Abstract
Pulmonary rehabilitation (PR) is effective in reducing symptoms and improving health status, and exercise tolerance of patients with chronic obstructive pulmonary disease (COPD). The coronavirus disease 19 (COVID-19) pandemic has greatly impacted PR programs and their delivery to patients. Owing to fears of viral transmission and resultant outbreaks of COVID-19, institution-based PR programs have been forced to significantly reduce enrolment or in some cases completely shut down during the pandemic. As a majority of COPD patients are elderly and have multiple co-morbidities including cardiovascular disease and diabetes, they are notably susceptible to severe complications of COVID-19. As such, patients have been advised to stay at home and avoid social contact to the maximum extent possible. This has increased patients’ vulnerability to physical deconditioning, depression, and social isolation. To address this major gap in care, some traditional hospital or clinic-centered PR programs have converted some or all of their learning contents to home-based telerehabilitation during the pandemic. There are, however, some significant barriers to this approach that have impeded its implementation in the community. These include variable access and use of technology (by patients), a lack of standardization of methods and tools for evaluation of the program, and inadequate training and resources for health professionals in optimally delivering telerehabilitation to patients. There is a pressing need for high-quality studies on these modalities of PR to enable the successful implementation of PR at home and via teleconferencing technologies. Here, we highlight the importance of telerehabilitation of patients with COPD in the post-COVID world and discuss various strategies for clinical implementation.
Keywords: pulmonary rehabilitation, COVID-19, telerehabilitation, COPD
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) is a novel betacoronavirus1,2 that was first identified in December of 2019 through a cluster of pneumonia with an unknown origin.2,3 The disease caused by this virus is now known as Coronavirus disease 19 (COVID-19), and has been characterized as a global pandemic by the World Health Organization since March 11th 2020. SARS-CoV-2 shares 80% sequence homology with its original counterpart SARS coronavirus that led to an outbreak in 2003.1,2 SARS-CoV-2, however, has evolved features that have enhanced its ability to bind to its receptor, angiotensin-converting enzyme-2 (ACE-2) on the cell surface and to penetrate inside the cells, causing cellular infection.4,5 The clinical manifestation of COVID-19 ranges from no symptoms to life-threatening pathologies, such as vasculitis, myocarditis, severe pneumonia, multi-organ failure, and death.1,6 Although the virus attacks most frequently the respiratory tract, COVID-19 can directly and indirectly through cytokine storms or vascular inflammation and thrombosis lead to many non-pulmonary complications.6,7
Chronic obstructive pulmonary disease (COPD) is a condition that is characterized by persistent airway inflammation and airflow limitation.8 It is the 3rd leading cause of mortality worldwide.8,9 There is an ongoing controversy on whether COPD is a risk factor for COVID-19. Although some have reported an increase in the risk,10,11 other studies have shown no significant association;12 and a few studies have even reported a protective relationship.11,13,14 As most of these studies have relied on self-report or a physician diagnosis of COPD, which is prone to measurement error, diagnostic misclassification may in part explain the variation in the results across these studies.
Studies of patients with severe COVID-19, however, have produced more consistent results. While there is still some variation in data, the totality of results indicates that COPD is a significant risk factor for severe COVID-19, increasing the risk by 50% to 100% and leading to poor outcomes including longer stays in the intensive care unit (ICU) and mortality.10,15 The reason for this observation is unclear. One possibility is that COPD patients have poor lung function reserves,16 which when challenged with SARS-CoV-2 pneumonia may lead to respiratory failure and death. Another possibility is the significant upregulation of the ACE-2 receptor in the small airway epithelium of individuals with COPD.17,18 As noted previously, SARS-CoV-2 primarily uses ACE-2 as its cognate receptor to invade cells in the respiratory tract. ACE-2 expression levels are highest in the nasal cavity and progressively decrease as the airways branch and become smaller in size.19 ACE-2 expression is generally very low in the gas exchange units of the lung.19 However, with COPD, airway expression levels of ACE-2 increase, even in the small airways17 This may allow the virus to propagate from the upper airways into the smaller airways, causing pneumonia. Once the lower airways become infected with the virus, the host down-regulates ACE-2, which may limit the spread of the virus.20 However, the “side effect” of this process is local vasoconstriction, and inflammation, which increases the risk of complications, such as diffuse alveolar damage, vascular endothelialitis, thrombosis, and hypoxemia.21 A third possibility is that in COPD patients, there may be up-regulation of ACE-2 in the myocardium.22 This may enable the virus to directly infect the heart and cause myocardial damage.23
Patients with severe COVID-19 experience a considerable amount of morbidity during hospitalization. Many of these deficits including lethargy, breathlessness, diffuse myalgias and cognitive dysfunction may remain post-recovery from the acute illness.24,25 Approximately 50% of patients with severe COVID-19 will require rehabilitation following hospital discharge.26–28 However, owing to constraints of social and physical distancing and concerns over SARS-CoV-2 transmission in the community, traditional modes of rehabilitation cannot be easily implemented during the pandemic. Here, we will discuss the PR for COPD patients in the COVID-19 era with a perspective of finding a new standard of PR delivery.29,30
Pulmonary Rehabilitation
Definition
Pulmonary rehabilitation (PR) is one of the most effective management strategies to improve shortness of breath, health status, and exercise tolerance of patients with COPD. It also leads to a reduction in symptoms of anxiety and depression. Many randomized controlled trials, meta-analyses, and evidence-based reviews have provided solid evidence for the benefits of PR programs in symptomatic COPD patients.8,31 The 2013 American Thoracic Society (ATS)/European Respiratory Society (ERS) Statement defines PR as
a comprehensive intervention based on a thorough patient assessment followed by patient-tailored therapies, which include, but are not limited to, exercise training, education, and behavior change, designed to improve the physical and emotional condition of people with chronic respiratory disease and to promote the long-term adherence of health-enhancing behaviors.32
More broadly, PR is considered as a critical component of integrated patient management, and usually includes a range of healthcare professionals to ensure optimal outcomes.33 The multidisciplinary team for PR generally consists of pulmonologists, physical medicine specialists, social workers, psychologists, nurses, respiratory therapists, occupational therapists, physiotherapists, general practitioners, pharmacists, and dieticians. Patients with a high symptom burden or those at increased risk of exacerbations are recommended to participate in a formal rehabilitation program that is structured and multi-disciplinary.34,35
PR Before COVID
Although there is tremendous variation in the structure and setting of PR across the world, the most common form of PR is implemented through an outpatient, hospital-based exercise program. According to a global survey completed by representatives of 430 centers from 40 countries, 262 (60.9%) were outpatient programs while 41 (9.5%) were inpatient programs, and 106 (24.7%) centers offered both. Only 21 (4.9%) programs were based at home or in a primary care setting.36 It should be noted that, to date, most of the evidence supporting the benefits of PR have been generated from studies or PR programs that were hospital-based.37–39
Three major US and international guidelines recommend that, for optimal therapeutic benefits, a PR program should consist of at least 3 to 5 supervised (exercise) sessions per week for 12 weeks (of at least 20 minutes per session) during which patients achieve >60% of maximal peak exercise capacity.33,37,39 Optimally, PR sessions should include endurance training, interval training, and resistance/strength training of upper and lower limbs in addition to walking exercises. Other key components of PR include self-management training. Self-management interventions are structured, often multi-component, and ideally personalized to achieve goals of patient motivation, and engagement to enable adoption of positive health behaviors and development of self-management skills and mastery of their disease.40 Randomized controlled studies (RCTs) have shown that self-management interventions when properly implemented improve patients’ health status and decrease their risk for hospitalization and emergency department visits.41–44
However, despite the known benefits of PR, in the real world, patients’ attendance and completion rate of PR are low. For example, in 2018, Keating et al reported in a systematic review that the percentage of referred participants who did not attend any PR sessions ranged from 8.3% to 49.6% and the percentage of non-completers ranged from 9.7% to 31.8%. The major barriers to PR compliance were 1) disruption to patients’ established routine; 2) a lack of enthusiasm for PR by patient’s physician; 3) a lack of perceived benefit, and 4) inconvenient timing. An often overlooked impediment is transportation. Indeed, some studies have shown that insufficient transportation to and from hospital-based PR programs to be the most common barrier to participation in PR.45,46 It has been reported that those who travelled more than 30 minutes to a PR center were significantly less likely to complete PR than those who travelled shorter distances.47,48 The authors of this review concluded that to enhance uptake and completion of PR programs, more attention is required to remove these barriers by subsidizing transportation, providing greater support for patients in enrolling and completing PR, and empowering patients to make informed decisions about their care.49 In 2015, the Official ATS/ERS Policy Statement on Enhancing Implementation, Use and Delivery of PR50 highlighted other common barriers to PR: insufficient funding for the programs; limited resources for PR programs; inadequate reimbursement for PR; a lack of awareness and knowledge of the benefits of PR by healthcare professionals, payers, patients, and caregivers; suboptimal use of PR by suitable patients;46,51 and limited training opportunities for PR professionals.
One method of addressing some of these gaps in care is by instituting home-based PR programs. There is a growing body of evidence to suggest that home-based exercise training is feasible and can be effective in improving exercise capacity, reducing breathlessness, and enhancing quality of life. Notably, home-based programs can significantly improve patients’ adherence and completion rates to PR.31,52–57 Maltais et al, for example, conducted an RCT of 252 patients with moderate to severe COPD in 10 academic and community medical centers across Canada. The study assessed whether self-monitored, home-based rehabilitation was as effective as outpatient, hospital-based rehabilitation in these patients. They found that home rehabilitation was non-inferior to hospital-based outpatient programs in patients with COPD.52 Mccarthy et al performed a meta-analysis and found that home-based PR resulted in significantly greater improvements in exercise capacity and health-related quality of life (HRQoL) compared with usual care in patients with COPD.31 Importantly, as outlined by Bourbeau et al,57 for optimal results, a home-based PR program must be capable of preserving all core components that define PR, including exercise program supervision, a multidimensional approach, and education with self-management interventions.
Telerehabilitation
Another method of addressing the limitations of traditional hospital-based PR programs is through telerehabilitation.28,58,59 Telerehabilitation (a subset of telehealth) is the use of information and communication technologies to provide clinical rehabilitation services from a distance.60 It can be provided in a variety of different ways, including two-way real-time visits with audio, video, or both; asynchronous e-visits; virtual check-ins; remote evaluations of recorded videos or images; or telephone assessment and management services.61 It may be delivered directly to a patient’s home or a nearby healthcare facility.62
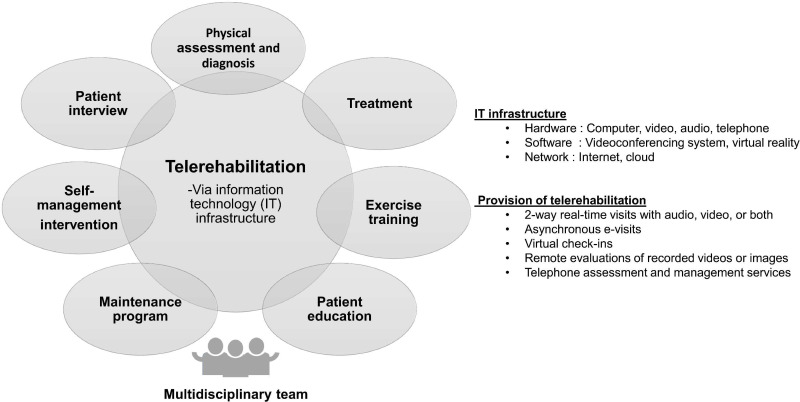
The common approach of building an information technology (IT) infrastructure to support telerehabilitation is to purchase IT systems that create similar experience as that which is experienced through traditional face-to-face encounters. Some examples of these IT infrastructure include a videoconferencing system, virtual reality, and electronic/portable devices.63 Essential telerehabilitation technology components include human factors (patients, providers, organizations, and society), economic factors, and technologies.64 The various components of telerehabilitation are shown in Figure 1.
Figure 1.
Various components of telerehabilitation. Telerehabilitation is delivered to patients via information technology (IT) infrastructure. The various components of the rehabilitation program are similar to those of conventional rehabilitation programs which have been demonstrated to improve health status of patients with COPD.
The earliest publication of telerehabilitation can be traced back to 1998.65 Since then, the use of technology in home-based telerehabilitation has gradually increased as an alternative to the conventional hospital-based PR. Telerehabilitation is currently used primarily in cardiac, musculoskeletal, and neurological rehabilitation.58,66 There have been a number of telerehabilitation studies in COPD.67–76 Two pilot RCTs evaluated a web-based exercise program consisting of smartphone activity coaching, self-management education, and teleconsultations compared with a control group that consisted of usual care.67,68 These studies showed that web-based rehabilitation program was associated with improvements in activity level, higher compliance to the activity coaching,65 and increased patient satisfaction with the exercise program.68 Tsai et al conducted an RCT in patients with COPD to determine the effects of home-based telerehabilitation that incorporated direct supervision of all exercise sessions using real-time videoconferencing. They found that this approach significantly improved exercise endurance and self-efficacy of patients. There was also a positive trend towards improvements in health-related quality of life (HRQoL) when compared with usual medical care.74
Recently, Hansen et al conducted a single-blinded, multicenter RCT that investigated the effects of a supervised telerehabilitation program (PTR) compared with a supervised conventional PR program. The authors hypothesized that telerehabilitation would be superior to a supervised conventional PR in improving 6 min walk distance, possibly by increasing patients’ adherence rate to the program. The telerehabilitation method was given via a videoconferencing software system that was implemented through a single touch screen. The investigators however failed to find a significant difference in outcomes between PTR and conventional PR; interestingly, more participants completed PTR.75 There is another assessor-blinded, multi-center RCT that is evaluating the effectiveness of home-based telerehabilitation versus traditional center-based pulmonary rehabilitation in patients with COPD (the REAcH trial);77 the results are pending. In summary, while there have been several clinical studies of telerehabilitation including RCTs, its implementation in the community has been slow and variable.78,79 Studies67–75,77 of telerehabilitation in COPD are shown in Table 1. There is tremendous heterogeneity in the design of these studies including measurement of clinical outcomes, such as activity level, endurance time in walking, and the number of hospital admissions pre- and post-PR and the technologies that were deployed in these studies.
Table 1.
Relevant Studies of Telerehabilitation in COPD Patients
| Reference (Year) Country | Study Design | Participants | Intervention | Control | Duration | Primary Outcome Parameter | Outcomes |
|---|---|---|---|---|---|---|---|
| Tabak et al67 (2014) Netherlands | Pilot RCT | 34 | Activity coach (3D-accelerometer with smartphone) for ambulant activity registration and real-time feedback, complemented by a web portal with a symptom diary for self-treatment of exacerbations. | Usual care (Details unknown) | 4 weeks | Activity level | The activity coach was used more than prescribed (108%) and compliance was related to the increase in activity level for the first 2 feedback weeks (r=0.62, p=0.03). Health status significantly improved within the intervention group (p=0.05). No significant difference in health status improvement. |
| Tabak et al68 (2014) Netherlands | Pilot RCT | 29 | Program consisted of 4 modules. 1) activity coach for ambulant activity monitoring and real-time coaching of daily activity behavior 2) web-based exercise program for home exercising 3) self-management of COPD exacerbations via a triage diary on the web portal, including self-treatment of exacerbations 4) teleconsultation |
Regular physiotherapy sessions, in the case of an impending exacerbation, the participants had to contact their medical doctor as usual. | 9 months | Satisfaction | The telehealth program with decision support showed good satisfaction. The program was accessed on 86% of the treatment days, especially the diary. Patient adherence with the exercise scheme was low (21%). |
| Paneroni et al69 (2015) Italy | Multicenter, prospective, controlled, non randomized pilot study | 36 | Home-based reinforcement telerehabilitation program. 28 sessions of strength exercises (60 min) and cycle training (40 min) using a satellite platform provided telemonitoring, tele-prescription, video-assistance and phone-calls. Patients were equipped with an oximeter, steps-counter, bicycle, remote control and interactive TV software. |
Standard outpatient rehabilitation program. 28 sessions of strength exercises (60 min) and cycle training (40 min). Program included strength exercises, cycle ergometer training, and an educational intervention to promote an appropriate lifestyle and self-management. |
Maximum 40 days | Feasibility, adherence and satisfaction | The telerehabilitation patients completed all sessions without side effects, used the remote control 1394 ± 2329 times being in the 84% of the cases satisfied with the service. In 22% of the cases patients found the technology unfriendly. |
| Zanaboni et al70 (2013) Norway | Feasibility study | 10 | A long-term telerehabilitation service comprising exercise training at home with a 2-year follow-up program by a physiotherapist. Equipment included a treadmill, a pulse oximeter and a tablet computer. Participants had weekly videoconference sessions with the physiotherapist. A website was used to access a training program and to fill in a daily diary and a training diary. |
None | Long-term intervention with a 2-year follow-up | Number of hospital admissions | There was a reduction of 27% in the COPD-related hospital costs. No drop-outs. Feedback from the participants was very positive. |
| Holland et al71 (2013) Australia | Feasibility study | 8 | Supervised aerobic training twice a week, with 2 participants and a physiotherapist attending each class via videoconferencing from separate locations. | None | 8 weeks | Adverse events, sessions attended, and system usability. | No significant adverse events occurred during the study. Participants attended 76% of possible sessions. System usability ratings were excellent when sessions were delivered via the university network (mean 94 out of 100) but lower when using the hospital network (mean 59 out of 100), with 67% of technical problems related to data network capability. |
| Marquis et al72 (2015) Canada | Pre-/postintervention study | 26 | 15 in-home teletreatment sessions via videoconference from a service center to their home. Education provided via self-learning health capsules. |
None | 8 weeks | Changes in exercise tolerance (6MWT, CET) and quality of life CRQ. | Significant improvements between pre-and postintervention on the 6MWT (32 m; p<0.001), CET (41 s; p=0.005), and three of four CRQ domains (dyspnea [p<0.001], fatigue [p=0.002], and emotion [p=0.002]). Participants’ satisfaction and adherence rate with telerehabilitation were very high. |
| Tousignant et al73 (2012) Canada | Pre-experimental pilot study | 3 | Telerehabilitation sessions (15 sessions) were conducted by 2 trained physiotherapists from a service center to the patient’s home. Locomotor function (walking performance) and quality of life were measured in person prior to and at the end of the treatment by an independent assessor. |
None | 8 weeks | Feasibility | Clinical outcomes improved for all subjects except for locomotor function in the first participant. All participants showed a trend towards better quality of life (dyspnea, fatigue, emotions, mastery). |
| Tsai et al74 (2017) Australia | RCT (assessor and statistician blinded) | 37 | The same physiotherapist who was based in the hospital supervised up to 4 participants remotely exercising at home in each session using real‐time desktop videoconferencing software. | Usual medical management including optimal pharmacological intervention and an action plan was provided. This group did not participate in any exercise training. |
8 weeks | Endurance exercise capacity | The telerehabilitation group showed a significant increase in endurance shuttle walk test time (mean difference = 340 s (95% CI: 153–526, p < 0.001)). |
| Hansen et al75 (2020) Denmark | RCT (assessor and statistician blinded), superiority, multicenter | 134 | A group-based, supervised and standardized program performed by the patients in their homes 3 times weekly via a videoconference software system installed on a single touch screen. The exercise sessions lasted 35 min with incorporated warm-up and high repetitive time-based muscle endurance training followed by 5 min’ rest before beginning a patient education session of 20 min. |
A group-based, supervised and standardized and was performed twice a week. The exercise sessions lasted 60 min and incorporated warm-up, endurance and resistance training and a cool-down period. The patient education sessions lasted 60 to 90 min and took place once a week after the exercise session. |
10 weeks | Change in the 6MWT on completion of the program | No statistically significant between-group differences for changes in 6MWT after intervention or at 22 weeks’ follow-up. |
| Cox et al77 (2018) Australia | RCT (assessor and statistician blinded), equivalence, multicenter | Unknown | Home-based training twice a week, in groups of 4–6 participants at a time, supervised by an experienced physiotherapist. Exercise training will comprise 30 min of lower limb aerobic training and individualized strength training exercises. Participants will undertake a total of 30 min of cycle training, in two or more bouts, in each session. Disease-specific education and collaborative self-management training. |
A standard outpatient pulmonary program at the center where they were recruited. Twice-weekly supervised sessions in groups of 8–12 participants, supervised by a health professional experienced in the delivery of pulmonary rehabilitation and qualified assistants. At least 30 min of lower limb aerobic training each session, which may be completed in shorter intervals if continuous training is limited by symptoms. |
8 weeks | Change in CRQ-D from baseline to end of intervention | Results have not been published. |
Abbreviations: RCT, randomized controlled study; COPD, chronic obstructive lung disease; TV, television; 6MWT, 6-minute walk test; CET, cycle endurance test; CRQ, Chronic Respiratory Questionnaire; CRQ-D, CRQ dyspnea domain.
PR During the COVID-19 Pandemic
COVID-19 pandemic has greatly impacted all aspects of health care delivery. Precautions related to COVID-19 have created a need for safer service delivery options for health services. To protect health care workers and patients across the country from disease transmission, rules, regulations, and reimbursement policies have been substantially altered to enable widespread use of telecommunications technology in lieu of in-person clinical visits.80 In response to this new landscape of medical practice, multiple organizations, such as the American Physical Therapy Association, Australian Physiotherapy Association, and Italian Physiotherapy Association, have expanded resources and advice for the implementation of telerehabilitation services.
According to the Report from the American Physical Therapy Association published in June 2020,81 physical therapy services have been deemed as essential by federal, state, and local authorities during the pandemic. Despite this, many physical therapists have proactively curtailed their hours of work to reduce patient contact time to “flatten the curve” of the pandemic. Overall, 54% of physiotherapists (PTs) have reduced their work-time and only 10% have increased their work-hours.
The American Physical Therapy Association recommends the implementation of video-conferencing technology to enable direct communication between PTs and clients during the pandemic. Prior to the pandemic, 98% of PTs surveyed were not providing live video consultations. Of the 2% who were, the vast majority (69%) reported seeing less than one patient per week. During the pandemic, 50% reported providing live video consultations. Of these, just over half (51%) treated between one and five patients per week, 17% treated six to 10 patients per week, and 17% treated more than 10 patients via live consultations. In terms of delivery, the most popular platform for video consultations was Zoom®, which was endorsed by 43% of the PTs, who participated in the survey. Doxy.me® was used by another 30%, and Epic® was used by 9%. Respondents identified more than two dozen platforms that were used to facilitate video-based care. Notably, 31% of those surveyed said that their patients and clients lacked adequate technology, and 21% said their facility’s technology was a limiting factor in delivering e-technology-based care.
As COPD patients are particularly vulnerable to severe complications of COVID-19, in-person PR should not be conducted during the pandemic, except in exceptional circumstances.82,83 In-person PR may be considered only when the community spread of COVID-19 is low. For those PTs, who must provide in-person care during the pandemic, acceptable personal protective equipment (PPE) should be worn at all times. Despite these recommendations, several dozens of those surveyed commented that they found their PPE supplies to be inadequate.81 One study found that 67% of PTs were provided PPE training by their employer since the advent of the pandemic; 8% reported not being provided PPEs, while 18% had to cancel appointments with their clients because they lacked PPEs. The Canadian Thoracic Society has identified several factors that are critical to reducing the risk of virus transmissions. This includes screening procedures for in-person PR, sufficiently large exercise space (to enable social distancing), equipment preparation and regular cleansing, and proper use of waiting areas. Hybrid models that include in-person assessment and exercise testing, and a combination of in-person and virtual exercise training, education, and self-management can be used to optimize exercise safety and training effectiveness while decreasing the risk of disease transmission and infection rates.84 It should be noted that modern telerehabilitation technologies should contain devices that will enable remote monitoring of physiological signs and symptoms during exercise in real-time or in a “store and forward” capacity. There should also be the ability for supervisors to “beam-in” when necessary to provide instant feedback to the patients and to halt the program when patients are physically struggling.85
Challenges and Unanswered Questions
Despite the rapid progress of telerehabilitation, there are still many challenges and impediments to its widespread adoption in the community. Prior to the COVID-19 pandemic, the use of telerehabilitation was low (~4.9% including all home-based PR).70 One major impediment is the lack of well developed, evidence-based statements to guide its set-up in the community. Another major impediment is a paucity of high-quality evidence indicating that telerehabilitation confers comparable or even greater clinical benefits to patients compared with traditional programs at a reasonable (or lower) cost.50,74 There is also a pressing need to understand which patients, if any, benefit the most from telerehabilitation.86 While there have been a number of telerehabilitation studies in COPD,67,75 the contextual heterogeneity of these studies (including the type of intervention, the participants involved, and the health care system in which they were deployed) makes clinical implementation extremely challenging.85 Since the introduction of telerehabilitation can be quite expensive, it is important to ensure that its implementation is grounded on sound clinical policy decisions and solid high-quality evidentiary data. If these challenges can be addressed, telerehabilitation holds great “therapeutic” promise for a large number of COPD patients during and post-COVID-19 pandemic.55 In most jurisdictions, telerehabilitation is poorly funded. Funding is important to build a solid foundation of evidence for its use and to enable the sustainability of these programs in the community. Before the COVID pandemic, many insurers around the world were willing to reimburse PR in the outpatient setting but not for telerehabilitation.85,87 Bierman et al reported in 2018 that the uptake of telerehabilitation in health systems across the United States was hampered by variation and restrictions in state regulations and reimbursement policies of Medicare and private insurers.87 Legislation providing funding for telerehabilitation would provide a permanent policy solution to expand its use in the community.
There are also challenges related to access and use of technology, optimal mode of assessment, and the ability to obtain outcome measures, as well as adequate supervision of patients during PR. During the pandemic, patients have been differentially affected by their access to the internet or broadband capabilities. Studies75,81,88 have found that people with less experience in digital technologies, such as the elderly population, were less likely to enjoy PR programs and thus more likely to leave the program. This is relevant for COPD patients, who tend to be older. Recently, Bentley et al developed a smartphone app and an activity tracker to help people with COPD to maintain (or increase) physical activity after undertaking PR. Their RCT showed that simplicity and usability of the technology were the two most important determinants of patient engagement with the intervention.89 It is not known whether PR adherence improves by adding online (support) groups to the videoconferencing technology.75 Remote assessment may be difficult, as the current technology does not fully enable “physical examination” of patients. This may limit the caregivers’ ability to establish an accurate functional status at baseline for the patient, and to prescribe the “right” intensity and duration of PR for the patient to achieve optimal outcomes. As there is no current technology that can ensure the safety of patients during unsupervised PR, adequate supervision from trained PR healthcare professionals is still necessary at this moment. Technology that enables accurate evaluation of remote PR and ensure the safety of patients in unsupervised settings is eagerly anticipated. Another current limitation is that in many jurisdictions, there is a shortage of qualified health professionals, who are trained in home-based PR or telerehabilitation.33
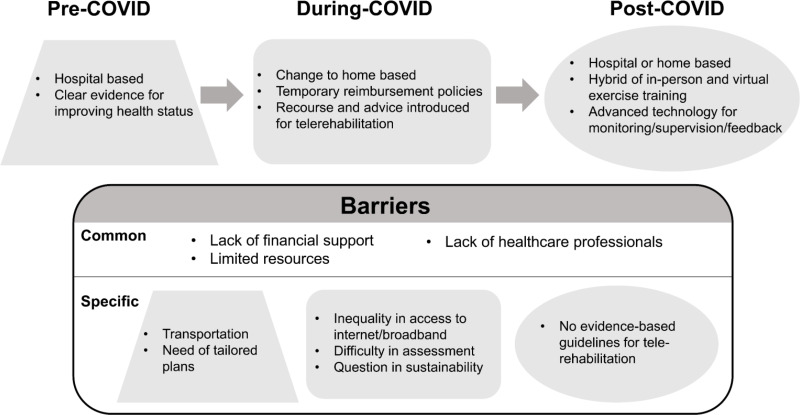
The COVID-19 pandemic has resulted in a dramatic change in our community and many losses of lives. These changes as well as barriers to pulmonary rehabilitation for COPD patients are summarized in Figure 2. Although traditionally PR has been hospital or clinic-centered, the pandemic has prompted discussions and debates on the merits of home-based PR as well as telerehabilitation. There is now a pressing need for high-quality studies on these modalities of PR to enable successful implementation of PR but supervised and unsupervised at home and via teleconferencing technologies.
Figure 2.
Practical changes and barriers in pulmonary rehabilitation for COPD patients in the post-COVID world. The COVID-19 pandemic has resulted in dramatic changes in the way in which pulmonary rehabilitation (PR) is delivered. These include changes in venue and increased use of telerehabilitation. The pandemic has also revealed the long-standing structural barriers of PR, such as the lack of financial support, resources, and healthcare professionals, which have been exacerbated during the COVID pandemic.
Acknowledgments
DDS is supported by a Tier 1 Canada Research Chair in COPD and the de Lazzari Family Chair at HLI. MT is supported by MITACS and Providence Airway Centre at St. Paul’s Hospital. This work was in part funded by St. Paul’s Foundation COVID-19 Rapid Response Fund.
Abbreviations
SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; COVID-19, Coronavirus disease 19; ACE-2, angiotensin-converting enzyme-2; COPD, chronic obstructive pulmonary disease; PR, pulmonary rehabilitation; RCTs, Randomized controlled studies; ATS, American Thoracic Society; ERS, European Respiratory Society; IT, information technology; HRQoL, health-related quality of life; PTR, telerehabilitation program; PPE, personal protective equipment.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- 7.Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;56(4):2003006. doi: 10.1183/13993003.03006-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 9.Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi: 10.1371/journal.pone.0233147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attaway AA, Zein J, Hatipoglu US. SARS-CoV-2 infection in the COPD population is associated with increased healthcare utilization: an analysis of Cleveland clinic’s COVID-19 registry. EClinicalMedicine. 2020;26:100515. doi: 10.1016/j.eclinm.2020.100515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calmes D, Graff S, Maes N, et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9(1):160–169. doi: 10.1016/j.jaip.2020.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JM, Niikura M, Yang CWT, et al. COVID-19 and COPD. Eur Respir J. 2020;56(2):2002108. doi: 10.1183/13993003.02108-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101–111. doi: 10.4104/pcrj.2013.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688. doi: 10.1183/13993003.00688-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne S, Yang CX, Timens W, et al. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir Med. 2020;8(6):e50–e1. doi: 10.1016/S2213-2600(20)30224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sungnak W, Huang N, Becavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciulla MM. SARS-CoV-2 downregulation of ACE2 and pleiotropic effects of ACEIs/ARBs. Hypertens Res. 2020;43(9):985–986. doi: 10.1038/s41440-020-0488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142(5):426–428. doi: 10.1161/CIRCULATIONAHA.120.047049 [DOI] [PubMed] [Google Scholar]
- 23.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceravolo MG, Arienti C, de Sire A, et al. Rehabilitation and COVID-19: the cochrane rehabilitation 2020 rapid living systematic review. Eur J Phys Rehabil Med. 2020;56(5):642–651. doi: 10.23736/S1973-9087.20.06501-6 [DOI] [PubMed] [Google Scholar]
- 25.de Sire A, Andrenelli E, Negrini F, Negrini S, Ceravolo MG. Systematic rapid living review on rehabilitation needs due to COVID-19: update as of April 30th, 2020. Eur J Phys Rehabil Med. 2020;56(3):354–360. doi: 10.23736/S1973-9087.20.06378-9 [DOI] [PubMed] [Google Scholar]
- 26.Curci C, Pisano F, Bonacci E, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 rehabilitation unit and proposal of a treatment protocol. Eur J Phys Rehabil Med. 2020;56(5):633–641. doi: 10.23736/S1973-9087.20.06339-X [DOI] [PubMed] [Google Scholar]
- 27.Ferraro F, Calafiore D, Dambruoso F, Guidarini S, de Sire A. COVID-19 related fatigue: which role for rehabilitation in post-COVID-19 patients? A case series. J Med Virol. 2020. doi: 10.1002/jmv.26717 [DOI] [PubMed] [Google Scholar]
- 28.Salawu A, Green A, Crooks MG, Brixey N, Ross DH, Sivan M. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int J Environ Res Public Health. 2020;17(13):4890. doi: 10.3390/ijerph17134890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TJ, Chau B, Lui M, Lam GT, Lin N, Humbert S. Physical medicine and rehabilitation and pulmonary rehabilitation for COVID-19. Am J Phys Med Rehabil. 2020;99(9):769–774. doi: 10.1097/PHM.0000000000001505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitacca M, Carone M, Clini EM, et al. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: the Italian position paper. Respiration. 2020;99(6):493–499. doi: 10.1159/000508399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 33.Vogiatzis I, Rochester CL, Spruit MA, Troosters T, Clini EM; American Thoracic Society/European Respiratory Society Task Force on Policy in Pulmonary R. Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J. 2016;47(5):1336–1341. doi: 10.1183/13993003.02151-2015 [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Thomas J, Sadatsafavi JM, FitzGerald,JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 35.Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–150. doi: 10.1378/chest.14-2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J. 2014;43(5):1326–1337. doi: 10.1183/09031936.00145613 [DOI] [PubMed] [Google Scholar]
- 37.Garvey C, Bayles MP, Hamm LF, et al. Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: AN OFFICIAL STATEMENT FROM THE AMERICAN ASSOCIATION OF CARDIOVASCULAR AND PULMONARY REHABILITATION. J Cardiopulm Rehabil Prev. 2016;36(2):75–83. doi: 10.1097/HCR.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 38.Alison JA, McKeough ZJ, Johnston K, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22(4):800–819. doi: 10.1111/resp.13025 [DOI] [PubMed] [Google Scholar]
- 39.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(5 Suppl):4S–42S. doi: 10.1378/chest.06-2418 [DOI] [PubMed] [Google Scholar]
- 40.Effing TW, Vercoulen JH, Bourbeau J, et al. Definition of a COPD self-management intervention: international Expert Group consensus. Eur Respir J. 2016;48(1):46–54. doi: 10.1183/13993003.00025-2016 [DOI] [PubMed] [Google Scholar]
- 41.Benzo R, Vickers K, Novotny PJ, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A Randomized Study. Am J Respir Crit Care Med. 2016;194(6):672–680. doi: 10.1164/rccm.201512-2503OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benzo R, McEvoy C. Effect of health coaching delivered by a respiratory therapist or nurse on self-management abilities in severe COPD: analysis of a Large Randomized Study. Respir Care. 2019;64(9):1065–1072. doi: 10.4187/respcare.05927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585–591. doi: 10.1001/archinte.163.5.585 [DOI] [PubMed] [Google Scholar]
- 44.Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182(7):890–896. doi: 10.1164/rccm.200910-1579OC [DOI] [PubMed] [Google Scholar]
- 45.Wadell K, Janaudis Ferreira T, Arne M, Lisspers K, Stallberg B, Emtner M. Hospital-based pulmonary rehabilitation in patients with COPD in Sweden–a national survey. Respir Med. 2013;107(8):1195–1200. doi: 10.1016/j.rmed.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 46.Thorpe O, Johnston K, Kumar S. Barriers and enablers to physical activity participation in patients with COPD: a systematic review. J Cardiopulm Rehabil Prev. 2012;32(6):359–369. doi: 10.1097/HCR.0b013e318262d7df [DOI] [PubMed] [Google Scholar]
- 47.Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD, Nett Research G. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105–116. doi: 10.1080/15412550801941190 [DOI] [PubMed] [Google Scholar]
- 48.Sabit R, Griffiths TL, Watkins AJ, et al. Predictors of poor attendance at an outpatient pulmonary rehabilitation programme. Respir Med. 2008;102(6):819–824. doi: 10.1016/j.rmed.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 49.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8(2):89–99. doi: 10.1177/1479972310393756 [DOI] [PubMed] [Google Scholar]
- 50.Rochester CL, Vogiatzis I, Holland AE, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 51.Jones SE, Green SA, Clark AL, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax. 2014;69(2):181–182. doi: 10.1136/thoraxjnl-2013-204227 [DOI] [PubMed] [Google Scholar]
- 52.Maltais F, Bourbeau J, Shapiro S, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008;149(12):869–878. doi: 10.7326/0003-4819-149-12-200812160-00006 [DOI] [PubMed] [Google Scholar]
- 53.Güell MR, de Lucas P, Gáldiz JB, et al. Home vs hospital-based pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: a Spanish multicenter trial. Arch Bronconeumol. 2008;44(10):512–518. doi: 10.1157/13126830 [DOI] [PubMed] [Google Scholar]
- 54.Fernández AM, Pascual J, Ferrando C, Arnal A, Vergara I, Sevila V. Home-based pulmonary rehabilitation in very severe COPD: is it safe and useful? J Cardiopulm Rehabil Prev. 2009;29(5):325–331. doi: 10.1097/HCR.0b013e3181ac7b9d [DOI] [PubMed] [Google Scholar]
- 55.Burkow TM, Vognild LK, Johnsen E, et al. Comprehensive pulmonary rehabilitation in home-based online groups: a mixed method pilot study in COPD. BMC Res Notes. 2015;8:766. doi: 10.1186/s13104-015-1713-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. 2017;72(1):57–65. doi: 10.1136/thoraxjnl-2016-208514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourbeau J, Gagnon S, Ross B. Pulmonary rehabilitation. Clin Chest Med. 2020;41(3):513–528. doi: 10.1016/j.ccm.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 58.Turolla A, Rossettini G, Viceconti A, Palese A, Geri T. Musculoskeletal physical therapy during the COVID-19 pandemic: is telerehabilitation the answer? Phys Ther. 2020;100(8):1260–1264. doi: 10.1093/ptj/pzaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton IJ. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi: 10.2340/16501977-2727 [DOI] [PubMed] [Google Scholar]
- 60.Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427–447. doi: 10.1080/09638280802062553 [DOI] [PubMed] [Google Scholar]
- 61.Prvu Bettger JRL. Telerehabilitation in the age of COVID-19: an opportunity for learning health system research. Phys Ther. 2020;100(11):1913–1916. doi: 10.1093/ptj/pzaa151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox NS, McDonald CF, Hill CJ, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2018;2018(6):CD013040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finkelstein J, Jeong IC, Doerstling M, Shen Y, Wei C, Karpatkin H. Usability of remote assessment of exercise capacity for pulmonary telerehabilitation program. Stud Health Technol Inform. 2020;275:72–76. doi: 10.3233/SHTI200697 [DOI] [PubMed] [Google Scholar]
- 64.Sarsak HI. Telerehabilitation services: a successful paradigm for occupational therapy clinical services? Int Phys Med Rehabil J. 2020;5(2):93–98. [Google Scholar]
- 65.Burns RB, Crislip D, Daviou P, et al. Using telerehabilitation to support assistive technology. Assist Technol. 1998;10(2):126–133. doi: 10.1080/10400435.1998.10131970 [DOI] [PubMed] [Google Scholar]
- 66.Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol. 2017;4(2):e7. doi: 10.2196/rehab.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tabak M, Vollenbroek-Hutten MM, van der Valk PD, van der Palen J, Hermens HJ. A telerehabilitation intervention for patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot trial. Clin Rehabil. 2014;28(6):582–591. doi: 10.1177/0269215513512495 [DOI] [PubMed] [Google Scholar]
- 68.Tabak M, Brusse-Keizer M, van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:935–944. doi: 10.2147/COPD.S60179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paneroni M, Colombo F, Papalia A, et al. Is telerehabilitation a safe and viable option for patients with COPD? A feasibility study. COPD. 2015;12(2):217–225. doi: 10.3109/15412555.2014.933794 [DOI] [PubMed] [Google Scholar]
- 70.Zanaboni P, Lien LA, Hjalmarsen A, Wootton R. Long-term telerehabilitation of COPD patients in their homes: interim results from a pilot study in Northern Norway. J Telemed Telecare. 2013;19(7):425–429. doi: 10.1177/1357633X13506514 [DOI] [PubMed] [Google Scholar]
- 71.Holland AE, Hill CJ, Rochford P, Fiore J, Berlowitz DJ, McDonald CF. Telerehabilitation for people with chronic obstructive pulmonary disease: feasibility of a simple, real time model of supervised exercise training. J Telemed Telecare. 2013;19(4):222–226. doi: 10.1177/1357633x13487100 [DOI] [PubMed] [Google Scholar]
- 72.Marquis N, Larivée P, Saey D, Dubois M, Tousignant M. In-home pulmonary telerehabilitation for patients with chronic obstructive pulmonary disease: a pre-experimental study on effectiveness, satisfaction, and adherence. Telemed J E Health. 2015;21(11):870–879. doi: 10.1089/tmj.2014.0198 [DOI] [PubMed] [Google Scholar]
- 73.Tousignant M, Marquis N, Pagé C, et al. In-home telerehabilitation for older persons with chronic obstructive pulmonary disease: a Pilot Study. Int J Telerehabil. 2012;4(1):7–14. doi: 10.5195/IJT.2012.6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsai LL, McNamara RJ, Moddel C, Alison JA, McKenzie DK, McKeough ZJ. Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: the randomized controlled TeleR Study. Respirology. 2017;22(4):699–707. doi: 10.1111/resp.12966 [DOI] [PubMed] [Google Scholar]
- 75.Hansen H, Bieler T, Beyer N, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax. 2020;75(5):413–421. doi: 10.1136/thoraxjnl-2019-214246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbosa MT, Sousa CS, Morais-Almeida M, Simoes MJ, Mendes P. Telemedicine in COPD: an Overview by Topics. COPD. 2020;17(5):601–617. doi: 10.1080/15412555.2020.1815182 [DOI] [PubMed] [Google Scholar]
- 77.Cox NS, McDonald CF, Alison JA, et al. Telerehabilitation versus traditional centre-based pulmonary rehabilitation for people with chronic respiratory disease: protocol for a randomised controlled trial. BMC Pulm Med. 2018;18(1):71. doi: 10.1186/s12890-018-0646-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stickland M, Jourdain T, Wong EY, Rodgers WM, Jendzjowsky NG, MacDonald,GF. Using Telehealth technology to deliver pulmonary rehabilitation to patients with chronic obstructive pulmonary disease. Can Respir J. 2011;18(4):216–220. doi: 10.1155/2011/640865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holland AE, Cox NS. Telerehabilitation for COPD: could pulmonary rehabilitation deliver on its promise? Respirology. 2017;22(4):626–627. doi: 10.1111/resp.13028 [DOI] [PubMed] [Google Scholar]
- 80.Centers for Medicare and Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers 2020; [Internet]. Available from: https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Accessed November9, 2020.
- 81.Impact of COVID-19 on the physical therapy profession: a report from the American Physical Therapy Association. [Internet]. Available from: https://www.naranet.org/uploads/userfiles/files/documents/APTAReportImpactOfCOVID-19OnThePhysicalTherapyProfession.pdf. Accessed November6, 2020.
- 82.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi: 10.1002/jmv.25889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dechman G, Aceron R, Beauchamp M et al. Delivering pulmonary rehabilitation during the COVID-19 pandemic: a Canadian Thoracic Society Position Statement. Available from: https://www.tandfonline.com/doi/full/10.1080/24745332.2020.1828683. Accessed February 11, 2021.
- 85.Garvey C, Singer JP, Bruun AM, Soong A, Rigler J, Hays S. Moving Pulmonary Rehabilitation Into the Home: A CLINICAL REVIEW. J Cardiopulm Rehabil Prev. 2018;38(1):8–16. doi: 10.1097/HCR.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez-Gerez JJ, Bernal-Utrera C, Anarte-Lazo E, Garcia-Vidal JA, Botella-Rico JM, Rodriguez-Blanco C. Therapeutic pulmonary telerehabilitation protocol for patients affected by COVID-19, confined to their homes: study protocol for a randomized controlled trial. Trials. 2020;21(1):588. doi: 10.1186/s13063-020-04494-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bierman RT, Kwong MW, Calouro C. State occupational and physical therapy telehealth laws and regulations: a 50-state survey. Int J Telerehabil. 2018;10(2):3–54. doi: 10.5195/IJT.2018.6269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ambrosino N, Fracchia C. The role of tele-medicine in patients with respiratory diseases. Expert Rev Respir Med. 2017;11(11):893–900. doi: 10.1080/17476348.2017.1383898 [DOI] [PubMed] [Google Scholar]
- 89.Bentley CL, Powell L, Potter S, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized Controlled Feasibility Study. JMIR mHealth uHealth. 2020;8(6):e16203. doi: 10.2196/16203 [DOI] [PMC free article] [PubMed] [Google Scholar]