Keywords: androgens, COVID-19, kidney, renin-angiotensin system, SARS-CoV-2
Abstract
Coronavirus disease 2019 (COVID-19) has reached pandemic proportions, affecting millions of people worldwide. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of COVID-19. Epidemiological reports have shown that the severity of SARS-CoV-2 infection is associated with preexisting comorbidities such as hypertension, diabetes mellitus, cardiovascular diseases, and chronic kidney diseases, all of which are also risk factors for acute kidney injury (AKI). The kidney has emerged as a key organ affected by SARS-CoV-2. AKI is associated with increased morbidity and mortality in patients with COVID-19. Male sex is an independent predictor for AKI, and an increased death rate has been reported in male patients with COVID-19 worldwide. The mechanism(s) that mediate the sex discrepancy in mortality due to COVID-19 remain(s) unknown. Angiotensin-converting enzyme (ACE)2 is the receptor for SARS-CoV-2. Alterations in the ACE-to-ACE2 ratio have been implicated in renal diseases. This perspective aims to discuss data that suggest that androgens, via alterations in the intrarenal renin-angiotensin system, impair renal hemodynamics, predisposing patients to AKI during COVID-19 infection, which could explain the higher mortality observed in men with COVID-19. Clinicians should ensure early and effective cardiometabolic control for all patients to ameliorate the compensatory elevation of ACE2 and alterations in the ACE-to-ACE2 ratio. A better understanding of the role of androgens in SARS-CoV-2-associated AKI and mortality is imperative. The kidney could constitute a key organ that may explain the sex disparities of the higher mortality and worst outcomes associated with COVID-19 in men.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has reached pandemic proportions, affecting more than 50 million people and causing more than 1.2 million deaths worldwide (1). Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of the COVID-19 pandemic. Early epidemiological reports have shown that the severity of SARS-CoV-2 infection is frequently associated with preexisting comorbidities such as hypertension, diabetes mellitus, cardiovascular diseases, and chronic kidney diseases (2), all of which are also risk factors for acute kidney injury (AKI) (3). Among the several organs affected by SARS-CoV-2, the kidney has emerged as a key one (4). AKI is defined as a reversible decline in the glomerular filtration rate (GFR) that results in the accumulation of waste products (5). AKI is associated with increased morbidity and mortality in patients with COVID-19 (6–9) and is more likely to occur in patients with elevated basal serum creatinine (7). AKI is also a risk factor for chronic kidney disease (10). Recent studies have shown that male sex is an independent predictor for AKI in patients with COVID-19 (8, 11, 12). An increased death rate has been reported in males compared with females worldwide (13, 14). Although still unclear, there are multiple plausible hypotheses for this sex discrepancy in mortality due to COVID-19. Some of the plausible explanations could be: 1) male sex is a risk factor per se; 2) COVID-19-associated comorbidities have a higher prevalence in males compared with females, before SARS-CoV-2 infection; 3) female patients with COVID-19 have more robust T-cell activation than male patients (15); or 4) male patients with COVID-19 have an increased prevalence of neutralizing autoantibodies against type I interferons than their female counterparts (16). This perspective aims to discuss data from experimental models that suggest that androgens via alterations in the intrarenal renin-angiotensin-aldosterone system (RAAS) impair renal hemodynamics, predisposing patients to AKI during COVID-19 infection, which could explain the higher mortality observed in men.
ANDROGENS, THE KIDNEY, AND COVID-19
The androgens testosterone and its most potent metabolite, dihydrotestosterone (DHT), bind to the androgen receptor (AR) to elicit an androgenic response. In the kidney, AR is highly expressed in proximal tubule cells (17), glomerular endothelial cells (18), and podocytes (19). Podocyte damage is a hallmark of renal injury and associated proteinuria (20). SARS-CoV-2 viral particles have been identified in glomeruli and more specifically in podocytes during SARS-CoV-2 infection (21, 22). Furthermore, SARS-CoV-2 viral particles have been isolated from autopsied kidneys and were able to replicate in cell culture (23). All these data strongly indicate that not only SARS-CoV-2 RNA, protein, and viral particles are found in human kidneys but also viable infective viral particles. Moreover, postmortem analysis of SARS-CoV-2-positive patients has showed that those in which SARS-CoV-2 RNA could be detected in the kidney present a reduction in survival time, supporting a positive association between SARS-CoV-2 renal infection and COVID-19 disease severity (23). Data from experimental animal models have shown that GFR is lower in females compared with males, due to higher renal vascular resistance in females. Thereby, the male kidney is vasodilated compared with the female kidney (24). Renal hyperfiltration is thought to be a key initiating factor in renal injury in patients with diabetes mellitus and obesity (25, 26). It has been hypothesized that renal hyperfiltration is an indication of generalized endothelial dysfunction (27). Afferent arterioles are resistance vessels in the kidney, which play a major role in regulating both the myogenic response and tubuloglomerular feedback. Those two are critical mechanisms for renal protection during insults (28). It has been recently demonstrated that testosterone via AR dilates norepinephrine-preconstricted mouse afferent arterioles in a dose-dependent manner (29) and also enhances tubuloglomerular feedback (30). Thereby, it could be hypothesized that androgen-mediated vasodilation of the afferent arteriole enhances the transmission of systemic pressure to the glomerulus, resulting in increases in intraglomerular pressure that finally lead to renal injury, which may be subclinical in many instances. Furthermore, androgens can increase efferent arteriolar resistance, by increasing renal angiotensin II (ANG II) levels, leading to an exacerbation of glomerular injury (31). Androgens are inversely associated with renal function in apparently healthy men without a history of cardiovascular disease (32). Moreover, testosterone has been reported to increase thromboxane A2 receptor density and responsiveness in rat aortas (33), a prostanoid that plays a potential role in the development of microvascular dysfunction (34). During the COVID-19 clinical course, there is generalized microvascular dysfunction (35), which could lead to renal hypoperfusion in the presence of androgen-mediated renal injury, resulting in AKI. Supporting this hypothesis, recent studies have shown that the most frequent finding in patients with AKI and COVID-19 is acute tubular necrosis (36–38) but, in another study, glomerular injury was reported (39). Recent studies have suggested that androgenetic alopecia, a clinical indicator of androgen excess biological activity, is present in an elevated proportion of hospitalized patients with COVID-19 (40) and also correlates with worsening respiratory status and death in patients with COVID-19 (41). Randomized controlled clinical trials testing the AR blocker bicalutamide (NCT04374279), the gonadotropin release hormone antagonist degarelix (NCT04397718), and the mineralocorticoid receptor and AR blocker spironolactone (NCT04345887) in patients with COVID-19 are currently underway. Whether AR blockers will decrease the mortality and prevalence of AKI in COVID-19 is unclear at present.
POLYCYSTIC OVARIAN SYNDROME, TRANSGENDER INDIVIDUALS, AND COVID-19
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder in reproductive-age women with a prevalence of 5%–20% in this population (42, 43). PCOS is characterized by clinical and/or biochemical signs of hyperandrogenism, oligo- or anovulation, and/or polycystic ovaries. Hyperandrogenism is present in more than 80% of women with PCOS and those women have a worse metabolic profile than normoandrogenic subjects with PCOS (43–47). Importantly, microvascular endothelial dysfunction is observed in women with PCOS and is driven by androgens (48). Moreover, serum testosterone positively correlates with markers of renal injury in patients with PCOS (49). Whether women with PCOS are prone to higher SARS-CoV-2 death rates, AKI, or long-term renal deleterious consequences due to COVID-19 is currently unknown but highly plausible. We have previously established an animal model of PCOS in female rats that mimics many of the metabolic and cardiovascular abnormalities observed in women with PCOS (50). Chronic administration of DHT to female rats causes an increase in food intake, obesity, insulin resistance, blood pressure, GFR, and renal hyperfiltration, as observed in women with PCOS (50–53). More research is needed to determine whether women with PCOS infected with SARS-CoV-2 have a higher prevalence of AKI and mortality compared with normal cycling women. Transgender men are persons who, due to their sexual secondary characteristics at birth, are assigned a female gender but identify themselves as males. Testosterone is used to stop menses and induce virilization, including a male pattern of sexual and facial hair, a change in voice, male body physical appearance, and testosterone levels similar to cisgender males. A recent publication showed that transgender men had increased endothelial dysfunction compared with cisgender women (54). Whether the presence of endothelial dysfunction in this population is associated with renal hemodynamic changes is unknown. It can be hypothesized that COVID-19 infection is associated with worsened morbidity and mortality in transgender men.
THE RAAS, ACE2, AND COVID-19
What could be the mechanism(s) that underline the abnormal androgen-mediated renal hemodynamics? The RAAS is a major regulator of blood pressure and it is composed of classical and nonclassical arms with opposite effects on renal physiology. In the classical RAAS arm, angiotensinogen is cleaved by circulating renin to generate angiotensin I, which, in turn, is converted to ANG II by angiotensin-converting enzyme (ACE). ANG II binds and activates the ANG II type 1 receptor (AT1R) to increase blood pressure, sodium reabsorption, and renal vasoconstriction. We and others have shown that androgens upregulate intrarenal angiotensinogen expression (55, 56). In the nonclassical RAAS arm, the membrane-bound carboxypeptidase enzyme ACE2 converts ANG II to angiotensin 1–7 (ANG 1–7). ANG 1–7 binds to the Mas receptor, opposing most of the (patho)physiological actions of ANG II in the kidney.
ACE2 is highly expressed in the kidney (57). ACE2 is the receptor for the coronavirus that causes COVID-19, SARS-CoV-2 (58). ACE2 is highly expressed in proximal tubule cells; however, recent single-cell transcriptome studies have shown that ACE2 is also expressed in glomerular epithelial cells and podocytes (59, 60). Moreover, AR regulates the transcription of TMPRSS2 (61), a required protease for SARS-CoV-2 entry into target cells that is coexpressed in those same cell types (59, 60). Although SARS-CoV2 enters cells through membrane-bound ACE2, there is also concern that it will downregulate ACE2 as SARS-CoV does (62, 63), which could lead to an increased ACE-to-ACE2 ratio. An elevated ACE-to-ACE2 ratio has been implicated in several renal diseases (63, 64). Plasma ACE2 activity is low in healthy subjects but elevated in patients with cardiovascular disease or renal diseases, especially in men (65–67). The increased plasma ACE2 activity in males (65–67) could be due to increased ACE2 expression or decreased a disintegrin and metalloproteinase 17 (ADAM17) activity; ADAM17 cleaves membrane-bound ACE2 to generate soluble (circulating) ACE2. ADAM17 expression has been reported to be decreased by androgens in LNCaP prostate cancer cell (68), which would result in decreased soluble ACE2 levels. Whether ADAM17 is subject to the same regulation by androgens in normal tissues is unknown and could help to understand sex differences in COVID-19 outcomes. Moreover, it is possible to speculate that the increase in renal ACE2 could represent a compensatory mechanism to endothelial dysfunction in response to several insults. More research needs to be done to demonstrate the direct effect of androgen upon intrarenal and extrarenal ACE-to-ACE2 ratios before and during COVID-19 infection.
A meta-analysis of more than 1.2 million subjects with previously normal estimated GFR (eGFR) showed that those with hypertension have an increased risk of developing AKI (69). Recent studies have highlighted the key role of ACE2 in mediating sexual dimorphism in blood pressure control (70). Ji et al. (70) showed using an elegant set of experiments that male mice have a faster increase in ANG II-induced increase in blood pressure than female mice and that ACE2 genetic ablation increased the rate of ANG II-induced hypertension in female mice with a negligible effect on male mice. Those studies supported the notion of a larger protective effect of ACE2 in female mice than in male mice. Interestingly, the ACE2 gene is located on the X chromosome (71), which raises the possibility that differences in sex chromosome dosage (2X vs. 1X) could impact ACE2 activity. The Four Core Genotypes rodent model allows dissecting the gonadal effects from sex chromosome effects. Recently, Liu et al. (72) reported that renal ACE2 activity, using optimized enzymatic conditions, and expression are higher in male mouse kidneys compared with female mouse kidneys. Moreover, estradiol reduced renal ACE2 activity, and the effect of estradiol was independent of sex and the sex chromosomal complement (72). In the same study, gonadectomy did not affect the expression or activity of ACE2 in male kidneys (72). We have previously reported that some of the renal actions of androgen excess in female rats persisted for months after androgen withdrawal (51); whether this is also the case in male mice is unknown. All these data highlight plenty of opportunities for further research to fully elucidate the role of sex steroids on ACE2 expression and activity regulation.
RAAS blockers such as ACE inhibitors or AT1R blockers are drug classes widely used to treat hypertension. ACE2 shares considerable homology with ACE but is not inhibited by ACE inhibitors. The recent report showing that ACE2 functions as the receptor for SARS-CoV-2 stirred unprecedented controversy in the field on the continuous use of RAAS blockers in patients who tested positive for COVID-19 as well as at-risk populations. RAAS blockers increase systemic and tissue ACE2 expression and activity in preclinical models (73, 74). However, there is no evidence to support an association between RAAS blockers use and more severe disease; some large studies and a systematic review found no relationship between the use of these drugs and severity of COVID-19 (75), whereas other data suggest that these drugs may actually attenuate the severity of disease (76, 77). Interestingly, men with hypertension are treated more frequently with RAAS blockers, whereas diuretics are more frequently prescribed in women (78). This difference in drug prescription is presumably due to RAAS blockers’ potential teratogenic effects (79). The role that RAAS blockers play in the prevalence of AKI and mortality in patients with COVID-19 is unclear at present.
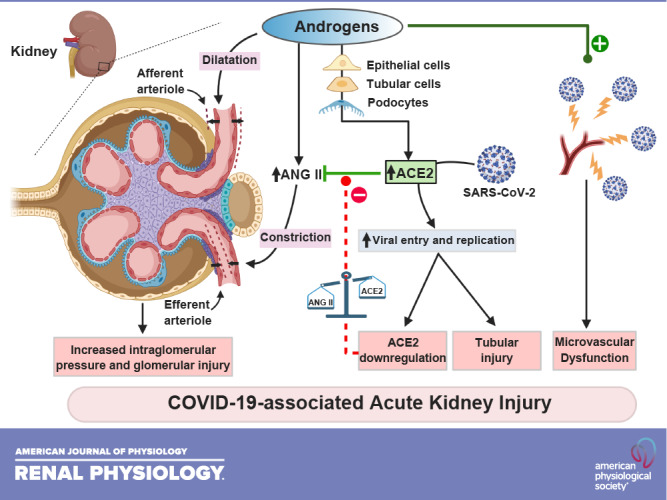
Figure 1 shows the proposed physiological, cellular, and molecular mechanisms by which androgens lead to COVID-19-associated AKI in SARS-CoV-2 infection.
Figure 1.
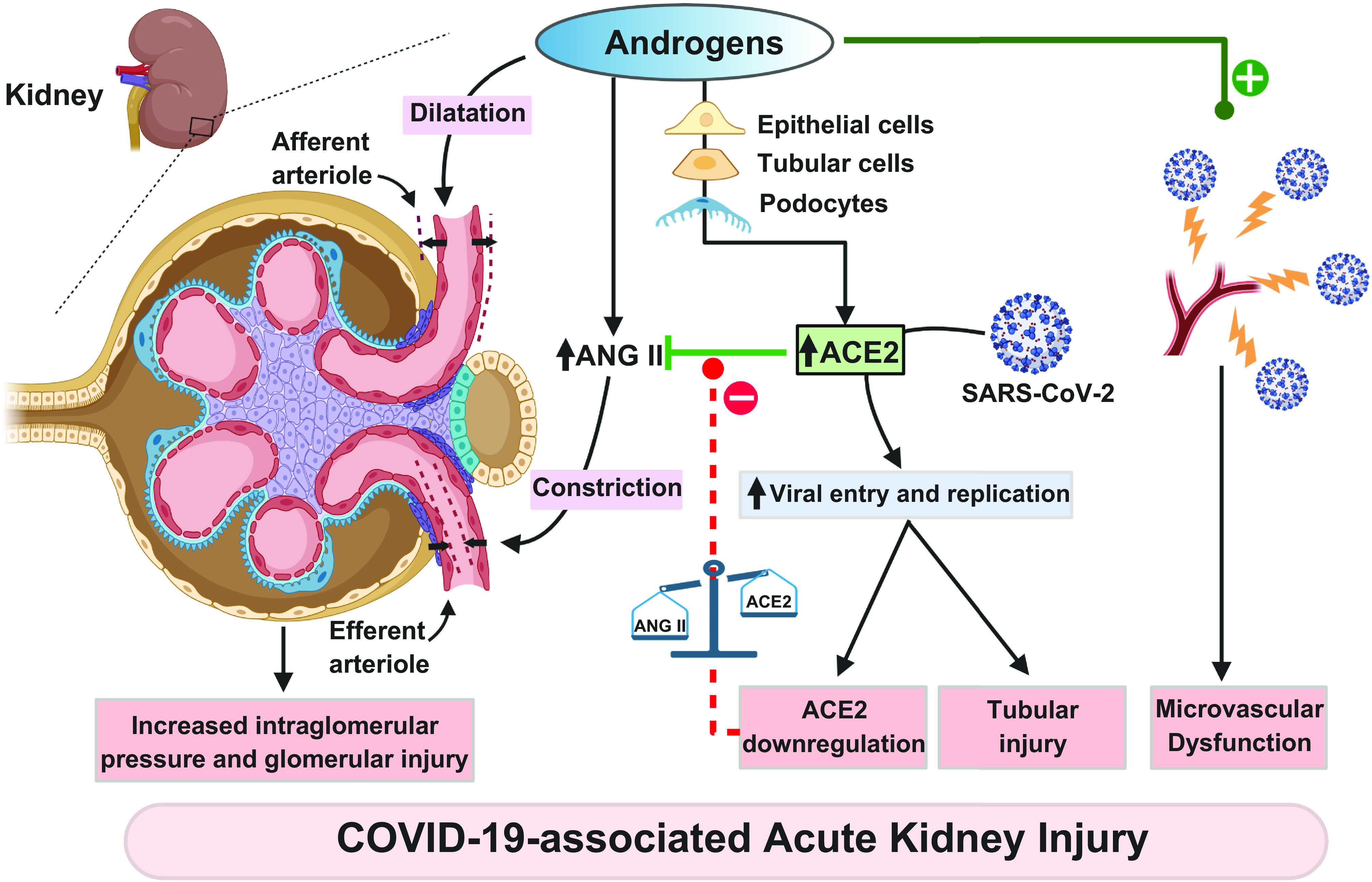
Physiological, cellular, and molecular mechanisms by which androgens lead to COVID-19-associated acute kidney injury in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. ACE2, angiotensin-converting enzyme 2; ANG II, angiotensin II.
CONCLUSIONS
At present, there is no cure for COVID-19, defining cure as the reversion of all affected organs and systems by SARS-CoV-2 to the predisease state (80–82). Clinicians need to ensure early and effective cardiometabolic control for all patients to ameliorate the compensatory elevation of ACE2 and alterations in the ACE-to-ACE2 ratio. A better understanding of the role of androgens underlying the mortality and acute renal injury associated with SARS-CoV-2 infection is imperative. The kidney could constitute a key organ that may explain the sex disparities of the higher mortality associated with COVID-19 in men.
GRANTS
This work was supported by National Institutes of Health Grants P20GM121334 (to L.L.Y.C. and D.G.R.) and R21DK113500 (to D.G.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
L.L.Y.C., S.R., J.E.P., and D.G.R. drafted manuscript; L.L.Y.C., S.R., J.E.P., and D.G.R. edited and revised manuscript; L.L.Y.C., S.R., J.E.P., and D.G.R. approved final version of manuscript.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20: 533–534, 2020. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Sole F, Farcomeni A, Loffredo L, Carnevale R, Menichelli D, Vicario T, Pignatelli P, Pastori D. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest 50: e13378, 2020. doi: 10.1111/eci.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesropian PD, Othersen J, Mason D, Wang J, Asif A, Mathew RO. Community-acquired acute kidney injury: a challenge and opportunity for primary care in kidney health. Nephrology (Carlton) 21: 729–735, 2016. doi: 10.1111/nep.12751. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med 26: 1017–1032, 2020. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Section 2: AKI definition. Kidney Int Suppl (2011) 2: 19–36, 2012. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, , et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32: 151–160, 2021. doi: 10.1681/asn.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, Abramowitz MK, Levy R, Kumar N, Mokrzycki MH, Coco M, Dominguez M, Prudhvi K, Golestaneh L. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 31: 2145–2157, 2020. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y-M, Xie J, Chen M-M, Zhang X, Cheng X, Li H, Zhou F, Qin J-J, Lei F, Chen Z, Lin L, Yang C, Mao W, Chen G, Lu H, Xia X, Wang D, Liao X, Yang J, Huang X, Zhang B-H, Yuan Y, Cai J, Zhang XJ, Wang Y, Zhang X, She Z-G, Li H. Kidney function indicators predict adverse outcomes of COVID-19. Med (NY) 2: 38–48.e2, 2021. doi: 10.1016/j.medj.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu RK, Hsu CY. The role of acute kidney injury in chronic kidney disease. Semin Nephrol 36: 283–292, 2016. doi: 10.1016/j.semnephrol.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med 17: e1003406, 2020. doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The sex Gender and COVID-19 Project. The COVID-19 sex-disaggregated data tracker. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/ [2021 Jan 21].
- 14.Pradhan A, Olsson P-E. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ 11: 53, 2020. doi: 10.1186/s13293-020-00330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, , et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588: 315–320, 2020. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, , et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370: eabd4585, 2020. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinkler M, Bujalska IJ, Kaur K, Onyimba CU, Buhner S, Allolio B, Hughes SV, Hewison M, Stewart PM. Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension 46: 787–798, 2005. doi: 10.1161/01.HYP.0000184362.61744.c1. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Estay V, Carreño DV, San Francisco IF, Sotomayor P, Godoy AS, Smith GJ. Androgen receptor in human endothelial cells. J Endocrinol 224: R131–R137, 2015. doi: 10.1530/JOE-14-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ, Karl M. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011. doi: 10.1038/ki.2010.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001. doi: 10.1172/JCI200114629. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbate M, Rottoli D, Gianatti A. COVID-19 attacks the kidney: ultrastructural evidence for the presence of virus in the glomerular epithelium. Nephron 144: 341–342, 2020. doi: 10.1159/000508430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, Kluge S, Gross O, Pueschel K, Schröder AS, Edler C, Aepfelbacher M, Puelles VG, Huber TB. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munger K, Baylis C. Sex differences in renal hemodynamics in rats. Am J Physiol 254: F223–F231, 1988. doi: 10.1152/ajprenal.1988.254.2.F223. [DOI] [PubMed] [Google Scholar]
- 25.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 26.Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6, 2012. doi: 10.4239/wjd.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherney DZ, Miller JA, Scholey JW, Nasrallah R, Hébert RL, Dekker MG, Slorach C, Sochett EB, Bradley TJ. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care 33: 1344–1346, 2010. doi: 10.2337/dc09-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Fu Y, Ge Y, Juncos LA, Reckelhoff JF, Liu R. The vasodilatory effect of testosterone on renal afferent arterioles. Gend Med 9: 103–111, 2012. doi: 10.1016/j.genm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y, Lu Y, Liu EY, Zhu X, Mahajan GJ, Lu D, Roman RJ, Liu R. Testosterone enhances tubuloglomerular feedback by increasing superoxide production in the macula densa. Am J Physiol Regul Integr Comp Physiol 304: R726–R733, 2013. doi: 10.1152/ajpregu.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol 289: F941–F948, 2005. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 32.Tomaszewski M, Charchar FJ, Maric C, Kuzniewicz R, Gola M, Grzeszczak W, Samani NJ, Zukowska-Szczechowska E. Inverse associations between androgens and renal function: the Young Men Cardiovascular Association (YMCA) study. Am J Hypertens 22: 100–105, 2009. doi: 10.1038/ajh.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda K, Ruff A, Morinelli TA, Mathur RS, Halushka PV. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am J Physiol 267: H887–H893, 1994. doi: 10.1152/ajpheart.1994.267.3.H887. [DOI] [PubMed] [Google Scholar]
- 34.Chiang C-Y, Chien C-Y, Qiou W-Y, Chang C, Yu I-S, Chang P-Y, Chien C-T. Genetic depletion of thromboxane A2/thromboxane-prostanoid receptor signalling prevents microvascular dysfunction in ischaemia/reperfusion Injury. Thromb Haemost 118: 1982–1996, 2018. doi: 10.1055/s-0038-1672206. [DOI] [PubMed] [Google Scholar]
- 35.Colantuoni A, Martini R, Caprari P, Ballestri M, Capecchi PL, Gnasso A, Lo Presti R, Marcoccia A, Rossi M, Caimi G. COVID-19 sepsis and microcirculation dysfunction. Front Physiol 11: 747, 2020. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D'Agati V, Markowitz G. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 31: 2158–2167, 2020. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, Bijol V, Northwell Nephrology COVID-19 Research Consortium. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol 31: 1948–1958, 2020. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, Yi F, Yang H-C, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, Canetta P, Ratner LE, Marasa M, Gharavi AG, Stokes MB, Markowitz GS, D'Agati VD. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 31: 1959–1968, 2020. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wambier CG, Vaño-Galván S, McCoy J, Gomez-Zubiaur A, Herrera S, Hermosa-Gelbard Á, Moreno-Arrones OM, Jiménez-Gómez N, González-Cantero A, Fonda-Pascual P, Segurado-Miravalles G, Shapiro J, Pérez-García B, Goren A. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The “Gabrin sign”. J Am Acad Dermatol 83: 680–682, 2020. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wambier CG, Vaño-Galván S, McCoy J, Pai S, Dhurat R, Goren A. Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. J Am Acad Dermatol 83: e453–e454, 2020. doi: 10.1016/j.jaad.2020.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev 36: 487–525, 2015. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106: 6–15, 2016. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91: 4237–4245, 2006. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 45.Chen M-J, Yang W-S, Yang J-H, Chen C-L, Ho H-N, Yang Y-S. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49: 1442–1447, 2007. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 46.Dapas M, Lin FTJ, Nadkarni GN, Sisk R, Legro RS, Urbanek M, Hayes MG, Dunaif A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med 17: e1003132, 2020. doi: 10.1371/journal.pmed.1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang A, Brennan K, Azziz R. Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril 93: 1938–1941, 2010. doi: 10.1016/j.fertnstert.2008.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Usselman CW, Yarovinsky TO, Steele FE, Leone CA, Taylor HS, Bender JR, Stachenfeld NS. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: role of the endothelin B receptor. J Physiol 597: 2853–2865, 2019. doi: 10.1113/JP277756. [DOI] [PubMed] [Google Scholar]
- 49.Song Y, Ye W, Ye H, Xie T, Shen W, Zhou L. Serum testosterone acts as a prognostic indicator in polycystic ovary syndrome-associated kidney injury. Physiol Rep 7: e14219, 2019. doi: 10.14814/phy2.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med 8: 103–115, 2011. doi: 10.1016/j.genm.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres Fernandez ED, Adams KV, Syed M, Maranon RO, Romero DG, Yanes Cardozo LL. Long-lasting androgen-induced cardiometabolic effects in polycystic ovary syndrome. J Endocr Soc 2: 949–964, 2018. doi: 10.1210/js.2018-00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres Fernandez ED, Huffman AM, Syed M, Romero DG, Yanes Cardozo LL. Effect of GLP-1 receptor agonists in the cardiometabolic complications in a rat model of postmenopausal PCOS. Endocrinology 160: 2787–2799, 2019. doi: 10.1210/en.2019-00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanes Cardozo LL, Romero DG, Reckelhoff JF. Cardiometabolic features of polycystic ovary syndrome: role of androgens. Physiology (Bethesda) 32: 357–366, 2017. doi: 10.1152/physiol.00030.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gulanski BI, Flannery CA, Peter PR, Leone CA, Stachenfeld NS. Compromised endothelial function in transgender men taking testosterone. Clin Endocrinol (Oxf) 92: 138–144, 2020. doi: 10.1111/cen.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 56.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87: E1–E9, 2000. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He Q, Mok TN, Yun L, He C, Li J, Pan J. Single-cell RNA sequencing analysis of human kidney reveals the presence of ACE2 receptor: a potential pathway of COVID-19 infection. Mol Genet Genomic Med 8: e1442, 2020. doi: 10.1002/mgg3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan X-W, Xu D, Zhang H, Zhou W, Wang L-H, Cui X-G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 46: 1114–1116, 2020. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wambier CG, Goren A, Vaño-Galván S, Ramos PM, Ossimetha A, Nau G, Herrera S, McCoy J. Androgen sensitivity gateway to COVID–19 disease severity. Drug Dev Res 81: 771–776, 2020. doi: 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46: 586–590, 2020. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol 4: 74–82, 2015. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One 13: e0198144, 2018. doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts MA, Velkoska E, Ierino FL, Burrell LM. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol Dial Transplant 28: 2287–2294, 2013. doi: 10.1093/ndt/gft038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 30: 375–383, 2012. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 68.McCulloch DR, Harvey M, Herington AC. The expression of the ADAMs proteases in prostate cancer cell lines and their regulation by dihydrotestosterone. Mol Cell Endocrinol 167: 11–21, 2000. doi: 10.1016/s0303-7207(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 69.James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, de Jong P, Gansevoort RT, Levey AS, Warnock DG, Sarnak MJ; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 66: 602–612, 2015. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ji H, de Souza AMA, Bajaj B, Zheng W, Wu X, Speth RC, Sandberg K. Sex-specific modulation of blood pressure and the renin-angiotensin system by ACE (angiotensin-converting enzyme) 2. Hypertension 76: 478–487, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komatsu T, Suzuki Y, Imai J, Sugano S, Hida M, Tanigami A, Muroi S, Yamada Y, Hanaoka K. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2). DNA Seq 13: 217–220, 2002. doi: 10.1080/1042517021000021608. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex dif 1: 6, 2010. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 74.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 296: F398–F405, 2009. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 382: 2441–2448, 2020. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, , et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 126: 1671–1681, 2020. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou F, Liu Y-M, Xie J, Li H, Lei F, Yang H, Qin J-J, Cai J, Zhang X-J, Wu B, Xia M, Xiang D, Yang C, Ma X, Xu Q, Lu Z, Lu H, Xia X, Wang D, Liao X, Peng G, Yang J, Huang X, Zhang B-D, Yuan Y, Wei X, Liu PP, Wang Y, Zhang P, She Z-G, Xia J, Li H. Comparative impacts of ACE (angiotensin-converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension 76: e15–e17, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15622. [DOI] [PubMed] [Google Scholar]
- 78.Ljungman C, Kahan T, Schiöler L, Hjerpe P, Hasselström J, Wettermark B, Boström KB, Manhem K. Gender differences in antihypertensive drug treatment: results from the Swedish Primary Care Cardiovascular Database (SPCCD). J Am Soc Hypertens 8: 882–890, 2014. doi: 10.1016/j.jash.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Cooper WO. Clinical implications of increased congenital malformations after first trimester exposures to angiotensin-converting enzyme inhibitors. J Cardiovasc Nurs 23: 20–24, 2008. doi: 10.1097/01.JCN.0000305052.73376.de. [DOI] [PubMed] [Google Scholar]
- 80.Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID-19. J Mol Med (Berl) 98: 789–803, 2020. doi: 10.1007/s00109-020-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumari P, Singh A, Ngasainao MR, Shakeel I, Kumar S, Lal S, Singhal A, Sohal SS, Singh IK, Hassan MI. Potential diagnostics and therapeutic approaches in COVID-19. Clin Chim Acta 510: 488–497, 2020. doi: 10.1016/j.cca.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Liu C, Liu G, Luo W, Xia N. COVID-19: progress in diagnostics, therapy and vaccination. Theranostics 10: 7821–7835, 2020. doi: 10.7150/thno.47987. [DOI] [PMC free article] [PubMed] [Google Scholar]


