Summary
RNA viruses are responsible for many zoonotic diseases that post great challenges for public health. Effective therapeutics against these viral infections remain limited. Here, we deployed a computational framework for host-based drug repositioning to predict potential antiviral drugs from 2,352 approved drugs and 1,062 natural compounds embedded in herbs of traditional Chinese medicine. By systematically interrogating public genetic screening data, we comprehensively cataloged host dependency genes (HDGs) that are indispensable for successful viral infection corresponding to 10 families and 29 species of RNA viruses. We then utilized these HDGs as potential drug targets and interrogated extensive drug-target interactions through database retrieval, literature mining, and de novo prediction using artificial intelligence-based algorithms. Repurposed drugs or natural compounds were proposed against many viral pathogens such as coronaviruses including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), flaviviruses, and influenza viruses. This study helps to prioritize promising drug candidates for in-depth evaluation against these virus-related diseases.
Subject areas: Molecular Biology, Bioinformatics, Pharmacoinformatics
Graphical abstract
Highlights
-
•
Host dependency genes for RNA viruses are systematically cataloged
-
•
Repositioned drug candidates target host dependency genes for antiviral purpose
-
•
Artificial intelligence-based algorithms help to predict drug-target interactions
-
•
Prioritized antiviral drug candidates are proposed against RNA viruses
Molecular Biology; Bioinformatics; Pharmacoinformatics
Introduction
The recent outbreak and spreading of coronavirus 2019 disease (also known as COVID-19) has become a severe public health crisis that threatens not only human health but also social lifestyle and global economy (Zhu et al., 2020). RNA virus termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the underlying pathogen for COVID-19 (Li et al., 2020). Despite some progresses in early diagnosis and clinical treatment, people still lack consistent and reliable solutions to defeat SARS-CoV-2 and halt COVID-19 pandemic globally (Altay et al., 2020). In addition to SARS-CoV-2 coronavirus, the outbreak of other pathogenic RNA viruses such as coronaviruses of various types (e.g., SARS-CoV and MERS-CoV), flaviviruses (e.g., West Nile virus, Dengue virus and Zika virus), and influenza viruses (e.g., H1N1 and H3N2 stains) also cause severe infectious diseases in human (Petersen et al., 2020; Pierson and Diamond, 2020).
Vaccination is one of the most effective approaches to prevent viral infection by conferring active immunity to the host and helping to establish herd immunity. However, it usually takes years for a successful vaccine to be developed and implemented. To achieve an immediate control of viral disease for the infected patients, therapeutic drug then serves as the primary option and is highly demanded especially for recently emerging pathogens without known therapeutic formula. Encouraging efforts have been made toward antiviral drug development or drug repositioning against the above mentioned RNA viruses and their related diseases (Dighe et al., 2019; Mottin et al., 2018; Zhang et al., 2019; Zumla et al., 2016). Most of these studies focused on various viral genes or proteins that are key mediators to complete the virus life cycle, for instance, targeting spike proteins to block cell entry or inhibiting RNA polymerase to interfere viral gene replication. Virus-centered strategy has been proved feasible in light of the successful development of antiviral drugs in recent years. This approach heavily relies on the specific knowledge about each viral pathogen and its virus-host interplay, which usually requires extensive investigation efforts and is preferable for de novo antiviral drug development spanning years of time (De Clercq and Li, 2016). In contrast, drug repositioning or drug repurposing that exploits existing “old” drugs for “new” purposes offers a quick solution and would be practical to respond to emerging contagious diseases, before valid vaccine and de novo drugs are available. For COVID-19, several known antiviral drugs or compounds previously designed for other RNA viruses have been proposed and tested in the first place, including Ebola virus-targeting drug remdesivir that demonstrated in vitro activity but had unsatisfactory response during following clinical trials (Wang et al., 2020a, 2020b). More rational drug repositioning strategies have been explored recently with the aim to identify potential drugs that can target important viral proteins, given the rapid progresses on SARS-CoV-2 protein structure characterization (Dai et al., 2020; Jin et al., 2020; Wu et al., 2020). However, these approaches usually neglect host effect, and the drugs proposed often exhibit significant in vitro activity but with less success in vivo.
Here, we interrogated a different drug repositioning strategy for COVID-19 and other notorious RNA virus-related diseases from the host-centered perspective. Viruses require key host genes (or factors) for infection and replication, and these host dependency genes (HDGs) serve as potential targets for drug repurposing. By a comprehensive literature collection and data mining, we cataloged HDGs and revealed their molecular features in virus-host interactions for 29 RNA virus species across 10 viral families. We then employed an integrative drug repositioning approach by combining known drug-target interactions (DTIs) from multiple databases with computational predictions for more potential DTIs. We identified candidate host-targeting drugs and natural compounds with broad-spectrum antiviral potentiality for diseases caused by pathogenic coronaviruses, flaviviruses, and influenza viruses.
Results
Strategic overview of host-centered antiviral drug repositioning
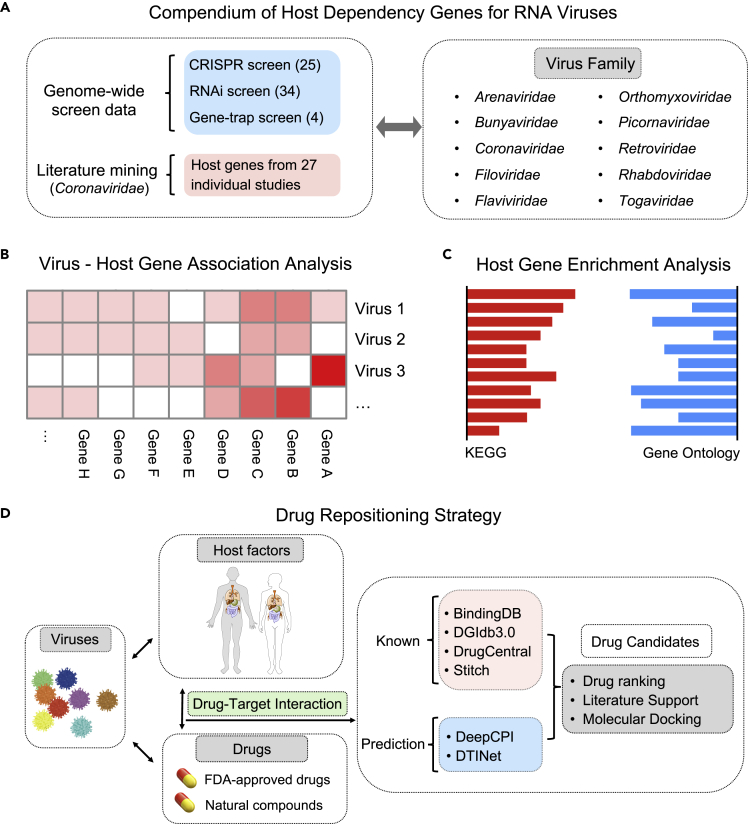
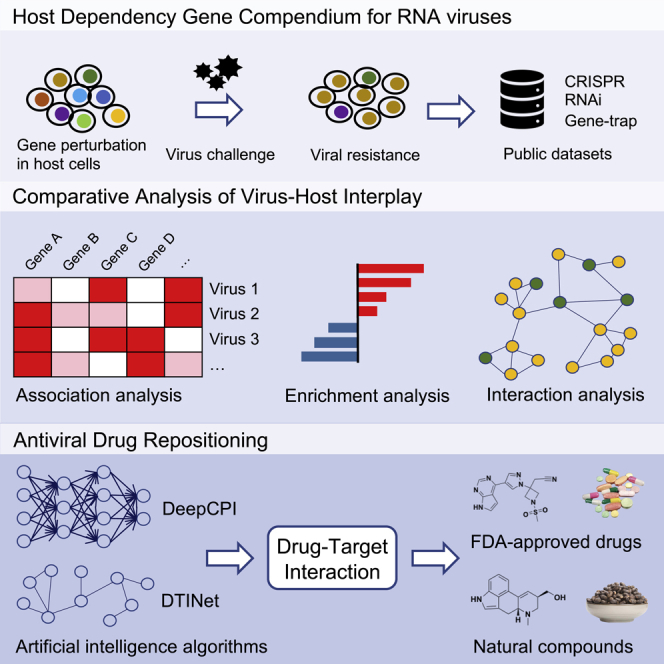
Although many host genes may interplay with viral genes within the host cells, only a few of them are essential for complete infection in a virus-specific manner. Blocking these host essential or dependency genes for viral infection with targeted drugs underlies the principle of host-centered drug repositioning. In the current study, we primarily focused on RNA viruses, especially SARS-CoV-2 and other recently prevalent species (Table S1). The overall workflow of this study is illustrated in Figure 1. Firstly, we sought to systematically catalog the virus-specific HDGs by comprehensively archiving and interrogating published studies that performed functional genetic screens in human cells challenged with RNA viruses (Figure 1A). These works employed multiple genetic perturbation platforms such as gene trap, RNA interference (RNAi), or clustered regularly interspaced palindromic repeats (CRISPR) to identify HDGs whose loss of function renders host resistance to specific viral infection. When genetically perturbed cell pool is challenged by the corresponding virus, the HDG-deficient cells tend to escape from virus-inflicted cell deterioration and positively selected in the final cell pool by which the HDG could be identified. Screening data from 63 independent studies spanning 10 families and 29 species of RNA viruses were collected (Figure 1A; Table S1). With higher priority for Coronaviridae due to the COVID-19 pandemic, we additionally performed in-depth literature mining to include individual HDGs identified from 34 Coronaviridae-focused studies (Table S1). Notably, we primarily considered studies using human-derived cells or tissues as host systems to better reflect the clinically relevant host response and for appropriate drug repurposing. Next, we performed comparative analysis of the host dependency features across multiple viruses to extract consensus HDGs for the following drug repositioning (Figures 1B and 1C). To establish the targeting relationship between drugs and genes, we not only considered the known DTIs from several related databases (e.g., DGIdb3.0 and BindingDB) but also conducted de novo DTI prediction with independent computational methods including DeepCPI and DTINet. Top drug candidates were examined in detail, and ranked lists with two scoring systems for marketed drugs or natural compounds were recommended as potential antiviral solutions (Figure 1D).
Figure 1.
Strategic workflow of this study
(A) Compiling of HDGs for ten families of RNA viruses. Human-specific HDGs were collected from related high-throughput genetic screening studies predominantly using CRISPR, RNAi, and haploid gene-trap techniques. For HDGs in Coronaviridae viruses, literatures specifically working on individual HDGs were also considered.
(B) Comparative analysis of HDGs across different RNA virus families.
(C) Functional enrichment analysis revealed molecular features of HDGs for corresponding virus families.
(D) Drug repositioning strategy in this study. We used high confident HDGs as host factors to be drugged. Two thousand three hundred fifty two FDA-approved drugs and 1,062 nature compounds selected from TCM herbs were interrogated. Potential DTIs were established by both known database information and de novo DTI prediction with AI-based computational methods. The top repurposed drug candidates were discussed in detail.
Cataloging virus-specific host dependency genes
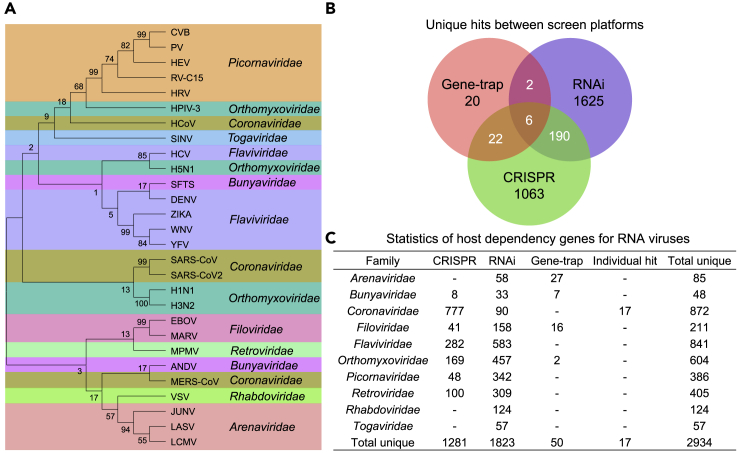
To generate a comprehensive compendium of HDGs for RNA viruses in an efficient manner, we primarily utilized the published studies to date performing functional genetic screens. In addition, to meet the urgent need for fighting SARS-CoV-2 and COVID-19, we also included individual HDGs identified from 34 focused studies for Coronaviridae. We established a human-specific HDG compendium for 29 RNA virus species across 10 families (Table S1). To make the compendium as inclusive as possible, we took a union of HDGs for a given virus species across different studies and screening platforms. Phylogenetic analysis based on the sequence evolution of viral RNA-dependent RNA polymerase (RdRp) gene among these species showed that RNA viruses in the same taxonomic families tend to cluster together (Figures 2A and S1A; Table S2), indicating a potentially coherent mechanism by which different but evolutionarily close viruses employ to live. RNAi represented the mostly adopted genetic perturbation technique, accounting for 54% (34 out of 63) of all these screening studies. Most of the rest studies mainly employed the recently emerging revolutionized genome editing tool CRISPR-Cas for gene loss of function, whereas only 4 studies utilized the traditional gene-trap screening strategy in haploid cells (Table S1). Accordingly, RNAi screens identified the most HDGs, and only a fraction of them were recapitulated in CRISPR screens and gene-trap screens (Figures 2B and 2C and S1B). The low-level concordance across the three types of screens may be partially explained by (1) the unbalanced number of studies using different platforms, (2) intrinsically technical biases between screening platforms or libraries, and (3) batch effect across independent studies.
Figure 2.
Systematically cataloging HDGs for different RNA viruses
(A) The phylogenetic tree for interrogated RNA viruses was constructed with nucleic acid sequence of viral RNA polymerase RdRp gene using neighbor-joining (NJ) method.
(B) The Venn diagram of HDGs retrieved from different screening platforms.
(C) Summary and statistics of HDG compendium for all the viruses under investigation.
To further examine the variations of HDG calling across different studies, we re-analyzed a part of these CRISPR screening data where raw sequencing or count data are available using the MAGeCK-VISPR pipeline we previously developed (Li et al., 2015). Each gene is assigned a “β score” by the pipeline to indicate the function of the gene in screens. The higher the “β score”, the more positive selection for the corresponding gene and the more likely for the gene to be an HDG hit in viral resistance screens. Re-analyzing of CRISPR screen data with a uniform β score criteria does not significantly affect HDG calling compared to the original analysis in corresponding studies, suggesting that computational algorithm bias here is minimal for such positive selection at least for CRISPR screens (Table S3). Different viruses across different studies exhibit variations on HDG profiles based on these re-analyzed CRISPR screen data (Figure S2A). The composite pool of HDGs identified by the re-analyzed CRISPR screens showed extensive protein-protein interactions and are enriched for infection-related pathways (Figures S2B and S2C; Table S3).
Crucial virus-host interplay revealed by functional host factors
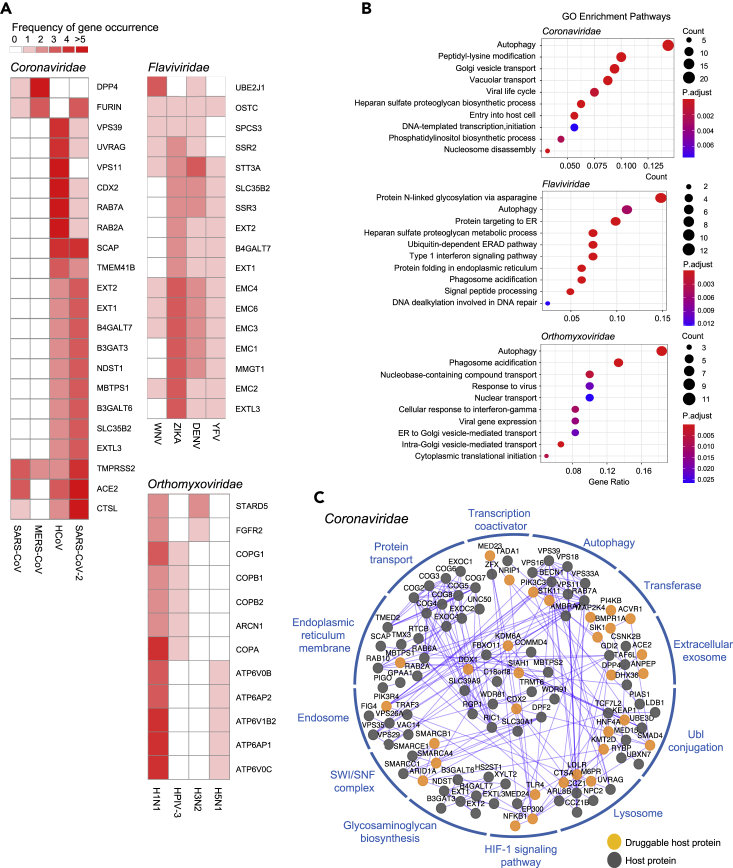
We next sought to look into the biological features of these virus-host interactions. Comparative analysis of HDGs showed that different families of RNA virus exhibit differential profiles of HDGs and some families have fewer HDGs identified because of either fewer data sources or biological difference per se (Figure S3A). To minimize the analytic bias due to data insufficiency and fluctuation, we primarily focused on Coronaviridae (e.g., severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2 viruses), Flaviviridae (e.g., dengue virus, Zika virus, and West Nile virus), and Orthomyxoviridae families (e.g., influenza A viruses H1N1, H3N2, and H5N1 subtypes) that have the most HDGs collected (Figure 2C; Table S1). We filtered all the HDGs by only keeping the one occurring more than once within respective families as high confidence HDGs to minimize the noises. We then depended on this refined list of HDGs for the following analysis as well as for drug repurposing. Comparative analysis indicated some common HDGs within these three viral families, posing a possibility to develop broad-spectrum antivirals when targeting these mutual targets (Figure 3A).
Figure 3.
Characterization of HDGs for corresponding RNA virus families
(A) Subsets of shared HDGs across different RNA viruses within corresponding virus families. HDGs present in at least two different viruses within a given virus family were shown. The frequency of HDG occurrence across studies was denoted in different colors.
(B) Gene ontology enrichment analysis of respective HDGs for corresponding virus families. The size of the dot indicates the number of HDGs in the corresponding pathway. The color of the dot represents the value of Benjamini and Hochberg FDR-adjusted p value.
(C) Protein-protein interaction network of HDGs in Coronaviridae family. Each HDG is presented as a node. The edge between two nodes indicates a protein-protein interaction. The druggable HGDs with targeted drug candidates predicted in this study were highlighted.
Pathway and functional gene category enrichment analysis with gene ontology and Kyoto Encyclopedia of Genes and Genomes tools showed that autophagy and infection-related processes are significantly enriched among HDGs for Coronaviridae viruses (Figures 3B and 3C and S3B; Table S4). On the other hand, Flaviviridae and Orthomyxoviridae viruses share several significantly enriched terms related to intracellular membrane system and its implicated functions (Figures 3B and S3B; Table S4). Network analysis demonstrated extensive protein-protein interactions between these HDGs and associated protein complexes with targetable HDGs highlighted (Figures 3C and S3C). These results indicated that a significant amount of host proteins encoded by HDGs may be physically associated to collectively function in certain complexes, organelles, signaling pathways, or cellular processes that are essential for viral responses.
Mining known drug-target interactions across multiple databases
To identify potential drugs for viral HDGs, we firstly collected known DTIs from multiple public databases such as DGIdb3.0 (covering data from DrugBank, ChEMBL, therapeutic target database TTD, PharmGKB and ClinicalTrials.gov, etc.), BindingDB, DrugCentral, and Stitch (Cotto et al., 2018; Gilson et al., 2016; Kuhn et al., 2010; Ursu et al., 2019). DTIs extracted from databases depend on multiple lines of evidence ranging from approved drug description, in vitro binding assay, text mining and manual inspection, etc. We primarily focused on 2,352 drugs approved by the Food and Drug Administration (FDA) since their safety is validated and could be readily tested and applicable. In addition, we also included a selected list of 1,062 natural compounds that are active ingredients of traditional Chinese medicine (TCM) herbs and pass special criteria for favorable druggability (Methods). With this database retrieval approach and further manual inspection, we investigated known drug-gene pairs and introduced 31~103 FDA-approved drugs targeting HDGs for respective Coronaviridae, Flaviviridae, and Orthomyxoviridae viral families (Tables S5 and S6).
Predicting drug-target interactions
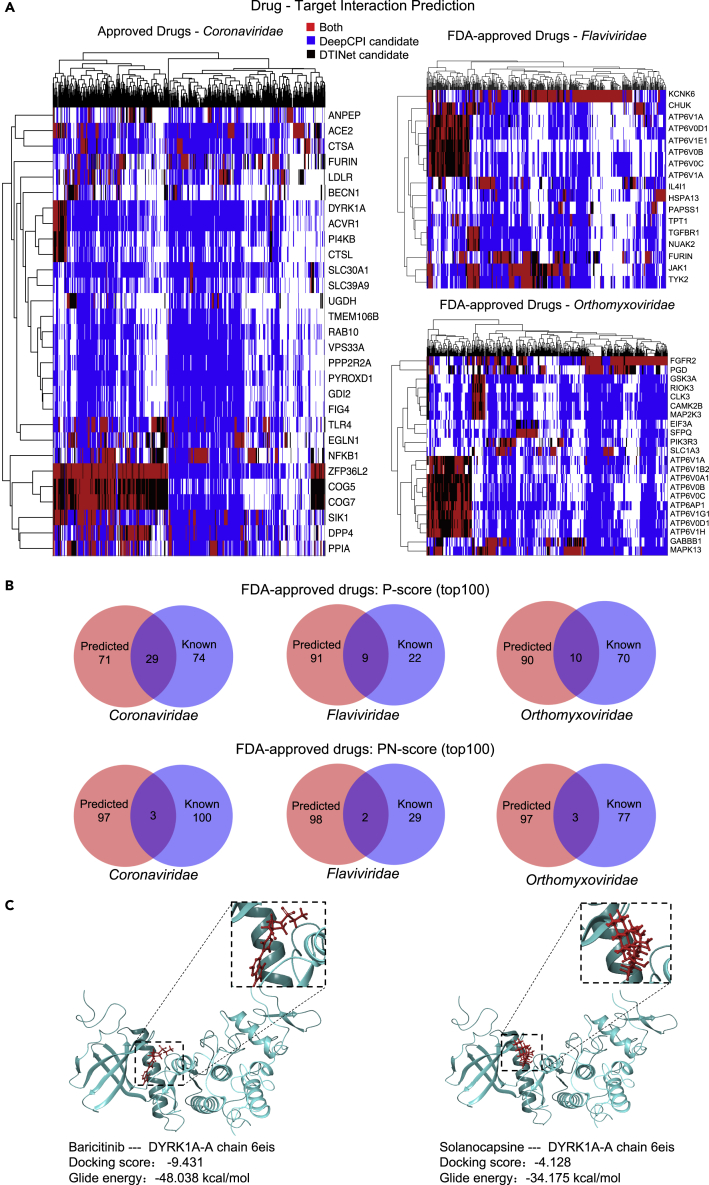
We employed two machine learning methods (DeepCPI and DTINet) to predict more potential drug-gene interactions in silico. DeepCPI, a high-throughput computational framework combining feature embedding and deep learning technique to predict compound-protein interactions (Wan et al., 2019), was adopted to extensively exploit potential DTIs between HDGs and FDA-approved drugs or natural compounds. Another independent method termed DTINet, a network-based machine learning pipeline for DTI prediction on a large scale (Luo et al., 2017), was also utilized. DeepCPI can be applied for the DTI prediction without much prior knowledge of drugs and targets, and it is superior in terms of computational speed and easy installment. In contrast, DTINet requires a heterogeneous network that is constructed using the known information from four domains such as drugs, proteins, diseases, and side effects. Although using different principles and strategies, both methods have been shown to perform well during cross-validation with large-scale DTI data (Luo et al., 2017; Wan et al., 2019) and may complement with each other for better DTI prediction. Either method depends on a calculated score (DeepCPI score or DTINet score) to quantify the confidence of predicted interaction for a given drug-target pair. We took the intersection of the prediction results from both methods with normalized Z score cutoffs for FDA-approved drug repurposing (Figure 4A). On the other hand, we primarily relied on DeepCPI results for natural compound analysis since DTINet does not perform well due to insufficient modeling data for natural compounds. Compared to known DTIs retrieved from databases, more DTIs are predicted for the three viral families with some consensus (Figure 4B; Table S5).
Figure 4.
Drug repositioning using multiple prediction models
(A) The heatmap showing DTI prediction by DeepCPI or DTINet methods. Each row represents a targetable HDG, and each column represents an FDA-approved drug. The top predicted DTI is color coded according to the color legend.
(B) The Venn diagram of FDA-approved drugs repurposed from known and de novo prediction sources of the top 100 hits by two ranking methods (P-score; PN-score) for the three indicated virus families.
(C) Molecular docking analysis showing the potential binding pocket of the repurposed drug baricitinib and natural compound solanocapsine with targeted host factors DYRK1A.
Prioritizing candidate drugs and natural compounds
In addition to known DTI annotation, we prioritized potential drug candidates primarily by the predicted DTIs. Since one drug may target multiple HDG targets that may produce enhanced antiviral response, we first ranked these repurposed drugs mainly according to their targeting number and potency of HDG targets reflected by DTI prediction scores. For FDA-approved drugs, the two DTI prediction scores from both DeepCPI and DTINet algorithms were considered and transformed into a joint P-score to rank the predicted drugs (Methods; Table S5). This ranking system puts emphasis on the HDG targeting effect but overlooks the negative impact from promiscuous non-HDG targets and cytotoxic essential gene targets. Thus, we also provided a second ranking system by incorporating the promiscuousness and cytotoxicity effect with a joint PN-score (Methods; Table S5). As shown in Tables 1, 2, S7, S8, S11, and S12, the two ranking systems shared a great portion of common hits from the top candidate drugs for antiviral purpose against Coronaviridae, Flaviviridae, and Orthomyxoviridae viruses, in agreement with the fact that the promiscuousness and side effect of approved drug are already tested clinically and usually controllable.
Table 1.
Joint P-score ranking: the top ten repurposed FDA-approved drugs against Coronaviridae viruses
| Drug candidate | Approved indication | PubChem CID | Top 10 predicted host targets | Known interaction | Joint P-score |
|---|---|---|---|---|---|
| Fostamatinib | Chronic immune thrombocytopenia | 11671467 | PI4KB DYRK1A ACVR1 CTSL SIK1 COG5 COG7 ZFP36L2 ACE2 | SYK | 1.078 |
| Baricitinib | Rheumatoid arthritis | 44205240 | CTSL PI4KB DYRK1A ACVR1 ACE2 COG5 SIK1 COG7 ZFP36L2 | JAK1 JAK2 JAK3 TYK2 |
0.407 |
| Simvastatin | Hypercholesterolemia | 54454 | ANPEP COG7 DPP4 COG5 ZFP36L2 CTSL PI4KB DYRK1A ACVR1 ACE2 | HMGCR | 0.363 |
| Tofacitinib | Rheumatoid arthritis | 9926791 | DYRK1A ACVR1 CTSL ACE2 SIK1 COG7 COG5 ZFP36L2 DPP4 | JAK3 JAK2 JAK1 | 0.362 |
| Etoricoxib | Rheumatoid arthritis | 123619 | DYRK1A ACVR1 CTSL PI4KB ACE2 COG5 SIK1 COG7 ZFP36L2 | COX2 | 0.340 |
| Bivalirudin | Angina | 16129704 | CTSL PI4KB ACVR1 DYRK1A SIK1 LDLR COG7 COG5 ZFP36L2 | F2 | 0.334 |
| Flurbiprofen | Arthritis | 3394 | CTSL PI4KB ACVR1 DYRK1A ACE2 SIK1 ZFP36L2 | COX1 COX2 | 0.311 |
| Lusutrombopag | Thrombocytopenia | 49843517 | PI4KB CTSL DYRK1A ACVR1 SIK1 COG7 COG5 ZFP36L2 | MPL | 0.309 |
| Bosutinib | Chronic Myelogenous Leukemia | 5328940 | BECN1 COG5 COG7 LDLR FURIN SIK1 PPIA DPP4 ZFP36L2 ACE2 | SRC ABL1 | 0.253 |
| Hydroxychloroquine | Rheumatoid arthritis | 3652 | ACE2 DYRK1A ACVR1 SIK1 ZFP36L2 | N/A | 0.252 |
N/A: not applicable.
Table 2.
Joint PN-score ranking: the top ten repurposed FDA-approved drugs against Coronaviridae viruses
| Drug candidate | Approved indication | PubChem CID | Top 10 predicted host targets | Known interaction | Joint PN-score |
|---|---|---|---|---|---|
| Baricitinib | Rheumatoid arthritis | 44205240 | CTSL PI4KB DYRK1A ACVR1 ACE2 COG5 SIK1 COG7 ZFP36L2 | JAK1 JAK2 JAK3 TYK2 |
0.155 |
| Lusutrombopag | Thrombocytopenia | 49843517 | PI4KB CTSL DYRK1A ACVR1 SIK1 COG7 COG5 ZFP36L2 | MPL | 0.127 |
| Bivalirudin | Thrombocytopenia | 16129704 | CTSL PI4KB ACVR1 DYRK1A SIK1 LDLR COG7 COG5 ZFP36L2 | F2 | 0.126 |
| Etoricoxib | Rheumatoid arthritis | 123619 | DYRK1A ACVR1 CTSL PI4KB ACE2 COG5 SIK1 COG7 ZFP36L2 | PTGS2 | 0.123 |
| Semaglutide | Type 2 diabetes | 56843331 | DPP4 COG5 COG7 ZFP36L2 ANPEP | GLP1R | 0.108 |
| Fostamatinib | Chronic immune thrombocytopenia | 11671467 | PI4KB DYRK1A ACVR1 CTSL SIK1 COG5 COG7 ZFP36L2 ACE2 | SYK | 0.105 |
| Histrelin | Prostate cancer | 25077993 | BECN1 EGLN1 COG5 COG7 ZFP36L2 | GNRH1 | 0.087 |
| Lopromide | X-ray contrast agent | 3736 | GDI2 RAB10 PYROXD1 FIG4 PPP2R2A VPS33A TMEM106B | PGP | 0.081 |
| Hydroxychloroquine | Rheumatoid arthritis | 3652 | ACE2 DYRK1A ACVR1 SIK1 ZFP36L2 | N/A | 0.079 |
| Vildagliptin | Type 2 diabetes | 6918537 | DPP4 COG7 ZFP36L2 | DPP4 | 0.073 |
N/A: not applicable.
Of note, among these top drug candidates for Coronaviridae viruses (Tables 1 and 2), baricitinib, a Janus kinase (JAK) inhibitor approved for rheumatoid arthritis treatment, has been shown to lower the cytokine effect and reduce the viral load in patients with COVID-19 by targeting Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling and numb-associated kinases, respectively (Stebbing et al., 2020). Several clinical trials have been launched globally to evaluate the therapeutic effect of baricitinib (ClinicalTrials.gov Identifier: NCT04358614, NCT04320277, and NCT04321993). Molecular docking analysis of baricitinib and its predicted targets showed high binding affinity between them, further supporting their potential interactions (Figures 4C and S4; Table S15). Another JAK inhibitor tofacitinib among the top ten repurposed Coronaviridae-targeting drugs is also being evaluated for COVID-19 treatment in an active clinical trial (NCT04415151). Moreover, tofacitinib was previously shown to be a potent inhibitor for immunodeficiency virus type 1 (HIV-1) replication in vitro, further supporting its antiviral activity (Gavegnano et al., 2014). Interestingly, hydroxychloroquine also stands out among the top ten candidates, consistent with its in vitro antiviral activity for SARS-CoV-2 albeit less effective in vivo (Maisonnasse et al., 2020; Wang et al., 2020a). Taken together, our analysis provides encouraging repurposing candidates for antiviral application.
Candidate natural compounds were also ranked with two systems by either DeepCPI P-score or DeepCPI PN-score (Methods; Tables 3 and 4; Tables S5, S9, S10, S13, and S14). We also summarized the herbs that include the corresponding compound as part of their active ingredients. Among the top ten predicted natural compounds against Coronaviridae viruses (Tables 3 and 4), some of them, such as lysergol, solanocapsine, picrasidine D, and effusol, have emerged under both ranking methods. Interestingly, sophocarpine has been reported to exhibit antiviral activity against enterovirus 71 (Jin et al., 2017). Moreover, Asari Radix et Rhizoma (Xi Xin) and Codonopsis Radix (Dang Shen), the TCM herbs that contain selected compounds picrasidine D and sophocarpine, respectively, are included in the current TCM formula to treat COVID-19 in China according to Chinese National Health Commission Guidelines for COVID-19 Treatment, eighth edition (http://www.nhc.gov.cn/yzygj/). Representative molecular docking analysis was also performed for compound solanocapsine and its predicted targets, and again, high-affinity interaction modules can be generated between the compound and predicted targets (Figures 4C and S4; Table S15). These results further supported the validity of our repurposing strategy, and it is worthy to evaluate these drug candidates for corresponding antiviral purposes in depth. In addition to HDGs, we also applied our drug prediction pipeline onto 38 SARS-CoV-2 viral proteins with DeepCPI algorithm. A tentative list of repurposed drugs with direct antiviral functions was provided and ready for further experimental validation (Table S5).
Table 3.
DeepCPI P-score ranking: the top ten repurposed natural compounds against Coronaviridae viruses
| Drug candidate | TCMSPa MOL ID | PubChem CID | Herb | Top 10 predicted host targets | DeepCPI P-score |
|---|---|---|---|---|---|
| Lysergol | MOL005261 | 14,987 | Pharbitidis Semen | ANGPT2 BECN1 COG2 COG6 PCBD1 RTCB TMED2 UGDH VPS29 | 4.059 |
| Atropine | MOL002219 | 174174 | Lycii Cortex, Hyoscyami Semen |
COG2 HNF4A KDM6A KEAP1 PGGT1B RABL3 RAD54L2 SIRT6 SMARCB1 UGDH | 3.842 |
| Solanocapsine | MOL007356 | 73,419 | Solanum Nigrum | ANGPT2 BECN1 COG2 COG7 CTSL EXOC1 KDM6A PCBD1 PGGT1B | 3.587 |
| Costaclavine | MOL008145 | 160462 | Ricini Semen | ANGPT2 BECN1 COG6 DOHH PCBD1 RTCB SAR1A TMED2 VPS26A VPS29 | 3.473 |
| Chanoclavine | MOL005260 | 5281381 | Semen Pharbitidis | ANGPT2 ANPEP COG2 PCBD1 RTCB SAR1A TMED2 UGDH VPS26A VPS29 | 3.299 |
| Triptofordin B1 | MOL003232 | 122391803 | Tripterygii Radix | BECN1 EP300 HIRA KEAP1 PCBD1 PGGT1B TADA1 TOM1 VPS11 VPS29 | 3.175 |
| Picrasidine D | MOL012140 | 5316876 | Asari Radix et Rhizoma | HIRA EP300 HNF4A RAD54L2 SCAP SIRT6 SMARCB1 TADA1 TOM1 WDR91 | 3.141 |
| 9alpha-hydroxysophoramine | MOL006570 | 50695119 | Sophorae Flavescentis Radix | CTSL DPF2 KEAP1 PIK3C3 RAD54L2 SCAP SIRT6 SMARCA4 UGDH WDR91 | 2.976 |
| Effusol | MOL007910 | 100801 | Junci Medulla | AKAP6 ANGPT2 COG6 HIRA KDM6A PIAS1 RLF RTCB SMARCB1 | 2.886 |
| Sophocarpine | MOL003627 | 115269 | Codonopsis Radix | DDX1 DPF2 GDI2 PIK3C3 SCAP SMARCA4 SMARCC1 TMEM106B TMPRSS2 UGDH | 2.833 |
TCMSP database: Traditional Chinese Medicine Systems Pharmacology Database.
Table 4.
DeepCPI PN-score ranking: the top ten repurposed natural compounds against Coronaviridae viruses
| Drug candidate | TCMSPa MOL ID | PubChem CID | Herb | Top 10 predicted host targets | DeepCPI PN-score |
|---|---|---|---|---|---|
| Solanocapsine | MOL007356 | 73,419 | Solanum Nigrum | ANGPT2 BECN1 COG2 COG7 CTSL EXOC1 KDM6A PCBD1 PGGT1B | 0.378 |
| Vitexifolin C | MOL011912 | 11033408 | Viticis Fructus | EIF4G2 HIRA KDM6A | 0.374 |
| Dehydroeffusal | MOL007904 | 101191858 | Junci Medulla | DPF2 KDM6A PIAS1 RLF | 0.341 |
| Lysergol | MOL005261 | 14,987 | Pharbitidis Semen | ANGPT2 BECN1 COG2 COG6 PCBD1 RTCB TMED2 UGDH VPS29 | 0.338 |
| Picrasidine D | MOL012140 | 5316876 | Asari Radix et Rhizoma | HIRA HNF4A RAD54L2 SCAP SIRT6 SMARCB1 TADA1 TOM1 WDR91 | 0.334 |
| Isolimonic acid | MOL013443 | 131752314 | Aurantii Fructus Immaturus | ANGPT2 ANPEP BECN1 COG2 COG7 EXOC1 RAB6A SMARCB1 UGDH | 0.329 |
| Methyl 15-hydroxydehydroabietate | MOL012165 | 11573479 | Solidaginis Herba | AKAP6 B4GALT7 | 0.305 |
| Neotigogenin | MOL008519 | 12304433 | Trigonellae Semen | AKAP6 ANGPT2 BECN1 COG2 COG4 EXOC1 PIAS1 VPS11 VPS29 | 0.301 |
| Cyclopamine | MOL009027 | 442972 | Fritiliariae Irrhosae Bulbus | ANGPT2 BECN1 DPF2 EP300 KDM6A LDLR PIAS1 RLF VPS11 | 0.298 |
| Effusol | MOL007910 | 100801 | Junci Medulla | AKAP6 ANGPT2 COG6 HIRA KDM6A PIAS1 RLF RTCB SMARCB1 | 0.291 |
TCMSP database: Traditional Chinese Medicine Systems Pharmacology Database.
Discussion
Given the limited number of de novo antiviral drugs approved during recent years, drug repositioning or repurposing has become a pivotal approach to combat pathogenic viruses and related diseases. In particular, when confronted with an emergent pandemic such as current COVID-19 caused by coronavirus SARS-CoV-2, people highly demand quick and effective solutions for disease control and therapeutic treatment. By systematically compiling the HDGs for RNA viruses and thoroughly digging tentative DTIs, we took host-centered angle to prioritize the potential FDA-approved drugs and natural products as repurposed antiviral candidates against a plethora of RNA viruses, including recently prevailing coronaviruses, Zika virus, dengue viruses, influenza viruses, etc. These recommended drugs or natural compounds are readily tested in the laboratory and clinical settings for their antiviral uses.
Compared to virus-centered antiviral strategy that targets viral genes to directly interfere with virus reproduction and infection, a host-centered antiviral approach has several advantages such as (1) functional host genes are more conserved and evolutionally stable than viral genes, which makes host-targeting drugs more tolerant to frequent viral mutations than those virus-targeting counterparts; 2) different viruses may share a similar set of host genes during certain stages of viral life cycle, which underlines the basis of developing broad-spectrum antivirals so that one host-targeting drug may treat multiple virus infection; and (3) there are significantly more targeted drugs approved for host genes than those for viral genes, thus likely increasing the success rate of drug repurposing by adopting host-centered strategy. Previous studies have extensively tried targeting host genes for developing antiviral solutions (Ackerman et al., 2018; Bosl et al., 2019; Li et al., 2019; Loganathan et al., 2020; Luo et al., 2017; Saiz et al., 2018; Zhou et al., 2020). Host receptors mediating viral entrance into the cells represent the most popular host targets for drugs to block viral infection. A wider range of host genes identified through protein-protein interaction with viral genes serves as the predominant source of host factors to be targeted. In addition, targeting the host transcriptome change resulted from viral infection can be viewed as another host-based drug repositioning strategy. Recent studies also identified SARS-CoV-2-associated human proteins, changed transcriptome, and proteome of human cells in response to SARS-CoV-2 infection to facilitate drug repurposing (Bojkova et al., 2020; Gordon et al., 2020). However, most of these host targets are not essentially required or functionally redundant for complete viral reproduction and infection, even though they are closely associated to the viral components or processes. In principal, effective host drugs should target those functional host genes or related processes on which the virus depends to hinder viral functions within a cell. Therefore, our work particularly focused on those HDGs identified primarily by recent genome-wide screening studies for multiple RNA viruses, which may greatly improve the success rate of drug repositioning compared to previous host-based approaches.
Given a set of host genes, how to evaluate the potential drug effect on specific genes becomes the major challenge for successful drug repurposing. Experimental evaluation of physical interaction strength and kinetics between a drug and a target is an ideal way to establish a definite drug-target relationship. Nevertheless, it tends to be exhausting and impractical when dealing with multiple drugs versus multiple targets. Although drug-related databases have annotated some DTIs from multiple lines of evidence including experimental data, marketed drug description, and literature mining, more systematic and logic approaches to define DTIs especially in a high-throughput manner are still highly demanded. Artificial intelligence such as machine learning and deep learning has been implemented in several computational tools to predict the potential DTIs at a large scale (D'Souza et al., 2020; Rifaioglu et al., 2019; Zhou et al., 2019). In addition to database-retrieved information, here we applied two independent computational pipelines to predict de novo DTIs with quantitative measures. We expect to improve DTI identification with these combinatorial approaches by prioritizing the consensus results. Furthermore, quantitative evaluation of DTI with interaction scores enables a likelihood ranking of potential drug candidates, which may provide better guidance for the following in-depth evaluation.
The repurposed drug candidates recommended by this study not only cover FDA-approved drugs but also include natural compounds especially present in TCM herbs. The active ingredients from the TCM herbs provide a wealth of resource by which new drugs for specific diseases can be discovered, including for antiviral purposes. As our approach is primarily based on targeting HDGs, the viral families that share common druggable host targets may occasionally result in similar repurposed drug or compounds (Tables 1, 2, 3, and 4, S7–S14). Although vaccination is a major strategy to build immune barrier among the population against viral spread, effective drugs are still quite crucial for those individuals already infected by the virus, especially for those detrimental ones investigated here. The fundamental difference of this study with previous drug repositioning work largely lies in target selection, DTI determination, and final repurposed drug candidates.
In summary, our study presents a host-based strategy by focusing on HDGs for a series of RNA viruses to identify potential candidate drugs or natural compounds against related viral diseases, with special emphasis on drug repositioning scheme toward SARS-CoV-2 and COVID-19. This work not only reveals key essential features of viral infection from the host perspective but also provides reasonable and promising antiviral drug candidates for further evaluations in hope of finally controlling these detrimental viral diseases.
Limitations of the study
There are several limitations in the current study. Firstly, we were unable to perform experimental evaluations of these proposed drugs for their antiviral effect at current stage, due to the restricted access to those highly pathogenic viruses. Secondly, the compiling of HDGs may not be complete enough for some viruses to infer the whole host dependency basis and perform appropriate drug repurposing since the currently available data for HDGs are still limited despite the studies collected in this work. Thirdly, we mainly relied on DeepCPI, DTINet, and database-retrieved information followed by manual inspection to assign drug-gene pairing relationship. Further application of more other computational DTI prediction tools may compensate or improve the outcomes of drug selection.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Teng Fei (feiteng@mail.neu.edu.cn).
Material availability
The study did not generate any unique reagents.
Data and code availability
This published article includes all data sets generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31871344, 32071441), the Fundamental Research Funds for the Central Universities (N2020001, N182005005), the 111 Project (B16009), XingLiao Talents Program (XLYC1807212) (to T.F.), the startup fund of the Center for Genetic Medicine Research (to W.L.), and the China Postdoctoral Science Foundation (2020T130012ZX) (to Y.Y.). The computational processing of CRISPR screens is partly supported by COVID-19 HPC Consortium (to W.L.).
Author contributions
Z.L., Y.Y., W.L., and T.F. conceived and designed the research. Z.L., Y.Y., X.C., and Q.C. performed the research. All the authors analyzed the data. Z.L., Y.Y., X.C., Q.C., W.L., and T.F. wrote the manuscript with the input of all the other authors. T.F. and W.L. supervised the study.
Declaration of interests
W.L. reports serving as a consultant of Tavros Therapeutics. Other authors declare no conflict of interest.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102148.
Contributor Information
Wei Li, Email: wli2@childrensnational.org.
Teng Fei, Email: feiteng@mail.neu.edu.cn.
Supplemental information
References
- Ackerman E.E., Kawakami E., Katoh M., Watanabe T., Watanabe S., Tomita Y., Lopes T.J., Matsuoka Y., Kitano H., Shoemaker J.E. Network-Guided discovery of influenza virus replication host factors. mBio. 2018;9:e02002. doi: 10.1128/mBio.02002-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altay O., Mohammadi E., Lam S., Turkez H., Boren J., Nielsen J., Uhlen M., Mardinoglu A. Current status of COVID-19 therapies and drug repositioning applications. iScience. 2020;23:101303. doi: 10.1016/j.isci.2020.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Münch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl K., Ianevski A., Than T.T., Andersen P.I., Kuivanen S., Teppor M., Zusinaite E., Dumpis U., Vitkauskiene A., Cox R.J. Common nodes of virus-host interaction revealed through an integrated network analysis. Front. Immunol. 2019;10:2186. doi: 10.3389/fimmu.2019.02186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto K.C., Wagner A.H., Feng Y.Y., Kiwala S., Coffman A.C., Spies G., Wollam A., Spies N.C., Griffith O.L., Griffith M. DGIdb 3.0: a redesign and expansion of the drug-gene interaction database. Nucleic Acids Res. 2018;46:D1068–D1073. doi: 10.1093/nar/gkx1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S., Prema K.V., Balaji S. Machine learning models for drug-target interactions: current knowledge and future directions. Drug Discov. Today. 2020;25:748–756. doi: 10.1016/j.drudis.2020.03.003. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe S.N., Ekwudu O., Dua K., Chellappan D.K., Katavic P.L., Collet T.A. Recent update on anti-dengue drug discovery. Eur. J. Med. Chem. 2019;176:431–455. doi: 10.1016/j.ejmech.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Gavegnano C., Detorio M., Montero C., Bosque A., Planelles V., Schinazi R.F. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob. Agents Chemother. 2014;58:1977–1986. doi: 10.1128/AAC.02496-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson M.K., Liu T., Baitaluk M., Nicola G., Hwang L., Chong J. BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016;44:D1045–D1053. doi: 10.1093/nar/gkv1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Yang L., Ding G., Yang G., Han Y., Zhang X., Li W. Sophocarpine against enterovirus 71 in vitro. Exp. Ther. Med. 2017;14:3792–3797. doi: 10.3892/etm.2017.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Zhao Y., Sun Y., Zhang B., Wang H., Wu Y., Zhu Y., Zhu C., Hu T., Du X. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat. Struct. Mol. Biol. 2020;27:529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Szklarczyk D., Franceschini A., Campillos M., von Mering C., Jensen L.J., Beyer A., Bork P. Stitch 2: an interaction network database for small molecules and proteins. Nucleic Acids Res. 2010;38:D552–D556. doi: 10.1093/nar/gkp937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.C., Wang X.J., Wang H.R. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov. Today. 2019;24:726–736. doi: 10.1016/j.drudis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Koster J., Xu H., Chen C.H., Xiao T., Liu J.S., Brown M., Liu X.S. Quality control, modeling, and visualization of CRISPR screens with MAGeCK-VISPR. Genome Biol. 2015;16:281. doi: 10.1186/s13059-015-0843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan T., Ramachandran S., Shankaran P., Nagarajan D., Mohan S.S. Host transcriptome-guided drug repurposing for COVID-19 treatment: a meta-analysis based approach. PeerJ. 2020;8:e9357. doi: 10.7717/peerj.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Zhao X., Zhou J., Yang J., Zhang Y., Kuang W., Peng J., Chen L., Zeng J. A network integration approach for drug-target interaction prediction and computational drug repositioning from heterogeneous information. Nat. Commun. 2017;8:573. doi: 10.1038/s41467-017-00680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., Naninck T., Pizzorno A., Lemaitre J., Gonçalves A. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- Mottin M., Borba J., Braga R.C., Torres P.H.M., Martini M.C., Proenca-Modena J.L., Judice C.C., Costa F.T.M., Ekins S., Perryman A.L. The A-Z of Zika drug discovery. Drug Discov. Today. 2018;23:1833–1847. doi: 10.1016/j.drudis.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S., Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson T.C., Diamond M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020;5:796–812. doi: 10.1038/s41564-020-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifaioglu A.S., Atas H., Martin M.J., Cetin-Atalay R., Atalay V., Dogan T. Recent applications of deep learning and machine intelligence on in silico drug discovery: methods, tools and databases. Brief Bioinform. 2019;20:1878–1912. doi: 10.1093/bib/bby061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz J.C., Oya N.J., Blazquez A.B., Escribano-Romero E., Martin-Acebes M.A. Host-directed antivirals: a realistic alternative to fight Zika virus. Viruses. 2018;10:453. doi: 10.3390/v10090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J., Monteil V., Lauschke V.M., Mirazimi A., Youhanna S. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020:e12697. doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu O., Holmes J., Bologa C.G., Yang J.J., Mathias S.L., Stathias V., Nguyen D.T., Schurer S., Oprea T. DrugCentral 2018: an update. Nucleic Acids Res. 2019;47:D963–D970. doi: 10.1093/nar/gky963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F., Zhu Y., Hu H., Dai A., Cai X., Chen L., Gong H., Xia T., Yang D., Wang M.W. DeepCPI: a deep learning-based framework for large-scale in silico drug screening. Genomics Proteomics Bioinformatics. 2019;17:478–495. doi: 10.1016/j.gpb.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Hu Y., Musharrafieh R., Yin H., Wang J. Focusing on the influenza virus polymerase complex: recent progress in drug discovery and assay development. Curr. Med. Chem. 2019;26:2243–2263. doi: 10.2174/0929867325666180706112940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Li Z., Yang J., Tian G., Liu F., Wen H., Peng L., Chen M., Xiang J., Peng L. Revealing drug-target interactions with computational models and algorithms. Molecules. 2019;24:1714. doi: 10.3390/molecules24091714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This published article includes all data sets generated or analyzed during this study.