Abstract
Purpose
This review aims to compare the magnitude of the effects of chronic consumption of fruits; specifically berries, citrus and cherries on cardiovascular disease (CVD) risk factors.
Methods
PubMed, Web of Science, Scopus, and psycARTICLES were searched from inception until January 2020. Forty-five chronic (≥ 1 week) randomised controlled trials assessing CVD risk factors including endothelial (dys)function, blood pressure (BP), blood lipids and inflammatory biomarkers were included.
Results
Investigated interventions reported improvements in endothelial function (n = 8), inflammatory biomarkers and lipid status (n = 14), and BP (n = 10). Berries including juice of barberry, cranberry, grape, pomegranate, powder of blueberry, grape, raspberry and freeze-dried strawberry significantly reduced SBP by 3.68 mmHg (95% CI − 6.79 to − 0.58; P = 0.02) and DBP by 1.52 mmHg (95% CI − 2.87 to − 0.18, P = 0.04). In subgroup analysis, these associations were limited to cranberry juice (SBP by 1.52 mmHg [95% CI − 2.97 to − 0.07; P = 0.05], DBP by 1.78 mmHg [95% CI − 3.43 to − 0.12, P = 0.04] and cherry juice (SBP by 3.11 mmHg [95% CI − 4.06 to − 2.15; P = 0.02]). Berries also significantly elevated sVCAM-1 levels by 14.57 ng/mL (85% CI 4.22 to 24.93; P = 0.02).
Conclusion
These findings suggest that supplementing cranberry or cherry juice might contribute to an improvement in blood pressure. No other significant improvements were observed for other specified fruits. More research is warranted comparing different classes of fruit and exploring the importance of fruit processing on their cardiovascular-protective effects.
Electronic supplementary material
The online version of this article (10.1007/s00394-020-02299-w) contains supplementary material, which is available to authorized users.
Keywords: Fruit, Intervention, Endothelial function, CVD risk factors, Systematic review, Meta-analysis
Introduction
Current World Health Organization (WHO) recommendations for fruit intake combined with vegetable intake are a minimum 400 g/day [1]. A recent meta-analysis indicated that the intake of 800 g/day of fruit was associated with a 27% reductions in relative risk of CVD [2]. It is well recognised that cardiovascular health can be affected by several dietary factors [3]. Epidemiological evidence has established strong inverse associations between flavonoid-rich fruit (e.g. strawberries, grapefruit) and coronary heart disease (CHD) mortality in CVD-free postmenopausal women after multivariate adjustment [4]. Endothelial function is a primary indicator of cardiovascular health, a damaged endothelium will cause disruption of vascular hemostasis and further lead to endothelial dysfunction, which is the manifestation underlying atherosclerosis, hypertension, and other CVDs [5, 6]. Intervention studies also provide evidence supporting the consumption of a range of fruit and fruit juice to reduce cardiovascular dysfunction risk factors. For example, consumption of fruit containing relatively high levels of anthocyanins and procyanidins, such as berries, has been shown to improve CVD risk factors, namely endothelial dysfunction, dyslipidaemia, platelet aggregation, and hypertension [7, 8], whereas flavanone-rich citrus, such as orange, were effective in improving hypercholesterolaemia [7]. The consumption of cherries was also suggested by interventions to promote cardiovascular health by preventing or decreasing lipid levels and inflammation [9]. One systematic review and epidemiological evidence have also revealed that the consumption of fruit juice including citrus, berries and cherry juice may benefit vascular health by affecting risk markers such as blood pressure and lipid profiles [10, 11].
Fruit juice and powder may be effective methods to increase overall fruit consumption, which may explain the emerging intervention studies investigating health benefits with fruit powder and juice supplementations. With regard to nutritional value, freeze-dried fruit powder that is devoid of water retains concentrated bio-accessible antioxidants, fibre and other components [10]. Research has suggested that the juicing process can lead to a lower content of fibre and certain bioactives such as polyphenols, vitamins, and minerals [12, 13], while other research suggests that processing can increase the bioavailability of carotenoids, such as lycopene [14]. A recent single-dose bioavailability study showed only minor differences between whole blueberry fruit and blueberry juice [15]; indeed a systematic review demonstrated that the intake of fruit and vegetable juice offered similar cardiovascular health benefits to the intake of whole fruit and vegetables [16].
Other systematic reviews have assessed the effect of fruit and vegetable intake on endothelial function or the effect of specific fruit juice intake on CVD risk factors [17, 18]. However, to the best of our knowledge, the effects of the fruit-delivery method (type and processed form) in relation to CVD risk factors including endothelial (dys)function, lipid profile (i.e. total cholesterol) and inflammatory biomarkers (i.e. C-reactive protein/CRP) has not been appraised. A review of this type is important to clarify the evidence base for the type and form of fruit that is most cardiovascular-protective. Therefore, the aim of this study was to systematically review and meta-analyse available human intervention studies to evaluate the potential effect of consumption of whole, freeze-dried, powdered, and juiced forms of fruit, and specifically berry, citrus and cherry fruit, on CVD risk factors in randomised controlled trials (RCTs) in line with the PICOS (population, intervention, comparator, outcome, study design) framework (Supplemental Table 1).
Methods
Study eligibility
We searched for studies assessing the effect of specific fruit supplementations on CVD risk factors including terms of “fruit”, “CVD risk factors”, “endothelial function”, “BP”, “lipid”, “inflammatory biomarker”. The following specific inclusion criteria were applied: (1) study design: RCTs; (2) subjects: adult subjects ≥ 18 years of age; (3) interventions: intervention RCTs providing or promoting berry, or cherry or citrus fruit or their juice or freeze-dried, or powdered fruit consumption; (4) intervention length: at least 1 week; (5) control: control groups without components of citrus fruit, cherry, or berries, likely placebo group; (6) outcomes: the primary outcomes were the whole body measurements: systolic and diastolic blood pressure (SBP and DBP) and the endothelial (dys)function assessed by flow-mediated dilation (FMD) and pulse wave velocity (PWV); the secondary outcomes were the blood biomarkers including circulating fatty acids triglycerides (TAGs) and total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein (HDL-C); inflammatory biomarkers such as high-sensitivity C-reactive protein (hsCRP), nitric oxide (NO), intercellular adhesion molecules (ICAMs) and vascular adhesion molecules (VCAMs) were also explored (described below); (7) only English-language and peer-reviewed articles were included. No restriction of publication year was applied.
Data sources
The present systematic review and meta-analysis was conducted in accordance with Cochrane [19] and Centre for Reviews and Dissemination guidelines [20] and was reported according to PRISMA guidelines [21] (Supplemental Table 2). The protocol has been registered with PROSPERO, the International Prospective Register of Systematic Reviews (Registration number CRD42018091896). Such protocol includes the investigation of the impact of these fruits on cognitive function, however this analysis will be reported elsewhere. Two researchers (YW, JLG) assessed articles independently for inclusion eligibility. The searches using PubMed, Web of Science, Scopus and psycARTICLES were conducted from inception until January 2020. No restriction of publication year was applied and the search result covered studies published between 1960 and 2020.
The search of the investigated themes in this review was undertaken using terms as following: (1) fruit; (2) citrus; (3) orange; (4) berry; (5) berries; (6) grape; (7) blueberry; (8) blueberries; (9) blackberry; (10) blackberries; (11) raspberry; (12) raspberries; (13) cranberry; (14) cranberries; (15) cherry; (16) cherries; (17) “endothelial function”; (18) “vascular function”; (19) “vascular risk factors”; (20) hypertension; (21) “blood pressure”; (22) BP; (23) “pulse wave velocity”; (24) PWV; (25) “flow-mediated dilation”; (26) FMD; (27) lipid; (28) cholesterol; (29) LDL; (30) HDL; (31) triglyceride; (32) biomarkers; (33) inflammatory; (34) “Nitric Oxide”; (35) NO; (36) ICAM; (37) VCAM; (38) hsCRP; (39) trial; (40) intervention. Search strategy was supplied (Supplemental Table 3).
Study selection
YW and JLG selected articles independently for eligibility. Articles were moved to the next screening phase or discarded when full disagreement was reached. JKL served as an arbitrator if any disagreements that were not resolved. No disagreements occurred during the selection phase. All records were exported to EndNote X8 reference management software. The selection of eligible studies was based on two steps. Firstly, the title and abstract of each study were screened for relevance; full texts were then reviewed for those without certainty for inclusion. Reference lists of included papers and relevant systematic reviews were also screened by hand-searching for additional articles.
Data abstraction
Data were extracted by YW and JLG independent of each other, their selections for accuracy were reviewed in meeting. Corresponding authors were contacted via e-mail to request information if there were missing data or for clarification. Data from endpoints and the baseline were obtained. A pre-defined data extraction form in Microsoft Excel 2016 was used to input studies data, which includes information on (1) author and published year; (2) study design; (3) population characteristics (ethnicity, mean age, sex, mean body mass index (BMI), health status and sample size at baseline); (4) treatment details (intervention type, length, dosage and frequency); (5) control group settings; (6) retention rate; (7) measured outcomes for both experimental group and placebo group at baseline and the longest post-intervention time point to avoid the bias of selectively choosing data.
Risk of bias assessments
Study quality for RCTs was assessed by Jadad Score (0–5), which takes into account whether a trial was randomised and blinded with appropriate procedure, and whether dropouts were well recorded; a score ≥ 3 indicates a high-quality trial [22].
Publication bias was assessed by Funnel plot and Egger’s test, ‘trim and fill’ method was implemented to identify and correct for funnel plot asymmetry arising from publication bias [23] (Supplemental Fig. 1).
Data synthesis
R studio version 3.5.2 [24] and the package “meta” [25] were used to pool and meta-analyse data from collected studies. Subgroup analysis with at least 10 studies supplementing berries was implemented to estimate separate effects of different types of berries and the heterogeneity for each berry intervention subgroup. There were 3 studies supplementing grapefruit juice and cherry juice as concentrate instead of 100% juice, which could cause variations to the juice quality and bioavailability [26–28]. For example, anthocyanins are better preserved in purees (57%) than in clarified juice (31%) when comparing different forms of processed blackberries [29]. Sensitivity analysis of juice quality was carried out to investigate the effect of juice on the meta-analysis results.
All pooled results were presented as weighted mean difference with 2-sided p values. 95% confidence intervals (CIs) and prediction intervals were both presented in the results. The FMD value was expressed in percentage unit and the PWV value was expressed in m/s; the conversion of cm/s to m/s for PWV value was applied when necessary for pooled mean differences in meta-analysis. For blood lipids, the conversion factor 1 mmol/L = 38.67 mg/dL was used for total, HDL, and LDL cholesterol level and 1 mmol/L = 88.57 mg/dL for triglycerides level [30] where applicable.
The Hartung–Knapp–Sidik–Jonkman method for random-effects meta-analysis [31] was applied. Heterogeneity was estimated by Cochrane Q statistics and the consistency of study results was assessed by I2 statistics as an extension of Cochrane Q statistics and an I2 > 50% is considered for a high heterogeneity level [32]. The effect sizes based on the weighted mean difference (WMD) between treatment groups were used when measurement units of assessed outcomes were comparable across studies. The standardised mean difference (SMD) was used when studies have used different measurement units and the conversion had failed.
Results
Literature search
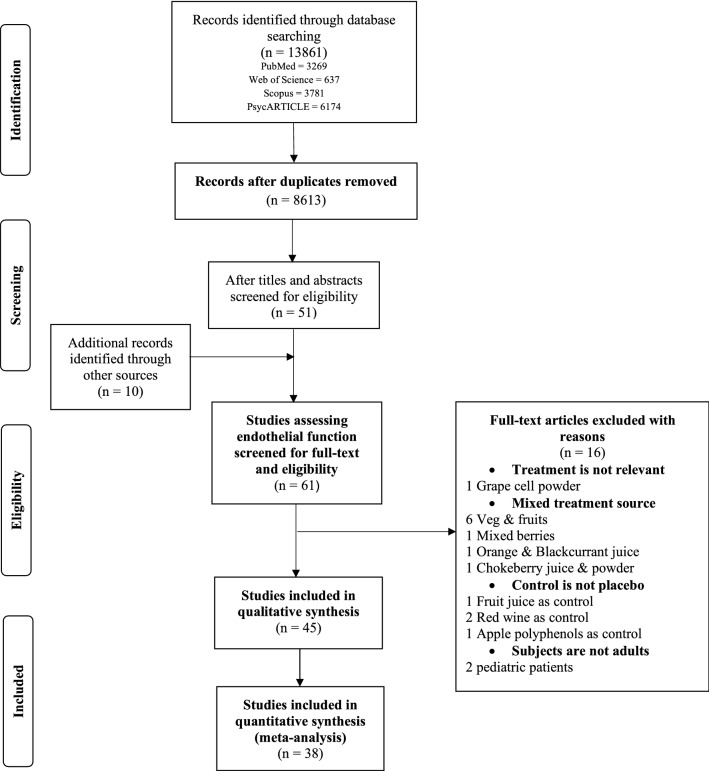
In accordance with PRISMA guidelines [21], Fig. 1 describes the selection process of included studies. The initial search produced 13,861 articles from the four databases, this record was reduced to 8613 articles after duplicates were removed. After screening of the titles and abstracts for eligibility, 51 articles were included and 10 additional articles were added from manual search through reference lists of initially identified articles. The final selection identified 61 trials assessing CVD risk factors, where 16 articles were further excluded after checking full-text eligibility (Supplemental Table 4). Finally 45 trials were included in this review, 38 trials from these were included in the meta-analysis.
Fig. 1.
Flow diagram of study selection for the review according to PRISMA guidelines
Study characteristics
Forty-five studies were included in this systematic review, of which 18 were crossover randomised controlled trials (RCT) [27, 33–49], and 27 were parallel RCTs [26, 28, 50–74] (see Table 3). The sample size of both experimental and control group in the interventions ranged from 5 to 63. The total sample size for the intervention group was 1130; the total sample size for the control group was 1109. Participants’ characteristics at baseline also vary across studies; most trials recruited healthy subjects (n = 13), while there were 7 studies with participants manifesting increased CVD risks (deteriorated lipid profiles and hypertension) and 3 with diagnosed CVD/CHD; 18 with metabolic syndrome (inclusive of overweight); 1 with mild‑to‑moderate dementia, 1 with chronic obstructive pulmonary disease, 1 with type 2 diabetes and 1 with end-stage renal disease (Table 3).
Table 3.
Summary of fruit interventions
| (Author/year) country of origin | Study design | Ethnicity (%) | Mean or range age (years) | Sex (%male) | Mean BMI (kg/m2) | Participants healthy status | Baseline sample size (total, intervention, control) | Intervention type | Intervention length (Hours(h), Days(d) or Weeks (wks)) | Dose and frequency of consumption | Control group | Retention rate | Outcomes | Total Jadad score (0–5) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bardagjy et al. (2018) US | Randomized, placebo controlled, double-blinded crossover trial | NA | 48.6 ± 15.4 | 20% | 37.0 ± 9.9 | Obese but otherwise healthy adults | 23– > 20 | 60 g equals 2.2 cups or 330 g of grapes | 4 wks | GP (60 g) was equivalent to 330 g or 2.2 cups of fresh grapes and contained 297 mg total polyphenols (as gallic acid equivalents) | PBO was matched to GP in calories, macronutrients, taste, and appearance but provided zero polyphenols/serving | 87% | SBP, DBP, TC, HDL, LDL, TG, CRP, ET-1, IL-6: oxLDL, sICAM, sVCAM, TNFc | 3 |
| Barona et al. (2012) Colombia | Double-blind cross-over RCT | N/A | 51.3 ± 9.6 | N/A | N/A | Metabolic syndrome | 25 | grape powder | 30d | 46 g/d = 2 serving of fresh grapes | Placebo | 96% | SBP, DBP, FMD, TG, HDL, Glucose, BMI, Nox, sICAM-1, sVCAM-1, E-selectin | 2 |
| Boldaji et al. (2019) Iran | Crossover RCT | Pomegranate juice | 47.8 ± 13.3 | 61% | 23.9 ± 4.8 | ESRD patients on dialysis treatment, dialysis 3 times a week for at least 3 months, | 40:38 | 100 mL PJ | 8 wks | 100 mL PJ three times a week after their dialysis session | No intervention as control | 97.6% | SBP, DBP, TC, HDL, LDL, TG, IL-6 | 2 |
| Buscemi et al. (2012) Italy | Single-blinded crossover RCT | N/A | 48 ± 13 | 53 | 32.1 ± 4.9 | subjects with increased CVD risk | 19 | red orange juice | 7d | 500 mL/d | Placebo drink (12 healthy non-diabetic subjects acting as control group) | 91% | FMD, GTN (glyceryl-trinitrate), hs-CRP, IL-6, TNF-α, NO, PCs (protein carbonyl) | 2 |
| Constans et al. (2015) France | Cross-over, single-blinded RCT | N/A | 53.8 ± 2 | 100 | 26 ± 1 | Mild hypercholesterolemic men (LDL-C between 130 and 190 mgL) | 25 | Blond orange juice | 4 wks | 200 mL (3×/d) | Control beverage | 96% | Glucose, TC, LDL, HDL,TC/HDL, TG, ApoA-1, ApoB, Lpa, hsCRP, Brachial FMD, sICAM-1, sVCAM-1, sE-selectin | 3 |
| Desai et al. (2018) UK | Single-blinded crossover RCT | Montmorency tart cherry juice (MTCJ) | 30 ± 10 years | 7/11 | BMI 24.43 ± 3.23 | Healthy | 11 | 30 mL MTCJ | 20 days | 30 mL | Placebo | 100% | SBP, DBP, TC, LDL, TG, IL-7 | 3 |
| Dohadwala et al. (2010) US | Crossover RCT | 42% black | 43 ± 12 | 69 | 28 ± 3.8 | Stage 1 hypertension | 63 | Concord grape juice | 8 wks | 490 mL (965 mg/d) | Placebo drink | 77% | SBP, DBP, TG TC, LDL, HDL | 3 |
| Dohadwala et al. (2011) US | Crossover double-blind RCT | Black 45.5% | 62 ± 10 | 68 | 29.5 ± 4.5 | Coronary heart disease | 44 | Double-strength cranberry juice | 4 wks | 480 mL/d | Calorie, taste, and appearance-matched placebo beverage containing no polyphenols | 91% | SBP, DBP, FMD, Baseline diameter, Dilation to nitroglycerin, Baseline flow, FMD, Hyperemic flow, InPAT ratio, Cartoid-radial PWV, Cartoid-femoral PWV | 4 |
| Habauzit et al. (2015) France | Crossover double-blind RCT | Caucasian | 58 ± 4 | 0 | 25.7 ± 2.3 | Postmenopausal woman | 52 | Concentrate blond grapefruit juice | 6 months | 340 mL/d (2 × 170) | Isocaloric Control drink | 92% | SBP, DBP, Pulse pressure, FMD dilation, Baseline brachial diameter, PAT ratio, Pulse pressure, NO, Endothelin 1 | 4 |
| Holland et al. (2018) UK | Open label crossover RCT | Blood orange juice | 52.2 ± 13.6 | Male 20/41 | 29.0 5.1 | Healthy | 45 (41) | 500 mL blood OJ providing 50 mg anthocyanins/d | 28 days | 2 × 250– > 500 mL | 500 mL blonde OJ without anthocyanins | 91% | SBP, DBP, ba PWV, cf PWV, NO, CRP, TG, HDL-C, LDL-C | 2 |
| Lamport et al. (2016) UK | Double-blind, crossover RCT | N/A | 43.2 ± 0.6 | 0 | 24.6 ± 0.5 | Healthy | 25(19) | Concord grape juice | 6 wks, 12 wks | 355 mL/d | Energy-, taste-, and appearance-matched placebo | 77% | SBP, DBP | 4 |
| Martin (2018) US | Crossover RCT | N/A | 38.1 ± 12.5 years | ### | 32.2 ± 4.6:32.2 ± 4.8 | Overweight and obese subjects | 13-- > 10 | 240 mL tart cherry juice | 4 weeks | 240 mL/d | Placebo juice | 77% | IL-6, IL-10, TNF-, MCP-1, hsCRP | 4 |
| Millar (2018) US | Double-blind crossover RCT | N/A | 53.5 ± 10.1 | ### | 33.0 ± 4.77 kg/m2 | Metabolic syndrome | 20 | Grape powder (contributing 195 mg total polyphenols) | 4 weeks | 60 g grape powder per day | Placebo powder | 100% | Total cholesterol, HDL-C and HDL particles, TG | 3 |
| Morand et al. (2011) French | Crossover RCT | N/A | 56 ± 1 | 100 | 27.4 ± 0.3 | Overweight healthy | 24 | Orange juice | 4 wks | 500 mL/d | Control drink + placebo capsules (starch) | 100% | SBP, DBP, Pulse pressure, Glucose, Insulin, Triglycerides, Total cholesterol, LDL, HDL, CRP, IL-6, vWF, sICAM-1, sVCAM-1, NOx | 5 |
| Riso et al. (2013) Italy | Crossover RCT | N/A | 47.8 ± 9.7 | 100 | 47.8 ± 9.7 | CVD risk factors | 18 | Freeze-dried wild blueberry powder 25 g | 6 wks | 250 mL/d | Placebo drink consisted of 250 mL water, 7.5 g fructose, 7 g glucose, 0.5 g citric acid and 0.03 g blueberry flavor | 89% | RHI, FRHI, AI, AI@75, Diastolic pressure, SBP, Total NO, sVCAM-1 | 2 |
| Ruel et al. (2013) Canada | Double-blind crossover RCT | N/A | 45 ± 10 | 100 | 28.3 ± 2.4 | Overweight | 35 | 27% cranberry juice | 4 wks | 500 mL/d | Placebo juice | 100% | Heart rate, Systolic BP, Diastolic BP, MAP, Resting AIx, Δ AIx salbutamol, Δ AIx GTN, Global endothelial function, NOx, Uric acid, Oxidized LDL, sICAM-1, sVCAM-1, sE-selectin | 3 |
| Siasos et al. (2014) Greece | Double-blind crossover RCT | N/A | 26.34 ± 4.93 | 38 | 23.21 ± 4.10 | Healthy | 26 | 100% concord grape juice | 7 d, 14 d | 7 cc/kg/d | The grapefruit placebo juice matched the flavor, color, calorie, and sugar profile of the CGJ but did not contain any polyphenols | 100% | FMD, PWV/carotid-femoral, Total cholesterol, LDL-C, TG, Serum glucose, SBP, DBP | 4 |
| Willems et al. (2015) UK | Double blind, randomized, placebo-controlled, and cross-over | N/A | 38 ± 8 | 62 | 23 ± 2 | Healthy | 13 | Blackcurrant powder | 7 d | 6 g/day (138.6 mg anthocyanins) | Blackcurrant juice 3–4 mg anthocyanins per dose | 77% | SBP, DBP, mean arterial BP, heart rate, Stroke volume, cardiac output, peripheral resistance | 2 |
| Basu et al. (2014) UK | RCT | N/A | 49 ± 10 y | 9.1 | 36 ± 6 5 | Obese adults with elevated serum lipids | 60 (15:15:15:15) | High-dose freeze dried strawberry (10% weight of fresh strawberries) and low-dose freeze-dried strawberry | 12 wks | 50 g/d for high dose; 25 g/d for low dose | High-dose calorie- and fiber-matched control 44 g/d; low-dose calorie- and fiber-matched control 24 g/d | 100% | BMI, SBP, DBP, glucose, insulin, Total cholesterol, LDL-C, HDL-C, LDL:HDL, VLDL-C, TGs, hs-CRP, sVCAM-1, sICAM-1 | 2 |
| Basu et al. (2010) US | RCT | N/A | 47.0 ± 3.0 | 7.4 | 37.5 ± 2.15 | Metabolic syndrome | 30 (15:12) | Freeze-dried strawberry beverage (50 g freeze-dried strawberries ∼ 3 cups fresh strawberries | 8 wks | 3 cups/d | 4 cups of water/d | 90% | BMI, SBP, DBP, glucose, Total cholesterol, HDL-C, LDL-C, VLDL-C, TGs, ICAM-1, VCAM-1 | 2 |
| Basu et al. (2011) US | RCT | N/A | 52.0 ± 8.0 | 0 | 40.0 ± 7.7 | Metabolic syndrome | 31 (15:16) | Cranberry juice | 8 wks | 240 mL/458 mg/d | Placebo drink | 97% | SBP, DBP, Total cholesterol,H DL-C, LDL-C, TGs | 3 |
| Basu et al. (2010) US | Single-blinded parallel RCT | N/A | 50.0 ± 3.0 SE | 8.3 | 37.8 ± 2.3 | Metabolic syndrome | 66 (25:23) | 480 mL freeze-dried blueberry beverage (50 g freeze-dried blueberries) | 8 wks | 50 g freeze-dried bb beverage | 480 mL water and vanilla extract | 73%:72% | SBP, DBP, TG, Total, LDL-, HDL-, oxLDL- cholesterol | 2 |
| Cerda (2006a) Spain | RCT | N/A | 60 ± 10.9 | N/A | 31.4 ± 4.8 | Chronic obstructive pulmonary disease | 30 (15:15) | 400 mL pomegranate juice (2660 mg/d) | 5 wks | 400 mL/d | Placebo drink | 100% | Total cholesterol, HDL-C, LDL-C, TGs | 3 |
| Curtis et al. (2019) UK | Double-blind, parallel RCT | NA | 63 ± 7 | ### | 31.2 ± 3.0 | Metabolic syndrome | 144– > 115(37:39:39) | 26 g equivalent to 1 cup (150 g) and 1/2 cup (75 g) milled blueberries powder | 6 months | Equivalent 150 g BB | Dextrose, maltodextrin, and fructose, which were produced as a purple powder, with blueberry aromatics generated from natural (nonanthocyanin) and artificial color and flavorings | 80% | SBP, DBP, TC, HDL, LDL, TG | 3 |
| Chew et al. (2019) US | Randomized, double-blind, placebo-controlled, parallel design trial | NA | 43.1 ± 1.1 | ### | 30.8 ± 0.4 k | Non-smoking overweight | 79– > 78(40:38) | 450 mL cranberry extract beverage (CEB) | 8 wks | 450 mL | The placebo beverage was designed to look, smell, and taste such as the CEB, but did not contain cranberry constituents. | 98% | CRP, IL-6, IL-10, IL-23, TNF-α; IFN-γ | 3 |
| Chai et al. (2019) US | Parallel RCT | Tart cherry juice | 28.5 ± 3.7:27.3 ± 4.2 | 40%:53% | 70.0 ± 3.7:(69.5 ± 3.9 | Older adults | 37– > (20:17) | 12 weeks | 68 mL of Montmorency tart cherry concentrate was diluted with 412 mL of water | Control drink was prepared by mixing unsweetened black cherry flavored Kool-Aid (Kraft Foods, Chicago, IL, USA) with water | 100% | TNF-α, CRP, ET-1, NO, OxLDL | 3 | |
| Duthie (2006b) Scotland | RCT | N/A | 18-40 y | 0 | N/A | Healthy | 20 (11:9) | Cranberry juice | 2 wks | 750 mL (852 mg/d) | Placebo drink (6.72 mg/d) | 100% | Total cholesterol, HDL-C, LDL-C, TGs | 2 |
| Dow et al. (2013) US | RCT | Non-Hispanic white race (62.3) | 41.8 ± 10.7 | 30 | 32.1 ± 4.1 | Obese or with additional MetS (42%) | 74 (37:32) | Low bioactive diet plus half of a fresh Rio red grapefruit × 3 times | 6 wks | Low bioactive diet plus 1.5 grapefruit/d | A low bioactive diet devoid of citrus | 93% | sVCAM-1, hsCRP | 1 |
| Flammer et al. (2013) US | Double blind RCT | N/A | 49.5 ± 16.2 | 45 | 27.7 ± 5.9:27.2 ± 5.5 | Peripheral endothelial dysfunction and CVD risk factors | 84 (32:37) | Cranberry juice ((double-strength Ocean Spray® light cranberry juice cocktail (54% cranberry juice)) | 4 months | 2*230 mL/d | Placebo juice beverage, an isocaloric formulation mimicking the flavor and color of the cranberry beverage | 82% | RHI, SBP, DBP, pulse pressure, heart rate, AI augmentation via EndoPAT, hsCRP, VCAM, ICAM, I1-6, TNF-alpha, oxLDL, Cholesterol, HDL, TG | 3 |
| Gonzalez-Ortiz (2011b) US | RCT | N/A | 25–55 y | N/A | 30.0–39.9 | Obesity | 20 (10:10) | Pomegranate juice | 1 month | 120 mL | Placebo drink | 100% | Total cholesterol, HDL-C, LDL-C, TGs | 3 |
| Hollis (2010a) US | RCT | N/A | 18–55 | N/A | 25.0–29.9 | Overweight | 51 (25:26) | Concord grape juice | 12 weeks | 480 mL (933.6 mg/d) | Placebo drink | 100% | Total cholesterol, HDL-C, LDL-C, TGs | 3 |
| Jeong et al. (2014) Korea | Double-blind parallel group RCT | N/A | 58.0 ± 9.2:60.1 ± 9.5 | 47 | 26.3 ± 4.3:25.1 ± 4.0 | Metabolic syndrome | 77(39:38) | Powdered black raspberry | 12 wks | 750 mg/d (4 capsules) | Placebo group-cellulose, isomalto, and corn powder. | 92% | Resting brachial artery diameter, reactive hyperemia brachial artery diameter, IL-6, NF-a, C-reactive protein, Adiponectin, sICAM-1, sVCAM | 3 |
| Jeong et al. (2016) Korea | Double-blind RCT | N/A | 56.4 ± 9.2:60.7 ± 10.4 | N/A | 25.9 ± 4.6:24.7 ± 3.9 | Metabolic syndrome | 51(26:25) | Black raspberry powder | 12 wks | 750 mg/d (4 capsules) | Placebo | 100% | SBP, DBP, heart rate, radial augmentation | 3 |
| Johnson et al. (2015) US | Double-blind parallel group RCT | N/A | 59.7 ± 4.58:57.3 ± 4.77 | 1 | 30.1 ± 5.94:32.7 ± 6.80 | Pre- and stage 1-hypertension | 49 (20:20) | Freeze-dried blueberry powder | 4 wks, 8 wks | 22 g/d | 22 g macronutrient-matched control powder consisted of maltodextrin, fructose, artificial and natural blueberry flavoring, artificial purple and red color, citric acid, and silica dioxide | 83% | SBP, DBP, Mean arterial pressure, Carotid-femoral pulse wave velocity, Brachial-ankle pulse wave velocity, Heart rate | 4 |
| Kent et al. (2017) Australia | parallel groups RCT | N/A | 78.9 ± 5.2:80.6 ± 6.6 | 51 | 25.7 ± 3.4:26.6 ± 3.5 | Mild-to-moderate dementia | 49 (24:25) | Cherry juice | 12 wks | 200 mL/d | Flavonoids-devoid apple juice | 86% | Letter verbal fluency (executive function), SBP, DBP, heart rate, IL 6, hsCRP | 4 |
| Khan et al. (2014) UK | Parallel groups RCT | N/A | 51 ± 11:51 ± 8 | 67 | 29.2 ± 6.9:28.9 ± 6.5 | Healthy | 66 (21:22:21) | High blackcurrant juice drink; low blackcurrant juice drink | 6 wks | 250 mL | Flavored water | 97% | SBP, DBP, FMD, GTN-mediated vasodilation | 3 |
| Kim (2018) US | Double-blinded, placebo-controlled RCT | N/A | 46.6 (11.5):42.0 (14.4) | 31.6%:27.8% | 33.5 ± 6.7 | Metabolic syndrome | 43– > 37(19:18) | açaí beverage (containing 1139 mg L − 1 gallic acid equivalents of total polyphenolics) | 12 weeks | 325 mL/d | 325 mL placebo beverage | 86% | Total cholesterol, TGs, hs-CRP, IL-6, TNF-a | 2 |
| Lynn et al. (2012) UK | Parallel groups, single blind RCT | N/A | 39 ± 1.24 vs 36.1 ± 0.92 | 33 | 24.99 ± 1.26 vs 24.99 ± 1.06 | Healthy | 51 (24:24) | Pomegranate juice | 4 wks | 330 mL/day | lemonade drink-devoid of bioactive plant compounds, antioxidants or vitamins, and contained only a trace amount of sodium, similar energy and carbohydrate | 100% | PWV, SBP, DBP, MAP, Heart rate | 3 |
| Lynn et al. (2014) UK | Parallel open-label RCT | N/A | 38.3 ± 6.16 vs 37.2 ± 5.78 | 38 | 24.6 ± 3.63 vs 23.5 ± 3 | Healthy | 47 (25:21) | Cherry juice trate (30 mL diluted with 220 mL of water; Cherry Active®) | 6 wks | 250 mL/d | Lemonade drink | 98% | PWV, hsCRP, SBP, DBP, Total cholesterol, HDL-C | 2 |
| Lazavi et al. (2018) Iran | Paralleled RCT | Barberry juice (BJ) | 56.86 ± 8.47 | 33.3%:38% | 29.22 ± 3.98:27.78 ± 3.45 | Patients with type 2 diabetes (T2DM) | 46 (23:23) | 200 mL/d PJ | 8 wks | 200 mL | No intervention | 100% | SBP, DBP, TC, HDL, LDL, TG, ApoB, ApoA | 5 |
| Leal et al. (2019) Brazil | Paralleled RCT | Grape juice | 64.9 ± 4.0:72.9 ± 5.6 | 38.5% | 24.7 ± 1.0:26.6 ± 1.1 | 22/31 hypertensive elderly | 10:10 | 200 mL of GJ | 12 wks | 200 mL | No intervention | 91% | SBP, DBP | 1 |
| McAnulty et al. (2014) US | Parallel groups RCT | N/A | 46.15 + 11.92 vs 39.92 + 13.38 | N/A | 27.8 ± 5.46 vs 24.23 ± 3.44 | Sedentary males and females | 25 (13:12) | Blueberry powder--equivalent to 250 g rehydrated berries | 6 wks | 38 g/d | placebo powder contained a blend of maltodextrin, fructose, BB flavoring, coloring, citric acid, and a flow agent (silica) | 100% | SBP, DBP, Aix (Augmentation Index), ASP (aortic systolic pressure), cPWV | 2 |
| Novotny et al. (2015) US | Parallel groups RCT | N/A | 49.8 ± 11.1 vs 50.0 ± 11.6 | 48 | 27.8 ± 3.8 vs 28.9 ± 4.5 | Healthy | 60 (30:30) | CRANBERRY juice | 8 wks | 480 mL/d | Flavor/color/energy-matched placebo beverage | 93% | Total cholesterol, LDL cholesterol, HDL cholesterol, TGs, apo A-I, apo A-II, apoB, sICAM, sVCAM, Diastolic BP, Systolic BP, CRP | 4 |
| Sumner et al. (2005) US | RCT | 86.67% white | 69 ± 11 | 89 | 28 ± 6 | CHD and myocardial ischemia patients | 45 (26:19) | Pomegranate juice | 3 months | 240 mL/d | Placebo drink | 93% | SBP, DBP, Total cholesterol, HDL-C, LDL-C, TGs | 4 |
| Stull et al. (2015) US | parallel group double-blind RCT | 52.27 | 55 ± 2:59 ± 2 | 36 | 35.2 ± 0.8 vs 36.0 ± 1.1 | Metabolic syndrome | 46(23:23) | Freeze-dried blueberry powder-- > 2 cups of fresh whole blueberries/consumed with 24-oz yogurt and skim milk-based smoothie | 6 wks | 45 g/d | Identical smoothie without the blueberry powder | 87% | Glucose, Insulin, Triglycerides, Cholesterol, LDL, HDL, 24 h-SBP, 24 h-DBP, RHI | 4 |
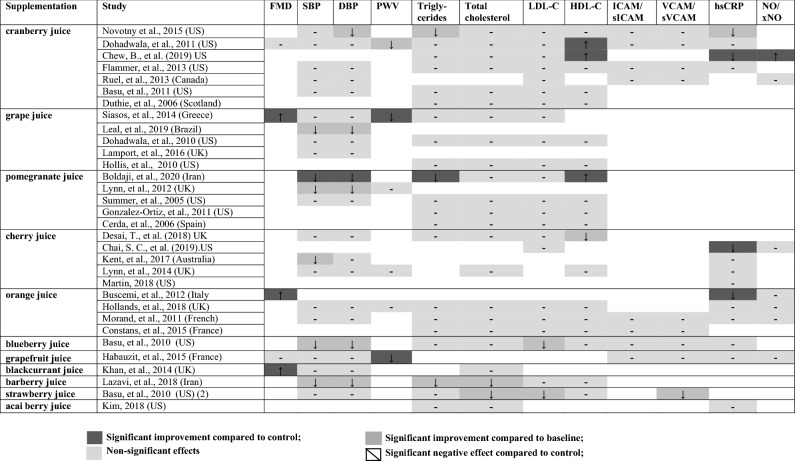
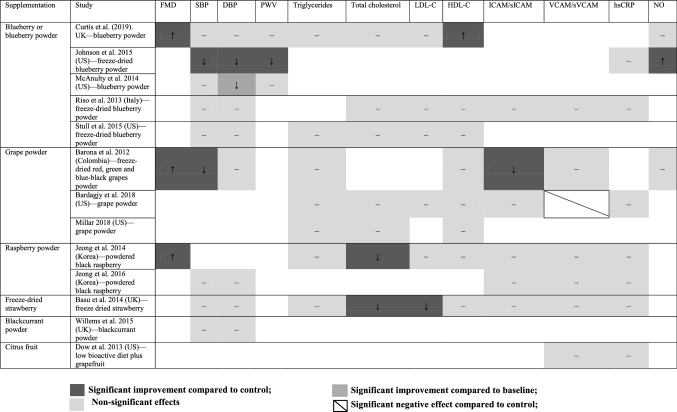
Results from 32 studies (71% of the interventions) that supplemented fruit juice are shown in Table 1, while studies supplementing whole fruit and fruit in other freeze-dried forms (13 studies), are presented in Table 2. Study effects are represented with greyscales in corresponding with reported positive, negative effects and no effect compared to either control or baseline data. Treatments were all delivered in arms of experimental and control groups. The mean chronic treatment duration was 57 days with a standard deviation (SD) of 43 days (ranged from 7 days to 180 days).
Table 1.
Qualitative summarisation for fruit juice interventions
Table 2.
Qualitative summarisation for whole fruit or freeze-dried or powdered fruit interventions
Among the fruit juice category, most studies evaluated the effect of cranberry juice, grape juice, pomegranate juice, cherry juice, orange juice (n = 7, 5, 5, 5, 4, respectively). The mean dosage applied for these types of juices was 480 mL, 353 mL, 238 mL, 173.6 mL and 425 mL, respectively. The remaining interventions included blueberry juice (n = 1), grapefruit juice (n = 1), barberry juice (n = 1), blackcurrant juice (n = 1), strawberry juice (n = 1) and acai berry juice (n = 1) (see Table 1). In Table 2, four trials supplemented freeze-dried blueberry powder. Portion conversion of powder to whole fruit was provided in each study; typically the mean dosage of blueberry powder supplementations was 32.75 g (equivalent to approximately 1.5 cups of fresh blueberries). Three trials supplemented freeze-dried grape powder. The mean dosage of grape powder supplemented was 55.33 g, which is equivalent to approximately 2.5 cups of fresh grapes. The remaining 5 studies supplemented other berries (powdered raspberry, powdered blackcurrant, freeze-dried strawberry) and citrus fruit (1.5 portion of grapefruit following a low bioactive diet).
Study quality
The average retention rate for all included trials was 92.64%, of which 30 out of 45 RCTs obtained no less than 3 points of total Jadad score (see Table 3). Trials generally provided adequate description of methods and procedures, although only 40% of RCTs implemented true randomisation with an adequate description of methods (i.e. computerised statistical randomisation) and 33.33% of RCTs reported implementing blinding processes, where the placebo were colour and taste matched to mask treatments, and the received treatment was not revealed until the statistical analysis was completed for double blinding. However, there was no report assessing participants’ blinding for instance by guessing the treatment they received (Supplemental Table 5).
Meta-analysis of CVD risk factors
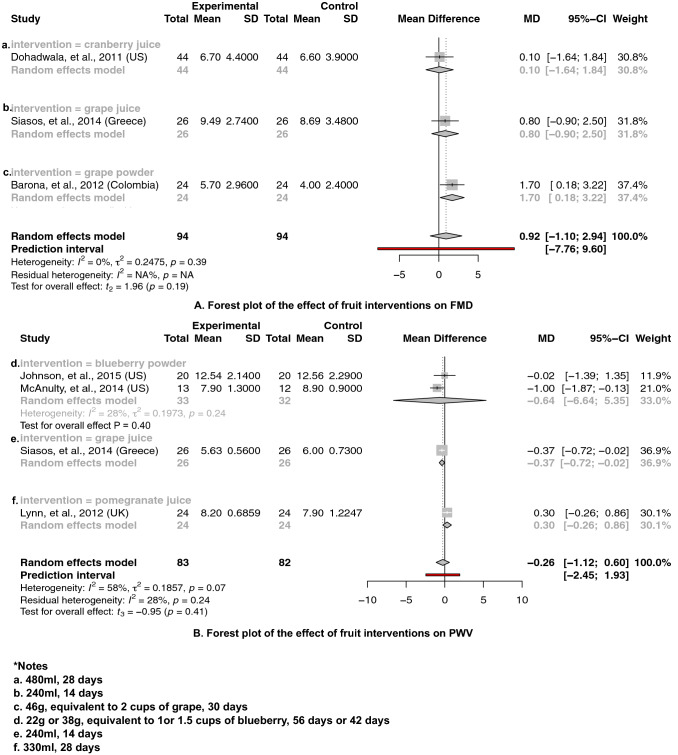
Thirty-eight trials were included in the meta-analysis. The meta-analysis of 38 studies assessing FMD, PWV, SBP, DBP, levels of TAG, TC, HDL-C and LDL-C and levels of vascular inflammatory biomarkers ICAMs, VCAMs, hsCRP and NO are displayed in forest plots (Figs. 2, 3, 4, 5 and Supplemental Figs. 2–17). The interventions used in these studies supplemented: blueberry powder, grape juice and grape powder, cranberry juice, orange juice, whole grapefruit, pomegranate juice, raspberry powder, freeze-dried strawberry, acai berry juice and barberry juice. Among investigated outcomes, no significant improvements were shown to either FMD or PWV in the treatment group relative to the control group (Fig. 2). The I2 test suggested no heterogeneity for interventions assessing the effect on FMD (I2 = 0%, P = 0.39) and non-significant moderate heterogeneities for interventions assessing the effect on PWV (I2 = 58%, P = 0.07).
Fig. 2.
The effect of berry interventions including a cranberry juice, b grape juice and c grape powder assessing FMD and d blueberry powder, e grape juice and f pomegranate juice assessing PWV
Fig. 3.
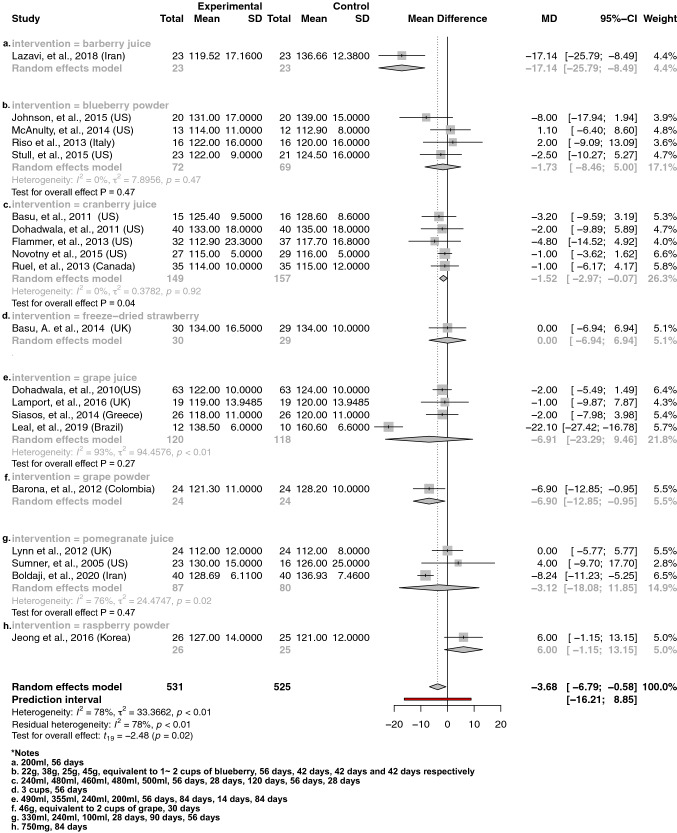
The effect of berry interventions including a barberry juice, b blueberry powder, c cranberry juice, d freeze-dried strawberry, e grape juice, f grape powder, g pomegranate juice and h raspberry powder assessing SBP
Fig. 4.
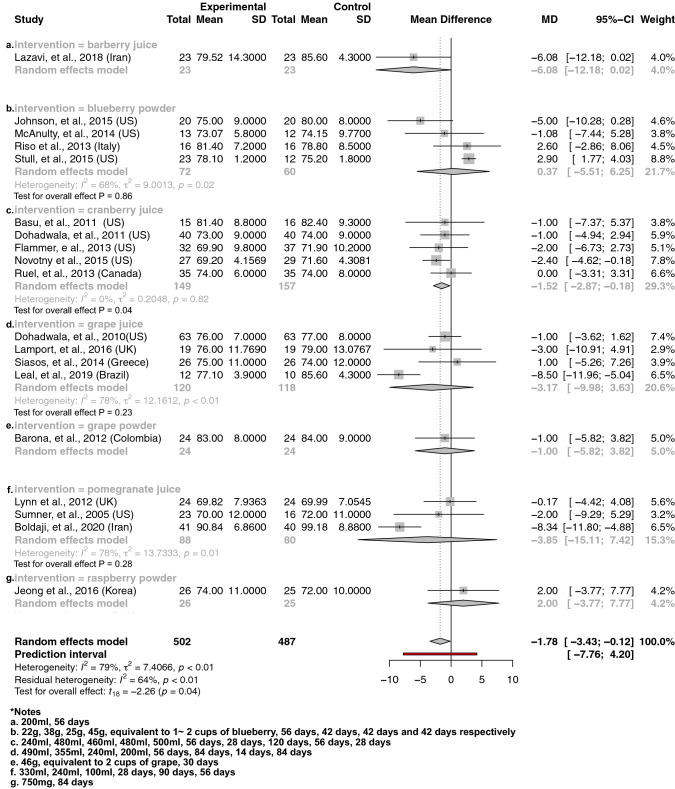
The effect of berry interventions including a barberry juice, b blueberry powder, c cranberry juice, d grape juice, e grape powder, f pomegranate juice and g raspberry powder assessing DBP
Fig. 5.
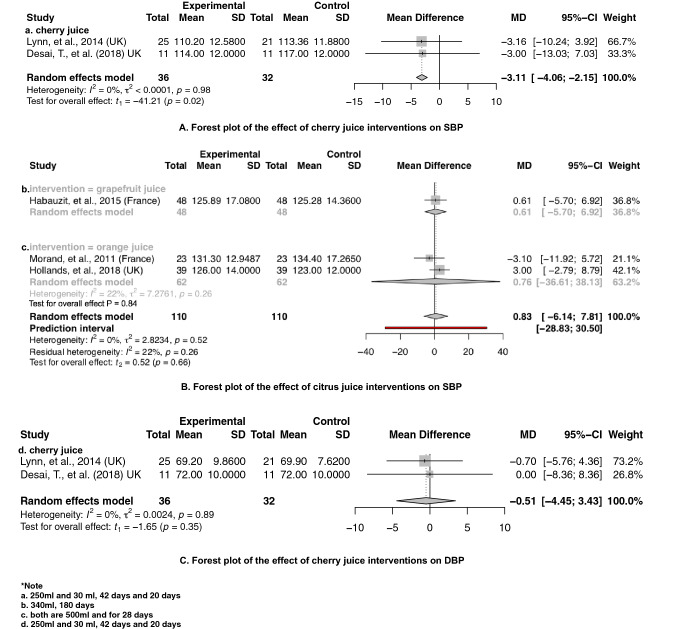
The effect of a cherry juice and b grapefruit juice, c orange juice interventions assessing SBP and d cherry juice interventions assessing DBP
Our principal findings from a meta-analysis of interventions supplementing with berries (including 531 and 502 treatment participants) including barberry juice, blueberry powder, cranberry juice, freeze-dried strawberry, grape juice, grape powder, pomegranate juice and raspberry powder suggested significantly reduced SBP by 3.68 mmHg [95% CI − 6.79 to − 0.58; P = 0.02] (Fig. 3) and DBP by 1.78 mmHg [95% CI − 3.43 to − 0.12; P = 0.04] (Fig. 4), respectively. Subgroup analysis showed that specific interventions using cranberry juice, with mean dosage of 432 mL and length of 8 weeks, included 149 treatment participants and significantly decreased SBP and DBP by 1.52 mmHg (95% CI − 2.97 to − 0.07; P = 0.05) (Fig. 3) and 1.52 mmHg (95% CI − 2.87 to − 0.18, P = 0.04) (Fig. 4), respectively. Two cherry juice interventions including 36 treatment participants with dosage of 30 mL for 20 days and 330 mL for 6 weeks separately also led to a significant reduction in SBP by 3.11 mmHg (95% CI − 4.06 to − 2.15; P = 0.02) (Fig. 5). Berry group including blueberry juice, cranberry juice, grape powder, pomegranate juice and raspberry powder was also shown to significantly increase sVCAM-1 level by 14.57 ng/mL (95% CI 4.22 to 24.93, P = 0.02) in the treatment group relative to the control (Supplemental Fig. 12). The sensitivity analysis suggested no effect of grapefruit concentrate juice on the result of SBP and no effect of cherry concentrate juice on the results of SBP, DBP (Supplemental Table 6). The I2 test suggested significant substantial heterogeneities for berry group investigating the effects on SBP (I2 = 78%, P < 0.01) (Fig. 3) and DBP (I2 = 78%, P < 0.01) (Fig. 4). Funnel plots and the Egger’s test for the berry group showed an overall symmetric distribution of the interventions around the standard error for the investigated outcomes of SBP; asymmetric distributions were shown for the berry group investigating the effect on DBP, trim and fill method was further implemented to adjust for the publication bias (Supplemental Fig. 1).
There were no significant effects of other included intervention groups on other vascular and inflammatory markers: TAG (Supplemental Figs. 2, 3), TC (Supplemental Figs. 4, 5), LDL-C (Supplemental Figs. 6, 7), HDL-C (Supplemental Figs. 8, 9), ICAMs (Supplemental Figs. 10, 11), VCAMs (Supplemental Figs. 12, 13), NO (Supplemental Figs. 14, 15), or hsCRP (Supplemental Figs. 16, 17). The I2 test suggested significant substantial and moderate heterogeneities for berry group (I2 = 71%, P < 0.01) and cherry juice (I2 = 55%, P = 0.14) investigating the effects on TC, respectively (Supplemental Figs. 4, 5). There are significant moderate heterogeneity for berry group investigating the effects on HDL-C (I2 = 56%, P < 0.01) (Supplemental Fig. 8); non-significant moderate heterogeneities were shown for berry group investigating the effects on TAG (I2 = 36%, P = 0.08) (Supplemental Fig. 2), LDL (I2 = 37%, P = 0.08) (Supplemental Fig. 6). Funnel plots and the Egger’s test for the berry group showed an overall symmetric distribution of the interventions around the standard error for the investigated outcomes of TAG, TC, LDL-C (Egger’s tests P > 0.05) (Supplemental Fig. 1). Asymmetric distributions were shown for the berry group investigating the effect on TAG, trim and fill method was further implemented to adjust for the publication bias (Supplemental Fig. 1).
Discussion
Principal findings
We are continually reminded of the health benefits of consuming more fruit and one consumer-friendly strategy to increase fruit consumption is through juice [12]. Even though the juicing process can influence the nutritional value of fruit; a systematic review has demonstrated that the intake of fruit and vegetable juice offered similar health benefits to the intake of whole fruit and vegetables [16]. The results from our review support the beneficial effects of juice and have revealed the potential of berries, in juiced form, to play a beneficial role in the diet to maintain cardiovascular health. High dose of 432 mL cranberry juice and small studies of cherry juice using up to 330 mL showed improvements to blood pressure in our meta-analysis, whereas the National Health Service adult portion size recommendation for fruit juice is no more than 150 mL per day [75], thus a downsized portion according to daily recommendation should be studied in intervention studies.
These findings suggest that interventions with berries, especially using juiced cranberries or cherries, as the most active substitutes for whole fruit, may effectively reduce SBP and DBP. However, the current analyses do not support the notion that the consumption of fruit powders or other fruit juices will confer a cardiovascular function-protective benefit.
Scientific analysis of findings
Our review showed that blueberry and grape in both juiced and freeze-dried forms have been frequently studied for their cardio and vascular protective effects, however, this quantitative analysis only supported an improvement on the outcomes by the consumption of cranberry juice and cherry juice.
A previous systematic review investigated the impact of fruit polyphenols on blood lipids (n = 17), platelet function (n = 9), BP (n = 9) and endothelium-dependent vasodilation (vascular function) (n = 7) and suggested that polyphenols from fruits such as pomegranate, purple grapes and berries are particularly effective at preventing hypertension compared to other CVD risk factors [7]. Berries in particular were shown to possess cardio-protective properties; the underlying mechanisms highlighted include inhibitory effects on inflammatory gene expression, oxidative stress, carbohydrate digestive enzymes and foam cell formation as well as increased effect on nitric oxide synthase following anthocyanins, the major polyphenol in berries [8].
A previous meta-analysis has grouped RCTs without separating the type of fruit, thus the magnitude of the effects of different fruit juice interventions were not compared. Their results supported the overall consumption of various fruit juices to significantly lower DBP by 2.07 mm Hg (95% CI − 3.75 to − 0.39; P = 0.02), whereas no improvement in SBP or lipid levels was obtained within 8 included RCTs [76]. In comparison with this previous report, the present report confirms the significant effects on DBP and reveals also significant effects on SBP.
In another meta-analysis of 95 prospective studies of fruit and vegetable intake, Aune et al. [2] found that fruit juice intake had little association with CVD and total cancer, while slight inverse associations were observed for CHD with RR (95% CI) 0.79 (0.63–0.98), stroke with RR (95% CI) 0.67 (0.60–0.76) and all-cause mortality with RR (95% CI) 0.87 (0.83–0.91) every 100 g/day increment, however, the very low number of studies (n = 2) makes these findings preliminary and more studies are needed before any firm conclusions can be drawn [2]. Furthermore, there is evidence showing that increasing the consumption of fruit juice by one serving per day was associated with a 7% greater incidence of type 2 diabetes (95% CI 0.8% to 14%) [77] and there is also greater risk of weight gain with higher consumption of fruit juice, probably because of the high sugar content and excess calories provided [78]. Fruit juice contains quantities of sugar classified as ‘free’ sugars like sucrose, compared with whole fruit in which the sugars are classified as intrinsic. Increased dietary fructose following sucrose intake is reported to increase de novo lipogenesis (DNL) levels and VLDL, which has been shown to increase the risk of developing non-alcoholic fatty liver disease (NAFLD) [79]. Therefore, cautious interpretations should be made when promoting fruit juice consumption as healthy options to increase fruit and vegetable intake.
Other epidemiological evidence has indicated an inverted association between fruit intake and CVD risk factors. Among 34,492 CVD-free postmenopausal women in the Iowa Women’s Health Study with 16 years of follow-up, a significantly reduced risk ratio of CVD mortality was associated with intake of at least once per week of apples and pears, oranges, grapefruit, blueberries, strawberries, grapes and raisins after adjustment for age and energy [4]. However, following adjustment for other confounding covariates, the significance was only retained for the intake of strawberries, apples and pears. In a further investigation of strawberry intake for its cross-sectional association with lipids and CRP profiles, only a borderline significance was reported for a reduced CRP levels [80]. Aune et al. [2] also reported an inverse association between high vs. low berry consumption and all-cause mortality in a meta-analysis, whereas no similar associations were observed for CHD and CVD [2].
Our review has also shown elevated sVCAM-1 level after the berries intervention, however, some authors have suggested that the magnitude of the increase in sVCAM-1 may not be clinically relevant, as the other vascular inflammatory markers did not change between the treatment and the control group after the interventions [41], which is also in line with the results of other inflammatory markers in our review. Aside from this, Bardagjy et al. [41] and Ruel et al. [44] reported significantly higher sVCAM-1 levels in the treatment group at the baseline compared to the control, which may have contributed to the elevated sVCAM level after the interventions in the berries-treated group. Although the consumption of a range of berries have been linked with improved cardiovascular health, considering the results from our review and previous evidence, current evidence is insufficient and inconsistent to substantiate the consumption of specific berries or other fruit as a cardiovascular-protective dietary strategy.
Implications for health and future research
Among our results, SBP improved significantly by over 3 mmHg after interventions with specific berries and cherry juice, which may likely have practical implications as blood pressure is an important indicator not just for endothelial function, but also for CVD mortality risk [81]. A report from the Joint National Committee and several meta-analyses have estimated that lowering SBP by 5 mmHg or more could decrease stroke risk by 13% [82], CVD risk by 3% to 38% [83], deaths from stroke by 14%, deaths from heart diseases by 9% and overall mortality by 7% [84].
However, we only analysed two cherry juice studies with relatively small sample sizes in this review and no other risk factors were improved by this intervention. It would be helpful to have more studies on this topic in order to inform policymakers in nutrition. Future studies on supplementing berries (i.e. cranberries, blueberries, grapes) with a sufficient sample size are warranted, as these appear to have the biggest potential to improve endothelial function and cardiovascular function. Further studies on this topic incorporating effect sizes with interpretation from CVD risk reduction are also required.
Strengths and limitations
To our knowledge, this is the first systematic review and meta-analysis to compare the impact of fruit in various delivery forms, on cardiovascular health. We also used the newly developed Hartung–Knapp–Sidik–Jonkman method for random-effects model in meta-analysis in addition to a comprehensive search of the literature in the topic. There are limitations to our review, however. As explored by the subgroup analysis, the significant moderate-to-substantial heterogeneity among the berry group majorly contributed to grape juice and pomegranate juice studies (Figs. 3, 4), however, the number of studies within grape juice and pomegranate juice interventions (n ranged from 3 to 4) were too few to perform subgroup analysis. The high heterogeneity could be explained by the different populations and regions and participant characteristics at baseline within these few studies. Physical activity level has been considered as cofactor, but no adjustments for physical activity level have been applied among the included studies in this review.
There is limited study data under some types of interventions investigating all risk factors (i.e. grape powder and cherry juice) to be meta-analysed; and even though studies supplementing cranberry juice have shown an significant effect, they are not accompanied by improvements to other risk factors and are limited to relatively small sample sizes within 2 studies, so the implications of our results should be treated with caution. Heterogeneities presented in our results, however, were explored by subgroup analyses of different intervention subgroups, due to the limited number of studies under each participants characteristic and country region, we were unable to further compare among different baseline-characterised subjects (i.e. physical activity, gender), regions (i.e. western and other countries) and juice qualities.
Conclusion
This review has highlighted a scarcity of intervention studies aimed at improving endothelial function and cardiovascular health by consuming berries, citrus and cherries in different forms such as freeze-dried and powdered fruit or as fruit juice. The quantitative analysis led us to further explore the potential of various berries, cherries and citrus-based interventions to improve endothelial function and cardiovascular health. There is a potential for berries in juiced forms to benefit cardio-health, however, these are only suggestive and raised from non-substantial evidence from a few studies within each intervention type. Inconsistent evidence was reported considering results from our analysis along with other reviews regarding the effect of fruit juice on CVD risk factors. More research supplementing summarised interventions in this review is warranted to reinforce the evidence and to further substantiate the health benefits of specific fruit-based interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors developed the study concept and design and contributed to the critical revision of the manuscript for important intellectual content; YW wrote the draft of manuscript, conducted data extraction and statistical analysis; all authors contributed to discussion and reviewed/edited the manuscript. All authors have read and approved the final version submitted for publication.
Funding
This review received no specific grant from any funding agency, commercial or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest in this review.
References
- 1.WHO . Diet, nutrition and the prevention of chronic diseases. Report of a Joint FAO, WHO Expert Consultation. WHO Technical Report Series. Geneva: World Helath Organization; 2003. [Google Scholar]
- 2.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73(1):61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 4.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 5.Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Münzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174(12):1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luscher TF, Vanhoutte PM. The endothelium: modulator of cardiovascular function. Boca Raton: CRC Press; 2020. [Google Scholar]
- 7.Chong MF-F, Macdonald R, Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr. 2010;104(S3):S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- 8.Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68(3):168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients. 2018;10(3):368. doi: 10.3390/nu10030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Zhou Y, Li S, Zhang P, Zhou T, Xu DP, Li HB. Effects and mechanisms of fruit and vegetable juices on cardiovascular diseases. Int J Mol Sci. 2017 doi: 10.3390/ijms18030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oude Griep LM, Stamler J, Chan Q, Van Horn L, Steffen LM, Miura K, Ueshima H, Okuda N, Zhao L, Daviglus ML, Elliott P, IRG Association of raw fruit and fruit juice consumption with blood pressure: the INTERMAP Study. Am J Clin Nutr. 2013;97(5):1083–1091. doi: 10.3945/ajcn.112.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruxton CHS. Smoothies: one portion or two? Nutr Bull. 2008;33:129–132. doi: 10.1111/j.1467-3010.2008.00696.x. [DOI] [Google Scholar]
- 13.Kamiloglu S, Demirci M, Selen S, Toydemir G, Boyacioglu D, Capanoglu E. Home processing of tomatoes (Solanum lycopersicum): effects on in vitro bioaccessibility of total lycopene, phenolics, flavonoids, and antioxidant capacity. J Sci Food Agric. 2014;94(11):2225–2233. doi: 10.1002/jsfa.6546. [DOI] [PubMed] [Google Scholar]
- 14.Porrini M, Riso P, Testolin G. Absorption of lycopene from single or daily portions of raw and processed tomato. Br J Nutr. 1998;80(4):353–361. doi: 10.1017/S000711459800141X. [DOI] [PubMed] [Google Scholar]
- 15.Langer S, Kennel A, Lodge JK. The influence of juicing on the appearance of blueberry metabolites 2 h after consumption: a metabolite profiling approach. Br J Nutr. 2018;119(11):1233–1244. doi: 10.1017/s0007114518000855. [DOI] [PubMed] [Google Scholar]
- 16.Ruxton CHS, Gardner EJ, Walker D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int J Food Sci Nutr. 2006;57(3–4):249–272. doi: 10.1080/0963748060085134. [DOI] [PubMed] [Google Scholar]
- 17.Blanch N, Clifton PM, Keogh JB. A systematic review of vascular and endothelial function: effects of fruit, vegetable and potassium intake. Nutr Metab Cardiovasc Dis. 2015;25(3):253–266. doi: 10.1016/j.numecd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Basu A, Penugonda K. Pomegranate juice: a heart-healthy fruit juice. Nutr Rev. 2009;67(1):49–56. doi: 10.1111/j.1753-4887.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacconelli E. Systematic reviews: CRD's guidance for undertaking reviews in health care. Lancet Infect Dis. 2010;10(4):226. doi: 10.1016/S1473-3099(10)70065-7. [DOI] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. London: Royal College of Psychiatrists; 2014. [DOI] [PubMed] [Google Scholar]
- 24.Core Team R. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 25.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid-Based Mental Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynn A, Mathew S, Moore CT, Russell J, Robinson E, Soumpasi V, Barker ME. Effect of a Tart Cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum Nutr. 2014;69(2):122–127. doi: 10.1007/s11130-014-0409-x. [DOI] [PubMed] [Google Scholar]
- 27.Habauzit V, Verny MA, Milenkovic D, Barber-Chamoux N, Mazur A, Dubray C, Morand C. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: a randomized, controlled, crossover trial. Am J Clin Nutr. 2015;102(1):66–74. doi: 10.3945/ajcn.114.104646. [DOI] [PubMed] [Google Scholar]
- 28.Chai SC, Davis K, Zhang Z, Zha L, Kirschner KF. Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. 2019 doi: 10.3390/nu11020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krga I, Milenkovic D. Anthocyanins: from sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J Agric Food Chem. 2019;67(7):1771–1783. doi: 10.1021/acs.jafc.8b06737. [DOI] [PubMed] [Google Scholar]
- 30.Weihrauch JL, Posati LP, Anderson BA, Exler J. Lipid conversion factors for calculating fatty acid contents of foods. J Am Oil Chem Soc. 1977;54(1):36–40. doi: 10.1007/BF02671370. [DOI] [PubMed] [Google Scholar]
- 31.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barona J, Aristizabal JC, Blesso CN, Volek JS, Fernandez ML. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J Nutr. 2012;142(9):1626–1632. doi: 10.3945/jn.112.162743. [DOI] [PubMed] [Google Scholar]
- 34.Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, Verga S, Rini G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012;95(5):1089–1095. doi: 10.3945/ajcn.111.031088. [DOI] [PubMed] [Google Scholar]
- 35.Constans J, Bennetau-Pelissero C, Martin JF, Rock E, Mazur A, Bedel A, Morand C, Bérard AM. Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clin Nutr. 2015;34(6):1093–1100. doi: 10.1016/j.clnu.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, Blumberg JB, Vita JA. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93(5):934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, Mazur A. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011;93(1):73–80. doi: 10.3945/ajcn.110.004945. [DOI] [PubMed] [Google Scholar]
- 38.Riso P, Klimis-Zacas D, Del Bo C, Martini D, Campolo J, Vendrame S, Møller P, Loft S, De Maria R, Porrini M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. 2013;52(3):949–961. doi: 10.1007/s00394-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 39.Siasos G, Tousoulis D, Kokkou E, Oikonomou E, Kollia M-E, Verveniotis A, Gouliopoulos N, Zisimos K, Plastiras A, Maniatis K, Stefanadis C. Favorable effects of concord grape juice on endothelial function and arterial stiffness in healthy smokers. Am J Hypertens. 2014;27(1):38–45. doi: 10.1093/ajh/hpt176. [DOI] [PubMed] [Google Scholar]
- 40.Willems ME, Myers SD, Gault ML, Cook MD. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int J Sport Nutr Exerc Metab. 2015;25(4):367–374. doi: 10.1123/ijsnem.2014-0233. [DOI] [PubMed] [Google Scholar]
- 41.Bardagjy AS, Hu Q, Giebler KA, Ford A, Steinberg FM. Effects of grape consumption on biomarkers of inflammation, endothelial function, and PBMC gene expression in obese subjects. Arch Biochem Biophys. 2018;646:145–152. doi: 10.1016/j.abb.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Barati Boldaji R, Akhlaghi M, Sagheb MM, Esmaeilinezhad Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: a randomized crossover trial. J Sci Food Agric. 2020;100(2):846–854. doi: 10.1002/jsfa.10096. [DOI] [PubMed] [Google Scholar]
- 43.Hollands WJ, Armah CN, Doleman JF, Perez-Moral N, Winterbone MS, Kroon PA. 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: a randomised controlled trial. Br J Nutr. 2018;119(4):415–421. doi: 10.1017/s0007114517003865. [DOI] [PubMed] [Google Scholar]
- 44.Ruel G, Lapointe A, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr Res (New York, NY) 2013;33(1):41–49. doi: 10.1016/j.nutres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Desai T, Bottoms L, Roberts M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur J Appl Physiol. 2018;118(12):2523–2539. doi: 10.1007/s00421-018-3978-9. [DOI] [PubMed] [Google Scholar]
- 46.Dohadwala MM, Hamburg NM, Holbrook M, Kim BH, Duess M-A, Levit A, Titas M, Chung WB, Vincent FB, Caiano TL, Frame AA, Keaney JF, Jr, Vita JA. Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. Am J Clin Nutr. 2010;92(5):1052–1059. doi: 10.3945/ajcn.2010.29905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamport DJ, Lawton CL, Merat N, Jamson H, Myrissa K, Hofinan D, Chadwick HK, Quadt F, Wightman JD, Dye L. Concord grape juice, cognitive function, and driving performance: a 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am J Clin Nutr. 2016;103(3):775–783. doi: 10.3945/ajcn.115.114553. [DOI] [PubMed] [Google Scholar]
- 48.Martin KR, Burrell L, Bopp J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: a randomized, crossover pilot study. Food Funct. 2018;9(10):5290–5300. doi: 10.1039/c8fo01492b. [DOI] [PubMed] [Google Scholar]
- 49.Millar CL, Duclos Q, Garcia C, Norris GH, Lemos BS, DiMarco DM, Fernandez ML, Blesso CN. Effects of freeze-dried grape powder on high-density lipoprotein function in adults with metabolic syndrome: a randomized controlled pilot study. Metab Syndr Relat Disord. 2018;16(9):464–469. doi: 10.1089/met.2018.0052. [DOI] [PubMed] [Google Scholar]
- 50.McAnulty LS, Collier SR, Landram MJ, Whittaker DS, Isaacs SE, Klemka JM, Cheek SL, Arms JC, McAnulty SR. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr Res. 2014;34(7):577–584. doi: 10.1016/j.nutres.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr. 2015;145(6):1185–1193. doi: 10.3945/jn.114.203190. [DOI] [PubMed] [Google Scholar]
- 52.Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, Johnson WD, Cefalu WT. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2015;7(6):4107–4123. doi: 10.3390/nu7064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynn A, Hamadeh H, Leung WC, Russell JM, Barker ME. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum Nutr. 2012;67(3):309–314. doi: 10.1007/s11130-012-0295-z. [DOI] [PubMed] [Google Scholar]
- 54.Khan F, Ray S, Craigie AM, Kennedy G, Hill A, Barton KL, Broughton J, Belch JJF. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: a randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radical Biol Med. 2014;72:232–237. doi: 10.1016/j.freeradbiomed.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Kent K, Charlton K, Roodenrys S, Batterham M, Potter J, Traynor V, Gilbert H, Morgan O, Richards R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur J Nutr. 2017;56(1):333–341. doi: 10.1007/s00394-015-1083-y. [DOI] [PubMed] [Google Scholar]
- 56.Johnson SA, Figueroa A, Navaei N, Wong A, Kalfon R, Ormsbee LT, Feresin RG, Elam ML, Hooshmand S, Payton ME, Arjmandi BH. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: a randomized, double-blind, placebo-controlled clinical trial. J Acad Nutr Diet. 2015;115(3):369–377. doi: 10.1016/j.jand.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Jeong HS, Hong SJ, Lee TB, Kwon JW, Jeong JT, Joo HJ, Park JH, Ahn CM, Yu CW, Lim DS. Effects of black raspberry on lipid profiles and vascular endothelial function in patients with metabolic syndrome. Phytother Res. 2014;28(10):1492–1498. doi: 10.1002/ptr.5154. [DOI] [PubMed] [Google Scholar]
- 58.Dow CA, Wertheim BC, Patil BS, Thomson CA. Daily consumption of grapefruit for 6 weeks reduces urine f2-isoprostanes in overweight adults with high baseline values but has no effect on plasma high-sensitivity c-reactive protein or soluble vascular cellular adhesion molecule 1. J Nutr. 2013;143(10):1586–1592. doi: 10.3945/jn.113.175166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basu A, Fu DX, Wilkinson M, Simmons B, Wu M, Betts NM, Du M, Lyons TJ. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res (New York, NY) 2010;30(7):462–469. doi: 10.1016/j.nutres.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140(9):1582–1587. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr. 2014;144(6):830–837. doi: 10.3945/jn.113.188169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basu A, Betts NM, Ortiz J, Simmons B, Wu M, Lyons TJ. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr Res (New York, NY) 2011;31(3):190–196. doi: 10.1016/j.nutres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cerdá B, Soto C, Albaladejo MD, Martínez P, Sánchez-Gascón F, Tomás-Barberán F, Espín JC. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2005;60:245. doi: 10.1038/sj.ejcn.1602309. [DOI] [PubMed] [Google Scholar]
- 64.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, Yap LS, Christen P, Duthie GG. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006;45(2):113–122. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 65.González-Ortiz M, Martínez-Abundis E, Espinel-Bermúdez MC, Pérez-Rubio KG. Effect of pomegranate juice on insulin secretion and sensitivity in patients with obesity. Ann Nutr Metab. 2011;58(3):220–223. doi: 10.1159/000330116. [DOI] [PubMed] [Google Scholar]
- 66.Hollis JH, Houchins JA, Blumberg JB, Mattes RD. Effects of concord grape juice on appetite, diet, body weight, lipid profile, and antioxidant status of adults. J Am Coll Nutr. 2009;28(5):574–582. doi: 10.1080/07315724.2009.10719789. [DOI] [PubMed] [Google Scholar]
- 67.Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, Raisin CJ, Ornish D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96(6):810–814. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 68.Curtis PJ, Van Der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, Evans M, Fernandez BO, Meiss MS, Minnion M, Potter J, Minihane AM, Kay CD, Rimm EB, Cassidy A. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr. 2019;109(6):1535–1545. doi: 10.1093/ajcn/nqy380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chew B, Mathison B, Kimble L, McKay D, Kaspar K, Khoo C, Chen CO, Blumberg J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: a randomized controlled trial. Eur J Nutr. 2019;58(3):1223–1235. doi: 10.1007/s00394-018-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazavi F, Mirmiran P, Sohrab G, Nikpayam O, Angoorani P, Hedayati M. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: a randomized clinical trial. Complement Ther Clin Pract. 2018;31:170–174. doi: 10.1016/j.ctcp.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Leal JB, Leal JB, Leal JBP, Borges YD, de Moura MIL, Teixeira-Araujo AA, da Costa VS, Carvalho FO. Grape juice and aerobic exercise on blood pressure. Nutr Food Sci. 2019 doi: 10.1108/nfs-08-2019-0256. [DOI] [Google Scholar]
- 72.Jeong HS, Hong SJ, Cho JY, Lee TB, Kwon JW, Joo HJ, Park JH, Yu CW, Lim DS. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: randomized, double-blinded, placebo-controlled clinical trial. Nutrition (Burbank, Los Angeles County, Calif) 2016;32(4):461–467. doi: 10.1016/j.nut.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Kim H, Simbo SY, Fang C, McAlister L, Roque A, Banerjee N, Talcott ST, Zhao H, Kreider RB, Mertens-Talcott SU. Acai (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018;9(6):3097–3103. doi: 10.1039/c8fo00595h. [DOI] [PubMed] [Google Scholar]
- 74.Flammer AJ, Martin EA, Goessl M, Widmer RJ, Lennon RJ, Sexton JA, Loeffler D, Khosla S, Lerman LO, Lerman A. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr. 2013;52(1):289–296. doi: 10.1007/s00394-012-0334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guide, NHS Choices Rough. Fruit and Vegetable Portion Sizes. NHS Choices. http://www.nhs.uk/livewell/5aday/documents/downloads/5aday_portion_guide.pdf. Accessed 25 Sept 2015 [WebCite Cache ID 6bokXyRXx].
- 76.Liu K, Xing A, Chen K, Wang B, Zhou R, Chen S, Xu H, Mi M. Effect of fruit juice on cholesterol and blood pressure in adults: a meta-analysis of 19 randomized controlled trials. PLoS ONE. 2013;8(4):e61420. doi: 10.1371/journal.pone.0061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 79.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Investig. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sesso HD, Gaziano JM, Jenkins DJ, Buring JE. Strawberry intake, lipids, C-reactive protein, and the risk of cardiovascular disease in women. J Am Coll Nutr. 2007;26(4):303–310. doi: 10.1080/07315724.2007.10719615. [DOI] [PubMed] [Google Scholar]
- 81.van den Hoogen PCW, Feskens EJM, Nagelkerke NJD, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. N Engl J Med. 2000;342(1):1–8. doi: 10.1056/nejm200001063420101. [DOI] [PubMed] [Google Scholar]
- 82.Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29(7):1253–1269. doi: 10.1097/HJH.0b013e3283469976. [DOI] [PubMed] [Google Scholar]
- 83.Karmali KN, Lloyd-Jones DM, van der Leeuw J, Goff DC, Jr, Yusuf S, Zanchetti A, Glasziou P, Jackson R, Woodward M, Rodgers A, Neal BC, Berge E, Teo K, Davis BR, Chalmers J, Pepine C, Rahimi K, Sundstrom J. Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: a meta-analysis of individual participant data. PLoS Med. 2018;15(3):e1002538. doi: 10.1371/journal.pmed.1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Institutes of Health The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–46. doi: 10.1001/archinte.1997.00440420033005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.