Abstract
Background:
In ISCHEMIA, an initial invasive strategy did not significantly reduce rates of cardiovascular events or all-cause mortality compared with a conservative strategy in patients with stable ischemic heart disease and moderate/severe myocardial ischemia. The most frequent component of composite cardiovascular endpoints was myocardial infarction.
Methods:
ISCHEMIA prespecified that the primary and major secondary composite endpoints of the trial be analyzed using two MI definitions. For procedural MI, the primary MI definition used CK-MB as the preferred biomarker whereas the secondary definition used cardiac troponin. Procedural thresholds were >5 times URL for PCI and >10 times for CABG. Procedural MI definitions included (i) a category of elevated biomarker only events with much higher biomarker thresholds (ii) new ST segment depression of ≥ 1mm for the primary and ≥ 0.5 mm for the secondary definition and (iii) new coronary dissections ≥ NHLBI grade 3. We compared MI type, frequency, and prognosis by treatment assignment using both MI definitions.
Results:
Procedural MI’s accounted for 20.1% of all MI events with the primary definition and 40.6% of all MI events with the secondary definition. Four-year MI rates in patients undergoing revascularization were more frequent with the invasive vs conservative strategy using the primary [2.7% vs. 1.1%; adjusted HR 2.98 (95% CI 1.87, 4.73)] and secondary [8.2% vs. 2.0%; adjusted HR 5.04 (95% CI 3.64, 6.97)] MI definitions. Type 1 MI’s were less frequent with the invasive vs conservative strategy using the primary [3.40% vs. 6.89%; adjusted HR 0.53 (95% CI 0.41,0.69); p<0.0001], and secondary [3.48% vs 6.89%; adjusted HR 0.53 (95% CI 0.41, 0.69); p<0.0001] definitions. The risk of subsequent cardiovascular death was higher after a type 1 MI compared to no MI using the primary [adjusted HR 3.38 (95% CI 2.03,5.61); p<0.001] or secondary MI definition [adjusted HR 3.52 (2.11, 5.88); p<0.001].
Conclusions:
In ISCHEMIA, type 1 MI events using the primary and secondary definitions during 5-year follow-up were more frequent with an initial conservative strategy and associated with subsequent cardiovascular death. Procedural MI rates were greater in the invasive strategy and using the secondary MI definition.
Clinical Trial Registration:
NCT01471522; https://clinicaltrials.gov/ct2/show/NCT01471522
Keywords: myocardial infarction, catheterization, optimal medical therapy, revascularization, stable ischemic heart disease
Introduction
Spontaneous (type 1) myocardial infarction (MI) is an important outcome in clinical trials used to assess efficacy and safety of different treatment strategies. The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches trial (ISCHEMIA) randomized patients with stable ischemic heart disease and moderate or severe myocardial ischemia on noninvasive testing to an initial invasive or conservative strategy.1–3 After a median 3.2 year follow-up, there was no statistical evidence of a difference in the primary composite endpoint of cardiovascular death, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest, or in the major secondary composite outcome of cardiovascular death or MI.1
MI was the main contributor to the primary and major secondary composite outcomes. When ISCHEMIA was designed, both the 3rd Universal Definition of Myocardial Infarction (UDMI 3) that gives precedence to cardiac troponin (cTn) values, and the Society for Cardiovascular Angiography and Interventions (SCAI) definition that gives precedence to CK-MB values for procedural-related MI had been introduced.4, 5 An abundance of published data had established a relationship between the magnitude of post procedural CK-MB elevation with a range of thresholds and short- and long-term mortality.4–18 A similar relationship was observed with cTn assays in some but not all studies.9–18
Given the uncertainty of whether a threshold exists above which a procedural MI confers an adverse prognosis, we developed two definitions of procedural MI with a plan to evaluate their impact on treatment outcomes. A clinical events committee (CEC) adjudicated all occurrences of elevated cardiac biomarkers after coronary revascularization, as well as any hospitalizations with elevated cardiac biomarkers using a primary and secondary MI definition. The aim of this report is to compare treatment comparisons of the primary and major secondary composite endpoints in the ISCHEMIA trial using the primary and secondary MI definitions. We also examined the treatment effect of the invasive and conservative strategies on MI event rates by type using both MI definitions and determined their prognostic significance.
METHODS
The ISCHEMIA Trial.
Deidentified participant data and a data dictionary will be available starting June 30, 2022. Methods of data sharing will be determined based on the National Institutes of Health data sharing policy and in discussion with the National Institutes of Health and the National Heart, Lung, and Blood Institute program officer. The ISCHEMIA trial design, protocol, baseline characteristics, and major clinical outcomes have been published.1–3 Briefly, the ISCHEMIA trial was a large international trial that tested two major treatment strategies in 5,179 patients with moderate or severe myocardial ischemia on stress testing, most of whom underwent coronary computed tomography angiography prior to randomization to confirm the presence of obstructive coronary disease and absence of unprotected left main disease ≥50%. Angiographic findings of the coronary computed tomography angiogram were blinded to the treating physicians. Patients were randomized to an initial invasive strategy with prompt angiography and coronary revascularization if feasible and intensive medical therapy or an initial conservative approach with intensive medical therapy, with angiography permitted for worsening symptoms. Exclusion criteria included acute coronary syndrome in the prior 2 months, angina that could not be controlled with medical therapy, ejection fraction <35%, estimated GFR <30 ml/min, or severe valvular disease. All patients were prescribed guideline-based medical therapy for secondary prevention. Baseline characteristics of the study population were similar in both treatment strategies (Table I in the Supplement).1, 2 The average age of the study population was 64 years and 22.6% were women.
Of the 2,588 patients randomized to the invasive strategy, 1524 (58.9%) received PCI, 530 (20.5%) CABG and 534 (20.6%) patients did not undergo a revascularization procedure, 59.6% of whom did not have obstructive coronary disease at cardiac catheterization. Of the 2,591 patients assigned to the conservative strategy, 369 (14.2%) patients underwent PCI, and 175 (6.8%) underwent CABG during the follow-up phase. The randomization period for ISCHEMIA started Aug 7, 2012 and ended January 31, 2018. The last patient visit was June 30, 2019. There were 112 (2.2%) patients who withdrew from the study or were lost to follow-up. The median duration of follow-up was 3.2 years (Q1, Q3: 2.2 to 4.4). The protocol was approved by the Institutional Review Board at each institution. All patients provided written informed consent. The trial was funded by the National Heart, Lung, and Blood Institute with industry support providing some free drugs and devices.
Definitions of Myocardial Infarction.
A detailed description of the MI definitions and Clinical Event Committee members can be found in the supplement. MI definitions for types 1,2, 4b and 4c were based on UDMI 3. The procedural MI definition used in ISCHEMIA included (i) a category of elevated biomarker only events with much higher biomarker thresholds than the level required when ancillary evidence of myocardial ischemia was present (supplement section v) (ii) new ST segment depression of ≥ 1mm for the primary and ≥ 0.5 mm for the secondary definition and (iii) new coronary dissections ≥ NHLBI grade 3. Procedural MI was diagnosed if it occurred within 48 hours and was a consequence of the procedure. Non-procedural MI events were diagnosed if they occurred later and included MI types 1, 2, 4b and 4c. For elective revascularization procedures, CK-MB and cTn measurements were protocol requirements pre-procedure, and between 8–16 hours ± 2 hours post-procedure or hospital discharge, whichever came first. Additional CK-MB or cTn measurements were acquired as needed for suspected myocardial ischemic events. All biomarker measurements available in the hospital records were available to the CEC for MI adjudication. If pre-procedural biomarkers were unavailable, they were assumed to be normal in the absence of a recent change in clinical symptoms or ECG evidence of acute myocardial ischemia indicating a pre-procedural acute MI.
The primary MI definition in ISCHEMIA used the site-determined MI decision limit or the upper limit of normal (ULN) for biomarkers, of which cTn was the preferred biomarker for non-procedural UDMI types 1, 2, 4b and 4c (unless only CK-MB values were available). For types 4a and 5 procedural-related MI, CK-MB was the preferred biomarker (unless only cTn values were available). A post-procedural CK-MB threshold >5 fold the ULN within 48 hours associated with specific ECG, angiographic, or imaging findings indicating myocardial ischemia defined a type 4a PCI-related MI. For type 5 CABG-related MI, a post-procedural CK-MB threshold > 10 fold the ULN within 48 hours associated with new Q waves or persistent left bundle branch block defined an event. In addition, post-procedure elevated biomarker only criteria were defined by higher thresholds but without supporting evidence of myocardial ischemia. Such extreme biomarker only elevations were also counted as type 4a and 5 MIs for the primary MI definition. The biomarker elevation only thresholds were a post-procedural rise in CK-MB >10-fold the ULN (cTn >70 fold the decision limit if CK-MB was unavailable) for PCI and CK-MB >15 fold the ULN (or cTn >100 fold the decision limit if CK-MB was unavailable) for CABG for the primary definition.
The secondary MI definition used cTn as the preferred biomarker for all MI types as well as the manufacturer’s recommended 99th percentile of the upper reference limit (URL), with specific clinical, angiographic, ECG and imaging criteria (supplement).19 The cTn thresholds for a type 4a MI and type 5 MI were the same as those used for the primary MI definition. As with the primary definition, cTn was the preferred biomarker for non-procedural UDMI types 1, 2, 4b and 4c (unless only CK-MB values were available), and similar to the primary MI definition, post-procedure elevated biomarker only criteria were defined by higher thresholds but without supporting evidence of myocardial ischemia and included in the secondary definitions of type 4a and 5 MI. For the secondary definition, a post-procedural rise in cTn >70 fold the manufacturer’s recommended 99th percentile URL (or CK-MB >10-fold the ULN if cTn was unavailable) for PCI and >100 fold the 99th percentile URL (or CK-MB >15 fold the ULN if cTn was unavailable) for CABG were used.
In addition to reviewing all procedural biomarker elevations, the CEC reviewed all post-randomization hospital admissions that were associated with elevated cardiac biomarkers. Thus, site-reported as well as the remaining “triggered” cardiac events were sent to the CEC to maximize sensitivity to capture unreported cardiac events in this open-label trial. Of the 2794 suspected MI events reviewed by the CEC, 1812 (65%) were triggered and 982 (35%) were site-reported as an ACS event. Of the 332 site-reported MI events, 258 (77.7%) were confirmed by the CEC. Of the 1812 triggered events, 318 (18%) were classified as an MI by the CEC. St Louis University core ECG laboratory reviewed all pre- and post-procedural ECG tracings, those associated with ACS admissions, and at 1 year, 3 years and study close-out to determine new ST segment, T-wave and Q wave findings that met protocol criteria.
Complicated and Large MI
An MI was classified by the CEC reviewers as complicated if after the MI, during the same admission, there was evidence of new or worsening heart failure, hemodynamic instability, cardiogenic shock, drop in left ventricular ejection fraction >10% from baseline, or electrical instability such as life-threatening ventricular tachycardia or ventricular fibrillation complicating the event.
Large MI was classified by peak cTn values. A Type 4a or 5 MI was considered large if it met the elevated biomarker only criteria (cTn >70 or >100 times 99th percentile URL for type 4a and 5 MI respectively) and for non-procedural MI’s if the peak cTn was > 70 times the 99th percentile URL
Statistical Analysis
Baseline characteristics, including demographics, cardiovascular risk factors, cardiovascular disease history, and selected labs are presented for all patients according to randomized treatment strategy. Continuous variables are presented as medians (Q1, Q3) and categorical variables are presented as counts (percentages). The number of MI events (first MI events; overall and by type for primary and secondary definitions) are summarized with counts and percentages among all randomized patients. To account for the competing risk of any type of death in the analysis of individual non-fatal MI endpoints, cumulative incidence rates (95% CI) were estimated for the invasive and conservative groups and Gray’s test20 was applied to compare incidence rates over the duration of follow-up.
Cox regression modeling was used to characterize the association between randomized treatment strategy and time to first occurrence of an MI. Unadjusted and adjusted hazard ratios (95% CI) and p-values are reported for comparing invasive vs. conservative strategies. Each model was adjusted for a set of pre-specified prognostically important baseline covariates that included age at randomization, sex, estimated glomerular filtration rate (eGFR), LVEF, and diabetes. To allow for non-linear covariate effects, the continuous variables of age, LVEF and eGFR were modeled as restricted cubic splines with knots at the approximate 10th, 50th, and 90th percentiles of each variable’s empirical distribution. The association of MI vs. no MI on subsequent events of death and hospitalization for heart failure, was characterized by reporting the adjusted hazard ratio (95% CI) and p-value from a Cox regression model in which MI during follow-up was treated as a time-dependent covariate. Models were adjusted for age at randomization, sex, eGFR, LVEF, diabetes, randomized treatment strategy, prior heart failure, prior MI, smoking status, LDL-C, and extent of myocardial ischemia. Continuous variables were modeled as restricted cubic splines with knots at the 10th, 50th, and 90th percentiles of each variable’s empirical distribution. Additional description of the statistical modeling discussion is found in the methods section of the supplement.
RESULTS
First MI events occurred in 443 (8.6%) of the 5,179 patients with the primary definition and 593 (11.5%) with the secondary definition (Table 1). The MI’s were fatal within 30 days in 32 (7.2%) and 35 (5.9%) patients with the primary and secondary definitions, respectively (Table II in the Supplement). The majority of fatal MI’s that occurred during follow-up were types 1 or 2. The number of fatal procedural MI’s was relatively small with the majority being type 5 MI’s. Procedural MI’s accounted for 20.1% of all MI events with the primary definition and 40.6% of all MI events with the secondary definition (Figure 1). Procedural MI’s were classified as complicated MI’s significantly less often than non-procedural MI’s for both the primary definition (p=0.037) and secondary definition (p<0.001)] (Table III in the Supplement). The number of non-procedural MI (types 1, 2, 4b, 4c) were similar regardless of whether the site-determined (primary MI definition) or manufacturers recommended 99th percentile URL (secondary MI definition) decision threshold was used. Three more first type 1 MI’s were detected using the manufacturer’s 99th percentile URL and 2 less first type 4B and 4C MI’s.
Table 1:
Distribution of First MI Events by Type and Randomized Treatment Arm
| Primary Definition | Secondary Definition | |||
|---|---|---|---|---|
| MI Type | INV (N=210) | CON (N=233) | INV (N=343) | CON (N=250) |
| Type 1 | 75/210 (35.7%) | 147/233 (63.1%) | 74/343 (21.6%) | 151/250 (60.4%) |
| Type 2 | 32/210 (15.2%) | 35/233 (15.0%) | 32/343 (9.3%) | 35/250 (14.0%) |
| Type 3 | 1/210 (0.5%) | 2/233 (0.9%) | 1/343 (0.3%) | 2/250 (0.8%) |
| Type 4a | 26/210 (12.4%) | 4/233 (1.7%) | 98/343 (28.6%) | 12/250 (4.8%) |
| Type 4b | 13/210 (6.2%) | 6/233 (2.6%) | 12/343 (3.5%) | 6/250 (2.4%) |
| Type 4c | 6/210 (2.9%) | 4/233 (1.7%) | 5/343 (1.5%) | 4/250 (1.6%) |
| Type 5 | 43/210 (20.5%) | 16/233 (6.9%) | 109/343 (31.8%) | 22/250 (8.8%) |
| Silent | 14/210 (6.7%) | 19/233 (8.2%) | 12/343 (3.5%) | 18/250 (7.2%) |
Figure 1. Distribution of first MI events by type.
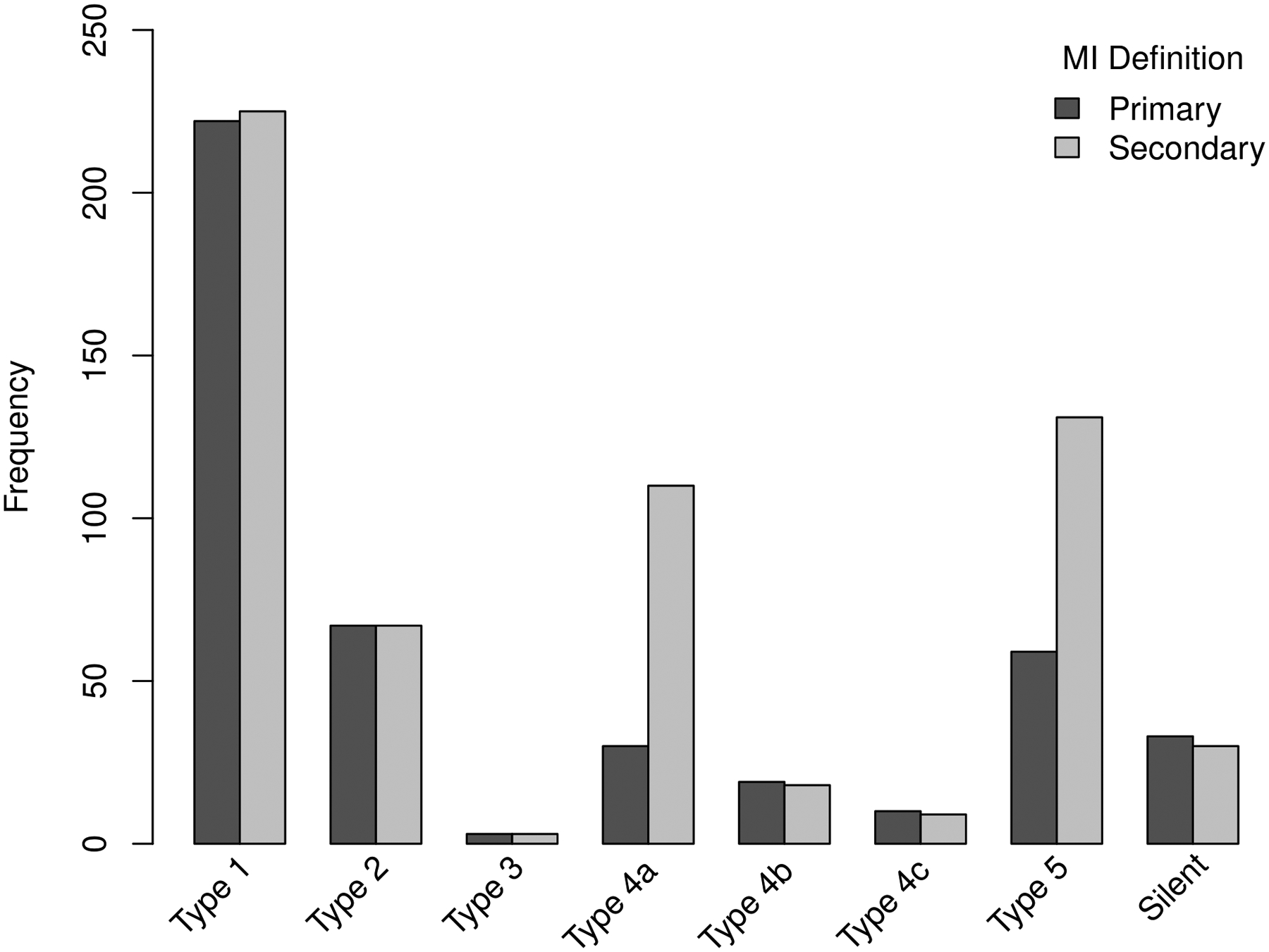
The difference in total MI rates between the primary and secondary MI definitions was primarily due to increased procedural MI events using the secondary MI definition. Type 4a and 5 MI’s accounted for 20.1% of all MI’s using the primary definition and 40.6% using the secondary definition.
Of the 289 type 1 or 2 MI events, 67 (23.2%) were type 2 MI’s and were associated with a greater rate of complications compared to the other MI types (Table III in the Supplement). The incident rates for type 2 MI were similar regardless of treatment strategy or MI definition (Table IV in the Supplement). Of the 100 patients with a large non-procedural MI during follow-up, 71%, 19%, and 10% were types 1, 2, and 4b or 4c MI events.
Treatment Strategy and MI Type
First MI events were more frequent with the conservative as compared with the invasive strategy using the primary definition, and less frequent with the conservative as compared with the invasive strategy using the secondary MI definition (Table 1, Figure 2). As expected, significantly more procedural MI’s (p<0.001) and stent-related type 4b and 4c MI’s occurred in the invasive strategy, regardless of MI definition. Table IV and Figure I in the Supplement provide a breakdown of procedural MI’s by treatment strategy according to whether they were classified by biomarker elevation only, or by MI criteria with supporting evidence of myocardial ischemia (types 4a and 5). The five-year cumulative incidence rates of the different MI subtypes by treatment strategy using the primary and secondary MI definition are found in Table IV in the Supplement. Of the 31 type 4a MI events classified using the primary definition, 24 (77%) had supporting evidence of myocardial ischemia. Of the 64 type 5 MI events classified using the primary definition, 15 (23%) had supporting evidence of myocardial ischemia. The frequency of supportive evidence for myocardial ischemia post-procedure was similar using the secondary MI definition.
Figure 2. Results for MI type according to treatment strategy.
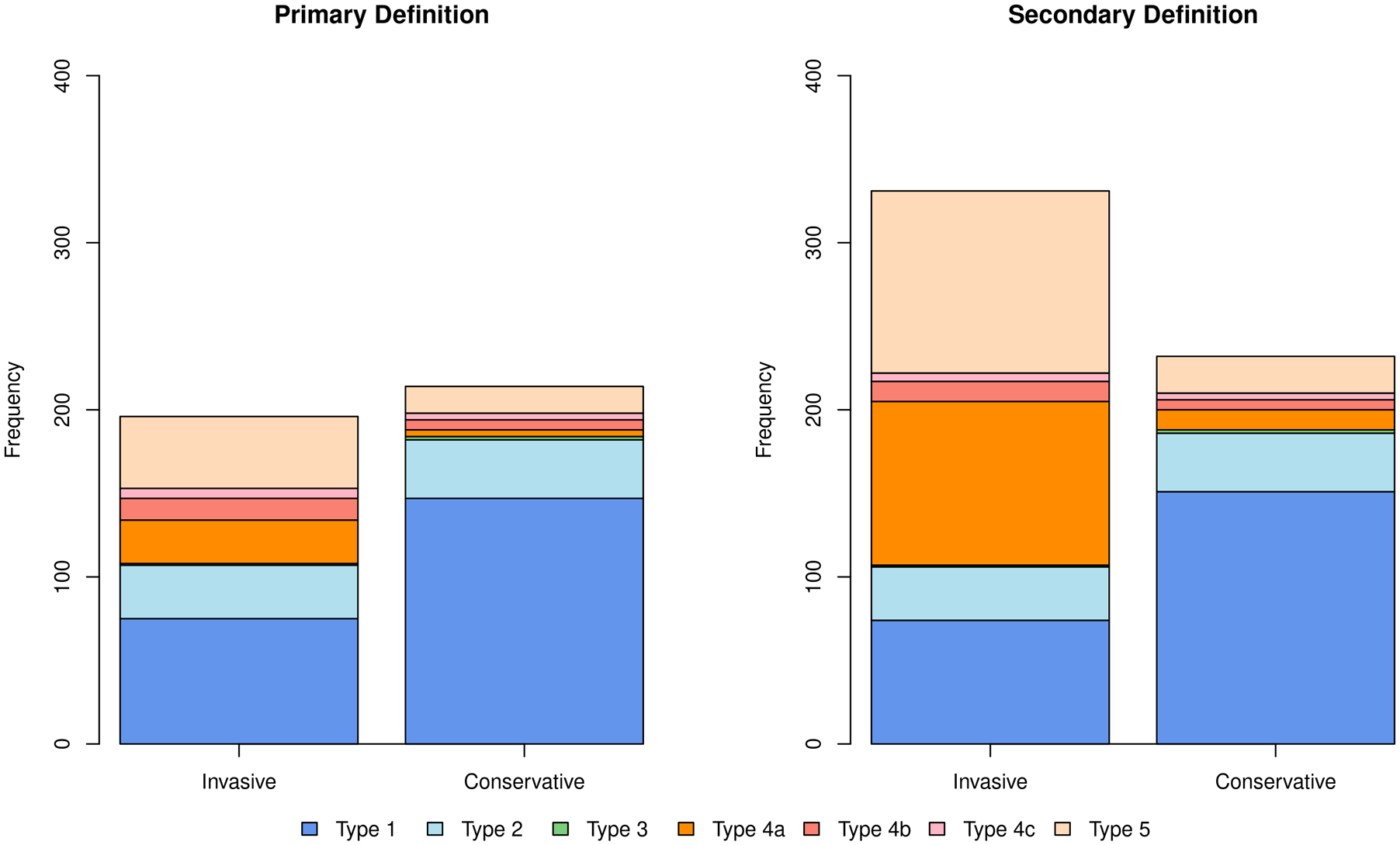
With the primary definition, there were slightly more first MI events in the conservative strategy whereas the opposite was true using the secondary MI definition. Dark blue shows spontaneous type 1 MI’s that were reduced in the invasive strategy regardless of which MI definition was used. The incidence of type 2 MI’s shown in light blue were similar. Procedural MI’s (orange) were more common in the invasive strategy and, as expected, occurred with greater frequency using the secondary definition (right panel). Stent related type 4b (stent thrombosis-related) and 4c MI’s (in-stent restenosis-related) shown in red were more frequent in the invasive strategy.
The cumulative incidence of type 1 MI by treatment assignment overall was significantly less in the invasive than the conservative strategy using either MI definition (p<0.0001) (Table 1, Figure 3). The decreased incidence of type 1 MI events in the invasive strategy was observed for those who underwent PCI or CABG, as well as in the subset that had no revascularization (59.6% of whom had no obstructive coronary disease at cardiac catheterization; p<0.001 for individual comparisons and p<0.0001 for the group comparison).
Figure 3. Spontaneous Type 1 MI by Strategy and by MI Definitions Management after Cath by Invasive Strategy vs Conservative Strategy.
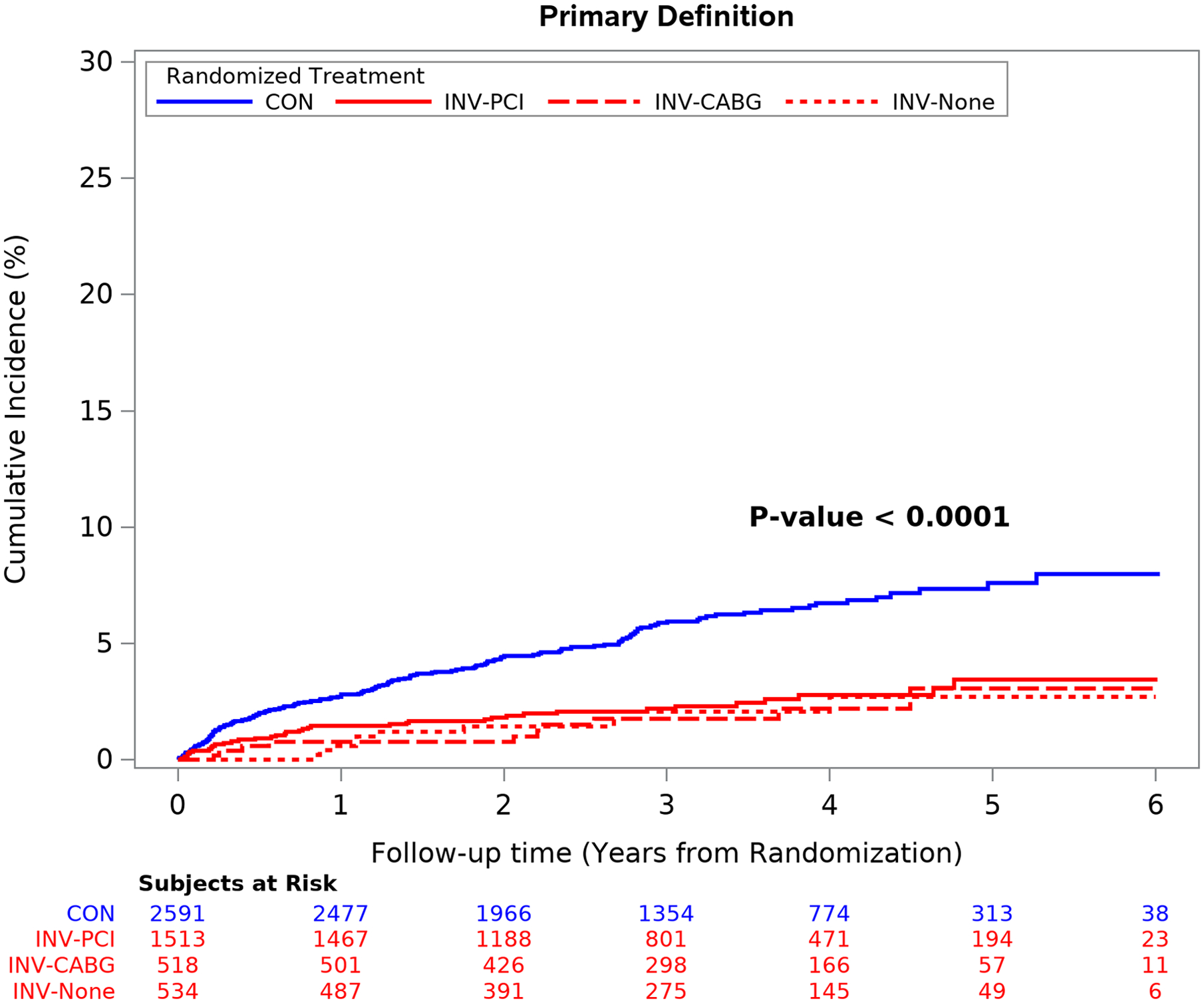
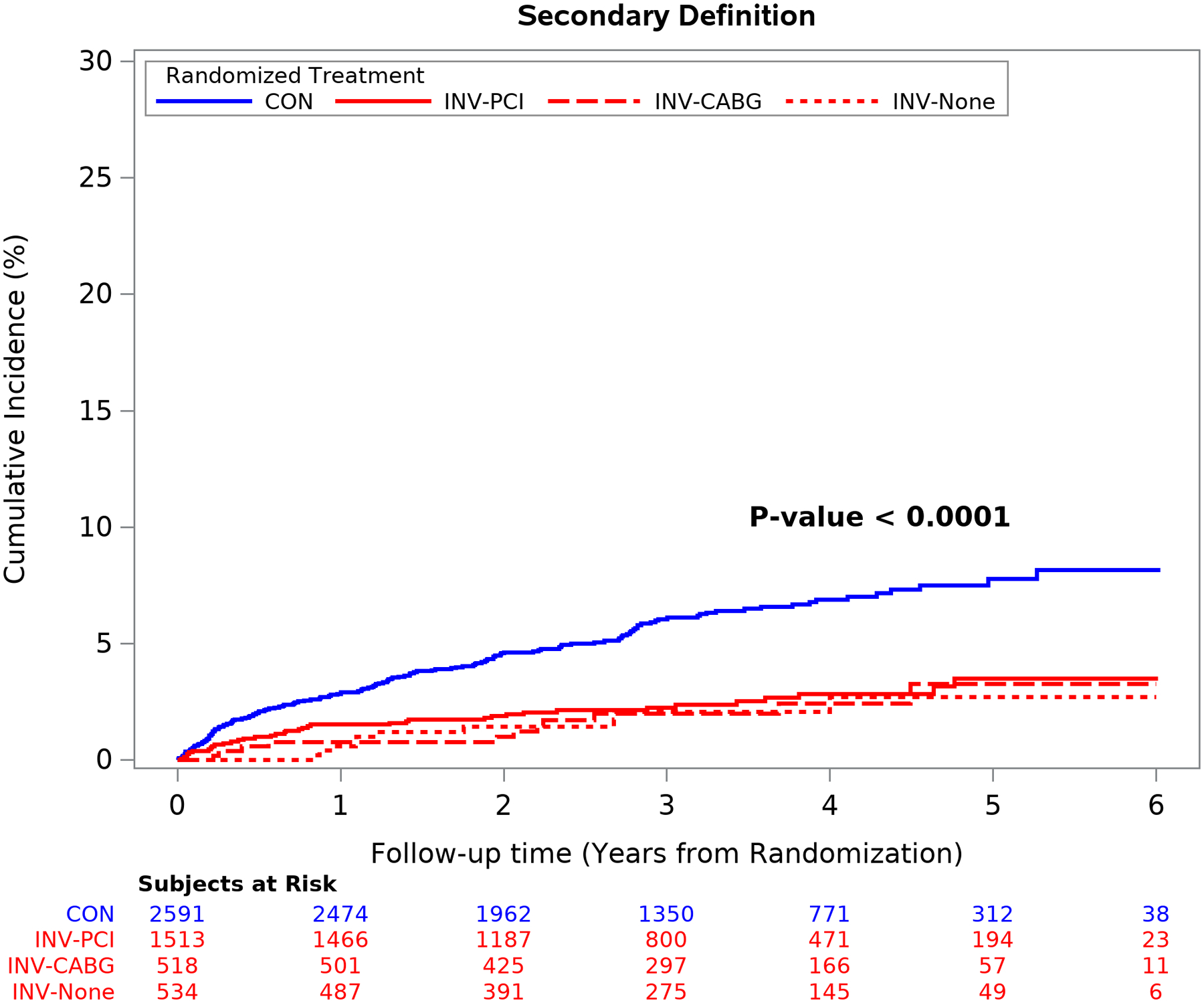
Spontaneous type 1 MI events were significantly more frequent in the conservative strategy regardless of type of revascularization procedure performed or MI definition used. Group differences are significant at p<0.001 and individual pairwise comparisons to the CON group are significant at p <0.001 after adjustment for multiple comparisons. CON = conservative strategy; INV-PCI = invasive strategy patients that received PCI; INV-CABG = invasive strategy patients that received coronary bypass surgery; INV-None = invasive strategy patients that did not receive coronary revascularization (59.6% had non-obstructive coronary disease at catheterization).
The mean time free from the composite of cardiovascular death, MI, admission for unstable angina, heart failure or resuscitated cardiac arrest over 5 years was similar between treatment groups using the primary definition of MI.1 Conversely, the difference in the composite end-point was significantly greater in the invasive group using the secondary MI definition (p <0.001) because of an increased number of procedural MI’s. (Figure 4). Similar findings were seen for the composite of cardiovascular death or MI and for all-cause death or MI (Figure II in the Supplement). The difference was the result of the greater number of procedural MI events classified by the secondary definition. The five-year estimated cumulative event rate for cardiovascular death was 5.2% for the invasive vs 6.5% for the conservative strategy [difference: −1.3% (95% CI: −3.1% to 0.6%)] [Cox model HR2: 0.87 (95% CI: 0.66 to 1.15)]
Figure 4. Outcomes according to treatment strategy and MI definition.
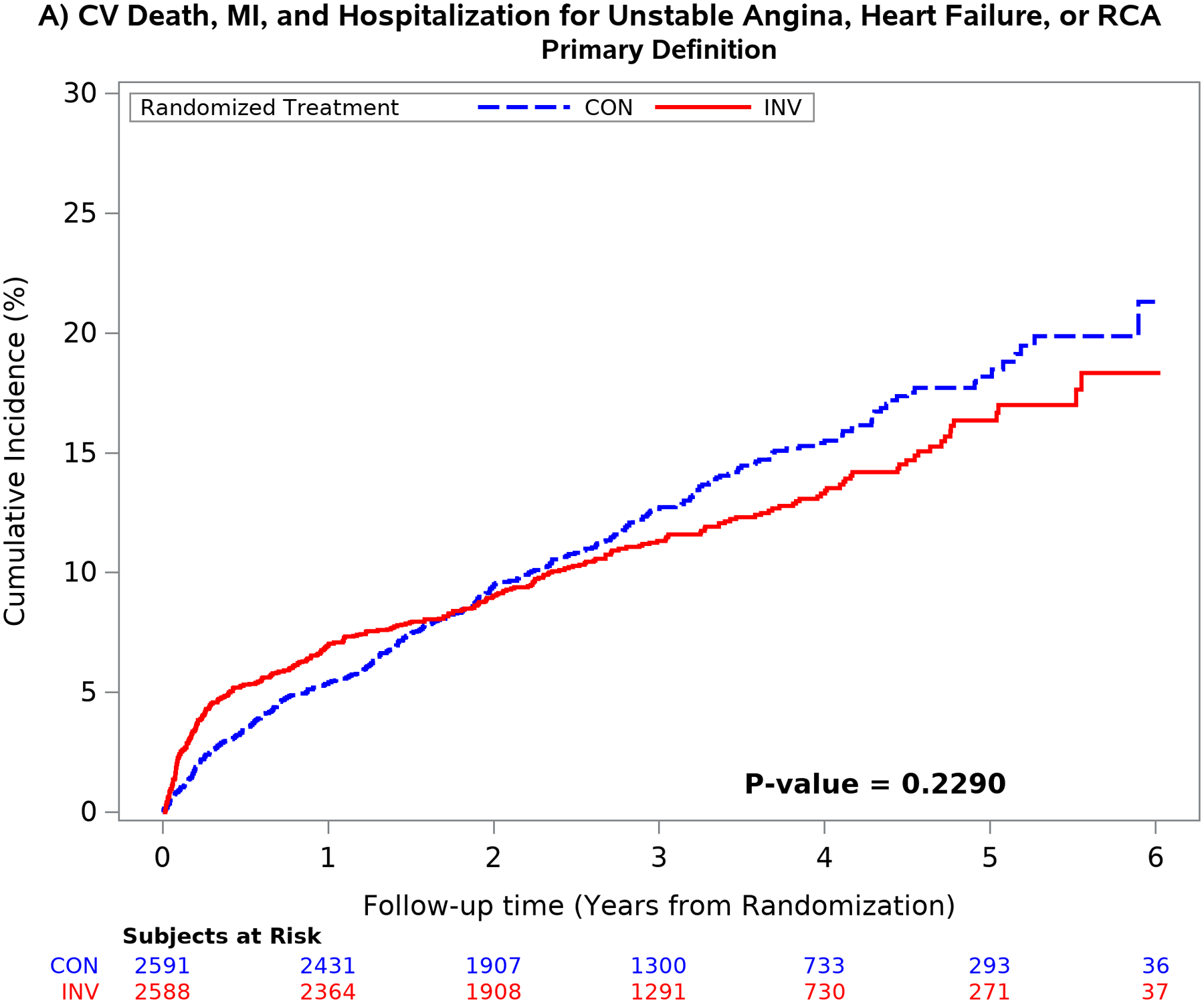
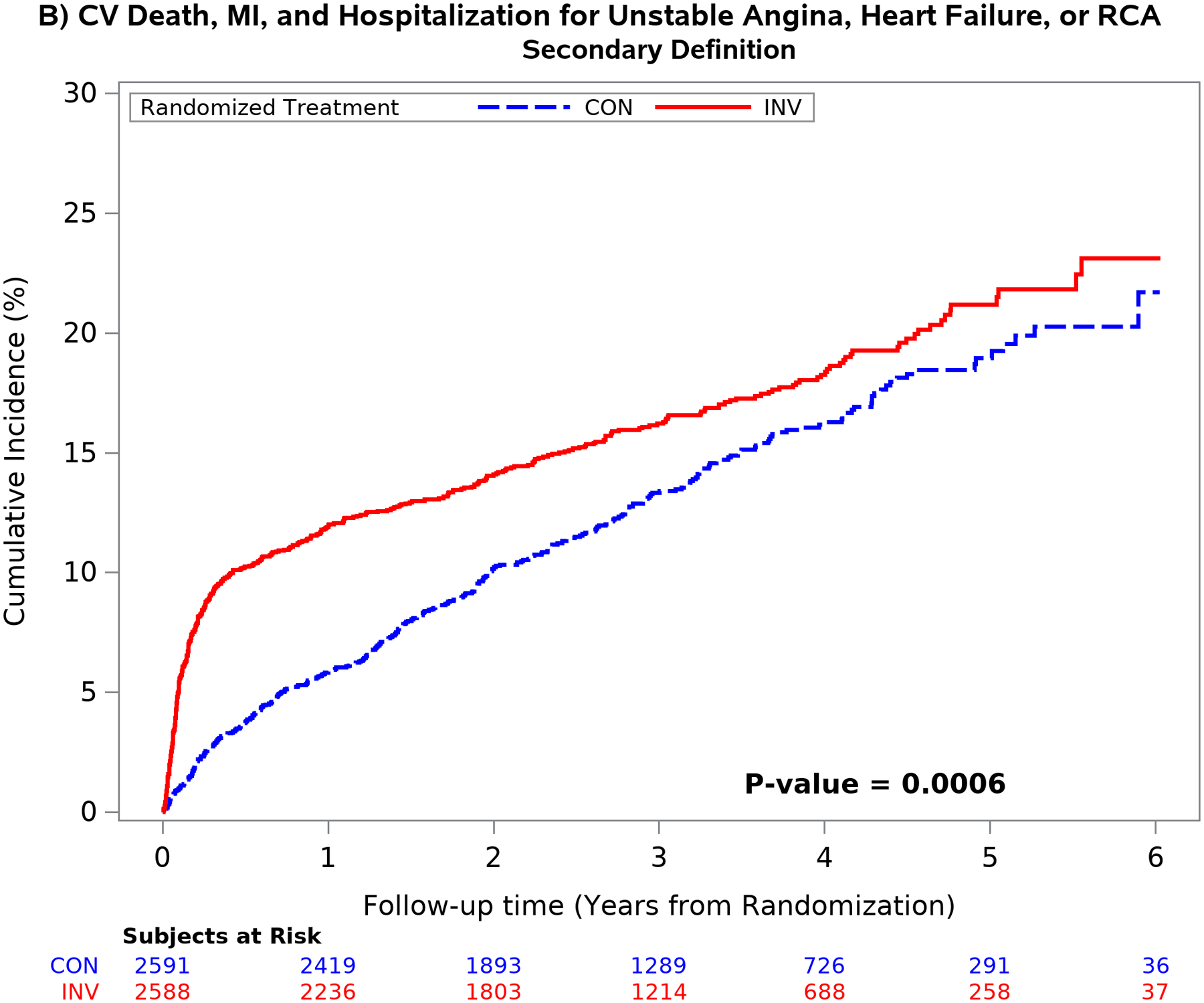
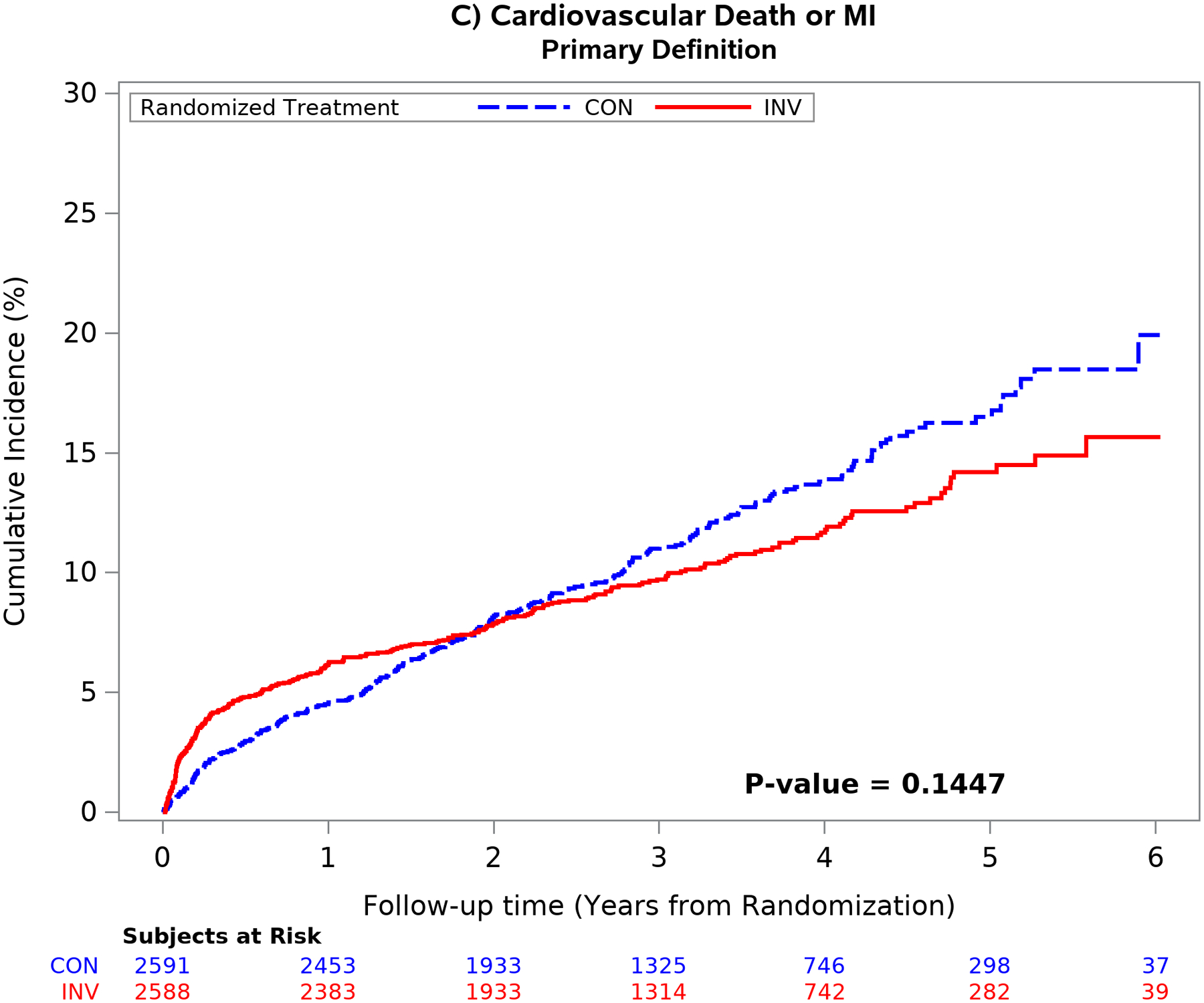
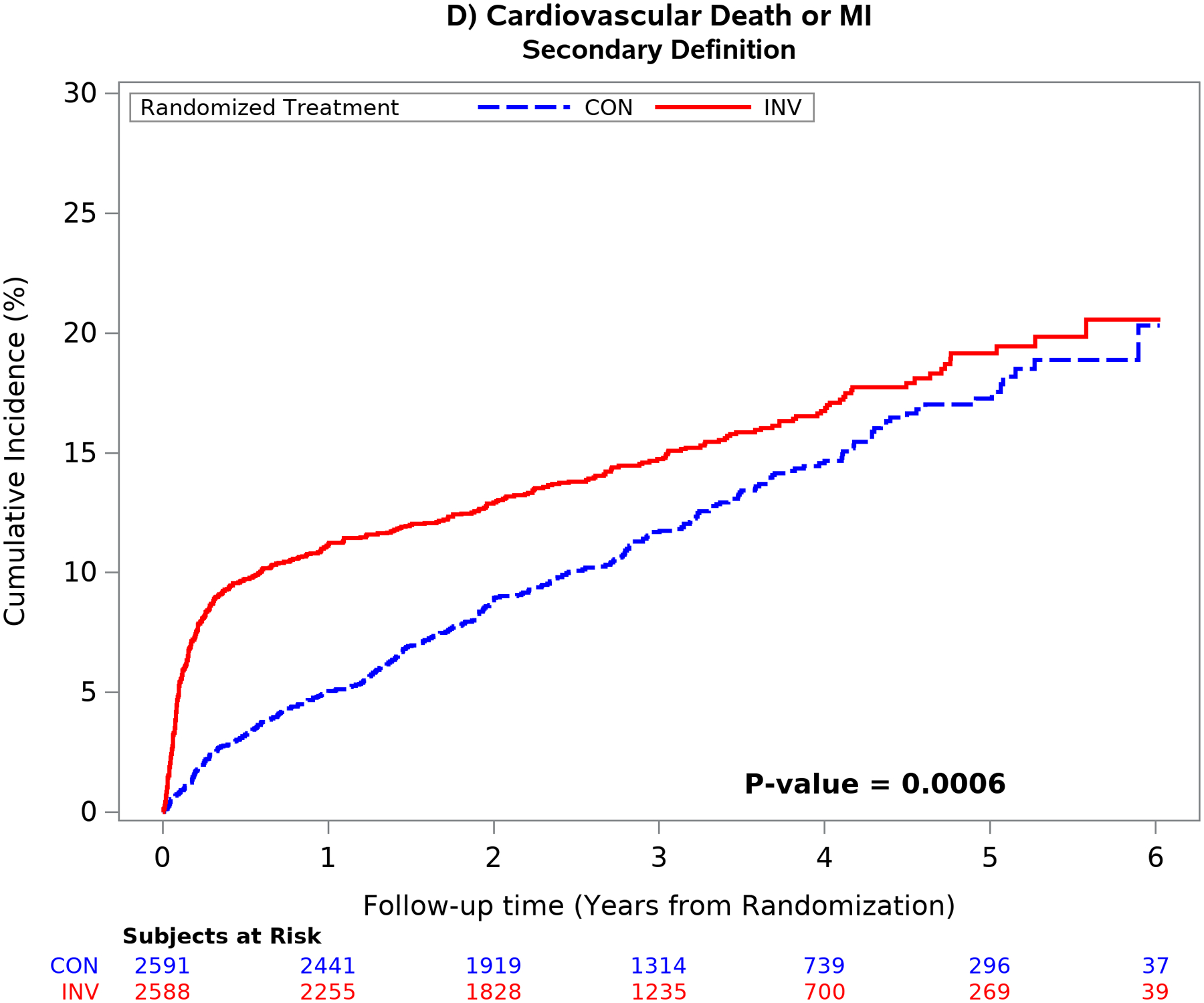
Unadjusted cumulative incidence plot of the 5-component primary ISCHEMIA end-point using the primary MI (left upper panel) and secondary MI (right upper panel) definitions and the composite end-point of cardiovascular death or MI using the primary (left lower panel) and secondary (right lower panel) MI definitions by randomized treatment strategy. Choice of MI definition had an important impact on the outcome results. The secondary MI definition (right panels) was associated with an increased number of early procedural MI events in the invasive as compared with the conservative strategy. The difference between treatment groups attenuated over time using the secondary definition (right panel) primarily due to the increased number of spontaneous type 1 MI events in the conservative strategy. Cardiovascular death rates were low and not statistically different between treatment groups.
Prognostic Association of MI Type According to the MI Definition
All-cause death subsequently occurred in 25 (11.5%) of 217 patients with a type 1 MI and 5 (5.6%) of 89 patients with a procedural MI using the primary definition (Table 2).The rates for all-cause death were 25 (11.2%) of 223 with a type 1 MI and 16 (6.5%) of 245 with a procedural MI using the secondary definition. All-cause death subsequently occurred in 17 (25.4%) of 67 patients with a type 2 MI; 15 deaths were attributed to cardiovascular causes. All-cause death subsequently occurred in 219 (4.8%) of 4585 patients who had no MI by either the primary or secondary MI definition. Of those 219 deaths, 144 (65.8%) were attributed to cardiovascular causes. The 5-year unadjusted death rate among patients who had no MI (by primary or secondary definition), was 7.7% (95% CI: 6.5%, 9.0%).
Table 2:
Death, Cardiovascular Death and the Composite of Cardiovascular Death or Hospitalization for Heart Failure Events Following an MI
| MI Type | No. MI Events | Event | No. Events Post-MI | HR (95% CI)* MI vs. No MI | P-value |
|---|---|---|---|---|---|
| Primary Definition | |||||
| Procedural MI | 89 | All-cause Death | 5 | 1.14 (0.42, 3.08) | 0.803 |
| Procedural MI (INV Only) | 67 | All-cause Death | 4 | 1.40 (0.51, 3.86) | 0.511 |
| Procedural MI (Excluding Stand-alone MI) | 36 | All-cause Death | 3 | 2.10 (0.67, 6.66) | 0.205 |
| Type 4B or 4C MI | 34 | All-cause Death | 5 | 3.99 (1.62, 9.83) | 0.003 |
| Type 1 MI | 217 | All-cause Death | 25 | 2.44 (1.54, 3.88) | <.001 |
| Procedural MI | 89 | Cardiovascular Death | 5 | 1.99 (0.73, 5.43) | 0.181 |
| Procedural MI (INV Only) | 67 | Cardiovascular Death | 4 | 2.77 (0.99, 7.76) | 0.052 |
| Procedural MI (Excluding Stand-alone MI) | 36 | Cardiovascular Death | 3 | 3.75 (1.17, 11.97) | 0.026 |
| Type 4B or 4C MI | 34 | Cardiovascular Death | 5 | 6.80 (2.73, 16.93) | <.001 |
| Type 1 MI | 217 | Cardiovascular Death | 21 | 3.38 (2.03, 5.61) | <.001 |
| Procedural MI | 89 | Cardiovascular Death or Hospitalization for Heart Failure | 5 | 1.45 (0.53, 3.93) | 0.469 |
| Procedural MI (INV Only) | 67 | Cardiovascular Death or Hospitalization for Heart Failure | 4 | 1.95 (0.71, 5.39) | 0.196 |
| Procedural MI (Excluding Stand-alone MI) | 36 | Cardiovascular Death or Hospitalization for Heart Failure | 3 | 2.61 (0.82, 8.28) | 0.103 |
| Type 4B or 4C MI | 34 | Cardiovascular Death or Hospitalization for Heart Failure | 8 | 7.82 (3.58, 17.84) | <.001 |
| Type 1 MI | 215 | Cardiovascular Death or Hospitalization for Heart Failure | 23 | 2.94 (1.82, 4.74) | <.001 |
| Secondary Definition | |||||
| Procedural MI | 245 | All-cause Death | 16 | 1.06 (0.56, 2.02) | 0.858 |
| Procedural MI (INV Only) | 204 | All-cause Death | 15 | 1.21 (0.63, 2.34) | 0.569 |
| Procedural MI (Excluding Stand-alone MI) | 115 | All-cause Death | 9 | 1.38 (0.61, 3.15) | 0.440 |
| Type 4B or 4C MI | 35 | All-cause Death | 5 | 3.69 (1.50, 9.09) | 0.005 |
| Type 1 MI | 223 | All-cause Death | 25 | 2.55 (1.60, 4.06) | <.001 |
| Procedural MI | 245 | Cardiovascular Death | 13 | 1.24 (0.57, 2.68) | 0.592 |
| Procedural MI (INV Only) | 204 | Cardiovascular Death | 12 | 1.54 (0.70, 3.43) | 0.286 |
| Procedural MI (Excluding Stand-alone MI) | 115 | Cardiovascular Death | 8 | 1.95 (0.79, 4.84) | 0.149 |
| Type 4B or 4C MI | 35 | Cardiovascular Death | 5 | 6.17 (2.48, 15.35) | <.001 |
| Type 1 MI | 223 | Cardiovascular Death | 21 | 3.52 (2.11, 5.88) | <.001 |
| Procedural MI | 245 | Cardiovascular Death or Hospitalization for Heart Failure | 15 | 1.16 (0.59, 2.30) | 0.661 |
| Procedural MI (INV Only) | 204 | Cardiovascular Death or Hospitalization for Heart Failure | 14 | 1.42 (0.70, 2.85) | 0.330 |
| Procedural MI (Excluding Stand-alone MI) | 115 | Cardiovascular Death or Hospitalization for Heart Failure | 10 | 2.00 (0.93, 4.31) | 0.077 |
| Type 4B or 4C MI | 35 | Cardiovascular Death or Hospitalization for Heart Failure | 8 | 7.07 (3.25, 15.38) | <.001 |
| Type 1 MI | 221 | Cardiovascular Death or Hospitalization for Heart Failure | 23 | 3.07 (1.90, 4.97) | <.001 |
Adjusted for the main ISCHEMIA trial covariates (age at randomization, sex, eGFR, ejection fraction, and diabetes) in addition to randomized treatment strategy, prior heart failure, prior MI, smoking status, LDL-C, and degree of ischemia. Continuous covariates are modeled as restricted cubic splines. The prognostic models flag an MI that occurs up to and on the day of the event of interest.
The hazard ratios for all-cause death, cardiovascular death, and cardiovascular death or admission for heart failure using the primary or secondary definition are shown in Table 2 and Figure 5. There were 11 type 1 and 6 type 4b/4c MI’s in which another MI type occurred prior to the MI of interest. After multivariable adjustment for baseline characteristics and treatment group, a type 1 MI was strongly associated with an increased risk for all-cause death, cardiovascular death, and the composite of cardiovascular death or admission for heart failure compared with patients that did not have an MI during follow-up regardless of which MI definition was used [(Table 2, Figure 5) (p<0.001)].
Figure 5. Adjusted risks of MI on subsequent all-cause and cardiovascular death according to MI definition.
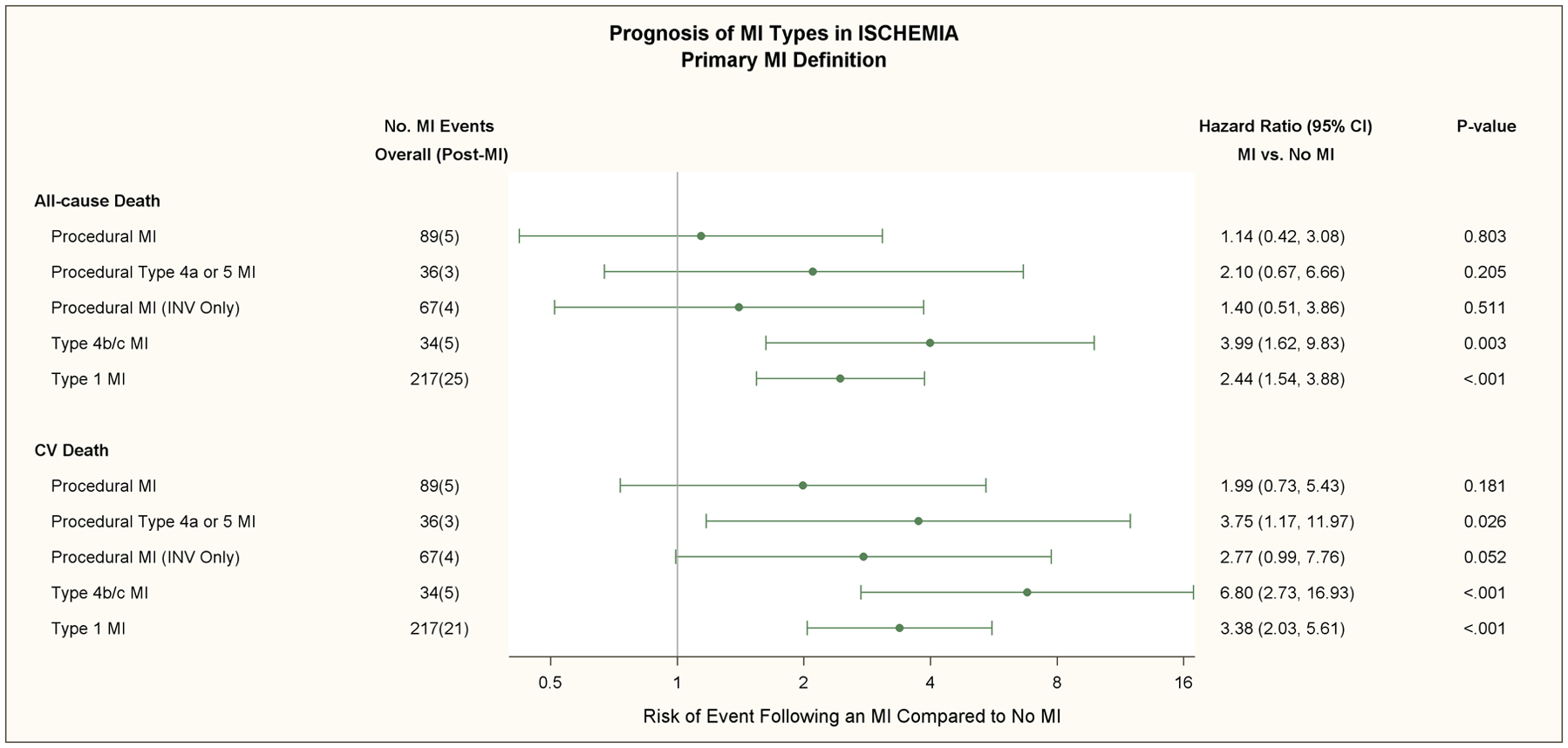
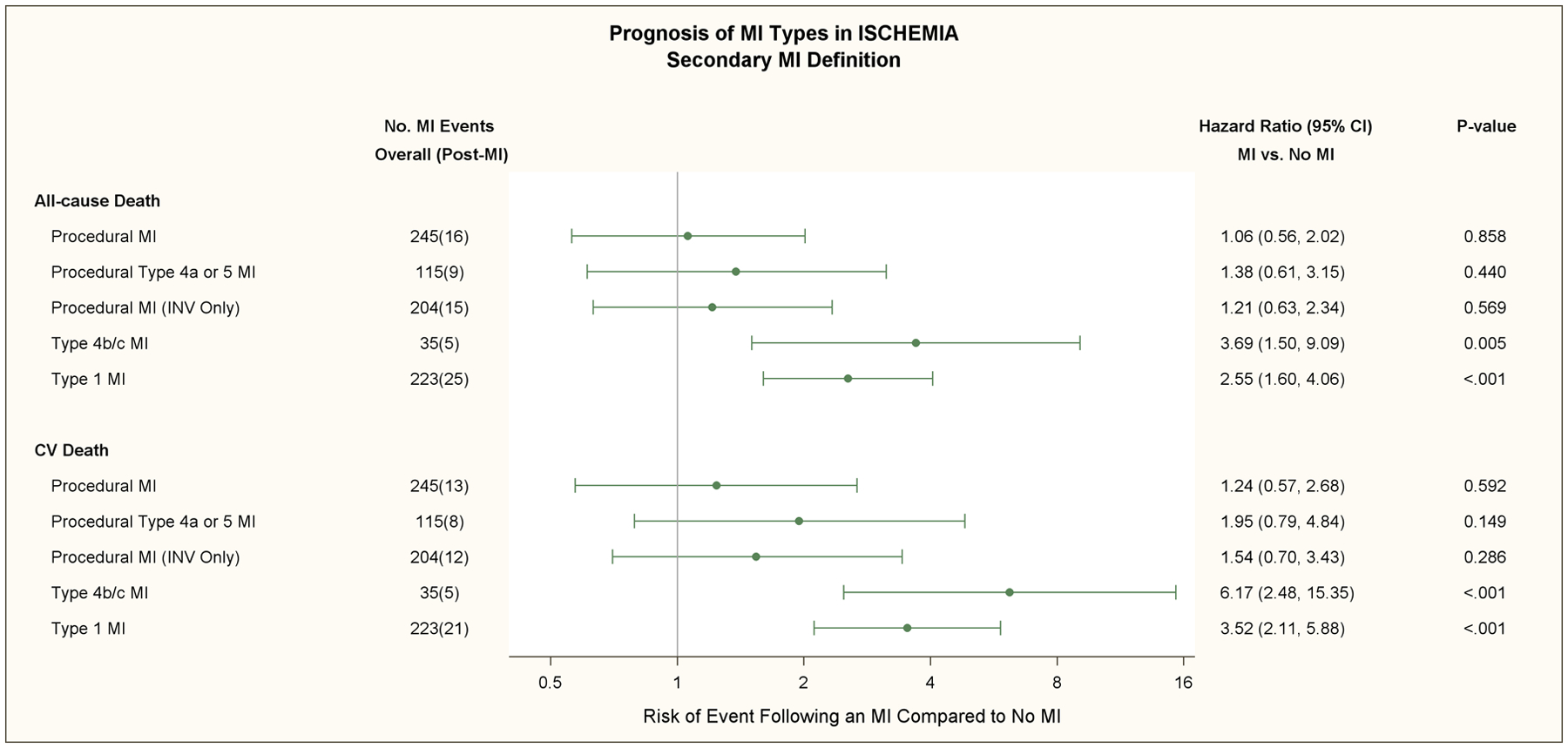
The multivariate adjusted relative risk of all-cause death and cardiovascular death for the primary (upper panel) and secondary (lower panel) MI definitions are shown in this forest plot for procedural MI, procedural type 4a or 5 MI with ancillary evidence of myocardial ischemia, procedural MI in the invasive strategy only (Procedural MI [INV Only]), types 4b/c stent related MI’s, and type 1 MI.’s Total number of MI events and subsequent deaths are shown in the second column. In patients assigned to the invasive strategy, the adjusted hazard ratio for cardiovascular death was 2.77 times greater in patients who had a procedural MI and no non-procedural MI with the primary MI definition compared to patients who had no MI during follow-up (p=0.052). The adjusted risk of subsequent all-cause death and cardiovascular death was increased for patients that had a type 1 MI and no procedural MI compared with patients that had no MI during follow-up with the primary and secondary MI definitions (p<0.001), respectively. The adjusted risk for cardiovascular death was greater for patients that sustained a type 4b/c (stent-related) MI (p<0.001).
Of the 67 procedural MI (INV) using the primary MI definition 4 patients died (Figure 5); 1 had type 4a MI and 3 had type 5 MI. Of the 204 procedural MI (INV) using the secondary definition 15 patients died. Of the 15, 8 had type 4a MI and 7 had type 5 MI. During follow-up, 4 patients with a type 4b MI (stent thrombosis) died and 1 patient with a type 4c MI died. MI types 4b,4c were pooled for analytic purposes since the number of this type of MI were relatively small and both are stent-related events. The hazard ratios for all-cause death, cardiovascular death, and cardiovascular death or admission for heart failure using the primary or secondary definition were greater for types 4b/4c MI compared to type 1 or procedural MI using both MI definitions. The impact of elevated pre-procedural biomarker values in subjects with a type 4a and 5 MI is shown in Table V in the Supplement. Most patients with a procedural MI had normal pre-procedure biomarkers. In those with elevated pre-procedural biomarkers, subsequent deaths were uncommon and did not occur with the primary MI definition. Post-procedural CK-MB ratio elevations > 10 times ULN were uncommon and occurred in <1% and 3.3% of patients post-PCI and post-CABG, respectively. One of the 19 patients with a post-procedural CK-MB elevation > 10 times ULN died.
DISCUSSION
MI events were the predominant component of the primary and major secondary outcomes in ISCHEMIA, and interpretation of the overall trial was sensitive to the definition of procedural MI used. The primary MI definition used CK-MB as the preferred biomarker for assessment of type 4a and 5 procedural MI whereas the secondary MI definition used similar thresholds of cTn. CK-MB is relatively insensitive compared to cTn, resulting in a relatively lower frequency of procedural MI events with the primary compared to the secondary procedural MI definition. As expected, procedural MIs were more common with the invasive strategy than the conservative strategy using both the primary and secondary definition. With the primary ISCHEMIA trial MI definition, there were no major long-term differences in the primary composite event rate and the major secondary composite rate of cardiovascular death or MI. In contrast, as a result of the increased rates of procedural MI with the secondary definition, a significant treatment difference in the primary trial endpoint and major secondary composite endpoint of cardiovascular death or MI was observed favoring the conservative strategy. Removal of biomarker elevation only criteria from types 4a and 5 MI’s did not produce a meaningful change in the treatment comparison results or conclusions with either MI definition. Finally, the pattern of association of procedural and non-procedural MI’s by the two MI definitions with subsequent death were relatively consistent.
Treatment Strategy and Myocardial Infarction Risk
In ISCHEMIA, the risk of type 1 MI’s was reduced for patients that had either a PCI or CABG procedure. In contrast, the COURAGE and BARI 2D trials did not show a reduction in spontaneous MI rates with initial PCI and optimal medical therapy vs. initial optimal medical therapy alone.21–24 In the BARI 2D trial, patients with diabetes were randomized after the coronary angiogram was performed and the treating physician then determined if the patient was more suitable for PCI or CABG. Biomarkers were not routinely collected after coronary revascularization in BARI 2D. However, type 1 MI events were significantly reduced by a strategy of prompt CABG as compared to a conservative strategy in BARI 2 D, particularly in higher risk patients.23, 24 Similar findings were reported in the FREEDOM trial when the comparator was a PCI strategy.25 FAME-2 did not show a significant reduction in spontaneous MI events with FFR-guided PCI after 3 years of follow-up although a trend towards decreased MI events was observed after 5 years.26, 27 The reason(s) why PCI conferred protection against type 1 MI is not clear since spontaneous MI events often occur in non-stented vessels or non-flow limiting lesions.28 Possible explanations include more effective revascularization than was previously possible, increased use of dual antiplatelet therapy, ascertainment bias, or other aspect of the invasive strategy.29–32 More potent dual antiplatelet therapy has been shown to reduce de novo atherothrombotic events in addition to preventing complications associated with stenting of the culprit lesion following ACS.30 However, DAPT usage for patients assigned to the invasive strategy in ISCHEMIA was greatest in the initial 18 months after the procedure, yet type 1 MI’s continued to occur with greater frequency in the conservative strategy throughout followup.1 It is also possible that clinicians would be more likely to admit a patient with chest pain if they were assigned to the conservative strategy and therefore more likely to have an MI diagnosed (ascertainment bias).1
Prognostic Impact of MI Type
The type 1 MI events that occurred in ISCHEMIA were associated with an increased risk for all-cause death, cardiovascular death, and the composite of cardiovascular death or heart failure admission compared to patients without an MI after adjustment for treatment strategy and regardless of MI definition. In contrast, the risk of subsequent all-cause death or cardiovascular death after procedural MI events compared to patients without an MI was less than type 1 MI’s, and the MI’s were less likely to be complicated. Our findings support previous reports comparing non-procedural to procedural MI events that show a higher mortality with spontaneous MI events.28,33–37 Procedural MI’s are often related to baseline risk, atherosclerosis burden, and procedural complexity.33 Patients with a type 1 MI are at higher risk of thrombotic complications due to acute evolving intracoronary thrombosis, later (out-of-hospital) presentation after symptom onset, and different mechanisms in that elective PCI-related infarcts tend to result from microembolism, dissection, or temporary occlusions that occur when the procedure is performed and can often be treated. Thus, type 1 MI events compared to procedural MI’s are generally associated with a worse prognosis. We did not observe substantial differences in the rates of type 2 MI between treatment strategies. The frequency of complications after a type 2 MI’s was greater than the other MI types and associated with a worse prognosis than type 1 or procedural MI’s. A higher incidence of adverse outcomes with type 2 MI compared to type 1 MI’s has been previously reported.38–40
Types 4b/c MI’s accounted for 9.1% of all first MI’s in patients assigned to the invasive strategy and were associated with a greater risk for all-cause death and cardiovascular death, than type 1 or procedural MI’s. The mortality rate for type 4b (stent thrombosis) was greater than for type 4c (restenosis) and accounted for 65% of the type 4b/4c MI’s. This finding is consistent with other reports, such as the CORONOR registry in patients with stable coronary disease and PCI, in which late stent thrombosis accounted for 20% of all MI types and was associated with a 4-fold increased mortality rate compared to spontaneous MI’s after 5 years of follow-up. Although stent-related MI events are a consequence of randomization to the invasive strategy, and are associated with a greater risk of death, they accounted for less than 10% of all post-randomization MI’s in the selected sites in the ISCHEMIA trial. Type 1 (spontaneous) MI’s that were unrelated to the stented lesion were also associated with increased mortality as compared to procedural MI’s and accounted for 51% of all MI’s with the primary MI definition.
MI Reference Limits
Bagai et al reported substantial variability in the decision limit performed for various cTn assays in a cohort study of 276 hospital laboratories in 31 countries participating in the ISCHEMIA trial.19 Twenty-one unique troponin assays from 9 manufacturers were in use at these sites. Approximately 1/3 of sites applied the suggested 99th percentile URL with the ratio of troponin value to the manufacturer’s recommended decision limit sometimes varying more than 10 fold, regardless of whether the sites were in the United States or whether the assay used was conventional or hs-cTn. In a large multinational trial, such as ISCHEMIA, laboratory sheets from individual institutions usually do not indicate which assay was used or if the MI decision limit used is the manufacturer’s 99th percentile. In fact, it was not rare that sites using the same assay had different URL’s. To minimize this type of variability, we used the manufacturer’s recommended 99th percentile URL for individual assays using the secondary MI definition in ISCHEMIA with sex-specific thresholds when available. We used site-determined local decision limits for the primary definition. The choice of site determined vs the suggested manufacturer’s 99th percentile URL did not have a substantial impact on incidence rates of non-procedural MI events. In many cases, the magnitude of biomarker release after MI usually exceeded both reference limits, and during the course of the trial, many hospital laboratories in this international study had adopted the manufacturer’s recommended 99th percentile URL, particularly for hs-cTn.
Elevated Pre-procedural Biomarkers
In the setting of elective PCI, an association of elevated pre-procedural hs-cTn T values and subsequent increased mortality has been reported (12). Elevated pre-procedural biomarkers usually indicate acute or chronic myocardial injury, both of which are known to adversely impact prognosis (14). In one retrospective series of 5,626 patients undergoing elective PCI, an increase in post-procedural hs-TnT level did not offer prognostic information beyond that provided by the baseline level of the biomarker. In this series, isolated biomarker increases only were reported and MI events were not classified (12). In ISCHEMIA, the CEC determined if the elevated pre-procedural biomarker value based on clinical, electrocardiographic, and imaging findings was the result of a type 1,2, 4b, or 4c MI. If the pre-procedural value was missing and the patient had no recent change in symptoms or clinical evidence of myocardial ischemia, the pre-procedure biomarkers were presumed to be normal. When pre-procedural values were elevated and post-procedural values increased >20% associated with clinical evidence of myocardial ischemia, the procedural MI was classified as a type 4a or type 5 MI. Pre-procedural biomarkers were normal in 75–86% of subjects in ISCHEMIA (Table V in the Supplement). A normal baseline biomarker value was usually based on the single protocol required sample for elective procedures. Determination of a stable baseline pre-procedure based on a single sample would impact the definition of normal more for the primary than the secondary MI definition given the relative insensitivity of CK-MB as compared to cTn.
Study Limitations
ISCHEMIA had a median follow-up of 3.2 years which is relatively short for a chronic disease process, and it may take longer to observe the association of spontaneous and procedural MI events on all-cause mortality, cardiovascular mortality, and heart failure and for differences between treatment strategies to emerge. In the Coronary Artery Surgery Study (CASS) that randomized mild-moderately symptomatic or asymptomatic patients with coronary artery disease to CABG or medical therapy, CABG neither prolonged life nor prevented MI after five years compared to medical therapy.41 However, a significant improvement in 7-year survival was reported in a relatively small subset of patients with 3-vessel disease and LVEF >34% and <50% with elective bypass surgery. Although that subset was <100 patients, and the trial was conducted in an era of minimal medical therapy, this result changed clinical practice.41–43 Similarly, the benefits of CABG in patients with LVEF ≤35% in the (STICH) trial only emerged with 10-year follow-up.44
Both ISCHEMIA definitions of procedural MI included biomarker elevation only criteria and not MI as defined in the UDMI 3 or 4.14 Thus, the rates of procedural MI’s in ISCHEMIA are greater than the rates that would have been observed had the UDMI definition been used (Table IV in the Supplement). In some studies, elevated biomarker only criteria have been associated with increased mortality.12 The implications of using different procedural MI definitions after coronary revascularization in terms of outcomes and prognosis has been well described.45–48 We did not observe a relationship between magnitude of CK-MB ratio and all-cause mortality. The patients enrolled in ISCHEMIA were stable at the time of randomization; only a small number of patients had post-procedural CK-MB values that exceeded 10 x ULN and the number of deaths was insufficient to test the relationship of larger post-procedural CK-MB elevations to mortality.
The number of patients with procedural MI’s that had elevated pre-procedural biomarkers were relatively small precluding a robust analysis of the prognostic value of elevated pre-procedural biomarkers and their relationship to post-procedural values and subsequent prognosis. Finally, deaths after procedural MI’s were relatively infrequent in ISCHEMIA, and prognostic correlation of subsequent death after a procedural MI should be interpreted with caution regardless of MI definition.
Conclusions
Our data show that choice of MI definition influences MI event rates, which were the most frequent component of the primary endpoint in the ISCHEMIA trial. Using the primary MI definition, the invasive and conservative strategies resulted in similar rates of the primary and secondary composite end-points, whereas using the secondary MI definition, we observed a greater frequency of the primary and secondary composite endpoints in patients assigned to the invasive strategy. In contrast to procedural MIs, spontaneous type 1 MI’s were more strongly associated with an increased risk of death and were significantly reduced in patients randomized to the invasive strategy. However, it is unclear whether this reduction was attributable to revascularization, DAPT, ascertainment bias, or some other mechanism. Type 4b/c MI’s were relatively infrequent but associated with a greater risk of subsequent death. Longer-term follow-up may determine if the differences in MI rate and type translate into differential treatment effects on cardiovascular mortality.
Supplementary Material
Clinical Perspective.
What is New?
This analysis of the ISCHEMIA trial demonstrated that procedural MI definition had an important impact on event frequency and subsequent prognosis.
When the pre-specified secondary MI definition was applied, the conservative strategy had significantly lower composite event rates for the primary and major secondary trial endpoints due to an increased number of procedural MI’s in the invasive strategy.
Spontaneous type 1 MI events, associated with increased risk of cardiovascular death, were reduced with an invasive strategy (PCI or CABG).
What are the Clinical Implications?
An early invasive strategy is associated with a reduced risk for spontaneous type 1 MI. The mechanism for the reduction requires further study.
The incidence of procedural MI was determined by the MI definition used. Using the biomarker-specific thresholds established for this trial, procedural MI’s were less frequent using CK-MB compared to cardiac troponin.
Sources of Funding
NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565
This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Non-standard Abbreviations and Acronyms
- ISCHEMIA
International Study of Comparative Health Effectiveness With Medical and Invasive Approaches
- UDMI
Universal Definition of Myocardial Infarction
- SCAI
Society for Cardiovascular Angiography and Interventions
- CEC
Clinical Events Committee
- ULN
Upper limit of normal
- URL
Upper reference limit
- ACS
Acute coronary syndrome
- INV
Invasive strategy
Footnotes
Disclosure Statements
Dr. Bernard R. Chaitman reports grants from National Heart, Lung and Blood Institute during the conduct of the study, personal fees from Merck, NovoNordisk, Sanofi, Lilly, Johnson and Johnson, Daiichi Sankyo, Tricida, Relypsa, Imbria, and Xylocor outside the submitted work;
Dr. Karen P. Alexander reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Derek Cyr reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Jeffrey S. Berger reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Harmony Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from Siemens, non-financial support from BioTelemetry, outside the submitted work;
Dr. Sripal Bangalore repots grants from National Heart, Lung, and Blood Institute during the conduct of the study; grants and personal fees from Abbott Vascular, personal fees from Biotronik, personal fees from Pfizer, personal fees from Amgen, personal fees from Reata, outside the submitted work;.
Dr. William E. Boden reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, personal fees from Janssen, outside the submitted work
Dr. Lopes reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; other from Bayer, other from Boehringer Ingleheim, grants and other from Bristol-Myers Squibb, other from Daiichi Sankyo, grants and other from Glaxo Smith Kline, grants and other from Medtronic, other from Merck, grants and other from Pfizer, other from Portola, grants and other from Sanofi, outside the submitted work;.
Dr. Marcin Demkow reports grants from National Heart, Lung and Blood Institute during the conduct of the study and receives proctoring honoraria from ABBOTT, EDWARDS, BOSTON and MEDTRONIC
Dr. Gian Piero Perna reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Robert K. Riezebos reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Edward O. McFalls reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Subhash Banerjee reports grants from National Heart, Lung and Blood Institute during the conduct of the study; reports consulting honoraria from Medtronic, Astra Zeneca, Livmor Inc.; Institutional research grants: Boston Scientific Corp., Chiesi
Dr. Akshay Bagai reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Gilbert Gosselin reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Sean M. O’Brien reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Frank W. Rockhold reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; grants and personal fees from Janssen, personal fees from Merck HeathCare KGaA, personal fees from Merck Research Labs, personal fees from Novo Nordisk, personal fees from KLSMC, personal fees from Aldeyra, personal fees from Rhythm , personal fees from Phathom, grants and personal fees from AstraZeneca, personal fees from Complexa, grants and personal fees from Eidos, other from Athira, other from Spencer Healthcare, outside the submitted work;
Dr. David D. Waters reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Kristian A. Thygesen reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Gregg W. Stone reports grants and personal fees from National Heart, Lung, and Blood Institute , during the conduct of the study; personal fees from Terumo, personal fees from Amaranth, personal fees from Shockwave, personal fees and other from Valfix, personal fees from TherOx, personal fees from Reva, personal fees from Vascular Dynamics, personal fees from Robocath, personal fees from HeartFlow, personal fees from Gore, personal fees from Ablative Solutions, personal fees from Matrizyme, personal fees from Miracor, personal fees from Neovasc, personal fees from V-wave, personal fees from Abiomed, personal fees from Claret, personal fees from Sirtex, personal fees and other from Ancora, personal fees and other from Qool Therapeutics, other from Cagent, other from Applied Therapeutics, other from Biostar family of funds, other from MedFocus family of funds, personal fees and other from SpectraWave, personal fees from MAIA Pharmaceuticals, personal fees and other from Orchestra Biomed, other from Aria, personal fees from Vectorious, other from Cardiac Success, outside the submitted work; .
Dr Harvey D. White reports grants from National Heart, Lung and Blood Institute during the conduct of the study; reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi-Aventis and Regeneron Pharmaceuticals, for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly, for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals, , for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study (A Study to Evaluate the Effect of Long-term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc, for the dal-GenE study (Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc, for the AEGIS-II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd, and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid [ETC-1002] or Placebo) from Esperion Therapeutics Inc. He was on the Advisory Board for Genentech, Inc. and received lecture fees from AstraZeneca.
Dr. David J. Maron reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Judith S. Hochman is Study Chair for the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA) trial for which, in addition to support by a National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular, Medtronic, Inc, St Jude Medical Inc, Volcano Corporation, Arbor Pharmaceuticals LLC, AstraZeneca, Merck Sharp and Dohme Corp, Omron Healthcare Inc, and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca.
Supplemental Materials
Clinical Event Committee Membership
Supplemental Tables I - V
Supplemental Figures I-II
Supplemental Statistical Methods
Primary and Secondary MI Definitions
REFERENCE
- 1.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Alexander KP, Senior R, Boden WE, Stone GW, Goodman SG, Lopes RD, et al. Baseline Characteristics and Risk Profiles of Participants in the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2019;4:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction, Authors/Task Force Members Chairpersons, Thygesen K, Alpert JS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 5.Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E and Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsey JB, Kennedy KF, Stolker JM, Gilchrist IC, Mukherjee D, Marso SP, Pencina MJ, Kleiman NS and Cohen DJ. Prognostic implications of creatine kinase-MB elevation after percutaneous coronary intervention: results from the Evaluation of Drug-Eluting Stents and Ischemic Events (EVENT) registry. Circ Cardiovasc Interv. 2011;4:474–80. [DOI] [PubMed] [Google Scholar]
- 7.Lim CC, van Gaal WJ, Testa L, Cuculi F, Arnold JR, Karamitsos T, Francis JM, Petersen SE, Digby JE, Westaby S, et al. With the “universal definition,” measurement of creatine kinase-myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. J Am Coll Cardiol. 2011;57:653–61. [DOI] [PubMed] [Google Scholar]
- 8.Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, Engoren M, Alexander JH, Levy JH, Chaitman BR, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA. 2011;305:585–91. [DOI] [PubMed] [Google Scholar]
- 9.Zeitouni M, Silvain J, Guedeney P, Kerneis M, Yan Y, Overtchouk P, Barthelemy O, Hauguel-Moreau M, Choussat R, Helft G, et al. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur Heart J. 2018;39:1100–1109. [DOI] [PubMed] [Google Scholar]
- 10.Thielmann M, Sharma V, Al-Attar N, Bulluck H, Bisleri G, Bunge JJH, Czerny M, Ferdinandy P, Frey UH, Heusch G, et al. ESC Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart Position Paper: Perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur Heart J. 2017;38:2392–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novack V, Pencina M, Cohen DJ, Kleiman NS, Yen CH, Saucedo JF, Berger PB and Cutlip DE. Troponin criteria for myocardial infarction after percutaneous coronary intervention. Arch Intern Med. 2012;172:502–8. [DOI] [PubMed] [Google Scholar]
- 12.Ndrepepa G, Colleran R, Braun S, Cassese S, Hieber J, Fusaro M, Kufner S, Ott I, Byrne RA, Husser O, et al. High-Sensitivity Troponin T and Mortality After Elective Percutaneous Coronary Intervention. J Am Coll Cardiol. 2016;68:2259–2268. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur Heart J. 2018;39:2192–2207. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD and Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial I. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 15.Cho MS, Ahn JM, Lee CH, Kang DY, Lee JB, Lee PH, Kang SJ, Lee SW, Kim YH, Lee CW, et al. Differential Rates and Clinical Significance of Periprocedural Myocardial Infarction After Stenting or Bypass Surgery for Multivessel Coronary Disease According to Various Definitions. JACC Cardiovasc Interv. 2017;10:1498–1507. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Yehuda O, Chen S, Redfors B, McAndrew T, Crowley A, Kosmidou I, Kandzari DE, Puskas JD, Morice MC, Taggart DP, et al. Impact of large periprocedural myocardial infarction on mortality after percutaneous coronary intervention and coronary artery bypass grafting for left main disease: an analysis from the EXCEL trial. Eur Heart J. 2019;40:1930–1941. [DOI] [PubMed] [Google Scholar]
- 17.Koskinas KC, Ndrepepa G, Raber L, Karagiannis A, Kufner S, Zanchin T, Hieber J, Hunziker L, Mayer K, Byrne RA, et al. Prognostic Impact of Periprocedural Myocardial Infarction in Patients Undergoing Elective Percutaneous Coronary Interventions. Circ Cardiovasc Interv. 2018;11:e006752. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen PH, Nybo M, Jensen MK, Mortensen PE, Poulsen TS, Diederichsen AC and Mickley H. Optimal cut-off value for cardiac troponin I in ruling out Type 5 myocardial infarction. Interact Cardiovasc Thorac Surg. 2014;18:544–50. [DOI] [PubMed] [Google Scholar]
- 19.Bagai A, Alexander KP, Berger JS, Senior R, Sajeev C, Pracon R, Mavromatis K, Lopez-Sendon JL, Gosselin G, Diaz A, et al. Use of troponin assay 99th percentile as the decision level for myocardial infarction diagnosis. Am Heart J. 2017;190:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk W, Knudtson M, Dada M, Casperson P, Harris CL, et al. The evolving pattern of symptomatic coronary artery disease in the United States and Canada: baseline characteristics of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial. Am J Cardiol. 2007;99:208–12. [DOI] [PubMed] [Google Scholar]
- 22.Bari 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks MM, Chaitman BR, Nesto RW, Hardison RM, Feit F, Gersh BJ, Krone RJ, Sako EY, Rogers WJ, Garber AJ, et al. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2012;126:2115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaitman BR, Hardison RM, Adler D, Gebhart S, Grogan M, Ocampo S, Sopko G, Ramires JA, Schneider D, Frye RL, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–84. [DOI] [PubMed] [Google Scholar]
- 26.Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, Juni P, Pijls NHJ, Hlatky MA and FAME 2 Trial Investigators. Clinical Outcomes and Cost-Effectiveness of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Patients With Stable Coronary Artery Disease: Three-Year Follow-Up of the FAME 2 Trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation. 2018;137:480–487. [DOI] [PubMed] [Google Scholar]
- 27.Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrom T, Kaab S, Dambrink JH, Rioufol G, et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. [DOI] [PubMed] [Google Scholar]
- 29.Tomaniak M, Chichareon P, Onuma Y, Deliargyris EN, Takahashi K, Kogame N, Modolo R, Chang CC, Rademaker-Havinga T, Storey RF, et al. Benefit and Risks of Aspirin in Addition to Ticagrelor in Acute Coronary Syndromes: A Post Hoc Analysis of the Randomized GLOBAL LEADERS Trial. JAMA Cardiol. 2019. doi: 10.1001/jamacardio.2019.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scirica BM, Bergmark BA, Morrow DA, Antman EM, Bonaca MP, Murphy SA, Sabatine MS, Braunwald E and Wiviott SD. Nonculprit Lesion Myocardial Infarction Following Percutaneous Coronary Intervention in Patients With Acute Coronary Syndrome. J Am Coll Cardiol. 2020;75:1095–1106. [DOI] [PubMed] [Google Scholar]
- 31.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800. [DOI] [PubMed] [Google Scholar]
- 33.Bangalore S, Pencina MJ, Kleiman NS and Cohen DJ. Prognostic implications of procedural vs spontaneous myocardial infarction: results from the Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry. Am Heart J. 2013;166:1027–34. [DOI] [PubMed] [Google Scholar]
- 34.Akkerhuis KM, Alexander JH, Tardiff BE, Boersma E, Harrington RA, Lincoff AM and Simoons ML. Minor myocardial damage and prognosis: are spontaneous and percutaneous coronary intervention-related events different? Circulation. 2002;105:554–6. [DOI] [PubMed] [Google Scholar]
- 35.Damman P, Wallentin L, Fox KA, Windhausen F, Hirsch A, Clayton T, Pocock SJ, Lagerqvist B, Tijssen JG and de Winter RJ. Long-term cardiovascular mortality after procedure-related or spontaneous myocardial infarction in patients with non-ST-segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II, ICTUS, and RITA-3 trials (FIR). Circulation. 2012;125:568–76. [DOI] [PubMed] [Google Scholar]
- 36.Pervaiz MH, Sood P, Sudhir K, Hermiller JB, Hou L, Hattori K, Su X, Cao S, Wang J, Applegate RJ, et al. Periprocedural myocardial infarction in a randomized trial of everolimus-eluting and Paclitaxel-eluting coronary stents: frequency and impact on mortality according to historic versus universal definitions. Circ Cardiovasc Interv. 2012;5:150–6. [DOI] [PubMed] [Google Scholar]
- 37.Prasad A, Gersh BJ, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Pocock SJ, McLaurin BT, Cox DA, et al. Prognostic significance of periprocedural versus spontaneously occurring myocardial infarction after percutaneous coronary intervention in patients with acute coronary syndromes: an analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;54:477–86. [DOI] [PubMed] [Google Scholar]
- 38.DeFilippis AP, Chapman AR, Mills NL, de Lemos JA, Arbab-Zadeh A, Newby LK and Morrow DA. Assessment and Treatment of Patients With Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation. 2019;140:1661–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thygesen K and Jaffe AS. The Gloomy Long-Term Prognosis of Patients With Type 2 Myocardial Infarction or Myocardial Injury. J Am Coll Cardiol. 2020;75:1014–1016. [DOI] [PubMed] [Google Scholar]
- 40.Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, Rihal CS, Gersh BJ, Lewis B, Lennon RJ, et al. Incidence, Trends, and Outcomes of Type 2 Myocardial Infarction in a Community Cohort. Circulation. 2020;141:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CASS Principal Investigators and Their Associates. Myocardial infarction and mortality in the coronary artery surgery study (CASS) randomized trial. N Engl J Med. 1984;310:750–8. [DOI] [PubMed] [Google Scholar]
- 42.Passamani E, Davis KB, Gillespie MJ and Killip T. A randomized trial of coronary artery bypass surgery. Survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–71. [DOI] [PubMed] [Google Scholar]
- 43.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–70. [DOI] [PubMed] [Google Scholar]
- 44.Howlett JG, Stebbins A, Petrie MC, Jhund PS, Castelvecchio S, Cherniavsky A, Sueta CA, Roy A, Pina IL, Wurm R, et al. CABG Improves Outcomes in Patients With Ischemic Cardiomyopathy: 10-Year Follow-Up of the STICH Trial. JACC Heart Fail. 2019;7:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregson J, Stone GW, Ben-Yehuda O, Redfors B, Kandzari DE, Morice M-C, Leon MB, Kosmidou I, Lembo NJ, Brown WM, et al. Implications of Alternative Definitions of Peri-Procedural Myocardial Infarction After Coronary Revascularization. J Am Coll Cardiol. 2020;76:1609–1621. [DOI] [PubMed] [Google Scholar]
- 46.Hara H, Serruys PW, Takahashi K, Kawashima H, Ono M, Gao C, Wang R, Mohr FW, Holmes DR, Davierwala PM, et al. Impact of Peri-Procedural Myocardial Infarction on Outcomes After Revascularization. J Am Coll Cardiol. 2020;76:1622–1639. [DOI] [PubMed] [Google Scholar]
- 47.Cutlip DE. Procedural Myocardial Infarction. Definitions Everywhere, But Not Any That May Fit. J Am Coll Cardiol. 2020;76:1640–1643. [DOI] [PubMed] [Google Scholar]
- 48.Silvain J, Zeitouni M, Paradies V, Zheng H, Ndrepepa G, Cavallini C, Feldman DN, Sharma SK, Mehilli J, Jaffe AS et al. Cardiac procedural myocardial injury, infarction and mortality in patients undergoing elective PCI: a pooled analysis of patient-level data. European Heart Journal (2020) 00, 1–13 doi: 10.1093/eurheartj/ehaa885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


