Abstract
An emerging concept is that quiescent mature skeletal cells provide an importantcellular source for bone regeneration. It has long been considered that a small number of resident skeletal stem cells are solely responsible for the remarkable regenerative capacity of adult bones. However, recent in vivolineage-tracing studies suggest that all stages of skeletal lineage cells, including dormant pre-adipocyte-like stromal cells in the marrow, osteoblast precursor cells on the bone surface and other stem and progenitor cells, are concomitantly recruited to the injury site and collectively participate in regeneration of the damaged skeletal structure. Lineage plasticity appears to play an important role in this process, by which mature skeletal cells can transform their identities into skeletal stem cell-like cellsin response to injury.These highly malleable, long-living mature skeletal cells, readily available throughout postnatal life,might represent an ideal cellular resource that can be exploited for regenerative medicine.
Keywords: Skeletal stem cells (SSCs), Bone marrow stromal cells (BMSCs), Mesenchymal stem cells (MSCs), Bone regeneration, Fracture repair, Cellular plasticity, In vivo cell lineage analysis
Introduction
Bones, characterized by strong and rigid structures owing to mineralized matrix, are surprisingly malleable organs that can maintain their structures throughout life. The primary functions of bonesin protecting vital organs and achieving locomotion render these tissuesparticularly susceptible to various degrees ofdamage, ranging in severity from microfractures to fractures that completelydisrupt tissue continuity.Most small and mechanically stable fractures heal byintramembranous bone formation, whereas large and unstable fractures alsoinvolve endochondral bone formation in which fibrocartilages and soft callusare newly generated near the fracture site to bridge bone fragments.[1, 2] Therefore, bonesrepair these damages withexcellentinherent capabilities for regeneration.Impaired regenerative capabilities due to aging or other systemic conditions cause delayed union or non-union of bone fractures[3, 4] and are associated with the increased mortality risk;[5, 6] therefore, understanding the mechanism of bone regeneration has significant impact on human health. The emerginghypothesis is that lineage plasticity of the skeletal lineage plays an important role in bone regeneration, wherein the full spectrum of skeletal lineage cells is mobilized to provide emergency cellular sources thatcollectively participate in regeneration of the damaged skeletal structure.
Boneregeneration requires highly coordinated processes of mobilization, proliferation, and differentiation of skeletal cellsto allow deposition of mineralized matrix at the injury site. It is generally considered that stem cells of the skeletal lineage termed skeletal stem cells (SSCs) are primarily responsible for generating new cells necessary for regeneration.[7] These SSCs, once categorized under the diffuse term of mesenchymal stem cells (MSCs), are posited to play important roles in growth, homeostasis and regeneration of bone tissues.[8] The prevailing idea is that SSCs stand at the pinnacle of the skeletal lineage, which has been largely extrapolated from other well-studied somatic stem cells such as hematopoietic and epithelial stem cells.[9–13] However, the challenge to this idea is that the current retrospective approach for identifying SSCs does not permittounambiguously define the in vivoidentity of these stem cells. In addition, the property and the function of SSCs are highlyvariable across different compartments, without any single master stem cells universally contributing to all compartments. In fact, each distinct compartment of bones, such as the growth plate, the periosteum and the bone marrow, maintain its own unique population of stem cells with distinct functionality.[8, 14–17] Therefore, roles that stem cells play in bone regeneration remain largely speculative; their roles may be highly context-dependent.
Robust regenerative potential does not necessarily mandate the maintenance of a small population of tissue-specific stem cells throughout postnatal life, particularly in organs with marked slow turnover.There are alternative ways to generate functionally mature cells necessary for regeneration. For example, the liver and the pancreas possess high regenerative capacity without any discernable population of tissue-specific stem cells; these organs primarily depend on mature cells for tissue maintenance in homeostasis and regeneration in response to injury.[18] The biological process by which mature cells revert into progenitor-like cells in response to injury, generally termed cellular plasticity, appears to play an important role in maintaining the regenerative potential of bones.[19]
In this essay, we discuss the relative contribution of skeletal stem cells and mature skeletal cell populations to bone regeneration.We argue that lineage plasticity of mature skeletal cells is an important mechanism underpinning bone regeneration, in a way much similar to other major slow turnover organs. It is intriguing to speculate that at least part of skeletal stem cells representstransient intermediate entities along the trajectory from one differentiated cell type to another(Figure 1).
Figure. 1. Two lineage models for bone regeneration.
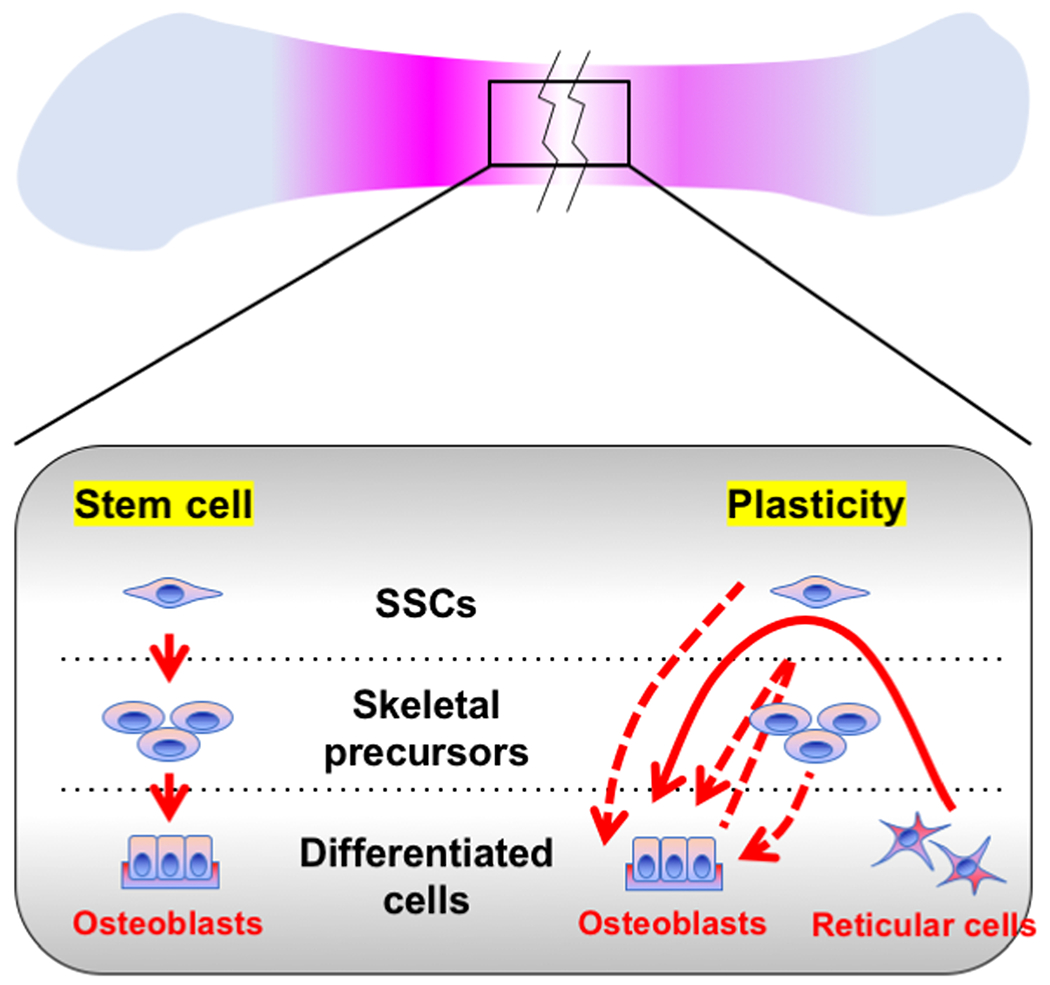
The prevailing model is that a small number of resident SSCs are responsible for the remarkable regenerative capacity of bones (left). The alternative model is that lineage plasticity of mature skeletal cells is a mechanism underpinning bone’s regenerative capacity (right). Note that these two proposed models are not mutually exclusive.
1. Skeletal stem cells: what are their in vivo correlates?
Skeletal stem cells (SSCs) are generally considered to play important roles in growth, homeostasis and regeneration of bone tissues. SSCs are primarily defined by their in vitro functions, as self-renewing cells withthe “trilineage” potential to differentiate into chondrocytes,osteoblasts and adipocytes in cultured conditions, as well as with the ability to establish bone and bone marrowassociated with marrow stromal cells after heterotopic transplantation. An in vitro colony forming unit-fibroblast (CFU-F) assay and the subsequent transplantation assay have long served as a gold standard to define SSCs. The concept of SSCs was originally developed in 1960s, based on the discovery that bulk or plastic-adherent bone marrow cells can establish ossicles containing bone and bone marrow after transplantationinto immunodeficient mice.[20–22] Decades later, the definition of SSCs was substantially sophisticated usingcell surface markers that are used to isolate these clonogeniccellsby fluorescence activated cell sorting (FACS). This approach was initially applied to the human bone marrow using CD146 as a marker to identifySSCs among perivascular stromal cells.[23] It was later identified that CD51 (αV integrin)+PDGFRα+ cells represent a small subset of CD146+ cells with even more enriched colony-forming activities.[24] In the mouse bone marrow, non-hematopoietic non-endothelial PDGFRα+Sca1+cells,[25] CD73+ cells,[26, 27] CD271+ cells[28–31] and CD106+ cells[32] have been identified to behighly enriched for SSCs. In recent years, this cell surface marker-based approach has been applied to isolate SSCs from other skeletal compartments, such as the growth plate and the periosteum.Chan et al. isolated “mouse SSCs”from the perinatal mouse growth plate as defined by CD51+CD90−CD105−CD200+ non-hematopoietic mesenchymal cells[33]; they subsequently isolated “human SSCs”from the fetal human growth plate as defined by PDPN+CD146−CD73+CD164+ non-hematopoietic mesenchymal cells.[34] Periosteal stem cells (PSCs) were isolated from the periosteum using the same panel of markers as mouse SSCs.[16] Further, highly clonogenic cells with greater growth and differentiation capacity than bone marrow SSCs were isolated from the periosteum, as defined by Sca1+CD29+ cells.[35] Therefore, these lines of studies lend credence to the hypothesis that a small population of highly clonogenic SSCs present in each bone compartments play important roles in tissue maintenance in homeostasis and regeneration in response to injury.
Although extremely powerful, these widely used ex vivo assays for SSCs have inevitable limitations, in that these “stem cells” can be evaluated only in artificial exogenous conditions after cell isolation. How these stem cells behave in their native environments cannot be concluded from these types of studies. In fact, hematopoietic stem cells (HSCs) have been stringently defined by a defined panel of cell surface markers and subsequent transplantation assays for several decades; however, the recent study demonstrates that these HSCs contribute little to native hematopoiesis under unperturbed conditions.[36] Moreover, cell populations identified by sets of markers are always composed of heterogeneous cell populations, presumably including not only highly clonogenic “stem cells”, but also at least some of their descendants including terminally differentiated cells. In addition, these “stem cells” rapidly change their gene expression profiles and exit from their original statuses under regenerative conditions; as a result, it is expected that expression of the utilized markers is not maintained over the course of regeneration. Therefore, in vivo correlates of SSCs identified by above-mentioned cell surface markers remain largely unclarified. It requires cautions to extrapolate these transplantation-based findings to the native process of bone regeneration.
2. In vivo lineage-tracing analysis: approaches to testing the stem cell hypothesisin bone regeneration
The widely-accepted method to interrogate cell fates and functions of stem cells in their native environments is in vivo lineage-tracing experiments using transgenic mice. This approach typicallyemploys the cre-loxP technology to permanently mark cells of interest using a double transgenic system (Figure 2). Crerecombinase is expressed in a promoter-specified mannerin the first transgenic line and acts on the reporter locus of the second transgenic line. The reporter construct is typically engineered in a ubiquitously active locus, such as in the Rosa26 locus; the “STOP” sequences, composed of multiple sequences directing the addition ofpolyA sequences and translation termination codons in all three readingframes, are flanked by loxP sitesto halt continued transcription and translation of reporter genes. When cre recombinase removes the “STOP” sequences, the reporter gene becomes expressed under the direction of a ubiquitously active promoter, such as the CAG promoter. In a modified “inducible” version, the crerecombinase is covalently bound to the ligand-binding domain of the estrogen receptor (creERT2) that has been mutated so that tamoxifen, but not estradiol,can bind and change its tertiary structure. Translocation of the creERT2 complexto the nucleus is dependent on the presence of 4-hydroxy-tamoxifen (4-OHT),which is an active form of tamoxifen produced after being metabolized in theliver. Therefore, in the creERT2 system, tamoxifen administration can temporarilyactivate cre-loxP recombination only for 24–48 hours until 4-OHT is cleared away from the cell. Recombination in the reporter locus is irreversible;therefore, the reporter gene is continually expressed in the targeted cell andits descendants, even after the promoter that droveexpression of cre recombinase becomes no longer active.Several different versions of the modified Rosa26 reporter locus (“R26R”) areavailable, including R26R-LacZ (encoding β-galactosidase), R26R-YFP (yellowfluorescent protein), R26R-tdTomato (encoding tandem dimer of redfluorescent protein, DsRed), and R26R-Confetti. These reporter alleles have different sensitivity to cre-loxP recombination. The Confetti locus encodes fourdifferent fluorescent proteins (nuclear GFP, YFP, tdTomato and CFP [cyanfluorescent protein]), in which one of them becomes stochastically expresseduponcre-loxP recombination.The in vivo lineage-tracing approach has been applied to defineprogenitor–descendant relationships in the native environment in essentially all organs in mice. Todraw meaningful conclusions from these experiments, it is essential to identify promoters that are active only ina narrow array of desirable cell types, and ideally, promoters without anyactivity in descendant cells.
Figure 2. CreERT2-loxP approach for in vivo lineage-tracing experiments.
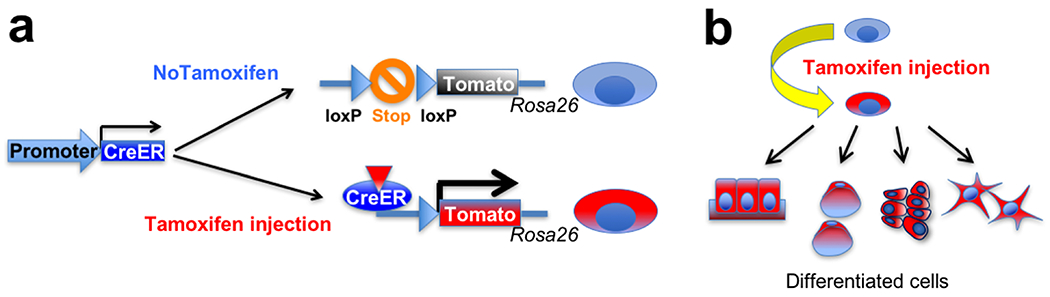
(a) Tamoxifen administration can temporarily activate cre-loxP recombination in a target cell population, which removes “STOP” sequences in the Rosa26 reporter locus. As a result, the reporter gene becomes permanently expressed in the targeted cells.
(b) The reporter gene is continually expressed in the targeted cell and its descendants, allowing permanent marking of a given cell population.
In recent years, this lineage-tracing approach has been applied to reveal the behaviors and functions of SSCs in tissue growth, homeostasis and regeneration.[10, 37–44] These genetic studies have provided important insights into the fundamental characteristics of SSCs and their potentially downstream skeletal progenitor cell populations. However, heterogeneity of cell populations marked by the promoter/enhancers of the given genes complicates overall interpretationof the findings, in a manner similar to those “stem cells” identified by a set of cell surface markers. Cells identified by most of the creERT2 transgenic lines referenced above assumingly involve not only “stem cells”, but also at least part of their descendants including those already terminally differentiated. Whether it is stem cells or their downstream progeny that robustly participate in the given process cannot be conclusively determined.
One of the skeletal stem cell populations that are clearlymaintained in a defined anatomical location is PTHrP+ cells, which are exclusively localized to the resting zone of the postnatal growth plate.[14] These PTHrP+ stem cells clonally establish columns of chondrocytes within the growth plate, and subsequently transform into osteoblasts and marrow stromal cells beneath the growth plate in a high sequential manner.[14, 45] Another example is Mx1+aSMA+ periosteal stem cells (P-SSCs) that are defined from their downstream progeny in a defined anatomical location of the periosteum.[43] Despite these advances, specific anatomical locations housing SSC populations have not been largely identified yet.[17] Therefore, the behaviors and functions of stem cells in a majority of skeletal compartments cannot be easily discerned due to the absence of “stem cell-specific” inducible genetic tools, particularly in the highly crowded skeletal tissues such as the bone marrow and the periosteum.
The in vivo lineage-tracing approach has not yet been extensivelyapplied to study the process ofbone fracture healing, primarily due to lack of highly cell type-specific inducible genetic tools. Some studies examining the function of potential skeletal stem cell populations rely ona “constitutivelyactive” version of cre recombinases, such as Prrx1-cre[35] and Ctsk-cre[16] for the periosteum, and LepR-cre[46] for the bone marrow.The fundamental difference between“constitutively active” cre and “inducible” creERT2requires close attention;unlike the latter, the former induces recombination whenever the promoterbecomes active, therefore there is no temporal factor that controls cre activities. If that promoterbecomes active in other cell types at a late phase during lineage development, the possible relationships between the different cell types marked by areporter gene cannot be delineated. Therefore, the contribution of native stem cells to inherent bone regeneration remains largely inconclusive, as roles that putative stem cells play in the process of bone regeneration cannot be completely defined based on the current sets of toolkits.
3. Unexpected roles of dormant marrow fat precursor cells in bone regeneration
Cells of the skeletal lineageat various stages of differentiation can be classified by a well-described set of marker genes. Importantly, cells at each defined stagestill demonstrate substantialcellular heterogeneity and functional diversity.The prime example is bone marrow stromal cells (BMSCs), which are undifferentiated mesenchymal cells residing in a perisinusoidal space of the bone marrow. BMSCs express important hematopoiesis-supporting cytokines such as C-X-C motif chemokine 12 (CXCL12, also known as stromal cell-derived factor 1, SDF1)[47] and stem cell factor (SCF, also known as KIT ligand).[48] In addition, BMSCs also express leptin receptor (LepR), a receptor for fat-specific hormone leptin. As a result, some of the BMSCs are termed as CXCL12+LepR+cells.[41] Lineage-tracing studiesrevealed that CXCL12+LepR+ BMSCsprovide a long-lasting source of osteoblasts in physiological conditions, while encompassing all colony forming-unit fibroblasts (CFU-Fs);[41, 46] these findings support the idea that there exists a small population of skeletal stem cells within CXCL12+LepR+ BMSCs. Recent single-cell RNA-sequencing studies revealed the substantial cellular heterogeneity within BMSCs in general,[49–51] and, more specifically, CXCL12-abudant reticular (CAR) cells.[19] In fact, CAR cells are composed of two major groups of pre-adipocyte-like “Adipo-CAR” cells and pre-osteoblast-like “Osteo-CAR” cells.[19, 52] Therefore, these studies have established the concept that CXCL12+LepR+ BMSCs, initially thought to be homogeneous, are indeed heterogeneous and composed of at least two populations of fat and bone precursor cell populations, in addition to a population of putative “stem cells” with unknown identities.
The next logical question is whether each cellular subset of CXCL12+LepR+ BMSCs possesses its own unique function in physiological and regenerative conditions. Our recent in vivo lineage-tracing studyusing a Cxcl12-creER transgenic line shed light on the unique functionality of a specific subset of BMSCs.[19] Importantly, we found that Cxcl12-creERpreferentially marks a quiescent subset of CXCL12+LepR+ BMSCs upon tamoxifen injection, which are exclusively located in a perisinusoidal space of the central bone marrow. Interestingly, these Cxcl12-creER+cells possess a pre-adipocyte-like state akin to Adipo-CAR cells with little colony-forming activities. These Cxcl12-creER+ BMSCs are highly dormant and do not contribute to cortical bone osteoblasts in physiological conditions. However,in regenerative conditions, these Cxcl12-creER+ BMSCs are actively recruited to the injury siteand robustly differentiate into osteoblasts and osteocytes to repair the cortical bone defect in regenerative conditions. Therefore, a highly quiescent subset of CXCL12+LepR+ BMSCs in the central bone marrow, which normally function asmarrow fat precursor cells, can be activated in response to injury and robustly contribute to cortical bone regeneration.
The important mechanistic question is how dormant marrow fat precursor cells can be enlisted for bone regeneration.To address this, we further performed combined lineage-tracing and single-cell RNA-seq analyses during injury responses. Cxcl12-creER+ BMSCs transformed their identities intoskeletal stem-like cells in response to injury, which represented an intermediate state between osteoblasts and marrow pre-adipocytes. These intermediate-state stem cell-like cells possessed robust colony-forming activities, and orderly differentiated into mature osteoblasts to fill the bone defect. Further, this transformative process was regulated by canonical Wnt signaling. Therefore, the quiescent fat precursor-like subset of CXCL12+LepR+ BMSCs can de-differentiate into skeletal stem cell-like cells in response to injury, and re-differentiate into osteoblasts to facilitate bone regeneration, in a manner mediated by canonical Wnt signaling. These findings shed light on the unexpected roles of non-skeletal stem cells, indicating the potential role of cellular plasticity in bone regeneration(Figure 3).
Figure 3. A Wnt-mediated transformation of bone marrow stromal cell identity coordinates cortical bone regeneration.
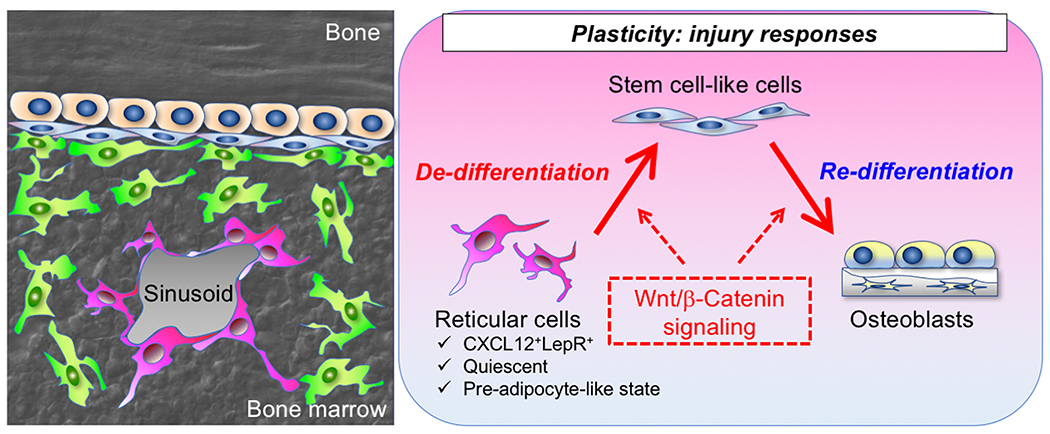
The quiescent fat precursor-like subset of CXCL12+LepR+ BMSCs can de-differentiate into skeletal stem cell-like cells in response to injury, and re-differentiate into osteoblasts to facilitate bone regeneration, in a manner mediated by canonical Wnt signaling.
4. Stem and mature cellscontribute cooperatively to bone regeneration
These findings raise a new hypothesis that so-called “skeletal stem cells (SSCs)” can be newly generated under regenerative conditions when the demand for cytogenesis is particularly elevated, supporting the presumptive role of cellular plasticity in bone regeneration. The next important question is whether this plasticity is unique to marrow fat precursor cells, or it also occurs to other mature skeletal cells abundantly present in the milieu, such as osteoblasts or their immediate precursor cells. To address this question, we closely examined our model for cortical bone regeneration and defined the relative contribution of various mature skeletal cell populations, by utilizing multiple tamoxifen-inducible creERT2 lines that are active in these mature cell types, namely Cxcl12-creER for marrow fat precursor cells, andOsx-creER for osteoblasts and their precursor cells.
As a quantitative model to define the relative contribution of various cell populations to bone regeneration, we employed the drill-hole injury model.In this model, a hole with the standardized size (typically up to 1mm in diameter) is created in the cortical bone using a bur or a drill bit, in a standardized position of the long bone. The drill hole is typically createdunilaterallyin the diaphysis (the middle shaft of long bones) to disrupt the endocortical surface. The drill-hole cortical defect is exclusively repaired by BMSCs through the intramembranous pathway, as the periosteum is completely removed from the surgical field.[53, 54] This mechanically stable drill-hole injury is an excellent model to interrogate regenerative potentials of BMSCs, together with a bone marrow ablation surgery that induces direct differentiation of BMSCs into osteoblasts within the bone marrow.[55] The injured area of the cortical bone can be easily identified by standard histology, and the total number of osteocytes present in the regenerated portion of the cortical bone serves as the denominator to lineage-traced osteocytes to determine the contribution of each cell type to cortical bone repair.
First, we defined the contribution of quiescent pre-adipocyte-like Cxcl12-creER+ cells to osteocytes in the regenerated portion of the cortical bone after 8 weeks of injury. These cells contributed to approximately 40% of osteocytes in the regenerated portion of the cortical bone. This number is substantial, and indicates that these dormant marrow fat precursor cells are indeed functionally important contributors to cortical bone repair; this conclusion is further supported by the additional functional assay that deletion of canonical Wnt signaling in these cells leads to insufficiencies in cortical bone repair. However, this number also points to another important factthat the remaining 60% of osteocytes in the regenerated portion of the cortical bone are not derived from Cxcl12-creER+ BMSCs.
Second, we defined the contribution of Osx-creER+ osteoblast precursor cells to osteocytes in the regenerated portion of the cortical bone after 8 weeks of injury. These cells provide a particularly important cellular source during bone development;[38, 56] however, these cells essentially lose their potential and its expression becomes more restrictive to mature osteoblasts in adulthood.Indeed, Osx-creER marks the vast majority of mature osteoblasts on the bone surface and osteocytes embedded in the bone matrix. Osx-creER+ cells contributed to approximately 12% of osteocytes in the regenerated portion of the cortical bone, indicating that these cells retain their ability to participate in cortical bone repair in adulthood particularly in response to injury.
The findings from lineage-tracing studies of Cxcl12-creER and Osx-creER raise another important question; that is, what is the source of the remaining 48% of osteocytes in the regenerated portion of the cortical bone? There are several potential sources that account for the remaining osteocytes of the regenerated cortical bone. The first potential source is other non-pre-adipocyte subsets of CXCL12+LepR+ BMSCs, including those pre-osteoblast-like cells termed as Osteo-CAR cells. These cells abundantly express pre-osteoblast markers such as alkaline phosphatase (Alpl) and periostin (Postn), therefore primed to provide a rapid source of osteoblasts under regenerative conditions. The second potential source is other immature BMSCs that do not express CXCL12or LepR. The identities of these BMSCs that may encompass bona fide SSCs have not yet been clearly identified, or are part of a separate heterogeneously labeled population such as byMx1-cre,[43] Gli1-creER[42] or Prrx1-cre.[35] The third potential source is other mature skeletal cells that are not marrow pre-adipocyte-like cells or osteoblast on the bone surface. The emerging concept is that cells that originate from multiple cellular sources collectively participate in bone regeneration under emergencies of bone injuries. How these cellular sources differentially contribute to bone regeneration will need to be clarified with novel cell type-specific inducible genetic tools in future studies.
As discussed above, we identified that dormant pre-adipocyte-like Cxcl12-creER+ BMSCs can transform their identities to skeletal stem cell-like cells in response to injury. The important question is whether this transformative capacity is unique to pre-adipocyte-like cells. It remains to be defined whetherOsx-creER+ cells can revert back to intermediate-state skeletal stem cell-like cells, or directly differentiate into osteoblasts in the process of cortical bone regeneration. It is interesting to speculate that bone regeneration utilizes multiple modes of cellular plasticity, wherein at least part of “skeletal stem cells” represent transient intermediate-state cells between the cycle of “de-differentiation” and “re-differentiation”. Whether there is a genuinely self-renewing skeletal cell population within highly diverse BMSCsremains to be clarified in vivo(Figure 4).The important caveat is that direct evidence demonstrating direct conversion of “mature” skeletal cells to stem cell-like cells is still lacking in the current studies. It would be important in future studies to take advantage of more rigorous approaches at a single-cell level, such as intravital imaging, to test this hypothesis.
Figure 4. Cooperative contribution of stem and mature cells to bone regeneration.
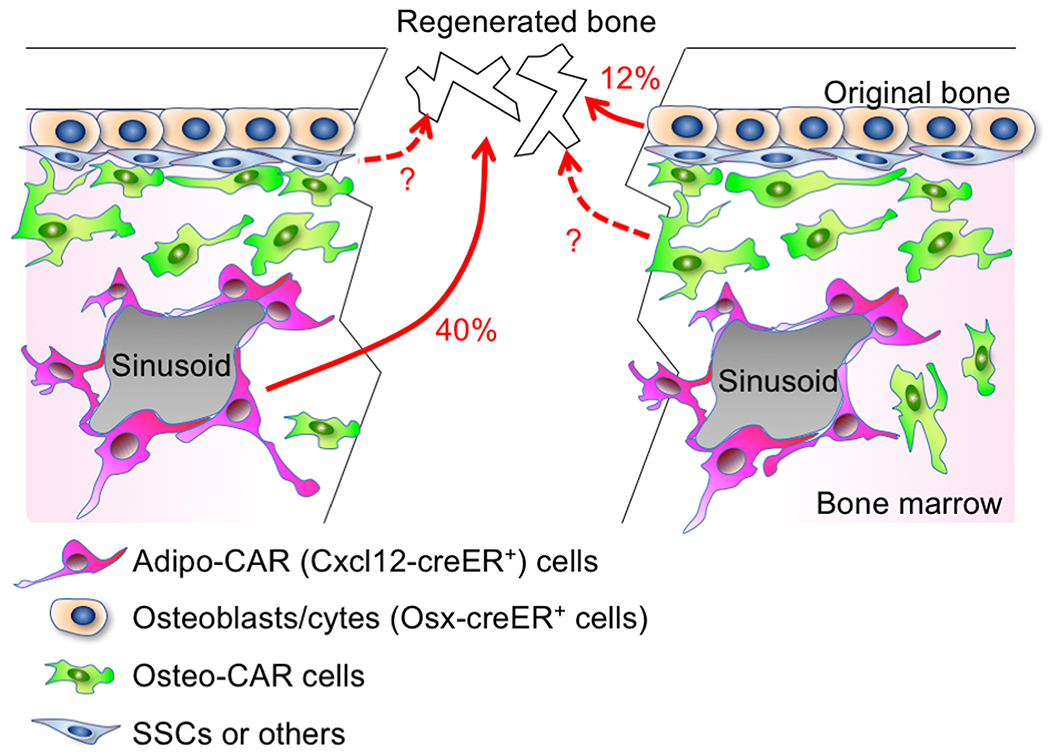
Various types of skeletal cells in bone marrow contribute to cortical bone regeneration. Cxcl12-creER+ Adipo-CAR cells contribute to 40%, whereas Osx-creER+ osteoblast precursors contribute to 12% of osteocytes in the regenerated bone. Other cell types, including Osteo-CAR cells, SSCs and others cells with unknown identities, may contribute to the remaining osteocytes of the regenerated bone.
5. Canonical Wnt signaling pathways play important roles in skeletal cell lineage plasticity
As discussed above, canonical Wnt signaling plays an important functional role in directing dormant pre-adipocyte-like BMSCs to the regenerative process, through converting these cells to a transient stem cell-like state. Indeed, canonical Wnt signaling has been widely recognized as an important pathway that critically regulates bone development and regeneration.[57–59] Transcriptional activation of canonical Wnt signaling pathways in cells of the skeletal lineage highly depends on the differentiation stage, indicating the context-dependent role of canonical Wnt signaling in vivo.[60, 61] We found that inactivation of canonical Wnt signaling in either Cxcl12-creER+ pre-adipocyte-like BMSCs or Dlx5-creER+ osteoblast precursors led to insufficiencies in cortical bone regeneration; therefore, canonical Wnt signaling has a unanimous role in promoting bone regeneration across different cellular subsets of the skeletal lineage.Transcription factors Sox9 and Runx2 cooperatively regulatecommitment to the osteoblast lineage in a manner regulated bycanonical Wnt signaling during skeletal development.[57, 58] Interestingly, this canonical Wnt-mediated cellular plasticity of quiescent Cxcl12-creER+BMSCs does not seem to be mediated by Sox9 or Runx2 function, underscoring the fundamental difference between canonical Wnt-regulated bone development and regeneration. In other major slow turnover organs such as in the liver, canonical Wnt signaling plays important roles in regulating the plasticity of mature cells both in homeostasis and regeneration.[62] Therefore, activation of canonical Wnt signaling may be a common mechanism in inducing lineage plasticity across many slow turnover organs.
Conclusions and prospects
Here, we have argued that skeletal cell lineage plasticity serves as an important mechanism for bone regeneration, during which mature skeletal cells, including dormant pre-adipocyte-like marrow stromal cells and osteoblast precursor cells are mobilized to the injury site together with other stem and progenitor cells, and collectively participate in regeneration. The recent in vivo lineage-tracing studies call for a revision on the prevailing skeletal stem cell-centric model of bone regeneration, to a more diversified model in which multiple classes of mature cells are involved for the regenerative process. It is currently unclear what is the relative contribution of cellular plasticity and stem cell recruitment; however, it appears that cellular plasticity may provide more than 50% of cells participating in regeneration under some settings.Cellular plasticity plays major roles in tissue regeneration across other organs, not only in relatively fast turnover organs such as the skin and the intestine,[12] but also in slow turnover organs such as the liver and the pancreas.[18] The common scheme is that lineage-restricted cells such as unipotent progenitors or differentiated cells revert to a stem cell-like state during injury responses to ensure proper tissue regeneration. Bones also appear to employ this mechanism to ensure that tissue regeneration occurs at a proper time and place. Current evidence on bone regeneration is only limited to BMSCs to repair a relatively small cortical bone defect; the remaining question is whether this process of skeletal lineage plasticity also occurs to periosteal cells to repair a much larger bone defect associated with complete bone fractures. This would require additional cell type-specific inducible genetic tools that allow interrogating the behaviors and functions of various mature cellular subsets of skeletal cells. Exploiting mature skeletal cells as a cellular source for “autotherapies” of bone defects represents an opportunity for regenerative medicine.
Acknowledgement
This research was supported by grants from National Institutes ofHealth (R01DE026666 to N.O., R03DE027421 to W.O.).
Footnotes
Conflict of interest
The authors declare no competing interests.
References
- [1].Claes LE, Heigele CA (1999). Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech, 32, 255–266. [DOI] [PubMed] [Google Scholar]
- [2].Claes L, Recknagel S, Ignatius A (2012). Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol, 8, 133–143. [DOI] [PubMed] [Google Scholar]
- [3].Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J (2014). Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res, 472, 3523–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clark D, Brazina S, Yang F, Hu D, Hsieh CL, Niemi EC, … Marcucio R (2020). Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell, 19, e13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Foulke BA, Kendal AR, Murray DW, Pandit H (2016). Fracture healing in the elderly: A review. Maturitas, 92, 49–55. [DOI] [PubMed] [Google Scholar]
- [6].Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009). Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA, 301, 513–521. [DOI] [PubMed] [Google Scholar]
- [7].Einhorn TA, Gerstenfeld LC (2015). Fracture healing: mechanisms and interventions. Nat Rev Rheumatol, 11, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bianco P (2014). “Mesenchymal” stem cells. Annu Rev Cell Dev Biol, 30, 677–704. [DOI] [PubMed] [Google Scholar]
- [9].Mendelson A, Frenette PS (2014). Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med, 20, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, … Frenette PS (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 466, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, … Dick JE (2016). Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science, 351, aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blanpain C, Fuchs E (2014). Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science, 344, 1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Merrell AJ, Stanger BZ (2016). Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol, 17, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, … Ono N (2018). Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature, 563, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Newton PT, Li L, Zhou B, Schweingruber C, Hovorakova M, Xie M, … Chagin AS (2019). A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature, 567, 234–238. [DOI] [PubMed] [Google Scholar]
- [16].Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, … Greenblatt MB (2018). Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature, 562, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matsushita Y, Ono W, Ono N (2020). Skeletal Stem Cells for Bone Development and Repair: Diversity Matters. Curr Osteoporos Rep, 18, 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kopp JL, Grompe M, Sander M (2016). Stem cells versus plasticity in liver and pancreas regeneration. Nat Cell Biol, 18, 238–245. [DOI] [PubMed] [Google Scholar]
- [19].Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, … Ono N (2020). A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun, 11, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966). Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol, 16, 381–390. [PubMed] [Google Scholar]
- [21].Tavassoli M, Crosby WH (1968). Transplantation of marrow to extramedullary sites. Science, 161, 54–56. [DOI] [PubMed] [Google Scholar]
- [22].Bianco P, Robey PG (2015). Skeletal stem cells. Development, 142, 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, … Bianco P (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell, 131, 324–336. [DOI] [PubMed] [Google Scholar]
- [24].Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS (2013). PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med, 210, 1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, … Matsuzaki Y (2009). Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med, 206, 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Breitbach M, Kimura K, Luis TC, Fuegemann CJ, Woll PS, Hesse M, … Fleischmann BK (2018). In Vivo Labeling by CD73 Marks Multipotent Stromal Cells and Highlights Endothelial Heterogeneity in the Bone Marrow Niche. Cell Stem Cell, 22, 262–276.e267. [DOI] [PubMed] [Google Scholar]
- [27].Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E (2012). Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy, 14, 431–440. [DOI] [PubMed] [Google Scholar]
- [28].Boxall SA, Jones E (2012). Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int, 2012, 975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Coutu DL, Kokkaliaris KD, Kunz L, Schroeder T (2017). Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat Biotechnol, 35, 1202–1210. [DOI] [PubMed] [Google Scholar]
- [30].Das B, Kashino SS, Pulu I, Kalita D, Swami V, Yeger H, … Campos-Neto A (2013). CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med, 5, 170ra113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Álvarez-Viejo M, Menéndez-Menéndez Y, Otero-Hernández J (2015). CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells, 7, 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, … Han ZC (2013). CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One, 8, e59354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, … Longaker MT (2015). Identification and specification of the mouse skeletal stem cell. Cell, 160, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, … Longaker MT (2018). Identification of the Human Skeletal Stem Cell. Cell, 175, 43–56.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Duchamp de Lageneste O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, … Colnot C (2018). Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun, 9, 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, … Camargo FD (2014). Clonal dynamics of native haematopoiesis. Nature, 514, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, … Wang TC (2015). Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell, 160, 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ono N, Ono W, Nagasawa T, Kronenberg HM (2014). A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol, 16, 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, … Frenette PS (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 502, 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grcevic D, Pejda S, Matthews BG, Repic D, Wang L, Li H, … Kalajzic I (2012). In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells, 30, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T (2018). Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev, 32, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shi Y, He G, Lee WC, McKenzie JA, Silva MJ, Long F (2017). Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun, 8, 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ortinau LC, Wang H, Lei K, Deveza L, Jeong Y, Hara Y, … Park D (2019). Identification of Functionally Distinct Mx1+αSMA+ Periosteal Skeletal Stem Cells. Cell Stem Cell, 25, 784–796.e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, … Scadden DT (2012). Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell, 10, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Matsushita Y, Ono W, Ono N (2020a). Growth plate skeletal stem cells and their transition from cartilage to bone. Bone, 136, 115359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell, 15, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T (2003). Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity, 19, 257–267. [DOI] [PubMed] [Google Scholar]
- [48].Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature, 481, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, … Scadden DT (2019). A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell, 177, 1915–1932.e1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, … Aifantis I (2019). The bone marrow microenvironment at single-cell resolution. Nature, 569, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wolock SL, Krishnan I, Tenen DE, Matkins V, Camacho V, Patel S, … Welner RS (2019). Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep, 28, 302–311.e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Baccin C, Al-Sabah J, Velten L, Helbling PM, Grünschläger F, Hernández-Malmierca P, … Haas S (2020). Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol, 22, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sakamoto K, Matsushita Y, Minamizato T, Katsuki Y, Katsube KI, Yamaguchi A (2017). The Bone Regeneration Model and Primary Osteoblastic Cell Culture Used in the Analysis of Ccn3 Transgenic and Knockout Mice. Methods Mol Biol, 1489, 309–324. [DOI] [PubMed] [Google Scholar]
- [54].Matsushita Y, Sakamoto K, Tamamura Y, Shibata Y, Minamizato T, Kihara T, … Yamaguchi A (2013). CCN3 protein participates in bone regeneration as an inhibitory factor. J Biol Chem, 288, 19973–19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ono N, Nakashima K, Schipani E, Hayata T, Ezura Y, Soma K, … Noda M (2012). Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation. J Cell Physiol, 227, 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, … Frenette PS (2014). Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell, 29, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Day TF, Guo X, Garrett-Beal L, Yang Y (2005). Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell, 8, 739–750. [DOI] [PubMed] [Google Scholar]
- [58].Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C (2005). Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell, 8, 727–738. [DOI] [PubMed] [Google Scholar]
- [59].Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, … Helms JA (2010). Wnt proteins promote bone regeneration. Sci Transl Med, 2, 29ra30. [DOI] [PubMed] [Google Scholar]
- [60].Ramakrishnan AB, Sinha A, Fan VB, Cadigan KM (2018). The Wnt Transcriptional Switch: TLE Removal or Inactivation? Bioessays, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Marcellini S, Henriquez JP, Bertin A (2012). Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. Bioessays, 34, 953–962. [DOI] [PubMed] [Google Scholar]
- [62].Kendall TJ, Duff CM, Boulter L, Wilson DH, Freyer E, Aitken S, … Hastie ND (2019). Embryonic mesothelial-derived hepatic lineage of quiescent and heterogenous scar-orchestrating cells defined but suppressed by WT1. Nat Commun, 10, 4688. [DOI] [PMC free article] [PubMed] [Google Scholar]


