Abstract
Orofacial clefts (OFCs) are among the most common birth defects and impart a significant burden on afflicted individuals and their families. It is increasingly understood that many non-syndromic OFCs are a consequence of extrinsic factors, genetic susceptibilities, and interactions of the two. Therefore, understanding environmental mechanisms of OFCs is important in the prevention of future cases. This review examines the molecular mechanisms associated with environmental factors that either protect against or increase the risk of OFCs. We focus on essential metabolic pathways, environmental signaling mechanisms, detoxification pathways, behavioral risk factors, and biological hazards that may disrupt orofacial development.
1. Introduction
Orofacial cleft (OFC) is among the most common birth defects affecting newborns and occurs at an average frequency of 1 out of 700 live births. Craniofacial structures are formed within the first ten weeks of human development, involving a tight coordination between molecular signaling pathways (Reynolds et al., 2020) (in this issue) and cellular processes such as proliferation, migration, differentiation, transition, and apoptosis (Ji et al., 2020) (in this issue). Perturbation of any of these processes could offset the delicate balance required for normal craniofacial morphogenesis and results in birth defects such as OFCs. There are significant personal, social, and financial burdens associated with OFCs among afflicted individuals and their families. Further, those suffering from OFCs may be at increased risk of associated morbidities (Silva et al., 2018). It is therefore a priority to understand all aspects of OFC etiology so that it may be prevented when possible. Syndromic OFCs are typically associated with genetic mutation or chromosomal abnormalities. Non-syndromic OFCs (to which “OFC” refers from here on) are believed to be multifactorial and frequently involve genetic susceptibility to environmental factors (gene-environment interactions, or GxE). Epigenetic processes such as DNA methylation or histone modification, as well as non-coding microRNAs that regulate gene expression, are also influenced by environmental factors and may account for OFCs (Garland et al., 2020) (in this issue).
Generally, OFCs are divided into two classes: cleft lip with or without cleft palate (CL/P) and cleft palate only (CPO). There is also evidence that cleft lip only (CLO) is a distinct class, although this can be difficult to discern in severe CLO cases since palatogenesis may depend on lip fusion (Harville, Wilcox, Lie, Vindenes, & Abyholm, 2005). Orofacial development involves several morphogenetic steps, molecular signaling pathways, and epithelial-mesenchymal interactions that can be affected by extrinsic factors (Ji et al., 2020; Reynolds et al., 2020) (in this issue). Lip development precedes palatogenesis, the latter of which can be generalized to involve palatal shelf growth, elevation, and fusion. During fusion, cells of the medial edge epithelium (MEE) from both shelves merge, becoming the medial epithelial seam (MES) and disappearing through apoptosis, migration, and epithelial-mesenchymal transition (EMT). These processes involve signaling through various pathways such as Wnt, fibroblast growth factor (Fgf), transforming growth factor beta (Tgf-β), epidermal growth factor (Egf), Notch, Hedgehog, and others. In some cases, these processes can be perturbed by environmental factors that alter gene expression and offset the delicate balance of developmental signaling (X. Z. Liu et al., 2020). In other cases, genetic susceptibilities can adversely affect orofacial development but only in the context of an environmental stressor. For example, a recent study found that deficiency of the transcription factor Msx1 does not affect murine lip fusion unless the mother endures hypoxic stress (Nakatomi et al., 2020). Other stressors, like oxidative stress mediated by reactive oxygen species (ROS), can directly damage biomolecules and do not necessarily require genetic susceptibility to cause OFCs (Larouche & Hales, 2009).
Understanding the downstream molecular effects of environmental risk factors may be informative for health professionals that issue guidance for protecting human developmental health. In this review, we present a survey on recent studies examining environmental effects on the risk of oral clefts. We begin with metabolism of the essential nutrients, folates and retinoids, and their role in orofacial development. We then describe three major environmental signaling pathways and their potential interactions in mediating teratogenicity. Next, we describe how detoxification pathways and associated genetic variants could influence OFCs. Behavioral risk factors, such as smoking tobacco, drinking alcohol, or occupational exposures, and possible mechanisms are also reviewed. Finally, we consider evidence for the involvement of pathogenic factors in OFC etiology.
2. Metabolism of folates and retinoids in orofacial clefts
2.1. Folic acid metabolism
Folate (vitamin B9) is an essential hydrophilic nutrient required for 1-carbon reactions in various cellular processes. Animals are unable to produce folate de novo and must obtain it through the diet. Some folate-dependent bioactivities include the synthesis of nucleotides, methionine, and polyamines, as well as the methylation of biomolecules including DNA, RNA, proteins, lipids, and other small molecules (Crider, Yang, Berry, & Bailey, 2012). Folate is therefore required for cell proliferation, differentiation, repair, and other processes necessary for development such as chromatin remodeling and genetic imprinting (Loenen, 2006). It is particularly critical for neural tube development, as folate deficiency is a well-described risk factor for neural tube defect (NTD) pathologies like spina bifida. There is increasing laboratory and epidemiological evidence that supplementation with folate, usually through the synthetic form of folic acid, is also protective against OFCs. For the average adult, the recommended daily intake of folate is 400 μg, while for pregnant women the recommended amount is > 450 μg per day based on meta-analysis (Marchetta et al., 2015).
Folate metabolism is a complex process with various regulatory mechanisms, feedback loops, and interacting pathways. While this is reviewed in greater detail elsewhere (Lucock, 2000), we provide a brief overview of the folate cycle and its relationship with the methionine cycle. Once obtained through the diet, folic acid is typically metabolized in the small intestine and liver (Crider et al., 2012). Metabolism starts with the sequential reduction of folic acid to dihydrofolate and tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (DHFR). THF is subsequently converted into 5,10-methylene-THF by serine hydroxymethyltransferase (SHMT), which uses pyridoxine (vitamin B6) as a cofactor. Finally, methylenetetrahydrofolate reductase (MTHFR) converts 5,10-methylene-THF into 5-methyl-THF, the primary folate metabolite utilized by non-hepatic cells.
Once in circulation, 5-methyl-THF is brought to the membrane of target cells where it enters the cytoplasm through the activities of folate carrier and binding proteins (Lucock, 2000). The 1-carbon cycle begins with conversion of 5-methyl-THF to THF by methionine synthetase, which uses cobalamin (vitamin B12) as a cofactor (Crider et al., 2012). This reaction creates methionine by transferring a methyl group from 5-methyl-THF to homocysteine. Methionine, in turn, can be converted to S-adenosylmethionine (SAM) by SAM synthetase (Loenen, 2006). Through the activities of methyltransferases, SAM is the methyl donor for most biomolecules and is required for epigenetic modifications such as DNA methylation and the post-translational methylation of histone residues. SAM becomes S-adenosylhomocysteine (SAH) after donating its methyl group, which is subsequently converted to homocysteine by S-adenosylhomocysteine hydrolase (SAHH). Homocysteine again becomes available as a substrate for methionine synthesis by using THF, thus completing the methionine cycle. Choline/betaine metabolism also interacts with the methionine cycle by providing trimethylglycine, which can donate methyl groups to homocysteine through the activity of betaine-homocysteine methyltransferase (BHMT) (Barak, Beckenhauer, Junnila, & Tuma, 1993).
In 1998, the United States Food and Drug Administration implemented mandatory fortification of grains with folic acid (Food & Administration, 1996). The goal was to protect the population against NTDs, but the decision may also have been beneficial for preventing OFCs. A cross-sectional study among the Californian population found a decreased prevalence of both CL/P (PR = 0.91, 95% CI: 0.82, 1.00) and CPO (PR = 0.81, 95% CI: 0.70, 0.93) following mandatory grain fortification with folic acid (Yang, Carmichael, & Shaw, 2016). However, a study on voluntary fortification in Australia observed a decrease risk only for NTDs and not for other birth defects among the West Australian population (Bower, Miller, Payne, & Serna, 2006). Similarly, a Cochrane review found no evidence for a protective effect of folate supplementation against OFCs, although the result was considered low quality evidence (De-Regil, Pena-Rosas, Fernandez-Gaxiola, & Rayco-Solon, 2015). Despite disagreement in the literature, there is some additional evidence that folate is protective against OFCs. A recent systematic review concluded that folic acid could be protective in high dosages (Jayarajan, Natarajan, & Nagamuttu, 2019). Folate appears to be most protective when supplement starts early relative to conception. Recent meta-analysis found that folic acid supplementation was only protective when administered periconceptionally (OR = 0.64, 95% CI: 0.56, 0.74) versus during pregnancy (OR = 0.90, 95% CI: 0.71, 1.14) (Jahanbin, Shadkam, Miri, Shirazi, & Abtahi, 2018). Similarly, another study found that supplementation during the first trimester was protective (OR = 0.14, 95% CI: 0.05, 0.41), but not during the second trimester (OR = 9.79, 95% CI: 3.47, 27.50) (Figueiredo et al., 2015). Because the metabolism and utilization of folate requires co-factors, it is likely that folic acid supplementation is augmented by the intake of multivitamins. A study in the Netherlands found that thiamine and pyridoxine protected against orofacial clefts, but only in the context of folic acid supplementation (Krapels et al., 2004). This is consistent with the role of pyridoxine in THF metabolism (Crider et al., 2012), while thiamine may be related to folates by common transport proteins (Zhao & Goldman, 2013).
Dozens of studies on genetic variants related to the folate cycle and risk for OFCs have been conducted, as previously reviewed (Bhaskar, Murthy, & Babu, 2011). Although evidence is often equivocal, human polymorphisms in folate-associated genes may either increase or decrease the risk of OFCs. Genetic variation has been hypothesized to explain why folic acid fortification has been protective against OFCs is some regions but not others (Nazer & Cifuentes, 2014). Recent studies in the Chilean population serve as an example of how GxE interactions affect the relationship between OFCs and folate metabolism. Among CL/P cases, a polymorphism of the SHTM1 gene was less frequent than in control cases (Salamanca et al., 2020b). The authors postulated that the mutation protected against CL/P by decreasing enzymatic activity, thereby increased cellular folate levels. Three intronic alleles of MTR, involved in SAM metabolism, may also be protective against CL/P (Salamanca et al., 2020a). In contrast, the most widely documented polymorphism in folate metabolism, MTHFR c.677C>T, decreases enzymatic activity and has been associated with increased risk for CL/P (Ramirez-Chau, Blanco, Colombo, Pardo, & Suazo, 2016). Interaction between maternal folic acid intake and this mutation has been previously described (I. A. van Rooij, Swinkels, Blom, Merkus, & Steegers-Theunissen, 2003). Meta-analysis of 15 studies indicated that maternal 677C>T mutation increased risk of CL/P, demonstrated an important role for maternal metabolism of folates in OFC pathology (Pan et al., 2015). Polymorphisms of other folate-related genes are associated with OFCs, as described in a companion review (Reynolds et al., 2020) (in this issue).
There are several possible mechanisms by which folates can be protective against OFCs. One is that the folate cycle supports nucleotide synthesis, allowing for the rapid genome replication and cell proliferation required in orofacial development. Because of this role, folate deficiency is also linked with aberrant DNA repair due to misincorporation of uracil, leading to a catastrophic repair cycle and destabilization of the genome (Duthie, Narayanan, Brand, Pirie, & Grant, 2002). Additionally, many folate-dependent downstream processes are mediated by SAM. Some of these include the synthesis of polyamines, which are involved in cell proliferation, differentiation, and apoptosis (Bjelakovic et al., 2017). SAM is also a substrate for DNA methyltransferases involved in epigenetic modifications, specifically DNA methylation and post-translational methylation of histone residues (Garland et al., 2020) (in this issue). Folate deficiency could additionally result in the accumulation of homocysteine to toxic levels (hyperhomocysteinemia), which has been linked to OFCs (Lonn et al., 2006; Wong et al., 1999). Hyperhomocysteinemia can increase cellular asymmetric dimethylarginine which generates ROS and oxidative stress (Tyagi et al., 2005). Thus, the protective effects of folate against OFCs likely stems from its various roles associated with cell proliferation, differentiation, and physiological homeostasis.
2.2. Retinoic acid metabolism
Retinoic acid (RA), a metabolite derived from retinol (vitamin A), is a small lipophilic molecule with critical roles in tissue patterning and cell fate specification during embryogenesis (Rhinn & Dolle, 2012). In most animals, retinoids must be obtained environmentally through the diet as retinol, retinyl esters, or carotenoids (e.g., β-carotene) (Finnell et al., 2004). Retinol, the primary circulating retinoid, is obtained by embryos through transplacental circulation in placental mammals (Spiegler, Kim, Wassef, Shete, & Quadro, 2012). Retinoid metabolism involves several steps mediated by binding proteins, transporters, and metabolic enzymes, many of which are controlled through feedback mechanisms intrinsic to RA-mediated signaling (Okano, Udagawa, & Shiota, 2014; Rhinn & Dolle, 2012). Overabundance (Dekker et al., 1994) or knockout (Halilagic et al., 2007) of these proteins has perturbed orofacial development in laboratory studies.
Once in circulation following dietary or pharmacological exposure, retinol is bound by retinol binding protein 4 (RBP4) and transported to the cell membrane (Rhinn & Dolle, 2012). Because maternal RBP4 cannot cross the placenta, retinol must diffuse across the yolk sac and placenta to bind with embryonic RBP4, which is synthesized in the latter structure (S. J. Ward, Chambon, Ong, & Bavik, 1997). Embryonic RBP4 facilitates retinol circulation to a target cell, where the transmembrane protein signaling receptor and transporter of retinol (STRA6) transports retinol across the cell membrane and into the cytoplasm (Kawaguchi et al., 2007). At this stage, the pathway splits—retinol may be processed for storage, or it may continue through the RA metabolic pathway for bioactivation. For storage, retinol is processed into retinyl ester by lecithin retinol acyltransferase (LRAT) (Ruiz et al., 1999). In target cells, retinol is instead oxidized into retinaldehyde by enzymes belonging to either of two enzyme families: cytosolic alcohol dehydrogenases (ADHs) or microsomal retinol dehydrogenases (RDHs) (Pares, Farres, Kedishvili, & Duester, 2008). Retinaldehyde serves as a substrate for one of three tissue-specific isoforms of retinaldehyde dehydrogenases (RALDHs; RALDH1, RALDH2, and RALDH3) which oxidize it into bioactive RA (Niederreither & Dolle, 2008).
RA signaling includes feedback mechanisms that alter the abundances of proteins that control the cellular RA pool across tissues. An important negative feedback loop is the increased expression of cytochrome P450 family member 26 enzymes (CYP26A1, CYP26B1, and CYP26C1), which oxidize RA into metabolites with increased polarity such as 4-hydroxy-retinoic acid and 4-oxo-retinoic acid (Chithalen, Luu, Petkovich, & Jones, 2002). While these are still bioactive metabolites, they are subject to conjugation reactions that facilitate their elimination (Rhinn & Dolle, 2012). In mice, double knockout of Raldh2 and Raldh3 resulted in severe craniofacial malformations including failure of frontonasal fusion (Halilagic et al., 2007), while knockout of Cyp26b1 prevented tongue depression and horizontal elevation of the palatal shelves (Okano et al., 2014). Upstream of these enzymes, knockout of Rdh10 demonstrated a role for RA synthesis in spontaneous neuromuscular movement of the tongue and mandible, which facilitates palatal shelf elevation (Friedl et al., 2019). Thus, the cellular pool of RA must be tightly regulated for appropriate orofacial morphogenesis.
In human populations, there is some evidence for polymorphisms of genes associated with RA metabolism and signaling that could increase the risk for OFCs. A study on Han and Uyghur populations in China found an association between offspring RBP4 polymorphism and increased risk for CL/P using a machine learning model (S. J. Zhang et al., 2018). Two other studies in Chinese populations and a study in an Indian population found correlation between RARA polymorphism and increased risk for CL/P (Fan, Li, & Wu, 2007; Peanchitlertkajorn, Cooper, Liu, Field, & Marazita, 2003; Xavier et al., 2013).
Teratogenic levels of retinoids have been reached during pregnancy through pharmacological and dietary sources. Exposure to the retinoid-based acne medication isotretinoin, for example, was associated with birth defects including cleft palate (Lammer et al., 1985). Excess vitamin A (hypervitaminosis A) or deficiency (hypovitaminosis A) can both adversely affect orofacial development. Daily supplementation of vitamin A in excess of 10000 IU (approximately 3000 μg) has been associated with cleft lip in humans (Rothman et al., 1995). High levels of dietary vitamin A have also induced cleft palate in mice (Inomata et al., 2005), rats (Mineshima et al., 2012), cats (Freytag, Liu, Rogers, & Morris, 2003), and dogs (Wiersig & Swenson, 1967). Conversely, some recent studies have found that vitamin A consumption is protective against OFCs. A population study in Norway found a decreased risk of CPO among pregnant women with daily intake of vitamin A ranging between 1911.1 - 9640.0 μg (OR = 0.47, 95% CI: 0.24, 0.94) (Johansen, Lie, Wilcox, Andersen, & Drevon, 2008). Similar protective effects were observed for β-carotene intake ranging between 4,531 – 26,903 μg (OR = 0.50, 95% CI: 0.31, 0.80). Consumption of cod liver oil, which contains large quantities of retinol as well as antioxidants like vitamin E and omega-3 fatty acids, is reportedly recommended to pregnant women in Norway (Johansen et al., 2008). This guideline is supported by evidence from a study in China that found cod liver oil supplementation during the first trimester had protective effects against both CL/P (OR = 0.12, 95% CI: 0.03, 0.52) and CPO (OR = 0.16, 95% CI: 0.02, 1.21) (Hao et al., 2015). The effect was even stronger among women who took it before conception (CL/P: OR = 0.04, 95% CI: 0.01, 0.31; CPO: OR = 0.11, 95% CI: 0.01, 0.82). It is unknown how exactly cod liver oil may protect against OFCs, but it may involve a combination of support for RA signaling and antioxidant activity. A study in Denmark similarly observed a protective effect of vitamin A, either through multivitamins use or liver consumption (Mitchell, Murray, O'Brien, & Christensen, 2003). Approaches using proteomic and metabolite analyses may better assess the relationship between vitamin A status and OFCs. One study found that serum levels of RBP4 were significantly lowered in CL/P cases versus controls (sample size n = 10-14) (J. Zhang et al., 2014). Metabolite analysis verified that vitamin A levels were correspondingly lower in the CL/P group.
Because hyper- and hypovitaminosis A are both associated with birth defects including OFCs, there is risk associated with the choice of supplementing during pregnancy. The World Health Organization (WHO) has stated that prenatal vitamin A supplementation is not recommended in populations where a well-balanced diet can be maintained during pregnancy (McGuire, 2012). However, in regions where hypovitaminosis A is a known public health issue, WHO advises that pregnant women take an oral liquid, oil-based supplement containing retinyl palmitate or retinyl acetate at either 10,000 IU daily or up to 25,000 IU weekly (McGuire, 2012).
3. Environmental signaling pathways in orofacial clefts
While environmental stressors like ROS can disrupt orofacial development by causing cellular damage (Larouche & Hales, 2009), some xenobiotics serve as ligands for receptors that modulate gene expression. Environmental signaling pathways can be inappropriately activated or blocked to disrupt tightly coordinated developmental processes. Among the best studied pathways interacting with orofacial development are those controlled by the retinoic acid receptor (RAR), aryl hydrocarbon receptor (AHR), and glucocorticoid receptor (GR). Extrinsic factors interacting with these pathways have helped to characterize potential environmental hazards that exist for OFCs. Additionally, they have been useful means of chemically inducing OFCs in the laboratory to better understand the molecular and cellular processes of orofacial development. In this section, we describe the signaling pathways mediated by RAR, AHR, and GR, as well as their downstream mechanisms that could cause OFCs. We also discuss how these signaling pathways can interact to increase teratogenicity.
3.1. Retinoic acid receptor signaling
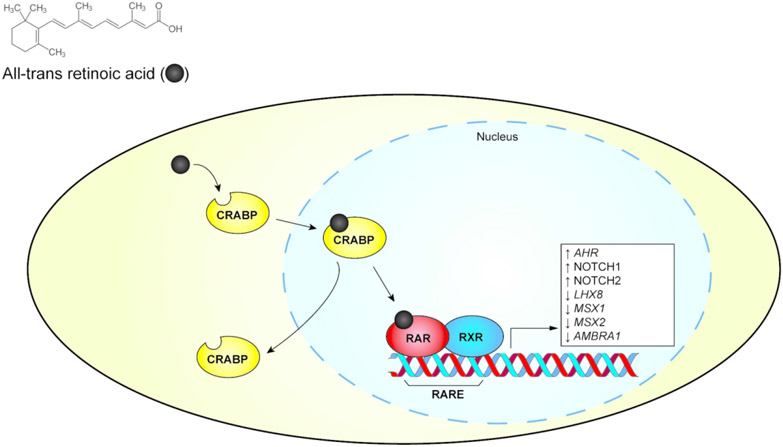
Once retinol is metabolized into RA, cellular retinoic acid binding protein (CRABP) transports RA into the nucleus (Okano et al., 2014) (Figure 1). There it serves as a ligand for retinoic acid receptors (RARs), a group of transcription factors consisting of three isotypes: RARα, RARβ, and RARγ. The RARs function as heterodimers by partnering with the retinoid X receptors (RXRs), similarly consisting of three isotypes: RXRα, RXRβ, and RXRγ. The RAR-RXR heterodimers modulate the expression of specific genes through binding of retinoic acid response elements (RAREs) in gene promoter regions. In the RA-unbound state, the RAR-RXR heterodimers recruit co-repressor proteins and prevent transcription. Upon binding RA, they alter conformation and recruit co-activator proteins to act as transcription activators.
Figure 1.
Simplified retinoic acid receptor (RAR) signaling pathway. The pathway’s modulation of gene expression and protein abundance associated with orofacial clefts is shown in the white box. CRABP, cellular retinoic acid binding protein. RARE, retinoic acid receptor response element. RXR, retinoid X receptor.
The importance of RA signaling in orofacial development is conserved in vertebrates, as demonstrated in studies using zebrafish (Laue, Janicke, Plaster, Sonntag, & Hammerschmidt, 2008), Xenopus (Kennedy & Dickinson, 2012), chicks (Shimomura et al., 2015), and mice (Rhinn, Schuhbaur, Niederreither, & Dolle, 2011). In rodents, studies on the role of RA signaling have utilized various approaches such as targeted knockout of RARs and RXRs, knockout or inhibition of enzymes involved in RA metabolism, or by addition of exogenous RA (Rhinn & Dolle, 2012). Laboratory exposures have utilized excess all-trans retinoic acid (ATRA) in the mouse model to generate cleft palate phenotypes with high frequency. These exposures could perturb development by disrupting concentration-dependent changes in gene expression and activating RA signaling beyond a normal spatiotemporal context. RA signaling via ATRA exposure can be mutually antagonistic toward Tgf-β, Wnt, and Hedgehog signaling during palatogenesis. The specifics of these interactions are discussed in a companion review (Reynolds et al., 2020) (in this issue). Additionally, ATRA exposure can modulate Notch signaling in palatal epithelial and mesenchymal cells, possibly affecting p21 signaling in both tissues. One study showed that ATRA exposure at embryonic day (E) 12 increased the abundance of Notch1 in MEE cells at E15, which was hypothesized to inhibit normal apoptotic processes (Y. D. Zhang et al., 2016). Another study found that ATRA exposure at E10 increased abundance of Notch2 and decreased that of CyclinD1 in mesenchymal cells between E12.5-14.5, thereby inhibiting proliferation (Y. D. Zhang et al., 2017). Because RA signaling intersects with several pathways, the balance of the endogenous RA gradient must be carefully managed through feedback mechanisms.
RA signaling can modulate the expression of transcription factors to control developmental processes, in some cases through its interactions with other pathways. In the upper jaw of chick embryos, ATRA exposure decreased expression of Lhx8, Msx1, and Msx2, the former of which is controlled by Fgf signaling (Shimomura et al., 2015). These genes appear to be similarly controlled through endogenous RA signaling. Knockdown of raldh2 and rary during orofacial development in Xenopus resulted in median facial cleft as well as decreased expression of lhx8 and msx2 (Kennedy & Dickinson, 2012). In mice, ATRA exposure may affect Lef1-mediated periderm trajectory toward EMT by decreasing expression of Ambra1 in a lncRNA-transcription factor-target gene regulatory network (Shu, Dong, & Shu, 2019). In mice, Cyp26b1 is particularly important for palatogenesis and is required for horizontal elevation of the palatal shelves as well as tongue depression.
3.2. Aryl hydrocarbon receptor signaling
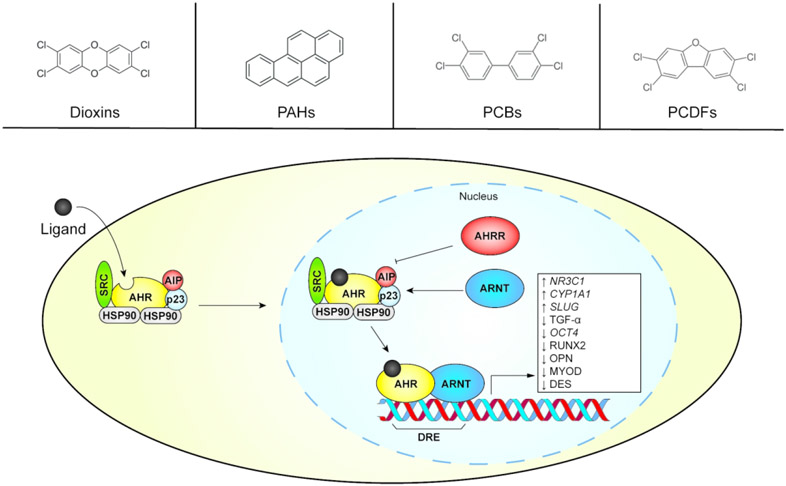
Activation of the aryl hydrocarbon receptor (AHR) during orofacial development is a reliable method for inducing cleft palate in the laboratory. Also known as the dioxin receptor, the AHR is a member of the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of transcription factors (Nebert, 2017). It is a promiscuous receptor that binds several xenobiotic chemicals as well as some endogenous compounds (Figure 2), as reviewed in (Denison & Nagy, 2003). Major ligands include industrial products and byproducts such as dioxins and polychlorinated biphenyls (PCBs); combustion products such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated dibenzofurans (PCDFs); and phytonutrients such as flavonoids and carotenoids. The AHR also responds to endogenous ligands including tryptophan derivatives, tetrapyrroles, and arachidonic acid metabolites. The AHR exhibits a structure-activity relationship (SAR) with its ligands (P. Shankar et al., 2019). While dioxins and PCBs are known to induce OFCs (Courtney & Moore, 1971; Watanabe & Sugahara, 1981), the endogenous ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) does not appear to affect orofacial development (Henry, Bemis, Henry, Kende, & Gasiewicz, 2006). Additionally, some AHR ligands are non-activating and can prevent AHR signaling through competitive inhibition (Brenerova et al., 2016; Guyot, Chevallier, Barouki, & Coumoul, 2013). AHR ligands often occur in the environment as a mixture of activating and non-activating ligands, each competing for AHR with different binding affinities and SARs (Geier et al., 2018). It is therefore challenging to understand how relevant exposure scenarios will affect human development including how they may impact the risk for OFCs.
Figure 2.
Simplified aryl hydrocarbon receptor (AHR) signaling pathway. Representative members of ligand classes are presented in the top panels, while the AHR transcriptional activation pathway is illustrated below. The pathway’s modulation of gene expression and protein abundance associated with orofacial clefts is shown in the white box. ARNT, aryl hydrocarbon receptor nuclear translocator. AHRR, aryl hydrocarbon receptor repressor. AIP, aryl hydrocarbon receptor interacting protein. DRE, dioxin response element. PAH, polycyclic aromatic hydrocarbon. PCB, polychlorinated biphenyl. PCDF, polychlorinated dibenzofuran.
While it has endogenous roles, the AHR acts as an environmental sensor. Its transcriptional activities usually result in an adaptive response ultimately intended for detoxification and/or elimination of the ligand(s) (Denison & Nagy, 2003; Nebert, 2017). To this end, its activation as a transcription factor strongly induces expression of genes encoding phase I and II metabolic enzymes (described in Section 3). During inactivity, the AHR resides in the cytosol in association with a chaperone complex. Upon binding a ligand, it translocates to the nucleus where it heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT). The AHR-ARNT heterodimer subsequently regulates gene expression through binding of dioxin response elements (DREs) within gene promoters, resulting in activation or repression of transcription. The phase I metabolic enzyme CYP1A1, along with a negative feedback regulator called the aryl hydrocarbon receptor repressor (AHRR), are well-described biomarkers for AHR activation. Polymorphisms in the ARNT (Kayano et al., 2004) and AHRR (Elizabeth J Leslie et al., 2017) (Linnenkamp, Raskin, Esposito, & Herai, 2020) genes have been associated with OFCs, indicating that allelic variation of genes in the AHR pathway may perturb orofacial development through GxE interactions (Kayano et al., 2004).
Much of what is known about the role of the AHR in OFCs is derived from dioxin exposure studies. The most potent is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which is often regarded as the most toxic anthropogenic substance known (Gallo, Scheuplein, & Heijden, 1991; Kao, Chen, Liu, & Wu, 2001; McAdams & Aquino, 1994). TCDD has been experimentally demonstrated to induce cleft palate and hydronephrosis in both mice and rats at dose-dependent levels (Huuskonen, Unkila, Pohjanvirta, & Tuomisto, 1994; R. M. Pratt, Dencker, & Diewert, 1984), as well as in ex vivo human embryonic palate cultures (Abbott & Birnbaum, 1991; Abbott, Probst, Perdew, & Buckalew, 1998). TCDD exposures do not increase the incidence of cleft lip in mice, even in the A/J strain that is susceptible to spontaneous cleft lip during development (Yamada, Mishima, Fujiwara, Imura, & Sugahara, 2006). Mouse embryos that lack functional AHR do not develop cleft palate following TCDD exposure, demonstrating absolute dependency of TCDD-induced cleft palate on AHR-mediated effects (Mimura et al., 1997; Peters et al., 1999). In contrast, the developing pups of Ahr-null dams are up to five times more sensitive to TCDD exposure, likely as a consequence of increased uptake by the pups (Thomae, Glover, & Bradfield, 2004).
TCDD exposure can affect gene expression in both epithelial and mesenchymal tissues, disrupting several stages of palatogenesis including palatal growth and fusion. Histological examination of developing TCDD-exposed mouse palate found that the MEE of the separate palatal shelves make contact, but this is followed by a post-fusional split (Yamada et al., 2006). One explanation for this could be the disruption of epithelial cell development. Rupture of the epithelial basement membrane has been observed in TCDD-exposed palates, suggesting that cell adhesion or integrity of the palatal shelves were compromised (Sakuma et al., 2018). AHR signaling also appears to disrupt growth factor signaling during palatogenesis. TCDD exposure reduces expression of transforming growth factor alpha (TGF-α), which normally is highly expressed in the epithelial cells of human palatal cultures (Citterio & Gaillard, 1994). This indicated that TCDD targets the Egf pathway, which was confirmed using knockout mice (Abbott et al., 2003), although the effect on Egf signaling appears to be counterintuitive to decreased ligand expression. In human fetal palatal epithelial cells (hFPECs), TCDD stimulated EGF receptor phosphorylation, resulting in the phosphorylation of ERK/p38 and subsequent cell proliferation (Z. Gao, Bu, Liu, et al., 2016). TCDD also promoted entry into the S and G2/M phases at least partly through the PI3K/AKT pathway (Z. Gao, Bu, Zhang, et al., 2016). While TCDD exposure decreased abundance of epithelial markers E-cadherin and keratin-14, it increased abundance of mesenchymal markers vimentin and fibronectin, signifying inappropriate progression through EMT. A likely mechanism was the increased expression of Slug, an inducer of EMT and repressor of E-cadherin, which has DREs in its promoter that could allow direct modulation by AHR. Co-immunoprecipitation demonstrated that, in addition to Slug induction, TCDD also promoted physical interaction between AHR and Slug. These studies provided strong evidence that a key mechanism of AHR-mediated cleft palate is inappropriate induction of EMT, with Slug as a likely intermediary. This is consistent with previous observations that exogenous Tgf-β3, a mediator of EMT during palatogenesis, protects against cleft palate following TCDD exposure (Thomae, Stevens, & Bradfield, 2005). A caveat is that Tgf-β signaling can repress expression of the AHR (Wolff et al., 2001), so the protective effect of Tgf-β3 may be independent of its effects on EMT. However, TCDD has been observed to decrease expression of Tgf-β3 in the MEE, concurrent with loss of filopodia and cell polarity that may interfere with apoptosis (Li, He, Meng, Lu, & Shi, 2014).
AHR can additionally disrupt mesenchymal development during palatogenesis. In mice, an immunohistological survey found that TCDD exposure prevented osteogenesis (decreased abundance of Runx2 and osteopontin) and myogenesis (decreased expression of MyoD and desmin) (Yamada et al., 2006). In human fetal palatal mesenchymal cells (hFPMCs), TCDD caused decreased proliferation, alkaline phosphatase activity, and deposition of calcium onto mineralizing tissue (X. Z. Liu et al., 2020). Further, TCDD exposure decreased TGF-β (SMAD2/3) and BMP (SMAD1/5/8) signaling, altering cellular function and fate. Co-treatment of hFPMCs with TGF-β1 and BMP2 restored appropriate SMAD signaling for both pathways and rescued the impaired osteogenesis.
Taken together, these studies demonstrated that AHR activation, as mediated by TCDD, disrupt both epithelial and mesenchymal development during palatogenesis. Specifically, AHR activation can increase proliferation and EMT of epithelial cells, while decreasing proliferation and preventing differentiation of mesenchymal cells. EMT and osteogenesis appear to be major processes affected by AHR activation. Additional studies have identified other potential mechanisms of AHR-mediated cleft palate, including altered expression of genes implicated in OFCs such as the lncRNA H19 (L. Y. Gao et al., 2017) and gamma aminobutyric acid type A receptor beta-3 subunit (GABRB3) (Lei et al., 2019). AHR activation can also alter the epigenetic state of palatal cells, inducing promoter hypermethylation of stemness genes like Oct4 (Tao et al., 2020; Wang, Yuan, Fu, & Zhai, 2016; Wang et al., 2017). While much has been characterized about the effect of AHR signaling on OFCs, more remains to be discovered. As an example, little is known of the full range of possible AHR ligands that could contribute to OFCs, as well as their relative potencies for inducing cleft palate. For example, in toxicity assays using the embryonic zebrafish, the screening of over a hundred AHR ligands revealed varying teratological effects based on chemical structure, ranging from craniofacial malformations to appendage duplication (Reynolds et al., 2020; Prarthana Shankar, Dasgupta, Hahn, & Tanguay, 2020; P. Shankar et al., 2019). Characterizing these hazards will be important for evaluating environmental risks for craniofacial defects mediated by AHR signaling. Additionally, a better understanding of AHR signaling could lead to therapeutic approaches toward preventing OFCs in higher risk cases, such as those who may be genetically or occupationally predisposed to having offspring with an OFC.
3.3. Glucocorticoid receptor signaling
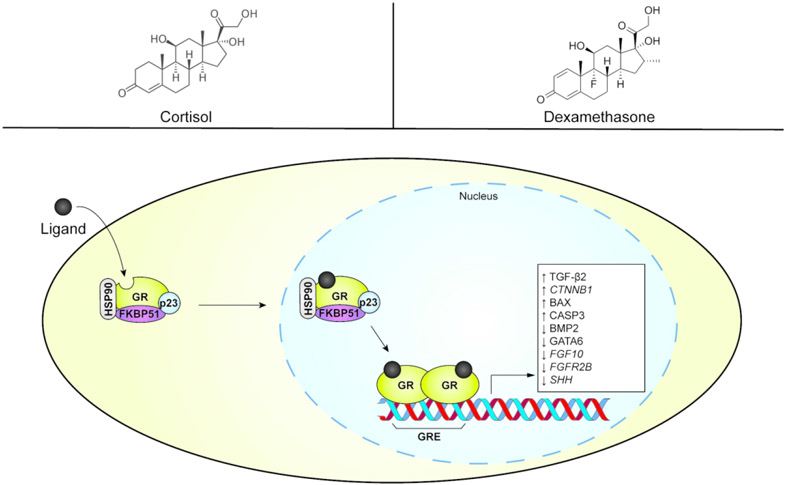
Glucocorticoids (GCs) are a class of corticosteroid hormones that include both endogenous and exogenous chemicals. Homeostatic levels of endogenous cortisol are required for appropriate craniofacial morphogenesis, but addition of exogenous GCs can cause cleft palate (Hu, Gao, Liao, Tang, & Lu, 2013; R. Pratt, 1980). Although excess of cortisol itself can cause cleft palate in mice (Jaskoll, Choy, Chen, & Melnick, 1996), some synthetic GCs may have a more potent effect of cleft palate induction. As an example, the synthetic GC dexamethasone is approximately 300 times more potent than cortisol for inducing cleft palate (Pinsky & Digeorge, 1965). The transcriptional response to GCs is mediated by the glucocorticoid receptor (GR), a member of the nuclear receptor family of transcription factors that is encoded by the gene NR3C1 (Weikum, Knuesel, Ortlund, & Yamamoto, 2017). Monomeric GR resides in the cytosol with a chaperone complex and becomes activated as a transcription factor upon binding a ligand (Vandevyver, Dejager, & Libert, 2012; Weikum et al., 2017) (Figure 3). After translocating to the nucleus, the GR homodimerizes and subsequently binds glucocorticoid response elements (GREs) within gene promoters to modulate gene expression. It can activate or repress the transcription of thousands of genes involved in immune response, inflammation, wound healing, energy metabolism, and others (Weikum et al., 2017).
Figure 3.
Simplified glucocorticoid receptor (GR) signaling pathway. Examples of the endogenous ligand (cortisol) and a synthetic ligand (dexamethasone) are presented in the top panels. The pathway’s direct or indirect modulation of gene expression and protein abundance associated with orofacial clefts is shown in the white box. FKBP51, FK506 binding protein 51 (representing immunophilins). GRE, glucocorticoid response element. HSP90, heat shock protein 90.
An early observation in GR-mediated cleft palate was decreased proliferation of mesenchymal cells and secretion of the ECM (Diewert & Pratt, 1981). This hypoplasia prevented contact between the shelves and blocked palatal fusion. One hypothesis was that GCs induced cleft palate through the same mechanism by which the GR regulates the inflammatory response. Activation of the GR induces expression of macrocortin and other anti-phospholipases, thereby preventing the Phospholipase A2-mediated cleavage of arachidonic acid from phospholipids (Grove, Willis, & Pratt, 1985). This prevents downstream metabolism of arachidonic acid into physiologically active derivatives such as leukotrienes, thromboxanes, and prostaglandins, the latter of which are believed to regulate cyclic AMP levels in palatal cells (Greene & Garbarino, 1984). However, a study investigating this hypothesis in mice showed that addition of exogenous arachidonic acid did not rescue pups from GC-mediated cleft palate (Grove et al., 1985). This demonstrated two possibilities: 1) inhibition of arachidonic acid does not affect palatogenesis; or 2) inhibited metabolism of arachidonic acid is still a valid mechanism, but additional mechanisms are involved that prevented rescue.
Most subsequent efforts have not considered the arachidonic acid hypothesis, instead focusing on GC-mediated changes to cellular properties or modulation of developmental signaling. Previous observations for decreased production of ECM proteins have been corroborated in mouse studies. Dexamethasone acetate was found to decrease the palatal area covered by glucosaminoglycan content (Fu, Li, & Huang, 1997). Both hyaluronate and collagen had decreased abundance following exposure to dexamethasone (Montenegro, Rojas, Dominguez, & Rosales, 1998). Each of these ECM component types is important for palatal shelf elevation and fusion (Ji et al., 2020) (in this issue). Additionally, dexamethasone increased the abundance of E-cadherin, which is normally suppressed during palatal fusion and EMT (Xiaoxiao, Li, Li, Qian, & Chenghao, 2015). Transmission electron microscopy of mouse palates exposed to dexamethasone revealed cellular swelling and loose cell connection, along with decreased abundance of mitochondria and extracellular matrix (Lan et al., 2016).
Mesenchymal proliferation may be inhibited by the GR through modulation of Tgf-β2, which inhibits cell proliferation by targeting G1 phase cyclins and cyclin-dependent kinases (Derynck, 1994; Jaskoll et al., 1996). During palatogenesis, exposure to exogenous cortisol maintained elevated levels of Tgf-β2 during a stage where it normally decreases at E14 (Jaskoll et al., 1996). The authors postulated that this sustained expression of Tgf-β2 occurred during a sensitive time for proliferating, perturbing cell cycle progression through the above mechanism. Another possible mechanism of reduced proliferation is through modulation of β-catenin, which was inappropriately upregulated in palatal tissues of mice exposed to dexamethasone (Xiaoxiao et al., 2015). In addition to exhibiting decreased proliferation, cells in the palatal shelves also undergo apoptosis following dexamethasone exposure with increased abundance of Bax and caspase-3 (Lan et al., 2016). Bmp2, which suppresses apoptosis, also had decreased abundance. This may have been due to decreased levels of Gata-6, which regulates Bmp2 expression.
Activation of the GR can have specific effects on the epithelium that contribute to the inability of palates to fuse. An in vitro study on cultured murine palatal cells found that dexamethasone induced the differentiation of MEE cells into squamous epithelial cells, perturbing development of the palate epithelium (Huang, Shi, Sun, & Wang, 2005). An in vivo study found that dexamethasone exposure decreased expression of Fgf10 in the mesenchyme as well as Fgfr2b and sonic hedgehog (Shh) expression in the epithelium, thereby perturbing Fgf-Hedgehog signaling axis (He et al., 2010). Interestingly, this study found that co-exposure with vitamin B12 restored gene expression and mesenchymal proliferation. More recently, a study found that dexamethasone exposure disrupted the PAR complex by decreasing transcription and translation of PAR3 and PAR6 in the epithelium, and aPKC in both epithelial and mesenchymal cells (Ma, Shi, & Zheng, 2018). This adversely affected epithelial cell polarity and contributed to the inability of palatal shelves to fuse.
These studies demonstrated that GR signaling can modulate several aspects of palatogenesis, particularly mesenchymal proliferation and epithelial differentiation. In general, GR signaling prevents palatal shelves from ever making contact, but fusion would likely be inhibited by GC exposure due to the altered developmental state of epithelial cells. The GR appears to modulate several developmental pathways important for palatogenesis including Wnt, Fgf, Tgf-β, and Bmp. The exact mechanisms behind this are not well understood, but at least in the case of Bmp signaling, this is a consequence of altered Gata6 expression. A better understanding of the mechanisms by which GR induces cleft palate would provide additional methods of protecting against OFCs. There is evidence from mouse studies that vitamins B6 and B12 could protect factor against OFCs caused by GCs, and it would be advantageous to know whether these or other compounds would be protective in a clinical setting (Lu et al., 2008; Yoneda & Pratt, 1982). There is also evidence for vertebrate-conserved SAR in GR signaling, which guides the development of novel GCs with the goal of preventing unwanted side effects (I. H. Song, Gold, Straub, Burmester, & Buttgereit, 2005). For example, some GCs like beclomethasone dipropionate increase expression of the feto-oncogene Cripto-1/Tdgf1 in embryonic mouse cells, which could perturb several developmental pathways relevant to orofacial development (Garland et al., 2019). The development of GCs with lower risk for teratogenicity would be beneficial for pregnant women, especially for those who may unexpectedly require immunosuppression in emergency situations.
3.4. Signaling interactions in orofacial development
There is evidence for substantial crosstalk between environmental signaling pathways and orofacial development, particularly in association with AHR signaling. For example, a mouse study found that the expression of Ahr the nasal mesenchyme depends on the expression of Raldh3 in the nasal epithelium (Jacobs et al., 2011b). The resulting model is that epithelium-derived RA transduced mesenchymal expression of Ahr through activation by RAR-γ. Knockout studies demonstrated that this regulatory network was required for TCDD-induced cleft palate. AHR signaling appears to interact with multiple hormone signaling pathways as well. TCDD exposure increased abundance of the GR in both epithelial and mesenchymal tissue in the palate (Abbott, Perdew, Buckalew, & Birnbaum, 1994). Co-exposure of TCDD and hydrocortisone during palatogenesis induced cleft palate in mice at 100% incidence using concentrations that could not induce cleft palate following exposure to either chemical alone (L. S. Birnbaum, Harris, Miller, Pratt, & Lamb, 1986). The AHR may also interact with thyroid signaling. Compared to TCDD exposure alone (approximately 8% incidence), co-exposure of TCDD and either of the thyroid hormones triiodothyronine (T3) or thyroxine (T4) increased incidence of cleft palate in mice to 31% and 27% at the maximum tested dosages, respectively (Lamb, Harris, McKinney, & Birnbaum, 1986). T3 or T4 alone induced cleft palate, but only at a dose-independent rate of 1%, indicating a synergy between TCDD exposure and thyroid hormone signaling. Additionally, during murine palatogenesis, TCDD exposure reduced abundance of estrogen receptor alpha (ER-α) (Yamada et al., 2014). Given that AHR has previously been associated with ER-α and osteoblast differentiation (Ge, Cui, Cheng, & Han, 2018), TCDD exposure may further promote cleft palate by antagonizing estrogen signaling and downstream palatal bone formation.
The GR, in addition to its interactions with AHR signaling, may also influence RA signaling. Polymorphisms associated with glucocorticoid-induced cleft palate appear to influence the role of vitamin A in exacerbating the risk (Tyan & Tyan, 1993). This indicates that sensitivity to excess retinoids could be linked to genetic variants affecting pathways other than retinoid metabolism.
4. Biotransformation and transport in OFCs
Developmental processes rely on the biotransforming activities of metabolic enzymes for synthesis, modification, and degradation of endogenous compounds. Similar activities are critical for detoxification and subsequent elimination of exogenous chemicals that may be teratogenic. Exposure to chemicals incidentally (e.g. solvents and biocides) or behaviorally (e.g. smoking, drinking, and diet) can elicit and/or interfere with chemical metabolism, as described below. Dysfunction and misregulation of biotransformation enzymes and transporters are associated with OFCs in the laboratory and in human populations (Beaty et al., 2010; Shi et al., 2007). Metabolic activity may inadvertently result in the generation of toxic intermediates that cause oxidative stress or other harmful effects (Zangar, Davydov, & Verma, 2004). Here we describe what is currently known about the involvement of biotransformation enzymes in orofacial development with a focus on their roles in detoxification.
Exposure to xenobiotics can trigger increased expression of metabolic and transport enzymes. Enzymatic activities can result in structural modifications that facilitate detoxification and subsequent renal or biliary excretion, usually through the addition of polar groups that also increase hydrophilicity (Gonzalez, 2005). This process is often divided into three parts: phase I (oxidation), phase II (conjugation), and phase III (transport). A review by Xu et al. (2005) provides an overview of these detoxification phases and mechanisms of their induction (C. J. Xu, Li, & Kong, 2005). Metabolic enzymes outside of this paradigm, such as aldehyde dehydrogenases, are also involved in the detoxification of some xenobiotics. Dysfunction of any of these processes have been linked with OFCs.
4.1. Phase I: Oxidation
Phase I metabolic enzymes include cytochrome P450s (CYPs), epoxide hydrolases (EHs), and flavin-containing monooxygenases (FMOs). The CYP and EH classes have been associated with OFC pathology through human population or knockout/knock-in studies. Within the human genome, there are 57 genes encoding putatively functional CYP enzymes that are categorized into subfamilies based on sequence similarity (Fujikura, Ingelman-Sundberg, & Lauschke, 2015). CYPs are primarily expressed in hepatic tissue but are also found in the intestine, kidney, lung, and hematopoietic tissues (Gonzalez, 2005). Some can be also induced in orofacial tissue, such as Cyp1a1 in mice (Jacobs et al., 2011a). While some CYPs can promiscuously metabolize thousands of endogenous or exogenous substrates, others are more specialized and direct their activities toward few chemicals. In general, CYPs oxidize carbon and nitrogen groups resulting in the formation of alcohol groups (Gonzalez, 2005). CYP-mediated oxidation of aromatic substrates can yield an epoxide, although these groups spontaneously hydrolyze into diols or are catalytically hydrolyzed into trans-dihydrodiols by EHs. In contrast, FMOs are not yet directly implicated in OFCs. Because they are not induced by xenobiotic exposure and must compete for substrates with CYPs (Krueger & Williams, 2005), it is possible that FMOs may have less importance for OFC pathology. However, because FMOs can yield metabolites distinct from CYP products, they warrant consideration in future studies.
Although CYPs generate electrophilic metabolites that may be more toxic than the original substrate, evidence suggests that CYPs ultimately play a protective role in OFC pathology. This could be through the detoxification pathway or by altering toxicokinetics via sequestration. Polymorphisms in both fetal and maternal CYP genes have been associated with OFCs, often in the context of smoking or drinking. CYP1A1 is considered one of the most important enzymes for metabolizing several combustion products in smoke, particularly polycyclic aromatic hydrocarbons (PAHs). In the context of maternal smoking, fetal polymorphisms in CYP1A1 had protective associations with CL/P in French (Chevrier et al., 2008), Danish, and Iowan (Shi et al., 2007) populations, at least one of which is thought to increase enzymatic function by stabilizing mRNA. Maternal polymorphism was also associated with protective effects in the French population (Chevrier et al., 2008). A knockout study demonstrated that altered expression of Cyp1a1 or Cyp1b1 does not significantly alter dioxin-induced OFCs during murine development (Dragin, Dalton, Miller, Shertzer, & Nebert, 2006). The same study showed that Cyp1a2 (or CYP1A2 in a humanized model) in the maternal liver had a protective effect, likely due to altered toxicokinetics. Thus, while there is evidence that CYP enzymes may be protective, the functional relevance for CYP1A1 or CYP1B1 in OFCs remains to be better characterized.
Fetal polymorphism of another CYP gene, CYP2E1, may also have a protective effect against OFCs as observed in a French population (Chevrier et al., 2008; Chevrier et al., 2007). CYP2E1 metabolizes organic solvents such as ethanol and benzene (Ingelman-Sundberg, 2004), as well as other toxicants including nitrosamines and tannins (Lieber, 1997). It may also convert retinoic acid into a less active metabolite (C. Liu, Russell, Seitz, & Wang, 2001; Stoilov, Jansson, Sarfarazi, & Schenkman, 2001), potentially offsetting the delicate balance required for appropriate retinoid signaling (Okano et al., 2014). This suggests that smoking or drinking could indirectly affect development by stimulating endogenous metabolism via phase I induction, although it is unclear how exactly the polymorphism affects this process (Chevrier et al., 2008; Shi, Wehby, & Murray, 2008).
CYP-metabolized PAHs may have reactive epoxide functionalities that are subsequently hydrolyzed by the microsomal EH enzyme, EPHX1. Data from an Iowan population suggested a protective interaction between maternal smoking and a fetal EPHX1 polymorphism that increased enzymatic activity (Shi et al., 2007). Since this implied that EPHX1 activity protects against OFCs, it is tempting to speculate that epoxidized chemicals derived from cigarette smoke are teratogenic. However, two other studies observed no interaction between smoking and EPHX1 polymorphisms in OFCs (Hartsfield et al., 2001; Ramirez et al., 2007). Despite mixed conclusions, a demonstrated role for EPHX1 in OFC pathology could improve our understanding of smoke-induced OFCs and deserves further investigation.
4.2. Phase II: Conjugation
Phase II enzymes covalently add small organic donor molecules to xenobiotics and intermediate metabolites. These conjugations are usually detoxifying mechanisms that may also facilitate excretion by polarizing the xenobiotic and increasing hydrophilicity. While several types of donor groups are used, some major modifications include arylamine N-acetylation, glutathionylation, UDP-glucuronidation, and sulfonation. Respectively, these are catalyzed by arylamine N-acetylases (NATs), glutathione S-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), and sulfotransferases (SULTs), with the former two families having implications in human OFCs.
Cigarette smoke is known to contain toxicants such as arylhydroxylamines (Lammer, Shaw, Iovannisci, Van Waes, & Finnell, 2004), and conjugating enzymes such as NATs provide an important mechanism for detoxifying these compounds. Humans possess two NAT genes, NAT1 and NAT2, that each have over two dozen known polymorphisms that may affect arylamine detoxification and OFCs (Shi et al., 2008). A study on a California population linked two fetal polymorphisms of NAT1 that increased the risk of CL/P by 4-fold when mothers smoked during early pregnancy (compared to reference genotype infants with non-smoking mothers) (Lammer et al., 2004). A study on a Danish population identified a protective effect of a maternal NAT2 allele (Shi et al., 2007), while maternal polymorphism imparting a slow acetylator phenotype increased risk of CL/P in an Argentine population (Santos, Ramallo, Muzzio, Camelo, & Bailliet, 2015). In a Chinese population, polymorphisms of both NAT1 and NAT2 were found to increase the risk of CL/P, with increased risk among those having polymorphisms in both isozymes (T. Song et al., 2013). These studies suggest that detoxification mediated by NATs may be an important protective factor in OFCs. Outside of its role in detoxification, NAT1 (and its murine ortholog, NAT2) may be involved in folate metabolism (R. P. Erickson, 2010; A. Ward, Summers, & Sim, 1995), implicating a possible endogenous function during orofacial development that could be altered by environmental factors.
GSTs, another class of conjugating enzymes that are associated with OFCs, detoxify and confer water solubility to compounds by covalently adding reduced glutathione. Human cytosolic GSTs are categorized into eight classes: alpha, kappa, mu, omega, pi, sigma, theta, and zeta (Strange, Spiteri, Ramachandran, & Fryer, 2001). In human populations, GST genes are highly polymorphic for alleles that can adversely affect enzyme function. Null mutations in two genes, GSTM1 and GSTT1, are rather frequent and can vary based on population ethnicity. One study, using an international database of Caucasians (n = 12,525), Asians (n = 2,136), and Africans and African-Americans (n = 996), found that the sex-averaged frequencies GSTM1 homozygous null genotypes were approximately 53% in both Caucasians and Asians, and 28% of Africans/African-Americans (Garte et al., 2001). GSTT1 is similar, having null alleles at sex-averaged frequencies of 20% in Caucasians and 52% in Asians (Malaysian population).
GSTM1 and GSTT1 are capable of conjugating reduced glutathione to epoxidized PAHs (Alexandrie et al., 2000; Guengerich, 1993) and are therefore important for detoxification of CYP-metabolized smoke products (Shi et al., 2008). A relationship between maternal smoking (at least 20 cigarettes per day) and fetal null GSTM1 genotype was identified in a California population (Lammer, Shaw, Iovannisci, & Finnell, 2005). On the other hand, maternal null GSTM1 genotype increased the risk of CL/P in a Polish population independent of maternal smoking (Hozyasz, Mostowska, Surowiec, & Jagodziński, 2005). This may signify a more ubiquitous role in detoxifying unspecified compounds, or perhaps an endogenous housekeeping role, for GSTM1 in the prevention of OFCs. Maternal and fetal null alleles for GSTT1 are also linked with OFCs. Dutch children whose mothers carried the GSTT1-null allele and smoked during pregnancy were at 3-fold increased risk for CL/P compared to smoking mothers with the reference genotype (I. A. L. M. van Rooij et al., 2001). When both fetal and maternal GSTT1 genotypes were null, there was a 5-fold increased risk of CL/P when compared to the reference group. Maternal GSTT1-null genotype with smoking during pregnancy were also linked with CL/P in a California population (Lammer et al., 2005), while fetal GSTT1-null genotype with maternal smoking was linked to CL/P in Iowan and Danish populations with skewed risk toward CPO (Shi et al., 2007). Shi et al. (2008) note that fetal GSTT1-null genotype also increased risk of CPO in mothers occupationally exposed to chemicals such as dyes, propellants, and insecticides (G. M. Shaw, Nelson, Iovannisci, Finnell, & Lammer, 2003; Shi et al., 2008). Fetal polymorphisms in other GST genes, such as GSTA4 and GSTP1, have also been linked with increased OFC risk in the context of maternal smoking (Krapels et al., 2008; Shi et al., 2007) with increased chance of CPO associated with the latter gene in a Danish population.
4.3. Phase III: Transport
Different families of efflux transporter proteins may be induced as part of a response to environmental exposure or may be constitutively expressed. Transporters are expressed in the placenta and have an important role in protecting the fetus from toxicants during development. Members of the ATP-binding cassette (ABC) family of proteins are involved in transporting chemicals across extra- and intracellular membranes. So far, two ABC members have been implicated in OFC pathology: ABCA4, involved in retinoid transport; and ABCB1, involved in folic acid and drug transport. ABCA4 is a flippase transporter of retinoids including retinaldehyde and N-retinyl-phosphatidylethanolamine (Beharry, Zhong, & Molday, 2004). Polymorphism mapping to the ABCA4 region was associated with CL/P in a GWAS study using case-parent trio data from individuals in the United States, Europe, China, Taiwan, Singapore, Korea, and the Philippines (Beaty et al., 2010). Association between ABCA4 polymorphism and OFCs has been corroborated in several studies using data from various populations (Beaty et al., 2013; Fontoura, Silva, Granjeiro, & Letra, 2012; Lennon et al., 2012; Mi et al., 2015; Wu et al., 2018; Wu-Chou et al., 2020; Yuan, Blanton, & Hecht, 2011; Z.-w. Zhou et al., 2013). Using in situ hybridization, the authors of the initial study noted that Abca4 is not expressed in the developing murine palate at E13.5-14.5 (Beaty et al., 2010).
Evidence to support involvement of ABCA4 in orofacial development is lacking, as it is a photoreceptor-specific transporter (Beharry et al., 2004; Lennon et al., 2012). A mouse study demonstrated that dysfunction of an adjacent gene, Arhgap29, causes CL/P likely through interacting with an Irf6 regulatory network (E. J. Leslie et al., 2012). The authors of that study proposed ARHGAP29 as the true etiological gene in ABCA4-associated polymorphisms that increase CL/P risk. Given these circumstances, further work is needed to determine whether ABCA4 truly plays a role in OFCs.
ABCB1, encoding the P-glycoprotein efflux pump, is expressed in the placenta and can regulate the movement of several drugs (Hitzl et al., 2004; von Richter et al., 2009), making it an important transporter for protecting embryo development. In a case-control study of a Dutch population, children whose mothers had the 3435TT genotype and took any medication during pregnancy had an over 6-fold risk of CL/P compared to children whose mothers had the 3435CC and did not take medication. (Bliek et al., 2009). Notably, folic acid supplementation around the time of conception ameliorated these effects by 30%. Previous work has established that P-glycoprotein can mediate the export of folates, and folate-rich conditions can increase the capacity of P-glycoprotein to transport substrate (Hooijberg et al., 2004). Consistent with a role for ABCB1 in orofacial development, mouse pups with an Abcb1-null genotype are susceptible to cleft palates following maternal exposure to the macrocyclic lactone, avermectin (Lankas, Wise, Cartwright, Pippert, & Umbenhauer, 1998; Macdonald & Gledhill, 2007).
5. Behavioral risk factors for OFCs
Behavioral risk factors are among the most modifiable, making them a useful target in the prevention of OFCs. Known behavioral risk factors include smoking, drinking alcohol, and consumption of pharmacological agents. Here we describe studies examining the correlation between these factors and OFC with a focus on tobacco smoke and alcohol consumption.
5.1. Tobacco smoke
Historically, the consensus regarding an association between maternal exposure to tobacco smoking during pregnancy and OFC was divergent. In 2004, meta-analysis of 24 case-control and cohort studies published in the World Health Organization Bulletin identified associations between maternal smoking and either CL/P [RR = 1.34; 95% CI: 1.25, 1.44] or CPO (RR = 1.22; 95% CI: 1.10, 1.35) (Little, Cardy, & Munger, 2004). Ten years later, the 50th Anniversary United States Surgeon General’s report declared sufficient evidence for a causal link between maternal active smoking and OFC ("The health consequences of smoking—50 years of progress: a report of the Surgeon General," 2014; Honein, Devine, Grosse, & Reefhuis, 2014). Since then, several studies in various regions of the world have implicated maternal active smoking in OFC pathology (Table 1). Even though the magnitude of the association was not large in many studies, increased statistical power through meta-analyses provides further validation of the risks associated with smoking. Including 29 case-control studies, meta-analysis by Xuan et al. (2016) identified increased risk for CL/P (OR = 1.37, 95% CI: 1.26, 1.49) and CPO (OR = 1.24, 95% CI: 1.12, 1.38) as a consequence of maternal active smoking (Xuan et al., 2016). There is also substantial evidence that environmental tobacco smoke (ETS, also called second-hand smoke or maternal passive smoking) poses an OFC risk which in some cases was found to exceed that of active smoking. Two meta-analyses identified increased risk for OFC due to maternal passive smoking (OR = 1.87, 95% CI: 1.47, 2.39 (Zheng, Xie, Yang, & Qin, 2019); OR = 1.51, 95% CI: 1.16–1.97 (Oldereid et al., 2018)). A case-control study on Norwegian and American populations found that combined maternal active and passive smoking elevated the risk of either CL/P, CPO, or CLO relative to active or passive smoking alone (Kummet et al., 2016), indicating an additive effect of tobacco smoke toward OFC risk. Consistently, the number of cigarettes consumed appears to be an additional factor and indicates a dose-response relationship between smoking and OFCs. In an American population, Chung et al. identified elevated risk of OFC when pregnant women smoked 21 or more cigarettes per day (OR = 1.78, 95% CI: 1.22, 2.59) relative to 1-10 per day (OR = 1.50, 95% CI: 1.28, 1.76) (Chung, Kowalski, Kim, & Buchman, 2000). A similar effect was observed in a Chinese study on passive smoking, where developing offspring of mothers exposed to ETS > 6 times/week (OR = 2.6, 95% CI: 1.8, 3.8) were at greater risk for OFC compared those whose mothers were exposed 1-6 times/week (OR: 1.2, 95% CI: 0.9, 1.7) (Pi et al., 2018). Biomarkers, such as the nicotine metabolite cotinine, may help elucidate the dose-relationship between tobacco smoke and OFCs. A retrospective case-control study on the cotinine levels of pregnant women found no dose-response relationship between cotinine and OFC risk (G. M. Shaw et al., 2009). The authors noted that a prospective study, with a larger sample size and consideration for the relatively fast rate of cotinine metabolism, would provide a better measure of the dose-response relationship.
Table 1.
Recent population studies on the effects of maternal active and passive smoking on the risk for OFCs. Final adjusted regression model statistics used where available. Sample sizes reflect totals for overall study. CI, confidence interval. CLO, cleft lip only. CL/P, cleft lip with or without cleft palate. CPO, cleft palate only. OFC, any orofacial cleft. OR, odds ratio. PR, prevalence ratio. RR, relative risk.
| Study | Design | Population | Sample | Exposure type | Statistic (95% CI) |
|---|---|---|---|---|---|
| (Regina Altoe et al., 2020) | Case-control (retrospective) | Brazil | OFC: n = 150 Control: n = 300 |
Maternal active smoking | OR: 1.53 (0.61, 3.87) |
| Maternal passive smoking | OR: 2.18 (1.21, 3.93) | ||||
| (Maranhao et al., 2020) | Relative risk | Brazil | CL/P n = 345 CPO: n = 148 CLO n = 153 |
Maternal active smoking | CL/P vs CPO RR: 0.94 (0.52, 1.71) |
| CLO vs CPO RR: 0.61 (0.17, 2.12) | |||||
| (L. Acs et al., 2020) | Case-control (retrospective; matched and population) | Hungary | CPO: n = 751 Matched control: n = 1196 Population control: n = 57231 |
Maternal active smoking | Matched control OR: 2.5 (1.9, 3.3) |
| Population control OR: 2.3 (1.9, 2.8) | |||||
| (Sato et al., 2020) | Case-control (prospective) | Japan | CL/P: n = 146 Control: n = 93987 |
Maternal active smoking | Smoked during pregnancy OR: 1.28 (0.77, 2.12) |
| Maternal passive smoking | Every day OR: 1.49 (0.93, 2.39) |
||||
| (Zheng et al., 2019) | Meta-analysis | Maternal passive smoking | OR: 1.87 (1.47, 2.39) | ||
| (Oldereid et al., 2018) | Meta-analysis | Maternal passive smoking | OR: 1.51 (1.16–1.97) | ||
| (Bui, Ayub, Ahmed, Taioli, & Taub, 2018) | Case-control (Retrospective) | Pakistan | CL/P: n = 225 CPO: n = 72 Control: n = 131 |
Maternal, paternal, or both parents smoking | OFC OR: 1.89 (1.10, 3.26) |
| CL/P OR: 2.00 (1.13, 3.53) | |||||
| CPO OR: 1.51 (0.71, 3.19) | |||||
| (Pi et al., 2018) | Case-control (retrospective) | China | OFC: n = 240 Control: n = 1420 |
Maternal passive smoking | 1-6 times/week OR: 1.2 (0.9, 1.7) |
| >6 times/week OR: 2.6 (1.8, 3.8) | |||||
| (Mbuyi-Musanzayi et al., 2018) | Case-control (retrospective) | DR Congo | CL/P: n = 172 Control: n = 162 |
Maternal passive smoking | OR: 1.46 (0.38, 5.57) |
| (D. P. Xu et al., 2018) | Case-control (retrospective) | China | CL/P: n = 239 Control: n = 209 |
Maternal active smoking | Without drinking OR: 1.67 (1.01, 2.75) |
| With drinking OR: 2.21 (1.09, 4.47) | |||||
| (Angulo-Castro et al., 2017) | Case-control (retrospective) | Mexico | CL/P n = 24 Control: n = 24 |
Maternal active smoking | OR: 8.1 (1.6-39.3) |
| (Ly et al., 2017) | Case-control (retrospective) | Vietnam, Philippines, Honduras, Morocco | OFC: n = 392 Control: n = 234 |
Maternal passive smoking | Frequency 1-3 OR: 0.40 (0.11–1.42) |
| Frequency 6-14 OR: 0.41 (0.11–1.49) | |||||
| Frequency 15-20 OR: 0.68 (0.19–2.52) | |||||
| Frequency 20+ OR: 0.33 (0.10–1.11) | |||||
| (Lili et al., 2017) | Case-control (retrospective) | China | CL/P: n = 453 Control: n = 452 |
Maternal passive smoking | OR: 413.94 (24.54, 984.00) |
| (Xuan et al., 2016) | Meta-analysis | Maternal active smoking | CL/P OR: 1.37 (1.26, 1.49) |
||
| CPO OR: 1.24 (1.12, 1.38) | |||||
| (Campos Neves, Volpato, Espinosa, Aranha, & Borges, 2016) | Cross-sectional | Brazil | CL/P: n = 64 CPO: n = 22 CLO: n = 30 |
Maternal active smoking | PR: 1.33 (1.16, 1.52) |
| Maternal passive smoking | PR: 1.21 (1.01, 1.45) | ||||
| (McKinney et al., 2016) | Case-control (retrospective) | Thailand | CL/P: n = 95 Control: n = 95 |
Maternal passive smoking | OR: 6.52 (1.98, 21.44) |
| (Kummet et al., 2016) | Case-control (retrospective) | Norway, United States | OFC: n = 4508 Control n = 9626 |
Maternal active smoking | CL/P OR: 1.28 (1.09, 1.51) |
| CPO OR: 1.25 (1.01, 1.55 | |||||
| CLO OR: 1.52 (1.19, 1.94) | |||||
| Maternal passive smoking | CL/P OR: 1.11 (0.98, 1.27) |
||||
| CPO OR: 1.18 (1.00, 1.39) | |||||
| CLO OR: 1.14 (0.93, 1.39) | |||||
| Maternal active and passive smoking | CL/P OR: 1.55 (1.36, 1.77) |
||||
| CPO OR: 1.43 (1.20, 1.70) | |||||
| CLO OR: 1.63 (1.33, 2.00) | |||||
| (Hoyt et al., 2016) | Case-control (retrospective) | United States | CL/P: n = 245 CPO: n = 125 CLO: n = 94 Control: n = 369 |
Maternal passive smoking | CL/P OR: 1.24 (1.05, 1.46) |
| CPO OR: 1.31 (1.06-1.63) | |||||
| CLO OR: 1.41 (1.10, 1.81) | |||||
| (Ebadifar et al., 2016) | Case-control (retrospective) | Iran | CL/P: n = 76 CPO: n = 29 Control: n = 210 |
Maternal active smoking | CL/P OR: 19.2 (6.2, 59.5) |
| CPO OR: 48.7 (8, 293) | |||||
| Maternal passive smoking | CL/P OR: 0.45 (0.2, 1.05) |
||||
| CPO OR: 11.2 (3.1, 40.3) | |||||
| Both parents smoking | CL/P OR: 6.0 (2.2, 16.6) |
||||
| CPO OR: 55.6 (12, 256) | |||||
| (Sabbagh et al., 2016) | Case-control (retrospective) | Saudi Arabia | CL/P: n = 112 Control: n = 138 |
Maternal Jorak smoking | OR: 14.07 (1.55, 128.1) |
| Maternal passive smoking | OR: 2.05 (1.05, 4.01) | ||||
| (Sabbagh et al., 2015) | Meta-analysis | Maternal passive smoking | OFC OR: 2.11 (1.54, 2.89) |
||
| CL/P OR: 2.05 (1.27, 3.30) | |||||
| CPO OR: 2.11 (1.23-3.62) | |||||
| (Hao et al., 2015) | Case-control (retrospective) | China | CL/P n = 362 CPO n = 137 |
Maternal active smoking | CL/P OR: 1.25 (0.72, 2.17) |
| CPO OR: 1.14 (0.52, 2.47) | |||||
| Maternal passive smoking | CL/P OR: 2.52 (1.90, 3.33) |
||||
| CPO OR: 2.14 (1.45, 3.15) | |||||
| Maternal passive smoking (paternal active) | CL/P OR: 2.17 (1.65, 2.87) |
||||
| CPO OR: 1.87 (1.28, 2.75) | |||||
| (Chung, Kowalski, Kim, & Buchman, 2000) | Case-control (retrospective) | United States | CL/P: 2207 Control: 4414 |
Maternal active smoking | 1-10/day OR: 1.50 (1.28, 1.76) |
| 11-20/day OR: 1.55 (1.23, 1.95) | |||||
| 21+/day OR: 1.78 (1.22, 2.59 | |||||
Because smoking is an established risk factor for OFCs, efforts must be made on individual and societal levels to prevent future incidence of tobacco smoke related OFCs. Health care providers and policymakers can advocate more effectively by knowing how many incidences of OFC can be prevented through modified behavior. Population attributable fraction (PAF) is a measure of the proportion of incidents that can be attributed to a given risk factor. Honein et al. found that that up to 6.1% of OFC cases in the United States could be prevented by ceasing maternal active smoking in the periconceptual period of pregnancy (Honein et al., 2014). A recent prospective case-control study on a Japanese population indicated that the PAF of CL/P due to maternal active smoking is 9.9%, and 10.8% for passive smoking (Sato et al., 2020).
There are thousands of compounds present in tobacco smoke (Rodgman & Perfetti, 2016). Correspondingly, there are several hypothesized mechanisms for how tobacco smoke increases the risk of OFCs. The expression of genes involved in cell cycle regulation, DNA repair, and oxidative stress response has been affected by tobacco smoke in fetal mouse tissue (Izzotti et al., 2003). Additionally, tobacco smoke can induce proteasome-mediated degradation of proteins that mediate DNA methylation in 1st branchial arch (BA1) cells (Mukhopadhyay, Greene, & Pisano, 2015). The smoke is known to contain endogenous tobacco plant compounds, such as nicotine, as well as pyrolysis products and added chemicals (Rodgman & Perfetti, 2016). Several teratogens such as polycyclic aromatic hydrocarbons (PAHs), dioxins, carbon monoxide, pesticides, and heavy metals such as cadmium may be present in tobacco smoke. One possible mechanism for smoke-induced OFC is AHR activation. Exposure to heavy metals can cause OFCs in rodent models and are believed to act teratogenically through induction of oxidative stress and perturbation of redox-sensitive signaling pathways (W. L. Ni et al., 2018). Both nicotine and carbon monoxide can cause fetal hypoxia, a condition known to induce CL/P in mouse embryos (Bailey, Johnston, & Billet, 1995). Nicotine is a vasoconstrictor that can impair uterine vascular function and affect blood flow and oxygen delivery to the fetus (Xiao, Huang, Yang, & Zhang, 2007), while carbon monoxide can prevent oxygen transport by forming carboxyhemoglobin in red blood cells. Fetal hemoglobin has greater affinity for carbon monoxide compared to adult hemoglobin, forming 10-15% more carboxyhemoglobin than the mother (Farrow, Davis, Roy, Mccloud, & Nichols, 1990; Omaye, 2002). Pesticide exposure has been associated with OFCs (Yang et al., 2014), and some biocides can cause OFCs through inhibiting steroid anabolism (Giavini & Menegola, 2010).
Genetic susceptibility plays a major role in the risk of tobacco smoke-induced OFCs. Polymorphism of genes encoding developmental signaling ligands, such as transforming growth factor alpha (TGFA), transforming growth factor beta 3 (TGFB3), and bone morphogenetic protein 4 (BMP4), have been associated with OFCs in the context of maternal smoke exposure. TGFA, or transforming growth factor alpha, is a ligand for epidermal growth factor receptors. It is expressed in the MEE cells during palatal fusion (Ebadifar, Hamedi, KhorramKhorshid, Kamali, & Moghadam, 2016; Mitchell, 1997; Vieira & Orioli, 2001), and is known to regulate extracellular matrix synthesis and mesenchymal migration in palatal cultures (Ebadifar et al., 2016; Holder, Vintiner, Farren, Malcolm, & Winter, 1992; Machida et al., 1999; Qian, Feingold, Stoll, & May, 1993; Shiang et al., 1993). Variants effecting TGFA-smoking GxE have been observed in several populations (Ebadifar et al., 2016; Hwang et al., 1995; Junaid, Narayanan, Jayanthi, Kumar, & Selvamary, 2018; Maestri et al., 1997; G. M. Shaw et al., 1996; Sull et al., 2009). TGFB3 encodes a ligand that regulates TGF-β signaling which is critical for lip and palatal development (Reynolds et al., 2020) (in this issue). Expressed in the epithelium, TGFB3 regulates fusion at the MES and cell proliferation. Variants in TGFB3, in the context of smoking, have been associated with CL/P (Guo, Huang, Ding, Lin, & Gong, 2010; Lin et al., 2010; Romitti et al., 1999), CPO (Romitti et al., 1999), and submucous cleft palate (SMCP) (Reiter et al., 2012). BMP4, a ligand for BMP signaling, has polymorphism associated with CL/P and is involved in fusion of the medial and lateral nasal processes (Reynolds et al., 2020) (in this issue). Polymorphism of BMP4, in conjunction with smoking, has been associated with CL/P (Lin et al., 2010).
In addition to growth factors, polymorphisms in other OFC-related genes have been implicated in smoking and orofacial clefts. Fetal homozygous mutation (allele 4) in MSX1, a transcription factor important for neural crest specification, was found to impart the greatest susceptibility for OFC when mothers were exposed to smoke during pregnancy in one study (van den Boogaard et al., 2008). The authors speculated a relationship with genes involved in cell cycle regulation. This is consistent with a hypoxic mechanism, as Msx1 deficiency in mice can induce cleft lip coinciding with maternal hypoxia (Nakatomi et al., 2020). Another possible hypoxic mechanism is through the depriving of oxygen from certain proteins with 20G oxygenase domains that have been associated with OFCs, such as the lysine demethylase PHF8 (Loenarz et al., 2010). Polymorphism in another transcription factor involved in regulating TGF-β during palatogenesis (Bjork, Turbe-Doan, Prysak, Herron, & Beier, 2010), PRDM16, was associated with OFCs and either active or passive smoking (Yin, Li, Li, & Zou, 2019). Variants of genes involved in detoxification have also been associated with tobacco smoke exposure and OFCs as previously discussed.
5.2. Electronic nicotine delivery systems
Health concerns have emerged over the recent surge in electronic nicotine delivery system (ENDS) usage such as e-cigarettes and nicotine vaporizing (vaping) devices, with little known about how exposure during the periconceptional period could impact human development. The use of ENDS as a perceived safer alternative to (or a method to quit) cigarette smoking among girls and women of reproductive age is of particular concern (Mark, Farquhar, Chisolm, Coleman-Cowger, & Terplan, 2015; Oncken et al., 2017; Wagner, Camerota, & Propper, 2017). Vapor is derived from an “e-liquid” that is usually heated by a metal filament, and chemical composition of the e-liquid depends on flavorings and other constituents. Impurities may be generated by vaporizing the e-liquid, or they may already be present in liquid form as contaminants (Hahn et al., 2014). Typical components of e-cigarette vapor, some of which pose a suspected OFC hazard, include nicotine, nitrosamines, aldehydes, carbon monoxide, heavy metals, and free radicals (Hahn et al., 2014; Rowell & Tarran, 2015). Although little is known about the developmental toxicity associated with e-cigarettes, some laboratory evidence suggests that vaping aerosols are toxic to craniofacial development. One study using Xenopus identified an oral cleft hazard in e-cigarette aerosols (Kennedy, Kandalam, Olivares-Navarrete, & Dickinson, 2017). The authors found that aerosol from a minimal e-liquid containing only propylene glycol, vegetable glycerin, and nicotine caused microstomia in larvae, while aerosol from e-liquids containing certain flavorings produced medial oral cleft. Exposure of murine O9–1 neural crest cells to cleft-inducing aerosols decreased expression of vascular and chondral markers, indicating a potential hazard for mammalian craniofacial development. The risk for human OFC due to vape exposure remains to be determined.
5.3. Alcohol consumption
Unlike tobacco smoking, there is less substantial evidence for a role of maternal alcohol consumption in OFC etiology (Table 2). A recent study in Brazil found elevated but non-significant risk associated with alcohol consumption at the highest frequency measured (OR = 1.55, 95% CI: 0.56, 4.26). Another recent study in Japan also observed no relationship between maternal drinking and OFC by two metrics: the mother quit drinking after pregnancy (OR = 0.87, 95% CI 0.63, 1.22) versus current drinker during pregnancy (OR = 0.71, 95% CI: 0.22, 2.27) (Sato et al., 2020). Meta-analysis by Yin et al. similarly found no relationship between any drinking and CL/P (OR = 1.00, 95% CI: 0.87, 1.15) or CPO (OR = 1.02, 95% CI: 0.92, 1.14) (Yin et al., 2019). However, some studies have identified an OFC risk related to alcohol consumption in China (OR = 1.79, 95% CI: 1.03, 3.12) (D. P. Xu et al., 2018), Democratic Republic of the Congo (OR = 19.30, 95% CI: 1.89, 197.10) (Mbuyi-Musanzayi et al., 2018), and Mexico (OR = 1.90, 95% CI: 1.17, 3.08) (Angulo-Castro et al., 2017). A pooled study by Deroo et al. (2016) observed an increased risk for CLO (OR = 1.95, 95% CI: 1.23, 3.11), but only for instances where the mother binged (5+ drinks) in at least three different sittings during pregnancy (Deroo et al., 2016). Another study in Brazil found an increased risk of CL/P due to drinking relative to CPO (RR: 1.54, 95% CI: 1.07, 2.38) (Maranhao et al., 2020).
Table 2.
Recent population studies on the effects of maternal drinking on the risk for OFCs. Final adjusted regression model statistics used where available. Sample sizes reflect totals for overall study. CI, confidence interval. CLO, cleft lip only. CL/P, cleft lip with or without cleft palate. CPO, cleft palate only. OFC, any orofacial cleft. OR, odds ratio. RR, relative risk.
| Study | Design | Population | Sample | Exposure type | Statistic |
|---|---|---|---|---|---|
| (Regina Altoe et al., 2020) | Case-control (retrospective) | Brazil | OFC: n = 150 Control: n = 300 |
1-7 times/week | OR: 1.18 (0.67, 2.05) |
| 1-2 times/month | OR: 0.20 (0.03, 1.57) | ||||
| 5+ drinks | OR: 1.55 (0.56, 4.26) | ||||
| 1-4 drinks | OR: 0.85 (0.46, 1.55) | ||||
| (Maranhao et al., 2020) | Relative risk | Brazil | CL/P n = 346 CPO: n = 237 CLO n = 151 |
Unspecified frequency | CL/P vs CPO RR: 1.54 (1.07, 2.38) |
| CLO vs CPO RR: 1.25 (0.51, 3.07 | |||||
| (Sato et al., 2020) | Case-control (prospective) | Japan | CL/P: n = 146 Control: n = 93987 |
Quit after pregnancy | OR: 0.87 (0.63, 1.22) |
| Current drinker | OR: 0.71 (0.22, 2.27) | ||||
| (Yin et al., 2019) | Meta-analysis | Any drinking | CL/P OR: 1.00 (0.87, 1.15) |
||
| CPO OR: 1.02 (0.92, 1.14) | |||||
| (D. P. Xu et al., 2018) | Case-control (retrospective) | China | CL/P: n = 239 Control: n = 209 |
Unspecified frequency | OR: 1.79 (1.03, 3.12) |
| (Mbuyi-Musanzayi et al., 2018) | Case-control (retrospective) | DR Congo | CL/P: n = 172 Control: n = 162 |
Unspecified frequency | OR: 19.30 (1.89, 197.10) |
| (Angulo-Castro et al., 2017) | Case-control (retrospective) | Mexico | CL/P n = 24 Control: n = 24 |
Unspecified frequency | OR: 1.9 (1.17-3.08) |
| (Deroo et al., 2016) | Pooled study | Non-binge (<5 drinks), 1-2 times | OFC OR: 1.07 (0.96, 1.20) |
||
| CL/P OR: 1.09 (0.93, 1.27) | |||||
| CPO OR: 0.97 (0.82, 1.15) | |||||
| CLO OR: 1.19 (0.98, 1.45) | |||||
| Non-binge (<5 drinks), 3+ times | OFC OR: 0.89 (0.79, 1.01) |
||||
| CL/P OR: 0.86 (0.72, 1.02) | |||||
| CPO OR: 0.88 (0.74, 1.06) | |||||
| CLO OR: 0.98 (0.79, 1.21) | |||||
| Binge (5+ drinks), 1-2 times | OFC OR: 1.00 (0.70, 1.45) |
||||
| CL/P OR: 0.91 (0.55, 1.51) | |||||
| CPO OR: 1.22 (0.73, 2.04) | |||||
| CLO OR: 0.94 (0.49, 1.85) | |||||
| Binge (5+ drinks), 3+ times | OFC OR: 1.23 (0.89, 1.69) |
||||
| CL/P OR: 1.02 (0.65, 1.60) | |||||
| CPO OR: 1.04 (0.63, 1.73) | |||||
| CLO OR: 1.95 (1.23, 3.11) | |||||
| (Hao et al., 2015) | Case-control (retrospective) | China | CL/P n = 362 CPO n = 137 |
Unspecified frequency | CL/P OR: 0.75 (0.50, 1.12) |
| CPO OR: 1.28 (0.77, 2.11) | |||||
| (Bell et al., 2014) | Meta-analysis | Any drinking | CL/P OR: 1.00 (0.86, 1.16) |
||
| CPO OR: 1.05 (0.92, 1.21) | |||||
| Binge (5+ drinks at any one time) | CL/P OR: 1.04 (0.87, 1.24) |
||||
| CPO OR: 0.94 (0.74, 1.21) | |||||
The exact mechanisms by which consuming alcoholic beverages increases the risk of OFC are not understood, but it has been intensely studied in the context of other developmental pathologies. Several hypotheses exist for teratological mechanisms of ethanol (EtOH) that could plausibly account for OFCs, such as antagonism of retinoic acid (RA) signaling (Pullarkat, 1991), altered epigenetics (F. C. Zhou et al., 2011), and oxidative stress (Zima et al., 2001). These mechanisms involve the oxidation of EtOH into a toxic intermediate, acetaldehyde (AcAL), before ultimately being metabolized into acetyl-CoA by aldehyde dehydrogenase (ALDH) (Deitrich, Zimatkin, & Pronko, 2006). Enzymes that form AcAL from EtOH include catalase, ADH, and the CYP enzyme CYP2E1. In the RA-EtOH competition model, AcAL competes with retinaldehyde for enzymes that biosynthesize RA. A recent study using Xenopus found that embryonic exposure to EtOH prevented RA synthesis due to the competitive binding of AcAL with Aldh1a2 (retinaldehyde dehydrogenase 2), which synthesizes RA from retinaldehyde (Shabtai, Bendelac, Jubran, Hirschberg, & Fainsod, 2018). Chemical inhibition of ADH prevented formation of AcAL, permitting normal RA synthesis by Aldh1a2. Kinetic analysis of human ALDH1A2 identified a preference for AcAL as a substrate over retinaldehyde, providing a biochemical basis for the RA-EtOH competition model. Protective CYP2E1 gene variants associated with decreased risk of OFC are consistent with differential ability to metabolize EtOH as well as RA (Chevrier et al., 2007).
While the RA-EtOH competition model seems like a straightforward mechanism, the effect of EtOH on orofacial development appears to be more complex. In zebrafish larvae, EtOH exposure disrupted hair cell development in the lateral line but these effects were partially rescued by co-exposure with RA or folic acid (Muralidharan, Sarmah, & Marrs, 2015). The rescue effects of folic acid could be manifold due to its supportive effects on cell proliferation, epigenetic changes, and oxidative stress. Indeed, EtOH exposure can alter the epigenetic state of neural stem cells by altering the DNA methylation of cell cycle genes (Hicks, Middleton, & Miller, 2010). Another zebrafish study found that craniofacial malformations in pdgfra mutants were exacerbated by EtOH, and pdgfra protected against EtOH through the PI3K-mTOR pathway (McCarthy et al., 2013). Additionally, in vitro studies found that metabolism of EtOH to AcAL by CYP2E1 can lead to formation of ROS to induce oxidative stress-mediated activation of JNK signaling, and apoptosis (Jin, Ande, Kumar, & Kumar, 2013). Vitamins C and E, like through antioxidant activity, could rescue these affects. In murine embryonic stem cells, EtOH and AcAL promoted differentiation through transcriptional activities of the RA receptor, RAR-γ, causing induction of target genes such as Hoxa1 and Cyp26a1 (Serio, Laursen, Urvalek, Gross, & Gudas, 2019). Thus, EtOH exposure may alter the stemness and/or fate trajectory of cells during embryogenesis (Serio & Gudas, 2020).
While these effects are seemingly consistent with disruption of orofacial development, there is little experimental evidence linking them together. Much work remains to be done regarding EtOH exposure and mechanisms of OFC, but there is enough knowledge to begin well-planned investigations.
5.4. Diet and pharmacological exposures
The relationship between maternal diet and risk for OFC has been studied intensively. Higher quality diet, from the standpoint of the Mediterranean diet and USDA Food Guide Pyramid, is associated with better outcomes for orofacial development (Carmichael et al., 2012). Some research indicates that vegetarian diets increase the risk of OFCs (Neogi et al., 2017), possibly because of a lack of vitamins (specifically, cobalamin), minerals, and fatty acids that are more prevalent in animal products (Pistollato et al., 2015). In a Chinese study, maternal consumption of tea and pickled food was associated with higher risk of OFCs, whereas soy consumption was protective (Lili et al., 2017). More studies will be necessary to determine the safety of select food items in the context of orofacial development. In these studies, food contamination should be a major consideration. For example, a study in the Democratic Republic of the Congo found a substantially increased risk for OFC in mothers that consumed Kapolowe fish, which was substantially contaminated with heavy metal (OR: 29.47, 95% CI: 7.44, 116.72) (Mbuyi-Musanzayi et al., 2018).
There is also evidence that supplementation with vitamins and minerals can protect against OFCs, but not all studies agree. A study in China found that multivitamin use decreased risk of both CL/P (OR = 0.04, 95% CI: 0.01, 0.11) and CPO (OR = 0.08, 95% CI: 0.02, 0.25), but only when taken preconceptionally (Hao et al., 2015). A recent study in Brazil, however, found no significant decreased risk of OFC among those that took a multivitamin (Regina Altoe et al., 2020). In some cases, use of pharmacological agents during pregnancy could increase risk of OFC. One of the best studied is phenytoin, an anti-convulsant that was previously believed to induce cleft palate through its interaction with the GR (Inayoshi, Ohsaki, Nagata, & Kurisu, 1989). It is now understood that phenytoin teratogenicity is more likely mediated through its inhibition of delayed rectifier K+ channels, causing cleft palate by altering heart pumping and inducing hypoxia (Azarbayjani & Danielsson, 2001).
5.5. Occupational and domestic exposures
Occupational exposure to solvents may be a risk factor for OFCs. An example is methanol, poisonous alcohol with the capacity for inducing cleft palate in mice (Siu, Shapiro, Wiley, & Wells, 2013). A recent meta-analysis found that glycol ethers specifically increased the risk for CL/P (OR = 1.95, 95% CI: 1.38, 2.75) (Spinder et al., 2019). Another study on a Chinese population associated unspecified solvent exposure to a large increase in risk for CL/P (OR = 6.07, 95% CI: 1.49, 24.76) (Hao et al., 2015). The evidence for an association is not always consistent, however, as multiple studies have failed to associated OFC risk with solvent exposure. In Texas, exposure to relevant environmental levels to any of the BTEX compounds (benzene, toluene, ethyl benzene, or xylene) was not associated with CL/P (Ramakrishnan et al., 2013). Maternal occupational exposure to chlorinated solvents was also not associated with OFCs based on data from the National Birth Defects Prevention Study (Desrosiers et al., 2012). If solvents are a risk factor for OFCs, it likely depends on the chemical class and whether the mother or fetus are genetically susceptible due to polymorphisms of detoxification genes (Chevrier et al., 2007).
Biocides, including agricultural pesticides, are potential OFC risk factors. One meta-analysis based on 19 studies found a slight increased risk associated with pesticide exposure (OR = 1.37; 95% CI: 1.04 to 1.81) (Romitti, Herring, Dennis, & Wong-Gibbons, 2007). A study in the Democratic Republic of the Congo identified a significantly increased CL/P risk associated with pesticides (OR = 130.306, 95% CI: 13.19, 1286.95) (Mbuyi-Musanzayi et al., 2018). In China, a case-control study identified an increased risk for both CL/P (OR = 23.99, 95% CI: 9.58, 60.06) and CPO (OR = 17.20, 95% CI: 6.35, 46.60) among mothers exposed to pesticides (Hao et al., 2015). Little is known about how specific pesticides could influence orofacial development. One class of biocides, azole fungicides, can induce cleft palate through their inhibitory effect on CYP51 (Giavini & Menegola, 2010). CYP51, or sterol 14α-demethylase, is a cholesterogenic enzyme that catalyzes an initial step required for steroid hormone synthesis. Homozygous Cyp51 knockout mouse embryos, while suffering lethality at E15, present with phenotypes correlating with Antley-Bixler syndrome that includes cleft palate (Keber et al., 2011).
Residential proximity to chemical emission may contribute to OFC incidence. In Texas, mothers over 35 were at increased risk to bear offspring with CL/P if they lived near sources of ethyl chloride (OR = 1.81, 95% CI: 1.06, 3.07), or CPO if they lived near sources of 1,2-dichloroethane (OR = 1.93, 95% CI: 1.05, 3.54) (Brender, Shinde, Zhan, Gong, & Langlois, 2014). A study in China found that residence near any factory also increased the risk for CL/P (OR = 1.90, 95% CI: 1.60, 2.25) (Lili et al., 2017). A possible contributing factor to the risk of OFCs for those who live near factories, or for those who work industrial professions, is the presence of AHR ligands in industrial or combustion products such as PAHs, polychlorinated biphenyls (PCBs), and polychlorinated dibenzofurans (PCDFs). Benzo[a]pyrene (BaP) is an example of a highly teratogenic and carcinogenic PAH that can induce cleft palate in mice (Shum, Jensen, & Nebert, 1979). A case control study using data from National Birth Defects Prevention Study identified a significant association between cleft lip with or without cleft palate and maternal occupational PAH exposure (Langlois et al., 2013). Another study in China measured PAH levels in umbilical cord samples from children with and without OFC as a surrogate for in utero exposure (W. Ni et al., 2019). In this analysis, the authors found no significant association between in utero PAH exposure and OFC. Few studies have examined the effects of PAH exposure on human orofacial development, and more are needed to understand if such an association exists.
Other persistent organic pollutants like PCBs and PCDFs interact with the AHR and are associated with cleft palate (Denison & Nagy, 2003). PCBs may have dioxin-like or non-dioxin-like structure (Van den Berg et al., 2006). They have been used primarily as electrical insulating, hydraulic, heat transfer, and lubricating fluids, with additionally utility as plasticizers, fire retardant mixtures, and carbonless copy paper (M. D. Erickson & Kaley, 2011). Although U.S. manufacturing of PCBs ended in 1977 due to concerns over their adverse health effects, they continue to be used and their environmental persistence is still a concern. In mice, subcutaneous injection of Kanechlor-500 (KC-500), a mixture of PCBs of which the main constituent was pentachlorobiphenyl, daily during E6-15 increased cleft palate incidence at doses of 10 – 50 mg total (not normalized to maternal body weight) (Watanabe & Sugahara, 1981). In another study, 2,2’, 4,4’, 5,5’-hexachlorobiphenyl (HCB) was found to cause hydronephrosis, but not cleft palate, in mouse embryos. Co-exposure of HCB and TCDD reduced the incidence of cleft palate compared to TCDD exposure alone, suggesting that HCB competes with TCDD for binding AHR (Morrissey, Harris, Diliberto, & Birnbaum, 1992). Non-dioxin-like PCBs have been shown to suppress the response to TCDD or PCB-126 (a dioxin-like PCB) exposure in rat liver cells in vitro (Brenerova et al., 2016). PCB exposures are therefore a possible risk for OFCs, but this depends on exposure context including presence or absence of other AHR ligands.
PCDFs, which are structurally similar to dioxins, are often found as combustion products of organic materials that contain chlorine such as PCB-containing substances. They are not intentionally produced for industrial purposes and have no known industrial or commercial utility (Fiedler, 1996). Among those that induce cleft palate in mice include 2,3,7,8-tetrachlorodibenzofuran (TCDF), 1,2,3,7,8-pentachlorodibenzofuran (1-PeCDF), 2,3,4,7,8-pentachlorodibenzofuran (4-PeCDF), and 1,2,3,4,7,8-hexachlorodibenzofuran (HCDF) (L. Birnbaum, Harris, Barnhart, & Morrissey, 1987; L. S. Birnbaum, Harris, Crawford, & Morrissey, 1987; Hassoun, Dargy, Dencker, Lundin, & Borwell, 1984; Weber, Lamb, Harris, & Moore, 1984). In Japan during 1968, rice oil was contaminated with PCBs and associated degradation products (i.e., PCDFs) resulting in a mass poisoning. Congenital effects of in utero exposure to contaminated rice oil included altered pigmentation and was known as fetal PCB syndrome (Mitoma et al., 2015). While no cases of OFC have been reported in association with the Yusho incident, experimental evidence in mouse has indicated a cleft palate hazard for PCBs and PCDFs.
6. Pathogens and orofacial clefts
While chemical exposures and genetic predisposition can influence the risk of OFCs, there is evidence that biotic factors such as pathogens may also be involved. In some cases, such as with cytomegalovirus, specific molecular pathways likely contribute to OFC pathology. For others, however, it is unclear whether the pathogen acts through a specific mechanism or if the maternal or fetal immune response causes a teratogen effect. Here we discuss pathogenic associations with OFCs and, where possible, describe specific mechanisms affecting orofacial development.
6.1. Viruses
Viral infection during the first trimester is a suspected cause of OFC. While some viruses are known teratogens, the evidence for an association with OFC is divergent. Among the viruses considered potential risk factors for OFC are varicella, rubella, rubeola, Epstein-Barr virus, herpesvirus, cytomegalovirus, common cold, and influenza (Acs, Banhidy, Puho, & Czeizel, 2005; Banhidy, Puho, Acs, & Czeizel, 2007b; Cohen, 2000; A. E. Czeizel, Puho, Acs, & Banhidy, 2008; Luteijn, Brown, & Dolk, 2014; Metneki, Puho, & Czeizel, 2005; Molnarova et al., 2018; W. Shaw, 2004; J. Zhang & Cai, 1993). In 1963, Leck detected an elevated incidence of OFC in relation to influenza infection (Leck, 1963). Based on observations, he speculated that if an influenza-induced syndrome of birth defects existed, cleft lip would be among the four major congenital abnormalities most likely observed. However, several studies have disagreed that influenza infection is teratogenic (A. Czeizel, Tusnady, Domany, & Borsy, 1967; Doll, Hill, & Sakula, 1960; Walker & Mc, 1959; Widelock, Csizmas, & Klein, 1963; Wilson & Stein, 1969). Notably, different groups have reached divergent conclusions from the 1957 Asian influenza pandemic. While Coffey and Jessop (1959) identified an elevated incidence of congenital abnormalities in Dublin, Doll and Hill (1960) found no such correlation in London (Coffey & Jessop, 1959; Doll et al., 1960). The latter group stated that if craniofacial malformations are a hazard of influenza infection during the first trimester, then the risk is so low that it is difficult to measure. One possible confounder of these types of studies is the consumption of drugs for symptom relief during acute illness (Saxén, 1975).
In spite of the divergent conclusions of the literature, more recent studies have identified a correlation between influenza and orofacial cleft (Acs et al., 2005; Metneki et al., 2005). The mechanisms involved are not fully understood, but research suggests that hyperthermia from fever induction may be an indirect driver of teratogenesis. A Hungarian case-control study by Acs et al. (2005) identified a significant correlation between influenza and cleft lip/palate among mothers that were diagnosed with influenza in the second and/or third month of the first trimester (Acs et al., 2005). Notably, this correlation disappeared among women that used antipyretics. Another study in Hungary found a correlation between OFC and other febrile infections that may be viral or bacterial, such as gastroenteritis, sinusitis, and bronchitis (Metneki et al., 2005). Herpesvirus infection during the second and/or third month of the first trimester has also been associated with congenital abnormalities including OFC, and antipyretic use in this context has been correlated with decreased OFC incidence (Banhidy, Puho, Acs, & Czeizel, 2007a). A separate study purposed for identifying non-pathogenic etiologies found a slight elevation of risk for OFC among mothers that used an electric blanket or heated waterbed in the periconceptional period (G. M. Shaw, Nelson, Todoroff, Wasserman, & Neutra, 1999). Further work must be undertaken to verify a hyperthermal etiology for OFC and pathogenic teratogenesis.
Cytomegalovirus (CMV), a herpes-related virus, is a suspected etiological agent of OFC and its mechanism of action appears to be independent of hyperthermia. CMV infection in pregnant women is a known teratogenic hazard. Weichert et al. (2010) reported a single case of cleft lip and micrognathia in a fetus whose mother was infected with CMV (Weichert, Vogt, Dudenhausen, & Kalache, 2010). In 2017, Divya et al. reported that CMV may be a potent inducer of cleft lip/palate, mental disability, and deafness (Divya et al., 2017). Animal models have shown that CMV impairs mesenchymal development, making orofacial development particularly vulnerable to CMV infection due to its effects on epithelial-mesenchymal interactions (Tsutsui, 1995; Weichert et al., 2010). Infection of murine BA1 culture demonstrated a tropism of CMV toward neural crest mesenchyme and disruption of the extracellular matrix (Melnick, Mocarski, Abichaker, Huang, & Jaskoll, 2006). The authors also suspected an EMT mechanism in CMV-exposed submandibular salivary glands, although this was difficult to ascertain in the ex vivo context. A follow-up study demonstrated that CMV infection of murine mandibular process culture from E11 resulted in mandibular hypoplasia stemming from decreased osteogenesis, as well as decreased chondrogenesis of Meckel’s cartilage (Jaskoll, Abichaker, Sedghizadeh, Bringas, & Melnick, 2008; Melnick et al., 2006). Both studies described a disruption of nuclear factor kappa B (NFκB) signaling. The latter paper also demonstrated increased abundance of the Tgf-β signaling inhibitor Smad7 in infected osteoblasts and periosteal cells, as well as an absence of Shh expression in Meckel’s cartilage. Given that both Smad7 and Shh are critical for normal orofacial development and are responsive to NFκB, the authors postulated that CMV may induce craniofacial malformations through an NFκB-dependent mechanism.
6.2. Bacteria
Like viral infections, there is some divergence as to whether bacterial infection can cause OFC. Some bacteria suspected of an association with OFC include mycoplasma (Mycoplasma pneumoniae), chlamydia (Chlamydia trachomatis), and syphilis (Treponema pallidum pallidum) (Gupta, Chotaliya, & Marfatia, 2012; LopezCamelo & Orioli, 1996; Molnarova et al., 2006). Based on clinical data from Campeche, Mexico, authors of a case-control study identified a possible association between bacterial infection during pregnancy and OFC, although information on specific pathogens was unavailable (Acuna-Gonzalez et al., 2011). A Slovak study published in 2006 compared the presence of antibodies to mycoplasma and chlamydia infection in newborns with OFC incidence (Molnarova et al., 2006). This study identified a high prevalence of mycoplasma antibodies in newborns with OFC and their mothers, in addition to a slightly elevated prevalence of chlamydia antibodies. The authors concluded that mycoplasma infection was more frequent in newborns with OFC and their mothers, and that chlamydia presented a comparatively lower and perhaps insignificant risk for OFC. They nonetheless state that this study could not identify a definitive association between infection with pathogen of OFC. Overall, the relationship between OFC and bacterial infection remains to be elucidated.
6.3. Protozoa
The only known protozoan associated with OFC is Toxoplasma gondii, the pathogen responsible for the disease toxoplasmosis. While usually asymptomatic, transmission of T. gondii from mother to fetus can result in serious birth defects, including craniofacial malformations such as microcephaly and hydrocephalus (J. Jones, Lopez, & Wilson, 2003). A survey published in 2001 indicated that 15% of women at childbearing age in the United States showed evidence of T. gondii infection (J. L. Jones et al., 2001). Latent maternal infection poses a low risk to the fetus since the parasites exist within tissue cysts as nonmigratory bradyzoites. However, ingestion of sporulated T. gondii oocytes from the environment is followed by transformation into the migratory tachyzoite stage. Only in this form can T. gondii cross the placental barrier to infect the fetus; thus, the hazard for congenital toxoplasmosis exists predominantly for women that are pregnant at the time of infection. Cats are the definitive host for the T. gondii reproductive stage, and congenital toxoplasmosis is one of the best-described hazards for maternal handling of used cat litter during pregnancy. Another entryway for T. gondii infection is through consuming contaminated food or water (Dubey, 2009).
Toxoplasmosis has been a suspected etiological agent of OFC since at least the 1950s (Gabka, 1953). In 1992, a hospital in Kunshan, China identified ten children with OFC that had T. gondii tachyzoites and bradyzoites within the cleft tissue (Gu, Lu, & Ma, 1992). A 1994 Slovak study found a correlation with serum antibodies for T. gondii in mothers and their children with OFC (Holková et al., 1994). In 2017, a hospital in Mexico City reported a single case of congenital toxoplasmosis where the infant presented with oblique oro-orbital cleft and associated anophthalmia (Arce-Estrada et al., 2017). While congenital rubella infection was also implicated, the defects were attributed to congenital toxoplasmosis. Despite this evidence, not all studies have identified an association between OFC and congenital toxoplasmosis (Gylling, 1967). Further study is required to understand the full teratogenic potential of T. gondii.
7. Conclusions
Orofacial development is an intricate biological process involving rapid growth, differentiation, tissue fusion, transformation, and many other processes (Ji et al., 2020) (in this issue). The possible environmental influences on these activities are correspondingly complex and can interact with various aspects of development. This is further complicated by genetic variation that could perturb (or enhance) both endogenous processes and cellular capacity for dealing with environmental insults (Shi et al., 2007). Understanding the mechanisms by which environmental factors impact orofacial development could help in the prevention of future OFC cases.
Among the most important metabolic processes in orofacial development are folate and retinoid metabolism. Folates promote cellular proliferation, differentiation, epigenetics, and can prevent oxidative stress by preventing hyperhomocysteinemia (Wahl et al., 2015; Wong et al., 1999). There is increasing evidence that folic acid supplementation protects against OFCs, but its effectiveness may vary on a population or individual basis. A major influence on this outcome may be allelic variation in genes related to folate metabolism (Ramirez-Chau et al., 2016). Studies that integrate, genetic, dietary, and metabolic data would help clarify whether folates are truly protective against OFCs. Given the importance of maternal folate metabolism in OFCs, personalized guidance based on genotype may help guide expecting parents in managing their dietary needs. A similar approach may also clarify whether supplementing with vitamin A (i.e., consuming cod liver oil) is protective or an unnecessary risk.
To address the environmental mechanisms of OFCs, there are several challenges that must be met. These including the identification of hazards, genetic susceptibilities, and preventive measures. While many environmental factors are already known to increase the risk OFCs, there are likely many others for which the hazard has yet to be identified. Robust chemical screens using in vivo (S. Liu, Narumi, Ikeda, Morita, & Tasaki, 2020), in vitro (Belair et al., 2018), and in silico (Baker et al., 2020) approaches will be needed to identify OFC-inducing teratogens. Such approaches may include novel chemical screens or utilize a priori knowledge of bioinformatic relationships. These screens will also facilitate the identification of therapeutic supplements that protect against environmentally-induced OFCs (Muralidharan et al., 2015). Incorporating genetic models will add a layer of complexity to these approaches. Genome-wide surveys for gene-environment interactions can assist in the developmental of such models (O. A. Haaland et al., 2018; Ø. A. Haaland et al., 2019). Taking full advantage of these approach will help identify hazards, mechanisms, and therapeutic approaches in the effort to prevent OFCs.
Acknowledgments
This work is supported by grants from the NIH (R01DE026737, R01NS102261, and R01DE021696 to C.J.Z.) and the Shriners Hospitals for Children (85105 to C.J.Z.). We are grateful to the rest of Zhou lab members for their general supports during the manuscript preparation. We apologize to colleagues whose important work we were unable to cite due to space constraints or inadvertently overlooking.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
DATA SHARING AND DATA ACCESSIBILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Abbott BD, & Birnbaum LS (1991). TCDD exposure of human embryonic palatal shelves in organ culture alters the differentiation of medial epithelial cells. Teratology, 43(2), 119–132. doi: 10.1002/tera.1420430205 [DOI] [PubMed] [Google Scholar]
- Abbott BD, Buckalew AR, DeVito MJ, Ross D, Bryant PL, & Schmid JE (2003). EGF and TGF-alpha expression influence the developmental toxicity of TCDD: dose response and AhR phenotype in EGF, TGF-alpha, and EGF + TGF-alpha knockout mice. Toxicol Sci, 71(1), 84–95. doi: 10.1093/toxsci/71.1.84 [DOI] [PubMed] [Google Scholar]
- Abbott BD, Perdew GH, Buckalew AR, & Birnbaum LS (1994). Interactive regulation of Ah and glucocorticoid receptors in the synergistic induction of cleft palate by 2,3,7,8-tetrachlorodibenzo-p-dioxin and hydrocortisone. Toxicol Appl Pharmacol, 128(1), 138–150. doi: 10.1006/taap.1994.1191 [DOI] [PubMed] [Google Scholar]
- Abbott BD, Probst MR, Perdew GH, & Buckalew AR (1998). AH receptor, ARNT, glucocorticoid receptor, EGF receptor, EGF, TGF alpha, TGF beta 1, TGF beta 2, and TGF beta 3 expression in human embryonic palate, and effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Teratology, 58(2), 30–43. doi: [DOI] [PubMed] [Google Scholar]
- Acs N, Banhidy F, Puho E, & Czeizel AE (2005). Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Research Part a-Clinical and Molecular Teratology, 73(12), 989–996. doi: 10.1002/bdra.20195 [DOI] [PubMed] [Google Scholar]
- Acuna-Gonzalez G, Medina-Solis CE, Maupome G, Escoffie-Ramirez M, Hernandez-Romano J, Marquez-Corona Mde L, … Villalobos-Rodelo JJ (2011). Family history and socioeconomic risk factors for non-syndromic cleft lip and palate: a matched case-control study in a less developed country. Biomedica, 31(3), 381–391. doi: 10.1590/S0120-41572011000300010 [DOI] [PubMed] [Google Scholar]
- Alexandrie AK, Warholm M, Carstensen U, Axmon A, Hagmar L, Levin JO, … Rannug A (2000). CYP1A1 and GSTM1 polymorphisms affect urinary 1-hydroxypyrene levels after PAH exposure. Carcinogenesis, 21(4), 669–676. doi: 10.1093/carcin/21.4.669 [DOI] [PubMed] [Google Scholar]
- Angulo-Castro E, Acosta-Alfaro LF, Guadron-Llanos AM, Canizalez-Roman A, Gonzalez-Ibarra F, Osuna-Ramirez I, & Murillo-Llanes J (2017). Maternal Risk Factors Associated with the Development of Cleft Lip and Cleft Palate in Mexico: A Case-Control Study. Iran J Otorhinolaryngol, 29(93), 189–195. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28819616 [PMC free article] [PubMed] [Google Scholar]
- Arce-Estrada GE, Gomez-Toscano V, Cedillo-Pelaez C, Sesman-Bernal AL, Bosch-Canto V, Mayorga-Butron JL, … Correa D (2017). Report of an unsual case of anophthalmia and craniofacial cleft in a newborn with Toxoplasma gondii congenital infection. BMC Infect Dis, 17(1), 459. doi: 10.1186/s12879-017-2565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbayjani F, & Danielsson BR (2001). Phenytoin-induced cleft palate: Evidence for embryonic cardiac bradyarrhythmia due to inhibition of delayed rectifier K+ channels resulting in hypoxia-reoxygenation damage. Teratology, 63(3), 152–160. doi: 10.1002/tera.1026 [DOI] [PubMed] [Google Scholar]
- Bailey LJ, Johnston MC, & Billet J (1995). Effects of Carbon-Monoxide and Hypoxia on Cleft-Lip in a/J Mice. Cleft Palate-Craniofacial Journal, 32(1), 14–19. doi: [DOI] [PubMed] [Google Scholar]
- Baker NC, Sipes NS, Franzosa J, Belair DG, Abbott BD, Judson RS, & Knudsen TB (2020). Characterizing cleft palate toxicants using ToxCast data, chemical structure, and the biomedical literature. Birth Defects Research, 112(1), 19–39. doi: 10.1002/bdr2.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banhidy F, Puho E, Acs N, & Czeizel AE (2007a). Possible Indirect Association Between Maternal Recurrent Orofacial Herpes in Pregnancy and Higher Risk for Congenital Abnormalities. Journal of the Turkish-German Gynecological Association, 8(2), 155–164. Retrieved from <Go to ISI>://WOS:000420587400004 [Google Scholar]
- Banhidy F, Puho E, Acs N, & Czeizel AE (2007b). Possible Indirect Association Between Maternal Recurrent Orofacial Herpes in Pregnancy and Higher Risk for Congenital Abnormalities. Journal of the Turkish-German Gynecological Association, 8(2), 155–164. Retrieved from <Go to ISI>://WOS:000420587400004 [Google Scholar]
- Barak AJ, Beckenhauer HC, Junnila M, & Tuma DJ (1993). Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcoholism-Clinical and Experimental Research, 17(3), 552–555. doi: 10.1111/j.1530-0277.1993.tb00798.x [DOI] [PubMed] [Google Scholar]
- Beaty TH, Murray JC, Marazita ML, Munger RG, Hetmanski IRJB, Liang KY, … Scott AF (2010). A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4 (vol 42, pg 525, 2010). Nature Genetics, 42(8), 727–727. doi: 10.1038/ng0810-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty TH, Taub MA, Scott AF, Murray JC, Marazita ML, Schwender H, … Ruczinski I (2013). Confirming genes influencing risk to cleft lip with/without cleft palate in a case-parent trio study. Human Genetics, 132(7), 771–781. doi: 10.1007/s00439-013-1283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry S, Zhong M, & Molday RS (2004). N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR). Journal of Biological Chemistry, 279(52), 53972–53979. doi: 10.1074/jbc.M405216200 [DOI] [PubMed] [Google Scholar]
- Belair DG, Wolf CJ, Moorefield SD, Wood C, Becker C, & Abbott BD (2018). A Three-Dimensional Organoid Culture Model to Assess the Influence of Chemicals on Morphogenetic Fusion. Toxicol Sci, 166(2), 394–408. doi: 10.1093/toxsci/kfy207 [DOI] [PubMed] [Google Scholar]
- Bhaskar LVKS, Murthy J, & Babu GV (2011). Polymorphisms in genes involved in folate metabolism and orofacial clefts. Archives of Oral Biology, 56(8), 723–737. doi: 10.1016/j.archoralbio.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Birnbaum L, Harris M, Barnhart E, & Morrissey R (1987). Teratogenicity of three polychlorinated dibenzofurans in C57BL6N mice. Toxicology and Applied Pharmacology, 90(2), 206–216. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Harris MW, Crawford DD, & Morrissey RE (1987). Teratogenic Effects of Polychlorinated Dibenzofurans in Combination in C57bl/6n Mice. Toxicology and Applied Pharmacology, 91(2), 246–255. doi: 10.1016/0041-008x(87)90105-0 [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Harris MW, Miller CP, Pratt RM, & Lamb JC (1986). Synergistic interaction of 2,3,7,8,-tetrachlorodibenzo-p-dioxin and hydrocortisone in the induction of cleft palate in mice. Teratology, 33(1), 29–35. doi: 10.1002/tera.1420330106 [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Stojanovic I, Stoimenov TJ, Pavlovic D, Kocic G, Bjelakovic GB, … Basic J (2017). Polyamines, folic acid supplementation and cancerogenesis. Pteridines, 28(3-4), 115–131. doi: 10.1515/pterid-2017-0012 [DOI] [Google Scholar]
- Bjork BC, Turbe-Doan A, Prysak M, Herron BJ, & Beier DR (2010). Prdm16 is required for normal palatogenesis in mice. Human Molecular Genetics, 19(5), 774–789. doi: 10.1093/hmg/ddp543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliek BJB, van Schaik RHN, van der Heiden IP, Sayed-Tabatabaei FA, van Duijn CM, Steegers EAP, … Interaction, E. G. E. (2009). Maternal Medication Use, Carriership of the ABCB1 3435C > T Polymorphism and the Risk of a Child With Cleft Lip With or Without Cleft Palate. American Journal of Medical Genetics Part A, 149a(10), 2088–2092. doi: 10.1002/ajmg.a.33036 [DOI] [PubMed] [Google Scholar]
- Bower C, Miller M, Payne J, & Serna P (2006). Folate intake and the primary prevention of non-neural birth defects. Australian and New Zealand Journal of Public Health, 30(3), 258–261. doi:DOI 10.1111/j.1467-842X.2006.tb00867.x [DOI] [PubMed] [Google Scholar]
- Brender JD, Shinde MU, Zhan FB, Gong X, & Langlois PH (2014). Maternal residential proximity to chlorinated solvent emissions and birth defects in offspring: a case-control study. Environmental Health, 13. doi: 10.1186/1476-069x-13-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenerova P, Hamers T, Kamstra JH, Vondracek J, Strapacova S, Andersson PL, & Machala M (2016). Pure non-dioxin-like PCB congeners suppress induction of AhR-dependent endpoints in rat liver cells. Environ Sci Pollut Res Int, 23(3), 2099–2107. doi: 10.1007/s11356-015-4819-6 [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Feldkamp ML, Munger RG, Siega-Riz AM, Botto LD, … Prevention, N. B. D. (2012). Reduced Risks of Neural Tube Defects and Orofacial Clefts With Higher Diet Quality. Archives of Pediatrics & Adolescent Medicine, 166(2), 121–126. doi: 10.1001/archpediatrics.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier C, Bahuau M, Perret C, Iovannisci DM, Nelva A, Herman C, … Cordier S (2008). Genetic susceptibilities in the association between maternal exposure to tobacco smoke and the risk of nonsyndromic oral cleft. American Journal of Medical Genetics Part A, 146a(18), 2396–2406. doi: 10.1002/ajmg.a.32505 [DOI] [PubMed] [Google Scholar]
- Chevrier C, Perret C, Bahuau M, Nelva A, Herman C, Francannet C, … Cordier S (2007). Fetal and maternal CYP2E1 genotypes and the risk of nonsyndromic oral clefts. American Journal of Medical Genetics Part A, 143a(12), 1382–1385. doi: 10.1002/ajmg.a.31779 [DOI] [PubMed] [Google Scholar]
- Chithalen JV, Luu L, Petkovich M, & Jones G (2002). HPLC-MS/MS analysis of the products generated from all-trans-retinoic acid using recombinant human CYP26A. Journal of Lipid Research, 43(7), 1133–1142. doi: 10.1194/jlr.M100343-JLR200 [DOI] [PubMed] [Google Scholar]
- Chung KC, Kowalski CP, Kim HM, & Buchman SR (2000). Maternal cigarette smoking during pregnancy and the risk of having a child with cleft lip/palate. Plast Reconstr Surg, 105(2), 485–491. doi: 10.1097/00006534-200002000-00001 [DOI] [PubMed] [Google Scholar]
- Citterio HL, & Gaillard DA (1994). Expression of Transforming Growth-Factor-Alpha (Tgf-Alpha), Epidermal Growth-Factor Receptor (Egf-R) and Cell-Proliferation during Human Palatogenesis - an Immunohistochemical Study. International Journal of Developmental Biology, 38(3), 499–505. Retrieved from <Go to ISI>://WOS:A1994PK96900011 [PubMed] [Google Scholar]
- Coffey VP, & Jessop WJE (1959). Maternal Influenza and Congenital Deformities - a Prospective Study. Lancet, 2(November28), 935–938. Retrieved from <Go to ISI>://WOS:A1959WB57700004 [DOI] [PubMed] [Google Scholar]
- Cohen M (2000). Etiology and pathogenesis of orofacial clefting. Oral and Maxillofacial Surgery Clinics of North America, 12(3), 379–398. [Google Scholar]
- Courtney KD, & Moore JA (1971). Teratology studies with 2,4,5-trichlorophenoxyacetic acid and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol, 20(3), 396–403. doi: 10.1016/0041-008x(71)90282-1 [DOI] [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ, & Bailey LB (2012). Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Advances in Nutrition, 3(1), 21–38. doi: 10.3945/an.111.000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel A, Tusnady G, Domany Z, & Borsy K (1967). Effects of Influenza on Pregnancy. Lancet, 2(7514), 516-+. Retrieved from <Go to ISI>://WOS:A196798448000454166424 [Google Scholar]
- Czeizel AE, Puho EH, Acs N, & Banhidy F (2008). Delineation of a multiple congenital abnormality syndrome in the offspring of pregnant women affected with high fever-related disorders: a population-based study. Congenit Anom (Kyoto), 48(4), 158–166. doi: 10.1111/j.1741-4520.2008.00202.x [DOI] [PubMed] [Google Scholar]
- De-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, & Rayco-Solon P (2015). Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews(12). doi: 10.1002/14651858.CD007950.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich R, Zimatkin S, & Pronko S (2006). Oxidation of ethanol in the brain and its consequences. Alcohol Res Health, 29(4), 266–273. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17718405 [PMC free article] [PubMed] [Google Scholar]
- Dekker EJ, Vaessen MJ, van den Berg C, Timmermans A, Godsave S, Holling T, … Durston A (1994). Overexpression of a cellular retinoic acid binding protein (xCRABP) causes anteroposterior defects in developing Xenopus embryos. Development, 120(4), 973–985. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7600972 [DOI] [PubMed] [Google Scholar]
- Denison MS, & Nagy SR (2003). Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annual Review of Pharmacology and Toxicology, 43, 309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828 [DOI] [PubMed] [Google Scholar]
- Deroo LA, Wilcox AJ, Lie RT, Romitti PA, Pedersen DA, Munger RG, … Wehby GL (2016). Maternal alcohol binge-drinking in the first trimester and the risk of orofacial clefts in offspring: a large population-based pooling study. European Journal of Epidemiology, 31(10), 1021–1034. doi: 10.1007/s10654-016-0171-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R (1994). TGF-beta-receptor-mediated signaling. Trends Biochem Sci, 19(12), 548–553. doi: 10.1016/0968-0004(94)90059-0 [DOI] [PubMed] [Google Scholar]
- Desrosiers TA, Lawson CC, Meyer RE, Richardson DB, Daniels JL, Waters MA, … Stud, N. B. D. P. (2012). Maternal occupational exposure to organic solvents during early pregnancy and risks of neural tube defects and orofacial clefts. Occupational and Environmental Medicine, 69(7), 493–499. doi: 10.1136/oemed-2011-100245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diewert VM, & Pratt RM (1981). Cortisone-induced cleft palate in A/J mice: failure of palatal shelf contact. Teratology, 24(2), 149–162. doi: 10.1002/tera.1420240206 [DOI] [PubMed] [Google Scholar]
- Divya D, Prasad MGS, Radhakrishna AN, Reddy SP, Pratyusha K, Kumar KS, & Sandeep R (2017). The serological evidence of cytomegalovirus infection as a potent aetiological factor for cleft lip/palate, mental retardation and deafness. Journal of clinical and diagnostic research: JCDR, 11(6), ZC51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Hill AB, & Sakula J (1960). Asian influenza in pregnancy and congenital defects. Br J Prev Soc Med, 14, 167–172. doi: 10.1136/jech.14.4.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Dalton TP, Miller ML, Shertzer HG, & Nebert DW (2006). For dioxin-induced birth defects, mouse or human CYP1A2 in maternal liver protects whereas mouse CYP1A1 and CYP1B1 are inconsequential. Journal of Biological Chemistry, 281(27), 18591–18600. doi: 10.1074/jbc.M601159200 [DOI] [PubMed] [Google Scholar]
- Dubey JP (2009). History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol, 39(8), 877–882. doi: 10.1016/j.ijpara.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Duthie SJ, Narayanan S, Brand GM, Pirie L, & Grant G (2002). Impact of folate deficiency on DNA stability. Journal of Nutrition, 132(8 Suppl), 2444S–2449S. doi: 10.1093/jn/132.8.2444S [DOI] [PubMed] [Google Scholar]
- Ebadifar A, Hamedi R, KhorramKhorshid HR, Kamali K, & Moghadam FA (2016). Parental cigarette smoking, transforming growth factor-alpha gene variant and the risk of orofacial cleft in Iranian infants. Iranian Journal of Basic Medical Sciences, 19(4), 366–373. Retrieved from <Go to ISI>://WOS:000377166600004 [PMC free article] [PubMed] [Google Scholar]
- Erickson MD, & Kaley RG 2nd. (2011). Applications of polychlorinated biphenyls. Environ Scl Pollut Reslnt, 18(2), 135–151. doi: 10.1007/s11356-010-0392-1 [DOI] [PubMed] [Google Scholar]
- Erickson RP (2010). Genes, Environment, and Orofacial Clefting: N-Acetyltransferase and Folic Acid. Journal of Craniofacial Surgery, 21(5), 1384–1387. doi: 10.1097/SCS.0b013e3181ec6992 [DOI] [PubMed] [Google Scholar]
- Fan G-Z, Li Y-L, & Wu P-A (2007). Association between retinoic acid receptor alpha gene polymorphisms and nonsyndromic cleft lip with or without cleft palate susceptibility. Zhonghua yi xue za zhi, 87(6), 396–398. [PubMed] [Google Scholar]
- Farrow JR, Davis GJ, Roy TM, Mccloud LC, & Nichols GR (1990). Fetal Death Due to Nonlethal Maternal Carbon-Monoxide Poisoning. Journal of Forensic Sciences, 35(6), 1448–1452. Retrieved from <Go to ISI>://WOS:A1990EJ64800026 [PubMed] [Google Scholar]
- Fiedler H (1996). Sources of PCDD/PCDF and impact on the environment. Chemosphere, 32(1), 55–64. doi: 10.1016/0045-6535(95)00228-6 [DOI] [PubMed] [Google Scholar]
- Figueiredo RF, Figueiredo N, Feguri A, Bieski I, Mello R, Espinosa M, & Damazo AS (2015). The role of the folic acid to the prevention of orofacial cleft: an epidemiological study. Oral Diseases, 21(2), 240–247. doi: 10.1111/odi.12256 [DOI] [PubMed] [Google Scholar]
- Finnell RH, Shaw GM, Lammer EJ, Brandl KL, Carmichael SL, & Rosenquist TH (2004). Gene-nutrient interactions: importance of folates and retinoids during early embryogenesis. Toxicology and Applied Pharmacology, 198(2), 75–85. doi: 10.1016/j.taap.2003.09.031 [DOI] [PubMed] [Google Scholar]
- Fontoura C, Silva RM, Granjeiro JM, & Letra A (2012). Further evidence of association of the ABCA4 gene with cleft lip/palate. European Journal of Oral Sciences, 120(6), 553–557. doi: 10.1111/eos.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food, & Administration, D. (1996). Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid; final rule (21 CFR Parts 136, 137, and 139). Federal Register, 61, 8781–8797. [Google Scholar]
- Freytag TL, Liu SM, Rogers QR, & Morris JG (2003). Teratogenic effects of chronic ingestion of high levels of vitamin A in cats. Journal of Animal Physiology and Animal Nutrition, 87(1-2), 42–51. doi: 10.1046/j.1439-0396.2003.00400.x [DOI] [PubMed] [Google Scholar]
- Friedl RM, Raja S, Metzler MA, Patel ND, Brittian KR, Jones SP, & Sandell LL (2019). RDH10 function is necessary for spontaneous fetal mouth movement that facilitates palate shelf elevation. Disease Models & Mechanisms, 12(7). doi: 10.1242/dmm.039073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li Z, & Huang H (1997). [Significance of glycosaminoglycans content in developing mechanism of cleft palate]. Zhonghua Kou Qiang Yi Xue Za Zhi, 32(1), 43–45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10677946 [PubMed] [Google Scholar]
- Fujikura K, Ingelman-Sundberg M, & Lauschke VM (2015). Genetic variation in the human cytochrome P450 supergene family. Pharmacogenetics and Genomics, 25(12), 584–594. doi: 10.1097/Fpc.0000000000000172 [DOI] [PubMed] [Google Scholar]
- Gabka J (1953). [Etiology of labial, oral and palatal fissures with special reference to toxoplasmosis. I..]. Dtsch Stomatol, 3(10), 294–300. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/13127562 [PubMed] [Google Scholar]
- Gallo MA, Scheuplein RJ, & Heijden KA (1991). Biological basis for risk assessment of dioxins and related compounds (Vol. 35): Cold Spring Harbor Laboratory. [Google Scholar]
- Gao LY, Zhang FQ, Zhao WH, Han GL, Wang X, Li Q, … Wu WD (2017). LncRNA H19 and Target Gene-mediated Cleft Palate Induced by TCDD. Biomedical and Environmental Sciences, 30(9), 676–680. doi: 10.3967/bes2017.090 [DOI] [PubMed] [Google Scholar]
- Gao Z, Bu Y, Zhang G, Liu X, Wang X, Ding S, … Yu Z (2016). Effect of TCDD on the fate of epithelial cells isolated from human fetal palatal shelves (hFPECs). Toxicol Appl Pharmacol, 305, 186–193. doi: 10.1016/j.taap.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Gao Z, Bu YJ, Liu XZ, Wang XG, Zhang GF, Wang EH, … Yu ZL (2016). TCDD promoted EMT of hFPECs via AhR, which involved the activation of EGFR/ERK signaling. Toxicology and Applied Pharmacology, 298, 48–55. doi: 10.1016/j.taap.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Garland MA, Sengupta S, Mathew LK, Truong L, de Jong E, Piersma AH, … Tanguay RL (2019). Glucocorticoid receptor-dependent induction of cripto-1 (one-eyed pinhead) inhibits zebrafish caudal fin regeneration. Toxicol Rep, 6, 529–537. doi: 10.1016/j.toxrep.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland MA, Sun B, Zhang S, Reynolds K, Ji Y, & Zhou CJ (2020). Role of epigenetics and miRNAs in orofacial clefts. Birth Defects Res. doi: 10.1002/bdr2.1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, … Taioli E (2001). Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiology Biomarkers & Prevention, 10(12), 1239–1248. Retrieved from <Go to ISI>://WOS:000173023100002 [PubMed] [Google Scholar]
- Ge L, Cui Y, Cheng K, & Han J (2018). Isopsoralen Enhanced Osteogenesis by Targeting AhR/ERalpha. Molecules, 23(10). doi: 10.3390/molecules23102600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier MC, Minick DJ, Truong L, Tilton S, Pande P, Anderson KA, … Tanguay RL (2018). Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicology and Applied Pharmacology, 354, 115–125. doi: 10.1016/j.taap.2018.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavini E, & Menegola E (2010). Are azole fungicides a teratogenic risk for human conceptus? Toxicology Letters, 198(2), 106–111. doi: 10.1016/j.toxlet.2010.07.005 [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ (2005). Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 569(1-2), 101–110. doi: 10.1016/j.mrfmmm.2004.04.021 [DOI] [PubMed] [Google Scholar]
- Greene RM, & Garbarino MP (1984). Role of Cyclic-Amp, Prostaglandins, and Catecholamines during Normal Palate Development. Current Topics in Developmental Biology, 19, 65–79. doi: 10.1016/S0070-2153(08)60395-6 [DOI] [PubMed] [Google Scholar]
- Grove RI, Willis WD, & Pratt RM (1985). Inhibition of arachidonic acid metabolism is not involved in dexamethasone-induced growth inhibition in embryonic palatal development. Prostaglandins, Leukotrienes and Medicine, 17(1), 85–95. [DOI] [PubMed] [Google Scholar]
- Gu S, Lu S, & Ma Y (1992). Study on the relationship between congenital toxoplasmosis and monsters of cleft lip and palate accompanied by multiple malformation. Shanghai kou qiang yi xue= Shanghai journal of stomatology, 1(2), 66–69. [PubMed] [Google Scholar]
- Guengerich FP (1993). The 1992 Brodie,Bernard,B. Award Lecture - Bioactivation and Detoxication of Toxic and Carcinogenic Chemicals. Drug Metabolism and Disposition, 21(1), 1–6. Retrieved from <Go to ISI>://WOS:A1993KR78100001 [PubMed] [Google Scholar]
- Guo ZQ, Huang CL, Ding KH, Lin JY, & Gong BZ (2010). Transforming Growth Factor Beta-3 and Environmental Factors and Cleft Lip with/without Cleft Palate. DNA and Cell Biology, 29(7), 375–380. doi: 10.1089/dna.2009.1009 [DOI] [PubMed] [Google Scholar]
- Gupta R, Chotaliya K, & Marfatia YS (2012). Cleft lip as a presentation of congenital syphilis. Indian Journal of Sexually Transmitted Diseases and AIDS, 33(1), 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot E, Chevallier A, Barouki R, & Coumoul X (2013). The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov Today, 18(9-10), 479–486. doi: 10.1016/j.drudis.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Gylling U (1967). Heredity of cleft lips and palates and factors influencing etiology. Paper presented at the Excerpta Medica International Congress Series. [Google Scholar]
- Haaland OA, Lie RT, Romanowska J, Gjerdevik M, Gjessing HK, & Jugessur A (2018). A Genome-Wide Search for Gene-Environment Effects in Isolated Cleft Lip with or without Cleft Palate Triads Points to an Interaction between Maternal Periconceptional Vitamin Use and Variants in ESRRG. Frontiers in Genetics, 9. doi: 10.3389/fgene.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland ØA, Romanowska J, Gjerdevik M, Lie RT, Gjessing HK, & Jugessur A (2019). A genome-wide scan of cleft lip triads identifies parent-of-origin interaction effects between ANK3 and maternal smoking, and between ARHGEF10 and alcohol consumption. F1000Res, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, … Lachenmeier DW (2014). Electronic cigarettes: overview of chemical composition and exposure estimation. Tobacco Induced Diseases, 12. doi: 10.1186/s12971-014-0023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dolle P, & Studer M (2007). Retinoids control anterior and dorsal properties in the developing forebrain. Developmental Biology, 303(1), 362–375. doi: 10.1016/j.ydbio.2006.11.021 [DOI] [PubMed] [Google Scholar]
- Hao Y, Tian S, Jiao X, Mi N, Zhang B, Song T, … Zhuang D (2015). Association of Parental Environmental Exposures and Supplementation Intake with Risk of Nonsyndromic Orofacial Clefts: A Case-Control Study in Heilongjiang Province, China. Nutrients, 7(9), 7172–7184. doi: 10.3390/nu7095328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsfield JK, Hickman TA, Everett ET, Shaw GM, Lammer EJ, & Finnell RA (2001). Analysis of the EPHX1 113 polymorphism and GSTM1 homozygous null polymorphism and oral clefting associated with maternal smoking. American Journal of Medical Genetics, 102(1), 21–24. doi: [DOI] [PubMed] [Google Scholar]
- Harville EW, Wilcox AI, Lie RT, Vindenes H, & Abyholm F (2005). Cleft lip and palate versus cleft lip only: Are they distinct defects? American Journal of Epidemiology, 162(5), 448–453. doi: 10.1093/aje/kwi214 [DOI] [PubMed] [Google Scholar]
- Hassoun E, Dargy R, Dencker L, Lundin LG, & Borwell P (1984). Teratogenicity of 2,3,7,8-Tetrachlorodibenzofuran in Bxd Recombinant Inbred Strains. Toxicology Letters, 23(1), 37–42. doi:Doi 10.1016/0378-4274(84)90007-9 [DOI] [PubMed] [Google Scholar]
- He W, Meng T, Wu M, Shi B, Lu SJ, & Li CH (2010). Perturbation of Fgf10 signal pathway in mouse embryonic palate by dexamethasone and vitamin B12 in vivo. J Pediatr Surg, 45(10), 2030–2035. doi: 10.1016/j.jpedsurg.2010.05.018 [DOI] [PubMed] [Google Scholar]
- The health consequences of smoking—50 years of progress: a report of the Surgeon General. (2014). In: Atlanta, GA: US Department of Health and Human Services, Centers for Disease; …. [Google Scholar]
- Henry EC, Bemis JC, Henry O, Kende AS, & Gasiewicz TA (2006). A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Archives of Biochemistry and Biophysics, 450(1), 67–77. doi: 10.1016/j.abb.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Hicks SD, Middleton FA, & Miller MW (2010). Ethanol-induced methylation of cell cycle genes in neural stem cells. J Neurochem, 114(6), 1767–1780. doi: 10.1111/j.l471-4159.2010.06886.x [DOI] [PubMed] [Google Scholar]
- Hitzl M, Schaeffeler E, Hocher B, Slowinski T, Halle H, Eichelbaum M, … Schwab M (2004). Variable expression of P-glycoprotein in the human placenta and its association with mutations of the multidrug resistance 1 gene (MDR1, ABCB1). Pharmacogenetics, 14(5), 309–318. doi: 10.1097/00008571-200405000-00006 [DOI] [PubMed] [Google Scholar]
- Holder SE, Vintiner GM, Farren B, Malcolm S, & Winter RM (1992). Confirmation of an Association between Rflps at the Transforming Growth Factor-Alpha Locus and Non-Syndromic Cleft-Lip and Palate. Journal of Medical Genetics, 29(6), 390–392. doi:DOI 10.1136/jmg.29.6.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holková R, Molnárová A, Nemec R, Brozman M, Fábry J, & Horáková E (1994). Relation between toxoplasmosis and orofacial clefts in children. Bratislavske lekarske listy, 95(2), 64–67. [PubMed] [Google Scholar]
- Honein MA, Devine O, Grosse SD, & Reefhuis J (2014). Prevention of orofacial clefts caused by smoking: implications of the Surgeon General's report. Birth Defects Res A Clin Mol Teratol, 100(11), 822–825. doi: 10.1002/bdra.23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijberg JH, Jansen G, Assaraf YG, Kathmann I, Pieters R, Laan AC, … Peters GJ (2004). Folate concentration dependent transport activity of the Multidrug Resistance Protein 1 (ABCC1). Biochemical Pharmacology, 67(8), 1541–1548. doi: 10.1016/j.bcp.2003.12.022 [DOI] [PubMed] [Google Scholar]
- Hozyasz KK, Mostowska A, Surowiec Z, & Jagodziński PP (2005). Genetic polymorphisms of GSTM1 and GSTT1 in mothers of children with isolated cleft lip with or without cleft palate. Przeglad lekarski, 62(10), 1019–1022. [PubMed] [Google Scholar]
- Hu X, Gao JH, Liao YJ, Tang SJ, & Lu F (2013). Dexamethasone alters epithelium proliferation and survival and suppresses Wnt/beta-catenin signaling in developing cleft palate. Food Chem Toxicol, 56, 67–7A. doi: 10.1016/j.fct.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Huang L, Shi B, Sun JH, & Wang Y (2005). [Influence of dexamethasone on fusion of embryonic palatal medial edge epithelium in mouse palatal shelves in vitro], Hua Xi Kou Qiang Yi Xue Za Zhi, 23(2), 103–105. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15952616 [PubMed] [Google Scholar]
- Huuskonen H, Unkila M, Pohjanvirta R, & Tuomisto J (1994). Developmental toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the most TCDD-resistant and -susceptible rat strains. Toxicol Appl Pharmacol, 124(2), 174–180. doi: 10.1006/taap.1994.1021 [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Beaty TH, Panny SR, Street NA, Joseph JM, Gordon S, … Francomano CA (1995). Association study of transforming growth factor alpha (TGF alpha) Taql polymorphism and oral clefts: indication of gene-environment interaction in a population-based sample of infants with birth defects. Am J Epidemiol, 141(7), 629–636. doi: 10.1093/oxfordjournals.aje.a117478 [DOI] [PubMed] [Google Scholar]
- Inayoshi K, Ohsaki Y, Nagata K, & Kurisu K (1989). The relation between phenytoin-receptor and glucocorticoid in the induction of cleft palate with phenytoin in mice. Japanese Journal of Oral Biology, 31(6), 718–723. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M (2004). Human drug metabolising cytochrome P450 enzymes: properties and polymorphisms. Naunyn-Schmiedebergs Archives of Pharmacology, 369(1), 89–104. doi: 10.1007/s00210-003-0819-z [DOI] [PubMed] [Google Scholar]
- Inomata T, Kiuchi A, Yoshida T, Hisamatsu S, Takizawa A, Kashiwazaki N, … Ninomiya H (2005). Hypervitaminosis A resulting in DNA aberration in fetal transgenic mice (Muta(TM) Mouse). Mutation Research-Genetic Toxicology and Environmental Mutagenesis, 586(1), 58–67. doi: 10.1016/j.mrgentox.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Izzotti A, Balansky RM, Cartiglia C, Camoirano A, Longobardi M, & De Flora S (2003). Genomic and transcriptional alterations in mouse fetus liver after transplacental exposure to cigarette smoke. Faseb Journal, 17(6), 1127-+. doi: 10.1096/fj.02-0967fje [DOI] [PubMed] [Google Scholar]
- Jacobs H, Dennefeld C, Feret B, Viluksela M, Hakansson H, Mark M, & Ghyselinck NB (2011a). Retinoic Acid Drives Aryl Hydrocarbon Receptor Expression and Is Instrumental to Dioxin-Induced Toxicity during Palate Development. Environmental Health Perspectives, 119(11), 1590–1595. doi: 10.1289/ehp.1003075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H, Dennefeld C, Feret B, Viluksela M, Hakansson H, Mark M, & Ghyselinck NB (2011b). Retinoic acid drives aryl hydrocarbon receptor expression and is instrumental to dioxin-induced toxicity during palate development. Environ Health Perspect, 119(11), 1590–1595. doi: 10.1289/ehp.1003075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanbin A, Shadkam E, Miri HH, Shirazi AS, & Abtahi M (2018). Maternal Folic Acid Supplementation and the Risk of Oral Clefts in Offspring. Journal of Craniofacial Surgery, 29(6), e534–e541. doi: 10.1097/SCS.0000000000004488 [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Abichaker G, Sedghizadeh PP, Bringas P Jr., & Melnick M (2008). Cytomegalovirus induces abnormal chondrogenesis and osteogenesis during embryonic mandibular development. Bmc Developmental Biology, 8, 33. doi: 10.1186/1471-213X-8-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskoll T, Choy HA, Chen HM, & Melnick M (1996). Developmental expression and CORT-regulation of TGF-beta and EGF receptor mRNA during mouse palatal morphogenesis: Correlation between CORT-induced cleft palate and TGF-beta 2 mRNA expression. Teratology, 54(1), 34–44. doi: [DOI] [PubMed] [Google Scholar]
- Jayarajan R, Natarajan A, & Nagamuttu R (2019). Efficacy of Periconceptional High-Dose Folic Acid in Isolated Orofacial Cleft Prevention: A Systematic Review. Indian J Plast Surg, 52(2), 153–159. doi: 10.1055/s-0039-1696864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Garland MA, Sun B, Zhang S, Reynolds K, McMahon M, … Zhou CJ (2020). Cellular and developmental basis of orofacial clefts. Birth Defects Res. doi: 10.1002/bdr2.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Ande A, Kumar A, & Kumar S (2013). Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis, 4, e554. doi: 10.1038/cddis.2013.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen AMW, Lie RT, Wilcox AJ, Andersen LF, & Drevon CA (2008). Maternal dietary intake of vitamin A and risk of orofacial clefts: A population-based case-control study in Norway. American Journal of Epidemiology, 167(10), 1164–1170. doi: 10.1093/aje/kwn035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Lopez A, & Wilson M (2003). Congenital toxoplasmosis. Am Fam Physician, 67(10), 2131–2138. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12776962 [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, & McAuley JB (2001). Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol, 154(4), 357–365. doi: 10.1093/aje/154.4.357 [DOI] [PubMed] [Google Scholar]
- Junaid M, Narayanan MBA, Jayanthi D, Kumar SGR, & Selvamary AL (2018). Association between maternal exposure to tobacco, presence of TGFA gene, and the occurrence of oral clefts. A case control study. Clin Oral Investig, 22(1), 217–223. doi: 10.1007/s00784-017-2102-6 [DOI] [PubMed] [Google Scholar]
- Kao CM, Chen SC, Liu JK, & Wu MJ (2001). Evaluation of TCDD biodegradability under different redox conditions. Chemosphere, 44(6), 1447–1454. doi: 10.1016/s0045-6535(00)00464-1 [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, … Sun H (2007). A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science, 315(5813), 820–825. doi: 10.1126/science.1136244 [DOI] [PubMed] [Google Scholar]
- Kayano S, Suzuki Y, Kanno K, Aoki Y, Kure S, Yamada A, & Matsubara Y (2004). Significant association between nonsyndromic oral clefts and arylhydrocarbon receptor nuclear translocator (ARNT). American Journal of Medical Genetics Part A, 130a(1), 40–44. doi: 10.1002/ajmg.a.30023 [DOI] [PubMed] [Google Scholar]
- Keber R, Motaln H, Wagner KD, Debeljak N, Rassoulzadegan M, Acimovic J, … Horvat S (2011). Mouse Knockout of the Cholesterogenic Cytochrome P450 Lanosterol 14 alpha-Demethylase (Cyp51) Resembles Antley-Bixler Syndrome. Journal of Biological Chemistry, 286(33), 29086–29097. doi: 10.1074/jbc.M111.253245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AE, & Dickinson AJG (2012). Median facial clefts in Xenopus laevis: Roles of retinoic acid signaling and homeobox genes. Developmental Biology, 365(1), 229–240. doi: 10.1016/j.ydbio.2012.02.033 [DOI] [PubMed] [Google Scholar]
- Kennedy AE, Kandalam S, Olivares-Navarrete R, & Dickinson AJG (2017). E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLoS One, 12(9). doi: 10.1371/journal.pone.0185729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapels IPC, Raijmakers-Eichhorn J, Peters WHM, Roelofs HMJ, Ras F, Steegers-Theunissen GPM, & Interactio EG-E (2008). The I105V polymorphism in glutathione S-transferase P1, parental smoking and the risk for nonsyndromic cleft lip with or without cleft palate. European Journal of Human Genetics, 16(3), 358–366. doi: 10.1038/sj.ejhg.5201973 [DOI] [PubMed] [Google Scholar]
- Krapels IPC, van Rooij IALM, Ocke MC, van Cleef BAGL, Kuijpers-Jagtman AMM, & Steegers-Theunissen RPM (2004). Maternal dietary B vitamin intake, other than folate, and the association with orofacial cleft in the offspring. European Journal of Nutrition, 43(1), 7–14. doi: 10.1007/s00394-004-0433-y [DOI] [PubMed] [Google Scholar]
- Krueger SK, & Williams DE (2005). Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacology & Therapeutics, 106(3), 357–387. doi: 10.1016/j.pharmthera.2005.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummet CM, Moreno LM, Wilcox AJ, Romitti PA, DeRoo LA, Munger RG, … Wehby GL (2016). Passive smoke exposure as a risk factor for oral clefts-A large international population-based study. Am J Epidemiol, 183(9), 834–841. doi: 10.1093/aje/kwv279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JC, Harris MW, McKinney JD, & Birnbaum LS (1986). Effects of thyroid hormones on the induction of cleft palate by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL6N mice. Toxicology and Applied Pharmacology, 84(1), 115–124. doi: 10.1016/0041-008x(86)90420-5 [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, … et al. (1985). Retinoic acid embryopathy. N Engl J Med, 313(14), 837–841. doi: 10.1056/NEJM198510033131401 [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Shaw GM, Iovannisci DM, & Finnell RH (2005). Maternal smoking, genetic variation of glutathione s-transferases, and risk for orofacial clefts. Epidemiology, 16(5), 698–701. doi: 10.1097/01.ede.0000172136.26733.4b [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Shaw GM, Iovannisci DM, Van Waes J, & Finnell RH (2004). Maternal smoking and the risk of orofacial clefts: Susceptibility with NAT1 and NAT2 polymorphisms. Epidemiology, 15(2), 150–156. doi: 10.1097/01.ede.0000112214.33432.cc [DOI] [PubMed] [Google Scholar]
- Lan SJ, Yang XG, Chen Z, Yang TY, Xiang CH, Zhang D, … Rong L (2016). Role of GATA-6 and Bone Morphogenetic Protein-2 in Dexamethasone-Induced Cleft Palate Formation in Institute of Cancer Research Mice. Journal of Craniofacial Surgery, 27(6), 1600–1605. doi: 10.1097/SCS.0000000000002844 [DOI] [PubMed] [Google Scholar]
- Langlois PH, Floyt AT, Lupo PJ, Lawson CC, Waters MA, Desrosiers TA, … Lammer EJ (2013). Maternal occupational exposure to polycyclic aromatic hydrocarbons and risk of oral cleft-affected pregnancies. Cleft Palate Craniofac J, 50(3), 337–346. doi: 10.1597/12-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankas GR, Wise LD, Cartwright ME, Pippert T, & Umbenhauer DR (1998). Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reproductive Toxicology, 12(4), 457–463. doi:Doi 10.1016/S0890-6238(98)00027-6 [DOI] [PubMed] [Google Scholar]
- Larouche G, & Hales BF (2009). The impact of human superoxide dismutase 1 expression in a mouse model on the embryotoxicity of hydroxyurea. Birth Defects Res A Clin Mol Teratol, 85(9), 800–807. doi: 10.1002/bdra.20595 [DOI] [PubMed] [Google Scholar]
- Laue K, Janicke M, Plaster N, Sonntag C, & Hammerschmidt M (2008). Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development, 135(22), 3775–3787. doi: 10.1242/dev.021238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek I (1963). Incidence of Malformations Following Influenza Epidemics. British Journal of Preventive & Social Medicine, 17(2), 70-+. Retrieved from <Goto ISI>://WOS:A19637631A00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei JQ, Qiu L, Ding XH, Fu YX, Yuan XG, & Liu Y (2019). [Expression of gamma-aminobutyric acid type A receptor beta3 subunit in murine cleft palate induced by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin], Zhonghua Kou Qiang Yi Xue Za Zhi, 54(5), 328–334. doi: 10.3760/cma.j.issn.1002-0098.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Lennon CJ, Birkeland AC, Nunez JAP, Su GH, Lanzano P, Guzman E, … Haddad J (2012). Association of candidate genes with nonsyndromic clefts in Honduran and Colombian populations. Laryngoscope, 122(9), 2082–2087. doi: 10.1002/lary.23394 [DOI] [PubMed] [Google Scholar]
- Leslie EJ, Carlson JC, Shaffer JR, Buxó CJ, Castilla EE, Christensen K, … Moreno L (2017). Association studies of low-frequency coding variants in nonsyndromic cleft lip with or without cleft palate. American Journal of Medical Genetics Part A, 173(6), 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, Mansilla MA, Biggs LC, Schuette K, Bullard S, Cooper M, … Murray JC (2012). Expression and mutation analyses implicate ARHGAP29 as the etiologic gene for the cleft lip with or without cleft palate locus identified by genome-wide association on chromosome 1p22. Birth Defects Research Part a-Clinical and Molecular Teratology, 94(11), 934–942. doi: 10.1002/bdra.23076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, He W, Meng T, Lu S, & Shi B (2014). Tetrachlorodibenzo-p-dioxin-induced cleft palate because of partial loss of cell polarity to interfere with apoptosis during early developmental stage. Zhonghua kou qiang yi xue za zhi= Zhonghua kouqiang yixue zazhi= Chinese journal of stomatology, 49(12), 719. [PubMed] [Google Scholar]
- Lieber CS (1997). Cytochrome P-4502E1: Its physiological and pathological role. Physiological Reviews, 77(2), 517–544. Retrieved from <Goto ISI>://WOS:A1997WV25700007 [DOI] [PubMed] [Google Scholar]
- Lili Y, Jian M, Junpeng G, Kun Z, Jinfang Z, & Yongqing H (2017). Association between non-syndromic cleft lip with or without cleft palate and environmental factors in Ningxia. Hua xi kou qiang yi xue za zhi= Huaxi kouqiang yixue zazhi= West China journal of stomatology, 35(3), 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Luan R, Guo Z, Lin X, Tang H, & Chen Y (2010). The correlation analysis between environmental factors, bone morphogenetic protein-4 and transforming growth factor beta-3 polymorphisms in nonsyndromic cleft lip with or without cleft palate. Zhonghua kou qiang yixue za zhi= Zhonghua kouqiang yixue zazhi= Chinese journal of stomatology, 45(10), 596–600. [PubMed] [Google Scholar]
- Linnenkamp BDW, Raskin S, Esposito SE, & Herai RH (2020). A comprehensive analysis of AHRR gene as a candidate for cleft lip with or without cleft palate. Mutation Research/Reviews in Mutation Research, 108319. [DOI] [PubMed] [Google Scholar]
- Little J, Cardy A, & Munger RG (2004). Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ, 82(3), 213–218. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15112010 [PMC free article] [PubMed] [Google Scholar]
- Liu C, Russell RM, Seitz HK, & Wang XD (2001). Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology, 120(1), 179–189. doi: 10.1053/gast.2001.20877 [DOI] [PubMed] [Google Scholar]
- Liu S, Narumi R, Ikeda N, Morita O, & Tasaki J (2020). Chemical-induced craniofacial anomalies caused by disruption of neural crest cell development in a zebrafish model. Dev Dyn, 249(7), 794–815. doi: 10.1002/dvdy.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Li X, Tao YC, Li N, Ji MM, Zhang XL, … Yu ZL (2020). TCDD inhibited the osteogenic differentiation of human fetal palatal mesenchymal cells through AhR and BMP-2/TGF-beta/Smad signaling. Toxicology, 431. doi: 10.1016/j.tox.2019.152353 [DOI] [PubMed] [Google Scholar]
- Loenarz C, Ge W, Coleman ML, Rose NR, Cooper CD, Klose RJ, … Schofield CJ (2010). PHF8, a gene associated with cleft lip/palate and mental retardation, encodes for an Nepsilon-dimethyl lysine demethylase. Human Molecular Genetics, 19(2), 217–222. doi: 10.1093/hmg/ddp480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen WAM (2006). S-adenosylmethionine: jack of all trades and master of everything? Biochemical Society Transactions, 34, 330–333. doi: 10.1042/Bst0340330 [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, … Investigators, H. (2006). Homocysteine lowering with folic acid and B vitamins in vascular disease. New England Journal of Medicine, 354(15), 1567–1577. Retrieved from <Go to ISI>://WOS:000236736900005 [DOI] [PubMed] [Google Scholar]
- LopezCamelo JS, & Orioli IM (1996). Heterogeneous rates for birth defects in Latin America: Hints on causality. Genetic Epidemiology, 13(5), 469–481. doi: [DOI] [PubMed] [Google Scholar]
- Lu SJ, He W, Shi B, Meng T, Li XY, & Liu YR (2008). A preliminary study on the teratogenesis of dexamethasone and the preventive effect of vitamin B12 on murine embryonic palatal shelf fusion in vitro. J Zhejiang Univ Sci B, 9(4), 306–312. doi: 10.1631/jzus.B0710625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucock M (2000). Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Molecular Genetics and Metabolism, 71(1-2), 121–138. doi: 10.1006/mgme.2000.3027 [DOI] [PubMed] [Google Scholar]
- Luteijn JM, Brown MJ, & Dolk H (2014). Influenza and congenital anomalies: a systematic review and meta-analysis. Hum Reprod, 29(4), 809–823. doi: 10.1093/humrep/det455 [DOI] [PubMed] [Google Scholar]
- Ma L, Shi B, & Zheng Q (2018). Cell Polarity and PAR Complex Likely to Be Involved in Dexamethasone-Induced Cleft Palate. Journal of Craniofacial Surgery, 29(2), 260–263. doi: 10.1097/Scs.0000000000004055 [DOI] [PubMed] [Google Scholar]
- Macdonald N, & Gledhill A (2007). Potential impact of ABCB1 (p-glycoprotein) polymorphisms on avermectin toxicity in humans. Archives of Toxicology, 81(8), 553–563. doi: 10.1007/s00204-007-0193-6 [DOI] [PubMed] [Google Scholar]
- Machida J, Yoshiura K, Funkhauser CD, Natsume N, Kawai T, & Murray JC (1999). Transforming growth factor-alpha (TGFA): Genomic structure, boundary sequences, and mutation analysis in nonsyndromic cleft lip/palate and cleft palate only. Genomics, 61(3), 237–242. doi: 10.1006/geno.1999.5962 [DOI] [PubMed] [Google Scholar]
- Maestri NE, Beaty TH, Hetmanski J, Smith EA, McIntosh I, Wyszynski DF, … VanderKolk C (1997). Application of transmission disequilibrium tests to nonsyndromic oral clefts: including candidate genes and environmental exposures in the models. Am J Med Genet, 73(3), 337–344. doi: [DOI] [PubMed] [Google Scholar]
- Maranhao SC, Sa J, Cangussu MCT, Coletta RD, Reis SRA, & Medrado A (2020). Nonsyndromic oral clefts and associated risk factors in the state of Bahia, Brazil. Eur Arch Paediatr Dent. doi: 10.1007/s40368-020-00522-0 [DOI] [PubMed] [Google Scholar]
- Marchetta CM, Devine OJ, Crider KS, Tsang BL, Cordero AM, Qi YP, … Hamner HC (2015). Assessing the Association between Natural Food Folate Intake and Blood Folate Concentrations: A Systematic Review and Bayesian Meta-Analysis of Trials and Observational Studies. Nutrients, 7(4), 2663–2686. doi: 10.3390/nu7042663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KS, Farquhar B, Chisolm MS, Coleman-Cowger VH, & Terplan M (2015). Knowledge, Attitudes, and Practice of Electronic Cigarette Use Among Pregnant Women. Journal of Addiction Medicine, 9(4), 266–272. doi: 10.1097/Adm.0000000000000128 [DOI] [PubMed] [Google Scholar]
- Mbuyi-Musanzayi S, Kayembe TJ, Kashal MK, Lukusa PT, Kalenga PM, Tshilombo FK, … Reychler H (2018). Non-syndromic cleft lip and/or cleft palate: Epidemiology and risk factors in Lubumbashi (DR Congo), a case-control study. J Craniomaxillofac Surg, 46(7), 1051–1058. doi: 10.1016/j.jcms.2018.05.006 [DOI] [PubMed] [Google Scholar]
- McAdams CL, & Aquino JT (1994). Dioxin: impact on solid waste industry uncertain. Waste Age, 25(11). [Google Scholar]
- McCarthy N, Wetherill L, Lovely CB, Swartz ME, Foroud TM, & Eberhart JK (2013). Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD. Development, 140(15), 3254–3265. doi: 10.1242/dev.094938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S (2012). WHO Guideline: Vitamin A supplementation in pregnant women. Geneva: WHO, 2011; WHO Guideline: Vitamin A supplementation in postpartum women. Geneva: WHO, 2011. Advances in Nutrition, 3(2), 215–216. doi: 10.3945/an.111.001701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick M, Mocarski ES, Abichaker G, Huang J, & Jaskoll T (2006). Cytomegalovirus-induced embryopathology: mouse submandibular salivary gland epithelial-mesenchymal ontogeny as a model. Bmc Developmental Biology, 6. doi: 10.1186/1471-213x-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metneki J, Puho E, & Czeizel AE (2005). Maternal diseases and isolated orofacial clefts in Hungary. Birth Defects Res A Clin Mol Teratol, 73(9), 617–623. doi: 10.1002/bdra.20177 [DOI] [PubMed] [Google Scholar]
- Mi N, Hao YR, Jiao XH, Zheng XD, Shi JN, & Chen YJ (2015). A polymorphic marker associated with non-syndromic cleft lip with or without cleft palate in a population in Heilongjiang Province, northern China. Archives of Oral Biology, 60(2), 357–361. doi: 10.1016/j.archoralbio.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, … Fujii-Kuriyama Y (1997). Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells, 2(10), 645–654. doi: 10.1046/j.1365-2443.1997.1490345.x [DOI] [PubMed] [Google Scholar]
- Mineshima H, Fukuta T, Kato E, Uchida K, Aoki T, Matsuno Y, & Mori C (2012). Malformation spectrum induced by ketoconazole after single administration to pregnant rats during the critical period - comparison with vitamin A-induced malformation spectrum. J Appl Toxicol, 32(2), 98–107. doi: 10.1002/jat.1636 [DOI] [PubMed] [Google Scholar]
- Mitchell LE (1997). Transforming growth factor alpha locus and nonsyndromic cleft lip with or without cleft palate: A reappraisal. Genetic Epidemiology, 14(3), 231–240. Retrieved from <Go to ISI>://WOS:A1997XC84200002 [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Murray JC, O'Brien S, & Christensen K (2003). Retinoic acid receptor alpha gene variants, multivitamin use, and liver intake as risk factors for oral clefts: A population-based case-control study in Denmark, 1991-1994. American Journal of Epidemiology, 158(1), 69–76. doi: 10.1093/aje/kwg102 [DOI] [PubMed] [Google Scholar]
- Mitoma C, Uchi H, Tsukimori K, Yamada H, Akahane M, Imamura T, … Furue M (2015). Yusho and its latest findings-A review in studies conducted by the Yusho Group. Environment International, 82, 41–48. doi: 10.1016/j.envint.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Molnarova A, Kovacova E, Majtan J, Fedeles J, Bielikova E, Cvachova S, … Repiska V (2006). Chlamydia and mycoplasma infections during pregnancy and their relationships to orofacial cleft. Biologia, 61(6), 719–723. doi: 10.2478/s11756-006-0147-0 [DOI] [Google Scholar]
- Molnarova A, Palencar D, Fekiacova D, Bielikova E, Ticha E, & Ujhazy E (2018). Orofacial clefts and infections during pregnancy. Biologia, 73(6), 629–635. doi: 10.2478/s11756-018-0065-y [DOI] [Google Scholar]
- Montenegro MA, Rojas M, Dominguez S, & Rosales CJ (1998). Differences in extracellular matrix components and cell density during normal and dexamethasone-treated secondary palate development in two strains of mice with different susceptibility to glucocorticoid induced-clefting. J Craniofac Genet Dev Biol, 18(2), 100–106. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9672842 [PubMed] [Google Scholar]
- Morrissey RE, Harris MW, Diliberto JJ, & Birnbaum LS (1992). Limited PCB antagonism of TCDD-induced malformations in mice. Toxicology Letters, 60(1), 19–25. doi: 10.1016/0378-4274(92)90043-j [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Greene RM, & Pisano MM (2015). Cigarette smoke induces proteasomal-mediated degradation of DNA methyltransferases and methyl CpG-/CpG domain-binding proteins in embryonic orofacial cells. Reproductive Toxicology, 58, 140–148. doi: 10.1016/j.reprotox.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, & Marrs JA (2015). Zebrafish retinal defects induced by ethanol exposure are rescued by retinoic acid and folic acid supplement. Alcohol, 49(2), 149–163. doi: 10.1016/j.alcohol.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi M, Ludwig KU, Knapp M, Kist R, Lisgo S, Ohshima H, … Peters H (2020). Msx1 deficiency interacts with hypoxia and induces a morphogenetic regulation during mouse lip development. Development, 147(21). doi: 10.1242/dev.189175 [DOI] [PubMed] [Google Scholar]
- Nazer J, & Cifuentes L (2014). Prevalence of congenital malformations at birth in chilean maternity hospitals. Revista Medica De Chile, 142(9), 1150–1156. doi: 10.4067/S0034-98872014000900009 [DOI] [PubMed] [Google Scholar]
- Nebert DW (2017). Aryl hydrocarbon receptor (AHR): "pioneer member" of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of "sensors" of foreign and endogenous signals. Prog Lipid Res, 67, 38–57. doi: 10.1016/j.plipres.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi SB, Singh S, Pallepogula DR, Pant H, Kolli SR, Bharti P, … Gudlavalleti VSM (2017). Risk factors for orofacial clefts in India: A case-control study. Birth Defects Res, 109(16), 1284–1291. doi: 10.1002/bdr2.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Yang W, Jin L, Liu J, Li Z, Wang B, … Ren A (2019). Levels of polycyclic aromatic hydrocarbons in umbilical cord and risk of orofacial clefts. Sci Total Environ, 678, 123–132. doi: 10.1016/j.scitotenv.2019.04.404 [DOI] [PubMed] [Google Scholar]
- Ni WL, Yang WL, Yu JH, Li ZW, Jin L, Liu JF, … Ren AG (2018). Umbilical Cord Concentrations of Selected Heavy Metals and Risk for Orofacial Clefts. Environmental Science & Technology, 52(18), 10787–10795. doi: 10.1021/acs.est.8b02404 [DOI] [PubMed] [Google Scholar]
- Niederreither K, & Dolle P (2008). Retinoic acid in development: towards an integrated view. Nature Reviews Genetics, 9(7), 541–553. doi: 10.1038/nrg2340 [DOI] [PubMed] [Google Scholar]
- Okano J, Udagawa J, & Shiota K (2014). Roles of retinoic acid signaling in normal and abnormal development of the palate and tongue. Congenit Anom (Kyoto), 54(2), 69–76. doi: 10.1111/cga.12049 [DOI] [PubMed] [Google Scholar]
- Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, … Bergh C (2018). The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Human Reproduction Update, 24(3), 320–389. doi: 10.1093/humupd/dmy005 [DOI] [PubMed] [Google Scholar]
- Omaye ST (2002). Metabolic modulation of carbon monoxide toxicity. Toxicology, 180(2), 139–150. doi: 10.1016/s0300-483x(02)00387-6 [DOI] [PubMed] [Google Scholar]
- Oncken C, Ricci KA, Kuo CL, Dornelas E, Kranzler HR, & Sankey HZ (2017). Correlates of Electronic Cigarettes Use Before and During Pregnancy. Nicotine & Tobacco Research, 19(5), 585–590. doi: 10.1093/ntr/ntw225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Wang P, Yin X, Liu X, Li D, Li X, … Yu, Z. (2015). Association between Maternal MTHFR Polymorphisms and Nonsyndromic Cleft Lip with or without Cleft Palate in Offspring, A Meta-Analysis Based on 15 Case-Control Studies. Int J Fertil Steril, 8(4), 463–480. doi: 10.22074/ijfs.2015.4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pares X, Farres J, Kedishvili N, & Duester G (2008). Medium- and short-chain dehydrogenase/reductase gene and protein families : Medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol Life Sci, 65(24), 3936–3949. doi: 10.1007/s00018-008-8591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peanchitlertkajorn S, Cooper ME, Liu YE, Field LL, & Marazita ML (2003). Chromosome 17: Gene mapping studies of cleft lip with or without cleft palate in Chinese families. Cleft Palate-Craniofacial Journal, 40(1), 71–79. doi: [DOI] [PubMed] [Google Scholar]
- Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, & Abbott BD (1999). Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol Sci, 47(1), 86–92. doi: 10.1093/toxsci/47.1.86 [DOI] [PubMed] [Google Scholar]
- Pi X, Li Z, Jin L, Liu J, Zhang Y, Zhang L, … Ren A (2018). Secondhand smoke during the periconceptional period increases the risk for orofacial clefts in offspring. Paediatr Perinat Epidemiol, 32(5), 423–427. doi: 10.1111/ppe.12497 [DOI] [PubMed] [Google Scholar]
- Pinsky L, & Digeorge AM (1965). Cleft Palate in the Mouse: A Teratogenic Index of Glucocorticoid Potency. Science, 147(3656), 402–403. doi: 10.1126/science.147.3656.402 [DOI] [PubMed] [Google Scholar]
- Pistollato F, Cano SS, Elio I, Vergara MM, Giampieri F, & Battino M (2015). Plant-Based and Plant-Rich Diet Patterns during Gestation: Beneficial Effects and Possible Shortcomings. Advances in Nutrition, 6(5), 581–591. doi: 10.3945/an.115.009126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt R (1980). Involvement of glucocorticoids and epidermal growth factor in secondary palate development. Current trends in prenatal craniofacial development, 235–252. [Google Scholar]
- Pratt RM, Dencker L, & Diewert VM (1984). 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced cleft palate in the mouse: evidence for alterations in palatal shelf fusion. Teratog Carcinog Mutagen, 4(5), 427–436. doi: 10.1002/tcm.1770040505 [DOI] [PubMed] [Google Scholar]
- Pullarkat RK (1991). Hypothesis - Prenatal Ethanol-Induced Birth-Defects and Retinoic Acid. Alcoholism-Clinical and Experimental Research, 15(3), 565–567. doi:DOI 10.1111/j.1530-0277.1991.tb00561.x [DOI] [PubMed] [Google Scholar]
- Qian JF, Feingold J, Stoll C, & May E (1993). Transforming Growth-Factor-Alpha - Characterization of the Bamhi, Rsai, and Taqi Polymorphic Regions. American Journal of Human Genetics, 53(1), 168–175. Retrieved from <Go to ISI>://WOS:A1993LJ38500020 [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan A, Lupo PJ, Agopian A, Linder SH, Stock TH, Langlois PH, & Craft E (2013). Evaluating the Effects of Maternal Exposure to Benzene, Toluene, Ethyl benzene, and Xylene on Oral Clefts Among Offspring in Texas: 1999-2008. Birth Defects Research Part a-Clinical and Molecular Teratology, 97(8), 532–537. doi: 10.1002/bdra.23139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez D, Lammer EJ, Iovannisci DM, Laurent C, Finnell RH, & Shaw GM (2007). Maternal smoking during early pregnancy, GSTP1 and EPHX1 variants, and risk of isolated orofacial clefts. Cleft Palate-Craniofacial Journal, 44(4), 366–373. doi: 10.1597/06-011.1 [DOI] [PubMed] [Google Scholar]
- Ramirez-Chau C, Blanco R, Colombo A, Pardo R, & Suazo J (2016). MTHFR c.677C > T is a risk factor for non-syndromic cleft lip with or without cleft palate in Chile. Oral Diseases, 22(7), 703–708. doi: 10.1111/odi.12533 [DOI] [PubMed] [Google Scholar]
- Regina Altoe S, Borges AH, Neves A, Aranha AMF, Borba AM, Espinosa MM, & Volpato LER (2020). Influence of Parental Exposure to Risk Factors in the Occurrence of Oral Clefts. J Dent (Shiraz), 21(2), 119–126. doi: 10.30476/DENTJODS.2019.77620.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R, Brosch S, Ludeke M, Fischbein E, Haase S, Pickhard A, … Maier C (2012). Genetic and environmental risk factors for submucous cleft palate. European Journal of Oral Sciences, 120(2), 97–103. doi: 10.1111/j.1600-0722.2012.00948.x [DOI] [PubMed] [Google Scholar]
- Reynolds K, Zhang S, Sun B, Garland MA, Ji Y, & Zhou CJ (2020). Genetics and signaling mechanisms of orofacial clefts. Birth Defects Res. doi: 10.1002/bdr2.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, & Dolle P (2012). Retinoic acid signalling during development. Development, 139(5), 843–858. doi: 10.1242/dev.065938 [DOI] [PubMed] [Google Scholar]
- Rhinn M, Schuhbaur B, Niederreither K, & Dolle P (2011). Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci U S A, 108(40), 16687–16692. doi: 10.1073/pnas.1103877108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgman A, & Perfetti TA (2016). The chemical components of tobacco and tobacco smoke: CRC press. [Google Scholar]
- Romitti PA, Herring AM, Dennis LK, & Wong-Gibbons DL (2007). Meta-analysis: Pesticides and orofacial clefts. Cleft Palate-Craniofacial Journal, 44(4), 358–365. doi: 10.1597/06-100.1 [DOI] [PubMed] [Google Scholar]
- Romitti PA, Lidral AC, Munger RG, Daack-Hirsch S, Burns TL, & Murray JC (1999). Candidate genes for nonsyndromic cleft lip and palate and maternal cigarette smoking and alcohol consumption: evaluation of genotype-environment interactions from a population-based case-control study of orofacial clefts. Teratology, 59(1), 39–50. doi: [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, & Milunsky A (1995). Teratogenicity of high vitamin A intake. N Engl J Med, 333(21), 1369–1373. doi: 10.1056/NEJM199511233332101 [DOI] [PubMed] [Google Scholar]
- Rowell TR, & Tarran R (2015). Will chronic e-cigarette use cause lung disease? American Journal of Physiology-Lung Cellular and Molecular Physiology, 309(12), L1398–L1409. doi: 10.1152/ajplung.00272.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, & Bok D (1999). Molecular and biochemical characterization of lecithin retinol acyltransferase. Journal of Biological Chemistry, 274(6), 3834–3841. doi: 10.1074/jbc.274.6.3834 [DOI] [PubMed] [Google Scholar]
- Sakuma C, Imura H, Yamada T, Sugahara T, Hirata A, Ikeda Y, & Natsume N (2018). Cleft palate formation after palatal fusion occurs due to the rupture of epithelial basement membranes. Journal of Cranio-Maxillofacial Surgery, 46(12), 2027–2031. doi: 10.1016/j.jcms.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Salamanca C, Gonzalez-Hormazabal P, Recabarren AS, Recabarren PA, Pantoja R, Leiva N, … Suazo J (2020a). Genetic variants in S-adenosyl-methionine synthesis pathway and nonsyndromic cleft lip with or without cleft palate in Chile. Pediatric Research. doi: 10.1038/s41390-020-0994-3 [DOI] [PubMed] [Google Scholar]
- Salamanca C, Gonzalez-Hormazabal P, Recabarren AS, Recabarren PA, Pantoja R, Leiva N, … Suazo J (2020b). A SHMT1 variant decreases the risk of nonsyndromic cleft lip with or without cleft palate in Chile. Oral Diseases, 26(1), 159–165. doi: 10.1111/odi.13229 [DOI] [PubMed] [Google Scholar]
- Santos MR, Ramallo V, Muzzio M, Camelo JSL, & Bailliet G (2015). Association between NAT2 polymorphisms with non-syndromic cleft lip with or without cleft palate in Argentina. Revista Medica De Chile, 143(4), 444–450. doi: 10.4067/S0034-98872015000400005 [DOI] [PubMed] [Google Scholar]
- Sato Y, Yoshioka E, Saijo Y, Miyamoto T, Sengoku K, Azuma H, … Children's Study, G. (2020). Population attributable fractions of modifiable risk factors for nonsyndromic orofacial clefts: a prospective cohort study from the Japan Environment and Children's Study. J Epidemiol. doi: 10.2188/jea.JE20190347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén I (1975). The association between maternal influenza, drug consumption and oral clefts. Acta odontologica Scandinavica, 33(5), 259–267. [DOI] [PubMed] [Google Scholar]
- Serio RN, & Gudas LJ (2020). Modification of stem cell states by alcohol and acetaldehyde. Chem Biol Interact, 316, 108919. doi: 10.1016/j.cbi.2019.108919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio RN, Laursen KB, Urvalek AM, Gross SS, & Gudas LJ (2019). Ethanol promotes differentiation of embryonic stem cells through retinoic acid receptor-gamma. Journal of Biological Chemistry, 294(14), 5536–5548. doi: 10.1074/jbc.RA118.007153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabtai Y, Bendelac L, Jubran H, Hirschberg J, & Fainsod A (2018). Acetaldehyde inhibits retinoic acid biosynthesis to mediate alcohol teratogenicity. Scientific Reports, 8. doi: 10.1038/s41598-017-18719-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P, Dasgupta S, Hahn ME, & Tanguay RL (2020). A Review of the Functional Roles of the Zebrafish Aryl Hydrocarbon Receptors. Toxicological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P, Geier MC, Truong L, McClure RS, Pande P, Waters KM, & Tanguay RL (2019). Coupling Genome-wide Transcriptomics and Developmental Toxicity Profiles in Zebrafish to Characterize Polycyclic Aromatic Hydrocarbon (PAH) Hazard. International Journal of Molecular Sciences, 20(10). doi: 10.3390/ijms20102570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Vollset SE, Yang W, Finnell RH, Blom H, … Ueland PM (2009). Mid-pregnancy cotinine and risks of orofacial clefts and neural tube defects. J Pediatr, 154(1), 17–19. doi: 10.1016/j.jpeds.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Nelson V, Iovannisci DM, Finnell RH, & Lammer EJ (2003). Maternal occupational chemical exposures and biotransformation genotypes as risk factors for selected congenital anomalies. American Journal of Epidemiology, 157(6), 475–484. doi: 10.1093/aje/kwg013 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Nelson V, Todoroff K, Wasserman CR, & Neutra RR (1999). Maternal periconceptional use of electric bed-heating devices and risk for neural tube defects and orofacial clefts. Teratology, 60(3), 124–129. doi: [DOI] [PubMed] [Google Scholar]
- Shaw GM, Wasserman CR, Lammer EJ, O'Malley CD, Murray JC, Basart AM, & Tolarova MM (1996). Orofacial clefts, parental cigarette smoking, and transforming growth factor-alpha gene variants. Am J Hum Genet, 58(3), 551–561. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8644715 [PMC free article] [PubMed] [Google Scholar]
- Shaw W (2004). Global strategies to reduce the health care burden of craniofacial anomalies: Report of WHO meetings on international collaborative research on craniofacial anomalies. Cleft Palate-Craniofacial Journal, 41(3), 238–243. Retrieved from <Go to ISI>://WOS:000221753600003 [DOI] [PubMed] [Google Scholar]
- Shi M, Christensen K, Weinberg CR, Romitti P, Bathum L, Lozada A, … Murray JC (2007). Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am J Hum Genet, 80(1), 76–90. doi: 10.1086/510518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Wehby GL, & Murray JC (2008). Review on genetic variants and maternal smoking in the etiology of oral clefts and other birth defects. Birth Defects Res C Embryo Today, 84(1), 16–29. doi: 10.1002/bdrc.20117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R, Lidral AC, Ardinger HH, Buetow KH, Romitti PA, Munger RG, & Murray JC (1993). Association of Transforming Growth-Factor Alpha-Gene Polymorphisms with Nonsyndromic Cleft-Palate Only (Cpo). American Journal of Human Genetics, 53(4), 836–843. Retrieved from <Go to ISI>://WOS:A1993LY62700007 [PMC free article] [PubMed] [Google Scholar]
- Shimomura T, Kawakami M, Okuda H, Tatsumi K, Morita S, Nochioka K, … Wanaka A (2015). Retinoic acid regulates Lhx8 expression via FGF-8b to the upper jaw development of chick embryo. Journal of Bioscience and Bioengineering, 119(3), 260–266. doi: 10.1016/j.jbiosc.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Shu X, Dong Z, & Shu S (2019). AMBRA1-mediated autophagy and apoptosis associated with an epithelial-mesenchymal transition in the development of cleft palate induced by all-trans retinoic acid. Annals of translational medicine, 7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum S, Jensen NM, & Nebert DW (1979). The murine Ah locus: in utero toxicity and teratogenesis associated with genetic differences in benzo[a]pyrene metabolism. Teratology, 20(3), 365–376. doi: 10.1002/tera.1420200307 [DOI] [PubMed] [Google Scholar]
- Silva H, Arruda TTS, Souza KSC, Bezerra JF, Leite GCP, Brito MEF, … Rezende AA (2018). Risk factors and comorbidities in Brazilian patients with orofacial clefts. Braz Oral Res, 32, e24. doi: 10.1590/1807-3107bor-2018.vol32.0024 [DOI] [PubMed] [Google Scholar]
- Siu MT, Shapiro AM, Wiley MJ, & Wells PG (2013). A role for glutathione, independent ofoxidative stress, in the developmental toxicity of methanol. Toxicol Appl Pharmacol, 273(3), 508–515. doi: 10.1016/j.taap.2013.09.020 [DOI] [PubMed] [Google Scholar]
- Song IH, Gold R, Straub RH, Burmester GR, & Buttgereit F (2005). New glucocorticoids on the horizon: repress, don't activate! J Rheumatol, 32(7), 1199–1207. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16041872 [PubMed] [Google Scholar]
- Song T, Wu D, Wang YQ, Li HD, Yin NB, & Zhao ZM (2013). Association of NAT1 and NAT2 genes with nonsyndromic cleft lip and palate. Molecular Medicine Reports, 8(1), 211–216. doi: 10.3892/mmr.2013.1467 [DOI] [PubMed] [Google Scholar]
- Spiegler E, Kim YK, Wassef L, Shete V, & Quadro L (2012). Maternal-fetal transfer and metabolism of vitamin A and its precursor beta-carotene in the developing tissues. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 1821(1), 88–98. doi: 10.1016/j.bbalip.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinder N, Prins JR, Bergman JEH, Smidt N, Kromhout H, Boezen HM, & de Walle HEK (2019). Congenital anomalies in the offspring of occupationally exposed mothers: a systematic review and meta-analysis of studies using expert assessment for occupational exposures. Hum Reprod, 34(5), 903–919. doi: 10.1093/humrep/dez033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov I, Jansson I, Sarfarazi M, & Schenkman JB (2001). Roles of cytochrome p450 in development. Drug metabolism and drug interactions, 18(1), 33–56. [DOI] [PubMed] [Google Scholar]
- Strange RC, Spiteri MA, Ramachandran S, & Fryer AA (2001). Glutathione-S-transferase family of enzymes. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis, 482(1-2), 21–26. doi: 10.1016/S0027-5107(01)00206-8 [DOI] [PubMed] [Google Scholar]
- Sull JW, Liang KY, Hetmanski JB, Wu T, Fallin MD, Ingersoll RG, … Beaty TH (2009). Evidence that TGFA influences risk to cleft lip with/without cleft palate through unconventional genetic mechanisms. Human Genetics, 126(3), 385–394. doi: 10.1007/s00439-009-0680-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Liu X, Cui L, Liu X, Chen Y, He Z, … Yu Z (2020). Oct4 plays a role in 2, 3, 7, 8 - tetrachlorobenzo-p-dioxin (TCDD) inducing cleft palate and inhibiting mesenchymal proliferation. Toxicology, 438, 152444. doi: 10.1016/j.tox.2020.152444 [DOI] [PubMed] [Google Scholar]
- Thomae TL, Glover E, & Bradfield CA (2004). A maternal Ahr null genotype sensitizes embryos to chemical teratogenesis. Journal of Biological Chemistry, 279(29), 30189–30194. doi: 10.1074/jbc.M403690200 [DOI] [PubMed] [Google Scholar]
- Thomae TL, Stevens EA, & Bradfield CA (2005). Transforming growth factor-beta 3 restores fusion in palatal shelves exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of Biological Chemistry, 280(13), 12742–12746. doi: 10.1074/jbc.M410780200 [DOI] [PubMed] [Google Scholar]
- Tsutsui Y (1995). Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection. Pathol Int, 45(2), 91–102. doi: 10.1111/j.1440-1827.1995.tb03428.x [DOI] [PubMed] [Google Scholar]
- Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, & Tyagi SC (2005). Mechanisms of homocysteine-induced oxidative stress. American Journal of Physiology-Heart and Circulatory Physiology, 289(6), H2649–H2656. doi: 10.1152/ajpheart.00548.2005 [DOI] [PubMed] [Google Scholar]
- Tyan ML, & Tyan DB (1993). Vitamin-a-Enhanced Cleft-Palate Susceptibility Gene Maps between C4 and B144 within the H-2-Complex. Proceedings of the Society for Experimental Biology and Medicine, 202(4), 482–486. Retrieved from <Go to ISI>://WOS:A1993KU63700015 [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, … Peterson RE (2006). The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci, 93(2), 223–241. doi: 10.1093/toxsci/kfl055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard MJH, de Costa D, Krapels IPC, Liu F, van Duijn C, Sinke RJ, … Steegers-Theunissen RPM (2008). The MSX1 allele 4 homozygous child exposed to smoking at periconception is most sensitive in developing nonsyndromic orofacial clefts. Human Genetics, 124(5), 525–534. doi: 10.1007/s00439-008-0569-6 [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Swinkels DW, Blom HJ, Merkus HM, & Steegers-Theunissen RP (2003). Vitamin and homocysteine status of mothers and infants and the risk of nonsyndromic orofacial clefts. Am J Obstet Gynecol, 189(4), 1155–1160. doi: 10.1067/s0002-9378(03)00592-1 [DOI] [PubMed] [Google Scholar]
- van Rooij IALM, Wegerif MJM, Roelofs HMJ, Peters WHM, Kuijpers-Jagtman AM, Zielhuis GA, … Steegers-Theunissen RPM (2001). Smoking, genetic polymorphisms in biotransformation enzymes, and nonsyndromic oral clefting: A gene-environment interaction. Epidemiology, 12(5), 502–507. doi: 10.1097/00001648-200109000-00007 [DOI] [PubMed] [Google Scholar]
- Vandevyver S, Dejager L, & Libert C (2012). On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic, 13(3), 364–374. doi: 10.1111/j.1600-0854.2011.01288.x [DOI] [PubMed] [Google Scholar]
- Vieira AR, & Orioli IM (2001). Candidate genes for nonsyndromic cleft lip and palate. Journal of Dentistry for Children, 68(4), 272-+. Retrieved from <Go to ISI>://WOS:000172932600021 [PubMed] [Google Scholar]
- von Richter O, Glavinas H, Krajcsi P, Liehner S, Siewert B, & Zech K (2009). A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn-Schmiedebergs Archives of Pharmacology, 379(1), 11–26. doi: 10.1007/s00210-008-0345-0 [DOI] [PubMed] [Google Scholar]
- Wagner NJ, Camerota M, & Propper C (2017). Prevalence and Perceptions of Electronic Cigarette Use during Pregnancy. Maternal and Child Health Journal, 21(8), 1655–1661. doi: 10.1007/s10995-016-2257-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SE, Kennedy AE, Wyatt BH, Moore AD, Pridgen DE, Cherry AM, … Dickinson AJG (2015). The role of folate metabolism in orofacial development and clefting. Developmental Biology, 405(1), 108–122. doi: 10.1016/j.ydbio.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WM, & Mc KA (1959). Asian influenza in pregnancy; relationship to fetal anomalies. Obstet Gynecol, 13(4), 394–398. doi: 10.1097/00006250-195904000-00002 [DOI] [PubMed] [Google Scholar]
- Wang C, Yuan X, Fu Y, & Zhai S (2016). [Global DNA methylation changes during palatal formation in fetal mice induced by 2,3,7,8-tetrachlrodibenzo-p-dioxin]. Zhonghua Zheng Xing Wai Ke Za Zhi, 32(5), 372–377. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30066996 [PubMed] [Google Scholar]
- Wang C, Yuan XG, Liu CP, Zhai SN, Zhang DW, & Fu YX (2017). Preliminary research on DNA methylation changes during murine palatogenesis induced by TCDD. Journal of Cranio-MaxillofacialSurgery, 45(5), 678–684. doi: 10.1016/j.jcms.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Ward A, Summers MJ, & Sim E (1995). Purification of Recombinant Human N-Acetyltransferase Type-1 (Nat1) Expressed in Escherichia-Coli and Characterization of Its Potential Role in Folate Metabolism. Biochemical Pharmacology, 49(12), 1759–1767. doi: 10.1016/0006-2952(95)00087-G [DOI] [PubMed] [Google Scholar]
- Ward SJ, Chambon P, Ong DE, & Bavik C (1997). A retinol-binding protein receptor-mediated mechanism for uptake of vitamin A to postimplantation rat embryos. Biology of Reproduction, 57(4), 751–755. doi:DOI 10.1095/biolreprod57.4.751 [DOI] [PubMed] [Google Scholar]
- Watanabe M, & Sugahara T (1981). Experimental formation of cleft palate in mice with polychlorinated biphenyls (PCB). Toxicology, 19(1), 49–53. doi: 10.1016/0300-483x(81)90064-0 [DOI] [PubMed] [Google Scholar]
- Weber H, Lamb JC, Harris MW, & Moore JA (1984). Teratogenicity of 2.3.7.8-tetrachlorodibenzofuran (TCDF) in mice. Toxicology Letters, 20(2), 183–188. doi: 10.1016/0378-4274(84)90145-0 [DOI] [PubMed] [Google Scholar]
- Weichert A, Vogt M, Dudenhausen JW, & Kalache KD (2010). Evidence in a Human Fetus of Micrognathia and Cleft Lip as Potential Effects of Early Cytomegalovirus Infection. Fetal Diagnosis and Therapy, 28(4), 225–228. doi: 10.1159/000320203 [DOI] [PubMed] [Google Scholar]
- Weikum ER, Knuesel MT, Ortlund EA, & Yamamoto KR (2017). Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat Rev Mol Cell Biol, 18(3), 159–174. doi: 10.1038/nrm.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelock D, Csizmas L, & Klein S (1963). Influenza, pregnancy, and fetal outcome. Public Health Rep, 78, 1–11. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14000477 [PMC free article] [PubMed] [Google Scholar]
- Wiersig D, & Swenson M (1967). Teratogenicity of vitamin A in canine. Paper presented at the Federation Proceedings. [Google Scholar]
- Wilson MG, & Stein AM (1969). Teratogenic Effects of Asian Influenza - an Extended Study. Journal of the American Medical Association, 210(2), 336-&. doi: 10.1001/jama.210.2.336 [DOI] [PubMed] [Google Scholar]
- Wolff S, Harper PA, Wong JM, Mostert V, Wang Y, & Abel J (2001). Cell-specific regulation of human aryl hydrocarbon receptor expression by transforming growth factor-beta(1). Mol Pharmacol, 59(4), 716–724. doi: 10.1124/mol.59.4.716 [DOI] [PubMed] [Google Scholar]
- Wong WY, Eskes TK, Kuijpers-Jagtman AM, Spauwen PH, Steegers EA, Thomas CM, … Steegers-Theunissen RP (1999). Nonsyndromic orofacial clefts: association with maternal hyperhomocysteinemia. Teratology, 60(5), 253–257. doi: [DOI] [PubMed] [Google Scholar]
- Wu N, Lu YP, Liu K, Li ZJ, Liu Q, & Lu L (2018). Associations of ABCA4 and MAFB with Nonsyndromic Cleft Lip with or without Cleft Palate in a Northeastern Chinese Population. Journal of Hard Tissue Biology, 27(3), 181–184. doi: 10.2485/jhtb.27.181 [DOI] [Google Scholar]
- Wu-Chou YH, Chen KTP, Lu YC, Lin YT, Chang HF, & Lo LJ (2020). The SNP rs560426 Within ABCA4-ARHGAP29 Locus and the Risk of Nonsyndromic Oral Clefts. Cleft Palate-Craniofacial Journal, 57(6), 671–677. doi: 10.1177/1055665619899764 [DOI] [PubMed] [Google Scholar]
- Xavier D, Arif Y, Murali R, Kishore Kumar S, Vipin Kumar S, Tamang R, … Bhaskar L (2013). Analysis of microsatellite polymorphisms in South Indian patients with non syndromic cleft lip and palate. Balkan J Med Genet, 16(1), 49–54. doi: 10.2478/bjmg-2013-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao DL, Huang XH, Yang SM, & Zhang LB (2007). Direct effects of nicotine on contractility of the uterine artery in pregnancy. Journal of Pharmacology and Experimental Therapeutics, 322(1), 180–185. doi: 10.1124/jpet.107.119354 [DOI] [PubMed] [Google Scholar]
- Xiaoxiao P, Li L, Li M, Qian Z, & Chenghao L (2015). [Preliminary study on E-cadherin expression in dexamethasone-induced palatal cleft in mouse]. Hua Xi Kou Qiang Yi Xue Za Zhi, 33(6), 581–584. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27051948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CJ, Li CYT, & Kong ANT (2005). Induction of phase I, II and III drug metabolism/transport by xenobiotics. Archives of Pharmacal Research, 28(3), 249–268. doi: 10.1007/Bf02977789 [DOI] [PubMed] [Google Scholar]
- Xu DP, Qu WD, Sun C, Cao RY, Liu DW, & Du PG (2018). A Study on Environmental Factors for Nonsyndromic Cleft Lip and/or Palate. Journal of Craniofacial Surgery, 29(2), 364–367. doi: 10.1097/Scs.0000000000004214 [DOI] [PubMed] [Google Scholar]
- Xuan Z, Zhongpeng Y, Yanjun G, Jiaqi D, Yuchi Z, Bing S, & Chenghao L (2016). Maternal active smoking and risk of oral clefts: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol, 122(6), 680–690. doi: 10.1016/j.oooo.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Yamada T, Hirata A, Sasabe E, Yoshimura T, Ohno S, Kitamura N, & Yamamoto T (2014). TCDD disrupts posterior palatogenesis and causes cleft palate. Journal of Cranio-Maxillofacial Surgery, 42(1), 1–6. doi: 10.1016/j.jcms.2013.01.024 [DOI] [PubMed] [Google Scholar]
- Yamada T, Mishima K, Fujiwara K, Imura H, & Sugahara T (2006). Cleft lip and palate in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: a morphological in vivo study. Congenit Anom (Kyoto), 46(1), 21–25. doi: 10.1111/j.1741-4520.2006.00097.x [DOI] [PubMed] [Google Scholar]
- Yang W, Carmichael SL, Roberts EM, Kegley SE, Padula AM, English PB, & Shaw GM (2014). Residential Agricultural Pesticide Exposures and Risk of Neural Tube Defects and Orofacial Clefts Among Offspring in the San Joaquin Valley of California. American Journal of Epidemiology, 179(6), 740–748. doi: 10.1093/aje/kwt324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Carmichael SL, & Shaw GM (2016). Folic Acid Fortification and Prevalences of Neural Tube Defects, Orofacial Clefts, and Gastroschisis in California, 1989 to 2010. Birth Defects Research Part a-Clinical and Molecular Teratology, 106(12), 1032–1041. doi: 10.1002/bdra.23514 [DOI] [PubMed] [Google Scholar]
- Yin X, Li J, Li Y, & Zou S (2019). Maternal alcohol consumption and oral clefts: a meta-analysis. Br J Oral Maxillofac Surg, 57(9), 839–846. doi: 10.1016/j.bjoms.2019.08.013 [DOI] [PubMed] [Google Scholar]
- Yoneda T, & Pratt RM (1982). Vitamin B6 reduces cortisone-induced cleft palate in the mouse. Teratology, 26(3), 255–258. doi: 10.1002/tera.1420260306 [DOI] [PubMed] [Google Scholar]
- Yuan QP, Blanton SH, & Hecht JT (2011). Association of ABCA4 and MAFB With Non-Syndromic Cleft Lip With or Without Cleft Palate. American Journal of Medical Genetics Part A, 155a(6), 1469–1471. doi: 10.1002/ajmg.a.33940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar RC, Davydov DR, & Verma S (2004). Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicology and Applied Pharmacology, 199(3), 316–331. doi: 10.1016/j.taap.2004.01.018 [DOI] [PubMed] [Google Scholar]
- Zhang J, & Cai WW (1993). Association of the common cold in the first trimester of pregnancy with birth defects. Pediatrics, 92(A), 559–563. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8414827 [PubMed] [Google Scholar]
- Zhang J, Zhou S, Zhang Q, Feng S, Chen Y, Zheng H,… Chen F (2014). Proteomic Analysis of RBP4/Vitamin A in Children with Cleft Lip and/or Palate. J Dent Res, 93(6), 547–552. doi: 10.1177/0022034514530397 [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Meng P, Zhang J, Jia P, Lin J, Wang X, … Wei X (2018). Machine Learning Models for Genetic Risk Assessment of Infants with Non-syndromic Orofacial Cleft. Genomics Proteomics Bioinformatics, 16(5), 354–364. doi: 10.1016/j.gpb.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YD, Dong SY, Wang JN, Wang M, Chen M, & Huang HZ (2017). Involvement of Notch2 in all-trans retinoic acid-induced inhibition of mouse embryonic palate mesenchymal cell proliferation. Molecular Medicine Reports, 16(3), 2538–2546. doi: 10.3892/mmr.2017.6940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YD, Dong SY, Wang WC, Wang JN, Wang M, Chen M, … Huang HZ (2016). Activation of Notch1 inhibits medial edge epithelium apoptosis in all-trans retinoic acid-induced cleft palate in mice. Biochemical and Biophysical Research Communications, 477(3), 322–328. doi: 10.1016/j.bbrc.2016.06.107 [DOI] [PubMed] [Google Scholar]
- Zhao RB, & Goldman ID (2013). Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Molecular Aspects of Medicine, 34(2-3), 373–385. doi: 10.1016/j.mam.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xie G, Yang T, & Qin J (2019). Congenital malformations are associated with secondhand smoke among nonsmoking women: A meta-analysis. Birth, 46(2), 222–233. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Balaraman Y, Teng M, Liu Y, Singh RP, & Nephew KP (2011). Alcohol alters DNA methylation patterns and inhibits neural stem cell differentiation. Alcoholism-Clinical and Experimental Research, 35(A), 735–746. doi: 10.1111/j.1530-0277.2010.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.-w., Yang X, Wan Y.-b., Xin Y.-h., Zhai K, Ma J, … Wang Y.-r. (2013). Associations of chromosomes 17q22, 10q25. 3 and ABCA4 gene polymorphisms with non-syndromic cleft lip/palate in Ningxia Hui and Han population. Journal of Shandong University (Health Sciences), 1. [Google Scholar]
- Zima T, Fialova L, Mestek O, Janebova M, Crkovska J, Malbohan I, … Popov P (2001). Oxidative stress, metabolism of ethanol and alcohol-related diseases. Journal of Biomedical Science, 8(1), 59–70. doi: 10.1007/Bf02255972 [DOI] [PubMed] [Google Scholar]