Abstract
Background and Aims
Sugar‐containing beverage intake is a major risk factor for obesity in both children and adults and appears to be associated with NAFLD in adults. The purpose of this study was to examine the associations between sugar‐containing beverage intake in infancy and liver fat accumulation and NAFLD among school‐aged children.
Approach and Results
In a population‐based prospective cohort study of 1,940 infants, we assessed sugar‐containing beverage intake at 1 year with a validated Food Frequency Questionnaire. Liver fat fraction and NAFLD (liver fat fraction ≥5.0%) were assessed with MR. Higher sugar‐containing beverage intake in infancy was not associated with higher liver fat accumulation at 10 years of age when assessed continuously (SD, 0.03; 95% CI, 0.02, 0.07, per one‐serving/day increase of sugar‐containing beverage intake) or categorically (P = 0.38). However, compared to infants with <1.0 serving/day, those with >2.0 servings/day had the highest odds of NAFLD at 10 years of age (OR, 3.02; 95% CI, 1.34, 6.83). These associations remained borderline significant after additional adjustment for sugar‐containing beverage intake and body mass index at school age (P = 0.13). Stratified analyses showed stronger associations between sugar‐containing beverage intake in infancy and NAFLD at 10 years of age among children of mothers with lower educational attainment (OR, 1.48; 95% CI, 1.12, 1.97) and among children with overweight or obesity (OR, 1.47; 95% CI, 1.05, 2.07).
Conclusions
Higher sugar‐containing beverage intake in infancy was associated with NAFLD in school‐aged children, independent of sugar‐containing beverage intake and body mass index at school age. Limiting the intake of sugar‐containing beverages in infancy may help prevent liver steatosis at school age.
Abbreviations
- BMI
body mass index
- FFQ
Food Frequency Questionnaire
- IDEAL‐IQ
Iterative Decomposition of water and fat with Echo Asymmetry and Least squares estimation
High intake of sugar‐containing beverages is a strong risk factor for obesity across the life course.( 1 , 2 , 3 ) Results from prospective studies show sugar‐containing beverage intake in infancy is related to body mass index (BMI) in adulthood.( 3 , 4 , 5 ) In addition, findings from randomized controlled trials suggest that higher consumption of sugar‐containing beverages increases adiposity in children.( 6 , 7 , 8 ) Recent studies in adults showed that higher intake of sugar‐containing beverages is not only associated with general adiposity but also with increased liver fat accumulation.( 9 , 10 ) Increased liver fat accumulation and NAFLD reflect a heterogeneous spectrum, ranging from liver steatosis to steatohepatitis, fibrosis, cirrhosis, and, eventually, end‐stage liver disease.( 11 ) We have recently reported associations between liver fat across the full spectrum and risk factors for cardiometabolic disease at school age.( 12 ) Early life exposures may contribute to the development of not only obesity but also of liver fat accumulation and NAFLD.( 11 , 13 , 14 ) It is not known whether sugar‐containing beverage intake in infancy leads to a higher risk of liver fat accumulation and subsequent NAFLD later in life. Insight into these associations might lead to preventive strategies focused on the earliest phase of life, to optimize liver conditions in later life.
In a population‐based prospective cohort study among 1,940 children, we examined the prospective associations between sugar‐containing beverage intake in infancy and liver fat accumulation and NAFLD, assessed with MR at 10 years of age. We additionally explored whether any association was explained by sociodemographics, lifestyle factors, or sugar‐containing beverage intake and BMI at school age.
Methods
Study Population
The study was embedded in the Generation R Study. This is a population‐based prospective cohort from early fetal life onward, based in Rotterdam, the Netherlands.( 15 ) The study has been approved by the Medical Ethics Committee of the Erasmus University Medical Center in Rotterdam (MEC 198.782/2001/31). Written, informed consent was obtained from all parents.( 15 ) All children were born between April 2002 and January 2006. The infant Food Frequency Questionnaire (FFQ) was implemented at a later stage during the study; therefore, this study was performed in a subgroup of the total population.( 16 ) Of the 5,088 mothers who received the FFQ, 3,643 completed it. A total of 3,614 infants had valid information on dietary intake assessed by the FFQ at the age of 1 year. A subgroup of 1,940 of these children were invited for MRI measurements at 10 years of age (Fig. 1).
FIG. 1.
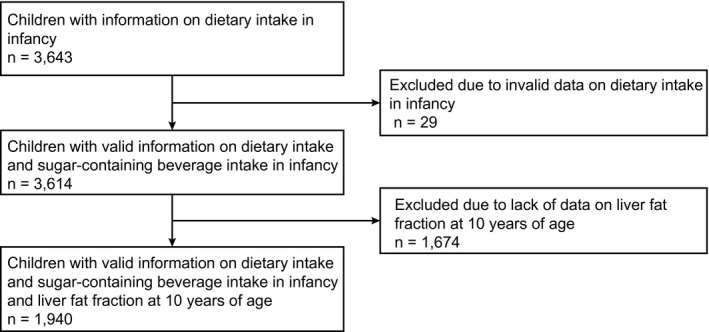
Flowchart of study participants.
Intake of Sugar‐Containing Beverages
Infant sugar‐containing beverage intake was assessed at a mean age of 13.6 months (SD, 1.8). Parents completed the FFQ using the last month as the reference period. The 211‐item semiquantitative FFQ was designed in cooperation with the Division of Human Nutrition of Wageningen University, the Netherlands, and based on an existing validated FFQ.( 17 ) It was modified to include foods frequently consumed during early life, according to a national Dutch food consumption survey among 941 Dutch children aged 9 to 18 months.( 16 ) The intraclass correlation coefficient for sugar‐containing beverage intake was calculated as 0.76; this was estimated comparing sugar‐containing beverage intake of the FFQ with a validation study with three 24‐hour recalls in a representative sample.( 3 ) Total sugar‐containing beverage intake included fruit juices, fruit concentrates, soft drinks, and lemonade. We converted the intake of sugar‐containing beverage consumption into the number of servings per day, with one serving equaling 150 g, as defined.( 18 ) The consumption of sugar‐containing beverages was assessed continuously and categorized into three categories: low, <1 serving/day; medium, 1‐2 servings/day; and high, >2 servings/day. The reference group was <1 serving/day, based on the cutoff in the diet quality score for preschool children.( 19 )
Liver Fat at 10 Years of Age
We measured liver fat using a 3T MR scanner (Discovery MR750w; GE Healthcare, Milwaukee, WI) as described.( 15 , 20 , 21 , 22 ) The children wore light clothing without metal objects while undergoing the body scan. A liver fat scan was performed using a single‐breath‐hold, 3‐dimensional volume and a special three‐point proton density–weighted Dixon technique (IDEAL‐IQ, Iterative Decomposition of water and fat with Echo Asymmetry and Least squares estimation) for generating a precise liver fat fraction image.( 23 ) The IDEAL‐IQ scan is based on a carefully tuned six‐echo echo planar imaging acquisition. The obtained fat fraction maps were analyzed by Precision Image Analysis (Kirkland, WA) using the sliceOmatic (TomoVision, Magog, Canada) software package. All extraneous structures and any image artifacts were removed manually.( 24 ) Liver fat fraction was determined by taking four samples of at least 4 cm2 from the central portion of the hepatic volume. Subsequently, the mean signal intensities were averaged to generate an overall mean liver fat estimation. Liver fat measured with IDEAL‐IQ using MR is reproducible, highly precise, and validated in adults.( 25 , 26 ) As described, NAFLD was defined as liver fat ≥5.0%.( 20 , 26 , 27 )
Covariates
At enrollment in the study, we obtained information on maternal age, parity, education level, smoking, net household income, and prepregnancy weight by questionnaires. We measured maternal height and calculated prepregnancy BMI. Information on child age, sex, and birth weight was obtained from medical records, and ethnicity was obtained by questionnaires and was defined based on country of birth of the parents.( 28 ) We categorized ethnicity into European (Dutch and other European) versus non‐European (African [Cape Verdean, other African, Dutch Antillean, and Surinamese‐Creole], American, Asian [Indonesian, other Asian, Surinamese‐Hindu, and Surinamese‐unspecified], Turkish, Moroccan, Oceanian). At the 10‐years‐of‐age follow‐up visit, we measured childhood height and weight, both without shoes and heavy clothing, and calculated BMI and sex‐adjusted and age‐adjusted childhood BMI SD scores based on Dutch reference growth charts (Growth Analyzer 4.0).( 29 ) Childhood BMI was categorized into normal weight versus overweight or obese.( 30 ) Physical activity and screen time were assessed with questionnaires at school age.( 31 ) The child diet quality score for preschool children was used, previously calculated with information from the FFQ at 1 year of age.( 19 ) Sugar‐containing beverage intake at 8 years of age was assessed with the validated 71‐item semiquantitative FFQ.( 32 , 33 ) The Dutch 2015 Guidelines for a Healthy Diet were used to calculate energy and nutrient intake at 8 years of age.( 34 )
Statistical Analysis
First, we conducted a nonresponse analysis among infants with a valid FFQ, comparing children with and without liver MR scans with Student t tests, Mann‐Whitney U tests, and chi‐square tests. Second, we examined the associations between sugar‐containing beverage intake and liver fat accumulation using linear regression models. Third, we used logistic regression models to assess the associations between sugar‐containing beverage intake and the odds of NAFLD. Analyses were performed using sugar‐containing beverage intake as a continuous measure and categorized (low, <1 serving/day (reference); medium, 1‐2 servings/day; and high, >2 servings/day). The basic model was adjusted for child at 10 years of age, sex, and total energy intake; the confounder model was additionally adjusted for maternal prepregnancy BMI, education, and net household income and child ethnicity, physical activity, and screen time; and the mediator model was additionally adjusted for sugar‐containing beverage intake at 8 years of age and BMI at 10 years of age. Included covariates were based on previous studies, strong correlations with consumption of sugar‐containing beverages and liver fat accumulation, and changes in effect estimates of >10%, as well as the directed acyclic graph we constructed with these covariates (Supporting Fig. S1).( 10 , 35 , 36 ) As secondary analysis, we examined the associations between sugar‐containing beverage intake at 8 years of age and liver fat accumulation at 10 years of age using similar models, with adjustment for total energy intake at 8 years of age. The distribution of liver fat was skewed, and natural log‐transformed values were used in all linear regression analyses. To assess whether the associations differed by type of sugar‐containing beverage, we repeated the analyses separately for intake of fruit juice and intake of soft drinks and lemonade. Based on previous findings, we hypothesized that the associations between sugar‐containing beverage intake and liver fat accumulation might differ by maternal educational level.( 36 ) Because we observed a statistically significant interaction between sugar‐containing beverage intake in infancy with maternal education and childhood BMI, we performed additional stratified analyses.( 12 , 36 ) We did not observe a statistically significant interaction between sugar‐containing beverage intake in infancy with child sex.( 3 , 35 ) As sensitivity analyses, we first repeated the confounder model with sugar‐containing beverage intake standardized for total daily energy intake using the residual method and without adjustment for total daily energy intake as a confounder, as energy standardization could possibly reduce the measurement error.( 37 ) Second, we examined the associations between sugar‐containing beverage intake in infancy and liver fat accumulation among children of Dutch ethnicity only, as the FFQ was originally developed for participants of Dutch ethnicity. Missing data in the covariates were multiple‐imputed using the Markov chain Monte Carlo approach. Five imputed data sets were created and analyzed together. All statistical analyses were performed using SPSS version 25.0 for Windows (IBM, Chicago, IL).
Results
Subject Characteristics
The median sugar‐containing beverage intake in infancy was 0.9 serving/day (95% range, 0.0‐3.7), and the median liver fat fraction at 10 years of age was 2.0% (95% range, 1.2‐4.6), 1.9% (95% range, 1.2‐4.3), and 2.0% (95% range, 1.2‐6.1) in the groups with low, medium, and high sugar‐containing beverage intake in infancy, respectively (Table 1). Children without liver fat measurement were less often of European ethnicity and had higher total daily energy intake in infancy (Supporting Table S1).
TABLE 1.
Subject Characteristics in Infancy
| Total Group | Sugar‐Containing Beverage Intake in Infancy | |||
|---|---|---|---|---|
| n = 1,940 | Low (<1 serving/day; n = 1,015) | Medium (1‐2 servings/day; n = 572) | High (>2 servings/day; n = 353) | |
| Maternal characteristics | ||||
| Age at enrollment, years | 31.8 ± 4.3 | 32.1 ± 4.3 | 31.5 ± 4.2 | 31.4 ± 4.7 |
| Prepregnancy BMI, kg/m2 | 23.4 ± 4.0 | 23.2 ± 3.8 | 23.6 ± 4.3 | 23.5 ± 3.9 |
| Parity, nulliparous | 1,154 (61.1) | 604 (61.2) | 343 (61.5) | 207 (60.3) |
| Education, higher | 1,181 (62.4) | 643 (65.3) | 341 (60.9) | 197 (56.8) |
| Smoking during pregnancy, continued | 188 (10.8) | 84 (9.1) | 51 (10.0) | 53 (16.8) |
| Net household income, ≥2,200 Euros/month | 1,176 (70.4) | 615 (70.9) | 356 (71.9) | 205 (66.3) |
| Child characteristics | ||||
| Sex, male | 937 (48.3) | 490 (48.3) | 275 (48.1) | 172 (48.7) |
| Ethnicity, European | 1,496 (77.4) | 767 (75.8) | 459 (80.5) | 270 (76.9) |
| Birth weight, g | 3,452 ± 574 | 3,427 ± 584 | 3,481 ± 559 | 3,477 ± 568 |
| Age at 1‐year FFQ, months | 13.6 ± 1.8 | 13.4 ± 1.6 | 13.7 ± 1.9 | 13.9 ± 2.1 |
| Total energy intake at 1 year, kcal/day | 1,306 ± 385 | 1,219 ± 357 | 1,323 ± 350 | 1,531 ± 421 |
| Diet quality score at 1 year, 0‐10 | 4.3 ± 1.4 | 4.4 ± 1.4 | 4.2 ± 1.3 | 4.2 ± 1.4 |
| Sugar‐containing beverages at 1 year, servings/day | ||||
| Total | 0.9 (0.0, 3.7) | 0.5 (0.0, 1.0) | 1.6 (1.0, 1.9) | 2.8 (2.0, 5.6) |
| Fruit juice | 0.1 (0.0, 1.9) | 0.0 (0.0, 0.9) | 0.1 (0.0, 1.9) | 0.4 (0.0, 3.7) |
| Soft drinks and lemonade | 0.7 (0.0, 2.8) | 0.3 (0.0, 0.9) | 1.1 (0.0, 1.9) | 1.9 (0.0, 4.6) |
| Age at 8‐year FFQ, years | 8.1 ± 0.1 | 8.1 ± 0.2 | 8.1 ± 0.1 | 8.1 ± 0.2 |
| Total energy intake at 8 years, kcal/day | 1,469 ± 341 | 1,445 ± 342 | 1,481 ± 326 | 1,517 ± 356 |
| Diet quality score at 8 years, 0‐10 | 4.6 ± 1.2 | 4.6 ± 1.2 | 4.6 ± 1.2 | 4.5 ± 1.3 |
| Sugar‐containing beverages at 8 years, servings/day | ||||
| Total | 2.2 (0.1, 8.9) | 2.0 (0.0, 7.8) | 2.4 (0.1, 7.9) | 2.6 (0.1, 10.4) |
| Fruit juice | 0.4 (0.0, 3.2) | 0.4 (0.0, 3.1) | 0.4 (0.0, 3.6) | 0.4 (0.0, 3.7) |
| Soft drinks and lemonade | 1.5 (0.0, 7.8) | 1.2 (0.0, 6.9) | 1.5 (0.0, 7.4) | 1.7 (0.0, 9.2) |
| Age at 10‐year visit, years | 9.8 ± 0.3 | 9.8 ± 0.2 | 9.8 ± 0.3 | 9.8 ± 0.3 |
| Physical activity, hours/day | 1.4 (0.3, 3.5) | 1.3 (0.3, 3.3) | 1.4 (0.3, 3.5) | 1.5 (0.4, 3.8) |
| Screen time, ≥2 hours/day | 796 (49.5) | 384 (46.5) | 239 (49.7) | 173 (57.5) |
| BMI, kg/m2 | 17.2 ± 2.4 | 17.1 ± 2.5 | 17.1 ± 2.3 | 17.6 ± 2.5 |
| Liver fat fraction, % | 2.0 (1.2, 4.7) | 2.0 (1.2, 4.6) | 1.9 (1.2, 4.3) | 2.0 (1.2, 6.1) |
| NAFLD | 36 (1.9) | 14 (1.4) | 8 (1.4) | 14 (4.0) |
Values, but not imputed data, are observed and represent numbers (valid percentage), means ± SD, or medians (95% range) shown for the total group and stratified for sugar‐containing beverage intake at 1 year. Maternal characteristics were obtained when they were enrolled in the study, mostly in early pregnancy. Child’s ethnicity based on the parent’s country of birth was categorized into European (Dutch and other European) and non‐European (African [Cape Verdean, other African, Dutch Antillean, and Surinamese‐Creole], American, Asian [Indonesian, other Asian, Surinamese‐Hindu, and Surinamese‐unspecified], Turkish, Moroccan, Oceanian).
Sugar‐Containing Beverage Intake in Infancy and Liver Fat Accumulation
Figure 2 shows that the distribution of liver fat fraction at 10 years of age (categories <2.0%, 2.0%‐2.9%, 3.0%‐3.9%, 4.0%‐4.9%, and ≥5.0%) differed per infant sugar‐containing beverage intake category. The percentage of children with liver fat fraction of ≥5.0% increased from 1.4% (n = 14 of 1,015) in the low‐intake group to 4.0% (n = 14 of 353) in the high‐intake group (P < 0.01). After adjusting for confounders, sugar‐containing beverage intake in infancy was neither continuously (SD, 0.03; 95% CI, 0.02, 0.07, per one‐serving‐per‐day increase in sugar‐containing beverage intake) nor categorically (P = 0.38) associated with liver fat fraction across the full range. There were no substantial differences in results among the basic, confounder, and mediator models (Table 2 and Supporting Figs. S2 and S3).
FIG. 2.
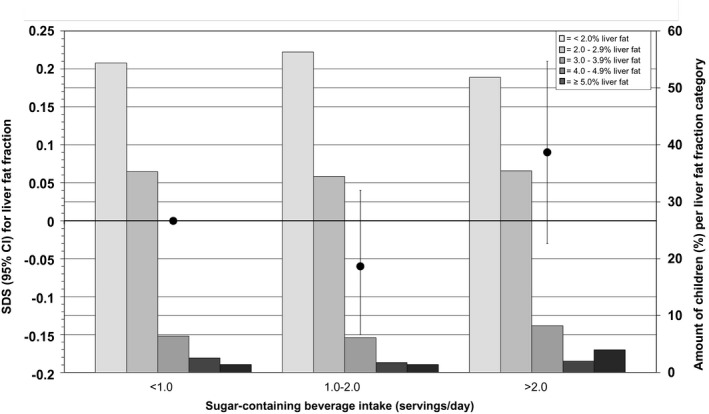
Associations between sugar‐containing beverage intake in infancy and liver fat fraction in school‐aged children. The black circles represent regression coefficients (95% CIs) from linear regression models that reflect differences in liver fat fraction (in SD) per sugar‐containing beverage intake category as compared with the reference group (children with less than one serving/day intake of sugar‐containing beverage), as scaled on the left y‐axis. These associations are adjusted for children at 10 years of age, sex, total energy intake, maternal prepregnancy BMI, education, net household income, child ethnicity, physical activity, and screen time. The bars present the amount of children (as a percentage) per liver fat fraction categories (<2.0%, 2.0%‐2.9%, 3.0%‐3.9%, 4.0%‐4.9%, and ≥5.0% liver fat) per sugar‐containing beverage intake, as scaled on the right y‐axis. Trend: 0.03 SD (95% CI, 0.03‐0.08) per one‐serving‐per‐day increase in sugar‐containing beverage intake. Abbreviation: SDS, SD score.
TABLE 2.
Associations Between Sugar‐Containing Beverage Intake in Infancy and Liver Fat Fraction and NAFLD in School‐Aged Children
| Sugar‐containing beverages (servings/day) at 1 year | MR‐Measured Liver Fat at School Age, n = 1,940 | |
|---|---|---|
| Liver fat fraction, SD | NAFLD, yes/no | |
| Basic model | 0.037 (0.02, 0.06) | 1.44 (1.27, 1.64)* |
| Confounder model | 0.026 (−0.02, 0.07) | 1.34 (1.06, 1.69) † |
| Mediator model | −0.004 (−0.05, 0.04) | 1.34 (0.97, 1.83) |
Values are regression coefficients (95% CIs) from linear regression models that reflect differences in liver fat fraction (in SD) per sugar‐containing beverage intake per day at 1 year. Values are ORs (95% CIs) that reflect the risk of NAFLD per sugar‐containing beverage intake per day at 1 year. The basic model is adjusted for children at 10 years of age, sex, and total energy intake. The confounder model is the basic model additionally adjusted for maternal prepregnancy BMI, education, net household income, child ethnicity, physical activity, and screen time. The mediator model is the confounder model additionally adjusted for sugar‐containing beverage intake at 8 years of age and BMI at 10 years of age. NAFLD was defined as “yes” when liver fat ≥5.0% and as “no” when liver fat <5.0%.
P value < 0.01.
P value < 0.05.
Higher sugar‐containing beverage intake in infancy was associated with higher odds of NAFLD, both continuously (OR, 1.34; 95% CI, 1.06, 1.69) and categorically (P < 0.05) (Fig. 3). Compared to infants with less than one serving per day, infants with over two servings per day had the highest odds of NAFLD (OR, 3.02; 95% CI, 1.34, 6.83). There were no differences in results between the basic and confounder models (Table 2). Also, the effect estimates were only slightly affected and of borderline significance after additional adjustment for sugar‐containing beverage intake at 8 years of age and BMI at 10 years of age (P = 0.13) (Table 2, Fig. 4, and Supporting Fig. S4).
FIG. 3.
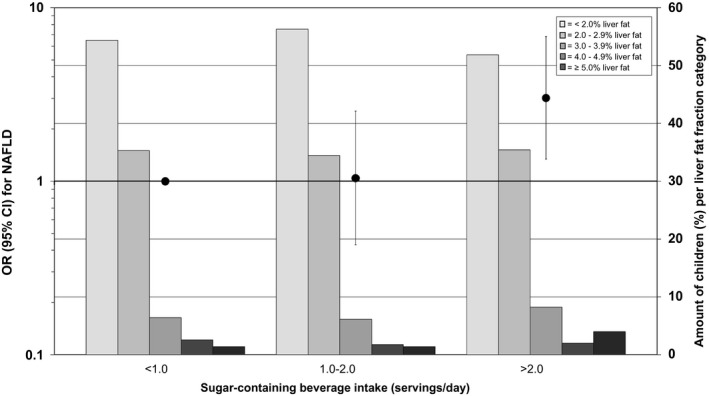
Associations between sugar‐containing beverage intake in infancy and odds of NAFLD in school‐aged children. The black circles represent ORs (95% CIs) that reflect the risk of NAFLD per sugar‐containing beverage intake category in infancy as compared with the reference group (children with less than one serving per day intake of sugar‐containing beverage), as scaled on the left y‐axis. These associations are adjusted for children at 10 years of age, sex, total energy intake, maternal prepregnancy BMI, education, net household income, child ethnicity, physical activity, and screen time. The bars present the amount of children (as a percentage) per liver fat fraction categories (< 2.0%, 2.0%‐2.9%, 3.0%‐3.9%, 4.0%‐4.9%, and ≥5.0% liver fat) per sugar‐containing beverage intake, as scaled on the right y‐axis. Trend: OR, 1.73 (95% CI, 1.12‐2.66) per one‐serving‐per‐day increase in sugar‐containing beverage intake.
FIG. 4.
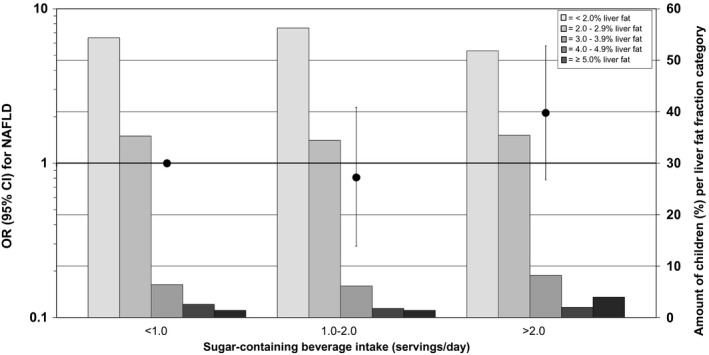
Associations between sugar‐containing beverage intake in infancy and odds of NAFLD in school‐aged children (mediator model). The black circles represent ORs (95% CIs) that reflect the risk of NAFLD per sugar‐containing beverage intake category in infancy as compared with the reference group (children with less than one serving per day intake of sugar‐containing beverage), as scaled on the left y‐axis. These associations are adjusted for children at 10 years of age, sex, total energy intake, maternal prepregnancy BMI, education, net household income, child ethnicity, physical activity, screen time, sugar‐containing beverage intake at 8 years of age, and BMI at 10 years of age. The bars present the amount of children (as a percentage) per liver fat fraction categories (< 2.0%, 2.0%‐2.9%, 3.0%‐3.9%, 4.0%‐4.9%, and ≥5.0% liver fat) per sugar‐containing beverage intake, as scaled on the right y‐axis. Trend: OR, 1.41 (95% CI, 0.83‐2.40) per one‐serving‐per‐day increase in sugar‐containing beverage intake.
Analyses stratified for maternal education level suggested that among children from mothers with a lower or medium level of educational attainment, higher sugar‐containing beverage intake in infancy was associated with increased liver fat fraction (SD, 0.09; 95% CI, 0.03, 0.16, per one‐serving‐per‐day increase in sugar‐containing beverage intake), whereas no association was observed in mothers with higher education (Supporting Table S2). Stratified analyses in sugar‐containing beverage intake in infancy with NAFLD suggested that the odds of NAFLD are stronger among children of mothers with a lower or medium level of educational attainment (OR, 1.48; 95% CI, 1.12, 1.97), compared to children of mothers with a higher level of educational attainment and among children with overweight or obesity (OR, 1.47; 95% CI, 1.05, 2.07) compared to children with normal weight (Supporting Table S2).
Sugar‐containing beverage intake in infancy categorized in either fruit juice or soft drinks and lemonade was not associated with liver fat fraction or NAFLD at school age (Supporting Table S3). Sugar‐containing beverage intake at 8 years of age was not associated with liver fat fraction or NAFLD at school age (Supporting Table S4).
Sensitivity Analyses
When we used sugar‐containing beverage intake standardized for total daily energy intake instead of unstandardized sugar‐containing beverage intake, the odds were largely similar for the associations with NAFLD but not statistically significant (Supporting Table S5). We also observed similar results to the main findings when we restricted our analyses to children of Dutch ethnicity only or among singleton children only (Supporting Table S5).
Discussion
We observed that higher sugar‐containing beverage intake in infancy is associated with an increased risk of NAFLD in children of school age. The associations remained borderline significant after additional adjustment for sugar‐containing beverage intake at 8 years of age and BMI at 10 years of age. The intake of sugar‐containing beverages in infancy showed stronger associations with NAFLD at 10 years of age among children of mothers with lower educational attainment as compared to those with high educational attainment and among children with overweight or obesity as compared to children with normal weight. The associations of sugar‐containing beverage intake in infancy with NAFLD at 10 years of age were present even with an overall low prevalence of NAFLD in the study population.
Interpretation of Main Findings
Sugar‐containing beverage consumption is the main source of added sugar intake in the total daily energy intake of children and adults the last decades.( 6 , 8 , 38 ) In adults, sugar‐containing beverage intake is strongly associated with the development of NAFLD.( 10 , 39 ) Increased liver fat accumulation and NAFLD reflect a heterogeneous spectrum, ranging from liver steatosis, to steatohepatitis, fibrosis, cirrhosis, and, eventually end‐stage liver disease.( 11 ) NAFLD is associated with an increased risk of cardiovascular disease, dyslipidemia, and type 2 diabetes in adults.( 40 ) Using data from the same cohort as the current study, we recently reported associations of liver fat across the full spectrum with risk factors for cardiometabolic disease already at 10 years.( 12 ) Dietary patterns in infancy have been shown to track into adulthood.( 39 ) Early lifestyle exposures are suggested to contribute to the development of not only obesity but also liver fat accumulation and NAFLD.( 11 , 13 , 14 ) We hypothesized that intake of sugar‐containing beverages in infancy is associated with liver fat accumulation in children of school age. We indeed observed that higher intake of sugar‐containing beverages in infancy is associated with increased odds of NAFLD at 10 years of age, but not with liver fat accumulation across the full range.
In the current study, we observed that infants who consumed more than two sugar‐containing beverage servings per day had the highest odds of NAFLD at 10 years of age. We also observed that the association of sugar‐containing beverage intake in infancy with NAFLD at school age was largely independent of sugar‐containing beverage intake at 8 years of age. Furthermore, childhood BMI, a known risk factor for NAFLD, did not appear to explain the observed associations. Our findings are important, as prospective data on sugar‐containing beverage intake during infancy and its relation with liver fat accumulation and NAFLD are lacking, and lifestyle exposures in early life are suggested to track into adulthood. Our findings are in line with previous studies in adults. Two large cross‐sectional studies among middle‐aged adults observed that sugar‐containing beverage consumption was, independently of BMI, associated with increased liver fat accumulation.( 9 , 10 ) A recent randomized controlled trial among 40 adolescent boys diagnosed with NAFLD demonstrated that restricting sugar intake reduces liver fat accumulation.( 35 )
When we stratified our analyses, we observed stronger associations between sugar‐containing beverage intake and both liver fat accumulation and NAFLD among children from mothers with a lower level of educational attainment. The combination of lower maternal education, seen as proxy for family socioeconomic status, and higher sugar‐containing beverage intake in infancy might track from infancy into childhood and exacerbate liver fat accumulation. Stratified analyses of BMI at 10 years of age also showed stronger associations between sugar‐containing beverage intake and NAFLD among children with overweight or obesity. Thus, our findings suggest that sugar‐containing beverage intake in infancy appears to be associated with the development of NAFLD at 10 years of age and that these associations are stronger among children with overweight or obesity.
We did not observe associations between sugar‐containing beverage intake in infancy and liver fat across the full range. It appears likely that, due to the relatively large group of infants with low sugar‐containing beverage intake in infancy, together with the limited and still healthy spectrum of liver fat across the full range at school age, the differences in sugar‐containing beverage intake in infancy are too small to observe an effect on liver fat fraction across the full range at 10 years of age. The differences observed for early‐life intake of sugar‐containing beverage with the risk of NAFLD at 10 years of age were not present for the sugar‐containing beverage intake at 8 years of age. The absence of association between sugar‐containing beverage intake at 8 years of age and liver fat might be explained by reverse causality, because parents of children who are overweight or obese might reduce or underreport total energy intake and sugar‐containing beverage intake, or it may indicate that sugar‐containing beverage intake does not track into childhood.
Multiple mechanisms underlying the associations between sugar‐containing beverage intake and liver fat accumulation have been proposed. It has been suggested that glucose, especially fructose and fructose‐containing sugars, which are all primarily metabolized in the liver, increase hepatic de novo lipogenesis.( 1 , 35 , 41 ) In addition, consumption of sugar‐containing beverages induces peaks in blood glucose, insulin, and triglyceride concentrations, which may lead to insulin resistance and, subsequently, to liver fat accumulation.( 1 , 9 , 10 , 39 ) Moreover, intake of liquid food leads to less satiety and more postprandial hunger and therefore to increased total daily energy intake.( 9 , 42 ) Based on our findings, future studies should explore lifestyle interventions from infancy onward, to reduce sugar‐containing beverage intake and maintain an adequate healthy total daily energy intake. Intervention studies from early life onward will provide important new insights into the effectiveness of these interventions as well as the causality of the observed associations between sugar‐containing beverage intake in infancy and NAFLD in later life.
Methodological Considerations
Major strengths of this study are the population‐based prospective longitudinal design with a large sample size, with information on sugar‐containing beverage intake in infancy and liver fat fraction measured with MR at 10 years of age. A subgroup of the study population underwent MR measurements at 10 years of age (54%; n = 1,940). Although the nonresponse at the outcome measurement could lead to biased effect estimates if associations were different between those included and not included in the analyses, this appears unlikely. To assess the average sugar‐containing beverage intake in infancy, the 211‐item semiquantitative FFQ , which may be subject to underreporting, was used. The study population contained a relatively small number of children with overweight or obesity, which indicates a selection toward a lean population that might affect the generalizability of our findings. The healthy and young study population might also explain the small number of cases with NAFLD, which could have limited our statistical power to detect significant associations. However, our findings are important because prospective data on sugar‐containing beverage intake in infancy and its relationship with liver fat accumulation are lacking, and lifestyle exposures in early life appear to track into adulthood. Because we had a young study population, our results are not likely biased by alcohol use or known history of jaundice, hepatitis, smoking, or medication use. We did not include breastfeeding as a covariate in our analyses, as previously we did not observe an association between breastfeeding and liver fat fraction.( 43 ) Finally, although many covariates were included, there still might be some residual confounding, as in any observational study.
In summary, higher sugar‐containing beverage intake in infancy was associated with NAFLD in school‐aged children, independently of sugar‐containing beverage intake and BMI at school age. These associations tended to be stronger among children of mothers with a lower level of educational attainment and among children with overweight or obesity. Future preventive strategies should focus on the intake of sugar‐containing beverages from infancy onward, to reduce the risk of NAFLD in later life.
Author Contributions
M.L.G. and V.W.V.J. were responsible for the study concept. M.L.G., S.S., and V.W.V.J. were responsible for the data collection and interpretation, statistical analysis, and manuscript draft. R.G. and J.F.F. were responsible for the manuscript review. All authors were responsible for reading and approving the final manuscript.
Supporting information
Supplementary Material
Acknowledgment
The authors thank the general practitioners, hospitals, midwives, and pharmacies in Rotterdam.
The general design of the Generation R Study was made possible by financial support from the Erasmus University Medical Center, the Netherlands Organization for Health Research and Development, and the Ministry of Health, Welfare, and Sport. The study was supported in part by the European Research Council (Consolidator Grant ERC‐2014‐CoG‐648916), the European Union’s Horizon 2020 Research and Innovation programme LifeCycle (733206), the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL, NutriPROGRAM project, and ZonMw the Netherlands project [529051022] and Precise project [529051023]), the Dutch Heart Foundation (2017T013), the Dutch Diabetes Foundation (2017.81.002), and the Netherlands Organization for Health Research and Development (543003109).
Potential conflict of interest: Nothing to report.
See Editorial on Page 483
References
Author names in bold designate shared co‐first authorship.
- 1. Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non‐alcoholic fatty liver disease. J Hepatol 2008;48:993‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar‐sweetened beverages and genetic risk of obesity. N Engl J Med 2012;367:1387‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leermakers ETM, Felix JF, Erler NS, Ćerimagić A, Wijtzes AI, Hofman A, et al. Sugar‐containing beverage intake in toddlers and body composition up to age 6 years: the Generation R Study. Eur J Clin Nutr 2015;69:314‐321. [DOI] [PubMed] [Google Scholar]
- 4. Quah PL, Kleijweg J, Chang YY, Toh JY, Lim HX, Sugianto R, et al. Association of sugar‐sweetened beverage intake at 18 months and 5 years of age with adiposity outcomes at 6 years of age: the Singapore GUSTO mother‐offspring cohort. Br J Nutr 2019;122:1303‐1312. [DOI] [PubMed] [Google Scholar]
- 5. Pan L, Li R, Park S, Galuska DA, Sherry B, Freedman DS. A longitudinal analysis of sugar‐sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014;134(Suppl. 1):S29‐S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, et al. A randomized trial of sugar‐sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar‐free or sugar‐sweetened beverages and body weight in children. N Engl J Med 2012;367:1397‐1406. [DOI] [PubMed] [Google Scholar]
- 8. Malik VS, Pan A, Willett WC, Hu FB. Sugar‐sweetened beverages and weight gain in children and adults: a systematic review and meta‐analysis. Am J Clin Nutr 2013;98:1084‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Eekelen E, Beulens JWJ, Geelen A, Schrauwen‐Hinderling VB, Lamb H, de Roos A, et al. Consumption of alcoholic and sugar‐sweetened beverages is associated with increased liver fat content in middle‐aged men and women. J Nutr 2019;149:649‐658. [DOI] [PubMed] [Google Scholar]
- 10. Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, et al. Sugar‐sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol 2015;63:462‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063‐2072. [DOI] [PubMed] [Google Scholar]
- 12. Geurtsen ML, Santos S, Felix JF, Duijts L, Vernooij MW, Gaillard R, et al. Liver fat and cardiometabolic risk factors among school‐age children. Hepatology 2020;72:119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ayonrinde OT, Oddy WH, Adams LA, Mori TA, Beilin LJ, de Klerk N, et al. Infant nutrition and maternal obesity influence the risk of non‐alcoholic fatty liver disease in adolescents. J Hepatol 2017;67:568‐576. [DOI] [PubMed] [Google Scholar]
- 14. Santos S, Monnereau C, Felix JF, Duijts L, Gaillard R, Jaddoe VWV. Maternal body mass index, gestational weight gain, and childhood abdominal, pericardial, and liver fat assessed by magnetic resonance imaging. Int J Obes (Lond) 2019;43:581‐593. [DOI] [PubMed] [Google Scholar]
- 15. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 2016;31:1243‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kiefte‐de Jong JC, de Vries JH, Bleeker SE, Jaddoe VWV, Hofman A, Raat H, et al. Socio‐demographic and lifestyle determinants of “Western‐like” and “Health conscious” dietary patterns in toddlers. Br J Nutr 2013;109:137‐147. [DOI] [PubMed] [Google Scholar]
- 17. Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker‐based validity of a food‐frequency questionnaire estimating intake of fats and cholesterol. Am J Clinical Nutr 1993;58:489‐496. [DOI] [PubMed] [Google Scholar]
- 18. NEVO‐tabel . Nederlands Voedingsstoffenbestand 2011. Den Haag: RIVM/Voedingscentrum; 2011. [Google Scholar]
- 19. Voortman T, Kiefte‐de Jong JC, Geelen A, Villamor E, Moll HA, de Jongste JC, et al. The development of a diet quality score for preschool children and its validation and determinants in the Generation R Study. J Nutr 2015;145:306‐314. [DOI] [PubMed] [Google Scholar]
- 20. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H‐MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta‐analysis. Eur Radiol 2011;21:87‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 2015;61:1887‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 2011;34:729‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev 2011;12:e504‐e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol 2014;12:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petaja EM, Yki‐Jarvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD: a systematic review. Int J Mol Sci 2016;17:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr 2016;170:e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Immigrants in the Netherlands 2004 (Allochtonen in Nederland 2004). Den Haag/Heerlen, the Netherlands: Statistics Netherlands (Centraal Bureau voor de statistiek); 2004. [Google Scholar]
- 29. Fredriks AM, van Buuren S, Wit JM, Verloove‐Vanhorick SP. Body index measurements in 1996‐7 compared with 1980. Arch Dis Child 2000;82:107‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sigman A. Time for a view on screen time. Arch Dis Child 2012;97:935‐942. [DOI] [PubMed] [Google Scholar]
- 32. van der Velde LA, Nguyen AN, Schoufour JD, Geelen A, Jaddoe VWV, Franco OH, et al. Diet quality in childhood: the Generation R Study. Eur J Nutr 2019;58:1259‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dutman AE, Stafleu A, Kruizinga A, Brants HAM, Westerterp KR, Kistemaker C, et al. Validation of an FFQ and options for data processing using the doubly labelled water method in children. Public Health Nutr 2011;14:410‐417. [DOI] [PubMed] [Google Scholar]
- 34. Health Council of the Netherlands (2015) Dutch Guidelines for a Healthy Diet 2015. The Hague, the Netherlands: The Health Council of the Netherlands; 2015. [Google Scholar]
- 35. Schwimmer JB, Ugalde‐Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA 2019;321:256‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wijtzes AI, Jansen W, Jansen PW, Jaddoe VW, Hofman A, Raat H. Maternal educational level and preschool children’s consumption of high‐calorie snacks and sugar‐containing beverages: mediation by the family food environment. Prev Med 2013;57:607‐612. [DOI] [PubMed] [Google Scholar]
- 37. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(Suppl. 4):1220S‐1228S. discussion 1229S‐1231S. [DOI] [PubMed] [Google Scholar]
- 38. Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999‐2016. JAMA 2019;322:1178‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol 2009;51:918‐924. [DOI] [PubMed] [Google Scholar]
- 40. Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014;510:84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beysen C, Ruddy M, Stoch A, Mixson L, Rosko K, Riiff T, et al. Dose‐dependent quantitative effects of acute fructose administration on hepatic de novo lipogenesis in healthy humans. Am J Physiol Endocrinol Metab 2018;315:E126‐E132. [DOI] [PubMed] [Google Scholar]
- 42. Bray GA, Nielsen SJ, Popkin BM. Consumption of high‐fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537‐543. [DOI] [PubMed] [Google Scholar]
- 43. Vogelezang S, Santos S, Toemen L, Oei EHG, Felix JF, Jaddoe VWV. Associations of fetal and infant weight change with general, visceral, and organ adiposity at school age. JAMA Netw Open 2019;2:e192843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


