Abstract
Aim
To estimate the cost‐effectiveness of sequential addition of empagliflozin versus sitagliptin after metformin in patients with type 2 diabetes (T2D) with or without cardiovascular disease (CVD) from the perspective of the US healthcare payer.
Methods
An individual simulation model predicted lifetime diabetes‐related complications, using UKPDS‐OM2 equations in patients without CVD, and EMPA‐REG OUTCOME equations in patients with CVD. Additional US‐based sources informed inputs for population characteristics, adverse events, non‐CV death, treatment escalation, quality of life and costs. Costs and quality‐adjusted life‐years (QALYs) were discounted 3.0% annually.
Results
The incremental cost‐effectiveness ratio (ICER) for second‐line empagliflozin versus sitagliptin in the overall T2D population was $6967/QALY. Empagliflozin led to longer CVD‐free survival (0.07 years) and an 11% reduction in CV death in patients with CVD compared with sitagliptin. Empagliflozin resulted in greater benefits with greater costs in patients with versus without baseline CVD, yielding ICERs of $3589/QALY versus $12 577/QALY, respectively. Results were consistent across a range of deterministic and probabilistic sensitivity analyses and scenarios.
Conclusion
Compared with sitagliptin, empagliflozin was cost‐effective (at $50 000/QALY US threshold) as a second‐line treatment to metformin for T2D patients with or without CVD in the United States. Our findings lend additional support for more widespread adoption of guidelines by healthcare decision‐makers for T2D treatment.
Keywords: cardiovascular disease, cost‐effectiveness, empagliflozin, sitagliptin, sodium‐glucose co‐transporter‐2 inhibitor, type 2 diabetes, United States
1. INTRODUCTION
People with type 2 diabetes (T2D) are at an increased risk of many related complications, including cardiovascular disease (CVD; conditions that affect the heart or circulation, including myocardial infarction [MI], stroke or ischaemic heart disease), kidney disease, neuropathy, blindness and lower‐extremity amputation. Among these factors, CVD is the most prevalent cause of morbidity and mortality in the T2D population. About 32% of people with T2D have CVD, with the remainder at an elevated risk of developing CVD. 1 CVD leads to ~50% of deaths in people with T2D, largely attributable to an increased risk of MI and stroke. 2
American Diabetes Association (ADA) guidelines for T2D management stress the importance of preventing and managing CVD, based on results from recent CV outcomes trials (CVOTs). 3 Metformin monotherapy is the standard initial therapy for T2D, followed by stepwise addition of other glucose‐lowering medications over time. Second‐line therapy choices in patients without CVD or heart failure (HF) may include a sodium‐glucose co‐transporter‐2 inhibitor (SGLT‐2i), glucagon‐like peptide‐1 receptor agonist (GLP‐1RA), dipeptidyl peptidase‐4 inhibitor (DPP‐4i), thiazolidinedione, sulphonylurea or basal insulin. For the T2D population with CVD, SGLT‐2is or GLP‐1RAs are recommended as second‐line therapy after metformin, given their proven CV benefits. Further escalation of treatment should be considered for patients not maintaining treatment targets.
Empagliflozin, an SGLT‐2i, has shown reductions in HbA1c, body weight and systolic blood pressure (SBP), 4 , 5 and marked benefits on CV outcomes in the landmark EMPA‐REG OUTCOME CVOT. Empagliflozin showed significant reduction in three‐point major adverse cardiac event (3P‐MACE; CV death, non‐fatal MI or non‐fatal stroke; hazard ratio [HR]: 0.86, 95% confidence interval [CI]: 0.74‐0.99), CV death (HR: 0.62, 95% CI: 0.49‐0.77) and hospitalization for HF (HHF; HR: 0.65, 95% CI: 0.50‐0.85) in patients with T2D and established CVD. Based on the EMPA‐REG OUTCOME CVOT, empagliflozin was approved for an additional indication of reducing the risk of CV death in this population. The DPP‐4i sitagliptin has shown reductions in HbA1c and SBP, a neutral effect on weight and CV outcomes, but no significant reduction in the 3P‐MACE composite (HR: 0.99, 95% CI: 0.89‐1.10), CV death (HR: 1.03, 95% CI: 0.89‐1.19) or HHF (HR: 1.00, 95% CI: 0.83‐1.20) between the treatment and placebo arms in the TECOS CVOT. 6
Despite ADA treatment guidelines, 3 the use of DPP‐4is (12.5% in a large US cohort from 2016 to 2018) 7 with no demonstrated CVD benefit continues to be higher than SGLT‐2is and GLP‐1RAs (9.0% and 7.9%, respectively), 7 agents with well‐documented CV benefits. Another study showed that drug choice in US patients with T2D did not vary by presence of CVD. 8 More closely aligning US prescribing practices with current treatment guidelines may lead to efficient utilization of healthcare resources. Although trials have shown clinical benefits of empagliflozin and sitagliptin, the long‐term economic impact of alternative treatment pathways is unknown to healthcare payers.
The objective of this study was to estimate the long‐term cost‐effectiveness of sequential therapy of empagliflozin versus sitagliptin to metformin for treatment in patients with T2D with or without CVD from the perspective of the US payer. Patients with T2D on metformin therapy who add empagliflozin as second‐line followed by third‐line sitagliptin were compared with a pathway where patients with T2D on metformin add sitagliptin as second‐line followed by third‐line empagliflozin.
2. MATERIALS AND METHODS
2.1. Model overview
An individual patient‐level simulation model was implemented in Microsoft Excel using the discretely integrated condition event platform (see Appendix Figure SA1 and text in the supporting information). 9 The model simulated US patients with T2D with or without CVD on metformin plus empagliflozin or metformin plus sitagliptin under two treatment escalation pathways (Figure 1). For both pathways, patients could add third‐line therapy (sitagliptin or empagliflozin, respectively) and eventually fourth‐line therapy as insulin. This strategy allowed for counterfactual simulation pathways, where the main difference was the drug added as second‐line therapy.
FIGURE 1.
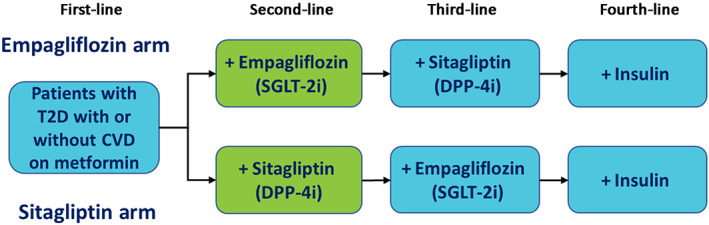
Modelled treatment pathways. Patients are on dual therapy at the beginning of the simulation model. CVD, cardiovascular disease; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor; T2D, type 2 diabetes
Patients could experience diabetes‐related complications and adverse events (AEs) when treated with different therapies, until death. Modelled diabetes‐related complications in patients with and without CVD correspond to endpoints from the EMPA‐REG OUTCOME CVOT and risk equations from the United Kingdom Prospective Diabetes Study Outcomes Model 2 (UKPDS‐OM2), respectively. 10 , 11 The occurrence of diabetes‐related complications was assumed to change over the course of the simulation as patients experienced CV events (Figure 2). CV history was updated as patients experienced events over time in the model (Figure SA2). Both patients with or without CVD could experience a fatal event.
FIGURE 2.
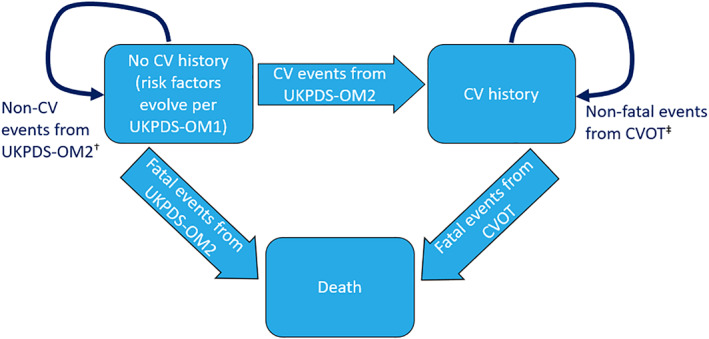
Disease progression. CV, cardiovascular; CVOT, CV outcomes trial; UKPDS, United Kingdom Prospective Diabetes Study; OM1, Outcomes Model 1; OM2, Outcomes Model 2. † UKPDS OM2 is an individual‐level state transition model with annual probability of events based on patient demographics, diabetes duration, biomarkers and history of diabetes‐related complications. ‡ An individual patient‐level, time‐to‐event approach was used to capture occurrence of each event based on event‐free survival curves with time‐dependent covariates
Cumulative events, life‐years (LYs), quality‐adjusted LYs (QALYs) and costs were accrued over a lifetime horizon for each patient. QALYs and costs were discounted at 3.0% per year.
2.2. Population
The modelled population represented US patients with T2D. Simulated patients had a profile of CV risk factors and other correlates that predict diabetes‐related complications with the UKPDS‐OM2 11 and EMPA‐REG OUTCOME CVOT 10 risk equations. Population characteristics were created using National Health and Nutrition Examination Survey data, 12 with supplementary data to complete the patient profiles (Appendix S1). 13 The mean age of the population was 61.4 (standard deviation [SD] 13.3) years, with a mean HbA1c of 9.4% (SD 3.5%), mean SBP of 144.8 (SD 27.1) mmHg, mean body mass index of 31.0 (SD 7.0) kg/m2, 50% female and 20% with CVD at baseline (full baseline characteristics are provided in Table SA1). CVD was defined as one or more previous MI, stroke, unstable angina (UA), multi‐vessel coronary artery disease (CAD), single‐vessel CAD or peripheral artery disease, aligned with the EMPA‐REG OUTCOME CVOT inclusion criteria. 5 Cohorts of 5000 patients with the same individual baseline characteristics were assigned to each comparison pathway.
2.3. Diabetes‐related complications and mortality
Simulated patients with or without CVD could experience an MI, stroke, HF, renal failure or CV death. Ischaemic heart disease (IHD), blindness, ulcer and amputation could occur in simulated patients without CVD, while UA, transient ischaemic attack (TIA), revascularization, macroalbuminuria and renal injury could occur in simulated patients with CVD. See definitions of the complications in Table SA2.
In patients with CVD at baseline or after they experience a relevant CV event during simulated follow‐up, published event‐free survival (EFS) curves from EMPA‐REG OUTCOME CVOT data were used. 10 Briefly, the EFS curves were derived by fitting the EMPA‐REG OUTCOME CVOT data to parametric distributions (e.g. Weibull, exponential) and conducting parametric proportional hazards regression analyses to predict event risks based on individual patient characteristics. 10 These equations rely on extrapolation of EMPA‐REG OUTCOME data on actual event rates, requiring no extrapolated changes in surrogate biomarker values (e.g. HbA1c). Therapeutic benefit on glycaemic control was not directly modelled in these patients, and risk of complications was projected independently from the ongoing progression of diabetes pathophysiology. The shape of the parametric survival functions was assumed to implicitly capture HbA1c and other evolving risk factors that contribute to changing event rates over time. The EMPA‐REG OUTCOME CVOT equations captured recurrent CV events, but renal events did not recur. Risks of diabetes‐related complications were predicted from patient baseline characteristics for those entering the model with CVD or characteristics at development of CVD and time‐dependent variables. Non‐CV death was based on US life tables. 14
The published UKPDS‐OM2 equations 11 applied in simulated patients without CVD predicted first events. For amputation, an equation to predict a second event was included. Death was estimated using four equations based on whether complications occurred, and which complications happened in the current annual cycle. One or more complications or death could occur annually. Likelihood of complications was based on patients' baseline demographic and clinical characteristics, evolving risk factors (e.g. age, HbA1c, SBP, smoking status, diabetes duration) and history of diabetes‐related complications. Time‐varying characteristics evolved per UKPDS Outcomes Model 1 longitudinal equations. 15 Occurrence of non‐fatal MI, non‐fatal stroke and IHD changed patients' CVD status. Patients' clinical history was preserved during this transition (Appendix S1).
Model predictions in patients without and with CVD were validated against the UKPDS‐OM2 study and EMPA‐REG OUTCOME CVOT data, respectively. Predicted 1‐year and 10‐year event probabilities were compared with those published in the UKPDS‐OM2 study. Outcomes predicted by applying the EMPA‐REG OUTCOME equations in the model over the 3‐year CVOT duration reproduced the within‐trial outcomes.
2.4. Treatment inputs
Timing of initiation of a new line of therapy was obtained from published trends in antidiabetes drug use in the US by Montvida et al. 16 This recent study used a large sample (n = 1 023 340) of adults with T2D who initiated an antidiabetes drug (including DPP‐4i and SGLT‐2i) from 2005 to 2016 in the US Centricity Electronic Medical Records database, which records diabetes prevalence (based on diagnostic codes) similar to the national prevalence. 16 A mean rate of initiations for third‐line (12.6 initiations per 100 person‐years) and fourth‐line (3.1 initiations per 100 person‐years) was applied for each treatment pathway. 16 Patients who added insulin were assumed to receive an appropriately titrated dose. Following treatment escalation, patients remained on therapy for life.
In patients without CVD, network meta‐analysis (NMA) of antidiabetic agents informed the treatment‐mediated changes in HbA1c, SBP and weight (Table SA3) in patients initiating empagliflozin and sitagliptin. 17 Efficacy of empagliflozin in patients with CVD was a covariate in each risk equation estimated from the EMPA‐REG OUTCOME CVOT data. 10 Relative efficacy data for sitagliptin from a published indirect treatment comparison (ITC) by Balijepalli et al. using published outcomes from the EMPA‐REG OUTCOME and TECOS CVOTs was applied to each equation. 18 An HR for empagliflozin versus sitagliptin on CV death (HR: 0.60; 95% CI: 0.46‐0.79) and HHF (HR: 0.65; 95% CI: 0.47‐0.90) was obtained. 18 Occurrence of other modelled complications with sitagliptin, where published outcomes from the TECOS trial were not available to inform the ITC, were assumed to resemble placebo from the EMPA‐REG OUTCOME CVOT. Patients who add on insulin were assumed to have the same complication risks they had prior to adding insulin.
AEs that were reported in at least 5% of patients using empagliflozin (urinary tract infection, genital mycotic infection) or sitagliptin (upper respiratory tract infection, nasopharyngitis and headache), per US prescribing labels, were modelled.
2.5. Health‐related quality of life
The impact of diabetes‐related complications and AEs on quality of life (QoL) was captured by applying event disutilities in the year a complication occurred, and where appropriate, in each subsequent year after the event. Utility values were based on published studies primarily of patients with T2D (Table SA4). 19 , 20 , 21 , 22 , 23 , 24 As patients accumulated multiple events, a correction factor estimated by Sullivan and Ghushchyan 22 adjusted patient utility upward to avoid overestimating the degree of QoL impairment in patients with a history of at least two complications.
2.6. Costs and perspective
Direct costs were accounted in 2018 US dollars from the US healthcare payer perspective (Tables SA5‐SA7). Costs were inflated from prior years, where applicable, using the medical component of the US consumer price index. 25 The overall population costs were estimated by applying the baseline age distribution (55% aged <65 years, 45% aged ≥65 years) to costs for a commercially insured and Medicare payer. Refer to Appendix S1 for additional details.
2.7. Sensitivity analyses
Parameter and statistical uncertainty were assessed in deterministic sensitivity analyses (DSA) and a probabilistic sensitivity analysis (PSA; see Appendix S1). Additional scenarios were considered. First, the model was run in two subpopulations: patients without CVD and patients with CVD at baseline. Next, scenarios considered differential payment rates by commercial insurance payers and Medicare payers. Finally, shorter time horizons (1, 3, 5 and 10 years) were explored in the overall population and CVD subpopulation.
3. RESULTS
The base‐case results (Table 1) over a lifetime horizon showed that empagliflozin compared with sitagliptin as second‐line treatment yielded an increase of 0.37 LYs and 0.19 QALYs. Clinical benefits resulted from a reduced cumulative incidence of diabetes‐related complications in the empagliflozin arm over patients' lifetimes (Figure 3). Empagliflozin was associated with longer CVD‐free survival (by 0.07 years), and an 11% reduction in CV death in patients with CVD compared with sitagliptin. Lower or similar rates of complications were estimated across treatment pathways in patients without CVD; however, fewer MI, HHF, TIA, revascularization, macroalbuminuria, renal injury and renal failure events were simulated with second‐line empagliflozin versus sitagliptin in patients with CVD . See additional base case results in Table SA8 and Table SA9.
TABLE 1.
Base case and scenario analyses cost‐effectiveness results
| A. Base case | Second‐line empagliflozin | Second‐line sitagliptin | Incremental | ICER ($/QALY) |
|---|---|---|---|---|
| Undiscounted LYs | 15.65 | 15.28 | 0.37 | $6967 |
| CVD‐free LYs a | 11.77 | 11.70 | 0.07 | |
| Discounted QALYs | 8.85 | 8.66 | 0.19 | |
| Discounted cost | ||||
| Total cost | $89 436 | $88 118 | $1318 | |
| Drug acquisition cost | $56 708 | $54 613 | $2095 | |
| Clinical event cost | $32 728 | $33 505 | –$777 |
| Undiscounted LYs | Discounted QALYs | Discounted cost ($) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. Scenarios | Empagliflozin | Sitagliptin | Incremental | Empagliflozin | Sitagliptin | Incremental | Empagliflozin | Sitagliptin | Incremental | ICER ($/QALY) |
| Baseline CVD status | ||||||||||
| CVD subpopulation | 12.36 | 11.40 | 0.96 | 7.53 | 7.00 | 0.53 | $86 272 | $84 380 | $1892 | $3589 |
| Non‐CVD subpopulation | 16.85 | 16.61 | 0.24 | 9.40 | 9.29 | 0.11 | $92 265 | $90 924 | $1341 | $12 577 |
| Perspective | ||||||||||
| Commercial payer | 20.64 | 20.27 | 0.37 | 11.10 | 10.93 | 0.17 | $111 048 | $109 459 | $1589 | $9081 |
| Medicare payer | 9.49 | 9.15 | 0.34 | 6.10 | 5.90 | 0.20 | $63 674 | $62 750 | $924 | $4799 |
| Time horizon | ||||||||||
| 1‐y, overall population | 0.98 | 0.98 | 0.0009 | 0.78 | 0.77 | 0.01 | $4929 | $4875 | $54 | $69 752 |
| 3‐y, overall population | 2.83 | 2.81 | 0.02 | 2.16 | 2.15 | 0.01 | $14 765 | $14 822 | ‐$57 | Dominates |
| 5‐y, overall population | 4.51 | 4.48 | 0.03 | 3.35 | 3.33 | 0.02 | $24 484 | $24 557 | ‐$73 | Dominates |
| 10‐y, overall population | 8.03 | 7.92 | 0.11 | 5.58 | 5.50 | 0.08 | $45 591 | $45 547 | $44 | $557 |
| 1‐y, CVD subpopulation | 0.99 | 0.98 | 0.01 | 0.78 | 0.78 | 0.003 | $5290 | $5664 | ‐$374 | Dominates |
| 3‐y, CVD subpopulation | 2.85 | 2.82 | 0.03 | 2.18 | 2.15 | 0.03 | $16 581 | $17 645 | ‐$1064 | Dominates |
| 5‐y, CVD subpopulation | 4.54 | 4.43 | 0.11 | 3.37 | 3.28 | 0.09 | $27 866 | $29 377 | ‐$1511 | Dominates |
| 10‐y, CVD subpopulation | 7.92 | 7.54 | 0.38 | 5.49 | 5.23 | 0.26 | $52 640 | $54 113 | ‐$1473 | Dominates |
Abbreviations: CVD, cardiovascular disease; ICER, incremental cost‐effectiveness ratio; LYs, life‐years; QALYs, quality‐adjusted life‐years.
Among patients without CVD at baseline.
FIGURE 3.
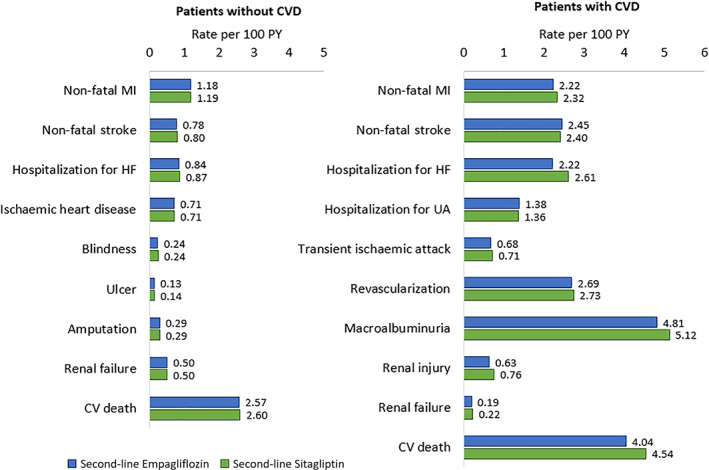
Estimated rates of lifetime diabetes‐related complications in the overall population. CVD, cardiovascular disease; HF, heart failure; MI, myocardial infarction; PY, patient‐years; UA, unstable angina
Evaluation of direct costs suggested that the total mean cost per patient was $1318 higher with second‐line empagliflozin versus sitagliptin over patients' lifetimes. This is largely because of higher drug costs ($2095/patient) with empagliflozin versus sitagliptin, partially related to increased survival. Empagliflozin was associated with reduced rates of diabetes‐related complications, which lowered clinical event costs ($777/patient). Clinical benefits and costs resulted in an incremental cost‐effectiveness ratio (ICER) of $6967/QALY, well below a $50 000‐$150 000 per QALY US willingness‐to‐pay (WTP) threshold range considered by the Institute for Clinical and Economic Review. 26
In all modelled scenarios of the DSA, second‐line empagliflozin was either cost‐effective or dominant (i.e. more effective with cost savings). The main ICER driver was variation in the rebate percentages applied to empagliflozin's wholesale acquisition cost (WAC) or sitagliptin's WAC (ranging from dominant to $21 364/QALY), still yielding an ICER below the lower‐range WTP threshold of $50 000/QALY. Variation in the ICER under all scenarios is shown in Figure 4.
FIGURE 4.
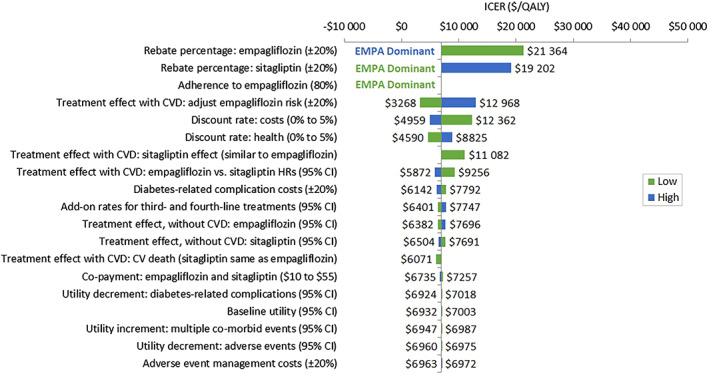
Tornado diagram of ICER ($/QALY). CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life‐year
The mean PSA ICER was consistent with the base case at $6865/QALY, with a relatively narrow 95% CI of $3004/QALY to $11 346/QALY. In all iterations, second‐line empagliflozin was cost‐effective against second‐line sitagliptin, even considering the most stringent WTP threshold ($50 000/QALY). A scatterplot depicting the variation in incremental costs and incremental QALYs for empagliflozin versus sitagliptin as second‐line treatment is shown in Figure SA3. Rates of diabetes‐related complications estimated in the PSA are provided in Table SA10.
Empagliflozin versus sitagliptin as second‐line resulted in greater benefits in patients with baseline CVD versus without CVD, with greater incremental costs, yielding ICERs of $3589/QALY versus $12 577/QALY, respectively (Table 1). Using payment rates by US commercial payers led to an ICER of $9081/QALY while the Medicare scenario produced an ICER of $4799/QALY. Restricting the analysis to shorter time horizons from 1 to 10 years in the overall population found that second‐line empagliflozin versus sitagliptin was either cost‐effective (ICERs ranged from $557/QALY to $69 752/QALY) or dominant. Second‐line empagliflozin versus sitagliptin was dominant in the CVD subpopulation over all short‐term time frames of 1 to 10 years.
4. DISCUSSION
This evaluation showed that for US payers, second‐line empagliflozin followed by addition of sitagliptin is a highly cost‐effective treatment for T2D compared with second‐line sitagliptin then empagliflozin in patients with or without CVD on metformin monotherapy, considering a $50 000‐$150 000 per QALY US WTP threshold range. 26 In the base case, scenarios varying the CV history population, and scenarios considering commercial payer and Medicare perspectives separately, second‐line empagliflozin improved QALYs and extended life expectancy with a modest lifetime cost increase. Together, these benefits yielded ICERs well below a strict WTP threshold of $50 000/QALY in the US. Over shorter timeframes of 1 to 10 years, second‐line empagliflozin versus sitagliptin was dominant in patients with T2D and CVD and was either cost‐effective at a US WTP of $100 000/QALY or dominant in overall T2D patients (with or without CVD). Exploratory DSA suggested that the ICER was robust to changes in the majority of input parameters. In a couple of modelled scenarios, second‐line empagliflozin was a dominant strategy over second‐line sitagliptin (i.e. QALY gains and cost savings) for treatment of T2D. The ICER was most sensitive to the rebate percentage applied to empagliflozin's WAC. With simultaneous variation in inputs according to an appropriate distribution of plausible values in PSA, the ICER was probable to remain within bounds considered cost‐effective in the US.
This study reports novel cost‐effectiveness results of second‐line empagliflozin versus sitagliptin, taking into account both treatment sequences and a broad T2D population. Previous treatment‐sequence cost‐effectiveness analyses in T2D have been reported, although not with sequences that compared empagliflozin with sitagliptin. 27 , 28 In a previous analysis that did compare empagliflozin plus standard of care (SoC) with sitagliptin plus SoC, sequences were not considered and only patients with established CVD were modelled. 29 Nonetheless, similar trends were observed in that study, as empagliflozin plus SoC was found to be cost‐effective compared with sitagliptin plus SoC (£6464/QALY). 29 In another study, although a comparison of empagliflozin and sitagliptin was not presented, the absolute cost and QALY results indicate that empagliflozin is cost‐effective versus sitagliptin (an ICER of $37 500/QALY was calculated based on the reported results). 30 In addition, the results of this analysis are consistent with comparisons of empagliflozin versus SoC (glucose‐lowering agents and therapies to manage CV risk factors as monotherapy or in combination) and competitors in patients with T2D and established CVD. 10 , 31 , 32 , 33 , 34 , 35
In patients with CVD, outcomes were based on CVOT data, providing the opportunity to use hard endpoint data to more fully capture empagliflozin cardioprotection. Another strength is that the model employed US‐specific population data, non‐CV death rates, treatment escalation rates, utilities and costs.
Results were dependent on key modelling assumptions. First, the model assumed changes in clinical biomarkers on rates of diabetes‐related complications estimated from the UKPDS data reflect rates observed in US clinical practice, and complication risks are applicable to a population without CVD. The applicability of the UKPDS‐OM2 equations to a US population without CVD is reasonable given the equations have been widely employed in economic analyses for diverse T2D populations globally, 36 and the majority of the UKPDS population (92%) did not have CVD. 37 Second, the treatment effect of sitagliptin on diabetes‐related complications other than HHF and CV death in patients with CVD was assumed to resemble placebo in the EMPA‐REG OUTCOME CVOT. This was assumed given the neutral effect of sitagliptin on the composite and individual CV endpoints in the TECOS CVOT, and because TECOS did not assess treatment benefit on renal function. However, even after applying the same treatment effect as empagliflozin for these outcomes resulted in an ICER of $11 082/QALY, still less than $50 000/QALY. Third, treatment escalation was based on published literature that considered HbA1c levels indirectly, rather than direct HbA1c evolution. Fourth, when patients with CVD initiated third‐line therapy, benefit was assumed to accumulate, that is, for outcomes improved with empagliflozin, the treatment effect of empagliflozin and sitagliptin in combination was assumed to be similar to the effect of empagliflozin. As described by van Baar et al., the net effect on the CV‐renal outcome of combining empagliflozin and sitagliptin together with metformin in clinical practice would be unlikely to be worse than treatment with empagliflozin plus metformin, based on evidence from the EMPA‐REG OUTCOME CVOT (i.e. subgroup analysis of DPP‐4i background therapy) and clinical evidence about DPP‐4i effects (i.e. modest effect on surrogate measures and no induced CV‐renal benefit). 38 Lastly, once a treatment was initiated, modelled patients remained on T2D therapy for life. Although as diabetes duration lengthens, patients may become contraindicated (e.g. because of renal dysfunction) or discontinue therapy for other reasons. This was a conservative assumption, partially attributable to empagliflozinʼs survival benefit, as discontinuation would reduce the higher pharmacy costs of empagliflozin compared with sitagliptin.
No direct, randomized comparison of clinical outcomes are available between empagliflozin and sitagliptin. Therefore, a published ITC 18 and NMA 17 were used to provide effectiveness inputs in patients with and without CVD, respectively. Although evidence synthesis methods have become widely used and accepted in health economic modelling when head‐to‐head clinical trials have not been conducted, this may be considered a limitation of the analysis. Furthermore, surrogate measures (e.g. HbA1c) were used to predict the occurrence of complications related to T2D in patients without CVD. Evidence suggests that these risk factors do not fully explain the cardioprotective benefits of empagliflozin, thus only part of this benefit may be captured in modelled patients without CVD. 39 Next, it was not possible to capture the same diabetes‐related complications in patients with and without CVD using the UKPDS‐OM2 and EMPA‐REG OUTCOME CVOT risk equations. For example, simulated patients were not at risk of blindness or amputation after developing CVD, although this is possible in real life. Next, although diabetic ketoacidosis (DKA) is a recognized complication of SGLT‐2i in T2D, there were few cases and no imbalance between treatment groups in placebo‐controlled clinical studies, thus DKA cases were not modelled. Neither did the model capture the hypoglycaemia risk of insulin. Incremental differences between treatment pathways are the focus of this pharmacoeconomic analysis and drive the ICER. AEs associated with insulin and metformin would be very similar across the compared pathways and marginally impact the model results. Last, results of these analyses are relevant to current practice in the US. Although regional costs can be tailored to the specific context of individual countries, generalizability to other healthcare settings beyond the US may be limited because of variations in treatment guidelines, and financial and organizational structures of healthcare in other markets.
A common approach in cost‐effectiveness analyses is to extrapolate long‐term outcomes from short‐term trial data to make predictions over a lifetime horizon, such as assuming that changes in surrogate measures or risks of diabetes‐related complications remain constant beyond the trial duration. However, simulation modelling is a feasible approach to efficiently synthesize multiple sources of evidence to forecast long‐term outcomes in the absence of long‐term clinical follow‐up data.
This study employed a pharmacoeconomic model to assess the cost‐effectiveness of empagliflozin versus sitagliptin as second‐line therapy after metformin. Results suggest that empagliflozin as second‐line treatment for T2D compared with second‐line sitagliptin leads to better health benefits for patients (lower rate of complications, higher LYs and improved QALYs) and is a highly cost‐effective treatment option from the perspective of US payers.
CONFLICT OF INTEREST
PP and SS are employees of Boehringer Ingelheim Pharmaceuticals Inc. OR and SB are salaried employees of Evidera, a research and consulting firm in the biopharmaceutical field. As such, they work with a variety of companies and are explicitly precluded from accepting any payment or honoraria directly from them for services rendered. During the study and article development, AK and VA‐R were employed by Evidera and are subject to the same restriction. Evidera received funding from Boehringer Ingelheim Pharmaceuticals Inc. for collaboration on this project and article. Boehringer Ingelheim Pharmaceuticals Inc. was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
AUTHOR CONTRIBUTIONS
OR wrote the manuscript and contributed to the model concept/development and analysis. AK contributed to the model concept/development and reviewed/edited the manuscript. SB contributed to the manuscript writing, model development and analysis. VA‐R contributed to the model development and reviewed/edited the manuscript. PP and SS contributed to the model concept and reviewed/edited the manuscript. The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE).
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
This study and article were funded by Boehringer Ingelheim Pharmaceuticals Inc. of Ridgefield, CT, USA.
Reifsnider O, Kansal A, Pimple P, Aponte‐Ribero V, Brand S, Shetty S. Cost‐effectiveness analysis of empagliflozin versus sitagliptin as second‐line therapy for treatment in patients with type 2 diabetes in the United States. Diabetes Obes Metab. 2021;23:791–799. 10.1111/dom.14268
Funding information This study and article were funded by Boehringer Ingelheim Pharmaceuticals Inc. of Ridgefield, CT, USA.
DATA AVAILABILITY STATEMENT
Our study data (which is based on de‐identified data from a clinical trial) is not in a repository, but is available upon reasonable request from the corresponding author.
REFERENCES
- 1. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association (ADA) . Standards of medical care in diabetes‐2019 abridged for primary care providers. Clin Diabetes. 2019;37(1):11‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haring HU, Merker L, Seewaldt‐Becker E, et al. Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2014;37(6):1650‐1659. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 6. Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232‐242. [DOI] [PubMed] [Google Scholar]
- 7. Arnold SV, de Lemos JA, Rosenson RS, et al. Use of guideline‐recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618‐620. [DOI] [PubMed] [Google Scholar]
- 8. Le P, Chaitoff A, Misra‐Hebert AD, Ye W, Herman WH, Rothberg MB. Use of antihyperglycemic medications in U.S. adults: an analysis of the National Health and Nutrition Examination Survey. Diabetes Care. 2020;43(6):1227‐1233. [DOI] [PubMed] [Google Scholar]
- 9. Caro JJ. Discretely integrated condition event (DICE) simulation for pharmacoeconomics. Pharmacoeconomics. 2016;34(7):665‐672. [DOI] [PubMed] [Google Scholar]
- 10. Kansal A, Reifsnider OS, Proskorovsky I, et al. Cost‐effectiveness analysis of empagliflozin treatment in people with type 2 diabetes and established cardiovascular disease in the EMPA‐REG OUTCOME trial. Diabet Med. 2019;36(11):1494‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925‐1933. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) . National Health and Nutrition Examination Survey data. 1999–2008. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed December 11, 2019.
- 13. Optum claims data in subjects with type 2 diabetes . 2019; https://www.optum.com/. Accessed December 11, 2019.
- 14. Arias E, Xu J. United States life tables, 2015. Natl Vital Stat Rep. 2018;67(7):1‐64. [PubMed] [Google Scholar]
- 15. Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747‐1759. [DOI] [PubMed] [Google Scholar]
- 16. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long‐term trends in antidiabetes drug usage in the U.S.: real‐world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 17. Mearns ES, Sobieraj DM, White CM, et al. Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta‐analysis. PLoS One. 2015;10(4):e0125879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balijepalli C, Shirali R, Kandaswamy P, et al. Cardiovascular safety of empagliflozin versus dipeptidyl peptidase‐4 (DPP‐4) inhibitors in type 2 diabetes: systematic literature review and indirect comparisons. Diabetes Ther. 2018;9(4):1491‐1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grandy S, Fox KM, Shield Study Group . Change in health status (EQ‐5D) over 5 years among individuals with and without type 2 diabetes mellitus in the SHIELD longitudinal study. Health Qual Life Outcomes. 2012;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindgren P, Graff J, Olsson AG, Pedersen TJ, Jonsson B, Ideal Trial Investigators . Cost‐effectiveness of high‐dose atorvastatin compared with regular dose simvastatin. Eur Heart J. 2007;28(12):1448‐1453. [DOI] [PubMed] [Google Scholar]
- 21. Sullivan PW, Ghushchyan V. Preference‐based EQ‐5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan PW, Ghushchyan VH. EQ‐5D scores for diabetes‐related comorbidities. Value Health. 2016;19(8):1002‐1008. [DOI] [PubMed] [Google Scholar]
- 23. van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ. The impact of pandemic influenza H1N1 on health‐related quality of life: a prospective population‐based study. PLoS One. 2011;6(3):e17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu R, Insinga RP, Golden W, Hu XH. EuroQol (EQ‐5D) health utility scores for patients with migraine. Qual Life Res. 2011;20(4):601‐608. [DOI] [PubMed] [Google Scholar]
- 25. Bureau of Labor Statistics . Medical Care Consumer Price Index 2018. http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed December 13, 2018.
- 26. Institute for Clinical and Economic Review (ICER) . Overview of the ICER value assessment framework and update for 2017‐2019. 2018. https://icer-review.org/wp-content/uploads/2018/03/ICER-value-assessment-framework-update-FINAL-062217.pdf. Accessed December 11, 2019.
- 27. Hung A, Jois B, Lugo A, Slejko JF. Cost‐effectiveness of diabetes treatment sequences to inform step therapy policies. Am J Manag Care. 2020;26(3):e76‐e83. [DOI] [PubMed] [Google Scholar]
- 28. Pawaskar M, Bilir SP, Kowal S, Gonzalez C, Rajpathak S, Davies G. Cost‐effectiveness of DPP‐4 inhibitor and SGLT2 inhibitor combination therapy for type 2 diabetes. Am J Manag Care. 2019;25(5):231‐238. [PubMed] [Google Scholar]
- 29. Ramos M, Foos V, Ustyugova A, Hau N, Gandhi P, Lamotte M. Cost‐effectiveness analysis of empagliflozin in comparison to sitagliptin and saxagliptin based on cardiovascular outcome trials in patients with type 2 diabetes and established cardiovascular disease. Diabetes Ther. 2019;10(6):2153‐2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rind D, Guzauskas G, Fazioli K, et al. Oral Semaglutide for Type 2 Diabetes: Effectiveness and Value. Evidence Report. 2019. https://icer-review.org/wp-content/uploads/2019/09/ICER_Diabetes_Evidence-Report_110119.pdf. Accessed September 29, 2020.
- 31. Gourzoulidis G, Tzanetakos C, Ioannidis I, et al. Cost‐effectiveness of empagliflozin for the treatment of patients with type 2 diabetes mellitus at increased cardiovascular risk in Greece. Clin Drug Investig. 2018;38(5):417‐426. [DOI] [PubMed] [Google Scholar]
- 32. Iannazzo S, Mannucci E, Reifsnider O, Maggioni AP. Cost‐effectiveness analysis of empagliflozin in the treatment of patients with type 2 diabetes and established cardiovascular disease in Italy, based on the results of the EMPA‐REG OUTCOME study. Farmeconomia Health Econ Ther Pathways. 2017;18:43‐53. [Google Scholar]
- 33. Kaku K, Haneda M, Sakamaki H, et al. Cost‐effectiveness analysis of Empagliflozin in Japan based on results from the Asian subpopulation in the EMPA‐REG OUTCOME trial. Clin Ther. 2019;41(10):2021‐2040. [DOI] [PubMed] [Google Scholar]
- 34. Kansal A, Reifsnider O, Lee J, et al. Cost‐effectiveness analysis of empagliflozin compared with canagliflozin or standard of care in patients with T2DM and established cardiovascular (CV) disease, poster presentation at a prior American Diabetes Association annual conference, thus the poster abstract number (1294‐P). Diabetes. 2018;67(suppl 1). Abstract. Poster 1294‐P abstract available at https://diabetes.diabetesjournals.org/content/67/Supplement_1/1294-P. [Google Scholar]
- 35. Mettam SR, Bajaj H, Kansal AR, Kandaswamy P. Cost effectiveness of empagliflozin in patients with T2DM and high CV risk in Canada. Abstract PDB52 at ISPOR 19th Annual European Congress, 29 October‐2 November 2016, Vienna, Austria. Value Health. 2016;19(7):A674. [Google Scholar]
- 36. Yi Y, Philips Z, Bergman G, Burslem K. Economic models in type 2 diabetes. Curr Med Res Opin. 2010;26(9):2105‐2118. [DOI] [PubMed] [Google Scholar]
- 37. Turner RC. The U.K. Prospective Diabetes Study. A review. Diabetes Care. 1998;21(suppl 3):C35‐C38. [DOI] [PubMed] [Google Scholar]
- 38. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, RG IJ, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41(8):1543‐1556. [DOI] [PubMed] [Google Scholar]
- 39. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41(2):356‐363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
Our study data (which is based on de‐identified data from a clinical trial) is not in a repository, but is available upon reasonable request from the corresponding author.


