Abstract
Objectives:
To characterize the outcomes of neoadjuvant chemotherapy (NAC) pre-treated patients found to be lymph node (LN)-positive at the time of radical cystectomy and pelvic lymph node dissection (RC/PLND) for urothelial carcinoma of the bladder (UCB).
Subjects and Methods:
Of 1484 patients treated with RC/PLND for UCB from 2000-2010, we analyzed 198 patients with clinically non-metastatic (cN0M0) muscle-invasive UCB who were found to be LN-positive at RC/PLND. As patients not receiving perioperative chemotherapy were significantly older and comorbid, we compared LN-positive patients previously treated with NAC (n=32) to LN-positive patients treated with adjuvant chemotherapy (AC, n=49) using Cox proportional hazards models. A sensitivity analysis was designed to account for the additional time to RC in NAC patients.
Results:
The three-year recurrence-free survival estimate for LN-positive NAC patients was 26%, compared with 60% for LN-positive AC patients. LN-positive patients treated with NAC had significantly higher risks of disease recurrence and cancer-specific mortality in univariate analyses (HR=2.86, 95%CI 1.58-5.19, p=0.001 and HR=2.50, 95%CI 1.34-4.65, p=0.004, respectively) and multivariable analyses adjusting for pathologic stage and LN density (HR=3.11, 95%CI 1.59-6.07, p=0.001 and HR=3.05, 95%CI 1.46-6.35, p=0.003, respectively). Sensitivity analyses similarly demonstrated worse outcomes for NAC pre-treated LN-positive patients.
Conclusion:
LN-positive patients previously treated with NAC have a poor prognosis, significantly worse than LN-positive patients subsequently treated with AC, and should be considered for protocols using sandwich chemotherapy approaches or novel agents. These results should be considered in the interpretation of and stratification for clinical trials.
Keywords: neoadjuvant chemotherapy, lymph node metastasis, bladder cancer, radical cystectomy, lymph nodes
Introduction
Recurrence rates for lymph node (LN)-positive bladder cancer patients following radical cystectomy (RC) and bilateral pelvic lymph node dissection (PLND) are high. Only one-third of LN-positive patients will survive 3 years following RC/PLND [1, 2] with the majority of recurrences occurring within 2 years. Up to 25% of patients undergoing RC/PLND for muscle-invasive bladder cancer will have LN metastases identified on final pathologic review [3-6].
Rates of utilization of neoadjuvant chemotherapy (NAC) in patients with muscle-invasive bladder cancer (cT2-4N0M0) have gradually increased following reports demonstrating survival benefit [7-10]. While the implementation of NAC has improved outcomes for muscle-invasive bladder cancer patients as a whole, outcomes for clinically non-metastatic patients treated with NAC who are found to be LN-positive at RC/PLND are not well studied. Prior publications have reported upon the outcomes of LN-positive patients undergoing induction chemotherapy followed by consolidative surgery [11] or have grouped clinically LN-negative patients (who receive true neoadjuvant chemotherapy) together with clinically LN-positive patients (who receive definitive or induction chemotherapy) [12].
We hypothesized that patients treated with NAC found to have LN-positive disease after RC/PLND may have a significantly worse outcome compared to chemotherapy-naïve LN-positive patients. The purpose of this study was to characterize the outcomes of NAC pre-treated, pathological LN-positive patients managed with RC/PLND.
Subjects and Methods
Study Population
After institutional review board approval, we queried our prospective institutional bladder cancer database. We identified 1484 consecutive patients who underwent RC between 2000 and 2010 for urothelial carcinoma of the bladder, of whom 306 (21%) were found to have LN metastasis on histopathologic analysis. Of these patients, 198 were treated with curative intent RC/PLND for muscle-invasive urothelial carcinoma of the bladder and had preoperative axial imaging demonstrating clinically LN-negative, non-metastatic (cN0M0) disease. The clinical staging of cN0M0 patients was initially determined by the treating physician and was verified by the investigators.
Perioperative systemic chemotherapy consisted primarily of cisplatin-based regimens, either gemcitabine/cisplatin (gemcitabine 1000 mg/m2 and cisplatin 70 mg/m2 on day 1, gemcitabine 1000 mg/m2 on day 8) in a 21-day cycle or methotrexate, vinblastine, adriamycin, and cisplatin (MVAC). Chemotherapy in patients who were clinically non-metastatic (cN0M0) prior to RC was classified as neoadjuvant chemotherapy (NAC), adjuvant chemotherapy (AC, administered within 3 months of RC/PLND with no evidence of disease following RC/PLND), and salvage chemotherapy (chemotherapy delivered for recurrent disease after RC/PLND). All patients with clinically positive LNs prior to RC were excluded as they constituted a group of regionally metastatic patients and therefore received definitive preoperative chemotherapy (not NAC) if eligible.
Forty-three of these patients (22%) were treated with NAC, 67 (34%) were treated with AC, and 93 (47%) did not receive any perioperative chemotherapy. Given their advanced age and significant additional comorbidities (p<0.01) compared to patients who received chemotherapy, patients who did not receive any perioperative systemic chemotherapy were excluded from further analyses (Supplemental Table 1) [13]. We further excluded 5 patients who received both NAC and AC, 13 who had a delay to initiation of AC (>3 months), and 6 patients who did not have 3-month follow-up. Our final comparative study cohorts consisted of 81 LN-positive patients treated with either NAC (n=32) or AC (n=49).
Surgical specimens were evaluated according to our standard institutional protocol. Lymph nodes were processed by a standard method that included dissection from the adipose tissue under bright light. No fat-clearing solutions were used. All identified LNs were sectioned and pathologically evaluated. Tumor stage was assigned according to the 2002 TNM American Joint Committee on Cancer classification [14]. Follow-up was performed according to institutional protocols, which included interval evaluations for clinical and radiographic evidence of disease. Disease recurrence was defined as any radiographic or pathologically verified documentation of disease. Cause of death was prospectively entered into the institutional database, verified by chart review, and corroborated by death certificates.
Statistical Analysis
Associations between categorical clinicopathologic variables were examined using Fisher’s exact test. Survival probabilities were estimated using Kaplan-Meier methods. Univariate and multivariable Cox proportional hazards models were used to estimate the risks of disease recurrence and cancer-specific mortality (CSM) after RC/PLND. Multivariable models adjusted for pathological T stage (≤pT2, pT3, pT4) and LN density.
Given the timing of NAC and AC relative to RC/PLND, a potential bias against NAC secondary to time lag from diagnosis to surgery was accounted for by using a previously published methodology as a sensitivity analysis [15]. Recurrence and cancer-specific mortality were assessed as dichotomous variables up to two years following RC. Patients censored prior to two years were excluded, leaving 77 patients for each model. The same predictors were then used to build univariate and multivariable logistic regression models for the dichotomous outcomes. All reported p values are two-sided, and statistical significance was set as p < 0.05. Analyses were conducted using Stata 12.0 (Stata Corp., College Station, TX).
Results
Median age of our cohort at time of RC was 64 (IQR 57, 71). The median number of positive LNs was two (IQR 1, 5), with a median number of LNs removed of 20 (IQR 12, 29). These clinicopathologic characteristics were similar in the NAC and AC groups. LN-positive patients treated with NAC had more advanced primary tumor stage than LN-positive patients treated with AC (p=0.02) and were more often female (50% vs. 24%, p=0.03) (Table 1). Median follow-up time for survivors was 7.3 years (IQR 5.0-9.5). Forty-six patients experienced disease recurrence.
Table 1.
Patient characteristics of 81 lymph node positive patients stratified by treatment with neoadjuvant or adjuvant chemotherapy. All values are median (IQR) or frequency (proportion).
| Neoadjuvant chemotherapy |
Adjuvant chemotherapy |
p-value | |
|---|---|---|---|
| Number of patients | 32 | 49 | |
| Age at surgery | 65 (58, 71) | 62 (56, 70) | 0.3 |
| Gender, male | 16 (50%) | 37 (76%) | 0.031 |
| Pathological stage | 0.023 | ||
| pT0 | 0 (0%) | 3 (6%) | |
| pTis | 2 (6%) | 1 (2%) | |
| pT1 | 0 (0%) | 1 (2%) | |
| pT2 | 4 (13%) | 7 (14%) | |
| pT3 | 19 (59%) | 14 (29%) | |
| pT4 | 7 (22%) | 23 (47%) | |
| Number of total LNs identified | 18 (12, 25) | 23 (12, 31) | 0.3 |
| Number of positive LNs detected | 2 (1, 5) | 3 (2, 5) | 0.2 |
| LN density | 0.16 (0.07, 0.29) | 0.14 (0.08, 0.30) | 0.8 |
| Histology | 0.8 | ||
| Urothelial carcinoma, NOS | 17 (53%) | 32 (65%) | |
| Micropapillary | 4 (13%) | 6 (12%) | |
| Squamous | 4 (13%) | 3 (6%) | |
| Glandular | 2 (6%) | 3 (6%) | |
| Other | 5 (16%) | 5 (10%) | |
| ACCI | 0.6 | ||
| 0 | 0 (0%) | 0 (0%) | |
| 1 | 1 (3%) | 3 (6%) | |
| 2 | 10 (31%) | 11 (22%) | |
| 3 | 8 (25%) | 11 (22%) | |
| 4 | 7 (22%) | 13 (27%) | |
| 5 | 3 (9%) | 5 (10%) | |
| 6 | 2 (6%) | 2 (4%) | |
| ≥7 | 1 (3%) | 4 (8%) |
ACCI, Age-adjusted Charlson Comorbidity Index; IQR, interquartile range; LN, lymph node; NOS, not otherwise specified
Most patients received platinum-based chemotherapy, representing 29 of 32 (91%) of patients treated with NAC, and 40 of 49 (82%) of patients treated with AC. The majority of patients treated with NAC received at least 3 cycles of platinum-based chemotherapy (72%), with 27 (84%) receiving at least 2 cycles. Similarly, 37 (76%) and 40 (82%) patients treated with AC received at least 3 and 2 cycles of platinum-based chemotherapy, respectively.
Our univariate Cox models demonstrated that clinically non-metastatic patients treated with NAC who were found to be LN-positive at RC were at a significantly higher risk of disease recurrence (HR=2.86; 95% CI 1.58-5.19; p=0.001) and cancer-specific mortality (HR=2.50; 95% CI 1.34-4.65; p=0.004) as compared to LN-positive patients treated with AC (Figure 1 and Table 2). After adjusting for pathologic stage and LN density in our multivariable models, LN-positive patients previously treated with NAC were still at greater risk for disease recurrence and cancer-specific mortality (HR=3.11, 95% CI 1.59-6.07, p=0.001 and HR=3.05, 95% CI 1.46-6.35, p=0.003, respectively).
Figure 1.
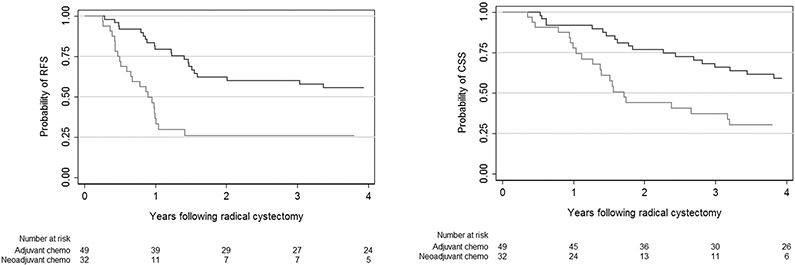
Kaplan-Meier recurrence-free survival (RFS) and cancer-specific survival (CSS) curves in 81 LN-positive patients. Gray line represents clinically non-metastatic neoadjuvant chemotherapy patients found to have LN metastases. Black line represents clinically non-metastatic chemotherapy-naïve patients found to be LN-positive at radical cystectomy and subsequently treated with adjuvant chemotherapy.
Table 2.
Comparison of cancer-specific outcomes in 81 lymph node-positive patients stratified by chemotherapy treatment.
| Univariate | Multivariable* | |||||
|---|---|---|---|---|---|---|
| Outcome | HR | 95% CI | p-value | HR | 95% CI | p- value |
| Disease Recurrence | ||||||
| Adjuvant chemotherapy | Ref. | Ref. | ||||
| Neoadjuvant chemotherapy | 2.86 | 1.58, 5.19 | 0.001 | 3.11 | 1.59, 6.07 | 0.001 |
| Cancer-Specific Mortality | ||||||
| Adjuvant chemotherapy | Ref. | Ref. | ||||
| Neoadjuvant chemotherapy | 2.50 | 1.34, 4.65 | 0.004 | 3.05 | 1.46, 6.35 | 0.003 |
Model adjusted for pathological stage (≤pT2, pT3, pT4) and LN density.
Given the relative timing of NAC, radical cystectomy, and AC, we performed a two-year outcomes logistic regression sensitivity analysis to eliminate any potential bias against LN-positive patients treated with NAC (“delay” to RC). The results of the sensitivity analysis confirmed the above findings, that LN-positive patients following NAC were at higher risk for disease recurrence (OR=5.29, 95% CI 1.89-14.83, p=0.002) and cancer-specific mortality (OR=4.28, 95% CI 1.59-11.50, p=0.004) than LN-positive patients treated with AC (Table 3). Again, these results remained significant after adjustment for pathologic stage and LN density in multivariable analyses (OR=5.59, 95% CI 1.95-16.01, p=0.001 and OR=5.24, 95% CI 1.78-15.46, p=0.003, respectively).
Table 3.
Sensitivity analysis: Logistic regression modeling for cancer-specific outcomes in 77 lymph node positive patients ≤ 2 years after radical cystectomy and bilateral pelvic lymph node dissection.
| Univariate | Multivariable* | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Disease Recurrence | ||||||
| Adjuvant chemotherapy | Ref. | Ref. | ||||
| Neoadjuvant chemotherapy | 5.29 | 1.89, 14.83 | 0.002 | 5.59 | 1.95, 16.01 | 0.001 |
| Cancer-Specific Mortality | ||||||
| Adjuvant chemotherapy | Ref. | Ref. | ||||
| Neoadjuvant chemotherapy | 4.28 | 1.59, 11.50 | 0.004 | 5.24 | 1.78, 15.46 | 0.003 |
Model adjusted for pathological stage (≤pT2, pT3, pT4) and LN density.
Discussion
We found that clinically non-metastatic muscle-invasive bladder cancer patients treated with NAC and found to be LN-positive had significantly worse outcomes as compared to chemotherapy-naïve LN-positive patients subsequently treated with AC. LN-positive NAC patients had a 3-year RFS survival estimate of 26%, as compared with 60% for LN-positive AC patients. By comparison, a 36% 3-year RFS was found in a historical series of 1550 LN-positive patients treated with RC/PLND alone [1].
While NAC has improved outcomes for patients with muscle-invasive bladder cancer, not all patients will harbor chemotherapy-sensitive disease. The majority of patients with significant residual disease following NAC will ultimately experience disease recurrence [8]. In our study, patients treated with NAC and found to have LN-positive disease represent an empirically defined chemotherapy-resistant cohort. Conversely, within a group of chemotherapy-naïve LN-positive patients, there exists a proportion with chemotherapy-sensitive disease that will benefit from AC. Therefore, it is not unexpected that chemotherapy non-response is associated with worse cancer-specific outcomes. In a study of 52 bladder cancer patients with pathologically-confirmed LN metastases, response to preoperative platinum-based chemotherapy was associated with improved survival, and lymph node status was more important than local tumor status [16].
Our findings that LN-positive patients previously treated with NAC have extremely poor outcomes are significant and have not previously been well described. Most studies investigating prognostic factors in LN-positive bladder cancer patients exclude patients treated with either neoadjuvant chemotherapy or adjuvant chemotherapy [2, 4, 17, 18]. While some studies have reported on the outcomes of LN-positive patients following preoperative chemotherapy, most either focus upon clinically LN-positive patients [11] or group clinically LN-negative patients (who receive true neoadjuvant chemotherapy) and clinically LN-positive patients (who receive full-course induction chemotherapy) together [12]. Retrospective studies including patients treated in an earlier era often do not have strict criteria for preoperative axial imaging. Further, although studies have identified that pathologic responders to NAC have better outcomes than non-responders, the comparison of NAC non-responders with chemotherapy-naïve non-responders has not been well characterized [19]. Another study reported on the incidence of LN metastases in clinically non-metastatic patients comparing those treated with NAC vs. RC alone, but focused on the effect of NAC upon the likelihood of LN metastases rather than the outcomes of the 16 LN-positive NAC patients [20].
By demonstrating the poor survival of LN-positive NAC patients, we provide supportive information that this characteristic be used in the stratification of LN-positive patients that are studied in clinical trials. Expected outcomes, power calculations and perhaps altered response patterns to study drugs may better be interpreted by recognizing the differential outcomes of the different LN-positive patient populations. Additionally, the poor observed outcomes provide strong rationale for enrolling LN-positive NAC patients in prospective clinical trials utilizing novel therapeutic agents or approaches to chemotherapy. Some examples of relevant ongoing clinical trials include those of adjuvant atezolizumab (ClinicalTrials.gov NCT02450331) and another examining adjuvant nivolumab (ClinicalTrials.gov NCT02632409) with high-risk features following radical cystectomy. Both of these trials include both patients without prior treatment and also patients who received neoadjuvant chemotherapy.
Genomic profiling of bladder cancer holds the promise to rationally define subsets of patients who might benefit from targeted therapies [21]. However, there are numerous challenges to this approach, as studies have begun to identify the scope of the genomic heterogeneity of urothelial carcinoma [22, 23] and intra-tumor heterogeneity [24], and initial trials of rational targeted therapy for metastatic bladder cancer have had disappointing results [25]. Another strategy involves the elucidation of molecular or genetic predictors of chemosensitivity. While studies have identified potential prognostic or predictive biomarkers of response to chemotherapy in bladder cancer and other malignancies, results have been mixed [26, 27].
The magnitude of the effects identified by our study should be interpreted with caution, as 5 patients who recurred before initiation of AC were excluded from the AC group as they were classified as having received salvage, rather than adjuvant, chemotherapy). The drastic difference in survival between these two treatment groups may not be quite as large if these patients were included. However, while the adjuvant LN-positive patients may have had slightly worse outcomes in an “intention to treat” analysis, they are comparable to those reported in the literature [12]. Furthermore, the outcomes for LN-positive NAC patients are poor even with respect to historical series of LN-positive patients not treated with AC.
Our study has several limitations, including those common to all retrospective analyses. Our sample size was limited by the inclusion/exclusion criteria we used to obtain comparable cohorts on LN-positive patients in terms of demographics and clinical disease staging. Nevertheless, there was a large effect size. Although we only included patients with muscle-invasive and clinically non-metastatic disease (cT2-4N0M0) confirmed by our institution’s pathologists and radiologists, we did not perform a re-review of histology and axial imaging. Finally, since patients in our study were treated by various oncologists and surgeons over a 10-year period, our data are heterogeneous and representative of practice at a large academic cancer center.
In summary, we found that clinically LN-negative, non-metastatic (cN0M0) muscle-invasive bladder cancer patients treated with NAC and found to have LN-positive disease at time of RC/PLND have poor outcomes, with a 3-year recurrence-free survival estimate of 26%. These outcomes were significantly poorer than those for chemotherapy-naïve cN0M0 patients found to have LN-positive disease and subsequently treated with AC.
Supplementary Material
Acknowledgments
Funding: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, the Michael and Zena Wiener for Therapeutics Program in Bladder Cancer, Pin Down Bladder Cancer, and the MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
References
- [1].International Bladder Cancer Nomogram C, Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006. August 20: 24:3967–72 [DOI] [PubMed] [Google Scholar]
- [2].Fajkovic H, Cha EK, Jeldres C, et al. Extranodal extension is a powerful prognostic factor in bladder cancer patients with lymph node metastasis. European urology. 2013. November: 64:837–45 [DOI] [PubMed] [Google Scholar]
- [3].Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001. February 1: 19:666–75 [DOI] [PubMed] [Google Scholar]
- [4].Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003. February 15: 21:690–6 [DOI] [PubMed] [Google Scholar]
- [5].Vazina A, Dugi D, Shariat SF, Evans J, Link R, Lerner SP. Stage specific lymph node metastasis mapping in radical cystectomy specimens. The Journal of urology. 2004. May: 171:1830–4 [DOI] [PubMed] [Google Scholar]
- [6].Tarin TV, Power NE, Ehdaie B, et al. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. European urology. 2012. May: 61:1025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999. August 14: 354:533–40 [PubMed] [Google Scholar]
- [8].Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. The New England journal of medicine. 2003. August 28: 349:859–66 [DOI] [PubMed] [Google Scholar]
- [9].Griffiths G, Canc MRCAB, Grp NBCS, Tratamiento CUE. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. Journal of Clinical Oncology. 2011. June 1: 29:2171–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reardon ZD, Patel SG, Zaid HB, et al. Trends in the Use of Perioperative Chemotherapy for Localized and Locally Advanced Muscle-invasive Bladder Cancer: A Sign of Changing Tides. European urology. 2014. January 23: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Meijer RP, Mertens LS, van Rhijn BW, et al. Induction chemotherapy followed by surgery in node positive bladder cancer. Urology. 2014. January: 83:134–9 [DOI] [PubMed] [Google Scholar]
- [12].Kassouf W, Agarwal PK, Grossman HB, et al. Outcome of patients with bladder cancer with pN+ disease after preoperative chemotherapy and radical cystectomy. Urology. 2009. January: 73:147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008. June: 112:2384–92 [DOI] [PubMed] [Google Scholar]
- [14].AJCC Cancer Staging Manual. 6th edn, New York: Springer, 2002 [Google Scholar]
- [15].Vickers AJ, Bianco FJ Jr., Boorjian S, Scardino PT, Eastham JA. Does a delay between diagnosis and radical prostatectomy increase the risk of disease recurrence? Cancer. 2006. February 1: 106:576–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nieuwenhuijzen JA, Bex A, Meinhardt W, et al. Neoadjuvant methotrexate, vinblastine, doxorubicin and cisplatin for histologically proven lymph node positive bladder cancer. The Journal of urology. 2005. July: 174:80–5 [DOI] [PubMed] [Google Scholar]
- [17].Herr HW. Superiority of ratio based lymph node staging for bladder cancer. The Journal of urology. 2003. March: 169:943–5 [DOI] [PubMed] [Google Scholar]
- [18].Rink M, Hansen J, Cha EK, et al. Outcomes and prognostic factors in patients with a single lymph node metastasis at time of radical cystectomy. BJU international. 2013. January: 111:74–84 [DOI] [PubMed] [Google Scholar]
- [19].Manoharan M, Katkoori D, Kishore TA, Kava B, Singal R, Soloway MS. Outcome after radical cystectomy in patients with clinical T2 bladder cancer in whom neoadjuvant chemotherapy has failed. BJU international. 2009. December: 104:1646–9 [DOI] [PubMed] [Google Scholar]
- [20].Mertens LS, Meijer RP, Meinhardt W, et al. Occult lymph node metastases in patients with carcinoma invading bladder muscle: incidence after neoadjuvant chemotherapy and cystectomy vs after cystectomy alone. BJU international. 2014. July: 114:67–74 [DOI] [PubMed] [Google Scholar]
- [21].Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012. October 12: 338:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014. March 20: 507:315–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim PH, Cha EK, Sfakianos JP, et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. European urology. 2015. February: 67:198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012. March 8: 366:883–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Milowsky MI, Iyer G, Regazzi AM, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU international. 2013. August: 112:462–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007. March: 18:522–8 [DOI] [PubMed] [Google Scholar]
- [27].Friboulet L, Olaussen KA, Pignon JP, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. The New England journal of medicine. 2013. March 21: 368:1101–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


