Abstract
The coupling between nitrification and N2 gas production to recycle ammonia back to the atmosphere is a key step in the nitrogen cycle that has been researched widely. An assumption for such research is that the products of nitrification (nitrite or nitrate) mix freely in the environment before reduction to N2 gas. Here we show, in oxic riverbeds, that the pattern of N2 gas production from ammonia deviates by ~3- to 16-fold from that predicted for denitrification or anammox involving nitrite or nitrate as free porewater intermediates. Rather, the patterns match that for a coupling through a cryptic pool, isolated from the porewater. A cryptic pool challenges our understanding of a key step in the nitrogen cycle and masks our ability to distinguish between sources of N2 gas that 20 years’ research has sought to identify. Our reasoning suggests a new pathway or a new type of coupling between known pathways in the nitrogen cycle.
Subject terms: Microbiology, Biogeochemistry, Environmental sciences
The N cycle involves complex, microbially-mediated shuttling between ammonium, nitrite and nitrate, with climatically important greenhouse gas byproducts. Here the authors use isotope labeling experiments in river sediments and find a cryptic new step in the N cycle between nitrification and the removal of fixed N through N2 gas production.
Introduction
Nitrogen is a key bio-element for life on Earth, integral to proteins and the very DNA that tells life what to do. A vast reservoir of nitrogen resides in the atmosphere as N2 gas, unavailable to the majority of life until being fixed by either biological or anthropogenic nitrogen fixation. Life’s organically-bound nitrogen in turn decays to ammonia following excretion or death. To complete the cycle, first nitrogen must be oxidised to nitrite or nitrate which can then be reduced back to atmospheric N2 gas. This process of ammonia oxidation—known as nitrification—typically occurs in two stages carried out by specialised aerobic chemoautotrophic ammonia- and nitrite-oxidising microbes, for example, in soils, sediments, freshwater, or marine ecosystems (Eqs. 1 and 2, respectively):
| 1 |
| 2 |
Nitrite and nitrate can then be reduced to N2 gas either alone, in a phylogenetically widespread form of microbial anaerobic respiration termed denitrification1 (Eq. 3a, b) or, in combination with ammonia, in a phylogenetically narrow respiratory pathway termed anaerobic ammonia oxidation, namely anammox2 (Eq. 4).
| 3a |
| 3b |
| 4 |
In addition, smaller amounts of N can be returned to the atmosphere as nitrous oxide (N2O) but we do not consider those further here3–5. Combinations of Eqs. (1) to (4) recycle ammonia back into atmospheric N2 gas and this coupling between aerobic nitrification and anaerobic N2 gas production is a key concept in the nitrogen cycle, controlling ecosystem production and the abundance of life on Earth6,7.
Besides the now accepted reactions described in Eqs. (1) to (4), Broda’s original thermodynamic predictions that drove the quest for anammox8,9 also included the potential for complete aerobic ammonia oxidation to N2 gas—that, to the best of our knowledge—has yet to be observed in nature:
| 5 |
In estuarine or coastal sea sediments, combinations of recognised aerobic and anaerobic metabolisms (Eqs. 1 to 4) buffer the flux of terrestrial nitrogen out to sea and are considered to be physically divided between the oxic and anoxic sediment layers—albeit by only a few tenths of millimetres10. In rivers, nitrite and nitrate borne from aerobic nitrification (Eqs. 1 and 2), in either the surrounding catchment soils or the riverbed itself, can be transported over large distances (1–100 km) before some 47 Tg N per year is removed from the fluvial network as N2 gas11–13. Regardless of the setting, the important point to appreciate here is that the products of aerobic nitrification (e.g., nitrate and nitrite) are assumed to be free to mix with any existing nitrate and nitrite in the surrounding porewater before they are subsequently metabolised, anaerobically, to N2 gas. That is, there is—in effect—only one pool of nitrate and nitrite awaiting reduction to N2 gas regardless of their origins. Indeed, this concept of free mixing between substrates lies at the very heart of the common 15N isotope pairing techniques used to disentangle and quantify the cycling of nitrogen in sediments that are major sources of N2 gas on Earth11,14,15.
Most research into the coupling between aerobic nitrification and anaerobic N2 gas production in sediments has studied the two separately using either oxic or anoxic incubations, respectively16, but now work including oxygen is increasing17. Previously we demonstrated18 that oxic (~30% to 100% of air-saturation for oxygen) gravel and sandy riverbed sediments harbour a coupling between aerobic nitrification and, seemingly, anaerobic N2 gas production with that production being attributed to a combination of denitrification and anammox18. We now show that the pattern of N2 gas production from ammonia in these oxic riverbeds violates the prevailing concept that coupled nitrification and N2 gas production is a two-step process with free nitrite or nitrate as intermediates. Not only does this challenge our understanding of a key coupling in the nitrogen cycle but it also masks our ability to distinguish between denitrification and anammox as sources of N2 gas. Indeed, it may actually suggest a new pathway or at least a new type of coupling between known pathways in the nitrogen cycle.
Results and discussion
N2 gas production is independent from porewater nitrite or nitrate
Following on from our original work18 on nitrification and putative anaerobic N2 gas production in oxic riverbeds, we wanted to explore further how these two processes are coupled. We began by collecting sediment from four rivers—two each of predominantly gravel and sand and then extended our sampling to a total of twelve rivers (Supplementary Figure 1 and Supplementary Table 1). We added 15N-ammonia to oxic sediment microcosms (see Methods) to trace the coupling between nitrification and N2 gas production both with and without the inhibitor of aerobic nitrification, allylthiourea19 (~80 µM ATU in the porewater, Treatments 1 & 2, Table 1 and Methods) that does not inhibit denitrification or anammox2,20. As before18, we measured the immediate production of 15N-N2-gas that was stopped by inhibiting the first step (Eq. 1) of aerobic 15N-ammonia oxidation with ATU (Fig. 1a, Table 1). The coupling between aerobic ammonia oxidation and N2 gas production was clearly strong, however it was not complete. For example, across the twelve rivers, approximately 60% (Fig. 1b) of the oxidised 15N-ammonia tracer was recovered from the porewater as 15NOx−, i.e., as either 15N-nitrite (Eq. 1) or the final product of nitrification, 15N-nitrate (Eq. 2) e.g., 15NOx− is the sum of 15NO2− and 15NO3−.
Table 1.
Summary of total 15N-N2 production in oxic incubations with 15NH4+ or 15NO2−. Mixed-effects models were used to estimate overall rates of total 15N-N2 production for the incubations in Fig. 1a. Treatments 1 to 6 were applied to sediments from the first set of 4 rivers, and then just treatments 1 and 2 for the subsequent set of 12 rivers. Model fitting was carried out in the lme4 package in R45 and rate estimates, standard errors (s.e.) and 95% confidence intervals derived using emtrends from the emmeans package (see Methods). Significant production (bold) of 15N-N2 was only measured with treatments 1 and 3.
| Code, Treatment | Rivers (replicates) | Total 15N-N2 (nmol N g−1 h−1) | s.e. | Lower 95% C.I. | Upper 95% C.I. |
|---|---|---|---|---|---|
| 1, 15NH4+ + ATU | 4 (5) | 0.110 | 0.337 | −0.667 | 0.886 |
| 2, 15NH4+ | 4 (5) | 1.855 | 0.326 | 1.078 | 2.631 |
| 3, 15NH4+ + 14NO2− + ATU | 4 (5) | 0.152 | 0.337 | −0.625 | 0.929 |
| 4, 15NH4+ + 14NO2− | 4 (5) | 1.941 | 0.326 | 1.165 | 2.717 |
| 5, 14NH4+ + 15NO2− + ATU | 4 (5) | 0.314 | 0.326 | −0.462 | 1.091 |
| 6, 14NH4+ + 15NO2− | 4 (5) | 0.279 | 0.326 | −0.497 | 1.055 |
| 1, 15NH4+ + ATU | 12 (5) | 0.129 | 0.178 | −0.249 | 0.506 |
| 2, 15NH4+ | 12 (5) | 1.465 | 0.176 | 1.091 | 1.839 |
Fig. 1. Oxic incubations with 15N-ammonia tracer produce both 15N2 gas and 15NOx−.
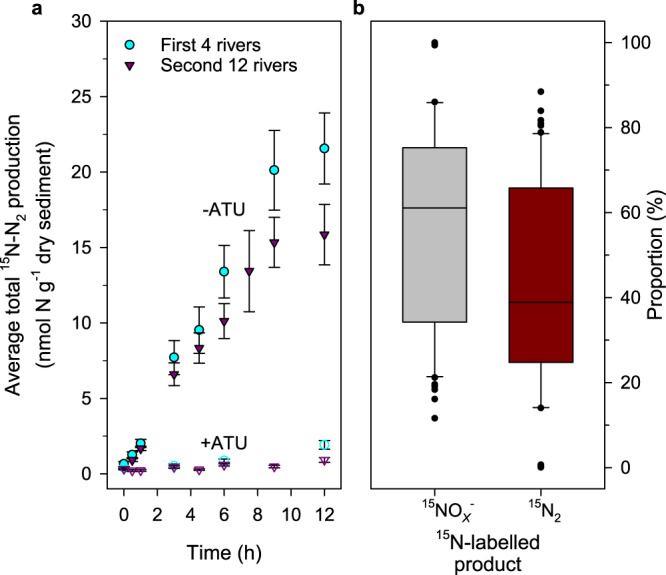
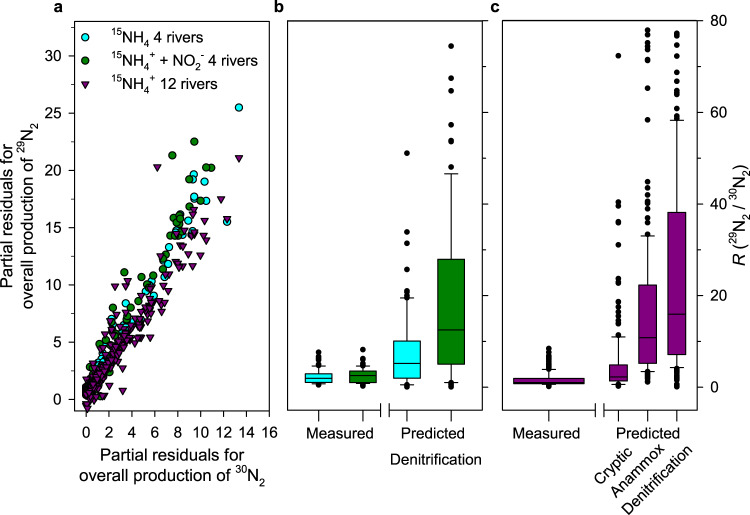
a Overall average production of total 15N-N2 (i.e., 29N2 and 30N2) over time in the presence or absence of the inhibitor of ammonia mono-oxygenase, allylthiourea (ATU). The first 4 rivers (cyan circles, n = 40, 4 rivers x 5 replicates x 2 treatments at each time point, ± 1 s.e.) and the follow-up across 12 rivers (purple triangles, n = 60, 12 rivers x 5 replicates at each time point, ± 1 s.e.); open coloured symbols are the same plus ATU (see Table 1). b Proportions of oxidised 15N-ammonia tracer from a, recovered as either 15NOx− or 15N2 across the 12 rivers (n = 60 as for a). Upper and lower box boundaries are 75th and 25th percentiles, respectively, upper and lower whiskers are 90th and 10th percentiles, respectively, the extreme outliers the maxima and minima and the horizontal line the centre, median value.
The presence of 15N-ammonia and 15N-NOx− together in the porewater generates two 15N-labelled substrate pools. The fraction of the pool labelled with 15N is termed FA for ammonia (NH3) and FN for NOx− (Eqs. 10 and 11 in Methods). Theoretically, combinations of Eqs. (1) to (4) can draw on these two substrate pools (FA and FN) to produce both the single-15N-labelled, 29N2 gas (e.g., 14N, 15N) and the double-15N-labelled, 30N2 gas (e.g., 15N, 15N) which we illustrate schematically in Fig. 2a. Note that denitrification can draw on NOx− as either NO2− or NO3− but anammox is solely fuelled by NO2−. The published and accepted mathematical framework21 (See derivation of equations in Supplementary Note 1) tells us that the fraction of 15N-labelling in each of the substrate pools (FA and FN) must influence the ratio of 29N2 to 30N2 (here termed R) and the overall fraction of 15N in the N2 gas produced e.g., the overall blend of 28N2, 29N2 and 30N2 (here termed FN2)21,22. While complex, the accepted framework also tells us that so long as we know what fraction of each component part (FA, FN and FN2) is labelled with 15N, then we can still calculate how the N2 gas is produced e.g., by anammox or denitrification and understand the nature of this key coupling in the nitrogen cycle21,22.
Fig. 2. Accepted and proposed cryptic couplings in oxic N cycling.
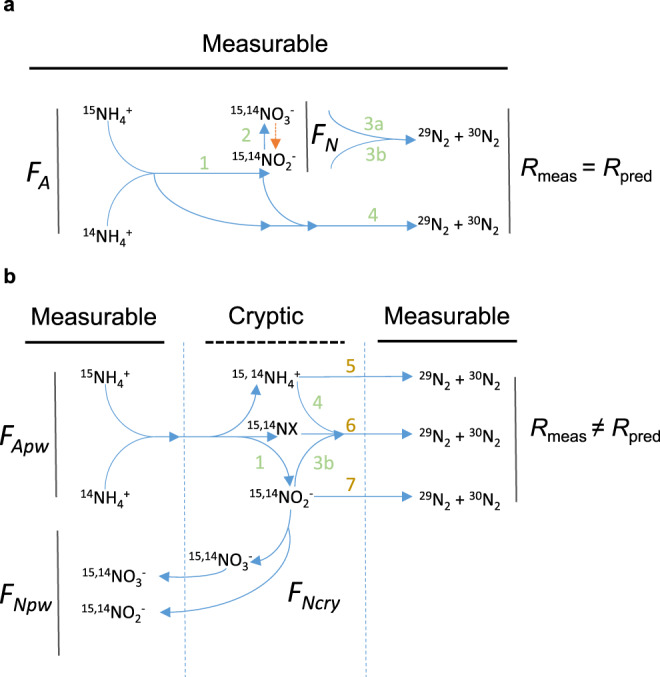
a 15NH4+ tracer is added to oxic sediments to mix with 14NH4+ in the porewater, with the fraction of 15N labelling known as FA. Through reactions 1 and 2, 14NH4+ and 15NH4+ are oxidised aerobically to 14,15NO2− and 14,15NO3− to generate a 14,15NOx− pool with 15N labelling known as FN. NO3− and/or NO2− can be denitrified to N2 gas (reactions 3a, 3b), or NO2− can oxidise NH4+ anaerobically through anammox to N2 gas (reaction 4). Regardless of the precise setting and combination of reactions, all substrates and products are free to mix and the measured ratio of 29N2 to 30N2 produced (R) can be predicted from the measured 15N labelling in the porewater. The downwards pointing orange arrow indicates NO3− respiration to NO2− that we do not consider further here. b In contrast, our measured values for R cannot be predicted using the measured fraction of 15N labelling in the porewater (FA and FN) and known combinations of reactions 1 to 4 but can only be approximated assuming a cryptic element (FNcry). A cryptic element could be a hidden substrate pool (6, novel or known) or novel parts of existing processes (7, e.g., complete nitrifier-denitrification beyond N2O to N2) and/or a completely new pathway (reaction 5 e.g., complete aerobic ammonia oxidation to N2) or cryptic combinations of known pathways after partial aerobic ammonia oxidation to nitrite (reactions, 1, 3b, 4).
We tested the validity of this accepted mathematical framework by changing the fraction of porewater NOx− labelled with 15N (FN) and looking for how this influenced the ratio of 29N2 to 30N2 produced (R). First we directly decreased FN by adding 14N-nitrite to dilute the 15N-nitrite accumulating in the porewater from the oxidation of 15N-ammonia (Treatments 3 and 4, Table 1). Surprisingly, diluting FN had no discernible effect on the values for R produced in the two sets of incubations (Fig. 3b. 2.32, 95% CI 2.01 to 2.64 versus 2.43, 95% CI 2.12 to 2.74, see Table 2 and Supplementary Table 2 for 29N2 and 30N2 production). We then repeated our incubations with just 15NH4+ (with and without ATU, Treatments 1 and 2) across twelve rivers and measured a similar value for R of 1.8 (95% CI, 1.41 to 2.20, Fig. 3c) at an even lower value for FN (see Table 1). Note, we might have expected R to increase steeply as an inverse function of FN (Supplementary Figure 3). We can predict what values for R we might have expected if our N2 gas had been produced by either denitrification or anammox fuelled by porewater nitrite and/or ammonia, respectively (Fig. 2a) and compare them to our measured R values to highlight the disparity between the two (Fig. 3b, c and Table 2):
| 6 |
| 7 |
Our measured R values were too low to be explained by either denitrification or anammox fuelled by porewater FN and/or FA (Fig. 2a) and even a mixture of these two processes couldn’t produce such low values for R on average. This consistent disparity between our measured and predicted values for R, according to the accepted model, along with the constancy in R, despite differences in FN (Table 2), strongly implies that porewater NOx− had little influence on the 15N-labelling of the N2 gas produced from the oxidation of 15N-ammonia. Further, in an analogous set of incubations where we added 15N-nitrite instead of 15N-ammonia, we measured no consistent production of 15N-N2 gas (Treatments 5 & 6 Table 1 and Methods). Hence, nitrogen for N2 formation was not drawn primarily from the porewater NOx− pool (Fig. 2a). Instead, we propose that any N2 producing pathways draw from a cryptic nitrogen pool (Fig. 2b) with 15N-labelled fraction, FNcry, instead of the familiar porewater pool with 15N-labelled fraction, FNpw. Indeed, if we invoke a cryptic pool by making the 15N-labelling of FN the same as 15N-ammonia in the porewater FA in Eqs. (6) and (7) and thereby force denitrification and/or anammox to draw on that FNcry pool, then the predicted R values come closer to our measured R values (R cryptic, Fig. 3c and Table 2).
Fig. 3. Ratios of 29N2 and 30N2 production consistently below those predicted.
a Consistent 29N2 production (nmol g−1 h−1) from 15N-ammonia added to oxic sediments, against each corresponding measure of 30N2 production at each time-point (>0.5 h < 10 h) in each incubation in Fig. 1a presented here as the partial residuals from mixed-effects models (n = 100 and n = 300, for the 4- and 12-river datasets, respectively). b The corresponding measured values for R from a, for the first 4 rivers incubated with either 15NH4+ (95% CI for R = 2.01 to 2.64) or 15NH4+ and additional 14NO2− (95% CI for R = 2.12 to 2.74), against those predicted for denitrification of porewater NO2−. c Measured R values for the 12 river sediments incubated with only 15NH4+ (95% CI for R = 1.41 to 2.20), against predicted R values for denitrification, anammox, and a cryptic coupling. See main text and Table 2. Upper and lower box boundaries are 75th and 25th percentiles, respectively, upper and lower whiskers are 90th and 10th percentiles, respectively, the extreme outliers the maxima and minima and the horizontal line the centre, median value.
Table 2.
Summary of the overall measured and predicted ratios of 29N2 to 30N2 production (R) for treatments 2 and 4 and the fraction of 15N labelling in each substrate pool for FN and FA, on average. Overall measured and predicted R estimates, standard errors (s.e.) and 95% confidence intervals were derived with mixed-effects models using lme4 and emmeans (See Methods) and similarly for FN and FA. See Supplementary Table 3 for further details.
| Code, Treatment | Rivers (replicates) | R (29N2/30N2) | Lower 95% C.I. | Upper 95% C.I. | P | FN‡ | FA‡ | |
|---|---|---|---|---|---|---|---|---|
| Measured | NO2− | NOx− | ||||||
| 2, 15NH4+ | 4 (5) | 2.32 (0.16) | 2.0 | 2.6 | 0.32 | 0.41 | 0.57 | |
| 4, 15NH4+ + 14NO2− | 4 (5) | 2.43 (0.16) | 2.1 | 2.7 | 0.27 | 0.36 | 0.51 | |
| 4 minus 2 | 4 (5) | 0.11 (0.08) | 0.20 | |||||
| 2 & 4 | 4 (10) | 2.38 (0.15) | 2.1 | 2.7 | ||||
| 2, 15NH4+ | 12 (5) | 1.81 (0.20) | 1.4 | 2.2 | 0.16 | 0.25 | 0.45 | |
| Predicted† | ||||||||
| 2, 15NH4+ | 4 (5) | 7.81 (1.36)D | 5.1 | 10.5 | ||||
| 4, 15NH4+ + 14NO2− | 4 (5) | 20.05 (1.49)D | 16.9 | 22.3 | ||||
| 2, 15NH4+ | 12 (5) | 29.4 (2.28)D | 24.8 | 33.9 | ||||
| 2, 15NH4+ | 12 (5) | 19.3 (2.28)A | 14.8 | 23.9 | ||||
| 2, 15NH4+ | 12 (5) | 9.3 (2.28)C | 4.8 | 13.8 | ||||
†Predicted R values, using Eqs. (6) and (7), for denitrification (D), anammox (A) and cryptic (C) processes fuelled by porewater NO2−, see Supplementary Table 3 for NOx−. Note also that the predicted values are derived using each individual measure of FN and FA in each vial, and that FN‡ are the overall mean values simply to illustrate the effect of adding 14NO2− to the incubations with sediments from the first 4 rivers and overall lower FN value for the 12 river incubations.
N2 is produced from ammonia through a cryptic intermediate
We can use both the accepted21 and a new mathematical framework to more formally justify our proposal for a cryptic intermediate pool or process. First, we define the proportion of N2 gas coming from anammox relative to denitrification that is conventionally known as ra15. ra has to lie between 0 and 1 and, in the accepted framework, is expressed as a function of porewater FA and FN and R according to21 (See Eq. (1) to (14) in Supplementary Note 1):
| 8 |
In the accepted framework, however, our measured values for R and porewater FA and FN generate nonsensical estimates for ra (e.g., −6.06 to 3.03, not > 0 < 1). Just as for Fig. 3c, we cannot apportion N2 gas between anammox and denitrification drawing on porewater FN and/or FA – in the conventional sense – to produce our measured R values (Fig. 2a). Next, we define the 15N- labelling of the N2 gas produced (FN2), which, like ra (Eq. 6), also has to lie between 0 and 1 (See Eq. (1) to (14) in Supplementary Note 1).
| 9 |
Unlike ra, which is expressed as a function of both porewater FA and FN, only FN is required to parameterise FN2 (Eq. 9cf. Eq. 8). That is not to say that FA has no influence on FN2, as FN—be it either the FNcry or FNpw pools—must result from ammonia oxidation drawing on FA (Fig. 2).
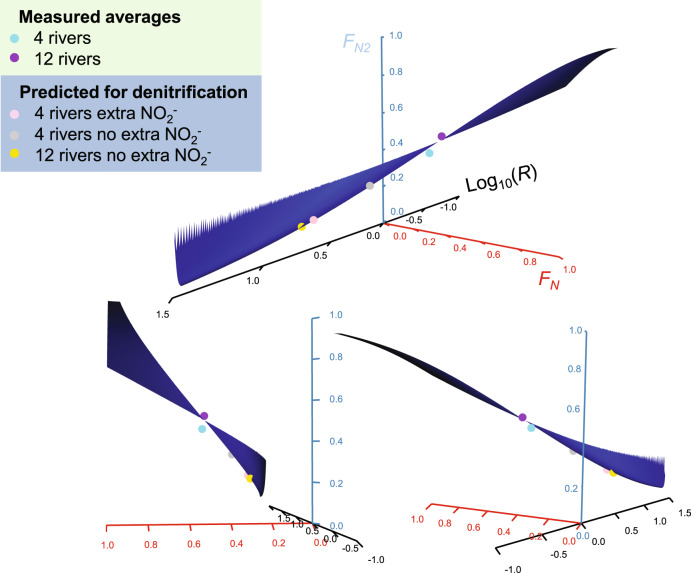
We can then use solutions to Eqs. (8) and (9) between > 0 < 1 to define a solution space for any combination of FN, FA, and realistic values for R (See Supplementary Figure 3 for R as a function of 15N atom %) that we can visualise as a 3D ribbon (Fig. 4). The height of the ribbon is defined in terms of FN2 and is depicted here for our average value for FA of 0.51 (Table 1 and see Supplementary Fig. 4 for FA at 0.1 and 0.9). Overall the ribbon is very narrow and where FA = FN there are no solutions and this singularity appears as a gap in the ribbon. If FNcry is isolated and derives solely from the oxidation of FA (Fig. 2b), then FNcry has to equal FA. Further, if FN2 is only dependent on FN (Eq. 9) and this FN is equivalent to FNcry, then our calculated values for FN2—plotted as functions of our measured values for R and FA (where FNcry equal FA)—should fall near the gap in the ribbon where FN equals FA. This is indeed what we observe and especially for the better parameterised 12 river estimate (Fig. 4). In contrast, if we again force denitrification to be the only source of N2, and calculate FN2 assuming that FN = FNpw (Fig. 2a), then the points fall away from our measured R values. Hence, in the presence of 15N-ammonia and oxygen, our measured R values only make sense if we assume FNcry = FA (Fig. 2b) i.e., the porewater nitrite pool essentially represents the left-overs of the cryptic transformations during which N2 is produced.
Fig. 4. Orientations of the solution space ribbon with both measured and predicted values for R.
Here we present all data in just one solution space for the average fraction of 15N in the ammonia pool (FA) of 0.51 and combinations of Eq. (8) (FN2) and 9 (ra) both yielding values between > 0 < 1. R is the ratio of 29N2 to 30N2 and FN and FN2 the fraction of 15N in the NOx− and N2 gas pools, respectively. To plot FN2 for each of our measured values of R we have to assume that FN equals FA measured in the porewater. In the solution space, there are no solutions where FA = FN (i.e., 0.51) and this singularity appears as a gap in the ribbon. Despite measurable changes in porewater FN, the average values for both the 4-river and 12-river study appear near to each other and the gap where FA = FN. Note that the better parameterised 12-river average touches the gap and by inference, FA ≈ FNcry (Fig. 2b). Denitrification fuelled by porewater NOx− predicts values away from our measured values for R. Note, for the single predicted denitrification R values we use the median FN values.
Internal NOx− cycling or a novel pathway or organism
We propose that the coupling between ammonia oxidation and N2 gas production in oxic, permeable riverbed sediments involves a cryptic intermediate pool derived solely from the oxidation of ammonia that remains isolated from the porewater prior to the production of N2 gas. In one scenario, a cryptic pool, similar to the porewater NOx− pool, is fed by the oxidation of ammonia to NOx−, or possibly NO (ref. 3,23,24), through nitrification. The pathway from FNcry to the production of N2 gas, however, branches off before that NOx− mixes with the ambient porewater NOx− (Fig. 2b) and would require internal NOx− cycling. Internal NOx− cycling is recognised as a potential source of interference for 15N isotope tracer studies in the ocean25,26 and is known in the consortia of ammonia oxidisers and anammox bacteria in wastewater CANON27 reactors (Complete Autotrophic Nitrogen removal Over Nitrite. Figure 2b, reactions 1 & 4) – though the actual mechanism in nature remains unknown.
Alternatively, some aerobic ammonia oxidising bacteria first produce nitrite (reaction 1) that they then reduce to N2O gas in a process known as nitrifier-denitrification3. Known nitrifier-denitrifier bacteria, however, lack a canonical N2O-reductase (NOS, nosZ) to reduce N2O to N2 gas, so are not currently recognised as complete denitrifiers (reaction 7, Fig. 2b). Nitrosocyanin, a soluble red Cu protein isolated from Nitrosomonas europaea28, is recognised as a plausible substitute to canonical N2O-reductase that could enable complete nitrifier-denitrification to N2 gas3. Our data enable us to test this hypothesis. For example, we know that 15NO2− from the initial oxidation of 15NH4+ exchanges with the porewater (reaction 1, Figs. 1b and 2a) and we would expect, therefore, that 15NO2− added to the porewater would be available to any nitrifying-denitrifying bacteria29. We have, however, already shown that adding 15NO2− to the porewater resulted in no consistent production of N2 gas (Treatments 5 & 6, Table 1) i.e., N2 gas production is dependent on the initial oxidation of 15N-ammonia. This fact, along with the clear discrepancy between the measured and predicted scenarios involving porewater NOx− (Figs. 3b, 3c & 4) make it hard to reconcile our N2 gas production with either nitrifier-denitrification or canonical denitrification (reactions 3a, 3b & 7, Fig. 2).
Finally, it is theoretically possible for ammonia to be completely oxidised by oxygen to N2 gas (equation 58) within a single, unknown organism. Such a reaction offers the simplest explanation for our results, with their strong dependency on aerobic ammonia oxidation and lack of influence from external porewater nitrite. Regardless of the actual pathway that produces the N2 gas (Fig. 2b), an isolated cryptic intermediate pool has to have the same 15N-labelling of the ammonia pool (FNcry = FA). As a consequence of this equality, we can no longer distinguish between sources of N2 gas, be it a denitrification-like pathway reductively combining N from an oxidised cryptic pool, an anammox-like process drawing on ammonia and cryptic N, or complete ammonia oxidation, as they would all produce 29N2 and 30N2 at the same ratio (Fig. 2b where R is equal for each process).
Our observations challenge the current understanding of a key coupling in the nitrogen cycle in permeable, oxic riverbed sediments that may also apply to other biomes where the oxidation of ammonia is tightly coupled to the production of N2 gas, such as continental shelf-sediments30,31 and groundwater aquifers17. Whether it transpires that our cryptic coupling is mediated by a novel organism or, as of yet, a masked combination of known players in the nitrogen cycle remains to be resolved.
Methods
Study sites and sediment sampling
We began by collecting sediment samples from four rivers which we subsequently widened to a total of twelve rivers in southern England, UK, between October 2015 and May 2016 (Supplementary Figure 1 and Supplementary Table 1). Among them, the Rivers Lambourn, Darent, Wylye, Rib, Pant, Stour (1) and Stour (2) have chalk-based, permeable gravel-dominated riverbeds, while the Rivers Marden, Hammer, Medway, Broadstone, and Nadder have less permeable, sand-dominated riverbeds as described elsewhere18,32,33. At each river, surface sediments (<5 cm) were collected from five different locations using Perspex corers (13-cm × 9-cm internal diameter, 827 mL and sealed at one end with an oil-seal stopper)) which were then transferred to plastic zip-lock bags (VWR International) and stored in a cool bag (Thermo) during transport back to the laboratory. Each sediment sample from each river was then homogenised in the laboratory for the experiments described below.
Aerobic ammonia oxidation in oxic sediment slurries
15N-NH4+ oxidation experiments were carried out with sediments first from four rivers (the rivers Lambourn, Wylye, Marden, and Hammer) and then all twelve. In a standard anoxic application of 15N isotope pairing techniques34–36, ambient porewater nitrite, nitrate, and any residual oxygen are removed by pre-incubating the anoxic sediment slurries for 12 h to 24 h before adding any 15N-tracers35,36. Here this was not possible as we were measuring the aerobic oxidation of NH4+ and so to avoid contamination from the high background 14NOx− (14NO3− + 14NO2−), which is typical for these rivers24, instead we used nitrite- and nitrate-free synthetic river water (0.12 g/l NaHCO3, 0.04 g/l KHCO3, 0.07 g/l MgSO4.7H2O, 0.09 g/l CaCl2 2H2O, pH = 7) to make the sediment slurries as before18.
Oxic slurries were prepared by adding approximately 3 g sediment (~0.75 ml of porewater) and 2.7 ml air-saturated synthetic river water into 12 ml gas-tight vials (Exetainer, Labco), leaving an approximate 6 ml headspace of air which is equivalent to ~58 µmol O2 per prepared vial. We know from previous incubations with similar sediments from 28 rivers37 respiration rates to be ~187 nmol O2 g−1 h−1, on average (±64.3, 95%, C.I.), that would consume ~12% of the total oxygen during a 12 h incubation. In addition, we also checked oxygen over time using a microelectrode (50 µm, Unisense) in parallel sets of scaled-up slurries (120 mL with the same ratio of sediment to water to headspace) for two rivers and found comparatively little consumption as before18 and see example in Supplementary Figure 2.
To trace the oxidation of ammonia to N2 gas, the prepared oxic slurry vials were then sealed and injected with 100 µl of 14 mM 15NH4+ stock-solutions (98 atom% 15N, Sigma-Aldrich) to generate final porewater concentrations of ~390 µM 15NH4+. This high 15N concentration ensured sufficient labelling of the ammonia pool (~50%) to enable quantifiable production of both single-labelled, 29N2, and dual-labelled, 30N2, in order to calculate R in Eqs. (6) to (9). To link the production of N2 gas to the initial aerobic oxidation of ammonia, an additional set of slurries were injected with 100 µl of 14 mM 15NH4+ (as above), along with 2.8 mM (stock-solution) of the ammonia mono-oxygenase inhibitor19, allylthiourea (ATU), to give final porewater concentrations of ~390 µM 15NH4+ and ~80 µM ATU. While we have shown previously that 80 µM ATU inhibits aerobic ammonia oxidation in gravel and sandy riverbed sediments18, higher concentrations maybe required in other settings38. All of the oxic slurry vials were then incubated on a shaker (120 rpm, Stuart SSL1) for up to 12 h (Table 1, Treatments 1 and 2) in a temperature-controlled room at 12 °C. Incubations amended with just 15NH4+ were terminated at 0 h, 0.5 h, 1 h, 3 h, 4.5 h, 6 h, 9 h, and 12 h while those amended with both 15NH4+ and ATU were terminated at 0 h, 3 h, 6 h, and 12 h by injecting 100 μl of formaldehyde (38%, w/v) through the vial septa. All vials were then stored upside down prior to quantification of 29N2 and 30N2 by mass-spectrometry and R is then simply 29N2/30N2 (see below).
In addition to measuring the production of 29N2 and 30N2 gases (R), the fraction of 15N in the inorganic nitrogen porewater pools (FA for ammonia and FN for NOx− e.g., NO2− plus NO3−) needed to be quantified too (see Eqs. 6 to 9). To avoid any potential interference from formaldehyde, on the analysis of the inorganic nitrogen species, a parallel set of 15NH4+ amended slurries was prepared solely for nutrient analyses. At each time point (as above for N2 gas analysis), vials were injected with 20 µL of 1.6 M NaOH to preserve nitrite before being frozen at −20 °C39. Samples were defrosted and centrifuged at 1200 rpm for 10 min and the collected supernatant analysed (see below).
Manipulating the degree of 15N-labelling in the porewater NO2− pool (FN as FNpw)
In typical anoxic sediment slurry incubations used to quantify N2 gas production from denitrification and anammox34,35, the fraction of porewater substrate labelled with 15N (FA or FN) influences the ratio of 29N2 to 30N2 produced. To characterise the influence of porewater NO2− on the coupling between 15N-NH4+ oxidation and 15N-N2 production in oxic sediment slurries, we manipulated the fraction of porewater NO2− labelled with 15N. Oxic sediment slurries from the first four riverbeds were injected (100 µl) with combinations of stock-solutions of 14 mM 15NH4+ and 840 µM 14NO2− or just 14 mM 15NH4+ and both with or without 2.8 mM ATU. This generated final porewater concentrations of ~390 µM 15NH4+, ~24 µM 14NO2− or ~80 µM ATU and the prepared vials were then incubated on a shaker as above (see Table 1, Treatments 3 and 4). As above, oxic slurry vials were sacrificed at different time points for 15N2 gas analysis and with a parallel set of 15NH4+ or 15NH4+ plus NO2− amended slurries solely for nutrient analyses.
To further test the dependency of N2 gas production on the initial oxidation of 15N-ammonia, we also performed a set of analogous incubations with sediments from the first four rivers with 15NO2− (Table 1, Treatments 5 and 6). Here everything was the same (amount of sediment, with or without ATU, incubation times, oxygen etc.,) except the 15N-labelling was added with nitrite rather than ammonia (as above) to final concentrations of ~390 µM 14NH4+ and ~24 µM 15NO2− (98 atom% 15N, Sigma-Aldrich). If active, we would have expected N2 gas production from reactions 3b and 4.
Analytical methods
Headspaces of the oxic slurry samples were analysed for 15N-N2 using a continuous-flow isotope ratio mass spectrometer (Sercon 20–22, UK) as described elsewhere18. The mass spectrometer has a sensitivity of 0.1 ‰ 15N which here translates to approximately 0.1 nmol 15N-N2 g−1 dry sediment. To determine porewater FN (NO2− or NOx−, below) the concentration of 15NO2− in the 15NH4+ treatments was measured, the preserved supernatants were diluted and 3 ml of sample transferred into a new 3 ml gas-tight vial (Exetainer, Labco), the vial capped and a 0.5 ml helium headspace (BOC) added. Samples were injected with 100 μl of sulfamic acid (4 mM in 4 M HCl) and placed on a shaker (120 rpm, Stuart SSL1) overnight to reduce 15NO2− to 15N-N2 and the headspaces subsequently analysed for 15N-N2 as above18,40. For 15NOx− (15NO2− plus 15NO3−) analysis, 0.3 g spongy cadmium and 200 µl of 1 M imidazole, along with 3.5 ml of sample were added to each gas-tight vial (12 ml, Exetainer, Labco) and the vials shaken (120 rpm, Stuart SSL1) for 2.5 h to reduce 15NO3− to 15NO2− and the samples then treated as above to convert 15NO2− to N218,41. The sensitivity for 15NOx− was approximately 0.4 nmol 15N g−1 dry sediment. FN was then calculated for NO2− or NOx− as:
| 10 |
And similarly for FA:
| 11 |
Where 15NH4+ was determined by the increase in concentration, measured by standard indophenol-blue wet-chemistry, above ambient background in controls after the addition of 15NH4+.
Sediment particle size was determined by sorting the dried sediments through a series of sieves (Endecotts Ltd, England) from 16 mm, 13.2, 8, 4, 1.4, 0.5, 0.25, 0.125, to 0.0625 mm and then weighing each size fraction. Grain size distributions were calculated and classified on the Wentworth scale as gravel (particles coarser than 2 mm), sand (particles between 0.0625 and 2 mm), mud (silt plus clay material finer than 0.0625 mm)42. For sediment organic C and N content, disaggregated samples were oven-dried, acidified by HCl (2 M) to remove inorganic carbonates43 and re-dried to a constant weight. Then ~50 mg of sediments were transferred to tin-cups, reweighed, and combusted at 1000 °C in an integrated elemental analyser and mass-spectrometer (Sercon, Integra 2, UK).
Statistical analysis
We used mixed-effects models to estimate overall rates of total 15N-N2 gas production during the incubations (Fig. 1a), treating each of either the first four or subsequent twelve rivers as genuine, independent replicates. Within each river, each of the 5 technical replicates were nested within each respective river and fitted as random effects on the slope and intercept in each case; though it was not always necessary to retain replicate or all the random effects in a model to get the best fit to the data – based on lowest AIC (Akaike Information Criterion). To visualise the consistent production of 29N2 to 30N2 across the incubations with 15N-ammonia, we regressed each measure of 29N2 on each measure of 30N2, at each time point, in each incubation and display (Fig. 3a) the partial residuals for the best fitting model44. To estimate the overall average measured and predicted ratios of 29N2 to 30N2 (R) we only used the data for the time points >0.5 h < 10 h i.e., when there was measurable (~0.1 nmol N2 g−1 dry sediment), steady-production of both 15N labelled gases, divided each measure of 29N2 by each respective measure of 30N2 at each time point, in each incubation and treated river and replicate as above. For the first 4 rivers, the ratio R was estimated by fitting each time point as a random-effect, but for the larger, 12 river dataset, time was fitted as a fixed-effect and R estimated for the middle time point in the incubations and similarly for FN (for both NO2− and NOx−) and FA. All statistical analyses were performed in R (version 3.6.3, 2020-02-29) under RStudio (version 1.2.5033). Model fitting was carried out in the “lme4” package (version 1.1-21) and parameter (marginal mean) estimates, standard errors, and confidence intervals derived using the “emmeans” package (version 1.4.5) with Kenwood-Roger degrees of freedom and Tukey correction where appropriate.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Ian Sanders and Katrina Lansdown for technical assistance and Axel Rossberg for help with the 3D imagery and Queen Mary University of London and the Chinese Scholarship Council for supporting the research.
Source data
Author contributions
M.T. and L.O. conceived the study and L.O. performed all of the experiments and B.T. formulated the mathematical framework. L.O. and M.T. analysed the data and M.T. and B.T. drafted the manuscript. All authors commented on and revised the manuscript.
Data availability
Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Robert Hall, Nicole Wrage-Mönnig, and other, anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-21400-3.
References
- 1.Shapleigh, J. P. in The Prokaryotes: Volume 2: Ecophysiology and Biochemistry (eds Dworkin, M. et al.) 769–792 (Springer New York, 2006).
- 2.van de Graaf AA, et al. Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 1995;61:1246–1251. doi: 10.1128/AEM.61.4.1246-1251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrage-Monnig N, et al. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018;123:A3–A16. doi: 10.1016/j.soilbio.2018.03.020. [DOI] [Google Scholar]
- 4.Goreau TJ, et al. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 1980;40:526–532. doi: 10.1128/AEM.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korner H, Zumft WG. Expression of denitrification enzymes in response to the dissolved-oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl. Environ. Microbiol. 1989;55:1670–1676. doi: 10.1128/AEM.55.7.1670-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkowski PG. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. doi: 10.1038/387272a0. [DOI] [Google Scholar]
- 7.Vlaeminck SE, Hay AG, Maignien L, Verstraete W. In quest of the nitrogen oxidizing prokaryotes of the early Earth. Environ. Microbiol. 2011;13:283–295. doi: 10.1111/j.1462-2920.2010.02345.x. [DOI] [PubMed] [Google Scholar]
- 8.Broda E. 2 kinds of lithotrophs missing in nature. Z. Allg. Mikrobiol. 1977;17:491–493. doi: 10.1002/jobm.19770170611. [DOI] [PubMed] [Google Scholar]
- 9.Mulder A, Vandegraaf AA, Robertson LA, Kuenen JG. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol. Ecol. 1995;16:177–183. doi: 10.1111/j.1574-6941.1995.tb00281.x. [DOI] [Google Scholar]
- 10.Nielsen OI, Gribsholt B, Kristensen E, Revsbech NP. Microscale distribution of oxygen and nitrate in sediment inhabited by Nereis diversicolor: spatial patterns and estimated reaction rates. Aquat. Microb. Ecol. 2004;34:23–32. doi: 10.3354/ame034023. [DOI] [Google Scholar]
- 11.Seitzinger S, et al. Denitrification across landscapes and waterscapes: a synthesis. Ecol. Appl. 2006;16:2064–2090. doi: 10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Mulholland PJ, et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature. 2008;452:202–206. doi: 10.1038/nature06686. [DOI] [PubMed] [Google Scholar]
- 13.Galloway JN, et al. Nitrogen cycles: past, present, and future. Biogeochem. 2004;70:153–226. doi: 10.1007/s10533-004-0370-0. [DOI] [Google Scholar]
- 14.Nielsen LP. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol. Ecol. 1992;86:357–362. doi: 10.1111/j.1574-6968.1992.tb04828.x. [DOI] [Google Scholar]
- 15.Risgaard-Petersen N, Nielsen LP, Rysgaard S, Dalsgaard T, Meyer RL. Application of the isotope pairing technique in sediments where anammox and denitrification coexist. Limnol. Oceanogr. Methods. 2003;1:63–73. doi: 10.4319/lom.2003.1.63. [DOI] [Google Scholar]
- 16.Zhu G, et al. Hotspots of anaerobic ammonium oxidation at land-freshwater interfaces. Nat. Geosci. 2013;6:103–107. doi: 10.1038/ngeo1683. [DOI] [Google Scholar]
- 17.Wang SY, et al. Anaerobic ammonium oxidation is a major N-sink in aquifer systems around the world. ISME J. 2020;14:151–163. doi: 10.1038/s41396-019-0513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lansdown K, et al. Importance and controls of anaerobic ammonium oxidation influenced by riverbed geology. Nat. Geosci. 2016;9:357–360. doi: 10.1038/ngeo2684. [DOI] [Google Scholar]
- 19.Hall GH. Measurement of nitrification rates in lake-sediments - comparison of the nitrification inhibitors nitrapyrin and allylthiourea. Microb. Ecol. 1984;10:25–36. doi: 10.1007/BF02011592. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MM, Thamdrup B, Dalsgaard T. Effects of specific inhibitors on anammox and denitrification in marine sediments. Appl. Environ. Microbiol. 2007;73:3151–3158. doi: 10.1128/AEM.01898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song GD, Liu SM, Kuypers MMM, Lavik G. Application of the isotope pairing technique in sediments where anammox, denitrification, and dissimilatory nitrate reduction to ammonium coexist. Limnol. Oceanogr. Meth. 2016;14:801–815. doi: 10.1002/lom3.10127. [DOI] [Google Scholar]
- 22.Jensen MM, et al. Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium. ISME J. 2011;5:1660–1670. doi: 10.1038/ismej.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z, Wessels HJCT, van Alen T, Jetten MSM, Kartal B. Nitric oxide-dependent anaerobic ammonium oxidation. Nat. Commun. 2019;10:1244. doi: 10.1038/s41467-019-09268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreiber F, Stief P, Kuypers MMM, de Beer D. Nitric oxide turnover in permeable river sediment. Limnol. Oceanogr. 2014;59:1310–1320. doi: 10.4319/lo.2014.59.4.1310. [DOI] [Google Scholar]
- 25.Nicholls JC, Davies CA, Trimmer M. High-resolution profiles and nitrogen isotope tracing pairs reveal a dominant source of nitrous oxide and multiple pathways of nitrogen gas formation in the central Arabian Sea. Limnol. Oceanogr. 2007;52:156–168. doi: 10.4319/lo.2007.52.1.0156. [DOI] [Google Scholar]
- 26.De Brabandere L, et al. Vertical partitioning of nitrogen-loss processes across the oxic-anoxic interface of an oceanic oxygen minimum zone. Environ. Microbiol. 2014;16:3041–3054. doi: 10.1111/1462-2920.12255. [DOI] [PubMed] [Google Scholar]
- 27.Sliekers AO, Haaijer SCM, Stafsnes MH, Kuenen JG, Jetten MSM. Competition and coexistence of aerobic ammonium- and nitrite-oxidizing bacteria at low oxygen concentrations. Appl. Microbiol. Biotechnol. 2005;68:808–817. doi: 10.1007/s00253-005-1974-6. [DOI] [PubMed] [Google Scholar]
- 28.Arciero DM, Pierce BS, Hendrich MP, Hooper AB. Nitrosocyanin, a red cupredoxin-like protein from Nitrosomonas europaea. Biochemistry. 2002;41:1703–1709. doi: 10.1021/bi015908w. [DOI] [PubMed] [Google Scholar]
- 29.Shaw LJ, et al. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ. Microbiol. 2006;8:214–222. doi: 10.1111/j.1462-2920.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 30.Christensen JP, Murray JW, Devol AH, Codispoti LA. Denitrification in continental shelf sediments has major impact on the oceanic nitrogen budget. Glob. Biogeochem. Cy. 1987;1:97–116. doi: 10.1029/GB001i002p00097. [DOI] [Google Scholar]
- 31.Trimmer M, Nicholls JC. Production of nitrogen gas via anammox and denitrification in intact sediment cores along a continental shelf to slope transect in the North Atlantic. Limnol. Oceanogr. 2009;54:577–589. doi: 10.4319/lo.2009.54.2.0577. [DOI] [Google Scholar]
- 32.Shen L, Ouyang L, Zhu Y, Trimmer M. Spatial separation of anaerobic ammonium oxidation and nitrite-dependent anaerobic methane oxidation in permeable riverbeds. Environ. Microbiol. 2019;21:1185–1195. doi: 10.1111/1462-2920.14554. [DOI] [PubMed] [Google Scholar]
- 33.Heppell CM, et al. Hydrological controls on DOC: nitrate resource stoichiometry in a lowland, agricultural catchment, southern UK. Hydrol. Earth Syst. Sci. 2017;21:4785–4802. doi: 10.5194/hess-21-4785-2017. [DOI] [Google Scholar]
- 34.Thamdrup B, Dalsgaard T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 2002;68:1312–1318. doi: 10.1128/AEM.68.3.1312-1318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trimmer M, Nicholls JC, Deflandre R. Anaerobic ammonium oxidation measured in sediments along the Thames Estuary, United Kingdom. Appl. Environ. Microbiol. 2003;69:6447–6454. doi: 10.1128/AEM.69.11.6447-6454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risgaard-Petersen N, et al. Anaerobic ammonium oxidation in an estuarine sediment. Aquat. Microb. Ecol. 2004;36:293–304. doi: 10.3354/ame036293. [DOI] [Google Scholar]
- 37.Shelley F, Grey J, Trimmer M. Widespread methanotrophic primary production in lowland chalk rivers. Proc. R. Soc. B. 2014;281:20132854. doi: 10.1098/rspb.2013.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens-Habbena W, et al. The production of nitric oxide by marine ammonia-oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ. Microbiol. 2015;17:2261–2274. doi: 10.1111/1462-2920.12677. [DOI] [PubMed] [Google Scholar]
- 39.Wolff JC, Ornemark U, Taylor PDP, De Bievre P. Stability studies and purification procedure for nitrite solutions in view of the preparation of isotopic reference materials. Talanta. 1998;46:1031–1040. doi: 10.1016/S0039-9140(97)00371-8. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Chalk PM, Freney JR. Release of dinitrogen from nitrite and sulfamic acid for isotope ratio analysis of soil extracts containing N-15 labeled nitrite and nitrate. Analyst. 1990;115:365–370. doi: 10.1039/an9901500365. [DOI] [Google Scholar]
- 41.McIlvin MR, Altabet MA. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal. Chem. 2005;77:5589–5595. doi: 10.1021/ac050528s. [DOI] [PubMed] [Google Scholar]
- 42.Folk RL. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J. Geol. 1954;62:344–359. doi: 10.1086/626171. [DOI] [Google Scholar]
- 43.Hedges JI, Stern JH. Carbon and nitrogen determination of carbonate-containing solids. Limnol. Oceanogr. 1984;29:657–663. doi: 10.4319/lo.1984.29.3.0657. [DOI] [Google Scholar]
- 44.Ehnes RB, Rall BC, Brose U. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 2011;14:993–1000. doi: 10.1111/j.1461-0248.2011.01660.x. [DOI] [PubMed] [Google Scholar]
- 45.R Development Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Source data are provided with this paper.