Abstract
Background:
Chronotropic incompetence (CI) is common in HFpEF and is linked to impaired aerobic capacity. Whether upstream autonomic signaling pathways responsible for raising exercise heart rate (HR) are impaired in HFpEF is unknown. We investigated the integrity of central command and muscle metaboreceptor function, two predominant mechanisms responsible for exertional increases in HR, in HFpEF and senior control subjects.
Methods:
Fourteen healthy, senior controls (7M,7F) and 20 carefully screened HFpEF patients (8M,12F) underwent cardiopulmonary exercise testing (peak VO2) and static handgrip exercise at 40% of maximal voluntary contraction (MVC) to fatigue with post-exercise circulatory arrest (PECA) for 2 minutes to assess central command and metaboreceptor function respectively.
Results:
Peak VO2 (13.1 ± 3.4 vs 22.7 ± 4.0 ml/kg/min; p<0.001) and HR (122 ± 20 vs 155 ± 14 bpm; p<0.001) were lower in HFpEF than senior controls. There were no significant differences in peak HR response during static handgrip between groups (HFpEF vs controls: 90 ± 13 vs 93 ± 10 bpm; p=0.49). Metaboreceptor function defined as mean arterial blood pressure at the end of PECA was also not significantly different between groups.
Conclusions:
Central command (vagally mediated) and metaboreceptor function (sympathetically mediated) in patients with HFpEF were not different from healthy senior controls despite significantly lower peak whole-body exercise heart rates. These results demonstrate key reflex autonomic pathways regulating exercise heart rate responsiveness are intact in HFpEF.
Keywords: HFpEF, autonomic function, exercise
Introduction
Exercise intolerance is common in patients with heart failure with preserved ejection fraction (HFpEF). Several purported mechanisms involving cardiac and peripheral pathways, either by impairing convective or diffusive oxygen delivery, are thought to play a role in limiting exercise capacity in these patients.1 One purported mechanism of decreased exercise capacity is chronotropic incompetence, the inability to increase heart rate during exercise. A blunted heart rate response limits increases in cardiac output which in turn reduces peak oxygen uptake (VO2). Mechanisms controlling heart rate responsiveness during exercise are regulated by afferent feedback from muscle metabo- and mechanoreceptors which are integrated with feedforward signals from higher motor cortical centers in the central nervous system (central command) to decrease parasympathetic and increase sympathetic output.2 These signals are then transduced at the sinus node to electro-mechanically increase heart rate and cardiac contractility. We have previously shown cardiac β-receptor transduction is impaired in patients with HFpEF, though this impairment does not fully explain the reduced peak HR in these patients.3 Whether upstream autonomic signaling pathways are also impaired in HFpEF is unknown.
The purpose of the present study was to assess the integrity of autonomic control over exercise heart rate, specifically central command and skeletal muscle metaboreceptor function, and whether abnormalities in either of these reflexes could account for apparent chronotropic incompetence in HFpEF. We used a static handgrip exercise model4 to assess integrity of central command (maximal heart rate during isometric handgrip) and muscle metaboreceptor (blood pressure during post-exercise circulatory arrest) reflexes. We hypothesized there would be no differences in central command feedforward control of heart rate but that skeletal muscle metaboreceptor function would be augmented compared to senior control subjects of similar age.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study recruitment
HFpEF patients were recruited from a university cardiology clinic. The Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Resources approved all study procedures. Subjects were invited to participate if they (1) were older than 60 years of age; (2) had a hospitalization for heart failure; (3) had evidence for congestion by either chest x-ray or elevated filling pressures (pulmonary capillary wedge or left ventricular end-diastolic pressures > 16 mmHg); and (4) LV ejection fraction > 50% at study entry. HFpEF subjects were excluded for history of EF < 50%, body mass index > 40 kg/m2, serum creatinine > 2.0 mg/dL, severe chronic obstructive pulmonary disease, permanent atrial fibrillation, constrictive or restrictive cardiomyopathy, severe valvular disease or history of valvular surgery and if they were unable to perform exercise testing. Control subjects were recruited from the Dallas Heart Study, a population-based cohort of over 6,000 individuals, enriched by a random sampling of employees of Texas Health Resources, a large healthcare provider in the Dallas-Fort Worth metroplex, as previously described.5
After providing written informed consent, all subjects underwent testing as outlined below. Control subjects were excluded if they had a history of hypertension or elevated 24-hour ambulatory blood pressure > 140/90 mmHg (Oscar 2, Suntech Medical) and if they had a history of cardiovascular disease (e.g. stroke, myocardial infarction, atrial fibrillation, etc.), diabetes, COPD, former or current smokers and body mass index (BMI) > 30 kg/m2. Both HFpEF and senior controls, underwent a screening maximal exercise stress echo prior to enrollment and were excluded if they had evidence of coronary ischemia by ECG and echocardiography. Atrioventricular (AV)-nodal blocking agents (e.g. non-dihydropyridine calcium channel blockers, beta-blockers) were held for at least five half-lives prior to testing days.
Isometric Handgrip Testing
Subjects arrived in a climate-controlled laboratory after a light breakfast or lunch. Subjects were studied in the supine position and rested quietly on a foam mattress for 15 minutes after instrumentation and prior to data collection. A handgrip dynamometer was placed in their dominant hand and rested against their hip. Subjects were instructed to keep the device handle held vertically to avoid supination or pronation of the forearm. Subjects then performed three maximal voluntary contractions in the supine position using their dominant hand. The highest MVC value was selected and a target line corresponding to the 40% of MVC target was displayed on a computer screen above the subject. A brief practice (<30 seconds) session was used to familiarize subjects with the grip strength needed to maintain the 40% MVC target. Subjects were instructed not to perform an involuntary Valsalva maneuver or hold their breath during handgrip exercise. After a fifteen-minute quiet rest period, subjects performed isometric handgrip testing at 40% of MVC to exhaustion. Just prior to exhaustion (defined as an inability to sustain > 38% MVC for more than 2 seconds) and before complete termination of handgrip exercise, a cuff on the same dominant arm was inflated to supra-systolic pressure of 250 mmHg (Hokanson Inc; Bellevue WA) to trap forearm metabolites. After two minutes, the cuff was deflated, and the subject monitored for an additional two minutes. Heart rate and beat by beat blood pressure (BMEye Nexfin, the Netherlands) were measured continuously on the non-exercising hand and recorded just prior to handgrip fatigue and every minute thereafter. Prior studies have validated changes in beat-by-beat blood pressure measurements taken by the BMEye methodology at rest and with exercise.6, 7 Arm cuff blood pressures (Tango, Suntech Medical, North Carolina, US) were measured at the beginning of exercise and at the end of the protocol and used to calibrate the beat by beat blood pressure readings in post-study processing. A calibration factor was obtained as the ratio of the brachial cuff systolic and diastolic pressures and the average beat-by-beat systolic and diastolic pressures over the time the brachial cuff inflation occurred. A calibration factor for both systolic and diastolic BP was calculated by dividing the brachial cuff pressure by the corresponding beat-by-beat pressure averages. The systolic and diastolic calibration factors were then applied to beat by beat BP measures during handgrip exercise and post-exercise cuff inflation and used to report pressures at peak exercise, 1-, 2- and 3- minutes after cessation of exercise. Mean arterial BP was calculated as (systolic BP)/3 + 2*(diastolic BP)/3. Peak heart rate was defined as the highest heart rate achieved just prior to the end of handgrip exercise. Peak blood pressure for assessment of muscle metaboreceptor function was taken as the blood pressure at the end of the 2-minute supra-systolic cuff occlusion.
Whole body exercise Testing
After handgrip exercise testing and sufficient rest, a modified Astrand-Saltin incremental treadmill protocol was used to determine peak exercise capacity and peak exercise heart rate. Measures of ventilatory gas exchange were made by use of the Douglas bag technique.8 Gas fractions were analyzed by mass spectrometry (Marquette MGA 1100), and ventilatory volume was measured by a Tissot spirometer. Maximum oxygen uptake was defined as the highest oxygen uptake measured from at least a 30s Douglas bag. Cardiac output was measured using a modified acetylene gas re-breathing technique.9
Statistical Analysis
Statistical analysis was performed using commercially available software (JMP, Cary NC). All reported variables are presented as means with standard deviations unless otherwise noted. Unpaired t-tests were used to determine group differences between senior controls and patients with HFpEF. Repeated measures mixed models were used to determine group differences in blood pressure during the various handgrip exercise stages. A p value less than 0.05 was considered statistically significant.
Results
Demographic information and whole-body exercise variables for patients with HFpEF (n=20) and senior control subjects (n=14) are shown in Table 1. Age and sex distribution were similar between groups. HFpEF patients were more likely to be obese. Peak exercise heart rate was 30 bpm lower in HFpEF. Both relative and absolute peak VO2 were also lower in patients with HFpEF. Peak lactate and RER were both lower in HFpEF suggesting incomplete activation of maximal skeletal muscle metabolism prior to exercise cessation.
Table 1:
Demographic and Exercise Performance Data
| HFpEF (n=20) | Controls (n=14) | p value | |
|---|---|---|---|
| Age (yr) | 69 ± 7 | 72 ± 5 | 0.17 |
| Women, n (%) | 12 (60%) | 7 (50%) | 0.58 |
| Body Mass Index (kg/m2) | 35.1 ± 4.4 | 27.0 ± 3.6 | <0.001 |
| Hypertension, n (%) | 19 (95%) | 0 (0%) | <0.001 |
| Diabetes mellitus, n (%) | 13 (65%) | 0 (0%) | <0.001 |
| Medications | |||
| ACEi/ARB, n (%) | 13 (65%) | 0 (0%) | |
| Beta-blocker, n (%) | 18 (90%) | 0 (0%) | |
| Calcium-channel blocker, n (%) | 6 (30%) | 0 (0%) | |
| Loop diuretic, n (%) | 19 (95%) | 0 (0%) | |
| Maximal exercise variables | |||
| Resting heart rate (bpm) | 76 ± 12 | 77 ± 15 | 0.85 |
| Peak heart rate (bpm) | 122 ± 20 | 155 ± 14 | <0.001 |
| Peak VO2 (ml/kg/min) | 13.1 ± 3.4 | 22.7 ± 4.0 | <0.001 |
| Peak VO2 (L/min) | 1.29 ± 0.45 | 1.71 ± 0.44 | 0.009 |
| Peak cardiac output (L/min) | 11.2 ± 2.8 | 12.4 ± 2.9 | 0.23 |
| Peak cardiac index (L/min/m2) | 5.2 ± 1.0 | 6.6 ± 1.2 | 0.001 |
| Peak stroke volume (ml) | 92 ± 18 | 81 ± 21 | 0.10 |
| RER | 1.02 ± 0.09 | 1.12 ± 0.08 | 0.001 |
| Peak Lactate (mmol/L) | 3.79 ± 1.97 | 5.87 ± 1.73 | 0.004 |
| Peak AVO2 difference (ml/dL) | 11.3 ± 2.1 | 14.0 ± 2.0 | 0.001 |
| Peak Ventilation (L/min) | 50.3 ± 13.8 | 73.2 ± 21.5 | 0.001 |
Maximal hand grip strength was similar between groups (Table 2). Total hand grip exercise duration was also similar between groups. Resting heart rate was higher in the patients with HFpEF but both groups reached a similar peak heart rate at the end of static handgrip exercise (HFpEF vs controls: 90 ± 13 vs 93 ± 10 bpm; p=0.49). On account of higher resting heart rate, heart rate change during handgrip was lower in the patients with HFpEF (19 ± 9 vs 30 ± 11 bpm; p=0.005).
Table 2:
Handgrip Exercise Performance in HFpEF and Senior Controls
| HFpEF | Controls | p value | |
|---|---|---|---|
| Maximal hand grip strength (kg) | 28.0 ± 6.7 | 29.3 ± 9.8 | 0.71 |
| Total hand grip duration (s) | 217 ± 111 | 224 ± 113 | 0.86 |
| Resting heart rate (bpm) | 71 ± 11 | 63 ± 9 | 0.05 |
| Peak heart rate (bpm) | 90 ± 13 | 93 ± 10 | 0.49 |
| Heart rate change (bpm) | 19 ± 9 | 30 ± 11 | 0.005 |
| Baseline mean arterial pressure (mmHg) | 86 ± 12 | 76 ± 10 | 0.03 |
| Peak mean arterial pressure (mmHg) | 140 ± 24 | 157 ± 25 | 0.07 |
| Mean arterial pressure at minute 2 of post-exercise cuff occlusion (mmHg) | 112 ± 15 | 120 ± 15 | 0.18 |
| Calibration factor | |||
| Systole | 1.02 ± 0.11 | 1.10 ± 0.09 | 0.06 |
| Diastole | 1.39 ± 0.21 | 1.38 ± 0.23 | 0.81 |
A calibration factor was used to correct beat-by-beat blood pressure obtained by finger plethysmography to the brachial cuff pressure.
Blood pressure responses to peak handgrip and post-exercise circulatory arrest (PECA) are shown in Figure 1 and Table 2. Control subjects had slightly higher mean arterial pressure (MAP) response during handgrip and PECA (group x stage p=0.002; group p=0.27, stage p<0.001). Beyond this significant interaction, there were no statistically significant differences between groups during peak handgrip exercise or within PECA stages. Peak MAP in patients with HFpEF was nominally lower (140 ± 24 vs 157 ± 25 mmHg; p=0.07). The two-minute mark of PECA was used to define peak metaboreceptor activity. There were no significant differences in MAP between groups at this post-exercise time point (HFpEF vs Controls: 112 ± 15 vs 120 ± 15 mmHg; p=0.18).
Figure 1:
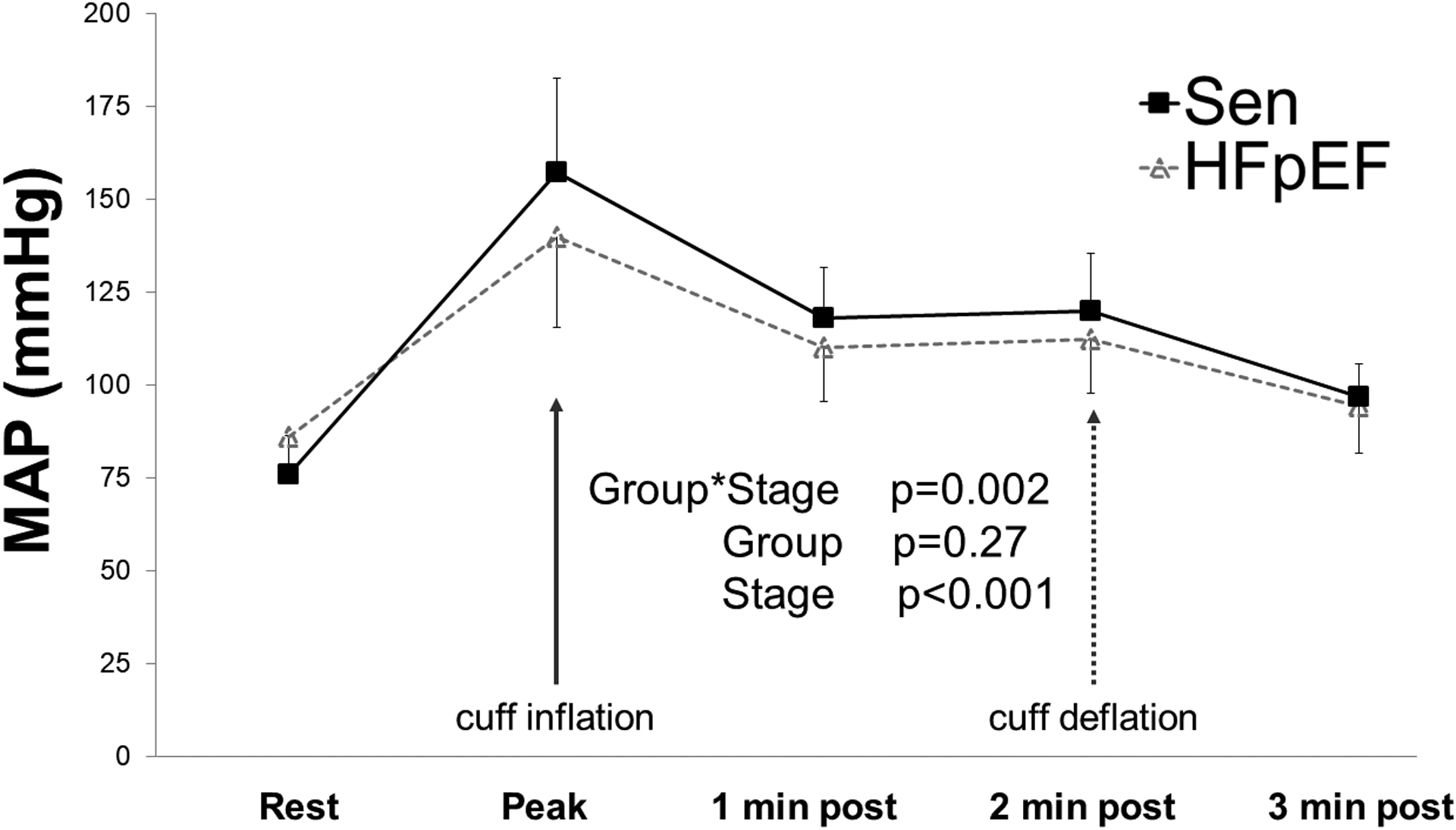
Mean arterial blood pressure (MAP) response during handgrip exercise and immediate post-exercise cuff inflation in patients with HFpEF and senior (Sen) controls. Cuff deflation occurred 2 minutes post exercise. Error bars are standard deviation. Peak MAP corresponds to pressures just prior to cessation of handgrip exercise.
To better understand the relationship between heart rate responses during handgrip and heart rate during whole body exercise, we ran a correlation between these two variables. As a group, heart rate response achieved during handgrip exercise was modestly correlated whole-body exercise (R2 0.33; p<0.001). Within the HFpEF patient group, the relationship between handgrip heart rate and whole body exercise heart rate was stronger (R2 0.57; p<0001).
Discussion
The primary findings of this study are that central command and metaboreceptor function in patients with HFpEF do not differ from healthy senior controls despite significantly lower peak whole-body exercise heart rates. Our evidence demonstrating both normal, healthy central command and metaboreceptor function are the similarity in peak HR during handgrip exercise and the similar metaboreceptor activity as measured in the forearm, respectively. Additionally, we observed a correlation between peak static handgrip HR and whole-body exercise heart rate, suggesting a lesser role for central command in determining heart rate responsiveness in HFpEF. Taken together, these results demonstrate key reflex autonomic pathways regulating exercise heart rate responsiveness are intact in HFpEF.
Increases in heart rate during exercise are regulated primarily by feedforward reflexes from cortical motor centers and afferent signaling from skeletal muscle metaboreceptors and mechanoreceptors. (Figure 2) Central command, a feed-forward signal originating from the anterior cingulate cortex, is activated at the onset of volitional exertion to increase heart rate in proportion to perceived effort.10 Activation of metaboreceptors, located within skeletal muscle in response to metabolites released from metabolic active muscle 11allows for careful matching of metabolic demand and oxygen delivery at a given exercise work rate whilst central command contextualizes the relative perception of effort onto the corresponding sympathetic outflow.12, 13 Activation of these reflexes results in parasympathetic withdrawal and increased sympathetic outflow leading to increases in heart rate, vasoconstriction of non-exercising vascular beds and cardiac contractility.14, 15 Both central command and metaboreceptor reflexes are highly influenced by the duration and intensity of exercise and thus are able to “fine tune” heart rate responses for a given exercise intensity.
Figure 2:
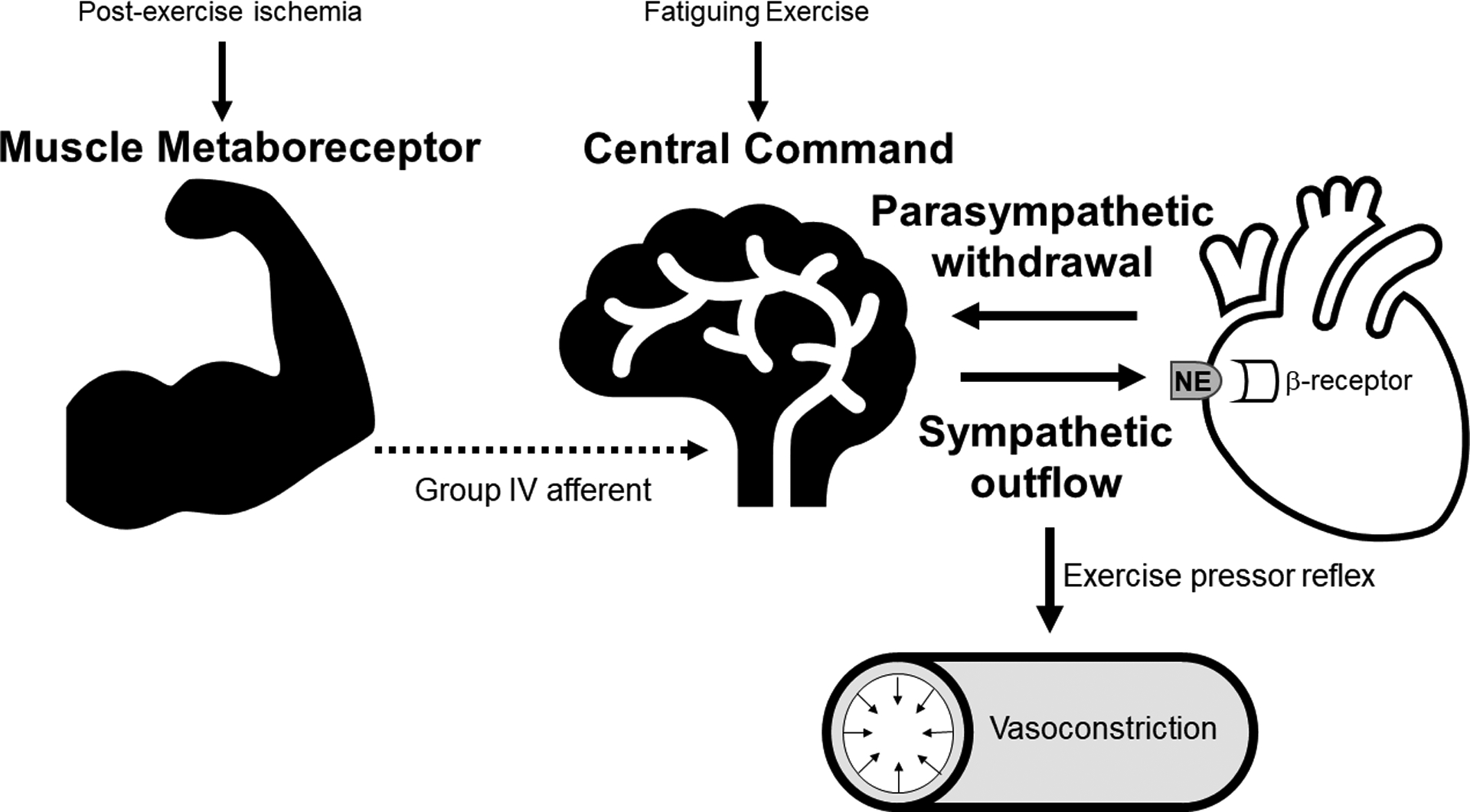
Schematic overview of autonomic reflex pathways controlling heart rate and blood response during exercise. Activation of central command is proportional to exercise intensity and increases heart rate early in exercise as a result of parasympathetic withdrawal. Metaboreceptors within skeletal muscle are activated by skeletal muscle metabolic by-products, sensed by group IV afferent receptors. The corresponding increase in sympathetic outflow from metaboreceptor activation are transduced via β- and α- receptors to increase in heart rate and vasoconstriction, further increasing heart rate and blood pressure during exercise.
There is limited understanding of central command and metaboreceptor function in heart failure, particularly HFpEF, in humans. Animal models of chronic systolic heart failure using decerebrate rats have shown increased sympathetic outflow after cerebral stimulation compared to control animals suggesting central command is increased for a given stimulus.16 Human studies have shown conflicting results with studies showing increased17 or no change18 in heart rate responses to static handgrip. Some of the differences in findings may be attributable to the degree of heart failure severity, with more severe heart failure stages showing heightened sympathetic outflow for similar metabolic work. Assessments of metaboreceptor function in systolic heart failure similarly shows heterogenous results with studies showing either heightened19, 20 or unchanged21–23 metaboreceptor activation highlighting the difficulties in studying a heterogenous population. The function of these reflexes in HFpEF, particularly as it relates to regulation of exercise heart rate has not been previously studied.
The static handgrip exercise to fatigue model with post-exercise circulatory arrest is a well validated approach to assessing the integrity of central command and metaboreceptor function. As shown in figure 2, the onset of exercise, handgrip in this case, activates central command leading to para-sympathetic withdrawal. The resultant increase in heart rate scales to the perception of effort, reaching a maximum just prior to muscle fatigue. Exercising to fatigue establishes a similar relative metabolic endpoint and helps to normalize differences in exercising forearm muscle mass.24 While we did not observe group significant differences between senior controls and patients with HFpEF in MVC strength or total duration of handgrip exercise to failure time, there was a wide range in MVC (range 16 – 44 kg) and handgrip duration (range 131 – 480 seconds) amongst subjects. Subjects with lower MVCs tended to have longer handgrip times to fatigue. Neither MVC nor handgrip duration were associated with peak handgrip heart rate suggesting subjects experienced similar perceptions of fatigue just prior to the cessation of exercise. Peak heart rates observed in our study were similar to other studies, with a typical reported increase of 15–24 bpm above baseline heart rate.25–27 While peak heart rates at the end of handgrip were similar between senior controls and patients with HFpEF, the change in heart rate from rest to peak handgrip exercise was lower by 11 bpm in HFpEF patients. One possible explanation for this difference is that resting heart rate prior to start of handgrip was 8 bpm higher in HFpEF than controls. The majority of static handgrip studies have shown similar peak handgrip heart rate of 80–90 bpm as we observed raising the possibility of a ceiling effect in heart rate responsiveness from forearm exercise. Differences in resting heart rate are therefore more likely to have an impact on changes in heart rate response during handgrip. The exact mechanism for the higher resting heart rate is unclear but may be an effect of β-blocker withdrawal.28 Ninety percent of patients with HFpEF in our study were taking β-blockers chronically and were asked to hold 5 doses prior to testing.
Forearm metaboreceptor function was also not different between groups. Mean arterial blood pressure after 2 minutes of PECA were similar between senior controls and patients with HFpEF. Our findings are consistent with reports of preserved metaboreceptor function in patients with heart failure with reduced ejection fraction (HFrEF).18, 21 Interestingly, there was a significant interaction effect of MAP during handgrip and PECA favoring higher MAPs in control subjects than HFpEF. This effect was largely driven by a nominally higher but variable MAP during peak handgrip exercise. One potential explanation for higher MAPs in the control subjects is the higher prevalence of anti-hypertensive therapies in the HFpEF group. Patients with HFpEF were more likely to be on vasodilatory medications which could have contributed to a blunting of blood pressure response during isometric exercise. Alternatively, metaboreceptor sensitivity could be reduced in chronic HFpEF similar to what has been observed in HFrEF.29
Overall our results demonstrate key autonomic reflexes controlling exercise heart rate response in HFpEF are preserved relative to healthy seniors and suggest afferent and efferent feedforward pathways during exercise are not compromised. These findings, in context of our previous work3 demonstrating a blunting of β-receptor stimulation in some patients with HFpEF, suggest that chronotropic incompetence is not a result of insufficient excitatory sympathetic drive or centrally mediated parasympathetic withdrawal. Rather decreased cardiac β-receptor transduction or premature cessation of exercise due to excessive dyspnea may be alternative mechanisms responsible for chronotropic incompetence.
There are limitations to our study that should be noted. First, we did not measure muscle sympathetic nerve activity (MSNA) in subjects. MSNA levels would have been helpful to establish whether the relative change in efferent signaling during handgrip exercise was similar between groups or whether patients with HFpEF had a larger increase in MSNA suggesting less efficient transduction of cardiac β-receptors. Second, we ignored the effects of muscle mechanoreceptors which also contribute to increased sympathetic signaling and cannot rule out abnormalities in mechanoreceptor contributions to exercise heart rate. Lastly, metaboreceptor sensitivity measured in the forearm may not be representative of metaboreceptor function in the legs which elicit larger heart rate and ventilatory responses to exercise.30 However in contrast to leg exercise, forearm exercise is less likely to alter venous return to the heart or introduce large changes in vascular conductance and may be better at isolating central command and metaboreceptor pathways.
Conclusion
Exercise tolerance is a prominent symptom in patients with HFpEF. Central command and metaboreceptor signaling are important neural reflexes regulating exercise heart rate responsiveness and have not been studied in HFpEF. Despite significantly lower peak whole-body exercise heart rate, our study demonstrated these key autonomic reflexes controlling exercise heart rate response in HFpEF were preserved relative to healthy seniors. Our results suggest afferent metaboreceptor function and efferent feedforward pathways arising from central command during exercise are not compromised and thus do not contribute to observed chronotropic incompetence in patients with HFpEF.
Clinical Perspective:
What is new?
We assessed the integrity of autonomic reflexes controlling exercise heart rate in patients with heart failure with preserved ejection fraction (HFpEF) and senior controls.
Afferent metaboreceptor function and efferent feedforward pathways arising from central command during exercise were not different between patients with HFpEF and senior controls.
What are the clinical implications?
Chronotropic incompetence is common in patients with HFpEF and thought to contribute to exercise intolerance.
Our findings demonstrate upstream autonomic control of exercise heart rate is not impaired in patients with HFpEF and thus does not contribute to observed chronotropic incompetence in these patients.
Our findings further emphasize the role of cardiac β-receptor responsiveness in transducing upstream sympathetic signaling in increasing exercise heart rate.
Acknowledgements:
We would like to sincerely thank Sheryl Livingston for providing nursing support throughout the testing procedures.
Sources of funding: This project was supported by the National Institutes of Health (AG017479).
Nonstandard abbreviations:
- MAP
mean arterial pressure
- VO2
oxygen consumption
- RER
respiratory exchange ratio
- MVC
maximal voluntary contraction
- PECA
post exercise circulatory arrest
Footnotes
Disclosure: None
References
- 1.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD and Lewis GD. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victor RG, Secher NH, Lyson T and Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circulation research. 1995;76:127–131. [DOI] [PubMed] [Google Scholar]
- 3.Sarma S, Stoller D, Hendrix J, Howden E, Lawley J, Livingston S, Adams-Huet B, Holmes C, Goldstein DS and Levine BD. Mechanisms of Chronotropic Incompetence in Heart Failure With Preserved Ejection Fraction. Circulation Heart failure. 2020;13:e006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandevia SC and Hobbs SF. Cardiovascular responses to static exercise in man: central and reflex contributions. J Physiol. 1990;430:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM and Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American journal of cardiology. 2004;93:1473–1480. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Zuckerman JH, Giller CA and Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. The American journal of physiology. 1998;274:H233–241. [DOI] [PubMed] [Google Scholar]
- 7.Stok WJ, Westerhof BE, Guelen I and Karemaker JM. Aortic pressure wave reconstruction during exercise is improved by adaptive filtering: a pilot study. Med Biol Eng Comput. 2011;49:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D and Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. [DOI] [PubMed] [Google Scholar]
- 9.Hardin EA, Stoller D, Lawley J, Howden EJ, Hieda M, Pawelczyk J, Jarvis S, Prisk K, Sarma S and Levine BD. Noninvasive Assessment of Cardiac Output: Accuracy and Precision of the Closed-Circuit Acetylene Rebreathing Technique for Cardiac Output Measurement. J Am Heart Assoc. 2020;9:e015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB and Morgan WP. Brain activation by central command during actual and imagined handgrip under hypnosis. Journal of applied physiology. 2002;92:1317–1324. [DOI] [PubMed] [Google Scholar]
- 11.Murphy MN, Mizuno M, Mitchell JH and Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. American journal of physiology Heart and circulatory physiology. 2011;301:H1191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell JH. Neural control of the circulation during exercise: insights from the 1970–1971 Oxford studies. Exp Physiol. 2012;97:14–9. [DOI] [PubMed] [Google Scholar]
- 13.Badrov MB, Olver TD and Shoemaker JK. Central vs. peripheral determinants of sympathetic neural recruitment: insights from static handgrip exercise and postexercise circulatory occlusion. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1013–R1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White DW and Raven PB. Autonomic neural control of heart rate during dynamic exercise: revisited. The Journal of physiology. 2014;592:2491–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A and Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc. 2003;35:221–228; discussion 229. [DOI] [PubMed] [Google Scholar]
- 16.Koba S, Gao Z, Xing J, Sinoway LI and Li J. Sympathetic responses to exercise in myocardial infarction rats: a role of central command. Am J Physiol Heart Circ Physiol. 2006;291:H2735–2742. [DOI] [PubMed] [Google Scholar]
- 17.Negrao CE, Rondon MU, Tinucci T, Alves MJ, Roveda F, Braga AM, Reis SF, Nastari L, Barretto AC, Krieger EM and Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart Circ Physiol. 2001;280:H1286–1292. [DOI] [PubMed] [Google Scholar]
- 18.Carrington CA, Fisher WJ, Davies MK and White MJ. Muscle afferent and central command contributions to the cardiovascular response to isometric exercise of postural muscle in patients with mild chronic heart failure. Clin Sci (Lond). 2001;100:643–651. [PubMed] [Google Scholar]
- 19.Piepoli MF and Coats AJ. Increased metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol. 1985. 2007;102:494–496; [DOI] [PubMed] [Google Scholar]
- 20.Notarius CF, Atchison DJ and Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–976. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MAH, Nativi-Nicolau J, Stehlik J, Richardson RS and Wray DW. Metaboreceptor activation in heart failure with reduced ejection fraction: Linking cardiac and peripheral vascular haemodynamics. Exp Physiol. 2018;103:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A and Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101:784–789. [DOI] [PubMed] [Google Scholar]
- 23.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB and Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. [DOI] [PubMed] [Google Scholar]
- 24.Seals DR, Chase PB and Taylor JA. Autonomic mediation of the pressor responses to isometric exercise in humans. Journal Appl Physiol. 1988;64:2190–2196. [DOI] [PubMed] [Google Scholar]
- 25.Fu Q, Levine BD, Pawelczyk JA, Ertl AC, Diedrich A, Cox JF, Zuckerman JH, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC Jr. Cooke WH, Robertson RM, Baisch FJ, Blomqvist CG, Eckberg DL, Robertson D and Biaggioni I. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J Physiol. 2002;544:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD and Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark AL, Victor RG, Nerhed C and Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. [DOI] [PubMed] [Google Scholar]
- 28.Gilligan DM, Chan WL, Stewart R and Oakley CM. Adrenergic hypersensitivity after beta-blocker withdrawal in hypertrophic cardiomyopathy. Am J Cardiol. 1991;68:766–772. [DOI] [PubMed] [Google Scholar]
- 29.Piepoli MF and Crisafulli A. Pathophysiology of human heart failure: importance of skeletal muscle myopathy and reflexes. Exp Physiol. 2014;99:609–615. [DOI] [PubMed] [Google Scholar]
- 30.Scott AC, Davies LC, Coats AJ and Piepoli M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci (Lond). 2002;102:23–30. [PubMed] [Google Scholar]


