Abstract
Background & Aims
Abnormal liver tests are common in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, but a possible direct role of the virus in liver injury and its association with short-term outcomes are controversial. Therefore, we aimed to compare the pattern of abnormal liver tests in patients with SARS-CoV-2 with those of patients infected with influenza, a non-hepatotropic respiratory virus, and their association with worse outcomes during hospitalisation.
Methods
We performed a retrospective cohort study of 1,737 hospitalised patients (865 with influenza and 872 with SARS-CoV-2) in a tertiary medical centre. We defined abnormal liver tests as alanine transaminase or aspartate transaminase ≥40 IU/ml at any time-point during hospitalisation.
Results
Abnormal liver tests were mild to moderate in most patients regardless of infection type, but the majority of patients with influenza had a transaminase peak earlier during hospitalisation compared with patients with SARS-CoV-2. Abnormal liver tests correlated with markers of severe disease in either influenza or SARS-CoV-2 infections, and were associated with death, occurring mainly in patients with severe liver test abnormalities (>200 IU/L) (38.7% and 60% of patients with influenza or SARS-CoV-2, respectively). In multivariate analysis, controlling for age, sex, lymphopaenia, and C-reactive protein, liver test abnormalities remained significantly associated with death for influenza (odds ratio 4.344; 95% CI 2.218–8.508) and SARS-CoV-2 (odds ratio 3.898; 95% CI 2.203–6.896). These results were confirmed upon propensity score matching.
Conclusions
Abnormal liver tests during hospitalisation with SARS-CoV-2 or influenza infections are common, may differ in their time course, and reflect disease severity. They are associated with worse outcomes, mainly in patients with severe liver test abnormalities, regardless of infection type.
Lay summary
Coronavirus disease 2019 (COVID-19) is a serious global health pandemic, the causative agent of which is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Abnormal liver tests are common among SARS-CoV-2 infected patients and are often associated with worse outcomes. Herein, we compare the pattern of abnormal liver tests and their association with disease severity between 2 major non-hepatotropic respiratory viruses: SARS-CoV-2 and influenza. We show that abnormal liver tests are common in both infections, may slightly differ in their kinetics, and are associated with worse outcomes, especially in patients with severe liver test abnormalities. These results strongly suggest that abnormal liver tests in SARS-CoV-2 patients reflect disease severity, rather than a virus-mediated direct liver injury, and should be closely followed in admitted patients.
Keywords: Respiratory tract infections, COVID-19, Liver injury
Abbreviations: ALKP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BOSmin, minimal blood oxygen saturation; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SBPmin, minimal systolic blood pressure; WBC count, white blood cell count
Graphical abstract
Highlights
-
•
Abnormal liver tests are common in patients infected with SARS-Cov-2 and are associated with worse outcomes.
-
•
Comparison of abnormal liver tests in SARS-CoV-2 and influenza infected patients show similarities in relation to disease severity and worse outcomes.
-
•
Abnormal liver tests may appear later during hospitalisation in SARS-CoV-2- compared to influenza-infected patients.
-
•
Abnormal liver tests most probably reflect disease severity, rather than direct liver injury.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the current coronavirus disease 2019 (COVID-19) pandemic, first emerging in China,1 but now affecting most countries in the world.2 Although the respiratory system is the main target of the virus, SARS-CoV-2 often triggers a systemic immune-mediated response that might result in injury to various organs, such as the kidneys, heart, or liver.3,4 Recent reports indicate that hepatic injury is not uncommon among patients with COVID-19,[5], [6], [7] and further suggest that abnormal liver tests are associated with increased mortality and should therefore be carefully monitored during hospitalisation.[8], [9], [10] However, the predictive value and the association of abnormal liver tests with worse outcomes in patients with COVID-19 is still controversial.11,12
Interestingly, SARS-Cov-associated hepatitis has been reported well before the present pandemic.13 Furthermore, the liver might also be collaterally damaged by infections with other respiratory viruses, such as influenza.14 Although few reports have suggested that certain strains of influenza A in children are possibly hepatotropic and might promote liver injury by directly targeting the hepatocytes,15 the widespread concept favours an immune-mediated mechanism,16 medication-related hepatotoxicity, or hypotension that might result in hepatic ischaemic injury.
We speculate that liver injury in COVID-19 is rather a marker of disease severity as in other viral respiratory infections.17 Accordingly, we aimed to investigate the prevalence and natural course of abnormal liver tests, as well as their association with the composite outcome of mechanical ventilation or death, in patients with COVID-19 as compared with patients with influenza infection.
Patients and methods
Study design and patients
This retrospective study is based on prospectively collected data of patients older than 18 years admitted to our medical centre from early 2018 to early January 2021, found to be positive for either influenza (A/B) or SARS-CoV-2 by RT-PCR. Positive RT-PCR patients for more than 1 of the examined viruses, or lacking full information on liver enzymes were excluded. Rabin Medical Center (RMC) Review Board (#RMC-20-0142; Petah-Tikva, Israel) approved the study, which was performed according to the declaration of Helsinki.
Definitions and outcomes
We defined abnormal liver tests, including aspartate transaminase (AST) or alanine transaminase (ALT) as levels ≥40 (U/L). These values are close to the accepted values defined in the literature (https://gi.testcatalog.org).
The primary outcomes of the study were defined as death (during hospitalisation or within 3 days after discharge) or a composite of mechanical ventilation or death during hospitalisation.
Data collection
The following data were prospectively collected from the electronic medical records of patients included in the study: basic characteristics, such as age, sex, BMI, date of admission, and outcomes (mechanical ventilation, length of hospitalisation, discharge, transfer to ICU, or death). Background liver disease was defined as documented cirrhosis, viral hepatitis, or fatty liver (either previously recorded in the electronic files or observed by imaging during hospitalisation), and major cardiovascular diseases were defined as known ischaemic heart disease, hypertension, or diabetes. Clinical parameters recorded during hospitalisation, including minimal blood oxygen saturation (BOSmin), maximal fever, minimal systolic blood pressure (SBPmin), as well as the presence of in-hospital pneumonia, treatment with antiviral or antimicrobial agents were collected from the electronic medical files. Laboratory data of liver enzymes were extracted at 4 time points: on admission, last test before discharge, as well as maximum and minimum levels during hospitalisation. In some cases, where there were fewer than 4 separate assessments, these time points overlapped. Maximum and minimum levels of the following laboratory parameters were collected for correlation analyses: white blood cell (WBC) count, C-reactive protein (CRP), lactate dehydrogenase, lymphocytes, alkaline phosphatase (ALKP), and liver synthetic functions.
Factors related to elevated liver enzymes, such as serology tests for CMV, EBV, HIV, herpes simplex virus (HSV), varicella zoster virus (VZV), hepatitis A, B, C, and relevant imaging tests during hospitalisation (abdominal sonography or abdominal computed tomography) were extracted from the patients’ electronic files.
Statistical analysis
We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and GraphPad PRISM 9 software (GraphPad Software, San Diego, CA, USA). Normality tests were conducted on all variables. Owing to the non-normal distribution of our variables, we used the non-parametric Wilcoxon and Kruskal–Wallis tests when appropriate and median and IQR are presented. Chi-square or Fisher’s exact tests were used to compare categorical variables between study groups, accordingly. Two-sided p <0.05 was considered statistically significant.
The patient cohort was divided into 2 RT-PCR-based viral diagnosis groups and then further divided according to their liver enzyme status. Baseline characteristics, laboratory tests, and basic information regarding hospitalisation were compared between the groups and subgroups. Uni- and multivariate regression models were constructed to estimate predictors for the primary outcome. Variables previously described found to be associated with the aforementioned outcomes, such as low lymphocyte counts, high CRP levels, age, or sex,[18], [19], [20] as well as variables that were found to significantly associate in the univariate analyses, were incorporated into a Cox regression model. Odd ratios (ORs) with 95% CIs were calculated. Logistic regression was used to calculate ORs.
As a sensitivity analysis, propensity-score matching was used to identify a cohort of patients with similar risk factors and baseline characteristics that were shown to impact the outcome and were used in the multivariate model. Matching was performed with the use of a 1:1 matching protocol without replacement, and a regression model was performed on propensity-matched cohort of patients with and without abnormal liver tests for each virus independently. Analyses of the primary outcomes were performed in a binary regression model.
Results
Patients’ characteristics according to infection type
We allocated all patients hospitalised in our medical centre between early 2018 to early January 2021 as a result of infection with either influenza (A/B) (2018–2020) or SARS-CoV-2 (during 2020–2021). Overall, following exclusion of patients for various reasons, 1,737 patients (865 with influenza and 872 with SARS-CoV-2) were included in the final analysis (Fig. S1).
Table 1 outlines the various demographics, as well as major clinical features and laboratory values. A higher proportion of SARS-CoV-2 patients were males with a higher BMI compared with patients with influenza infection. The rates of death or the composite of mechanical ventilation or death were higher in SARS-CoV-2 patients compared with influenza patients (15.9% vs. 6.4% for death and 20.3% vs. 8.6% for mechanical ventilation or death, respectively).
Table 1.
Major characteristics of patients according to the type of infection.
| Influenza (n = 865) | SARS-CoV-2 n = 872) | p value | |
|---|---|---|---|
| Age (median, IQR) | 69 (51–80) | 67 (53–78) | 0.191 |
| Sex – females (%) | 55.8 | 45.4 | <0.001 |
| BMI (median, IQR) | 26.26 (22.68–30.06) | 27.55 (24.45–24.45) | <0.001 |
| Prior liver disease (%) | 4.4 | 2.8 | 0.087 |
| Cardiovascular diseases | 45.8 | 49.4 | 0.141 |
| In-hospital pneumonia (%) | 17.5 | 64.2 | <0.001 |
| Antibiotic treatment (%) | 67.3 | 50.5 | <0.001 |
| Antiviral treatment (%) | 80.6 | 20.3 | <0.001 |
| Saturation (%)min (median, IQR) | 94.00 (90.00–97.00) | 92.00 (86.00–96.00) | <0.001 |
| Systolic BPmin (median, IQR) | 108.00 (95.00–127.00) | 107.00 (98.00–121.00) | 0.364 |
| Albuminmin (median, IQR) | 3.60(3.20–4.00) | 3.30 (2.80–3.80) | <0.001 |
| Lymphmin (median, IQR) | 0.60 (0.40–1.00) | 0.80 (0.50–1.20) | <0.0001 |
| CRPmax (median, IQR) | 6.22 )2.95–13.98) | 9.10 (2.92–19.36) | <0.001 |
| ALTmax (median, IQR) | 23.00 (16.00–37.50) | 26.20 (17.00–53.00) | <0.001 |
| ASTmax (median, IQR) | 30.00 (22.00–46.00) | 33.05 (22.87–62.65) | <0.001 |
| ALKPmax (median, IQR) | 82.00 (65.00–114.00) | 88.00 (68.00–128.00) | 0.004 |
| GGTmax (median, IQR) | 32.50 (18.00–75.50) | 50.00 (24.00–115.00) | <0.001 |
| ALTmax 40–200 (%) | 20.6 | 32.1 | |
| ALTmax 200–400 (%) | 0.8 | 0.9 | |
| ALTmax >400 (%) | 0.8 | 1.2 | |
| ASTmax 40–200 (%) | 28.6 | 37.0 | |
| ASTmax 200–400 (%) | 2.4 | 3.1 | |
| ASTmax >400 (%) | 0.8 | 1.1 | |
| TBilmax (median, IQR) | 0.45 (0.30–0.70) | 0.50 (0.35–0.80) | <0.0001 |
| INRmax (median, IQR) | 1.09 (0.97–1.26) | 1.15 (1.06–1.30) | <0.001 |
| Death (%) | 6.4 | 15.9 | <0.001 |
| Mechanical ventilation or death (%) | 8.6 | 20.3 | <0.001 |
Variables were compared between groups using the non-parametric Wilcoxon rank-sum test or Chi-square test for independence. ALKP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; INR, international normalised ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TBil, total bilirubin.
The ALT level was elevated in 22.2% and 34.2%, and AST was elevated in 31.8% and 41.2% of patients with influenza and SARS-CoV-2, respectively. Only 1.6% and 3.2% of influenza patients had severe elevation in ALT or AST (>200 IU/L) vs. 2.1% and 4.2% of SARS-CoV-2 patients, respectively. Median values of ALT, AST, ALKP, and gamma-glutamyl transferase (GGT) were significantly higher in patients infected with SARS-CoV-2 compared with patients infected with influenza.
The temporal pattern of abnormal liver tests according to the type of infection
To validate that the abnormal liver tests were largely transient and likely related to the acute illness, we compared the highest with the lowest values during hospitalisation. There was a clear and statistically significant difference between the highest and the lowest levels of liver tests during hospitalisation, for patients with abnormal values, for both influenza and SARS-CoV-2 infections (Fig. S2).
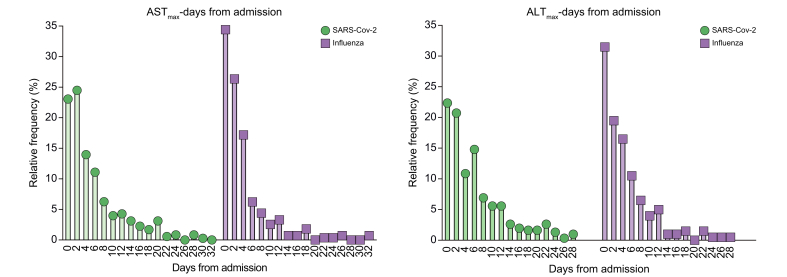
We next analysed the timing of peak abnormal liver enzymes during hospitalisation (Fig. 1). AST level peaked by day 4 of hospitalisation in ~80% of patients with influenza vs. ~60% of patients with SARS-CoV-2 and between days 6 and 16 of hospitalisation in ~16% of patients with influenza vs. ~30% of patients with SARS-CoV-2. The ALT peak was recorded by day 4 in ~70% of patients with influenza vs. ~55% of patients with SARS-CoV-2 and between days 6 and 16 in ~27% of patients with influenza vs. ~35% of patients with SARS-CoV-2.
Fig 1.
Time course of elevated liver tests in influenza or SARS-CoV-2 infections.
Patients with abnormal liver tests during hospitalisation were allocated and the timing of their maximal value of liver tests (days post admission) was plotted on a frequency distribution plot for each type of infection (influenza or SARS-CoV-2). ALT, alanine transaminase; AST, aspartate transaminase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Characteristics of patients and disease severity as a function of liver tests
Patients’ demographics and the severity of their disease according to liver tests were subsequently analysed (Table 2). The percentage of males among patients with abnormal liver tests was significantly higher compared with patients with normal liver tests in both influenza or SARS-CoV-2 infected patients, however, patients with abnormal liver tests did not have a significantly higher rate of prior liver disease. SARS-CoV-2, but not influenza infected patients, with abnormal liver tests had a significantly higher BMI compared with patients with normal tests.
Table 2.
Major characteristics and outcomes of patients according to the presence or absence of abnormal liver tests (ALTmax ≥40, ASTmax ≥40).
| Parameter | Influenza |
p value | SARS-CoV-2 |
p value | ||
|---|---|---|---|---|---|---|
| Abnormal liver tests n = 293 | Normal liver tests n = 572 | Abnormal liver tests n = 396 | Normal liver tests n = 476 | |||
| Age | 71.00 (56.00–81.00) | 68.00 (41.00–80.00) | 0.059 | 67.00 (56.00–78.00) | 67.00 (49.75–77.25) | 0.124 |
| Sex – female % | 43.3 | 62.2 | <0.001 | 34.6 | 54.4 | <0.001 |
| BMI (median, IQR) | 25.83 (22.49–29.76) | 26.51 (22.89–30.12) | 0.411 | 27.78 (24.81–31.18) | 27.34 (23.91–30.80) | 0.039 |
| Prior liver disease (%) | 5.8 | 3.7 | 0.203 | 4.0 | 1.7 | 0.056 |
| Major illness (DM, HTN) (%) | 48.1 | 44.6 | 0.359 | 53.3 | 46.2 | 0.044 |
| In-hospital pneumonia (%) | 19.1 | 16.6 | 0.242 | 61.8 | 68.4 | 0.852 |
| Antibiotics treatment (%) | 75.8 | 62.9 | <0.001 | 63.4 | 39.7 | <0.0001 |
| Antiviral treatment (%) | 80.2 | 80.8 | 0.716 | 31.1 | 11.3 | <0.001 |
| Systolic BP (min) (median, IQR) | 105.00 (92.00, 123.00) | 110.00 (96.00–129.00) | 0.005 | 104.00 (94.00–117.00) | 110.00 (100.00–125.00) | <0.001 |
| Saturation% (min) (median, IQR) | 93.00 (88.00–96.00) | 95.00 (91.00–97.00) | <0.0001 | 88.00 (82.00–93.00) | 94.00 (90.50–97.00) | <0.0001 |
| Albumin (mg/dl) (median, IQR) | 3.30 (2.80–3.70) | 3.70 (3.30–4.05) | <0.0001 | 3.00 (2.40–3.42) | 3.60 (3.20–3.92) | <0.0001 |
| Lymphmin (median, IQR) | 0.60 (0.30–0.90) | 0.70 (0.50–1.00) | <0.0001 | 0.60 (0.30–0.90) | 1.00 (0.70–1.40) | <0.0001 |
| CRPmax (median, IQR) | 10.58 (4.72–21.13) | 5.04 (2.56–10.33) | <0.0001 | 16.27 (7.80–28.36) | 5.11 (1.55–10.72) | <0.0001 |
| ALTmax (median, IQR) | 48.00 (33.00–76.00) | 18.00 (14.00–23.25) | <0.0001 | 57.20 (41.00–94.28) | 18.00 (14.00–23.12) | <0.0001 |
| ASTmax (median, IQR) | 60.00 (46.00–98.00) | 24.00 (19.00–31.00) | <0.0001 | 68.70 (48.00–110.55) | 23.15 (18.58–29.08) | <0.0001 |
| ALKPmax (median, IQR) | 95.00 (72.00, 154.00) | 78.00 (63.00–103.00) | <0.0001 | 103.00 (74.00–163.00) | 80.00 (63.00–104.00) | <0.0001 |
| GGTmax (median, IQR) | 66.00 (35.00–176.00) | 24.00 (15.00–43.25) | <0.0001 | 100.00 (50.00-234.00) | 28.00 (17.00–56.00) | <0.0001 |
| TBilmax (mg/dl) (median, IQR) | 0.56 (0.37–0.89) | 0.40 (0.28–0.59) | <0.0001 | 0.63 (0.44–1.10) | 0.41 (0.30–0.60) | <0.001 |
| INRmax (median, IQR) | 1.16 (1.02–1.36) | 1.05 (0.96–1.20) | <0.0001 | 1.21 (1.10–1.40) | 1.10 (1.00–1.20) | <0.0001 |
| Death (%) | 14.0 | 2.4 | <0.001 | 29.0 | 5.0 | <0.001 |
| Mechanical ventilation or death (%) | 17.7 | 3.8 | <0.001 | 37.1 | 6.3 | <0.001 |
| ICU admission (%) | 5.5 | 0.9 | <0.001 | 11.4 | 1.1 | <0.001 |
| Length of hospital stay (days) (median, IQR) | 5.00 (3.00–10.00) | 3.00 (2.00–5.00) | <0.0001 | 10.00 (5.00–17.00) | 4.00 (2.00–7.00) | <0.001 |
| Remdesivir (%) | 21.0 | 5.5 | <0.001 | |||
| Enoxaparin (%) | 68.2 | 43.3 | <0.001 | |||
| Dexamethasone (%) | 54.8 | 20.2 | <0.001 | |||
Variables were compared between groups using the non-parametric Wilcoxon rank-sum test or Chi-square test for independence. ALKP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; DM, diabetes mellitus; GGT, gamma-glutamyl transferase; HTN, hypertension; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TBil, total bilirubin.
Patients with liver test abnormalities had a higher rate of antibiotics use compared with patients with normal liver tests regardless of infection type (75.8% vs. 62.9%, p <0.001, for influenza infected patients and 63.4% vs. 39.7%, p <0.0001, for SARS-CoV-2 infected patients), but the use of antiviral agents was significantly higher only among SARS-CoV-2 infected patients with abnormal liver tests compared with those with normal liver tests (31.1% vs. 11.3%, p <0.001).
In addition, treatment with either remdesivir, dexamethasone, or enoxaparin, was significantly more common in SARS-CoV-2 infected patients with abnormal liver tests compared with patients with normal tests (21.0% vs. 5.5% p <0.001 for remdesivir, 68.2% vs. 43.3%, p <0.001 for enoxaparin, and 54.8% vs. 20.2%, p <0.001 for dexamethasone), most probably reflecting their disease severity.
There were significant differences in clinical and laboratory parameters of disease severity, including BOSmin, CRPmax, lymphocytes min, and albumin min between patients with or without liver test abnormalities, regardless of infection type.
Consistently, patients with liver test abnormalities had a higher rate of death (14% vs. 2.4%, p <0.001 for influenza, and 29% vs. 5.0%, p <0.001 for SARS-CoV-2) and admission to ICU (5.5% vs. 0.9%, p <0.001 for influenza, 11.4% vs. 1.1%, p <0.001 for SARS-CoV-2), as well as longer hospitalisation days (5.0 [3.0–10.0] vs. 3.0 [2.0–5.0], p <0.001 for influenza, and 10.0 [5.0–17.0] vs. 4.0 [2.0–7.0], p <0.001 for SARS-CoV-2), compared with patients with normal liver tests.
Association of patients’ characteristics and parameters of disease severity with poor outcomes
A univariate analysis to test possible association of patients’ demographic, clinical, or laboratory parameters with a composite outcome of mechanical ventilation or death during hospitalisation was performed. Patients’ age, use of antibiotics, hospitalisation length, and admission to ICU as well as other disease severity parameters, including BOSmin and SBPmin, were associated with the composite outcome in both influenza and SARS-CoV-2 infections (Table S1).
Routinely used laboratory parameters to evaluate disease severity, CRP, and albumin levels, were associated with the composite outcome in both infections. Importantly, the same was true for abnormal liver tests (ALT, AST, or GGT), all strongly associated with the composite outcome in the 2 groups of infected patients.
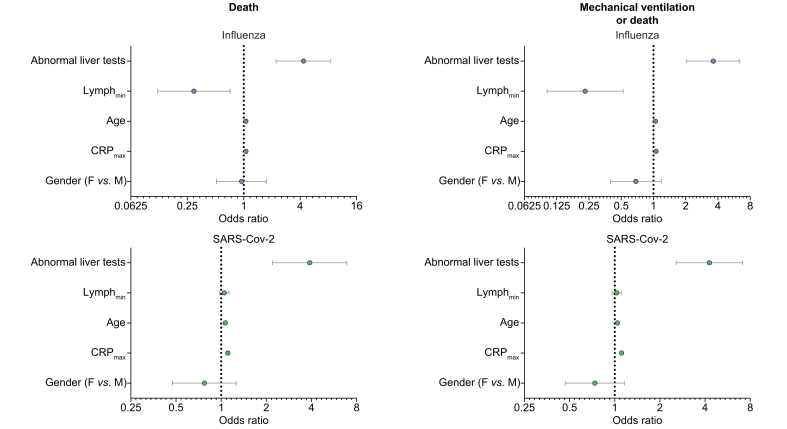
Liver test abnormalities incorporated in a multivariate regression analysis, utilising patients’ age and sex as well as CRP and lymphocyte count (Fig. 2), were found to be significantly associated with the composite outcome of mechanical ventilation or death (OR = 3.62, 95% CI 2.04–6.40 for influenza and OR = 4.28, 95% CI 2.57–7.11 for SARS-CoV-2), or an outcome of death (OR = 4.34, 95% CI 2.21–8.50 for influenza and OR = 3.89, 95% CI 2.20–6.89 for SARS-CoV-2) in patients with either influenza or SARS-CoV-2 infections.
Fig 2.
A multivariate analysis (a Cox regression model) of various parameters related to patients’ basic demographics and disease severity and their association with a composite outcome of mechanical ventilation or death, or death only.
The odds ratios (OR) for each parameter incorporated in the analysis and 95% confidence limits are plotted. CRP, C-reactive protein; F, females; M, males; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Secondary analysis for verification was done using propensity-score matching. Liver enzymes were predictive for an outcome of intubation or death in both viruses in the matched cohorts. We next plotted the peak levels of AST or ALT recorded during hospitalisation against minimal albumin or maximal CRP values for each patient in our cohort (Figs. S3 and S4). There was an inverse statistically significant correlation between liver tests and albumin levels and a linear statistically significant correlation between liver tests and CRP levels for AST or ALT. This suggests that liver test abnormalities correlate well with other markers of disease severity.
Correlation of patients’ poor outcomes with the severity of liver test abnormalities
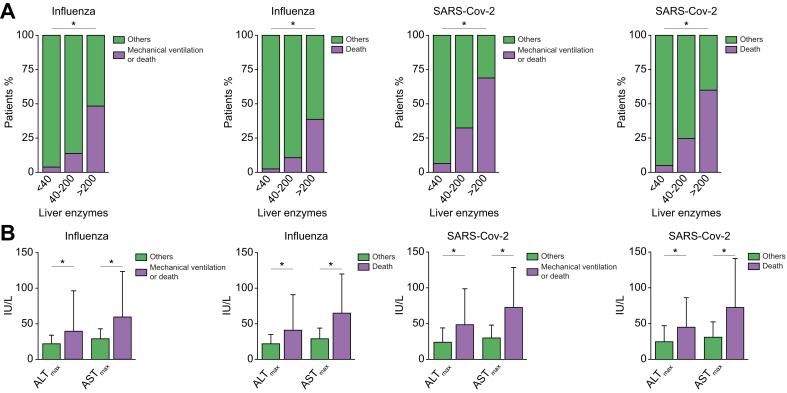
The percentage of patients who achieved the composite outcome of mechanical ventilation or death, or an outcome of death, correlated well with the severity of liver tests abnormalities (Fig. 3A). We categorised patients as having normal liver enzymes (ALT or AST <40 IU/L), mild to moderate liver test abnormalities (at least 1 enzyme ≥40 IU/L but <200 IU/L) or severe liver test abnormalities (at least 1 of the enzymes ≥200 IU/L). The percentage of patients with the composite outcome of mechanical ventilation or death was 3.9%, 13.8%, and 48.4% (p <0.001) for influenza and 6.4%, 32.5%, and 68.9% (p <0.001) for SARS-CoV-2, for patients with mild, moderate, or severe liver tests abnormalities, respectively. The percentage of patients with the outcome of death was 2.5%, 10.8%, and 38.7% for influenza, and 5.1%, 24.6%, and 60% for SARS-CoV-2, for patients with mild, moderate, or severe liver tests abnormalities, respectively.
Fig 3.
Association of disease severity with the magnitude of liver tests abnormalities.
(A) Stacked graphs presenting the percentage of patients that either achieved or not achieved (others) the endpoint of either mechanical ventilation or death or death only, as a function of their liver tests (ALT and AST <40 IU/L, at least 1 of the enzymes ≥40 IU/L but <200 IU/L or at least one of the enzymes ≥200 IU/L) for influenza or SARS-CoV-2 infections (∗p <0.001). (B) Median values and range (upper/lower limits) of liver enzymes for patients that either achieved or not (others) the composite endpoint (mechanical ventilation or death or death only) for influenza or SARS-CoV-2 infections (∗p <0.001). ALT, alanine transaminase; AST, aspartate transaminase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Consistently, ALTmax or ASTmax levels of patients with the composite outcome of mechanical ventilation or death or death alone were significantly higher compared with all other patients, regardless of infection type (Fig. 3B).
Discussion
Here, we show that abnormal liver tests are common among admitted patients with either influenza or SARS-CoV-2 infections, and that the percentage of patients with abnormal liver tests at some point during infection does not differ much between infections, ranging from ~20% to 40% of patients. Several recent studies have highlighted the relatively high frequency of abnormal liver tests among patients infected with SARS-CoV-26,21 Our study suggests that this phenomenon is not unique to this infection but rather similarly occurs within other major respiratory viral infections. However, the kinetics of abnormal liver tests may differ between infections, with a delayed elevation of liver tests in the case of SARS-CoV-2 compared with influenza, compatible with the well-established observation that clinical deterioration of SARS-CoV-2 patients is typically delayed until days 7–10 after symptom onset.3
Disease severity or worse outcomes parameters were significantly more common among patients with abnormal liver tests regardless of the type of infection, in line with other parameters, both clinical or laboratory, known to be associated with disease severity.
Therefore, it is reasonable to speculate that abnormal liver tests are a surrogate marker of disease severity and worse outcomes in patients hospitalised as a result of acute respiratory viral infection, and that this is not a property unique to a particular virus. In support of this, liver enzymes were all found to be inversely correlated with the minimal value of serum albumin and linearly correlated with maximal CRP levels recorded during hospitalisation, two other well-established markers for disease severity.
Our study indicates that patients with severe abnormal liver tests (≥200 IU/L) are at the highest risk of worse outcomes, regardless of infection type. This is in line with a recent report from a large cohort of SARS-CoV-2 patients in the US, indicating that in the 6.4% of patients with severe abnormal liver tests (>5 times normal range), worse outcomes should be anticipated.22 However, the majority of our patients with abnormal liver tests presented with only mild to moderate liver enzyme abnormalities.
Several mechanisms may explain liver biochemical abnormalities during non-hepatotropic viral infection, but the leading theory is that liver injury is immune mediate, resulting from molecular mimicry between viral and hepatocyte antigens.16 However, a recent study has suggested that SARS-CoV-2 contributes to hepatic impairment by directly infecting the liver resulting in a typical histological signature.23 These authors related relatively mild alterations in transaminase levels to hepatic impairment, reflected by a low albumin level, and to massive apoptosis observed in few liver biopsies. Nevertheless, this study has recently been criticised,24,25 arguing that the relatively mild abnormal liver tests do not reflect true liver injury, usually defined by much higher elevations in transaminases.26 Moreover, the observed low albumin levels could reflect the severity of the disease rather than liver synthetic dysfunction. Indeed, our study suggests that abnormal liver tests correlate well with low albumin levels as well as with other markers of disease severity, such as CRP and that in most infected patients with abnormal liver tests, the impairment is mild and is not virus specific.
Although this study includes a relatively large number of patients with an ample of clinical and laboratory data, it also has several limitations: First, it is retrospective and not all patients were systematically tested for liver enzymes, and we had no control on the timing by which these tests were taken for each patient. In addition, as the COVID-19 pandemic began only in 2020 and there were no recorded cases of influenza infections during the following winter of 2020/2021, we compared SARS-CoV-2 to influenza infected patients admitted to our medical centre during separate time periods. Second, we had no access to laboratory results prior to hospitalisation and could not accurately assess whether the observed abnormal liver tests during hospitalisation were entirely new. We have partially overcome this issue by showing that the peak in abnormal liver tests is significantly higher than the nadir during hospitalisation, suggesting that at least in the majority of patients, the maximal abnormal liver tests values are related to the acute disease and not to an underlying liver problem. Third, the cut-off value of 40 IU/L for a definition of elevated ALT and AST levels is largely arbitrary and was not adjusted for age, weight, and sex. However, these cut-offs were used in previous studies,23 and largely represent values in the range observed in 95% of the healthy population. In addition, the reason that we have not included the ALKP and GGT values in our primary analysis is that ALKP is not necessarily liver-specific and GGT does not necessarily reflect a direct liver injury.
In summary, in this study we show that abnormal liver tests are quite common and appear with similar frequency among hospitalised patients with either influenza or SARS-CoV-2 infections, although the timing of abnormal liver tests peak may be somewhat delayed in patients with SARS-CoV-2. The abnormal liver tests correlate with other markers of disease severity in the 2 types of infection and are associated with worse outcomes. Although our results suggest that abnormal liver tests do not necessarily reflect a direct and significant liver injury, but is rather a consequence of a systemic inflammatory response, we believe that liver tests should be routinely taken in such hospitalised patients, and that abnormal values should alert the treating physician to monitor the patient more intensively.
Financial support
There is no funding to declare regarding this work.
Authors’ contributions
Conceptualised and designed the study: N.S., A.I., M.B., A.S.
Collected and organised the data: N.S.
Analysed the data: T.S., I.H.S., A.S.
Wrote the paper: A.S., N.S.
Edited the paper: A.S., A.I., M.B.
Data availability
Anonymous data on which the analysis in the paper is based on, will be provided upon request.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100258.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. Our World in Data; 2020. Coronavirus Pandemic (COVID-19)https://ourworldindata.org/coronavirus [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer E.A.K., Arvind A., Bloom P.P., Chung R.T. Interrelationship between coronavirus infection and liver disease. Clin Liver Dis (Hoboken) 2020;15:175–180. doi: 10.1002/cld.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundt M.A., Deng Y., Ciarleglio M.M., Nathanson M.H., Lim J.K. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei F., Liu Y.M., Zhou F., Qin J.J., Zhang P., Zhu L. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi X., Liu C., Jiang Z., Gu Y., Zhang G., Shao C. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73:455–458. doi: 10.1016/j.jhep.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponziani F.R., Del Zompo F., Nesci A., Santopaolo F., Ianiro G., Pompili M. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060–1068. doi: 10.1111/apt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vespa E., Pugliese N., Piovani D., Capogreco A., Danese S., Aghemo A. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73:1275–1276. doi: 10.1016/j.jhep.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papic N., Pangercic A., Vargovic M., Barsic B., Vince A., Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir Viruses. 2012;6:e2–e5. doi: 10.1111/j.1750-2659.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitworth J.R., Mack C.L., O'Connor J.A., Narkewicz M.R., Mengshol S., Sokol R.J. Acute hepatitis and liver failure associated with influenza A infection in children. J Pediatr Gastroenterol Nutr. 2006;43:536–538. doi: 10.1097/01.mpg.0000232332.00677.3d. [DOI] [PubMed] [Google Scholar]
- 16.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorburn K., Fulton C., King C., Ramaneswaran D., Alammar A., McNamara P.S. Transaminase levels reflect disease severity in children ventilated for respiratory syncytial virus (RSV) bronchiolitis. Sci Rep. 2018;8:1803. doi: 10.1038/s41598-018-20292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhargava A., Fukushima E.A., Levine M., Zhao W., Tanveer F., Szpunar S.M. Predictors for severe COVID-19 infection. Clin Infect Dis. 2020;71:1962–1968. doi: 10.1093/cid/ciaa674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertolini A., van de Peppel I.P., Bodewes F., Moshage H., Fantin A., Farinati F. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phipps M.M., Barraza L.H., LaSota E.D., Sobieszczyk M.E., Pereira M.R., Zheng E.X. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Liu S., Liu H., Li W., Lin F., Jiang L. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangash M.N., Patel J.M., Parekh D., Murphy N., Brown R.M., Elsharkawy A.M. SARS-CoV-2: is the liver merely a bystander to severe disease? J Hepatol. 2020;73:995–996. doi: 10.1016/j.jhep.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philips C.A., Ahamed R., Augustine P. SARS-CoV-2 related liver impairment – perception may not be the reality. J Hepatol. 2020;73:991–992. doi: 10.1016/j.jhep.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufour D.R., Lott J.A., Nolte F.S., Gretch D.R., Koff R.S., Seeff L.B. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous data on which the analysis in the paper is based on, will be provided upon request.