacemaker of the brain, the SCN, along with circadian-based behavior (Ciarleglio et al., 2011a; Ciarleglio et al., 2011b; Tackenberg and McMahon, 2018). In addition, developmental photoperiod can impact brain regions downstream of the SCN, by enduringly programming neurons (Green et al., 2015; Siemann et al., 2019; Giannoni-Guzman, 2020), monoamine content (Otsuka et al., 2014; Goda et al., 2015), gene expression levels (Siemann, 2020), and affective behaviors associated with the DRN and serotonin system (Prendergast and Nelson, 2005; Einat et al., 2006; Pyter and Nelson, 2006; Krivisky et al., 2011). In addition, we have highlighted the sensitive periods of prenatal and postnatal development that are necessary for these effects (Takai et al., 2018; Siemann et al., 2019), and the first evidence, in humans, of prenatal photoperiodic exposure being associated with decreased risk for mood disorders in the offspring later in life (Devore et al., 2018). In preclinical and clinical studies, even relatively brief exposures to photoperiod result in lasting changes to dopaminergic firing rate (Domínguez-López et al., 2014; Meng et al., 2018), monoamine content (Dulcis et al., 2013; Aumann et al., 2016; Itzhacki et al., 2018), and reward-like behaviors (Sorg et al., 2011; Young et al., 2018). These findings not only provide evidence that photoperiodic exposure can enduringly impact brain regions across multiple neurotransmitter systems, but also highlights that circadian photoperiod exposure may produce significant circuit-level changes (Figure 2). Further investigations are needed to critically evaluate and determine the neural mechanisms underlying the interactions responsible for producing the system-level changes observed between photoperiod, mood, and reward.
Future studies should first evaluate the direct effects of circadian photoperiod on dopaminergic physiology, signaling, and reward-based behaviors. These investigations would then allow for several lines of questioning into how a pervasive environmental factor such as the duration of daylight or photoperiod can potentially have an enduring impact on circuits (i.e., serotonin and dopamine) that are critically intertwined with disorders of mood and reward. Monoaminergic dysregulation has been implicated to be central in the development of depression-like behavioral states, and the mesolimbic dopamine system, comprising the VTA and NAc, is a major target of the DRN (Soghomonian et al., 1989; Brown and Molliver, 2000). Elegant circuit-based studies have demonstrated how these underlying connections and specific neural populations are responsible for aspects of mood and reward-based behaviors (Cohen et al., 2012; Friedman et al., 2014; Liu et al., 2014; Ogawa et al., 2014; Cohen et al., 2015; Li et al., 2016; You et al., 2016; Wong-Lin et al., 2017; Wang et al., 2019; Nagai et al., 2020). However, there is limited evidence of studies evaluating the circadian-drive on serotonergic and dopaminergic circuitry. Specifically, the effects of photoperiodic programming of DRN 5-HT neurons in the VTA and NAc is unexplored. These findings demonstrate a clear opportunity to pursue circuit-based studies investigating the effects of circadian photoperiodic modulation of the serotonergic and dopaminergic systems. By utilizing tools such as electrophysiology, chemogenetics and optogenetics it would be possible to identify the molecular underpinnings and neuronal populations that are necessary and sufficient to produce these changes, and to further understand the underlying circuitry that is responsible for photoperiodic programming, with the ultimate goal that this would lead to more novel and specific therapeutic targets for affective and reward-based disorders.
Conclusions
Circadian influences directly impact neural circuits associated with both mood and reward-based disorders. Studies have investigated both the genetic components along with environmental factors such as circadian photoperiod to provide further understanding into the underlying mechanisms needed to produce these changes. Emerging evidence demonstrates that photoperiodic exposure results in lasting effects to the underlying neurophysiology, brain regions, and associated behaviors in the serotonin and dopamine systems, when measured in isolation. While evaluating each system individually has been informative in determining the influence of photoperiod on serotonin and dopamine signaling, it is most relevant to then utilize this knowledge to determine how photoperiod impacts the underlying mechanisms and connections between these two systems. More focused circuit-based studies investigating the role of photoperiod in multiple neurotransmitter systems would lead to further understanding into these effects, and may allow for the identification of specific neuronal populations responsible for the resultant behaviors underlying mood and reward. Therefore, evaluating the effects of circadian photoperiod in both the serotonin and dopamine systems, along with the known overlapping circuitry, could provide key insights into the development, pathophysiology, and eventually therapeutic treatments for mood and reward-related disorders.
Rhythms, Reward, and Blues: Consequences of Circadian Photoperiod on Affective & Reward Circuit Function – Highlights.
The brain’s central biological clock is connected to key nuclei involved in mood and reward
Circadian factors influence the serotonin and dopamine systems and affective and reward disorders
Circadian photoperiod may play an underappreciated role in affective and addictive disorders
Acknowledgments
This research was supported by National Institutes of Health Grants NIH R01 MH108562 (DM), NIH R01 DA040630 (BAG), 5T32MH018921-24: Development of Psychopathology: From Brain and Behavioral Science to Intervention (JS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare they have no competing interests.
References
- (CDC), C.f.D.C.a.P. (2016). Depression.
- (NIDA), N.I.o.D.A. (2020). Costs of Substance Abuse.
- (NIMH), N.I.o.M.H. (2016). Substance Use and Mental Health.
- (NIMH), N.I.o.M.H. (2017). Any Anxiety Disorder.
- (NIMH), N.I.o.M.H. (2019). Major Depression Among Adults.
- (WHO), W.H.O. (2019). Depression.
- Abarca C, Albrecht U, and Spanagel R (2002). Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences 99(13), 9026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addy N, Nunes E, and Wickham R (2015). Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test. Behavioural brain research 288, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL (2015). Exposure to early adversity: points of cross-species translation that can lead to improved understanding of depression. Development and psychopathology 27(2), 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Chaney S, and Hirsh J (1999). Requirement of circadian genes for cocaine sensitization in Drosophila. Science 285(5430), 1066–1068. [DOI] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, and Turecki G (2003). A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Molecular psychiatry 8(6), 574–591. [DOI] [PubMed] [Google Scholar]
- Arias B, Gutierrez B, Pintor L, Gasto C, and Fananas L (2001). Variability in the 5-HT 2A receptor gene is associated with seasonal pattern in major depression. Molecular psychiatry 6(2), 239–242. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Raabus M, Tomas D, Prijanto A, Churilov L, Spitzer NC, et al. (2016). Differences in number of midbrain dopamine neurons associated with summer and winter photoperiods in humans. PloS one 11(7), e0158847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC (2001). Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain research bulletin 56(5), 413–424. [DOI] [PubMed] [Google Scholar]
- Baimel C, McGarry LM, and Carter AG (2019). The projection targets of medium spiny neurons govern cocaine-evoked synaptic plasticity in the nucleus accumbens. Cell reports 28(9), 2256–2263. e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TJ, and Gauvin DV (2000). Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacology Biochemistry and Behavior 65(2), 289–299. [DOI] [PubMed] [Google Scholar]
- Barnard AR, and Nolan PM (2008). When clocks go bad: neurobehavioural consequences of disrupted circadian timing. PLoS Genet 4(5), e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian T, and Nelson R (2017). Timing of light exposure affects mood and brain circuits. Translational psychiatry 7(1), e1017–e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Haim A, and Nelson RJ (2011). Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36(7), 1062–1069. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, and Nelson RJ (2013). Influence of the modern light environment on mood. Molecular psychiatry 18(7), 751–757. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Weil ZM, and Nelson RJ (2012). Chronic citalopram treatment ameliorates depressive behavior associated with light at night. Behavioral neuroscience 126(5), 654. [DOI] [PubMed] [Google Scholar]
- Belujon P, and Grace AA (2015). Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proceedings of the Royal Society B: Biological Sciences 282(1805), 20142516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, and Smeraldi E (2008). A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neuroscience letters 445(2), 184–187. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, et al. (2003). Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 123(1), 23–26. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. (2002). Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular psychiatry 7(1), 118. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, and Rhoades RW (1993). Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proceedings of the National Academy of Sciences 90(1), 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bildt C, and Michélsen H (2002). Gender differences in the effects from working conditions on mental health: a 4-year follow-up. International archives of occupational and environmental health 75(4), 252–258. [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. (2013). Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nature neuroscience 16(5), 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. (2011). A transient placental source of serotonin for the fetal forebrain. Nature 472(7343), 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, and Levitt P (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, and Molliver ME (2000). Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. Journal of Neuroscience 20(5), 1952–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CJ, Abela AR, Chu D, Li Z, Ji X, Lambe EK, et al. (2019). Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition. Neuropsychopharmacology 44(4), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney B, Li J, Walsh D, Stein R, Vawter M, Cartagena P, et al. (2015). Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Molecular psychiatry 20(1), 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Serati M, Grassi S, Pergoli L, Cantone L, Altamura AC, et al. (2018). The role of clock genes in the etiology of Major Depressive Disorder: Special Section on “Translational and Neuroscience Studies in Affective Disorders” Section Editor, Nobile Maria. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. Journal of Affective Disorders 234, 351–357. [DOI] [PubMed] [Google Scholar]
- Cagampang F, Yamazaki S, Otori Y, and Inouye S-I (1993). Serotonin in the raphe nuclei: regulation by light and an endogenous pacemaker. Neuroreport 5(1), 49–52. [DOI] [PubMed] [Google Scholar]
- Cagampang FRA, and Inouye S-IT (1994). Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: regulation by light and an endogenous pacemaker. Brain research 639(1), 175–179. [DOI] [PubMed] [Google Scholar]
- Canli T, and Lesch K-P (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nature neuroscience 10(9). [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, et al. (1995). Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science (New York, NY) 268(5218), 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, and Gaspar P (1996). Lack of barrels in the somatosensory cortex of monoamine oxidase A–deficient mice: role of a serotonin excess during the critical period. Neuron 16(2), 297–307. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301(5631), 386–389. [DOI] [PubMed] [Google Scholar]
- Castrogiovanni P, Iapichino S, Pacchierotti C, and Pieraccini F (1998). Season of birth in psychiatry. Neuropsychobiology 37(4), 175–181. [DOI] [PubMed] [Google Scholar]
- Chang B, Daniele CA, Gallagher K, Madonia M, Mitchum RD, Barrett L, et al. (2011). Nicotinic excitation of serotonergic projections from dorsal raphe to the nucleus accumbens. Journal of neurophysiology 106(2), 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493(7433), 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotai J, and Adolfsson R (2002). Converging evidence suggests that monoamine neurotransmitter turnover in human adults is associated with their season of birth. European Archives of Psychiatry and Clinical Neuroscience 252(3), 130–134. [DOI] [PubMed] [Google Scholar]
- Chotai J, Serretti A, Lattuada E, Lorenzi C, and Lilli R (2003). Gene–environment interaction in psychiatric disorders as indicated by season of birth variations in tryptophan hydroxylase (TPH), serotonin transporter (5-HTTLPR) and dopamine receptor (DRD4) gene polymorphisms. Psychiatry research 119(1), 99–111. [DOI] [PubMed] [Google Scholar]
- Choy M, and Salbu RL (2011). Jet lag: current and potential therapies. Pharmacy and Therapeutics 36(4), 221. [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio C, Resuehr H, and McMahon D (2011a). Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 197, 8–16. [DOI] [PubMed] [Google Scholar]
- Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, and McMahon DG (2011b). Perinatal photoperiod imprints the circadian clock. Nature neuroscience 14(1), 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Resuehr HE, Axley JC, Deneris ES, and McMahon DG (2014). Pet-1 deficiency alters the circadian clock and its temporal organization of behavior. PloS one 9(5), e97412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, and Rosenwasser AM (2007). Repeated Light–Dark Phase Shifts Modulate Voluntary Ethanol Intake in Male and Female High Alcohol-Drinking (HAD1) Rats. Alcoholism: Clinical and Experimental Research 31(10), 1699–1706. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, and Uchida N (2015). Serotonergic neurons signal reward and punishment on multiple timescales. Elife 4, e06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, and Uchida N (2012). Neuron-type-specific signals for reward and punishment in the ventral tegmental area. nature 482(7383), 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, and Leventhal BL (1996). The serotonin system in autism. Curr Opin Pediatr 8(4), 348–354. [DOI] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao J-L, Spencer S, Marvin M, Falcon E, et al. (2011). Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology 36(7), 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Mendoza J, Clesse D, Pévet P, and Challet E (2008). Serotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodent. Experimental neurology 210(2), 501–513. [DOI] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Renard CE, and Bourin M (2004). Dopamine, depression and antidepressants. Fundamental & clinical pharmacology 18(6), 601–607. [DOI] [PubMed] [Google Scholar]
- Daut RA, and Fonken LK (2019). Circadian regulation of depression: a role for serotonin. Frontiers in neuroendocrinology 54, 100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, and Spampinato U (1999). Role of serotonin2A and serotonin2B/2C receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. Journal of neurochemistry 73(3), 1033–1042. [DOI] [PubMed] [Google Scholar]
- Destoop M, Morrens M, Coppens V, and Dom G (2019). Addiction, anhedonia, and comorbid mood disorder. A narrative review. Frontiers in psychiatry 10, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, and Semba K (2008). “Reciprocal connections between the suprachiasmatic nucleus and the midbrain raphe nuclei: A putative role in the circadian control of behavioral states,” in Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Springer; ), 103–131. [Google Scholar]
- Devore EE, Chang S-C, Okereke OI, McMahon DG, and Schernhammer ES (2018). Photoperiod during maternal pregnancy and lifetime depression in offspring. Journal of psychiatric research 104, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G, Morahan JM, Lacey MV, DeLuca GC, Giovannoni G, Ebers GC, et al. (2012). Seasonal distribution of psychiatric births in England. PloS one 7(4), e34866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, and Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501(7466), 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-López S, Howell RD, López-Canúl MG, Leyton M, and Gobbi G (2014). Electrophysiological characterization of dopamine neuronal activity in the ventral tegmental area across the light–dark cycle. Synapse 68(10), 454–467. [DOI] [PubMed] [Google Scholar]
- Dorrian J, and Skinner N (2012). Alcohol consumption patterns of shiftworkers compared with dayworkers. Chronobiology international 29(5), 610–618. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, and Spitzer NC (2013). Neurotransmitter switching in the adult brain regulates behavior. Science 340(6131), 449–453. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, and Nemeroff CB (2007). The role of dopamine in the pathophysiology of depression. Archives of general psychiatry 64(3), 327–337. [DOI] [PubMed] [Google Scholar]
- Einat H, Kronfeld-Schor N, and Eilam D (2006). Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behavioural brain research 173(1), 153–157. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. (2004). Gene–environment interaction analysis of serotonin system markers with adolescent depression. Molecular psychiatry 9(10), 908–915. [DOI] [PubMed] [Google Scholar]
- Enoch M, Goldman D, Barnett R, Sher L, Mazzanti C, and Rosenthal N (1999). Association between seasonal affective disorder and the 5-HT 2A promoter polymorphism,– 1438G/A. Molecular psychiatry 4(1), 89–92. [DOI] [PubMed] [Google Scholar]
- Even C, Schröder CM, Friedman S, and Rouillon F (2008). Efficacy of light therapy in nonseasonal depression: a systematic review. Journal of affective disorders 108(1–2), 11–23. [DOI] [PubMed] [Google Scholar]
- Falcón E, and McClung CA (2009). A role for the circadian genes in drug addiction. Neuropharmacology 56, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. (2014). Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proceedings of the National Academy of Sciences 111(26), E2751–E2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, et al. (2009). Influence of light at night on murine anxiety-and depressive-like responses. Behavioural brain research 205(2), 349–354. [DOI] [PubMed] [Google Scholar]
- Fonken LK, and Nelson RJ (2011). Illuminating the deleterious effects of light at night. F1000 medicine reports 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, and Nelson RJ (2013). Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behavioural brain research 243, 74–78. [DOI] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, and Mainen ZF (2015). Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Current Biology 25(3), 306–315. [DOI] [PubMed] [Google Scholar]
- Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C, and Roenneberg T (2013). “Sleep and circadian rhythm disruption in social jetlag and mental illness,” in Progress in molecular biology and translational science. Elsevier; ), 325–346. [DOI] [PubMed] [Google Scholar]
- Foster RG, and Roenneberg T (2008). Human responses to the geophysical daily, annual and lunar cycles. Current biology 18(17), R784–R794. [DOI] [PubMed] [Google Scholar]
- Fox ME, and Lobo MK (2019). The molecular and cellular mechanisms of depression: a focus on reward circuitry. Molecular psychiatry, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T, Chandra R, Gaynor A, Konkalmatt P, Metzbower S, Evans B, et al. (2017). Molecular basis of dendritic atrophy and activity in stress susceptibility. Molecular psychiatry 22(11), 1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Gaynor A, Chandra R, Fox ME, and Lobo MK (2019). The selective RhoA inhibitor rhosin promotes stress resiliency through enhancing D1-medium spiny neuron plasticity and reducing hyperexcitability. Biological psychiatry 85(12), 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. (2014). Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344(6181), 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, and Maroteaux L (2003). The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews Neuroscience 4(12), 1002–1012. [DOI] [PubMed] [Google Scholar]
- Gauvin D, Baird T, Vanecek S, Briscoe R, Vallett M, and Holloway F (1997). Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcoholism: Clinical and Experimental Research 21(5), 817–825. [PubMed] [Google Scholar]
- Geoffray M, Nicolas A, Speranza M, and Georgieff N (2016). Are circadian rhythms new pathways to understand Autism Spectrum Disorder? Journal of Physiology-Paris 110(4), 434–438. [DOI] [PubMed] [Google Scholar]
- Gervais J, and Rouillard C (2000). Dorsal raphe stimulation differentially modulates dopaminergic neurons in the ventral tegmental area and substantia nigra. Synapse 35(4), 281–291. [DOI] [PubMed] [Google Scholar]
- Giannoni-Guzman M, Kamitakahara A, Magalong V, Levitt P, McMahon DG (2020). Circadian photoperiod alters TREK-1 channel function and expression in dorsal raphe serotonergic neurons. bioRxiv. doi: 10.1101/2020.06.24.169532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, DiNardo LA, and Ehlen JC (2000). Dorsal raphe nuclear stimulation of SCN serotonin release and circadian phase-resetting. Brain research 859(2), 224–232. [DOI] [PubMed] [Google Scholar]
- Glickman G (2010). Circadian rhythms and sleep in children with autism. Neuroscience & Biobehavioral Reviews 34(5), 755–768. [DOI] [PubMed] [Google Scholar]
- Goda R, Otsuka T, Iwamoto A, Kawai M, Shibata S, Furuse M, et al. (2015). Serotonin levels in the dorsal raphe nuclei of both chipmunks and mice are enhanced by long photoperiod, but brain dopamine level response to photoperiod is species-specific. Neuroscience letters 593, 95–100. [DOI] [PubMed] [Google Scholar]
- Green NH, Jackson CR, Iwamoto H, Tackenberg MC, and McMahon DG (2015). Photoperiod programs dorsal raphe serotonergic neurons and affective behaviors. Current Biology 25(10), 1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo RM, and Güler AD (2019). Focus: Clocks and Cycles: Dopamine Signaling in Circadian Photoentrainment: Consequences of Desynchrony. The Yale Journal of Biology and Medicine 92(2), 271. [PMC free article] [PubMed] [Google Scholar]
- Grippo RM, Purohit AM, Zhang Q, Zweifel LS, and Güler AD (2017). Direct midbrain dopamine input to the suprachiasmatic nucleus accelerates circadian entrainment. Current Biology 27(16), 2465–2475.e2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. (2002). Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416(6879), 396–400. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, and Malenka RC (2012). Integrating synaptic plasticity and striatal circuit function in addiction. Current opinion in neurobiology 22(3), 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Nakao K, and Nakamura K (2015). Appetitive and aversive information coding in the primate dorsal raphe nucleus. Journal of Neuroscience 35(15), 6195–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Salgado JS, Taylor MD, Hoerbelt P, Pinto DFC, Steinberg EE, et al. (2019). Distinct neural mechanisms for the prosocial and rewarding properties of MDMA. Science translational medicine 11(522). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, and Binder EB (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental neurology 233(1), 102–111. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, et al. (2003). Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37(2), 233–247. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, and Crawley JN (2003). Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biological psychiatry 54(10), 953–959. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, and Crawley JN (2002). Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27(6), 914–923. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Otaka M, Neumann PA, Wang Z, Cook JM, Schlüter OM, et al. (2013). Exposure to cocaine regulates inhibitory synaptic transmission from the ventral tegmental area to the nucleus accumbens. The Journal of physiology 591(19), 4827–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhacki J, Clesse D, Goumon Y, Van Someren EJ, and Mendoza J (2018). Light rescues circadian behavior and brain dopamine abnormalities in diurnal rodents exposed to a winter-like photoperiod. Brain Structure and Function 223(6), 2641–2652. [DOI] [PubMed] [Google Scholar]
- Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppä T, et al. (2003). Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology 28(4), 734–739. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Nöthen MM, Gustavsson JP, Neidt H, Bunzel R, Propping P, et al. (1998). Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Psychiatry research 79(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Kalbitzer J, Erritzoe D, Holst KK, Nielsen FÅ, Marner L, Lehel S, et al. (2010). Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. Biological psychiatry 67(11), 1033–1039. [DOI] [PubMed] [Google Scholar]
- Kalueff A, Fox M, Gallagher P, and Murphy D (2007). Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes, Brain and Behavior 6(4), 389–400. [DOI] [PubMed] [Google Scholar]
- Ketchesin KD, Becker-Krail D, and McClung CA (2018). Mood-related central and peripheral clocks. European Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchesin KD, Becker-Krail D, and McClung CA (2020). Mood-related central and peripheral clocks. European Journal of Neuroscience 51(1), 326–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby L, Zeeb F, and Winstanley C (2011). Contributions of serotonin in addiction vulnerability. Neuropharmacology 61(3), 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, Li S, and Mabrouk G (2004). GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. Journal of Comparative Neurology 469(2), 170–184. [DOI] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, et al. (2011). A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. Journal of Neuroscience 31(8), 2756–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lönnqvist J, et al. (2010). Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol & Alcoholism 45(4), 303–311. [DOI] [PubMed] [Google Scholar]
- Krishnan V, and Nestler EJ (2008). The molecular neurobiology of depression. Nature 455(7215), 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivisky K, Ashkenazy T, Kronfeld-Schor N, and Einat H (2011). Antidepressants reverse short-photoperiod-induced, forced swim test depression-like behavior in the diurnal fat sand rat: further support for the utilization of diurnal rodents for modeling affective disorders. Neuropsychobiology 63(3), 191–196. [DOI] [PubMed] [Google Scholar]
- Kurlansik SL, and Ibay AD (2012). Seasonal affective disorder. American Family Physician 86(11), 1037–1041. [PubMed] [Google Scholar]
- Lam RW, Bowering T, Tam E, Grewal A, Yatham L, Shiah I, et al. (2000). Effects of rapid tryptophan depletion in patients with seasonal affective disorder in natural summer remission. Psychological Medicine 30(1), 79–87. [DOI] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, et al. (2006). The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. American Journal of Psychiatry 163(5), 805–812. [DOI] [PubMed] [Google Scholar]
- Lam RW, Zis AP, Grewal A, Delgado PL, Charney DS, and Krystal JH (1996). Effects of rapid tryptophan depletion in patients with seasonal affective disorder in remission after light therapy. Archives of General Psychiatry 53(1), 41–44. [DOI] [PubMed] [Google Scholar]
- Lambert G, Reid C, Kaye D, Jennings G, and Esler M (2002). Effect of sunlight and season on serotonin turnover in the brain. The Lancet 360(9348), 1840–1842. [DOI] [PubMed] [Google Scholar]
- Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, and Welsh DK (2016a). Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biological psychiatry 80(11), 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long JE, and Welsh DK (2016b). Depression-like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. European Journal of Neuroscience 43(10), 1309–1320. [DOI] [PubMed] [Google Scholar]
- Landgraf D, McCarthy MJ, and Welsh DK (2014a). Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Current psychiatry reports 16(10), 483. [DOI] [PubMed] [Google Scholar]
- Landgraf D, McCarthy MJ, and Welsh DK (2014b). The role of the circadian clock in animal models of mood disorders. Behavioral neuroscience 128(3), 344. [DOI] [PubMed] [Google Scholar]
- Lee A, Myung S-K, Cho JJ, Jung Y-J, Yoon JL, and Kim MY (2017). Night shift work and risk of depression: meta-analysis of observational studies. Journal of Korean Medical Science 32(7), 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Newschaffer CJ, Lessler JT, Lee BK, Shah R, and Zimmerman AW (2008). Variation in season of birth in singleton and multiple births concordant for autism spectrum disorders. Paediatric and perinatal epidemiology 22(2), 172–179. [DOI] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee H-K, Yang S, Zhao H, et al. (2012). Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491(7425), 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274(5292), 1527–1531. [DOI] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. (2013). Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences 110(24), 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li C-Y, Xi W, Jin S, Wu Z-H, Jiang P, et al. (2019). Rostral and caudal ventral tegmental area GABAergic inputs to different dorsal raphe neurons participate in opioid dependence. Neuron 101(4), 748–761. e745. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, et al. (2016). Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nature communications 7, 10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, and Malenka RC (2012). Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487(7406), 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, et al. (2014). Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81(6), 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. (2010). Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330(6002), 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Williams III WP, and McClung CA (2014). Circadian rhythms and addiction: mechanistic insights and future directions. Behavioral neuroscience 128(3), 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, and Aston-Jones G (2009). Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. European Journal of Neuroscience 29(4), 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, and Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69(4), 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, et al. (2018). Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. The Lancet Psychiatry 5(6), 507–514. [DOI] [PubMed] [Google Scholar]
- Magnusson A, and Boivin D (2003). Seasonal affective disorder: an overview. Chronobiology international 20(2), 189–207. [DOI] [PubMed] [Google Scholar]
- Mahmood T, and Silverstone T (2001). Serotonin and bipolar disorder. Journal of affective disorders 66(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Manz KM, Ghose D, Turner BD, Taylor A, Becker J, Grueter CA, et al. (2020). Calcium-Permeable AMPA Receptors Promote Endocannabinoid Signaling at Parvalbumin Interneuron Synapses in the Nucleus Accumbens Core. Cell reports 32(4), 107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens G, and van Loo K (2007). Genetic and environmental factors in complex neurodevelopmental disorders. Current genomics 8(7), 429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W, Murphy J, Gatto G, Levy A, Yoshimoto K, Lumeng L, et al. (1993). CNS mechanisms of alcohol self-administration. Alcohol and alcoholism supplement 2, 463–467. [PubMed] [Google Scholar]
- McClung CA (2007a). Circadian genes, rhythms and the biology of mood disorders. Pharmacology & therapeutics 114(2), 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA (2007b). Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. TheScientificWorldJournal 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA (2013). How might circadian rhythms control mood? Let me count the ways. Biological psychiatry 74(4), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. (2005). Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences 102(26), 9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RA, et al. (2014). Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell reports 8(6), 1857–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Li H. q., Deisseroth K, Leutgeb S, and Spitzer NC (2018). Neuronal activity regulates neurotransmitter switching in the adult brain following light-induced stress. Proceedings of the National Academy of Sciences 115(20), 5064–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliarini S, Pacini G, Pelosi B, Lunardi G, and Pasqualetti M (2013). Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Molecular psychiatry 18(10), 1106–1118. [DOI] [PubMed] [Google Scholar]
- Modai I, Kikinzon L, and Valevski A (1994). Environmental factors and admission rates in patients with major psychiatric disorders. Chronobiology international 11(3), 196–199. [DOI] [PubMed] [Google Scholar]
- Montange MF, Cauter EV, Refetoff S, Désir D, Tourniaire J, and Copinschi G (1981). Effects of “jet lag” on hormonal patterns. II. Adaptation of melatonin circadian periodicity. The Journal of Clinical Endocrinology & Metabolism 52(4), 642–649. [DOI] [PubMed] [Google Scholar]
- Moriam S, and Sobhani ME (2013). Epigenetic effect of chronic stress on dopamine signaling and depression. Genetics & epigenetics 5, GEG. S11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao J-L, Kumar J, Chakravarty S, Asaithamby A, et al. (2010). Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological psychiatry 68(6), 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, and Homberg JR (2015). The role of serotonin in drug use and addiction. Behavioural brain research 277, 146–192. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Takayama K, Nishitani N, Andoh C, Koda M, Shirakawa H, et al. (2020). The Role of Dorsal Raphe Serotonin Neurons in the Balance between Reward and Aversion. International journal of molecular sciences 21(6), 2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K (2013). The role of the dorsal raphé nucleus in reward-seeking behavior. Frontiers in integrative neuroscience 7, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara KJ, and Nelson RJ (2007). The dark side of light at night: physiological, epidemiological, and ecological consequences. Journal of pineal research 43(3), 215–224. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, and Monteggia LM (2002). Neurobiology of depression. Neuron 34(1), 13–25. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, and Carlezon WA Jr (2006). The mesolimbic dopamine reward circuit in depression. Biological psychiatry 59(12), 1151–1159. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Praschak-Rieder N, Heβelmann B, Rao M-L, Glück J, and Kasper S (1997). Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Archives of General Psychiatry 54(2), 133–138. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Turner EH, Matthews JR, Postolache TT, Barnett RL, Rauh M, et al. (1998). Effects of tryptophan depletion vs catecholamine depletion in patients with seasonal affective disorder in remission with light therapy. Archives of General Psychiatry 55(6), 524–530. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Willeit M, Praschak-Rieder N, Asenbaum S, Stastny J, Hilger E, et al. (2001). Dopamine transporter availability in symptomatic depressed patients with seasonal affective disorder and healthy controls. Psychological medicine 31(8), 1467. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. (2006). Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 141(3), 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, and Parsons LH (2004). Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. Journal of Pharmacology and Experimental Therapeutics 311(2), 711–719. [DOI] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Hwang D, Uchida N, and Watabe-Uchida M (2014). Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell reports 8(4), 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Mitchell AC, and McMahon DG (2006). Constant light disrupts the developing mouse biological clock. Pediatric research 60(3), 304–308. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, and McMahon DG (2005). Constant light desynchronizes mammalian clock neurons. Nature neuroscience 8(3), 267–269. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Kawai M, Togo Y, Goda R, Kawase T, Matsuo H, et al. (2014). Photoperiodic responses of depression-like behavior, the brain serotonergic system, and peripheral metabolism in laboratory mice. Psychoneuroendocrinology 40, 37–47. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, et al. (2013). The role of clock in ethanol-related behaviors. Neuropsychopharmacology 38(12), 2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L, Porcella A, and Gessa G (2000). The role of stress in the pathophysiology of the dopaminergic system. Molecular psychiatry 5(1), 14. [DOI] [PubMed] [Google Scholar]
- Parekh PK, Becker-Krail D, Sundaravelu P, Ishigaki S, Okado H, Sobue G, et al. (2018). Altered GluA1 (Gria1) function and accumbal synaptic plasticity in the ClockΔ19 model of bipolar mania. Biological psychiatry 84(11), 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh PK, Logan RW, Ketchesin KD, Becker-Krail D, Shelton MA, Hildebrand MA, et al. (2019). Cell-type-specific regulation of nucleus accumbens synaptic plasticity and cocaine reward sensitivity by the circadian protein, NPAS2. Journal of Neuroscience 39(24), 4657–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh PK, Ozburn AR, and McClung CA (2015). Circadian clock genes: effects on dopamine, reward and addiction. Alcohol 49(4), 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L, and Justice J Jr (1993). Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. Journal of neurochemistry 61(5), 1611–1619. [DOI] [PubMed] [Google Scholar]
- Partonen T (2012). Clock gene variants in mood and anxiety disorders. Journal of Neural Transmission 119(10), 1133–1145. [DOI] [PubMed] [Google Scholar]
- Partonen T (2015). Clock genes in human alcohol abuse and comorbid conditions. Alcohol 49(4), 359–365. [DOI] [PubMed] [Google Scholar]
- Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, et al. (2007). Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Annals of medicine 39(3), 229–238. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, and Lüscher C (2015). Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88(5), 1054–1066. [DOI] [PubMed] [Google Scholar]
- Paulus EV, and Mintz EM (2012). Developmental disruption of the serotonin system alters circadian rhythms. Physiology & behavior 105(2), 257–263. [DOI] [PubMed] [Google Scholar]
- Paulus EV, and Mintz EM (2013). Photic and nonphotic responses of the circadian clock in serotonin-deficient Pet-1 knockout mice. Chronobiology international 30(10), 1251–1260. [DOI] [PubMed] [Google Scholar]
- Peeters F, Berkhof J, Delespaul P, Rottenberg J, and Nicolson NA (2006). Diurnal mood variation in major depressive disorder. Emotion 6(3), 383. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Zghoul T, De Fonseca FR, Spanagel R, and Bilbao A (2009). PRECLINICAL STUDY: Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addiction Biology 14(3), 253–259. [DOI] [PubMed] [Google Scholar]
- Persico AM, Baldi A, Dell’Acqua ML, Moessner R, Murphy DL, Lesch K-P, et al. (2003). Reduced programmed cell death in brains of serotonin transporter knockout mice. Neuroreport 14(3), 341–344. [DOI] [PubMed] [Google Scholar]
- Pietraszek M, Urano T, Sumiyoshi K, Takada Y, Takada A, Ohara K, et al. (1992). Diurnal variations of whole blood serotonin content in patients with depression and neurosis. Journal of neurology, neurosurgery, and psychiatry 55(4), 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirraglia PA, Stafford RS, and Singer DE (2003). Trends in prescribing of selective serotonin reuptake inhibitors and other newer antidepressant agents in adult primary care. Primary care companion to the Journal of clinical psychiatry 5(4), 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisansky MT, Lefevre EM, Retzlaff CL, Trieu BH, Leipold DW, and Rothwell PE (2019). Nucleus accumbens fast-spiking interneurons constrain impulsive action. Biological Psychiatry 86(11), 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu A, Vaughan M, Nilsson A, Arimoto N, Lamia K, and Welsh DK (2020). Vulnerability to helpless behavior is regulated by the circadian clock component CRYPTOCHROME in the mouse nucleus accumbens. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, and Willeit M (2011). “Imaging of seasonal affective disorder and seasonality effects on serotonin and dopamine function in the human brain,” in Brain Imaging in Behavioral Neuroscience. Springer; ), 149–167. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Willeit M, Wilson AA, Houle S, and Meyer JH (2008). Seasonal variation in human brain serotonin transporter binding. Archives of general psychiatry 65(9), 1072–1078. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, and Nelson RJ (2005). Affective responses to changes in day length in Siberian hamsters (Phodopus sungorus). Psychoneuroendocrinology 30(5), 438–452. [DOI] [PubMed] [Google Scholar]
- Pyter LM, and Nelson RJ (2006). Enduring effects of photoperiod on affective behaviors in Siberian hamsters (Phodopus sungorus). Behavioral neuroscience 120(1), 125–134. [DOI] [PubMed] [Google Scholar]
- Quello SB, Brady KT, and Sonne SC (2005). Mood disorders and substance use disorder: a complex comorbidity. Science & practice perspectives 3(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio J, Pévet P, and Masson-Pévet M (1996). Serotonergic modulation of photically induced increase in melatonin receptor density and Fos immunoreactivity in the suprachiasmatic nuclei of the rat. Journal of neuroendocrinology 8(11), 839–845. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, et al. (2011). Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. Journal of Neuroscience 31(16), 6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HL, and Reilly SM (2002). A survey of the health experiences of international business travelers: Part one—Physiological aspects. Aaohn Journal 50(10), 449–459. [PubMed] [Google Scholar]
- Rosenthal NE, Mazzanti CM, Barnett RL, Hardin TA, Turner EH, Lam GK, et al. (1998). Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Molecular psychiatry 3(2), 175–177. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, and Foster JA (2010). Effects of repeated light–dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol 44(3), 229–237. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. (2007). Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences 104(15), 6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, and Nestler EJ (2013). The brain reward circuitry in mood disorders. Nature Reviews Neuroscience 14(9), 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J, et al. (1995). Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats—influence on dopaminergic stimulation. Chronobiology International 12(2), 87–99. [DOI] [PubMed] [Google Scholar]
- Schall TA, Wright WJ, and Dong Y (2020). Nucleus accumbens fast-spiking interneurons in motivational and addictive behaviors. Molecular Psychiatry, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh K-R, Chu Y-S, and Pan J-T (1997). Circadian change of dopaminergic neuron activity: effects of constant light and melatonin. Neuroreport 8(9), 2283–2287. [DOI] [PubMed] [Google Scholar]
- Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, et al. (2015). Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Molecular psychiatry 20(11), 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann J, Williams P, Malik TN, Jackson C, Green NH, Emeson R, Levitt P, McMahon DG (2020). Photoperiodic Effects on Monoamine Signaling & Gene Expression Throughout Development in the Serotonin & Dopamine Systems. bioRxiv. doi: 10.1101/2020.06.25.171470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann JK, Green NH, Reddy N, and McMahon DG (2019). Sequential photoperiodic programing of serotonin neurons, signaling and behaviors during prenatal and postnatal development. Frontiers in neuroscience 13, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä T, Kananen L, Greco D, Donner J, Silander K, Terwilliger JD, et al. (2010). An association analysis of circadian genes in anxiety disorders. Biological psychiatry 67(12), 1163–1170. [DOI] [PubMed] [Google Scholar]
- Sjöholm LK, Kovanen L, Saarikoski ST, Schalling M, Lavebratt C, and Partonen T (2010). CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. Journal of circadian rhythms 8(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, and Jansen HT (2005). Time of day alters long-term sensitization to cocaine in rats. Brain research 1065(1–2), 132–137. [DOI] [PubMed] [Google Scholar]
- Small KM, Nunes E, Hughley S, and Addy NA (2016). Ventral tegmental area muscarinic receptors modulate depression and anxiety-related behaviors in rats. Neuroscience letters 616, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian J-J, Descarries L, and Watkins KC (1989). Serotonin innervation in adult rat neostriatum. II. Ultrastructural features: a radioautographic and immunocytochemical study. Brain research 481(1), 67–86. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Stark G, Sergeeva A, and Jansen HT (2011). Photoperiodic suppression of drug reinstatement. Neuroscience 176, 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. (2005). The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature medicine 11(1), 35–42. [DOI] [PubMed] [Google Scholar]
- Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, et al. (2013). Circadian genes P eriod 1 and P eriod 2 in the nucleus accumbens regulate anxiety-related behavior. European Journal of Neuroscience 37(2), 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindelegger C, Stein P, Wadsak W, Fink M, Mitterhauser M, Moser U, et al. (2012). Light-dependent alteration of serotonin-1A receptor binding in cortical and subcortical limbic regions in the human brain. The World Journal of Biological Psychiatry 13(6), 413–422. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Allen AJ, Glod CA, Clark CH, Teicher MH, Richter D, et al. (1997). A controlled trial of light therapy for the treatment of pediatric seasonal affective disorder. Journal of the American Academy of Child & Adolescent Psychiatry 36(6), 816–821. [DOI] [PubMed] [Google Scholar]
- Tackenberg MC, and McMahon DG (2018). Photoperiodic Programming of the SCN and Its Role in Photoperiodic Output. Neural plasticity 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Kawai M, Ogo T, Ichinose T, Furuya S, Takaki N, et al. (2018). Early-life Photoperiod Influences Depression-like Behavior, Prepulse Inhibition of the Acoustic Startle Response, and Hippocampal Astrogenesis in Mice. Neuroscience 374, 133–143. [DOI] [PubMed] [Google Scholar]
- Terman M, and Terman JS (2005). Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS spectrums 10(8), 647–663. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS, Quitkin FM, McGrath P, Stewart J, and Rafferty B (1989). Light therapy for seasonal affective disorder. Neuropsychopharmacology 2(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Fabbri M, Martoni M, and Natale V (2012). Season of birth and mood seasonality in late childhood and adolescence. Psychiatry research 195(1), 66–68. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Miller J, Rawlings R, and Yolken RH (1997). Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophrenia research 28(1), 1–38. [DOI] [PubMed] [Google Scholar]
- Treadway MT, and Zald DH (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews 35(3), 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkoff AM, and Storr CL (1998). Work schedule characteristics and substance use in nurses. American journal of industrial medicine 34(3), 266–271. [DOI] [PubMed] [Google Scholar]
- Turner BD, Kashima DT, Manz KM, Grueter CA, and Grueter BA (2017). Synaptic plasticity in the nucleus accumbens: Lessons learned from experience. ACS chemical neuroscience 9(9), 2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuunainen A, Kripke DF, and Endo T (2004). Light therapy for non-seasonal depression. Cochrane Database of Systematic Reviews (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J, et al. (2013). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493(7433), 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer AE, Levitan RD, Houle S, Wilson AA, Nobrega JN, and Meyer JH (2016). Increased seasonal variation in serotonin transporter binding in seasonal affective disorder. Neuropsychopharmacology 41(10), 2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, and McGuffin P (2008). “The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis”. Nature Publishing Group; ). [DOI] [PubMed] [Google Scholar]
- Upton A, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I, et al. (1999). Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. The Journal of Neuroscience 19(16), 7007–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg RI, Serlie MJ, Kalsbeek A, and la Fleur SE (2015). Serotonin, a possible intermediate between disturbed circadian rhythms and metabolic disease. Neuroscience 301, 155–167. [DOI] [PubMed] [Google Scholar]
- Vertes RP, and Linley SB (Year). “Comparison of projections of the dorsal and median raphe nuclei, with some functional considerations”, in: International Congress Series: Elsevier; ), 98–120. [Google Scholar]
- Vogel M, Braungardt T, Meyer W, and Schneider W (2012). The effects of shift work on physical and mental health. Journal of neural transmission 119(10), 1121–1132. [DOI] [PubMed] [Google Scholar]
- Volkow ND (2004). The reality of comorbidity: depression and drug abuse. Biological psychiatry. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, and Telang F (2011). Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences 108(37), 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, de la Cruz F, Köhler S, and Bär K-J (2017). Treatment associated changes of functional connectivity of midbrain/brainstem nuclei in major depressive disorder. Scientific reports 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Borniger JC, Gaudier-Diaz MM, Meléndez-Fernández OH, Pascoe JL, DeVries AC, et al. (2020a). Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Molecular psychiatry 25(5), 1080–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Walton JC, DeVries AC, and Nelson RJ (2020b). Circadian rhythm disruption and mental health. Translational Psychiatry 10(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-L, Zhang S, Qi J, Wang H, Cachope R, Mejias-Aponte CA, et al. (2019). Dorsal raphe dual serotonin-glutamate neurons drive reward by establishing excitatory synapses on VTA mesoaccumbens dopamine neurons. Cell reports 26(5), 1128–1142.e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gallegos DA, Pogorelov VM, O’Hare JK, Calakos N, Wetsel WC, et al. (2018). Parvalbumin interneurons of the mouse nucleus accumbens are required for amphetamine-induced locomotor sensitization and conditioned place preference. Neuropsychopharmacology 43(5), 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC (2017). Circadian rhythms and substance abuse: chronobiological considerations for the treatment of addiction. Current psychiatry reports 19(2), 12. [DOI] [PubMed] [Google Scholar]
- Weber M, Lauterburg T, Tobler I, and Burgunder J-M (2004). Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neuroscience letters 358(1), 17–20. [DOI] [PubMed] [Google Scholar]
- Westrin Å, and Lam RW (2007). Seasonal affective disorder: a clinical update. Annals of Clinical Psychiatry 19(4), 239–246. [DOI] [PubMed] [Google Scholar]
- Willeit M, Sitte HH, Thierry N, Michalek K, Praschak-Rieder N, Zill P, et al. (2008). Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology 33(7), 1503–1513. [DOI] [PubMed] [Google Scholar]
- Winter C, von Rumohr A, Mundt A, Petrus D, Klein J, Lee T, et al. (2007). Lesions of dopaminergic neurons in the substantia nigra pars compacta and in the ventral tegmental area enhance depressive-like behavior in rats. Behavioural brain research 184(2), 133–141. [DOI] [PubMed] [Google Scholar]
- Winthorst WH, Roest AM, Bos EH, Meesters Y, Penninx BW, Nolen WA, et al. (2017). Seasonal affective disorder and non-seasonal affective disorders: results from the NESDA study. BJPsych open 3(4), 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschläger A, Karne H, Jordan D, Lowe MJ, Jones SE, and Anand A (2018). Spectral dynamics of resting state fMRI within the ventral tegmental area and dorsal raphe nuclei in medication-free major depressive disorder in young adults. Frontiers in psychiatry 9, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Lin K, Wang D-H, Moustafa AA, Cohen JY, and Nakamura K (2017). Toward a multiscale modeling framework for understanding serotonergic function. Journal of Psychopharmacology 31(9), 1121–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L-Z, Liu L-J, Yuan M, Li S-X, Yue X-D, Lai J-L, et al. (2016). Short photoperiod condition increases susceptibility to stress in adolescent male rats. Behavioural brain research 300, 38–44. [DOI] [PubMed] [Google Scholar]
- You I-J, Wright SR, Garcia-Garcia AL, Tapper AR, Gardner PD, Koob GF, et al. (2016). 5-HT 1A Autoreceptors in the Dorsal Raphe Nucleus Convey Vulnerability to Compulsive Cocaine Seeking. Neuropsychopharmacology 41(5), 1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Cope ZA, Romoli B, Schrurs E, Joosen A, van Enkhuizen J, et al. (2018). Mice with reduced DAT levels recreate seasonal-induced switching between states in bipolar disorder. Neuropsychopharmacology 43(8), 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauderer C, and Ganzer CA (2015). Seasonal affective disorder: an overview. Mental Health Practice 18(9). [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, and Spanagel R (2007). Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1 Brdm1 mutant mice. Psychopharmacology 190(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai T, Zwanzger P, Schüle C, Eser D, Rupprecht R, et al. (2004). SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Molecular psychiatry 9(11), 1030. [DOI] [PubMed] [Google Scholar]


