Abstract
BACKGROUND:
Autism spectrum disorder (ASD) is a multifactorial neurodevelopmental disorder that encompasses a complex and heterogeneous set of traits. Subclinical traits that mirror the core features of ASD, referred to as the broad autism phenotype (BAP), have been documented repeatedly in unaffected relatives and are believed to reflect underlying genetic liability to ASD. The BAP may help inform the etiology of ASD by allowing the stratification of families into more phenotypically and etiologically homogeneous subgroups. This study explores polygenic scores related to the BAP.
METHODS:
Phenotypic and genotypic information were obtained from 2614 trios from the Simons Simplex Collection. Polygenic scores of ASD (ASD-PGSs) were generated across the sample to determine the shared genetic overlap between the BAP and ASD. Maternal and paternal ASD-PGSs were explored in relation to BAP traits and their child’s ASD symptomatology.
RESULTS:
Maternal pragmatic language was related to child’s social communicative atypicalities. In fathers, rigid personality was related to increased repetitive behaviors in children. Maternal (but not paternal) ASD-PGSs were related to the pragmatic language and rigid BAP domains.
CONCLUSIONS:
Associations emerged between parent and child phenotypes, with more associations emerging in mothers than in fathers. ASD-PGS associations emerged with BAP in mothers only, highlighting the potential for a female protective factor, and implicating the polygenic etiology of ASD-related phenotypes in the BAP.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that is estimated to occur in 1 in 59 children below 8 years of age in the United States (1). In their pioneering twin study, Folstein and Rutter (2) evaluated monozygotic (i.e., identical) and dizygotic (i.e., fraternal) twins for infantile ASD, finding higher concordance for infantile ASD (i.e., both twins had the same diagnosis) in monozygotic twins (36%) than in dizygotic twins (0%) and suggesting that there is a genetic, heritable basis for ASD. A follow-up study (3) using an expanded sample revealed that 92% of monozygotic twin pairs were concordant for a broader spectrum of ASD-related social and cognitive atypicalities (compared with only 10% concordance in dizygotic pairs), suggesting that ASD and ASD traits are highly heritable. Indeed, a recent meta-analysis of ASD twin studies (4) presented almost perfect concordance of ASD between monozygotic twins (98%), adding to the evidence of the strong heritability of ASD. A substantial body of work has since identified a subclinical set of traits, including social reticence, rigid personality, and pragmatic (i.e., social) language differences, collectively known as the broad autism phenotype (BAP), that mirror the core symptoms of ASD in unaffected relatives.
Component features of the BAP may constitute candidate endophenotypes, or heritable traits linked to a disorder that are observed in affected individuals and their unaffected relatives. Though many endophenotypes are likely polygenic themselves, they may be useful for study in complex, heterogeneous conditions such as ASD, by helping to identify etiologically homogeneous subgroups based on shared endophenotypes (5–7). Evidence that features of the BAP constitute candidate endophenotypes comes from studies showing significantly higher rates of BAP features in unaffected relatives of individuals with ASD than in the general population (8–11), and even higher rates in multiplex families (12,13), suggesting that BAP features are sensitive indices of genetic loading. ASD symptomatology in children was shown to correlate with BAP features in parents (14–16), even when BAP traits were noted before a parent went on to have a child with ASD (17). Further, BAP features have been shown to cosegregate with distinct patterns of neurocognitive performance in clinically unaffected relatives (12,18–22), suggesting that there are links with underlying neural substrates affected by ASD genetic risk. Finally, unlike ASD, which by definition requires the presence of impairments in social interaction and communication and the presence of restricted and repetitive interests and behaviors, traits of the BAP have been observed to segregate independently in unaffected relatives (22–24), potentially reflecting distinct genetic contributions to the component features of ASD (25–27). Taken together, evidence suggests that the BAP may therefore help to leverage studies of ASD etiology by providing a distilled phenotypic expression of genetic liability to ASD with potentially more straightforward ties to underlying etiology than the complex and heterogeneous ASD phenotype (28). Characterizing endophenotypes among clinically unaffected relatives may also provide insights into familial patterns of transmission, permitting focus on transmitting relatives for more refined analysis of ASD-risk loci (11,28).
Family studies of ASD show an aggregation of cases within families, suggesting that there is an inherited genetic component to the disorder (29,30). Family and population genetic studies revealed a complex architecture with genetic liability originating from rare, structural, de novo, and common variation (29,31). However, rare, structural, and de novo variants collectively explain less than 5% of the total liability of ASD (32,33). In contrast, common variants are estimated to explain most of the genetic contribution to ASD (32,34). Genome-wide association studies estimate that heritability due to common variation is approximately 12% for ASD (35). One method to assess associations with ASD genetics is by harnessing common variants through polygenic scoring.
Polygenic scoring is a method of calculating an individual’s underlying genetic liability to a complex trait using weights derived from large-scale genome-wide association studies. A major benefit of polygenic scores (PGSs) is that a score can be calculated for any genotyped individual, regardless of diagnostic status. PGSs of ASD have been previously shown to imperfectly, but significantly, associate with ASD case-control status and ASD-related features (34), suggesting that PGS is a reliable measurement of inherited ASD genetic factors. Additionally, the range of derived PGSs allows for continuous measurement of use in correlational analyses with other complex phenotypes. For example, ASD-PGSs associate with general cognitive ability, logical memory, and verbal intelligence in the general population (36). Given the polygenic and heterogeneous nature of ASD, polygenic analyses in family members of individuals with ASD can also help to further elucidate the relationship between polygenic scores and symptom domains (28).
This study examined PGSs within families of individuals with ASD, with the hypothesis that ASD-PGSs underlie both aggregate ASD expression and subclinical BAP features. Specifically, the study aimed to examine 1) whether ASD-PGS is correlated with BAP features in parents of individuals with ASD; 2) whether ASD-PGS is correlated with the same symptom domain features in individuals with ASD; and 3) whether these correlations also reflect significant parent–child phenotypic associations. Importantly, ours is not an analysis of transmission that seeks to make causal claims, but instead is one of correlation that seeks to understand the relationship between symptom domains and genetic scores within families.
METHODS AND MATERIALS
Participants
Participants were drawn from the Simons Simplex Collection (SSC), and the sample included a maximum of 2618 mothers, 2614 fathers, and 2621 children with ASD. Required inclusion criteria for SSC were that probands were between 4 and 18 years of age; had a nonverbal mental age above 18 months; had no history of neurological deficits, birth trauma, or perinatal complications; had no genetic evidence of fragile X syndrome or Down syndrome (37); and had no ASD within third-degree relatives. Inclusion of both parents when available was preferred. All children included met DSM-IV or DSM-5 diagnostic criteria for ASD. The child sample included 348 female and 2273 male subjects. Parent and child participant characteristics are further described in Tables 1 and 2.
Table 1.
Sample Characteristics for Parents of Individuals With ASD
| Measure | Role | n | Mean | Range | SD | Average Scores, Mean (Range) | Clinical Cutoffs, Score (% Met BAP)a | Group Difference |
|---|---|---|---|---|---|---|---|---|
| BAPQ Aloof Total Score | Mother | 2618 | 28.62 | 12–63 | 9.41 | 2.39 (1.00–5.25) | 3.45 (10) | t5180.50 = 19.53, p < .0001 |
| Father | 2614 | 33.97 | 12–68 | 10.26 | 2.83 (1.00–5.67) | 4.13 (8) | ||
| BAPQ Pragmatic Total Score | Mother | 2618 | 25.04 | 11–60 | 7.33 | 2.09 (1.00–5.00) | 2.94 (8) | t5217.74 = 14.57, p < .0001 |
| Father | 2614 | 28.07 | 12–59 | 7.69 | 2.34 (1.00–4.92) | 3.23 (9) | ||
| BAPQ Rigid Total Score | Mother | 2618 | 32.08 | 12–68 | 8.80 | 2.68 (1.00–5.67) | 3.70 (8) | t5230 = 9.76, p < .0001 |
| Father | 2614 | 34.49 | 12–69 | 9.04 | 2.88 (1.00–5.75) | 3.91 (10) | ||
| BAPQ Total Score | Mother | 2618 | 85.75 | 36–169 | 20.72 | 2.38 (1.00–4.69) | 3.17 (10) | t5216.62 = 18.34, p < .0001 |
| Father | 2614 | 96.53 | 40–179 | 21.77 | 2.68 (1.11–4.97) | 3.55 (8) |
While total scores were used in analyses for the BAPQ, means for average scores are additionally reported along with clinical cutoffs to aid in interpretation.
ASD, autism spectrum disorder; BAPQ, Broad Autism Phenotype Questionnaire Self Rating.
Based on Sasson et al. (70) updated norms.
Table 2.
Sample Characteristics of Individuals With ASD
| Measure | Domain | n | Mean | Range | SD | T Scores, Mean (Range) | Clinical Cutoff, Scorea |
|---|---|---|---|---|---|---|---|
| Age, Years | – | 2621 | 8.93 | 2.80–17.90 | 3.57 | – | – |
| IQ | FSIQ | 2006 | 92.00 | 38–167 | 19.89 | – | – |
| ADOS | CSS total | 2548 | 7.45 | 4–10 | 1.67 | – | 4+ |
| CSS RRB | 2561 | 7.82 | 1–10 | 1.84 | – | 6+ | |
| CSS SA | 2561 | 7.22 | 2–10 | 1.74 | – | 4+ | |
| ADI-R | a total score | 2619 | 20.30 | 8–30 | 5.69 | – | 10+ |
| b verbal total score | 2307 | 16.50 | 6–26 | 4.28 | – | 8+ | |
| b nonverbal total score | 2619 | 9.24 | 0–14 | 3.45 | – | 7+ | |
| c total score | 2619 | 6.52 | 0–12 | 2.50 | – | 3+ | |
| RBS-Rb | Total score | 2615 | 27.06 | 0–105 | 17.29 | – | 33.14+ |
| SRSb | Total score | 2610 | 97.93 | 11–177 | 26.95 | 79.51 (39–91) | 60+ (T score) |
a, reciprocal social interaction domain; ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; b, communication abnormalities domain; c, restricted and repetitive behavior domain; CSS, calibrated severity score; FSIQ, Full Scale IQ; RBS-R, Repetitive Behavior Scale–Revised; SRS, Social Responsiveness Scale.
Based on Sasson et al. (70) updated norms.
While total scores were used in analyses for the SRS and RBS-R, means for T scores are additionally reported along with clinical cutoffs to aid in interpretation.
Phenotypic Measures
Data Quality Control.
Parent and child phenotypes were derived based on clinical assessment and questionnaire measures aimed at characterizing BAP and ASD traits, respectively. Given the large sample size of the SSC, as well as the number of different measures included in the repository, a series of a priori analyses were completed to determine those measures that best captured a range of ASD-related traits within the constraints of a continuous distribution for PGS predictive analyses. For each parent measure, distributions were explored with all parents combined, as well as for mothers and fathers separately. For each child measure, distributions were similarly examined, though sex remained collapsed because of the smaller female sample. All variables were normally distributed, with only two measures demonstrating minimal positive skew (Repetitive Behavior Scale–Revised total scores and Autism Diagnostic Interview–Revised nonverbal scores). Parent–child correlations were examined to determine whether child phenotypes related to BAP traits in parents.
Parent BAP Measures.
The Broad Autism Phenotype Questionnaire (BAPQ) (10) is a 36-item questionnaire that assesses the personality and pragmatic language traits that are associated with the core deficits of ASD and that are known to be associated with the BAP. Patterns of sex differences for parents of children with ASD emerged across BAPQ self-report subscales and total scores, which is consistent with prior findings (10,38). The BAPQ scores of mothers and fathers were significantly different from one another, showing elevated scores in fathers compared with those in mothers across domains (see Table 1). Mothers and fathers were therefore examined separately for all analyses. Both BAPQ self-report total score and the aloof, pragmatic language, and rigid subdomain scores were examined. Also see Assessing Use of Parent BAP Measures in the Supplement.
Proband ASD Measures.
The following measures were explored in relationship with paternal and maternal BAPQ subscale and total scores: 1) Autism Diagnostic Observation Schedule (ADOS) calibrated severity scores (CSSs) (39,40), a diagnostic tool that measures ASD symptom severity across the domains of social reciprocity, language use, and restricted and repetitive behaviors and interests (RRBs); 2) Autism Diagnostic Interview-Revised (ADI-R) (41), a parent interview that assesses the child’s symptoms related to ASD; 3) Repetitive Behavior Scale-Revised (RBS-R) (42), a parent-report questionnaire used to measure the breadth of RRBs in individuals with ASD; 4) Social Responsiveness Scale (SRS) (43) parent report (44), a measure of social ability in individuals with ASD; 5) Social Communication Questionnaire (45), a short parent-report measure that evaluates communication skills and social functioning in individuals with ASD; 6) Aberrant Behavior Checklist (46), an informant-report measure that measures the severity of a range of problem behaviors; 7) Child Behavior Checklist (47), a parent-report questionnaire used to assess emotional and behavioral problems in children; and 8) Vineland Adaptive Behavior Scales (48), a measure of adaptive functioning completed by parents. The Social Communication Questionnaire, Aberrant Behavior Checklist, Child Behavior Checklist, and Vineland Adaptive Behavior Scales were dropped from subsequent analyses given the lack of associations observed between these measures and parent BAPQ, to reduce the number of variables contributing to the regression models.
As part of the SSC data-collection procedures, different IQ assessments were administered to participants based on age and cognitive ability and are described in SSC IQ Measurements in the Supplement.
Genetic Data Quality Control
SSC samples were genotyped on one of three Illumina platforms: 1Mv1, 1Mv3, or Omni2.5 (Illumina, San Diego, CA). Preimputation quality control (see Genotyping Quality Control and Imputation in the Supplement for details) was performed on each of the platforms separately using PLINK version 1.9 (49). All of the arrays were combined to perform imputation using the Michigan Imputation Server with the Haplotype Reference Consortium reference panel. Final sample size included 7,650,164 single nucleotide polymorphisms on 7254 individuals.
Polygenic Score Generation
Summary statistics were obtained from the Psychiatric Genomics Consortium’s meta-analysis of ASD (35) after removing the SSC sample. PGSs were generated using PRScs-auto (50), using the CEU sample from 1000 Genomes as the linkage disequilibrium reference panel. PRScs uses a Bayesian framework to model linkage disequilibrium from an external reference and applies a continuous shrinkage prior on single nucleotide polymorphism effect sizes to adjust for linkage disequilibrium. Using PLINK version 1.9 with the PRScs-adjusted summary statistics, PGSs were generated. PGSs were calculated on 7254 individuals with 1,113,041 single nucleotide polymorphisms. The PGSs showed no significant differences between arrays (p = .89); therefore, no further adjustment for array platform was made (Figures S1 and S2). PGSs were z score scaled for subsequent analyses so that the effect estimates were per standard deviation increase in PGS.
Statistical Analysis
A series of linear regression analyses were conducted across all analyses, unless otherwise specified, using base-R or the lm package for RStudio (Integrated Development for R; RStudio, PBC, Boston, MA). The p value significance thresholds were corrected for multiple testing across the entire study using both Bonferroni (0.05/195 = 3 × 10−4) and false discovery rate. False discovery rate was used in addition to Bonferroni because of the nonindependence of phenotypic variables.
Parent–Child Phenotype Associations.
All measures reported above were included in phenotypic analyses. Each parent–child association was explored using linear models in R for mothers and fathers separately, so there were single observations per parent–child pair. No covariates were added to the regression model (51). All measures are age corrected, and there was no significant difference between male and female subjects with ASD along any phenotypic measures. However, because proband IQ correlated with all proband and parent phenotypic variables (p values <.05), analyses were rerun with a subset of probands with IQ <70 (n = 269) and $70 (n = 1737) to examine potential differences in parent BAPQ and proband phenotypic associations by low- and high-IQ proband groups, respectively (for results, see IQ-Stratified Phenotype-Phenotype Associations in the Supplement). For significant associations that emerged between parent–child pairs, we used Fisher z transformation tests to empirically test whether detected parent–child associations were stronger in mother–child versus father–child dyads (for results, see Fisher’s z Transformation Tests Assessing the Strength of Phenotypic Associations in the Supplement).
Genotype–Phenotype Analyses.
All phenotypic data reported above were used for polygenic analyses; however, sample sizes differed by measure as follows: BAPQ aloof, pragmatic, rigid, and total scores (nmothers = 1812, nfathers = 1808), ADOS total CSS (n = 1765), ADOS RRB CSS (n = 1782), ADOS social affect CSS (n = 1782), ADI-R reciprocal social interaction domain total (n = 1826), ADI communication abnormalities domain nonverbal total (n = 1826), ADI-R communication abnormalities domain verbal total (n = 1644), ADI-R restricted and repetitive behavior domain total (n = 1826), RBS-R total (n = 1824), SRS total (n = 1827), and IQ scores (n = 1443). All scores were z score scaled for analysis so that the odds ratios were per 1 SD increase in PGS.
Parental BAPQ Phenotypes and Parental PGSs.
The association between parental PGSs and parental BAPQ phenotypes was explored between maternal or paternal PGSs and maternal or paternal BAPQ phenotypic scores, respectively, adjusting for top-10 principal components to adjust for ancestry. Sex differences in the association between ASD-PGS and BAPQ phenotypes were formally assessed using a linear regression with interaction term between ASD-PGSs and sex, controlled for the main effects of sex, ASD-PGSs, and top-10 principal components.
Proband Phenotypes and Proband PGSs.
Proband PGSs and proband phenotypic associations adjusted for proband sex and top-10 principal components to account for ancestry.
Parental BAPQ Phenotypes and Proband PGSs.
Associations between parent phenotypes and proband PGSs were examined by parent sex between maternal and/or paternal BAPQ scores and the proband PGSs.
Associations Between Proband Phenotypes and Parental PGSs.
Separate analyses were performed between each proband phenotype and maternal and paternal PGSs to determine associations between proband phenotypes and parent PGSs.
Associations Between High-BAPQ Parents and Male Probands PGSs.
To assess potential sex differences in associations between BAPQ and common genetic variation, we selected high-BAPQ mothers and fathers and tested their ASD-PGSs against male proband ASD-PGSs. Within each BAPQ domain, individuals with high BAPQs were defined as those in the fourth quartile of scores. Differences in ASD-PGSs with probands were determined separately in mothers and fathers using t tests. Results are presented in Figures S5 and S6 and Associations Between High BAPQ Parents and Male Probands Polygenic Scores in the Supplement.
RESULTS
Detailed statistical reporting is presented in Table 3 and Tables S1 and S2, with findings summarized below.
Table 3.
Associations Between Parental ASD-PGSs and BAPQ Phenotypes
| Phenotype | PGS Subject | p Value | β | SE |
|---|---|---|---|---|
| Aloof | Mother | .019 | 0.53 | 0.23 |
| Father | .095 | 0.42 | 0.25 | |
| Pragmatic | Mother | .001a | 0.58 | 0.18 |
| Father | .090 | 0.32 | 0.19 | |
| Rigid | Mother | .058 | 0.40 | 0.21 |
| Father | .360 | 0.20 | 0.22 | |
| Total | Mother | .002a | 1.52 | 0.50 |
| Father | .075 | 0.93 | 0.52 |
ASD, autism spectrum disorder; BAPQ, Broad Autism Phenotype Questionnaire; PGS, polygenic score.
Associations passing false discovery rate testing correction.
Parent–Child Phenotypic Associations
Further details of parent–child phenotypic associations are provided in Figure 1 and Tables S1 and S2.
Figure 1.
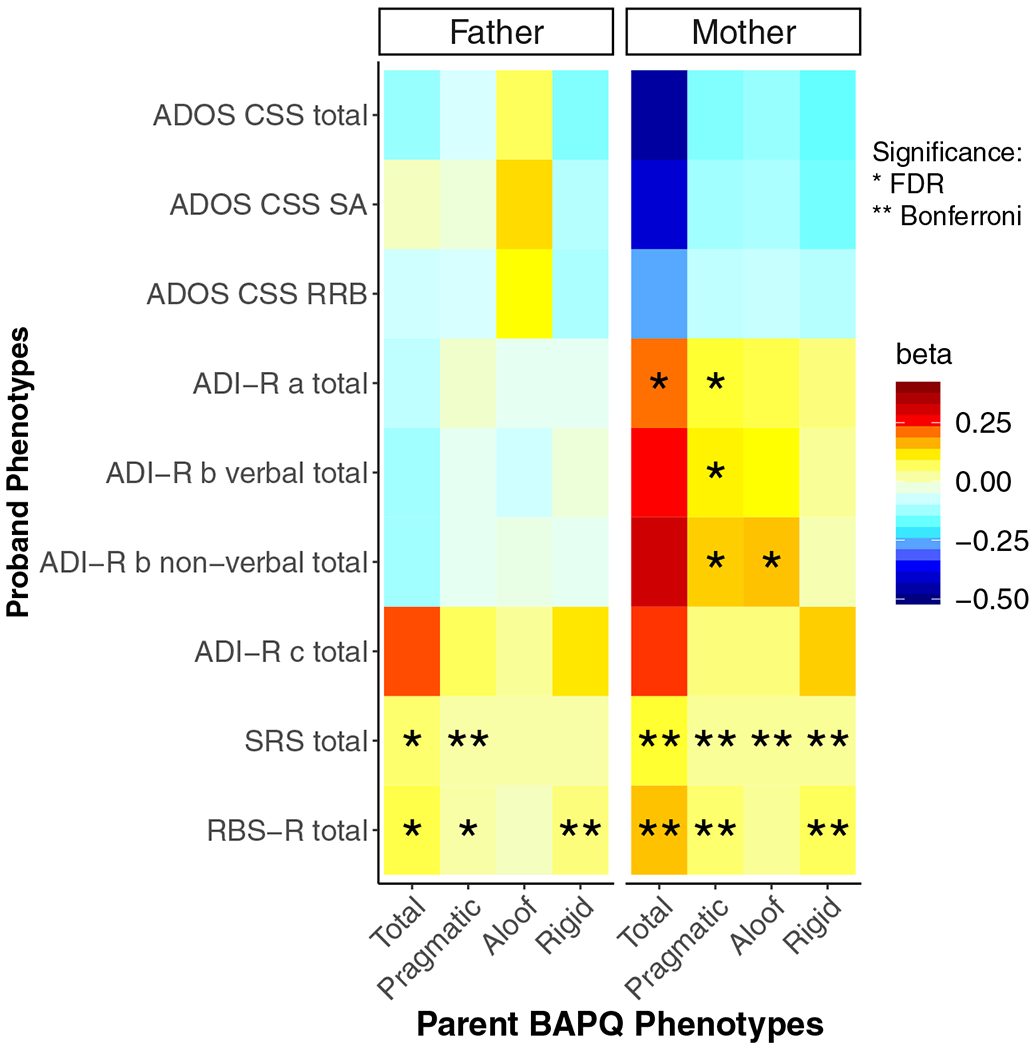
Heat map of associations between (left panel) paternal and (right panel) maternal Broad Autism Phenotype Questionnaire (BAPQ) scores and proband clinical-behavioral scores. a, reciprocal social interaction domain; ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; b, communication abnormalities domain; c, restricted and repetitive behavior domain; CSS, calibrated severity score; FDR, false discovery rate; RBS-R, Repetitive Behavior Scale–Revised; RRB, restricted and repetitive behaviors and interests domain; SA, social affect subscale; SRS, Social Responsiveness Scale. *Associations passing the FDR-corrected significance threshold. **Associations passing the Bonferroni-corrected significance threshold.
Autism Diagnostic Observation Schedule.
There was no significant association between maternal or paternal BAPQ scores and proband ADOS total CSS or any of the ADOS subscales (social affect CSS, RRB CSS).
Autism Diagnostic Interview–Revised.
A significant positive association was detected between maternal scores on the BAPQ pragmatic subscale and proband ADI-R reciprocal social interaction and nonverbal communication total scores (Figure S3A). There were additional significant associations between maternal BAPQ aloof and proband ADI-R total nonverbal communication scores as well as between maternal BAPQ total scores and proband ADI-R reciprocal social interaction scores. There were no other significant associations between maternal BAPQ aloof, rigid, or total scores and proband ADI-R scores. There were no significant associations with paternal BAPQ scores and proband ADI-R scores overall.
Social Responsiveness Scale.
All maternal BAPQ scores were significantly positively associated with proband SRS total scores. In contrast, only paternal BAPQ pragmatic and BAPQ total scores related to SRS total scores in probands overall. No association was observed between paternal BAPQ rigid or BAPQ aloof and proband SRS scores overall.
Repetitive Behavior Scale–Revised.
Maternal BAPQ pragmatic, rigid, and total scores were all associated with proband RBS-R total scores overall. In contrast, paternal BAPQ rigid, total, and pragmatic scores overall (Figure S3B) were significantly positively associated with proband RBS-R total scores. No significant association between paternal or maternal BAPQ aloof scores and proband RBS-R total scores emerged.
Genotype–Phenotype Associations
Further details of associations between genotype and phenotype are given in Figure 2 and Table 3.
Figure 2.
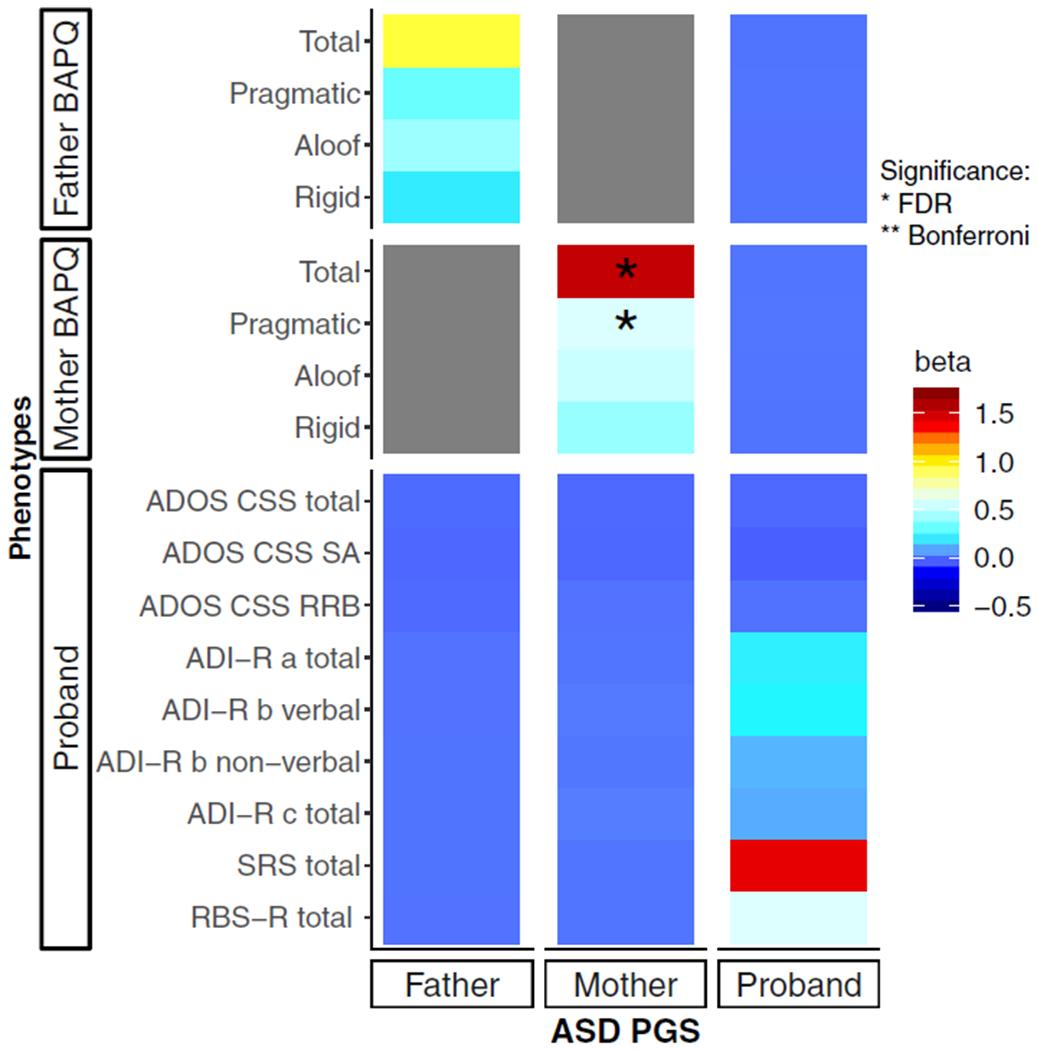
Heat map of associations between paternal, maternal, and proband polygenic scores with clinical-behavioral features of autism spectrum disorder and the broad autism phenotype. a, reciprocal social interaction domain; ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; b, communication abnormalities domain; BAPQ, Broad Autism Phenotype Questionnaire; c, restricted and repetitive behavior domain; CSS, calibrated severity score; FDR, false discovery rate; PGS, polygenic score; RBS-R, Repetitive Behavior Scale–Revised; RRB, restricted and repetitive behaviors and interests domain; SA, social affect subscale; SRS, Social Responsiveness Scale. *Associations passing the FDR-corrected significance threshold.
Parental BAPQ Phenotypes and Parental ASD-PGSs.
Maternal polygenic scores were associated with BAPQ pragmatic and total scores. However, paternal ASD-PGSs were not associated with any phenotype tested. Additionally, the effect estimates of maternal PGSs on maternal pragmatic, rigid, and total BAPQ scores were increased compared with the effect estimates of paternal PGSs on paternal BAPQ scores. No BAPQ domain showed a significant interaction between sex and ASD-PGS (aloof p = .489, pragmatic p = .243, rigid p = .331, total p = .249). See Figure S4.
Proband Phenotypes and Proband ASD-PGSs.
Proband ASD-PGSs were not associated with any phenotype tested.
Parental BAPQ Phenotypes and Proband ASD-PGSs.
Proband ASD-PGSs did not show any association with mother’s or father’s BAPQ scores.
Proband Phenotypes and Parental ASD-PGSs.
None of the tested proband phenotypes showed an association with maternal or paternal ASD-PGS.
DISCUSSION
This study examined PGSs within families of individuals with ASD, with the goal of exploring parent and child genotype–phenotype and phenotype–phenotype associations, as well as familial polygenic liability associated with ASD. While previous studies examined transmission disequilibrium in ASD genetic variants (52), this study evaluated the role of the familial relationship as an explanation for phenotypic similarity. Overall, results demonstrated relationships between parent and child clinical-behavioral phenotypes regardless of IQ, as well as associations between maternal PGSs and features of the BAP. Given the likelihood of increased de novo mutations in simplex families studied here, our results further emphasize the role of inherited genetic risk associated with ASD and the constituent features of ASD and the BAP.
Consistent with prior work (52,53), despite elevated scores of BAP trait expression emerging in fathers versus mothers, results revealed more robust relationships between maternal BAPQ scores and child clinical-behavioral traits (see Fisher’s z Transformation Tests Assessing the Strength of Phenotypic Associations in the Supplement). Maternal language-related phenotypes were consistently associated with more severe ASD symptomatology in probands, including strong associations between maternal pragmatic language scores and children’s social and nonverbal communication skills measured by the ADI-R, particularly in those children with lower IQ. Similar parent–child associations have been observed in other studies examining language-related phenotypes, where subtle differences in language fluency in mothers with the BAP were related to more severe symptoms in their children with ASD (19) [although a recent study in a smaller sample of mothers and fathers showed no relationships between maternal pragmatic language and child language features (54)]. Such a pattern of lineality may suggest a stronger inherited maternal effect for language-related phenotypes in ASD (though parents and children influence one another’s language patterns as well). Whereas the maternal effect appeared to be centered around language-related phenotypes, paternal BAP features appeared to be more associated with the RRB/rigid domain, particularly in those children with higher IQ. In contrast, all domains of the BAP in mothers were related to RRBs in probands regardless of proband IQ. Despite there being no prior family history of ASD in the families included in this study, these patterns of familiality may be further evidence that constituent features related to ASD combine additively to increase ASD risk (55).
One important consideration is that all associations between parent and child phenotypes emerged in parent-report measures (i.e., SRS, RBS-R, ADI-R) where mothers were typically the informant. It is possible that reporting bias existed that may have influenced results (although this possibility is true for most studies examining parent–child correlates, where data are generally derived from questionnaires completed by parents). It is also possible that because questionnaire measures typically provide a wider range of scores, variability is better captured (e.g., the RBS-R total scores in the present sample range from 0 to 105 [Table 2] compared with the ADOS severity scores, which range from 1 to 10). Significant associations emerging from parent–child association analyses of the ADI-R (and not the ADOS) could also be due to the different age ranges tapped by these measures —i.e., the ADI-R emphasizes early development, when ASD traits are often most apparent, whereas the ADOS captures a small window of current behaviors during the 40- to 60-minute direct-assessment period.
In line with findings from phenotypic analyses within families, analyses of parents’ ASD-PGSs also demonstrated a robust maternal genetic effect on mothers’ phenotypes, such that polygenic variants associated with ASD predicted maternal BAP features, including pragmatic language differences. The effect estimates of maternal PGSs on BAPQ scores were in some cases almost double the effect of paternal PGSs on paternal BAPQ scores, which could indicate an effect of sex on ASD-risk genes on the BAP. However, interaction tests showed that the ASD-PGSs of mothers and fathers did not have statistically different effect estimates with BAPQ domains. Since interaction terms require a substantially larger sample size to achieve statistical significance than main effects, the lack of a significant interaction could be due to low power rather than lack of true biologic differences. Thus, larger studies with greater power to detect sex interactions should consider conducting similar analyses. Sex differences are well documented in both diagnostic rates and phenotypic expression in male and female subjects with ASD (56,57), and they have been hypothesized to result from a female protective effect (58), where female subjects require greater inherited risk than males to exhibit ASD. Although large-scale studies have shown no appreciable difference in the common variant liability between male and female subjects with ASD (59), studies have shown an enrichment of loss-of-function de novo variants and rare copy number variants in female cases compared with controls (60). The different phenotypic and genotypic–phenotypic associations that emerged among mothers versus fathers may provide evidence for a female genetic protection for common variation as it relates to the ASD phenotypic spectrum. Mothers with increased BAPQ scores showed no difference in ASD-PGSs compared with male probands with ASD, whereas fathers with increased BAPQ scores showed significantly lower ASD-PGSs than male probands with ASD (see Figures S5 and S6 and Associations Between High BAPQ Parents and Male Probands Polygenic Scores in the Supplement). Moreover, father BAPQ scores were correlated with some phenotypes in children with IQ >70, while mother BAPQ scores were associated more broadly with phenotypes among all children (i.e., both low- and high-IQ groups) (see IQ-Stratified Phenotype-Phenotype Associations in the Supplement). Taken together, though they should be interpreted with caution, these findings are consistent with the possibility of a female protective effect (60,61) and warrant further investigation in additional cohorts. The discrepancy between studies may be due to the generally smaller female ASD sample sizes included in prior work (56) in comparison with the larger sample sizes of unaffected mothers with increased genetic liability to ASD included in this study, likely increasing power to detect a female protective effect on domain phenotypes. As such, this study may inform understanding of familial transmission of ASD-related traits, which may help to enhance power to detect genetic phenotypic variants associated with ASD.
Finally, the lack of association of proband ASD-PGSs or parental BAP scores with proband clinical-behavioral features, as well as of parental PGSs with proband clinical-behavioral phenotypes, were somewhat surprising. Lack of association may be a reflection of the phenotypic homogeneity present in the SSC sample (given the stringent selection criteria of the SSC) and/or potentially higher intellectual ability of parents in the SSC (though this metric was not directly assessed in parents in the SSC sample), which has been found to be associated with greater polygenic risk (36,62–64). Additionally, the large age range in ASD probands may affect the ability to find genetic associations with social communication traits, which have been shown to vary during adolescence (65). Inclusion of only simplex families from the SFARI (Simons Foundation Autism Research Initiative) Base SSC may additionally explain the lack of findings, with theorized additive genetic risk more commonly occurring in multiplex versus simplex families (66). Furthermore, the SSC excluded families in which ASD was suspected in parents, potentially reducing the variance of BAP features in the sample and reducing the ability to find associations with BAP features. A final explanation may be linked to the differential genetic architectures underlying ASD traits more broadly and social communication traits more specifically (65,67–69), which diminishes the likelihood of associations between ASD-risk genes and communication phenotypic traits. As such, future studies should consider the inclusion of multiplex families and may also benefit from the inclusion of additional BAP assessments in analyses, as questionnaires may limit the sensitivity of BAP trait detection given potential reporting biases in self-report measures of BAP traits (38).
In sum, this study revealed key associations of ASD-related features in probands with ASD and the parental BAP, with effects on mothers emerging in the language-related social communication domain, and paternal phenotypic effects in the rigid/RRB domain. Additionally, ASD-PGS associations with the BAP emerged only in mothers, highlighting the potential for a female protective factor that may also be expressed among first-degree relatives of individuals with ASD. Together, findings from this study underscore the significance of the BAP in parents, which may reflect more influences from common genetic variation and polygenic risk rather than rare variation that contributes to aggregate and heterogeneous ASD (26).
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Deposited Data; Public Database | Researchers can request access to phenotypic, genetic, or imaging data on the SSC through the SFARI Base (https://www.sfari.org/resource/sfari-base/) | The SSC is described in this manuscript by Fischbach et al, Neuron 2010 (https://doi.org/10.1016/j.neuron.2010.10.006) | All individuals in the SSC who met our filtering requirements described in the manuscript were included in the analysis. |
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by grants from the National Institutes of Health (Grant Nos. R01DC010191 and R01MH091131 [to principal investigator ML; KN, LB] and Grant Nos. U54MD010722-04, R01NS102371,R01MH113362, U01HG009086, R01MH118223, DP2HD98859, R01DC16977, R01NS105746, R56MH120736, R21HG010652, and RM1HG009034 [to LKD]) and the National Institutes of Health National Institute of General Medical Sciences (Grant No. 5T32GM080178–12 [JMS; principal investigator, Nancy J. Cox]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ML, LKD, and EHC conceived and designed the study and oversaw all analyses and writing of the manuscript. KN and JMS led data processing, analysis, and manuscript preparation. NM and LB contributed to data processing and analysis. NM also contributed to manuscript preparation. All authors read and approved the final manuscript.
We are grateful to all of the families at the participating SSC sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R. Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). We appreciate obtaining access to phenotypic and genetic data on SFARI Base. Approved researchers can obtain the SSC population dataset described in this study (https://www.sfari.org/resource/simons-simplex-collection/) by applying at https://base.sfari.org/ We also thank Dr. Richard Anney for providing the summary statistics from the ASD working group of the Psychiatric Genomics Consortium excluding the SSC samples.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://doi.org/10.1016/j.biopsych.2020.08.029.
REFERENCES
- 1.Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. (2018): Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folstein S, Rutter M (1977): Genetic influences and infantile autism. Nature 265:726–728. [DOI] [PubMed] [Google Scholar]
- 3.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995): Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med 25:63–77. [DOI] [PubMed] [Google Scholar]
- 4.Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F (2016): Heritability of autism spectrum disorders: A meta-analysis of twin studies. J Child Psychol Psychiatry 57:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong EHF, Fox JC, Ng MYM, Lee C-M (2011): Toward personalized medicine in the neuropsychiatric field. Int Rev Neurobiol 101:329–349. [DOI] [PubMed] [Google Scholar]
- 6.Beauchaine TP, Constantino JN (2017): Redefining the endophenotype concept to accommodate transdiagnostic vulnerabilities and etiological complexity. Biomark Med 11:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodbury-Smith M, Nicolson R, Zarrei M, Yuen RKC, Walker S, Howe J, et al. (2017): Variable phenotype expression in a family segregating microdeletions of the NRXN1 and MBD5 autism spectrum disorder susceptibility genes. NPJ Genomic Med 2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop DVM, Maybery M, Wong D, Maley A, Hill W, Hallmayer J (2004): Are phonological processing deficits part of the broad autism phenotype? Am J Med Genet 128B:54–60. [DOI] [PubMed] [Google Scholar]
- 9.Frazier TW, Youngstrom EA, Hardan AY, Georgiades S, Constantino JN, Eng C (2015): Quantitative autism symptom patterns recapitulate differential mechanisms of genetic transmission in single and multiple incidence families. Mol Autism 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurley RSE, Losh M, Parlier M, Reznick JS, Piven J (2007): The Broad Autism Phenotype Questionnaire. J Autism Dev Disord 37:1679–1690. [DOI] [PubMed] [Google Scholar]
- 11.Sasson NJ, Lam KSL, Parlier M, Daniels JL, Piven J (2013): Autism and the broad autism phenotype: Familial patterns and intergenerational transmission. J Neurodev Disord 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Losh M, Esserman D, Piven J (2010): Rapid automatized naming as an index of genetic liability to autism. J Neurodev Disord 2:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdts JA, Bernier R, Dawson G, Estes A (2013): The broader autism phenotype in simplex and multiplex families. J Autism Dev Disord 43:1597–1605. [DOI] [PubMed] [Google Scholar]
- 14.Stern YS, Maltman N, Roberts MY (2017): The influence of maternal pragmatics on the language skills of children with autism. J Dev Behav Pediatr 38:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klusek J, Martin GE, Losh M (2014): Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. J Intellect Disabil Res 58:940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin-Decanini T, Maltman N, Francis SM, Guter S, Anderson GM, Cook EH, Jacob S (2013): Parental broader autism subphenotypes in ASD affected families: Relationship to gender, child’s symptoms, SSRI treatment, and platelet serotonin. Autism Res 6:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Losh M, Martin GE, Lee M, Klusek J, Sideris J, Barron S, Wassink T (2017): Developmental markers of genetic liability to autism in parents: A longitudinal, multigenerational study. J Autism Dev Disord 47:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Losh M, Adolphs R, Piven J (2011): The broad autism phenotype In: Dawson G, Amaral D, Geschwind D, editors. Autism Spectrum Disorders. Oxford: Oxford University Press, 457–476. [Google Scholar]
- 19.Nayar K, Gordon PC, Martin GE, Hogan AL, La Valle C, McKinney W, et al. (2018): Links between looking and speaking in autism and first-degree relatives: Insights into the expression of genetic liability to autism. Mol Autism 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan-Brown AL, Hoedemaker RS, Gordon PC, Losh M (2014): Eye-voice span during rapid automatized naming: Evidence of reduced automaticity in individuals with autism spectrum disorder and their siblings. J Neurodev Disord 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, Piven J (2009): Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiatry 66:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Losh M, Childress D, Lam K, Piven J (2008): Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet 147:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantino JN, Todd RD (2003): Autistic traits in the general population: A twin study. Arch Gen Psychiatry 60:524–530. [DOI] [PubMed] [Google Scholar]
- 24.Piven J, Wzorek M, Landa R, Lainhart J, Bolton P, Chase G, Folstein S (1994): Personality characteristics of the parents of autistic individuals. Psychol Med 24:783–795. [DOI] [PubMed] [Google Scholar]
- 25.Happé F, Ronald A, Plomin R (2006): Time to give up on a single explanation for autism. Nat Neurosci 9:1218–1220. [DOI] [PubMed] [Google Scholar]
- 26.Gottesman II, Gould TD (2003): The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry 160:636–645. [DOI] [PubMed] [Google Scholar]
- 27.Davidson J, Goin-Kochel RP, Green-Snyder LA (2014): Expression of the broad autism phenotype in simplex autism families from the Simons Simplex Collection. J Autism Dev Disord 44:2392–2399. [DOI] [PubMed] [Google Scholar]
- 28.Woodbury-Smith M, Paterson AD, O’Connor I, Zarrei M, Yuen RKC, Howe JL, et al. (2018): A genome-wide linkage study of autism spectrum disorder and the broad autism phenotype in extended pedigrees. J Neurodev Disord 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De La Torre-Ubieta L, Won H, Stein JL, Geschwind DH (2016): Advancing the understanding of autism disease mechanisms through genetics. Nat Med 22:345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A (2014): The familial risk of autism. JAMA 311:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vorstman JAS, Parr JR, Moreno-De-Luca D, Anney RJL, Nurnberger JI, Hallmayer JF (2017): Autism genetics: Opportunities and challenges for clinical translation. Nat Rev Genet 18:362–376. [DOI] [PubMed] [Google Scholar]
- 32.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. (2014): Most genetic risk for autism resides with common variation. Nat Genet 46:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, et al. (2016): Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet 48:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anney R, Klei L, Pinto D, Almeida J, Bacchelli E, Baird G, et al. (2012): Individual common variants exert weak effects on the risk for autism spectrum disorders. Hum Mol Genet 21:4781–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. (2019): Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 51:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke TK, Lupton MK, Fernandez-Pujals AM, Starr J, Davies G, Cox S, et al. (2016): Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol Psychiatry 21:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischbach GD, Lord C (2010): The Simons Simplex Collection: A resource for identification of autism genetic risk factors. Neuron 68:192–195. [DOI] [PubMed] [Google Scholar]
- 38.Sasson NJ, Nowlin RB, Pinkham AE (2013): Social cognition, social skill, and the broad autism phenotype. Autism 17:655–667. [DOI] [PubMed] [Google Scholar]
- 39.Lord C, Risi S, Lambrect L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. (2000): The Autism Diagnostic Observation Schedule–Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223. [PubMed] [Google Scholar]
- 40.Gotham K, Risi S, Pickles A, Lord C (2007): The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. J Autism Dev Disord 37:613–627. [DOI] [PubMed] [Google Scholar]
- 41.Lord C, Rutter M, Le couteur A (1994): Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:1–27. [DOI] [PubMed] [Google Scholar]
- 42.Lam KSL, Aman MG (2007): The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. J Autism Dev Disord 37:855–866. [DOI] [PubMed] [Google Scholar]
- 43.Constantino JN (2013): Social Responsiveness Scale In: Volkmar FR, editor. Encyclopedia of Autism Spectrum Disorders. New York, NY: Springer, 2919–2929. [Google Scholar]
- 44.Constantino JN, Todd RD (2005): Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry 57:655–660. [DOI] [PubMed] [Google Scholar]
- 45.Rutter M, Bailey A, Lord C (2003): The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- 46.Aman MG (2013): Aberrant Behavior Checklist In: Volkmar FR, editor. Encyclopedia of Autism Spectrum Disorders. New York, NY: Springer, 10–17. [Google Scholar]
- 47.Achenbach TM, Rescorla LA (2001): Manual for the ASEBA School-Age Forms and Profile. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- 48.Sparrow SS, Cicchetti DV (1989): The Vineland Adaptive Behavior Scales In: Newmark CS, editor. Major Psychological Assessment Instruments, vol 2 Boston, MA: Allyn and Bacon, 199–231. [Google Scholar]
- 49.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. (2007): PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge T, Chen CY, Ni Y, Feng YCA, Smoller JW (2019): Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun 10:1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennis M, Francis D, Cirino P, Schachar R, Barnes M, Fletcher J (2009): Why IQ is not a covariate in cognitive studies of neuro-developmental disorders. J Int Neuropsychol Soc 15:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner DJ, Wigdor EM, Ripke S, Walters RK, Kosmicki JA, Grove J, et al. (2017): Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet 49:978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubenstein E, Chawla D (2018): Broader autism phenotype in parents of children with autism: A systematic review of percentage estimates. J Child Fam Stud 27:1705–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasson NJ, Faso DJ, Parlier M, Daniels JL, Piven J (2014): When father doesn’t know best: Selective disagreement between self-report and informant report of the broad autism phenotype in parents of a child with autism. Autism Res 7:731–739. [DOI] [PubMed] [Google Scholar]
- 55.Flippin M, Watson LR (2018): Parental broad autism phenotype and the language skills of children with autism spectrum disorder. J Autism Dev Disord 48:1895–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pohl A, Jones WR, Marrus N, Zhang Y, Klin A, Constantino JN (2019): Behavioral predictors ofautism recurrence are genetically independent and influence social reciprocity: Evidence that polygenic ASD risk is mediated by separable elements of developmental liability. Transl Psychiatry 9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S (2015): Sex/genderdifferences and autism: Setting the sceneforfuture research. J Am Acad Child Adolesc Psychiatry 54:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young H, Oreve MJ, Speranza M (2018): Clinical characteristics and problems diagnosing autism spectrum disorder in girls. Arch Pediatr 25:399–403. [DOI] [PubMed] [Google Scholar]
- 59.Tsai L, Stewart MA, August G (1981): Implication of sex differences in the familial transmission of infantile autism. J Autism Dev Disord 11:165–173. [DOI] [PubMed] [Google Scholar]
- 60.Mitra I, Tsang K, Ladd-Acosta C, Croen LA, Aldinger KA, Hendren RL, et al. (2016): Pleiotropic mechanisms indicated for sex differences in autism [published correction appears in PLoS Genet 2017 13: e1006831]. PLoS Genet 12:e1006425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, et al. (2014): A higher mutational burden in females supports a “female protective model” in neurodevelopmental disor-ders. Am J Hum Genet 94:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Constantino JN (2016): Data from the Baby Siblings Research Consortium confirm and specify the nature of the female protective effect in autism: A commentary on Messinger et al. Mol Autism 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. (2015): An atlas of genetic correlations across human diseases and traits. Nat Genet 47:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. (2016): Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie SJ, et al. (2016): Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol Psychiatry 21:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.St Pourcain B, Skuse DH, Mandy WP, Wang K, Hakonarson H, Timpson NJ, et al. (2014): Variability in the common genetic architecture of social-communication spectrum phenotypes during childhood and adolescence. Mol Autism 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klei L, Sanders SJ, Murtha MT, Hus V, Lowe JK, Willsey AJ, et al. (2012): Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Constantino JN (2018): Deconstructing autism: from unitary syndrome to contributory developmental endophenotypes. Int Rev Psychiatry 30:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ronald A, Happé F, Plomin R (2005): The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Dev Sci 8:444–458. [DOI] [PubMed] [Google Scholar]
- 70.Sasson NJ, Lam KSL, Childress D, Parlier M, Daniels JL, Piven J (2013): The Broad Autism Phenotype Questionnaire: Prevalence and diagnostic classification. Autism Res 6:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


