Summary
Carotenoids are isoprenoid compounds synthesized by all photosynthetic and some non‐photosynthetic organisms. They are essential for photosynthesis and contribute to many other aspects of a plant's life. The oxidative breakdown of carotenoids gives rise to the formation of a diverse family of essential metabolites called apocarotenoids. This metabolic process either takes place spontaneously through reactive oxygen species or is catalyzed by enzymes generally belonging to the CAROTENOID CLEAVAGE DIOXYGENASE family. Apocarotenoids include the phytohormones abscisic acid and strigolactones (SLs), signaling molecules and growth regulators. Abscisic acid and SLs are vital in regulating plant growth, development and stress response. SLs are also an essential component in plants’ rhizospheric communication with symbionts and parasites. Other apocarotenoid small molecules, such as blumenols, mycorradicins, zaxinone, anchorene, β‐cyclocitral, β‐cyclogeranic acid, β‐ionone and loliolide, are involved in plant growth and development, and/or contribute to different processes, including arbuscular mycorrhiza symbiosis, abiotic stress response, plant–plant and plant–herbivore interactions and plastid retrograde signaling. There are also indications for the presence of structurally unidentified linear cis‐carotene‐derived apocarotenoids, which are presumed to modulate plastid biogenesis and leaf morphology, among other developmental processes. Here, we provide an overview on the biology of old, recently discovered and supposed plant apocarotenoid signaling molecules, describing their biosynthesis, developmental and physiological functions, and role as a messenger in plant communication.
Keywords: abscisic acid, anchorene, apocarotenoids, β‐cyclocitral, β‐ionone, LCDAs, carotenoids, strigolactones, volatiles, zaxinone
Significance Statement
Apocarotenoids are carotenoid‐derived small molecules with regulatory functions, including cellular signaling, growth regulation and communication with surrounding organisms. Owing to these functions, apocarotenoids can influence plant physiology, development and plant–plant/plant–microorganisms interactions, making them a major focus of study in agriculture and fundamental research.
INTRODUCTION
Carotenoids are C40 isoprenoids with well‐described functions in photosynthesis, pollination, photoprotection and hormone biosynthesis (Krinsky, 1989; Hirschberg, 2001; Dall'Osto et al., 2007; Han et al., 2008). They are synthesized in all photosynthetic organisms and several non‐photosynthetic fungi and bacteria (Ruiz‐Sola and Rodriguez‐Concepcion, 2012; Moise et al., 2014; Nisar et al., 2015; Zheng et al., 2020). Plant carotenoid biosynthesis (Figure 1) takes place in plastids and relies on the methylerythritol phosphate pathway that provides the building blocks (isopentenyldiphosphate and dimethylallyl diphosphate) for different isoprenoid pathways, which also include gibberellin, chlorophyll side chain and tocopherol biosynthesis (Rodriguez‐Concepcion, 2010). Carotenoids, as well as these isoprenoids, originate from geranylgeranyl diphosphate that is formed by repeated condensation reactions of these building blocks (Figure 1). The initial step in carotenoid biosynthesis is the formation of 15‐cis‐phytoene, which is synthesized by the PHYTOENE SYNTHASE (PSY) that catalyzes the condensation of two geranylgeranyl diphosphate molecules (Dogbo et al., 1988). This is followed by two‐desaturation reactions mediated by the PHYTOENE DESATURASE (PDS), which lead to 9,15,9′‐tri‐cis‐ζ‐carotene via 9,15‐di‐cis‐phytofluene (Li et al., 1996). The ζ‐CAROTENE ISOMERASE (Z‐ISO) converts 9,15,9′‐tri‐cis‐ζ‐carotene to 9,9′‐di‐cis‐ζ‐carotene, which can be partially replaced by photoisomerization (Li et al., 2007; Chen et al., 2010). Next, the 9,9′‐di‐cis‐ζ‐carotene undergoes an additional two‐step desaturation catalyzed by the ζ‐CAROTENE DESATURASE (ZDS) that yields 7,9,9′‐tri‐cis‐neurosporene followed by 7,9,9′,7′‐tetra‐cis‐lycopene (pro‐lycopene), respectively (Bartley et al., 1999; Matthews et al., 2003). Finally, CRTISO catalyzes the conversion of pro‐lycopene to all‐trans‐lycopene. Similar to the tri‐cis to di‐cis conversion of ζ‐carotene, isomerization of pro‐lycopene to its all‐trans form can be partially complemented by photoisomerization in photosynthetic tissues (Isaacson et al., 2002; Park et al., 2002). In most prokaryotes and fungi, the steps leading from 15‐cis‐phytoene to all‐trans‐lycopene are mediated by a single polypeptide, CrtI, which replaces the four plant enzymes (Schaub et al., 2012). This capability explains the common employment of CrtI in generating transgenic, carotenoid‐biofortified crops (Zheng et al., 2020). Following the linear all‐trans‐lycopene, the pathway diverges into two main branches starting with the bicyclic α‐ or β‐carotenes and leading to the corresponding oxygen‐containing carotenoids (i.e., xanthophylls; Figure 1). β‐Carotene serves as the precursor of strigolactones (SLs), an important phytohormone involved in plant growth and development (Al‐Babili and Bouwmeester, 2015; Beltran and Stange, 2016; Shen et al., 2017). On the one hand, LYCOPENE β‐ and ɛ‐CYCLASES (LCY band LCYe) convert lycopene into α‐carotene, which carries an α‐ and a β‐ionone ring, and is subsequently hydroxylated into lutein by the action of cytochrome P450 enzyme CYP97A and CYP97C (Figure 1). On the other hand, LCYb introduces two β‐ionone rings into all‐trans‐lycopene, which are hydroxylated by NON‐HEME DIIRON OXIDASE (HYD)/CYP97A yielding zeaxanthin. The latter is the substrate of the ZEAXANTHIN EPOXIDASE that produces violaxanthin via antheraxanthin through sequential epoxidation. The reverse reactions back to zeaxanthin are catalyzed by the VIOLAXANTHIN DE‐EPOXIDASE (Dall'Osto et al., 2007; Neuman et al., 2014). Violaxanthin is then converted into neoxanthin (Neuman et al., 2014). Both violaxanthin and neoxanthin are precursors of the phytohormone abscisic acid (ABA; Figure 1). Plants utilize only the 9‐cis isoforms of epoxy‐xanthophyll precursors to produce ABA (Tan et al., 2003). The all‐trans‐ to 9‐cis‐ isomerization of violaxanthin and neoxanthin was poorly understood; however, a recent study suggested the involvement of ABA4 enzyme with the unknown cofactor(s) (Perreau et al., 2020).
Figure 1.
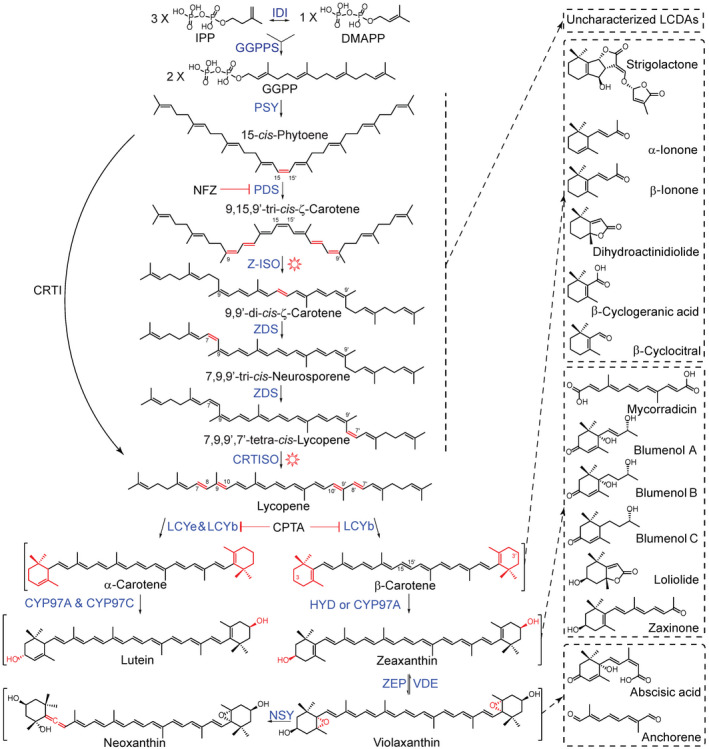
Carotenoid biosynthetic pathway and plant apocarotenoid regulatory metabolites.
Geranylgeranyl pyrophosphate (GGPP) is synthesized by the GGPP SYNTHASE (GGPPS) in plastids through the condensation of three isopentenyl diphosphates (IPP) and one dimethylallyl diphosphate (DMAPP). Sequentially, two GGPP molecules are used by the PHYTOENE SYNTHASE (PSY) to produce 15‐cis‐phytoene, which is an important rate‐limiting step in the synthesis of carotenoids. Then 15‐cis‐phytoene is converted into lycopene via multiple steps of desaturation and isomerization catalyzed by enzymes, including PHYTOENE DESATURASE (PDS), ζ‐CAROTENE ISOMERASE (Z‐ISO), ζ‐CAROTENE DESATURASE (ZDS), and CAROTENOID ISOMERASE (CRTISO). These multiple reactions are performed by a single enzyme, CRTI, in non‐photosynthetic bacteria. The enzymatic activity of Z‐ISO and CRTISO can be partially replaced by photoisomerization in plants. Cyclization of lycopene is catalyzed by LYCOPENE β‐ and ɛ‐CYCLASE (LCYb and LCYe) that form the β‐ or ε‐ionone rings of carotenes, respectively. β‐ and α‐Carotenes are subsequently converted into their downstream xanthophylls, such as zeaxanthin by NON‐HEME DIIRON OXIDASE (HYD)/CYP97A, and lutein by the action of CYP97A and CYP97C, respectively. Then zeaxanthin and violaxanthin can be interconverted in each other by the action of ZEAXANTHIN EPOXIDASE (ZEP) and VIOLAXANTHIN DE‐EPOXIDASE (VDE). Afterwards, violaxanthin is transformed into neoxanthin by NEOXANTHIN SYNTHASE (NSY). Modified bonds or moieties are colored red. Norflurazon (NFZ) and 2‐(4‐chlorophenylthio) ethyl‐diethylammonium chloride (CPTA) are inhibitors of carotenoid biosynthesis, affecting the enzymes PDS, LCYb and LCYe. Carotenoids can be metabolized into various apocarotenoids with important biological functions, via different processes. Representative bioactive apocarotenoids are boxed with a rectangular dash box. Sun image represents photoisomerization. CYP97, cytochrome P450 type carotenoid hydroxylase; IDI, isopentenyl diphosphate isomerase; IPP, isopentenyl diphosphate; LCDAs, linear cis‐carotene‐derived apocarotenoids; LCYb, LYCOPENE β‐CYCLASE; LCYe, LYCOPENE ɛ‐CYCLASE.
Owing to their electron‐rich, conjugated double bond system, carotenoids are susceptible to oxidation, causing the breakage of their backbone and leading to diverse carbonyl products generally called apocarotenoids. Initiated by reactive oxygen species (ROS), this process can occur without enzymatic catalysis in vitro as well as in planta. For instance, in vitro treatment of carotenoid solutions with photosensitizers generating singlet oxygen (1O2) leads to carotenoid degradation and the formation of a wide range of products, such as aldehydes, ketones, endoperoxides, epoxides and lactones (Stratton et al., 1993; Yamauchi et al., 1998; Fiedor et al., 2001; Bando et al., 2004; Fiedor et al., 2005; Ramel et al., 2012a). Interestingly, carotenoid peroxides themselves promote the oxidation of carotenoids as well as of other metabolite species, which makes them suitable for the propagation of oxidative stress signals in cells (Fiedor et al., 2005). Carotenoid backbone cleavage can also be catalyzed enzymatically by CAROTENOID CLEAVAGE DIOXYGENASEs (CCDs) (Al‐Babili and Bouwmeester, 2015; Beltran and Stange, 2016). CCDs are a ubiquitous family of non‐heme iron enzymes, which convert carotenoids into apocarotenoids acting as signaling molecules or hormone precursors (Giuliano et al., 2003; Hou et al., 2016; Felemban et al., 2019; Fiorilli et al., 2019; Wang et al., 2020b). In Arabidopsis, the CCD family is comprised of nine members, including five 9‐cis‐EPOXYCAROTENOID DIOXYGENASEs (NCED2, NCED3, NCED5, NCED6 and NCED9) and four CCDs (CCD1, CCD4, CCD7 and CCD8) (Tan et al., 2003; Sui et al., 2013). NCEDs are involved in ABA biosynthesis (Schwartz et al., 1997). CCD1 is involved in the cleavage of several carotenoids and apocarotenoids at different positions along their carbon structure (Schwartz et al., 2001; Vogel et al., 2008; Ilg et al., 2009; Ilg et al., 2014) leading to the production of volatiles responsible for flavor and aroma in various species, and dialdehydes with different chain lengths. CCD4 cleaves carotenoids either at the C7–C8 double bond in cryptoxanthin and zeaxanthin or at the C9–C10 double bond in bicyclic carotenoids (Rubio‐Moraga et al., 2014; Bruno et al., 2015; Bruno et al., 2016). In citrus, CCD4 is involved in carotenoid turnover in different tissues and in the production of citraurin (Pan et al., 2012). CCD7 and CCD8 are involved in SL biosynthesis by cleaving 9‐cis‐β‐carotene and converting 9‐cis‐β‐apo‐10′‐carotenal, respectively (see SL functions and biosynthesis and Figure 3) (Alder et al., 2012; Abe et al., 2014; Bruno et al., 2014; Zhang et al., 2014; Haider et al., 2018). Recently, other types of CCDs were reported, including CCD2 in Crocus species, and ZAXINONE SYNTHASE (ZAS), which represents an overlooked CCD clade (Frusciante et al., 2014; Ahrazem et al., 2016; Wang et al., 2019; Zhong et al., 2020).
Figure 3.
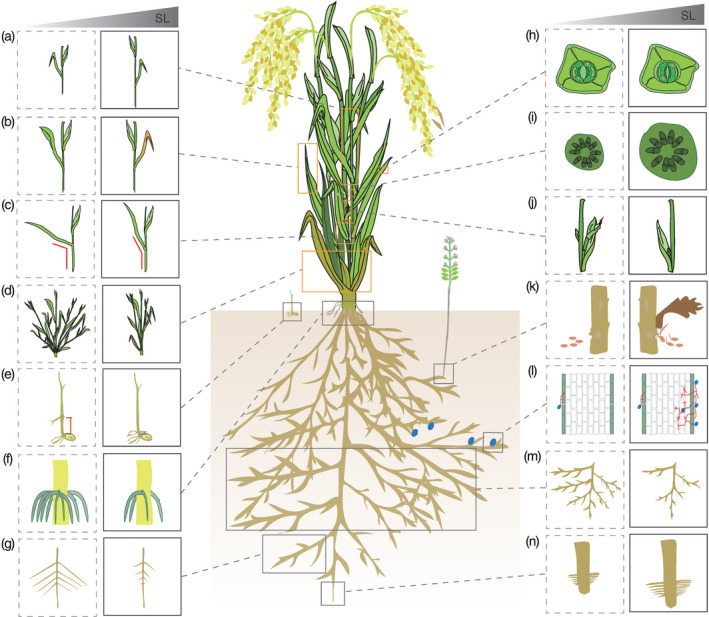
Functions of strigolactones (SLs) in plant development and plant‐rhizospheric communication.
SLs modulate many different plant growth and developmental processes, including (a) internode growth, (b) leaf senescence, (c) leaf angle, (d) tillering and tiller angle, (e) mesocotyl elongation, (f) adventitious roots formation, (g) secondary lateral root formation, (h) stomatal closure, (i) stem thickness increase and secondary growth, (j) axillary buds outgrowth, (k) parasitic seeds germination, (l) symbiotic interactions of roots with arbuscular mycorrhiza fungi, (m) lateral roots of formation and (n) root hair elongation and primary root growth.
Both enzymatic and non‐enzymatic oxidation processes may contribute to the formation of apocarotenoid signaling (ACS) molecules. For instance, the volatile β‐cyclocitral (β‐cc) is formed from β‐carotene by some CCDs, for example, the citrus fruit CCD4b, lipoxygenases, such as the tomato TomLocX (Gao et al., 2019), and, particularly under high‐light conditions, by 1O2 in photosynthetic tissues (Felemban et al., 2019). β‐cc is involved in 1O2 signaling, high light, drought and salt tolerance, and acts as a root growth regulator (Dabbagh et al., 1990; D'Alessandro et al., 2019; Dickinson et al., 2019). Furthermore, the oxidation of β‐cc leads to β‐cyclogeranic acid (or β‐cyclocitric acid [β‐ccA]; Figure 1), an apocarotenoid conferring drought tolerance in plants (D'Alessandro et al., 2019). Enzymatic and non‐enzymatic oxidation of β‐carotene also gives rise to other volatile compounds (Figure 1), including β‐ionone and dihydroactinidiolide (dhA) that are involved in a plant–herbivore interaction and 1O2 signaling and photoacclimation, respectively (Wei et al., 2011; Shumbe et al., 2014). The action of other CCDs on zeaxanthin (or lutein) led to the production of apocarotenoids (Figure 1) involved in arbuscular mycorrhiza (AM) symbiosis (i.e., mycorradicin, blumenols) (Lopez‐Raez et al., 2015; Wang et al., 2018), plant–plant and plant–herbivore interaction (i.e., loliolide) (Kong et al., 2018; Murata et al., 2019b), and hormone metabolism and growth regulation (i.e., zaxinone) (Wang et al., 2019; Ablazov et al., 2020). In addition, cleavage of violaxanthin (and probably all carotenoids downstream of ζ‐carotene) produces anchorene (Figure 1), a diapocarotenoid involved in growth stimulation of anchor roots (ANR; Jia et al., 2019). Here, we summarize important aspects of ABA and SLs metabolism, and highlight recently discovered apocarotenoids and linear cis‐carotene‐derived apocarotenoids (LCDAs) influencing plant growth, development and metabolism.
ABA FUNCTIONS AND BIOSYNTHESIS
ABA has been extensively studied over the last few decades due to its primary importance for fundamental plant science and its essential role in the coordination of agronomically important traits, such as root and shoot development, hypocotyl elongation, and fruit development and ripening (see LCDA‐associated phenotypes might be linked with the lack of downstream apocarotenoids) (Galpaz et al., 2008; Felemban et al., 2019). ABA is also necessary to generate and coordinate the plant’s response against abiotic and biotic stress factors (i.e., drought, salt and pathogens) through synergistic and antagonistic interactions with other hormones (i.e., gibberellins, ethylene and auxins) (De Vleesschauwer et al., 2010). A recent study showed that enhanced ABA and gibberellin content accumulation through the expression of the Daucus carota (carrot) LYCOPENE β‐CYCLASE1 (DcLCYb1) leads to increased plant yield and enhanced photosynthetic efficiency in tobacco (Moreno et al., 2020). ABA‐mediated coordination of the ripening process determines the harvest‐time and shelf‐life of horticulturally important crop plants. The enhanced ABA accumulation through LCYb overexpression leads to an extended shelf‐life of the tomato fruit (Diretto et al., 2020). The ABA‐mediated mechanism of stomatal closure is essential to control leaf transpiration (Malcheska et al., 2017) and to generate an immune response upon the perception of pathogen‐associated molecular patterns (Lim et al., 2015). High levels of ABA trigger ROS generation, which leads to oxidative stress followed by leaf senescence and cell death (Figure 2a) (An et al., 2019; Kim et al., 2019).
Figure 2.
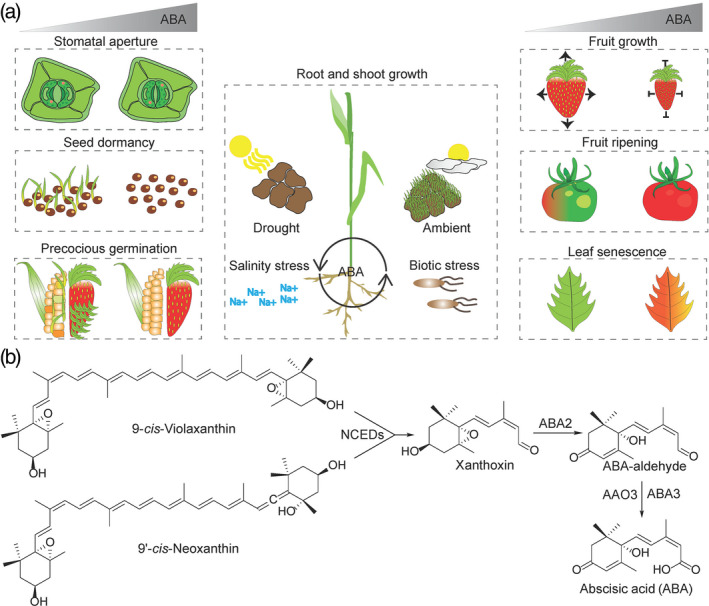
Abscisic acid (ABA) biosynthesis and functions in plants.
(a) ABA modulates plant growth and development. Altered ABA abundance modulates the plant root and shoot growth during biotic and abiotic stress conditions.
(b) ABA is a phytohormone synthesized by 9‐cis‐epoxycarotenoid dioxygenase (NCED)‐mediated cleavage of epoxycarotenoid precursors, 9‐cis‐violaxanthin and 9′‐cis‐neoxanthin. AAO3, ABSCISIC ALDEHYDE OXIDASE; ABA2;3, ABA‐DEFICIENT 2;3.
ABA biosynthesis starts with the NCED‐mediated enzymatic cleavage of 9‐cis‐violaxanthin or 9′‐cis‐neoxanthin (9‐cis‐epoxy‐xanthophylls) into xanthoxin (C15) and the corresponding C25‐apocarotenoid in plastids (Figure 2b) (Qin and Zeevaart, 1999; Tan et al., 2003). Xanthoxin is then transported to the cytosol for subsequent modifications to form ABA (Finkelstein, 2013). The short‐chain alcohol dehydrogenase, ABA‐DEFICIENT 2 (ABA2), converts xanthoxin to abscisic aldehyde, which is oxidized by ABSCISIC ALDEHYDE OXIDASE to ABA (Schwartz et al., 1997). The ABSCISIC ALDEHYDE OXIDASE catalytic activity requires a molybdenum cofactor supplied by sulfurase ABA‐DEFICIENT 3 (ABA3) (Seo et al., 2000; Li et al., 2013). ABA3 is also shown to contribute to the tolerance against oxidative stress in Arabidopsis in both an ABA‐dependent and an ABA‐independent manner (Watanabe et al., 2018). ABA can be further metabolized by some other enzymes to generate a relatively unstable isomer 8′‐OH‐ABA (Saito et al., 2004), and other modified forms of ABA, including catabolites, such as phaseic acid (PA), dihydrophaseic acid (DPA) and DPA‐4‐O‐β‐d‐glucoside (DPAG) (Cutler and Krochko, 1999). In a recent study, PA is shown to interact with some of the ABA receptors (PYLs), suggesting that PA functions as a phytohormone with relatively lower activity (Weng et al., 2016). Plants can store the esterified form of ABA, ABA glucosyl ester, in vacuoles (Lee et al., 2006; Xu et al., 2012). The ABA‐glucose esters can be hydrolyzed by β‐glucosidase BGLU18 to liberate ABA in case of immediate demand under drought stress conditions (Wade et al., 1999; Watanabe et al., 2014). ABA is involved, directly or indirectly, in several regulatory processes to modulate development, growth and stress response throughout the life cycle of plants. Here, we briefly explained the biosynthesis and function of ABA in plants. Further information describing the recent advances in ABA research can be found elsewhere (Felemban et al., 2019; Chen et al., 2020; Wang et al., 2020b).
SL FUNCTIONS AND BIOSYNTHESIS
In the last two decades, SLs have increasingly attracted the attention of biologists, in particular plant scientists, due to their versatile functions as rhizospheric, interspecific signaling molecules and plant hormones regulating development and adaptation to environmental changes (Brewer et al., 2013; Zwanenburg and Pospisil, 2013; Seto and Yamaguchi, 2014; Smith and Li, 2014; Waldie et al., 2014; Waters et al., 2017; Jia et al., 2018; Lanfranco et al., 2018; Yao et al., 2018; Burger and Chory, 2020). SLs were discovered in root exudates as germination stimulants of parasitic root weeds, and were shown to induce hyphal branching in symbiotic AM fungi (AMF; Cook et al., 1966; Akiyama et al., 2005). Additionally, SLs determine several aspects of plant physiology and shape plant architecture according to nutrient availability (Figure 3). For instance, Arabidopsis, rice, and pea SL‐deficient and ‐insensitive mutants showed a higher degree of shoot branching/tillering than the wild type (Gomez‐Roldan et al., 2008; Umehara et al., 2008). Furthermore, SLs are involved in many other shoot‐related developmental processes such as regulation of rice tiller angle by attenuating shoot gravitropism (Sang et al., 2014), promotion of shoot secondary growth, elongation of internodes (Agusti et al., 2011; de Saint et al., 2013), inhibition of hypocotyl and mesocotyl growth (Hu et al., 2010; Tsuchiya et al., 2010; Hu et al., 2014; Jia et al., 2014; Sun et al., 2018; Wang et al., 2020c), induction of leaf senescence (Snowden et al., 2005; Yamada et al., 2014) and decreasing the rice leaf angle in response to nutrient deficiencies (Sun et al., 2014; Shindo et al., 2020). They also play key roles in determining root architecture. For instance, SL‐deficient and ‐insensitive mutants have shorter primary roots and root hairs, shorter crown roots, higher secondary lateral roots (LRs), a higher density of LRs, and more adventitious roots compared with the wild type (Guan et al., 2012; Kohlen et al., 2012; Rasmussen et al., 2012a; Rasmussen et al., 2012b; Sun et al., 2019). SLs are utilized to adapt plants against nutrient deficiency (e.g., shortage in phosphate supply) through modulating root and shoot shape (Sun et al., 2014; Al‐Babili and Bouwmeester, 2015; Matthys et al., 2016). In addition, SLs modulate Arabidopsis seed germination (Tsuchiya et al., 2010; Toh et al., 2012) and play essential roles in plant development in the moss Physcomitrella patens (Proust et al., 2011; Hoffmann et al., 2014; Decker et al., 2017). Moreover, SLs trigger stomatal closure through an ABA‐independent pathway (Lv et al., 2018) and enhance plant resistance to abiotic and biotic stresses (Ha et al., 2014; Torres‐Vera et al., 2014; Lopez‐Raez et al., 2017; Nasir et al., 2019).
Natural SLs (Figure 4) are characterized by a butenolide ring (D ring) linked through an enol–ether bridge to a second moiety that consists of a tricyclic lactone (ABC ring) in the case of canonical SLs or a less defined structure in non‐canonical ones (Al‐Babili and Bouwmeester, 2015; Jia et al., 2018). Canonical SLs are further divided intostrigol‐ and orobanchol‐type, according to the stereochemistry of asymmetric carbon atoms at C3a and C8b: C3a R and C8b S configuration in strigol‐type SLs, including 5‐deoxystrigol (5DS, 18) and its derivatives, such as strigol (19), strigyl acetate (20), strigone (21), sorgolactone (22), sorgomol (23), ent‐2′‐epi‐orobanchol (24) and ent‐2′‐epi‐orobanchol acetate (25), and C3a S and C8b R configuration in orobanchol‐type SLs such as 4‐deoxyorobanchol (4DO, 26) and 4DO derivatives, including orobanchol (27), 7‐hydroxyorobanchol (28), orobanchyl acetate (29), 7‐OXO‐orobanchol (30), 7‐hydroxyorobanchyl acetate (31), 7‐OXO‐orobanchyl acetate (32), fabacol (33), fabacyl acetate (34), solanacol (35) and solanacyl acetate (36) (Yokota et al., 1998; Xie et al., 2008; Ueno et al., 2011; Kohlen et al., 2012; Kisugi et al., 2013; Kohlen et al., 2013; Xie et al., 2013; Xie, 2016). Non‐canonical SLs with a β‐ionone ring (A ring), including carlactone (CL, 5), carlactonic acid (CLA, 6), hydroxyl CLs (i.e., 3‐OH‐CL [14] and 4‐OH‐CL [10]), hydroxyl CLA (i.e., 18‐OH‐CLA [11]), methyl carlactonoate (MeCLA, 7) and its derivatives (i.e., 18‐OH‐MeCLA [12], 1″‐OH‐MeCLA [8], and heliolactone [9]) have been identified in plants (Baz et al., 2018; Iseki et al., 2018; Mori et al., 2020). In addition, plants produce several non‐canonical SLs with higher structural complexity, for example, Avenaol (17), Lotuslactone (13), Zealactone (15) and Zeapyranolactone (16) (Charnikhova et al., 2017, 2018).
Figure 4.
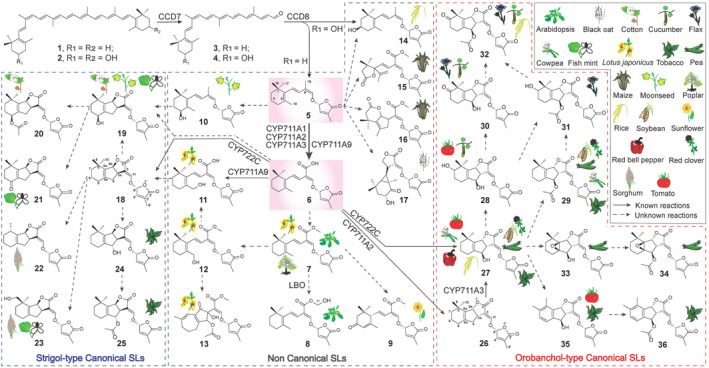
Strigolactone (SL) biosynthesis and structural diversity.
SL biosynthesis starts with isomerization of all‐trans‐ into 9‐cis‐β‐carotene (1) that is cleaved by CCD7 at the C9′–C10′ double bond, yielding 9‐cis‐β‐apo‐10′‐carotenal (3) and β‐ionone. The former is further converted by CCD8 into carlactone (5), a central intermediate of SL biosynthesis (Alder et al., 2012). 3‐OH‐carlactone (14), a presumed precursor of unidentified SLs, is formed from 9‐cis‐zeaxanthin (2) also by CCD7 and CCD8 (Bruno et al., 2014; Baz et al., 2018). Other enzymes, such as MAX1 and lateral branching oxidoreductase, further catalyze the post‐modification of carlactone (5), such as hydroxylation and oxidation, leading to divergent SLs, including canonical and non‐canonical SLs, which differ among various species (Jia et al., 2018; Yoneyama et al., 2020). 1, 9‐cis‐β‐Carotene; 2, 9‐cis‐Zeaxanthin; 3, 9‐cis‐β‐Apo‐10′‐carotenal; 4, 3‐OH‐9‐cis‐β‐Apo‐10′‐carotenal; 5, Carlactone; 6, Carlactonic acid; 7, Methyl carlactonoate; 8, 1′′‐OH‐methyl carlactonoate; 9, Heliolactone; 10, 4‐OH‐Carlactone; 11, 18‐OH‐Carlactonic acid; 12, 18‐OH‐Methyl carlactonoate; 13, Lotuslactone; 14, 3‐OH‐Carlactone; 15, Zealactone; 16, Zeapyranolactone; 17, Avenaol; 18, 5‐Deoxystrigol; 19, Strigol; 20, Strigyl acetate; 21, Strigon; 22, Sorgolactone; 23, Sorgomol; 24, ent‐2′‐epi‐Orobanchol; 25, ent‐2′‐epi‐Orobanchyl acetate; 26, 4‐Deoxyorobanchol; 27, Orobanchol; 28, 7‐OH‐Orobanchol; 29, Orobanchyl acetate, 30, 7‐OXO‐Orobanchol; 31, 7‐OH‐Orobanchyl acetate; 32, 7‐OXO‐Orobanchyl acetate; 33, Fabacol; 34, Fabacyl acetate; 35, Solanacol; 36, Solanacyl acetate; CCD7, carotenoid cleavage dioxygenase 7; CCD8, carotenoid cleavage dioxygenase 8; MAX1, more axillary growth 1; LBO, lateral branching oxidoreductase.
SL biosynthesis starts with the reversible isomerization of all‐trans‐β‐carotene to 9‐cis‐β‐carotene (1) by the carotene isomerase DWARF27 (Alder et al., 2012; Bruno and Al‐Babili, 2016; Abuauf et al., 2018). The 9‐cis‐β‐carotene (1) is then cleaved by CCD7 at the C9′–C10′ double bond to yield 9‐cis‐β‐apo‐10′‐carotenal (3) and β‐ionone. The 9‐cis‐β‐apo‐10′‐carotenal (3) is subsequently converted by CCD8 into CL (5), a key center intermediate of SL biosynthesis, and ω‐OH‐(4‐CH3)‐heptanal (Alder et al., 2012; Bruno et al., 2017). The following modifications of CL (5), such as oxidation, yield different types of SLs, including canonical and non‐canonical ones (Jia et al., 2018). It was also shown that the two CCD enzymes sequentially convert 9‐cis‐zeaxanthin (2) into 3‐OH‐CL (14), which might be a precursor of yet unidentified SLs (Bruno et al., 2014; Baz et al., 2018). The oxidation of CL (5) is catalyzed by Arabidopsis MORE AXILLARY GROWTH 1 (AtMAX1)/CYP711A1 to form CLA (6) (Abe et al., 2014), which is a common reaction catalyzed by CYP711 enzymes (Iseki et al., 2018). However, the biosynthetic pathway downstream of CLA (6) to divergent SLs, differ among various species. In Arabidopsis, CLA (6) can be converted by an unidentified methyltransferase into MeCLA (7), a substrate of the LATERAL BRANCHING OXIDOREDUCTASE that forms a metabolite with a mass of [MeCLA+ 16 Da] (Brewer et al., 2016). In Arabidopsis, [MeCLA+ 16 Da] was recently identified as hydroxymethyl carlactonoate (1″‐OH‐MeCLA [8]) (Yoneyama et al., 2020). MeCLA (7) is also the precursor of heliolactone (9), a non‐canonical SL formed in sunflower (Iseki et al., 2018). It was shown that Lotus japonicus MAX1 (LjMAX1/CYP711A9) expressed in yeast microsomes can convert CL (5) into 18‐OH‐CLA (11) via the intermediate CLA (6) (Mori et al., 2020). Moreover, feeding experiments indicated that 18‐OH‐MeCLA (12) is transformed to the canonical SL 5DS (18), directly or via 18‐OH‐CLA (11) as an intermediate, and to the non‐canonical SL lotuslactone (13) in L. japonicus (Zhang et al., 2014; Yoneyama et al., 2018). In rice, the MAX1 homolog Os900 (CYP711A2) catalyzes the repeated oxygenation and ring closures of CL to form 4DO (26) via CLA (6) (Zhang et al., 2014; Yoneyama et al., 2018). Then Os1400 (CYP711A3) catalyzes the subsequent hydroxylation of 4DO (26) to produce orobanchol (27) (Zhang et al., 2014; Yoneyama et al., 2018). CLA (6) is also a precursor of 5DS (18), the parent molecule of strigol‐type SLs, in sorghum and cotton, where it is converted into sorgomol (23), strigol (19) and strigyl acetate (20) (Iseki et al., 2018). The formation of 5DS (18) from CLA (6) in cotton is catalyzed by CYP722C, as shown by in vitro assays (Wakabayashi et al., 2020). In moonseed, strigol (19) can be produced either directly from CLA (6) or through a less understood route leading from CL (5) to strigol (19) via the intermediate 4‐OH‐CL (10), skipping the 5DS (18) formation (Iseki et al., 2018). Similarly, in cowpea, tomato, red bell pepper (Capsicum annuum), red clover and pea, exogenously applied rac‐CLA (6) is directly metabolized into orobanchol (27) without passing through 4DO (26) (Iseki et al., 2018; Ueno et al., 2018; Wakabayashi et al., 2019). The direct conversion of CLA (6) to orobanchol (27) is further illustrated by the functional characterization of cowpea VuCYP722C and tomato SlCYP722C in an in vitro assay and by using a tomato SlCYP722C mutant (Wakabayashi et al., 2019). These results show that the biosynthetic pathways of monohydroxylated SLs, such as strigol (19) and orobanchol (27), in some species can bypass the formation of the presumed parent SLs 4DO (26) and 5DS (18) (Ueno et al., 2018; Wakabayashi et al., 2019).
UNCHARACTERIZED LCDAs
As described above, plant carotenoid biosynthesis starts with the formation of 15‐cis‐phytoene and proceeds via several linear (acyclic) cis‐carotene intermediates that lead to all‐trans‐lycopene. The linear cis‐carotenes usually undergo fast conversion but can accumulate if there is a perturbation at the upstream biosynthesis pathway (Alagoz et al., 2018). Previous studies with cis‐carotene mutants demonstrated the presence of metabolic feedback signals influencing nuclear gene expression and plant phenotype. These metabolic signals were described as putative cis‐carotene cleavage products referred to as LCDAs. These supposed signaling molecules are among the less‐studied metabolites with a carotenoid origin. They have unknown chemical structures and a yet to be uncovered biosynthesis pathway, making them an exciting niche within the carotenoid field.
LCDAs as retrograde signals coordinating metabolic and phenotypic traits
The proposed LCDAs are involved in several metabolic and morphological processes, including the modulation of fruit, leaf and root pigmentation (Kachanovsky et al., 2012; Alvarez et al., 2016), plastid biogenesis at early seedling development (Cazzonelli et al., 2020), and determination of leaf morphology (Avendano‐Vazquez et al., 2014). Here, we explain the functions of LCDAs according to the reported metabolic feedback responses described in cis‐carotene mutants.
PSY is described as the “bottleneck” of the carotenoid biosynthesis pathway, which limits the overall metabolic flux, production and accumulation of downstream carotenoids (Alagoz et al., 2018). Epistatic interaction between the PSY and the downstream CRTISO genes was shown to influence the fruit pigmentation in tomato fruits (Kachanovsky et al., 2012). A recessive mutation in yellow‐flesh (r2997) tomato impairs the PSY1 gene expression and reduces the overall carotenoid accumulation. The tomato loss‐of‐function crtiso mutant, tangerine (t3002, t3406), hyper‐accumulates poly‐cis‐carotenes and has an enhanced PSY1 gene expression in fruits (Figure 5a). The fruits of tangerine yellow‐flesh (t × r) double mutants have a carotenoid profile similar to that of tangerine but an enhanced expression of PSY1. The epistatic interaction between yellow‐flesh and tangerine recovered the PSY1 expression; however, no epistatic relationship is observed in the ziso (zeta) psy double mutant (r2997/z2803). Thus, the recovery of PSY1 expression level in tangerine is attributed to a feedback response generated by cis‐carotenes synthesized between ZDS and CRTISO metabolic steps. An LCDA with 7,9,9′‐tri‐cis‐neurosporene (pro‐neurosporene) or 7,9,9′,7′‐tetra‐cis‐lycopene (pro‐lycopene or tetra‐cis‐lycopene) origin is proposed to arise through CCD‐mediated cleavage and to act as a feedback signal enhancing PSY1 gene expression and carotenogenesis in tomato fruits. Whether tomato CCDs can convert pro‐neurosporene or pro‐lycopene to biosynthesize an LCDA in vivo requires further investigation. Nevertheless, it was shown that tomato CCD1A and 1B enzymes cleave pro‐lycopene at different positions in vitro (Ilg et al., 2014).
Figure 5.
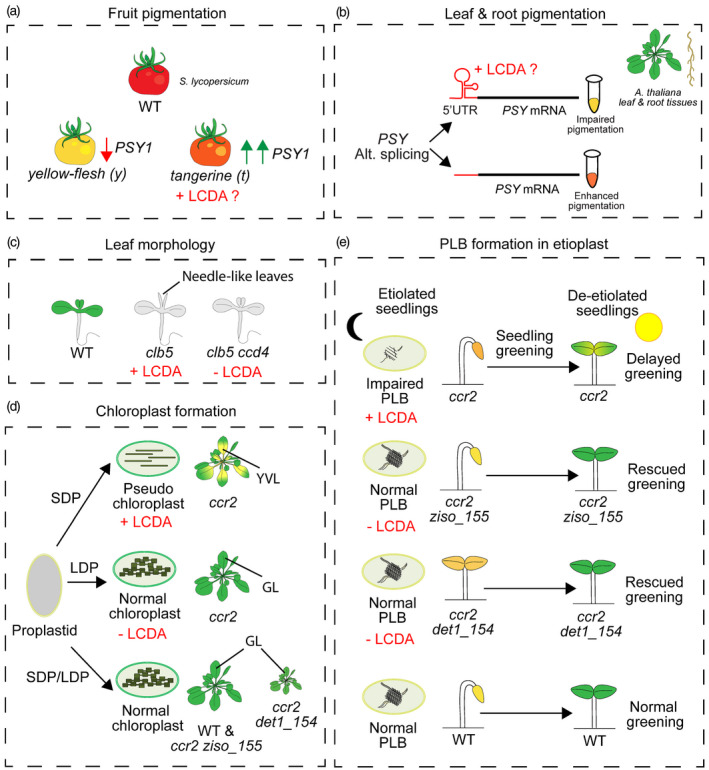
Uncharacterized linear cis‐carotene‐derived apocarotenoids (LCDAs) involved in the regulation of phenotypic processes, including organ pigmentation, leaf morphogenesis and chloroplast development in plants.
(a) An unknown LCDA generates a feedback response to enhance PHYTOENE SYNTHASE (PSY)1 gene expression in tomato tangerine fruits to modulate pigmentation. The yellow‐flesh mutant has impaired PSY1 expression, which results in reduced accumulation of carotenoids in fruits. Red and green arrows represent downregulation and upregulation of gene expression, respectively.
(b) An alternative splicing event of PSY modulates the carotenoid accumulation in leaves and roots of Arabidopsis.
(c) Longer PSY transcript with a regulatory hairpin motif supposedly interacts with a putative LCDA signal and impairs the carotenogenesis, whereas the shorter transcript variant enhances the carotenogenesis in case of an immediate demand.
(d) The clb5 mutation in Arabidopsis generates an unknown LCDA signal that induces needle‐like leaf development, reversible by the ccd4. An unknown LCDA promotes yellow virescent leaf (YVL) development in juvenile leaves of ccr2 grown under short‐day photoperiod (SDP). Long‐day photoperiod (LDP) and ziso_155 eliminate the LCDA and reverse the YVL phenotype in seedlings with ccr2 background.
(e) The same LCDA signal is found to impair the prolamellar body (PLB) formation, and so the cotyledon greening in dark‐grown etiolated ccr2 seedlings. The ziso_155 and det1_154 impair the accumulation of the PLB‐regulatory LCDA, thus, rescue the PLB formation and cotyledon greening phenotypes in etiolated seedlings. 5′ UTR, 5′ untranslated region; GL, green leaves; WT, wild type.
Besides transcriptional regulation, the PSY protein level in Arabidopsis post‐transcriptionally regulated by an alternative splicing event in the 5′ untranslated region (5′ UTR) of PSY transcripts (Alvarez et al., 2016). According to a described model, a hairpin loop motif in the secondary structure of the PSY mRNA 5′ UTR acts as a switch that responds to a putative ACS and mediates the PSY translation. The long PSY transcript variant that harbors the hairpin loop inhibits the translation and is necessary to maintain the carotenoid homeostasis. The shorter transcript variant missing the hairpin motif does not interact with the ACS and has a translation‐permissive secondary structure, thus, enhancing the translation in case of immediate demand for carotenoid production, such as in the dark to light transition (Figure 5b). The PSY‐regulatory ACS is described as a plastid‐derived apocarotenoid that can be produced by the cleavage of any carotenoid downstream of 15‐cis‐phytoene and 9,15‐di‐cis‐phytofluene. The identity of the ACS and its interaction with the PSY 5′ UTR is yet to be shown.
PDS activity in interaction with the PLASTID TERMINAL OXIDASE modulates the redox state of the thylakoid membranes and modulates tetrapyrrole retrograde signaling to coordinate the expression of PHOTOSYNTHESIS‐ASSOCIATED NUCLEAR GENEs (PhANGs) required in chloroplast biogenesis (Foudree et al., 2010; Wang and Fu, 2016). PDS loss‐of‐function mutations are lethal, and homozygous pds mutants have impaired chloroplast biogenesis, which results in an albino phenotype (Qin et al., 2007). PDS enzymatic activity can be impaired by chemical inhibitors, such as the herbicide Norflurazon (NFZ), and give rise to phytoene and phytofluene accumulation. Similar to pds mutation, the chemical inhibition of PDS activity by NFZ generates a tetrapyrrole‐derived retrograde signal (Mg‐Proto IX), which impairs the chlorophyll and carotenoid accumulation, and reduces the expression of PhANGs (Song et al., 2018). There are no reports that show the presence or association of a phytoene or phytofluene‐derived apocarotenoid in the formation of phenotypes attributed to the impaired PDS activity. Besides, recent research showed that 15‐cis‐phytoene and 9,15‐di‐cis‐phytofluene are not targeted by CCD‐mediated enzymatic degradation in Arabidopsis (Schaub et al., 2018). However, a member of the CCD family, annotated as ZmCCD10a, was recently discovered in maize and shown to cleave the C9–C10 (C9′–C10′) double bond in phytoene to generate geranylacetone (C13) when exogenously expressed in Escherichia coli (Zhong et al., 2020). However, this enzyme belongs to the zaxinone synthase clade (Wang et al., 2019), and it remains to be shown whether it catalyzes phytoene cleavage in planta. The in planta activity of a phytoene and/or phytofluene‐derived LCDA, including geranylacetone, remains to be uncovered.
LCDAs are also considered to determine the plant leaf morphology. The Arabidopsis ZDS/CHLOROPLAST BIOGENESIS5 (CLB5) mutant clb5 accumulates putative LCDA signals that are involved in the regulation of plastid biogenesis and cause needle‐like leaf formation during early seedling development (Avendano‐Vazquez et al., 2014). Molecular and biochemical analyses demonstrated that the change in PhANGs expression, as well as the needle‐like leaf phenotype, is associated with the accumulation of an LCDA signal proposed to be derived from 9,15‐di‐cis‐phytofluene or 9,15,9′‐tri‐cis‐ζ‐carotene. NFZ treatment and generation of clb5ccd4 double mutant restored the PhANG expression and rescued the leaf phenotype caused by clb5 mutation (Figure 5c). This indicates that CCD4 might be involved in the formation of the LCDA coordinating PhANG expression and leaf morphology. However, both in vivo and in vitro analysis showed that CCD4 is not capable of cleaving cis‐ζ‐carotene isomers due to their stereochemical configuration not allowing CCD4 to target C9–C10 and/or the C9′–C10′ double bond (Huang et al., 2009; Bruno et al., 2016). It was previously shown that CCD4 interacts with other regulatory proteins to determine plant leaf morphology, plastid development and leaf senescence through interacting with other core regulators located in the plastoglobuli (Naested et al., 2004; Bhuiyan et al., 2016). This shows that the reversion of the needle‐like leaf phenotype in the clb5ccd4 double mutant might be associated with a secondary effect caused by ccd4 mutation rather than CCD4’s direct involvement in the generation of the LCDA signal. The biochemical and molecular mechanisms coordinating the formation of the clb5 needle‐like leaf phenotype require further investigation.
Mutations in CRTISO impair plastid development and give rise to the formation of varying degrees of the yellow virescent leaf (YVL) phenotypes in plants, including tomato (tangerine) (Isaacson et al., 2002), melon (yofi) (Galpaz et al., 2013), rice (zebra2) (Chai et al., 2011) and Arabidopsis (ccr2) (Park et al., 2002). A previous study also showed that the etiolated cotyledons of ccr2 seedlings hyper‐accumulate cis‐carotenes and have impaired prolamellar body (PLB) formation, associated with the impaired photomorphogenic development (Park et al., 2002). Recent research with Arabidopsis ccr2 seedlings demonstrated that the virescence phenotype develops only in the juvenile leaves grown under the short‐day photoperiod. The short‐day photoperiod limits the photoisomerization and promotes the accumulation of cis‐carotenes and an unknown LCDA signal, which coordinates chloroplast formation in photosynthetic tissues of ccr2 seedlings (Cazzonelli et al., 2020). The YVLs of ccr2 have pseudo‐chloroplast rather than a normal chloroplast, suggesting an impaired photomorphogenic development. The epistatic interaction between ziso (zic) and ccr2, in ccr2 ziso_155 seedlings, eliminated the unknown LCDA signal and rescued the YVL phenotype (Figure 5d). The ccr2ziso_155 mutation restored the PLB formation in etiolated seedlings and rescued the cotyledon greening phenotype; thus, it revealed the link between the unknown LCDA and the regulation of PLB formation (Figure 5e). Both etiolated and light‐grown leaves of ccr2 ziso_155 have impaired expression of genes that repress photomorphogenesis (i.e., CONSTITUTIVE PHOTOMORPHOGENIC 1/COP1, DE‐ETIOLATED 1/DET1) and have enhanced expression of PhANGs (i.e., ELONGATED HYPOCOTYL 5/HY5, LIGHT‐HARVESTING CHLOROPHYLL A/B‐PROTEIN 1.3/LHCB1.3, RIBULOSE BISPHOSPHATE CARBOXYLASE SMALL CHAIN 1A/RBCS1a), suggesting a possible interaction between the unknown LCDA and photomorphogenic development.
Further metabolic analysis with etiolated ccr2_det1_154 and D15‐treated seedlings confirmed that the PLB‐regulatory unknown LCDA is possibly derived from 7,9,9′‐tri‐cis‐neurosporene or 9,9′‐di‐cis‐ζ‐carotene. DET1 is a repressor of photomorphogenesis, and its impairment downregulated the PROTOCHLOROPHYLLIDE OXIDOREDUCTASE (POR) gene expression and decreased the PHYTOCHROME‐INTERACTING FACTOR 3 (PIF3) protein abundance while enhancing HY5, thus induced PhANG expression and promoted plastid biogenesis in the ccr2 background. The PLB‐regulatory LCDA and det1 complemented each other by restoring PLB formation and rescued the delayed greening and YVL phenotypes in ccr2 det_154 seedlings (Figure 5d,e). The characterization of the PLB‐regulatory LCDA and the level of its interaction with PIF3 and HY5 transcription factors requires further research.
LCDA‐associated phenotypes might be linked with the lack of downstream apocarotenoids
Perturbations at the upstream carotenoid pathway, through genetic mutations or altered gene expression, affect the production of apocarotenoids synthesized at the downstream pathway. For instance, the overexpression of endogenous PSY in Arabidopsis enhanced the accumulation of β‐apocarotenoids (i.e., β‐apo‐10′‐carotenal, retinal), C13 apocarotenoid glycosides (i.e., 3‐oxo‐α‐ionol, 3‐hydroxy‐5,6‐epoxy‐β‐ionone) (Latari et al., 2015) and short‐chain apocarotene‐dialdehydes (i.e., glyoxal, methylglyoxal) (Schaub et al., 2018) in leaves and callus tissues. Another study showed that the overexpression of PSY‐ORFs from maize, rice and Arabidopsis itself did not significantly affect the accumulation of the carotenoid‐derived phytohormone ABA in 4‐week‐old Arabidopsis leaves (Alvarez et al., 2016). However, a PSY paralog PSY3 gene was previously shown to be induced by the abiotic stress conditions (i.e., drought and salinity) and responsible for increased ABA accumulation in rice and maize (Li et al., 2008; Welsch et al., 2008). Besides, expression of a PSY gene (PSY3) in the dicots Medicago truncatula and Solanum lycopersicum is highly upregulated during plant root–symbiotic AM interactions and associated with the accumulation of SLs and mycorrhiza‐induced α‐ionol (C13) and mycorradicin (C14) (Stauder et al., 2018). Similarly, the expression of saffron PSY3 (a close relative of dicot PSY3) was shown to be involved in the production of mycorrhizal‐induced apocarotenoids (Ahrazem et al., 2019). The increased abundance of apocarotenoids in plants with induced PSY expression or overexpression results from the enhanced metabolic flux and increased precursor availability reinforcing apocarotenoid biosynthesis at the downstream part of the pathway.
Genetic mutation or impaired expression of linear cis‐carotene biosynthetic genes has a negative impact on ABA biosynthesis and accumulation in plant tissues, giving rise to phenotypes associated with ABA deficiency. For instance, the sunflower nd1 (zds) (Conti et al., 2004), maize vp5 (pds) (Hable et al., 1998), vp9 (zds) (Matthews et al., 2003; Ma et al., 2014; Chen et al., 2017) and y9 (ziso) (Li et al., 2007) and rice phs‐1 (pds), phs‐2 (zds) and phs‐3 (crtiso) (Fang et al., 2008) mutants develop viviparous seeds that lack dormancy. Precocious seed/grain development reduces the overall yield and quality of crops and causes economic loss (Nonogaki and Nonogaki, 2017). Similarly, RNA interference‐mediated downregulation of ZDS reduces ABA accumulation and causes the development of ABA‐specific delayed fruit‐ripening phenotype in tomato fruits (McQuinn et al., 2020). The reduction of ABA in plants with impaired linear cis‐carotene biosynthesis is due to blockage of the metabolic flux at the upstream pathway, limiting the precursor availability to produce ABA.
The linear cis‐carotene pathway is a bottleneck for the production of downstream apocarotenoids and some phytohormones (i.e., ABA, SLs). Therefore, the LCDA‐associated phenotypes defined in cis‐carotene mutants might be partially linked with the impaired production or accumulation of downstream apocarotenoids functioning as either metabolic signals or phytohormones. The contribution of downstream signaling components to the emergence of the LCDA‐associated phenotypes and the feedback interaction between the linear cis‐carotene biosynthesis pathway and other apocarotenoids require further investigation.
Prospects for future LCDA research
Our current knowledge about the biosynthesis and function of LCDAs is mostly limited to data available on their precursor molecules, the acyclic cis‐carotenes. Previous studies defined several functions for putative LCDA signals, but the direct evidence showing the relevance between the described phenotypic and metabolic traits with LCDAs is missing. The molecular structures of LCDAs are unknown, but they might be predictable to a degree as their precursor is well characterized. In fact, some of the theoretically predicted LCDAs and their modified forms (i.e., farnesyl‐ and geranylacetone, pseudoionone, sulcatone, citral) were previously identified in plants (Shi et al., 2020). A recent study showed that LCDAs accumulate in a tissue‐specific manner, suggesting the possibility of having particular functions in plant development or environmental response (Rivers et al., 2019). The question remains unanswered, whether the primary LCDAs or their modified products cause the phenotypes described in the literature, or are they only the metabolic byproducts of the carotenoid biosynthesis pathway? Being specifically biosynthesized by the carotenoid pathway and having a biological function may increase the possibility of an LCDA to be a signaling molecule rather than a byproduct. The LCDAs can be produced by enzymatic cleavage or by ROS‐mediated degradation of cis‐carotenes. There are a few studies that show the possible involvement of CCDs in the generation of LCDAs in plants (Simkin et al., 2004; Yahyaa et al., 2013; Jing et al., 2015). In fact, the capability of CCDs to cleave acyclic cis‐carotenes to produce LCDAs is very limited (Vogel et al., 2008; Ilg et al., 2014; Bruno et al., 2016). Therefore, the majority of the LCDAs might be generated through the ROS‐mediated degradation of cis‐carotene precursors. Light quality and quantity determine ROS accumulation in plants (El‐Esawi et al., 2017; Waszczak et al., 2018). In addition, light‐ or enzyme‐mediated isomerization might be rate‐limiting the production of LCDAs during the day/night cycle, which might be a component in a regulatory network influencing metabolic and morphological traits according to light quality. Additional and more profound research is required to identify LCDAs structurally and explain the molecular mechanisms associated with the perception and transduction of these signals, which provoke the generation of responses and influence plant metabolism and phenotype.
CYCLIC AND ACYCLIC APOCAROTENOIDS WITH SIGNALING PROPERTIES
In recent years, various apocarotenoids involved in photoacclimation, drought tolerance, plant growth and development, AM symbiosis and plant defense against herbivores were reported. The still ongoing discovery of apocarotenoids with signaling and regulatory functions highlights the importance of carotenoids in coordinating different processes in the life of plants. For instance, there are cyclic and acyclic apocarotenoids that act as retrograde signals involved in high light and drought tolerance (e.g., β‐cc, β‐ccA and dhA), plant herbivory tolerance (e.g., β‐ionone, loliolide and α‐ionone), AM symbiosis (e.g., blumenols, mycorradicins), and plant development and parasitic control (e.g., anchorene and zaxinone). These apocarotenoids may have multiple functions, such as β‐ccA, which is involved in growth and development, and zaxinone, which is also shown to be involved in AM symbiosis.
Cyclic apocarotenoids as retrograde signals mediating high light and drought tolerance
High‐light stress is accompanied by the generation of 1O2 in the photosystem II, which attacks β‐carotene molecule and gives rise to the formation of β‐cc that can further be converted into the corresponding acid β‐ccA, or to β‐ionone, which is the precursor of dhA (Figure 6a). The lipid‐soluble nature of β‐cc and dhA might allow them to pass through plastid membranes and convey the stress message to the cytosol and the nucleus (D'Alessandro and Havaux, 2019). The retrograde signaling triggered by β‐cc is independent of canonical tetrapyrrole signals, but also independent of the EXECUTER 1 and 2 proteins (EX1, EX2), which mediates 1O2‐induced cell death (Ramel et al., 2012b; D'Alessandro et al., 2018; Dogra et al., 2018; D'Alessandro and Havaux, 2019). The protein level of 3′(2′),5′‐BISPHOSPHATE NUCLEOTIDASE (SAL1) decreased in β‐cc‐treated plants. SAL1 is responsible for the degradation of the well‐known retrograde signal 3′‐phosphoadenosine‐5′‐phosphate (PAP) (Estavillo et al., 2011; Ramel et al., 2012b). In addition, the β‐cc application enhanced the protein level of the enzyme SULFOTRANSFERASE (ST2A) that uses PAPS as a sulfate donor generating PAP (D'Alessandro and Havaux, 2019). Thus, β‐cc might induce PAP accumulation and trigger PAP retrograde signaling in response to an affected photosynthetic process in plants exposed to high light (Figure 6a). In line with this, transcriptome analysis of β‐cc‐ and dhA‐treated plants showed a reprogramming in gene expression, which is usually associated with enhanced photooxidative stress tolerance, for example, higher photosystem II photochemical efficiency and lower lipid peroxidation (Ramel et al., 2012b; Shumbe et al., 2014, 2017). In fact, β‐cc‐treated plants showed increased high‐light tolerance compared with mock‐treated or β‐ionone‐treated plants (Figure 6b).
Figure 6.
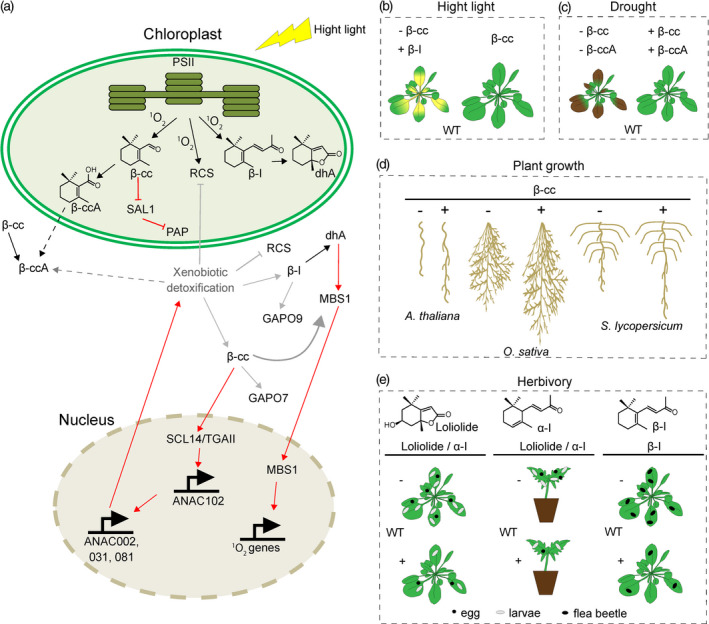
Apocarotenoids involved in high‐light and drought stress response, and in herbivore defense.
(a) 1O2 signaling cascade triggered by high‐light stress in plant leaves. Apocarotenoids (e.g., β‐cc, dhA and β‐ionone [β‐I]) are produced through the oxidation (black arrows) of β‐carotene in photosystem II (PSII). Further oxidation of these compounds (black arrows) forms, for instance, β‐cyclocitric acid (β‐ccA) and dihydroactinidiolide (dhA). β‐ccA might be transported to the cytosol by unknown transporters (dotted black arrow) or can be produced in the cytosol from the volatile β‐cc. 1O2 can produce reactive carbonyl species (RCS), which can be detoxified by the xenobiotic detoxification system (shown in gray). The volatile apocarotenoids can passively diffuse to the cytoplasm to activate at least two signaling cascades (red arrows) mediated by MBS1 or by the interaction between the SCL14 and the TGAII transcription factor. These signaling pathways induce 1O2‐responsive genes and genes involved in cellular detoxification. Xenobiotic detoxification system targets RCS and might be involved in β‐cc, β‐I and β‐ccA detoxification (gray arrows). β‐cc is interconnected with the 3‐phosphoadenosine 5‐phosphate (PAP)‐signaling by inducing PAP accumulation under excess light (red lines inside the chloroplast).
(b) β‐cc confers high‐light tolerance in Arabidopsis plants subjected to high‐light stress. WT, wild type.
(c) β‐cc promotes plant growth in Arabidopsis, rice and tomato roots.
(d) β‐cc and β‐ccA confer drought tolerance to Arabidopsis plants subjected to drought stress.
(e) α‐ and β‐ionone, and loliolide influence plant–herbivore interactions. Exogenous application of these volatiles confers protection against plant herbivores by reducing egg depositions and larvae numbers.
METHYLENE BLUE SENSITIVE (MBS1) is a small zinc finger protein that was previously identified in a genetic screen of Chlamydomonas reinhardtii mutants defective in response to 1O2 (Shao et al., 2013). MBS1 participates in the regulation of 1O2‐responsive‐genes, which resemble the effect of β‐cc/dhA in Arabidopsis. The absence of the MBS1 protein in Arabidopsis resulted in dramatic perturbations of 1O2 marker gene expression (Shao et al., 2013). An Arabidopsis MBS1 overexpressing line shows increased photoacclimation response and high‐light tolerance, while the mbs1 mutant is insensitive to β‐cc treatment (Shumbe et al., 2017). These results demonstrate the involvement of MBS1 in the signaling cascade downstream of β‐cc/dhA. The involvement of MBS1 in the 1O2 signaling pathway was further substantiated by a stronger dual localization of the protein in the cytosol and the nucleus after β‐cc treatment or high light. Taken together, it can be assumed that the high‐light stress signal is transmitted by β‐cc/dhA to MBS1, enabling it to enter the nucleus to activate 1O2‐responsive genes (Figure 6a).
An MBS‐independent activity of β‐cc, known as xenobiotic detoxification system, is present in all eukaryotes and comprises a great number of proteins (e.g., transcription factors, transporters and redox enzymes) (Sandermann, 1992). This system is partly coordinated at the transcriptional level by the interaction between the TGAII transcription factors and the GRAS protein SCARECROW‐LIKE 14/SCL14 (Fode et al., 2008). D'Alessandro et al. (2018) demonstrated that the xenobiotic detoxification coordinated by SCL14 is induced by both β‐cc and photooxidation (Figure 6a). This detoxification system is activated by endogenous toxicants (e.g., reactive carbonyl species) that derive from the decomposition of lipid peroxides (Mano, 2012). Upon excessive light exposure and by the action of β‐cc, plant cells are prepared for the accumulation of lipid peroxides by increasing the detoxification of reactive carbonyl species (D'Alessandro et al., 2018). As part of the xenobiotic response or by diffusing to the cytosol, β‐cc increases the SCL14 expression level and promotes its interaction with TGAII, a bZIP transcription factor. This interaction modulates expression levels of the chloroplast transcription factor ANAC102 (Inze et al., 2012). ANAC102 induces downstream transcription regulators (ANAC002, ANAC031 and ANAC081), which activate redox enzymes involved in the xenobiotic detoxification process (Figure 6a) (D'Alessandro et al., 2018). Similar to the β‐cc application in Arabidopsis, rice overexpressing the SCL14 rice homolog OsGRAS23 show increased high light/drought tolerance (Xu et al., 2015). Intriguingly, β‐cc and 1O2 also strongly induce the expression of several Arabidopsis glycosyltransferases in leaves (Ramel et al., 2012b, 2013). These enzymes are likely involved in the conversion of hydroxylated apocarotenoids that were shown, together with their glycosylated derivatives (glycosylated apocarotenoids [GAPO]), to increase significantly in Arabidopsis plants subjected to high‐light conditions (Mi et al., 2018). Mi et al. (2018) showed that β‐cc and β‐ionone concentrations were similar under control conditions, while the level of glycosylated β‐ionone was about 20 times higher than that of glycosylated β‐cc. Extremely high β‐ionone glycosylation (GAPO9) content could explain that β‐ionone shows similar unconjugated levels to those of β‐cc. Interestingly, GAPO7 content is just a small fraction of β‐cc, while conjugated β‐ionone (GAPO9) represents the majority of the β‐ionone concentration. Uneven production of the glycosylated β‐cc (GAPO7) and β‐ionone (GAPO9) forms suggests that β‐carotene derivatives have a detoxifying mechanism (D'Alessandro and Havaux, 2019). In addition, hydroxy‐β‐cc was not detectable (unlike hydroxylated β‐ionone), suggesting a quick conversion to its glycosylated form, pointing to an essential role of this process in the regulation of β‐cc signaling (Mi et al., 2018). This concludes that β‐cc metabolization likely limits its signal role through the aforementioned negative feedback mechanism, thereby returning 1O2‐induced signaling to unstressed levels.
Besides its role as a retrograde signal in the network regulating the cellular oxidative stress response, β‐cc has recently been shown to be a growth regulator (Dickinson et al., 2019). Dickinson et al. used a targeted chemical genetic approach to identify apocarotenoids that enhance root branching in the presence of N‐(4‐fluoro‐benzyl)‐N‐hydroxy‐3‐(4‐methoxy‐phenyl)‐propionamide (D15), a CCD inhibitor that reduces primary root length and inhibits LR capacity by 50% in Arabidopsis. Treatment of β‐cc under these conditions increased LR formation by approximately 40% in the presence of D15 in Arabidopsis (Figure 6c, left panel). In the absence of D15, exogenous β‐cc application at a concentration <1 µm increased primary root length and LR branching by 30%, suggesting that β‐cc acts as a growth promoter in Arabidopsis roots. In addition, experiments at the cellular level revealed that β‐cc promotes cell division in LR primordia after initiation and induces root growth by stimulating cell division (Dickinson et al., 2019). Moreover, experiments with mutant lines of pathways related to auxin, brassinosteroids and ROS signaling, which are known to modulate root growth and development, confirm an independent mechanism of action for the β‐cc effect. Further experiments with tomato and rice roots (Figure 6c, middle and right panel) confirmed the role of β‐cc as a conserved plant growth regulator (Dickinson et al., 2019).
Recently, D'Alessandro et al. (2019) showed that β‐cc is further metabolized into water‐soluble β‐ccA in leaves. Indeed, β‐ccA was found in Arabidopsis leaves subjected to drought stress or exposed to the β‐cc treatment. Moreover, exogenous application of β‐ccA was shown to protect plants from drought stress (Figure 6d) and to induce the expression of key water stress‐responsive genes (e.g., ANAC72, ATAF1, RD29B) (D'Alessandro et al., 2019). The use of Arabidopsis stomatal regulation (ost2‐2), ABA (abi1) and jasmonate receptor (coi1) mutants suggests a different, independent mechanism of action for β‐ccA (D'Alessandro et al., 2019). Interestingly, the β‐ccA drought‐protective effect was observed in pansy flower, pepper and tomato plants, indicating a general, conserved function of β‐ccA in drought response. It was also shown that the application of β‐cc to Arabidopsis plants grown in the greenhouse increased their drought tolerance (Figure 6d). However, while β‐cc treatment mimics the effect of β‐ccA, application of β‐ccA induced only one branch of the β‐cc signaling, suggesting that β‐cc exerts a β‐ccA‐independent function and is perceived by a different signaling pathway.
Role of β‐ionone, loliolide and α‐ionone in plant defense to herbivores
The sessile lifestyle of plants necessitates a great arsenal of metabolites that protect against herbivores and pathogens. This arsenal includes the so‐called plant activators, such as the phytohormone jasmonic acid (JA), which is involved in plant–pathogen protection through the activation of defense mechanisms that do not harm/kill the microbes or insects (Wei et al., 2011; Caceres et al., 2016; Murata et al., 2019a, 2019b,2019a, 2019b; Li et al., 2020). In addition, plants defend themselves by using metabolites that affect pathogens and exert antimicrobial or insecticidal activity. Interestingly, several cyclic apocarotenoids that induce herbivore resistance in plants without exhibiting insecticidal activity have been reported in the last years (Wei et al., 2011; Caceres et al., 2016; Murata et al., 2019a, 2019b,2019a, 2019b; Li et al., 2020). Examples of such apocarotenoids are α‐ionone, β‐ionone and loliolide, which arise through the degradation of α‐ and β‐carotene (Schwab et al., 2008).
β‐Ionone, a C13 β‐carotene‐derived volatile, is a component of fragrances released from various species of flowering plants (Wei et al., 2011). Plant–insect interaction is frequently characterized by plant volatile emissions that can act as attractants or repellents (Pivnick et al., 1992; Bartlet et al., 1997; Wang et al., 1999; Omura et al., 2000; Gruber et al., 2009). β‐Ionone has been reported as one of the components of the volatile emission by Trifolium strictumand leaves. β‐Ionone was previously shown to affect (repellent effect against insects and reduced egg deposition) several insects, such as earth mites, cabbage butterfly and crucifer flea beetle (Wang et al., 1999; Omura et al., 2000; Gruber et al., 2009). Moreover, β‐ionone exerted the highest deterrence activity against the redlegged earth mite compared with all the other volatile compounds emitted by the leaves of T. strictumand (Wang et al., 1999). β‐Ionone was present only in the volatiles emitted by Trifolium strictum but not by T. glanduliferum and T. subterraneum, suggesting specific defense mechanisms in different plant species. Moreover, β‐ionone was found in mature leaves and flowers of Brassica napus variety AC Excel (but not in any other cultivar) and showed one of the most potent inhibitory effects on the crucifer flea beetle (Figure 6e, right panel) (Gruber et al., 2009). The non‐repellent effect of β‐ionone against the cabbage butterfly (Omura et al., 2000), suggests a certain degree of volatile specificity and raises the possibility of different insects having different volatile receptors. These data point to a specificity on both the plant side and the insect side. Genetic and molecular studies with Arabidopsis plants overexpressing the AtCCD1 showed that higher transcription of the gene is reflected in higher β‐ionone levels and, therefore, in stronger plant defense to herbivores (Wei et al., 2011; Caceres et al., 2016). Leaves from AtCCD1 overexpressing plants were less eaten by about 50% by the flea beetle and the spider mite and showed reduced oviposition by whiteflies, pointing towards carotenoid pathway engineering as an emergent tool for pest control.
Loliolide is a β‐carotene derivative isolated from leaves of mosaic virus‐infected tobacco plants showing a hypersensitive response, and shown to induce resistance to herbivores upon exogenous application (Repeta, 1989; Rios et al., 2008; Murata et al., 2019b) (Figure 6e, left and middle panel). For instance, tomato leaves treated with loliolide showed reduced survival rate and egg deposition of the two‐spotted spider mite and survival rate of the larvae of the common cutworm (Figure 6e, middle panel) without exhibiting toxicity against these herbivores (Murata et al., 2019b). Interestingly, loliolide cellular content is not only increased after an infestation by the common cutworm larvae but also after the application of their oral secretions in tomato, suggesting that a molecular interaction between a component of the insect secretion and a plant receptor must be occurring. The same interaction is most likely the trigger that activates a signaling cascade resulting in the activation of key defense genes. A combination of microarray analysis and quantitative reverse transcription–polymerase chain reaction revealed the identity of two loliolide‐responsive genes, LIT8 and LIT13, involved in plant defense. LIT8 is a gene‐encoding cell wall invertase, while LIT13 is predicted to encode the WALL‐ASSOCIATED RECEPTOR KINASE 2 (Murata et al., 2019b). Both genes were upregulated in response to the exogenous application of loliolide but not in response to JA or salicylic acid (SA). Interestingly, JA‐ and SA‐responsive genes such as PROTEINASE INHIBITOR II (PIN2; downregulated), LEUCINE AMINOPEPTIDASE (LAPA1; downregulated), and basic β‐1,3‐GLUCANASE (GLUB; unchanged) did not respond to exogenous loliolide application, suggesting a JA‐/SA‐independent mechanism for gene activation after loliolide application. Furthermore, JA and SA levels remained unchanged after loliolide exogenous application supporting the activation of plant defense genes independently of the canonical JA signaling. (–)‐Loliolide was also recently reported as a soil‐borne signaling chemical conserved in dozens of plants (Kong et al., 2018). For instance, (–)‐loliolide was detected in the barnyard grass–rice allelopathic interaction (Li et al., 2020). The interaction between rice and five biotypes of barnyard grass was characterized by the higher levels of the allelochemicals momilactone B (a diterpenoid) and tricin (a flavonoid), measured in rice, in response to (–)‐loliolide, which was detected in the root exudates of all five biotypes of barnyard grass. Interestingly, the exogenous application of loliolide (and JA) elicited momilactone B and tricin production. Moreover, comparative transcriptomic analysis unveils the regulatory activity of (–)‐loliolide on the diterpenoid (upregulation of CPS4, KSL4 and MAS) and flavonoid (upregulation of CYP75B3 and CYP75B4) pathways. These data suggest that loliolide acts as a soil‐borne signal that enhances allelochemical production to coordinate plant–plant interaction.
The volatile apocarotenoid α‐ionone was recently reported as a protective molecule against plant herbivores (Murata et al., 2019a). Tomato plants pretreated with α‐ionone vapor decreased the survival rate and egg deposition of western flower thrips (Figure 6e, left and middle panel) without exerting insecticidal activity, as was previously reported for β‐ionone and loliolide. In addition, Arabidopsis plants pretreated with α‐ionone vapor showed a decreased survival rate of common cutworm (Figure 6e, left and middle panel). Opposite to JA or loliolide, exogenous application of α‐ionone induced the expression of GLUB and BASIC CHITINASE (CHI9) genes, suggesting a different mechanism of action than JA or loliolide.
Interestingly, exogenous β‐cc application in African spider plants exhibited protective properties against the two‐spotted spider mite, and also anti‐pathogenic properties against the oomycete Plasmopara viticola in grapevines (Nyalala et al., 2013; Lazazzara et al., 2018). This suggests that β‐apocarotenoid volatiles might have multiple functions affecting plant physiology.
Cyclic and acyclic apocarotenoids involved in AM symbiosis
Approximately 70% of all higher plants establish symbiotic associations with AMF (Fiorilli et al., 2015, 2019; Brundrett and Tedersoo, 2018; Lanfranco et al., 2018). This symbiotic association secures phosphorus and nitrogen for the plant and reduced carbon for the fungus (Helber et al., 2011; Bravo et al., 2017; Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017). AM symbiosis influences plant growth (Rooney et al., 2009; Adolfsson et al., 2015) and confers tolerance to abiotic and biotic stresses (Pineda et al., 2010; Vannette et al., 2013; Adolfsson et al., 2015; Sharma et al., 2017). AMF colonization establishment and maintenance have been associated with specific apocarotenoids (Figure 7a) (Walter et al., 2007; Floss et al., 2008a, 2008b,2008a, 2008b; Hill et al., 2018; Wang et al., 2018; Fiorilli et al., 2019), which accumulate after AMF inoculation (Schweiger et al., 2014; Aliferis et al., 2015; Adolfsson et al., 2017). Based on their structure, these apocarotenoids can be divided into two types, i.e., (i) so‐called blumenols (C13 derivatives), and (ii) so‐called mycorradicins (C14 derivatives) (Figure 7a) (Hill et al., 2018; Wang et al., 2018). The two types arise through sequential, two‐step cleavage, most likely from a C40 carotenoid precursor. Studies using ccd7 mutants in tomato and peas suggested the involvement of CCD7 in cleaving the C40 carotenoid precursor to form a C27 apocarotenoid and C13cyclohexenone, β‐ or α‐ionone (Vogel et al., 2010; Walter et al., 2010). In the next step, the C27 apocarotenoid is cleaved by CCD1, leading to rosafluene‐dialdehyde (C14), the mycorradicin precursor, and another cyclohexanone (C13) (Floss et al., 2008b; Walter et al., 2010; Hou et al., 2016). Blumenols are C13 cyclohexanone derivatives that accumulate in the roots of AFM‐colonized plants in direct correlation with the fungal colonization rate (Maier et al., 1995; Fester et al., 1999; Walter et al., 2000; Strack and Fester, 2006). Although information about blumenols is scarce, recently, a group of blumenols was found in shoots and roots (Figure 7a) of mycorrhizal plants (e.g., tomato, barley and potato) using a combination of targeted and untargeted metabolomics (Wang et al., 2018). This group is composed of five blumenols (11‐hydroxyblumenol C‐9‐O‐Glc, 11‐carboxyblumenol C‐9‐O‐Glc, 11‐hydroxyblumenol C‐9‐O‐Glc‐Glc, blumenol C‐9‐O‐Glc‐Glc and blumenol C‐9‐O‐Glc), and their abundance correlates with the AMF colonization rate, as reflected in changes in the transcript profile of canonical mycorrhization marker genes. However, their biological effect is not clear. For the moment, blumenols can be used as foliar markers that allow rapid detection of AM symbiosis and for the screening of functional AMF associations (Wang et al., 2018). Experiments to find their receptors, or generation of mutant lines with reduced/increased blumenol content, or exogenous treatments are required to shed light on the biological effect of these compounds.
Figure 7.
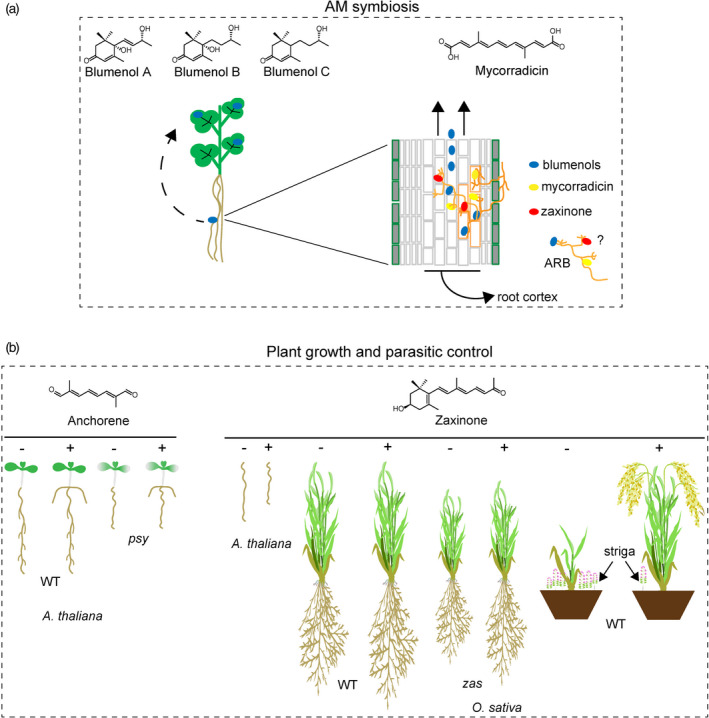
Apocarotenoids are involved in arbuscular mycorrhiza (AM) symbiosis and plant growth and development.
(a) AM symbiosis in the Medicago truncatula showing how blumenols produced in the root can be transported to the leaves to serve as an AM fungi colonization marker. Blumenols and mycorradicins are synthesized in the AM and contribute to its establishment and proper function. The involvement of zaxinone in this process has been reported, but its function is not fully characterized. ARB, arbuscule.
(b) Exogenous anchorene application into Arabidopsis roots stimulates the development of anchor roots. Exogenous zaxinone application to Arabidopsis roots reduces hypocotyl growth, while its application in rice promotes root and shoot growth and reduces Striga infestation.
Mycorradicins were detected as a mycorrhizal‐specific apocarotenoid mixture causing a yellow/orange pigmentation of mycorrhizal roots after AMF colonization (Scannerini and Bonfantefasolo, 1977; Klingner et al., 1995a, 1995b,1995a, 1995b; Floss et al., 2008a). Genetic evidence based on knockdown mutants affected in carotenoid metabolism demonstrated a positive correlation between C13 and C14 apocarotenoid content and mycorrhizal functionality. These apocarotenoids are synthesized in fungal arbuscules (Figure 7a), which contains the enzymes for their biosynthesis (Fester et al., 2002a, 2002b,2002a, 2002b; Hans et al., 2004; Walter et al., 2007; Walter et al., 2010). In M. truncatula, AMF colonization induced the 1‐DEOXY‐D‐XYLULOSE 5‐PHOSPHATE SYNTHASE 2 (MtDXS2) expression but not MtDXS1, which correlated with the accumulation of C13 and C14 apocarotenoids. Moreover, transgenic roots with repressed MtDXS2 expression showed a reduction in the level of both apocarotenoid types as well as in mycorrhization and the expression level of plant AM marker genes (e.g., phosphate transporter MtPT4). The reduction in expression of AM‐induced markers was accompanied by an increased ratio of degenerating/dead arbuscules to mature arbuscules. Interestingly, the reduction in the MtCCD1 expression level was reflected in differential reductions of C13 (30–47% residual expression) and C14 (3–6% residual expression) apocarotenoids. In contrast with the MtDXS2 mutant, MtCCD1 reduction did not cause major alteration in AM molecular markers but led to a moderate increase in the relative ratio of degenerated arbuscules. These phenotypes indicate a more prominent role for C13 (blumenols) than C14 (mycorracidins) derivatives in AM establishment and functioning. Accumulation of blumenols that derive from β‐ or α‐ionone cleavage products was also detected in roots after AMF inoculation (Maier et al., 1995; Walter et al., 2000; Strack and Fester, 2006).
The rice ZAS is a CCD representing an emergent clade in the plant CCD family. In vitro studies showed that this enzyme forms the apocarotenoid zaxinone that acts as a growth regulator (s. Zaxinone). The loss‐of‐function Oszas mutant shows a higher SL content and reduced root and shoot growth. Despite the high SL content, this mutant displays a lower level of AM colonization and no changes in arbuscule morphology when compared with the wild type. Moreover, OsZAS expression is induced during early (7‐day post‐inoculation) and during later stages (35‐day post‐inoculation) of mycorrhizal colonization in rice roots, indicating its involvement in mycorrhization (Fiorilli et al., 2015; Wang et al., 2019) (Figure 7a). The latter assumption is supported by the absence of ZAS orthologs in genomes of non‐AM plants (e.g., Arabidopsis) (Wang et al., 2019). However, more evidence is needed to understand the specific function of zaxinone in AM symbiosis.
Anchorene
Anchorene belongs to a group of less‐studied carotenoids, the so‐called diapocarotenoids. Diapocarotenoids can be found in some bacteria and plants (Giuliano et al., 2003; Perez‐Fons and Fraser, 2012; Frusciante et al., 2014; Demurtas et al., 2018). They are formed by the conjugation of two C15 molecules (farnesyl diphosphate) and are usually known as 4,4′‐diapocarotenoids (e.g., C30 diapophytoene and C30 diapolycopene) (Perez‐Fons and Fraser, 2012). Further oxygenation, glycosylation, methylation or acyl transfer reactions can greatly diversify diapocarotenoid species. The diapocarotenoid anchorene (C10) is found in several plant species (e.g., tomato, carrot, spinach). Although it seems possible that the cleavage of C11–C12 and C11′–C12′ double bonds from carotenoids downstream of ζ‐carotene results in the production of anchorene, it is still unclear how it is formed (Jia et al., 2019; Mi et al., 2019). Anchorene was shown to promote the development of ANR (Figure 7b, left panel) (Jia et al., 2019). ANR develop from the collet region situated at the root hypocotyl junction (Lucas et al., 2011). The regulation of ANR development by anchorene seems to be structure‐dependent as (i) exogenous application of structural isomers showed no effect on ANR development, and (ii) functional group substitutions in the anchorene molecule led to the loss of activity (Jia et al., 2019). Interestingly, the carotenoid‐deficient Arabidopsis psy mutant is deficient in ANR formation; however, this phenotype can be rescued by external anchorene application (Figure 7b, left panel). This result suggests that ANR formation requires a signal derived from carotenoids, which might be the anchorene in this case. Further investigation of anchorene activity demonstrated an effect on auxin homeostasis and indicated its involvement in nitrogen deficiency response (Jia et al., 2019).
Zaxinone
Recent phylogenetic analysis of plant CCD orthologs led to the discovery of ZAS, which is present in many plants but not in Arabidopsis or other members of Brassicaceae (Wang et al., 2019). In vitro characterization of this CCD member in rice revealed that ZAS cleaves a molecule of apo‐10′‐zeaxanthinal (3‐OH‐β‐apo‐10′‐carotenal, C27) at the C13–C14 double bond, generating a C18‐ketone (zaxinone) and an unstable C9‐dialdehyde (Wang et al., 2019). Interestingly a Zea mays CCD10a was recently shown to cleave phytoene, lycopene, δ‐carotene, ε‐carotene and β‐carotene, producing C8 (6‐methyl‐5‐hepten‐2‐one) and C13 (geranylacetone, α‐ionone and β‐ionone) apocarotenoids when expressed in E. coli (Zhong et al., 2020). However, in that study, the authors did not quantify zaxinone in a catalytic reaction involving the ZmCCD10a; thus, ZAS activity remains to be determined.
Zaxinone is produced by various plant species, including rice (Mi et al., 2018; Wang et al., 2019). Exogenous application of zaxinone to rice increased overall growth and biomass, while the corresponding mutant (Oszas) showed growth retardation (Figure 7b, right panel) (Wang et al., 2019). Compared with wild type, Oszas mutant contains a similar level of zaxinone in leaves but decreased content in roots, confirming the ZAS enzymatic activity in vivo but also suggesting an additional zaxinone biosynthetic route(s) (Wang et al., 2019). In addition, Oszas mutants showed reduced crown root length and number, lower tiller and panicle number (Wang et al., 2019). The hormone quantification analysis revealed that Oszas roots and root exudates have enhanced SL content compared with the wild type. The exogenous application of zaxinone to the Oszas mutant reverted most of the mutant growth phenotypes and reduced SL level and impaired its release. Moreover, zaxinone application resulted in the decreased transcript level of SL biosynthetic genes such as OsDWARF 27/D27, OsCCD8/D10, OsCCD7/D17 and OsCARLACTONE OXIDASE/CO, confirming its role as a negative regulator of rice SL biosynthesis. In addition, exogenous zaxinone application reduced the Striga infestation in a Striga‐susceptible rice variety by decreasing the amount of released SLs (Figure 7b, right panel). This suggests a potential application in alleviating Striga infestation and improving crop growth (Wang et al., 2019). In contrast to rice, a very recent publication by Ablazov et al. showed that zaxinone is a positive regulator of SL and ABA biosynthesis and does not promote Arabidopsis root growth (Ablazov et al., 2020). The same study also showed that exogenous zaxinone application reduces Arabidopsis hypocotyl growth by increasing ABA content (Ablazov et al., 2020). The presence of zaxinone in Arabidopsis and its effect on hormone homeostasis shows the occurrence of ZAS‐independent routes for zaxinone synthesis and indicates a general growth regulatory activity in AM and non‐AM plant species. Interestingly, phenyl‐based compounds mimicking zaxinone activity were recently synthesized (Wang et al., 2020a). Mimics of zaxinone (MiZax) MiZax3 and MiZax5 showed zaxinone activity. These compounds rescued the root growth phenotype of a (zaxinone‐deficient) rice mutant, by promoting growth, and reduced SL accumulation in roots and root exudates of wild‐type plants (Wang et al., 2020a). MiZax might be a valuable tool to study zaxinone function and its involvement in rice development and growth‐related processes further. The use of MiZax in agricultural applications might be an alternative approach in combating Striga infestation.
CONCLUDING REMARKS
Carotenoids are isoprenoid compounds that serve as precursors of a wide variety of signaling molecules (e.g., apocarotenoids) and hormones involved in almost all aspects of plant physiology and development. Plant apocarotenoids are produced from either spontaneous carotenoid oxidation or enzymatic‐assisted cleavage, carried out by the CCDs. Apocarotenoids might act as immediate signaling molecules (β‐cc) or growth regulators (zaxinone), while further modifications of some apocarotenoids result in the production of the phytohormones ABA and SLs. However, ACS properties and their ability to modulate plant physiology, architecture and development resemble the functions of phytohormones and raise the question of whether these small molecules are indeed phytohormones. In fact, some of them act independently of canonical hormone signaling pathways (e.g., β‐cc and dhA), while others interact with hormones to fulfill their function (e.g., anchorene, zaxinone). Considering these functions, the recent discoveries and further characterization of ACS molecules/hormone candidates may shed light on the mechanistic explanation of the emergence of these previously overlooked phenotypes related to plant development, stress tolerance and biotic interactions. Nevertheless, whether apocarotenoids are hormones or hormone‐like molecules, the characterization of the mechanism of action of these molecules will contribute to the understanding of the plant’s life and its interaction with the environment.
AUTHOR CONTRIBUTIONS
JM: wrote the SLs section and designed Figures 1, 3, and 4. YA: wrote LCDAs and ABA sections and designed Figures 2 and 5. JCM: wrote the abstract, introduction (with YA), cyclic apocarotenoids with signaling properties chapter, and designed Figures 6 and 7. JM and YA: prepared the figures with the input of JCM and SAB, JCM, and SAB structured the manuscript and prepared the final version with substantial input from JM and YA
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was supported by King Abdullah University of Science and Technology (KAUST), Competitive Research Grant (CRG2017), and baseline funding given to Salim Al‐Babili. We thank Dr. Hendrik N. J. Kuijer for valuable comments.
REFERENCES
- Abe, S. , Sado, A. , Tanaka, K. et al (2014) Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl Acad. Sci. USA, 111, 18084–18089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablazov, A. , Mi, J. , Jamil, M. , Jia, K.P. , Wang, J.Y. , Feng, Q. and Al‐Babili, S. (2020) The apocarotenoid zaxinone is a positive regulator of strigolactone and abscisic acid biosynthesis in Arabidopsis roots. Front. Plant Sci. 11, 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuauf, H. , Haider, I. , Jia, K.P. , Ablazov, A. , Mi, J. , Blilou, I. and Al‐Babili, S. (2018) The Arabidopsis DWARF27 gene encodes an all‐trans‐/9‐cis‐beta‐carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Sci. 277, 33–42. [DOI] [PubMed] [Google Scholar]
- Adolfsson, L. , Nziengui, H. , Abreu, I.N. et al (2017) Enhanced secondary‐ and hormone metabolism in leaves of arbuscular mycorrhizal Medicago truncatula. Plant Physiol. 175, 392–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfsson, L. , Solymosi, K. , Andersson, M.X. , Keresztes, A. , Uddling, J. , Schoefs, B. and Spetea, C. (2015) Mycorrhiza symbiosis increases the surface for sunlight capture in Medicago truncatula for better photosynthetic production. PLoS One, 10, e0115314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti, J. , Herold, S. , Schwarz, M. et al (2011) Strigolactone signaling is required for auxin‐dependent stimulation of secondary growth in plants. Proc. Natl Acad. Sci. USA, 108, 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrazem, O. , Diretto, G. , Argandona Picazo, J. et al (2019) The specialized roles in carotenogenesis and apocarotenogenesis of the phytoene synthase gene family in saffron. Front. Plant Sci. 10, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrazem, O. , Rubio‐Moraga, A. , Berman, J. , Capell, T. , Christou, P. , Zhu, C. and Gomez‐Gomez, L. (2016) The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 209, 650–663. [DOI] [PubMed] [Google Scholar]
- Akiyama, K. , Matsuzaki, K. and Hayashi, H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature, 435, 824–827. [DOI] [PubMed] [Google Scholar]
- Al‐Babili, S. and Bouwmeester, H.J. (2015) Strigolactones, a novel carotenoid‐derived plant hormone. Annu. Rev. Plant Biol. 66, 161–186. [DOI] [PubMed] [Google Scholar]
- Alagoz, Y. , Nayak, P. , Dhami, N. and Cazzonelli, C.I. (2018) cis‐carotene biosynthesis, evolution and regulation in plants: the emergence of novel signaling metabolites. Arch. Biochem. Biophys. 654, 172–184. [DOI] [PubMed] [Google Scholar]
- Alder, A. , Jamil, M. , Marzorati, M. , Bruno, M. , Vermathen, M. , Bigler, P. , Ghisla, S. , Bouwmeester, H. , Beyer, P. and Al‐Babili, S. (2012) The path from beta‐carotene to carlactone, a strigolactone‐like plant hormone. Science, 335, 1348–1351. [DOI] [PubMed] [Google Scholar]
- Aliferis, K.A. , Chamoun, R. and Jabaji, S. (2015) Metabolic responses of willow (Salix purpurea L.) leaves to mycorrhization as revealed by mass spectrometry and H‐1 NMR spectroscopy metabolite profiling. Front. Plant Sci. 6, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, D. , Voss, B. , Maass, D. , Wust, F. , Schaub, P. , Beyer, P. and Welsch, R. (2016) Carotenogenesis is regulated by 5'UTR‐mediated translation of phytoene synthase splice variants. Plant Physiol. 172, 2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, J.P. , Zhang, X.W. , Bi, S.Q. , You, C.X. , Wang, X.F. and Hao, Y.J. (2019) MdbHLH93, an apple activator regulating leaf senescence, is regulated by ABA and MdBT2 in antagonistic ways. New Phytol. 222, 735–751. [DOI] [PubMed] [Google Scholar]
- Avendano‐Vazquez, A.O. , Cordoba, E. , Llamas, E. et al (2014) An uncharacterized apocarotenoid‐derived signal generated in zeta‐carotene desaturase mutants regulates leaf development and the expression of chloroplast and nuclear genes in Arabidopsis. Plant Cell, 26, 2524–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando, N. , Hayashi, H. , Wakamatsu, S. , Inakuma, T. , Miyoshi, M. , Nagao, A. , Yamauchi, R. and Terao, J. (2004) Participation of singlet oxygen in ultraviolet‐a‐induced lipid peroxidation in mouse skin and its inhibition by dietary beta‐carotene: an ex vivo study. Free Radic. Biol. Med. 37, 1854–1863. [DOI] [PubMed] [Google Scholar]
- Bartlet, E. , Blight, M.M. , Lane, P. and Williams, I.H. (1997) The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol. Exp. Appl. 85, 257–262. [Google Scholar]
- Bartley, G.E. , Scolnik, P.A. and Beyer, P. (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta‐carotene desaturase, expressed in Escherichia coli, catalyze a poly‐cis pathway to yield pro‐lycopene. Eur. J. Biochem. 259, 396–403. [DOI] [PubMed] [Google Scholar]
- Baz, L. , Mori, N. , Mi, J. et al (2018) 3‐hydroxycarlactone, a novel product of the strigolactone biosynthesis core pathway. Mol. Plant, 11, 1312–1314. [DOI] [PubMed] [Google Scholar]
- Beltran, J.C.M. and Stange, C. (2016) Apocarotenoids: a new carotenoid‐derived pathway. Carotenoids in nature: biosynthesis, regulation and function. Subcell Biochem. 79, 239–272. [DOI] [PubMed] [Google Scholar]
- Bhuiyan, N.H. , Friso, G. , Rowland, E. , Majsec, K. and van Wijk, K.J. (2016) The plastoglobule‐localized metallopeptidase PGM48 is a positive regulator of senescence in Arabidopsis thaliana. Plant Cell, 28, 3020–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo, A. , Brands, M. , Wewer, V. , Dormann, P. and Harrison, M.J. (2017) Arbuscular mycorrhiza‐specific enzymes FatM and RAM2 fine‐tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 214, 1631–1645. [DOI] [PubMed] [Google Scholar]
- Brewer, P.B. , Koltai, H. and Beveridge, C.A. (2013) Diverse roles of strigolactones in plant development. Mol. Plant, 6, 18–28. [DOI] [PubMed] [Google Scholar]
- Brewer, P.B. , Yoneyama, K. , Filardo, F. et al (2016) LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc. Natl Acad. Sci. USA, 113, 6301–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett, M.C. and Tedersoo, L. (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115. [DOI] [PubMed] [Google Scholar]
- Bruno, M. and Al‐Babili, S. (2016) On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27. Planta, 243, 1429–1440. [DOI] [PubMed] [Google Scholar]
- Bruno, M. , Beyer, P. and Al‐Babili, S. (2015) The potato carotenoid cleavage dioxygenase 4 catalyzes a single cleavage of beta‐ionone ring‐containing carotenes and non‐epoxidated xanthophylls. Arch. Biochem. Biophys. 572, 126–133. [DOI] [PubMed] [Google Scholar]
- Bruno, M. , Hofmann, M. , Vermathen, M. , Alder, A. , Beyer, P. and Al‐Babili, S. (2014) On the substrate‐ and stereospecificity of the plant carotenoid cleavage dioxygenase 7. FEBS Lett. 588, 1802–1807. [DOI] [PubMed] [Google Scholar]
- Bruno, M. , Koschmieder, J. , Wuest, F. , Schaub, P. , Fehling‐Kaschek, M. , Timmer, J. , Beyer, P. and Al‐Babili, S. (2016) Enzymatic study on AtCCD4 and AtCCD7 and their potential to form acyclic regulatory metabolites. J. Exp. Bot. 67, 5993–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, M. , Vermathen, M. , Alder, A. , Wust, F. , Schaub, P. , van der Steen, R. , Beyer, P. , Ghisla, S. and Al‐Babili, S. (2017) Insights into the formation of carlactone from in‐depth analysis of the CCD8‐catalyzed reactions. FEBS Lett. 591, 792–800. [DOI] [PubMed] [Google Scholar]
- Burger, M. and Chory, J. (2020) The many models of strigolactone signaling. Trends Plant Sci. 25, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres, L.A. , Lakshminarayan, S. , Yeung, K.K. , McGarvey, B.D. , Hannoufa, A. , Sumarah, M.W. , Benitez, X. and Scott, I.M. (2016) Repellent and attractive effects of alpha‐, beta‐, and dihydro‐beta‐ ionone to generalist and specialist herbivores. J. Chem. Ecol. 42, 107–117. [DOI] [PubMed] [Google Scholar]
- Cazzonelli, C.I. , Hou, X. , Alagoz, Y. , Rivers, J. , Dhami, N. , Lee, J. , Marri, S. and Pogson, B.J. (2020) A cis‐carotene derived apocarotenoid regulates etioplast and chloroplast development. eLife, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, C. , Fang, J. , Liu, Y. et al (2011) ZEBRA2, encoding a carotenoid isomerase, is involved in photoprotection in rice. Plant Mol. Biol. 75, 211–221. [DOI] [PubMed] [Google Scholar]
- Charnikhova, T.V. , Gaus, K. , Lumbroso, A. , Sanders, M. , Vincken, J.P. , De Mesmaeker, A. , Ruyter‐Spira, C.P. , Screpanti, C. and Bouwmeester, H.J. (2017) Zealactones. Novel natural strigolactones from maize. Phytochemistry, 137, 123–131. [DOI] [PubMed] [Google Scholar]
- Charnikhova, T.V. , Gaus, K. , Lumbroso, A. , Sanders, M. , Vincken, J.P. , De Mesmaeker, A. , Ruyter‐Spira, C.P. , Screpanti, C. and Bouwmeester, H.J. (2018) Zeapyranolactone ‐ a novel strigolactone from maize. Phytochem. Lett. 24, 172–178. [DOI] [PubMed] [Google Scholar]
- Chen, K. , Li, G.J. , Bressan, R.A. , Song, C.P. , Zhu, J.K. and Zhao, Y. (2020) Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 62, 25–54. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Li, F. and Wurtzel, E.T. (2010) Isolation and characterization of the Z‐ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 153, 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Li, J.K. , Fan, K.J. et al (2017) Mutations in the maize zeta‐carotene desaturase gene lead to viviparous kernel. PLoS One, 12, e0174270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, A. , Pancaldi, S. , Fambrini, M. , Michelotti, V. , Bonora, A. , Salvini, M. and Pugliesi, C. (2004) A deficiency at the gene coding for zeta‐carotene desaturase characterizes the sunflower non dormant‐1 mutant. Plant Cell Phys. 45, 445–455. [DOI] [PubMed] [Google Scholar]
- Cook, C.E. , Whichard, L.P. , Turner, B. , Wall, M.E. and Egley, G.H. (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science, 154, 1189–1190. [DOI] [PubMed] [Google Scholar]
- Cutler, A.J. and Krochko, J.E. (1999) Formation and breakdown of ABA. Trends Plant Sci. 4, 472–478. [DOI] [PubMed] [Google Scholar]
- D'Alessandro, S. and Havaux, M. (2019) Sensing beta‐carotene oxidation in photosystem II to master plant stress tolerance. New Phytol. 223, 1776–1783. [DOI] [PubMed] [Google Scholar]
- D'Alessandro, S. , Ksas, B. and Havaux, M. (2018) Decoding beta‐cyclocitral‐mediated retrograde signaling reveals the role of a detoxification response in plant tolerance to photooxidative stress. Plant Cell, 30, 2495–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro, S. , Mizokami, Y. , Legeret, B. and Havaux, M. (2019) The apocarotenoid beta‐cyclocitric acid elicits drought tolerance in plants. iScience, 19, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh, S. , Epley, M. , Diven, W. and Ellis, D. (1990) Aminoaciduria of phosphate depletion manifests at the renal brush border membrane. Miner Electrolyte Metab. 16, 216–223. [PubMed] [Google Scholar]
- Dall'Osto, L. , Fiore, A. , Cazzaniga, S. , Giuliano, G. and Bassi, R. (2007) Different roles of alpha‐ and beta‐branch xanthophylls in photosystem assembly and photoprotection. J. Biol. Chem. 282, 35056–35068. [DOI] [PubMed] [Google Scholar]
- de Saint, G.A. , Bonhomme, S. , Boyer, F.D. and Rameau, C. (2013) Novel insights into strigolactone distribution and signalling. Curr. Opin. Plant Biol. 16, 583–589. [DOI] [PubMed] [Google Scholar]
- De Vleesschauwer, D. , Yang, Y. , Cruz, C.V. and Hofte, M. (2010) Abscisic acid‐induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase‐mediated repression of ethylene signaling. Plant Physiol. 152, 2036–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, E.L. , Alder, A. , Hunn, S. et al (2017) Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol. 216, 455–468. [DOI] [PubMed] [Google Scholar]
- Demurtas, O.C. , Frusciante, S. , Ferrante, P. et al (2018) Candidate enzymes for saffron crocin biosynthesis are localized in multiple cellular compartments. Plant Physiol. 177, 990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, A.J. , Lehner, K. , Mi, J. , Jia, K.P. , Mijar, M. , Dinneny, J. , Al‐Babili, S. and Benfey, P.N. (2019) beta‐Cyclocitral is a conserved root growth regulator. Proc. Natl Acad. Sci. USA, 116, 10563–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diretto, G. , Frusciante, S. , Fabbri, C. et al (2020) Manipulation of beta‐carotene levels in tomato fruits results in increased ABA content and extended shelf life. Plant Biotechol. J. 18, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogbo, O. , Laferriere, A. , D'Harlingue, A. and Camara, B. (1988) Carotenoid biosynthesis: isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc. Natl Acad. Sci. USA, 85, 7054–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V. , Rochaix, J.D. and Kim, C. (2018) Singlet oxygen‐triggered chloroplast‐to‐nucleus retrograde signalling pathways: an emerging perspective. Plant Cell Environ. 41, 1727–1738. [DOI] [PubMed] [Google Scholar]
- El‐Esawi, M. , Arthaut, L.D. , Jourdan, N. , d'Harlingue, A. , Link, J. , Martino, C.F. and Ahmad, M. (2017) Blue‐light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 7, 13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estavillo, G.M. , Crisp, P.A. , Pornsiriwong, W. et al (2011) Evidence for a SAL1‐PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell, 23, 3992–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, J. , Chai, C.L. , Qian, Q. et al (2008) Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre‐harvest sprouting and photo‐oxidation in rice. Plant J. J54, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felemban, A. , Braguy, J. , Zurbriggen, M.D. and Al‐Babili, S. (2019) Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 10, 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester, T. , Hause, B. , Schmidt, D. , Halfmann, K. , Schmidt, J. , Wray, V. , Hause, G. and Strack, D. (2002a) Occurrence and localization of apocarotenoids in arbuscular mycorrhizal plant roots. Plant Cell Physiol. 43, 256–265. [DOI] [PubMed] [Google Scholar]
- Fester, T. , Maier, W. and Strack, D. (1999) Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co‐inoculation with rhizosphere bacteria. Mycorrhiza, 8, 241–246. [Google Scholar]
- Fester, T. , Schmidt, D. , Lohse, S. , Walter, M.H. , Giuliano, G. , Bramley, P.M. , Fraser, P.D. , Hause, B. and Strack, D. (2002b) Stimulation of carotenoid metabolism in arbuscular mycorrhizal roots. Planta, 216, 148–154. [DOI] [PubMed] [Google Scholar]
- Fiedor, J. , Fiedor, L. , Haessner, R. and Scheer, H. (2005) Cyclic endoperoxides of beta‐carotene, potential pro‐oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta, 1709, 1–4. [DOI] [PubMed] [Google Scholar]
- Fiedor, J. , Fiedor, L. , Winkler, J. , Scherz, A. and Scheer, H. (2001) Photodynamics of the bacteriochlorophyll‐carotenoid system. 1. Bacteriochlorophyll‐photosensitized oxygenation of beta‐carotene in acetone. Photochem. Photobiol. 74, 64–71. [DOI] [PubMed] [Google Scholar]
- Finkelstein, R. (2013) Abscisic acid synthesis and response. Arabidopsis Book, 11, e0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorilli, V. , Vallino, M. , Biselli, C. , Faccio, A. , Bagnaresi, P. and Bonfante, P. (2015) Host and non‐host roots in rice: cellular and molecular approaches reveal differential responses to arbuscular mycorrhizal fungi. Front. Plant Sci. 6, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorilli, V. , Wang, J.Y. , Bonfante, P. , Lanfranco, L. and Al‐Babili, S. (2019) Apocarotenoids: old and new mediators of the arbuscular mycorrhizal symbiosis. Front. Plant Sci. 10, 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss, D.S. , Hause, B. , Lange, P.R. , Kuster, H. , Strack, D. and Walter, M.H. (2008a) Knock‐down of the MEP pathway isogene 1‐deoxy‐D‐xylulose 5‐phosphate synthase 2 inhibits formation of arbuscular mycorrhiza‐induced apocarotenoids, and abolishes normal expression of mycorrhiza‐specific plant marker genes. Plant J. 56, 86–100. [DOI] [PubMed] [Google Scholar]
- Floss, D.S. , Schliemann, W. , Schmidt, J. , Strack, D. and Walter, M.H. (2008b) RNA interference‐mediated repression of MtCCD1 in mycorrhizal roots of Medicago truncatula causes accumulation of C27 apocarotenoids, shedding light on the functional role of CCD1. Plant Physiol. 148, 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode, B. , Siemsen, T. , Thurow, C. , Weigel, R. and Gatz, C. (2008) The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress‐inducible promoters. Plant Cell, 20, 3122–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudree, A. , Aluru, M. and Rodermel, S. (2010) PDS activity acts as a rheostat of retrograde signaling during early chloroplast biogenesis. Plant Signal. Behav. 5, 1629–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frusciante, S. , Diretto, G. , Bruno, M. et al (2014) Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc. Natl Acad. Sci. USA, 111, 12246–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpaz, N. , Burger, Y. , Lavee, T. et al (2013) Genetic and chemical characterization of an EMS induced mutation in Cucumis melo CRTISO gene. Arch. Biochem. Biophys. 539, 117–125. [DOI] [PubMed] [Google Scholar]
- Galpaz, N. , Wang, Q. , Menda, N. , Zamir, D. and Hirschberg, J. (2008) Abscisic acid deficiency in the tomato mutant high‐pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 53, 717–730. [DOI] [PubMed] [Google Scholar]
- Gao, L. , Gonda, I. , Sun, H. et al (2019) The tomato pan‐genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 51, 1044–1051. [DOI] [PubMed] [Google Scholar]
- Giuliano, G. , Al‐Babili, S. and von Lintig, J. (2003) Carotenoid oxygenases: cleave it or leave it. Trends Plant Sci. 8, 145–149. [DOI] [PubMed] [Google Scholar]
- Gomez‐Roldan, V. , Fermas, S. , Brewer, P.B. et al (2008) Strigolactone inhibition of shoot branching. Nature, 455, 189–194. [DOI] [PubMed] [Google Scholar]
- Gruber, M.Y. , Xu, N. , Grenkow, L. , Li, X. , Onyilagha, J. , Soroka, J.J. , Westcott, N.D. and Hegedus, D.D. (2009) Responses of the crucifer flea beetle to brassica volatiles in an olfactometer. Environ. Entomol. 38, 1467–1479. [DOI] [PubMed] [Google Scholar]
- Guan, J.C. , Koch, K.E. , Suzuki, M. , Wu, S. , Latshaw, S. , Petruff, T. , Goulet, C. , Klee, H.J. and McCarty, D.R. (2012) Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching‐specific subnetwork. Plant Physiol. 160, 1303–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, C.V. , Leyva‐Gonzalez, M.A. , Osakabe, Y. et al (2014) Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl Acad. Sci. USA, 111, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hable, W.E. , Oishi, K.K. and Schumaker, K.S. (1998) Viviparous‐5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol. Gen. Genet. 257, 167–176. [DOI] [PubMed] [Google Scholar]
- Haider, I. , Andreo‐Jimenez, B. , Bruno, M. et al (2018) The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 69, 2403–2414. [DOI] [PubMed] [Google Scholar]
- Han, H.P. , Li, Y.X. and Zhou, S.F. (2008) Overexpression of phytoene synthase gene from Salicornia europaea alters response to reactive oxygen species under salt stress in transgenic Arabidopsis. Biotechnol. Lett. 30, 1501–1507. [DOI] [PubMed] [Google Scholar]
- Hans, J. , Hause, B. , Strack, D. and Walter, M.H. (2004) Cloning, characterization, and immunolocalization of a mycorrhiza‐inducible 1‐deoxy‐d‐xylulose 5‐phosphate reductoisomerase in arbuscule‐containing cells of maize. Plant Physiol. 134, 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helber, N. , Wippel, K. , Sauer, N. , Schaarschmidt, S. , Hause, B. and Requena, N. (2011) A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell, 23, 3812–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, E.M. , Robinson, L.A. , Abdul‐Sada, A. , Vanbergen, A.J. , Hodge, A. and Hartley, S.E. (2018) Arbuscular mycorrhizal fungi and plant chemical defence: effects of colonisation on aboveground and belowground metabolomes. J. Chem. Ecol. 44, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg, J. (2001) Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 4, 210–218. [DOI] [PubMed] [Google Scholar]
- Hoffmann, B. , Proust, H. , Belcram, K. , Labrune, C. , Boyer, F.D. , Rameau, C. and Bonhomme, S. (2014) Strigolactones inhibit caulonema elongation and cell division in the moss Physcomitrella patens. PLoS One, 9, e99206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, X. , Rivers, J. , Leon, P. , McQuinn, R.P. and Pogson, B.J. (2016) Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 21, 792–803. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , Yamauchi, T. , Yang, J. et al (2014) Strigolactone and cytokinin act antagonistically in regulating rice mesocotyl elongation in darkness. Plant Cell Physiol. 55, 30–41. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , Yan, H. , Yang, J. , Yamaguchi, S. , Maekawa, M. , Takamure, I. , Tsutsumi, N. , Kyozuka, J. and Nakazono, M. (2010) Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol. 51, 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, F.C. , Molnar, P. and Schwab, W. (2009) Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 60, 3011–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg, A. , Beyer, P. and Al‐Babili, S. (2009) Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J. 276, 736–747. [DOI] [PubMed] [Google Scholar]
- Ilg, A. , Bruno, M. , Beyer, P. and Al‐Babili, S. (2014) Tomato carotenoid cleavage dioxygenases 1A and 1B: Relaxed double bond specificity leads to a plenitude of dialdehydes, mono‐apocarotenoids and isoprenoid volatiles. FEBS Open Bio, 4, 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inze, A. , Vanderauwera, S. , Hoeberichts, F.A. , Vandorpe, M. , Van Gaever, T. and Van Breusegem, F. (2012) A subcellular localization compendium of hydrogen peroxide‐induced proteins. Plant Cell Environ. 35, 308–320. [DOI] [PubMed] [Google Scholar]
- Isaacson, T. , Ronen, G. , Zamir, D. and Hirschberg, J. (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta‐carotene and xanthophylls in plants. Plant Cell, 14, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki, M. , Shida, K. , Kuwabara, K. , Wakabayashi, T. , Mizutani, M. , Takikawa, H. and Sugimoto, Y. (2018) Evidence for species‐dependent biosynthetic pathways for converting carlactone to strigolactones in plants. J. Exp. Bot. 69, 2305–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, K.P. , Baz, L. and Al‐Babili, S. (2018) From carotenoids to strigolactones. J. Exp. Bot. 69, 2189–2204. [DOI] [PubMed] [Google Scholar]
- Jia, K.P. , Dickinson, A.J. , Mi, J. et al (2019) Anchorene is a carotenoid‐derived regulatory metabolite required for anchor root formation in Arabidopsis. Sci. Adv. 5, eaaw6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, K.P. , Luo, Q. , He, S.B. , Lu, X.D. and Yang, H.Q. (2014) Strigolactone‐regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis. Mol. Plant, 7, 528–540.24126495 [Google Scholar]
- Jiang, Y.N. , Wang, W.X. , Xie, Q.J. et al (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science, 356, 1172–1175. [DOI] [PubMed] [Google Scholar]
- Jing, G.X. , Li, T.T. , Qu, H.X. , Yun, Z. , Jia, Y.X. , Zheng, X.L. and Jiang, Y.M. (2015) Carotenoids and volatile profiles of yellow‐ and red‐fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes. Postharvest Biol. Technol. 109, 114–119. [Google Scholar]
- Kachanovsky, D.E. , Filler, S. , Isaacson, T. and Hirschberg, J. (2012) Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis‐carotenoids. Proc. Natl Acad. Sci. USA, 109, 19021–19026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keymer, A. , Pimprikar, P. , Wewer, V. et al (2017) Lipid transfer from plants to arbuscular mycorrhiza fungi. elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T. , Kang, K. , Kim, S.H. , An, G. and Paek, N.C. (2019) OsWRKY5 promotes rice leaf senescence via senescence‐associated NAC and abscisic acid biosynthesis pathway. Int. J. Mol. Sci. 20(18), 4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisugi, T. , Xie, X. , Kim, H.I. et al (2013) Strigone, isolation and identification as a natural strigolactone from Houttuynia cordata . Phytochemistry, 87, 60–64. [DOI] [PubMed] [Google Scholar]
- Klingner, A. , Bothe, H. , Wray, V. and Marner, F.J. (1995a) Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry, 38, 53–55. [Google Scholar]
- Klingner, A. , Hundeshagen, B. , Kernebeck, H. and Bothe, H. (1995b) Localization of the yellow pigment formed in roots of gramineous plants colonized by arbuscular fungi. Protoplasma, 185, 50–57. [Google Scholar]
- Kohlen, W. , Charnikhova, T. , Bours, R. , Lopez‐Raez, J.A. and Bouwmeester, H. (2013) Tomato strigolactones: a more detailed look. Plant Signal. Behav., 8, e22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlen, W. , Charnikhova, T. , Lammers, M. et al (2012) The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol. 196, 535–547. [DOI] [PubMed] [Google Scholar]
- Kong, C.H. , Zhang, S.Z. , Li, Y.H. , Xia, Z.C. , Yang, X.F. , Meiners, S.J. and Wang, P. (2018) Plant neighbor detection and allelochemical response are driven by root‐secreted signaling chemicals. Nat. Commun. 9, 3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky, N.I. (1989) Antioxidant functions of carotenoids. Free Radic. Biol. Med. 7, 617–635. [DOI] [PubMed] [Google Scholar]
- Lanfranco, L. , Fiorilli, V. , Venice, F. and Bonfante, P. (2018) Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. J. Exp. Bot. 69, 2175–2188. [DOI] [PubMed] [Google Scholar]
- Latari, K. , Wust, F. , Hubner, M. , Schaub, P. , Beisel, K.G. , Matsubara, S. , Beyer, P. and Welsch, R. (2015) Tissue‐specific apocarotenoid glycosylation contributes to carotenoid homeostasis in Arabidopsis leaves. Plant Physiol. 168, 1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazazzara, V. , Bueschl, C. , Parich, A. , Pertot, I. , Schuhmacher, R. and Perazzolli, M. (2018) Downy mildew symptoms on grapevines can be reduced by volatile organic compounds of resistant genotypes. Sci. Rep. 8, 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.H. , Piao, H.L. , Kim, H.Y. , Choi, S.M. , Jiang, F. , Hartung, W. , Hwang, I. , Kwak, J.M. , Lee, I.J. and Hwang, I. (2006) Activation of glucosidase via stress‐induced polymerization rapidly increases active pools of abscisic acid. Cell, 126, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Li, F. , Murillo, C. and Wurtzel, E.T. (2007) Maize Y9 encodes a product essential for 15‐cis‐zeta‐carotene isomerization. Plant Physiol. 144, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Vallabhaneni, R. and Wurtzel, E.T. (2008) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress‐induced root carotenogenesis. Plant Physiol. 146, 1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L.L. , Zhao, H.H. and Kong, C.H. (2020) (‐)‐Loliolide, the most ubiquitous lactone, is involved in barnyardgrass‐induced rice allelopathy. J. Exp. Bot. 71, 1540–1550. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zhang, J. , Zhang, J. , Hao, L. , Hua, J. , Duan, L. , Zhang, M. and Li, Z. (2013) Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotech. J. 11, 747–758. [DOI] [PubMed] [Google Scholar]
- Li, Z.H. , Matthews, P.D. , Burr, B. and Wurtzel, E.T. (1996) Cloning and characterization of a maize cDNA encoding phytoene desaturase, an enzyme of the carotenoid biosynthetic pathway. Plant Mol. Biol. 30, 269–279. [DOI] [PubMed] [Google Scholar]
- Lim, C.W. , Baek, W. , Jung, J. , Kim, J.H. and Lee, S.C. (2015) Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 16, 15251–15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Raez, J.A. , Fernandez, I. , Garcia, J.M. , Berrio, E. , Bonfante, P. , Walter, M.H. and Pozo, M.J. (2015) Differential spatio‐temporal expression of carotenoid cleavage dioxygenases regulates apocarotenoid fluxes during AM symbiosis. Plant Sci. 230, 59–69. [DOI] [PubMed] [Google Scholar]
- Lopez‐Raez, J.A. , Shirasu, K. and Foo, E. (2017) Strigolactones in plant interactions with beneficial and detrimental organisms: the yin and yang. Trends Plant Sci. 22, 527–537. [DOI] [PubMed] [Google Scholar]
- Lucas, M. , Swarup, R. , Paponov, I.A. et al (2011) Short‐root regulates primary, lateral, and adventitious root development in Arabidopsis. Plant Physiol. 155, 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuehl, L.H. , Menard, G.N. , Kurup, S. , Van Erp, H. , Radhakrishnan, G.V. , Breakspear, A. , Oldroyd, G.E.D. and Eastmond, P.J. (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science, 356, 1175–1178. [DOI] [PubMed] [Google Scholar]
- Lv, X. , Li, H. , Chen, X. , Xiang, X. , Guo, Z. , Yu, J. and Zhou, Y. (2018) The role of calcium‐dependent protein kinase in hydrogen peroxide, nitric oxide and ABA‐dependent cold acclimation. J. Exp. Bot. 69, 4127–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, N.N. , Feng, H.L. , Meng, X. , Li, D. , Yang, D.Y. , Wu, C.G. and Meng, Q.W. (2014) Overexpression of tomato SlNAC1 transcription factor alters fruit pigmentation and softening. BMC Plant Biol. 14, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, W. , Peipp, H. , Schmidt, J. , Wray, V. and Strack, D. (1995) Levels of a terpenoid glycoside (blumenin) and cell wall‐bound phenolics in some cereal mycorrhizas. Plant Physiol. 109, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcheska, F. , Ahmad, A. , Batool, S. et al (2017) Drought‐enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant Physiol. 174, 798–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano, J. (2012) Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 59, 90–97. [DOI] [PubMed] [Google Scholar]
- Matthews, P.D. , Luo, R. and Wurtzel, E.T. (2003) Maize phytoene desaturase and zeta‐carotene desaturase catalyse a poly‐Z desaturation pathway: implications for genetic engineering of carotenoid content among cereal crops. J. Exp. Bot. 54, 2215–2230. [DOI] [PubMed] [Google Scholar]
- Matthys, C. , Walton, A. , Struk, S. , Stes, E. , Boyer, F.D. , Gevaert, K. and Goormachtig, S. (2016) The whats, the wheres and the hows of strigolactone action in the roots. Planta, 243, 1327–1337. [DOI] [PubMed] [Google Scholar]
- McQuinn, R.P. , Gapper, N.E. , Gray, A.G. , Zhong, S.L. , Tohge, T. , Fei, Z.J. , Fernie, A.R. and Giovannoni, J.J. (2020) Manipulation of ZDS in tomato exposes carotenoid‐ and ABA‐specific effects on fruit development and ripening. Plant Biotech. J. 18, 2210–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi, J. , Jia, K.P. , Balakrishna, A. , Wang, J.Y. and Al‐Babili, S. (2019) An LC‐MS profiling method reveals a route for apocarotene glycosylation and shows its induction by high light stress in Arabidopsis. Analyst, 144, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Mi, J. , Jia, K.P. , Wang, J.Y. and Al‐Babili, S. (2018) A rapid LC‐MS method for qualitative and quantitative profiling of plant apocarotenoids. Anal. Chim. Acta, 1035, 87–95. [DOI] [PubMed] [Google Scholar]
- Moise, A.R. , Al‐Babili, S. and Wurtzel, E.T. (2014) Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 114, 164–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, J.C. , Mi, J. , Agrawal, S. , Kossler, S. , Tureckova, V. , Tarkowska, D. , Thiele, W. , Al‐Babili, S. , Bock, R. and Schottler, M.A. (2020) Expression of a carotenogenic gene allows faster biomass production by redesigning plant architecture and improving photosynthetic efficiency in tobacco. Plant J. 103, 1967–1984. [DOI] [PubMed] [Google Scholar]
- Mori, N. , Sado, A. , Xie, X.N. , Yoneyama, K. , Asami, K. , Seto, Y. , Nomura, T. , Yamaguchi, S. , Yoneyama, K. and Akiyama, K. (2020) Chemical identification of 18‐hydroxycarlactonoic acid as an LjMAX1 product and in planta conversion of its methyl ester to canonical and non‐canonical strigolactones in Lotus japonicus . Phytochemistry, 174, 112349. [DOI] [PubMed] [Google Scholar]
- Murata, M. , Kobayashi, T. and Seo, S. (2019a) alpha‐ionone, an apocarotenoid, induces plant resistance to western flower thrips, Frankliniella occidentalis, independently of jasmonic acid. Molecules, 25, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, M. , Nakai, Y. , Kawazu, K. et al (2019b) Loliolide, a carotenoid metabolite, is a potential endogenous inducer of herbivore resistance. Plant Physiol. 179, 1822–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naested, H. , Holm, A. , Jenkins, T. et al (2004) Arabidopsis VARIEGATED 3 encodes a chloroplast‐targeted, zinc‐finger protein required for chloroplast and palisade cell development. J. Cell Sci. 117, 4807–4818. [DOI] [PubMed] [Google Scholar]
- Nasir, F. , Tian, L. , Shi, S. , Chang, C. , Ma, L. , Gao, Y. and Tian, C. (2019) Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Biochem. 142, 106–116. [DOI] [PubMed] [Google Scholar]
- Neuman, H. , Galpaz, N. , Cunningham, F.X. Jr , Zamir, D. and Hirschberg, J. (2014) The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J. 78, 80–93. [DOI] [PubMed] [Google Scholar]
- Nisar, N. , Li, L. , Lu, S. , Khin, N.C. and Pogson, B.J. (2015) Carotenoid metabolism in plants. Mol. Plant, 8, 68–82. [DOI] [PubMed] [Google Scholar]
- Nonogaki, M. and Nonogaki, H. (2017) Prevention of preharvest sprouting through hormone engineering and germination recovery by chemical biology. Front. Plant Sci. 8, 90 10.3389/fpls.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalala, S.O. , Petersen, M.A. and Grout, B.W.W. (2013) Volatile compounds from leaves of the African spider plant (Gynandropsis gynandra) with bioactivity against spider mite (Tetranychus urticae). Ann. Appl. Biol. 162, 290–298. [Google Scholar]
- Omura, H. , Honda, K. and Hayashi, N. (2000) Floral scent of Osmanthus fragrans discourages foraging behavior of cabbage butterfly, Pieris rapae. J. Chem. Ecol. 26, 655–666. [Google Scholar]
- Pan, Z.Y. , Zeng, Y.L. , An, J.Y. , Ye, J.L. , Xu, Q. and Deng, X.X. (2012) An integrative analysis of transcriptome and proteome provides new insights into carotenoid biosynthesis and regulation in sweet orange fruits. J. Proteomics, 75, 4879–4880. [DOI] [PubMed] [Google Scholar]
- Park, H. , Kreunen, S.S. , Cuttriss, A.J. , DellaPenna, D. and Pogson, B.J. (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell, 14, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Fons, L. and Fraser, P.D. (2012) Analysis of diapocarotenoids found in pigmented Bacillus species. Methods Mol. Biol. 892, 335–345. [DOI] [PubMed] [Google Scholar]
- Perreau, F. , Frey, A. , Effroy‐Cuzzi, D. , Savane, P. , Berger, A. , Gissot, L. and Marion‐Poll, A. (2020) ABSCISIC ACID‐DEFICIENT4 has an essential function in both cis‐violaxanthin and cis‐neoxanthin synthesis. Plant Physiol. 84, 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda, A. , Zheng, S.J. , van Loon, J.J.A. , Pieterse, C.M.J. and Dicke, M. (2010) Helping plants to deal with insects: the role of beneficial soil‐borne microbes. Trends Plant Sci. 15, 507–514. [DOI] [PubMed] [Google Scholar]
- Pivnick, K.A. , Lamb, R.J. and Reed, D. (1992) Response of flea beetles, Phyllotreta spp., to mustard oils and nitriles in field trapping experiments. J. Chem. Ecol. 18, 863–873. [DOI] [PubMed] [Google Scholar]
- Proust, H. , Hoffmann, B. , Xie, X. , Yoneyama, K. , Schaefer, D.G. , Yoneyama, K. , Nogue, F. and Rameau, C. (2011) Strigolactones regulate protonema branching and act as a quorum sensing‐like signal in the moss Physcomitrella patens. Development, 138, 1531–1539. [DOI] [PubMed] [Google Scholar]
- Qin, G. , Gu, H. , Ma, L. , Peng, Y. , Deng, X.W. , Chen, Z. and Qu, L.J. (2007) Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 17, 471–482. [DOI] [PubMed] [Google Scholar]
- Qin, X. and Zeevaart, J.A. (1999) The 9‐cis‐epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water‐stressed bean. Proc. Natl Acad. Sci. USA, 96, 15354–15361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel, F. , Birtic, S. , Cuine, S. , Triantaphylides, C. , Ravanat, J.L. and Havaux, M. (2012a) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 158, 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel, F. , Birtic, S. , Ginies, C. , Soubigou‐Taconnat, L. , Triantaphylides, C. and Havaux, M. (2012b) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl Acad. Sci. USA, 109, 5535–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel, F. , Mialoundama, A.S. and Havaux, M. (2013) Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 64, 799–805. [DOI] [PubMed] [Google Scholar]
- Rasmussen, A. , Beveridge, C.A. and Geelen, D. (2012a) Inhibition of strigolactones promotes adventitious root formation. Plant Signal. Behav. 7, 694–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, A. , Mason, M.G. , De Cuyper, C. et al (2012b) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol. 158, 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repeta, D.J. (1989) Carotenoid diagenesis in recent marine‐sediments. 2. degradation of fucoxanthin to loliolide. Geochim. Cosmochim. Acta, 53, 699–707. [Google Scholar]
- Rios, J.J. , Fernandez‐Garcia, E. , Minguez‐Mosquera, M.I. and Perez‐Galvez, A. (2008) Description of volatile compounds generated by the degradation of carotenoids in paprika, tomato and marigold oleoresins. Food Chem. 106, 1145–1153. [Google Scholar]
- Rivers, J.Y. , Truong, T.T. , Pogson, B.J. and McQuinn, R.P. (2019) Volatile apocarotenoid discovery and quantification in Arabidopsis thaliana: optimized sensitive analysis via HS‐SPME‐GC/MS. Metabolomics, 15, 79. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Concepcion, M. (2010) Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 504, 118–122. [DOI] [PubMed] [Google Scholar]
- Rooney, D.C. , Killham, K. , Bending, G.D. , Baggs, E. , Weih, M. and Hodge, A. (2009) Mycorrhizas and biomass crops: opportunities for future sustainable development. Trends Plant Sci. 14, 542–549. [DOI] [PubMed] [Google Scholar]
- Rubio‐Moraga, A. , Rambla, J.L. , Fernandez‐de‐Carmen, A. , Trapero‐Mozos, A. , Ahrazem, O. , Orzaez, D. , Granell, A. and Gomez‐Gomez, L. (2014) New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress‐induced carotenoid cleavage dioxygenase gene from Crocus sativus . Plant Mol. Biol. 86, 555–569. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Sola, M.A. and Rodriguez‐Concepcion, M. (2012) Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book, 10, e0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, S. , Hirai, N. , Matsumoto, C. , Ohigashi, H. , Ohta, D. , Sakata, K. and Mizutani, M. (2004) Arabidopsis CYP707As encode (+)‐abscisic acid 8'‐hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 134, 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann, H. Jr (1992) Plant metabolism of xenobiotics. Trends Biochem. Sci. 17, 82–84. [DOI] [PubMed] [Google Scholar]
- Sang, D. , Chen, D. , Liu, G. et al (2014) Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl Acad. Sci. USA, 111, 11199–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannerini, S. and Bonfantefasolo, P. (1977) Unusual plastids in an endomycorrhizal root. Can. J. Bot., 55, 2471–2474. [Google Scholar]
- Schaub, P. , Rodriguez‐Franco, M. , Cazzonelli, C.I. , Alvarez, D. , Wust, F. and Welsch, R. (2018) Establishment of an Arabidopsis callus system to study the interrelations of biosynthesis, degradation and accumulation of carotenoids. PLoS One, 13, e0192158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub, P. , Yu, Q. , Gemmecker, S. , Poussin‐Courmontagne, P. , Mailliot, J. , McEwen, A.G. , Ghisla, S. , Al‐Babili, S. , Cavarelli, J. and Beyer, P. (2012) On the structure and function of the phytoene desaturase CRTI from Pantoea ananatis, a membrane‐peripheral and FAD‐dependent oxidase/isomerase. PLoS One, 7, e39550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, W. , Davidovich‐Rikanati, R. and Lewinsohn, E. (2008) Biosynthesis of plant‐derived flavor compounds. Plant J. 54, 712–732. [DOI] [PubMed] [Google Scholar]
- Schwartz, S.H. , Qin, X. and Zeevaart, J.A. (2001) Characterization of a novel carotenoid cleavage dioxygenase from plants. J. Biol. Chem. 276, 25208–25211. [DOI] [PubMed] [Google Scholar]
- Schwartz, S.H. , Tan, B.C. , Gage, D.A. , Zeevaart, J.A. and McCarty, D.R. (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science, 276, 1872–1874. [DOI] [PubMed] [Google Scholar]
- Schweiger, R. , Baier, M.C. , Persicke, M. and Muller, C. (2014) High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat. Commun. 5, 3886. [DOI] [PubMed] [Google Scholar]
- Seo, M. , Peeters, A.J. , Koiwai, H. , Oritani, T. , Marion‐Poll, A. , Zeevaart, J.A. , Koornneef, M. , Kamiya, Y. and Koshiba, T. (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl Acad. Sci. USA, 97, 12908–12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto, Y. and Yamaguchi, S. (2014) Strigolactone biosynthesis and perception. Curr. Opin. Plant Biol. 21, 1–6. [DOI] [PubMed] [Google Scholar]
- Shao, N. , Duan, G.Y. and Bock, R. (2013) A mediator of singlet oxygen responses in Chlamydomonas reinhardtii and Arabidopsis identified by a luciferase‐based genetic screen in algal cells. Plant Cell, 25, 4209–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, E. , Anand, G. and Kapoor, R. (2017) Terpenoids in plant and arbuscular mycorrhiza‐reinforced defence against herbivorous insects. Ann. Bot. 119, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Jiang, C.Q. , Yan, Y.F. , Liu, B.R. and Zu, C.L. (2017) Effect of increased UV‐B radiation on carotenoid accumulation and total antioxidant capacity in tobacco (Nicotiana tabacum L.) leaves. Genet. Mol. Res. 16 10.4238/gmr16018438 [DOI] [PubMed] [Google Scholar]
- Shi, J.A. , Cao, C. , Xu, J.Y. and Zhou, C.H. (2020) Research advances on biosynthesis, regulation, and biological activities of apocarotenoid aroma in horticultural plants. J. Chem. 2020, 1–11. [Google Scholar]
- Shindo, M. , Yamamoto, S. , Shimomura, K. and Umehara, M. (2020) Strigolactones decrease leaf angle in response to nutrient deficiencies in rice. Front. Plant Sci. 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumbe, L. , Bott, R. and Havaux, M. (2014) Dihydroactinidiolide, a high light‐induced beta‐carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis. Mol. Plant, 7, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Shumbe, L. , D'Alessandro, S. , Shao, N. , Chevalier, A. , Ksas, B. , Bock, R. and Havaux, M. (2017) METHYLENE BLUE SENSITIVITY 1 (MBS1) is required for acclimation of Arabidopsis to singlet oxygen and acts downstream of beta‐cyclocitral. Plant Cell Environ. 40, 216–226. [DOI] [PubMed] [Google Scholar]
- Simkin, A.J. , Schwartz, S.H. , Auldridge, M. , Taylor, M.G. and Klee, H.J. (2004) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta‐ionone, pseudoionone, and geranylacetone. Plant J. 40, 882–892. [DOI] [PubMed] [Google Scholar]
- Smith, S.M. and Li, J. (2014) Signalling and responses to strigolactones and karrikins. Curr. Opin. Plant Biol. 21, 23–29. [DOI] [PubMed] [Google Scholar]
- Snowden, K.C. , Simkin, A.J. , Janssen, B.J. , Templeton, K.R. , Loucas, H.M. , Simons, J.L. , Karunairetnam, S. , Gleave, A.P. , Clark, D.G. and Klee, H.J. (2005) The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell, 17, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L.J. , Chen, Z.F. and Larkin, R.M. (2018) The genomes uncoupled mutants are more sensitive to norflurazon than wild type. Plant Physiol. 178, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder, R. , Welsch, R. , Camagna, M. , Kohlen, W. , Balcke, G.U. , Tissier, A. and Walter, M.H. (2018) Strigolactone levels in dicot roots are determined by an ancestral symbiosis‐regulated clade of the PHYTOENE SYNTHASE gene family. Front. Plant Sci. 9, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack, D. and Fester, T. (2006) Isoprenoid metabolism and plastid reorganization in arbuscular mycorrhizal roots. New Phytol. 172, 22–34. [DOI] [PubMed] [Google Scholar]
- Stratton, S.P. , Schaefer, W.H. and Liebler, D.C. (1993) Isolation and identification of singlet oxygen oxidation products of beta‐carotene. Chem. Res. Toxicol. 6, 542–547. [DOI] [PubMed] [Google Scholar]
- Sui, X. , Kiser, P.D. , Lintig, J. and Palczewski, K. (2013) Structural basis of carotenoid cleavage: from bacteria to mammals. Arch. Biochem. Biophys. 539, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Tao, J. , Liu, S. , Huang, S. , Chen, S. , Xie, X. , Yoneyama, K. , Zhang, Y. and Xu, G. (2014) Strigolactones are involved in phosphate‐ and nitrate‐deficiency‐induced root development and auxin transport in rice. J. Exp. Bot. 65, 6735–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Xu, F. , Guo, X. , Wu, D. , Zhang, X. , Lou, M. , Luo, F. , Zhao, Q. , Xu, G. and Zhang, Y. (2019) A strigolactone signal inhibits secondary lateral root development in rice. Front. Plant Sci. 10, 1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S. , Wang, T. , Wang, L. , Li, X. , Jia, Y. , Liu, C. , Huang, X. , Xie, W. and Wang, X. (2018) Natural selection of a GSK3 determines rice mesocotyl domestication by coordinating strigolactone and brassinosteroid signaling. Nat. Commun. 9, 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, B.C. , Joseph, L.M. , Deng, W.T. , Liu, L. , Li, Q.B. , Cline, K. and McCarty, D.R. (2003) Molecular characterization of the Arabidopsis 9‐cis epoxycarotenoid dioxygenase gene family. Plant J. 35, 44–56. [DOI] [PubMed] [Google Scholar]
- Toh, S. , Kamiya, Y. , Kawakami, N. , Nambara, E. , McCourt, P. and Tsuchiya, Y. (2012) Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol. 53, 107–117. [DOI] [PubMed] [Google Scholar]
- Torres‐Vera, R. , Garcia, J.M. , Pozo, M.J. and Lopez‐Raez, J.A. (2014) Do strigolactones contribute to plant defence? Mol. Plant Pathol. 15, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya, Y. , Vidaurre, D. , Toh, S. , Hanada, A. , Nambara, E. , Kamiya, Y. , Yamaguchi, S. and McCourt, P. (2010) A small‐molecule screen identifies new functions for the plant hormone strigolactone. Nat. Chem. Biol. 6, 741–749. [DOI] [PubMed] [Google Scholar]
- Ueno, K. , Nakashima, H. , Mizutani, M. , Takikawa, H. and Sugimoto, Y. (2018) Bioconversion of 5‐deoxystrigol stereoisomers to monohydroxylated strigolactones by plants. J. Pestic. Sci. 43, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, K. , Nomura, S. , Muranaka, S. , Mizutani, M. , Takikawa, H. and Sugimoto, Y. (2011) Ent‐2'‐epi‐Orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J. Agric. Food Chem. 59, 10485–10490. [DOI] [PubMed] [Google Scholar]
- Umehara, M. , Hanada, A. , Yoshida, S. et al (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature, 455, 195–200. [DOI] [PubMed] [Google Scholar]
- Vannette, R.L. , Hunter, M.D. and Rasmann, S. (2013) Arbuscular nnycorrhizal fungi alter above‐ and below‐ground chemical defense expression differentially among Asclepias species. Front. Plant Sci. 4, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.T. , Tan, B.C. , McCarty, D.R. and Klee, H.J. (2008) The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 283, 11364–11373. [DOI] [PubMed] [Google Scholar]
- Vogel, J.T. , Walter, M.H. , Giavalisco, P. et al (2010) SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza‐induced apocarotenoid formation in tomato. Plant J. 61, 300–311. [DOI] [PubMed] [Google Scholar]
- Wade, P.A. , Gegonne, A. , Jones, P.L. , Ballestar, E. , Aubry, F. and Wolffe, A.P. (1999) Mi‐2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat. Genet. 23, 62–66. [DOI] [PubMed] [Google Scholar]
- Wakabayashi, T. , Hamana, M. , Mori, A. et al (2019) Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis. Sci. Adv. 5, eaax9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi, T. , Shida, K. , Kitano, Y. , Takikawa, H. , Mizutani, M. and Sugimoto, Y. (2020) CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5‐deoxystrigol. Planta, 251, 97. [DOI] [PubMed] [Google Scholar]
- Waldie, T. , McCulloch, H. and Leyser, O. (2014) Strigolactones and the control of plant development: lessons from shoot branching. Plant J. 79, 607–622. [DOI] [PubMed] [Google Scholar]
- Walter, M.H. , Fester, T. and Strack, D. (2000) Arbuscular mycorrhizal fungi induce the non‐mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the 'yellow pigment' and other apocarotenoids. Plant J. 21, 571–578. [DOI] [PubMed] [Google Scholar]
- Walter, M.H. , Floss, D.S. , Hans, J. , Fester, T. and Strack, D. (2007) Apocarotenoid biosynthesis in arbuscular mycorrhizal roots: contributions from methylerythritol phosphate pathway isogenes and tools for its manipulation. Phytochemistry, 68, 130–138. [DOI] [PubMed] [Google Scholar]
- Walter, M.H. , Floss, D.S. and Strack, D. (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta, 232, 1–17. [DOI] [PubMed] [Google Scholar]
- Wang, D. and Fu, A. (2016) The plastid terminal oxidase is a key factor balancing the redox state of thylakoid membrane. Enzymes, 40, 143–171. [DOI] [PubMed] [Google Scholar]
- Wang, J.Y. , Haider, I. , Jamil, M. et al (2019) The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 10, 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.Y. , Jamil, M. , Lin, P.Y. et al (2020a) Efficient mimics for elucidating zaxinone biology and promoting agricultural applications. Mol. Plant, 11, 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.Y. , Lin, P.Y. and Al‐Babili, S. (2020b) On the biosynthesis and evolution of apocarotenoid plant growth regulators. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2020.07.007 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Xu, Q. , Yu, H. et al (2020c) Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis thaliana. Plant Cell, 32, 2251–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Schafer, M. , Li, D. et al (2018) Blumenols as shoot markers of root symbiosis with arbuscular mycorrhizal fungi. eLife, 7, e37093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.F. , Ghisalberti, E.L. and Ridsdill‐Smith, J. (1999) Volatiles from Trifolium as feeding deterrents of redlegged earth mites. Phytochemistry, 52, 601–605. [Google Scholar]
- Waszczak, C. , Carmody, M. and Kangasjarvi, J. (2018) Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 69, 209–236. [DOI] [PubMed] [Google Scholar]
- Watanabe, S. , Kounosu, Y. , Shimada, H. and Sakamoto, A. (2014) Arabidopsis xanthine dehydrogenase mutants defective in purine degradation show a compromised protective response to drought and oxidative stress. Plant Biotechnol. 31, 173–178. [Google Scholar]
- Watanabe, S. , Sato, M. , Sawada, Y. , Tanaka, M. , Matsui, A. , Kanno, Y. , Hirai, M.Y. , Seki, M. , Sakamoto, A. and Seo, M. (2018) Arabidopsis molybdenum cofactor sulfurase ABA3 contributes to anthocyanin accumulation and oxidative stress tolerance in ABA‐dependent and independent ways. Sci. Rep. 8, 16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, M.T. , Gutjahr, C. , Bennett, T. and Nelson, D.C. (2017) Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 68, 291–322. [DOI] [PubMed] [Google Scholar]
- Wei, S. , Hannoufa, A. , Soroka, J. , Xu, N. , Li, X. , Zebarjadi, A. and Gruber, M. (2011) Enhanced beta‐ionone emission in Arabidopsis over‐expressing atccd1 reduces feeding damage in vivo by the crucifer flea beetle. Environ. Entomol. 40, 1622–1630. [DOI] [PubMed] [Google Scholar]
- Welsch, R. , Wust, F. , Bar, C. , Al‐Babili, S. and Beyer, P. (2008) A third phytoene synthase is devoted to abiotic stress‐induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 147, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, J.K. , Ye, M. , Li, B. and Noel, J.P. (2016) Co‐evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell, 166, 881–893. [DOI] [PubMed] [Google Scholar]
- Xie, X. , Yoneyama, K. , Kisugi, T. , Uchida, K. , Ito, S. , Akiyama, K. , Hayashi, H. , Yokota, T. , Nomura, T. and Yoneyama, K. (2013) Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant, 6, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X. , Yoneyama, K. , Kusumoto, D. , Yamada, Y. , Yokota, T. , Takeuchi, Y. and Yoneyama, K. (2008) Isolation and identification of alectrol as (+)‐orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry, 69, 427–431. [DOI] [PubMed] [Google Scholar]
- Xie, X.N. (2016) Structural diversity of strigolactones and their distribution in the plant kingdom. J. Pestic. Sci. 41, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, K. , Chen, S. , Li, T. , Ma, X. , Liang, X. , Ding, X. , Liu, H. and Luo, L. (2015) OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress‐responsive genes. BMC Plant Biol. 15, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z.Y. , Lee, K.H. , Dong, T. et al (2012) A vacuolar beta‐glucosidase homolog that possesses glucose‐conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell, 24, 2184–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyaa, M. , Bar, E. , Dubey, N.K. et al (2013) Formation of norisoprenoid flavor compounds in carrot (Daucus carota L.) roots: characterization of a cyclic‐specific carotenoid cleavage dioxygenase 1 gene. J. Agric. Food Chem. 61, 12244–12252. [DOI] [PubMed] [Google Scholar]
- Yamada, Y. , Furusawa, S. , Nagasaka, S. , Shimomura, K. , Yamaguchi, S. and Umehara, M. (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta, 240, 399–408. [DOI] [PubMed] [Google Scholar]
- Yamauchi, R. , Tsuchihashi, K. and Kato, K. (1998) Oxidation products of beta‐carotene during the peroxidation of methyl linoleate in the bulk phase. Biosci. Biotechnol. Biochem. 62, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Yao, R. , Li, J. and Xie, D. (2018) Recent advances in molecular basis for strigolactone action. Sci. China Life Sci. 61, 277–284. [DOI] [PubMed] [Google Scholar]
- Yokota, T. , Sakai, H. , Okuno, K. , Yoneyama, K. and Takeuchi, Y. (1998) Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry, 49, 1967–1973. [Google Scholar]
- Yoneyama, K. , Akiyama, K. , Brewer, P.B. et al (2020) Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct, 4, e00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama, K. , Mori, N. , Sato, T. et al (2018) Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. New Phytol. 218, 1522–1533. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , van Dijk, A.D. , Scaffidi, A. et al (2014) Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 10, 1028–1033. [DOI] [PubMed] [Google Scholar]
- Zheng, X. , Giuliano, G. and Al‐Babili, S. (2020) Carotenoid biofortification in crop plants: citius, altius, fortius. Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 1865(11), 158664. [DOI] [PubMed] [Google Scholar]
- Zhong, Y. , Pan, X. , Wang, R. et al (2020) ZmCCD10a encodes a distinct type of carotenoid cleavage dioxygenase and enhances plant resistance to low phosphate. Plant Physiol. 184, 374–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanenburg, B. and Pospisil, T. (2013) Structure and activity of strigolactones: new plant hormones with a rich future. Mol. Plant, 6, 38–62. [DOI] [PubMed] [Google Scholar]


