Replying to F. Peeters & H. Hofmann Nature Communications 10.1038/s41467-021-21215-2 (2021)
The prevailing paradigm in methane research is that biological methane production is exclusive to anoxic or near-anoxic habitats such as sediments and oxygen-deficient bottom waters in lakes. Paradoxically, methane supersaturation in oxic lake waters is widely reported. To resolve this paradox while preserving the paradigm, some researchers assume this methane originates entirely from anoxic sources and is then transported to the oxic waters through physical processes1–3. However, multiple recent studies have repeatedly shown, methane production can and does occur under oxic conditions on land, in the seas and in freshwaters, driven by diverse organisms within different life domains (Table 1 and references therein) and via photochemical conversion4. These findings raise legitimate questions about the nature of the environmental dynamics and global budget of methane. Because oxic methane production (OMP) is a recent discovery, its contribution to atmospheric emission is unknown. We conducted a whole-lake basin methane mass balance and analysed relevant literature data to estimate the contribution of OMP to surface emission versus lake morphometry.
Table 1.
Literature examples of oxic methane production (OMP) in different habitats and by different domains of life.
| Organism | Domain | CH4 production rate | Evidence | Reference |
|---|---|---|---|---|
| TERRESTRIAL | ||||
| Plants | Eukaryote | INC, ISO | Keppler et al. (2006)20 | |
| Plants | Eukaryote | INC | Messenger et al. (2009)21 | |
| Methanogens | Archaea | INC, ISO, OMIC | Angel et al. (2011)22 | |
| Fungi | Eukaryote | INC, ISO | Lenhart et al. (2012)23 | |
| Plants | Eukaryote | INC, ISO | Althoff et al. (2014)24 | |
| Methanogens | Archaea | MB, OMIC, PHYS | Angle et al. (2017)14 | |
| Cyanobacteria | Prokaryote | INC, ISO | Bizic et al. (2020)15 | |
| MARINE | ||||
| Mixed assemblage | INC, OMIC | Karl et al. (2008)17 | ||
| Bacteria | Prokaryote | INC, STAT | Damm et al. (2010)25 | |
| Cyanobacteria | Prokaryote | INC | White et al. (2010)26 | |
| α-Proteobacteria | Prokaryote | INC, OMIC | Carini et al. (2014)27 | |
| Haptophytes | Eukaryote | INC, ISO | Lenhart et al. (2016)28 | |
| Bacteria | Prokaryote | INC, OMIC | Repeta et al. (2016)29 | |
| Haptophytes | Eukaryote | INC, ISO | Klintzsch et al. (2019)30 | |
| Cyanobacteria | Prokaryote | INC, ISO | Bizic et al. (2020)15 | |
| γ-Proteobacteria | Prokaryote | INC | Ye et al. (2020)32 | |
| Haptophytes | Eukaryote | INC | Klintzsch et al. (2020)31 | |
| FRESHWATER | ||||
| Methanogens, algae | Archaea, Eukaryote | 38–58 nmol l−1 day−1 (Lake Stechlin) | INC | Grossart et al. (2011)11 |
| Methanogens, algae | Archaea, Eukaryote | 210–240 nmol l−1 day−1 (Lake Cromwell) | ISO, MB | Bogard et al. (2014)33 |
| α-, γ-proteobacteria | Prokaryote | INC, OMIC | Yao et al. (2016)13 | |
| Mixed assemblage | 110 nmol l−1 day−1 (Lake Hallwil) | MB | Donis et al. (2017)7 | |
| γ-Proetobacteria | Prokaryote | 0.2–0.7 nmol l−1 day−1 (Yellowstone Lake) | INC, ISO, OMIC | Wang et al. (2017)34 |
| Mixed assemblage | ISO, MB, PHYS | DelSontro et al. (2018)35 | ||
| Proteobacteria | Prokaryote | 54–257 nmol l−1 day−1 (Lake Bonney) | INC, OMIC | Li et al. (2019)36 |
| Cyanobacteria | Prokaryote | INC, OMIC | Khatun et al. (2019)37 | |
| Mixed assemblages |
72–88 nmol l−1 day−1 (Lake Stechlin) 78–138 nmol l−1 day−1 (Lake Hallwil) |
MB | Günthel et al. (2019)39 | |
| Cyanobacteria | Prokaryote | INC, ISO | Bizic et al. (2020)15 | |
| Cyanobacteria | Prokaryote | STAT | Khatun et al. (2020)38 | |
| Green algae, diatoms, cryptophytes | Eukaryote | 50–210 nmol l−1 day−1 (Lake Stechlin) | INC, ISO, MB, STAT | Hartmann et al. (2020)18 |
| Picoeukaryotes, diatoms | Eukaryote | STAT | Leon-Palmero et al. (2020)41 | |
| Proteobacteria | Prokaryote | 24–547 nmol l−1 day−1 (5 Lakes) | INC, ISO, OMIC | Perez-Coronel and Beman (2020)42 |
OMP evidence type: INC incubation experiments, ISO isotope techniques, MB mass balance approaches, OMIC molecular biological methods, PHYS physical modelling, STAT statistical analyses.
OMP has been observed in different limnic systems, e.g. temperate and arctic regions (DelSontro et al. 201835, Li et al. 2019)36, high-elevation (Perez-Coronel and Beman, 2020)42, and throughout the oligo-to-eutrophic nutrient spectrum (DelSontro et al., 201835, Khatun et al., 202038, Ye et al., 2020)32.
Because the dynamics of methane concentration and isotope signal in lake waters are influenced by different and opposing processes simultaneously, one cannot meaningfully deduce the presence or absence of OMP without properly accounting for modulations by physical and biological processes. For example, underestimating surface emission or ignoring oxidation would lead to incorrect interpretation of methane concentration and isotope data and incorrect dismissal of OMP (Supplementary Note 1).
By balancing the gains and losses of epilimnetic methane in a stratified water column, we estimated the contribution of oxic versus anoxic methane to surface emission (Supplementary Fig. 1). Epilimnetic methane may originate from lateral and vertical transport from anoxic zones, ebullition, and internal oxic production (OMP); surface emission and oxidation are the loss terms.
Surface methane emission can be measured directly using a flux chamber, or, in the absence of direct measurements, it is often modelled from surface-water methane concentrations and wind speeds. Both methods are commonly used but the results can differ considerably, and there exist many different wind-based models (for a more detailed discussion we refer readers to the literature5,6). Notably in their manuscript, Peeters and Hofmann excluded our direct measurements of methane fluxes to the atmosphere and exclusively rely on modelling approaches (Supplementary Note 2). We instead combined direct measurements with models that were established for the target lake. Therefore, we consider that our direct measurement approach minimises methodological and model biases, and better represents reality.
For Lake Hallwil, we used the littoral sediment-to-water methane flux as determined by Donis et al.7 who implemented two littoral sediment core measurements sampled at 3 and 7 m depth and applying Fick’s law. In contrast, Peeters and Hofmann implemented only the upper sediment core into their re-analysis. They justify this choice by stating the cores’ methane isotope signature vary. As the depth of Lake Hallwil’s surface mixed layer increased over the seasonal progression7, both sediment cores should be considered in the mass balance especially in the light of natural variability. For Lake Stechlin, we used data from two mesocosms and the open-water to resolve littoral methane input (Supplementary Notes 3 and 4). We estimated ebullitive methane fluxes as negligible in Lake Stechlin8,9. We further applied an ebullitive flux of 1.2 ± 0.8 mmol m−2 d−1 to Lake Hallwil10, giving a total sediment methane input of 3 mmol m−2 d−1 when combined with the diffusive flux, which is higher than the value assumed by Peeters and Hofmann. Vertical diffusive input was calculated from empirically measured methane concentration profiles and turbulent diffusivities. We parameterised methane oxidation as 30% of internal production for Lake Stechlin; in a sensitivity analysis, we evaluated this assumption and also considered the most conservative scenario, e.g., OMP set to minimum. For Lake Hallwil, methane oxidation rates were measured by experiments.
By balancing the different input and output fluxes, we produced the first system-wide OMP estimate for Lake Stechlin, which agrees well with direct bottle incubation measurements reported earlier11. To further account for (seasonal) variabilities and measurement uncertainties, we conducted Monte Carlo simulations and sensitivity analysis applying various conservative scenarios to the mass balance. It is, however, worth noting that the mass balance is sensitive to the flux parameterisation and the accuracy of its result is hinged on how reliably one accounts for these fluxes. To better resolve OMP and allow for more general and firm statements about OMP (including different lake systems), future studies should aim to reduce uncertainties associated with the littoral methane input (e.g. methodological uncertainty in sediment core measurements12) and methane oxidation—two key parameters in the epilimnetic methane budget.
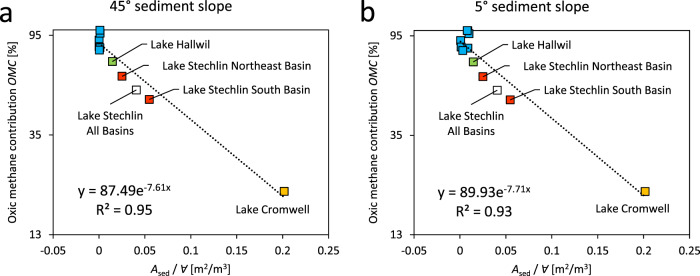
OMP by diverse organisms (Table 1) and pathways13–15 point to its wider potential relevance on a global scale. To examine how OMP may vary according to lake characteristics, we combined our results with analysis of literature data to estimate OMP contribution in relation to basin morphometry (Supplementary Note 5). The epilimnetic methane sources considered here are littoral sediment and OMP. On a whole-system level, the relative contributions of these sources are proportional to the total littoral sediment area and the epilimnion volume, respectively. Because the ratio of littoral sediment area to epilimnion volume decreases with increasing lake size, the contribution of OMP to surface emission is expected to increase with lake size. This trend does not change even when we assume a larger littoral sediment area by decreasing the sediment slope as suggested by Peeters and Hofmann (Fig. 1). As the current OMP dataset is limited to only a few lakes (four data points based on mass balance and seven based on transport modelling), future studies should aim to increase the number and types of lakes to verify the trend on a larger scale.
Fig. 1. Oxic methane contribution (OMC) to surface emission in relation to lake morphology.
Comparison of (a) the original relationship and (b) the alternative parameterisation using a smaller sediment slope angle. Ased is the littoral sediment area and ∀ is the surface mixed layer volume. Note, OMC is defined as in our original study; the x-axis is linearly scaled, and the y-axes is scaled to log2.7.
Note, as Peeters’ and Hofmann’s re-analysis excludes internal methane modulation, their OMP estimates reflect net rates while our study presents gross rates. Accordingly, their contribution pattern of oxic versus anoxic methane source to surface emission (NOMC) cannot be directly compared to our estimates (OMC) (further discrepancy is explained by Supplementary Note 5).
Oxic methane production defies the century-old teaching of anoxic methanogenesis and the convention of considering only anoxic sources in methane research; as such, skepticism is expected. While some may dismiss OMP as irrelevant16, others take a more practical approach and investigate the phenomenon at the ecological, organismal, and molecular levels13,17. However, the novelty of OMP also means researchers are still trialling different methods, each with their limitations (Table 2).
Table 2.
Overview on approaches to investigate oxic methane production (OMP) in lake waters.
| Approach | Description | Caveats | Reference examples |
|---|---|---|---|
|
Incubation of • Lake water • Enrichment cultures |
Cultivating microbes in closed containers and recording CH4 concentration over time. Additionally, the change in 13C/12C carbon isotope ratio in dissolved methane can be measured. | Bottle enclosure may alter the light and nutrient conditions versus in situ. Long-term incubations (exceeding hours) may not reflect in situ conditions due to changes to the production-consumption equilibrium (e.g., nutrient depletion, community alterations). |
Grossart et al. (2011)11, Bizic et al. (2020)15, Günthel et al. (2020)40, Hartmann et al. (2020)18, |
| Metagenomics | Molecular analysis of relevant enzyme machinery or genes. | Qualitative evidence. Presence of relevant genes and enzymes indicates production potential, but actual production rate can be affected by inhibitors, missing precursors, unfavourable conditions, epigenetic modulation, etc. |
Carini et al. (2014)27, Yao et al. (2016)13, Perez-Coronel and Beman (2020)42 |
| Statistical analysis | Methane concentration is measured together with other lake parameters. Statistical models are applied to test for correlative significance and predictive power. | Individual methane sources and sinks can be overlooked due to the complex lake water methane cycling. Results lack mechanistic understanding of the underlying processes. |
Fernandez et al. (2016)3, Günthel et al. (2020)40, Khatun et al. (2020)38, Leon-Palmero et al. (2020)41 |
| Physical modelling | Combining physical mechanistic aspects with correlative analysis. | Underrepresentation of internal biological modulation (oxidation and OMP). | Peeters et al. (2019)16 |
|
Mass balance of epilimnion in • Whole-lake basin or • Mesocosms/enclosures |
Methane input and output fluxes for the epilimnion are experimentally determined and balanced. Discrepancy is attributed to OMP. | Accuracy of OMP production rates depends on how reliably methane fluxes have been determined. Spatio-temporal data resolution is often limited. |
Bogard et al. (2014)33, Donis et al. (2017)7, Günthel et al. (2019)39, Peeters et al. (2019)16, Hartmann et al. (2020)18 |
|
Methane isotope analysis • Comparing ambient signatures or • Isotope budgets |
Analysing carbon (and hydrogen) stable isotope signatures of methane sources and considering isotope fractionation by biochemical and physical reactions (e.g., oxidation, OMP, phase exchange). Analogue to mass balance. | This analysis requires knowing (i) the quantity of all mass fluxes, (ii) isotope characteristics of all methane sources, (iii) isotope fractionation by biochemical and physical processes. Different precursors and biochemical production/consumption pathways can result in different isotope signatures. |
Tang et al. (2014)9, DelSontro et al. (2018)35, Günthel et al. (2020)40, Hartmann et al. (2020)18, Tsunogai et al. (2020)19 |
A better understanding of production, storage, consumption, and distribution processes of methane, including methane produced in oxic environments, is needed to improve the assessment of the global methane cycle. This requires better spatio-temporal data resolution and better constraints of data uncertainties by using multiple methods. For instance, OMP rates determined by bottle incubations can complement results based on mass budgets, as we did in our study. The incorporation of methane carbon18 and hydrogen19 isotope data into mass budgets is a promising way to further tease apart the different methane sources. Omic approaches can be used to investigate the different OMP pathways and the organisms involved.
We have discussed the caveats of our mass balance analysis, such as the limited amount of OMP and littoral flux data, limited types of lakes being considered, and the influence by other compounding factors. The global significance of OMP can only be fully assessed when more relevant data become available, but this also requires researchers to look beyond the anoxic paradigm and consider OMP in future methane measurements. We hope our and others’ work will continue to stimulate more research and constructive discussions on this topic.
Supplementary information
Acknowledgements
We thank Frank Peeters and Hilmar Hofmann for taking an interest in our study and raising several interesting discussion points about oxic methane production.
Source data
Author contributions
M.G., D.D., G.K. and D.F.M. analysed the data. M.G., D.D., G.K., D.I., M.B., D.F.M., H.-P.G. and K.W.T. discussed and wrote the manuscript.
Data availability
Data are made available in graphical or tabular form throughout the paper and Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks John Melack and other, anonymous, reviewers for their contributions to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marco Günthel, Email: marcoguenthel@gmail.com.
Daniel F. McGinnis, Email: daniel.mcginnis@unige.ch
Hans-Peter Grossart, Email: hgrossart@igb-berlin.de.
Kam W. Tang, Email: k.w.tang@swansea.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-21216-1.
References
- 1.Bastviken D, Cole J, Pace M, Tranvik L. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles. 2004;18:GB4009. doi: 10.1029/2004GB002238. [DOI] [Google Scholar]
- 2.Murase J, Sakai Y, Kametani A, Sugimoto A. Dynamics of methane in mesotrophic Lake Biwa, Japan. Ecol. Res. 2005;20:377–385. doi: 10.1007/s11284-005-0053-x. [DOI] [Google Scholar]
- 3.Fernandez JE, Peeters F, Hofmann H. On the methane paradox: transport from shallow water zones rather than in situ methanogenesis is the major source of CH4 in the open surface water of lakes. J. Geophys. Res. Biogeosci. 2016;121:2717–2726. doi: 10.1002/2016JG003586. [DOI] [Google Scholar]
- 4.Li Y, Fichot CG, Geng L, Scarratt MG, Xie H. The contribution of methane photoproduction to the oceanic methane paradox. Geophys. Res. Lett. 2020;47:e2020GL088362. [Google Scholar]
- 5.Schilder J, Bastviken D, van Hardenbroek M, Heiri O. Spatiotemporal patterns in methane flux and gas transfer velocity at low wind speeds: Implications for upscaling studies on small lakes. J. Geophys. Res. Biogeosci. 2016;121:1456–1467. doi: 10.1002/2016JG003346. [DOI] [Google Scholar]
- 6.Klaus M, Vachon D. Challenges of predicting gas transfer velocity from wind measurements over global lakes. Aquat. Sci. 2020;82:53. doi: 10.1007/s00027-020-00729-9. [DOI] [Google Scholar]
- 7.Donis D, et al. Full-scale evaluation of methane production under oxic conditions in a mesotrophic lake. Nat. Commun. 2017;8:1661. doi: 10.1038/s41467-017-01648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casper P. Methane production in littoral and profundal sediments of an oligotrophic and a eutrophic lake. Arch. Hydrobiol. Spec. Issues Adv. Limnol. 1996;48:253–259. [Google Scholar]
- 9.Tang KW, et al. Paradox reconsidered: Methane oversaturation in well‐oxygenated lake waters. Limnol. Oceanogr. 2014;59:275–284. doi: 10.4319/lo.2014.59.1.0275. [DOI] [Google Scholar]
- 10.Flury S, McGinnis DF, Gessner MO. Methane emissions from a freshwater marsh in response to experimentally simulated global warming and nitrogen enrichment. J. Geophys. Res. 2010;115:G01007. [Google Scholar]
- 11.Grossart H-P, Frindte K, Dziallas C, Eckert W, Tang KW. Microbial methane production in oxygenated water column of an oligotrophic lake. Proc. Natl Acad. Sci. USA. 2011;108:19657–19661. doi: 10.1073/pnas.1110716108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussmann I. Methane release through resuspension of littoral sediment. Biogeochemistry. 2005;74:283–302. doi: 10.1007/s10533-004-2223-2. [DOI] [Google Scholar]
- 13.Yao MC, Henny C, Maresca JA. Freshwater bacteria release methane as a by-product of phosphorus acquisition. J. Appl. Environ. Microbiol. 2016;82:6994–7003. doi: 10.1128/AEM.02399-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angle JC, et al. Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions. Nat. Commun. 2017;8:1567. doi: 10.1038/s41467-017-01753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bizic M, et al. Aquatic and terrestrial cyanobacteria produce methane. Sci. Adv. 2020;6:eaax5343. doi: 10.1126/sciadv.aax5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters F, Fernandez EJ, Hofmann H. Sediment fluxes rather than oxic methanogenesis explain diffusive CH4 emissions from lakes and reservoirs. Sci. Rep. 2019;9:243. doi: 10.1038/s41598-018-36530-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karl D, et al. Aerobic production of methane in the sea. Nat. Geosci. 2008;1:473–478. doi: 10.1038/ngeo234. [DOI] [Google Scholar]
- 18.Hartmann JF, et al. High spatiotemporal dynamics of methane production and emission in oxic surface water. Environ. Sci. Technol. 2020;54:1451–1463. doi: 10.1021/acs.est.9b03182. [DOI] [PubMed] [Google Scholar]
- 19.Tsunogai U, et al. Dual stable isotope characterization of excess methane in oxic waters of a mesotrophic lake. Limnol. Oceanogr. 2020;9999:1–16. [Google Scholar]
- 20.Keppler F, Hamilton J, Brass M, Röckmann T. Methane emissions from terrestrial plants under aerobic conditions. Nature. 2006;439:187–191. doi: 10.1038/nature04420. [DOI] [PubMed] [Google Scholar]
- 21.Messenger DJ, McLeod AR, Fry SC. The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ. 2009;32:1–9. doi: 10.1111/j.1365-3040.2008.01892.x. [DOI] [PubMed] [Google Scholar]
- 22.Angel R, Matthies D, Conrad R. Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen. PLoS ONE. 2011;6:e20453. doi: 10.1371/journal.pone.0020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenhart K, et al. Evidence for methane production by saprotrophic fungi. Nat. Commun. 2012 doi: 10.1038/ncomms2049. [DOI] [PubMed] [Google Scholar]
- 24.Althoff F, et al. Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat. Commun. 2014;5:4205. doi: 10.1038/ncomms5205. [DOI] [PubMed] [Google Scholar]
- 25.Damm E, et al. Methane production in aerobic oligotrophic surface water in the central Arctic Ocean. Biogeosciences. 2010;7:1099–1108. doi: 10.5194/bg-7-1099-2010. [DOI] [Google Scholar]
- 26.White AE, Karl DM, Björkman KM, Beversdorf LJ, Letelier RM. Phosphonate metabolism by Trichodesmium IMS101 and the production of greenhouse gases. Limnol. Oceanogr. 2010;55:1755–1767. doi: 10.4319/lo.2010.55.4.1755. [DOI] [Google Scholar]
- 27.Carini P, White A, Campbell E, Giovannoni SJ. Methane production by phosphate-starved SAR11 chemoheterotrophic marine bacteria. Nat. Commun. 2014;5:4346. doi: 10.1038/ncomms5346. [DOI] [PubMed] [Google Scholar]
- 28.Lenhart K, et al. Evidence for methane production by the marine algae Emiliania huxleyi. Biogeosciences. 2016;13:3163–3174. doi: 10.5194/bg-13-3163-2016. [DOI] [Google Scholar]
- 29.Repeta DJ, et al. Marine methane paradox explained by bacterial degradation of dissolved organic matter. Nat. Geosci. 2016;9:884–887. doi: 10.1038/ngeo2837. [DOI] [Google Scholar]
- 30.Klintzsch T, et al. Methane production by three widespread marine phytoplankton species: release rates, precursor compounds, and potential relevance for the environment. Biogeosciences. 2019;16:4129–4144. doi: 10.5194/bg-16-4129-2019. [DOI] [Google Scholar]
- 31.Klintzsch T, et al. Effects of temperature and light on methane production of widespread marine phytoplankton. J. Geophys. Res. Biogeosci. 2020;125:e2020JG005793. doi: 10.1029/2020JG005793. [DOI] [Google Scholar]
- 32.Ye WW, Wang XL, Zhang XH, Zhang GL. Methane production in oxic seawater of the western North Pacific and its marginal seas. Limnol. Oceanogr. 2020;65:2352–2365. doi: 10.1002/lno.11457. [DOI] [Google Scholar]
- 33.Bogard MJ, et al. Oxic water column methanogenesis as a major component of aquatic CH4 fluxes. Nat. Commun. 2014;5:5350. doi: 10.1038/ncomms6350. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Dore JE, McDermott TR. Methylphosphonate metabolism by Pseudomonas sp. populations contributes to the methane oversaturation paradox in an oxic freshwater lake. Environ. Microbiol. 2017;19:2366–2378. doi: 10.1111/1462-2920.13747. [DOI] [PubMed] [Google Scholar]
- 35.DelSontro T, del Giorgio PA, Prairie YT. No longer a paradox: The interaction between physical transport and biological processes explains the spatial distribution of surface water methane within and across lakes. Ecosystems. 2018;21:1073–1087. doi: 10.1007/s10021-017-0205-1. [DOI] [Google Scholar]
- 36.Li W, et al. Methane production in the oxygenated water column of a perennially ice‐covered Antarctic lake. Limnol. Oceanogr. 2019;65:143–156. doi: 10.1002/lno.11257. [DOI] [Google Scholar]
- 37.Khatun S, et al. Aerobic methane production by planktonic microbes in lakes. Sci Total Environ. 2019;696:133916. doi: 10.1016/j.scitotenv.2019.133916. [DOI] [Google Scholar]
- 38.Khatun S, et al. Linking stoichiometric organic carbon–nitrogen relationships to planktonic cyanobacteria and subsurface methane maximum in deep freshwater lakes. Water. 2020;12:402. doi: 10.3390/w12020402. [DOI] [Google Scholar]
- 39.Günthel M, et al. Contribution of oxic methane production to surface methane emission in lakes and its global importance. Nat. Commun. 2019;10:5497. doi: 10.1038/s41467-019-13320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Günthel M, et al. Photosynthesis‐driven methane production in oxic lake water as an important contributor to methane emission. Limnol. Oceanogr. 2020;65:2853–2865. doi: 10.1002/lno.11557. [DOI] [Google Scholar]
- 41.Leon-Palmero E, Contreras-Ruiz A, Sierra A, Morales-Baquero R, Reche I. Dissolved CH4 coupled to photosynthetic picoeukaryotes in oxic waters and to cumulative chlorophyll a in anoxic waters of reservoirs. Biogeosciences. 2020;17:3223–3245. doi: 10.5194/bg-17-3223-2020. [DOI] [Google Scholar]
- 42.Perez-Coronel E, Beman JM. Biogeochemical and omic evidence for multiple paradoxical methane production mechanisms in freshwater lakes. bioRxiv. 2020 doi: 10.1101/2020.07.28.225276. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are made available in graphical or tabular form throughout the paper and Supplementary Information. Source data are provided with this paper.