Abstract
In plants, stomata control water loss and CO2 uptake. The aperture and density of stomatal pores, and hence the exchange of gases between the plant and the atmosphere, are controlled by internal factors such as the plant hormone abscisic acid (ABA) and external signals including light and CO2. In this study, we examine the importance of ABA catabolism in the stomatal responses to CO2 and light. By using the ABA 8′-hydroxylase-deficient Arabidopsis thaliana double mutant cyp707a1 cyp707a3, which is unable to break down and instead accumulates high levels of ABA, we reveal the importance of the control of ABA concentration in mediating stomatal responses to CO2 and light. Intriguingly, our experiments suggest that endogenously produced ABA is unable to close stomata in the absence of CO2. Furthermore, we show that when plants are grown in short day conditions ABA breakdown is required for the modulation of both elevated [CO2]-induced stomatal closure and elevated [CO2]-induced reductions in leaf stomatal density. ABA catabolism is also required for the stomatal density response to light intensity, and for the full range of light-induced stomatal opening, suggesting that ABA catabolism is critical for the integration of stomatal responses to a range of environmental stimuli.
Keywords: stomata, CO2, carbon dioxide, guard cells, abscisic acid, light
1. Introduction
Stomata are pores found on the aerial surfaces of land plants. Each stoma is bound by a pair of guard cells which, through changes in turgor, regulate the aperture of the central pore. Controlling stomatal apertures allows the plant to regulate water loss and the uptake of CO2 from the atmosphere [1,2]. Thus, stomata play important roles in optimizing plant growth, survival and reproductive capacity through regulating photosynthesis and water loss and influencing nutrient uptake and leaf cooling. The optimization of stomatal gas exchange relies on two important processes, one physiological and the other developmental. Firstly, changes in guard cell turgor bring about stomatal opening in the light when soil water is plentiful and closure in the dark or when soil water availability is limited [3,4]. Secondly, plants are able to adjust their stomatal density so that when they are growing under conditions where high levels of gas exchange could be advantageous (e.g. at high light levels or low [CO2]) they produce leaves with a high density of stomata, and when growing under conditions where lower levels of gas exchange could be advantageous (e.g. at low light levels or high [CO2]) they produce leaves with a lower density of stomata [5]. Light intensity and quality regulate stomatal density through the red/far-red-absorbing phytochromes [6] and the cryptochrome blue light photoreceptors [5,7,8]. How plants integrate the multitude of environmentally variable signals to optimally adjust their stomatal apertures, and also over a longer time frame their stomatal density, are current topics of research which have wide implications for responses to predicted anthropogenic changes in global [CO2] and water availability. Furthermore, such studies have the potential to identify useful targets for future crop breeding under climate change.
The plant hormone abscisic acid (ABA) is a major regulator of plant responses to environmental stresses, and has long been known to reduce stomatal apertures under drought conditions [8]. ABA-induced guard cell closure mechanisms and their cross-talk with CO2 signalling are becoming well characterized [1,9,10]. Arabidopsis thaliana (Arabidopsis) plants deficient in ABA biosynthesis or ABA perception and signalling can be compromised in their ability to shut their stomatal pores in response to elevated [CO2] [10,11]; although other studies, while supporting a role for ABA, suggest that there is not a role for ABA receptors and some ABA signalling proteins [12–15]. ABA has also emerged as an important regulator of stomatal development [1,13,16–18]. Growth of plants at elevated [CO2] or in the presence of exogenous ABA generally results in plants with lower stomatal densities [17,19–22]. Mutants deficient in ABA biosynthesis and signalling have high stomatal densities [13] and can fail to reduce stomatal densities in response to elevated [CO2] [10,23]. In Arabidopsis, [CO2] perception feeds into the core signalling pathway controlling stomatal development [17] via a pathway that remains to be fully characterized but involves the activity of putative β-keto acyl CoA synthase encoded by the HIC gene [21], the epidermal patterning factor 2 (EPF2) peptide, the CO2 response secreted protease (CRSP) and the carbonic anhydrases CA1 and CA2 [24,25]. ABA's role in the stomatal density response to [CO2] has been shown [10,17], but the mechanisms by which it acts are unknown.
Although ABA and ABA signalling are required for stomatal [CO2] signalling [10,11], the role of ABA catabolism in [CO2] perception and stomatal responses remains contentious [14,15,26]. Plants are known to tightly control the metabolism of ABA to modulate responses to changes in humidity and water availability [17], and ABA catabolism is important for reducing endogenous ABA concentrations upon the cessation of water deficit [27]. Under water deficit stress, ABA is synthesized in and imported to guard cells where it induces stomatal closure and inhibits re-opening [28]. This reduces transpirational water loss, but also restricts the uptake of CO2 through stomata, thus inhibiting photosynthesis and growth. Upon rehydration, the export of ABA, the breakdown of ABA in the guard cell by ABA 8′-hydroxylases, or the conjugation of ABA by ABA glucosyl transferase activity must occur to permit re-opening of stomata and increased take-up of CO2 [6,14]. ABA is catabolized to phaseic acid (PA) and dihydrophaseic acid (DPA) by the action of CYP707A family cytochrome P450 monooxygenases (P450s) [29,30]. PA and DPA accumulate under prolonged water deficit and may underpin the plant's resilience to longer-term drought stress by maintaining some of the biological activity of non-hydroxylated ABA [23,29,31].
Humidity has been shown to be a strong regulator of the ABA catabolic enzyme, ABA 8′-hydroxylase [27,32]. In Arabidopsis, two ABA 8′-hydroxylases, CYP707A1 and CYP707A3, play key roles in modulating ABA levels in stomata and vascular tissues, respectively, in response to water stress and humidity [32–34]. The double mutant cyp707a1 cyp707a3 (cyp707a1/a3) has approximately 30% [34] to 400% [27] higher levels of ABA, and a lower stomatal index and density in comparison to wild-type Arabidopsis [13,16]. Merilo et al. reported wild-type-like aperture responses to [CO2] in cyp707a1 and cyp707a3 single mutants [15], and in cyp707a1/a3 double mutants [13], despite the fact that they contain substantially higher levels of ABA. The authors argue that because they observed no differences in aperture widths between cyp707a1/a3 and wild-type, other traits beside aperture must be responsible for the reduced stomatal conductances they reported in these mutants [13]. Merilo et al. [14] also show that other mutants in ABA transport and recycling are not impaired in their stomatal responses to [CO2] or humidity, including ABA exporter mutants abcg22, abcg25, abcg27 and abcg40, ABA importer mutant ait, and ABA recycling mutants bg1 and bg2 [14].
Here, we use the double mutant cyp707a1/a3 to further investigate the role of ABA catabolism in stomatal responses to CO2 and light. We show that the capacity for ABA catabolism is required for elevated [CO2]-induced stomatal closure and elevated [CO2]-induced reductions in leaf stomatal density. We also report that ABA 8′-hydroxylases CYP707A1 and CYP707A are required for the stomatal density response to light intensity, suggesting that the level of ABA in vegetative tissues is critical for the integration of several environmental responses.
2. Results and discussion
2.1. Stomatal aperture response to [CO2]
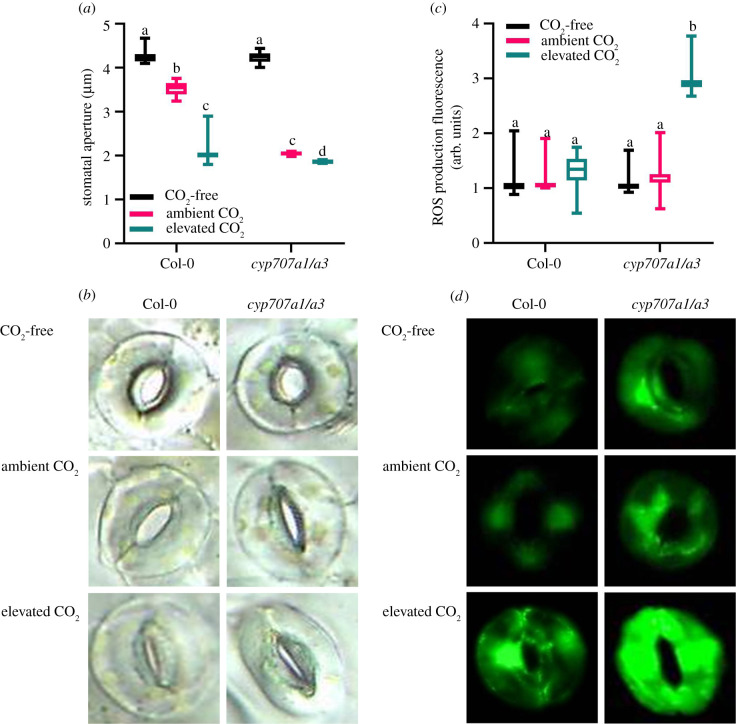
We measured the apertures of stomatal pores following exposure of isolated epidermal strips to differing levels of [CO2] using a previously described bioassay [35]. The results in figure 1a,b show that stomata of the cyp707a1/a3 mutant, which has impaired ability to break down ABA, were significantly more closed at both ambient and elevated levels of CO2 than wild-type (ANOVA, p = 0.0211, p = 0.0240, respectively). As the stomatal apertures of both wild-type and cyp707a1/a3 mutant were open to the same extent under low CO2, these data suggest that the cyp707a1/a3 mutant exhibits a hypersensitive response to [CO2] because of higher endogenous levels of ABA [27,32]. This stomatal hypersensitivity appears to be in contrast to previous reports of relatively normal [CO2]-mediated stomatal responses in this and other ABA catabolism mutants. The discrepancies between our results and the investigations of Merilo et al. [15] may arise from differences between whole rosette gas exchange measurements and epidermal peel measurements of apertures (although both labs used peels), but it more probably arises from the different plant growth conditions. In the single mutants, wild-type stomatal aperture responses to CO2 could be attributed to their ability to break down ABA within guard cells in the case of cyp707a3 and within the vasculature in the case of cyp707a1 [27], this spatially distinct activity potentially masking the effect of high ABA concentrations on guard cell [CO2] responses. However, this does not account for the cyp707a1/a3 aperture differences. Given the guard cell [CO2] hypersensitivity observed in our experiments at ambient and elevated [CO2], it is surprising that cyp707a1/a3 apertures were indistinguishable from wild-type in [CO2]-free air conditions (figure 1). This observation is intriguing as exogenous ABA has previously been shown to close stomata in many similar experiments using epidermal strips provided with CO2-free air [36]. This further suggests that the precise location of the ABA signal is important for the guard cell [CO2] response, and that under CO2-limiting conditions exogenous application of ABA may be more potent in triggering stomatal closure than endogenous ABA accumulated within guard cells. These results may reflect the relative contributions of mesophyll versus guard cell ABA production and breakdown [37] and suggest that the location of ABA biosynthesis is likely to be as important as the location of degradation, for aperture control. This is supported not only by vascular localized NCED3 for ABA production, but also by the relative contributions of vascular localized CYP707A3 versus guard cell localized CYP707A1 to whole leaf ABA breakdown [27].
Figure 1.
Impaired ABA catabolism sensitizes cyp707a1 cyp707a3 stomatal closure in response to [CO2], but does not induce aperture closure in the absence of CO2. (a) [CO2] induction of stomatal closure is hypersensitive in the cyp707a1 cyp707a3 mutant (labelled as cyp707a1/a3; ANOVA, p = 0.0211), but there is no significant (ANOVA, p = 0.1001) difference in stomatal apertures in the absence of CO2. (b) Representative bright-field images used to determine stomatal apertures from (a), showing differences between cyp707a1 cyp707a3 and wild-type guard cells under zero, ambient and elevated [CO2]. (c) [CO2] induction of reactive oxygen species (ROS) is hypersensitive in the cyp707a1 cyp707a3 mutant (ANOVA, p = 0.0240), but there is no significant (ANOVA, p = 0.9990, p = 0.9978) difference in ROS production in the absence of CO2 or in ambient [CO2]. (d) Representative images showing fluorescence of cyp707a1 cyp707a3 and wild-type guard cells under zero, ambient and elevated [CO2] as in (c).
The importance of ABA levels in the modulation of stomatal [CO2] responses was first demonstrated almost 40 years ago in a series of experiments performed by Raschke, Farquhar, Dubbe and others. Raschke observed that open stomata of Xanthium strumarium are insensitive to [CO2] but become [CO2] responsive when either supplied with ABA or subjected to mild water stress [38]. The work of Raschke and co-workers revealed that, under some conditions, stomatal [CO2] responsiveness is conditional on the presence, or level of, ABA [38–42]. Our results not only support the role of ABA levels in modulating stomatal aperture response to CO2 but also show that the inability to reduce ABA levels interferes with these responses. We suggest that minor excursions of vapour pressure deficit or other environmental conditions could result in semi-irreversible increases in ABA in the cyp707a1/a3. This dependence on ABA levels is the most likely reason for the differences observed between this work and the results of Merilo et al.
An increase in guard cell reactive oxygen species (ROS) has been shown to be required in both ABA- and [CO2]-induced stomatal closure [10,43–46]. To determine the role of ROS in the cyp707a1/a3 mutant's response to elevated [CO2], we used the fluorescent ROS indicator H2DCF-DA (see §4.3) [43]. Challenge of guard cells with elevated [CO2] resulted in a significant (ANOVA, p = 0.0093) increase in H2DCF-DA fluorescence, consistent with a significantly greater ABA- and [CO2]-induced increase in guard cell ROS production in the double mutant (figure 1c,d). Thus, the inability to catabolize ABA causes both an increased production of ROS at elevated [CO2] and an enhanced stomatal closure response to [CO2]. Although, in the experiment reported here, the increase in ROS was not significant in the Co-0 controls, these data support previous work suggesting that guard cell sensitivity to [CO2] is associated with an ABA-induced signalling pathway involving ROS [47,48].
2.2. Stomatal density response to [CO2]
Stomatal densities of mature leaves are influenced by both ABA and [CO2]. To determine the extent to which the ABA 8′-hydroxylase mutations affect stomatal density, we grew the single cyp707a1 and cyp707a3 mutants and the double cyp707a1/a3 mutant and counted the number of stomata that developed on the abaxial surface of mature leaves grown under ambient CO2 (figure 2a). Interestingly, while neither the cyp707a1 nor the cyp707a3 single mutants showed significantly (ANOVA, p = 0.2258 and p = 0.4763, respectively) altered stomatal density in comparison to wild-type, the cyp707a3 single mutant had significantly lower stomatal density than the cyp707a1 mutant (ANOVA, p = 0.0163). The cyp707a1/a3 double mutant, however, displayed a more significant (ANOVA, p = 0.0001) reduction in stomatal density compared to the single mutants and the wild-type, presumably because of its greatly elevated endogenous ABA levels [27].
Figure 2.
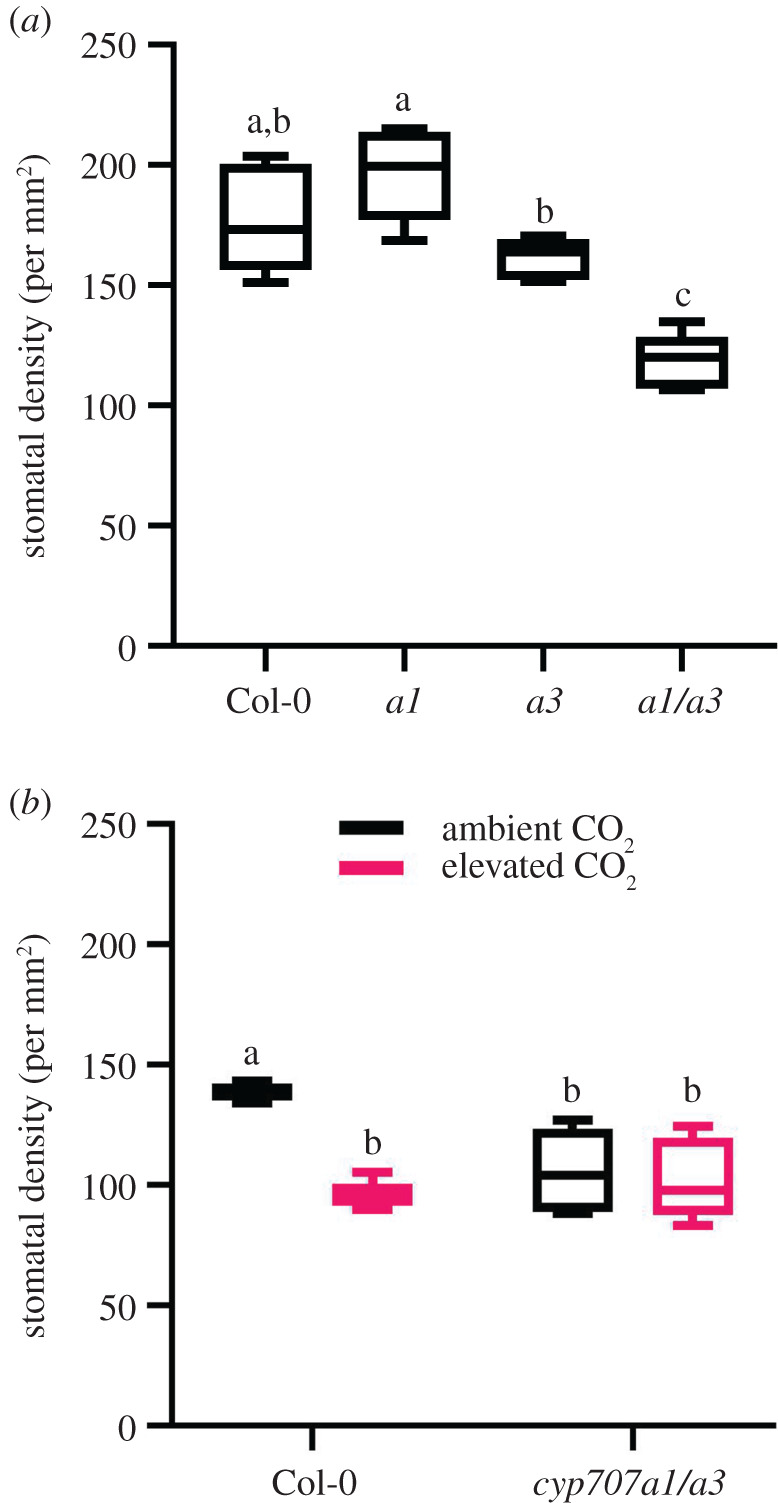
The stomatal density response to [CO2] requires ABA catabolism. (a) Mean stomatal density was significantly (ANOVA, p = 0.0001) lower in the double mutant cyp707a1 cyp707a3 leaves compared to wild-type grown under ambient conditions. Mean stomatal density was significantly (ANOVA, p = 0.0163) lower in the single cyp707a3 compared to the single cyp707a1 mutant. (b) Mean stomatal density was significantly (ANOVA, p = 0.0010) lower in wild-type leaves grown under 1000 ppm [CO2] compared to 400 ppm, but the stomatal density of cyp707a1 cyp707a3 mutant was unaffected by [CO2]. Stomatal densities of the cyp707a1 cyp707a3 mutant were significantly (ANOVA, p = 0.0094) lower than wild-type when grown under ambient [CO2] and did not reduce further when grown at elevated [CO2].
We then grew the double mutant and wild-type at ambient and elevated [CO2] to look at any possible interaction between endogenous ABA and [CO2]. We found that, unlike the wild-type plants, the cyp707a1/a3 plants that were deficient in ABA breakdown were unable to further reduce their leaf stomatal density in response to elevated [CO2] (figure 2b). Furthermore, we found that the cyp707a1/a3 plants had 24% lower stomatal density than wild-type when grown at ambient [CO2]. Presumably, this reflects higher levels of ABA in the double mutant. Thus, we conclude that the ability to break down ABA is essential for the maintenance of wild-type density responses to [CO2], and that ABA levels regulate leaf stomatal density.
Comparison of the density responses of the single mutants suggests that vascular-specific CYP707A3 expression contributes more strongly to wild-type modulation of stomatal density than the guard cell associated expression of CYP707A1. As vascular CYP707A3 expression contributes more greatly to ABA turnover than guard cell CYP707A1 expression [27] the single mutant phenotypes could be attributable to the respective plants' total ABA pool. Together, these findings strongly support and add to previous reports which suggest a role for ABA both in stomatal density [13,16,18,49] and in the stomatal density response to [CO2] [10,22].
2.3. Stomatal density response to light intensity
ABA turnover is also implicated in the interaction of stomatal CO2 responses and light signalling [18]. Having shown that the stomatal density response of mutants with defective ABA catabolism is unresponsive to [CO2] we carried out an experiment to test whether the inability to synthesize or break down ABA also impaired stomatal density responses to different light intensities (figure 3). Growth of plants under reduced light intensities (photosynthetic photon flux density, PPFD of 100 µmol m−2 s−1 versus 200 µmol m−2 s−1) resulted in significantly (ANOVA, p = 0.0356) lower stomatal densities in wild-type. However, cyp707a1/a3 plants maintained a consistently low stomatal density and were not able to significantly (ANOVA, p = 0.7162) reduce their stomatal density under lower irradiances. This result contrasts with the density response to low light in mutants that produce reduced ABA; the nced3 nced5 double mutant, which produces very low levels of ABA, displayed high stomatal densities and maintained a strong density response to light intensity (electronic supplementary material, figure S1). We also observed an interaction between elevated ABA levels and the stomatal aperture response to light; although cyp707a1/a3 was not significantly affected in the extent of light-induced stomatal opening (figure 3a) or ROS production at any individual time point compared with wild-type (electronic supplementary material, figure S2), the mutant's dynamic range of stomatal movement (or speed of stomatal movement) was significantly reduced in response to light across the experimental time course (t-test, T0 versus T135, p = 0.0001). For example, after 90 min in the light, wild-type stomatal apertures had increased by an average of 3.1 µm while cyp707a1/a3 apertures had only increased by 1.3 µm. Thus, ABA catabolism, but not an attenuation of ABA-induced ROS production, appears to be required for maximal light-induced stomatal opening.
Figure 3.
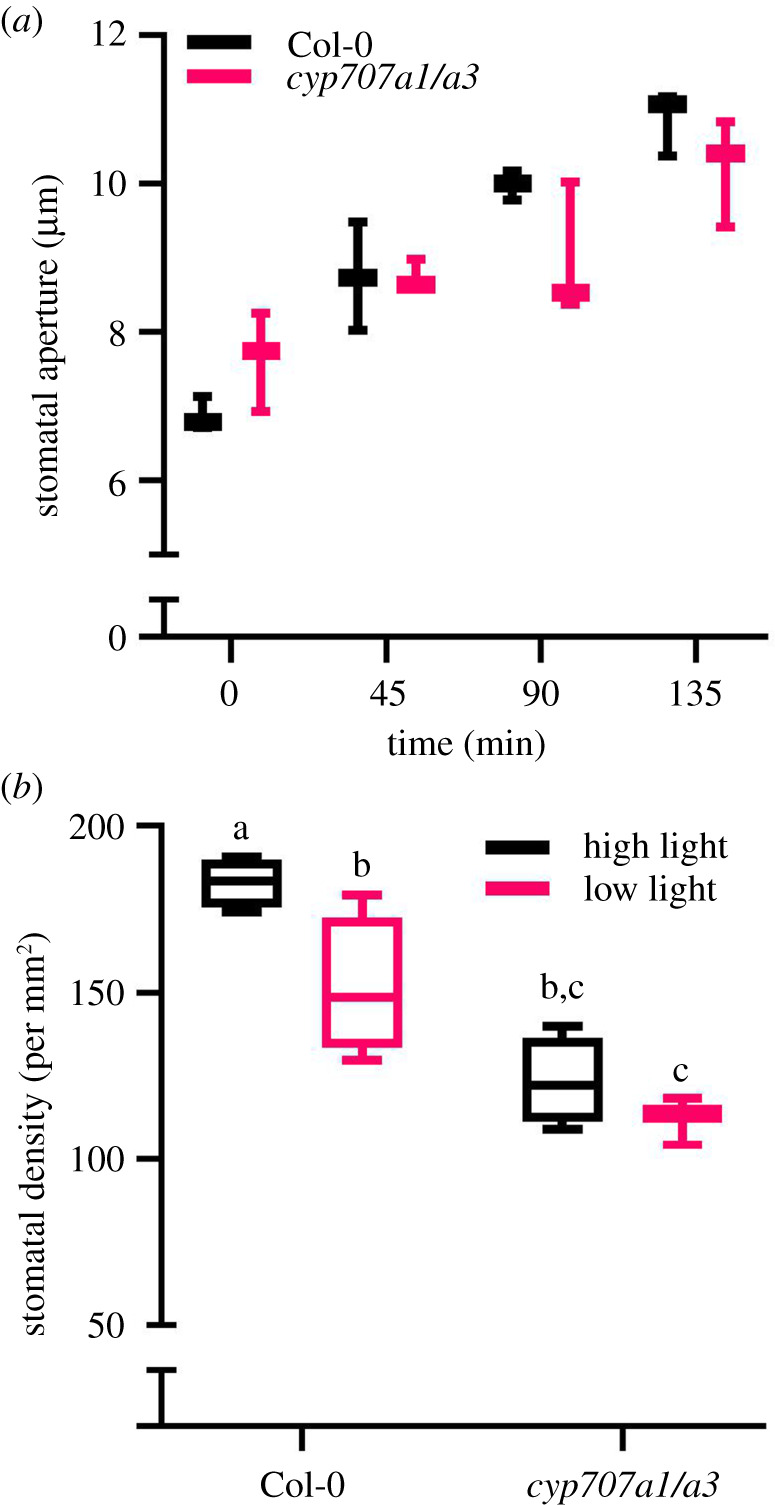
The dynamic range of stomatal aperture response to light is reduced, and the stomatal density response to light intensity is inhibited, when ABA catabolism is impaired. (a) The range of light-induced stomatal opening is reduced in the cyp707a1/a3 mutant. (b) Mean stomatal density was significantly (ANOVA, p = 0.0356) higher in wild-type leaves grown under 200 µmol m−2 s−1 PPFD than under 100 µmol m−2 s−1, but did not increase significantly (ANOVA, p = 0.7162) in the cyp707a1 cyp707a3 mutant at the higher light intensity.
Next, we investigated the differential expression of CYP707A transcripts in response to light intensity by quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. Interestingly, CYP707A1 as well as CYP707A2 are upregulated in wild-type Col-0 in response to transfer from growth at low light (50 µmol m−2 s−1) to high light (250 µmol m−2 s−1) conditions, as shown by qRT-PCR (electronic supplementary material, figure S3). This suggests that guard cell, and whole plant, ABA catabolism is involved in acclimation to high light levels, and as shown in figure 3a is required for appropriate light-induced stomatal opening dynamics. This is in broad agreement with the result of Huang et al. [50] who found that all four CYP707s and nine ABA biosynthesis genes including three NCEDs are upregulated under high light stress (1200 mmol m−2 s−1) [50]. Our results indicate that ABA biosynthesis is not required to reduce stomatal density at low light intensity, but that active ABA catabolism is required to increase stomatal density at high light. This impaired density response also suggests that the effect of ABA on stomatal density is stronger than the effect of light intensity, with ABA signals overriding light signals. In this way, water limitation could be a stronger driver of the stomatal density response than light. Although this is evidence for the primacy of water conservation (in contrast to carbon acquisition) in leaf development, there remains the possibility that endogenous ABA levels in the mutant could interfere with the integration of light and ABA signalling pathways. In addition, the restricted dynamic range of light-mediated stomatal opening (figure 3a), and the possible influence of day length, underlines the complexity of stomatal controls and the need for further deconstruction of closely linked pathways.
3. Conclusion and future directions
In summary, our results suggest that plants exhibiting a lesion in the ability to break down ABA exhibit hypersensitivity to CO2 in their stomatal closure most likely because of high endogenous levels of ABA. We also show that ABA catabolism is required for optimal stomatal opening in the absence of CO2. Similarly, these plants with impaired ABA catabolism exhibit markedly lower stomatal densities than normal (a result consistent with high endogenous levels of ABA) and fail to exhibit elevated CO2-induced reductions in stomatal density. Furthermore, we demonstrate that these plants also fail to increase stomatal density when grown under high irradiance. This suggests that ABA signals and water limitation override other environmental signals in leaf development, or because of a requirement for ABA in plant adaptation to high light (figure 4). The disruption of ABA catabolism therefore prevents modulation, and integration, of various environmental signals and thereby partially uncouples growth and abiotic stress response (figure 4). It remains to be seen whether ABA levels themselves or the failure to produce PA and DPA is responsible for some of these CO2 and light phenotypes and/or whether their absence further disrupts responses to prolonged abiotic signals. In conclusion, by using mutants impaired in ABA breakdown and synthesis we show that elevated [CO2]-mediated control of stomatal aperture and density and the control of stomatal density by light require precise control of both the level and cellular location of ABA. Furthermore, the reduced stomatal apertures and densities observed in this study suggest that ABA turnover provides a potential target for improving water use efficiency in crops under future predicted CO2 environments.
Figure 4.
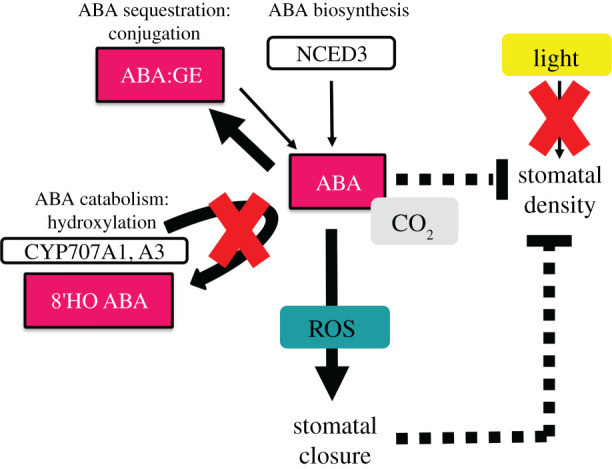
Disruption of abscisic acid (ABA) catabolism and feedback on stomatal phenotypes. The disruption of the cytochrome P450 CYP707A1 and CYP707A3 prevents the vascular and guard cell hydroxylation of ABA, respectively. ABA biosynthesis, regulated principally by the activity of the 9-cis-epoxycarotenoid dioxygenase (NCED), remains intact. ABA concentrations are therefore maintained at a higher level and the balance of the free ABA pool can be altered by reversible production of the less physiologically active ABA-glucose ester (ABA-GE). The resulting imbalance of ABA metabolism results in defective guard cell physiology and stomatal density responses to [CO2] and light intensity. The effect of elevated ABA appears to cancel out the effect of high light on stomatal density. Thick solid lines show upregulation of specific pathways and thin solid lines show downregulation, whereas dashed lines show inhibition of processes.
4. Material and methods
4.1. Plant material and growth conditions
Arabidopsis thaliana ecotype Col-0 was used in this investigation. Seeds of cyp707a1 cyp707a3 in the Col-0 background were obtained from Dr Eiji Nambara (University of Toronto), and have been previously described [27,34]. Seeds for experiments shown in figures 1–3a and S2 were sown onto M3 and perlite in a 4 : 1 ratio in plug trays and transferred into a growth cabinet (Conviron) with growth conditions: 22°C (day)/16°C (night) temperature cycle; 9/15 h light/dark cycle; 70% relative humidity; PPFD 150 µmol m−2 s−1; at ambient [CO2] (approx. 400 ppm) or elevated [CO2] (approx. 1000 ppm). Seeds for experiments shown in figure 3b and electronic supplementary material, figure S1 were sown and grown under either PPFD 100 or 200 µmol m−2 s−1 at ambient [CO2]. Plants used in the qRT-PCR light transfer experiment shown in electronic supplementary material, figure S3 were grown in a 12 h photoperiod at 22°C, 60% relative humidity, with 50 µmol m−2 s−1 light at ambient [CO2].
4.2. Measurement of stomatal aperture responses
Abaxial epidermis was removed from the youngest, fully expanded leaves of 5 to 6 week old plants and floated, cuticle-side up, on CO2-free 10 mM MES/KOH (pH 6.15) in 5 cm Petri dishes (Sterilin, UK) at 22°C. For [CO2]-concentration sensitivity experiments (figure 1) epidermal peels were transferred to fresh Petri dishes and incubated for 2 h in the light under a PPFD of 150 µmol m−2 s−1 in 50 mM KCl, 10 mM MES/KOH (pH 6.15) at 22°C while being aerated with CO2-free air by bubbling through the buffer solution. This treatment brought about stomatal opening. The use of CO2-free air treatment is a long-standing and standard technique in stomatal bioassays to induce maximal stomatal opening [51,52]. Peels were then either aerated with lab air (ambient, approx. 400 ppm CO2 as measured by IRGA), CO2 scrubbed air (0 ppm), or CO2 from a pressurized cylinder containing 1000 ppm CO2 in air (BOC, Special Gases, UK) by bubbling directly into the buffer. To determine the effect of calcium ion supplementation on [CO2]-concentration sensitivity, experiments were performed as above with and without the addition of 10 or 50 µM CaCl2 in the buffer solutions; no effect of CaCl2 was found at these concentrations, suggesting that endogenous guard cell calcium levels or residual calcium in buffer solutions are sufficient to ensure stomatal closure (electronic supplementary material, figure S4). For the light-induced opening assay plants were kept in the dark overnight and in the morning before the lights came on epidermal strips were prepared in dimlight and stored in 10 mM MES/KOH (pH 6.15) for one hour in the dark. Strips were then transferred to 50 mM KCl, 10 mM MES/KOH (pH 6.15) supplied with CO2-free air in the light. Peels were removed at 0, 45, 90 and 135 min time points, mounted on slides and measurements of stomatal aperture recorded using an inverted microscope (Leica DM-IRB, Leica, UK) or a microscope (Olympus BX51), fitted camera (Olympus DP70) and ImageJ software v. 1.43u. The maximal widths of 40 stomatal pores were measured per treatment in three separate replicated experiments (total stomatal number = 120; n = 3). To avoid experimenter bias, measurements were performed without the researcher being aware of the sample identity. Data were tested for normality and analysed using one-way or two-way ANOVA (MINITAB and Sigmaplot 12).
4.3. Measurement of ROS
To estimate ROS generation abaxial epidermal peels were prepared as described above and incubated in 50 mM KCl, 10 mM MES/KOH (pH 6.15) at 22°C, PPFD of 100 µmol m−2 s−1 while being aerated with CO2-free air for 2 h. For the experiments shown in figure 1, they were then transferred to 50 mM KCl, 10 mM MES/KOH (pH 6.15) at 22°C, PPFD of 100 µmol m−2s−1 and aerated with either ambient [CO2] (400 ppm) or 1000 ppm CO2 for 2 h. For the experiment shown in figure 3a and electronic supplementary material, figure S2, the peels were removed at 0, 45 and 90 min time points. For both experiments, the epidermal peels were then loaded (by pipetting) with 25 µM (final concentration) H2DCF-DA (2′,7'-dichlorodihydrofluorescein diacetate) (Invitrogen, UK) from a 25 mM stock in DMSO for 10 min in the dark. They were then washed in 50 mM KCl, 10 mM MES/KOH (pH 6.15) at 22°C for 10 min in the light (PPFD 150 µmol m−2 s−1) to remove excess H2DCF-DA and the fluorescence intensity was measured using an Olympus BX51 fluorescence microscope and ImageJ as described in [53]. For ROS analysis, pixel intensities of 40 stomatal areas (fluorescence zone of two guard cells) relative to their background intensities (four equivalent sized areas surrounding each stoma) were measured per treatment in three separate replicated experiments (total stomatal number = 120; n = 3). Fluorescence intensities were normalized to those of controls. Data were tested for normality and analysed using two-way ANOVA (MINITAB and Sigmaplot 12).
4.4. Stomatal density measurements
Dental impression medium (Coltene Whaledent, Switzerland) was applied to the abaxial surfaces of fully expanded leaves and nail varnish peels were taken from set impression medium. Cell counts were taken from four fields of view from the widest area of four leaves each from five plants of each genotype from both growth conditions (400 and 1000 ppm CO2 for experiments shown in figure 2 conditions, and PPFD of 100 or 200 µmol m−2 s−1 for experiments shown in figure 3b and electronic supplementary material, figure S1). Data were tested for normality and analysed using two-way ANOVA (MINITAB and Sigmaplot 12).
4.5. Quantitative RT-PCR analyses
For qRT-PCR analysis, RNA was extracted from plants grown for 12 days post-germination in 50 µmol m−2 s−1 light before being transferred to 250 µmol m−2 s−1 light. Tissue was collected at 3 and 6 h after transfer and RNA extracted using the Quick-RNA™ MiniPrep (Zymo Research) plant RNA extraction kit with on-column DNase treatment according to the manufacturer's instructions. RNA was reverse transcribed with High Capacity Reverse Transcriptase (Applied Biosystems). Transcript abundance of target genes was assayed using SYBR Green/JumpStart Taq Ready Mix qPCR Master Mix (Sigma Aldrich) on a Biorad CFX qPCR machine. The ACT2 and UBC21 genes were used as controls, as transcript levels remained constant under all treatments and relative expression levels were calculated using the ΔΔCt method. All primers used are in table 1. Expression was calculated relative to that of Col-0 seedlings grown at 50 µmol m−2 s−1 light. Three biological repeats and three technical repeats were performed for each sample and used to calculate s.e.m. values. Reaction conditions were (1 × 95°C–10 min; 40 × 95°C–15 s/57°C–20 s/72°C–30 s).
Table 1.
Details of primer sequences.
| target | forward primer sequence (5′-3′) | reverse primer sequence (5′-3′) |
|---|---|---|
| ACT2 | TCAGATGCCCAGAAGTCTTGTTCC | CCGTACAGATCCTTCCTGATA |
| UBC21 | GAATGCTTGGAGTCCTGCTTG | CTCAGGATGAGCCATCAATGC |
| HT1 kinase | GGGCTAAGCTTGAACAACAGT | GCGAGTAAGGCTCTTTCTTG |
| CYP707A1 | CCATCAAGATTCGAGGTGGCT | GGACACGAGTGGGTTCCATT |
| CYP707A2 | GCTCTCAGACCAACCGTCTC | CGAGGGTGTTGATGGACTTT |
| CYP707A3 | CAATTCTTGGGATGGAACTCAA | GTCTTTGCCGAGTATTGAGATT |
| CYP707A4 | CTATCCATGCGTGATGTTGG | GTGCAGAGGGTCCTATCAGC |
Supplementary Material
Supplementary Material
Acknowledgements
The authors gratefully thank Dr Eiji Nambara (University of Toronto) for the gift of the ABA catabolism mutants and Dr Annie Marion-Poll (INRA) for the gift of the ABA biosynthesis mutants.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
M.M. and C.C.C.C. contributed equally to this work by designing and performing experiments and analysing data. N.Z. and S.A.C. performed reciprocal light and qRT-PCR experiments. P.S. and Y.-K.L. performed CaCl2 bioassays. M.M., C.C.C.C., J.E.G., N.Z., S.A.C. and A.M.H. wrote the manuscript and interpreted data. C.C.C.C., J.E.G. and A.M.H. conceived the project. All authors reviewed the manuscript.
Competing interests
The authors declare no competing financial interests.
Funding
A.M.H. and J.E.G. acknowledge the Biotechnology and Biological Sciences Research Council (BBSRC) and the Leverhulme Trust for supporting the work described in this paper. C.C.C.C. would like to acknowledge funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 700867.
References
- 1.Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordström M, Azoulay-Shemer T, Rappel W-J, Iba K, Schroeder JI. 2016. CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci. 21, 16–30. ( 10.1016/j.tplants.2015.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. ( 10.1038/nature01843) [DOI] [PubMed] [Google Scholar]
- 3.Kim T-H, Böhmer M, Hu H, Nishimura N, Schroeder JI. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61, 561–591. ( 10.1146/annurev-arplant-042809-112226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawson T, Matthews J. 2020. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 71, 273–302. ( 10.1146/annurev-arplant-050718-100251) [DOI] [PubMed] [Google Scholar]
- 5.Casson SA, Gray JE. 2008. Influence of environmental factors on stomatal development. New Phytol. 178, 9–23. ( 10.1111/j.1469-8137.2007.02351.x) [DOI] [PubMed] [Google Scholar]
- 6.Casson SA, Hetherington AM. 2014. Phytochrome B is required for light-mediated systemic control of stomatal development. Curr. Biol. 24, 1216–1221. ( 10.1016/j.cub.2014.03.074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casson SA, Hetherington AM. 2010. Environmental regulation of stomatal development. Curr. Opin Plant Biol. 13, 90–95. ( 10.1016/j.pbi.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 8.Kang C-Y, Lian H-L, Wang F-F, Huang J-R, Yang H-Q. 2009. Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 21, 2624–2641. ( 10.1105/tpc.109.069765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI. 2015. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin Plant Biol. 28, 154–162. ( 10.1016/j.pbi.2015.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chater C, et al. 2015. Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr. Biol. 25, 2709–2716. ( 10.1016/j.cub.2015.09.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittrich M, et al. 2019. The role of Arabidopsis ABA receptors from the PYR/PYL/RCAR family in stomatal acclimation and closure signal integration. Nat. Plants 5, 1002–1011. ( 10.1038/s41477-019-0490-0) [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Takahashi Y, Hsu P-K, Kollist H, Merilo E, Krysan PJ, Schroeder JI. 2020. FRET kinase sensor development reveals SnRK2/OST1 activation by ABA but not by MeJA and high CO2 during stomatal closure. eLife 9, e56351 ( 10.7554/eLife.56351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalakas P, Merilo E, Kollist H, Brosché M. 2018. ABA-mediated regulation of stomatal density is OST1-independent. Plant Direct 2, e00082 ( 10.1002/pld3.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merilo E, Jalakas P, Kollist H, Brosché M. 2015. The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Molec. Plant 8, 657–659. ( 10.1016/j.molp.2015.01.014) [DOI] [PubMed] [Google Scholar]
- 15.Merilo E, et al. 2013. PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 62, 1652–1668. ( 10.1104/pp.113.220608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka Y, Nose T, Jikumaru Y, Kamiya Y. 2013. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 74, 448–457. ( 10.1111/tpj.12136) [DOI] [PubMed] [Google Scholar]
- 17.Chater CCC, Oliver J, Casson S, Gray JE. 2014. Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. New Phytol. 202, 376–391. ( 10.1111/nph.12713) [DOI] [PubMed] [Google Scholar]
- 18.Allen J, Guo K, Zhang D, Ince M, Jammes F. 2019. ABA-glucose ester hydrolyzing enzyme ATBG1 and PHYB antagonistically regulate stomatal development. PLoS ONE 14, e0218605 ( 10.1371/journal.pone.0218605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodward FI 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327, 617–618. ( 10.1038/327617a0) [DOI] [Google Scholar]
- 20.Woodward FI, Kelly CK. 1995. The influence of CO2 concentration on stomatal density. New Phytol. 131, 311–327. ( 10.1111/j.1469-8137.1995.tb03067.x) [DOI] [Google Scholar]
- 21.Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM. 2000. The HIC signalling pathway links CO2 perception to stomatal development. Nature 408, 713–716. ( 10.1038/35047071) [DOI] [PubMed] [Google Scholar]
- 22.Lake JA, Woodward FI. 2008. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytol. 179, 397–404. ( 10.1111/j.1469-8137.2008.02485.x) [DOI] [PubMed] [Google Scholar]
- 23.Sharkey TD, Raschke K. 1980. Effects of phaseic acid and dihydrophaseic acid on stomata and the photosynthetic apparatus. Plant Physiol. 65, 291–297. ( 10.1104/pp.65.2.291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engineer CB, Ghassemian M, Anderson JC, Peck SC, Hu H, Schroeder JI. 2014. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 513, 246–250. ( 10.1038/nature13452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matrosova A, Bogireddi H, Mateo-Peñas A, Hashimoto-Sugimoto M, Iba K, Schroeder JI. 2015. The HT1 protein kinase is essential for red light-induced stomatal opening and genetically interacts with OST1 in red light and CO2-induced stomatal movement responses. New Phytol. 208, 1126–1137. ( 10.1111/nph.13566) [DOI] [PubMed] [Google Scholar]
- 26.Merilo E, Jalakas P, Laanemets K, Mohammadi O, Hõrak H, Kollist H. 2015. Abscisic acid transport and homeostasis in the context of stomatal regulation. Molec. Plant 8, 1321–1333. ( 10.1016/j.molp.2015.06.006) [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E. 2009. High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol. 149, 825–834. ( 10.1104/pp.108.130823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer H, et al. 2013. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 23, 53–57. ( 10.1016/j.cub.2012.11.022) [DOI] [PubMed] [Google Scholar]
- 29.Pedro LR 2016. Abscisic acid catabolism generates phaseic acid, a molecule able to activate a subset of ABA receptors. Molec. Plant 9, 1448–1450. ( 10.1016/j.molp.2016.09.009) [DOI] [PubMed] [Google Scholar]
- 30.Kushiro T, et al. 2004. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8'-hydroxylases: key enzymes in ABA catabolism. EMBO J. 23, 1647–1656. ( 10.1038/sj.emboj.7600121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison MA, Walton DC. 1975. Abscisic acid metabolism in water-stressed bean leaves. Plant Physiol. 56, 250–254. ( 10.1104/pp.56.2.250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arve LE, Kruse OMO, Tanino KK, Olsen JE, Futsæther C, Torre S. 2015. Growth in continuous high air humidity increases the expression of CYP707A-genes and inhibits stomatal closure. Environ. Exp. Bot. 115, 11–19. ( 10.1016/j.envexpbot.2015.02.004) [DOI] [Google Scholar]
- 33.Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. 2006. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141, 97–107. ( 10.1104/pp.106.079475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umezawa T, et al. 2006. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 46, 171–182. ( 10.1111/j.1365-313X.2006.02683.x) [DOI] [PubMed] [Google Scholar]
- 35.McAinsh MR, Clayton H, Mansfield TA, Hetherington AM. 1996. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 111, 1031–1042. ( 10.1104/pp.111.4.1031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeiger E, Farquhar GD, Cowan IR (eds). 1987. Stomatal function. Stanford: Stanford University Press. [Google Scholar]
- 37.McAdam SAM, Brodribb TJ. 2018. Mesophyll cells are the main site of abscisic acid biosynthesis in water-stressed leaves. Plant Physiol. 177, 911–917. ( 10.1104/pp.17.01829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raschke K 1974. Abscisic acid sensitizes stomata to CO2 in leaves of Xanthium strumarium. In Proceedings of the 8th International Conference on Plant Growth Substances, Tokyo, pp. 1151–1158. [Google Scholar]
- 39.Drake B, Raschke K. 1974. Prechilling of Xanthium strumarium L. reduces net photosynthesis and, independently, stomatal conductance, while sensitizing the stomata to CO2. Plant Physiol. 53, 808–812. ( 10.1104/pp.53.6.808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansfield TA, Davies WJ. 1981. Stomata and stomatal mechanisms. In The physiology and biochemistry of drought resistance in plants (eds Aspinall D, Paleg LG), pp. 315–346. New York, NY: Academic Press. [Google Scholar]
- 41.Talbott LD, Rahveh E, Zeiger E. 2003. Relative humidity is a key factor in the acclimation of the stomatal response to CO2. J. Exp. Bot. 54, 2141–2147. ( 10.1093/jxb/erg215) [DOI] [PubMed] [Google Scholar]
- 42.Farquhar GD, Dubbe DR, Raschke K. 1978. Gain of the feedback loop involving carbon dioxide and stomata: theory and measurement. Plant Physiol. 62, 406–412. ( 10.1104/pp.62.3.406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwak JM, et al. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. ( 10.1093/emboj/cdg277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. ( 10.1038/35021067) [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P. 2001. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. ( 10.1104/pp.126.4.1438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolla VA, Vavasseur A, Raghavendra AS. 2007. Hydrogen peroxide production is an early event during bicarbonate induced stomatal closure in abaxial epidermis of Arabidopsis. Planta 225, 1421–1429. ( 10.1007/s00425-006-0450-6) [DOI] [PubMed] [Google Scholar]
- 47.Qi J, Song C-P, Wang B, Zhou J, Kangasjärvi J, Zhu J-K, Gong Z. 2018. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60, 805–826. ( 10.1111/jipb.12654) [DOI] [PubMed] [Google Scholar]
- 48.He J, Zhang R-X, Kim DS, Sun P, Liu H, Liu Z, Hetherington AM, Liang Y-K. 2020. ROS of distinct sources and salicylic acid separate elevated CO2-mediated stomatal movements in Arabidopsis. Front. Plant Sci. 11, 542 ( 10.3389/fpls.2020.00542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B, Kilk K, Kollist H. 2018. Stomatal VPD response: there is more to the story than ABA. Plant Physiol. 176, 851–864. ( 10.1104/pp.17.00912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Zhao X, Chory J. 2019. The Arabidopsis transcriptome responds specifically and dynamically to high light stress. Cell Rep. 29, 4186–4199. ( 10.1016/j.celrep.2019.11.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freudenberger H 1940. Die Reaktion der Schliesszellen auf Kohlensäure und Sauerstoffentzug. Protoplasma 35, 15–54. ( 10.1007/BF02807306) [DOI] [Google Scholar]
- 52.Heath OVS. 1948. Studies in stomatal action. Control of stomatal movement by a reduction in the normal carbon dioxide content of the air. Nature 161, 179 ( 10.1038/161179a0) [DOI] [PubMed] [Google Scholar]
- 53.Gavet O, Pines J. 2010. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell 18, 533–543. ( 10.1016/j.devcel.2010.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.