Abstract
Preclinical opioid research using animal models not only provides mechanistic insights into the modulation of opioid analgesia and its associated side effects, but also validates drug candidates for improved treatment options for opioid use disorder. Non-human primates (NHPs) have served as a surrogate species for humans in opioid research for more than five decades. The translational value of NHP models is supported by the documented species differences between rodents and primates regarding their behavioral and physiological responses to opioid-related ligands and that NHP studies have provided more concordant results with human studies. This review highlights the utilization of NHP models in five aspects of opioid research, i.e., analgesia, abuse liability, respiratory depression, physical dependence, and pruritus. Recent NHP studies have found that (1) mixed mu opioid and nociceptin/orphanin FQ peptide receptor partial agonists appear to be safe, non-addictive analgesics and (2) mu opioid receptor- and mixed opioid receptor subtype-based medications remain the only two classes of drugs that are effective in alleviating opioid-induced adverse effects. Given the recent advances in pharmaceutical sciences and discoveries of novel targets, NHP studies are posed to identify the translational gap and validate therapeutic targets for the treatment of opioid use disorder. Pharmacological studies using NHPs along with multiple outcome measures (e.g., behavior, physiologic function, and neuroimaging) will continue to facilitate the research and development of improved medications to curb the opioid epidemic.
Keywords: Analgesia, Drug abuse, Itch, Macaque, Nociceptin/Orphanin FQ, Opioid receptor, Physical dependence, Respiratory depression, Spinal cord
1. Introduction
Recent marked increase in misuse and abuse of prescription and illicit opioids and the epidemic of opioid overdose mortality have unprecedentedly affected the United States (Brady et al., 2016; Volkow and Blanco, 2020). Although mu opioid peptide (MOP) receptor agonists remain the most widely used analgesics, their adverse effects such as abuse liability and respiratory arrest have contributed to escalating medical and economic burdens in the global community (Degenhardt et al., 2014; Rudd et al., 2016). Several scientific strategies have been initiated to develop safe and effective medications for pain and opioid addiction, indicating an urgent and unmet need for the treatment of opioid use disorder (Volkow and Blanco, 2020; Volkow and Collins, 2017).
Based on the International Union of Basic and Clinical Pharmacology Nomenclature Committee, there are four opioid receptor subtypes, i.e., MOP, delta opioid peptide (DOP), kappa opioid peptide (KOP), and nociceptin/orphanin FQ peptide (NOP) receptors, in the opioid receptor family (Cox et al., 2015). Although the NOP receptor is considered a subcategory of the opioid receptor family due to its atypical low affinity for the classical endogenous opioid peptides and insensitivity to naloxone antagonism, all four receptors share similar signal transduction pathways. These four receptors are coupled to pertussis toxin-sensitive Gi/o proteins which inhibit adenylate cyclase and modulate the conductance of voltage-gated calcium channels and inward rectifying potassium channels (Al-Hasani and Bruchas, 2011). However, these receptor subtypes have different anatomical distributions in the central nervous system. Other than shared therapeutic benefit analgesia, they exhibit distinct functional roles in regulating physiological functions and mood (Gavioli et al., 2019; Lutz and Kieffer, 2013; Mansour et al., 1988; Tejeda and Bonci, 2019).
This review highlights the functional profiles of opioid receptor-related ligands in non-human primates (NHPs). In specific, we discuss species differences in pharmacological profiles of opioid-related ligands between rodents and NHPs; and we summarize how NHP models are used to evaluate novel experimental compounds’ functional profiles and efficacies to modulate MOP receptor agonist-associated adverse effects including abuse potential, respiratory depression, physical dependence, and pruritus.
2. Species differences between rodents and primates
NHPs, such as rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fascicularis), vervet monkeys (Chlorocebus aethiops sabaeus), marmosets (Callithrix jacchus), and squirrel monkeys (Saimiri sciureus), are used as essential animal models in biomedical research due to their close genetic, physiological and behavioral similarities to humans when compared with other commonly used rodent models (Phillips et al., 2014). In particular, rhesus macaques play a critical role in modeling human diseases and biological responses that are not well simulated in rodents (Liao and Zhang, 2008; Seok et al., 2013). First whole-genome analysis of rhesus macaques revealed fundamental genetic similarities to the human genome (Rhesus Macaque Genome et al., 2007). Recently updated macaque reference genome assembly and annotation will advance the biomedical utility of rhesus macaques and facilitate the development of new genetic NHP models of human diseases (Warren et al., 2020). NHP brain and cellular imaging techniques further promote exciting opportunities to compare brain structure and neuro-connectivity and to study the neurobiological influence of chronic drug use for the translational impact (Izpisua Belmonte et al., 2015; Macknik et al., 2019). For instance, magnetic resonance imaging-based methodologies demonstrate that a large number of human and macaque striatal voxels were not matched to any mouse cortico-striatal circuit (e.g., mouse->human: 85% unassigned) (Balsters et al., 2020). In addition, positron emission tomography (PET) neuroimaging in NHPs defines in vivo biodistribution and pharmacokinetics of abused drugs and drug interactions. The longevity and complex social behaviors of NHPs and their similar drug metabolism to humans make PET imaging a powerful tool to conduct longitudinal translational studies in drug abuse research (Banks et al., 2017; Howell and Murnane, 2011). Furthermore, due to the similar functions of the hypothalamic-pituitary axis between macaques and humans, the effects of opioid use and stress on neuroendocrine functions could be modeled in NHPs (Fountas et al., 2018; Meyer and Hamel, 2014). For example, KOP receptor agonists increased the levels of prolactin, adrenocorticotropic hormone and cortisol in both NHPs and humans (Butelman et al., 2004a; Ko and Husbands, 2020; Pascoe et al., 2008; Ur et al., 1997).
Evidently, species differences contributes to different results and interpretations regarding the receptor functions and drug effects. For instance, functional outcomes of drugs targeting serotonin system and neurobiology of serotonin receptors are markedly different between rats and NHPs (Li et al., 2010). There is now an extensive body of literature documenting that neuroanatomical, neurochemical, and neuropharmacological aspects of opioid and non-opioid receptors are comparable between humans and NHPs (Bianchi et al., 2012; Hawkinson et al., 2007; Phillips et al., 2014; Yu et al., 2019). In addition, humans and NHPs have the same thresholds for detecting a variety of stimuli and the nervous systems responsible for the somatosensory functions in the two species are fundamentally similar (Courtine et al., 2007; Kenshalo et al., 1989; LaMotte and Campbell, 1978; Rozsa et al., 1985). While rodent studies play a pivotal role in discovering novel targets as potential therapeutics, NHP studies establish a translational bridge toward human studies.
Rhesus macaques have been used for behavioral and pharmacological studies on opioids for more than five decades (Ding et al., 2020; Goldberg et al., 1969; Thompson and Schuster, 1964). Mounting evidence indicates that humans and NHPs have similar responses to opioid-related ligands (Lin and Ko, 2013; Mello et al., 1993; Woods and Winger, 1987). However, there are many examples that behavioral and physiological responses to opioid-related ligands in rodents cannot be translated to NHPs. Herein we briefly discuss four ligands to illustrate a translational gap.
First, a major metabolite of naltrexone, 6β-naltrexol, was demonstrated as a neutral MOP receptor antagonist in mice (Raehal et al., 2005). Unlike classic opioid antagonist naloxone, neutral antagonists do not alter basal MOP receptor signaling and could be used to block MOP receptor-medicated agonist and inverse agonist actions (Connor and Traynor, 2010; Wang et al., 2001). 6β-naltrexol did not significantly precipitate withdrawal in morphine-dependent mice and it reduced naloxone-precipitated withdrawal signs in mice (Raehal et al., 2005). In contrast, 6β-naltrexol precipitated withdrawal in morphine-dependent NHPs and it enhanced naltrexone-precipitated withdrawal symptoms in NHPs (Ko et al., 2006a). These findings indicate that the constitutive activities of the MOP receptor are different between rodents and primates and no neutral MOP receptor antagonist has yet been found in primates. Second, in searching for alternatives to buprenorphine, BU72 was identified with high affinity and efficacy for MOP receptors and it displayed a wide window between antinociception and respiratory depression in mice (Neilan et al., 2004). However, the antinociceptive dose of BU72 rapidly caused respiratory depression and arrest in NHPs, raising a safety concern that was not manifested in mice (Neilan et al., 2004). Third, while KOP receptor agonists produce antipruritic effects across species (Cowan and Ko, 2020; Inan and Cowan, 2020), a prototypical KOP receptor antagonist, nor-binaltorphimine, elicited robust scratching responses in mice, indicating a tonic inhibition of itch by endogenous KOP dynorphins (Kamei and Nagase, 2001; Kardon et al., 2014). In contrast, nor-binaltorphimine did not elicit scratching responses in NHPs following systemic and intrathecal administration (Ko et al., 2003; Lee et al., 2007), indicating different tonic activities of endogenous dynorphins between rodents and primates. Fourth, a NOP receptor-selective antagonist, J-113397, enhanced buprenorphine-induced antinociception in mice, indicating that activation of NOP receptors counteracted MOP receptor-mediated antinociception (Lutfy et al., 2003). However, J-113397 did not modulate buprenorphine-induced antinociception in NHPs. Instead, NOP receptor agonists synergistically enhanced MOP receptor agonist-induced antinociception in different NHP pain models (Cremeans et al., 2012; Hu et al., 2010). These findings clearly indicate that NOP receptor activation has opposite pain modulation between rodents and primates. Collectively, these examples pinpoint a critical need of NHP models to validate the functional profiles of ligand-receptor systems discovered in rodents and translate the therapeutic profiles of newly developed compounds to future human studies.
3. Analgesia
3.1. NHP pain models
In order to evaluate the analgesic effects of opioids, NHP researchers commonly use the warm water tail-withdrawal assay (Dykstra and Woods, 1986), which is similar to thermal stimuli used in rodents (Deuis et al., 2017), for basic pharmacological explorations. However, this assay is not highly relevant to human clinical pain, as it measures an evoked response from a noxious stimulus, i.e., sensory dimension with minimum affective component. In contrast, patients experience pain without overt stimulation. Clinical pain is multi-dimensional and accompanied by affective changes and comorbidities, a phenomenon that is not accounted for in the acute thermal nociceptive assay (Gracely et al., 1978; Mao, 2012). Nonetheless, this assay allows researchers to determine to what degree opioid-related ligands can prolong the NHP’s tail-withdrawal latency and whether detected antinociceptive doses produce any adverse effects. In general, following systemic administration, MOP receptor agonists produced thermal antinociceptive effects, which were accompanied by respiratory depression, scratching activity, and/or disruption of food-maintained operant behavior (France et al., 1992; Ko et al., 2002b; Negus et al., 2003). KOP receptor agonists produced antinociceptive effects at doses that induced sedation, diuresis, and discriminative stimulus effects (i.e., dysphoria and psychotomimesis) (Butelman et al., 2001; Butelman et al., 1993b; Dykstra et al., 1987; Ko et al., 1999b). This finding is consistent with other studies, showing that centrally-penetrating KOP receptor agonists produced sedative and psychotomimetic effects in humans (Pfeiffer et al., 1986; Walsh et al., 2001). DOP receptor agonists could not produce antinociception; instead, causing convulsions in NHPs (Negus et al., 1998; Sukhtankar et al., 2014). NOP receptor agonists produced antinociceptive effects at doses that did not cause respiratory depression and sedation (Ko et al., 2009; Podlesnik et al., 2011). However, NOP receptor agonists did not consistently increase the NHP’s tail-withdrawal latency across different groups of NHPs (Kiguchi and Ko, 2019). It is worth noting if NHPs are not well adapted to the NHP chair and the measurement of the tail-withdrawal responses, higher doses of opioid agonists are required to suppress NHP’s tail-withdrawal responses. Consequently, observed antinociceptive doses of opioids are higher than clinically used doses and do not display behavioral selectivity, i.e., antinociceptive doses also suppress other behaviors (Cowan and Ko, 2020; Kiguchi and Ko, 2019). Moreover, the analgesic effects of MOP, KOP, DOP, and NOP receptor agonists against different pain modalities (e.g., mechanical and neuropathic pain) warrant further investigation.
NHP researchers further developed other pain models to better assess the analgesic action of opioids. For example, capsaicin evokes pain sensation by activating the transient receptor potential vanilloid type 1, which is implicated in humans under diverse pain states (Brito et al., 2014). Capsaicin-induced allodynia has been utilized as a pain model in humans to evaluate and distinguish the analgesic efficacy of different classes of drugs (Eisenach et al., 2000; Park et al., 1995). In similar contexts, capsaicin-induced thermal allodynia in NHPs has been used to distinguish central versus peripheral sites of action (Butelman et al., 2004b; Ko et al., 1998; 1999a; Ko and Woods, 1999) and compare the antiallodynic efficacy among opioid and non-opioid ligands (Butelman et al., 2003; Ko et al., 2002a; Ko et al., 2000). Furthermore, carrageenan causes inflammation via cyclooxygenase-1/2 enzyme-mediated prostaglandin release (Dirig et al., 1998). Carrageenan-induced inflammatory pain in NHPs lasted for more than 6 hours post-injection and it is sensitive to detect potential antihyperalgesia of nonsteroidal anti-inflammatory drugs (NSAIDs), endogenous and synthetic opioids (Lee and Ko, 2015; Sukhtankar et al., 2014). These chemical-induced pain models document that opioid-related ligands are more potent in attenuating allodynia-/hyperalgesia-like responses in NHPs and their effective doses are close to clinically used doses (Kiguchi and Ko, 2019; Sukhtankar et al., 2014).
3.2. Strategies to enhance opioid analgesia
Pharmacological studies in NHP have identified viable strategies to develop safer opioid analgesics and/or to produce opioid-sparing effect, i.e., decreasing exposure to classic MOP receptor agonists. For example, NOP receptor agonists have been demonstrated to synergistically enhance the antinociceptive effects of MOP receptor agonists without causing respiratory depression and pruritus (Cremeans et al., 2012; Hu et al., 2010). Compounds with mixed MOP/NOP receptor agonist activities potently produced antinociceptive effects with fewer side effects, such as reinforcing effects (abuse potential), respiratory depression, pruritus, physical dependence and slower tolerance development (Ding et al., 2016; Ding et al., 2018b; Kiguchi et al., 2019). More importantly, a mixed NOP/opioid receptor agonist, cebranopadol, produced effective analgesia in patients with acute or chronic pain and showed reduced side effects including lower drug-liking effect, respiratory depression, and physical dependence (Calo and Lambert, 2018; Kiguchi et al., 2020b; Tzschentke et al., 2019). These findings exemplify that NHP studies represent a translational bridge and show functional similarities of ligands with mixed MOP/NOP receptor agonist activities between NHPs and humans.
Another strategy is to combine cannabinoid-related compounds with opioid analgesics. A series of NHP pharmacological studies demonstrates that cannabinoid receptor agonists, such as CP 55,940, WIN 55,212, and Δ9-tetrahydrocannabinol, enhanced the antinociceptive effects of MOP receptor agonists (Li et al., 2008; Maguire and France, 2014; Maguire et al., 2013). Importantly, these cannabinoid receptor agonists did not increase the respiratory depressant, discriminative stimulus, and reinforcing effects of MOP receptor agonists (Li et al., 2012; Maguire and France, 2018; Maguire et al., 2013; Weed et al., 2018). These findings support the notion that cannabinoid receptor agonists may produce opioid-sparing effects, i.e., to dispense opioid medications at lower doses, in humans (Abrams et al., 2011; Cooper et al., 2018; Lynch and Clark, 2003).
3.3. Limitations and opportunities of NHP analgesic studies
The development of chronic or neuropathic pain models in NHPs using surgical procedures is limited by ethical issues. Nonetheless, behavioral pharmacological studies in NHPs allow researcher to identify which ligand-receptor systems are involved in pain processing and how they are different from or similar to findings in rodents. NHP analgesic studies have used reflex-based assays for decades. Recently, an operant-based nociceptive assay has been developed in squirrel monkeys (Kangas and Bergman, 2014). This advance provides a valuable means to study how different classes of analgesics can restore the disruptive effects of nociceptive stimuli, rather than suppression of evoked nociceptive behavior. NHP investigators also found that neuroinflammation and dysregulation of multiple ligand-receptor systems involved in pain modulation appear to be permanent in the spinal cord of naturally occurring type 2 diabetic NHPs (Ding et al., 2018a; Kiguchi et al., 2017). This NHP disease model not only opens new avenues to study the functional roles of pain and neuroinflammation mediators, but also offers a valuable opportunity to explore potential treatments for diabetic neuropathy. Furthermore, rodent studies incorporating NHP data can enhance the translational relevance. For instance, nivolumab, a programmed cell death protein-1 (PD-1) inhibitor, attenuated morphine-induced antinociception in mice and NHPs, indicating that PD-1 regulates MOP receptor signaling in nociceptive neurons and anti-PD-1 immunotherapy may diminish opioid analgesia (Wang et al., 2020a). Through the assay improvement and studying neural substrates of NHPs under different states, NHP studies integrating multiple outcome measures will facilitate the research and development of innovative analgesics.
4. Abuse liability
Prescription and illicit opioids, primarily MOP receptor agonists, produce euphoria and are widely abused in our society. In the past few decades, the intravenous drug self-administration procedure is considered a gold standard to evaluate a drug’s abuse liability (i.e., reinforcing effect and strength) and potential medications for substance use disorders (Ator and Griffiths, 2003; Mello and Negus, 1996). In particular, NHP studies, not rodent models, have provided more concordant results with both human laboratory studies and clinical trials; and a series of empirical data supports the continued use of NHPs in drug abuse research (Banks et al., 2017; Ling et al., 2016; Wee et al., 2012; Weerts et al., 2007). Early NHP studies have demonstrated that MOP, not DOP, KOP, or NOP, receptor agonists produced reinforcing effects (Ko et al., 2009; Nakao et al., 2016; Negus et al., 1994). As KOP receptor activation decreases dopamine release in the nucleus accumbens (Spanagel et al., 1992), KOP receptor agonists are expected to counteract the reinforcing effects of abused drugs. However, KOP receptor agonists decreased self-administration of both drug and non-drug reinforcers in NHPs, indicating lack of functional selectivity (Cosgrove and Carroll, 2002; Mello and Negus, 1998).
Several treatment strategies have been initiated to tackle opioid abuse. First, anti-opioid immunopharmacotherapies have emerged as potential therapeutics for opioid use disorder. For example, fentanyl vaccines blunted the reinforcing effects of fentanyl in rats (Townsend et al., 2019). While fentanyl vaccines have great potential to prevent fentanyl from reaching the brain, it is not clear how NHPs would self-administer different MOP receptor agonists following fentanyl vaccine administration. It is challenging for a specific vaccine to protect individuals from experiencing reinforcing effects derived from abused opioids with diverse chemical structures or taking a much larger amount. Second, the development of G protein-biased MOP receptor agonists has emerged to mitigate MOP receptor-mediated adverse effects (Azzam et al., 2019). For example, a novel G protein-signalling biased MOP receptor agonist PZM21 produced antinociceptive effects without rewarding effects in mice (Manglik et al., 2016). However, PZM21 produced oxycodone-like reinforcing effects and strength in NHPs (Ding et al., 2020). Biased opioids may offer other advantages, but current studies in NHPs indicate they are not posed to be non-addictive analgesics.
Third, compounds with mixed opioid actions may retain analgesia with reduced side effects. For example, by decreasing the dopaminergic activity, NOP receptor activation is known to reduce the rewarding effects of abused drugs in rodents (Ciccocioppo et al., 2019; Toll et al., 2016). Buprenorphine is a MOP receptor partial agonist with binding affinity at KOP and DOP receptors (Lewis, 1985; Spagnolo et al., 2008). As a buprenorphine analog, BU08028 shows buprenorphine-like MOP receptor efficacy with additional affinity and efficacy at NOP receptors (Cami-Kobeci et al., 2011). BU08028 displayed lower reinforcing strength than buprenorphine in NHPs (Ding et al., 2016). Other mixed MOP/NOP receptor partial agonists (AT-121 and BU10038) or full agonist (cebranopadol) in different chemical structures also produced antinociception with decreased reinforcing strength (Ding et al., 2018b; Kiguchi et al., 2019; Kiguchi and Ko, 2019). More importantly, AT-121 was further tested to show its effectiveness to attenuate oxycodone-, not food-, induced reinforcing effects in NHPs (Ding et al., 2018b). These findings support the notion that compounds with mixed MOP/NOP receptor partial agonist activities have relatively lower abuse potential and can be used to attenuate opioid abuse-related effects (Kiguchi et al., 2020b; Lin and Ko, 2013). In a similar approach, given that MOP and KOP receptors have opposite modulation of dopaminergic neurons (Spanagel et al., 1992), mixed MOP/KOP receptor agonists are worth to be investigated in NHP models. In particular, mixtures of KOP receptor agonists (nalfurafine and salvinorin A) with oxycodone showed reduced self-administration in NHPs (Zamarripa et al., 2020). It is important to develop compounds with mixed MOP/KOP receptor agonists in different efficacies at MOP versus KOP receptors (Bidlack and Knapp, 2013; Greedy et al., 2013) and investigate their functional efficacy, selectivity, and tolerability in NHP models.
Finally, MOP receptor antagonist-based compounds have been proven to be a potential treatment option based on studies in NHPs. For instance, BU10119 with mixed MOP/KOP receptor antagonist activities showed therapeutic potential for the treatment of neuropsychiatric disorders (Almatroudi et al., 2018). BU10119 was recently demonstrated to not only block MOP receptor agonist-induced reinforcing effects, but also attenuate heroin-primed reinstatement of extinguished responding in NHPs, indicating of relapse prevention (Maguire et al., 2020a). The limitation of BU10119 could be its naltrexone-like property to elicit withdrawal signs in morphine-dependent NHPs (Maguire et al., 2020a). Another compound is methocinnamox, which was initially characterized as a pseudo-irreversible MOP receptor antagonist with a long duration of action in mice (Broadbear et al., 2000). A single dose of methocinnamox selectively blocked the reinforcing effects of MOP receptor agonists for several days without changing the NHP’s physiological functions (Maguire et al., 2019). More importantly, repeated administration (i.e., once per 12 days for five injections) of methocinnamox significantly decreased fentanyl self-administration for more than two months in NHPs (Maguire et al., 2020b). These exciting findings indicate that methocinnamox could be a safe, effective, and long-acting treatment option for opioid use disorder. Other than four treatment strategies mentioned above, there are other targets and approaches (France et al., 2020; Rasmussen et al., 2019) that have not yet been evaluated in NHP models. Preclinical studies using NHPs will continue to facilitate the research and development of effective medications for opioid use disorder.
5. Respiratory depression
Opioid-induced respiratory depression is one of the most concerning complications in pain treatment with opioid analgesics and overdose in persons with opioid use disorder. Researchers have established different NHP models to study opioid receptor mechanisms underlying respiratory depression and test strategies to ameliorate the respiratory depressant effects of opioids. Previous model used head plethysmograph to measure respiratory endpoints in restrained, conscious NHPs (Butelman et al., 1993a; Butelman et al., 2001; France et al., 1992). Recently, implantation of telemetry device has made it practical to measure real-time respiratory parameters in freely moving NHPs (Ding et al., 2016). Utilization of these NHP models demonstrated that MOP receptor, but not KOP, DOP or NOP receptors, is the main contributor to opioid-induced respiratory depression (France et al., 1994; Ko et al., 2009; Negus et al., 1994). A single injection of fentanyl at 2-fold of its antinociceptive dose in NHPs produced a rapid and severe decrease in respiration rate, which was reversed by a MOP receptor antagonist naltrexone (Ding et al., 2016). This observation in NHPs recapitulates the rapid onset and severity of fentanyl overdose in humans (Grell et al., 1970; Kuczyńska et al., 2018). In contrast, in the same NHP telemetry model, novel compounds with mixed opioid partial agonist actions did not compromise respiratory functions (Ding et al., 2016; Ding et al., 2018b; Kiguchi et al., 2019). Therefore, NHPs provide a valuable tool to distinguish clinically used opioids and new investigational compounds in their impacts on the respiration system. More importantly, it allows researchers to validate strategies to prevent or reverse respiratory depression, which can be translated to human studies.
One strategy to mitigate respiratory depression is to develop novel opioid agonists with reduced liability of respiratory depression. Mixed MOP/NOP receptor partial agonists showed potent antinociceptive effects without inducing respiratory depression (Ding et al., 2016; Ding et al., 2018b; Kiguchi et al., 2019). A mixed NOP/opioid receptor full agonist, cebranopadol, did not affect NHP’s respiratory function at a dose 3 times higher than its antinociceptive dose, which is consistent with observations in human studies (Dahan et al., 2017; Kiguchi and Ko, 2019). These findings support the research strategy to develop MOP/NOP receptor agonists as innovative analgesics with improved safety profile in humans. The other strategy is to develop new agents that can prevent or reverse opioid-induced respiratory depression. Although the opioid receptor antagonist naloxone is a quick-acting antidote for opioid overdose, its short duration of action limits it clinical utility. A recent NHP study demonstrates that a novel opioid antagonist methocinnamox effectively attenuated and reversed the respiratory depressant effects of heroin (Gerak et al., 2019). The striking advantage of methocinnamox is its long duration of action which lasts for a few days. It is important to further compare the duration of protection against opioid-induced respiratory depression between methocinnamox and a slow release formulation of naltrexone (Vivitrol). Nevertheless, these recent NHP findings have encouraging implications about methocinnamox being able to provide sustained protection from opioid overdose.
Direct activation of TREK1 potassium channel downstream of the MOP receptor induced antinociception but not opioid-induced respiratory depression in rodents (Busserolles et al., 2020). It would be interesting to further investigate whether TREK1 activators produce similar effects in NHPs. Several other pharmaceutical agents, such as serotonin receptor agonist, nicotinic receptor agonist, monoclonal antibodies with high affinities for opioids, have been studied in rodents to show potential effectiveness in reducing opioid-induced respiratory depression (Baehr et al., 2020; Ren et al., 2015; 2020). However, their side effect profiles and lack of thorough studies of efficacies hinder the therapeutic use of these agents (Dahan et al., 2018). Future studies of these newly developed compounds and other countermeasures for opioid toxicity (France et al., 2020) in NHPs will provide functional evidence of their potential utilities in humans.
6. Physical dependence
Physical dependence on opioids tends to perpetuate compulsive drug use and is considered an important component of opioid use disorder (Brady, 1991; Kosten and Baxter, 2019). It is responsible for the emergence of spontaneous withdrawal upon abrupt discontinuation of opioids or rapid dose reduction (Pergolizzi et al., 2020; Volkow and McLellan, 2016). In addition, following acute or repeated exposure to MOP agonists, humans and NHPs quickly develop physical dependence which is revealed by emergence of withdrawal signs after administration of an opioid receptor antagonist (Azolosa et al., 1994; Heishman et al., 1990; Ko et al., 2006a). The neurobiological mechanism underlying opioid physical dependence and withdrawal could be the binding to opioid receptors in the locus coeruleus. Acute effects of opioids lead to the decreased level of cyclic adenosine monophosphate (cAMP) and reduced release of norepinephrine. Upon spontaneous or precipitated withdrawal, the cAMP level and neuronal firing rates in the locus coeruleus increase dramatically above normal levels, causing excessive release of norepinephrine and severe opioid withdrawal symptoms (Cao et al., 2010; Kosten and George, 2002; Rasmussen et al., 1990).
NHP models have been established to quantitate opioid withdrawal signs and investigate how different strategies could modulate opioid withdrawal. In early studies, withdrawal signs designated as lying down, avoiding contact, vocalizing, crawling, holding abdomen, salivation, and tremors were noted in NHPs receiving chronic administration of morphine (Aceto et al., 1998; Beardsley et al., 2004; Martin and Eades, 1964). Recently, NHPs implanted with telemetry device were established to study antagonist-precipitated withdrawal (Ding et al., 2016; Kiguchi et al., 2019). Naltrexone quickly increased respiratory rate, minute volume, heart rate, and blood pressure in morphine-treated NHPs, validating that respiratory and cardiovascular parameters are reliable and quantitative indicators of antagonist-precipitated withdrawal. In addition, suppression of food-maintained operant responding was established as additional quantitative measure of precipitated withdrawal signs along with changes in physiological parameters and behavioral signs (Maguire et al., 2020a).
The NHP model has demonstrated its value in distinguishing various opioid full agonists versus partial agonists in their potentials to produce physical dependence. For example, neither the NOP receptor agonists nor mixed MOP/NOP receptor partial agonists produced physical dependence (Ding et al., 2016; Ding et al., 2018b; Kiguchi et al., 2019). These results support the notion that unlike MOP receptor agonists, NOP-related agonists have less liability to develop physical dependence following the same period of repeated exposure. Given that physical dependence is one of the major concerns associated with the use of opioid analgesics (Saxon, 2013; Schuckit, 2016; Volkow and Blanco, 2020), the development of mixed MOP/NOP receptor partial agonists is a viable approach to curb the opioid epidemic.
Another strategy for combating opioid withdrawal is to offer effective treatment options for suppressing withdrawal symptoms. In the clinic, opioid withdrawal is often managed by the utility of buprenorphine, a MOP receptor partial agonist (Gowing et al., 2017; Kosten and Baxter, 2019; Schuckit, 2016). Numerous clinical studies have documented that buprenorphine treatment can significantly reduce craving and relapse risk and suppress opioid withdrawal (Gowing et al., 2017; Kakko et al., 2019; Reimer et al., 2020). However, buprenorphine can acutely produce euphoric effects and mild-to-moderate physical dependence with prolonged use or precipitate withdrawal in individuals dependent on a higher efficacy MOP agonist; and it has the additional risk of diversion and misuse or abuse of medication (Eissenberg et al., 1996; Johanson et al., 2012; Lavonas et al., 2014; Saxon, 2013). Given the improved functional profiles of mixed MOP/NOP receptor partial agonists displayed in NHPs, it is important to determine and compare the effectiveness and potency of buprenorphine with those of BU08028 and AT-121 in alleviating opioid withdrawal in NHP models described above. Positive outcomes from such studies will open a new avenue of treatment for managing opioid withdrawal.
Besides the involvement of neurons in the underlying mechanisms of opioid withdrawal, interactions between glial cells and neurons are proposed to play an important role as well (Burma et al., 2017b). The microglial pannexin-1 channel was identified as a therapeutic target for opioid withdrawal in rodents. Pannexin-1-blocking peptide or the clinically used nonselective pannexin-1 channel blocker mefloquine or probenecid reduced the severity of opioid withdrawal without compromising antinociceptive effects (Burma et al., 2017a). Preclinical studies of pannexin-1 channel blockers in NHPs will shed light on their potential effectiveness in treating opioid dependence.
7. Pruritus
Pruritus (itch sensation) is a common side effect of spinal opioids often experienced by obstetric and postoperative patients. This problem significantly compromises the value of spinal opioid analgesics in providing pain relief (Ganesh and Maxwell, 2007). The neurobiological mechanisms of opioid-induced pruritus are not fully understood. Studies in laboratory animals and humans demonstrate that opioid-induced pruritus is mainly mediated by MOP receptors (Ko, 2015). While MOP receptors are expressed in both inhibitory and excitatory interneurons in the mouse spinal dorsal horn, a recent study demonstrates that intrathecal morphine elicited itch via MOP receptors on spinal inhibitory interneurons (Wang et al., 2020b).
To prevent or treat spinal opioid-induced itch, a variety of pharmacological agents have been evaluated in both preclinical animal models and clinical studies. In NHPs, intrathecal morphine simultaneously induced antinociception and robust scratching responses (Ko and Naughton, 2000). Unlike morphine, intrathecal administration of KOP, DOP, and NOP receptor agonists all produced antinociception without scratching responses, indicating that the MOP receptor, not other opioid receptor subtypes, mainly mediates spinal opioid-induced itch (Ko, 2014; Ko et al., 2006b). In addition, spinally elicited scratching response in NHPs is a valid in vivo endpoint for determining the efficacy of MOP receptor activation. For example, MOP receptor agonists with different intrinsic efficacy, such as DAMGO versus morphine, elicited different magnitudes of scratching activities (Ko et al., 2004). It is important to note that intrathecal morphine produces long-lasting itch sensation and pain relief for hours in humans and NHPs (Ko and Naughton, 2000; Palmer et al., 1999). However, intrathecal morphine only elicited mild and transient scratching lasting for 10–15 min or vehicle-like responses in rodents (Liu et al., 2011; Sukhtankar and Ko, 2013). Such drastic species differences between rodents and primates support the translational value of NHP models for the exploration and validation of drugs without itch sensation and drugs that can potentially treat spinal opioid-induced itch (Cowan and Ko, 2020; Ko, 2015).
Indeed, NHP studies have identified mixed MOP/NOP receptor agonists as a new class of analgesics without eliciting itch sensation (Kiguchi et al., 2020a). With regard to alleviating spinal-opioid induced itch, both opioid ligands and non-opioid ligands have been investigated for their effectiveness in NHPs (Ko, 2015). MOP antagonists equally blocked intrathecal morphine-induced itch scratching and antinociception (Ko and Naughton, 2000). It supports the clinical findings that MOP receptor antagonists are not ideal drugs for treating pruritus in obstetric patients (Dominguez and Habib, 2013; Ganesh and Maxwell, 2007). Compared with MOP antagonists, a mixed MOP/KOP receptor partial agonist, butorphanol, is effective in alleviating MOP agonist-induced itch without reversing analgesia in NHPs (Lee et al., 2007). These observations are in line with clinical studies demonstrating that mixed MOP/KOP receptor partial agonists could decrease incidence of pruritus without other side effects (Dominguez and Habib, 2013; Waxler et al., 2005). Furthermore, KOP receptor agonists, at non-sedating doses, can attenuate intrathecal morphine-induced scratching without affecting antinociception (Ko and Husbands, 2009; Ko et al., 2003). These findings facilitated the development of a KOP receptor agonist, nalfurafine, as an antipruritic. Clinical trials have reported that nalfurafine is a safe and effective antipruritic in hemodialysis patients suffering from uremic pruritus (Kumagai et al., 2012; Kumagai et al., 2010).
On the other hand, non-opioid ligands, such as serotonin 5-HT3 receptor antagonist (ondansetron), antihistamines, and NASIDs failed to attenuate intrathecal morphine-induced scratching in NHPs (Ko, 2015; Ko et al., 2004). These results to certain degree recapitulate the varied effectiveness of ondansetron and the ineffectiveness of antihistamines and NSAIDs in relieving spinal opioid-induced itch in clinical studies (Ko, 2015). Collectively, these pharmacological studies suggest that NHPs could serve as a surrogate species for humans in preclinical studies to identify effective treatments for spinal opioid-induced itch.
Furthermore, NHPs could also prove useful in studying the neurobiological mechanisms underlying spinal opioid-induced itch. In mice, intrathecal morphine-induced scratching was inhibited by co-administration with a gastrin-releasing peptide receptor (GRPR) antagonist (Liu et al., 2011). However, in NHPs, the GRPR antagonist could not attenuate spinal MOP agonist-elicited scratching activities (Lee and Ko, 2015). Recently, Wang et al demonstrated that intrathecal administration of neuropeptide Y suppressed morphine-induced itch in mice (Wang et al., 2020b). It will be interesting to examine whether neuropeptide Y plays a functional role in morphine-induced itch in NHPs as well. Rodent studies continue to provide mechanistic insights to the neurocircuitry for modulating itch (Kardon et al., 2014; Liu et al., 2011; Wang et al., 2020b). Future NHP studies can further define the functional profile of discovered ligand-receptor systems in sensory processing and determine if these targets modulate spinal opioid-induced itch in primates.
8. Conclusions
Similarities between NHPs and humans and species differences between rodents and NHPs in functional profiles of opioid-related pharmacological agents support the translational value of NHPs in opioid research (Banks et al., 2017; Kiguchi and Ko, 2019; Lin and Ko, 2013). Research in NHPs has led to the development of novel analgesic compounds that display improved side-effect profiles when compared with opioids currently used in the clinic. Furthermore, NHPs have served as a surrogate species to examine the therapeutic potentials of experimental compounds in the treatment of opioid use disorder. Figure 1 summarizes the main adverse effects of MOP receptor agonists discussed in this review and strategies to ameliorate such effects. Mixed MOP/NOP receptor partial agonists are being developed as safe, non-addictive analgesics (Kiguchi et al., 2020a). NHP studies have found that MOP receptor- or mixed opioid receptor subtype-based meditations, but not non-opioid ligands, show effectiveness in reducing opioid-induced adverse effects (Gerak et al., 2019; Kiguchi et al., 2020b; Maguire et al., 2020b). With recent advances in medicinal chemistry, there are other targets and treatment strategies (France et al., 2020; Rasmussen et al., 2019) which are worth investigating in NHP models. Behavioral pharmacological studies using NHPs will continue to facilitate the research and development of effective medications to address the challenges from the opioid crisis (Volkow and Collins, 2017).
Figure 1.
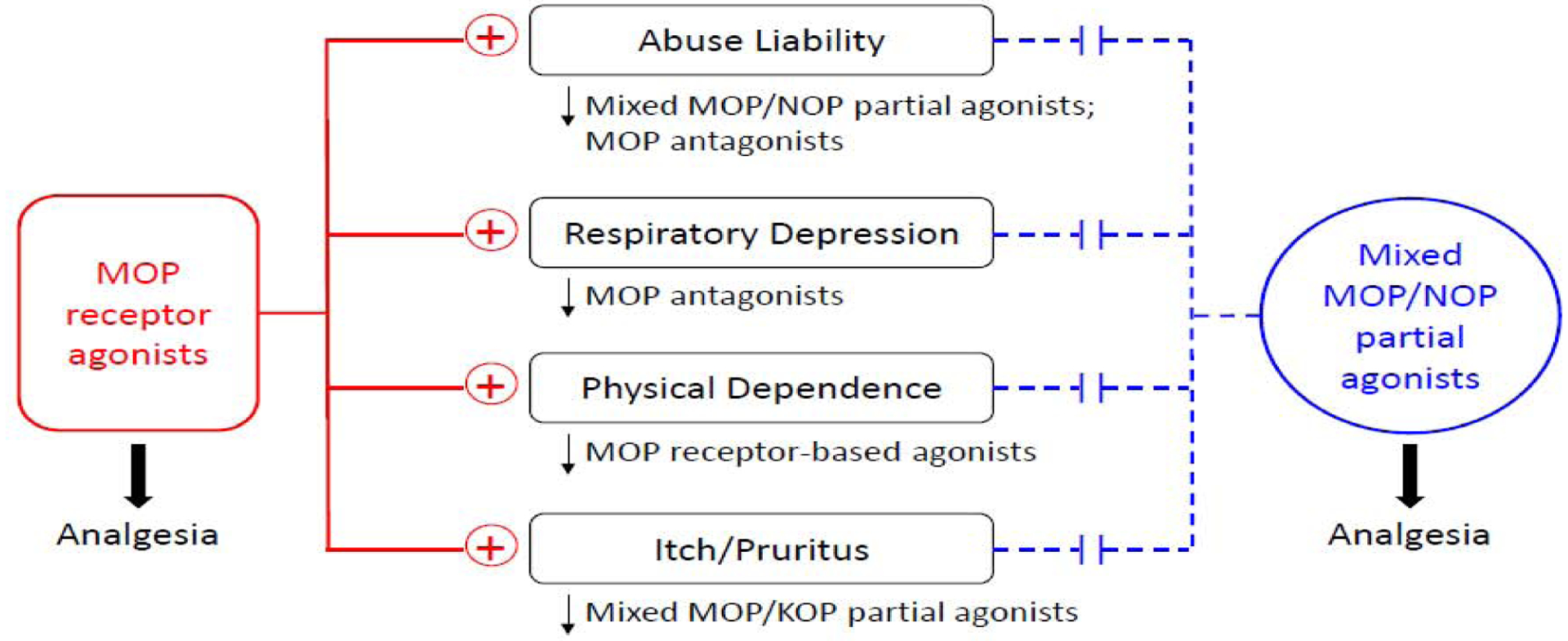
Simplified scheme to summarize the functional profiles of two classes of opioid-related agonists based on human and/or non-human primate studies. + symbols represent the adverse effects associated with MOP receptor agonists. Dashed lines indicate reduced or lack of adverse effects associated with mixed MOP/NOP receptor partial agonists. ↓ symbols represent that the adverse effect can be ameliorated by a specific category of pharmacological agents.
Supplementary Material
Acknowledgments
Funding by the U.S. National Institutes of Health, National Institute on Drug Abuse (DA032568, DA044775, DA044450, and DA049580) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR064456 and AR069861) is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. federal agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
H.D. and M.C.K. declare that there is no conflict of interest.
References
- Abrams DI, Couey P, Shade SB, Kelly ME and Benowitz NL (2011) Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 90:844–851. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Bowman ER, Harris LS and May EL (1998) Dependence studies of new compounds in the rhesus monkey, rat and mouse (1997). NIDA Res Monogr 178:363–407. [PubMed] [Google Scholar]
- Al-Hasani R and Bruchas MR (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroudi A, Ostovar M, Bailey CP, Husbands SM and Bailey SJ (2018) Antidepressant-like effects of BU10119, a novel buprenorphine analogue with mixed κ/μ receptor antagonist properties, in mice. Br J Pharmacol 175:2869–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA and Griffiths RR (2003) Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70:S55–72. [DOI] [PubMed] [Google Scholar]
- Azolosa JL, Stitzer ML and Greenwald MK (1994) Opioid physical dependence development: effects of single versus repeated morphine pretreatments and of subjects’ opioid exposure history. Psychopharmacology (Berl) 114:71–80. [DOI] [PubMed] [Google Scholar]
- Azzam AAH, McDonald J and Lambert DG (2019) Hot topics in opioid pharmacology: mixed and biased opioids. Br J Anaesth 122:e136–e145. [DOI] [PubMed] [Google Scholar]
- Baehr CA, Huseby Kelcher A, Khaimraj A, Reed DE, Pandit SG, AuCoin D, Averick S and Pravetoni M (2020) Monoclonal antibodies counteract opioid-induced behavioral and toxic effects in mice and rats. J Pharmacol Exp Ther: DOI: 10.1124/jpet.1120.000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Zerbi V, Sallet J, Wenderoth N and Mars RB (2020) Primate homologs of mouse cortico-striatal circuits. Elife 9: DOI: 10.7554/eLife.53680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW and Negus SS (2017) Utility of Nonhuman Primates in Substance Use Disorders Research. Ilar j:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL and Harris LS (2004) Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12:163–172. [DOI] [PubMed] [Google Scholar]
- Bianchi BR, Zhang XF, Reilly RM, Kym PR, Yao BB and Chen J (2012) Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J Pharmacol Exp Ther 341:360–368. [DOI] [PubMed] [Google Scholar]
- Bidlack JM and Knapp BI (2013) Mixed Mu/Kappa Opioid Agonists, in Research and Development of Opioid-Related Ligands (Ko MC and Husbands SMeds) pp 257–272, American Chemical Society, Washington, DC, USA. [Google Scholar]
- Brady JV (1991) Animal models for assessing drugs of abuse. Neurosci Biobehav Rev 15:35–43. [DOI] [PubMed] [Google Scholar]
- Brady KT, McCauley JL and Back SE (2016) Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am J Psychiatry 173:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito R, Sheth S, Mukherjea D, Rybak LP and Ramkumar V (2014) TRPV1: A Potential Drug Target for Treating Various Diseases. Cells 3:517–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH and Traynor JR (2000) Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: comparison with clocinnamox, beta-funaltrexamine, and beta-chlornaltrexamine. J Pharmacol Exp Ther 294:933–940. [PubMed] [Google Scholar]
- Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, Antle MC, Zamponi GW, Cahill CM, Borgland SL, De Koninck Y and Trang T (2017a) Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 23:355–360. [DOI] [PubMed] [Google Scholar]
- Burma NE, Kwok CH and Trang T (2017b) Therapies and mechanisms of opioid withdrawal. Pain Manag 7:455–459. [DOI] [PubMed] [Google Scholar]
- Busserolles J, Ben Soussia I, Pouchol L, Marie N, Meleine M, Devilliers M, Judon C, Schopp J, Clemenceau L, Poupon L, Chapuy E, Richard S, Noble F, Lesage F, Ducki S, Eschalier A and Lolignier S (2020) TREK1 channel activation as a new analgesic strategy devoid of opioid adverse effects. Br J Pharmacol 177:4782–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Harris TJ and Kreek MJ (2003) Topical capsaicin-induced allodynia in unanesthetized primates: pharmacological modulation. J Pharmacol Exp Ther 306:1106–1114. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW and Kreek MJ (2004a) Peripheral selectivity and apparent efficacy of dynorphins: comparison to non-peptidic kappa-opioid agonists in rhesus monkeys. Psychoneuroendocrinology 29:307–326. [DOI] [PubMed] [Google Scholar]
- Butelman ER, France CP and Woods JH (1993a) Apparent pA2 analysis on the respiratory depressant effects of alfentanil, etonitazene, ethylketocyclazocine (EKC) and Mr2033 in rhesus monkeys. J Pharmacol Exp Ther 264:145–151. [PubMed] [Google Scholar]
- Butelman ER, Harris TJ and Kreek MJ (2004b) Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther 311:155–163. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ and Woods JH (2001) GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. J Pharmacol Exp Ther 298:1049–1059. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR and Woods JH (1993b) Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther 267:1269–1276. [PubMed] [Google Scholar]
- Calo G and Lambert DG (2018) Nociceptin/orphanin FQ receptor ligands and translational challenges: focus on cebranopadol as an innovative analgesic. Br J Anaesth 121:1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami-Kobeci G, Polgar WE, Khroyan TV, Toll L and Husbands SM (2011) Structural determinants of opioid and NOP receptor activity in derivatives of buprenorphine. J Med Chem 54:6531–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Vialou VF, Lobo MK, Robison AJ, Neve RL, Cooper DC, Nestler EJ and Han MH (2010) Essential role of the cAMP-cAMP response-element binding protein pathway in opiate-induced homeostatic adaptations of locus coeruleus neurons. Proc Natl Acad Sci U S A 107:17011–17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N and Weiss F (2019) NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharmacol 254:187–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M and Traynor J (2010) Constitutively active μ-opioid receptors. Methods Enzymol 484:445–469. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD and Haney M (2018) Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 43:2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP and Carroll ME (2002) Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. J Pharmacol Exp Ther 301:993–1002. [DOI] [PubMed] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME and Edgerton VR (2007) Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med 13:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A and Ko MC (2020) Opioid Receptors in Itch and Pain Processing, in Itch and Pain: Similarities, Interactions, and Differences (Yosipovitch G, Andersen HH and Arendt-Nielsen L eds) pp 203–213, Wolters Kluwer N.V., Philadelphia, USA. [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L and Traynor JR (2015) Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol 172:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ and Ko MC (2012) Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Boom M, Sarton E, Hay J, Groeneveld GJ, Neukirchen M, Bothmer J, Aarts L and Olofsen E (2017) Respiratory Effects of the Nociceptin/Orphanin FQ Peptide and Opioid Receptor Agonist, Cebranopadol, in Healthy Human Volunteers. Anesthesiology 126:697–707. [DOI] [PubMed] [Google Scholar]
- Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M and Niesters M (2018) Averting Opioid-induced Respiratory Depression without Affecting Analgesia. Anesthesiology 128:1027–1037. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N and Vos T (2014) The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109:1320–1333. [DOI] [PubMed] [Google Scholar]
- Deuis JR, Dvorakova LS and Vetter I (2017) Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci 10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM and Ko MC (2016) A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113:E5511–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Kishioka S, Ma T, Peters CM and Ko MC (2018a) Differential mRNA expression of neuroinflammatory modulators in the spinal cord and thalamus of type 2 diabetic monkeys. J Diabetes 10:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Perrey DA, Nguyen T, Czoty PW, Hsu FC, Zhang Y and Ko MC (2020) Antinociceptive, reinforcing, and pruritic effects of the G-protein signalling-biased mu opioid receptor agonist PZM21 in non-human primates. Br J Anaesth 125:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT and Ko MC (2018b) A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10:eaar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirig DM, Isakson PC and Yaksh TL (1998) Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther 285:1031–1038. [PubMed] [Google Scholar]
- Dominguez JE and Habib AS (2013) Prophylaxis and treatment of the side-effects of neuraxial morphine analgesia following cesarean delivery. Curr Opin Anaesthesiol 26:288–295. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G and Woods JH (1987) Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther 242:413–420. [PubMed] [Google Scholar]
- Dykstra LA and Woods JH (1986) A tail withdrawal procedure for assessing analgesic activity in rhesus monkeys. J Pharmacol Methods 15:263–269. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD and Curry R (2000) Relative potency of epidural to intrathecal clonidine differs between acute thermal pain and capsaicin-induced allodynia. Pain 84:57–64. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE and Stitzer ML (1996) Buprenorphine’s physical dependence potential: antagonist-precipitated withdrawal in humans. J Pharmacol Exp Ther 276:449–459. [PubMed] [Google Scholar]
- Fountas A, Chai ST, Kourkouti C and Karavitaki N (2018) MECHANISMS OF ENDOCRINOLOGY: Endocrinology of opioids. Eur J Endocrinol 179:R183–R196. [DOI] [PubMed] [Google Scholar]
- France CP, Ahern GP, Averick S, Disney A, Enright HA, Esmaeli-Azad B, Federico A, Gerak LR, Husbands SM, Kolber B, Lau EY, Lao V, Maguire DR, Malfatti MA, Martinez G, Mayer BP, Pravetoni M, Sahibzada N, Skolnick P, Snyder EY, Tomycz N, Valdez CA and Zapf J (2020) Countermeasures for Preventing and Treating Opioid Overdose. Clin Pharmacol Ther:doi: 10.1002/cpt.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France CP, Medzihradsky F and Woods JH (1994) Comparison of kappa opioids in rhesus monkeys: behavioral effects and receptor binding affinities. J Pharmacol Exp Ther 268:47–58. [PubMed] [Google Scholar]
- France CP, Winger G, Seggel MR, Rice KC and Woods JH (1992) Pharmacological profile of a potent, efficacious fentanyl derivative in rhesus monkeys. Psychopharmacology (Berl) 109:291–298. [DOI] [PubMed] [Google Scholar]
- Ganesh A and Maxwell LG (2007) Pathophysiology and management of opioid-induced pruritus. Drugs 67:2323–2333. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Holanda VAD and Ruzza C (2019) NOP Ligands for the Treatment of Anxiety and Mood Disorders. Handb Exp Pharmacol 254:233–257. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, Woods JH, Husbands SM, Disney A and France CP (2019) Reversal and Prevention of the Respiratory-Depressant Effects of Heroin by the Novel mu-Opioid Receptor Antagonist Methocinnamox in Rhesus Monkeys. J Pharmacol Exp Ther 368:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH and Schuster CR (1969) Morphine: conditioned increases in self-administration in rhesus monkeys. Science 166:1306–1307. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM and Mbewe D (2017) Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev 2:Cd002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, McGrath F and Dubner R (1978) Ratio scales of sensory and affective verbal pain descriptors. Pain 5:5–18. [DOI] [PubMed] [Google Scholar]
- Greedy BM, Bradbury F, Thomas MP, Grivas K, Cami-Kobeci G, Archambeau A, Bosse K, Clark MJ, Aceto M, Lewis JW, Traynor JR and Husbands SM (2013) Orvinols with mixed kappa/mu opioid receptor agonist activity. J Med Chem 56:3207–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell FL, Koons RA and Denson JS (1970) Fentanyl in anesthesia: a report of 500 cases. Anesth Analg 49:523–532. [PubMed] [Google Scholar]
- Hawkinson JE, Szoke BG, Garofalo AW, Hom DS, Zhang H, Dreyer M, Fukuda JY, Chen L, Samant B, Simmonds S, Zeitz KP, Wadsworth A, Liao A, Chavez RA, Zmolek W, Ruslim L, Bova MP, Holcomb R, Butelman ER, Ko MC and Malmberg AB (2007) Pharmacological, pharmacokinetic, and primate analgesic efficacy profile of the novel bradykinin B1 Receptor antagonist ELN441958. J Pharmacol Exp Ther 322:619–630. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE and Liebson IA (1990) Acute opioid physical dependence in humans: effect of naloxone at 6 and 24 hours postmorphine. Pharmacol Biochem Behav 36:393–399. [DOI] [PubMed] [Google Scholar]
- Howell LL and Murnane KS (2011) Nonhuman primate positron emission tomography neuroimaging in drug abuse research. J Pharmacol Exp Ther 337:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R and Ko MC (2010) Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 148:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S and Cowan A (2020) Antipruritic Effects of Kappa Opioid Receptor Agonists: Evidence from Rodents to Humans. Handb Exp Pharmacol: DOI: 10.1007/1164_2020_1420 [DOI] [PubMed] [Google Scholar]
- Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J and Zhang F (2015) Brains, genes, and primates. Neuron 86:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Arfken CL, di Menza S and Schuster CR (2012) Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug Alcohol Depend 120:190–195. [DOI] [PubMed] [Google Scholar]
- Kakko J, Alho H, Baldacchino A, Molina R, Nava FA and Shaya G (2019) Craving in Opioid Use Disorder: From Neurobiology to Clinical Practice. Front Psychiatry 10:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J and Nagase H (2001) Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol 418:141–145. [DOI] [PubMed] [Google Scholar]
- Kangas BD and Bergman J (2014) Operant nociception in nonhuman primates. Pain 155:1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ and Ross SE (2014) Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo DR Jr., Anton F and Dubner R (1989) The detection and perceived intensity of noxious thermal stimuli in monkey and in human. J Neurophysiol 62:429–436. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, Hsu FC, Toll L, Husbands SM and Ko MC (2019) BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br J Anaesth 122:e146–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Kishioka S and Ko MC (2020a) Nociceptin/orphanin FQ peptide receptor-related ligands as novel analgesics. Curr Top Med Chem. [DOI] [PMC free article] [PubMed]
- Kiguchi N, Ding H and Ko MC (2020b) Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J Neurosci Res: Online ahead of print DOI: 10.1002/jnr.24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Peters CM, Kock ND, Kishioka S, Cline JM, Wagner JD and Ko MC (2017) Altered expression of glial markers, chemokines, and opioid receptors in the spinal cord of type 2 diabetic monkeys. Biochim Biophys Acta 1863:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N and Ko MC (2019) Effects of NOP-Related Ligands in Nonhuman Primates. Handb Exp Pharmacol 254:323–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC (2014) Roles of Central Opioid Receptor Subtypes in Regulating Itch Sensation, in Itch: Mechanisms and Treatment (Carstens E and Akiyama T eds), CRC Press/Taylor & Francis © 2014 by Taylor & Francis Group, LLC., Boca Raton (FL). [PubMed] [Google Scholar]
- Ko MC (2015) Neuraxial opioid-induced itch and its pharmacological antagonism. Handb Exp Pharmacol 226:315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER and Woods JH (1998) The role of peripheral mu opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther 286:150–156. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER and Woods JH (1999a) Activation of peripheral kappa opioid receptors inhibits capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther 289:378–385. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Divin MF, Lee H, Woods JH and Traynor JR (2006a) Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther 316:772–779. [DOI] [PubMed] [Google Scholar]
- Ko MC and Husbands SM (2009) Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther 328:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC and Husbands SM (2020) Pleiotropic Effects of Kappa Opioid Receptor-Related Ligands in Non-human Primates. Handb Exp Pharmacol: DOI: 10.1007/1164_2020_1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI and Woods JH (1999b) Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH and Naughton NN (2003) Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther 305:173–179. [DOI] [PubMed] [Google Scholar]
- Ko MC and Naughton NN (2000) An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology 92:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN, Traynor JR, Song MS, Woods JH, Rice KC and McKnight AT (2002a) Orphanin FQ inhibits capsaicin-induced thermal nociception in monkeys by activation of peripheral ORL1 receptors. Br J Pharmacol 135:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Song MS, Edwards T, Lee H and Naughton NN (2004) The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther 310:169–176. [DOI] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH and Winger G (2002b) Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther 301:698–704. [DOI] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH and Kennedy RT (2006b) Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther 318:1257–1264. [DOI] [PubMed] [Google Scholar]
- Ko MC, Willmont KJ, Burritt A, Hruby VJ and Woods JH (2000) Local inhibitory effects of dynorphin A-(1–17) on capsaicin-induced thermal allodynia in rhesus monkeys. Eur J Pharmacol 402:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC and Woods JH (1999) Local administration of delta9-tetrahydrocannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid action. Psychopharmacology (Berl) 143:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J and Prinssen EP (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR and Baxter LE (2019) Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am J Addict 28:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR and George TP (2002) The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 1:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczyńska K, Grzonkowski P, Kacprzak Ł and Zawilska JB (2018) Abuse of fentanyl: An emerging problem to face. Forensic Sci Int 289:207–214. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T and Suzuki H (2012) Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol 36:175–183. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H and Suzuki H (2010) Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 25:1251–1257. [DOI] [PubMed] [Google Scholar]
- LaMotte RH and Campbell JN (1978) Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol 41:509–528. [DOI] [PubMed] [Google Scholar]
- Lavonas EJ, Severtson SG, Martinez EM, Bucher-Bartelson B, Le Lait MC, Green JL, Murrelle LE, Cicero TJ, Kurtz SP, Rosenblum A, Surratt HL and Dart RC (2014) Abuse and diversion of buprenorphine sublingual tablets and film. J Subst Abuse Treat 47:27–34. [DOI] [PubMed] [Google Scholar]
- Lee H and Ko MC (2015) Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep 5:11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH and Ko MC (2007) Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology 107:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW (1985) Buprenorphine. Drug Alcohol Depend 14:363–372. [DOI] [PubMed] [Google Scholar]
- Li JX, Koek W and France CP (2012) Interactions between Δ(9)-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol 23:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Koek W, Rice KC and France CP (2010) Differential effects of serotonin 5-HT1A receptor agonists on the discriminative stimulus effects of the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rats and rhesus monkeys. J Pharmacol Exp Ther 333:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL and France CP (2008) Interactions between Delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology (Berl) 199:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao BY and Zhang J (2008) Null mutations in human and mouse orthologs frequently result in different phenotypes. Proc Natl Acad Sci U S A 105:6987–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP and Ko MC (2013) The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci 4:214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse MP, Saxon AJ, Mooney LJ, Thomas CM, Ang A, Matthews AG, Hasson A, Annon J, Sparenborg S, Liu DS, McCormack J, Church S, Swafford W, Drexler K, Schuman C, Ross S, Wiest K, Korthuis PT, Lawson W, Brigham GS, Knox PC, Dawes M and Rotrosen J (2016) Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction 111:1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH and Chen ZF (2011) Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT and Evans CJ (2003) Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE and Kieffer BL (2013) Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME and Clark AJ (2003) Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manage 25:496–498. [DOI] [PubMed] [Google Scholar]
- Macknik SL, Alexander RG, Caballero O, Chanovas J, Nielsen KJ, Nishimura N, Schaffer CB, Slovin H, Babayoff A, Barak R, Tang S, Ju N, Yazdan-Shahmorad A, Alonso JM, Malinskiy E and Martinez-Conde S (2019) Advanced Circuit and Cellular Imaging Methods in Nonhuman Primates. J Neurosci 39:8267–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR and France CP (2014) Impact of efficacy at the mu-opioid receptor on antinociceptive effects of combinations of mu-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther 351:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR and France CP (2018) Reinforcing effects of opioid/cannabinoid mixtures in rhesus monkeys responding under a food/drug choice procedure. Psychopharmacology (Berl) 235:2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Cami-Kobeci G, Husbands SM, France CP, Belli B and Flynn P (2020a) OREX-1019: A Novel Treatment of Opioid Use Disorder and Relapse Prevention. J Pharmacol Exp Ther 372:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Sanchez JJ, Javors MA, Disney A, Husbands SM and France CP (2020b) Effects of acute and repeated treatment with methocinnamox, a mu opioid receptor antagonist, on fentanyl self-administration in rhesus monkeys. Neuropsychopharmacology 45:1986–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Woods JH, Husbands SM, Disney A and France CP (2019) Long-Lasting Effects of Methocinnamox on Opioid Self-Administration in Rhesus Monkeys. J Pharmacol Exp Ther 368:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Yang W and France CP (2013) Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther 345:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL and Shoichet BK (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H and Watson SJ (1988) Anatomy of CNS opioid receptors. Trends Neurosci 11:308–314. [DOI] [PubMed] [Google Scholar]
- Mao J (2012) Current challenges in translational pain research. Trends Pharmacol Sci 33:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR and Eades CG (1964) A compariosn between acute and chronic physical dependence in the chronic spinal dog. J Pharmacol Exp Ther 146:385–394. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Lukas SE, Gastfriend DR, Teoh SK and Holman BL (1993) Buprenorphine treatment of opiate and cocaine abuse: clinical and preclinical studies. Harv Rev Psychiatry 1:168–183. [DOI] [PubMed] [Google Scholar]
- Mello NK and Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424. [DOI] [PubMed] [Google Scholar]
- Mello NK and Negus SS (1998) Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther 286:812–824. [PubMed] [Google Scholar]
- Meyer JS and Hamel AF (2014) Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. ILAR J 55:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Hirakata M, Miyamoto Y, Kainoh M, Wakasa Y and Yanagita T (2016) Nalfurafine hydrochloride, a selective κ opioid receptor agonist, has no reinforcing effect on intravenous self-administration in rhesus monkeys. J Pharmacol Sci 130:8–14. [DOI] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Gatch MB and Mello NK (2003) Effects of heroin and its metabolites on schedule-controlled responding and thermal nociception in rhesus monkeys: sensitivity to antagonism by quadazocine, naltrindole and beta-funaltrexamine. Drug Alcohol Depend 70:17–27. [DOI] [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, DeCosta B, Winger G and Woods JH (1994) Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther 270:1025–1034. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X and Rice K (1998) Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther 286:362–375. [PubMed] [Google Scholar]
- Neilan CL, Husbands SM, Breeden S, Ko MC, Aceto MD, Lewis JW, Woods JH and Traynor JR (2004) Characterization of the complex morphinan derivative BU72 as a high efficacy, long-lasting mu-opioid receptor agonist. Eur J Pharmacol 499:107–116. [DOI] [PubMed] [Google Scholar]
- Palmer CM, Emerson S, Volgoropolous D and Alves D (1999) Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology 90:437–444. [DOI] [PubMed] [Google Scholar]
- Park KM, Max MB, Robinovitz E, Gracely RH and Bennett GJ (1995) Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain 63:163–172. [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH and Ko MC (2008) Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology 33:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi JV Jr., Raffa RB and Rosenblatt MH (2020) Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J Clin Pharm Ther 45:892–903. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A and Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA and Voytko ML (2014) Why primate models matter. Am J Primatol 76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP and Woods JH (2011) The effects of nociceptin/orphanin FQ receptor agonist Ro 64–6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology (Berl) 213:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W and Bilsky EJ (2005) In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther 313:1150–1162. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK and Nestler EJ (1990) Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci 10:2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, White DA and Acri JB (2019) NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology 44:657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Vogelmann T, Trümper D and Scherbaum N (2020) Impact of Buprenorphine Dosage on the Occurrence of Relapses in Patients with Opioid Dependence. Eur Addict Res 26:77–84. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X and Greer JJ (2015) 5-HT1A receptor agonist Befiradol reduces fentanyl-induced respiratory depression, analgesia, and sedation in rats. Anesthesiology 122:424–434. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X and Greer JJ (2020) Countering Opioid-induced Respiratory Depression in Male Rats with Nicotinic Acetylcholine Receptor Partial Agonists Varenicline and ABT 594. Anesthesiology 132:1197–1211. [DOI] [PubMed] [Google Scholar]
- Rhesus Macaque Genome S, Analysis C, Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE and Zwieg AS (2007) Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234. [DOI] [PubMed] [Google Scholar]
- Rozsa AJ, Molinari HH, Greenspan JD and Kenshalo DR Sr. (1985) The primate as a model for the human temperature-sensing system: 1. Adapting temperature and intensity of thermal stimuli. Somatosens Res 2:303–314. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F and Scholl L (2016) Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65:1445–1452. [DOI] [PubMed] [Google Scholar]
- Saxon AJ (2013) Treatment of Opioid Dependence, in Research and Development of Opioid-Related Ligands (Ko MC and Husbands SM eds) pp 61–102. DOI: 110.1021/bk-2013-1131.ch1005, American Chemical Society, Washington, DC, USA. [Google Scholar]
- Schuckit MA (2016) Treatment of Opioid-Use Disorders. N Engl J Med 375:357–368. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation and Host Response to Injury LSCRP (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110:3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L and Zaveri NT (2008) Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol 153:609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A and Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89:2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD and Ko MC (2013) Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS One 8:e67422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC and Ko MC (2014) Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 231:1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA and Bonci A (2019) Dynorphin/kappa-opioid receptor control of dopamine dynamics: Implications for negative affective states and psychiatric disorders. Brain Res 1713:91–101. [DOI] [PubMed] [Google Scholar]
- Thompson T and Schuster CR (1964) MORPHINE SELF-ADMINISTRATION, FOOD-REINFORCED, AND AVOIDANCE BEHAVIORS IN RHESUS MONKEYS. Psychopharmacologia 5:87–94. [DOI] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo G, Cox BM and Zaveri NT (2016) Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev 68:419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Blake S, Faunce KE, Hwang CS, Natori Y, Zhou B, Bremer PT, Janda KD and Banks ML (2019) Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology 44:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Linz K, Koch T and Christoph T (2019) Cebranopadol: A Novel First-in-Class Potent Analgesic Acting via NOP and Opioid Receptors. Handb Exp Pharmacol 254:367–398. [DOI] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM and Grossman A (1997) The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol 120:781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND and Blanco C (2020) The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry: DOI: 10.1038/s41380-41020-40661-41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND and Collins FS (2017) The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377:391–394. [DOI] [PubMed] [Google Scholar]
- Volkow ND and McLellan AT (2016) Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med 374:1253–1263. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME and Bigelow GE (2001) Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 157:151–162. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ and Sadée W (2001) Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem 77:1590–1600. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang C, He Q, Matsuda M, Han Q, Wang K, Bang S, Ding H, Ko MC and Ji RR (2020a) Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci Transl Med 12:eaaw6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang C, Yao H, Chen O, Rahman S, Gu Y, Huh Y and Ji R-R (2020b) Central opioid receptors mediate morphine-induced itch and chronic itch. bioRxiv:2020.2005.2031.126805. [DOI] [PubMed]
- Warren WC, Harris RA, Haukness M, Fiddes IT, Murali SC, Fernandes J, Dishuck PC, Storer JM, Raveendran M, Hillier LW, Porubsky D, Mao Y, Gordon D, Vollger MR, Lewis AP, Munson KM, DeVogelaere E, Armstrong J, Diekhans M, Walker JA, Tomlinson C, Graves-Lindsay TA, Kremitzki M, Salama SR, Audano PA, Escalona M, Maurer NW, Antonacci F, Mercuri L, Maggiolini FAM, Catacchio CR, Underwood JG, O’Connor DH, Sanders AD, Korbel JO, Ferguson B, Kubisch HM, Picker L, Kalin NH, Rosene D, Levine J, Abbott DH, Gray SB, Sanchez MM, Kovacs-Balint ZA, Kemnitz JW, Thomasy SM, Roberts JA, Kinnally EL, Capitanio JP, Skene JHP, Platt M, Cole SA, Green RE, Ventura M, Wiseman RW, Paten B, Batzer MA, Rogers J and Eichler EE (2020) Sequence diversity analyses of an improved rhesus macaque genome enhance its biomedical utility. Science 370:eabc6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxler B, Dadabhoy ZP, Stojiljkovic L and Rabito SF (2005) Primer of postoperative pruritus for anesthesiologists. Anesthesiology 103:168–178. [DOI] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE and Koob GF (2012) A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med 4:146ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed PF, Gerak LR and France CP (2018) Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur J Pharmacol 833:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE and Goodwin AK (2007) The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol 15:309–327. [DOI] [PubMed] [Google Scholar]
- Woods JH and Winger G (1987) Behavioral characterization of opioid mixed agonist-antagonists. Drug Alcohol Depend 20:303–315. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhao T, Liu S, Wu Q, Johnson O, Wu Z, Zhuang Z, Shi Y, Peng L, He R, Yang Y, Sun J, Wang X, Xu H, Zeng Z, Zou P, Lei X, Luo W and Li Y (2019) MRGPRX4 is a bile acid receptor for human cholestatic itch. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Naylor JE, Huskinson SL, Townsend EA, Prisinzano TE and Freeman KB (2020) Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys. Psychopharmacology (Berl) 237:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


