Abstract
In cartilage tissue engineering, one key challenge is for regenerative tissue to recapitulate the biomechanical functions of native cartilage while maintaining normal mechanosensitive activities of chondrocytes. Thus, it is imperative to discern the micromechanobiological functions of the pericellular matrix, the ~ 2-4 μm-thick tissue domain in immediate contact with chondrocytes. In this study, we discovered that decorin, a small leucine-rich proteoglycan, is a key determinant of cartilage pericellular matrix micromechanics and chondrocyte mechanotransduction in vivo. The pericellular matrix of decorin-null murine cartilage developed reduced content of aggrecan, the major chondroitin sulfate proteoglycan of cartilage and a mild increase in collagen II fibril diameter vis-à-vis wild-type controls. As a result, decorin-null pericellular matrix showed a significant reduction in micromodulus, which became progressively more pronounced with maturation. In alignment with the defects of pericellular matrix, decorin-null chondrocytes exhibited decreased intracellular calcium activities, [Ca2+]i, in both physiologic and osmotically evoked fluidic environments in situ, illustrating impaired chondrocyte mechanotransduction. Next, we compared [Ca2+]i activities of wild-type and decorin-null chondrocytes following enzymatic removal of chondroitin sulfate glycosaminoglycans. The results showed that decorin-mediated chondrocyte mechanotransduction primarily through regulating the integrity of the aggrecan network, and thus, aggrecan-endowed negative charge microenvironment in the pericellular matrix. Collectively, our results provide robust genetic and biomechanical evidence that decorin is an essential constituent of the native cartilage matrix, and suggest that modulating decorin activities could improve cartilage regeneration.
Keywords: Pericellular matrix, decorin, chondrocyte, nanomechanics, mechanotransduction
INTRODUCTION
A key paradox in cartilage regeneration is that while a soft mechanical environment is required for maintaining chondrocyte phenotype [1], a much higher modulus is needed for engineered products to recapitulate the function of native tissue [2]. When cultured in vitro, chondrocytes are encapsulated in soft hydrogels with modulus ~ 10 kPa or less to maintain cell viability and prevent de-differentiation [1]. In this environment, while chondrocytes can synthesize major cartilage extracellular matrix (ECM) constituents, namely type II collagen and aggrecan, these molecules do not assemble into the hierarchical structure of the native ECM, thereby failing to fully restore the biomechanical properties of native tissue [3]. In vivo, chondrocytes reside within the pericellular matrix (PCM), a structurally distinctive, ~ 2-4 μm-thick cell-ECM intermediary [4]. The PCM is pivotal in transmitting biomechanical, biophysical and biological signals between the ECM and cells [5, 6], and is where the initial assembly of newly synthesized matrix molecules takes place [7, 8]. In healthy human cartilage, the modulus of PCM is ~ 50 kPa [9], much higher than that of the canonical hydrogel environment [1]. Despite being surrounded by a much stiffer matrix, residing chondrocytes can sustain their normal metabolic activities during development and maintenance in vivo. In osteoarthritis (OA), degeneration of the PCM is one of the earliest events upon disease initiation, leading to aberrant chondrocyte mechanotransduction, which contributes to the vicious loop of irreversible cartilage degeneration [9-11]. Understanding the biomechanical and biophysical characteristics of the native PCM will provide a much needed benchmark for engineered tissues to better recapitulate the native microniche of chondrocytes [12]. In addition, it is increasingly evident that changes in the ECM are the driving force of most human diseases, both congenital and acquired [13-15]. Knowledge about cartilage PCM could thus provide new insights into the roles of immediate cell micro-niche in other diseases as well [16-18].
In the past decades, there have been significant advances in understanding the roles of individual PCM biomolecules in cartilage health and disease [19], including type VI collagen [20-22], perlecan [23-25], biglycan [26, 27] and matrilins [28-31]. Despite these efforts, the assembly, structure and mechanobiological functions of PCM remain poorly understood [5]. It is unclear how the native PCM maintains normal chondrocyte activities despite being much stiffer than the in vitro chondrogenic microenvironment. One distinctive feature of the native PCM is the preferential localization of specific proteoglycans [32]. In particular, aggrecan, the major proteoglycan of cartilage, is more concentrated in the PCM [33], where it undergoes faster turnover [34], and with newly synthesized aggrecan mainly localized there [35]. Aggrecan has a bottle-brush architecture, consisting of ~ 400 nm-long core protein decorated with >100 densely packed, ~ 40 nm-long chondroitin sulfate glycosaminoglycan (CS-GAG) side chains. In cartilage, aggrecan contributes to > 90% of CS-GAGs and total fixed charges [19], and endows the matrix with its highly negatively charged environment [36]. Although the contribution of aggrecan to cartilage tissue-level biomechanics has been well documented [37], it is unclear whether or how aggrecan and its fixed negative charges impact the pericellular microenvironment and chondrocyte mechanosensing in vivo. It is also unclear how the structural integrity of aggrecan is maintained in the PCM, for that the primary assembly mechanism of aggrecan network, the link protein-assisted aggrecan-hyaluronan (HA) aggregation [38], does not fully address the diversity or changes in the retention of aggrecan at different development stages and disease states, or the preferential distribution of aggrecan in the PCM [35].
Decorin, a small leucine-rich proteoglycan, could play an important role in regulating the integrity of aggrecan in the PCM. Our recent studies showed that in decorin-null (Dcn−/−) mice, loss of decorin leads to markedly reduced retention of aggrecan in the ECM, resulting in impaired cartilage biomechanical properties [39] and increased susceptibility to surgery-induced OA [40]. We further showed that decorin primarily functions as a “physical linker” to increase the molecular adhesion between aggrecan-aggrecan and aggrecan-collagen II fibrils, thereby strengthening the integration of aggrecan networks without directly affecting chondrocyte biology or aggrecan biosynthesis [39]. In adult cartilage ECM, decorin and aggrecan are present in both the PCM and the territorial/interterritorial ECM (T/IT-ECM) that is further removed from cells [39]. We thus hypothesize that decorin is required for maintaining the integrity of aggrecan networks in the PCM. We tested this hypothesis by studying the nanostructural and micromechanical phenotype of Dcn−/− cartilage PCM. We further queried if loss of decorin disrupts chondrocyte mechanotransduction by assessing the intracellular calcium signaling activities, [Ca2+]i, in situ, which are one of the earliest, fundamental cell responses to mechanical stimuli [41]. Then, by studying [Ca2+]i signaling under enzymatic removal of CS-GAGs, we tested if the impact of decorin on chondrocytes is manifested through its regulation of aggrecan assembly in the PCM. Our findings suggest that the aggrecan-rich, highly negatively charged PCM microenvironment is essential for maintaining normal chondrocyte mechanosensitive activities, and decorin plays an important role in regulating the integrity of aggrecan in this critical microdomain.
RESULTS
Dcn−/− cartilage pericellular matrix exhibits altered nanostructure and sGAG content.
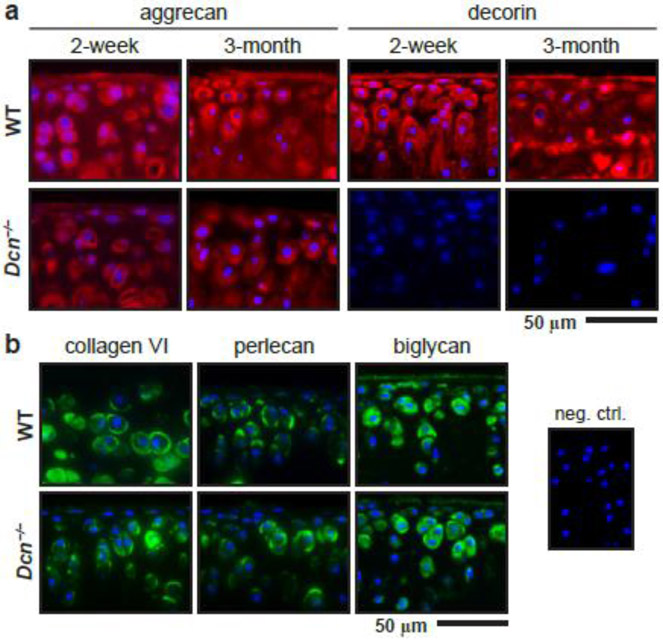
We first studied the impact of decorin loss on the morphology and structure of cartilage PCM. Specifically, we compared the distributions of key PCM biomolecules and the morphology of PCM between age-matched Dcn−/− and wild-type (WT) mice via immunofluorescence (IF) staining. In WT cartilage, aggrecan and decorin were distributed throughout the ECM, as show for mice at both 2 weeks and 3 months of ages (Fig. 1a). The staining intensity of aggrecan was stronger in the pericellular domain, corroborating literature showing higher concentration of aggrecan in the PCM [33, 42, 43]. Similarly, the staining of decorin was also stronger in the pericellular domain, which was in alignment with the more intense staining of decorin reported in the middle zone of adult bovine cartilage [42] and adult rabbit knee cartilage [44]. To this end, additional imaging assays would help delineating the localization pattern of decorin in the PCM versus T/IT-ECM at different ages and within different tissue regions. In addition, we imaged the three canonical biomarkers of cartilage PCM, collagen VI, perlecan and biglycan. All three molecules were found to localized in the PCM in both WT and Dcn−/− cartilage at 2 weeks of age (Fig. 1b).
Figure 1.
Distribution of extracellular matrix biomolecules in wild-type (WT) and decorin-null (Dcn−/−) cartilage via immunofluorescence (IF) imaging. a) IF images illustrate the reduced staining of aggrecan core protein and the absence of decorin in Dcn−/− cartilage at 2 weeks and 3 months of ages. b) IF images show similar distribution of pericellular matrix (PCM) biomarkers, type VI collagen, perlecan and biglycan, in WT and Dcn−/− cartilage at 2 weeks of age (blue: DAPI; shown together was the negative control).
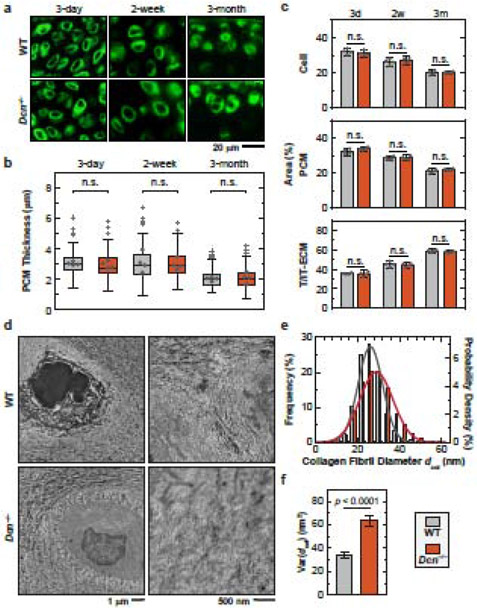
Applying immunolabeling of collagen VI to unfixed cartilage cryo-sections, we estimated the thickness of PCM in WT and Dcn−/− cartilage at 3 days (newborn), 2 weeks (immature) and 3 months (adult) of ages (Fig. 2a,b). We also calculated the areas occupied by cells, PCM and T/IT-ECM on the sections, which approximately correspond to the volume proportions of the three domains (Fig. 2c). During maturation, the thickness of cartilage PCM, as well as volume proportions of the cell and PCM, decreased gradually, while the proportion of the T/IT-ECM increased (Fig. 2c, Table S1). These results illustrate that during post-natal growth, cartilage evolves from a highly cellularized, PCM-rich soft composite to a stiffer tissue that is more dominated by the T/IT-ECM, which is directly responsible for its specialized biomechanical functions at the tissue-level.
Figure 2.
Decorin-null (Dcn−/−) cartilage develops normal PCM morphology and moderate changes of collagen fibrillar nanostructure in the PCM. a) Representative immunofluorescence (IF) images on fresh, unfixed cartilage cryo-sections immunolabeled with type VI collagen at 3 days, 2 weeks and 3 months of ages. b) Box-and-whisker plot of the distribution of cartilage PCM thickness (≥ 120 cells from n = 4 animals for each genotype). c) Comparison of the proportions of areas occupied by the cell, PCM and T/IT-ECM in WT and Dcn−/− cartilage (n = 4). See Table S1 for the complete list of descriptive statistics and adjusted p-values. d) Representative TEM images of collagen fibril structure on the sagittal sections of 3-month-old wild-type (WT) and Dcn−/− cartilage PCM. e) Histogram of fibril diameter distribution (> 1,400 fibrils from n = 4 animals). Shown together were the normal distribution, N(μ, σ2), fits to fibril diameters (for each fit, values of μ and σ correspond to the mean and standard deviation of fibril diameters shown in Table S2). f) Comparison of fibril diameter heterogeneity (variance) between the two genotypes (mean ± 95% CI).
In Dcn−/− cartilage, we did not observe marked up-regulation or altered distribution of collagen VI, perlecan or biglycan (Fig. 1b). We also did not detect any appreciable differences in the thickness of PCM (Fig. 2b), or the volume proportions of cell, PCM and T/IT-ECM domains (Fig. 2c). This suggests that with the loss of decorin, the PCM retains its compositional and morphological distinction from the T/IT-ECM, and PCM-specific molecules are not substantially up-regulated to compensate for the loss of decorin. However, with the loss of decorin, the staining of aggrecan protein core and sGAGs were reduced in both the PCM and T/IT-ECM at 2 weeks and 3 months of age (Fig. 1a). This reduction of aggrecan is consistent with our recent observation that Dcn−/− cartilage exhibits decreased aggrecan and sGAG content at the tissue level [39]. Thus, decorin plays a crucial role in regulating the structural integrity of aggrecan network assembly not only at the tissue level, but also in the pericellular matrix at the microscale.
Next, given that decorin can bind to collagen II fibril surfaces [45], we tested if loss of decorin alters the nanostructure of collagen fibrillar network in the PCM. As measured by TEM on cartilage from 3-month-old mice, for both genotypes, the PCM consists of significantly thinner fibrils relative to the T/IT-ECM (Fig. 2d, Table S2, p < 0.0001). In comparison to the WT, the PCM of Dcn−/− cartilage showed a mild increase in average collagen fibril diameter, dcol (Fig. 2e) but a salient increase in fibril heterogeneity (Fig. 2f). This observation is also consistent with our previous study reporting that the collagen fibrils in Dcn−/− cartilage T/IT-ECM showed no appreciable changes in average dcol, but a substantial increase in heterogeneity (Table S2). Taken together, loss of decorin did not alter the morphology or compositional distinction of cartilage PCM, but significantly reduced the aggrecan content therein, and had a minor effect on collagen fibrillar nanostructure.
Dcn−/− cartilage pericellular matrix develops reduced modulus during post-natal growth.
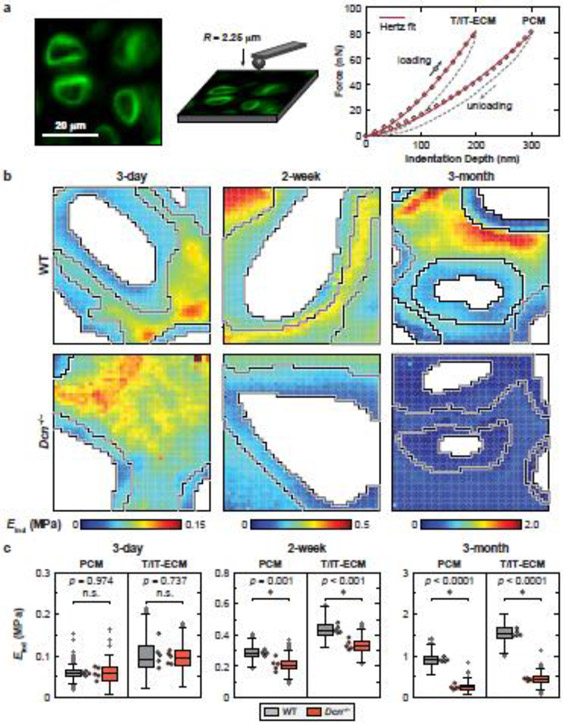
To determine if loss of decorin would affect the micromodulus of PCM, we applied the IF-guided AFM-nanomechanical mapping [10] on fresh, 6-μm-thick, unfixed sagittal cryo-sections of tibial cartilage prepared via the Kawamoto’s film-assisted cryo-sectioning method (Fig. 3a) [46]. Guided by the IF-labelled images of collagen VI, we separated the micromoduli of the PCM and T/IT-ECM, and excluded the values corresponding to cell remnants (Fig. 3b). In WT cartilage, the modulus of PCM was significantly lower than that of the T/IT-ECM at all tested ages (Fig. 3b,c, Table S3). This suggests that as early as 3 days of age, the PCM has become distinct from the tissue bulk, providing chondrocytes with a specialized micromechanical niche that persists throughout post-natal growth and maintenance. This salient micromechanical heterogeneity is consistent with previous findings in porcine [47], human [9] and murine [10, 48] cartilage. From newborn to adult ages, the moduli of both PCM and T/IT-ECM increased significantly, e.g., for the PCM, from 0.058 ± 0.005 MPa (mean ± 95% CI, n = 5) at 3 days of age to 0.285 ± 0.019 MPa at 2 weeks to 0.907 ± 0.056 MPa at 3 months (Fig. 3c), confirming the expected matrix stiffening with skeletal maturation [49].
Figure 3.
Decorin-null (Dcn−/− cartilage exhibits reduced micromodulus in the PCM and T/IT-ECM. a) Left panel: Schematic illustration of immunofluorescence (IF)-guided AFM nanomechanical mapping on the cryo-section of 2-week-old wild-type (WT) murine cartilage using a microspherical tip (R ≈ 2.25 μm); the PCM is immunolabeled with collagen VI. Right panel: Two representative indentation force versus depth (F-D) curves obtained on the 2-week-old WT cartilage cryo-section (measured in PBS, 10 μm/s rate), solid line: finite thickness-corrected Hertz model fit to the entire loading portion of the F-D curve. b) Representative indentation modulus maps of the PCM and T/IT-ECM partitioned for WT and Dcn−/− cartilage at 3 days, 2 weeks and 3 months of ages. Moduli corresponding to cell remnants were removed (white voids). c) Box-and-whisker plots of the PCM and T/IT-ECM micromodulus for WT versus Dcn−/− cartilage at each age (> 1,100 locations for each region, n = 5 animals). Each circle represents the average modulus of one animal, *: p < 0.05 between WT and Dcn−/− cartilage; n.s.: not significant. See Table S3 for the complete list of descriptive statistics and adjusted p-values.
In newborn mice, loss of decorin did not significantly alter the modulus of PCM (0.059 ± 0.018 MPa for Dcn−/− mice, p = 0.974 versus WT), indicating that decorin does not play a critical role in the embryonic development of cartilage matrix. In contrast, at 2 weeks of age, the modulus of Dcn−/− PCM was significantly reduced (0.212 ± 0.048 MPa) in comparison to WT (26 ± 10% lower, p = 0.001), which became more substantial by 3 months of age (0.252 ± 0.072 MPa, 72 ± 13% lower, p < 0.0001). Also, in Dcn−/− mice, from 2 weeks to 3 months of age, the maturation-associated stiffening effect was absent for the PCM (p = 0.167). Similarly, the modulus of T/IT-ECM in Dcn−/− cartilage was also significantly reduced at 2 weeks (23 ± 6%) and 3 months (72 ± 4%) of ages, but not at 3 days of age (Fig. 3c, Table S3). This observation is consistent with our recent finding at the tissue level, in which, the loss of decorin leads to reduced cartilage modulus at more than 1 week of age [39]. Taken together, the marked impairment of PCM micromechanics and lack of maturation-associated stiffening in Dcn−/− cartilage underscore that decorin is indispensable for the establishment of cartilage PCM during post-natal growth.
Dcn−/− chondrocytes exhibit decreased intracellular [Ca2+]i responses in situ.
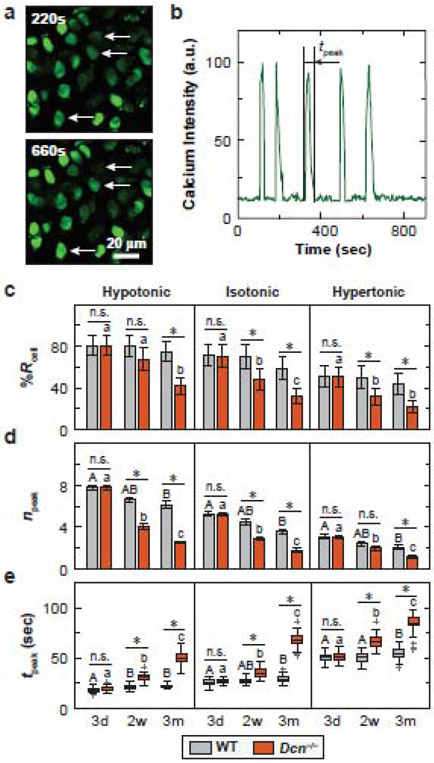
Next, we tested if the impaired PCM, as a result of decorin loss, disrupts chondrocyte mechanotransduction. To directly connect the phenotype of native cartilage PCM with the mechanosensing of chondrocytes in situ, we studied the intracellular calcium signaling, [Ca2+]i, activities of freshly dissected tibia cartilage explants in both physiological (isotonic) and osmotically instigated (hypo- and hypertonic) Dulbecco’s Modified Eagle Medium (DMEM). For both genotypes, we observed spontaneous [Ca2+]i oscillations at all three tested ages, from which, we extracted the temporal [Ca2+]i parameters, including the percentage of responding cells, %Rcell, the total number of [Ca2+]i peaks, npeak, and the duration of averaged peaks, tpeak, from each responding cell over a 15 minute observation period (Fig. 4a,b). In isotonic DMEM, comparing the two genotypes, Dcn−/− chondrocytes had similar [Ca2+]i responses to that of WT at 3 days of age, but showed significantly reduced activities (decreased %Rcell, npeak, and increased tpeak) at both 2 weeks and 3 months of ages, with more pronounced changes at 3 months (Fig. 4c-e, Table S4). The two genotypes also exhibited differentiated responses to maturation. For WT cartilage, there was only a moderate decrease of [Ca2+]i responses with age, where the 3-month group showed significantly lower npeak and longer tpeak than the 3-day group, but no changes were found between other pairs for npeak and tpeak, or among all three ages for %Rcell. In contrast, Dcn−/− cartilage exhibited significantly decreased [Ca2+]i responses between all age pairs and for all three parameters (Fig. 4c-e, Table S5).
Figure 4.
Decorin-null (Dcn−/−) chondrocytes exhibit decreased chondrocyte intracellular Ca2+ activities in situ. a) Representative confocal images of [Ca2+]i signaling in isotonic DMEM for 2-week-old wild-type (WT) cartilage explants. Chondrocytes were labeled with Ca-520™ AM and time series images were recorded using a confocal microscope with a 20× objective submerged in DMEM at 37 °C. b) Representative [Ca2+]i oscillation intensity curve of a single cell over a 15-min time frame illustrating the definition of tpeak, the duration of each peak. c-e) Comparison of [Ca2+]i signaling characteristics between WT and Dcn−/− chondrocytes at each age and osmolarity: c) percentage of responding cells, %Rcell (mean ± 95% CI), d) number of peaks within the 15-min testing time frame, npeak (mean ± 95% CI), e) duration of each peak, tpeak (box-and-whisker plot). Data represent > 40 responding cells pooled from n = 4 animals for each group. *: p < 0.05 between genotypes; n.s.: not significant. For longitudinal comparisons, in WT cartilage, there was no significant difference among all three ages for %Rcell in all three osmolarities. Different letters indicate significant differences between ages within each genotype. See Tables S4-S6 for the complete list of descriptive statistics and adjusted p-values.
Limited by the microscale dimension and irregular shape of murine knee cartilage, we were unable to directly apply well-defined compressive strains. Since the PCM is characterized by high proteoglycan concentration and consequently high negative fixed charge density [5], we applied osmotic stimuli instead. Under hypotonic stimuli, the electrical double layer (EDL) repulsion within the matrix is amplified [50]. At the same time, in situ, the stiffer surrounding PCM limits the spontaneous swelling response of chondrocytes to hypotonicity [51]. Thus, chondrocytes experience enhanced compressive stress, similar to the case of physiologic compression, which decreases the GAG-GAG spacing, resulting in enhanced EDL repulsion and osmotic pressure in the PCM. Conversely, hyper-osmotic stimuli lessen the EDL repulsion in the PCM, and reduce the compressive stress experienced by chondrocytes in situ.
For both genotypes at all three ages, chondrocyte [Ca2+]i responses were significantly enhanced by hypo-osmolarity (increased %Rcell, npeak and/or shorter tpeak), and reduced by hyper-osmolarity (Table S6). Under these osmotically instigated stimuli, Dcn−/− chondrocytes again showed similar [Ca2+]i responses as WT at 3 days of age, but decreased activities at 2 weeks and 3 months (Fig. 4c-e, Table S4). Furthermore, contrasts between the two genotypes were amplified by hypo-osmotic stimuli, as illustrated by a larger percentage of decrease in npeak of Dcn−/− chondrocytes relative to WT (e.g., 59 ± 4% in hypotonic versus 51 ± 3% in isotonic DMEM at 3 months of age), and lessened by hyper-osmolarity (42 ± 9% decrease). For tpeak, the differences were less pronounced, as Dcn−/− chondrocytes showed similar degrees of increase relative to WT in hypotonic (130 ± 6%) and isotonic (135 ± 6%) conditions, and a lesser degree in hypertonic condition (56 ± 6%). Thus, chondrocyte [Ca2+]i responses, as well as their extent of demotion in Dcn−/− cartilage, were both highly correlated with the degree of EDL repulsion and osmotic pressure present in the PCM.
Enzymatic removal of chondroitin sulfate suppresses chondrocyte [Ca2+]i responses in situ.
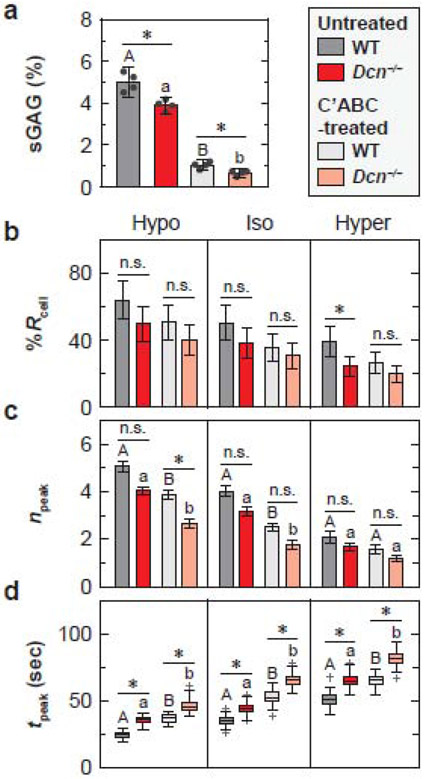
To determine if aggrecan and its CS-GAG chains are a major determinant of chondrocyte mechanosensing in situ, we tested chondrocyte [Ca2+]i signaling between the two genotypes after enzymatic removal of CS-GAGs from 2-week-old murine tibial cartilage. We employed a 12-hour chondroitinase ABC (C’ABC) treatment to induce maximal sGAG removal without significantly affecting cell viability [52]. In addition, using femoral head cartilage of 2-week-old mice, we assessed the impact of C’ABC treatment on sGAG content for both genotypes. In untreated, freshly dissected tissue, Dcn−/− cartilage (3.9 ± 0.4% wet wt., n = 4) showed 22 ± 8% lower sGAG content relative to the WT (5.0 ± 0.7%, p = 0.029, Fig. 5a). Treatment of C’ABC resulted in 84 ± 14% and 80 ± 13% decrease in sGAG content in Dcn−/− (0.7 ± 0.2%) and WT (1.0 ± 0.3%) cartilage, respectively (Fig. 5a). After the treatment, the amount of residual sGAGs in Dcn−/− cartilage was 36 ± 1% lower than that in WT (p = 0.029, Fig. 5a).
Figure 5.
Impact of enzymatic removal of chondroitin-sulfate glycosaminoglycans (CS-GAGs) on chondrocyte intracellular Ca2+ activities in situ. a) Comparison of the amount of sGAGs in chondroitinase ABC (C’ABC)-treated and untreated cartilage explants from wild-type (WT) and decorin-null (Dcn−/−) mice subjected to 12 hours C’ABC treatment at 37°C. b-d) Comparison of [Ca2+]i signaling characteristics between the untreated and C’ABC-treated cartilage at 2 weeks of age in DMEM: b) percentage of responding cells, %Rcell (mean ± 95% CI), c) number of peaks within the 15-min testing timeframe, npeak (mean ± 95% CI), and d) duration of each peak, tpeak (box-and-whisker plot). Data represent > 40 responding cells pooled from n = 4 animals for each group. *: p < 0.05 between genotype within each treatment group (n.s.: not significant). For both WT and Dcn−/− cartilage, there was no significant difference between treatment groups for %Rcell in all three osmolarities. Different letters indicate significant differences between treatment groups within each genotype. See Tables S7-S8 for the complete list of descriptive statistics and adjusted p-values.
For both genotypes, enzymatic removal of sGAGs did not alter %Rcell of [Ca2+]i responses, but significantly reduced temporal characteristics, e.g., lower npeak in hypo- and isotonic DMEM, and longer tpeak in all three osmolarities (Fig. 5b-d, Tables S7 and S8). This confirms that CS-GAGs on aggrecan, and their associated fixed charges regulates chondrocyte mechanosensing in situ. As such, chondrocytes in Dcn−/− cartilage still exhibited decreased [Ca2+]i responses in comparison to WT after the CS-GAG removal, as signified by lower npeak in hypo- and isotonic conditions, and longer tpeak in all three osmolarities (Fig. 5b-d, Tables S7 and S8). This effect was more conspicuous when EDL repulsion was amplified by hypo-osmolarity, as illustrated by the more pronounced differences in npeak (Fig. 5c). Thus, the contrasts between C’ABC-treated and untreated groups for both genotypes, and between WT and Dcn−/− groups for both treatment conditions, both suggest that fixed charges in cartilage matrix, as endowed by aggrecan, contribute to maintaining the normal [Ca2+]i activities of chondrocytes in situ.
DISCUSSION
Role of decorin in the integrity and micromechanobiology of cartilage PCM.
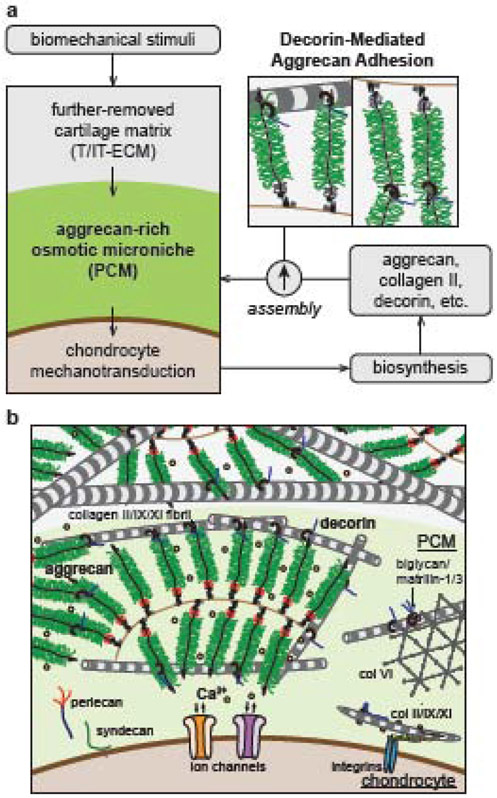
This study highlights a key role of decorin in regulating the integrity and mechanobiological functions of cartilage PCM, the immediate microenvironment of chondrocytes (Fig. 6). Previously, we found that decorin regulates the assembly of aggrecan networks and tissue-level biomechanics of cartilage ECM by increasing the molecular adhesion between aggrecan-aggrecan and aggrecan-collagen II fibrils [39]. This study shows that this regulatory role of decorin is essential for not only cartilage biomechanics at the tissue level, but also the integrity and mechanobiological functions of PCM at the microscale. In Dcn−/− cartilage, loss of decorin does not affect the compositional distinction between PCM and T/IT-ECM, the thickness of PCM or the PCM-to-T/IT-ECM volume ratio (Figs. 1b, 2a-c), but results in reduced aggrecan staining (Fig. 1a) and micromodulus (Fig. 3) of the PCM, as well as decreased chondrocyte [Ca2+]i activities in situ (Fig. 4). Here, the loss of aggrecan and impairment of PCM micromechanics are resulted from decorin directly regulating the aggrecan network assembly, rather than a secondary effect from the moderate changes of collagen fibril nanostructure (Fig. 2) [39]. In other genetically modified mice deficient of key collagen fibrillar constituents, such as collagens IX [53], XI [54] and III [48], despite the presence of much more pronounced structural defects in collagen fibrils, the amount of aggrecan and sGAGs in cartilage appeared to be normal.
Figure 6.
Schematic illustration of the working hypothesis on the structural role of decorin in cartilage pericellular matrix (PCM). a) Decorin regulates the structural integrity of PCM by mediating the molecular adhesion of aggrecan-aggrecan and aggrecan-collagen II fibrils therein. As a result, decorin regulates the PCM-mediated transmission of biomechanical stimuli from the extracellular matrix to chondrocytes, and thus, the mechanotransduction of chondrocytes. b) Schematic illustration of cartilage matrix molecular constituents, highlighting the crucial structural role of decorin in regulating both the PCM and T/IT-ECM. The schematics are inspired by Ref. [5, 19, 39].
In comparison to the T/IT-ECM that is further-removed from chondrocytes, the PCM has thinner collagen fibrils (Table S2), which provides a greater surface-to-volume ratio for decorin to interact with. This interaction could facilitate the physical connection between aggrecan and collagen fibrils through decorin, thereby enhancing the retention of aggrecan in the PCM (Fig. 6). Such mechanism could thus help explain why aggrecan is more concentrated in the PCM despite its ubiquitous presence throughout cartilage matrix. In addition, decorin binds to aggrecan through PCM-specific molecules including collagen VI and matrilin-1 [55, 56], which may further strengthen decorin-aggrecan interactions in the PCM. These interactions, however, may not be essential, for that neither Col6a1−/− [21] nor matrilin-null murine cartilage (Matn1−/− [28, 29], Matn3−/− [30] and Matn1-4−/− [31]) yielded the same phenotype of salient aggrecan reduction as in Dcn−/− cartilage. Meanwhile, the lack of phenotype in newborn Dcn−/− cartilage PCM (Fig. 3b,c) suggests that decorin is not required for initiating the aggrecan network assembly during embryonic development. However, during post-natal growth, the “physical linkage” provided by decorin becomes increasingly important, when cartilage experiences extensive joint loading, and the associated interstitial fluid flow could aggravate the diffusive loss of aggrecan in the absence of decorin and its stabilizing effect on aggrecan network.
We further showed that the integrity of aggrecan network in the PCM, as regulated by decorin, is required for normal mechanotransduction of chondrocytes. Chondrocytes are highly sensitive to their mechanical environment, and [Ca2+]i activities are one of the earliest, fundamental events in their responses to mechanical stimuli [41]. Transmembrane ion channels, such as transient receptor potential vanilloid-4 (TRPV4) [57] and mechanosensitive ion channels Piezo1/2 [58], are activated when chondrocyte cell membrane experiences external stimuli under deformation or osmotic stress. These activities are critical in mediating cartilage homeostasis and disease pathogenesis [59]. In situ, their activation is regulated by the immediate cellular microniche, i.e., the PCM. In the PCM, aggrecan and its CS-GAGs provide strain shielding for chondrocytes, and couple mechanical loading with interstitial osmolarity [60]. Meanwhile, the negatively charged environment sequesters counter ions, providing the immediate extracellular Ca2+ sources to mediate the activation of ion channels [61]. Here, the decreased chondrocyte [Ca2+]i responses in situ under CS-GAG removal (Fig. 5) highlights the importance of PCM aggrecan in maintaining chondrocyte mechanotransduction. In alignment with this effect, in the absence of decorin, when the reduction of aggrecan and impairment of PCM micromechanics are amplified with maturation (Fig. 3), demotion of chondrocyte [Ca2+]i responses also becomes increasingly pronounced (Fig. 4). These results together support an important role of decorin in regulating chondrocyte mechanotransduction in vivo by mediating its immediate microenvironment. To this end, while the exact biological pathways by which [Ca2+]i activities influence downstream signaling are not fully understood, [Ca2+]i activities have been shown to be positively correlated with chondrocyte anabolism [62], and demotion of [Ca2+]i signaling is one of the earliest events preceding the onset of post-traumatic OA [10]. Therefore, although decorin appears not to directly influence chondrocyte biosynthesis in the absence of mechanical stimuli in both alginate [39] and explant [40] cultures, it is possible that, by regulating the integrity of the PCM, decorin could impact the mechanosensitive activities of chondrocytes, and thus, the establishment and homeostasis of cartilage matrix in vivo (Fig. 6).
Comparison to the activities of other PCM-specific molecules in cartilage.
This structural role of decorin is distinct from the known activities of other PCM-specific biomolecules. In the PCM, collagen VI forms a hexagonal beaded network, and is found to regulate PCM micromodulus, chondrocyte swelling, [Ca2+]i signaling in situ [22], as well as integrin α1β1-mediated chondrocyte-matrix interactions [63]. However, loss of collagen VI does not alter the properties of T/IT-ECM [63], and leads to variable responses to OA in Col6a1−/− mice [20-22], indicating its impact is largely limited to the integrity of PCM, rather than the ECM as a whole. In contrast, decorin impacts both the PCM and T/IT-ECM (Fig. 3), and loss of decorin aggravates cartilage fibrillation and degradation in OA [40], suggesting a more essential role of decorin in the overall health of cartilage. Perlecan is a heparan-sulfate proteoglycan that directly regulates cell surface mechanosensing [64] and activation of fibroblast growth factor-2 (FGF-2) [65], Reduction of perlecan in Hspg2+/− mice results in impaired matrix development in embryonic joints [66]. Decorin, on the other hand, is not required for embryonic development, and only becomes more important during post-natal growth. Biglycan, another class I SLRP, is highly structurally homologous to decorin, but harbors two, instead of one, CS/DS-GAG chains near its N-terminus [67]. While decorin and biglycan have coordinated and compensatory activities in tendon [68, 69] and cornea [70], their roles in cartilage are distinct. The importance of biglycan to cartilage health is illustrated by accelerated spontaneous OA in Bgn−/− mice [26, 27], while soluble biglycan in cartilage may have an adverse effect by evoking inflammation through toll-like receptor-4 (TLR-4) [71]. Unlike decorin, biglycan primarily plays biological roles in regulating chondrocyte signaling. The absence of biglycan in the T/IT-ECM of Dcn−/− cartilage (Fig. 1b) also suggests that biglycan is not up-regulated to compensate for the loss of decorin, unlike the cases of tendon and cornea [68-70].
Comparison to other studies of Dcn−/− murine cartilage.
Our finding that Dcn−/− cartilage has lower micromodulus in both the PCM and T/IT-ECM than the WT control (Fig. 3) is different from the previous work by Gronau et al., which reported a higher modulus of Dcn−/− cartilage [72]. We attribute such contrast to differences in sample preparation, storage, length scale and geometry of AFM indenters, as well as the data analysis methods used in these studies [39]. In Gronau et al., AFM-nanoindentation was performed on 30-μm-thick cartilage cryo-sections using pyramidal tips (nominal radius R ≈ 20 nm, half open angle ≈ 20°), and the stiffness values are suggested to represent local micromechanics of individual ECM proteoglycans and collagen fibrils [73]. In this study, AFM-nanomechanical mapping was performed using microspherical indenter tips (R ≈ 2.25 μm) with a maximum depth of Dmax ≈ 0.5 μm, yielding a ≈ 1.4 μm effective tip-sample contact radius, or ≈ 6.3 μm2 contact area. Given that each aggrecan molecule consists of a ≈ 400 nm-long core protein with ≈ 40 nm-long CS-GAGs [37], the micromodulus of PCM reflects the integrated response of a few aggrecan molecules, collagen fibrils (≈ 30-80 diameter [39]) and other minor matrix constituents at the microscale, rather than that of individual matrix molecules. In addition, guided by the immunolabelling of collagen VI, we partitioned the micromodulus map into three distinct domains, i.e., the T/IT-ECM, PCM and cell remnants. As discussed in our recent study, this partition is necessary for accurately assessing the local micromodulus of biological tissues with high cell density and spatial heterogeneity [10]. For example, moduli measured on domains corresponding to chondrocytes represent cytoplasma and nucleus remnants damaged during cryo-sectioning, and inclusion of these data would substantially underestimate the micromodulus of cartilage matrix [10]. With this IF-guided partitioning, for both WT and Dcn−/− cartilage, the micromodulus of T/IT-ECM measured here (Table S3) are in quantitative agreement with the tissue-level modulus reported in our previous study (WT: 0.22 ± 0.08 MPa, 0.60 ± 0.06 MPa, 1.45 ± 0.21 MPa, Dcn−/− 0.19 ± 0.05 MPa, 0.39 ± 0.05 MPa, 0.46 ± 0.06 MPa at 3 days, 2 weeks and 3 months of ages, respectively) [39]. Furthermore, the micromodulus of 3-month-old WT cartilage T/IT-ECM measured here (1.54 ± 0.10 MPa) is also similar to the tissue-level modulus of young adult, healthy murine cartilage previously measured by AFM-nanoindentation [74-77] and instrumented microindentation [20, 78, 79], which are all in the range of ~ 1-2 MPa.
Potential application of decorin as a target for cartilage regeneration.
In cartilage tissue engineering, biosynthesis of aggrecan and collagen II is often elevated by external biochemical and/or biophysical cues [80]. Given the mechanosensitive nature of chondrocytes, dynamic loading is one most commonly used biomechanical stimulus [81]. In this regard, both partially preserved native PCM or pre-deposited neo-PCM can help transmit mechanical signals, enhancing chondrocyte anabolic responses to dynamic loading [82]. On the other hand, dynamic loading and associated interstitial fluid flow often aggravate the loss of newly synthesized aggrecan from developing neo-PCM, resulting in impaired tissue assembly [83]. This represents a major challenge in mesenchymal stem cell (MSC)-based cartilage regeneration, for that chondrogenically differentiated MSCs are less capable of retaining newly synthesized aggrecan relative to primary chondrocytes [84]. According to this study, decorin has the potential to enhance aggrecan retention in the neo-PCM formed under dynamic loading for both cell types [85]. Furthermore, transforming growth factor (TGF-β) is one most commonly used biochemical cue to elevate chondrocyte anabolism [86]. While decorin is shown to sequester TGF-β signaling through binding to TGF-β in other tissues [87], according to our recent work, decorin does not directly impact chondrocyte response of TGF-β stimuli when cultured in alginate [39]. Therefore, modulation of decorin activities in vitro is a promising path for improving the assembly of cartilage neo-matrix and harnessing chondrocyte mechanotransduction without having a detrimental impact on chondrocyte responses to biochemical or biomechanical cues.
In murine cartilage, the modulus of PCM is up to ~ 1 MPa (Fig. 2), which is more than ten-fold stiffer than the soft environment required for chondrocyte culture in vitro [1]. In comparison to various biopolymer systems, including hyaluronan-based hydrogels [88], one distinctive biophysical feature of the native PCM is the high concentration of aggrecan and associated high density of negative fixed charges. As shown here, this negatively charged environment is required for maintaining chondrocyte mechanosensing in situ (Fig. 5). Thus, if we can recreate the osmo-microenvironment in vitro, either using native aggrecan or biomimetic molecules, there is a path for us to restore the high stiffness of native cartilage while maintaining normal chondrocyte mechanotransduction [89, 90]. In this process, decorin could be a key player.
Limitations and outlook.
This study has several limitations. First, in Dcn−/− mice, both the PCM and T/IT-ECM are impaired at the same time. It is possible that the decreased [Ca2+]i signaling in Dcn−/− chondrocytes can be partially attributed to the altered T/IT-ECM. However, given the immediate contact of PCM with cells, we expect the integrity of PCM to have a more direct impact. To address this, our ongoing studies aim to elucidate the role of decorin in regulating the mechanosensing of chondrons extracted from native cartilage and chondrocytes integrated with neo-PCM, which will eliminate any secondary effects of the T/IT-ECM. We will assess how the modulation of decorin gene expression and/or exogenous decorin availability alter chondrocyte biosynthesis and neo-matrix assembly when cultured in hydrogels under applied oscillatory dynamic compression using the bioreactor equipped with a compressive loading device [91]. Second, to maintain cell viability, we did not completely remove sGAGs from cartilage explants (Fig. 5). We thus cannot conclude if the impact of decorin on chondrocyte [Ca2+]i activities is due to its regulation of aggrecan integrity or other mechanisms. Given the wide interactome of decorin with other cytokines, growth factors and receptors [92-95], it is possible for decorin to also regulate chondrocyte signaling through other biological activities. For example, soluble decorin and its core protein has been shown to mediate the intracellular calcium levels of squamous carcinoma cells by regulating epidermal growth factor receptors (EGFRs) [96, 97]. We therefore cannot rule out the possibility that decorin regulates chondrocyte [Ca2+]i through mechanisms other than mediating the local fixed charge density of PCM, which may contribute to the observation that Dcn−/− chondrocytes still show reduced [Ca2+]i activities relative to WT even after most CS-GAGs have been removed (Fig. 5).
CONCLUSIONS
In summary, this study shows that decorin, a small leucine-rich proteoglycan, functions as an important regulator of the micromechanics and mechanobiological function of cartilage pericellular matrix. In vivo, as chondrocyte resides in an aggrecan-rich, highly negative charged osmo-microenvironment, decorin influences chondrocyte mechanotransduction mainly through mediating the integrity of aggrecan in the PCM. These findings extend our recent discoveries on decorin activities in cartilage tissue-level biomechanics and OA pathogenesis, and highlight decorin as an indispensable constituent of cartilage PCM. Based on this understanding, modulation of decorin has the potential to enhance the retention and assembly of aggrecan in the neo-matrix of regenerative cartilage, thereby improving the quality of engineered cartilage while maintaining cell chondrogenic phenotype.
METHODS
Animal model.
Decorin-null (Dcn−/−) mice in the C57BL/6 strain were generated as previously described [98], and were housed in the Calhoun animal facility at Drexel University. Tissues were harvested from WT and Dcn−/− mice at newborn (3-day old), immature (2-week old) and adult (3-month old) ages from littermates of Dcn+/− breeders. Both male and female mice were included, for that we did not observe sex-associated variations in the phenotype of Dcn−/− cartilage [39]. All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) at Drexel University.
Immunofluorescence imaging.
Whole joints were harvested from WT and Dcn−/− mice, fixed in 4% paraformaldehyde, and then, decalcified in 10% EDTA for 7 days for joints at 2 weeks and 3 months of ages, respectively (n = 4 for each genotype at each age). Samples were embedded in paraffin and serial 6-μm-thick sagittal sections were cut across the joint. Following established procedures [99], sections were incubated with the primary antibodies for aggrecan (AB1031, MilliporeSigma, Burlington, MA, 1:100), decorin (LF-114, Kerafast, Boston, MA, 1:100), collagen VI (70R-CR009X, Fitzgerald, Acton, MA, 1:200 dilution), perlecan (A7L6, Santa Cruz Biotech, Dallas, TX, 1:200) and biglycan (ABT271, MilliporeSigma, 1:100) overnight in 4°C. Sections were then incubated with secondary antibodies directed against the primary antibodies for collagen VI (28903, Rockland, Pottstown, PA, 1:200), perlecan (A11006, Invitrogen, Carlsbad, CA, 1:200), as well as for aggrecan, decorin and biglycan (RE234142, Invitrogen, 1:500), respectively, for 1hr at room temperature. All imaging was performed on an Olympus FV1000 laser scanning microscope (Olympus, Center Valley, PA).
Transmission electron microscopy.
Transmission electron microscopy (TEM) was applied to the cross-sections of 3-month-old murine cartilage, following established procedures [100]. Thin cross-sections (≈ 90 nm thick) were prepared on freshly dissected condyles fixed with Karnovsky’s fixative for 15 min, followed by 1% osmium tetroxide for 1 hr, dehydrated in graded ethanol solutions, infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenylsuccinic anhydride and DMP-30 (EM Sciences, Fort Washington, PA), and polymerized at 60°C overnight, as described previously [39]. High resolution images were taken in the pericellular regions (< 2 μm from cell surface) of uncalcified cartilage middle/deep zone at 80 kV using a JEOL 1400 TEM (JEOL, Tokyo, Japan). For each region, collagen fibril diameter was measured in ImageJ (≥ 1,400 fibrils from n = 4 animals for each genotype).
Immunofluorescence-guided AFM nanomechanical mapping.
Cryo-sections of fresh, unfixed tibia cartilage at ≈ 6-μm-thickness in the sagittal plane were prepared in the optimal cutting temperature (OCT) media using the Kawamoto’s film-assisted method [46], as described previously (n = 5 for each group) [10]. Cryo-sections were fluorescently labeled by cartilage PCM biomarker, collagen VI [4] (primary antibody: 70R-CR009X, Fitzgerald, 1:50; secondary antibody: goat anti-rabbit IgG, Lot 28903, Rockland, 1:200, for 20 minutes each). Samples were then tested using the Total Internal Reflection Fluorescence (TIRF) guided-AFM (MFP-3D, Asylum Research, Goleta, CA) in PBS with protease inhibitors (Pierce A32963, ThermoFisher, Waltham, MA). Within each 20 × 20 μm2 region of interest (ROI) with well-defined, ring-shaped PCM terrains [47], AFM nanomechanical mapping was performed in a 40 × 40 grid (1,600 indents) using polystyrene microspherical tips (R ≈ 2.25 μm, nominal k ≈ 0.6 N/m, HQ:NSC36/tipless/Cr-Au, cantilever C, NanoAndMore, Watsonville, CA) up to ≈ 100 nN maximum indentation force at 10 μm/s effective indentation depth rate (≥ 3-4 ROIs for each joint). The effective indentation modulus, Eind, was calculated by fitting the entire loading portion of the indentation force-depth (F-D) curve to the finite thickness-corrected Hertz model (Fig. 3b) [101]. The modulus of Kawamoto’s film was assumed to be infinite, as it was > 10 × higher than that of cartilage (≈ 17.0 ± 4.2 MPa) [10]. Using corresponding IF images of collagen VI, we partitioned each map into three domains: the PCM, T/IT-ECM, and the domain corresponding to cellular remnants using a custom MATLAB (MathWorks, Natick, MA) code. We then analyzed the moduli of PCM and T/IT-ECM separately and excluded values corresponding to cell remnants.
Intracellular [Ca2+]i signaling under osmotic stimulation.
At each age, for additional mice (n = 4 for each group), we labeled cells within the tibia with intracellular calcium indicator Ca-520™ AM (15 μM, AAT Bioquest, Sunnyvale, CA) in isotonic DMEM at 37°C for 1 hr, and then, gently washed three times with DMEM. Time-series of confocal images were taken at 37°C on the same group of cells every 2 seconds for up to 15 min using a LSM 700 laser scanning confocal microscope with a 20× objective (Zeiss, Oberkochen, Germany) in hypotonic (165 mOsm, ionic strength (IS) = 0.075 M), isotonic (330 mOsm, IS = 0.15 M), and hypertonic (550 mOsm, IS = 0.23 M) DMEMs with protease inhibitors (Pierce 88266, ThermoFisher). For each genotype, age and osmolarity, 50-60 chondrocytes in each sample were analyzed, following established procedures [102]. The pattern of [Ca2+]i oscillation in each cell was characterized by the intensity of the fluorescent signal and its transient behavior. A responsive cell was considered as any cell in which the magnitude of the peak signal exceeded four times the maximum fluctuation of the baseline signal. The percentage of responding cells, %Rcell, was calculated by determining the proportion of responsive cells relative to the total cell number within a region of interest. For every cell that was responsive, the total number of [Ca2+]i peaks, npeak, was counted during the 15-minute observation period, and the average duration of peaks, tpeak, was extracted from each [Ca2+]i transient of responding cells.
Intracellular [Ca2+]i signaling with the enzymatic removal of sGAGs.
Additional tibia joints were harvested from 2-week-old WT and Dcn−/− mice (n = 4 for each group), and treated with 0.5 units/mL of protease-free chondroitinase ABC (C’ABC, C3667, Sigma-Aldrich) for 12 hours, with the contralateral knee cultured in serum-free DMEM for the same duration as the untreated control [52]. Samples were then labeled with Ca-520™ AM and time series images were obtained and analyzed at three different osmolarities, as described in the previous section. To estimate the degree of sGAG depletion, we repeated the same C’ABC treatment process on femoral head cartilage explants extracted from 2-week-old mice (n = 4 for each group), and measured the amount of sGAGs in the explants for both C’ABC-treated and untreated control using the standard dimethylmethylene blue (DMMB) dye assay [103].
Statistical analysis.
Linear statistical models were applied to analyze all quantitative outcomes using the R package lme4 (version 1.1-19) [104]. For continuous dependent variables including Eind, tpeak, sGAG content, PCM thickness, as well as the area proportions of cell, PCM and T/IT-ECM domains, the linear mixed effect model was applied. For non-continuous variables, the generalized linear model was applied to npeak (count) with the Poisson family, and to %Rcell (binary) with the binomial family, respectively. In these tests, genotype (WT versus Dcn−/−), age (3 days to 3 months), treatment type (untreated versus C’ABC treated), tissue region (PCM versus T/IT-ECM) and osmolarity condition (hypo-, iso- and hypertonic) were treated as fixed effect factors when applicable, with interaction terms between genotype and age, or between genotype and treatment type, while the individual animal effect was treated as a randomized factor. Prior to the test, likelihood ratio test was applied to the data to determine the choice of two covariance structures, unstructured versus compound symmetry. For all the variables, we found significant interactions between genotype and age (p < 0.0001). In contrast, for sGAG content and [Ca2+]i signaling outcomes, we did not find significant interactions between genotype and treatment conditions (p > 0.10). Holm-Bonferroni family-wise error correction was applied to adjust for multiple contrasts when testing the effect of genotype at each age or for each treatment type, or the effect of treatment for each genotype, in which, each region or osmolarity condition was analyzed separately. Within each genotype, Tukey-Kramer post-doc multiple comparison was applied to perform pair-wise comparisons among the tested ages, osmolarity conditions or tissue regions. For collagen fibril diameter data, since > 200 measurements were repeated for each group, according to the central limit theorem, unpaired two-sample z-test was used to compare the average values of dcol between the two genotypes, and two-sample F-test was applied to compare the variances of dcol, followed by Holm-Bonferroni family-wise error correction. Statistical outcomes on the effects of genotype, age and treatment were summarized in Tables S1-S8. In all the tests, the significance level was set at α = 0.05, and all the p-values have been adjusted for family-wise type I errors.
Supplementary Material
Highlights.
Decorin regulates the aggrecan network integrity and micromechanics of cartilage pericellular matrix.
The highly negative charged osmotic microenvironment of pericellular matrix is required for normal chondrocyte mechanotransduction in situ.
Decorin affects the intracellular calcium signaling of chondrocytes by mediating the aggrecan-endowed osmotic microenvironment of pericellular matrix.
The impact of decorin loss on the disruption of chondrocyte mechanobiology is increasingly aggravated during maturation.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NIH) Grant AR074490 to LH, the National Science Foundation (NSF) Grant CMMI-1662544 and CMMI-1751898 to LH, Drexel Interdisciplinary Collaboration and Research Enterprise (iCARE) for Healthcare by the U.S. Department of Education’s Graduate Assistance in Areas of National Need (GAANN) Program to DRC, as well as NIH Grant P30 AR069619 to the Penn Center for Musculoskeletal Disorders (PCMD). The IF-guided AFM nanomechanical mapping was carried out using the TIRF MFP-3D at the Singh Center for Nanotechnology at the University of Pennsylvania, part of the National Nanotechnology Coordinated Infrastructure Program, which is supported by the NSF Grant NNCI-1542153.
ABBREVIATIONS USED
- AFM
atomic force microscopy
- CS
chondroitin sulfate
- C’ABC
chondroitinase ABC
- DMEM
Dulbecco’s Modified Eagle Medium
- DMMB
dimethylmethylene blue
- DS
dermatan sulfate
- ECM
extracellular matrix
- EDL
electrical double layer
- EGFR
epidermal growth factor receptor
- GAG
glycosaminoglycan
- HA
hyaluronan
- IF
immunofluorescence
- IS
ionic strength
- MFP
molecular force probe
- MSC
mesenchymal stem cell
- OA
osteoarthritis
- PBS
phosphate buffered saline
- PCM
pericellular matrix
- ROI
region of interest
- sGAG
sulfated glycosaminoglycan
- T/IT-ECM
territorial/interterritorial extracellular matrix
- TEM
transmission electron microscopy
- TIRF
total internal reflection fluorescence
- TLR
toll-like receptor
- WT
wild-type
Footnotes
DECARATIONS OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wang T, Lai JH, Yang F, Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo, Tissue Eng. A 22 (23-24) (2016) 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maroudas A, Physicochemical properties of articular cartilage, in: Freeman MAR (Ed.), Adult Articular Cartilage, Pitman, England, 1979, pp. 215–290. [Google Scholar]
- [3].Huey DJ, Hu JC, Athanasiou KA, Unlike bone, cartilage regeneration remains elusive, Science 338 (6109) (2012) 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilusz RE, Sanchez-Adams J, Guilak F, The structure and function of the pericellular matrix of articular cartilage, Matrix Biol. 39 (2014) 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I, Osteoarthritis as a disease of the cartilage pericellular matrix, Matrix Biol. 71-72 (2018) 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Krishnan Y, Grodzinsky AJ, Cartilage diseases, Matrix Biol. 71-72 (2018) 51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Birk DE, Brückner P, Collagens, suprastructures, and collagen fibril assembly, in: Mecham RP (Ed.), The Extracellular Matrix: an Overview, Springer-Verlag, Berlin, 2011, pp. 77–115. [Google Scholar]
- [8].Knudson W, Ishizuka S, Terabe K, Askew EB, Knudson CB, The pericellular hyaluronan of articular chondrocytes, Matrix Biol. 78-79 (2019) 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wilusz RE, Zauscher S, Guilak F, Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage, Osteoarthritis Cartilage 21 (12) (2013) 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chery DR, Han B, Li Q, Zhou Y, Heo SJ, Kwok B, Chandrasekaran P, Wang C, Qin L, Lu XL, Kong D, Enomoto-Iwamoto M, Mauck RL, Han L, Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis, Acta Biomater. 111 (2020) 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Danalache M, Kleinert R, Schneider J, Erler AL, Schwitalle M, Riester R, Traub F, Hofmann UK, Changes in stiffness and biochemical composition of the pericellular matrix as a function of spatial chondrocyte organisation in osteoarthritic cartilage, Osteoarthritis Cartilage 27 (5) (2019) 823–832. [DOI] [PubMed] [Google Scholar]
- [12].Urbanczyk M, Layland SL, Schenke-Layland K, The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues, Matrix Biol. 85-86 (2020) 1–14. [DOI] [PubMed] [Google Scholar]
- [13].Iozzo RV, Gubbiotti MA, Extracellular matrix: the driving force of mammalian diseases, Matrix Biol. 71-72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases, Matrix Biol. 75-76 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rousselle P, Montmasson M, Garnier C, Extracellular matrix contribution to skin wound re-epithelialization, Matrix Biol. 75-76 (2019) 12–26. [DOI] [PubMed] [Google Scholar]
- [16].Christensen G, Herum KM, Lunde IG, Sweet, yet underappreciated: proteoglycans and extracellular matrix remodeling in heart disease, Matrix Biol. 75-76 (2019) 286–299. [DOI] [PubMed] [Google Scholar]
- [17].Salustri A, Campagnolo L, Klinger FG, Camaioni A, Molecular organization and mechanical properties of the hyaluronan matrix surrounding the mammalian oocyte, Matrix Biol. 78-79 (2019) 11–23. [DOI] [PubMed] [Google Scholar]
- [18].Wunderli SL, Blache U, Beretta Piccoli A, Niederöst B, Holenstein CN, Passini FS, Silván U, Bundgaard L, Auf dem Keller U, Snedeker JG, Tendon response to matrix unloading is determined by the patho-physiological niche, Matrix Biol. 89 (2020) 11–26. [DOI] [PubMed] [Google Scholar]
- [19].Heinegård D, Proteoglycans and more – from molecules to biology, Int. J. Exp. Pathol 90 (6) (2009) 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alexopoulos LG, Youn I, Bonaldo P, Guilak F, Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix, Arthritis Rheum. 60 (3) (2009) 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Christensen SE, Coles JM, Zelenski NA, Furman BD, Leddy HA, Zauscher S, Bonaldo P, Guilak F, Altered trabecular bone structure and delayed cartilage degeneration in the knees of collagen VI null mice, PLoS One 7 (3) (2012) e33397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zelenski NA, Leddy HA, Sanchez-Adams J, Zhang J, Bonaldo P, Liedtke W, Guilak F, Type VI collagen regulates pericellular matrix properties, chondrocyte swelling, and mechanotransduction in mouse articular cartilage, Arthritis Rheumatol. 67 (5) (2015) 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kaneko H, Ishijima M, Futami I, Tomikawa-Ichikawa N, Kosaki K, Sadatsuki R, Yamada Y, Kurosawa H, Kaneko K, Arikawa-Hirasawa E, Synovial perlecan is required for osteophyte formation in knee osteoarthritis, Matrix Biol. 32 (3-4) (2013) 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sadatsuki R, Kaneko H, Kinoshita M, Futami I, Nonaka R, Culley KL, Otero M, Hada S, Goldring MB, Yamada Y, Kaneko K, Arikawa-Hirasawa E, Ishijima M, Perlecan is required for the chondrogenic differentiation of synovial mesenchymal cells through regulation of Sox9 gene expression, J. Orthop. Res 35 (4) (2017) 837–846. [DOI] [PubMed] [Google Scholar]
- [25].Shu CC, Jackson MT, Smith MM, Smith SM, Penm S, Lord MS, Whitelock JM, Little CB, Melrose J, Ablation of perlecan domain 1 heparan sulfate reduces progressive cartilage degradation, synovitis, and osteophyte size in a preclinical model of posttraumatic osteoarthritis, Arthritis Rheumatol. 68 (4) (2016) 868–879. [DOI] [PubMed] [Google Scholar]
- [26].Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF, Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis, FASEB J. 16 (7) (2002) 673–680. [DOI] [PubMed] [Google Scholar]
- [27].Nuka S, Zhou W, Henry SP, Gendron CM, Schultz JB, Shinomura T, Johnson J, Wang Y, Keene DR, Ramirez-Solis R, Behringer RR, Young MF, Hoeoek M, Phenotypic characterization of epiphycan-deficient and epiphycan/biglycan double-deficient mice, Osteoarthritis Cartilage 18 (1) (2010) 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aszódi A, Bateman JF, Hirsch E, Baranyi M, Hunziker EB, Hauser N, Bösze Z, Fässler R, Normal skeletal development of mice lacking matrilin 1: redundant function of matrilins in cartilage?, Mol. Cell. Biol 19 (11) (1999) 7841–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, Cossman J, Jayasuriya CT, Li X, Guan Y, Fonseca V, Yang K, Charbonneau C, Yu H, Kanbe K, Ma P, Darling E, Chen Q, Deficient mechanical activation of anabolic transcripts and post-traumatic cartilage degeneration in matrilin-1 knockout mice, PLoS One 11 (6) (2016) e0156676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ko Y, Kobbe B, Nicolae C, Miosge N, Paulsson M, Wagener R, Aszódi A, Matrilin-3 is dispensable for mouse skeletal growth and development, Mol. Cell. Biol 24 (4) (2004) 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li P, Fleischhauer L, Nicolae C, Prein C, Farkas Z, Saller MM, Prall WC, Wagener R, Heilig J, Niehoff A, Clausen-Schaumann H, Alberton P, Aszodi A, Mice lacking the matrilin family of extracellular matrix proteins develop mild skeletal abnormalities and are susceptible to age-associated osteoarthritis, Int. J. Mol. Med 21 (2) (2020) 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brézillon S, Götte M, Passi A, Vigetti D, Ricard-Blum S, Sanderson RD, Neill T, Iozzo RV, Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics, Chem. Rev 118 (18) (2018) 9152–9232. [DOI] [PubMed] [Google Scholar]
- [33].Poole AR, Pidoux I, Reiner A, Rosenberg L, An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage, J. Cell Biol 93 (3) (1982) 921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Quinn TM, Maung AA, Grodzinsky AJ, Hunziker EB, Sandy JD, Physical and biological regulation of proteoglycan turnover around chondrocytes in cartilage explants. Implications for tissue degradation and repair, Ann. N. Y. Acad. Sci 878 (1) (1999) 420–441. [DOI] [PubMed] [Google Scholar]
- [35].Bayliss MT, Howat S, Davidson C, Dudhia J, The organization of aggrecan in human articular cartilage. Evidence for age-related changes in the rate of aggregation of newly synthesized molecules, J. Biol. Chem 275 (9) (2000) 6321–6327. [DOI] [PubMed] [Google Scholar]
- [36].Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C, Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy, J. Struct. Biol 143 (3) (2003) 242–257. [DOI] [PubMed] [Google Scholar]
- [37].Han L, Grodzinsky AJ, Ortiz C, Nanomechanics of the cartilage extracellular matrix, Annu. Rev. Mater. Res 41 (2011) 133–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hardingham TE, Muir H, The specific interaction of hyaluronic acid with cartilage proteoglycans, Biochim. Biophys. Acta 279 (2) (1972) 401–405. [DOI] [PubMed] [Google Scholar]
- [39].Han B, Li Q, Wang C, Patel P, Adams SM, Doyran B, Nia HT, Oftadeh R, Zhou S, Li CY, Liu XS, Lu XL, Enomoto-Iwamoto M, Qin L, Mauck RL, Iozzo RV, Birk DE, Han L, Decorin regulates the aggrecan network integrity and biomechanical functions of cartilage extracellular matrix, ACS Nano 13 (10) (2019) 11320–11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li Q, Han B, Wang C, Tong W, Tseng WJ, Han LH, Liu XS, Enomoto-Iwamoto M, Mauck RL, Qin L, Iozzo RV, Birk DE, Han L, Mediation of cartilage matrix degeneration and fibrillation by decorin in post-traumatic osteoarthritis, Arthritis Rheumatol. 72 (8) (2020) 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clapham DE, Calcium signaling, Cell 131 (6) (2007) 1047–1058. [DOI] [PubMed] [Google Scholar]
- [42].Hayes AJ, Tudor D, Nowell MA, Caterson B, Hughes CE, Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells, J. Histochem. Cytochem 56 (2) (2008) 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kvist AJ, Nyström A, Hultenby K, Sasaki T, Talts JL, Aspberg A, The major basement membrane components localize to the chondrocyte pericellular matrix — a cartilage basement membrane equivalent?, Matrix Biol. 27 (1) (2008) 22–33. [DOI] [PubMed] [Google Scholar]
- [44].Kavanagh E, Ashhurst DE, Development and aging of the articular cartilage of the rabbit knee joint: distribution of biglycan, decorin, and matrilin-1, J. Histochem. Cytochem 47 (12) (1999) 1603–1616. [DOI] [PubMed] [Google Scholar]
- [45].Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H, Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan, Biomacromolecules 7 (8) (2006) 2388–2393. [DOI] [PubMed] [Google Scholar]
- [46].Kawamoto T, Kawamoto K, Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot's film method (2012), Methods Mol. Biol 1130 (2014) 149–164. [DOI] [PubMed] [Google Scholar]
- [47].Wilusz RE, DeFrate LE, Guilak F, Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage, J. Royal Soc. Interface 9 (76) (2012) 2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang C, Brisson BK, Terajima M, Li Q, Hoxha K, Han B, Goldberg AM, Liu XS, Marcolongo MS, Enomoto-Iwamoto M, Yamauchi M, Volk SW, Han L, Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus, Matrix Biol. 85-86 (2020) 47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Williamson AK, Chen AC, Sah RL, Compressive properties and function-composition relationships of developing bovine articular cartilage, J. Orthop. Res 19 (6) (2001) 1113–1121. [DOI] [PubMed] [Google Scholar]
- [50].Dean D, Han L, Grodzinsky AJ, Ortiz C, Compressive nanomechanics of opposing aggrecan macromolecules, J. Biomech 39 (14) (2006) 2555–2565. [DOI] [PubMed] [Google Scholar]
- [51].Hing WA, Sherwin AF, Poole CA, The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge, Osteoarthritis Cartilage 10 (4) (2002) 297–307. [DOI] [PubMed] [Google Scholar]
- [52].Otsuki S, Brinson DC, Creighton L, Kinoshita M, Sah RL, D'Lima D, Lotz M, The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants, Arthritis Rheum. 58 (4) (2008) 1076–1085. [DOI] [PubMed] [Google Scholar]
- [53].Fässler R, Schnegelsberg PNJ, Dausman J, Shinya T, Muragaki Y, McCarthy MT, Olsen BR, Jaenisch R, Mice lacking α1(IX) collagen develop noninflammatory degenerative joint disease, Proc. Natl. Acad. Sci. USA 91 (11) (1994) 5070–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, Li Y, Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho), Arthritis Rheum. 48 (9) (2003) 2509–2518. [DOI] [PubMed] [Google Scholar]
- [55].Wiberg C, Hedbom E, Khairullina A, Lamande SR, Oldberg A, Timpl R, Mörgelin M, Heinegård D, Biglycan and decorin bind close to the N-terminal region of the collagen VI triple helix, J. Biol. Chem 276 (22) (2001) 18947–18952. [DOI] [PubMed] [Google Scholar]
- [56].Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegård D, Mörgelin M, Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan, J. Biol. Chem 278 (39) (2003) 37698–37704. [DOI] [PubMed] [Google Scholar]
- [57].O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F, TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading, Proc. Natl. Acad. Sci. USA 111 (4) (2014) 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Wu J, Beicker KN, Coles J, Zauscher S, Grandl J, Sachs F, Guilak F, Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage, Proc. Natl. Acad. Sci. USA 111 (47) (2014) E5114–E5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].O'Conor CJ, Ramalingam S, Zelenski NA, Benefield HC, Rigo I, Little D, Wu CL, Chen D, Liedtke W, McNulty AL, Guilak F, Cartilage-specific knockout of the mechanosensory ion channel TRPV4 decreases age-related osteoarthritis, Sci. Rep 6 (2016) 29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA, The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage, Ann. N. Y. Acad. Sci 1068 (2006) 498–512. [DOI] [PubMed] [Google Scholar]
- [61].Zhou Y, Lv M, Li T, Zhang T, Duncan R, Wang L, Lu XL, Spontaneous calcium signaling of cartilage cells: from spatiotemporal features to biophysical modeling, FASEB J. 33 (4) (2019) 4675–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weber JF, Waldman SD, Calcium signaling as a novel method to optimize the biosynthetic response of chondrocytes to dynamic mechanical loading, Biomech. Model. Mechanobiol 13 (6) (2014) 1387–1397. [DOI] [PubMed] [Google Scholar]
- [63].Loeser RF, Sadiev S, Tan L, Goldring MB, Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for α1β1 and α2β1 integrins in mediating chondrocyte adhesion to types II and VI collagen, Osteoarthritis Cartilage 8 (2) (2000) 96–105. [DOI] [PubMed] [Google Scholar]
- [64].Gubbiotti MA, Neill T, Iozzo RV, A current view of perlecan in physiology and pathology: a mosaic of functions, Matrix Biol. 57-58 (2017) 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J, FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer, Osteoarthritis Cartilage 15 (7) (2007) 752–763. [DOI] [PubMed] [Google Scholar]
- [66].Xu X, Li Z, Leng Y, Neu CP, Calve S, Knockdown of the pericellular matrix molecule perlecan lowers in situ cell and matrix stiffness in developing cartilage, Dev. Biol 418 (2) (2016) 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Iozzo RV, Goldoni S, Berendsen AD, Young MF, Small leucine-rich proteoglycans, in: Mecham RF (Ed.), The Extracellular Matrix: an Overview, Springer-Verlag, Berlin, 2011, pp. 197–231. [Google Scholar]
- [68].Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE, Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development, J. Cell. Biochem 98 (6) (2006) 1436–1449. [DOI] [PubMed] [Google Scholar]
- [69].Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, Lin L, Saez D, Adams SM, Iozzo RV, Soslowsky LJ, Birk DE, Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons, Matrix Biol. 64 (2017) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE, Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan, J. Biol. Chem 284 (13) (2009) 8879–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Barreto G, Soininen A, Ylinen P, Sandelin J, Konttinen YT, Nordstrom DC, Eklund KK, Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis, Arthritis Res. Ther 17 (1) (2015) 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gronau T, Kruger K, Prein C, Aszodi A, Gronau I, Iozzo RV, Mooren FC, Clausen-Schaumann H, Bertrand J, Pap T, Bruckner P, Dreier R, Forced exercise-induced osteoarthritis is attenuated in mice lacking the small leucine-rich proteoglycan decorin, Ann. Rheum. Dis 76 (2) (2017) 442–449. [DOI] [PubMed] [Google Scholar]
- [73].Loparic M, Wirz D, Daniels AU, Raiteri R, VanLandingham MR, Guex G, Martin I, Aebi U, Stolz M, Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: validation with a gel-microfiber composite, Biophys. J 98 (11) (2010) 2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Stolz M, Gottardi R, Raiteri R, Miot S, Martin I, Imer R, Staufer U, Raducanu A, Düggelin M, Baschong W, Daniels AU, Friederich NF, Aszodi A, Aebi U, Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy, Nat. Nanotechnol 4 (3) (2009) 186–192. [DOI] [PubMed] [Google Scholar]
- [75].Nia HT, Gauci SJ, Azadi M, Hung H-H, Frank E, Fosang AJ, Ortiz C, Grodzinsky AJ, High-bandwidth AFM-based rheology is a sensitive indicator of early cartilage aggrecan degradation relevant to mouse models of osteoarthritis, J. Biomech 48 (2015) 162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Batista MA, Nia HT, Önnerfjord P, Cox KA, Ortiz C, Grodzinsky AJ, Heinegård D, Han L, Nanomechanical phenotype of chondroadherin-null murine articular cartilage, Matrix Biol. 38 (2014) 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Doyran B, Tong W, Li Q, Jia H, Zhang X, Chen C, Enomoto-Iwamoto M, Lu XL, Qin L, Han L, Nanoindentation modulus of murine cartilage: a sensitive indicator of the initiation and progression of post-traumatic osteoarthritis, Osteoarthritis Cartilage 25 (1) (2017) 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cao L, Youn I, Guilak F, Setton LA, Compressive properties of mouse articular cartilage determined in a novel micro-indentation test method and biphasic finite element model, J. Biomech. Eng 128 (5) (2006) 766–771. [DOI] [PubMed] [Google Scholar]
- [79].Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y, Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen, Arthritis Rheum. 54 (9) (2006) 2891–2900. [DOI] [PubMed] [Google Scholar]
- [80].Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT, Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering, Tissue Eng. 9 (4) (2003) 597–611. [DOI] [PubMed] [Google Scholar]
- [81].Anderson DE, Johnstone B, Dynamic mechanical compression of chondrocytes for tissue engineering: a critical review, Front. Bioeng. Biotechnol 5 (2017) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang Z, Chondrons and the pericellular matrix of chondrocytes, Tissue Eng. B Rev 21 (3) (2015) 267–277. [DOI] [PubMed] [Google Scholar]
- [83].Huang AH, Farrell MJ, Kim M, Mauck RL, Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel, Eur. Cell Mater 19 (2010) 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Babalola OM, Bonassar LJ, Effects of seeding density on proteoglycan assembly of passaged mesenchymal stem cells, Cell. Mol. Bioeng 3 (3) (2010) 197–206. [Google Scholar]
- [85].Salinas CN, Anseth KS, Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells, J. Biomed. Mater. Res. A 90 (2) (2009) 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ, The role of growth factors in cartilage repair, Clin. Orthop. Relat. Res 469 (10) (2011) 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, R Twardzik D, Border WA, Ruoslahti E, Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β, Biochem. J 302 (1994) 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vega SL, Kwon MY, Burdick JA, Recent advances in hydrogels for cartilage tissue engineering, Eur. Cell Mater 33 (2017) 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ashworth JC, Thompson JL, James JR, Slater CE, Pijuan-Galitó S, Lis-Slimak K, Holley RJ, Meade KA, Thompson A, Arkill KP, Tassieri M, Wright AJ, Farnie G, Merry CLR, Peptide gels of fully-defined composition and mechanics for probing cell-cell and cell-matrix interactions in vitro, Matrix Biol. 85-86 (2020) 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Berger AJ, Renner CM, Hale I, Yang X, Ponik SM, Weisman PS, Masters KS, Kreeger PK, Scaffold stiffness influences breast cancer cell invasion via EGFR-linked Mena upregulation and matrix remodeling, Matrix Biol. 85-86 (2020) 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao PHG, Valhmu WB, Hung CT, Ateshian GA, Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels, J. Biomech. Eng 122 (3) (2000) 252–260. [DOI] [PubMed] [Google Scholar]
- [92].Gubbiotti MA, Vallet SD, Ricard-Blum S, Iozzo RV, Decorin interacting network: a comprehensive analysis of decorin-binding partners and their versatile functions, Matrix Biol. 55 (2016) 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pietraszek-Gremplewicz K, Karamanou K, Niang A, Dauchez M, Belloy N, Maquart FX, Baud S, Brézillon S, Small leucine-rich proteoglycans and matrix metalloproteinase-14: key partners?, Matrix Biol. 75-76 (2019) 271–285. [DOI] [PubMed] [Google Scholar]
- [94].Buraschi S, Neill T, Iozzo RV, Decorin is a devouring proteoglycan: remodeling of intracellular catabolism via autophagy and mitophagy, Matrix Biol. 75-76 (2019) 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cianfarani F, De Domenico E, Nyström A, Mastroeni S, Abeni D, Baldini E, Ulisse S, Uva P, Bruckner-Tuderman L, Zambruno G, Castiglia D, Odorisio T, Decorin counteracts disease progression in mice with recessive dystrophic epidermolysis bullosa, Matrix Biol. 81 (2019) 3–16. [DOI] [PubMed] [Google Scholar]
- [96].Patel S, Santra M, McQuillan DJ, Iozzo RV, Thomas AP, Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 carcinoma cells, J. Biol. Chem 273 (6) (1998) 3121–3124. [DOI] [PubMed] [Google Scholar]
- [97].Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnóczky G, Iozzo RV, Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo, J. Biol. Chem 275 (42) (2000) 32879–32887. [DOI] [PubMed] [Google Scholar]
- [98].Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV, Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility, J. Cell Biol 136 (3) (1997) 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Vanderploeg EJ, Wilson CG, Imler SM, Ling CH, Levenston ME, Regional variations in the distribution and colocalization of extracellular matrix proteins in the juvenile bovine meniscus, J. Anat 221 (2) (2012) 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE, Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice, J. Biol. Chem 284 (13) (2009) 8427–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS, Determination of elastic moduli of thin layers of soft material using the atomic force microscope, Biophys. J 82 (5) (2002) 2798–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Han WM, Heo SJ, Driscoll TP, Delucca JF, McLeod CM, Smith LJ, Duncan RL, Mauck RL, Elliott DM, Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage, Nat. Mater 15 (4) (2016) 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Farndale RW, Buttle DJ, Barrett AJ, Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue, Biochim. Biophys. Acta 883 (2) (1986) 173–177. [DOI] [PubMed] [Google Scholar]
- [104].Bates D, Mächler M, Bolker BM, Walker SC, Fitting linear mixed-effects models using lme4, J. Stat. Softw 67 (1) (2015) 1–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.