Abstract
Background/aims:
In obese youth, it is not clear what degree of β-cell impairment translates to glucose dysregulation commensurate with shifts from normal glucose tolerance (NGT) to impaired glucose tolerance (IGT) to type 2 diabetes. We aimed to investigate the quantitative relationship between β-cell (clamp-measured disposition index [DI]) and OGTT glucose area under the curve (G-AUC) in obese youth across the spectrum of glucose tolerance.
Methods:
Data from 152 youth (58 African-American [AA] and 94 American-White [AW]; 73 NGT, 48 IGT, and 31 type 2 diabetes) who completed a 3-hr hyperinsulinemic (80 mu/m2/min)-euglycemic clamp, and a 2-hr hyperglycemic (225 mg/dL) clamp synchronized with a 2-hr OGTT were examined.
Results:
In IGT vs. NGT, 36% lower DI corresponded to 27% higher G-AUC; in type 2 diabetes vs. IGT, 65% lower DI related to 25% higher G-AUC, and in type 2 diabetes vs. NGT, 78% lower DI paralleled 59% higher G-AUC. Although AA vs. AW youth had larger decrements in DI, from NGT to IGT and from NGT to type 2 diabetes, they displayed comparable increments in G-AUC.
Conclusion:
At least ~35–50% recovery in β-cell function might be needed to have clinically meaningful improvement in G-AUC commensurate with conversion to better glucose tolerance. Mechanism(s) protective against dysglycemia might be operative in AA vs. AW youth despite greater declines in DI. Treatments aiming to improve β-cell function should focus on degree of change in DI commensurate with clinically meaningful changes in glycemia, reflective of restoration of glucose tolerance.
Keywords: β-cell function, Clamp, Glucose area under the curve, OGTT, Glucose tolerance, Youth
1. Introduction
Once thought to be an adult disease, type 2 diabetes has emerged as an increasingly prevalent health condition in pediatrics, with a 30.5% increase in prevalence between 2001 and 2009 and an annual 7.1% increase in incidence [1,2]. Consistent with the natural history of type 2 diabetes in adults [3,4], the key pathophysiologic factor in youth-onset prediabetes and type 2 diabetes is pancreatic β-cell dysfunction against the backdrop of insulin resistance [5,6]. Our previous studies demonstrated that impaired β-cell function predates the onset of type 2 diabetes, is present in obese youth with prediabetes and even in obese youth with normal glucose tolerance (NGT) compared with their normal-weight peers, and worsens with increasing fasting and 2-hr glucose concentrations that are considered to be in the normal range [5,7–10]. This is in line with the finding that lower β-cell function relative to insulin sensitivity (i.e., disposition index [DI]) is the strongest metabolic predictor of future type 2 diabetes in adults [11].
Given the important role of β-cell function in the pathophysiology of youth-onset type 2 diabetes, two large-scale multi-center clinical therapeutic intervention studies, TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) and RISE (Restoring Insulin Secretion), have focused on improvement of β-cell function in response to their therapeutic approaches in obese youth with prediabetes and/or type 2 diabetes [12–15]. In the TODAY trial, oral glucose tolerance test (OGTT)-derived DI deteriorated progressively and rapidly in obese youth with type 2 diabetes who failed to maintain glycemic control (i.e., persistent elevation in HbA1c ≥8% for 6 months or inability to be weaned off insulin) [14]. Further, in youth with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes in the RISE trial, neither 3 months of glargine followed by 9 months of metformin, nor 12 months of metformin alone restored the impaired β-cell function [13]. However, limited scientific knowledge of the relationship between β-cell function and glucose regulation hinders our understanding of the results derived from the aforementioned clinical trials. Although there is evidence of deterioration in β-cell function with elevated fasting and 2-hr glucose concentrations in the abnormal range and even in the range that is considered normal [8,10], it is still not clear what degree of impairment in β-cell function translates to deterioration in glucose regulation due to the limitation of a single time point measurement of glucose concentration. We postulate that the glucose area under the curve (AUC) would seem to be a more physiological measure of glucose tolerance than the classic definition, which for convenience is based on a single arbitrary time point of 120 min. In other words, it is clinically germane to examine the magnitude of β-cell deterioration or improvement that is commensurate with worsening or recovery of glucose homeostasis based on integrated glucose responses measured with OGTT glucose-AUC. To date, no studies in youth prediabetes or type 2 diabetes have addressed this issue. Furthermore, in designing any intervention trial, it is critical to determine a priori the extent of β-cell improvement that is hypothesized to be achieved in order to accomplish reversal from type 2 diabetes to IGT or from IGT to NGT.
Therefore, the purpose of this study was to investigate the degree of impairment in β-cell function corresponding to escalations in OGTT glucose-AUC that differentiate NGT from IGT and from type 2 diabetes in obese youth.
2. Material and methods
2.1. Participants
Data from 152 adolescents (58 African-American [AA] and 94 American-White [AW]; ages 10 to <20 years old; Tanner stages II-V), who had an OGTT synchronized with a hyperglycemic and a hyperinsulinemic-euglycemic clamp, as participants in our National Institutes of Health-funded studies of Childhood Insulin Resistance and Childhood Metabolic Markers of Adult Morbidity, were included in the present study. Information resulting from these grants but unrelated to the relationship between clamp DI and OGTT glucose-AUC have been published [8,10,16–19]. There were 7 who were overweight with body mass index (BMI) ≥85th percentile for age and sex but <95th, and 145 obese with BMI ≥95th percentile. From here on, we will refer to the overweight and obese youth as “obese” for simplicity purposes. There were 73 participants with NGT, 48 with IGT, and 31 with type 2 diabetes. Glucose tolerance status was determined with a 2-hr OGTT [20]. Participants with isolated impaired fasting glucose (IFG) (n=9) were excluded because IGT alone and combined IFG and IGT are characterized with insulin resistance whereas isolated IFG is not, while both have impaired β-cell function [21]. All obese youth with type 2 diabetes were negative for glutamic acid decarboxylase (GAD) and insulinoma associated protein-2 (IA2) autoantibody. They all had adequate metabolic control, with an average HbA1c of 6.6 ± 0.2 [SE] % (range 4.7–8.3%) and average duration of diabetes of 7.7 ± 1.7 months (0–39 months). They were on treatment consisting of lifestyle modification (n=6), metformin alone (n=15), metformin together with insulin (n=7), and insulin alone (n=3). None received degludec or insulin glargine 300 IU/ml. Metformin was discontinued 36 h prior to the OGTT. Patients did not receive long- or intermediate-acting insulin for 24 hr prior to the OGTT. The last dose of short-acting insulin was given 6–8 hr prior to the OGTT. The same applied for the clamp experiments. Participants classified as NGT or IGT were not taking any medications known to affect glucose metabolism. Smoking status was not collected in this study. Participants were recruited through newspaper advertisements, flyers posted in the medical campus, city bus routes and the outpatient clinics in the Weight Management and Wellness Center and the Division of Pediatric Endocrinology. The study was approved by the institutional review board of the University of Pittsburgh, and written informed parental consent and child assent were obtained from all participants before any research procedures were performed.
2.2. Procedures
All procedures were performed at the Pediatric Clinical and Translational Research Center (PCTRC) of UPMC Children’s Hospital of Pittsburgh. All participants underwent medical history, physical examination, and hematologic and biochemical tests. Height and weight were measured to the nearest 0.1cm and 0.1kg, respectively, and used to calculate BMI. Pubertal development was assessed using Tanner criteria [22]. Body composition was evaluated with dual energy X-ray absorptiometry (DEXA) for the measurement of total body fat mass and percent body fat.
2.3. Metabolic studies
After a 10- to12-hr of overnight fast, participants underwent a 2-hr OGTT (1.75 g/kg, maximum 75 g), and blood samples were obtained at −15, 0, 15, 30, 60, 90 and 120 min for the measurement of glucose and insulin. Fasting blood samples were obtained for the determination of HbA1c. Participants were admitted twice to the PCTRC, either the day after the OGTT or on a separate visit within a 1- to 4-week period, to undergo a hyperinsulinemic-euglycemic clamp to assess in vivo insulin sensitivity, and a hyperglycemic clamp to assess insulin secretion, in random order [5]. Each clamp evaluation was performed after a 10- to 12-hr overnight fast. Fasting hepatic glucose production was measured before the start of the hyperinsulinemic-euglycemic clamp, with a primed (2.2 μmol/Kg) constant infusion of [6,6-2H2]glucose at 0.22 μmol/Kg/min for a total of 2 hours as described [5]. After the 2-hr baseline isotope infusion period, in vivo insulin sensitivity was evaluated during a 3-hr hyperinsulinemic (80 mu/m2/min)-euglycemic clamp (100 mg/dL) [5]. 1st-phase insulin secretion was assessed during a 2-hr hyperglycemic (225 mg/dL) clamp as described before [5]. Plasma glucose was increased rapidly to 225 mg/dL by a bolus infusion of dextrose and maintained at that level by a variable rate infusion of 20% dextrose for 2 hours, with frequent measurement of glucose and insulin concentrations.
2.4. Biochemical measurements
During the 2-hr OGTT, blood was collected in chilled aprotinin/EDTA tubes for the measurement of insulin. Blood samples were immediately separated in a refrigerated centrifuge. Plasma samples were divided into aliquots and stored at −80°C until analysis. Plasma glucose was determined by the glucose oxidase method using a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH, USA), and insulin by commercially available radioimmunoassay (Millipore, St. Charles, MO, USA), as previously reported [16]. HbA1c was measured by high-performance liquid chromatography (Tosoh Medics, Inc., San Francisco, CA, USA).
2.5. Calculations of OGTT glucose-AUC and clamp DI
OGTT-AUC for glucose and insulin was calculated with the use of the trapezoidal method. Fasting hepatic glucose production was calculated during the last 30 min of the baseline 2-hr isotope infusion (-30 to 0 min) of the hyperinsulinemic-euglycemic clamp, according to steady-state tracer dilution equations [5]. Hepatic insulin sensitivity was calculated as the inverse of the product of hepatic glucose production and fasting plasma insulin concentration [5,23]. During the hyperinsulinemic-euglycemic clamp, insulin-stimulated glucose disposal (Rd) was calculated to be equal to the rate of exogenous glucose infusion during the final 30 min of the clamp. Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin concentration multiplied by 100 [5]. During the hyperglycemic clamp, 1st-phase insulin concentration was calculated during the first 10 min as described before [5,19]. β-cell function relative to insulin sensitivity, i.e., clamp DI, was calculated as the product of insulin sensitivity, measured by the hyperinsulinemic-euglycemic clamp, and 1st-phase insulin secretion measured during the hyperglycemic clamp [5,8,10,19].
2.6. Statistical analysis
Univariate ANOVA using Bonferroni post hoc test and chi-square analyses were used to compare physical and metabolic characteristics across the 3 groups of obese youth with NGT, IGT and type 2 diabetes. Because youth-onset type 2 diabetes is more common in females and African American youth, we used two-way ANOVA to examine the effect of race (AA vs. AW) (race × glucose tolerance), or sex (male vs. female) (sex × glucose tolerance) (NGT, IGT and type 2 diabetes) on clamp DI and glucose-AUC. Additionally, this allowed us to assess whether there is a differential effect of glucose tolerance on clamp DI and glucose-AUC by race (interaction effect). Spearman’s correlation analysis as a nonparametric measure was used to examine bivariate relationships between clamp DI and glucose-AUC. More importantly, the magnitude of differences in clamp DI was compared with the degree of alterations in glucose-AUC, to provide a clinically meaningful translation of glycemic variations during a physiological test, the OGTT, imparted by changes in β-cell function. Data that did not meet the assumptions for normality were log10 transformed; untransformed data are presented for ease of interpretation. Data were analyzed using PASW 24.0 statistical software package with significance set at P ≤0.05. Data are mean ± SEM.
3. Results
3.1. Physical and metabolic characteristics of obese youth with NGT, IGT and type 2 diabetes
There were no differences in age, sex, race, Tanner stage, BMI, total body fat mass and percent body fat among the 3 groups, despite higher BMI percentile in obese youth with type 2 diabetes vs. NGT (Table 1). There was progressive increase in fasting & 2-hr glucose concentrations (Table 1) and glucose-AUC (Figure 1A) from NGT to IGT to type 2 diabetes. While fasting insulin increased from NGT to IGT to type 2 diabetes, 2-hr insulin and insulin-AUC were highest in IGT compared with NGT and type 2 diabetes (Table 1).
Table 1.
Physical and metabolic characteristics of obese youth with NGT, IGT and Type 2 diabetes.
| Ob-NGT (1) | Ob-IGT (2) | Ob-T2D (3) | P | P post hoc | |||
|---|---|---|---|---|---|---|---|
| n=73 | n=48 | n=31 | ANOVA | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| Age (yrs) | 15 ± 0.2 | 14.7 ± 0.3 | 15.0 ± 0.3 | NS | - | - | - |
| Race (% AA/AW) | 40/60 | 29/71 | 48/52 | NS | - | - | - |
| Sex (% M/F) | 45/55 | 31/69 | 45/55 | NS | - | - | - |
| Tanner stage (% II/III/IV/V) | 2/14/14/70 | 4/8/17/71 | 6/3/26/65 | NS | - | - | - |
| BMI (kg/m2) | 35.6 ± 0.7 | 37.1 ± 1.0 | 36.6 ± 1.0 | NS | - | - | - |
| BMI% | 98.0 ± 0.3 | 98.7 ± 0.2 | 99.0 ± 0.1 | 0.009 | 0.052 | 0.022 | NS |
| FM (kg) | 42.2 ± 1.4 | 44.1 ± 1.8 | 41.2 ± 1.9 | NS | - | - | - |
| % body fat | 43.4 ± 0.7 | 44.5 ± 0.7 | 42.6 ± 1.2 | NS | - | - | - |
| HbA1c (%) | 5.31 ± 0.05 | 5.33 ± 0.06 | 6.57 ± 0.15 | <0.0001 | NS | <0.0001 | <0.0001 |
| Fasting glucose (mg/dL) | 87.5 ± 0.9 | 93.1 ± 1.1 | 115.3 ± 4.9 | <0.0001 | 0.022 | <0.0001 | <0.0001 |
| Fasting insulin (μU/mL) | 32.9 ± 2.1 | 44.0 ± 3.6 | 62.5 ± 10.1 | 0.001 | 0.055 | <0.0001 | 0.038 |
| 2-hr glucose (mg/dL) | 112.2 ± 1.8 | 157.9 ± 2.6 | 205.8 ± 9.3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 2-hr insulin (μU/mL) | 169.5 ± 17.7 | 363.5 ± 30.5 | 200.2 ± 31.0 | <0.0001 | <0.0001 | NS | <0.0001 |
| Insulin-AUC (μU·mL−1·h−1) | 21597.1 ± 1734.5 | 31516.6 ± 2305.4 | 20510.1 ± 3650.0 | <0.0001 | 0.001 | NS | <0.0001 |
| Hepatic IS (mg/kg/min per μU/mL)−1 | 17.7 ± 1.1 | 11.8 ± 0.9 | 12.5 ± 2.1 | <0.0001 | 0.001 | <0.0001 | NS |
| Peripheral IS (mg/kg/min per μU/mL) | 2.2 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.2 | <0.0001 | 0.009 | <0.0001 | NS |
| 1st-phase insulin (μU/mL) | 270.7 ± 25.5 | 230.1 ± 18.8 | 124.5 ± 27.4 | <0.0001 | NS | <0.0001 | <0.0001 |
Data are mean ± SEM. Ob: obese; NGT: normal glucose tolerance; IGT: impaired glucose tolerance; T2D: type 2 diabetes; AA: African-American; AW: American-White; BMI: body mass index; FM: fat mass; FFM: fat free mass; AUC: area under the curve; IS: insulin sensitivity.
Figure 1:
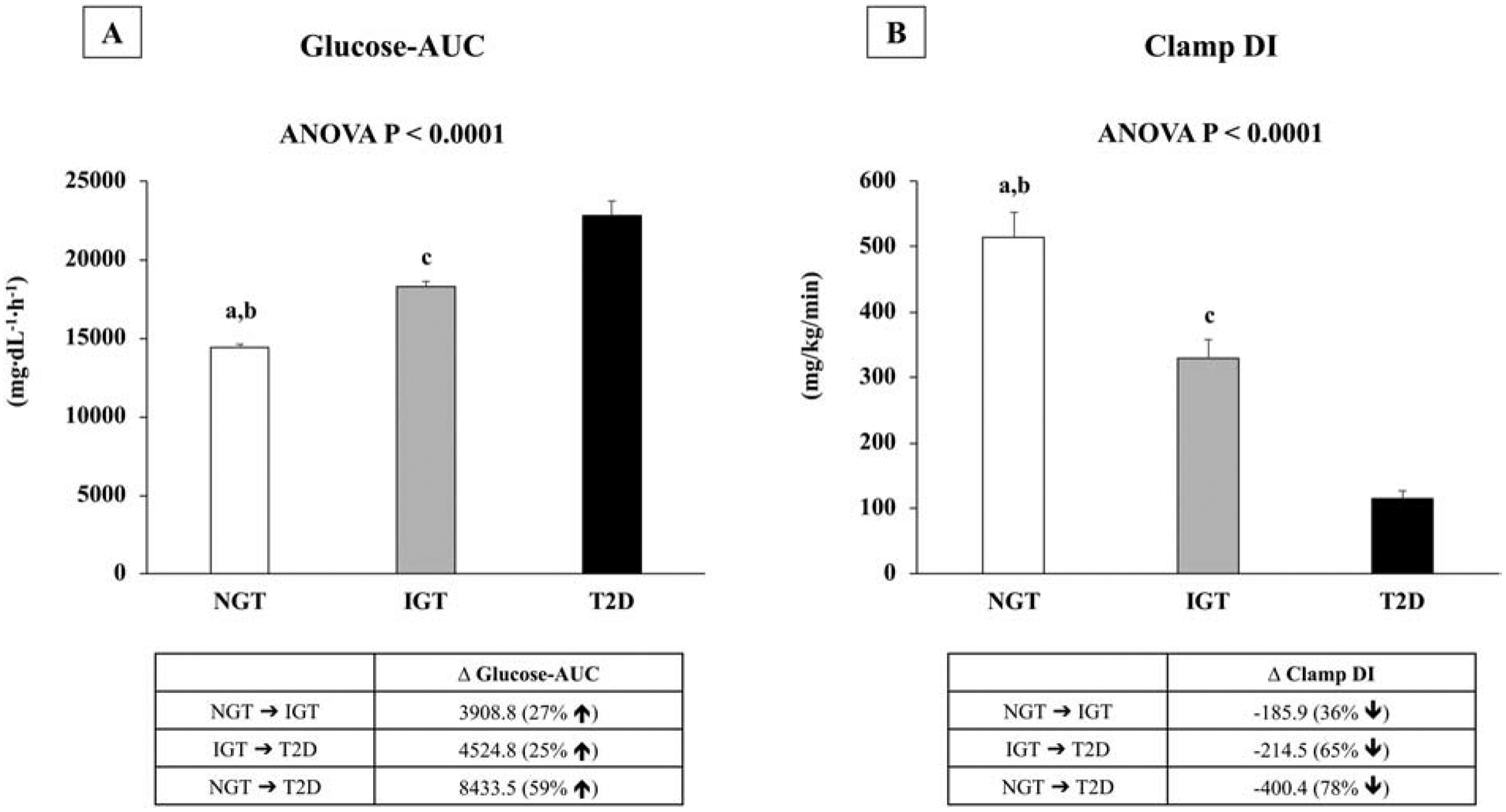
Glucose-AUC (A) and Clamp DI (B) in obese youth with NGT, IGT, and Type 2 diabetes. aP <0.001between NGT vs. IGT; bP <0.001 between NGT vs. Type 2 diabetes; cP <0.001 between IGT vs. Type 2 diabetes.
For the clamp-derived pathophysiological components, in vivo hepatic and peripheral insulin sensitivity were lower in obese youth with IGT and type 2 diabetes compared with NGT (Table 1). Obese youth with type 2 diabetes had the lowest 1st-phase insulin compared with the other two groups, with no difference between NGT and IGT (Table 1). However, DI or β-cell function relative to insulin sensitivity decreased progressively from NGT to IGT to type 2 diabetes (Figure 1B).
3.2. Quantitative relationship between clamp DI and glucose-AUC
Spearman’s correlation analysis showed an inverse relationship between clamp DI and glucose-AUC in the total cohort (r=−.693), and in each group of NGT (r=−.437), IGT (r=−.483), and type 2 diabetes (r=−.694, all P<0.0001). When the magnitude of differences in clamp DI was compared with the degree of alterations in glucose-AUC, a 36% lower DI corresponded to a 27% higher glucose-AUC in IGT vs. NGT. In type 2 diabetes vs. IGT, 65% lower DI related to 25% higher glucose-AUC, and in type 2 diabetes vs. NGT, 78% lower DI paralleled 59% higher glucose-AUC (Figure 1 A & B).
3.3. Sex- and race-specific relationship between clamp DI and glucose-AUC
Sex-specific analyses for clamp DI and glucose-AUC did not reveal any significant results; i.e., no interaction effect of sex and glycemic status on DI and glucose-AUC. However, race-specific analyses with two-way ANOVA (race × glycemic status), unraveled important observations as shown in Table 2 and Figure 2. Both clamp DI and glucose-AUC were significantly different by race and across glucose tolerance (Table 2; main effects, all p<0.01). Clamp DI was significantly higher in AA vs. AW youth with NGT and type 2 diabetes (Table 2). Glucose-AUC was significantly lower in AA vs. AW youth with NGT, with no race differences in IGT and type 2 diabetes (Table 2). There was a significant interaction effect of race × glycemic status on clamp DI (p=0.025), but no interaction effect on glucose-AUC (p=0.119). Figure 2 describes interaction effects by 3 categories, i.e., NGT to IGT (Figure 2: A & B); IGT to Type 2 diabetes (Figure 2: C & D); and NGT to Type 2 Diabetes (Figure 2: E & F). Despite significantly larger decrements in DI from NGT to IGT in AA vs. AW youth (Figure 2A; ΔDI = −296.2 ± 102.2 vs. −98.7 ± 36.6, respectively, p=0.04), the increment in glucose-AUC was similar between AA and AW youth (Figure 2B; ΔGlucose-AUC = 4311.8 ± 989.6 vs. 3557.5 ± 646.0, respectively, p=0.37). The findings were similar in NGT to type 2 diabetes; with AA youth having greater decrements in DI compared with AW youth (Figure 2E; ΔDI = −545.1 ± 99.9 vs. −311.7 ± 46.8, respectively, p=0.03) but comparable increases in glucose-AUC between AA and AW youth (Figure 2F; ΔGlucose-AUC = 7474.3 ± 967.1 vs. 9541.4 ± 826.0, respectively, p=0.131). There was no significant race interaction of clamp DI in youth with type 2 diabetes vs. IGT.
Table 2.
Race-specific metabolic characteristics of obese youth with NGT, IGT and Type 2 diabetes.
| NGT (29B/44W) | IGT (14B/34W) | T2D (15B/16W) | ANOVA P | ||
|---|---|---|---|---|---|
| Hepatic IS (mg/kg/min per μU/mL)−1 | |||||
| AA | 16.8 ± 1.8 | 10.9 ± 1.3 | 11.5 ± 3.1 | 0.012 | |
| AW | 18.3 ± 1.4 | 12.1 ± 1.2 | 13.5 ± 2.8 | 0.002 | |
| Peripheral IS (mg/kg/min per uU/mL) | |||||
| AA | 2.1 ± 0.2 | 1.3 ± 0.2* | 1.2 ± 0.2 | 0.001 | |
| AW | 2.3 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.3 | 0.024 | |
| 1st-phase insulin (uU/mL) | |||||
| AA | 379.9 ± 52* | 292.9 ± 36.7* | 187.6 ± 51.7* | 0.001 | |
| AW | 198.7 ± 18.4 | 204.3 ± 20.5 | 65.4 ± 9.6 | 0.0001 | |
| DI (mg/kg/min) | |||||
| AA | 683.9 ± 72.3* | 387.7 ± 78.2 | 138.8 ± 19.1* | 0.0001 | |
| AW | 403.7 ± 29.5 | 305.0 ± 24.1 | 92.0 ± 12.8 | 0.0001 | |
| Glucose-AUC (mg·dL−1·h−1) | |||||
| AA | 13618.2 ± 364.6* | 17930.1 ± 368.8 | 21092.5 ± 1337.8 | 0.0001 | |
| AW | 14860.8 ± 295.1 | 18418.3 ± 446.2 | 24402.2 ± 1190.8 | 0.0001 | |
AA: African American; AW: American White; IS: insulin sensitivity; DI: disposition index; AUC: area under the curve.
P<0.05 AA vs. AW in each glycemic group.
Figure 2:
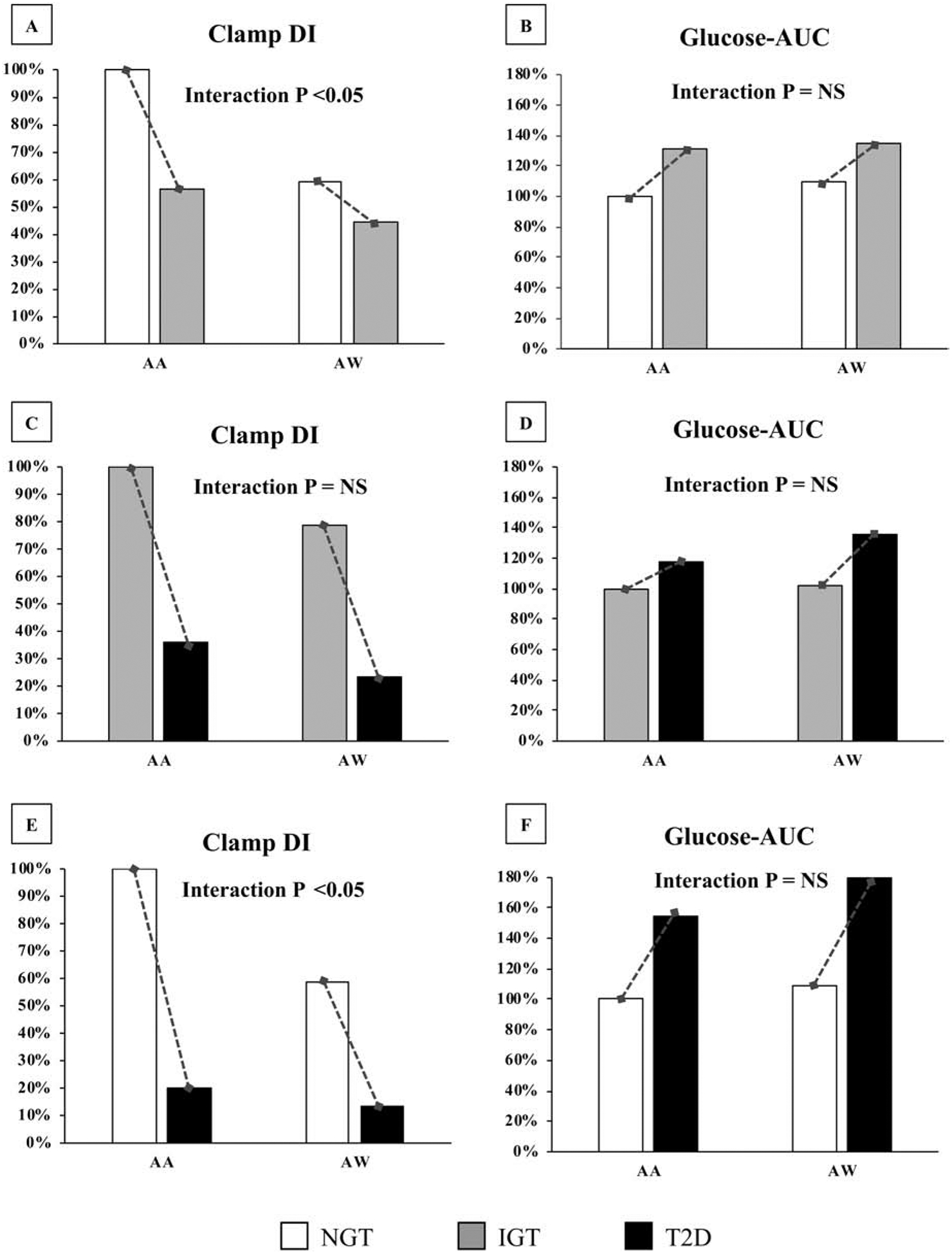
Interaction effects of race on Clamp DI and Glucose-AUC by 3 categories, i.e., NGT to IGT (A & B); IGT to Type 2 diabetes (C & D); and NGT to Type 2 Diabetes (E & F). Data are presented as percentages relative to the AA youth as reference group for both DI and AUC (ΔDI from NGT to IGT is −296.2 ± 102.2 in AA vs. −98.7 ± 36.6 in AW. ΔDI from IGT to Type 2 diabetes is −248.9 ± 116.8 in AA vs. −213.0 ± 36.6 in AW. ΔDI from NGT to Type 2 diabetes is −545.1 ± 99.9 in AA vs. −311.7 ± 46.8 in AW; ΔAUC from NGT to IGT is 4311.8 ± 989.6 in AA vs. 3557.5 ± 646.0 in AW. ΔAUC from IGT to Type 2 diabetes is 3162.4 ± 1130.0 in AA vs. 5983.8 ± 857.7 in AW. ΔAUC from NGT to Type 2 diabetes is 7474.3 ± 967.1 in AA vs. 9541.4 ± 826.0 in AW).
4. Discussion
The present study examined the relationship between β-cell function and OGTT glucose-AUC in obese youth along the spectrum of glycemia from NGT to IGT to type 2 diabetes. Based on our data, the degree of impairment in β-cell function was generally greater than the corresponding magnitude of deterioration in glucose-AUC. Such data may provide clinically useful information for intervention trials that aim to restore or improve β-cell function and reverse dysglycemia in IGT or type 2 diabetes. A 60% impairment in β-cell function in youth with type 2 diabetes compared with IGT reflects ~25% higher glucose-AUC. This implies that any intervention that aspires to revert type 2 diabetes to IGT in youth should expect at least a 60% improvement in DI.
In both adults and youth, it is well established that β-cell function relative to insulin sensitivity is a key pathophysiological component of type 2 diabetes [3–6]. This is the case in our present study exhibiting that clamp DI decreases progressively along the spectrum of glucose tolerance from NGT to IGT (36%), from IGT to type 2 diabetes (65%), and from NGT to type 2 diabetes (78%). Moreover, previous studies showed a significant inverse relationship of β-cell function, measured by the clamp experiment, with HbA1c [9], fasting glucose [10], and 2-hr glucose concentrations [5,8] in overweight/obese adolescents. Additionally, in the TODAY study of youth with established type 2 diabetes, initial β-cell reserve at randomization was an independent predictor of glycemic durability [14]. Irrespective of treatment, youth who failed to maintain glycemic control had significantly lower β-cell function at randomization compared with those who did not fail [14]. For these reasons, improvement in β-cell function is of utmost objective in any therapeutic intervention that aims to recover glucose tolerance [12–14]. However, glucose tolerance status is traditionally determined by a single cut-point of either the fasting or the 2-hr glucose concentration during the OGTT, which could limit proper interpretation of intervention effects along with β-cell improvement. Just two time points of the OGTT, a fasting and a 2-hr glucose concentration, may not harbor as much information as the AUC, which takes into account the additional time points for the glucose concentrations. We previously demonstrated that 1-hr glucose concentration exhibits an important relationship to β-cell function [24]. Obese youth, whether NGT or IGT, with 1-hr glucose ≥155 mg/dL had ~35–40% lower DI than those with 1-hr glucose <155 mg/dL [24]. Furthermore, β-cell function deteriorates as the fasting and the 2-hr glucose concentrations increase even in the range of what is considered to be normal [8,10]. Youth with a fasting plasma glucose ≥90-<100 mg/dL, had 25% lower β-cell function relative to insulin sensitivity compared with those with a fasting glucose ≤90 mg/dL, and youth with a fasting glucose of ≥100-<126 mg/dL, had ~ 55% lower DI than those with a fasting glucose of ≤90 mg/dL [10]. With respect to the 2-hr glucose concentration, the highest DI was in youth with a 2-hr glucose <120 mg/dL with a significant decline of ~40% in those with glucose concentrations between 120 and <140 mg/dL [8]. Taken collectively, the glucose-AUC provides an integrated view of the glucose concentrations at different time points irrespective of normal or abnormal cut offs. Thus, our findings add novel information to the existing sparse pediatric literature by demosntrating: 1) significant inverse relationship between clamp DI and glucose-AUC in the total cohort, and in each glucose tolerance group; and 2) by providing for the first time, a quantitative measure of the magnitude of impairment in DI that is coupled to glucose tolerance status, assessed by the OGTT glucose-AUC. Collectively, our data would suggest that around 30%, 60% and 75% improvement in β-cell function or DI is associated with restoration of glycemia from IGT to NGT, or from type 2 diabetes to IGT or from type 2 diabetes to NGT respectively in obese youth (Figure 1). While the RISE study aimed to restore insulin secretion in youth with prediabetes and early type 2 diabetes [12], the outcome of the intervention revealed that after 12 months of treatment neither metformin alone nor glargine followed by metformin, improved or maintained β-cell function [13]. Changes in OGTT glucose-AUC were not reported, but unpublished data suggest no changes (personal communications). However, no significant differences were observed between the two treatment groups at baseline, 12 and 15 months in HbA1c, fasting glucose and OGTT 2-hr glucose [13].
Another important information that emerges from the current analysis is the race divergence in the magnitude between the impairment in DI and the elevation in glucose-AUC. Our impetus to probe for race-related differences was the well-known higher rates of youth-onset type 2 diabetes in AA vs. AW [1,2,25]. In agreement with previous findings in pediatrics [26–29], obese AA youth exhibited hyperinsulinemia evidenced by higher 1st-phase insulin concentrations (measured by the hyperglycemic clamp) compared with their AW peers, and consequently higher DI in NGT and type 2 diabetes (Table 2). It is notable that hepatic insulin sensitivity was comparable between AA and AW youth across the spectrum of glucose tolerance while peripheral insulin sensitivity was significantly lower in AA vs. AW youth with IGT (Table 2). More importantly however, there was a race-contrast in the relationship between the magnitude of the decrement in β-cell function and the corresponding increment in glucose-AUC. As such, despite a greater reduction in DI in AA compared with AW youth with IGT vs. NGT (43% and 24% respectively) and type 2 diabetes vs. NGT (80% and 77% respectively), the increase in glucose-AUC was comparable between AA and AW youth (Figure 2). The present 2×2 ANOVA (race × glycemic status) confirmed that there are interaction effects of race on clamp DI from NGT to IGT and from NGT to type 2 diabetes, with no race interaction on glucose-AUC. Thus, our data suggest that loss of β-cell function might be less detrimental in AA compared with AW youth because ~3-fold greater impairment in clamp DI in AA youth corresponds to a similar increase in glucose-AUC to that of AW youth. Potential explanations for such observations would be: a) an adaptive mechanism of enhanced glucose-stimulated glucose uptake protective against dysglycemia in the face of larger declines in β-cell function in AA compared with AW youth, and/or b) the possibility of a threshold of β-cell function below which dysglycemia ensues. Since AA youth are hyperinsulinemic with increased DI compared with their AW peers, it might take a greater magnitude of decline in DI to reach this “presumed” threshold for dysglycemia. At the moment, the mechanism(s) behind why AA youth compared with their AW peers have similar degrees of deterioration in glucose-AUC despite greater declines in β-cell function are not known and need to be investigated.
The strengths of the present investigation include: 1) a first-time quantification of the relationship between clamp-measured DI and OGTT glucose-AUC in obese youth across the glucose tolerance spectrum from NGT to IGT to type 2 diabetes; and 2) the novel characterization of race-specific differences in the magnitude of impairment in clamp DI together with glucose-AUC. Such information is critical in designing intervention trials that aim to restore or improve β-cell function and insulin secretion in the hopes of improving and restoring glucose tolerance. Potential limitations would be the cross-sectional nature of our evaluation. A longitudinal study would have provided data in the same individuals as they transitioned from one glucose tolerance status to another commensurate with changes in their DI. However to date, intervention trials such as the RISE study in youth, which has comprehensive data on clamp-measured β-cell function, have not shown preservation of β-cell function [13]. Thus, considering the total lack of such data and the increased efforts for interventions to reverse and salvage β-cell function, we believe our cross-sectional data still provides much needed information and potential guidance with respect to power calculations in designing such trials. Another limitation is the inclusion of youth with type 2 diabetes who were on different treatment modalities (i.e., metformin, insulin, metformin plus insulin, or lifestyle modification). However, this diversity in therapy is inevitable as therapeutic approaches in the clinical setting are dictated by the severity of hyperglycemia [30]. Also, it would be unethical to hold back treatment in youth with type 2 diabetes for purposes of research when therapeutic options are available. Of note, there was no significant difference in the treatment modalities between AA and AW youth. Further, it is possible that despite negative GAD and IA2 autoantibodies, some may have been positive for zinc transporter 8 (ZnT8) antibody. However, the likelihood of this is quite low especially that in the TODAY study only 2 out of 687 youth were ZnT8 positive (0.29%) at screening with negative GAD and IA2 antibodies (personal communication). Another limitation of the study would be the relatively small sample size in the groups of IGT and type 2 diabetes. However, contrary to adults, IGT and type 2 diabetes remain relatively less in youth as was the case in RISE [31,32]. Lastly, the observed magnitude of β-cell dysfunction and the corresponding alterations in glucose metabolism may have intra- and inter-individual variations in repeated experiments. Undoubtedly, longitudinal studies in the same subjects will provide a better picture. However, until then, the present information might prove beneficial in designing large-scale interventional trials in youth with diverse ethnic backgrounds.
5. Conclusions
Our data suggest that large improvements in β-cell function might be needed to correct glucose regulation from dysglycemia to normoglycemia in obese youth. Furthermore, the quantitative relationship between β-cell impairment and glucose dysregulation varies by race in favor of AAs compared with AW youth. Intervention trials that aim to reverse or improve β-cell function in obese youth at high risk for type 2 diabetes, should not only focus on statistical improvement in β-cell function, rather on the degree of improvement needed to translate to clinically meaningful changes in glycemia reflective of recovery and conversion from type 2 diabetes to IGT or from IGT to NGT.
Highlights.
A significant inverse relationship between clamp DI and glucose-AUC across the spectrum of glucose tolerance
A clinically meaningful translation of glycemic variations imparted by changes in β-cell function from NGT to IGT, from IGT to type 2 diabetes, and from NGT to type 2 diabetes
Race divergence (African-American vs. American-White) in the magnitude between the impairment in clamp DI and the elevation in glucose-AUC
Acknowledgements
The authors thank the children who participated in this study and their parents; Nancy Guerra, C.R.N.P., for her assistance; Resa Stauffer for her laboratory expertise; and the nursing staff of the Pediatric Clinical and Translational Research Center for their outstanding care of the participants and meticulous attention to the research.
Funding
This study was supported by grants from the National Institute of Child Health and Human Development K24-HD01357 and R01-HD27503 to S.A., the American Diabetes Association (7-08-JF-27) and 1R21DK083654-01A1 to S.L., National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1TR000005, and National Center for Research Resources grant UL1RR024153 to the General Clinical Research Center.
Abbreviations:
- AA
African American
- AUC
area under the curve
- AW
American White
- BMI
body mass index
- DI
disposition index
- DEXA
dual energy X-ray absorptiometry
- Rd
glucose disposal rate
- GAD
glutamic acid decarboxylase
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- IA2
insulinoma associated protein-2
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- PCTRC
Pediatric Clinical and Translational Research Center
- RISE
Restoring Insulin Secretion
- TODAY
Treatment Options for Type 2 Diabetes in Adolescents and Youth
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Duality of interest
No potential conflicts of interest relevant to this article were reported.
References
- [1].Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;377:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. [DOI] [PubMed] [Google Scholar]
- [5].Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;1353:113–37. [DOI] [PubMed] [Google Scholar]
- [7].Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care. 2010;33:2225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining beta-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care. 2011;34:2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tfayli H, Lee S, Arslanian S. Declining beta-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care. 2010;33:2024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].RISE Consortium. Restoring Insulin Secretion (RISE): design of studies of beta-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care. 2014;37:780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Consortium RISE. Impact of insulin and metformin versus metformin alone on beta-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36:1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim JY, Michaliszyn SF, Nasr A, Lee S, Tfayli H, Hannon T, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care. 2016;39:1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim JY, Bacha F, Tfayli H, Michaliszyn SF, Yousuf S, Arslanian S. Adipose tissue insulin resistance in youth on the spectrum from normal weight to obese and from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes. Diabetes Care. 2019;42:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. The Journal of pediatrics. 2012;161:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sjaarda LA, Michaliszyn SF, Lee S, Tfayli H, Bacha F, Farchoukh L, et al. HbA(1c) diagnostic categories and beta-cell function relative to insulin sensitivity in overweight/obese adolescents. Diabetes Care. 2012;35:2559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13–S27. [DOI] [PubMed] [Google Scholar]
- [21].Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430–5. [DOI] [PubMed] [Google Scholar]
- [22].Tanner JM. Growth and maturation during adolescence. Nutr Rev 1981;39:43–55. [DOI] [PubMed] [Google Scholar]
- [23].Arslanian S, Kim JY, Nasr A, Bacha F, Tfayli H, Lee S, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: Who is worse off? Pediatr Diabetes. 2018;19:205–11. [DOI] [PubMed] [Google Scholar]
- [24].Tfayli H, Lee SJ, Bacha F, Arslanian S. One-hour plasma glucose concentration during the OGTT: what does it tell about beta-cell function relative to insulin sensitivity in overweight/obese children? Pediatr Diabetes. 2011;12:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, et al. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes. 2016;17:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bacha F, Gungor N, Lee S, Arslanian SA. Type 2 diabetes in youth: are there racial differences in beta-cell responsiveness relative to insulin sensitivity? Pediatr Diabetes. 2012;13:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31:1445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Uwaifo GI, Nguyen TT, Keil MF, Russell DL, Nicholson JC, Bonat SH, et al. Differences in insulin secretion and sensitivity of Caucasian and African American prepubertal children. The Journal of pediatrics. 2002;140:673–80. [DOI] [PubMed] [Google Scholar]
- [29].Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–9. [DOI] [PubMed] [Google Scholar]
- [30].Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2648–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Consortium RISE. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care. 2018;41:1696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Consortium RISE. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care. 2018;41:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


