Abstract
Objective
To determine the pain intensity of cluster headache through a large survey by comparing it to other painful disorders. Furthermore, to investigate the relationship between maximal pain, autonomic, and other clinical symptoms, as well as demographic attributes of cluster headache.
Background
The pain of cluster headache is anecdotally considered to be one of the worst pains in existence. The link between pain and autonomic features of cluster headache is understood mechanistically through the trigeminovascular reflex, though it is not clear if this is a graded response. Links between pain and other features of cluster headache are less well understood.
Methods
This Internet‐based cross‐sectional survey included questions on cluster headache diagnostic criteria, which were used as part of the inclusion/exclusion criteria for the study. Respondents were asked to rate a cluster headache attack on the 0–10 numerical rating scale. Additionally, they were asked if they had experienced a list of other painful conditions such as labor pain or nephrolithiasis; if so they were asked to rate that pain as well. The survey also included demographics, mood scores, and treatment responses.
Results
A total of 1604 cluster headache respondents were included in the analysis. Respondents rated cluster headache as significantly (p < 0.001) more intense than every other pain condition examined. Cluster headache attacks were rated as 9.7 ± 0.6 (mean ± standard deviation) on the numerical rating scale, followed by labor pain (7.2 ± 2.0), pancreatitis (7.0 ± 1.5), and nephrolithiasis (6.9 ± 1.9). The majority of cluster headache respondents rated a cluster headache attack at maximal or 10.0 pain (72.1%, 1157/1604). Respondents with maximal pain were statistically significantly more likely to have cranial autonomic features compared to respondents with less pain: conjunctival injection or lacrimation 91% (1057/1157) versus 85% (381/447), eyelid edema 77% (887/1157) versus 66% (293/447), forehead/facial sweating 60% (693/1157) versus 49% (217/447), fullness in the ear 47% (541/1157) versus 35% (155/447), and miosis/ptosis 85% (1124/1157) versus 75% (426/447) (all p values <0.001). Respondents with maximal pain also had other statistically significant findings: more frequent attacks (4.0 ± 2.0 attacks per day vs. 3.5 ± 2.0 attacks per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 ± 16.9 vs. 21.1 ± 15.2), decreased overall effectiveness from calcium channel blockers (on a 5‐point Likert scale), and more likely female: 34% (389/1157) versus 24% (108/447) (all p values <0.001). Pain intensity was not associated with restlessness, headache duration, age of onset, episodic/chronic status, or the effectiveness of any acute or preventive medication other than calcium channel blockers.
Conclusions
Cluster headache is an intensely painful disorder, even in the context of other disorders considered intensely painful. Maximal pain intensity is associated with more cranial autonomic features, suggesting a graded response between pain and autonomic features. Maximal pain intensity is also associated with headache frequency but not duration, suggesting a relationship between pain intensity and mechanisms controlling headache onset, but not between pain intensity and mechanisms controlling headache offset.
Keywords: cluster headache, cranial autonomic features, pain intensity, survey, trigeminal autonomic cephalalgia, trigeminal autonomic reflex
Abbreviations
- BDI
Beck Depression Inventory
- CHQ
Cluster Headache Questionnaire
- HDSQ
Hopelessness Depression Symptom Questionnaire
INTRODUCTION
A cluster headache attack is considered one of the most painful human experiences. The data on the remarkable intensity of cluster headache are primarily anecdotal, 1 , 2 , 3 though recent large studies have supported these findings with high pain intensity ratings. 4 , 5 Within cluster headache, patients with more intense pain may be at higher risk for suicide. 6 Thus, it is important to understand not only the magnitude of cluster headache pain, but also which cluster headache patients are at the highest risk for the most pain.
Physiologically the trigeminal distribution of pain and autonomic features of cluster headache 7 are linked through the trigeminal autonomic reflex, a pathway between the trigeminal nerve, trigeminocervical complex, superior salivatory nucleus, major petrosal nerve, and sphenopalatine ganglion. 8 It is not clear if this reflexive mechanism is an on/off response or a graded response. It is also not clear if pain intensity is related to other clinical features, such as headache duration, frequency, restlessness, or treatment response.
This study is part of the Cluster Headache Questionnaire (CHQ), a large international online survey. 9 In this study, we investigate pain intensity in cluster headache. We focus on the level of pain in comparison to other disorders considered intensely painful and examine demographic factors that might be predictive of increased pain intensity. We also examine the pain intensity of cluster headache in relation to other symptoms of cluster headache. We hypothesized that cluster headache pain intensity would be more painful than other extremely painful disorders and that a higher pain score would be associated with more cranial autonomic features.
METHODS
Methods are summarized below, with additional details in Table S1 and our previous publication. 9 The CHQ was a self‐administered internet‐based cross‐sectional survey of 152 items organized into eight separate sections: (1) Sign up and Verification, (2) Symptom Screening, (3) Demographics, (4) Experience, (5) Medications/Treatment, (6) Beck Depression Inventory II (BDI), (7) Hopelessness Depression Symptom Questionnaire (HDSQ), and (8) End of Survey – Contact Options. A previous publication focused primarily on the acute treatments in section 5 (“Medication/Treatment”). 9 This manuscript includes data from Sections 2 to 7. Institutional review board approval was obtained from the University of West Georgia. Informed consent was obtained at the beginning of the online survey: respondents were given a summary of the intent and purpose of the research and a brief summary of each section, then were required to verify their age (18 years or older) as well as agree to participate in the survey by clicking on the appropriate link.
The verbatim survey questions are listed in Figure S1 (including examples for questions that require branching logic). For pain level, respondents were given a variety of painful conditions and asked if they had experienced them; if so they were asked to rate the pain 0.0–10.0 on the numerical rating scale. Labor pain was asked only to women but did not address if intravenous or spinal/epidural anesthesia was used. Of note, there is some debate whether severe pain should be rated as “7 and higher” or as “8 and higher” on the numerical rating scale 10 , 11 , 12 and we chose the less stringent “7 and higher” a priori. For age of onset calculations, respondents were excluded if their stated age of onset was older than either their current age or their age of diagnosis. For calculations of frequency and duration, respondents occasionally provided two responses: one for episodic cluster headache and one for chronic cluster headache. The diagnosis of episodic or chronic was made as above, and for frequency and duration the average of these two responses was used. The study did not inquire about tobacco use so we are unable to comment on relationships between pain and tobacco.
Statistical analyses
Comparisons of the pain intensity of cluster headache to the pain intensities of other conditions were performed using a linear regression model with repeated measures. A heterogeneous Toeplitz matrix was chosen to be the covariance structure after comparing the Akaike Information Criteria values with several other covariance structures. For studying the associations of the pain intensity of cluster headache with other features, cluster headache respondents were divided into two groups: pain score equal to 10 and pain score less than 10. Continuous and ordinal variables were compared via the Wilcoxon rank test (two‐sided), while nominal variables were compared via Fisher's exact test. Ordinal and continuous variables were summarized as medians and interquartile ranges. In addition, the means and standard deviations of continuous variables were provided. Nominal variables were summarized as counts and percentages to the total. For treatments, the effectiveness of medication was ranked from 1 to 5 with 1 being “Completely Ineffective” and 5 being “Completely Effective.” Bonferroni correction was applied to adjust for multiple comparisons; since 49 variables were compared, p < 0.001 was considered significant. Full statistical results are included as Tables [Link], [Link], [Link]. All statistical analyses were performed using Statistical Analysis Software (SAS, Cary, NC) version 9.4.
No statistical calculation of power was performed prior to the study. The sample size was based on a previous study. 13 All other analyses were planned either before the survey began (by authors SMP and LIS) or after the survey started but before analysis began (by authors MJB, RES, and WZ) with two exceptions. First, after noting that cluster headache attacks were significantly more painful than all other conditions tested, an additional analysis using Fisher's exact test was performed to determine if cluster headache pain intensity changed with the presence or absence of the experience of other pain conditions. A Bonferroni correction was again applied, and since 16 comparisons were performed, a p < 0.003 was considered significant. Second, during the review process, detailed analysis of pain intensity by sex and country was recommended. For the calculation of pain intensity by gender we performed the Wilcoxon rank test. For the calculation of pain intensity by the country, we selected all countries with at least 50 respondents and performed a Kruskal–Wallis test. Further investigation of country together with sex was performed using regression analysis. p < 0.05 was considered significant.
Missing data are included in Table S4. Pain intensity for cluster headache had no missing data, but the full range of missing data for pain intensity of other pain conditions is unknown: in our survey design, a blank response could mean the respondent did not experience a particular pain condition, but could also mean they forgot if they experienced that condition. A similar concept applies for treatments, but some missing data can be assumed based on respondents who answered questions related to complications and access to treatments but did not answer the question about effectiveness. For caffeine, compared to our original paper, 9 an additional two responses were found for definite cluster headache that was not included in the original paper from 2019 (one completely ineffective and one minimally effective). These were included in this paper.
RESULTS
A total of 4876 IP addresses were recorded on the website (representing 4876 potential subjects); 3251 subjects agreed to participate in the questionnaire, and 1604 met inclusion and exclusion criteria 9 (see Figure S2). A comparison of painful disorders is shown in Figure 1. Cluster headache was the most severe pain with a mean of 9.7, followed by labor pain at 7.2, pancreatitis at 7.0, and nephrolithiasis at 6.9. There was a notable gap between cluster headache (9.7) and labor pain (7.2), while the rest of the disorders fell in a more continuous spectrum between 4.0 and 7.2. Cluster headache was significantly more painful than every other disorder examined (p < 0.001). The majority of respondents (72.1%, 1157/1604) rated pain as 10.0. Pain intensity was rated between 9.0 and 9.9 in 18.3% (294/1605), between 8.0 and 8.9 in 8.5% (136/1604), and between 7.0 and 7.9 in only 1.1% (12/1604) (Figure 2). Respondents experiencing other types of pain did not consider cluster headache less intense (Table S5). Conversely, respondents who experienced nephrolithiasis, migraine, or arthritis were more likely to give their cluster headache pain a score of 10 than those that had not experienced those pain conditions, and there was no significant difference for other pain types.
FIGURE 1.
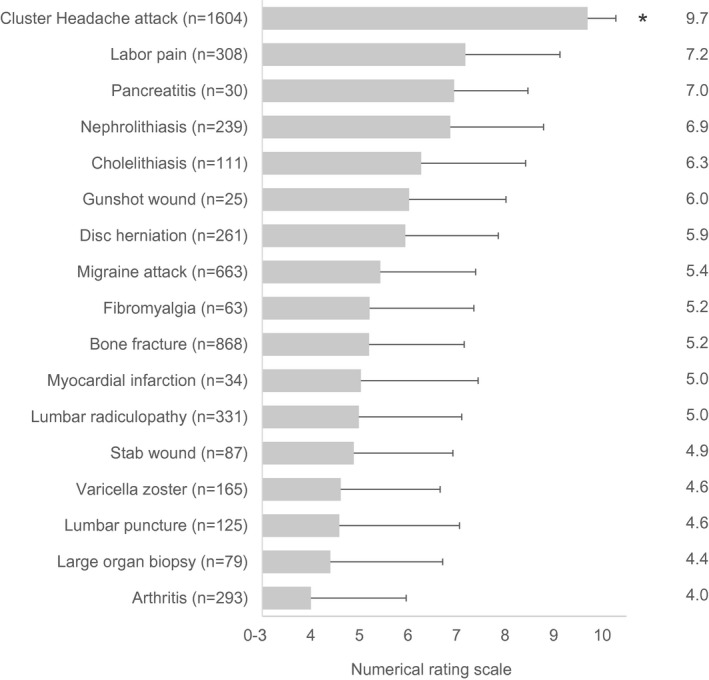
Pain scores for various pain conditions. All respondents were asked to rate their cluster headache pain using the Numerical Rating Scale (0–10, with 10 being the worst pain). Respondents were also asked if they had experienced any of a list of painful conditions; if they had, they were also asked to rate that pain. Numbers to the right indicate mean; error bars indicate standard deviation. The pain of cluster headache was individually compared to all other conditions, and in all cases was significantly more painful (*p < 0.0001)
FIGURE 2.
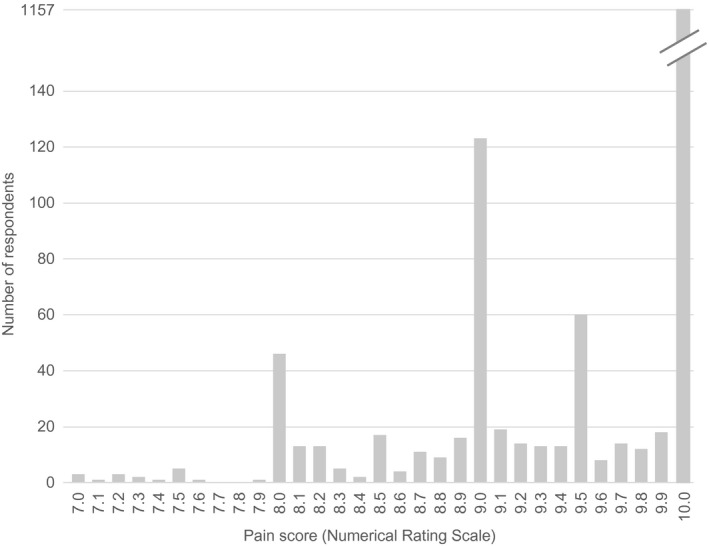
Individual responses (n = 1604) for cluster headache attack intensity, binned by 0.1 intervals on the 0–10 Numerical Rating Scale. An attack was rated as 10.0 pain in most patients. By definition, cluster headache patients have severe pain (7.0 or higher)
Maximal pain (10/10) was associated with a higher proportion of most cranial autonomic features as well as females, higher headache frequency, lower calcium channel blocker effectiveness, and a higher HDSQ score (Table 1). A higher BDI score did not meet significance (p = 0.003). The only cranial autonomic feature that was not associated with maximal pain was nasal congestion (p = 0.029), though of note rhinorrhea was not asked in our questionnaire. The maximal pain group had higher percentages of nasal congestion and restlessness/agitation; however, p values were not significant (p = 0.029 and 0.088, respectively). Maximal pain was not associated with several migrainous features that are sometimes reported in cluster headache, such as nausea/vomiting, photophobia/phonophobia, or aggravation by physical activity. There was no association between maximal pain and the effectiveness of any medication we examined with the exception of calcium channel blockers: on a 5‐point Likert scale from 1 (completely ineffective) to 5 (completely effective), respondents with maximal pain had an average effectiveness of 2.2 ± 1.1, where respondents with less pain had an average effectiveness of 2.5 ± 1.1 (p < 0.001, see Table S4).
TABLE 1.
Comparison of pain score (numerical rating scale) with cluster headache features
| CH pain score <10 (n = 447) | CH pain score = 10 (n = 1157) | p value | |
|---|---|---|---|
| Demographic characteristics | |||
| Sex | Female 108 (24%) | Female 389 (34%) | <0.001* |
| Age of onset | 28.2 ± 12.2 | 27.0 ± 12.6 | 0.037 |
| Age at first diagnosis | 34.2 ± 11.8 | 33.3 ± 11.5 | 0.302 |
| Current age | 45.3 ± 13.4 | 46.4 ± 12.8 | 0.083 |
| Episodic versus chronic cluster headache | Episodic 364 (81%) | Episodic 881 (76%) | 0.053 |
| Family history | Yes 36 (8%) | Yes 132 (11%) | 0.128 |
| Maybe 59 (13%) | Maybe 139 (12%) | ||
| No 352 (79%) | No 885 (77%) | ||
| Cluster headache features | |||
| Headache duration (in hours) | 83.1 ± 44.8 | 86.2 ± 44.3 | 0.130 |
| Headache frequency (attacks/day) | 3.5 ± 2.0 | 4.0 ± 2.0 | <0.001* |
| Unilateral conjunctival injection or lacrimation | Yes 381 (85%) | Yes 1057 (91%) | <0.001* |
| Unilateral nasal congestion | Yes 392 (88%) | Yes 1058 (91%) | 0.029 |
| Unilateral eyelid edema | Yes 293 (66%) | Yes 887 (77%) | <0.001* |
| Unilateral forehead and facial sweating | Yes 217 (49%) | Yes 693 (60%) | <0.001* |
| Unilateral sensation of fullness in the ear | Yes 155 (35%) | Yes 541 (47%) | <0.001* |
| Unilateral miosis and/or ptosis | Yes 334 (75%) | Yes 983 (85%) | <0.001* |
| A sense of restlessness or agitation | Yes 426 (95%) | Yes 1124 (97%) | 0.088 |
| Migrainous features | |||
| Nausea/vomiting | Yes 103 (23%) | Yes 338 (29%) | 0.013 |
| Photophobia or phonophobia | Yes 208 (47%) | Yes 596 (52%) | 0.075 |
| Worsening with physical activity | Yes 135 (30%) | Yes 368 (32%) | 0.549 |
| Mood scores | |||
| HDSQ score | 21.1 ± 15.2 | 24.5 ± 16.9 | <0.001* |
| BDI score | 12.9 ± 10.1 | 14.9 ± 11.3 | 0.003 |
| Treatment response—acute medications a | |||
| Triptans (n = 1193) | 3.4 ± 1.2 | 3.4 ± 1.1 | 0.346 |
| Oxygen (n = 1082) | 3.4 ± 1.0 | 3.4 ± 1.1 | 0.634 |
| Dihydroergotamine (n = 170) | 2.2 ± 1.2 | 2.4 ± 1.3 | 0.398 |
| Cafergot/ergotamine (n = 303) | 2.0 ± 1.3 | 2.1 ± 1.1 | 0.213 |
| Intranasal ketamine (n = 37) | 1.7 ± 1.1 | 2.3 ± 1.1 | 0.217 |
| Opioids (n = 541) | 1.9 ± 1.0 | 1.8 ± 1.0 | 0.217 |
| Intranasal capsaicin (n = 151) | 1.8 ± 0.9 | 1.6 ± 0.9 | 0.155 |
| Caffeine & energy drinks (n = 43) | 2.7 ± 0.9 | 2.8 ± 0.7 | 0.820 |
| Intranasal lidocaine (n = 241) | 1.7 ± 0.7 | 1.6 ± 0.8 | 0.496 |
| Treatment response—preventive medications a | |||
| Corticosteroids (n = 753) | 2.8 ± 1.2 | 2.6 ± 1.2 | 0.098 |
| Calcium channel blockers (n = 987) | 2.5 ± 1.1 | 2.2 ± 1.1 | <0.001* |
| Methysergide/methylergonavine (n = 161) | 2.2 ± 1.4 | 1.8 ± 1.1 | 0.291 |
| Anti‐epileptics (n = 586) | 1.8 ± 1.0 | 1.7 ± 0.9 | 0.218 |
| Lithium (n = 304) | 1.6 ± 0.9 | 1.7 ± 1.0 | 0.824 |
| Testosterone (n = 53) | 1.7 ± 1.1 | 1.6 ± 1.0 | 0.834 |
| Beta‐blockers (n = 372) | 1.6 ± 0.8 | 1.4 ± 0.8 | 0.012 |
Data reported as either “average ± standard deviation” or as “total number (% of total).”
Treatment responses were scaled from 1 to 5 (1. completely ineffective, 2. minimally effective, 3. somewhat effective, 4. very effective, 5. completely effective); means (between 1 and 5), standard deviations, and p values for treatment are shown here, full data are shown in Table S4.
A p value <0.001 was considered significant after Bonferroni correction.
Abbreviations: BDI, Beck Depression Inventory‐II; CH, Cluster Headache; HDSQ, Hopelessness Depression Symptom Questionnaire.
An in‐depth analysis of average pain intensity (not maximal pain intensity) was performed for sex and country (Table S6). Average pain intensity was higher in females and differed significantly (p = 0.0319) by country. The data for country, however, were confounded by sex: pain intensity differed by country for females but not for males.
DISCUSSION
Cluster headache pain is more intense than any other pain disorder we examined at 9.7, with the next most painful disorder, labor pain at 7.2, a full 2.5 points less on a 0–10 scale. With severe pain being rated 7 and higher on the numerical rating scale, it is interesting to note that only cluster headache attacks (9.7), labor pain (7.2), and pancreatitis (7.0) were considered severe pain. Nephrolithiasis, often considered extremely painful, was rated at the high end of moderate pain (6.9).
In our dataset, cranial autonomic features were more likely to be activated in respondents with more intense pain. This suggests that the trigeminal autonomic reflex in cluster headache is a graded response, with more intense pain leading to more autonomic features. Similarly, a recent functional imaging study suggests that the anterior hypothalamus, which may play a role in the trigeminal autonomic reflex along with the nucleus locus coeruleus and ventral posteromedial nucleus of the thalamus, is only activated when an intranasal trigeminal stimulus is regarded as painful. 14 Thus, in cluster headache, different brain regions may be activated based on pain. Interestingly, restlessness was not more common in patients with maximal pain. Restlessness has been proposed to originate in the hypothalamus 15 , 16 or the cerebellum. 17 Regardless of the system responsible for restlessness, our data suggest that the connection with agitation and pain is not a graded response.
The role of the trigeminal autonomic reflex in cluster headache is still unclear. Cranial autonomic activation does occur in other headache disorders such as migraine, 18 but robust autonomic activation appears to be unique to the trigeminal autonomic cephalalgias. 19 As Möller and May discuss, 8 the trigeminal autonomic reflex may not be a true reflex in cluster headache because some cluster headache patients do not experience autonomic features, and cluster headache patients with complete trigeminal nerve sectioning may experience painless attacks with preserved autonomic features. The trigeminal and autonomic features may be centrally activated, possibly through connections between the hypothalamus, trigeminal nucleus caudalis, and superior salivatory nucleus and other parasympathetic nuclei. 17 Our data suggest that pain intensity is associated with either increased neural activity in, or increased recruitment of, autonomic areas, resulting in an increased likelihood of autonomic features.
Maximal pain was associated with several other cluster headache features including: calcium channel blocker effectiveness, female sex, attacks per day, and mood disturbance scores. The significance of the association between maximal pain and decreased response to calcium channel blockers is unclear: some of the antiepileptic medications used for cluster headache also inhibit calcium channels, and antiepileptics had no association with maximal pain, suggesting more detailed evaluation is necessary to determine how calcium channel inhibition might be related to pain intensity in cluster headache. Maximal pain was also more likely in females. One possible explanation is general sex differences in pain processing. 20 , 21 However, there may be other disease‐specific reasons, such as an expanded location of pain in females with cluster headache especially in additional trigeminal distributions, 22 , 23 or increased allodynia in females with cluster headache. 24 Gender‐specific circadian features may also be important: a previous study of 1134 cluster headache patents showed increased pain intensity in women at nighttime but not during the daytime. 22 Finally, there may be cultural differences that affect females more than males: in our dataset, pain intensity differed by country for females but not for males.
Maximal pain was also more likely in respondents with more attacks per day but not with headache duration, suggesting that neural circuits necessary for attack initiation may be more linked to pain intensity than the circuits for attack termination. Finally mood disturbance scores were higher in respondents with maximal pain. The HDSQ is specifically designed to evaluate the hopelessness aspect of depression, 25 while BDI evaluates more general aspects of depression. 26 In our dataset, the HDSQ was higher in respondents with maximal pain, while the BDI was not significant, suggesting that hopelessness is particularly elevated in cluster headache patients with maximal pain. Hopelessness is considered a core component of suicidality, 27 , 28 and our data suggest hopelessness is higher when pain is maximal. Additionally, a previous study in cluster headache found an association between higher attack intensity and suicidal behavior. 6 Additional studies are needed to investigate whether hopelessness and pain intensity are dependent or independent risk factors for suicide in cluster headache.
Our data suggest that cluster headache attacks are more painful than several disorders widely considered to have very severe pain. However, cluster headache's ranking in the pantheon of the most painful human conditions is not clear. For example, some of the highest scoring disorders on the McGill Pain Questionnaire included complex regional pain syndrome, finger amputations, central post‐stroke pain, and pain associated with spinal cord injury, 29 which all have low prevalence like cluster headache. Thus, direct comparisons are difficult because of the insufficient number of people who have reported experiences of two or more of these disorders. These pain disorders could be standardized or “anchored” relative to a more common disorder such as nephrolithiasis or to an imagined event such as slamming a car door onto a finger, 30 but these may also be problematic because the amount of pain in nephrolithiasis is variable (in our study the standard deviation was 1.9 on a 0–10 scale). Additionally, an imagined event depends on the patient's imagination, 31 and it is not yet clear if an imagined anchor applies to all chronic pain conditions. 30 Also, pain intensity is not the only important factor in measuring pain. Duration and frequency are also important: migraine pain, for example, was rated less intense than cluster headache in our study but by definition has a longer duration.
There are several limitations to this study. Some are mentioned in our previous publication 9 but warrant a brief mention here including recall bias, the grouping of medications (such as all calcium channel blockers) without the assessment of doses, and the fact that the diagnoses of cluster headache were not confirmed by physician interview (only by survey questions addressing International Classification of Headache Disorders criteria). In this study, recall bias could potentially differ by disease state: recalled labor pain may be an average of peak and end pain, 32 which may not be true of other disorders. There are also limitations specific to this study. First, in evaluating the effectiveness of cluster headache medications, not all participants trialed all medications; some medications were trialed in less than 100 respondents (such as ketamine and testosterone), while others were trialed by over 1000 respondents (triptans and oxygen). Second, we did not confirm the diagnoses of labor pain, nephrolithiasis, or any other disorder. Nor did we inquire into what pain medications respondents received: a respondent receiving epidural anesthesia for labor pain, for example, may in fact rate pain as higher. 33 However, we also did not ask about current cluster headache treatments. Third, all respondents were asked to rate the pain of cluster headache before rating any other disorders; our reasoning for this was that patients anecdotally report needing to revise their 0–10 pain scale to account for the pain of cluster headache. However, putting cluster headache as the first pain question for all respondents, and putting it in the context of a study focused on cluster headache, could bias respondents into placing extra emphasis on cluster headache and thus rating it higher. Similarly, there may be selection bias for cluster headache but not other pain disorders, in that respondents filling out a survey focused on cluster headache could have more severe cluster headache symptoms than the general population. However, they might not have more severe labor pain or pancreatitis than the general population. Reducing this bias would likely involve adding cluster headache to a general study on pain intensity, but this would require a very large enrollment to include a sufficient number of cluster headache respondents. Finally, we should note that cluster headache is defined as “severe or very severe” pain, meaning that all respondents rating cluster headache less than 7/10 are excluded from this study because they do not meet the full criteria for cluster headache. This strict cluster headache definition might be considered an overestimation of the amount of pain. However, severe or very severe pain is found in the vast majority of cluster headache patients: in respondents with probable cluster headache, only 4.4% (26/589) stated pain less than 7/10 in our study, 9 and only 4.2% (12/289) had pain of less‐than‐severe intensity in a Dutch study. 34 However, it is possible that some of these respondents had less than 7/10 pain because their preventive medication effectively reduced the intensity of the attacks.
In conclusion, cluster headache is reported by a large group of international respondents to be more intense than every other pain disorders examined. Maximal pain corresponds to an increased frequency of cranial autonomic features and increased frequency of attacks, suggesting connections between pain and other brain areas in cluster headache.
CONFLICT OF INTEREST
SM Pearson and MJ Burish report no conflicts of interest. RE Shapiro has served as a paid consultant to Eli Lilly as a member of the Data Monitoring Committees for galcanezumab multi‐center clinical trials for both cluster headache and migraine, as well as on the advisory committee for the OVERCOME study. W Zhang and LI Schor report no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: Stuart M. Pearson and Larry I. Schor. Acquisition of data: Stuart M. Pearson and Larry I. Schor. Analysis and interpretation of data: Stuart M. Pearson, Mark J. Burish, Robert E. Shapiro, Wei Zhang, and Larry I. Schor. Drafting of the manuscript: Stuart M. Pearson, Mark J. Burish, and Wei Zhang. Revising it for intellectual content: Stuart M. Pearson, Mark J. Burish, Robert E. Shapiro, Wei Zhang, and Larry I. Schor. Final approval of the completed manuscript: Stuart M. Pearson, Mark J. Burish, Robert E. Shapiro, Wei Zhang, and Larry I. Schor.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
ACKNOWLEDGMENTS
We thank Robert Wold and Stewart Tepper for insights into study design as well as Clusterbusters and the International Headache Society for promoting the questionnaire. We also thank the Center for Biostatistics Collaboration and Data Services at the Department of Biostatistics and Data Science at UTHealth School of Public Health. This study received funding support from Autonomic Technologies, Inc. and Clusterbusters. Autonomic Technologies, Inc. had no role in the analysis or interpretation of results. Clusterbusters did not have a direct role in analysis or interpretation but the following should be noted: (a) Robert Wold is a founding member of Clusterbusters, (b) two of the authors (RES and LIS) have served in advisory roles for Clusterbusters, (c) preliminary data from this study were presented at a Clusterbusters annual conference.
Mark J. Burish and Stuart M. Pearson contributed equally to this work as first authors.
REFERENCES
- 1. Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1(4):251‐257. [DOI] [PubMed] [Google Scholar]
- 2. May A. Cluster headache: pathogenesis, diagnosis, and management. Lancet. 2005;366(9488):843‐855. [DOI] [PubMed] [Google Scholar]
- 3. Kudrow L. Cluster Headache. New York: Oxford University Press; 1980:1‐162. [Google Scholar]
- 4. Moon H‐S, Cho S‐J, Kim B‐K, et al. Field testing the diagnostic criteria of cluster headache in the third edition of the International Classification of Headache Disorders: a cross‐sectional multicentre study. Cephalalgia. 2019;39(7):900‐907. [DOI] [PubMed] [Google Scholar]
- 5. Moon H‐S, Park JW, Lee K‐S, et al. Clinical features of cluster headache patients in Korea. J Korean Med Sci. 2017;32(3):502‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji Lee M, Cho S‐J, Wook Park J, et al. Increased suicidality in patients with cluster headache. Cephalalgia. 2019;39(10):1249‐1256. [DOI] [PubMed] [Google Scholar]
- 7. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. [DOI] [PubMed] [Google Scholar]
- 8. Möller M, May A. The unique role of the trigeminal autonomic reflex and its modulation in primary headache disorders. Curr Opin Neurol;2019;32(3):438‐442. [DOI] [PubMed] [Google Scholar]
- 9. Pearson SM, Burish MJ, Shapiro RE, Yan Y, Schor LI. Effectiveness of oxygen and other acute treatments for cluster headache: results from the Cluster Headache Questionnaire, an International Survey. Headache. 2019;59:235‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li KK, Harris K, Hadi S, Chow E. What should be the optimal cut points for mild, moderate, and severe pain? J Palliat Med. 2007;10(6):1338‐1346. [DOI] [PubMed] [Google Scholar]
- 11. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277‐284. [DOI] [PubMed] [Google Scholar]
- 12. Hirschfeld G, Zernikow B. Variability of “optimal” cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain. 2013;154(1):154‐159. [DOI] [PubMed] [Google Scholar]
- 13. Rozen TD, Fishman RS. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52(1):99‐113. [DOI] [PubMed] [Google Scholar]
- 14. Möller M, Mehnert J, May A. Hypothalamic activation discriminates painful and non‐painful initiation of the trigeminal autonomic reflex—an fMRI study. Cephalalgia. 2020;40(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 15. Hoffmann J, May A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018;17(1):75‐83. [DOI] [PubMed] [Google Scholar]
- 16. Burish MJ, Chen Z, Yoo S‐H. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiol. 2018;25:e13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. May A, Schwedt TJ, Magis D, Pozo‐Rosich P, Evers S, Wang S‐J. Cluster headache. Nat Rev Dis Prim. 2018;4:1‐17. [DOI] [PubMed] [Google Scholar]
- 18. Riesco N, Pérez‐Alvarez AI, Verano L, et al. Prevalence of cranial autonomic parasympathetic symptoms in chronic migraine: usefulness of a new scale. Cephalalgia. 2016;36(4):346‐350. [DOI] [PubMed] [Google Scholar]
- 19. Goadsby PJ, Lipton RB. A review of paroxysmal hemicranias, SUNCT syndrome and other short‐lasting headaches with autonomic feature, including new cases. Brain. 1997;120(1):193‐209. [DOI] [PubMed] [Google Scholar]
- 20. Melchior M, Poisbeau P, Gaumond I, Marchand S. Insights into the mechanisms and the emergence of sex‐differences in pain. Neuroscience. 2016;338:63‐80. [DOI] [PubMed] [Google Scholar]
- 21. Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132:S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rozen TD, Fishman RS. Female cluster headache in the United States of America: what are the gender differences? J Neurol Sci. 2012;317(1‐2):17‐28. [DOI] [PubMed] [Google Scholar]
- 23. Allena M, De Icco R, Sances G, et al. Gender differences in the clinical presentation of cluster headache: a role for sexual hormones? Front Neurol. 2019;10:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilbrink LA, Louter MA, Teernstra OP, et al. Allodynia in cluster headache. Pain. 2017;158(6):1113‐1117. [DOI] [PubMed] [Google Scholar]
- 25. Metalsky GI, Joiner TE. The hopelessness depression symptom questionnaire. Cognit Ther Res. 1997;21(3):359‐384. [Google Scholar]
- 26. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561‐571. [DOI] [PubMed] [Google Scholar]
- 27. Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gvion Y, Horesh N, Levi‐Belz Y, Apter A. A proposed model of the development of suicidal ideations. Compr Psychiatry. 2015;1(56):93‐102. [DOI] [PubMed] [Google Scholar]
- 29. Melzack R, Katz J. Pain measurements in adult patients In: McMahon S, Koltzenburg M, Tracey I, Turk D, eds. Wall and Melzack’s Textbook of Pain. 6th ed. Philadelphia: Elsevier; 2013:301‐314. [Google Scholar]
- 30. Amir R, Leiba R, Eisenberg E. Anchoring the numeric pain scale changes pain intensity reports in patients with chronic but not with acute pain. Pain Pract. 2019;19(3):283‐288. [DOI] [PubMed] [Google Scholar]
- 31. Walton DM, Elliott JM, Salim S, Al‐Nasri I. A reconceptualization of the pain numeric rating scale: anchors and clinically important differences. J Hand Ther. 2018;31(2):179‐183. [DOI] [PubMed] [Google Scholar]
- 32. Chajut E, Caspi A, Chen R, Hod M, Ariely D. In pain thou shalt bring forth children: the peak‐and‐end rule in recall of labor pain. Psychol Sci. 2014;25(12):2266‐2271. [DOI] [PubMed] [Google Scholar]
- 33. Waldenström U, Schytt E. A longitudinal study of women’s memory of labour pain—from 2 months to 5 years after the birth. BJOG. 2009;116(4):577‐583. [DOI] [PubMed] [Google Scholar]
- 34. van Vliet J, Eekers P, Haan J, Ferrari M, Dutch RUSSH Study Group . Evaluating the IHS criteria for cluster headache—a comparison between patients meeting all criteria and patients failing one criterion. Cephalalgia. 2006;26(3):241‐245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6


