Abstract
Rationale & Objective:
Obesity has been related to risk for chronic kidney disease. However, the associations of different measures of midlife obesity with long-term kidney function trajectories and whether they differ by sex and race are unknown.
Study Design:
Observational study.
Setting & Participants:
13,496 participants from the Atherosclerosis Risk in Communities (ARIC) Study.
Predictors:
Midlife obesity status as measured by body mass index (BMI), waist-to-hip ratio, and predicted percent fat at baseline.
Outcomes:
Estimated glomerular filtration rate (eGFR) calculated using serum creatinine level measured at 5 study visits, and incident kidney failure with replacement therapy (KFRT).
Analytical Approach:
Mixed models with random intercepts and random slopes for eGFR. Cox proportional hazards models for KFRT.
Results:
Baseline mean age was 54 years, median eGFR was 103 mL/min/1.73 m2, and median BMI was 27 kg/m2. Over 30 years of follow-up, midlife obesity measures were associated with eGFR decline in White and Black women but not consistently in men. Adjusted for age, center, smoking, and coronary heart disease, the differences in eGFR slope per 1-SD higher BMI, waist-to-hip ratio, and predicted percent fat were 0.09 (95% CI, −0.18 to 0.36), −0.25 (95% CI, −0.50 to 0.01), and −0.14 (95% CI, −0.41 to 0.13) mL/min/1.73 m2 per decade for White men; −0.91 (95% CI, −1.15 to −0.67), −0.82 (95% CI, −1.06 to −0.58), and −1.02 (95% CI, −1.26 to −0.78) mL/min/1.73 m2 per decade for White women; −0.70 (95% CI, −1.54 to 0.14), −1.60 (95% CI, −2.42 to −0.78), and −1.24 (95% CI, −2.08 to −0.40) mL/min/1.73 m2 per decade for Black men; and −1.24 (95% CI, −2.08 to −0.40), −1.50 (95% CI, −2.05 to −0.95), and −1.43 (95% CI, −2.00 to −0.86) mL/min/1.73 m2 per decade for Black women. Obesity indicators were independently associated with risk for KFRT for all sex-race groups except White men.
Limitations:
Loss to follow-up during 3 decades of follow-up with 5 eGFR assessments.
Conclusions:
Obesity status is a risk factor for future decline in kidney function and development of KFRT in Black and White women, with less consistent associations among men.
Kidney function trajectories have long been used in the estimation of time to kidney failure.1 Recently, kidney function decline over time has been related not only to kidney failure but also to all-cause mortality and cardiovascular disease risk.2–4 Understanding risk factors for different patterns of kidney function trajectories is important so that individuals at risk for rapid progression may be targeted early for interventions. Successful interventions may prevent the development of disease among individuals with normal kidney function and slow the progression among those with kidney disease.
Obesity may be a targetable risk factor in the prevention of kidney function decline. Higher body mass index (BMI) has been associated with increased risk for incident chronic kidney disease (CKD), including greater kidney function decline among healthy young adults.5–7 However, BMI may not be the best marker of obesity-related risk and associations may differ across sex and race.8–12 Much less is known about the relationship between other obesity indicators, such as waist-to-hip ratio and the recently developed predicted percent fat,13 and long-term kidney function decline.
This study evaluated the associations of midlife obesity with subsequent trajectories of estimated glomerular filtration rate (eGFR) and risks for developing kidney failure with replacement therapy (KFRT) across sex-race groups in a community-based cohort of 13,496 middle-aged White and Black men and women during 30 years of follow-up. We examined several different obesity measures given the controversy over the optimal method of estimating obesity, with the goal of exploring whether obesity may precede faster kidney function decline.
Methods
Study Design and Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort study designed to evaluate risk factors for the development of cardiovascular disease.14 It enrolled a total of 15,792 middle-aged (45-64 years old at baseline) predominantly White and Black men and women from 4 communities in the United States: Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD. The first examination took place in 1987 to 1989 (baseline; study visit 1), with follow-up examinations initially at approximately 3-year intervals: 1990 to 1992 (study visit 2), 1993 to 1995 (study visit 3), 1996 to 1998 (study visit 4), and more recently, in 2011 to 2013 (study visit 5) and 2016 to 2017 (study visit 6). During each study visit, an extensive questionnaire was administered, a clinical examination was conducted, and blood and urine specimens were collected.
In the present study, we excluded study participants who at baseline had KFRT (n = 150), eGFR < 60 mL/min/1.73 m2 (n = 341), diabetes (n = 1,797), or BMI < 18.5 kg/m2 (n = 143). Thus, the analytic sample size was 13,496 (85.5% of the original cohort). We excluded participants with prevalent diabetes because clinical diabetes can lead to intentional and unintentional weight loss. Study participants provided written documentation of informed consent and study protocols were approved by the institutional review board at each study site.
Assessment of Obesity Status
BMI was calculated as measured weight (in kilograms) divided by measured height (in meters) squared. Waist-to-hip ratio, which has been shown by some studies to be the more appropriate metric for obesity-related risk stratification among older adults,15 was calculated from measurements of the circumference at umbilical level (waist) and maximum buttocks (hip) to the nearest centimeter. Predicted percent fat was derived using sex-specific anthropometric prediction equations including information on age, race, weight, height, and waist circumference (WC). The equations have been reported to explain a large amount of the variation in percent fat (R2 of 0.73 for men and 0.65 for women).13 We evaluated predicted percent fat as an indicator of obesity because it was more strongly correlated with obesity-related biomarkers compared with BMI in previous studies.13 The anthropometric prediction equations are:
Men: Percent fat (%) = 0.02 + (0.00 × age in years) − (0.07 × height in cm) − (0.08 × weight in kg) + (0.48 × WC in cm) + (0.32 if Mexican) + (0.02 if Hispanic) − (0.65 if Black) + (1.12 if other ethnicity)
Women: Percent fat (%) = 50.46 + (0.07 × age in years) − (0.26 × height in cm) + (0.27 × weight in kg) + (0.10 × WC in cm) + (0.89 if Mexican + (0.49 if Hispanic) − (1.57 if Black) + (0.43 if other ethnicity)
We modeled all obesity measurements both as continuous variables and in tertiles.
Assessment of Kidney Function
Kidney function was assessed by measuring creatinine in serum or plasma specimens collected during each study visit, except for study visit 3. A modified kinetic Jaffé method was adapted to measure creatinine and it was standardized to the National Institute of Standards and Technology standard and calibrated across study visits using repeat measurements from a sample of 200 ARIC Study participants.16–18 The CKD Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR based on creatinine level.19 For participants who developed incident KFRT (ascertained through linkage to the US Renal Data System [USRDS]), eGFR of 15 mL/min/1.73 m2 was imputed on the date of initiation of kidney replacement therapy (transplantation or dialysis).
Assessment of Other Variables
Demographic characteristics (date of birth for the calculation of age, sex, and race) and medical history (coronary heart disease [CHD]) were ascertained by a questionnaire administered by trained interviewers at the baseline study visit. Systolic blood pressure was measured 3 times using a random-zero sphygmomanometer. The average of the second and third measurements was used in the analysis. Study participants brought medications to the study visit and the names of all medications were transcribed. Blood samples that were collected from study participants during the baseline study visit were assayed for the measurement of high-density lipoprotein (HDL) cholesterol using an enzymatic method after precipitation with dextran sulfate-magnesium. Bioelectrical impedance (BIA), measured using the BIA 101-F device (Akern/RJL), was used to measure percent body fat and fat mass at visit 5.
Statistical Analysis
Baseline characteristics of the study population were compared by baseline BMI tertile, sex, and racial group using descriptive statistics and differences were tested using analysis of variance for continuous variables and χ2 tests for categorical variables. Spearman correlations between BIA-measured percent fat at visit 5 and BMI, waist-to-hip ratio, or predicted percent fat at visit 5 were examined within each sex and racial group. Scatterplots of waist-to-hip ratio and predicted percent fat against BMI tertile at baseline were shown by sex and race. We estimated the differences in annual eGFR decline slope according to baseline obesity status tertiles.20 Kernel density plots were used to illustrate the distribution of unadjusted annual predicted change in eGFR. Mixed models were used to evaluate the association between obesity status at baseline and eGFR trajectories using random intercepts and random slopes to account for individual variation in eGFR at baseline and its change. Cox proportional hazards models were used to estimate the association between baseline obesity status and KFRT. All models were stratified by sex and race (White/Black) because the association of eGFR decline showed an interaction with baseline obesity across race-sex groups (P < 0.001 for all obesity measurements), as well as larger variance in Blacks.
Two models were constructed. Model 1 was adjusted for age (continuous), center (Minneapolis, MN/Washington County, MD/Jackson, MS/Forsyth County, NC), current smoker (yes/no), and history of CHD (yes/no) at baseline. For model 2, we further adjusted for hypertension medication use (yes/no), systolic blood pressure (continuous), HDL cholesterol level (continuous), and eGFR (continuous; KFRT model only) at baseline to assess the associations of obesity and kidney function decline independent of other obesity-associated comorbid conditions. Because socioeconomic status may be associated with both weight change and kidney function, we also additionally adjusted for family income (annual income ≥ $25,000/<$25,000/not reported), and education (graduated high school/not graduated) and tested their interactions with obesity measures and time.
In sensitivity analyses, we categorized baseline obesity measures (BMI, waist-to-hip ratio, and predicted percent fat) into tertiles by sex and race and examined their associations with eGFR trajectories using the same mixed models as the main analysis. We examined the associations of interest only among participants with valid information on visit 6 using the same methods to test the robustness of our main results. Because smoking can lead to weight loss and modify the associations with obesity-related health conditions, we conducted a sensitivity analysis excluding current smokers.21 Because obesity is a risk factor for increased mortality,22 we conducted a Fine-Gray competing-risks analysis.23 All analyses were conducted using R, version 3.3.3 (R Development Core Team).
Results
Baseline Characteristics
Baseline characteristics of the 13,496 study participants (10,222 White and 3,274 Black) according to baseline BMI tertile, sex, and racial group are shown in Table 1. For all 4 sex and racial groups, participants with higher baseline BMI, particularly those in the highest tertile, were more likely to have higher waist-to-hip ratio, predicted percent fat, and systolic blood pressure and a history of CHD. They were also more likely to take antihypertensive medication and have lower HDL cholesterol levels and less likely to be current smokers (P < 0.001 for all comparisons).
Table 1.
Characteristics of the Study Population According to Baseline BMI Tertiles by Sex and Race (in 1987-1989)
| White (n = 10,222) |
Black (n = 3,274) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 4,802) |
Women (n = 5,420) |
Men (n = 1,270) |
Women (n = 2,004) |
|||||||||
| Characteristic | T1a (n = 1,601) |
T2a (n = 1,600) |
T3aaa (n = 1,601) |
T1a (n = 1,809) |
T2a (n = 1,803) |
T3a (n = 1,808) |
T1a (n = 423) |
T2a (n = 424) |
T3aA (n = 423) |
T1a (n = 668) |
T2a (n = 668) |
T3a (n = 668) |
| BMI, kg/m2 | 23.5 (1.5) | 26.8 (0.8) | 31.4 (3.1) | 21.6 (1.2) | 25.3 (1.1) | 32.2 (4.2) | 22.7 (1.7) | 26.9 (1.1) | 32.2 (3.5) | 24.2 (2.0) | 29.3 (1.4) | 37.4 (5.2) |
| WHRb | 0.93 (0.05) | 0.97 (0.04) | 1.00 (0.04) | 0.84 (0.07) | 0.88 (0.07) | 0.93 (0.07) | 0.90 (0.05) | 0.93 (0.04) | 0.97 (0.05) | 0.85 (0.07) | 0.90 (0.07) | 0.94 (0.07) |
| Predicted % fatb | 25.1 (2.3) | 28.2 (2.0) | 32.2 (3.2) | 35.4 (1.5) | 39.1 (1.4) | 45.5 (3.9) | 22.3 (2.6) | 25.9 (2.3) | 30.8 (4.1) | 36.1 (2.1) | 40.9 (1.6) | 48.5 (4.8) |
| eGFR, mL/min/1.73 m2b | 99.5 (11.1) | 97.8 (11.0) | 970 (11.6) | 103.0 (10.3) | 101.2 (11.4) | 100.4 (12.0) | 112.3 (15.5) | 107.9 (15.4) | 105.4 (16.7) | 115.4 (16.3) | 114.8 (15.9) | 115.4 (16.2) |
| Age, yb | 55.3 (5.7) | 55.1 (5.7) | 54.9 (5.6) | 53.9 (5.7) | 54.5 (5.6) | 54.5 (5.7) | 54.6 (6.0) | 53.8 (5.9) | 53.6 (5.7) | 53.3 (5.8) | 53.4 (5.6) | 53.2 (5.7) |
| Current smokerb | 510 (31.9%) | 358 (22.4%) | 325 (20.3%) | 558 (30.9%) | 438 (24.3%) | 339 (18.8%) | 217 (51.3%) | 157 (37.1%) | 113 (26.7%) | 226 (33.9%) | 158 (23.7%) | 126 (18.9%) |
| HTN med useb | 203 (12.7%) | 265 (16.7%) | 380 (23.9%) | 169 (9.4%) | 283 (15.7%) | 471 (26.2%) | 89 (21.1%) | 127 (30.1%) | 166 (39.4%) | 199 (29.9%) | 255 (38.4%) | 310 (46.8%) |
| SBP, mm Hgb | 116.6 (15.6) | 119.2 (15.6) | 122.0 (15.2) | 111.6 (16.3) | 115.7 (17.1) | 121.3 (16.8) | 127.7 (22.6) | 129.4 (21.6) | 130.7 (20.5) | 123.3 (20.8) | 125.2 (18.4) | 129.9 (20.3) |
| HDL-C, mg/Lb | 47.1 (13.9) | 42.6 (11.5) | 39.9 (10.2) | 64.8 (17.6) | 58.6 (16.3) | 51.7 (14.0) | 57.6 (20.3) | 50.0 (14.2) | 46.1 (12.6) | 63.9 (18.7) | 59.0 (17.3) | 55.3 (14.2) |
| Prevalent CHDb | 123 (79%) | 120 (7.6%) | 132 (8.4%) | 17 (1.0%) | 27 (1.5%) | 35 (2.0%) | 15 (3.6%) | 17 (4.1%) | 22 (5.2%) | 14 (2.2%) | 12 (1.8%) | 12 (1.8%) |
| HS graduateb | 1,329 (83.2%) | 1,316 (82.4%) | 1,321 (82.6%) | 1,625 (89.9%) | 1,553 (86.1%) | 1,416 (78.4%) | 225 (53.4%) | 253 (60.0%) | 251 (59.6%) | 487 (73.0%) | 411 (61.6%) | 375 (56.2%) |
| Annual family income < $25kb | 335 (20.9%) | 284 (17.8%) | 308 (19.2%) | 423 (23.4%) | 496 (27.5%) | 612 (33.8%) | 253 (59.8%) | 217 (51.2%) | 209 (49.4%) | 384 (57.5%) | 440 (65.9%) | 484 (72.5%) |
Note: N = 13,496. Mean (standard deviation) for continuous variables and number (percent) for categorical variables.
Abbreviations: BMI, body mass index; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HS, high school; HTN, hypertension; med, medication; SBP, systolic blood pressure; T, tertile; WHR, waist-to-hip ratio.
The cutoff points between the low and mid tertiles and between mid and high tertiles are 25.4 and 28.3 kg/m2 for White men, 23.4 and 27.5 kg/m2 for White women, 25.1 and 28.8 kg/m2 for Black men, and 27.0 and 32.0 kg/m2 for Black women.
P < 0.001 for comparing BMI groups; P value calculated by analysis of variance for continuous variables and X2 test for categorical variables.
Correlations of Obesity Measures
Percent fat measured by BIA at visit 5 was strongly correlated with all concomitant obesity measures overall and in all 4 sex-race groups (all P < 0.001; Fig S1). However, the strength of the correlation and differences between sex-race groups varied substantially. BMI and predicted percent fat had the highest correlations with BIA-measured percent fat (ranging from 0.62 to 0.85 across sex-race categories). In contrast, waist-to-hip ratio has a weaker overall correlation of −0.15 (ranging from 0.18 to 0.46 across sex-race categories). Predicted percent fat was the only measure that captured the sex difference in obesity measurements, resulting in convergence of regression lines for men and women. Density plots of distributions across baseline BMI tertiles showed that although waist-to-hip ratio and predicted percent fat increased with BMI, there was substantial overlap across tertiles, indicating that the measures would not classify the obesity status of individuals identically (Fig S2).
Variation in Annual Change in eGFR by Race and Sex
Among women, annual decline in eGFR was more rapid among participants in tertiles 2 and 3 of all 3 baseline obesity measurements; however, there was substantial overlap across categories. Black men in tertiles 2 and 3 of waist-to-hip ratio and predicted percent fat also had more substantial declines in eGFR, but we did not observe such a trend with BMI tertiles in this group (Fig 1A–C). Median annual eGFR declines in the low, mid, and high tertiles of baseline waist-to-hip ratio were 1.31 (interquartile range [IQR], 1.10-1.53), 1.37 (IQR, 1.16-1.61), and 1.32 (IQR, 1.11-1.52) mL/min/1.73 m2 per year for White men; 1.32 (IQR, 1.14-1.49), 1.43 (IQR, 1.27-1.59), and 1.51 (IQR, 1.35-1.67) mL/min/1.73 m2 per year for White women; 1.61 (IQR, 1.41-1.77), 1.69 (IQR, 1.45-1.85), and 1.90 (IQR, 1.60-2.07) mL/min/1.73 m2 per year for Black men; and 1.76 (IQR, 1.51-2.02), 1.94 (IQR, 1.67-2.18), and 2.10 (IQR, 1.86-2.28) mL/min/1.73 m2 per year for Black women, and those for baseline predicted percent fat were 1.29 (IQR, 1.08-1.51), 1.38 (IQR, 1.16-1.62), and 1.34 (IQR, 1.14-1.55) mL/min/1.73 m2 per year for White men; 1.32 (IQR, 1.13-1.49), 1.44 (IQR, 1.26-1.59), and 1.52 (IQR, 1.36-1.68) mL/min/1.73 m2 per year for White women; 1.62 (IQR, 1.40-1.82), 1.70 (IQR, 1.43-1.89), and 1.92 (IQR, 1.66-2.04) mL/min/1.73 m2 per year for Black men; and 1.81 (IQR, 1.53-2.07), 1.88 (IQR, 1.62-2.12), and 2.13 (IQR, 1.88-2.33) mL/min/1.73 m2 per year for Black women.
Figure 1.
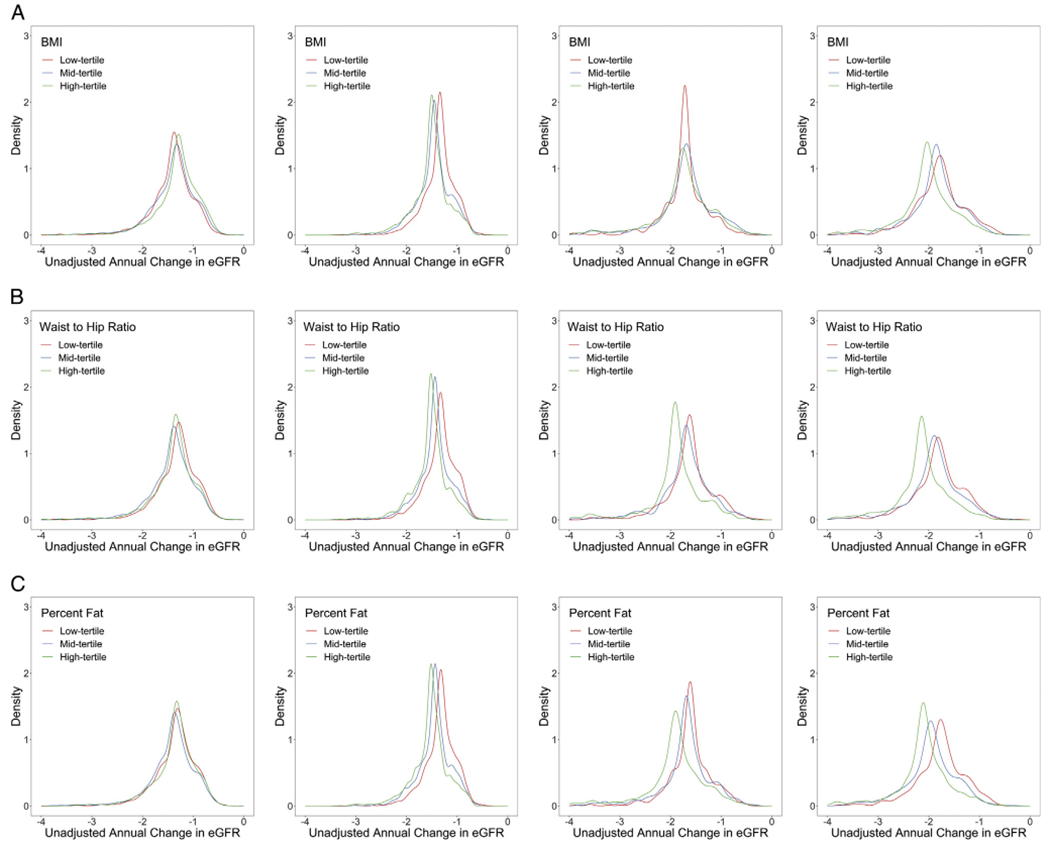
Distribution of unadjusted predicted average annual change in estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) within the Atherosclerosis Risk in Communities (ARIC) population according to baseline obesity marker tertile by sex and race. (A) Annual eGFR change according to body mass index (BMI) tertile among (left to right) White men, White women, Black men, and Black women. (B) Annual eGFR change according to waist-to-hip ratio tertile among (left to right) White men, White women, Black men, and Black women. (C) Annual eGFR change according to baseline predicted percent fat tertile among (left to right) White men, White women, Black men, and Black women.
Difference in eGFR Decline by Markers of Obesity
In women, all obesity indicators were associated with eGFR decline during 30 years’ follow-up. In Black men, only waist-to-hip ratio and predicted percent fat were associated with eGFR decline; in White men, no measures of obesity were associated with eGFR decline. Adjusted for age, center, smoking, and CHD, the difference in eGFR slope per 1-SD higher baseline BMI, waist-to-hip ratio, and predicted percent fat were 0.09 (95% CI, −0.18 to 0 36), −0.25 (95% CI, −0.50 to 0.01), and −0.14 (95% CI, −0.41 to 0.13) mL/min/1.73 m2 per decade for White men; −0.91 (95% CI, −1.15 to −0.67), −0.82 (95% CI, −1.06 to −0.58), and −1.02 (95% CI, −1.26 to −0.78) mL/min/1.73 m2 per decade for White women; −0.70 (95% CI, −1.54 to 0.14), −1.60 (95% CI, −2.42 to −0.78), and −1.24 (95% CI, −2.08 to −0.40) mL/min/1.73 m2 per decade for Black men; and −1.24 (95% CI, −2.08 to −0.40), −1.50 (95% CI, −2.05 to −0.95), and −1.43 (95% CI, −2.00 to −0.86) mL/min/1.73 m2 per decade for Black women (Table 2). None of the interactions between family income or education and obesity measurements with kidney function decline were significant after adjusting for multiple comparisons. Results were similar to the main analysis in sensitivity analyses that examined the association between tertiles of obesity indicators and eGFR decline to check the impact of potential nonlinearity (Table S1), that only included participants who attended visit 6 (Table S2), that excluded current smokers (Table S3), and that accounted for the competing risk for death before KFRT (Table S4).
Table 2.
Association of eGFR Slope With 3 Measures of Baseline Obesity by Sex and Race
| White (n = 10,222) |
Black (n = 3,274) |
|||
|---|---|---|---|---|
| Men (n = 4,802) | Women (n = 5,420) | Men (n = 1,270) | Women (n = 2,004) | |
| BMI, kg/m2 | 27.23 ± 3.86 | 26.33 ± 5.09 | 27.28 ± 4.53 | 30.29 ± 6.36 |
| eGFR slope per 1-SD greater BMIa,b | ||||
| Model 1c,d | 0.09 (−0.18 to 0.36) | −0.91 (−1.15 to −0.67)e | −0.70 (−1.54 to 0.14) | −1.35 (−1.92 to −0.78)e |
| Model 2c,d | 0.52 (0.23 to 0.80)e | −0.27 (−0.53 to −0.02)f | 0.03 (−0.85 to 0.91) | −0.85 (−1.45 to −0.25)g |
| WHR | 0.97 ± 0.05 | 0.89 ± 0.08 | 0.94 ± 0.05 | 0.90 ± 0.08 |
| eGFR slope per 1-SD greater WHRa,b | ||||
| Model 1 c,d | −0.25 (−0.50 to 0.005) | −0.82 (−1.06 to −0.58)e | −1.60 (−2.42 to −0.78)e | −1.50 (−2.05 to −0.95)e |
| Model 2c,d | 0.15 (−0.13 to 0.43) | −0.16 (−0.41 to 0.09) | −0.40 (−1.32 to 0.52) | −1.07 (−1.68 to −0.46)e |
| Predicted percent fat, % | 28.49 ± 3.88 | 39.99 ± 4.92 | 26.31 ± 4.65 | 41.83 ± 5.99 |
| eGFR slope per 1-SD greater predicted % fata,b | ||||
| Model 1 c,d | −0.14 (−0.41 to 0.13) | −1.02 (−1.26 to −0.78)e | −1.24 (−2.08 to −0.40)g | −1.43 (−2.00 to −0.86)e |
| Model 2c,d | 0.30 (0.02 to 0.59)f | −0.32 (−0.58 to −0.06)f | −0.26 (−1.17 to 0.65) | −0.90 (−1.5 to −0.29)g |
Note: The unit for eGFR slope was mL/min/1.73 m2 per decade; all such values given as estimate (95% CI).
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; SD, standard deviation; WHR, waist-to-hip ratio.
Centered at median of each race-sex group.
In an overall unadjusted model combining sex-race groups, P < 0.001 for all obesity measurements for interaction between sex-race and the association of eGFR decline with baseline obesity.
Model 1 adjusted for age (continuous), center (categorical), current smoker (yes/no), and prevalent coronary heart disease (yes/no) at baseline; model 2 additionally adjusted for hypertension medication (yes/no), systolic blood pressure (continuous), total cholesterol level (continuous), high-density lipoprotein cholesterol level (continuous), triglyceride level (continuous, log transformed), education level (high school graduate/not), and annual family income (categorical) at baseline.
Black participants in the Minnesota and Washington County centers were excluded in the model because of small numbers.
P < 0.001.
P < 0.05.
P < 0.01.
Risk for Developing KFRT
All obesity indicators were associated with increased risk for KFRT for all sex-race groups except among White men. Adjusted for age, center, smoking, and CHD, the hazard ratios for KFRT per 1-SD greater BMI, waist-to-hip ratio, and predicted percent fat were 1.26 (95% CI, 0.98-1.62), 1.12 (95% CI, 0.86-1.48), and 1.19 (95% CI, 0.91-1.54) for White men; 1.51 (95% CI, 1.14-2.01), 1.79 (95% CI, 1.26-2.53), and 1.72 (95% CI, 1.31-2.26) for White women; 1.75 (95% CI, 1.29-2.36), 1.99 (95% CI, 1.38-2.87), and 1.86 (95% CI, 1.36-2.55) for Black men; and 1.68 (95% CI, 1.33-2.13), 1.78 (95% CI, 1.32-2.40), and 1.68 (95% CI, 1.32-2.14) for Black women (Table 3). The 30-year difference in risk for KFRT across tertiles ranged from 0.8% to 5.8% (Fig S3).
Table 3.
HRs for KFRT According to Baseline Obesity Status by Sex and Race
| White (n/N = 95/10,222) |
Black (n/N = 95/3,274) |
|||
|---|---|---|---|---|
| Men (n/N = 57/4,802) | Women (n/N = 43/5,420) | Men (n/N = 39/1,270) | Women (n/N = 56/2,004) | |
| BMI, kg/m2 | 27.23 ± 3.86 | 26.33 ± 5.09 | 27.28 ± 4.53 | 30.29 ± 6.36 |
| HR per 1-SD greater BMIa | ||||
| Model 1 b,c | 1.26 (0.98-1.62) | 1.51 (1.14-2.01)d | 1.75 (1.29-2.36)e | 1.68 (1.33-2.13)e |
| Model 2b,c | 1.04 (0.79-1.39) | 1.06 (0.76-1.46) | 1.51 (1.09-2.11)f | 1.65 (1.29-2.13)e |
| WHR | 0.97 ± 0.05 | 0.89 ± 0.08 | 0.94 ± 0.05 | 0.90 ± 0.08 |
| HR per 1-SD greater WHRa | ||||
| Model 1 b,c | 1.12 (0.86-1.48) | 1.79 (1.26-2.53)d | 1.99 (1.38-2.87)e | 1.78 (1.32-2.40)e |
| Model 2b,c | 0.92 (0.69-1.23) | 1.26 (0.86-1.84) | 1.73 (1.18-2.53)d | 1.74 (1.26-2.40)d |
| Predicted % fat | 28.49 (3.88) | 39.99 (4.92) | 26.31 (4.65) | 41.83 (5.99) |
| HR per 1-SD greater predicted % fata | ||||
| Model 1 b,c | 1.19 (0.91-1.54) | 1.72 (1.31-2.26)d | 1.86 (1.36-2.55)d | 1.68 (1.32-2.14)d |
| Model 2b,c | 0.94 (0.71-1.25) | 1.27 (0.92-1.74) | 1.63 (1.16-2.30)e | 1.61 (1.24-2.09)d |
Note: Values in parentheses are 95% confidence intervals.
Abbreviations: BMI, body mass index; HR, hazard ratio; KFRT, kidney failure with replacement therapy; SD, standard deviation; WHR, waist-to-hip ratio.
Centered at median of each race-sex group.
Model 1 is random-effects model showing rate of decline (the interaction of each variable with follow-up time) adjusted for age (continuous), center (categorical), current smoker (yes/no), and prevalent coronary heart disease (yes/no) at baseline; model 2 additionally adjusted for hypertension medication (yes/no), systolic blood pressure (continuous), total cholesterol level (continuous), high-density lipoprotein cholesterol level (continuous), triglyceride level (continuous, log transformed), estimated glomerular filtration rate (continuous), education level (high school graduate/not graduated), and annual family income (categorical) at baseline.
Black participants in the Minnesota and Washington County centers were excluded in the model because of small numbers.
P < 0.01.
P < 0.001.
P < 0.05.
Discussion
In this community-based population of 13,496 middle-aged adults, we observed that obesity status, measured by BMI, waist-to-hip ratio, and predicted percent fat, was generally associated with more rapid future decline in kidney function and higher risk for developing KFRT during 30 years of follow-up. Associations were observed in White and Black women, as well as Black men; however, there was no evidence supporting associations between markers of obesity and eGFR decline or KFRT in White men. The more novel measure of obesity, predicted percent fat, a sex-specific equation that incorporates age, race, weight, height, and WC, was highly correlated with BIA-measured percent fat. Our study further documents the possible benefits of having normal BMI and obesity measures in midlife by suggesting that they are associated with slower rates of kidney function decline, at least in White and Black women and Black men. Documenting the full range of benefits of having normal BMI and obesity measures is important because obesity prevention may require significant effort and cost. The stronger associations with KFRT suggest that the overall benefit of having normal BMI and obesity measurements may be greater for higher risk groups.
The current study adds to the existing literature by demonstrating that midlife obesity is a risk factor for kidney function decline and the development of KFRT in later life in women and Black men and by quantifying the mean rate and range of decrease in kidney function during 30 years of follow-up. Existing research on kidney function trajectories has been more focused on individuals with kidney diseases rather than those with preserved kidney function.24,25 Our study addressed this gap by evaluating baseline obesity categories as a predictor of kidney function decline among individuals with preserved kidney function. We examined kidney function from midlife to older age, when the prevalence of kidney disease is highest and KFRT most often occurs.
Our study demonstrated that the association of midlife obesity with decline in kidney function differed by race and sex. Measures of obesity were not associated with kidney function decline in White men and BMI—but not waist-to-hip ratio or predicted percent fat—predicted kidney outcomes in Black men. Differences by sex in susceptibility to kidney outcomes associated with obesity among middle-aged adults has been described in previous literature. For example, previous studies suggested that higher BMI was a significant risk factor for the development of CKD in women, but not men, in a Japanese community cohort.11 However, the underlying mechanisms for this difference are unknown.
The lack of association in White men may be due to the combination of lower obesity and lower kidney disease progression among Whites, reducing power. Alternatively, there may be greater variation in muscle mass as a non-GFR influence of creatinine level among men compared with women.26–30 Given the greater magnitude and range of measurements of obesity in women than in men, it is also possible that the power to observe associations between obesity and future kidney function decline was larger and obesity explained more variance in rates of decline in women than in men.
In general, the ARIC cohort follows the expected trends from previous studies, with weight gain dominating in midlife and weight loss increasing at older age.31–34 The mix of intentional and unintentional weight loss is unknown. Therefore, we focused on baseline weight to provide clear temporality and minimize reverse causation. Longitudinal tracking of obesity rank simplifies the interpretation of midlife obesity as a risk factor for kidney disease progression over the subsequent decades.35
Our study used 3 indicators to model obesity: BMI, waist-to-hip ratio, and predicted percent fat. BMI has been most widely used in clinical and public health settings; however, whether it is the most suitable measure for all scenarios has been debated.8–10 Some studies have proposed that waist-to-hip ratio is more appropriate than BMI for gauging risk in middle- to older-age adults because generalized obesity in older ages has been thought to provide protection against injury, nutritional reserve against illness, and better weight-bearing bone formation.36–39 Also, BMI has been criticized for not being able to discriminate individuals with different body composition of fat mass and lean body mass, which has been suggested as the reason for the “obesity paradox,” a phenomenon that overweight and obese individuals have better health outcomes compared with normal-weight counterparts in some settings.40,41 In our study, we observed high sex/race-specific correlations between BMI and BIA-measured percent fat. Waist-to-hip ratio was distinct from BMI and was relatively weakly correlated with BIA-measured percent fat, although their associations with kidney outcomes were similar. The novel obesity marker, predicted percent fat, appeared to be not only highly correlated with BIA-measured percent fat but also a risk factor with considerable magnitude of both kidney function decline and risk for KFRT in women and Black men. However, associations between different indicators of obesity and kidney outcomes were in general similar. This is probably because our study population was generally healthy, while BMI loses its value mostly at advanced disease stage when loss of lean mass is important.41–43 Our results suggested that BMI is a good measure in a general population cohort for kidney outcomes and the improvement with more sophisticated measures in this setting is likely marginal.
This study has several strengths. The ARIC Study is a large prospective cohort. The long duration of the study (30 years of follow-up) permits characterization of kidney function decline in a population in which health was originally generally good. Given the inclusion of both Whites and Blacks and both men and women from 4 distinct US communities, we were able to examine associations between obesity and kidney outcomes by sex and race. BMI was measured and not self-reported. Also, multiple established risk factors were collected in a standardized manner according to a research protocol.
The main limitation of this study is that there were only up to 5 eGFR assessments for the estimation of long-term trajectories. However, there are few longitudinal population-based cohorts with more frequent assessments of eGFR over 30 years. Participants reaching KFRT were less likely to survive to participate in subsequent study visits; we included an estimate of their trajectory by imputing an eGFR of 15 mL/min/1.73 m2 at the time of KFRT onset. The potential differential loss to follow-up may also occur for people in higher tertiles of obesity at baseline. Although we measured and adjusted for many potential confounders, we cannot exclude the possibility of residual confounding.
In conclusion, we observed in community-dwelling adults that midlife obesity status was a risk factor for future decline in kidney function and the development of KFRT in all sex-race subgroups except for White men. The lack of associations in White men suggests that the role of obesity and its optimal quantification for kidney disease risk requires further study.
Supplementary Material
Figure S1: Scatterplot of BIA-measured percent fat and BMI, waist-to-hip ratio, or predicted percent fat at visit 5 with generalized additive regression line.
Figure S2: Distributions of waist-to-hip ratio and predicted percent fat according to baseline BMI tertile, by sex and race.
Figure S3: Kaplan-Meier survival free of KFRT by tertile of baseline obesity measure within each sex-race group, showing absolute risk of KFRT.
Table S1: Difference in eGFR slope according to baseline obesity status tertile by sex and race.
Table S2: Difference in eGFR slope according to baseline obesity status tertile by sex and race, among participants who had visit 6 information.
Table S3: Association of eGFR slope with 3 measures of baseline obesity by sex and race, excluding current smokers.
Table S4: Subhazard ratios for KFRT according to baseline obesity status by sex and race, using a Fine-Gray competing-risk model.
PLAIN-LANGUAGE SUMMARY.
Obesity is a targetable risk factor in the prevention of kidney function decline. However, although it is most commonly used, body mass index may not be the best marker of obesity-related risk, and the associations between obesity and kidney function decline may differ across sex and race. In the Atherosclerosis Risk in Communities Study, we evaluated associations between different measures of obesity at baseline and estimated glomerular filtration rate trajectories using mixed models with random intercepts and random slopes and estimated the associations between obesity and kidney failure with replacement therapy (KFRT) using Cox proportional hazards models. Obesity status is a risk factor for future decline in kidney function and development of KFRT in Black and White women, with less consistent associations among men.
Acknowledgements:
The authors thank the staff and participants of the ARIC Study for important contributions.
Support: The ARIC Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Funding for laboratory testing and biospecimen collection at ARIC visit 6 was supported by grant R01DK089174 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. None of the funders had any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit this report for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: Some of the data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Peer Review: Received November 19, 2019. Evaluated by 2 external peer reviewers and a statistician, with direct editorial input from an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form July 21, 2020. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Walser M, Drew HH, LaFrance ND. Creatinine measurements often yielded false estimates of progression in chronic renal failure. Kidney Int. 1988;34(3):412–418. [DOI] [PubMed] [Google Scholar]
- 2.Turin TC, Coresh J, Tonelli M, et al. Short-term change in kidney function and risk of end-stage renal disease. Nephrol Dial Transplant. 2012;27(10):3835–3843. [DOI] [PubMed] [Google Scholar]
- 3.Turin TC, Coresh J, Tonelli M, et al. Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int. 2013;83(4):684–691. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrington WG, Smith M, Bankhead C, et al. Body-mass index and risk of advanced chronic kidney disease: prospective analyses from a primary care cohort of 1.4 million adults in England. PloS One. 2017;12(3):e0173515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopple JD. Obesity and chronic kidney disease. J Ren Nutr. 2010;20(5)(suppl):S29–S30. [DOI] [PubMed] [Google Scholar]
- 7.Grubbs V, Lin F, Vittinghoff E, et al. Body mass index and early kidney function decline in young adults: a longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis. 2014;63(4):590–59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahima RS, Lazar MA. Physiology. The health risk of obesity–better metrics imperative. Science. 2013;341(6148):856–858. [DOI] [PubMed] [Google Scholar]
- 9.Mahadevan S, Ali I. Is body mass index a good indicator of obesity? Int J Diabetes Dev Countries. 2016;36(2):140–142. [Google Scholar]
- 10.Ortega FB, Sui X, Lavie CJ, Blair SN. Body mass index, the most widely used but also widely criticized index: would a criterion standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin Proc. 2016;91(4):443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komura H, Nomura I, Kitamura K, Kuwasako K, Kato J. Gender difference in relationship between body mass index and development of chronic kidney disease. BMC Res Notes. 2013;6:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobo G, Hecking M, Port FK, et al. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci (Lond). 2016;130(14):1147–1163. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Keum N, Hu FB, et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Br J Nutr. 2017;118(10):858–866. [DOI] [PubMed] [Google Scholar]
- 14.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 15.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19(10):724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 17.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929. [DOI] [PubMed] [Google Scholar]
- 18.Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in the Atherosclerosis Risk in Communities Study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61(7): 938–94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci. 1991;6(1):15–32. [Google Scholar]
- 21.Song M, Giovannucci E. Estimating the influence of obesity on cancer risk: stratification by smoking is critical. J Clin Oncol. 2016;34(27):3237–3239. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 24.Huang WH, Chen CY, Lin JL, Lin-Tan DT, Hsu CW, Yen TH. High body mass index reduces glomerular filtration rate decline in type II diabetes mellitus patients with stage 3 or 4 chronic kidney disease. Medicine. 2014;93(7):e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burckhardt P, Nagin DS, Padman R. Multi-trajectory models of chronic kidney disease progression. AMIA Annu Symp Proc. 2016;2016:1737–1746. [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. [DOI] [PubMed] [Google Scholar]
- 27.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Foster MC, Tighiouart H, et al. Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ 3rd. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75(10):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBlanc ES, Rizzo JH, Pedula KL, et al. Long-term weight trajectory and risk of hip fracture, falls, impaired physical function, and death. J Am Geriatr Soc. 2018;66(10):1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobb LK, McAdams-DeMarco MA, Gudzune KA, et al. Changes in body mass index and obesity risk in married couples over 25 years: the ARIC Cohort Study. Am J Epidemiol. 2016;183(5):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei GS, Coady SA, Reis JP, et al. Duration and degree of weight gain and incident diabetes in younger versus middle-aged black and white adults: ARIC, CARDIA, and the Framingham Heart Study. Diabetes Care. 2015;38(11):2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens J, Tyroler HA, Cai J, et al. Body weight change and carotid artery wall thickness. The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1998;147(6): 563–573. [DOI] [PubMed] [Google Scholar]
- 35.Ndumele CE, Cobb L, Lazo M, et al. Weight history and subclinical myocardial damage. Clin Chem. 2018;64(1): 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rissanen PM, Laakkonen EI, Suntioinen S, Penttila IM, Uusitupa MI. The nutritional status of Finnish home-living elderly people and the relationship between energy intake and chronic diseases. Age Ageing. 1996;25(2):133–138. [DOI] [PubMed] [Google Scholar]
- 37.Hedlund J Community-acquired pneumonia requiring hospitalisation. Factors of importance for the short-and long term prognosis. Scand J Infect Dis Suppl. 1995;97:1–60. [PubMed] [Google Scholar]
- 38.Tosteson AN, Gottlieb DJ, Radley DC, Fisher ES, Melton LJ 3rd. Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18(11):1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumgartner RN, Stauber PM, Koehler KM, Romero L, Garry PJ. Associations of fat and muscle masses with bone mineral in elderly men and women. Am J Clin Nutr. 1996;63(3): 365–372. [DOI] [PubMed] [Google Scholar]
- 40.Lee DH, Keum N, Hu FB, et al. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk: two large prospective studies in US men and women. Eur J Epidemiol. 2018;33(11):1113–1123. [DOI] [PubMed] [Google Scholar]
- 41.Lee DH, Keum N, Hu FB, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55–61. [DOI] [PubMed] [Google Scholar]
- 43.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370(3):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Scatterplot of BIA-measured percent fat and BMI, waist-to-hip ratio, or predicted percent fat at visit 5 with generalized additive regression line.
Figure S2: Distributions of waist-to-hip ratio and predicted percent fat according to baseline BMI tertile, by sex and race.
Figure S3: Kaplan-Meier survival free of KFRT by tertile of baseline obesity measure within each sex-race group, showing absolute risk of KFRT.
Table S1: Difference in eGFR slope according to baseline obesity status tertile by sex and race.
Table S2: Difference in eGFR slope according to baseline obesity status tertile by sex and race, among participants who had visit 6 information.
Table S3: Association of eGFR slope with 3 measures of baseline obesity by sex and race, excluding current smokers.
Table S4: Subhazard ratios for KFRT according to baseline obesity status by sex and race, using a Fine-Gray competing-risk model.


