Abstract
Vaccine platforms have been critical for accelerating the timeline of COVID-19 vaccine development. Faster vaccine timelines demand further development of these technologies. Currently investigated platform approaches include virally vectored and RNA-based vaccines, as well as DNA vaccines and recombinant protein expression system platforms, each featuring different advantages and challenges. Viral vector-based and DNA vaccines in particular have received a large share of research funding to date. Platform vaccine technologies may feature dual-use potential through informing or enabling pathogen engineering, which may raise the risk for the occurrence of deliberate, anthropogenic biological events. Research on virally vectored vaccines exhibits relatively high dual-use potential for two reasons. First, development of virally vectored vaccines may generate insights of particular dual-use concern such as techniques for circumventing pre-existing anti-vector immunity. Second, while the amount of work on viral vectors for gene therapy exceeds that for vaccine research, work on virally vectored vaccines may increase the number of individuals capable of engineering viruses of particular concern, such as ones closely related to smallpox. Other platform vaccine approaches, such as RNA vaccines, feature relatively little dual-use potential. The biosecurity risk associated with platform advancement may be minimised by focusing preferentially on circumventing anti-vector immunity with non-genetic rather than genetic modifications, using vectors that are not based on viruses pathogenic to humans, or preferential investment into promising RNA-based vaccine approaches. To reduce the risk of anthropogenic pandemics, structures for the governance of biotechnology and life science research with dual-use potential need to be reworked. Scientists outside of the pathogen research community, for instance those who work on viral vectors or oncolytic viruses, need to become more aware of the dual-use risks associated with their research. Both public and private research-funding bodies need to prioritise the evaluation and reduction of biosecurity risks.
Keywords: Vaccine, Platform vaccine, Viral vector, Dual-use, Biosecurity, COVID-19
1. Introduction
The COVID-19 pandemic has demonstrated the importance of vaccine platforms that can be rapidly tailored to novel biological threats. The perception that a widely available vaccine will put an end to the pandemic has directed global attention to COVID-19 vaccine development. Vaccine platforms have been critical in accelerating COVID-19 vaccine development, shortening processes previously taking years to only months. Going forward, we will likely see increased investment in such technologies in order to be able to tackle future pandemics even more rapidly.
However, certain kinds of biotechnologies have the potential to enable pathogen engineering, raising the risk for biological events including pandemics of the largest scale with the potential to destabilise society [1]. This article evaluates the dual-use potential of platform vaccines, analyses past and future research and funding of such approaches, and recommends how to manage associated biosecurity risks emerging from this research.
2. An evaluation of platform vaccine approaches
The term “platform vaccine” refers to vaccine approaches where an underlying, nearly identical mechanism, device, delivery vector, or cell line can be easily adapted and employed for targeting novel pathogens on the basis of their genetic sequence [2]. Vaccine scientists hope that such platform approaches will improve the speed with which vaccines against novel pathogens can be developed, tested in clinical trials, licensed, and manufactured. Virally vectored vaccines, nucleic acid vaccines (including RNA vaccines and DNA vaccines), and recombinant protein expression platforms are the most prominent examples of such platform vaccines. In contrast to this, traditional approaches such as inactivated or attenuated vaccines or vaccines based on subunit proteins isolated from viruses require extensive optimisation for each new pathogen, leading to longer development timelines.
The application of platform vaccine approaches to COVID-19 demonstrates how this technology may speed up vaccine development. RNA-based approaches have been able to leverage insights from the previous optimisation of vaccine platforms for MERS-CoV to rapidly advance through preclinical development for tackling COVID-19 [72]. For instance, the U.S.-based company Moderna’s COVID-19 vaccine candidate entered phase I clinical trials within 10 weeks after publication of the SARS-CoV-2 sequence [3]. Similarly, the virally vectored ChAdOx1 vaccine developed by the University of Oxford and AstraZeneca has been able to quickly enter large-scale clinical trials enabled by existing safety data of previous use of this vector for the development of a MERS vaccine [4]. Despite requiring isolation of viral samples, more traditional vaccine approaches such as inactivated virus vaccines and protein subunit vaccines have also advanced to clinical testing at great speed. Previous work on MERS-CoV has enabled the relatively rapid development of beta-propiolactone inactivated SARS-CoV-2 vaccine candidates [5].
Table 1 provides an overview of platform vaccine approaches and more traditional approaches, including an evaluation of their potential to tackle emerging pandemics and potential for dual-use. The utility of a given vaccine approach for tackling emerging diseases is not only dependent on the rapid development of an efficacious vaccine, but furthermore also depends on criteria such as production cost, thermostability, and cold chain requirement, and the achievement of a single-dose injection regimen [6].
Table 1.
Application, utility, and dual-use potential of different vaccine approaches. Presentation of leading COVID-19 vaccine candidates based on the WHO Draft landscape of COVID-19 candidate vaccines as of 9 Jan 2021[30].
| Approach | Description and COVID-19 use | Utility for tackling emerging diseases | Estimated dual-use potential |
|---|---|---|---|
| Platform approaches | |||
| Virally vectored vaccines | Antigen is inserted into genome of well-defined, non-pathogenic, usually replication-defective viral vectors, such as adenovirus (Ad), inducing expression in vaccine recipient’s cells [7] Manufactured in mammalian cell culture.
|
High:
|
Relatively high:
|
| mRNA vaccines | Antigen is encoded as messenger RNA, synthesised in cell-free in vitro transcription process, and formulated, e.g. with lipid nanoparticle (LNP) coat, to prevent degradation and improve cell entry. mRNA induces expression of the antigen in vaccine recipient’s cells for induction of an immune response.[7]
|
High:
|
Low:
|
| Self-amplifying RNA vaccines | Similar to mRNA vaccines, RNA replicon based on the alphavirus genome mediates amplification of the antigen-encoding mRNA within the recipient’s cells.
|
Medium-High:
|
Low(-Medium):
|
| DNA vaccines | Antigen is encoded as DNA plasmid, synthesised in cell-free in vitro transcription process, and injected to induce expression of the antigen in vaccine recipient’s cells. Cell uptake may be improved by electroporation.[7]
|
Medium:
|
Low:
|
| Recombinant protein expression platform | Antigen gene inserted into the expression system for protein production, e.g. insect baculovirus-mediated insertion into insect cell lines, modified to exhibit human glycosylation patterns; resulting proteins may be administered as virus-like particles (VLPs) or with adjuvant.[18]
|
Medium-High:
|
Low-Medium:
|
| Traditional approaches | |||
| Inactivated virus vaccines | Exposure of virulent virus to heat or chemical agents, for example, formalin or β-propiolactone, to “inactivate” the virus, to prevent infectivity while retaining immunogenicity.[5], [22] Virus for vaccination may be grown in embryonated chicken eggs or cell lines.[23]
|
Medium:
|
Medium:
|
| Live attenuated virus vaccine (empirical attenuation through serial passaging) | Classic empirical approach: Serial passaging of virus, e.g. through cells, for loss of virulence. Virus for vaccination may be grown in embryonated chicken eggs or cell lines.[23]
|
Low:
|
Low-Medium:
|
| Live attenuated virus vaccines (rational attenuation) | Rational attenuation approach: Based on viral engineering, e.g. synonymous codon replacement[25]
|
|
Medium-High:
|
3. Past funding and application of different platform vaccine approaches
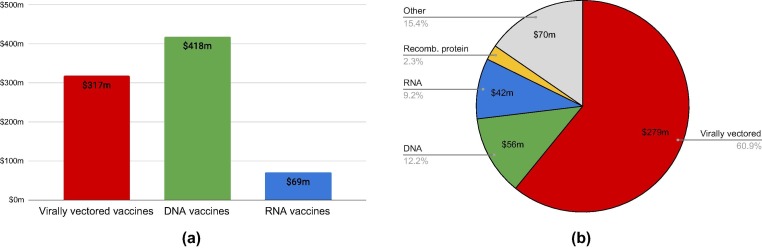
To date, most publicly funded research was directed towards virally vectored and DNA vaccine platforms, while work on RNA vaccines has received less public funding. Between 2009 and 2019, a relatively large amount of U.S. federal funding for research on vaccine platforms was awarded to virally vectored vaccines and DNA vaccines (Fig. 1 a). Ongoing challenges around nucleic acid stability and delivery have kept DNA vaccines from living up to the multiple decade-old hope of this technology as a fast-response platform.
Fig. 1.
Past funding of platform vaccine research. a) U.S. National Institutes of Health (NIH) funding between 2009 and 2019 as reported on federalreporter.nih.gov. b) CEPI Vaccine Funding until Nov 2019 as described in Bernasconi et al 2020 [26].
The Coalition for Epidemic Preparedness Innovations (CEPI), founded in 2017 in the aftermath of the Ebola epidemic, is a major international, non-governmental grantmaker for vaccine research. As of November 2019, CEPI had awarded $279m to viral vector-based approaches and $56m to DNA vaccines (Fig. 1b). This distribution reflects the high interest in the viral vector-based platform approach for tackling neglected pathogens after the 2014–2016 Ebola epidemic in West Africa, which led to the rapid development of two efficacious virally vectored vaccines which have now been licensed [28], [29]. On the other hand, CEPI funding allocated specifically for the advancement of rapid-response platforms went towards RNA ($42.4m) and recombinant protein platform advancement ($10.6m) [27].
The relative proportion of the number of COVID-19 candidate vaccines in clinical trials demonstrates the popularity of different vaccine approaches for tackling the ongoing pandemic. As of 9 January 20211, out of 63 candidate vaccines in clinical trials, seventeen are viral vector-based, eight are DNA-based, and seven are RNA-based [30]. While twenty-one vaccine candidates are protein-based, only a few vaccines such as the candidates by Novavax and Kentucky Bioprocessing leverage novel platform-like expression systems rather than traditional mammalian expression systems. Nine inactivated virus vaccines and only a single live attenuated vaccine are in clinical trials. Frontrunner candidates for each of the different approaches are listed in Table 1. Overall, developers are employing a balanced portfolio of vaccine approaches for tackling the coronavirus pandemic. It is notable that next to virally vectored vaccines, multiple RNA vaccines are among the frontrunner candidates despite receiving less public funding than virally vectored vaccines in the past. According to recent phase 3 trial data, both Pfizer-BioNTech’s and Moderna’s mRNA vaccine candidates reduce the chance of symptomatic COVID-19 infection by around 95% [31], [32]. The viral vector-based ChAdOx1 vaccine reports an efficacy of around 70% [33]. Evaluation of the data from these and future phase 3 trials will determine which approaches will succeed in inducing protective responses against COVID-19, and subsequent deployment of these different vaccines will shed further light on the manufacturing characteristics of the different platform approaches.
4. Dual-use potential of platform vaccine technologies
There are three dimensions of biological dual-use risks: 1) misuse of ostensibly civilian facilities, 2) misuse of equipment and agents, and 3) generation and dissemination of scientific knowledge with risk of misuse [34]. Historically, the dual-use potential of vaccines was tied to the dual-use potential of vaccine production facilities and equipment for production of threat agents for traditional inactivated or attenuated vaccines. This dual-use potential is illustrated by the historical example of Iraq repurposing fermenters from a veterinary vaccine production plant for botulinum toxin production in 1988.[34], [35] The Soviet Union also planned to use ostensibly civilian vaccine production facilities to produce biological warfare agents in the event of a war with the United States [36].
While over recent decades dual-use risks have generally been considered a niche concern in vaccine development, the focus of dual-use risk from vaccine research has shifted from the misuse of civilian facilities to the generation and dissemination of scientific insights with dual-use potential. One prominent example of vaccine research leading to the dissemination of dual-use insights was the synthesis of horsepox virus and its publication in 2018. This research was directed at the development of a better smallpox vaccine. However, as this was the first account of the synthesis of an orthopoxvirus, a virus in the same family and closely related to variola virus, the agent that causes smallpox, this research has lowered the barrier for individuals seeking to acquire the variola virus which has been eradicated from nature and is only known to exist at two secure repositories in the United States and Russia [37].
The increased concern around dual-use knowledge is driven by the fact that rapid advances in molecular biology, including DNA synthesis and gene-editing, continue to lower the barrier for viral engineering and synthesis [34]. Therefore, the risk from dissemination of dual-use insights on the modification of viral properties like transmissibility and immune evasion is amplified, as such insights might allow actors to create transmissible agents posing global or even existential threats. In comparison, the ability to produce large batches of toxins and non-transmissible viruses could be used to create harm at large but limited scale. While large-scale production has long been considered a key barrier to the weaponisation of existing viruses on the basis of knowledge on historical biological weapons programs, the potential for misuse of production technology would be limited to the proprietor of the facility in question [38], [39]. In contrast, once released into the public, scientific knowledge may inform malicious actors around the globe.
Dual-use aspects of research on novel platform vaccine technologies, which leverage recent advances in viral and nucleic acid synthesis and modification, have not been evaluated sufficiently to date. Hence, we here assess and compare the dual-use risk of different novel and traditional vaccine approaches (Table 1) and propose strategies to minimise biosecurity risks posed by vaccine platform technologies.
Compared to other vaccine approaches, we identify research on viral vector-based platforms as exhibiting relatively high dual-use potential. This is in particular due to the high concern we associate with the generation of knowledge on the modification of viral properties. As we discuss in more detail below, research on viral vector-based vaccines involves viral engineering which may inform modification of concerning agents such as variola virus and creates incentives for the generation of potentially concerning insights on conferring viral immune evasion. We classify research on rational attenuation approaches, which aims to create live attenuated viral vaccines with better genomic stability, as exhibiting medium to high dual-use potential. Research on synonymous codon replacement may not only lead to potential insights on enhancement of virulence, but importantly generates the synthetic biology tools necessary to conduct such enhancement, for example the ability to introduce many mutations simultaneously with high precision [26].
As discussed above, the traditional inactivated vaccine approach creates the facilities and uniquely advances capabilities for culturing large batches of virus. As this ability is mostly limited to the proprietor of the facility in question and minimal knowledge on the modification of viruses is gained, we classify this approach as exhibiting medium dual-use potential. Similarly, traditional empirically attenuated vaccines may also require large-scale culture of virus, however it does not involve culture of wild-type virus from the outset. Hence, empirically attenuated vaccines exhibit low to medium dual-use potential. Given the non-replicating nature of toxins and interest in protein production for a wide range of uses, we classify recombinant protein platforms, which may be leveraged for toxic protein production, as exhibiting low to medium dual-use potential.
Notably, we find that the promising nucleic acid-based vaccine platforms exhibit little obvious dual-use potential (Table 1). RNA and DNA vaccines commonly leverage nucleic acids encapsulated in lipid nanoparticles to induce expression of the target antigen in the vaccine recipient’s cells, removing the need of engineering viruses to fulfil the same function. However, such approaches might still increase biosecurity risk through mechanisms not yet identified, for instance through the advancement of enabling technologies such as bench-top DNA synthesis. Therefore, while some research into virally vectored vaccines creates dual-use insights with potential to lead to existential threats which exceeds the threat of dual-use insights from conventional vaccine approaches, novel nucleic acid vaccine platforms may exhibit even less dual-use potential than conventional approaches by removing the need for direct work on or production of viruses.
In the following section, we examine the dual-use potential of virally vectored vaccines to identify which particular lines of research raise concerns and how these concerns may be mitigated. Virally vectored vaccines may increase biosecurity risk through two routes: (1) generating particular insights with more direct dual-use potential and (2) spreading viral engineering capabilities which could enable misuse. Assessing this danger means looking at the incremental risk of further research into virally vectored vaccines, but also at the pre-existing margin of similar work and research. If vaccine platforms are a small part of all viral engineering or all immune-evasive work, the additional risk is commensurately less.
5. Dual-use potential of specific insights: Evading pre-existing immunity
The foremost source of biosecurity risk from research on virally vectored vaccines is the creation of insights and knowledge with dual-use potential. For instance, work on virally vectored vaccines may lead to insights into the evasion of pre-existing anti-vector immunity. Such knowledge may be leveraged to engineer pathogens to evade pre-existing, potentially vaccine-induced, immunity.
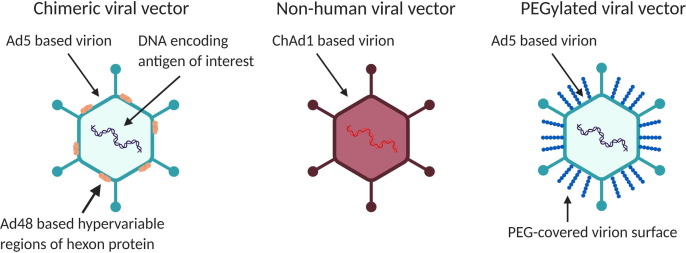
Pre-existing anti-vector immunity is one of the major limitations of viral vector-based vaccines and therapeutics. Pre-existing vector-specific antibodies may neutralise viral vectors before their entry of host cells and hence may prevent the induction of immune responses against the encoded antigens [12], [13]. Such pre-existing immunity may be induced by natural infection or by previous administration of a vector-based vaccine or therapeutic, therefore limiting the reusability of a given vector-based platform [14]. Accordingly, there exists a strong incentive to overcome this limitation, and many different approaches to circumventing pre-existing anti-vector immunity have been explored [40]. For instance, while adenovirus serotype 5 (Ad5) features desirable properties as a vaccine vector with regard to immunogenicity, there is a high prevalence of pre-existing anti-Ad5 immunity in the human population [41]. In order to circumvent this immunity, chimeric vectors have been created where hypervariable regions of Ad5 hexon protein are replaced with those from a less seroprevalent adenovirus serotype such as Ad48 (Fig. 2 ) [15]. One could readily imagine how experience with and knowledge of the creation of chimeric vectors to evade pre-existing anti-vector immunity could be leveraged to create pathogens able to evade vaccine-induced immunity. The example of the creation of chimeric Ad5 may only be of limited concern from a dual-use perspective as adenovirus is a relatively less concerning pathogen and this approach of creating chimeric vectors is relatively pathogen specific. However, similar modifications of attenuated versions of highly pathogenic viruses may be readily translatable to modifying pathogenic versions of these viruses for evasion of pre-existing or vaccine-induced immunity. For instance, Miest et al created a recombinant oncolytic measles virus capable of evading pre-existing neutralising antibodies through exchange of envelope glycoproteins with those of canine distemper virus [42]. Similar insights into the creation of new “serotypes” of pathogenic viruses may emerge from research on overcoming anti-vector immunity in the context of virally vectored vaccines. Furthermore, efforts to overcome pre-existing anti-vector immunity may lead to more universal insights into strategies for evading pre-existing immunity that are applicable and translatable to a wide range of pathogens.
Fig. 2.
Approaches for circumventing anti-vector immunity. Different strategies have been explored to evade pre-existing anti-vector immunity: The creation of chimeric viral vectors in which hypervariable regions of the Ad5 hexon protein are replaced by those of less seroprevalent adenovirus serotypes such as Ad48; the use of non-human viral vectors such as the chimpanzee adenovirus ChAd1; chemical surface modification of vectors with synthetic polymers such as polyethylene glycol (PEG).
There are certain strategies for circumventing anti-vector immunity which may exhibit less dual-use potential than genetic engineering of vectors. One such strategy is the use of non-human viral vectors, as is the case in the chimpanzee adenovirus-vectored ChAdOx1 COVID-19 vaccine or the rVSV-based vaccine for Ebola. Another strategy with potentially less dual-use potential than genetic approaches might be non-genetic virion modifications, such as covering the surface with synthetic polymers (e.g. through PEGylation) (Fig. 2). While such non-genetic approaches could be used to formulate a broad array of biological agents for immune evasion, these modifications are not passed onto viral progeny and hence do not increase the risk for the largest-scale catastrophic threats posed by transmissible, genetically engineered viruses [33], [43], [44].
In addition to work on virally vectored vaccines for infectious diseases, other applications of viral vectors, such as gene therapy or cancer vaccines, will drive research into overcoming the limitation of anti-vector immunity [45], [46]. Non-genetic methods might be a promising low dual-use potential solution to the problem of anti-vector immunity in cancer vaccines and gene therapy. However, stronger incentives for rapidly scalable and low-cost manufacturing for infectious disease vaccines may favour research into genetic methods that require fewer manufacturing steps but pose greater dual-use concern. Similarly, the creation of recombinant measles virus able to evade measles immunity in humans indicates that genetic approaches for evading anti-viral immunity that are passed onto viral progeny might also be investigated as part of oncolytic virus research [42].
6. Risk from spreading viral engineering capabilities
Another source of biosecurity risk from biotechnological research involving viral engineering is the inherent dual-use potential of the experience, skills, and equipment required for and developed through such work. Creating viral vector-based vaccines involves the creation and amplification of recombinant DNA plasmids for transfection into cells and viral rescue [47], [48], [49]. However, while these skills exhibit dual-use potential, they are common molecular biology capabilities. Indeed, there is a large overlap between work on viral vectors for infectious disease vaccines and work on viral vectors for gene therapy and cancer vaccines, as well as work on oncolytic viruses [45], [46]. Furthermore, similar capabilities may be advanced through a range of other synthetic biology research.
The marginal effect of viral vector-based vaccine research on the total number of individuals capable of engineering viral pathogens depends on the relative amount of research on virally vectored vaccines compared to other activities which increase access to similar capabilities. The fraction of publications on virally vectored vaccines out of publications on all applications of viral vectors and oncolytic virus research may be a good indicator of the marginal contribution of the work on virally vectored vaccines to overall viral engineering capability. Based on a search of the Scopus database, the 716 publications on virally vectored vaccines for infectious disease make up 10% of the 7007 publications on all applications of viral vectors and oncolytic viruses since 2015. The fact that only 10% of research on viral vectors is associated with virally vectored vaccines for infectious disease demonstrates that work on vaccines is not a major driver for spreading related technical capabilities.
While on the margin most work on viral vector-based vaccines won’t be associated with the spread of engineering capabilities with significant dual-use risk, one exception to this may be cases where research on vaccines based on particular vectors may inform work on particularly concerning pathogens. In particular, viral family-specific approaches such as the ability to rescue virus from plasmid-transfected cells, otherwise a potential bottleneck for actors with nefarious intent, may be lowered through work on less concerning, but related viruses. For instance, while vaccine vectors are commonly based on viruses with limited pathogenicity in humans like adenovirus, vaccinia virus has emerged as a popular vaccine platform due to its ability to stably incorporate large segments of foreign DNA [37], [38]. Given the high degree of genetic homology between orthopoxviruses, the Soviet biological weapons program conducted experiments inserting foreign DNA into vaccinia and other orthopoxviruses as a prelude to conducting such work with variola [50], [51]. Similarly, the development of techniques to synthesise horsepox virus and vaccinia virus could also be used to synthesise variola virus [52], [53]. Therefore, there may be particular dual-use risk associated with vaccine efforts on vectors related to pathogens that pose biosecurity risks. Despite this, it is worth noting that the current U.S. dual-use research of concern policy only covers a list of 15 pathogens which means that identical types of experiments on closely related pathogens, even if these might be directly applicable to the covered pathogens, are not subject to oversight and review [54]. For instance, while variola virus is included on this list, research on related mousepox, horsepox, and vaccinia viruses is currently not covered despite the potential for translatable insights.
7. Dual-use assessment of virally vectored vaccines: Limitations and takeaways
Overall, research on virally vectored vaccines seems to feature some dual-use potential, with certain approaches – such as efforts to evade antiviral immunity – raising particularly salient biosecurity concerns. Aside from insights into strategies for immune evasion, there might also be other insights of dual-use concern associated with further work on virally vectored vaccines which have not been identified here. Furthermore, our keyword-based search of the literature for the fractions of viral vector research for different applications might under- or overestimate the role of vaccines in driving viral engineering capabilities. In particular, this search might not capture the role and effect of privately funded research, some of which may be proprietary and therefore not part of the public literature. The synthesis of horsepox virus, for example, was funded by an American biotech company [55]. The proportion of dual-use research that is privately funded is likely to grow as companies account for a growing share of research in the life sciences. Since 2013, federal funding has accounted for less than half of national spending on scientific research in the United States [56].
Risk from dual-use insights is not just limited to work on virally vectored vaccines for infectious disease but is also associated with other applications of viral vectors and work on oncolytic viruses. Therefore, the additional risk from increased work on virally vectored vaccines might be commensurately less in the presence of continuing advances in these other areas. At the same time, this finding also demonstrates that individuals working in research areas such as gene therapy and oncolytic cancer therapy, which have not been traditionally associated with such biosecurity risks, should be aware of the dual-use potential of their research and accordingly make decisions on project choice and execution. Indeed, we do not lay claim to how the overall dual-use risk from vaccine research compares to that of other fields in the life sciences. Rather, we believe it is incumbent on each respective field to identify high risk research in order to prioritise the preferential pursuit of safer alternatives.
8. Managing biosecurity risks of platform vaccine advancement
Dual-use assessments need to weigh the risks associated with a given technology with its benefits. Platform vaccine approaches are hugely valuable for addressing emerging diseases as demonstrated by their application to tackling COVID-19 and Ebola. Novel platform vaccine approaches such as RNA vaccines will likely see a large increase in funding over the coming years having demonstrated promising properties such as fast development timelines and efficacy when tackling COVID-19 [16], [57], [58]. Depending on the situation and pathogen in question, different platforms may have more suitable properties than others, including with regard to immunogenicity and efficacy as well as practical considerations such as speed of development, cold chain requirements, number of injections required, and cost, speed, and scalability of manufacturing.
The unique upsides of virally vectored vaccines need to be considered when assessing and managing associated dual-use concerns. Some of the most promising and most advanced COVID-19 vaccines are based on viral vectors (Table 1) [30]. Importantly, virally vectored vaccines demonstrated fast development timelines and efficacy in the Ebola epidemics in West Africa and the Democratic Republic of Congo (DRC) [10], [28]. Merck’s VSV-based Ebola vaccine showed 97.5% efficacy in the DRC Ebola outbreak and was administered to over 303,000 individuals, playing a key role in the containment of the outbreak [28], [59], [60]. Additionally, viral vector-based vaccines are a technology that is relatively accessible to research groups and commercial developers around the globe, as demonstrated by 17 out of 63 COVID-19 vaccine candidates in clinical trials being based on this approach [30]. Importantly, virally vectored vaccines induce robust T cell responses which might be needed for inducing effective immunity against enveloped viruses with complex pathogenesis like poxviruses or filoviruses [9]. Among the available vaccine platforms, viral vector-based platforms may be the most promising for inducing protective immune responses with a single injection [10], [11]. Additionally, virally vectored vaccines do not require subzero transport and storage, which currently seems to be a limitation of RNA-based vaccines, as demonstrated by the COVID-19 vaccine efforts [19]. Hence, virally vectored vaccines are an important component of our current portfolio of vaccine approaches.
Nevertheless, risks associated with the development of virally vectored vaccines should be reduced where possible. Without question, the upsides of applying existing technologies, including vector-based vaccines, to countering the ongoing COVID-19 pandemic outweigh the current associated dual-use concerns. However, managing dual-use risk from research on this platform technology should be a consideration when decisions are made on how to advance the vaccine technology landscape over the coming years. To minimise biosecurity risks, funders and researchers should consider the dual-use potential of different approaches to advance viral vector-based platforms. Work should be prioritized for vectors which exhibit relatively low dual-use potential of associated technical capabilities and insights. Research on vectors which are homologous or related to potential biological threat agents such as variola or influenza virus should be minimized. Additionally, vectors based on non-human viruses with low pathogenicity in humans should be pursued preferentially.
To overcome specific technical challenges such as anti-vector immunity, finding low dual-use solutions should be prioritised. For instance, this might include choosing non-genetic over genetic methods for circumventing anti-vector immunity. Such non-genetic methods may include the expansion of the vector portfolio to viruses with low seroprevalence such as non-human adenoviruses or improving strategies for synthetic surface modifications which cannot be passed onto viral progeny. Additionally, prime-boost regimens with heterologous vectors or exploring different routes of administration may be low-risk dual-use strategies to reduce the effect of pre-existing anti-vector immunity on immunogenicity [61]. The success of the rVSV-based Ebola vaccine and the ChAdOx1 vaccine for COVID-19 demonstrate that non-human viruses can exhibit excellent properties as vectors for safe and efficacious vaccines [10], [33].
Additionally, preferential investment into promising low dual-use platform vaccine approaches such as RNA vaccines might reduce dual-use risk from platform vaccines in the long-run. There are two indications that the potential of RNA-based platforms to tackle most emerging pandemics may eventually exceed that of viral vector-based ones.
First, preclinical and early-stage clinical data on SARS-CoV-2 vaccine candidates suggests that mRNA-based vaccines such as the one developed by Moderna induce stronger neutralising antibody responses than those induced by the virally vectored ChAdOx1 vaccine [33], [62]. Indeed, early results from phase 3 clinical trials showed that two mRNA-based vaccines showed an efficacy around 95%, while data on the ChAdOx1 viral vector-based vaccine suggests that efficacy of this vaccine may only be around 70% [16], [17], [63]. Specific antibody responses are the critical correlate of protection for most viruses and hence the potential for RNA-based platforms to tackle most emerging viruses with pandemic potential may exceed that of viral vector-based ones [64].
Second, current practical limitations around the widespread manufacturing and deployment of RNA-based vaccines will likely be solved in the wake of the COVID-19 pandemic. Facilities and experience with large-scale manufacturing of RNA vaccines will be able to be leveraged for future iterations of the same platforms and the development of thermostable RNA vaccines seems within reach [6], [65], [66]. While viral vector-based platforms are limited in reusability by anti-vector immunity after widespread deployment, insights from development and deployment of RNA-based platforms for COVID-19 will be able to directly inform the application of the very same platforms to future targets.
The development of efficacious RNA vaccines that could be deployed quickly and widely for most pathogens would reduce the need to develop and deploy virally vectored vaccines. Not only would this prevent the buildup of anti-vector immunity to conserve the immunogenicity of virally vectored vaccines for when their unique immunogenic properties are needed, but this would also prevent such anti-vector immunity from interfering with the potency of cancer immunotherapy and gene therapy based on similar vectors. Importantly, conserving the deployment of virally vectored vaccines for when their unique properties are needed, might reduce the incentive to conduct dual-use research on overcoming anti-vector immunity.
9. Lessons for the governance of biotechnology
The Nuclear Threat Initiative has identified the need to “reduce biotechnology risks and implement global norms for life science research” as a key strategy to reducing the occurrence of global catastrophic biological risks, large-scale biological events with the potential to destabilise society [67]. The finding that certain research on viral vector-based vaccines may exhibit dual-use potential demonstrates that scientists outside of the pathogen research community need to become more aware of dual-use risks associated with their work and research-funding bodies need to prioritise the evaluation and reduction of biosecurity risks across a broader swathe of the life sciences research enterprise. To this end, education programs on such risks need to become part of university life science teaching. In the U.S., the National Science Advisory Board for Biosecurity should take the lead on creating guidance on this. Both public and private research-funding bodies should follow the example of the Wellcome Trust, the UK Biotechnological and Biological Research Council, and the UK Medical Research Council to require grant applicants to evaluate risks of misuse in applications and to notify funders of any unanticipated changes in the dual-use risk status of their research [68]. Furthermore, there is a need to expand the coverage of the U.S. policy on oversight of dual-use research of concern to pathogens closely related to those already listed given the transferability of knowledge on the engineering and synthesis of these agents. Indeed, given the transnational nature of modern life sciences research, global norms for responsible dual-use research should be developed by respected international scientific authorities such as the InterAcademy Partnership and World Health Organization. In addition, as company-based research plays a key role in advancing biotechnology, not only academic but also proprietary research needs to be the subject of future biosecurity oversight mechanisms. Indeed, the private sector could take a leading role in building robust governance for biotechnology to establish a secure foundation for their work and to retain public trust in their practices. Such practices could follow the example of the industry-led effort to universalise the screening of DNA synthesis orders through formation of the International Gene Synthesis Consortium [69].
When weighing biosecurity risks against the humanitarian benefits of research, the importance of a given scientific question may be used as a benchmark to determine the acceptable level of dual-use risk from associated experiments [70]. For instance, platform vaccines and specifically virally vectored vaccines are an important pandemic countermeasure with the potential to significantly reduce the harm caused by emerging pathogens. These upsides likely outweigh some of the associated dual-use concerns. Nevertheless, even if the benefits of a given experiment or technology are substantial, the approach with the least associated dual-use risk should be pursued to achieve a given goal. This is reflected in the U.S. Health and Human Services guide for funding of research on enhanced potential pandemic pathogens [71]. This consideration should also be applied to research which has not traditionally been associated with dual-use risks. As demonstrated, such research includes work on viral vectors and oncolytic viruses. Aside from new guidelines being needed to guide funding and work in these fields, grantmakers and researchers in these areas need to become aware of the biosecurity risks associated with their work to be able to evaluate if and how a given line of research should be conducted to minimise these risks.
10. Conclusions
Platform-based vaccines will likely play a key role in containing the COVID-19 pandemic. Further advancement of fast-response platform vaccine technologies is needed to build capacity to swiftly tackle future novel pathogens [72]. At the same time, the advancement of biotechnology with dual-use potential may lead to increased risk for anthropogenic pandemics. Therefore, all stakeholders involved in biotechnology and life science research need to become aware of associated biosecurity risks and foster a culture of thorough evaluation and minimisation of such risks. This is especially important for researchers in areas which have not traditionally been associated with dual-use concerns, such as in vaccinology, gene therapy, and oncolytic virus immunotherapy. For instance, further research on virally vectored vaccines which may involve viral engineering to overcome anti-vector immunity may exhibit relatively high dual-use potential. Hence, research into non-genetic methods rather than genetic methods for evading pre-existing anti-vector immunity should be prioritised. While there is potential for not yet identified mechanisms of dual-use risk, approaches such as RNA and DNA vaccines seem to feature relatively little dual-use potential. Therefore, preferential investment into promising RNA-based approaches might reduce the total biosecurity risk of the vaccine technology portfolio and the incentive to develop high dual-use potential solutions to circumvent anti-vector immunity.
11. Methods
U.S. funding of platform vaccine approaches: federalreporter.nih.gov was searched for U.S. National Institutes of Health (NIH) and Congressionally Directed Medical Research Programs (CDMRP) funding reported between 2009 and 2019. Queries used: ‘“Viral vector” AND vaccine NOT cancer’; ‘“DNA vaccine” NOT cancer’; ‘“RNA vaccine” NOT cancer’. No search terms for capturing funding of recombinant protein expression platform-based vaccine research were identified.
Scopus database review: The search was conducted on 19 August 2020 and included papers published since 2015. 109 papers attributed to cancer vaccine research (based on the keywords ‘“virus vector” AND “cancer vaccine”’) were subtracted from the 825 publications on all research on virally vectored vaccines (keywords ‘“virus vector” AND vaccine’). There were 5,065 publications on viral vectors (keyword ‘“virus vector”’), the majority of which is work on viral vectors for gene therapy, and 1942 papers on oncolytic virus research (keyword ‘“oncolytic virus”’). The scopus database can be found at www.scopus.com.
12. Data statement
All utilised data is publicly available. Methods and references have been provided.
CRediT authorship contribution statement
Jonas B. Sandbrink: Conceptualization, Investigation, Writing - original draft. Gregory D. Koblentz: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank Kevin Esvelt, Andrew Snyder-Beattie, Jacob Swett, James Wagstaff, and Brian Wang for discussions leading up to the writing of the manuscript and Gregory Lewis and Joshua Monrad for comments on the manuscript. The majority of this work was produced while JBS was a Summer Research Fellow with the Future of Humanity Institute, University of Oxford. This article reflects the views only of the authors.
Fig. 2 was created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.02.023.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Bibliography
- 1.Millett P., Snyder-Beattie A. Human agency and global catastrophic biorisks. Health Secur. 2017;15:335–336. doi: 10.1089/hs.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adalja A.A., Watson M., Cicero A., Inglesby T.V. Vaccine Platforms: State of the Field and Looming Challenges. Johns Hopkins Center for Health Security; 2019.
- 3.Fuller D.H., Berglund P. Amplifying RNA vaccine development. N Engl J Med. 2020;382:2469–2471. doi: 10.1056/NEJMcibr2009737. [DOI] [PubMed] [Google Scholar]
- 4.Covid Vaccine Front-Runner Is Months Ahead of Her Competition. Bloomberg. 2020 https://www.bloomberg.com/news/features/2020-07-15/oxford-s-covid-19-vaccine-is-the-coronavirus-front-runner [Google Scholar]
- 5.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandbrink J.B., Shattock R.J. RNA vaccines: a suitable platform for tackling emerging pandemics? Front Immunol. 2020;11:608460. doi: 10.3389/fimmu.2020.608460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebastian S., Lambe T. Clinical advances in viral-vectored influenza vaccines. Vaccines. 2018;6(2):29. doi: 10.3390/vaccines6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham B.S., Sullivan N.J. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nat Immunol. 2018;19:20–28. doi: 10.1038/s41590-017-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M., et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) The Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed]
- 12.Pichla-Gollon S.L., Lin S.-W., Hensley S.E., Lasaro M.O., Herkenhoff-Haut L., Drinker M., et al. Effect of preexisting immunity on an adenovirus vaccine vector. In vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J Virol. 2009;83:5567–5573. doi: 10.1128/JVI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pine S.O., Kublin J.G., Hammer S.M., Borgerding J., Huang Y., Casimiro D.R., et al. Pre-existing adenovirus immunity modifies a complex mixed Th1 and Th2 cytokine response to an Ad5/HIV-1 vaccine candidate in humans. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0018526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxena M., Van T.T.H., Baird F.J., Coloe P.J., Smooker P.M. Pre-existing immunity against vaccine vectors – friend or foe? Microbiology. 2013;159:1–11. doi: 10.1099/mic.0.049601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts D.M., Nanda A., Havenga M.J.E., Abbink P., Lynch D.M., Ewald B.A., et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 16.Herper M, Branswell H. Moderna’s Covid-19 vaccine is strongly effective, early look at data show. STAT 2020. https://www.statnews.com/2020/11/16/modernas-covid-19-vaccine-is-strongly-effective-early-look-at-data-show/ (accessed November 16, 2020).
- 17.Pfizer and BioNTech Covid-19 vaccine is 95% effective, full results show. STAT 2020. https://www.statnews.com/2020/11/18/pfizer-biontech-covid19-vaccine-fda-data/ (accessed November 23, 2020).
- 18.Kis Z, Shattock R, Shah N, Kontoravdi C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. vol. 14. Wiley-VCH Verlag; 2019. https://doi.org/10.1002/biot.201800376.
- 19.Will supply chain demands freeze Pfizer and BioNTech out of a big chunk of the Covid-19 market? Endpoints News n.d. https://endpts.com/will-supply-chain-demands-freeze-pfizer-and-biontech-out-of-a-big-chunk-of-the-covid-19-market/ (accessed August 31, 2020).
- 20.Vogel A.B., Lambert L., Kinnear E., Busse D., Erbar S., Reuter K.C., et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther. 2018;26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohsen M.O., Gomes A.C., Vogel M., Bachmann M.F. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines. 2018;6 doi: 10.3390/vaccines6030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders B., Koldijk M., Schuitemaker H. Inactivated viral vaccines. Vacc Anal Strateg Princ Control. 2014:45–80. doi: 10.1007/978-3-662-45024-6_2. [DOI] [Google Scholar]
- 23.Gallo-Ramírez L.E., Nikolay A., Genzel Y., Reichl U. Bioreactor concepts for cell culture-based viral vaccine production. Expert Rev Vaccines. 2015;14:1181–1195. doi: 10.1586/14760584.2015.1067144. [DOI] [PubMed] [Google Scholar]
- 24.Hanley K.A. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evolution. 2011;4:635–643. doi: 10.1007/s12052-011-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Nouën C., Collins P.L., Buchholz U.J. Attenuation of human respiratory viruses by synonymous genome recoding. Front Immunol. 2019;10:1250. doi: 10.3389/fimmu.2019.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Academies of Sciences, Engineering, and Medicine. Biodefense in the Age of Synthetic Biology. Washington, DC: The National Academies Press; 2018. [PubMed]
- 27.Bernasconi V., Kristiansen P.A., Whelan M., Román R.G., Bettis A., Yimer S.A., et al. Developing vaccines against epidemic-prone emerging infectious diseases. Bundesgesundheitsblatt. 2020;63:65–73. doi: 10.1007/s00103-019-03061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inungu J., Iheduru-Anderson K., Odio O.J. Recurrent ebolavirus disease in the democratic Republic of Congo: update and challenges. AIMS Public Health. 2019;6:502–513. doi: 10.3934/publichealth.2019.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staines R. J&J’s Ebola vaccine approved in EU 2020. https://pharmaphorum.com/news/jjs-ebola-vaccine-approved-in-eu/ (accessed November 20, 2020).
- 30.World Health Organisation. Draft landscape of COVID-19 candidate vaccines n.d. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed January 9, 2020).
- 31.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2020;0:null. https://doi.org/10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed]
- 32.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;0 doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atlas R.M., Dando M. The dual-use dilemma for the life sciences: perspectives, conundrums, and global solutions. Biosecurity Bioterrorism Biodefense Strategy Pract Sci. 2006;4:276–286. doi: 10.1089/bsp.2006.4.276. [DOI] [PubMed] [Google Scholar]
- 35.Central Intelligence Agency. Comprehensive Report of the Special Advisor to the DCI on Iraq’s WMD, with Addendums (Duefler Report). vol. 3. Washington DC: U.S. Government Office; 2005.
- 36.Leitenberg M., Zilinskas R.A. Harvard University Press; Cambridge: 2012. The Soviet Biological Weapons Program: A History. [Google Scholar]
- 37.Inglesby T. Horsepox and the need for a new norm, more transparency, and stronger oversight for experiments that pose pandemic risks. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Ouagrham-Gormley S. Barriers to Bioweapons: The Challenges of Expertise and Organization for Weapons Development. Ithaca: Cornell University Press; 2014.
- 39.Vogel K.M. Johns Hopkins University Press; Baltimore, MD: 2012. Phantom Menace or Looming Danger? A New Framework for Assessing Bioweapons Threats. [Google Scholar]
- 40.Thacker E.E., Timares L., Matthews Q.L. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev Vac. 2009;8:761–777. doi: 10.1586/erv.09.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu B., Zhou Y., Wu H., Wang Z., Zhan Y., Feng X., et al. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J Med Virol. 2012;84:1408–1414. doi: 10.1002/jmv.23325. [DOI] [PubMed] [Google Scholar]
- 42.Miest T.S., Yaiw K.-C., Frenzke M., Lampe J., Hudacek A.W., Springfeld C., et al. Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol Ther. 2011;19:1813–1820. doi: 10.1038/mt.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suder E., Furuyama W., Feldmann H., Marzi A., de Wit E. The vesicular stomatitis virus-based Ebola virus vaccine: from concept to clinical trials. Hum Vac Immunother. 2018;14:2107–2113. doi: 10.1080/21645515.2018.1473698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver E.A., Barry M.A. Effects of shielding adenoviral vectors with polyethylene glycol on vector-specific and vaccine-mediated immune responses. Hum Gene Ther. 2008;19:1369–1382. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sack B.K., Herzog R.W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr Opin Mol Ther. 2009;11:493–503. [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson M.S., Lemoine N.R., Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:e805629. doi: 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki M., Kondo S., Pei Z., Maekawa A., Saito I., Kanegae Y. Preferable sites and orientations of transgene inserted in the adenovirus vector genome: the E3 site may be unfavorable for transgene position. Gene Ther. 2015;22:421–429. doi: 10.1038/gt.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alharbi N.K. Poxviral promoters for improving the immunogenicity of MVA delivered vaccines. Hum Vaccines Immunother. 2018;15:203–209. doi: 10.1080/21645515.2018.1513439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatsis N., Ertl H.C.J. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilsdorf J.R., Zilinskas R.A. New considerations in infectious disease outbreaks: the threat of genetically modified microbes. Clin Infect Dis. 2005;40:1160–1165. doi: 10.1086/428843. [DOI] [PubMed] [Google Scholar]
- 51.Tucker J.B. Biological weapons in the former Soviet Union: an interview with Dr. Kenneth Alibek. Nonproliferation Rev. 1999;6:1–10. doi: 10.1080/10736709908436760. [DOI] [Google Scholar]
- 52.Koblentz G.D. The de novo synthesis of horsepox virus: implications for biosecurity and recommendations for preventing the reemergence of smallpox. Health Secur. 2017;15:620–628. doi: 10.1089/hs.2017.0061. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organisation. The Independent Advisory Group on Public Health Implications of Synthetic Biology Technology Related to Smallpox. A Report to the Director-General of the WHO. Geneva: 2015.
- 54.The United States Government. United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern. 2012.
- 55.Koblentz G.D. A critical analysis of the scientific and commercial rationales for the de novo synthesis of horsepox virus. MSphere. 2018;3 doi: 10.1128/mSphere.00040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mervis J. Data check: U.S. government share of basic research funding falls below 50% Science. 2017 doi: 10.1126/science.aal0890. [DOI] [PubMed] [Google Scholar]
- 57.Herper M. Covid-19 vaccine from Pfizer and BioNTech is strongly effective, data show. STAT 2020. https://www.statnews.com/2020/11/09/covid-19-vaccine-from-pfizer-and-biontech-is-strongly-effective-early-data-from-large-trial-indicate/ (accessed November 14, 2020).
- 58.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organisation. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. 2019.
- 60.Gavi, the Vaccine Alliance. First-of-its-kind vaccine agreement helps end Ebola outbreak in eastern DRC 2020. https://www.gavi.org/news/media-room/first-its-kind-vaccine-agreement-helps-end-ebola-outbreak-eastern-drc (accessed November 20, 2020).
- 61.Fausther-Bovendo H., Kobinger G.P. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccines Immunother. 2014;10:2875–2884. doi: 10.4161/hv.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA Vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/nejmoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirka D., Lawless J. 3rd major COVID-19 vaccine shown to be effective and cheaper. AP NEWS. 2020 https://apnews.com/article/oxford-coronavirus-pandemic-c99d26eb2946f6fde45a1edc002ff028 [Google Scholar]
- 64.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stitz L., Vogel A., Schnee M., Voss D., Rauch S., Mutzke T., et al. A thermostable messenger RNA based vaccine against rabies. PLoS Negl Trop Dis. 2017;11:e0006108. doi: 10.1371/journal.pntd.0006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang N.-N., Li X.-F., Deng Y.-Q., Zhao H., Huang Y.-J., Yang G., et al. A Thermostable mRNA vaccine against COVID-19. Cell. 2020;182(1271–1283):e16. doi: 10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cameron B., Yassif J., Jordan J., Eckles J. Preventing global catastrophic biological risks – lessons and recommendations from a Tabletop exercise held at the 2020 Munich security conference. Nuclear Threat Initiative. 2020 [Google Scholar]
- 68.Biotechnology and Biological Sciences Research Council, Medical Research Council and The Wellcome Trust. Managing Risks of Misuse Associated with Grant Funding Activities: A joint Biotechnology and Biological Sciences Research Council, Medical Research Council and Wellcome Trust Policy Statement. 2005.
- 69.Diggans J., Leproust E. Next steps for access to safe, secure DNA synthesis. Front Bioeng Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imperiale M.J., Casadevall A. A new approach to evaluating the risk-benefit equation for dual-use and gain-of-function research of concern. Front Bioeng Biotechnol. 2018;6 doi: 10.3389/fbioe.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.U.S. Department of Health and Human Services. Framework for Guiding Funding Decisions about Proposed Research Involving Enhanced Potential Pandemic Pathogens. 2017.
- 72.Monrad J.T., Sandbrink J.B., Cherian N.G. Promoting versatile vaccine development for emerging pandemics. npj Vaccines. 2021;6 doi: 10.1038/s41541-021-00290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.