Abstract
Background
The older population has been especially affected by the severe acute respiratory syndrome coronavirus 2 pandemic (COVID-19).
Objective
The aim of the study was to explore the incidence, severity, mortality rate, clinical features, and risk factors of symptoms of COVID-19 in home-dwelling older people, and its association with type of residence, cognitive deterioration, and neurodegenerative diseases.
Methods
Data about symptoms of COVID-19 were collected through a telephone survey in the cohort of 913 older volunteers of the Vallecas Project, aged 75–90 years, most of them (902) home-dwelling, in Madrid, Spain. The association of demographic and anthropometric measures, genetic polymorphisms, comorbidities, life habits, type of residence, and frailty surrogates were explored as potential risk factors for the incidence, severity, and mortality of COVID-19 in the older population.
Findings
Sixty-two cases reported symptoms compatible with COVID-19; 6 of them had died, 4 in their home and 2 in the nursing home. Moderate/severe cases were significantly older and more frequently males. The APOE ε4 allele was associated with the presence of symptoms of COVID-19. Higher systolic blood pressure, more intense smoking habit, more alcohol intake, lower consumption of coffee and tea, and cognitive impairment were associated with disease severity.
Conclusions
The estimated incidence of symptomatic COVID-19 in this older cohort of Madrid was 6.8%, with an overall mortality rate of 0.7% (18.2% in those living in a nursing home) and a fatality rate of 9.9%. Our exploratory study indicates that life habits, other clinical conditions and, the ε4 variant of the APOE gene are associated with the presence and clinical severity of coronavirus infection.
Keywords: COVID-19, Incidence, Mortality, Clinical features, Risk factors, Nursing home, Older people, APOE gene, Cognitive impairment, Madrid, Vallecas Project
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (COVID-19) has produced millions of infected symptomatic patients and hundreds of thousands of deaths around the world [1]. Most cases have been reported in the USA and several European countries. A large number of patients with COVID-19 have been reported in Spain, mainly in the conurbations of Madrid and Barcelona. By April 27, 2020, when the data of this study were collected, 209,465 cases of COVID-19 and 15,175 deaths had been registered [2]. Everywhere the older population has been the most severely affected [3], with a higher mortality rate [4].
The CIEN Foundation is working for almost a decade on the longitudinal assessment of a cohort of older people to identify early biomarkers and clinical profiles of cognitive impairment [5]. This cohort of home-dwelling volunteers, the Vallecas Project (VP), is representative of the older population of the city of Madrid and the participants have provided during several years of follow-up a substantial amount of demographic, clinical, physical, neurological, and biological data. We have now performed a phone survey in this cohort to estimate the incidence, severity, mortality, clinical features, and risk factors of symptomatic COVID-19 in the older population, as well as its relationships with cognitive state.
Methods
Settings and Subjects
The VP is a single-center, multidisciplinary, observational, longitudinal study of a cohort of 1,213 volunteers, aged 69–86 years and home-dwelling at baseline, recruited between 2011 and 2013 in Madrid, Spain [5]. Volunteers had no previous relevant psychiatric, neurologic, or systemic disorders, and after signing informed consent, they undertook a yearly systematic assessment including sociodemographic data, medical history, lifestyle habits, neurological and neuropsychological exams, blood collection, and brain MRI scans. During the follow-up of the VP, 200 volunteers have been lost because of death (75) or dropout (125), and regular contact by phone or by periodic assessments is maintained with 1,013 participants who have been approached for this survey. The attrition rate (16.5%) is associated with age (active 81.9 ± 3.9 vs. lost 83.6 ± 4.5 years), gender (active females 65.1% vs. lost 55.3%), and education (less than primary school: active 19.8% vs. loss 25.0%). The VP was approved by the Ethics Committee of the Carlos III Institute of Health.
Survey
All the available participants were contacted by phone call between April 22 and April 28, 2020, 15 days after the peak period of COVID-19 incidence in Spain, and asked about their potential symptoms of COVID-19. The staff of the CIEN Foundation performed this survey after a prior training on a standard simple questionnaire. At least 3 phone calls were performed before considering a case as nonresponder. The answers were obtained from the volunteers themselves or a proxy informant and were immediately collected using an electronic form.
Variables
The following data were ascertained in the survey to explore the incidence, clinical features, and severity of COVID-19: current residence (living at home or in a nursing home), presence of a clinical syndrome consistent with SARS-CoV-2 infection within the last 3 months, general and neurologic symptoms of COVID-19, hospitalization, oxygen therapy, mechanical ventilation, admission to intensive care unit, disease duration, and current clinical status. Cases with symptoms of COVID-19 were classified according to their clinical severity as mild (patients were cared for at home) or moderate/severe (patients admitted to hospital or died). Since our search was mainly focused on the constitutional features associated with the infection, the following baseline data, recorded in every case several years before the disease outbreak [5], were selected from the datasets of the VP to explore potential risk factors for the incidence and severity of COVID-19:
demographics: age, sex, educational attainment (less than primary school, primary school, high school, and more than high school), and estimated yearly income (<20,000, 20,000–50,000, >50,000 EUR/year);
anthropometric measures: abdominal circumference, weight, height, and BMI;
genetic polymorphisms: APOE (rs429358 and rs7412) genotype;
comorbidities: hypertension, diabetes, hypercholesterolemia, hypertriglyceridemia, ischemic heart disease, atrial fibrillation, cerebrovascular disease, lung diseases, obstructive sleep apnea, and major depression;
life habits: history, age at onset and duration of smoking and alcohol intake, coffee and tea consumption, number of drugs, and intake of any statin, angiotensin-converting enzyme inhibitor, and anticoagulant or antiplatelet agent;
frailty surrogates: up and go test [6], gait disturbances, present cognitive state (normal, mild cognitive impairment, and dementia), and Functional Activities Questionnaire (FAQ) [7].
Statistical Analysis
The incidence, mortality, and clinical features of COVID-19 in this cohort were described as either frequencies or means and standard deviations for categorical and quantitative variables, respectively. Logistic and Poisson regression models were used when appropriate, depending on the nature of the dependent variable, to (a) compare the demographics, anthropometric measures, comorbidities, life habits, frailty surrogates, and APOE genotype between individuals with and without symptoms of COVID-19 to identify those variables associated with the disease; (b) compare the same data between the subsample of symptomatic volunteers with a clinical ascertainment of the diagnosis of COVID-19 and those without symptoms trying to reduce the noise of other eventual conditions; and (c) compare the individuals with mild and those with moderate/severe symptoms to identify variables associated with disease severity. Since the incidence and severity of symptoms of COVID-19 showed a trend to be associated with older age and male sex, both variables were included in regression analyses as covariates. To assess the impact of the model upon data, we reported odds ratios (OR) and their corresponding 95% confidence intervals (CI).
Since this is an exploratory study, correction for multiple comparisons was not performed. R (version 3.4.2) was used for statistical analyses; bilateral p values were reported.
Results
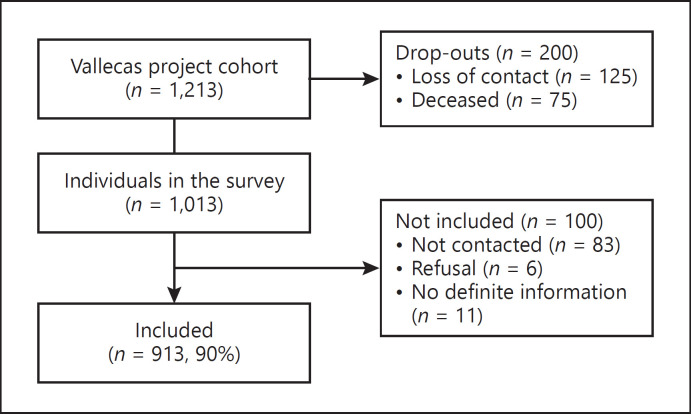
The survey was responded by 710 volunteers (70%) and 214 informants (21%), and valid responses were obtained from 913 volunteers (response rate 90%); 84 participants did not respond to 3 phone calls, 6 rejected the survey, and 11 were unable to give definite information about symptoms of COVID 19. The response rate was not related to the age, sex, or socioeconomic level (Fig. 1).
Fig. 1.
Individuals in the survey.
There were 590 women (64.6%) and 323 men (35.4%); their mean (SD) age was 81.9 (3.8) (range 75–94) years; and their education was as follows: 189, less than primary school (19.7%); 283, primary school (31%); 217, high school (23.8%); 232, more than high school (25.4%); and 1, unknown. Eleven individuals are currently living in nursing homes.
Sixty-two volunteers (incidence 6.8%), 38 females (incidence 6.4%) and 24 males (incidence 7.4%), reported symptoms compatible with COVID-19. Definite information about diagnostic ascertainment by reverse transcription PCR in the acute stage or serologic tests in late stages was obtained in 24 cases and in the symptomatic spouses of other 3 (43.6%); doubtful or negative serologic tests were reported by 4 cases, and no analytical data were available for the other cases. Of those 62 symptomatic cases, 38 (61.3%) had been ascertained by medical staff through clinical exams (i.e., tests for COVID-19, radiological exams, and other analyses), 18 were admitted to hospital (29%), and 24 (38.7%) were monitored by telephone at home by health-care staff. Oxygen therapy was administered to 17 (27.4%) during 17.6 (SD 3.3) days, but only 1 was admitted to the intensive care unit (1.6%) and none received mechanical ventilation. The reported symptoms had a mean duration of 15.6 (13.1) days and were still active in 11 cases. Six patients aged 81–90 years (3 men and 3 women) had died (fatality rate 9.9% and mortality rate 0.7%; for males, 12.5 and 0.9%; for females, 8.1 and 0.5%, respectively); 2 of them were living in nursing homes (mortality rate at nursing home 18.2%, in home-dwelling individuals 0.4%, χ2; p = 0.043, OR, 13.5; 95% CI, 1.49–122.8]).
Forty-two cases with symptoms of COVID-19 were classified as mild (67.7%) and 20 cases as moderate/severe (32.3%). Their demographic characteristics and reported symptoms are presented in Table 1. They had 5 (SD 3) symptoms of COVID-19 (range, 1–13). Moderate/severe cases were significantly older, more frequently males, and significantly more of them showed dyspnea, diarrhea, and confusion and required oxygen therapy.
Table 1.
Clinical features of symptomatic COVID-19 patients
| Mild (n = 42) mean (SD) [range] | Moderate/severe (n = 20) mean (SD) [range] | OR [95% CI] | p value* | |
|---|---|---|---|---|
| Age, years | 80.5 (3.2) [75–88] | 82.9 (4.2) [76–90] | 11.00 [1.70–71.10] | 0.014 |
| Duration of symptoms, days | 14.3 (12.6) [3–70] | 18.2 (14.2) [4–50] | 1.27 [1.10–1.46] | <0.001 |
| Number of symptoms | 4.7 (3.2) [1–12] | 5.5 (3.5) [1–13] | 1.10 [0.86–1.39] | 0.442 |
| N (%) | N (%) | OR [95% CI] | p value** | |
|---|---|---|---|---|
| Gender (males) | 12 (28.6) | 12 (60) | 3.75 [1.22–11.46] | 0.020 |
| Residence | ||||
| Home-dwelling | 40 (69) | 18 (31) | 2.22 [0.29–17.45] | 0.588 |
| Nursing home | 2 (50) | 2 (50) | ||
| General symptoms | ||||
| Fever | 29 (69) | 16 (80) | 1.79 [0.50–6.42] | 0.370 |
| Tiredness | 23 (54.8) | 13 (65) | 1.53 [0.51–4.62] | 0.446 |
| Cough | 22 (52.4) | 13 (65) | 1.69 [0.56–5.07] | 0.351 |
| Throat soreness | 15 (35.7) | 8 (40) | 1.20 [0.40–3.59] | 0.744 |
| Headache | 18 (42.9) | 4 (20) | 0.33 [0.09–1.17] | 0.086 |
| Dyspnea | 11 (26.2) | 14 (70) | 6.58 [2.03–21.36] | 0.002 |
| Myalgia | 17 (40.5) | 5 (25) | 0.49 [0.15–1.60] | 0.238 |
| Appetite loss | 17 (40.5) | 9 (45) | 1.20 [0.41–3.53] | 0.736 |
| Nausea | 10 (23.8) | 5 (25) | 1.07 [0.31–3.67] | 0.919 |
| Diarrhea | 11 (26.2) | 10 (50) | 2.82 [0.93–8.59] | 0.068 |
| Skin lesions | 3 (7.1) | 1 (5) | 0.68 [0.07–7.02] | 0.749 |
| Neurologic symptoms | ||||
| Loss of taste or smell | 19 (45.2) | 4 (20) | 0.30 [0.09–1.06] | 0.062 |
| Weakness | 1 (2.4) | 1 (5) | 2.16 [0.13–36.37] | 0.594 |
| Confusion | 0 (0) | 3 (15) | – | 0.995 |
| Memory loss | 2 (4.8) | 3 (15) | 3.53 [0.54–23.06] | 0.188 |
| Oxygen therapy | 2 (4.9) | 15 (75) | 60.0 [10.49–343.14] | <0.0001 |
OR, odds ratio; CI, confidence interval.
Linear regression model with Poisson distribution (mild as reference category).
Logistic regression (mild as reference category).
The variables associated with the presence of symptoms of COVID-19 are shown in Table 2. Symptomatic cases were more frequent at nursing homes (OR, 8.31; 95% CI, 2.37–29.23; p = 0.004), carried more frequently the APOE ε4 allele (OR, 2.40; 95% CI, 1.37–4.21, p = 0.002) and had also a tendency to have more obstructive sleep apnea. No differences were found in other anthropometric and frailty measures, comorbidities, or cognitive state. The same analyses were performed with the subsample of 38 patients who were diagnosed with COVID-19 by medical staff and similar results were found for APOE ε4 (OR, 2.417; 95% CI, 1.186–4.925; p = 0.012); in addition, the blood pressure recorded in positive cases years ago (147.2 SD [22.1] mm Hg) was higher than in controls (143.5 SD [20.8] mm Hg; OR, 1.07, 95% CI, 1.02–1.13; p = 0.01).
Table 2.
Variables associated with symptoms of COVID-19
| Variable | No symptoms (n = 851) mean (SD) | Symptoms of COVID-19 (n = 62) mean (SD) | OR [95%CI] | p value* |
|---|---|---|---|---|
| Age | 81.9 (3.84) | 81.2 (3.7) | 0.51 [0.19–1.36] | 0.178 |
| N (%) | N (%) | p value** | ||
|---|---|---|---|---|
| Sex (male) | 299 (35.1) | 24 (38.7) | 1.17 [0.69–1.98] | 0.570 |
| Education | ||||
| <Primary school | 165 (19.5) | 13 (21) | 0.98 [0.79–1.20] | 0.828 |
| Primary school | 259 (0.7) | 21 (33.9) | ||
| High school | 206 (24.4) | 11 (17.7) | ||
| >High school | 215 (25.4) | 17 (27.4) | ||
| Residence | ||||
| Home-dwelling | 844 (93.6) | 7 (63.6) | 8.31 [2.37–29.23] | 0.004 |
| Nursing home | 59 (6.4) | 4 (36.4) | ||
| Hypertension (yes) | 441 (52.1) | 31 (50) | 0.92 [0.55–1.54] | 0.753 |
| Hypercholesterolemia (yes) | 417 (49.5) | 26 (41.9) | 0.86 [0.61–1.22] | 0.537 |
| Diabetes (yes) | 105 (12.6) | 10 (16.1) | 1.34 [0.66–2.72] | 0.419 |
| Obstructive sleep apnea (yes) | 77 (9.1) | 10 (16.1) | 1.96 [0.96–4.02] | 0.065 |
| Smoking (yes) | 308 (36.4) | 27 (43.5) | 1.35 [0.80–2.27] | 0.259 |
| Alcohol (yes) | 394 (46.5) | 30 (48.4) | 1.08 [0.64–1.81] | 0.776 |
| APOE ε4 allele (yes) | 140 (16.6) | 20 (32.3) | 2.40 [1.37–4.21] | 0.002 |
| APOE4 alleles | ||||
| None | 705 (83.4) | 42 (67.7) | 1.85 [1.13–2.88] | 0.010 |
| Heterozygous | 133 (15.7) | 20 (32.3) | ||
| Homozygous | 7 (0.8) | 0 |
OR, odds ratio; CI, confidence interval.
Linear regression model with Poisson distribution (no symptoms as reference category).
Logistic regression (no symptoms as reference category).
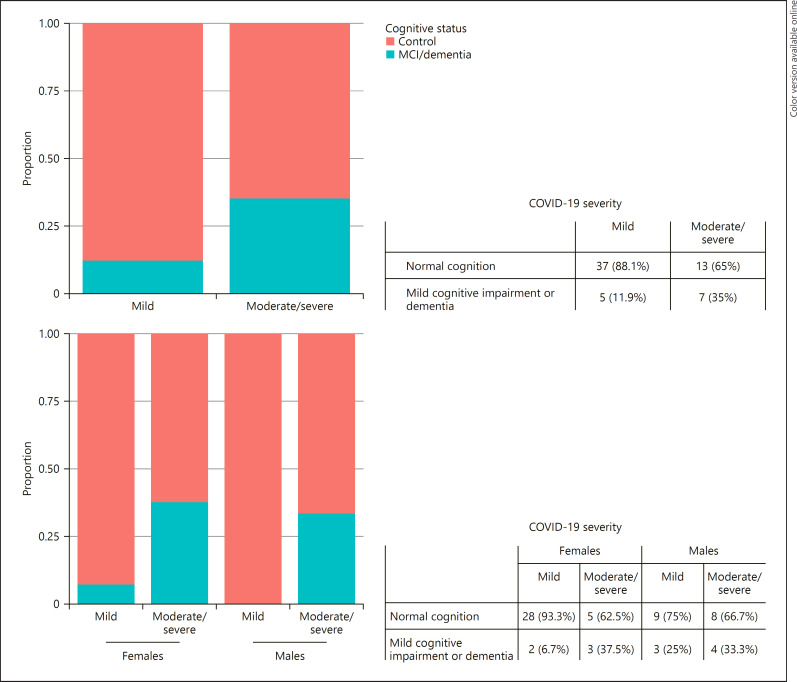
The disease severity was significantly associated with higher systolic blood pressure, more intense smoking habit, more alcohol intake, and lower consumption of coffee and tea (Table 3). Living in a nurse home and vascular risk factors (especially hypercholesterolemia, diabetes, and smoking) were more frequent but not significantly in cases with moderate/severe disease. Finally, patients with mild cognitive impairment or dementia were more prone to suffer moderate/severe symptoms of COVID-19 (Fisher's exact test = 0.043, OR, 3.88; 95% CI, 1.04–14.73). Further analysis by sex revealed that this finding was specifically associated with female sex (Fisher's exact test = 0.053, OR, 7.74; 95% CI, 0.97–76.81) (Fig. 2). No differences were found in other anthropometric and frailty measures or drug intake.
Table 3.
Variables related to the severity of symptoms
| Variable | Mild (n = 42) N (%) | Moderate/severe (n = 20) N (%) | OR [95% CI] | p value* |
|---|---|---|---|---|
| Education | ||||
| <Primary school | 11 (26.2) | 2 (10) | 0.84 [0.50–1.36] | 0.486 |
| Primary school | 9 (21.4) | 12 (60) | ||
| High school | 7 (16.7) | 4 (20) | ||
| >High school | 15 (35.7) | 2 (10) | ||
| Residence | ||||
| Home-dwelling | 40 (69) | 18 (31) | 2.22 [0.29–17.45] | 0.588 |
| Nursing home | 2 (50) | 2 (50) | ||
| Hypertension (yes) | 19 (45.2) | 12 (60) | 1.72 [0.52–5.65] | 0.371 |
| Hypercholesterolemia (yes) | 15 (38.1) | 10 (50) | 2.29 [0.65–7.91] | 0.224 |
| Diabetes (yes) | 5 (11.9) | 5 (25) | 3.31 [0.67–16.33] | 0.141 |
| Obstructive sleep apnea (yes) | 6 (14.3) | 4 (20) | 1.21 [0.23–6.41] | 0.826 |
| Smoking (yes) | 14 (33.3) | 13 (65) | 2.97 [0.79–11.17] | 0.106 |
| Alcohol (yes) | 18 (42.9) | 12 (60) | 1.52 [0.37–6.15] | 0.561 |
| APOE4 allele (yes) | 14 (33.3) | 6 (31.6) | 0.73 [0.20–2.67] | 0.632 |
| Mean (SD) [N] | Mean (SD) [N] | OR [CI 95%] | p value** | |
|---|---|---|---|---|
| Systolic BP, mm Hg | 142.8 (21.9) [42] | 148.00 (20.38) [20] | 1.07 [1.02–1.13] | 0.005 |
| Diastolic BP, mm Hg | 77.2 (11) [42] | 79.45 (13.05) [20] | 1.04 [0.99–1.10] | 0.091 |
| Smoking severity*** | 6,665 (6,237) [14] | 11,070 (13,341) [13] | 0.95 [0.91–0.99] | 0.015 |
| Alcohol intake, g/week | 75.5 (53.8) [22] | 93 (56.8) [11] | 1.08 [0.99–1.18] | 0.082 |
| Coffee intake, cups/week | 4.2 (4.7) [36] | 2 (2.9) [17] | 0.37 [0.24–0.55] | <0.0001 |
| Tea intake, cups/week | 1.8 (3.1) [36] | 0.6 (1.4) [17] | 0.44 [0.20–0.86] | 0.024 |
OR, odds ratio; CI, confidence interval; BP, blood pressure.
Logistic regression (mild as reference category); covariates: age and sex.
Linear regression with Poisson distribution (mild as reference category); covariates: age and sex.
Estimated total number of consumed packs of cigarettes as packs/day × years of active smoking × 365 days.
Fig. 2.
Cases with mild and moderate/severe symptoms of COVID-19 by cognitive status and sex.
Discussion
Although older people are more severely affected by the pandemic infection with SARS-CoV-2 [1], there are few studies describing the clinical features of COVID-19 in this subpopulation [3, 8] or the risk factors for its incidence and severity [9]. We have explored the incidence, severity, mortality, and risk factors by means of a phone survey in a cohort of older volunteers aged 75–94 years, whose demographic, clinical, and genetic features were available.
The incidence of symptoms diagnosed as, or suggestive of, COVID-19 in this Spanish cohort during the outbreak of the pandemic was 6.7%, close to the figures of incidence [10] or seroprevalence [11, 12, 13] reported by other international and Spanish epidemiological surveys. Interestingly, the incidence in this cohort of older people is similar to that of the overall population.
The case fatality rate in our older cohort (9.9%) is higher than that reported in Korea (3.1–3.7%) [4], but much lower than the figures reported for the whole population older than 70 years in Spain (13.5–20.5%) [2], which are calculated only on the cases verified by PCR test, and also include cases from the nursing homes, where both severity and mortality have been especially high. The higher risk, severity, and mortality of COVID-19 at nursing homes [14] is also confirmed in our survey, where mortality rate (18.2%) is extremely much higher than that in home-dwelling individuals (0.7%). The incidence, mortality, and case fatality rates are slightly higher for males, as reported for Spanish [2] and other populations [4, 15].
Although the epidemiological parameters observed in our cohort are rather consistent with those of other sources, they may be affected by the fact that the diagnosis was ascertained by medical staff only in 61.3% of positive cases. Notwithstanding the overall figures of incidence are close to those recently reported by the National Health Authority on the seroprevalence [16] and the diagnostic error can be expected mainly in mild cases.
The frequency of general [1, 17] and neurologic symptoms [18] is similar in our patients to those described in many previous reports. Cases classified as moderate/severe were more frequently males and older, as described in other series [2, 4, 9, 15, 19], and we controlled for these variables in the search for other associations.
A relevant finding is the association of APOE ε4 allele with the presence of symptoms of COVID-19 both in the sample of symptomatic patients and in those with medical assessment and diagnosis. These data agree with recent findings on a large study in the UK Biobank Community Cohort, where the APOE ε4 allele increases the risk of being hospitalized with COVID-19 [20].
This association suggests that APOE may be involved in the pathophysiology of the disease. The 3 major polymorphic alleles (ε2, ε3, and ε4) of this human gene encode 3 ApoE protein isoforms (E2, E3, and E4) [21] with markedly different effects on lipid metabolism [22]. Additionally, the APOE ε4 allele is the main genetic risk factor for sporadic Alzheimer's disease [23, 24], is associated with vascular disease [25], and is also related to the innate immune system [26] and to some infectious diseases [27, 28, 29, 30]. The role of APOE in viral infections seems to be due to its binding to heparan sulfate proteoglycans (HSPG), functioning either as a Trojan horse (HCV) or competing for the binding to the HSPG with the viral particles (HSV and HIV). APOE is expressed in lung cells modulating normal lung health and the pathogenesis of respiratory diseases, including asthma, acute lung injury, cancer, emphysema, pulmonary fibrosis, and pulmonary hypertension [31, 32]. Interestingly, although the SARS-CoV-2 enters into the cell by the binding of the viral spike protein to the ACE2 cellular receptor, the initial attachment seems to be facilitated by the binding of this protein to the cell surface HSPGs [33]. Thus, according to our results and other similar findings [20], we postulate that ApoE4 may increase the risk of adverse effects of SARS-CoV-2 infection through impaired competition with the viral particles for the binding to HSPG, rendering the epithelial pulmonary tissue more susceptible to the viral propagation.
The disease severity was associated with other comorbidities, such as high blood pressure, and toxic life habits, such as heavy smoking or alcohol intake, as previously reported [34, 35, 36]. Interestingly, other habits such as coffee and tea consumption were negatively associated. Whereas smoking and alcohol are deleterious agents for cardiovascular system and lung integrity that may facilitate or aggravate pulmonary infectious diseases, the explanation for the benefit of coffee or tea consumption is unclear. Coffee consumption is associated with some positive effects on the respiratory system [37], but perhaps in this cohort is also a surrogate of social habits with impact on the risk of viral transmission. Mild cognitive impairment and dementia, mainly due to neurodegenerative disease, were also found to be associated with the severity of COVID-19 in accordance with previous reports [15].
Our study has limitations. The most important is that the information has been gathered by phone call from the participants or informants without direct access to medical records; therefore, clinical data may be incomplete or include some errors, mainly in the mild cases who did not seek medical diagnosis and care. The diagnosis of SARS-CoV-2 infection was confirmed with biological tests in one half of cases, because testing was restricted during this period to hospital in-patients and because several informants were unaware of some of the procedures performed during their clinical workup. On the other hand, a few cases reported negative results in serologic tests; since the false-negative rate of these tests is sizable, clinical and epidemiologic criteria have been maintained. A second limitation is that data about chronic comorbidities considered in our analyses were recorded in the past and may have increased in the last years in some individuals; we have probably underestimated the association of present comorbidities and functional variables. In addition, the number of positive cases detected in our survey is small, and the statistical power of the study is low. For these limitations and because the exposure to the SARS-CoV-2, the most important determinant of infection, cannot be estimated and is probably heterogeneous, we emphasize the exploratory nature of our findings, especially for mild cases, that must be confirmed in other cohorts.
Notwithstanding, we can provide an estimate of the incidence, clinical features, and mortality rate of symptomatic COVID-19 in the older population in the region of Madrid. We also provide some evidence indicating that genetic polymorphisms such as APOE ε4 may facilitate the infection with this virus and its clinical severity.
Statement of Ethics
The Vallecas Project was approved by the Ethics Committee of the Carlos III Institute of Health (CEI PEI 46_2011-v2014). All the participants signed a written informed consent before their inclusion in the Vallecas Project, and all the data were analyzed anonymously, and clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
Conflict of Interest Statement
A.R. and M.C. are cofounders of Biocross SL and own stock options from the company, which is developing blood tests for the diagnosis of Alzheimer's disease, including the e4Risk® test, which is a method to detect the presence of the protein ApoE4 in human blood plasma, using high-throughput chemistry analyzers.
O.C. and M.C. participate in the European patent application EP 16 794 966.8-1111, entitled “Methods for Apolipoprotein Detection” that was granted by the European patent Agency on January 09, 2019, which is licensed to Biocross SL and directly related to the e4Risk® test.
The other authors do not report any financial or personal conflicts of interest. They do not have any employment, consultancies, stock ownership, honoraria, paid expert testimony, patents or patent applications, or travel grants related to the content of this research.
Funding Sources
This work was partially founded by the Institute of Health Carlos III, the Queen Sofia Foundation, and the Spanish Ministry of Science (SAF2016-78603-R to M.M. and M.C.).
Author Contributions
T.S., M.A.F.-B., and M.C.: study concept and design, acquisition, analysis and interpretation of data, study supervision, statistical analysis supervision, and drafting/revision of the manuscript. M.V., M.A.Z.-S., and B.F.: acquisition, analysis and interpretation of data, and revision of the manuscript. E.A., L.S., and O.C.: acquisition and analyses of data and revision of the manuscript. F.J.G.-L., A.R., and M.M.: analysis and interpretation of data and revision of the manuscript.
Author Disclosures
This work was sponsored by the Queen Sofia Foundation and Carlos III Institute of Health. All the authors collaborate or are employees at the Alzheimer's Disease Research Unit funded by these institutions in Madrid (Spain) and have worked in the Vallecas Project, where all the data were collected for this study. The Vallecas Project is registered at the JPND Global Cohort Portal.
Acknowledgements
We are indebted to all the volunteers who participated in the Vallecas Project and to many workers of the Fundación CIEN who collaborated in the performance of the survey and the collection of data. Laboratory analyses were partially supported by funding from the Spanish Ministry of Science (SAF2016-78603-R to M.M. and M.C.).
References
- 1.Rothan HA, Byrareddy SN, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Instituto de Salud Carlos III; RENAVE, Red Nacional de Vigilancia Epidemiológica. Informe nº 26. Situación de COVID-19 en España a 27 de abril de 2020. Equipo COVID-19.Centro Nacional de Epidemiología. Centro Nacional de Microbiología. Avalable from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%c2%ba%2026.%20Situaci%c3%b3n%20de%20COVID-19%20en%20Espa%c3%b1a%20a%2027%20de%20abril%20de%202020.pdf. [Google Scholar]
- 3.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020 Jun;80((6)):639–45. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020 Mar 16;35((10)):e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olazarán J, Valentí M, Frades B, Zea-Sevilla MA, Ávila-Villanueva M, Fernández-Blázquez MÁ, et al. Identifying early markers and mechanisms In Alzheimer's disease: methods and inclusion results of the Vallecas Project. Frontiers in Aging Neuroscience. 2015;7:181. doi: 10.3389/fnagi.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37((3)):323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020 Jun;80((6)):e14–8. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: a review of clinical data in China. Mech Ageing Dev. 2020 Apr 27;188:111255. doi: 10.1016/j.mad.2020.111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 National Incident Room Surveillance Team COVID-19, Australia: epidemiology report 6 (reporting week ending 19:00 AEDT 7 March 2020) Commun Dis Intell. 20182020 Mar 11;:44. doi: 10.33321/cdi.2020.44.21. [DOI] [PubMed] [Google Scholar]
- 11.Bendavid E, Mulaney B, Sood N, Shah S, Ling E, Bromley-Dulfano R, et al. COVID-19 antibody seroprevalence in Santa Clara county, California. medRxiv Preprint. 2020 Apr 4;:20062463. doi: 10.1093/ije/dyab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levesque J, Maybury DW. A note on COVID-19 seroprevalence studies: a meta-analysis using hierarchical modelling. medRxiv Preprint. 2020 May 03;:20089201. [Google Scholar]
- 13.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020 Aug 22;396((10250)):535–44. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMichael TM, Clark S, Pogosjans S, Kay M, Lewis J, Baer A, et al. COVID-19 in a long-term care facility: King County, Washington, February 27–March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020 Mar 27;69((12)):339–42. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv Preprint. 2020 Apr 23;:20076042. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Instituto de Salud Carlos III Estudio ENE-COVID19: Primera ronda estudio nacional de sero-epidemiología de la infección por SARS-CoV-2 en España. Informe preliminar. 13. De Mayo De 2020. Available from: https://www.ciencia.gob.es/stfls/MICINN/Ministerio/FICHEROS/ENECOVID_Informe_preliminar_cierre_primera_ronda_13Mayo2020.pdf.
- 17.Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020 Apr 13;99((9)):569. doi: 10.1177/0145561320920762. [DOI] [PubMed] [Google Scholar]
- 18.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020 Jul;163((1)):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease. J Clin Invest. 2020 May 1;130((5)):2620–9. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo CL, Pilling LC, Atkins JL, Masoli JAH, Delgado J, Kuchel GA, et al. APOE E4 genotype predicts severe COVID-19 in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020 Oct 15;75((11)):2231–32. doi: 10.1093/gerona/glaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emi M, Wu LL, Robertson MA, Myers RL, Hegele RA, Williams RR, et al. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3((4)):373–9. doi: 10.1016/0888-7543(88)90130-9. [DOI] [PubMed] [Google Scholar]
- 22.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 23.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261((5123)):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 24.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 25.van Bockxmeer FM, Mamotte CD. Apolipoprotein epsilon 4 homozygosity in young men with coronary heart disease. Lancet. 1992;340((8824)):879–80. doi: 10.1016/0140-6736(92)93288-x. [DOI] [PubMed] [Google Scholar]
- 26.Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172((4)):825–e18. doi: 10.1016/j.cell.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price DA, Bassendine MF, Norris SM, Golding C, Toms GL, Schmid ML, et al. Apolipoprotein epsilon3 allele is associated with persistent hepatitis C virus infection. Gut. 2006;55((5)):715–8. doi: 10.1136/gut.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon-4/epsilon-4 genotype accelerates HIV disease progression. Proc Nat Acad Sci U S A. 2008;105:8718–23. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgos JS, Ramirez C, Sastre I, Valdivieso F. Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. J Virol. 2006;80((11)):5383–7. doi: 10.1128/JVI.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1, apolipoprotein E, and cholesterol: a dangerous liaison in Alzheimer's disease and other disorders. Prog Lipid Res. 2006;45:73–90. doi: 10.1016/j.plipres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Yao X, Gordon EM, Figueroa DM, Barochia AV, Levine SJ. Emerging roles of apolipoprotein E and apolipoprotein A-I in the pathogenesis and treatment of lung disease. Am J Respir Cell Mol Biol. 2016;55((2)):159–69. doi: 10.1165/rcmb.2016-0060TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulminski AM, Barochia AV, Loika Y, Raghavachari N, Arbeev KG, Wojczynski MK, et al. The APOE ε4 allele is associated with a reduction in FEV1/FVC in women: a cross-sectional analysis of the Long Life Family Study. PLoS One. 2018;13:e0206873. doi: 10.1371/journal.pone.0206873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Chopra P, Li X, Wolfert MA, Tompkins SM, Boons G-J. SARS-CoV-2 spike protein binds heparan sulfate in a length- and sequence-dependent manner. BioRxiv Preprint. 2020 May 10;:2020.05.10.087288. [Google Scholar]
- 34.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8((4)):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vardavas C, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020 Mar 20;18((March)):20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109((5)):531–8. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfaro TM, Monteiro RA, Cunha RA, Cordeiro CR. Chronic coffee consumption and respiratory disease: a systematic review. Clin Respir J. 2018;12((3)):1283–94. doi: 10.1111/crj.12662. [DOI] [PubMed] [Google Scholar]