Abstract
Purpose:
Eating disorders and their core symptoms (e.g., binge eating, body weight/shape concerns) disproportionately affect females, and these sex-differentiated effects become prominent during and after puberty. Although psychosocial influences, like heightened sociocultural pressures for thinness in girls/women, contribute to this sex imbalance, biological factors could also play an important role.
Methods:
In this narrative review, we summarize evidence of biological factors underlying the sex-differentiated prevalence of eating pathology as well as within-sex variability in risk.
Findings:
There are sex differences in the pubertal emergence of genetic effects on eating pathology (adrenarche in males; gonadarche in females), and at least some genetic contributions to eating pathology appear to vary between the sexes. Furthermore, sex steroid hormones (e.g., testosterone, estradiol, progesterone) are leading contributors to differential risk for eating pathology in males and females across the lifespan. Emerging data suggest that between-sex and within-sex variability in risk might occur via hormone-driven modulation (activation/deactivation) of genetic influences and neural responsiveness to food-related cues.
Implications:
There is a biological basis to heightened risk for eating pathology in females, relative to males, as well as unique biological influences within each sex. Findings from this review highlight the importance of studying both sexes and considering sex-specific biological mechanisms that may underlie differential risk for eating pathology.
Keywords: sex difference, eating disorders, eating pathology, hormones, genetics, development
Introduction
Females are at greater risk than males for eating disorders and their core symptoms (e.g., body weight/shape concerns; binge eating), and onset often coincides with adolescence, particularly pubertal maturation. Indeed, prevalence rates are sex-differentiated during/after puberty, with the female:male ratio ranging from ~2:1 to ~10:1 across eating disorders, and effect sizes are in the medium-to-large range for eating pathology symptoms.1–5 It is recognized that psychosocial factors, such as increased appearance and weight pressures in females, contribute to developmental and sex-based differences in eating disorders; however, biological factors also play a critical role. For example, animal studies of over-controlled (e.g, activity-based anorexia) and under-controlled (e.g., binge eating paradigms) eating have also detected female predominance and enhanced adolescent/pubertal risk.3,6–8 Animals are not subject to the appearance/beauty ideals present in humans and thus, these data broadly confirm biological contributions to developmental and sex-differentiated patterns of risk.9
This narrative review summarizes evidence of biological factors related to sex differences in eating pathology. We have made a concerted effort to incorporate findings from animal and human studies since this translational approach balances ecological validity (from human research) with experimental control (from animal work).9,10 We have also conducted this review using a developmental lens, and when possible, highlight the ways in which identified biological factors may contribute to vulnerability to eating disorder symptomatology across different life stages. Because of the sex difference in eating disorder prevalence, a large proportion of biological research on eating pathology in humans has either exclusively focused on females or used samples that are predominantly comprised of females. Consequently, males are often either combined with females in analyses and/or sex differences are not tested (or are tested with inadequate statistical power). The general lack of statistical comparisons between the sexes has contributed to a limited understanding of potential sex-differentiated biological mechanisms underlying eating pathology. This review focused on biologically informative studies (behavioral genetics) and studies that have explored biological factors known to be sex-differentiated (e.g., sex steroids, neural function) since this is the largest research base on which conclusions can be drawn. Notably, sex-specific biological factors/effects can contribute to differentiation of the sexes and may therefore inform sex differences in eating pathology.
Methods
Terminology
Sex/Gender:
Sex and gender are not synonymous terms. Although self-identified gender often aligns with one’s chromosomal sex, this is not always the case. Herein we use the terminology sex, as oppose to gender, because most studies examining male-female differences have done so using chromosomal sex; however, we acknowledge that gender identity could result in different effects– an area of research that is vastly understudied but is needed.11
Eating Pathology:
Eating disorders are broadly characterized by disturbances in eating and weight focused cognitions (e.g., undue influence of weight/shape on one’s self-evaluation) and behaviors (e.g., over-control or under-control of eating). Despite distinct diagnostic classifications (e.g., anorexia nervosa, bulimia nervosa, binge eating disorder)1, several eating disorder symptoms, like binge eating and body weight/shape concerns, are transdiagnostic and can be measured dimensionally across a spectrum of severity.12 In this review, we have synthesized findings across studies that used various measurement approaches (e.g., diagnoses vs. dimensional scales in humans; behavioral phenotypes in animals) and use the term “eating pathology” to broadly discuss eating disorders diagnoses and/or their dimensional symptoms. We refer to distinct diagnoses or symptoms if/when findings have been unique to certain outcomes (e.g., binge eating vs. weight/shape concerns).
Puberty:
Puberty is a critical developmental period marked by substantial biological changes (e.g., hormonal secretion; neural changes) and encompasses two separate, but overlapping, phases of maturation: adrenarche and gonadarche.13 In humans, adrenarche begins in middle childhood, with the awakening of the adrenal glands in the hypothalamic-pituitary-adrenal (HPA) axis, and corresponds to a rise in adrenal androgens (e.g., dehydroepiandosterone; dehydroepiandosterone-sulphate; androstenedione) in boys and girls.13 Gonadarche in humans and animals begins in pre-to-early adolescence, with the activation of the hypothalamic-pituitary-gonadal (HPG) axis and corresponds to a rise in gonadal steroid hormones, particularly testosterone (in males) and estradiol and progesterone (in females).13 Notably, a form of adrenarche may also occur in rodents and non-human primates, but it is not well understood, so pubertal research in animals has rested on HPG-driven changes representative of gonadarche.14,15 Moreover, within the broad literature, gonadarche has traditionally been referred to as “puberty” because most physical changes (e.g., secondary sex characteristics) become apparent during this phase.13 Herein, we distinguish between andrenarche and gonadarche, and use puberty when broadly referring to effects that encompass both phases.
Study Criteria
Although this is a narrative review, it is important to specify the search strategy we used. The following search terms were entered into the PubMed database: eating disorder OR disordered eating, eating pathology, eating, anorexia nervosa, bulimia nervosa, binge eating disorder, AND sex difference OR sex effect, sexually-dimorphic, sex distribution, gender difference, gender effect, gender distribution, female, male, girls/women, boys/men. Articles were also identified from references within published articles. Article abstracts were screened for relevance, and studies were included if the study design and findings addressed some biological aspect of sex differences in the etiology of pathological eating symptoms or related behavior (e.g., hedonic eating). Given the interest in identifying biological effects that could contribute to etiology, as opposed to biological alterations representative of disease sequalae, studies that focused exclusively on disturbances in pathophysiological effects (e.g., case-control comparisons of hormonal alterations) during the ill-state of an eating disorder were excluded from our review. We also refrained from reviewing some factors (e.g., appetite/gastrointestional hormones) that exert sex-differentiated effects on general eating behavior16 but have not yet been established as having an etiological role in sex-differentiated risk for pathological forms of eating.
Discussion
Sex Differences in Genetic Effects
Twin studies are a powerful methodological approach for broadly estimating genetic influences on eating pathology and for illuminating any sex-differentiated effects. By comparing trait/disorder similarity between identical (who share 100% of their genes) and fraternal (who share, on average, 50% of their genes) twins, it is possible to estimate the proportion of additive genetic effects (i.e., effects that add across genes) contributing to the variance in eating pathology; if additive genetic effects are present, then identical twin correlations are approximately double the fraternal twin correlations.17,18 The magnitude of genetic influences can then be compared across female and male twins to reveal sex differences. Additional identification of sex differences can be attained from model estimates of the male-female genetic correlation – an estimate of the extent to which the same genetic variants contribute to eating pathology in both sexes.19–21
To date, most twin studies have been conducted in females and relatively few have formally tested sex differences, but some notable patterns have emerged. Adolescent and adult studies generally point to sex similarities in the magnitude of genetic effects on eating pathology symptoms (e.g., binge eating, weight/shape concerns, composite scores), with heritability estimates typically ~40–70% in both sexes.19–25 However, sex differences have been found for the timing of the developmental emergence of genetic contributions to eating pathology symptoms, as well as the types of genetic factors contributing to heritability estimates within each sex. For example, genetic effects on eating pathology become prominent during puberty in both sexes18,25–27; however, the emergence occurs at an earlier maturational point in males (i.e., during adrenarche) than females (i.e., during gonadarche). In boys, genetic effects on overall levels of eating pathology symptoms are 0% in pre-adrenarche, 44% in late adrenarche, and 57% in early gonadarche and into young adulthood.18,28 In girls, genetic effects on eating pathology are 0% in pre-gonadarche and ~50% in mid-gonadarche and beyond.26,27,29–31 These developmental patterns indirectly suggest that at least some of the underlying genetic mechanisms (e.g., timing of activation of protective/risk genes) likely differ for males versus females.
Twin studies have also revealed differences in types of genetic factors influencing eating pathology between the sexes. Across studies, moderate male-female genetic correlations (rg ~.50–.70) have been found for eating pathology symptoms (e.g., weight/shape concerns, binge eating); thus, although some of the genetic factors that contribute to eating pathology are shared between the sexes, a proportion of genes are distinct.19–21 The presence of sex-specific genetic factors on eating pathology and related phenotypes (e.g., food intake, food preferences, weight maintenance functions) was also confirmed in a recent meta-analysis.32 This meta-analysis explored 50 phenotypic categories/clusters (comprised from 2,608 individual trait statistics) and found that only 25% of the tested phenotypes showed evidence for sex-specific genetic processes.32 Pathological eating is therefore among a relatively small proportion of conditions that have, to some extent, different genetic factors contributing to phenotypic expression in males versus females.
Although twin study findings cannot speak to the specific genes contributing to eating pathology, these data highlight the importance of considering sex in molecular genetic studies (e.g., Genome-Wide Association Studies and polygenic risk score approaches). Indeed, sex-specific considerations in genetic variants/expression and the role of genes/proteins in CNS activity may be necessary for fully elucidating the biological basis of eating pathology. In the subsequent sections, we review some of the specific biological factors, including sex-specific processes, that may contribute to sex differences in the etiology of eating pathology and may explain some of the sex-differentiated etiologic effects observed in twin research.
Sex Steroid Hormones: Between-Sex and Within-Sex Effects Impact Eating Pathology
Sex steroid concentrations vary between the sexes throughout the lifespan. In the prenatal/perinatal period, males naturally produce heightened levels of the primary androgen, testosterone, whereas sex steroid production at this time remains low in females.33 Around 6–8 years of age, adrenarche begins – the adrenal cortex starts secreting adrenal androgens in both sexes.13,34 These steroids continue to incrementally rise through late adolescence, and become higher in males than females around mid-gonadarche.34 During gonadarche, the testes secrete increasing levels of testosterone in boys, and the ovaries produce increasing levels of estradiol in girls; ovarian production of progesterone comes online in girls during late gonadarche (i.e., at first ovulation).13 Additionally, girls/women produce small amounts of testosterone in their ovaries and adrenal glands, and boys/men have detectable levels of estradiol, primarily from testosterone aromatization.35 Age-related reductions in hormone production occur in adulthood; by age 30, androgen levels incrementally decline in both sexes and women experience low estradiol and progesterone once menopause is reached.36
Differential exposure to sex steroid hormones may be important for understanding sex-based differences in risk for eating pathology. Indeed, sex steroids facilitate organizational (i.e., long-lasting, permanent changes) and activational (i.e., transient alterations) effects on the central nervous system (CNS) that are known to drive between-sex differences and within-sex variation in the development of several sex-differentiated phenotypes (e.g., aggression, food intake, body weight).16,33,37 Hormone-dependent organization begins during prenatal/perinatal development, ends with the refinement of the CNS during puberty, and alters CNS responsiveness to the activational effects of circulating hormones on behavioral outcomes during adolescence/adulthood.33,38,39 Notably, because steroid hormones exert some of their action via intracellular genomic signaling (e.g., activation/inhibition of target genes)33,37,39, they are one set of biological factors that could uniquely contribute to the observed developmental and qualitative sex-based differences in genetic effects on eating pathology described above. In the following sections, we review evidence of organizational and activational influences of sex steroid hormones on variability in risk for eating pathology across different life stages.
Androgens
Prenatal/Perinatal Development
The natural exposure to testosterone during prenatal/perinatal in males results in organizational effects on the body and CNS that drive the development of several male-typical physical, physiological, and behavioral phenotypes, including eating behavior.16,33,37 Conversely, the relative absence of prenatal/perinatal testosterone exposure in normal-developing females prevents their CNS from becoming masculinized and allows for more female-typical physical, physiological, and behavioral phenotypes to develop. The strongest evidence of perinatal testosterone’s organizational effects on eating behavior come from non-human animal data, where exposure to exogenous testosterone can be experimentally manipulated and behavioral outcomes can be monitored across development. These experimental animal studies test whether female rodents perinatally exposed to testosterone show more male-typical eating behavior.
Using this experimental approach, animal studies have shown that perinatal testosterone contributes to post-pubertal sex-differentiated patterns of eating behavior, including sweet-taste preferences40,41, palatable food intake (PF: high fat/high sweet food)7, and binge eating propensity (males < females for all measures).7 For example, female rats treated with exogenous testosterone during perinatal development showed rates of binge eating proneness that were on par with male controls and were significantly lower than rates of binge eating proneness in female controls; however, consistent with epidemiological data in humans,9 these effects did not emerge until gonadarche.7 No sex or treatment group differences in rates of binge eating proneness were detected in pre-gonadarche.7 Thus, the lower post-pubertal prevalence of binge eating in males, relative to females, at least partially arises from the fact that males are naturally exposed to testosterone during prenatal/perinatal development.
Establishing that these organizational effects extend to humans is challenging. Hormone exposure cannot be directly manipulated in humans. Medical conditions that involve elevated prenatal/perinatal androgen exposure (e.g., congenital adrenal hyperplasia) are rare and have been linked to a number of other outcomes (e.g., overweight/obesity, HPA-axis dysregulation, body/appearance-based concerns) that might affect eating disorder risk beyond the effects of early testosterone.10 Given these methodological challenges, researchers have primarily relied on two biomarkers that capture between-person variability in prenatal testosterone exposure and can be assessed in the general population. Specifically, the index finger (2D)/ring finger (4D) ratio is posited to be a biomarker of prenatal testosterone exposure since it is sexually-dimorphic very early in fetal development (2D:4D in males < females)42,43, it is altered by prenatal androgen and estrogen signaling/receptors44, and it correlates with prenatal testosterone levels acquired from amniocentesis.42 Opposite-sex twins have been used as an additional methodological approach since females from opposite-sex twin pairs are thought to be exposed to higher testosterone as a result of sharing their in utero environment with a male co-twin.2,45 Direct evidence of hormonal transfer in humans is lacking, but intrauterine position effects are well-documented in animals46, and females from opposite-sex twins pairs have been found to exhibit more male-like characteristics, including physical traits (e.g., auditory systems) that would not be impacted by other factors (e.g., socialization from being raised with a male sibling).47
Some mixed results have emerged across studies using these indirect methods.10 For example, while early work found lower levels of eating pathology in females with lower 2D:4D ratios48 and females from opposite-sex twin pairs49, results were not consistently replicated.50–52 However, differential developmental windows of expression have been posited as one possible explanation for between-study discrepancies.45 Indeed, studies varied in the age range of participants or have relied on lifetime histories of eating disorders which could muddle results, particularly if there are developmental effects at play. Notably, consistent with animal findings and developmental shifts in risk, females from opposite-sex twin pairs begin to show more male-typical (i.e., lower) levels of eating pathology, as compared to female controls (i.e., females from same-sex twin pairs and non-twin females reared with a close-in-age brother) after mid-gonadarche.2 Furthermore, while there is evidence of prenatal testosterone’s protective effects on eating pathology during puberty and young adulthood45,48,49, null results have been found in opposite-sex twin and/or digit ratio studies that assessed girls during late adolescence45,51; this pattern has led to the speculation that prenatal testosterone’s protective effects on eating pathology in girls might become attenuated during the peak period of eating disorder risk (i.e., late adolescence), presumably from other psychosocial and/or biological risk effects that may emerge or predominant during this phase of development.45 Additional research exploring moderators of prenatal/perinatal testosterone’s organizational effects on eating pathology across the lifespan are, however, needed to explicitly test this possibility.
Puberty and Young Adulthood
The natural testosterone exposure during prenatal/perinatal development in males appears to impact sex differences in eating pathology by reducing risk in males relative to females; however, between-person variation in levels of circulating sex steroids, during and after puberty, could exert sex-specific effects that impact individual variation in risk among males and females and further contribute to sex-based differentiation in risk. Our below discussion focuses on within-sex effects of androgens on eating pathology during puberty and adulthood.
Within-Sex Effects in Males:
If circulating androgens (e.g., testosterone) exert protective effects on eating pathology in males, as occurs prenatally/perinatally, then boys/men with lower androgen levels would be at greatest risk for pathological eating, compared to other males. Despite the rise in androgen secretion during adrenarche13,34 and an emergence of genetic effects on eating pathology in boys18, only one study has explored associations between androgens and eating pathology during this developmental phase.53 Negligible associations were found between dehydroepiandrosterone, dehydroepiandrosterone-sulfate, and body image in boys, and the small positive association between testosterone and body image was attenuated after adjusting for BMI.53 This lack of hormone-body image associations in boys during adrenarche seems counterintuitive given the linear emergence of genetic effects on eating pathology in boys across adrenarche and gonadarche (independent of BMI).18 However, this pattern could mean that circulating levels of androgens are predictive of other forms of eating pathology (e.g., binge eating) that have not yet been examined during adrenarche and/or that genetic effects on eating pathology, from adrenarche to early gonadarche, reflect the presence of organizational androgen processes.10,18,54,55 Specifically, if adrenarche is a period of androgen-induced organization, then androgen mediated genomic changes could permanently alter the CNS during adrenarche and subsequently lead to differential risk for eating pathology during gonadarche and adulthood; the CNS would be expected to become responsive to the activational effects of circulating androgens on eating pathology, only after organization has occurred (e.g., when the magnitude of the heritability estimate plateaus: mid-to-late gonadarche and beyond).
Consistent with this pubertal organizational-activational framework, phenotypic associations between testosterone and eating pathology (e.g., overall scores and distinct symptoms, like binge eating) have been observed during gonadarche and adulthood, even after adjusting for BMI, depressive symptoms, and age.56,57 Specifically, lower levels of circulating testosterone predicted higher levels of pathological eating symptoms in boys, but this effect only became evident with advancing gonadarcheal maturation.57 Prior to mid-gonadarche, there was no association between testosterone and eating pathology in boys.57 Importantly, the significant, inverse associations between circulating testosterone and eating pathology in males continues into adulthood, as men with lower levels of circulating testosterone reported significantly higher levels of dysregulated eating symptoms (e.g., binge eating, loss-of-control eating, eating concerns).56 Moreover, gonadectomy of adult male rodents, which removes circulating testosterone, results in subsequent changes in their eating behavior, including increases in sucrose consumption.41 Taken together, these initial data highlight the pivotal role of testosterone in male eating pathology and the importance of considering maturational status when exploring sex steroid effects on eating pathology in boys.
Within-sex Effects in Females:
Studies have also explored links between androgens and eating pathology in females. Like boys, negligible phenotypic associations were detected between androgens (e.g., dehydroepiandrosterone, dehydroepiandrosterone-sulfate, testosterone) and body image in girls during adrenarche (after accounting for BMI).53 In adulthood, the direction of testosterone effects on eating pathology in women appear to be opposite to findings in boys/men – higher, as opposed to lower, levels may contribute to risk. For example, women with polycystic ovarian syndrome (PCOS), i.e., a condition associated with elevated testosterone, have increased rates of binge eating syndromes (i.e. bulimia nervosa, binge eating disorder) relative to controls.58 Disturbances in appetite regulation (e.g., increased food cravings, blunted satiety), which could promote binge eating symptomatology, have also been found in women with PCOS and have been linked to hormonal impairments in testosterone.59
Pharmacological manipulation of androgens in women with BN provides further evidence of positive testosterone-pathological eating associations. Following administration of an antiandrogenic oral contraceptive, women with BN showed significant reductions in circulating testosterone levels, and this hormonal change was related to decreases in appetite (e.g., hunger) and binge-purge symptoms, particularly in women who had hyperandrogenic levels and greater severity of bulimic symptoms at baseline.60 Similarly, reduction in binge-purge symptoms have also been found in patients with BN following treatment with a testosterone blocker (i.e., the androgen receptor antagonist, flutamide)61,62, whereas relapse of symptoms occurred when the drug was withdrawn.61 Together, findings suggest that high testosterone may enhance risk for pathological eating in adult women, and systematic reductions in testosterone may be effective at reducing vulnerability to binge-purge behavior in some women with BN.
Summary
Taken together, testosterone exposure during prenatal/perinatal development provides protective effects that reduce risk for eating pathology in males, relative to females, and the activation of testosterone during puberty and in adulthood may facilitate additional protective effects in males. Little is known about the potential role of other androgens (e.g., dehydroepiandrosterone; androstenedione) in either sex; however, it is notable that testosterone may have differential activational effects in males versus females. Lower testosterone is predictive of more eating pathology in males, whereas in adult females, higher testosterone might be related to increased eating pathology risk. These differential effects may seem inconsistent, but testosterone also exerts opposite effects in men versus women for other health outcomes (e.g., Type 2 diabetes; body composition).63 Relatedly, experimental animal data have shown that the stimulatory effects of testosterone on appetite and preference for a high-fat diet in female rodents is dependent upon an estrogenic milieu.64–66 Chronic administration of testosterone increased appetite, body weight, and preference for a high-fat diet in female rats that were either gonadally-intact or ovariectomized (OVX) combined with estradiol-treatment, whereas reductions in eating and body weight occurred in OVX female rats without supplemental estradiol.64–66 Consequently, sex-differentiated actions of testosterone may vary as a function of sex-typical hormonal milieus (e.g., presence/absence of estradiol).
Ovarian Hormones
In contrast to the prenatal/perinatal organization (e.g., masculinizing and defeminizing effects) from testosterone, ovarian hormones are not elevated in prenatal/perinatal development and do not play a major role at this stage of life.54 Thus, our review of ovarian hormones focuses on their organizational and activational actions on eating pathology during puberty, young adulthood, and midlife (perimenopause/menopause), and we only cover within-sex effects in females given a general absence of male data. These within-sex ovarian hormone effects could further inform understanding of sex-based differences in the biological basis of eating pathology.
Puberty and Young Adulthood
Most research on ovarian hormones and eating pathology has focused on the pubertal or young adulthood periods of development. Given that genetic contributions to eating pathology in girls emerge during gonadarche (independent of BMI)18,26–30 and the fact that steroid hormones can regulate gene transcription, recent research has explored whether pubertal ovarian hormones moderate genetic effects on eating pathology. This work has focused on estradiol since the developmental emergence of genetic influences on eating pathology occur earlier (e.g., mid-gonadarche) than the timing of progesterone activation (i.e., late gonadarche; first ovulation).26,27,29 Furthermore, because estradiol is associated with decreased food intake and binge eating in adulthood (a point we return to below)9,16, it has been hypothesized that lower activation of estradiol in girls during gonadarche may drive increases in heritability of binge eating.67 Indeed, stronger genetic effects on binge eating were found in girls with lower (heritability ~70%), as opposed to higher (heritability ~2%), levels of estradiol, even after accounting for age, BMI, and stage of pubertal development; progesterone did not exert contributory influences.67 It has been posited that these effects may reflect pubertal organizational effects on the CNS9,10,67, and data support this possibility. First, like testosterone effects in boys, phenotypic associations between ovarian hormones and eating pathology become evident in females only after gonadarcheal shifts in genetic effects have occurred; estradiol’s activational effects on eating pathology are not detected until late adolescence/adulthood (see below for more details). Second, pre-pubertal OVX in female rats increases the proportion of binge eating prone phenotypes during adolescence and adulthood68, whereas adult OVX increases PF consumption in all female rats but the relative proportion classified as binge eating prone versus binge eating resistant is not changed.69 This maintenance of classification status (binge eating prone vs binge eating resistant) even after adult OVX suggests that individual differences in binge-proneness might be permanently organized earlier in development.68,69
In regards to activational effects of ovarian hormones, early work was critical in demonstrating that estradiol exerts direct and anorexic effects on general eating behavior, whereas progesterone’s stimulatory effects largely occur indirectly via its antagonism of estradiol.16 More recent research has demonstrated that ovarian hormones also influence pathological eating symptoms. Moreover, while the propensity for binge eating appears to be organized during gonadarche (see above), OVX in adult female rats and mice leads to immediate increases in PF consumption whereas estradiol treatment reverses the effect.69–72 Pathological eating also varies with natural fluctuations in ovarian hormones across the estrous (in rats) and menstrual (in women) cycle. Intact female rats show higher binge-like eating when estradiol levels low (i.e., diestrous) and lower binge-like eating when estradiol levels are high (i.e., estrous).72 Similarly, women show higher binge eating and emotional eating in post-ovulatory phases (midluteal and premenstrual) when estradiol action is low1 and lower binge/emotional eating in pre-ovulatory phases (follicular/ovulatory) when estradiol action is high, independent of BMI or negative affect.73–77 Furthermore, data indicate that ovarian hormones exert direct effects on dysregulated eating symptoms (e.g., emotional eating, binge eating)73–76, whereas cycle/hormone influences on body-related concerns (e.g, body dissatisfaction, weight preoccupation) have been less robust78–82 and occur indirectly, e.g., secondary to emotional/binge eating or mediated by other cycle-based changes (e.g., water retention; overestimation of body size).79,80,82Together, these data highlight ovarian hormones as sex-specific biological factors that can enhance eating pathology, particularly emotional/binge eating, in women.
Nonetheless, not all females are impacted by ovarian hormones to the same extent; studies have therefore begun to consider factors that may influence variability and/or could interact with ovarian hormones to enhance or attenuate eating pathology risk. Despite the detection of some null moderation effects (e.g., BMI, affective/personality, magnitude of hormonal shift)83–86, significant moderators have also been identified.72,84,87–89 Most notably, differential levels of ovarian hormones alter within-person changes and between-person differences in genetic vulnerability to symptom expression; the heritability of emotional eating was two times higher in post-ovulatory versus pre-ovulatory phases87 and was stronger in women with higher progesterone levels (61%) than women with lower progesterone levels (37%).88 Additionally, animal and human data suggest that negative affect and dietary restraint/restriction may be key moderators: emotional eating was highest in women with high trait negative affect in combination with a hormonal milieu marked by low estradiol or high progesterone84, estradiol’s effects on emotional eating were somewhat enhanced at higher levels of dietary restraint83, and adult OVX female rats exposed to food restriction and stress (a form of negative affect) showed the highest binge-like behavior whereas treatment with ovarian hormones reversed the effects.72 Thus, consideration of moderating factors is likely important for fully illuminating hormonal effects on eating pathology.
Perimenopause/Menopause
Although most studies have focused on earlier developmental periods, researchers have begun to explore the perimenopause-menopause transition since this stage of life corresponds to dramatic hormonal shifts and weight gain, which may heighten risk for eating pathology.90,91 Moreover, during perimenopause, progesterone levels drop (and remain low in the absence of ovulation) whereas estradiol can show substantial variation (e.g., erratic high-low fluctuations).91 Consistent with the notion of enhanced eating pathology risk (for exceptions, see92–94), eating disorder prevalence and body shape/weight concerns are higher in perimenopausal as compared to premenopausal women95 and eating pathology symptoms (e.g., dietary restraint, disinhibited eating) are higher in postmenopausal versus premenopausal women.96,97 Additionally, using daily assessments, significant associations have been found between ovarian hormones and eating pathology in perimenopausal women; estradiol was predictive of binge eating at high, but not low, progesterone levels, whereas body dissatisfaction was highest when both estradiol and progesterone were low.98 These data provide initial evidence that the perimenopause-menopause transition may enhance risk for eating pathology symptoms in women, at least partially due to changes in ovarian hormone secretion.
Summary:
Data suggest that estradiol may exert organizational effects on binge eating during gonadarche; lower levels enhance genetic influences and may lead to increased risk for eating pathology after puberty. Both estradiol and progesterone play a role in late adolescence and adulthood, and their activational and interactive effects (especially hormonal milieus that result in low estradiol action) further facilitate risk for eating pathology in women. Ovarian hormones are therefore another set of biological factors that likely contribute to a sex-differentiated trajectory of risk and prevalence of eating pathology.
Neural Differences to Food-Related Stimuli
There is some indication that sex differences in susceptibility to body weight/shape concerns may be linked to differences in neural processing of body image stimuli99; however, most sex-based brain research has explored sex-differentiated neural responses to more basic eating/feeding behaviors. Research on sex-based differences in neural food-cue responsivity has, however, given very little consideration to developmental effects and has largely been conducted using animals or humans from the general population, as opposed to individuals identified as having pathological eating behavior. Nonetheless, these data likely have relevance to sex-differentiated risk for eating pathology since core symptoms (e.g., binge eating) occur on a dimensional spectrum12 and neural disruptions in food-cue and appetite-related processes have been found in eating disorder samples.100,101
Non-human animal data indicate that female rodents have a greater propensity to overconsume PF, independent of their physiological state (hunger vs. satiated)102,103, and may experience PF as inherently more rewarding than males.103,104 For example, female rats worked harder to obtain PF than males and were also more sensitive to the stimulatory/palatability effects of an opioid agonist (morphine).103 Compared to males, female rats have also been found to show stronger motivation for PF, and show greater neural activation of mesocoticolimibic reward circuit regions (e.g., nucleus accumbens, medial prefrontal cortex) following PF consumption.104 Although some sex similarities in neural activation in response to PF do occur (e.g., increased activation in hypothalamic and amygdala regions; orexin/hypocretin neurons in hypothalamus)102,104, sex-differentiated action in key neural substrates (e.g., reward regions) likely underlie sex differences in hedonic and motivational responses to PF.
Sex differences in neural responsivity to food cues have also been found in humans. The two studies conducted in children/pre-adolescents found some indication that sex may interact with weight status to enhance neural reactivity to visual cues of PF (e.g., higher weight girls > boys in parahippocampal gyrus and fusiform gyrus).105,106 Given sex-differentiated pubertal changes in body composition, namely increases in adiposity in girls vs lean muscle in boys,107 weight status may have inadvertently served as a proxy for pubertal maturation and findings might reflect interactions between sex and pubertal status; however, additional research is needed to directly test this possibility. Data in adults have been fairly consistent in showing that, following exposure to visual and taste PF stimuli, women tend to have greater activation than men in brain regions involved in visual processing (e.g., fusiform gyrus, occipital cortex108–111), taste and interoceptive processing (e.g., insula110–112), executive functioning and inhibitory control (e.g., prefrontal cortex108,110,112,113), and reward (e.g., caudate110) during fasted109–111,113 and/or satiated110–112 states. Furthermore, when men were directed to suppress their desire for food (i.e., a form of cognitive inhibition) while exposed to food stimuli, they had decreased activation in brain regions involved in emotional regulation, decision making, interoceptive processing, and reward and motivation (e.g., amygdala, insula, orbitofrontal cortex, striatum), whereas women were unable to suppress brain activation using directive cognitive inhibition.114 Thus, in addition to heightened brain responsivity to PF, women may have a decreased ability to suppress cue-induced food cravings at a neural level.114 This combination could contribute to sex differences in vulnerability to nonhomeostatic eating behaviors (e.g., overeating, binge eating).
Interestingly, the detected sex-differentiated effects may be driven, in part, by estradiol modulation of neural responsivity to food cues. For example, heightened binge eating behavior in OVX adult female rats was linked to increased neuronal activity in brain regions involved in affective processing and eating (e.g., amygdala, periventricular nucleus of the hypothalamus, bed nucleus of stria terminalis); however, estradiol treatment prevented these behavioral and neural effects.72 Additionally, reduced binge eating in OVX adult female mice was mediated by estrogen stimulation (via the estrogen receptor-α) in serotonin neurons of the dorsal raphe nuclei.70 Neural reactivity to food cues has also been shown to vary across the menstrual cycle in women; women had greater neural reactivity to images of PF in brain regions associated with craving/reward (e.g., orbitofrontal cortex) during the luteal phase of the cycle (when risk of emotional/binge eating is highest), as compared to follicular phase.115 Taken together, estradiol’s actions on neuronal response to food cues may contribute to heightened risk for pathological eating in vulnerable women and contribute to sex differences overall.
Conclusions
Animal and human data provide evidence of biological contributions to sex-based differences in risk for eating pathology. A general summary of effects is displayed in Figure 1. Twin data have revealed sex differences in the timing of the pubertal emergence of genetic effects on eating pathology (adrenarche in males; gonadarche in females) and have highlighted that some genetic variants contributing to eating pathology differ between the sexes. Given that sex steroids vary between the sexes and across the lifespan and act as key regulators of gene transcription, biological research exploring sex and developmental differences in vulnerability to eating pathology have heavily focused on these hormones.
Figure 1. Tentative Model of Developmental and Sex-Differentiated Sex Steroid and Genetic Effects on Eating Pathology Risk.
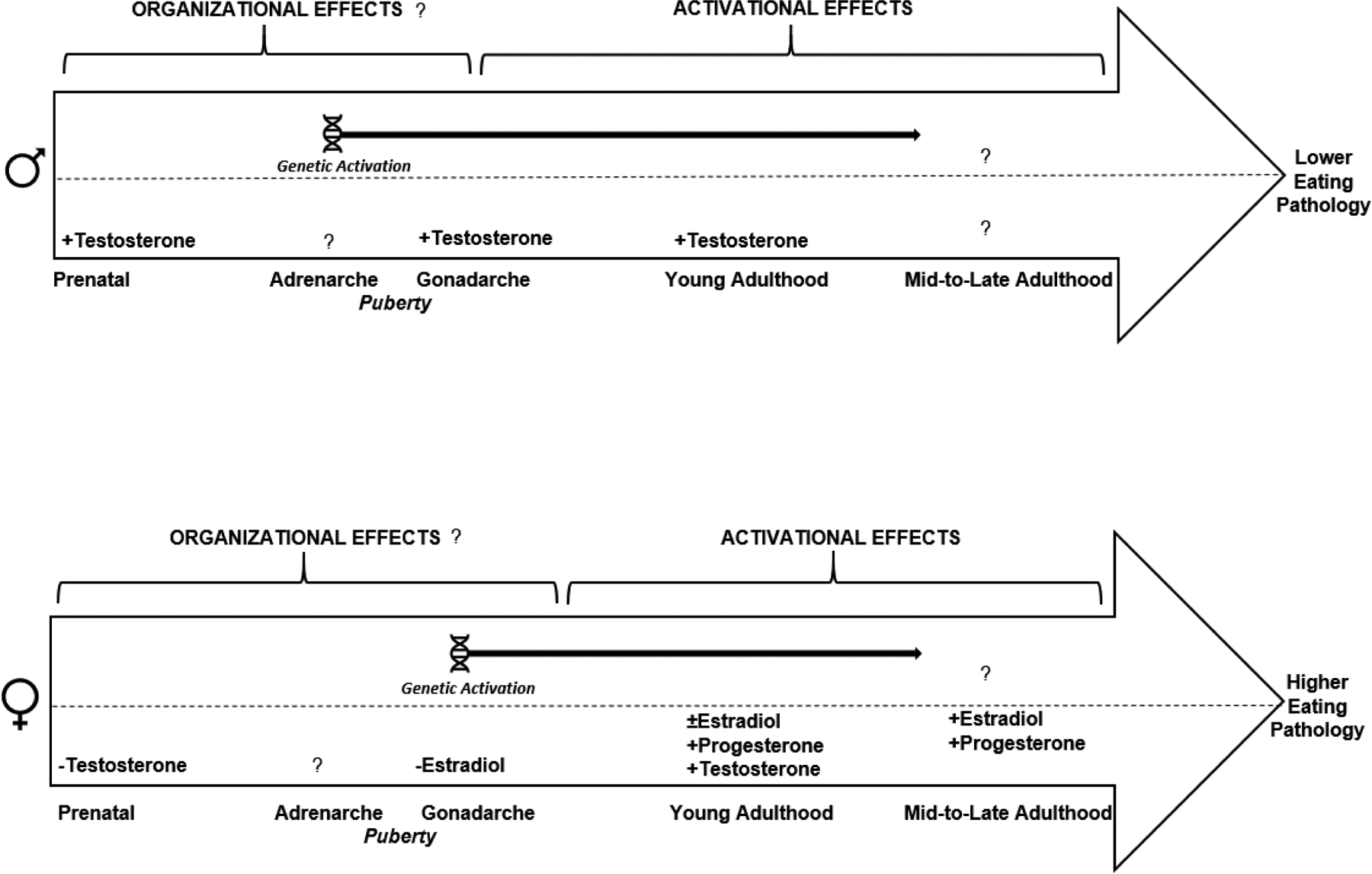
This figure synthesizes key findings from animal and human research exploring sex steroid and genetic effects on eating pathology in males (displayed at top) and females (displayed at bottom) across the lifespan. Genetic activation is reflected at the top half of each figure and refers to the developmental emergence of genetic influences (via heritabilty estimates) on eating pathology in boys, i.e., during adrenarche, or girls, i.e., during gonadarche. The specific sex steroids that have been implicated in eating pathology, within each sex, are indicated on the bottom half of each figure, and the corresponding sign (+ or −) reflects the direction of the hormonal influence on the outcome (i.e., lower eating pathology in males versus higher eating pathology in females). Question marks denote that the effect has not yet been studied or definitively established. Notably, organizational effects (and the corresponding critical window of sensitivity) are generaly more challenging to establish than the presence of activational effects; hence, our inclusion of question marks on organizational but not activational headings.
Lower risk for eating pathology among males appears to arise from an initial bout of testosterone exposure during prenatal/perinatal development and subsequent exposure to moderate-to-high levels of circulating testosterone after pubertal onset and in young adulthood. Thus, higher levels of circulating testosterone during/after gonadarche appear to be protective against pathological eating behavior in boys and men, whereas lower testosterone may increase risk; whether other androgens (e.g., androstadiene, dehydroepiandrosterone) also play a role, at the genetic or phenotypic level, remains to be determined. Studies have also not yet explored whether natural reductions in androgens during mid-to-late life influence differential risk for eating pathology among men.
In females, the lack of early testosterone exposure in combination with lower estradiol during gonadarche and in young adulthood, increases genetic and phenotypic risk for eating pathology. Progesterone then has stimulatory effects in adulthood only, largely via its antagonizing effects on estradiol. Data also suggest that higher (rather than lower) levels of circulating testosterone may enhance risk for eating pathology in young adult women; thus, the direction of testosterone’s activational effects on eating pathology in women appear to be opposite to males. Additionally, perimenopausal shifts in estradiol and progesterone secretion have been associated with eating pathology and may enhance risk in women during midlife. Animal and human data also point to notable sex differences in neural responsivity to highly palatable food cues that may contribute to sex-differentiated risk for eating pathology. While no studies have explored if/how development (e.g., puberty) may impact these neural processes, emerging data in females indicates that brain activity is modulated, in part, via estradiol. Consequently, between-sex and within-sex differences in risk for eating pathology seem to arise from a combination of, and interplay between, genetic, hormonal, and brain-driven processes.
Despite the promising findings, gaps exist in the literature. There has been a paucity of research exploring biological risk for eating pathology in males. The male-specific literature is largely comprised of single study reports (in contrast to the replicability observed in several female studies), and data in males have generally lagged behind work conducted in females. Furthermore, it is expected that sociocultural and psychological factors intersect with biological factors to impact individual and sex differences in risk for eating pathology. Although these effects (e.g., moderators of ovarian hormone effects) have been explored in several female studies, researchers have yet to explore sociocultural/psychological moderators of biological risk factors for eating pathology in boys/men. Thus, despite a growing literature, much more research is needed to further elucidate how biological mechanisms contribute to differential risk for eating pathology in males and females across the lifespan.
Highlights.
Some genetic effects on eating pathology vary between the sexes
Sex steroids impact sex-specific genetic and phenotypic effects on eating pathology
Testosterone exerts protective effects that reduce eating pathology in males
Lower estradiol enhances genetic and phenotypic eating pathology risk in girls/women
Neural responses to palatable food differ between the sexes, possibly via estradiol
Disclosure of Funding Support
Funding: This work was supported by the National Institute of Mental Health (NIMH) Grant R01 MH 117940-01 awarded to K.M.C., C.L.S., and K.L.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None.
During the mid-luteal phase, it is likely that high levels of progesterone serve to antagonize the typical protective action of elevated estradiol, and in the premenstrual phase, low progesterone likely permits the behavioral effects of low estradiol to be expressed. Consequently, in both postovulatory phases, low estradiol action may drive heightened binge/emotional eating. Conversely, in preovulatory phases of the cycle, estradiol levels increase while progesterone remains low – a hormonal milieu that would permit estradiol’s protective effects on binge/emotional eating to be expressed.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5). American Psychiatric Association; 2013. [Google Scholar]
- 2.Culbert KM, Breedlove SM, Sisk CL, Burt SA, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: a role for prenatal testosterone exposure. J Abnorm Psychol. 2013;122(2):420–432. doi: 10.1037/a0031791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klump KL, Racine S, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. Int J Eat Disord. 2013;46(7):729–736. doi: 10.1002/eat.22139 [DOI] [PubMed] [Google Scholar]
- 4.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udo T, Grilo CM. Prevalence and Correlates of DSM-5–Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol Psychiatry. 2018;84(5):345–354. doi: 10.1016/j.biopsych.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge Eating Proneness Emerges during Puberty in Female Rats: A Longitudinal Study. J Abnorm Psychol. 2011;120(4):948–955. doi: 10.1037/a0023600.Binge [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culbert KM, Sinclair EB, Hildebrandt BA, Klump KL, Sisk CL. Perinatal testosterone contributes to mid-to-post pubertal sex differences in risk for binge eating in male and female rats. J Abnorm Psychol. 2018;127(2):239–250. doi: 10.1037/abn0000334 [DOI] [PubMed] [Google Scholar]
- 8.Klenotich SJ, Dulawa SC. The activity-based anorexia mouse model In: Methods in Molecular Biology. Vol 829 Humana Press; 2012:377–393. doi: 10.1007/978-1-61779-458-2_25 [DOI] [PubMed] [Google Scholar]
- 9.Klump KL, Culbert KM, Sisk CL. Sex Differences in Binge Eating: Gonadal Hormone Effects Across Development. Annu Rev Clin Psychol. 2017;13(1):183–207. doi: 10.1146/annurev-clinpsy-032816-045309 [DOI] [PubMed] [Google Scholar]
- 10.Culbert KM, Sisk CL, Klump KL. Sex steroid hormones and differential risk for eating pathology: a review of genetic and phenotypic effects across development. Curr Opin Behav Sci. 2018;23:124–130. doi: 10.1016/j.cobeha.2018.06.005 [DOI] [Google Scholar]
- 11.Ristori J, Cocchetti C, Romani A, et al. Brain sex differences related to gender identity development: Genes or hormones? Int J Mol Sci. 2020;21(6). doi: 10.3390/ijms21062123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X, Donnellan MB, Burt SA, Klump KL. The dimensional nature of eating pathology: Evidence from a direct comparison of categorical, dimensional, and hybrid models. J Abnorm Psychol. 2016;125(5):715–726. doi: 10.1037/abn0000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the Boundaries of Early Adolescence: A User’s Guide to Assessing Pubertal Status and Pubertal Timing in Research With Adolescents. Appl Dev Sci. 2006;10(1):30–56. doi: 10.1207/s1532480xads1001_3 [DOI] [Google Scholar]
- 14.Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. Adrenarche in the rat. J Endocrinol. 2006;191(1):301–308. doi: 10.1677/joe.1.06972 [DOI] [PubMed] [Google Scholar]
- 15.Conley AJ, Bernstein RM, Nguyen AD. Adrenarche in nonhuman primates: The evidence for it and the need to redefine it. J Endocrinol. 2012;214(2):121–131. doi: 10.1530/JOE-11-0467 [DOI] [PubMed] [Google Scholar]
- 16.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc B Biol Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol Med. 2010;40(10):1745–1753. doi: 10.1017/S0033291709992236.Preliminary [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culbert KM, Burt SA, Klump KL. Expanding the developmental boundaries of etiologic effects: The role of adrenarche in genetic influences on disordered eating in males. J Abnorm Psychol. 2017;126(5):593–606. doi: 10.1037/abn0000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, Kendler KS. Genetic Risk Factors for Disordered Eating in Adolescent Males and Females. J Abnorm Psychol. 2009;118(3):576–586. doi: 10.1037/a0016314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichborn-Kjennerud T, Bulik CM, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: A population-based twin study. Int J Eat Disord. 2004;36(3):307–314. doi: 10.1002/eat.20047 [DOI] [PubMed] [Google Scholar]
- 21.Reichborn-Kjennerud T, Bulik CM, Kendler KS, et al. Gender differences in binge-eating: A population-based twin study. Acta Psychiatr Scand. 2003;108(3):196–202. doi: 10.1034/j.1600-0447.2003.00106.x [DOI] [PubMed] [Google Scholar]
- 22.Root TL, Ph D, Thornton L, et al. NIH Public Access. 2011;11(2):92–98. doi: 10.1016/j.eatbeh.2009.10.004.Shared [DOI] [Google Scholar]
- 23.Keski-Rahkonen A, Neale BM, Bulik CM, et al. Intentional weight loss in young adults: Sex-specific genetic and environmental effects. Obes Res. 2005;13(4):745–753. doi: 10.1038/oby.2005.84 [DOI] [PubMed] [Google Scholar]
- 24.Keski-Rahkonen A, Bulik CM, Neale BM, Rose RJ, Rissanen A, Kaprio J. Body dissatisfaction and drive for thinness in young adult twins. Int J Eat Disord. 2005;37(3):188–199. doi: 10.1002/eat.20138 [DOI] [PubMed] [Google Scholar]
- 25.Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: Evidence for a sex difference. Psychol Med. 2012;42(3):627–637. doi: 10.1017/S0033291711001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klump KL, Culbert KM, O’Connor S, Fowler N, Burt SA. The significant effects of puberty on the genetic diathesis of binge eating in girls. Int J Eat Disord. 2017;50(8):984–989. doi: 10.1002/eat.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychol Med. 2007;37(05):627. doi: 10.1017/S0033291707000189 [DOI] [PubMed] [Google Scholar]
- 28.Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: evidence for a sex differences. Psychol Med. 2012;42(3):627–637. doi: 10.1017/S0033291711001541.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. J Abnorm Psychol. 2009;118(4):788–796. doi: 10.1037/a0017207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. Int J Eat Disord. 2003;33:287–292. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor SM, Culbert KM, Mayhall LA, Burt SA, Klump KL. Differences in genetic and environmental influences on body weight and shape concerns across pubertal development in females. J Psychiatr Res. 2020;121(June 2019):39–46. doi: 10.1016/j.jpsychires.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringer S, Polderman T, Posthuma D. Majority of human traits do not show evidence for sex-specific genetic and environmental effects. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-09249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55(5):570–578. doi: 10.1016/j.yhbeh.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehman KS, Carr BR. Sex differences in adrenal androgens. Semin Reprod Med. 2004;22(4):349–360. doi: 10.1055/s-2004-861551 [DOI] [PubMed] [Google Scholar]
- 35.Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest. 2019;129(5):1818–1826. doi: 10.1172/JCI125755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGavack TH. Hormones and the aging process. J Am Geriatr Soc. 2003;51:S333–S337. [DOI] [PubMed] [Google Scholar]
- 37.Asarian L, Geary N. Sex differences in the physiology of eating. AJP Regul Integr Comp Physiol. 2013;305(11):R1215–R1267. doi: 10.1152/ajpregu.00446.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz KM, Sisk CL. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev. 2016;70:148–158. doi: 10.1016/j.neubiorev.2016.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraga-Amaro R, van Waarde A, Doorduin J, de Vries EFJ. Sex steroid hormones and brain function: PET imaging as a tool for research. J Neuroendocrinol. 2018;30(2):1–12. doi: 10.1111/jne.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wade GN, Zucker I. Taste preferences of female rats: modification by neonatal hormones, food deprivation and prior experience. Physiol Behav. 1969;4(6):935–943. doi: 10.1016/0031-9384(69)90044-4 [DOI] [Google Scholar]
- 41.Zucker I Hormonal determinants of sex differences in saccharin preference, food intake and body weight. Physiol Behav. 1969;4(4):595–602. doi: 10.1016/0031-9384(69)90160-7 [DOI] [Google Scholar]
- 42.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd To 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77(1–2):23–28. doi: 10.1016/j.earlhumdev.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 43.Malas MA, Dogan S, Hilal Evcil E, Desdicioglu K. Fetal development of the hand, digits and digit ratio (2D : 4D). Early Hum Dev. 2006;82(7):469–475. doi: 10.1016/j.earlhumdev.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 44.Zheng U, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci U S A. 2011;108(39):16289–16294. doi: 10.1073/pnas.1108312108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Culbert KM, Breedlove SM, Sisk CL, et al. Age differences in prenatal testosterone’s protective effects on disordered eating symptoms: Developmental windows of expression? Behav Neurosci. 2015;129(1):18–36. doi: 10.1037/bne0000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26(6):665–678. doi: 10.1016/S0149-7634(02)00038-6 [DOI] [PubMed] [Google Scholar]
- 47.McFadden D Masculinization effects in the auditory system. Arch Sex Behav. 2002;31(1):99–111. doi: 10.1023/A:1014087319682 [DOI] [PubMed] [Google Scholar]
- 48.Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med. 2006;36(4):539–546. doi: 10.1017/S0033291705006653 [DOI] [PubMed] [Google Scholar]
- 49.Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry. 2008;65(3):329–336. doi: 10.1001/archgenpsychiatry.2007.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raevuori A, Kaprio J, Hoek HW, Sihvola E, Rissanen A, Keski-Rahkonen A. Anorexia and bulimia nervosa in same-sex and opposite-sex twins: Lack of association with twin type in a nationwide study of Finnish twins. Am J Psychiatry. 2008;165(12):1604–1610. doi: 10.1176/appi.ajp.2008.08030362 [DOI] [PubMed] [Google Scholar]
- 51.Baker JH, Lichtenstein P, Kendler KS. Intrauterine testosterone exposure and risk for disordered eating. Br J Psychiatry. 2009;194(4):375–376. doi: 10.1192/bjp.bp.108.054692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lydecker JA, Pisetsky EM, Mitchell KS, et al. Association between co-twin sex and eating disorders in opposite sex twin pairs: Evaluations in North American, Norwegian, and Swedish samples. J Psychosom Res. 2012;72(1):73–77. doi: 10.1016/j.jpsychores.2011.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes EK, Mundy LK, Romaniuk H, et al. Body Image Dissatisfaction and the Adrenarchal Transition. J Adolesc Heal. 2018;63(5):621–627. doi: 10.1016/j.jadohealth.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 54.Sisk CL. Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Curr Opin Neurobiol. 2016;38:63–68. doi: 10.1016/j.conb.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev Cogn Neurosci. 2017;25:12–28. doi: 10.1016/j.dcn.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Culbert KM, Shope MM, Sisk CL, Klump KL. Low testosterone is associated with dysregulated eating symptoms in young adult men. Int J Eat Disord. 2020;53(9):1469–1479. doi: 10.1002/eat.23320 [DOI] [PubMed] [Google Scholar]
- 57.Culbert KM, Burt SA, Sisk CL, Nigg JT, Klump KL. The effects of circulating testosterone and pubertal maturation on risk for disordered eating symptoms in adolescent males. Psychol Med. 2014;44(11):2271–2286. doi: 10.1017/S0033291713003073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thannickal A, Brutocao C, Alsawas M, et al. Eating, sleeping and sexual function disorders in women with polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2020;92(4):338–349. doi: 10.1111/cen.14153 [DOI] [PubMed] [Google Scholar]
- 59.Hirschberg AL, Naessén S, Stridsberg M, Bystrom B, Holte J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol Endocrinol. 2004;19(2):79–87. doi: 10.1080/09513590400002300 [DOI] [PubMed] [Google Scholar]
- 60.Naessén S, Carlström K, Byström B, Pierre Y, Lindén Hirschberg A. Effects of an antiandrogenic oral contraceptive on appetite and eating behavior in bulimic women. Psychoneuroendocrinology. 2007;32(5):548–554. doi: 10.1016/j.psyneuen.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 61.Bergman L, Eriksson E. Marked symptom reduction in two women with bulimia nervosa treated with the testosterone receptor antagonist flutamide. Acta Psychiatr Scand. 1996;94:137–139. [DOI] [PubMed] [Google Scholar]
- 62.Sundblad C, Landén M, Eriksson T, Bergman L, Eriksson E. Effects of the androgen antagonist flutamide and the serotonin reuptake inhibitor citalopram in bulimia nervosa: A placebo-controlled pilot study. J Clin Psychopharmacol. 2005;25(1):85–88. doi: 10.1097/01.jcp.0000150222.31007.a9 [DOI] [PubMed] [Google Scholar]
- 63.Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. doi: 10.1038/s41591-020-0751-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasa T, Matsuzaki T, Tungalagsuvd A, et al. Effects of chronic testosterone administration on body weight and food intake differ among pre-pubertal, gonadal-intact, and ovariectomized female rats. Behav Brain Res. 2016;309:35–43. doi: 10.1016/j.bbr.2016.04.048 [DOI] [PubMed] [Google Scholar]
- 65.Iwasa T, Matsuzaki T, Yano K, et al. The effects of chronic testosterone administration on body weight, food intake, and adipose tissue are changed by estrogen treatment in female rats. Horm Behav. 2017;93:53–61. doi: 10.1016/j.yhbeh.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 66.Iwasa T, Matsuzaki T, Yano K, et al. Effects of chronic testosterone administration on the degree of preference for a high-fat diet and body weight in gonadal-intact and ovariectomized female rats. Behav Brain Res. 2018;349(February):102–108. doi: 10.1016/j.bbr.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 67.Klump KL, Fowler N, Mayhall L, Sisk CL, Culbert KM, Burt SA. Estrogen moderates genetic influences on binge eating during puberty: Disruption of normative processes? J Abnorm Psychol. 2018;127(5):458–470. doi: 10.1037/abn0000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klump KL, Sinclair EB, Hildebrandt BA, et al. The Disruptive Effects of Estrogen Removal Before Puberty on Risk for Binge Eating in Female Rats. Clin Psychol Sci. 2020;8(5):839–856. doi: 10.1177/2167702620921343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Horm Behav. 2011;59(4):585–593. doi: 10.1016/j.yhbeh.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao X, Xu P, Oyola MG, et al. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J Clin Invest. 2014;124(10):4351–4362. doi: 10.1172/JCI74726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Z, Geary N, Corwin RL. Individual Effects of Estradiol and Progesterone on Food Intake and Body Weight in Ovariectomized Binge Rats. Physiol Behav. 2011;104(5):687–693. doi: 10.1002/nbm.3066.Non-invasive [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Micioni Di Bonaventura MV, Lutz TA, Romano A, et al. Estrogenic suppression of binge-like eating elicited by cyclic food restriction and frustrative-nonreward stress in female rats. Int J Eat Disord. 2017;50(6):624–635. doi: 10.1002/eat.22687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2006;37:131–141. doi: 10.1017/S0033291706008956 [DOI] [PubMed] [Google Scholar]
- 74.Klump KL, Keel PK, Racine SE, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122(1):131–137. doi: 10.1037/a0029524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. 2008;38(12):1749–1757. doi: 10.1002/nbm.3066.Non-invasive [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klump KL, Racine SE, Hildebrandt B, et al. Influences of Ovarian Hormones on Dysregulated Eating: A Comparison of Associations in Women With Versus Women Without Binge Episodes. Clin Psychol Sci. 2014;2(5):545–559. doi: 10.1177/2167702614521794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry. 1987;144(12):1592–1595. [DOI] [PubMed] [Google Scholar]
- 78.Jappe LM, Gardner RM. Body-image perception and dissatisfaction throughout phases of hte female menstrual cycle. Percept Mot Skills. 2009;108:74–80. [DOI] [PubMed] [Google Scholar]
- 79.Altabe M, Thompson JK. Menstrual cycle, body image, and eating disturbance. Int J Eat Disord. 1990;9:395–401. [Google Scholar]
- 80.Hildebrandt BA, Racine SE, Keel PK, et al. The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. Int J Eat Disord. 2015;48(5):477–486. doi: 10.1002/eat.22326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Racine SE, Culbert KM, Keel PK, Sisk CL, Alexandra Burt S, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord. 2012;45(3):333–344. doi: 10.1002/eat.20941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carr-Nangle R, Johnson WG, Bergeron KC, Nangle DW. Body image changes over the menstrual cycle in normal women. Int J Eat Disord. 1994;16:267–273. [DOI] [PubMed] [Google Scholar]
- 83.Klump KL, Keel PK, Burt SA, et al. Ovarian hormones and emotional eating associations across the menstrual cycle: An examination of the potential moderating effects of body mass index and dietary restraint. Int J Eat Disord. 2013;46(3):256–263. doi: 10.1002/eat.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mikhail ME, Keel PK, Burt SA, et al. Trait Negative Affect Interacts With Ovarian Hormones to Predict Risk for Emotional Eating. Clin Psychol Sci. Published online 2020. doi: 10.1177/2167702620951535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Racine SE, Keel PK, Burt SA, Sisk CL, Neale M. Hormones and Emotional Eating Across the Menstrual Cycle : A. Eat Behav. 2014;14(2):161–166. doi: 10.1016/j.eatbeh.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fowler N, Keel PK, Burt SA, et al. Associations between ovarian hormones and emotional eating across the menstrual cycle: Do ovulatory shifts in hormones matter? Int J Eat Disord. 2019;52(2):195–199. doi: 10.1002/eat.22985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klump KL, Hildebrandt BA, O’Connor SM, et al. Changes in genetic risk for emotional eating across the menstrual cycle: a longitudinal study. Psychol Med. 2015;45(15):3227–3237. doi: 10.1017/S0033291715001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klump KL, O’Connor SA, Hildebrandt BA, et al. Differential effects of estrogen and progesterone on genetic and environmental risk for emotional eating in women. Clin Psychol Sci. 2016;4(5):895–908. doi: 10.1177/2167702616641637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forney KJ, Keel PK, O’Connor S, Sisk C, Burt SA, Klump KL. Interaction of hormonal and social environments in understanding body image concerns in adolescent girls. J Psychiatr Res. 2019;109(November 2018):178–184. doi: 10.1016/j.jpsychires.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baker JH, Runfola CD. Eating disorders in midlife women: A perimenopausal eating disorder? Maturitas. 2016;85(2016):112–116. doi: 10.1016/j.maturitas.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 91.Tepper PG, Randolph JF, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872–2880. doi: 10.1210/jc.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baker JH, Peterson CM, Thornton LM, et al. Reproductive and Appetite Hormones and Bulimic Symptoms during Midlife. Eur Eat Disord Rev. 2017;25(3):188–194. doi: 10.1002/erv.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thompson KA, Bardone-Cone AM. Menopausal status and disordered eating and body image concerns among middle-aged women. Int J Eat Disord. 2019;52(3):314–318. doi: 10.1002/eat.23030 [DOI] [PubMed] [Google Scholar]
- 94.Deeks AA, McCabe MP. Menopausal stage and age and perceptions of body image. Psychol Heal. 2001;16:367–379. [Google Scholar]
- 95.Mangweth-Matzek B, Hoek HW, Rupp CI, Kemmler G, Pope HG, Kinzl J. The menopausal transition - A possible window of vulnerability for eating pathology. Int J Eat Disord. 2013;46(6):609–616. doi: 10.1002/eat.22157 [DOI] [PubMed] [Google Scholar]
- 96.Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Predictors of pretreatment attrition from smoking cessation among pre- and postmenopausal, weight-concerned women. Eat Behav. 2006;7(3):243–251. doi: 10.1016/j.eatbeh.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 97.Drobnjak S, Atsiz S, Ditzen B, Tuschen-Caffier B, Ehlert U. Restrained eating and self-esteem in premenopausal and postmenopausal women. J Eat Disord. 2014;2(1):1–10. doi: 10.1186/s40337-014-0023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baker JH, Eisenlohr-moul T, Wu Y, Crystal E, Bulik CM, Girdler SS. Ovarian hormones influence eating disorder symptom varibility during the menopause transition: a pilot study. Eat Behav. 2019;35:101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shirao N, Okamoto Y, Mantani T, Okamoto Y, Yamawaki S. Gender differences in brain activity generated by unpleasant word stimuli concerning body image: An fMRI study. Br J Psychiatry. 2005;186(JAN.):48–53. doi: 10.1192/bjp.186.1.48 [DOI] [PubMed] [Google Scholar]
- 100.Frank GKW. Neuroimaging and eating disorders. Curr Opin Psychiatry. 2019;32(6):478–483. doi: 10.1097/YCO.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berner LA, Marsh R. Frontostriatal circuits and the development of bulimia nervosa. Front Behav Neurosci. 2014;8(NOV):1–12. doi: 10.3389/fnbeh.2014.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buczek L, Migliaccio J, Petrovich GD. Hedonic Eating: Sex Differences and Characterization of Orexin Activation and Signaling. Neuroscience. 2020;436:34–45. doi: 10.1016/j.neuroscience.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tapia MA, Lee JR, Weise VN, Tamasi AM, Will MJ. Sex differences in hedonic and homeostatic aspects of palatable food motivation. Behav Brain Res. 2019;359(November 2018):396–400. doi: 10.1016/j.bbr.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 104.Sinclair EB, Hildebrandt BA, Culbert KM, Klump KL, Sisk CL. Preliminary evidence of sex differences in behavioral and neural responses to palatable food reward in rats. Physiol Behav. 2017;176(January):165–173. doi: 10.1016/j.physbeh.2017.03.042 [DOI] [PubMed] [Google Scholar]
- 105.Keller KL, Kling SMR, Fuchs B, et al. A biopsychosocial model of sex differences in children’s eating behaviors. Nutrients. 2019;11(3). doi: 10.3390/nu11030682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo S, Alves J, Hardy K, et al. Neural processing of food cues in pre-pubertal children. Pediatr Obes. 2019;14(2):1–14. doi: 10.1111/ijpo.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):10–15. doi: 10.1097/MED.0b013e328320d54c [DOI] [PubMed] [Google Scholar]
- 108.Del Parigi A, Chen K, Gautier JF, et al. Sex differences in the human brain’s response to hunger and satiation. Am J Clin Nutr. 2002;75(6):1017–1022. doi: 10.1093/ajcn/75.6.1017 [DOI] [PubMed] [Google Scholar]
- 109.Frank S, Laharnar N, Kullmann S, et al. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 110.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243(1):91–96. doi: 10.1016/j.bbr.2012.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behav Brain Res. 2006;169(1):111–119. doi: 10.1016/j.bbr.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 112.Killgore WDS, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport. 2010;21(5):354–358. doi: 10.1097/WNR.0b013e32833774f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99(4):538–543. doi: 10.1016/j.physbeh.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang GJ, Volkow ND, Telang F, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. 2010;1363:81–92. doi: 10.1016/j.brainres.2010.09.071 [DOI] [PubMed] [Google Scholar]


