Abstract
Outdoor air pollution is a major contributor to the burden of disease worldwide. Most of the global population resides in places where air pollution levels, due to emissions from industry, power generation, transportation and domestic burning, considerably exceed the World Health Organization’s health-based air quality guidelines. Outdoor air pollution poses an urgent worldwide public health challenge because it is ubiquitous and has numerous serious adverse human health effects including cancer. Currently, there is substantial evidence from studies of humans and experimental animals, as well as mechanistic evidence to support a causal link between outdoor (ambient) air pollution, and especially particulate matter (PM) in outdoor air, with lung cancer incidence and mortality. It is estimated that hundreds of thousands of lung cancer deaths annually worldwide are attributable to PM air pollution. Epidemiological evidence on outdoor air pollution and risk of other types of cancer, such as bladder cancer or breast cancer is more limited. Outdoor air pollution may also be associated with poorer cancer survival though further research is needed. This report presents an overview of outdoor air pollutants, sources and global levels, as well as a description of epidemiological evidence linking outdoor air pollution with cancer incidence and mortality. Biological mechanisms of air pollution-derived carcinogenesis are also described. This report concludes by summarizing public health/policy recommendations, including multi-level interventions aimed at individual, community, and regional scales. Specific roles for medical and healthcare communities with regard to prevention and advocacy, and recommendations for further research are also described.
Keywords: particulate matter, lung cancer, cancer survival, bladder cancer, breast cancer
Introduction
Outdoor air pollution is a major contributor to the burden of disease worldwide.1 Most of the global population currently resides in places where air pollution levels, due to emissions from major sources such as industry, power generation, transportation and domestic burning, considerably exceed the World Health Organization’s (WHO) health-based air quality guidelines. This report presents an overview of outdoor air pollutants, sources and global levels, as well as a description of epidemiological evidence linking outdoor ambient air pollution with lung cancer incidence and mortality, followed by studies of other types of cancers in adults as well as childhood cancers, and biological mechanisms of air pollution-derived carcinogenesis. This report concludes by summarizing public health/policy recommendations, including multi-level interventions aimed at the individual, community, and regional scales. The specific role for the medical and healthcare community regarding prevention and advocacy, and recommendations for further research are also described.
Sources and Levels of Outdoor Air Pollution
Exposure to outdoor air pollution poses an urgent public health challenge worldwide because it is ubiquitous, affecting everyone, and has numerous serious adverse human health effects including cancer.2 Major primary air pollutants, those emitted directly into the environment largely as a result of combustion of fossil and biomass fuels, include gaseous pollutants (such as sulfur dioxide: SO2, nitrogen dioxide: NO2, carbon monoxide: CO, and volatile organic compounds: VOCs) and particulate matter (PM) (including carbonaceous aerosol particles, such as black soot). While CO is often low outdoors in the developed world today (due to the use of emissions controls such as catalytic converters on automobiles), high levels can be experienced near biomass burning sources, including wildfires.3 In addition, secondary air pollutants are formed in the atmosphere from primary pollutants, and include gaseous ozone (O3), a major component of photochemical smog, formed in the atmosphere when nitrogen oxides (NOx) and hydrocarbons such as VOCs react in the presence of sunlight. Similarly, particulate sulfate (e.g., sulfuric acid (H2SO4)) and nitrate (e.g., ammonium nitrate (NH4NO3)) aerosols are commonly created in the atmosphere from SO2 and NOx, respectively. Primary combustion particles and secondary particles are small in diameter, and are often referred to as fine particulate matter, or PM2.5 (particles ≤ 2.5 μm in aerodynamic diameter). Submicron combustion-related PM2.5 are of particular health concern because they contain numerous toxic compounds (e.g., acids and heavy metals) and can penetrate deeper into the lung than the larger PM generated by natural processes, such as most windblown soil particle mass.4
Air pollutants are emitted and/or formed both outdoors and indoors, resulting in personal pollutant exposure levels that can differ from levels measured by routine ambient air pollution measurements at centrally located air monitoring stations. The most common health-related air pollutants of greatest concern are summarized in Table 1 and are categorized into three classes: 1) pollutants primarily emitted into the outdoor environment; 2) pollutants primarily emitted into the indoor environment; and, 3) pollutants emitted into both outdoor and indoor environments. These pollutants and their typical sources are noted, including: PM2.5, SO2, NO2, O3, and CO. Subsequent discussions herein will focus on outdoor air pollutants that are associated with cancer, especially PM and its constituents.
Table 1.
Common health impacting air pollutants, grouped by origin
| Air Pollutant | Typical Sources |
|---|---|
| 1: Predominantly Outdoor Air Pollutants | |
| Sulfur dioxide (SO2) | Fuel combustion, smelters |
| Ozone (O3) | Generated via photochemical reactions in the atmosphere from nitrogen oxides (NOx) and volatile organic compounds (VOCs), as well as natural processes (e.g., stratosphere) |
| Arsenic (As), Chromium (Cr) | Coal combustion fine particulate matter (PM2.5) |
| Nickel (Ni), Vanadium (V) | Residual oil combustion fine particulate matter (PM2.5) |
| 2. Predominantly Indoor Air Pollutants | |
| Radon | Building materials (concrete, stone), ground water |
| Asbestos, mineral, synthetic fibers | Fire-retardant, acoustic, thermal, or electrical insulation |
| Biological contaminant | Infections, dust mites, animal dander, allergens |
| 3: Both Outdoor and Indoor Air Pollutants | |
| Suspended particulate matter (PM) | |
| Fine PM (PM2.5) | Outdoor: Fossil fuel combustion, gas-to-particle conversion, biomass burning Indoor: Biomass fuel combustion, tobacco smoking |
| Coarse PM (PM2.5–10) | Outdoor: Dust storms, windblown soil, pollens Indoor: Mold spores, re-suspended dust |
| Nitrogen dioxide (NO2) | Outdoor: Fossil fuel combustion (e.g., diesel vehicle emissions) Indoor: Tobacco smoking, gas cooking stoves |
| Volatile organic compounds (VOCs) | Outdoor: Petrochemical solvents, evaporated fuels, biogenics Indoor: Fuel and paint vapors, combustion, adhesives, cosmetics, solvents, particleboard (formaldehyde), insulation, furnishings, tobacco smoke |
| Carbon monoxide (CO) | Outdoor: Fossil fuel combustion, biomass burning, wildfires Indoor: Tobacco smoke, unvented gas heaters |
| Lead (Pb) | Outdoor: Industrial emissions, leaded fuel combustion, lead processing Indoor: Leaded paint wear |
| Mercury (Hg) | Outdoor: Coal combustion, ore refining Indoor: Fungicides in paints, thermometer breakage, ritual use |
| Pesticides | Outdoor: Agricultural Indoors: Home applications of herbicides, insecticides, fungicides, etc. |
| Ammonia | Outdoor: Livestock yards Indoor: Metabolic activity, cleaning products |
| Hazardous air pollutants (HAPs) (e.g., benzene, 1,3-butadiene, formaldehyde, acids) | Outdoor: Incomplete combustion, chemical processing Indoor: Solvent use |
Adapted from: World Health Organization (WHO). Estimating Human Exposures to Air Pollutants. Offset Pub. No. 69. Geneva: World Health Organization; 1982 and International Agency for Research on Cancer (IARC). Outdoor Air Pollution. Volume 109. Lyon: International Agency for Research on Cancer; 2013.
PM represents a broad class of chemically and physically diverse aerosols comprised of solid particles or liquid droplets suspended in the air. Such aerosols can be characterized by their size (discussed below), formation mechanism, origin, chemical composition, atmospheric behavior and method of measurement. The concentration of particles in the air varies across space and time and reflects the source of the particles and the pollutant transformations that occur in the atmosphere. PM air pollution can also be viewed in two major components: “primary” PM, including “soot” emitted directly into the atmosphere by combustion pollution sources such as industry, electric power plants, diesel buses, and automobiles, and; “secondary” PM, formed in the atmosphere from primary gaseous pollutants, such as SO2 and NOx gases (discussed above). Other primary sources include non-exhaust traffic emissions and windblown dusts from roadways, construction sites, agriculture, and deserts. Desert dust clouds have been documented to be capable of impacting population centers by being transported long distances.5
PM is commonly characterized according to the following size fractions:
PM10 (PM ≤ 10 μm in aerodynamic diameter) are the largest inhalable particles. Particles larger than 10 μm are generally not inhaled past the trachea, and are caught in the nose and throat, and not deposited in the lung. PM10 also includes all the fractions described below;
PM2.5–10, also known as coarse fraction particles (PM with an aerodynamic diameter > 2.5 μm, but ≤ 10 μm); and
PM2.5, also known as fine particles (PM with an aerodynamic diameter ≤ 2.5 μm), can be inhaled into the deepest recesses of the lung, including to the alveoli sacs, where oxygen exchange to the bloodstream occurs. As such, PM2.5 has increasingly become a major research focus of adverse human health impacts of outdoor air pollution exposure over recent decades.
The smallest fraction of PM2.5 are nanoparticles, also known as ultrafine particles (UFPs), generally defined as particles ≤ 0.1 μm in aerodynamic diameter.
The mass concentration (as μg/m3) is the common metric used to evaluate and regulate PM pollution, though some constituents, such as lead (Pb) concentration, have been separately regulated. While UFPs usually make up only a small fraction of PM2.5 mass, UFPs commonly account for a majority of the number concentration of particles in PM2.5. It has been hypothesized based on toxicological studies that UFPs may be an especially toxic component of PM2.5 because of their small size, large numbers, and large surface area to mass ratio, but epidemiological evidence is currently sparse.6
PM2.5 is directly emitted from combustion sources, and is also formed from gaseous precursors, such as SO2 and NOx, or organic compounds (discussed above). In some areas and under some conditions, these secondary particles make up a substantial proportion of the PM2.5 mass. Secondary fine particles are commonly composed of sulfate, nitrate, chloride and ammonium compounds, organic carbon, and condensed metals. Combustion of fossil fuels, and especially coal, further results in PM2.5 that is highly enriched in multiple moderately volatile, and potentially toxic elements. These include the chalcophile elements such as zinc (Zn), arsenic (As), selenium (Se), molybdenum (Mo), and cadmium (Cd).7 Indeed, coal combustion has been found to account for approximately one-quarter of the world’s emissions of both As and mercury (Hg).8,9 PM2.5 can remain in the atmosphere for days to weeks, and travel through the atmosphere hundreds to thousands of kilometers;5 conversely most coarse particles typically deposit to the earth within minutes to hours and travel within only tens of kilometers from the emission source.
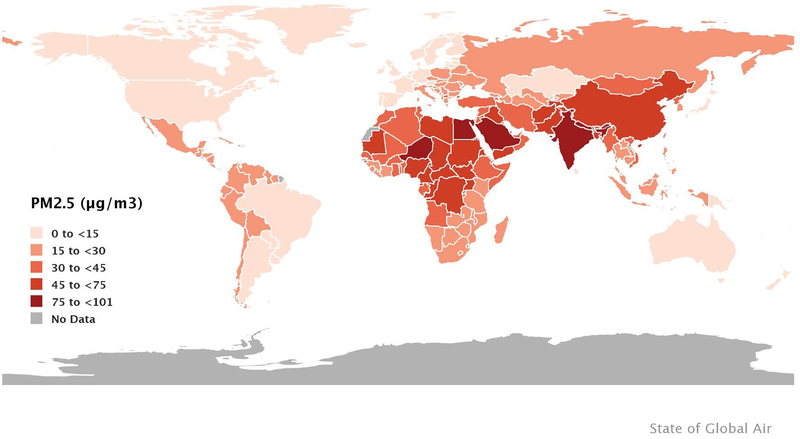
The global population-weighted mean annual average PM2.5 concentration was 46 μg/m3 in 2017, four-fold greater than the WHO’s health-based world air quality guideline of 10 μg/m3 (Figure 1).10 Ninety-two percent of the global population worldwide lives in areas where ambient PM2.5 concentrations exceed the WHO guideline and large percentages of the populations of China, Bangladesh, India, Pakistan and Nigeria have exposures above the WHO’s highest interim target guideline of 35 μg/m3. Among the ten largest countries by population, population-weighted ambient PM2.5 in 2017 varied by more than 12-fold; from 7 μg/m3 in the United States to 91 μg/m3 in India. For NO2, global population-weighted mean concentrations were estimated to be 1.6 ppb during 1996–2012, and were observed to have increased by 0.9% (95% confidence interval (CI) 0.6–1.1) per year during that time.11 Areas with the highest population-weighted mean concentrations were high-income Asia Pacific (4.9 ppb), Western Europe (4.1 ppb), and high income North America (3.7 ppb), though there was a decreasing trend ranging from 2.1 to 4.7% per year. Population-weighted mean concentrations in East Asia were 2.9 ppm and increasing at the highest rate of 6.7% per year. In contrast, population-weighted mean concentrations in areas of South and Southeast Asia, Africa, and the Caribbean were ≤ 0.5 ppb. The global population-weighted mean O3 concentration worldwide was 57 ppb in 2017, unchanged from 1990.10
Figure 1.
Average annual population-weighted PM2.5 concentrations in 2017. Source: Health Effects Institute. 2019. State of Global Air 2019. Data source: Global Burden of Disease Study 2017. IHME, 2018.
An overview of the epidemiological evidence linking outdoor ambient air pollution with lung cancer incidence and mortality is provided below, followed by studies of other types of cancers in adults and children. Studies were identified through literature searches of Medline through June 2020, reference lists of identified studies and authoritative reports, as well as via personal correspondence. Although numerous epidemiological studies have evaluated some aspect of the association of outdoor air pollution and cancer, here we sought to highlight key contributions including meta-analyses and large-scale original studies, with a focus on the most recent and informative published literature. Methodological considerations and research needs are also discussed.
Epidemiological Studies of Outdoor Air Pollution and Lung Cancer
Lung cancer is the most commonly diagnosed cancer worldwide and is the leading cause of cancer death, with an estimated 2.1 million new cases and 1.8 million deaths occurring in 2018, representing 11.6% of all new cancer diagnoses and 18.4% of all cancer deaths.12 In the United States, approximately 234,030 new lung cancer cases occurred and 154,050 deaths were estimated in the same year.13 Lung cancer is highly fatal, with an overall 5-year survival rate of only 18%.13 Rates of lung cancer incidence and mortality vary substantially within and between countries, depending largely on historical patterns of cigarette smoking,12 with long latency periods of up to approximately 30 years between the start of the smoking epidemic and the rise of lung cancer incidence. The highest incidence rates for lung cancer among men are currently observed in Micronesia/Polynesia, Eastern Asia, and Eastern Europe, and for women in North America, Northern and Western Europe, and Australia/New Zealand.12 In several European countries, lung cancer incidence rates are beginning to converge in men and women as increasing rates in women are approaching declining rates in men.12
Although cigarette smoking accounts for more than 80% of lung cancers, substantial numbers of lung cancer cases are observed among never smokers. Outdoor ambient air pollution and exposure to other inhalable agents, such as household burning of solid fuels, residential radon, second hand tobacco smoke, asbestos, certain metals and organic chemicals, and work in rubber manufacturing, paving, roofing, painting, or chimney sweeping, and other occupational exposures have also been associated with lung cancer risk.12–14
Based on sufficient evidence in studies of humans and experimental animals, as well as strong mechanistic evidence, the International Agency for Research on Cancer (IARC) in 2013 classified both outdoor air pollution and PM in outdoor air pollution as Group 1 human carcinogens for lung cancer.15 The IARC evaluation noted that general population cohort studies with quantitative data on long-term estimates of outdoor air pollution exposure, including the large-scale American Cancer Society (ACS) Cancer Prevention Study-II (CPS-II) and the European Study of Cohorts for Air Pollution Effects (ESCAPE), were particularly informative in their evaluation with a broad range of exposures considered and detailed information on potential confounders, notably cigarette smoking.16–18 Because the possibility of residual confounding by cigarette smoking of reported air pollution effects had remained a concern, the analysis of thousands of never-smokers in the ACS CPS-II study, which observed increased lung cancer mortality associated with long-term PM2.5 exposure, was particularly influential.17 Interestingly, the IARC conclusion of a causal link between outdoor air pollution and PM in outdoor air with increased lung cancer risk was long ago foreshadowed, given the presence of carcinogens in ambient air. Indeed, in the introduction to their landmark report on the preliminary findings of their case-control study of lung cancer in London, Doll and Hill19 commented: “Two main causes have from time to time been put forward: (1) a general atmospheric pollution from the exhaust fumes of cars, from the surface dust of tarred roads, and from gas-works, industrial plants, and coal fires; and (2) the smoking of tobacco.” In the ensuing 70 years, the dominance of tobacco smoking as a cause of lung cancer perhaps distracted attention away from the role of outdoor air pollution as another avoidable cause.
IARC has also classified household burning of coal as a Group 1 human carcinogen and household burning of biomass fuel as a Group 2A (probably carcinogenic) for lung cancer.20,21 Household burning of solid fuels, both coal and biomass, contribute significantly to high levels of outdoor air pollution and hence burden of disease in low- and middle-income countries.22–25
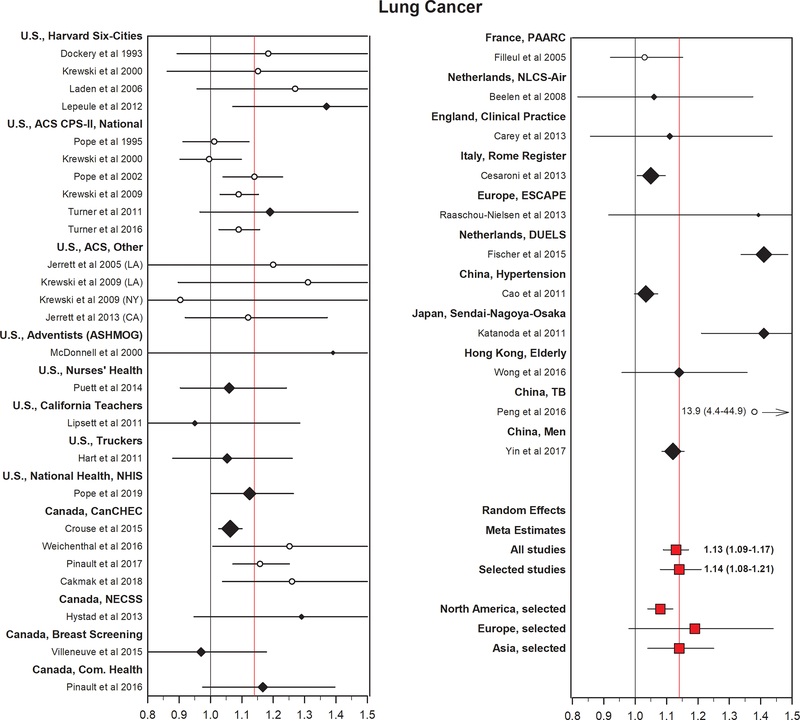
Meta-analysis of findings from 14 studies of outdoor air pollution conducted largely in North America and Europe reported a statistically significant 9% (95% CI 4–14%) increase in risk for lung cancer incidence or mortality per each 10 μg/m3 increase in PM2.5 concentrations and in 9 studies of PM10, an 8% (95% CI 0–17%) increase in risk per 10 μg/m3.26 Lung cancer incidence and mortality were considered together here, since due to the highly fatal nature of the disease, mortality is considered a valid indicator of incidence. Although significant heterogeneity in findings by continent was not observed, there were few studies conducted in Asia or other regions of the world. Findings were also generally similar by exposure assessment method, among studies using either fixed site monitoring or model-based indicators of outdoor air pollution exposure, as well as by covariate adjustment for cigarette smoking or other sociodemographic variables. In an even more recent updated meta-analysis of findings from 20 cohort studies, a somewhat larger increase in lung cancer incidence or mortality (i.e., 14%, 95% CI 8–21% per 10 μg/m3 PM2.5) was observed with similar findings again in studies from different regions (Figure 2).27 When extrapolated to the global population-weighted mean annual average PM2.5 concentration (46 μg/m3) relative to the WHO’s health-based world air quality guideline (10 μg/m3), this represents an approximately 60% excess risk of lung cancer mortality.
Figure 2.
Estimated adjusted HRs (and 95% CIs) for lung cancer mortality per 10 μg/m3 elevation in PM2.5 from multiple cohort studies. Black diamonds represent selected studies with the size of the diamond proportional to the relative weight in the random effect estimate using selected studies. The red squares represent random effects meta-estimates. The black line is a reference line at HR = 1. The red line is a reference line at HR equals the random effects meta-estimate using the selected studies. Source: modified and updated figure from, Pope CA III, Coleman N, Pond ZA, Burnett RT. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res. 2020;183:108924. Reprinted under a CC BY-NC-ND 4.0 creative commons license.
There were also significant adverse associations in meta-analyses of studies of NO2 exposure, a marker of traffic-related air pollution, for lung cancer mortality (relative risks (RRs) of 1.04 to 1.05 per 10 μg/m3), although results were attenuated somewhat in studies that adjusted for individual-level cigarette smoking status and were no longer significant.28,29 Additional research in Asia and in other understudied and more highly polluted regions is needed,27 as well as with improved data on individual and lifetime outdoor air pollution exposures, including time-varying estimates of outdoor air pollution exposures over long time periods and consideration of individual and residential mobility over time.
Results from several recent large-scale epidemiological studies also showed adverse findings. There was a significant adverse association of ambient PM2.5 and lung cancer mortality among 635,539 United States National Health Interview Survey (NHIS) participants (hazard ratio (HR) per 10 μg/m3 = 1.13, 95% CI 1.00–1.26, n = 7,420 lung cancer deaths).30 There were suggestive adverse associations of both PM2.5 and PM10 with lung cancer mortality in analysis of 49,564 participants in the Danish Diet, Cancer and Health Cohort but no associations with black carbon, NO2, or O3.31 However, there was no clear association of PM2.5, PM10, or NO2 and lung cancer mortality among Dutch national health survey participants (n = 339,633), possibly due to the short follow-up or other methodological characteristics of this study.32 Among studies without individual-level information on cigarette smoking history, in analysis of ~4.9 million individuals in the Ontario Population Health and Environment Cohort (ONPHEC), there were significant adverse associations of both ambient PM2.5 (HR per 5.3 μg/m3 = 1.02, 95% CI 1.01–1.05) and NO2 (HR per 14 ppb = 1.05, 95% CI 1.03–1.07) but not O3 or Ox (combined oxidant capacity of NO2 and O3) and incident lung cancer.33 In analysis of 18.9 million United States Medicare beneficiaries, there were significant adverse associations particularly with longer-term moving average PM2.5 exposure and lung cancer mortality (HR per 10 μg/m3 60 month moving average = 1.33, 95% CI 1.24–1.40).34 There were also some significant adverse associations with O3 and NO2.35,36 Although analysis extended to 53 million Medicare beneficiaries reported no association of PM2.5 and lung cancer mortality, there may be confounding by cigarette smoking status in rural populations included here.37
Worldwide, ambient PM2.5 air pollution was estimated to have contributed to 265,267 lung cancer deaths (95% uncertainty interval (UI) 182,903–350,835) in 2017, or 14.1% (UI 9.8–18.7) of all lung cancer deaths.1 The global proportion of lung cancer deaths attributable to ambient PM2.5 was second only to tobacco smoking (14.1% vs. 63.2%)1.
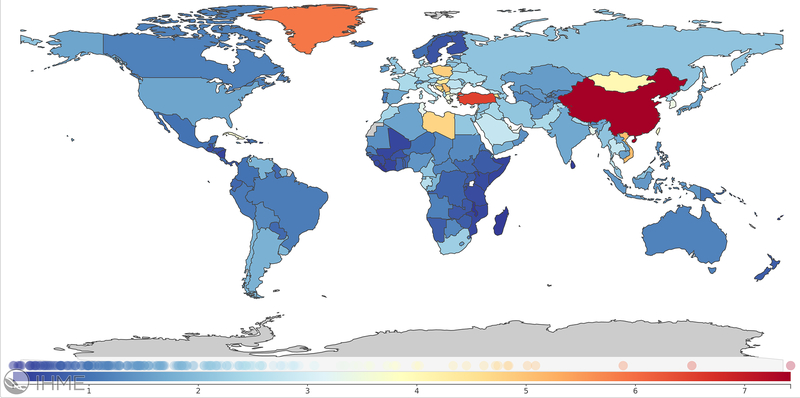
Mortality attributable to PM2.5 depends not only on patterns in ambient pollutant levels, but also on other factors including underlying population dynamics, ageing, mortality rates, access to health care and other racial and socioeconomic disparities38 and as such, the number of estimated attributable lung cancer deaths has increased by nearly 30% since 2007. These factors may also explain, at least in part, the wide variation in country-specific estimates. Age-standardized PM2.5-attributable lung cancer mortality rates and population-attributable fractions in the United States for example were 1.6/100,000 (UI 0.65–2.91) and 4.7% (UI 1.9–8.5) compared to 7.4/100,000 (UI 5.4–9.5) and 20.5% (UI 14.8–25.9) in China (Figure 3).
Figure 3.
Global age-standardized PM2.5-attributable trachea, bronchus, and lung cancer mortality rates per 100,000 in 2017. Source: https://vizhub.healthdata.org/gbd-compare/; accessed April 29, 2020.
Despite such major advances in knowledge surrounding associations of outdoor air pollution and lung cancer, additional questions remain. For example, less is known regarding associations for specific histologic types of lung cancer, of relevance to treatment and prognosis, an area of active investigation with regard to tobacco smoking. The increasing risk of adenocarcinoma over the last four decades is considered as reflecting changes in cigarettes and the delivery of carcinogens.39 A mechanistic basis for a link of air pollution to particular histological types is uncertain though some studies have suggested stronger findings with adenocarcinoma. In the meta-analysis of Hamra et al.26 results for both PM2.5 and PM10 were somewhat stronger for adenocarcinoma (RRs per 10 μg/m3 = 1.40, 95% CI 1.07–1.83 and 1.29, 95% CI 1.02–1.63 respectively), though there were few studies. Among more recent work, in an analysis of 89,234 women in the Canadian National Breast Screening Study, there was a significant adverse association of PM2.5 and incident lung cancer overall (HR per 10 μg/m3 = 1.34, 95% CI 1.10–1.65), which strengthened somewhat for both small cell carcinoma (HR = 1.53, 95% CI 0.93–2.53) and adenocarcinoma (HR = 1.44, 95% CI 1.06–1.97).40 In analysis of 80,285 participants in the Adventist Health and Smog Study-2, there was a significant adverse association of PM2.5 and total lung cancer incidence (HR per 10 ug/m3 = 1.43, 95% CI 1.11–1.84).41 There was also an adverse association with adenocarcinoma (HR = 1.31, 95% CI 0.87–1.97) which strengthened in participants reporting spending >1h/day outdoors.42 There was also an adverse association of ambient PM10 concentrations and total lung cancer incidence in the EAGLE study, consisting of 2,099 cases and 2,120 controls in the Lombardy Region of Italy (odds ratio (OR) per 10 μg/m3 = 1.28, 95% CI 0.95–1.72) with somewhat stronger findings for squamous cell carcinoma (OR = 1.44, 95% CI 0.90–2.29).43 In a large-scale South Korean study, including 6,567,909 participants from a National Health Insurance database, there was no overall association of either PM10 or NO2 concentrations and incident lung cancer but an adverse association of PM10 and adenocarcinoma in male smokers (HR > 60.9 vs < 50.40 μg/m3 = 1.14; 95% CI 1.03–1.25).44 Further research on air pollution and lung cancer by histological type is needed.
Knowledge regarding effects of differing PM components for lung cancer is also limited.26,45 In analysis of 669,046 ACS CPS-II participants, there was a significant adverse association of total PM2.5 and lung cancer mortality (HR per 10 ug/m3 = 1.09, 95% CI 1.03–1.16), as well as with both near source (largely traffic-related) and regional PM2.5 components.46 There were also stronger lung cancer mortality associations in the ACS CPS-II with coal combustion-related PM2.5 as well as with Se, a coal combustion tracer, and S elemental components specifically.47 In an analysis of 193,300 participants in the Canadian Census Health and Environment Cohort (CanCHEC) there were significant adverse associations of glutathione- but not ascorbate-related PM2.5 oxidative burden (the product of PM2.5 mass and oxidative potential (the ability of regional filter extracts to deplete antioxidants glutathione or ascorbate in a synthetic respiratory tract lining fluid)) and lung cancer mortality.48 In an analysis of 2.6 million CanCHEC participants, associations of total PM2.5 and lung cancer mortality were similar by spatial climatic zone.49 The ESCAPE study of 245,782 participants from 14 cohorts reported elevated RRs for incident lung cancer risk associated with various PM2.5 or PM10 components, particularly for S (long-range transport, secondary combustion-related components) and nickel (Ni) (oil-burning, industry).50 IARC also concluded that there is sufficient evidence in humans for the carcinogenicity of diesel engine exhaust, as well as some PM constituents (e.g., Ni, chromium (Cr), Cd, silica dust) for lung cancer.51,52 There was also sufficient evidence in experimental animals for the carcinogenicity of condensates of gasoline engine exhaust.51 Therefore while limited, the strongest evidence to date implicates fine PM of fossil fuel combustion origins.
There is also limited information regarding modification of outdoor air pollution associations by other individual or lifestyle factors. Hamra et al.26 reported that associations with PM2.5 were somewhat stronger among former smokers (RR = 1.44, 95% CI 1.04–2.01) and never smokers (RR = 1.18, 95% CI 1.00–1.39) than in current smokers (RR = 1.06, 95% CI 0.97–1.15). However, few studies have examined possible joint effects of air pollution and cigarette smoking on an additive scale, which may be most relevant for public health. In analysis in the ACS CPS-II, there was some evidence for an interaction between ambient PM2.5 and cigarette smoking for lung cancer mortality, with risk among those with both exposures greater than what was expected from the sum of the effects of both exposures alone.53 It was estimated that 14% (95% CI 0–25%) of lung cancer deaths in that study were attributable to the interaction between these two factors. The ESCAPE lung cancer study reported no interaction between ambient PM2.5 or PM10 concentrations and fruit consumption.18 Future studies with individual-level information on potential confounding and modifying factors, including cigarette smoking and diet, captured over time are needed.
Finally, ambient PM2.5, PM10 and NO2 were associated with poorer lung cancer survival, particularly early-stage non-small cell cancers, among 352,053 California lung cancer patients.54 There was also an adverse association of long-term exposure to PM2.5 and first hospital admission for lung cancer in a cohort of 11 million Medicare beneficiaries in the South-Eastern United States, indicating a potential association with exacerbation of disease.55 Further research is needed to better understand the impact of outdoor air pollution on patterns of morbidity and mortality following lung cancer diagnosis.
Epidemiological Studies of Outdoor Air Pollution and Other Types of Cancer
Epidemiological evidence for associations of outdoor air pollution with other types of cancer than lung is more limited, although adverse associations have been reported in an increasing number of studies. Previous studies are typically limited by small numbers of cancer cases, the use of fatal rather than incident disease endpoints (particularly relevant for cancers with more favorable prognoses), the use of recent as opposed to historical estimates of long-term outdoor air pollution concentrations, as well as some conflicting findings. Outdoor air pollution might cause cancer at sites other than the lung through absorption, metabolism, and distribution of inhaled carcinogens.
Following lung cancer, the subsequent leading causes of cancer diagnoses worldwide include female breast cancer (11.6%), prostate cancer (7.1%), and colorectal cancer (6.1%).12 For mortality, cancer of the colorectum (9.2%), stomach (8.2%), and liver (8.2%) account for the next greatest numbers of cancer deaths.12 In addition to lung cancer, cigarette smoking is also considered an IARC Group 1 carcinogen for cancers of the oral cavity, nasal cavity, pharynx, nasopharynx, larynx, esophagus, stomach, colorectum, pancreas, liver/bile duct, kidney, renal pelvis/ureter, bladder, ovary, cervix, and myeloid leukemia with limited evidence for other types of cancer, such as breast cancer.21 Second hand tobacco smoke has also been suggestively associated with many of these types of cancer.39
Other inhalable pollutants have also been associated with multiple types of cancer. A meta-analysis of household air pollution from burning of solid fuels also noted adverse associations with oral, cervical, and esophageal cancer.56 Occupational exposure to various agents have also been associated with cancer at different sites, including for example diesel and gasoline exhaust, polyaromatic hydrocarbons (PAHs), inhalable dusts (metals, silica), or work in trucking, mining, foundries, carbon black production, or work with asphalt.14,51,57–60
The IARC evaluation noted that beyond lung cancer, some adverse associations with outdoor air pollution were observed for bladder cancer in studies using different metrics of exposure to outdoor air pollution, traffic, or occupation as a surrogate indicator of exposure.15 Bladder cancer shares several risk factors with lung cancer. However, results from more recent studies are mixed. In analysis of 623,048 ACS CPS-II participants, there was a significant adverse association of PM2.5 and bladder cancer mortality (HR per 4.4 μg/m3 = 1.13, 95% CI 1.03–1.23, n = 1,324) but no association with NO2 or O3.61 There was also a significant adverse association of PM2.5 and bladder cancer mortality in the NHIS (HR per 10 μg/m3 = 1.48, 95% CI 1.00–2.20, n = 589).30 Although an early hospital-based study of 1,219 incident bladder cancer cases and 1,271 controls in Spain reported an adverse association of living more than 40 years in a city of > 100,000 inhabitants and bladder cancer risk (OR = 1.30, 95% CI 1.04–1.63),62 an updated analysis including estimates of ambient PM2.5 and NO2 at the participant residence based on European land-use regression models, showed no clear association.63 There was also no association of ambient PM10, PM2.5–10, PM2.5, PM2.5 absorbance, NO2, NOx, other elemental PM components, organic carbon, or traffic density with bladder cancer incidence in the ESCAPE study.64
Previous studies have suggested some adverse associations of both NO2 and NOx and breast cancer, with fewer clear associations with PM.65 Among most recent studies, in analysis of 47,433 women in the United States Sister Study there were adverse associations of both NO2 (HR per 5.8 ppb = 1.06, 95% CI 1.02–1.11) and PM2.5 (HR per 3.6 μg/m3 = 1.05, 95% CI 0.99–1.11) and breast cancer incidence overall (n = 2,848).66 There were also adverse associations of PM2.5 concentrations characterized by low S and high sodium (Na) and NO3- fractions and invasive breast cancer incidence in California participants, and of PM2.5 characterized by high fractions of silicon (Si), calcium (Ca), potassium (K), and aluminium (Al) among participants in the Western United States. There were also adverse associations of several non-metallic air toxics, including methylene chloride and breast cancer incidence observed.67 In analysis of 57,589 women in the Multiethnic Cohort, significant adverse associations of NOx, NO2, PM2.5, and PM10 with breast cancer incidence were observed among those living within 500 m of major roads, with stronger associations for NOx and NO2 among African American and Japanese American women overall.68 In the Canadian National Breast Screening Study (n=89,247) there were adverse associations of both PM2.5 (HR per 10 μg/m3 = 1.26, 95% CI 0.99–1.61) and NO2 (HRs per 9.7 ppb ranging from 1.13–1.17) and risk of incident premenopausal but not postmenopausal disease.69,70 However, results from other recent studies have reported no clear associations with incident breast cancer risk.33,71,72 Further, in one case-control study of 4,059 breast cancer cases and 4,059 matched controls nested in the French E3N cohort, there were significant inverse associations of ambient Cd and risk of both incident estrogen receptor (ER) - and ER-/progesterone receptor (PR)- disease.73 In the Nurses Health studies, there was an adverse association of PM and all-cause mortality among women diagnosed with breast cancer, as well as greater breast cancer specific mortality among women with stage 1 disease.74 Results of studies of mammographic density, a breast cancer risk factor, are also mixed.75–77
For other types of cancer, there are fewer studies and results are also inconsistent. Although there was an adverse association of NOx and brain tumour incidence in analysis of 54,304 participants in the Danish Diet Cancer and Health cohort (incidence rate ratio (IRR) per 100 μg/m3 = 2.28, 95% CI 1.25–4.19, n = 95),78 findings were not replicated in subsequent studies.79,80 There was an adverse, but non-significant association of PM2.5 absorbance and malignant (HR per 10–5/m3 = 1.67, 95% CI 0.89–3.14; n=466) but not non-malignant (n=366) brain tumour incidence in ESCAPE, though there were no data on brain tumour histology or morphology.81 Analysis of 103,308 Multiethnic Cohort participants reported significant adverse associations of both outdoor benzene and PM10 exposure and malignant brain tumor risk in men (n = 94), particularly among Latino men, but not in women.82 There was also a significant adverse association of O3 and meningioma risk among men (n = 130). There was a significant adverse association of within-city ambient UFP concentration and malignant brain tumour incidence in analysis of 1.9 million CanCHEC participants in Montreal and Toronto (HR per 10,000/cm3 = 1.11, 95% CI 1.04–1.19, n = 1,400).83
Among other cancers of the digestive organs and urinary tract, in the ACS CPS-II cohort, there were significant adverse associations of PM2.5 with kidney cancer mortality (HR per 4.4 μg/m3 = 1.14, 95% CI 1.03–1.27, n = 927) and of NO2 with colorectal cancer mortality (HR per 6.5 ppb = 1.06, 95% CI 1.02–1.10, n = 6,475).61 The NHIS study reported significant adverse associations of PM2.5 and stomach (HR per 10 μg/m3 = 1.87, 95% CI 1.20–2.92, n = 525) and colorectal (HR per 10 μg/m3 = 1.29, 95% CI 1.05–1.59, n = 2,572) cancer mortality.30 There were also some suggestive adverse associations in analysis of both kidney (n=697) and liver (n= 279) cancer incidence in ESCAPE, though there were small numbers of cancer cases.84,85 Total PM2.5 and PM2.5 S were also associated with incident gastric cancer risk.86,87 A Hong Kong cohort of 66,820 participants reported significant adverse associations of PM2.5 with both upper digestive tract (HR per 10 μg/m3 = 1.42, 95% CI 1.06–1.89, n = 323) and accessory organ (HR = 1.35, 95% CI 1.06–1.71, n = 676) cancer mortality.88 A Taiwan cohort including 23,820 participants and 464 incident hepatocellular carcinoma (HCC) cases, accounting for 85–90% of primary liver cancer cases, reported adverse associations with PM2.5 mediated by alanine transaminase levels, an indicator of chronic liver inflammation.89 In a study including 56,245 HCC cases in the United States Surveillance, Epidemiology, and End Results (SEER) database, there was a significant adverse association with PM2.5 (IRR per 10 μg/m3 = 1.26, 95% CI 1.08–1.47).90 PM2.5 was also related with reduced HCC survival.91
Results of studies of hematopoietic cancers, leukemias and lymphomas, are also limited and mixed, with few studies having power to consider specific hematopoietic cancer subtypes. In the ACS CPS-II, there were no clear associations of ambient air pollutant exposure and non-Hodgkinʼs lymphoma (NHL), Hodgkinʼs lymphoma (HL), multiple myeloma, or leukemia mortality.61 However, in a more recent analysis among 115,996 ACS CPS-II Nutrition cohort participants including 2,595 incident hematologic cancer cases, there were significant adverse associations of outdoor benzene exposure and incident myelodysplastic syndromes and T-cell lymphoma overall, and follicular lymphoma among men.92 The NHIS study reported significant adverse PM2.5 associations with HL (HR per 10 μg/m3 = 4.18, 95% CI 1.02–14.60, n = 59), NHL (HR = 1.48, 95% CI 1.10–1.98, n = 1,016), and leukemia (HR = 1.43, 95% CI 1.05–1.97, n = 970) mortality.30 A case-control study including 1,064 total incident leukemia cases and 5,039 controls across Canada observed no clear association with PM2.5 overall or when examining chronic lymphocytic leukemia specifically.93 Studies in Denmark have reported no clear associations of ambient air pollutant exposure and incident NHL,78,94 though in one study significant adverse associations of primary carbonaceous and secondary organic aerosols were observed.95 A Danish case-control study of 1,967 incident leukemia cases and 3,381 controls reported significant adverse associations of ambient NO2 (OR per 10 μg/m3 = 1.31, 95% CI 1.02–1.68) and NOx (OR per 20 μg/m3 = 1.20, 95% CI 1.04–1.38) and incident acute myeloid leukemia (AML).96
Epidemiological Studies of Outdoor Air Pollution and Childhood Cancer
The incidence of childhood cancers is increasing, based on a recent report of data from 68 countries and over 100 population-based registries.97 An average of 215,000 children aged younger than 15 years, and 85,000 children aged 15–19 years were diagnosed with cancer each year from 1990–2017, which is likely an underestimate due to a lack of data in low income countries. In children, leukemia and lymphoma account for almost half of all cancers, followed by central nervous system (CNS) tumors and tumors originating in embryonic tissues, such as neuroblastoma, retinoblastoma and nephroblastoma.
The literature on outdoor air pollution and childhood cancers is limited. Most studies have examined leukemias, CNS tumors, or all childhood cancers combined, and few had sufficient sample sizes to stratify by more specific cancer subtypes. Most studies considered outdoor ambient air pollution exposure at birth or during childhood, while fewer examined prenatal exposure. Most early studies relied on metrics of traffic density, and were unable to examine concentrations of specific air pollutants. For example, a nationwide cohort in Switzerland observed that the risk of leukemia in children who lived < 100 m from a highway was 1.43 (95% CI 0.79–2.61) times greater than that of children who lived ≥ 500 m away, particularly for children aged younger than 5 years.98
Despite these limitations, there is some suggestive evidence for an adverse association of traffic-related air pollution and acute childhood leukemia.15,99–103 IARC noted that a weak adverse association with childhood leukemia, particularly acute lymphoblastic leukemia (ALL), could not be ruled out, but noted that results were inconsistent with evidence of potential publication bias.15 In a meta-analysis of 12 studies of traffic-related benzene exposure there was a nearly 1.5-fold higher risk of ALL and a 2-fold higher risk of AML.100 In an even more recent meta-analysis of 29 studies, benzene exposure was adversely and linearly associated with risk of childhood leukemia, particularly AML, and most consistently among children < 6 years.102 There was also no association observed of NO2 and leukemia risk, except at the highest exposure levels, as well as no association with traffic density or PM2.5, though there were some possible associations with ALL.
Few studies have examined the relationship between air pollution and childhood CNS tumors.104–107 One difficulty is the potential for etiologic heterogeneity among phenotypes (e.g., astrocytomas and medulloblastomas), as few studies have data to examine these rare CNS subtypes. Danysh et al.104 in a study of 1,949 children diagnosed with a CNS tumor in Texas, reported significant adverse associations of both medium and medium-high 1,3-butadiene concentrations and medium diesel particulate matter (DPM) concentrations with astrocytomas (IRRs = 1.46; 95% CI 1.05–2.01; 1.69; 95% CI 1.22–2.33; and 1.42; 95% CI 1.05–1.94 respectively), as well as of medium DPM concentrations and medulloblastoma (IRR = 1.46; 95% CI 1.01–2.12) compared to low concentrations. Other studies reported no clear associations of traffic-related air pollution and childhood CNS tumors.105–107
Among studies of prenatal outdoor air pollution exposure, studies of child cancer in California have observed significant adverse associations of exposure to traffic pollution during gestation and risk of ALL, germ-cell tumors and retinoblastoma.106 In another California study, each 25 ppb increase in average maternal exposure to NO, NO2, and NOx during pregnancy increased the risk of ALL in offspring by 9, 23, and 8% respectively.105 Bilateral retinoblastoma was also associated with second and third trimesters exposures. Prenatal exposure to acetaldehyde, 1,3-butadiene, benzene, and toluene were adversely associated with central nervous system primitive neuroectodermal tumor (PNET) and PAHs with medulloblastoma.108 A Texas study reported an adverse association of embryonal tumors in children whose mothers lived < 500 m from a major road during pregnancy compared with 500 m or more (OR = 1.24; 95% CI 1.00–1.54), with the strongest findings observed for unilateral retinoblastoma (OR = 1.67; 95% CI 0.96–2.93).109 In a study of more than two million Canadian children followed-up from birth to 4 years, PM2.5 exposure during the first trimester was associated with a significant adverse association of astrocytoma (HR per 4.0 μg/m3 = 1.40; 95% CI 1.05–1.86, n = 94) and first trimester NO2 with ALL (HR per 13.3 ppb =1.20; 95% CI 1.02–1.41, n = 302).110
Lastly, a Utah study reported significant adverse PM2.5 - cancer mortality associations among pediatric patients with lymphoma and CNS tumors as well as among adolescent and young adult patients with CNS tumors, carcinomas, melanomas, breast, and colorectal cancers.111 Further research of mortality in childhood cancer patients is needed.
Biological Mechanisms of Air Pollution-Derived Carcinogenesis
The biological mechanisms behind air pollution-related carcinogenesis remain to be elucidated. Still, extensive evidence from indirect models show how outdoor air pollution contributes to abnormal cell proliferation and cancer.112 Post-inhalation, air pollutants may generate effects along the respiratory tract, in locations such as the extra-thoracic, tracheobronchial, or alveolar airways. Retained particles and gas can have significant consequences on both the local and systemic level, generating low-grade and long-term inflammation and oxidative stress.113 Air pollution contains several mutagens and carcinogens, including PAHs (e.g., benzo(a)pyrene and polar compounds),114 dioxins,115 sulfur-containing compounds (SO3, H2SO4),116 and 3-nitrobenzanthrone.117 PAHs are a class of compounds associated with human cancer risk due to their ability to generate DNA adducts.118 One meta-analysis has confirmed the nonlinear dose-response relationship between air pollution PAH and DNA adducts,119 and several studies have indicated that carcinogen-DNA adducts are closely associated with cancer risk.120–122 However, an individual’s repair capacity may determine if DNA adducts are eliminated by the repair machinery, potentially inducing DNA mutations.123
Gene mutations and gene silencing are particularly relevant during carcinogenic processes when they can affect tumor suppressor genes (TSGs).124 Several studies have shown that there are fractions of outdoor air that contain mutagenic particulate and volatile matter.125 Also, mice exposed to industrial ambient air pollution showed higher heritable mutation at tandem-repeat DNA loci.126 TP53 is a TSG involved in cell proliferation, apoptosis, and damage repair, and its mutation/inactivation contributes to the pathogenesis of lung cancer.127 Studies have shown that low‐dose PM2.5 may induce epigenetic silencing of TP53 in human alveolar epithelial cells.128 Remarkably, studies from Yu et al.129 showed that the number of mutations was three times higher in air pollution-related lung cancers than in lung cancers from low-exposed regions. These mutations were seen across hundreds of genes, including TP53.
Outdoor air pollution has also been linked to several epigenetic modifications,130 including changes to post-translational modifications of histones,131 5-hydroxymethylation,130 and most notably DNA methylation (DNAm). DNAm is a biochemical change that occurs in cytosines, particularly at the CpG context, and modifies gene expression as well as several other functions. As mentioned for TP53, hypermethylation contributes to gene silencing,132 but DNA hypomethylation contributes to chromosome instability133 and activation of retrotransposon sequences and repetitive elements such as LINE-1134 and Alu.135 DNA hypomethylation also affects critical chromosome regions such as the subtelomeric and pericentromeric regions.136 Exposure to ambient air pollution, whether short-term or long-term, is associated with abnormal DNA methylation.137–139 Other studies have also shown that human epithelial cells exposed to PM2.5 are more susceptible to hypomethylation and transcriptional activation of several genes and also microRNAs (miRNAs), modifying cancer-related signaling pathways.140 PM2.5 is also able to induce changes in long non-coding RNAs (lncRNA) such as loc146880 via reactive oxygen species (ROS), promoting autophagy and malignancy of lung cells.141
Transcriptional changes in miRNAs have also been described in human bronchial cells exposed to ambient PM2.5, including downregulation of miR-182 and miR-185, potentially deregulating oncogenes (SLC30A1, SERPINB2, and AKR1C1) and facilitating neoplastic transformation.142 Other studies have found that dysregulation of actin cytoskeleton and down-regulation of miR-802 expression is present in the A549 cell line after particulate matter exposure.143 Human bronchial epithelial cells exposed to various concentrations of PM2.5 also show transcription changes in hundreds of genes, affecting some involved in inflammatory and immune response, oxidative stress, and DNA damage, as well as decreased cell viability in a dose-dependent manner.144 Several other studies have found that air pollution compounds induce the release of pro‐inflammatory cytokines, including IL‐6, TNF‐α and granulocyte‐macrophage colony-stimulating factor (GM‐CSF), resulting in low-grade, chronic inflammation in the airway and throughout the body.145–147 Another critical driver of carcinogenesis associated with air pollution is oxidative stress (OS).148 OS is characterized by the increase in free radicals (reactive oxygen species [ROS] and reactive nitrogen species [RNS]). The most-studied air pollutants concerning the intracellular formation of free radicals are ozone (O3),149 nitrogen oxides (NO and NO2), and metals.150 Early studies demonstrated that mouse fibroblasts exposed to ROS could lead to carcinogenic transformation of cells.151 ROS are considered pro-neoplastic factors, they stimulate cell proliferation, invasiveness, angiogenesis, and metastasis, and inhibit apoptosis.152
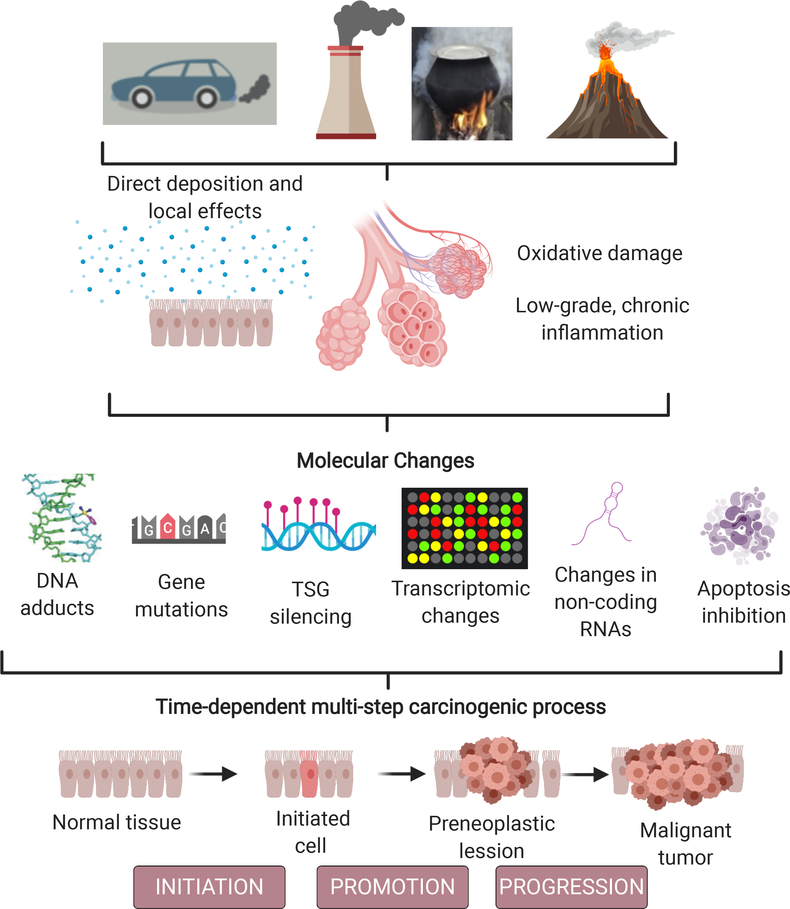
Air pollution-related carcinogenesis is expected to follow a multi-step process that includes initiation, promotion, and progression (Figure 4).153 Although not completely understood, individual and time-dependent dose influences the mechanisms by which environmental air pollutants result in cancer cell transformation. The impact of air pollution particular carcinogens and their mixtures disrupt several molecular processes via direct or indirect (inflammation and OS) damage, inducing TSG inactivation and the activation of oncogenes,154 cell cycle alterations dependent on TP53 activation,155 activation of energetic dysregulation,156 chromosome instability,157–158 the inhibition of apoptosis,159 and the induction of cell proliferation in somatic cells.160 Further research will clarify which mechanisms are most relevant and can be used as early biomarkers of air pollution-related cancer.
Figure 4.
Air pollution-related cancer: Potential pathways and mechanisms. TSG: Tumor suppressor genes.
Public Health/Policy Recommendations
Few cancers have been as well characterized as lung cancer from the perspective of etiology, leading to the well-documented predominant role of environmental factors in causing this highly fatal malignancy. As mentioned above, outdoor air pollution and specifically PM was classified by IARC as a causal agent (Group 1 carcinogen) for lung cancer.15 Despite this, the United States Environmental Protection Agency (EPA) in its most recent review of the evidence on PM, the Integrated Science Assessment, found the weight-of-evidence to indicate that PM2.5 is only “likely to be causal”.161 Nonetheless, when the IARC conclusion was published,162 its policy implications figured prominently in media reports.
From a public health policy perspective, the addition of outdoor air pollution to the list of causes of lung cancer, and potentially also to a growing list of cancers at other sites, offers another imperative for air quality management. Given widespread recognition that lung cancer is highly fatal, the IARC conclusion may prove a more powerful motivator than other less well understood, adverse effects of air pollution.
Implementing measures to reduce cancer due to outdoor air pollution is challenging, as there are typically numerous combustion sources with emissions including both specific carcinogens and other agents that may increase cancer risk. Based on understanding of carcinogenesis and considering the agents known to be in air pollution, a linear non-threshold relationship between exposure and risk can be reasonably assumed.26,163,164 From a regulatory perspective, this implies that any exposure conveys some risk and that lowering exposure to the maximum extent feasible should be the goal.
The definition of “acceptable risk” by Lowrance165 is a useful starting point for considering management of cancer risk caused by air pollution: “A thing is safe if its risks are judged to be acceptable.” For lung cancer, using PM as the indicator of exposure, risks have been quantified with sufficient certainty for carrying out a risk assessment, but any consensus societal judgment as to the acceptability of lung cancer risk from air pollution is lacking. Estimates of the burden of lung cancer attributable to air pollution have been made at the population level (above). The global proportion of lung cancer deaths attributable to ambient PM2.5 is second only to tobacco smoking.
Interventions to reduce air pollution exposure may be considered at various scales including the individual, community, industrial, and broader regional scales.166 In the United States, the Clean Air Act calls on the Administrator of the EPA to set National Ambient Air Quality Standards (NAAQS) that protect public health “with an adequate margin of safety.” For PM, that goal cannot be absolutely achieved as adverse effects of air pollution have been demonstrated at levels well below current NAAQS and for some adverse effects, including lung cancer, non-threshold risk relationships are biologically plausible. Acknowledging that risk cannot be fully avoided through regulatory action, the EPA uses risk assessment methods and scenarios of exposure reduction under different changes to the NAAQS. The adverse health effects considered have been those for which the agency has found the relationship to be causal. Thus, in the current revision of the PM NAAQS, lung cancer will likely not be an element of the risk assessment considered. Nonetheless, the many organizations concerned with lung cancer, should use the mounting evidence and IARC finding to advocate for accurate air pollution monitoring and tighter air quality management and for specific consideration of sources that most prominently contribute to the dose of inhaled carcinogens, including controlling fine PM from combustion, especially from fossil fuel sources.
Multiple interventions occurring over long time scales have led to improvements in outdoor air quality in many higher-income and some middle-income countries and improvements in health.23,24,167–170 Further research to evaluate the effectiveness of specific interventions in low- and middle- income countries, where air quality continues to worsen, is needed.167 Reductions in biomass burning, which can contribute to high levels of air pollution outdoors, as well as improvements in cooking stoves and indoor ventilation are important air quality improvement strategies worldwide.171,172 Impacts in terms of reduction of lung cancer incidence, as noted above, requires long-term and sustained intervention over multiple years and decades. Although potential impacts on cancer survival post-diagnosis have also been suggested, further research is required to evaluate impacts of reducing patient-level outdoor air pollution exposure on survival.54
Available research regarding interventions to reduce outdoor air pollution levels has resulted in subsequent calls for cities to pursue more compact and mixed-use urban designs with a transport modal shift from private vehicles to active transport.173 Specific interventions may relate to destination accessibility, employment distribution, residential density, availability and cost of parking, and enhancement of active travel networks.174 Interventions related with road-traffic emissions have also included planning and development management, car-free policies, clean air zones, vehicle technologies and reducing emissions from public sector transport services, smooth driving and speed reduction, public transportation provision, and raising public awareness of the adverse human health impacts of outdoor air pollution.169,175 The key role of the medical and healthcare community in raising public and patient awareness, including of monitoring of local air quality indices and guidelines,172 motivating action on air quality management, and involvement in the policy process has been described.171,173,175,176 The support of the medical and healthcare community in the further conduct of relevant etiological and innovative intervention studies is also needed.173 There is also increasing interest in the use of green spaces and green infrastructure in air pollution mitigation, though further research in terms of specific infrastructure deployment is needed to optimize health benefits, reduce unintended consequences, and develop evidence-based guidelines for implementation.177
Individual-level interventions have also been described including the use of personal respirators, though impacts on exposure and health are difficult to evaluate in the general population.171,178 Reductions in exposure to PM2.5 and other particle pollutants have been reported in some studies,179 though the overall evidence remains inadequate.178 The use of personal respirators in combination with avoidance behaviour, such as route selection for example, has been recommended.178 Reductions in indoor levels of PM2.5 have been observed with the use of some household filtration systems.180,181 In terms of commuting mode, in a review of studies of travel microenvironments in Europe, pedestrians experienced the lowest exposure to air pollution and car users the highest, though results may not be applicable to other areas.182 Personal mobile monitoring technologies, including mobile phones, may support avoidance behaviours in the future.183
Lastly, the suggestion of possible greater-than-additive joint effects of cigarette smoking and PM2.5 concentrations for lung cancer mortality may also imply that public health efforts in tobacco control and air quality management may result in greater than expected reductions in lung cancer rates due to the reduction of cases attributable to the interaction of both factors.53
Conclusion
In conclusion, there is clear and substantial evidence of a link between outdoor ambient air pollution, and particularly PM in outdoor air, with lung cancer incidence and mortality causing hundreds of thousands of lung cancer deaths annually worldwide. This burden represents an urgent worldwide public health challenge requiring multiple multi-level public health and policy interventions for cancer prevention. Epidemiological evidence on outdoor air pollution and other types of cancer is more limited. Further research on cancer incidence and survival at other cancer sites is needed, as well as on the effectiveness of specific interventions for cancer prevention, particularly in low- and middle- income countries.
Acknowledgements
Nathan Coleman for helpful comments on an earlier version of the manuscript. Julia Knox for contributing to an earlier draft of the section on Biological Mechanisms.
Funding: MCT is funded by a Ramón y Cajal fellowship (RYC-2017-01892) from the Spanish Ministry of Science, Innovation and Universities and cofunded by the European Social Fund. ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. GT was supported in part by Center Grant ES 00260 from the National Institute of Environmental Health Sciences (NIEHS).
REFERENCES
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lelieveld J, Evans J, Fnais M, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. [DOI] [PubMed] [Google Scholar]
- 3.Santos LR, Alves-Correia M, Câmara M, et al. Multiple victims of carbon monoxide poisoning in the aftermath of a wildfire: a case series. Acta Med Port. 2018;31:146–151. [DOI] [PubMed] [Google Scholar]
- 4.Thurston G Outdoor air pollution: Sources, atmospheric transport, and human health effects. International Encyclopedia of Public Health (Second Edition). 2017;367–377. [Google Scholar]
- 5.Lall R, Thurston G. Identifying and quantifying transported vs. local sources of New York City PM2.5 fine particulate matter air pollution. Atmos Environ. 2006;40:S333–S346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leikauf GD, Kim SH, Jang AS. Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med. 2020;52:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Research Council (NRC). Controlling Airborne Particles. Washington, DC: The National Academies Press; 1980. [Google Scholar]
- 8.Matschullat J Arsenic in the geosphere—A review. Sci Total Environ. 2000;249:297–312. [DOI] [PubMed] [Google Scholar]
- 9.UN Environment. Global Mercury Assessment 2018. Geneva: UN Environment Programme, Chemicals and Health Branch; 2019. [Google Scholar]
- 10.Health Effects Institute (HEI). State of Global Air 2019 Special Report. Boston, MA: Health Effects Institute; 2019. [Google Scholar]
- 11.Geddes JA, Martin RV, Boys BL, van Donkelaar A. Long-term trends worldwide in ambient NO2 concentrations inferred from satellite observations. Environ Health Perspect. 2016;124:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 14.Loomis D, Guha N, Hall AL, Straif K. Identifying occupational carcinogens: an update from the IARC Monographs. Occup Environ Med. 2018;75:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer (IARC). Outdoor Air Pollution. Volume 109 Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- 16.Krewski D, Jerrett M, Burnett RT, et al. Extended Follow-up and Spatial Analysis of the American Cancer Society Study Linking Particulate Air Pollution and Mortality. Boston, MA: Health Effects Institute; 2009. [PubMed] [Google Scholar]
- 17.Turner MC, Krewski D, Pope CA III, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never smokers. Am J Respir Crit Care Med. 2011;184:1374–1381. [DOI] [PubMed] [Google Scholar]
- 18.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14:813–822. [DOI] [PubMed] [Google Scholar]
- 19.Doll R, Hill AB. Smoking and carcinoma of the lung. Br Med J. 1950;2:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer (IARC). Household Use of Solid Fuels and High-Temperature Frying. Volume 95 Lyon: International Agency for Research on Cancer; 2006. [Google Scholar]
- 21.International Agency for Research on Cancer (IARC). A Review of Human Carcinogens. Part E: Personal Habits and Indoor Combustions. Volume 100 Lyon: International Agency for Research on Cancer; 2009. [Google Scholar]
- 22.Chafe ZA, Brauer M, Klimont Z, et al. Household cooking with solid fuels contributes to ambient PM2.5 air pollution and the burden of disease. Environ Health Perspect. 2014;122:1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD MAPS Working Group. Burden of Disease Attributable to Coal-Burning and Other Major Sources of Air Pollution in China. Special Report 20. Boston, MA: Health Effects Institute; 2016. [Google Scholar]
- 24.GBD MAPS Working Group. Burden of Disease Attributable to Major Air Pollution Sources in India. Special Report 21. Boston, MA: Health Effects Institute; 2018. [Google Scholar]
- 25.HEI Household Air Pollution–Ghana Working Group. Contribution of Household Air Pollution to Ambient Air Pollution in Ghana. Communication 19. Boston, MA: Health Effects Institute; 2019. [Google Scholar]
- 26.Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. 2014;122:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pope CA III, Coleman N, Pond ZA, Burnett RT. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res. 2020;183:108924. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson RW, Butland BK, Anderson HR, Maynard RL. Long-term concentrations of nitrogen dioxide and mortality. Epidemiology. 2018;29:460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamra GB, Laden F, Cohen AJ, Raaschou-Nielsen O, Brauer M, Loomis D. Lung cancer and exposure to nitrogen dioxide and traffic: a systematic review and meta-analysis. Environ Health Perspect. 2015;123:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman NC, Burnett RT, Higbee JD, et al. Cancer mortality risk, fine particulate air pollution, and smoking in a large, representative cohort of US adults. Cancer Causes Control 2020;31:767–776. [DOI] [PubMed] [Google Scholar]
- 31.Hvidtfeldt UA, Geels C, Sørensen M, et al. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ Int. 2019;133:105268. [DOI] [PubMed] [Google Scholar]
- 32.Klompmaker JO, Hoek G, Bloemsma LD, et al. Surrounding green, air pollution, traffic noise exposure and non-accidental and cause-specific mortality. Environ Int. 2020;134:105341. [DOI] [PubMed] [Google Scholar]
- 33.Bai L, Shin S, Burnett RT, et al. Exposure to ambient air pollution and the incidence of lung cancer and breast cancer in the Ontario Population Health and Environment Cohort. Int J Cancer. 2020;146:2450–2459. [DOI] [PubMed] [Google Scholar]
- 34.Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol. 2017;186:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eum KD, Kazemiparkouhi F, Wang B, et al. Long-term NO2 exposures and cause-specific mortality in American older adults. Environ Int. 2019;124:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazemiparkouhi F, Eum KD, Wang B, Manjourides J, Suh HH. Long-term ozone exposures and cause-specific mortality in a US Medicare cohort. J Expo Sci Environ Epidemiol 2019. April 16 (Online ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Eum KD, Kazemiparkouhi F, et al. The impact of long-term PM2.5 exposure on specific causes of death: exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ Health. 2020;19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowe B, Xie Y, Yan Y, Al-Aly Z. Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw Open. 2019;2:e1915834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Department of Health and Human Services. The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 40.Tomczak A, Miller AB, Weichenthal SA, et al. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int J Cancer. 2016;139:1958–1966. [DOI] [PubMed] [Google Scholar]
- 41.Gharibvand L, Lawrence Beeson W, Shavlik D, et al. The association between ambient fine particulate matter and incident adenocarcinoma subtype of lung cancer. Environ Health. 2017;16:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gharibvand L, Shavlik D, Ghamsary M, et al. The association between ambient fine particulate air pollution and lung cancer incidence: results from the AHSMOG-2 study. Environ Health Perspect. 2017;125:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consonni D, Carugno M, De Matteis S, et al. Outdoor particulate matter (PM10) exposure and lung cancer risk in the EAGLE study. PLoS One. 2018;13:e0203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moon DH, Kwon SO, Kim SY, Kim WJ. Air pollution and incidence of lung cancer by histological type in Korean adults: a Korean national health insurance service health examinee cohort study. Int J Environ Res Public Health. 2020;17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US National Academy of Sciences, Engineering, and Medicine. Using 21st Century Science to Improve Risk-Related Evaluations. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 46.Turner MC, Jerrett M, Pope CA III, et al. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med. 2016;193:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurston GD, Ito K, Lall R, et al. NPACT Study 4. Mortality and Long-Term Exposure to PM2.5 and Its Components in the American Cancer Society’s Cancer Prevention Study II Cohort In: National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components. Boston: Health Effects Institute; 2013, pp. 127–166. [PubMed] [Google Scholar]
- 48.Weichenthal S, Crouse DL, Pinault L, et al. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Res. 2016;146:92–99. [DOI] [PubMed] [Google Scholar]
- 49.Cakmak S, Hebbern C, Pinault L, et al. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ Int. 2018;111:200–211. [DOI] [PubMed] [Google Scholar]
- 50.Raaschou-Nielsen O, Beelen R, Wang M, et al. Particulate matter air pollution components and risk for lung cancer. Environ Int. 2016;87:66–73. [DOI] [PubMed] [Google Scholar]
- 51.International Agency for Research on Cancer (IARC). Diesel and Gasoline Engine Exhausts and some Nitroarenes. Volume 105 Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 52.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner MC, Cohen A, Jerrett M, et al. Interactions between cigarette smoking and fine particulate matter in the risk of lung cancer mortality in Cancer Prevention Study II. Am J Epidemiol. 2014;180:1145–1149. [DOI] [PubMed] [Google Scholar]
- 54.Eckel SP, Cockburn M, Shu YH, et al. Air pollution affects lung cancer survival. Thorax. 2016;71:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int. 2019;130:104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Josyula S, Lin J, Xue X, et al. Household air pollution and cancers other than lung: a meta-analysis. Environ Health. 2015;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo J, Kauppinen T, Kyyrönen P, et al. Risk of esophageal, ovarian, testicular, kidney and bladder cancers and leukemia among Finnish workers exposed to diesel or gasoline engine exhaust. Int J Cancer. 2004;111:286–292. [DOI] [PubMed] [Google Scholar]
- 58.Peters CE, Parent MÉ, Harris SA, et al. Occupational exposure to diesel and gasoline engine exhausts and the risk of kidney cancer in Canadian men. Ann Work Expo Health. 2018;62:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rota M, Bosetti C, Boccia S, Boffetta P, La Vecchia C. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: an updated systematic review and a meta-analysis to 2014. Arch Toxicol. 2014;88:1479–1490. [DOI] [PubMed] [Google Scholar]
- 60.International Agency for Research on Cancer (IARC). Arsenic, Metals, Fibres, and Dusts. Volume 100C Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 61.Turner MC, Krewski D, Diver WR, et al. Ambient air pollution and cancer mortality in the Cancer Prevention Study-II. Environ Health Perspect. 2017;125:087013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castaño-Vinyals G, Cantor KP, Tardon A, et al. Air pollution and risk of urinary bladder cancer in a case-control study in Spain. Occup Environ Med. 2008;65:56–60. [DOI] [PubMed] [Google Scholar]
- 63.Turner MC, Gracia-Lavedan E, Cirac M, et al. Ambient air pollution and incident bladder cancer risk: updated analysis of the Spanish Bladder Cancer Study. Int J Cancer. 2019;145:894–900. [DOI] [PubMed] [Google Scholar]
- 64.Pedersen M, Stafoggia M, Weinmayr G, et al. Is there an association between ambient air pollution and bladder cancer incidence? Analysis of 15 European cohorts. Eur Urol Focus. 2018;4:113–120. [DOI] [PubMed] [Google Scholar]
- 65.White AJ, Bradshaw PT, Hamra GB. Air pollution and breast cancer: a review. Curr Epidemiol Rep. 2018;5:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, Sandler DP. Air pollution, clustering of particulate matter components, and breast cancer in the Sister Study: a U.S.-wide cohort. Environ Health Perspect. 2019;127:107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niehoff NM, Gammon MD, Keil AP, et al. Airborne mammary carcinogens and breast cancer risk in the Sister Study. Environ Int. 2019;130:104897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng I, Tseng C, Wu J, et al. Association between ambient air pollution and breast cancer risk: The multiethnic cohort study. Int J Cancer. 2020;146:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldberg MS, Villeneuve PJ, Crouse D, et al. Associations between incident breast cancer and ambient concentrations of nitrogen dioxide from a national land use regression model in the Canadian National Breast Screening Study. Environ Int. 2019;133:105182. [DOI] [PubMed] [Google Scholar]
- 70.Villeneuve PJ, Goldberg MS, Crouse DL, et al. Residential exposure to fine particulate matter air pollution and incident breast cancer in a cohort of Canadian women. Environ Epidemiol 2018;2:e021. [Google Scholar]
- 71.Hart JE, Bertrand KA, DuPre N, et al. Exposure to hazardous air pollutants and risk of incident breast cancer in the nurses’ health study II. Environ Health. 2018;17:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andersen ZJ, Ravnskjær L, Andersen KK, et al. Long-term exposure to fine particulate matter and breast cancer incidence in the Danish nurse cohort study. Cancer Epidemiol Biomarkers Prev. 2017;26:428–430. [DOI] [PubMed] [Google Scholar]
- 73.Amadou A, Praud D, Coudon T, et al. Chronic long-term exposure to cadmium air pollution and breast cancer risk in the French E3N cohort. Int J Cancer. 2020;146:341–351. [DOI] [PubMed] [Google Scholar]
- 74.DuPré NC, Hart JE, Holmes MD, et al. Particulate matter and traffic-related exposures in relation to breast cancer survival. Cancer Epidemiol Biomarkers Prev. 2019;28:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DuPré NC, Hart JE, Bertrand KA, Kraft P, Laden F, Tamimi RM. Residential particulate matter and distance to roadways in relation to mammographic density: results from the Nurses’ Health Studies. Breast Cancer Res. 2017;19:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yaghjyan L, Arao R, Brokamp C, et al. Association between air pollution and mammographic breast density in the Breast Cancer Surveillance Consortium. Breast Cancer Res. 2017;19:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huynh S, von Euler-Chelpin M, Raaschou-Nielsen O, et al. Long-term exposure to air pollution and mammographic density in the Danish Diet, Cancer and Health cohort. Environ Health. 2015;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, et al. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poulsen AH, Sorensen M, Andersen ZJ, Ketzel M, Raaschou-Nielsen O. Air pollution from traffic and risk for brain tumors: a nationwide study in Denmark. Cancer Causes Control. 2016;27:473–480. [DOI] [PubMed] [Google Scholar]
- 80.Jørgensen JT, Johansen MS, Ravnskjær L, et al. Long-term exposure to ambient air pollution and incidence of brain tumours: the Danish nurse cohort. Neurotoxicology. 2016;55:122–130. [DOI] [PubMed] [Google Scholar]
- 81.Andersen ZJ, Pedersen M, Weinmayr G, et al. Long-term exposure to ambient air pollution and incidence of brain tumor: the European Study of Cohorts for Air Pollution Effects (ESCAPE). Neuro Oncol. 2018;20:420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu AH, Wu J, Tseng C, et al. Association between outdoor air pollution and risk of malignant and benign brain tumors: the Multiethnic Cohort Study. JNCI Cancer Spectr. 2020;4:pkz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weichenthal S, Olaniyan T, Christidis T, et al. Within-city spatial variations in ambient ultrafine particle concentrations and incident brain tumors in adults. Epidemiology. 2020;31:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raaschou-Nielsen O, Pedersen M, Stafoggia M, et al. Outdoor air pollution and risk for kidney parenchyma cancer in 14 European cohorts. Int J Cancer. 2017;140:1528–1537. [DOI] [PubMed] [Google Scholar]
- 85.Pedersen M, Andersen ZJ, Stafoggia M, et al. Ambient air pollution and primary liver cancer incidence in four European cohorts within the ESCAPE project. Environ Res. 2017;154:226–233. [DOI] [PubMed] [Google Scholar]
- 86.Nagel G, Stafoggia M, Pedersen M, et al. Air pollution and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Int J Cancer. 2018;143:1632–1643. [DOI] [PubMed] [Google Scholar]
- 87.Weinmayr G, Pedersen M, Stafoggia M, et al. Particulate matter air pollution components and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts of Air Pollution Effects (ESCAPE). Environ Int. 2018;120:163–171. [DOI] [PubMed] [Google Scholar]
- 88.Wong CM, Tsang H, Lai HK, et al. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol Biomarkers Prev. 2016;25:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan WC, Wu CD, Chen MJ, et al. Fine particle pollution, alanine transaminase, and liver cancer: a Taiwanese prospective cohort study (REVEAL-HBV). J Natl Cancer Inst. 2015;108(3). [DOI] [PubMed] [Google Scholar]
- 90.VoPham T, Bertrand KA, Tamimi RM, Laden F, Hart JE. Ambient PM 2.5 air pollution exposure and hepatocellular carcinoma incidence in the United States. Cancer Causes Control. 2018;29:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng H, Eckel SP, Liu L, Lurmann FW, Cockburn MG, Gilliland FD. Particulate matter air pollution and liver cancer survival. Int J Cancer. 2017;141:744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Teras LR, Diver WR, Deubler EL, et al. Residential ambient benzene exposure in the United States and subsequent risk of hematologic malignancies. Int J Cancer. 2019;145:2647–2660. [DOI] [PubMed] [Google Scholar]
- 93.Winters N, Goldberg MS, Hystad P, et al. Exposure to ambient air pollution in Canada and the risk of adult leukemia. Sci Total Environ. 2015;526:153–176. [DOI] [PubMed] [Google Scholar]
- 94.Taj T, Poulsen AH, Ketzel M, et al. Long-term exposure to air pollution and risk of non-Hodgkin lymphoma in Denmark: a population-based case-control study. Int J Cancer. 2020;doi: 10.1002/ijc.32978. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Taj T, Poulsen AH, Ketzel M, et al. Long-term exposure to PM2.5 and its constituents and risk of Non-Hodgkin lymphoma in Denmark: a population-based case-control study. Environ Res. 2020;188:109762. doi: 10.1016/j.envres.2020.109762. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 96.Raaschou-Nielsen O, Ketzel M, Harbo Poulsen A, Sorensen M. Traffic-related air pollution and risk for leukaemia of an adult population. Int J Cancer. 2016;138:1111–1117. [DOI] [PubMed] [Google Scholar]
- 97.Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spycher BD, Feller M, Röösli M, et al. Childhood cancer and residential exposure to highways: a nationwide cohort study. Eur J Epidemiol. 2015;30:1263–1275. [DOI] [PubMed] [Google Scholar]
- 99.Boothe VL, Boehmer TK, Wendel AM, Yip FY. Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am J Prev Med. 2014;46:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C. Parental, in utero, and early-life exposure to benzene and the risk of childhood leukemia: a meta-analysis. Am J Epidemiol. 2016;183:1– 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:36–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Filippini T, Hatch EE, Rothman KJ, et al. Association between outdoor air pollution and childhood leukemia: a systematic review and dose-response meta-analysis. Environ Health Perspect. 2019;127:46002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun XX, Zhang SS, Ma XL. No association between traffic density and risk of childhood leukemia: A meta-analysis. Asian Pacific J Cancer Prev. 2014;15:5229–5232. [DOI] [PubMed] [Google Scholar]
- 104.Danysh HE, Mitchell LE, Zhang K, Scheurer ME, Lupo PJ. Traffic-related air pollution and the incidence of childhood central nervous system tumors: Texas, 2001–2009. Pediatr Blood Cancer. 2015;62:1572–1578. [DOI] [PubMed] [Google Scholar]
- 105.Ghosh JK, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol. 2013;178:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heck JE, Wu J, Lombardi C, et al. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect. 2013;121:1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A. Residential exposure to traffic in California and childhood cancer. Epidemiology. 2004;15:6–12. [DOI] [PubMed] [Google Scholar]
- 108.von Ehrenstein OS, Heck JE, Park AS, Cockburn M, Escobedo L, Ritz B. In utero and early-life exposure to ambient air toxics and childhood brain tumors: a population-based case-control study in California, USA. Environ Health Perspect. 2016;124:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar SV, Lupo PJ, Pompeii LA, Danysh HE. Maternal residential proximity to major roadways and pediatric embryonal tumors in offspring. Int J Environ Res Public Health. 2018;15:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavigne E, Bélair MA, Do MT, et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario, Canada. Environ Int. 2017;100:139–147. [DOI] [PubMed] [Google Scholar]
- 111.Ou JY, Hanson HA, Ramsay JM, et al. Fine particulate matter air pollution and mortality among pediatric, adolescent, and young adult cancer patients. Cancer Epidemiol Biomarkers Prev. 2020;doi: 10.1158/1055-9965.EPI-19-1363. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pitot HC. The molecular biology of carcinogenesis. Cancer. 1993;72:962–970. [DOI] [PubMed] [Google Scholar]
- 113.Brauer M, Avila-Casado C, Fortoul TI, Vedal S, Stevens B, Churg A. Air pollution and retained particles in the lung. Environ Health Perspect. 2001;109:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Castano-Vinyals G, D’Errico A, Malats N, Kogevinas M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup Environ Med. 2004;61:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health 2020;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamagishi K, Onuma K, Chiba Y, et al. Generation of gaseous sulfur-containing compounds in tumour tissue and suppression of gas diffusion as an antitumour treatment. Gut 2012;61:554–561. [DOI] [PubMed] [Google Scholar]
- 117.Arlt VM. 3-Nitrobenzanthrone, a potential human cancer hazard in diesel exhaust and urban air pollution: a review of the evidence. Mutagenesis 2005;20:399–410. [DOI] [PubMed] [Google Scholar]
- 118.Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci. 2015;145:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peluso M, Ceppi M, Munnia A, Puntoni R, Parodi S. Analysis of 13 (32)P-DNA postlabeling studies on occupational cohorts exposed to air pollution. Am J Epidemiol. 2001;153:546–558. [DOI] [PubMed] [Google Scholar]
- 120.Dunn BP. Carcinogen adducts as an indicator for the public health risks of consuming carcinogen-exposed fish and shellfish. Environ Health Perspect. 1991;90:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peluso M, Airoldi L, Armelle M, et al. White blood cell DNA adducts, smoking, and NAT2 and GSTM1 genotypes in bladder cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1998;7:341–346. [PubMed] [Google Scholar]
- 122.Tang D, Santella RM, Blackwood AM, et al. A molecular epidemiological case-control study of lung cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:341–346. [PubMed] [Google Scholar]
- 123.Demetriou CA, Vineis P. Carcinogenicity of ambient air pollution: Use of biomarkers, lessons learnt and future directions. J Thorac Dis. 2015;7:67–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang LH, Wu CF, Rajasekaran N, Shin YK. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem. 2018;51:2647–2693. [DOI] [PubMed] [Google Scholar]
- 125.Claxton LD, Matthews PP, Warren SH. The genotoxicity of ambient outdoor air, a review: salmonella mutagenicity. Mutat Res. 2004;567:347–399. [DOI] [PubMed] [Google Scholar]
- 126.Somers CM, Yauk CL, White PA, Parfett CL, Quinn JS. Air pollution induces heritable DNA mutations. Proc Natl Acad Sci U S A 2002;99:15904–15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deben C, van den Bossche J, van der Steen N, et al. Deep sequencing of the TP53 gene reveals a potential risk allele for non-small cell lung cancer and supports the negative prognostic value of TP53 variants. Tumour Biol. 2017;39:1010428317694327. [DOI] [PubMed] [Google Scholar]
- 128.Zhou W, Tian D, He J, et al. Repeated PM2.5 exposure inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation. Oncotarget 2016;7:20691–20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu XJ, Yang MJ, Zhou B, et al. Characterization of somatic mutations in air pollution-related lung cancer. EBioMedicine 2015;2:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sanchez-Guerra M, Zheng Y, Osorio-Yanez C, et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: Results from the Beijing truck driver air pollution study. Epigenetics. 2015;10:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gondalia R, Baldassari A, Holliday KM, et al. Methylome-wide association study provides evidence of particulate matter air pollution-associated DNA methylation. Environ Int. 2019;132:104723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clark SJ, Melki J. DNA methylation and gene silencing in cancer: which is the guilty party? Oncogene 2002;21:5380–5387. [DOI] [PubMed] [Google Scholar]
- 133.Zhang W, Klinkebiel D, Barger CJ, et al. Global DNA hypomethylation in epithelial ovarian cancer: passive demethylation and association with genomic instability. Cancers (Basel) 2020;12:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ponomaryova AA, Rykova EY, Azhikina T, et al. Long interspersed nuclear element-1 methylation status in the circulating DNA from blood of patients with malignant and chronic inflammatory lung diseases. Eur J Cancer Prev. 2020;doi: 10.1097/CEJ.0000000000000601. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.Ye D, Jiang D, Zhang X, Mao Y. Alu methylation and risk of cancer: a meta-analysis. Am J Med Sci. 2020;359:271–280. [DOI] [PubMed] [Google Scholar]
- 136.Prada D, Gonzalez R, Sanchez L, Castro C, Fabian E, Herrera LA. Satellite 2 demethylation induced by 5-azacytidine is associated with missegregation of chromosomes 1 and 16 in human somatic cells. Mutat Res. 2012;729:100–105. [DOI] [PubMed] [Google Scholar]
- 137.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo L, Byun HM, Zhong J, et al. Effects of short-term exposure to inhalable particulate matter on DNA methylation of tandem repeats. Environ Mol Mutagen. 2014;55:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tarantini L, Bonzini M, Apostoli P, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hesselbach K, Kim GJ, Flemming S, et al. Disease relevant modifications of the methylome and transcriptome by particulate matter (PM2.5) from biomass combustion. Epigenetics 2017;12:779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deng X, Feng N, Zheng M, et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim Biophys Acta Gen Subj. 2017;1861:112–125. [DOI] [PubMed] [Google Scholar]
- 142.Liu C, Guo H, Cheng X, et al. Exposure to airborne PM2.5 suppresses microRNA expression and deregulates target oncogenes that cause neoplastic transformation in NIH3T3 cells. Oncotarget 2015;6:29428–29439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li X, Lv Y, Gao N, et al. microRNA-802/Rnd3 pathway imposes on carcinogenesis and metastasis of fine particulate matter exposure. Oncotarget 2016;7:35026–35043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding X, Wang M, Chu H, et al. Global gene expression profiling of human bronchial epithelial cells exposed to airborne fine particulate matter collected from Wuhan, China. Toxicol Lett. 2014;228:25–33. [DOI] [PubMed] [Google Scholar]
- 145.Zhou Z, Liu Y, Duan F, et al. Transcriptomic analyses of the biological effects of airborne PM2.5 exposure on human bronchial epithelial cells. PLoS One. 2015;10:e0138267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baulig A, Blanchet S, Rumelhard M, Lacroix G, Marano F, Baeza-Squiban A. Fine urban atmospheric particulate matter modulates inflammatory gene and protein expression in human bronchial epithelial cells. Front Biosci. 2007;12:771–782. [DOI] [PubMed] [Google Scholar]
- 147.Gualtieri M, Mantecca P, Cetta F, Camatini M. Organic compounds in tire particle induce reactive oxygen species and heat-shock proteins in the human alveolar cell line A549. Environ Int. 2008;34:437–442. [DOI] [PubMed] [Google Scholar]
- 148.Strzelczyk JK, Wiczkowski A. Oxidative damage and carcinogenesis. Contemp Oncol (Pozn). 2012;16:230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54. [DOI] [PubMed] [Google Scholar]
- 150.Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by cd4(+)t cells in neurodegenerative diseases. Front Cell Neurosci. 2018;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zimmerman R, Cerutti P. Active oxygen acts as a promoter of transformation in mouse embryo C3H/10T1/2/C18 fibroblasts. Proc Natl Acad Sci U S A. 1984;81:2085–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Halliwell B Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. [DOI] [PubMed] [Google Scholar]
- 153.Puisieux A, Pommier RM, Morel AP, Lavial F. Cellular pliancy and the multistep process of tumorigenesis. Cancer Cell. 2018;33:164–172. [DOI] [PubMed] [Google Scholar]
- 154.Bai J, Meng Z. Effect of sulfur dioxide on expression of proto-oncogenes and tumor suppressor genes from rats. Environ Toxicol. 2010;25:272–283. [DOI] [PubMed] [Google Scholar]
- 155.Abbas I, Verdin A, Escande F, et al. In vitro short-term exposure to air pollution pm2.5–0.3 induced cell cycle alterations and genetic instability in a human lung cell coculture model. Environ Res. 2016;147:146–158. [DOI] [PubMed] [Google Scholar]
- 156.Reyes-Caballero H, Rao X, Sun Q, et al. Air pollution-derived particulate matter dysregulates hepatic krebs cycle, glucose and lipid metabolism in mice. Sci Rep. 2019;9:17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Santibanez-Andrade M, Quezada-Maldonado EM, Osornio-Vargas A, Sanchez-Perez Y, Garcia-Cuellar CM. Air pollution and genomic instability: The role of particulate matter in lung carcinogenesis. Environ Pollut. 2017;229:412–422. [DOI] [PubMed] [Google Scholar]
- 158.Taghizadeh S, Najmabadi H, Kamali K, Behjati F. Evaluation of chromosomal aberrations caused by air pollutants in some taxi drivers from two polluted districts of urban Tehran and its comparison with drivers from rural areas of Lahijan: A pilot study. J Environ Health Sci Eng. 2014;12:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dagher Z, Garcon G, Billet S, et al. Activation of different pathways of apoptosis by air pollution particulate matter (pm2.5) in human epithelial lung cells (l132) in culture. Toxicology. 2006;225:12–24. [DOI] [PubMed] [Google Scholar]
- 160.Novack L, Yitshak-Sade M, Landau D, et al. Association between ambient air pollution and proliferation of umbilical cord blood cells. Environ Res. 2016;151:783–788. [DOI] [PubMed] [Google Scholar]
- 161.US Environmental Protection Agency (US EPA). Integrated Science Assessment (ISA) for Particulate Matter. Research Triangle Park: U.S. Environmental Protection Agency; 2019. [PubMed] [Google Scholar]
- 162.Loomis D, Grosse Y, Lauby-Secretan B, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. [DOI] [PubMed] [Google Scholar]
- 163.Burnett R, Chen H, Szyszkowicz M, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. 2018;115:9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pope CA 3rd, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lowrance WW. Of Acceptable Risk; Science and the Determination of Safety. Los Altos: William Kaufmann; 1976. [Google Scholar]
- 166.Annesi-Maesano I The air of Europe: where are we going? Eur Respir Rev. 2017;26(146). pii: 170024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Burns J, Boogaard H, Polus S, et al. Interventions to reduce ambient air pollution and their effects on health: an abridged Cochrane systematic review. Environ Int. 2020;135:105400. [DOI] [PubMed] [Google Scholar]
- 168.Correia AW, Pope CA, Dockery DW, Wang Y, Ezzati M, Dominici F. The effect of air pollution control on life expectancy in the United States: an analysis of 545 US counties for the period 2000 to 2007. Epidemiology. 2013;24:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Glazener A, Khreis H. Transforming our cities: best practices towards clean air and active transportation. Curr Environ Health Rep. 2019;6:22–37. [DOI] [PubMed] [Google Scholar]
- 170.Pope CA, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schraufnagel DE, Balmes JR, De Matteis S, et al. Health benefits of air pollution reduction. Ann Am Thorac Soc. 2019;16:1478–1487. [DOI] [PubMed] [Google Scholar]
- 172.Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388:1939–1951. [DOI] [PubMed] [Google Scholar]
- 173.Sallis JF, Bull F, Burdett R, et al. Use of science to guide city planning policy and practice: how to achieve healthy and sustainable future cities. Lancet. 2016;388:2936–2947. [DOI] [PubMed] [Google Scholar]
- 174.Giles-Corti B, Vernez-Moudon A, Reis R, et al. City planning and population health: a global challenge. Lancet. 2016;388:2912–2924. [DOI] [PubMed] [Google Scholar]
- 175.Vardoulakis S, Kettle R, Cosford P, et al. Local action on outdoor air pollution to improve public health. Int J Public Health. 2018:63:557–565. [DOI] [PubMed] [Google Scholar]
- 176.Wang L, Zhong B, Vardoulakis S, et al. Air quality strategies on public health and health equity in Europe—a systematic review. Int J Environ Res Public Health. 2016;13:1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kumar P, Druckman A, Gallagher J, et al. The nexus between air pollution, green infrastructure and human health. Environ Int. 2019;133:105181. [DOI] [PubMed] [Google Scholar]
- 178.ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the “Assessment of the expected health benefits of anti-pollution respirators”. Maisons-Alfort: French Agency for Food, Environmental and Occupational Health & Safety; 2018. [Google Scholar]
- 179.Pacitto A, Amato F, Salmatonidis A, et al. Effectiveness of commercial face masks to reduce personal PM exposure. Sci Total Environ. 2019;650:1582–1590. [DOI] [PubMed] [Google Scholar]
- 180.Fisk WJ, Chan WR. Health benefits and costs of filtration interventions that reduce indoor exposure to PM2.5 during wildfires. Indoor Air. 2017;27:191–204. [DOI] [PubMed] [Google Scholar]
- 181.Huang G, Zhou W, Qian Y, Fisher B. Breathing the same air? Socioeconomic disparities in PM2.5 exposure and the potential benefits from air filtration. Sci Total Environ. 2019;657:619–626. [DOI] [PubMed] [Google Scholar]
- 182.de Nazelle A, Bode O, Orjuela JP. Comparison of air pollution exposures in active vs. passive travel modes in European cities: a quantitative review. Environ Int. 2017;99:151–160. [DOI] [PubMed] [Google Scholar]
- 183.Nyarku M, Mazaheri M, Jayaratne R, et al. Mobile phones as monitors of personal exposure to air pollution: Is this the future? PLoS One. 2018;13:e0193150. [DOI] [PMC free article] [PubMed] [Google Scholar]