Abstract
Alzheimer’s disease (AD) is the most common form of dementia in the elderly. Together with cerebral amyloid accumulation, several factors contribute to AD pathology including vascular alterations, systemic inflammation, genetic/epigenetic status and mitochondrial dysfunction. Much is now being devoted to neuroinflammation. However, anti-inflammatory drugs as numerous other therapies, mainly targeted on β-amyloid, have failed to show efficacious effects in AD. Timing, proper selection of patients, and the need for a multitarget approach appear to be the main weak points of current therapeutic efforts. The efficacy of a treatment could be better evaluate if efficient biomarkers are available. We propose here the application of precision medicine principles in AD to simultaneously verify the efficacy of a treatment and the reliability of specific biomarkers according to individually tailored biomarker-guided targeted therapies. People at risk of developing AD or in the very early phase of the disease should be stratified according to: (1) neuropsychological tests; (2) apolipoprotein E (ApoE) genotyping; (3) biochemical analysis of plasma and cerebrospinal fluid (CSF); (4) MRI and positron emission tomography and (5) assessment of their inflammatory profile by an integration of various genetic and biochemical parameters in plasma, CSF and an analysis of microbiota composition. The selected population should be treated with antiamyloidogenic and anti-inflammatory drugs in randomised, longitudinal, placebo-controlled studies using ad hoc profiles (eg, vascular profile, mitochondrial profile, etc…) If these criteria are adopted widely and the results shared, it may be possible to rapidly develop innovative and personalised drug treatment protocols with more realistic chances of being efficacious.
Keywords: alzheimer's disease, amyloid, pharmacology, immunology
Introduction
Alzheimer’s disease (AD) and other dementias affects about 45 million people worldwide and the ageing of the world population foreseen for the next decades will increase its incidence. AD is the main form of age-related dementia and is one of the principal causes of disability.1 AD is neuropathologically characterised by cerebral extracellular deposition of β-amyloid (Aβ) and intracellular formation of neurofibrillary tangles (NFT) by hyperphosphorylated tau, along with associated parenchymal inflammatory reactions. Neuronal dysfunction and synaptic loss in cortical and hippocampal regions2 and tau deposition in specific regions correlate well with the cognitive decline observed in prodromal and active stages of the disease. The neurodegenerative process at clinical onset is advanced, therapeutic approaches, mainly focused on Aβ deposition, until now, have not shown any efficacy in slowing or halting disease progression. The identification Aβ deposition as a pharmacological target in AD is supported by genetic and experimental data. The familial forms of AD (FAD) are associated with mutations the amyloid precursor protein (APP) gene or in genes encoding proteins involved in APP metabolism (presenilin 1 and 2).3 AD experimental models have been developed by transgenic expression of single or multiple mutated sequences of human genes associated to AD.4 The amyloid cascade hypothesis recently updated by Selkoe and Hardy5 has driven AD studies at various levels for decades, but it undoubtedly is time for further evolution of antiamyloidogenic therapies. A recent revision of the results of phase III clinical trial with aducanumab, an anti-Aβ antibody purified directly from human biological fluid,6 stopped for futility it seem to give some hope in terms of efficacy with the higher dosage. However, more information is required to establish the consistency of results and in any case the positive effects shown appeared limited.7
The progress of AD better correlate with tau pathology, NFT initially accumulates in the entorhinal region and subsequently in the limbic system and neocortical regions.8 The structural association between brain atrophy and tau accumulation is now investigated by positron emission tomography (PET) analysis using new [18F] radioligands, conclusive results are not yet available, but some evidence support this relationship in mild cognitive impairment (MCI) and AD subjects.9–11 As therapeutic target the accumulation of pathological tau received recently more attention in part due to the failure of anti-Aβ treatment, the initially antitau therapy was based on inhibition of tau aggregation and phosphorylation, or on stabilisation of microtubules, but most of these approaches have been discontinued because of toxicity and/or lack of efficacy.12 Tau-targeted immunotherapy and oligonucleotide antisense against MAPT sequences are promising approaches currently under the investigation.13 14
The prion-like distribution of Aβ and tau pathology is a recent acquisition supported by numerous experimental data in AD models and in several other neurodegenerative disorders where specific proteins act as seed to diffuse the neuronal damage.15 The mechanisms responsible of this cell to cell spreading might directly involve soluble aggregates, oligomers, of pathological proteins but the proteins can be also vehiculated by extracellular microvesicles like exosomes.16 The possible pathogenic role played by exosomes in the neurodegenerative disorders is still elusive but it is undoubted that they attract the attention as possible therapeutic target and diagnostic tool.17
Higher level of iron has been observed in specific brain regions of patients with AD, the contribution of iron overload in the pathogenesis of AD has been considered from different points of view. The presence of iron has been associated directly with Aβ plaques and its influence on Aβ aggregation toxicity and inflammation has been shown.18–20 In different cerebral cell types iron accumulation is combined with an increase of oxidative stress and mitochondrial dysfunction and many common polymorphisms associated with high levels of iron are also associated with AD.21 Thus, iron chelators molecules and neuroprotective drugs with chelator properties have been proposed for therapeutic approach to AD. Although some iron chelators are already in clinical practice, several aspects need to be considered to develop and optimise the use of these molecules in the neurodegenerative disorders22 23
In the last two decades, the complex scenario that characterises neurodegenerative disorder pathogenesis, including that of AD, has been carefully analysed. The results of these investigations can be summarised along two main directions, which apparently conflict. On one side, numerous pathogenic factors, including mitochondrial failure, oxidative stress, inflammation, iron accumulation, cellular trafficking impairment, lipid metabolism alterations and genetic risk factors, have been found to contribute to the pathological process. All these alterations combined with more specific elements, for example, protein misfolding and toxic oligomer production, have been recognised as common pathogenic mechanisms in neurodegenerative processes. Evidence for this has been observed in virtually all neurodegenerative disorders. On the other side, each disorder can be subcategorised not only through classical clinical distinctions—sporadic versus genetic, age of onset, progression—but also according to specific pathogenetic profile. The heterogeneity of AD cases has been evaluated by a systemic review meta-analysis based on neuropathology and neuroimaging results.24 Interindividual variability has been found in differential susceptibility to single pathological components. Pharmacological tools interfering with common neurotoxic mechanisms could be useful in attenuating many aspects of the neurodegenerative process. In contrast, only an appropriate genetic and biological profile of a single patient in the early phase of the disease could obtain efficacious treatment. These considerations have important implications in therapeutic strategy assessment and in clinical trial design. Translation of results from experimental or epidemiological studies into treatments has produced only modest, or negative, effects. Possible explanations of these failures are numerous. However, relative to the concepts enunciated above, two fundamental things are necessary: better characterisation of the patients and a multitarget approach.
Together with correct timing of treatment, this should decrease the number of subjects required to power an AD trial and improve the chances for success. In recent unsuccessful phase III AD therapeutic trials, designed as a single treatment administered to a large population of patients, the statistic power of the study was high but no effect of the treatment was found. This approach is questionable from many points of view. For instance, if thousands of patients are required to obtain minimal changes of neuropsychological scales, even if they are statistically significant, the possibility that the positive result reflects a real improvement of patient quality of life is remote. A relatively small but homogeneous group of patients should provide more chances to obtain positive and relevant results.25
One of the possible components modulating AD pathogenesis is the inflammatory state. To examine the relevance of inflammation, it is necessary to identify biological markers for an ‘inflammatory profile’. In fact, the contribution of inflammation in early phases of AD pathogenesis might be individually and temporally different among patients. According to the precision medicine principle, the identification of a common profile in a selected group of subjects should help the therapeutic approach. On the other hand, it is commonly accepted that AD is a multifactorial disorder, thus multiple therapeutic agents should be tested together. Regulatory agency guidelines specifying that multitreatment trials do not require comparison with monotherapy groups means that trials can be reduced at two arms—treatment and placebo (European Medicines Agency guidelines).26
Biological markers in AD
Together with the therapeutic agents themselves, developing efficacious therapies depends strongly on the availability of biomarkers for monitoring treatment efficacy and progression. The presence of validated biomarkers capable of assessing disease before the appearance of the clinical symptoms is particularly important when both the presymptomatic period and disease itself are characterised by slow progression, as occurring in AD. In two last decades, cognitive decline in AD was associated with reduction of cerebrospinal fluid (CSF) Aβ levels, increases in Tau and pTau levels, Aβ-PET cerebral deposition, hippocampal volumetric reduction determined by MRI analysis, and cerebral hypometabolism determined by [18F]-fluorodeoxyglucose (FDG)-PET. Jack et al27 proposed a model where changes in these parameters were temporally correlated to AD pathophysiology. Revisions to the original model incorporated interindividual variability in cognitive impairment associated with progression of AD pathophysiology, as well as modifications of the specific temporal ordering of some biomarkers. It also has been recognised that Aβ and tau deposition might be initiated independently in sporadic AD, in which Aβ pathophysiology can accelerate antecedent limbic and brainstem tauopathy. According to evidence initially obtained from Down syndrome subjects, where APP gene triplication invariably leads to AD, cerebral Aβ deposition anticipated the development of frank AD syndrome two decades before observation of clinical symptoms.28 These data have been recently confirmed using PiB (Pittsburgh compound B) binding in postmortem frontal cortex across the lifespan.29 Similar results were found in longitudinal analysis of pre-symptomatic subjects carrying FAD mutations where the reduction of Aβ in CSF and the incremental increase of cerebral Aβ deposition determined by PET analysis preceded the clinical manifestations of disease by 10–15 years.30 31 The authors observed that Aβ accumulation is largely complete before progressive neurodegeneration and cognitive decline occur in subjects carrying different AD associated mutations. Similar conclusions were reached by Landau et al,32 who studied subjects from the Alzheimer's Disease Neuroimaging Initiative study and concluded that amyloid deposition has an early and subclinical impact on cognitive function that precedes metabolic changes. Hypometabolism becomes more pronounced later in the course of the disease when it is closely linked temporally to overt cognitive symptoms.
Jack et al33 have proposed three categories of biomarkers based on pathophysiology. ‘A’ refers to the value of Aβ biomarkers (amyloid PET or CSF Aβ 42); ‘T,’ the value of a tau biomarker (CSF phospho tau or tau PET); and ‘N,’ biomarkers of neurodegeneration (FDG-PET, structural MRI or CSF total tau). A recent longitudinal study to evaluate the prediction capacity of various biological markers was developed within A-T-N frame adopted by the National Institute on Aging and Alzheimer's Association.34 In this longitudinal study of subjects without dementia, the addition of amyloid PET, tau PET and MRI cortical thickness to a model that included clinical and genetic variables resulted in a small but statistically significant improvement in predictive accuracy for memory decline. Although a staging of Aβ deposits has been recently proposed,35 Aβ accumulation was varied little after AD onset while tau aggregation was better associated with progressive neurodegeneration.36 Although in specific conditions autoptic data seem to indicate an independent evolution of Aβ deposits and NFT,37 a temporal sequence between the two main injuries in AD is more convincing. He et al38 proposed an intriguing model connecting Aβ deposits and tau fibrillar tangles based on the capacity of Aβ plaques to facilitate the rapid amplification of tau seeds into large tau aggregates, creating an environmental for tau precipitation. In any case, these conventional biomarkers have been profitably used in various trials to improve diagnostic criteria and better select patients for clinical trials. The capacity of biomarkers to indicate treatment efficacy was less evident. In some circumstances, the biomarker changes during the trial indicated a positive trend, although the clinical outcome was unsatisfactory. Bapineuzumab, a monoclonal antibody specific to the N-terminus of Aβ, positively influenced biological parameters, including a decrease of CSF tau and p-tau and PIB-PET signal,39 40 but in the absence of any clinical effects. An increase of CSF and plasma Aβ was observed in patients treated with Solamezumab, a humanised monoclonal antibody that binds to the mid-domain of the Aβ peptide that was designed to slow AD progression by increasing clearance of soluble Aβ from the brain.41 High-affinity antibody-Aβ peptide complexes in plasma might induced cerebral drainage (‘peripheral sink’), which has been proposed as a possible mechanism by which passive immunisation might reduce Aβ burden in AD experimental models. Unifying these data is difficult, especially when biomarker changes occur in the absence of therapeutic effects. Changes in plasma and CSF Aβ levels with drugs interfering with amyloid metabolism also may occur in the absence of positive clinical results. Explaining the reduction of CSF Tau levels after treatment with bapineuzumab without clinical consequence is problematic. One possible explanation of this discrepancy is that positive biological changes occurred too late to influence the cognitive performance of the patients. Nevertheless, it is too early to consider these biomarkers useless. Efforts to optimise treatment conditions should first be pursued. In these two specific cases, a more extensive phase II trial might lead to more positive phase III results. As pointed out by Gold,42 correct design of a phase II trial associated with an independent evaluation of the results might save time and money in developing improved therapies. In this context, new biomarkers that reflect changes in other aspects of AD pathophysiology should be developed, as noted by Moreth et al43 and more extensively investigated by Molineuvo et al.44 These authors correctly suggest that biomarkers used in drug development programmes should be selected based on their capacity to monitor the biological events influenced by the treatment. Selection criteria should consider assessment of disease state and/or prognosis, definition of a drug’s mechanism of action, and identification of the right parameter(s) to monitor efficacy. Identifying additional fluid biomarkers reflecting other aspects of AD pathophysiology is critical for monitoring new therapeutic approaches. Several novel fluid biomarkers have been proposed, but their role in AD pathology and their use as AD biomarkers have yet to be validated. In a recent study of unimpaired aged subjects, Merluzzi et al45 did a cross-sectional correlation of markers of neurodegeneration in CSF with cognitive subclinical decline. Neurofilament light protein (NFL) was sensitive to cognitive alterations in the observed population, more so than neurogranin or total tau.
Extensive studies have been performed to identify possible AD biological markers in more accessible fluids, such as blood, but also in saliva and urine.46 47 Serum and plasma levels of Aβ1–40 and Aβ1–42, or their ratio, have been proposed as peripheral markers of AD and AD prodromal states for twenty years. Although a large meta-analysis demonstrated the diagnostic inconsistency of these determinations,48 two recent studies have reproposed the blood-based Aβ1–42 and Aβ1–40 ratio as an important component of a diagnostic process including other blood markers.49 50 Both studies emphasised the limited information derived from a screening exclusively based blood Aβ ratio, but also the low cost and the practical advantages of this measurement. In another recent study, a longitudinal cohort study in older individuals with subjective memory complaints was performed to determine whether plasma Aβ1–40/Aβ1–42 ratios might correlate with positive brain Aβ-PET. The results showed that plasma Aβ1–40/Aβ1–42 ratio was is indeed a useful predictor of cerebral amyloidosis (accuracy 81% at baseline). This finding is reproduced (balanced accuracy: 71%) when using data collected at subsequent time points (after 1-year and 3-year follow-up). The authors indicate the need for cohort studies to test Aβ1–40/Aβ1–42 ratios as biomarkers, as they may be a cost-effective biomarker useful for assessing brain amyloidosis in individuals who are at risk for AD.51 Similar results with even more promising evidence were shown by Schindler et al.52 Here the alteration of Aβ 1–42/Aβ1–40 ratio in plasma predicted the cerebral accumulation of amyloid, determined by cerebral PET analysis, with an accuracy of 85% in apolipoprotein E (ApoE) negative subjects and 94% in ApoE positive cases. A further goal of these investigations might be screening, in primary care settings, all elderly people over 70 years old to identify those at risk for future disease development. These determinations are close to being an ideal fluid biomarker for AD as they are reliable, reproducible, non-invasive, simple to measure and inexpensive. β-site amyloid precursor protein cleaving enzyme (BACE) activity, Aβ oligomers (AβOs), tau and p-tau in blood were also investigated as biomarker of AD in prodromal phases of AD. Longitudinally, higher levels of plasma total tau have been associated with greater cognitive decline and risk of MCI. This relationship was independent of elevated brain Aβ concentration. Recent work suggests that the contribution of tau level determination in blood to predicting cognitive decline is modest, while a good relationship occurred with CSF tau and brain atrophy in frank AD.53 However, plasma pTau, especially p-tau-217 has been found to strongly correlate with specific modifications, amyloidosis and tau phosphorylation, at the cerebral level.54 NLF levels in plasma have been studied to determine if they might serve as a marker of neurodegeneration. Meta-analysis confirms that plasma NFL levels are elevated in the prodromal phase of AD.55 Plasma NFL levels correlate with hypometabolism in bilateral parahippocampal and middle temporal gyri and are a good diagnostic marker for AD in Down syndrome subjects, more recently serurm NLF levels have been proposed as a marker for frontotemporal dementia.56 Furthermore, the methodological evolution enables the simultaneous determination of tau, p-tau, NLF and Aβ 1–42 in plasma, this combination might give the best condition to assess in plasma the risk of developing dementia.57
Small non-coding RNA (miRNA), through binding to the 3′-untranslated region region in messenger RNA, control gene expression. Deregulated miRNA expression in brain tissue and biological fluids from AD and MCI subjects has been shown.58 Although their expression have been found more stable and consistent in brain tissue, the possibility that a specific blood miRNA could be associated with early phases of a pathological condition has been investigated and promising candidates were identified.59 The possibility that specific miRNAs are directly involved in AD pathogenesis and in neurodegenerative processes makes these elements and their identification in peripheral fluids extremely interesting. Targets of miRNA deregulation in peripheral blood have been found and grouped according to the types of genes involved. Groups include inflammation, apoptosis, amyloid and tau signalling pathways, but half of the miRNAS have undetermined targets.59 miRNAs, thus, could be very useful biomarkers, but methodological conditions and timing of determination need to be harmonised to get reliable tools. Promising results in terms of biomarker tools have been found in plasma neural cell-derived exosomes, circulating microvescicles derived from cerebral tissue. Aβ, tau protein and numerous other proteins are associated with exosomes, thus their composition might reflect pathophysiological conditions.60 The purification and analysis of neuronal derived exosomes would be useful for monitoring AD progression, interindividual differences and pharmacological activities.61
The determination of Aβ1–42 in saliva samples has been proposed as a biomarker in AD43 as several other components of saliva, including tau, appeared to have diagnostic utility f or establishing or predicting AD. The possibility of finding useful urine biomarkers associable with AD or its prodromal phases has been investigated by measuring levels of various metabolites related to cellular membranes, oxidative pathways or metals and aminoacids.62 The levels of numerous elements, including isoprostane 8,12-iso-iPF2a-VI, total free amino acids, 8-hydroxy-2'-deoxyguanosine, glycine and enzymatic activity of NaCl-stimulated PON1, have been determined in AD, MCI and controls,63 but none had the reliability necessary for a biomarker for AD studies.
Neuroinflammation in AD
Inflammation appears to occur in all neurodegenerative disorders and distinct immune factors are associated with each type of disorder. Similarities and differences in inflammatory activation have been explored by comparing rare and common neurodegenerative disorders.64 A broad variety of mechanisms are involved in changing the original protective role of glial cells into a damaging ones. Alterations of mechanisms controlling inflammation, change of activation state, persistent production of cytokines, recruitment of peripheral cells are all processes observed in neurodegenerative tissue.65 Using a simple experimental model to evaluate histopathological, biochemical and behavioural consequences of Aβ or α-synuclein oligomers, we demonstrated that different oligomers induced similar inflammatory reactions, but through distinct molecular pathways.66 Along this line, King et al,67 at the clinical level, have found that subjects exhibiting MCI in the early stages of AD or Lewy body dementia all displayed inflammatory activation, although with some differences.
The evidence that inflammation may be involved in early phases of AD has generated new hypotheses of pathogenesis that involve the combination of Aβ deposits, gliosis and neuronal dysfunction. In terms of therapeutic strategy, the possible beneficial effects derived from immunoreactivity control must be considered before the use of immunotherapy as an antiamyloidosis strategy, but the results of this latter therapy are equally unsatisfactory.68 69 For example, indomethacin, a classical non-steroidal anti-inflammatory drug (NSAID), was tested in a small group of patients, producing results that were partially positive but with substantial gastrointestinal side effects that likely will preclude chronic treatment with this drug. However, a trial combining indomethacin with a gastroprotective drug found that cognitive deterioration was not significantly different in treated patients compared with those receiving a placebo, although a positive trend was observed. These results cannot be considered conclusive for several reasons, including the small number of subjects treated and the possible influence of gastroprtective on risk of developing dementia. Valid conclusions can be reached when an appropriate protocol taking in consideration all aspects will be applied. Similar results have been reported for treatments targeting tumour necrosis factor in AD.70 Numerous epidemiological studies have demonstrated the protective effects of anti-inflammatory agents in a preclinical period. In the Rotterdam study, it has been shown that when the exposure to NSAIDs was 5 years before diagnosis, the risk of developing AD was reduced by a factor of five.71 We have shown that in an AD population the use of NSAIDs was extremely low compared with age-matched controls.72 As expected, the translation of epidemiological knowledge into effective treatments is complicated. However, for AD, the availability of numerous molecules already characterised for other clinical uses has facilitated the design of trials. Starting with the antimalarial drug hydroxychloroquine,73 a large number of anti-inflammatory drugs has been tested against AD in formal clinical trials. NSAIDs have been tested under different conditions, including preventive treatment, with modest or negative results. However, the dozens of genes with potential roles in AD pathogenesis can be limited to a restricted number of biological functions, the immune system, together with lipid metabolism and vesicle traffic, is included. Polymorphisms in the genes encoding TREM2, CD33 or CR1, and several missense mutations on genes for immune factors, have been identified as risk factors in AD. Furthermore, since TREM2 is expressed essentially only in microglia, the association between TREM2 polymorphisms and AD adds a non-neuronal element to its pathogenesis. Inflammation in cerebral parenchyma has been traditionally considered a secondary event due to Aβ deposits and neurodegeneration, but recently, glial activation has been recognised as a primary contributor to pathology.74 Numerous observations in physiological and pathological conditions have proved how intimately connected is synaptic activity with microglia and astroglia.75 Both glial cell types affect synapse modelling and neuronal function with combined or independent actions. Thus, in AD, alteration of glial activities might induce synaptic loss or damage in the absence of amyloid plaques or neurodegeneration.76 The formation of misfolded soluble Aβ aggregates, oligomers (AβOs), is the first pathogenic step in AD, followed by the formation of insoluble aggregates, protofibrils and fibrils and plaques. Oligomers are considered the neurotoxic form of Aβ,77 their presence has been associated with uncontrolled inflammation, and thus the combined deleterious effects of immune factors and AβOs on the neuronal system appear critical in the early phase of the disease. Understanding the balance between the positive effects of glial activation (exerting surveillance to eliminate pathogenic elements, including protein aggregates) and the negative effects (overproduction of immune factors with detrimental effects on neuronal function) is essential for establishing the pathogenic role of inflammation. This subtle equilibrium could be altered by genetic profile, general condition of the organism and specific elements. Peripheral conditions altering inflammation state, like obesity, metabolic syndrome or diabetes, can substantially facilitate the detrimental effects on neuronal function78 and reduce resilience.79 In contrast, physical exercise, recognised as protective in AD, interferes with inflammation, reduces microglia activation and produces a specific cytokine profile that might increase neuronal resilience.80 Furthermore, AD pathogenesis can be also influenced by gut microbiota.81 The changes of inflammation markers in blood or CSF, in combination with the profiles of genes encoding inflammatory factors and analysis of the microbiota, have been studied in AD subjects.82 These changes most likely reflect neuroinflammation in the brain, as verified by PET analysis and activation of cerebral microglia.83 In some cases, the levels of TREM2 and other immune factors in AD CSF correlate with disease severity84 and drug activity.85 However, biological variability and low consistency of the inflammatory marker determinations in plasma and CSF make the correlation with disease progression poor.86 This variability may have its origin in the heterogeneity of disease manifestations rather than inevitable biological oscillations, especially in early phases of AD. This deserves further investigation. The contribution of inflammation to AD pathogenesis, as mentioned in the introduction, might be individually and temporally different, suggesting the possibility that this variability, rather than being a problem, becomes an interesting element worth analysing carefully.
Precision medicine approach
Precision medicine, here considered synonymous with precision pharmacology, could be considered an aspect of the wider concept of personalised medicine. Precision medicine is based on the identification of the most appropriate pharmacological tool to interrupt or antagonise the pathological process, while personalised medicine involves not only the treatment but the complex of specific elements that contribute to the quality of care. Precision medicine has been proposed in the field of oncology, where the response to treatment is often a delicate balance between positive effects and toxicity and individual responses may vary significantly. The application of precision medicine in neurodegenerative disorders was formally proposed recently.87 The principle is the same—individually tailored biomarker-guided targeted therapies88 and the adoption of the best practice based on individual patient characteristics. The stratification of subjects by a selection of pathophysological profiles within the complex scenario of the neurodegenerative disorders is proposed to implement the efficacy of pharmacological treatments. This approach is a necessary consequence of the numerous clinical trial failures that have been reported. At the moment, it remains a potential innovative intervention that needs to be tested under appropriate conditions. Several different molecular mechanisms can contribute to AD pathogenesis and, in the early phase of the disease, the relative importance of one or the other can be decisive in precipitating the pathological condition. Systems biology approaches might be useful to interrogate the input coming from numerous parameters that need to be considered to obtain a subject profile.88 89 Surrogate or direct biomarkers, together with the genetic profiles, can assess the neuroprotective or at risk profile, in particular, the contribution of inflammation.
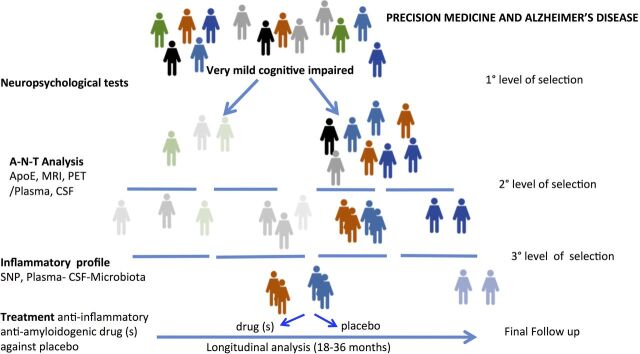
The application of precision medicine principles driven by individual inflammatory states was proposed by Wilcock ’s group.90 This initial approach can be further developed through consideration of the levels of numerous factors in plasma and CSF, including a large panel of cytokines, the microbiota metagenomics composition, exosome analysis, genetic and epigenetic factors. These factors should be considered for cluster analysis and included in machine learning algorithms to stratify the subjects according to ‘inflammatory profile’.91 This procedure should be applied as third level of selection in prodromal AD subjects initially characterised by neuropsychological tests to distinguish amnestic and non-amnestic cognitive decline and successively classified by biomarkers described in A-T-N framing. This complex approach must be developed longitudinally and associated with an intervention to directly evaluate the consistency of the parameters considered in association with possible drug efficacy,92–94 an initial validation step. As illustrated in figure 1, the assessment of inflammation profile must be immediately tested with pharmacological treatment, however the manipulation of the immune system will not be sufficient to interfere with AD progression. A multifactorial approach for effective antidementia action will be necessary that focuses on several targets, including inflammation, amyloid aggregation and probably neuroprotection. Single drugs with multitarget activity or more drugs with different mechanism of action can be tested in formal clinical trial. Doxycyline could be a good example of a multitarget drug because, in addition to the antibiotic effect of tetracyclines, doxycycline combines both antiamyloidogenic and anti-inflammatory activities.95 96 The efficacy of the drug can be dissected according to inflammation profile, biomarkers in plasma can be evaluated several times during the study, and comparison of CSF and microbiota can be done prior to and at the end of the study. The patients can be stratified according to a different physiopathological profile oriented by other therapeutic targets. Alterations of mitochondrial function97 or vascular contribution can be specifically examined if the available therapeutic tools are targeted on these functions.
Figure 1.
Flow chart of hypothetical clinical trial protocol with three levels of selection of patients with mild cognitive impairment: (1) neuropsychological tests to distinguish amnestic and non-amnestic MCI (2) CSF analyses to identify subjects with high propensities to convert to AD and (3) inflammatory profiles assessed by the consideration of numerous parameters in plasma, CSF and microbiota, together with genetic background. In this model, the combination of antiamyloidogenic and anti-inflammatory drugs versus placebo is investigated. AD, Alzheimer’s disease; ApoE, apolipoprotein E, CSF, cerebrospinal fluid; MCI, mild cognitive impairment; PET, positron emission tomography; SNP, single nucleotide polymorphisms.
Conclusions and perspectives
The accumulation of information on AD pathogenesis in the last two decades has produced scientific knowledge and consciousness of the complexity of the pathological scenario. On the other hand, excess simplification has characterised the translation of this complexity into therapeutic approaches, much as expressed in the quotation ‘There is always a well-known solution to every human problem—neat, simple and wrong.’ For AD, this solution is a single pharmacological approach to treating a heterogeneous, complex disorder. It now is time to face this complexity and create more accurate and precise therapeutic approach. As illustrated here, there is evidence to consider Aβ deposition and inflammation as initial phenomena of the complex pathological process. However, pharmacological interventions targeting both amyloid and inflammation have not shown positive results. Timing of treatment, selection of the patients, and the lack of multifactor approach might be the main reasons of these failures. Reliable biomarkers qualified for a specific context of use is another fundamental aspect that needs to be considered in therapy. The application of precision medicine principles to well characterised patients and target therapy and the use of appropriate biomarkers likely will provide the best chance to develop effective therapies. Here, we have proposed a model of study that could be applied to any kind of pharmacological approach. It comprises initial rigorous selection of early symptomatic subjects or even non-symptomatic at-risk cases, definition of primary clinical outcomes in combination with an appropriate battery of biomarkers to simultaneously test drug efficacy and marker reliability. With application of innovative calculation tools, the assessment of biomarkers can be progressively improved and refined according to possible relationship with cognitive state and drug activity to design trials with new compounds to prevent disease before it clinical phase. This would substantially reduce the costs and time of patient care. Similar processes can be applied to different pharmacological combination using ad hoc profiling (eg, vascular profile, mitochondrial profile, etc) according to which individually-tailored biomarker-guided targeted therapies can be implemented. It is essential to arrive at be go no go decision prior to the initiation of phase III trials. Furthermore, when the selection of patients is accurate according to target therapy, the optimal number of recruited subjects could be in the order of hundreds, not thousands. Our model, although not really new, brings into sharp focus the need for more rational use of existing scientific information and for wide dissemination of both positive and negative results to validate protocols with the potential to improve the quality of life of patients and their families.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.GBD 2015 Neurological Disorders Collaborator Group Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol 2017;16:877–97. 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol 1990;27:457–64. 10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- 3.Guerreiro R, Hardy J. Genetics of Alzheimer's disease. Neurotherapeutics 2014;11:732–7. 10.1007/s13311-014-0295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balducci C, Forloni G. App transgenic mice: their use and limitations. Neuromolecular Med 2011;13:117–37. 10.1007/s12017-010-8141-7 [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016;8:595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 2016;537:50–6. 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 7.Schneider L A resurrection of aducanumab for Alzheimer's disease. Lancet Neurol 2020;19:111–2. 10.1016/S1474-4422(19)30480-6 [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. 10.1007/s00401-006-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neddens J, Temmel M, Flunkert S, et al. Phosphorylation of different tau sites during progression of Alzheimer's disease. Acta Neuropathol Commun 2018;6:52. 10.1186/s40478-018-0557-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G, Zheng S, Zhu Z, et al. Association of tau accumulation and atrophy in mild cognitive impairment: a longitudinal study. Ann Nucl Med 2020;34:815–23. 10.1007/s12149-020-01506-2 [DOI] [PubMed] [Google Scholar]
- 11.Pascoal TA, Therriault J, Benedet AL, et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 2020;143:2818–30. 10.1093/brain/awaa180 [DOI] [PubMed] [Google Scholar]
- 12.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 2018;14:399–415. 10.1038/s41582-018-0013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadhav S, Avila J, Schöll M, et al. A walk through tau therapeutic strategies. Acta Neuropathol Commun 2019;7:22. 10.1186/s40478-019-0664-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarthy M, Chen S, Wang T, et al. Development of Novel Chemically-Modified Nucleic Acid Molecules for Efficient Inhibition of Human MAPT Gene Expression. Genes 2020;11:667. 10.3390/genes11060667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jucker M, Walker LC. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat Neurosci 2018;21:1341–9. 10.1038/s41593-018-0238-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamlett ED, Ledreux A, Potter H, et al. Exosomal biomarkers in Down syndrome and Alzheimer's disease. Free Radic Biol Med 2018;114:110–21. 10.1016/j.freeradbiomed.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson LS, Hamlett ED, Stone TD, et al. Neuronally derived extracellular vesicles: an emerging tool for understanding Alzheimer's disease. Mol Neurodegener 2019;14:22. 10.1186/s13024-019-0317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayton S, Diouf I, Bush AI, et al. Evidence that iron accelerates Alzheimer's pathology: a CSF biomarker study. J Neurol Neurosurg Psychiatry 2018;89:456–60. 10.1136/jnnp-2017-316551 [DOI] [PubMed] [Google Scholar]
- 19.Joppe K, Roser A-E, Maass F, et al. The contribution of iron to protein aggregation disorders in the central nervous system. Front Neurosci 2019;13:15. 10.3389/fnins.2019.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding B, Chen K-M, Ling H-W, et al. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer's disease. J Magn Reson Imaging 2009;29:793–8. 10.1002/jmri.21730 [DOI] [PubMed] [Google Scholar]
- 21.Ali-Rahmani F, Schengrund C-L, Connor JR. Hfe gene variants, iron, and lipids: a novel connection in Alzheimer's disease. Front Pharmacol 2014;5:165. 10.3389/fphar.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuñez MT, Chana-Cuevas P. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals 2018;11:109. 10.3390/ph11040109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagwe-Parab S, Kaur G. Molecular targets and therapeutic interventions for iron induced neurodegeneration. Brain Res Bull 2020;156:1–9. 10.1016/j.brainresbull.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 24.Ferreira D, Nordberg A, Westman E. Biological subtypes of Alzheimer disease: a systematic review and meta-analysis. Neurology 2020;94:436–48. 10.1212/WNL.0000000000009058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider LS Pragmatic trials and repurposed drugs for Alzheimer disease. JAMA Neurol 2020;77:162. 10.1001/jamaneurol.2019.3784 [DOI] [PubMed] [Google Scholar]
- 26.EMA, Committee for Medicinal Products for Human Use (CHMP) Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease. Available: https://www.ema.europa.eu/en/clinical-investigation-medicines-treatment-alzheimers-disease
- 27.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–28. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giaccone G, Tagliavini F, Linoli G, et al. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett 1989;97:232–8. 10.1016/0304-3940(89)90169-9 [DOI] [PubMed] [Google Scholar]
- 29.LeVine H, Spielmann HP, Matveev S, et al. Down syndrome: age-dependence of PiB binding in postmortem frontal cortex across the lifespan. Neurobiol Aging 2017;54:163–9. 10.1016/j.neurobiolaging.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moonis M, Swearer JM, Dayaw MPE, et al. Familial Alzheimer disease: decreases in CSF Abeta42 levels precede cognitive decline. Neurology 2005;65:323–5. 10.1212/01.wnl.0000171397.32851.bc [DOI] [PubMed] [Google Scholar]
- 31.Yau W-YW, Tudorascu DL, McDade EM, et al. Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer's disease: a prospective cohort study. Lancet Neurol 2015;14:804–13. 10.1016/S1474-4422(15)00135-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 2012;72:578–86. 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–47. 10.1212/WNL.0000000000002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–62. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattsson N, Palmqvist S, Stomrud E, et al. Staging β -Amyloid Pathology With Amyloid Positron Emission Tomography. JAMA Neurol 2019;76:1319 10.1001/jamaneurol.2019.2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Wiste HJ, Therneau TM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 2019;321:2316–25. 10.1001/jama.2019.7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol 2011;121:171–81. 10.1007/s00401-010-0789-4 [DOI] [PubMed] [Google Scholar]
- 38.He Z, Guo JL, McBride JD, et al. Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med 2018;24:29–38. 10.1038/nm.4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blennow K, Zetterberg H, Rinne JO, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 2012;69:1002–10. 10.1001/archneurol.2012.90 [DOI] [PubMed] [Google Scholar]
- 40.Liu E, Schmidt ME, Margolin R, et al. Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology 2015;85:692–700. 10.1212/WNL.0000000000001877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis BA, Sundell K, Lachno DR, et al. Central pharmacodynamic activity of solanezumab in mild Alzheimer's disease dementia. Alzheimers Dement 2018;4:652–60. 10.1016/j.trci.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold M Phase II clinical trials of anti-amyloid β antibodies: when is enough, enough? Alzheimers Dement 2017;3:402–9. 10.1016/j.trci.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreth J, Mavoungou C, Schindowski K. Is Abeta a sufficient biomarker for monitoring anti-Abeta clinical studies? A critical review. Front Aging Neurosci 2013;5:25. 10.3389/fnagi.2013.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molinuevo JL, Ayton S, Batrla R, et al. Current state of Alzheimer's fluid biomarkers. Acta Neuropathol 2018;136:821–53. 10.1007/s00401-018-1932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merluzzi AP, Vogt NM, Norton D, et al. Differential effects of neurodegeneration biomarkers on subclinical cognitive decline. Alzheimers Dement 2019;5:129–38. 10.1016/j.trci.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao F, Hong X, Li S, et al. Urine-Based biomarkers for Alzheimer's disease identified through coupling computational and experimental methods. J Alzheimers Dis 2018;65:421–31. 10.3233/JAD-180261 [DOI] [PubMed] [Google Scholar]
- 47.Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer's disease in saliva: a systematic review. Dis Markers 2019;2019:1–11. 10.1155/2019/4761054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer's disease and vascular disease. Sci Rep 2016;6:26801. 10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsson B, Lautner R, Andreasson U, et al. Csf and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol 2016;15:673–84. 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- 50.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature 2018;554:249–54. 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 51.Vergallo A, Mégret L, Lista S, et al. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer's disease. Alzheimers Dement 2019;15:764–75. 10.1016/j.jalz.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 52.Schindler SE, Bollinger JG, Ovod V, et al. High-Precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019;93:10.1212/WNL.0000000000008081–1659. 10.1212/WNL.0000000000008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira JB, Westman E, Hansson O, et al. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer's disease. Neurobiol Aging 2017;58:14–29. 10.1016/j.neurobiolaging.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Barthélemy NR, Horie K, Sato C, et al. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease. J Exp Med 2020;217:e20200861. 10.1084/jem.20200861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019. 10.1001/jamaneurol.2019.1534. [Epub ahead of print: 17 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol 2019;18:1103–11. 10.1016/S1474-4422(19)30354-0 [DOI] [PubMed] [Google Scholar]
- 57.de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 2020;143:1220–32. 10.1093/brain/awaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takousis P, Sadlon A, Schulz J, et al. Differential expression of microRNAs in Alzheimer's disease brain, blood, and cerebrospinal fluid. Alzheimers Dement 2019;15:1468–77. 10.1016/j.jalz.2019.06.4952 [DOI] [PubMed] [Google Scholar]
- 59.Nagaraj S, Laskowska-Kaszub K, Dębski KJ, et al. Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer's disease patients from non-demented subjects. Oncotarget 2017;8:16122–43. 10.18632/oncotarget.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mustapic M, Eitan E, Werner JK, et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci 2017;11:278. 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang L, Dong H, Cao H, et al. Exosomes in pathogenesis, diagnosis, and treatment of Alzheimer's disease. Med Sci Monit 2019;25:3329–35. 10.12659/MSM.914027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hampel H, Goetzl EJ, Kapogiannis D, et al. Biomarker-Drug and liquid biopsy Co-development for disease staging and targeted therapy: Cornerstones for Alzheimer's precision medicine and pharmacology. Front Pharmacol 2019;10:310. 10.3389/fphar.2019.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartmann S, Ledur Kist TB. A review of biomarkers of Alzheimer's disease in noninvasive samples. Biomark Med 2018;12:677–90. 10.2217/bmm-2017-0388 [DOI] [PubMed] [Google Scholar]
- 64.Hickman S, Izzy S, Sen P, et al. Microglia in neurodegeneration. Nat Neurosci 2018;21:1359–69. 10.1038/s41593-018-0242-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forloni G, Artuso V, La Vitola P, et al. Oligomeropathies and pathogenesis of Alzheimer and Parkinson's diseases. Mov Disord 2016;31:771–81. 10.1002/mds.26624 [DOI] [PubMed] [Google Scholar]
- 66.La Vitola P, Balducci C, Cerovic M, et al. Alpha-Synuclein oligomers impair memory through glial cell activation and via Toll-like receptor 2. Brain Behav Immun 2018;69:591–602. 10.1016/j.bbi.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 67.King E, O'Brien J, Donaghy P, et al. Inflammation in mild cognitive impairment due to Parkinson's disease, Lewy body disease, and Alzheimer's disease. Int J Geriatr Psychiatry 2019;34:1244–50. 10.1002/gps.5124 [DOI] [PubMed] [Google Scholar]
- 68.McGeer PL, Rogers J. Anti-Inflammatory agents as a therapeutic approach to Alzheimer's disease. Neurology 1992;42:447–9. 10.1212/WNL.42.2.447 [DOI] [PubMed] [Google Scholar]
- 69.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999;400:173–7. 10.1038/22124 [DOI] [PubMed] [Google Scholar]
- 70.Forloni G, Balducci C. Alzheimer's disease, oligomers, and inflammation. J Alzheimers Dis 2018;62:1261–76. 10.3233/JAD-170819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.in t' Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med 2001;345:1515–21. 10.1056/NEJMoa010178 [DOI] [PubMed] [Google Scholar]
- 72.Lucca U, Tettamanti M, Forloni G, et al. Nonsteroidal antiinflammatory drug use in Alzheimer's disease. Biol Psychiatry 1994;36:854–6. 10.1016/0006-3223(94)90598-3 [DOI] [PubMed] [Google Scholar]
- 73.Van Gool WA, Weinstein HC, Scheltens P, et al. Effect of hydroxychloroquine on progression of dementia in early Alzheimer's disease: an 18-month randomised, double-blind, placebo-controlled study. Lancet 2001;358:455–60. 10.1016/S0140-6736(01)05623-9 [DOI] [PubMed] [Google Scholar]
- 74.Chung W-S, Welsh CA, Barres BA, et al. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci 2015;18:1539–45. 10.1038/nn.4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris GP, Clark IA, Zinn R, et al. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem 2013;105:40–53. 10.1016/j.nlm.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 76.Balducci C, Santamaria G, La Vitola P, et al. Doxycycline counteracts neuroinflammation restoring memory in Alzheimer's disease mouse models. Neurobiol Aging 2018;70:128–39. 10.1016/j.neurobiolaging.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 77.Balducci C, Beeg M, Stravalaci M, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A 2010;107:2295–300. 10.1073/pnas.0911829107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Felice FG, Ferreira ST, Inflammation FST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014;63:2262–72. 10.2337/db13-1954 [DOI] [PubMed] [Google Scholar]
- 79.Du Z, Li Y, Li J, et al. Physical activity can improve cognition in patients with Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging 2018;13:1593–603. 10.2147/CIA.S169565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barroeta-Espar I, Weinstock LD, Perez-Nievas BG, et al. Distinct cytokine profiles in human brains resilient to Alzheimer's pathology. Neurobiol Dis 2019;121:327–37. 10.1016/j.nbd.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marizzoni M, Provasi S, Cattaneo A, et al. Microbiota and neurodegenerative diseases. Curr Opin Neurol 2017;30:630–8. 10.1097/WCO.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 82.Janelidze S, Mattsson N, Stomrud E, et al. Csf biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018;91:e867–77. 10.1212/WNL.0000000000006082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerami C, Iaccarino L, Perani D. Molecular imaging of neuroinflammation in neurodegenerative dementias: the role of in vivo PET imaging. Int J Mol Sci 2017;18:pii: E993 10.3390/ijms18050993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baldacci F, Lista S, Palermo G, et al. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: advances in development. Expert Rev Proteomics 2019;16:593–600. 10.1080/14789450.2019.1628643 [DOI] [PubMed] [Google Scholar]
- 85.Ross J, Sharma S, Winston J, et al. CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: a 12-week, double-blind, placebo-controlled study. Curr Alzheimer Res 2013;10:742–53. 10.2174/13892037113149990144 [DOI] [PubMed] [Google Scholar]
- 86.Martin E, Boucher C, Fontaine B, et al. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer's disease models: effects of aging and amyloid pathology. Aging Cell 2017;16:27–38. 10.1111/acel.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hampel H, Vergallo A, Perry G, et al. The Alzheimer precision medicine initiative. J Alzheimers Dis 2019;68:1–24. 10.3233/JAD-181121 [DOI] [PubMed] [Google Scholar]
- 88.Reitz C Toward precision medicine in Alzheimer's disease. Ann Transl Med 2016;4:107. 10.21037/atm.2016.03.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hampel H, O'Bryant SE, Durrleman S, et al. A precision medicine initiative for Alzheimer's disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric 2017;20:107–18. 10.1080/13697137.2017.1287866 [DOI] [PubMed] [Google Scholar]
- 90.Wilcock DM Neuroinflammatory phenotypes and their roles in Alzheimer's disease. Neurodegener Dis 2014;13:183–5. 10.1159/000354228 [DOI] [PubMed] [Google Scholar]
- 91.Morgan AR, Touchard S, O'Hagan C, et al. The Correlation between Inflammatory Biomarkers and Polygenic Risk Score in Alzheimer's Disease. J Alzheimers Dis 2017;56:25–36. 10.3233/JAD-160889 [DOI] [PubMed] [Google Scholar]
- 92.Morgan AR, Touchard S, Leckey C, et al. Inflammatory biomarkers in Alzheimer's disease plasma. Alzheimers Dement 2019;15:776–87. 10.1016/j.jalz.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lai KSP, Liu CS, Rau A, et al. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 2017;88:876–82. 10.1136/jnnp-2017-316201 [DOI] [PubMed] [Google Scholar]
- 94.Nordengen K, Kirsebom B-E, Henjum K, et al. Glial activation and inflammation along the Alzheimer's disease continuum. J Neuroinflammation 2019;16:46. 10.1186/s12974-019-1399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Forloni G, Colombo L, Girola L, et al. Anti-Amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett 2001;487:404–7. 10.1016/S0014-5793(00)02380-2 [DOI] [PubMed] [Google Scholar]
- 96.Santa-Cecília FV, Socias B, Ouidja MO, et al. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox Res 2016;29:447–59. 10.1007/s12640-015-9592-2 [DOI] [PubMed] [Google Scholar]
- 97.Albensi BC Dysfunction of mitochondria: implications for Alzheimer's disease. Int Rev Neurobiol 2019;145:13–27. 10.1016/bs.irn.2019.03.001 [DOI] [PubMed] [Google Scholar]