Abstract
Ischemic stroke is still a serious threat to human life and health, but there are few therapeutic options available to treat stroke because of limited blood-brain penetration. The development of nanotechnology may overcome some of the problems related to traditional drug development. In this review, we focus on the potential applications of nanotechnology in stroke. First, we will discuss the main molecular pathological mechanisms of ischemic stroke to develop a targeted strategy. Second, considering the important role of the blood-brain barrier in stroke treatment, we also delve mechanisms by which the blood-brain barrier protects the brain, and the reasons why the therapeutics must pass through the blood-brain barrier to achieve efficacy. Lastly, we provide a comprehensive review related to the application of nanomaterials to treat stroke, including liposomes, polymers, metal nanoparticles, carbon nanotubes, graphene, black phosphorus, hydrogels and dendrimers. To conclude, we will summarize the challenges and future prospects of nanomedicine-based stroke treatments.
Keywords: Stroke, Nanomaterials, Blood-brain barrier
Graphical abstract
Highlights
-
•
Discussed the main molecular pathological mechanisms of ischemic stroke.
-
•
Reviewed several treatments for stroke.
-
•
Discussed the blood-brain barrier in detail.Summarized the applications of various nanomaterials in stroke.
-
•
Summarized the challenges and future prospects of nanomedicine-based stroke treatment.
1. Introduction
Stroke has received widespread attention because of its serious threat to human health and well-being [1]. It is expected that by 2030, the number of stroke cases and related deaths will rise to 23 million and 7.8 million, respectively. Stroke is caused by an abrupt reduction of blood flow to the brain because of an embolus, a blood clot. Cerebral ischemia triggers a complex cascade of biochemical reactions due to reduced glucose and oxygen delivery, resulting in a substantial decrease in mitochondrial adenosine triphosphate (ATP) levels. The stroke cascade immediate glutamate excitotoxicity, oxidative stress and inflammation that contribute to tissue injury [[2], [3], [4]]. Because of the complexity of pathophysiological responses following a stroke, few therapeutic approaches have clinical efficacy [5]. The only approved treatment for embolic stroke is recanalization induced by the application of recombinant tissue plasminogen activator (rt-PA) [6,7]. However, this approach is limited by a narrow therapeutic window (<4.5 h) and complications in some patients.
According to the recent literature, only 3 positive phase III randomized clinical trials (RCTs) have been conducted for acute ischemic stroke. They validate the clinical effect of intravenous (IV) tPA for acute ischemic stroke within 3 h; Prolyse in Acute Cerebral Thromboembolism (PROACT) II demonstrated efficacy of IV tPA in selected patients out to 4.5 h; European Cooperative Acute Stroke Study (ECASS III) demonstrated the clinical and recanalization effect of intra-arterial (IA) prourokinase in the treatment of M1 or M2 middle cerebral artery occlusion within 6 h of a stroke.
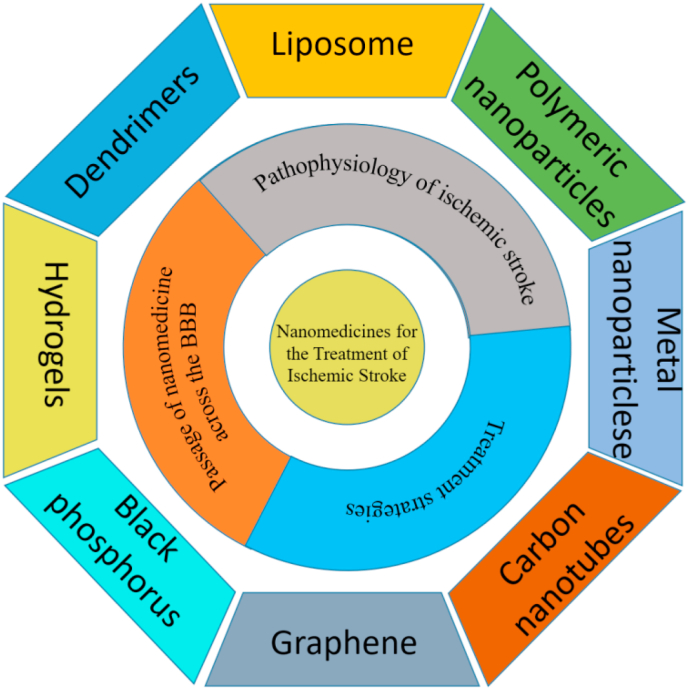
Recently, nanomedicine delivery systems have gained some attention as effective and safe systems for the delivery of therapeutic agents across the BBB [8]. Limited success is due to their ability to penetrate the blood brain barrier via both passive and active targeting mechanisms, resulting in enhanced accumulation in brain (see Fig. 1).
Fig. 1.
Nano-medicine for the treatment of ischemic stroke.
2. Pathophysiology of ischemic stroke
2.1. Excitotoxicity
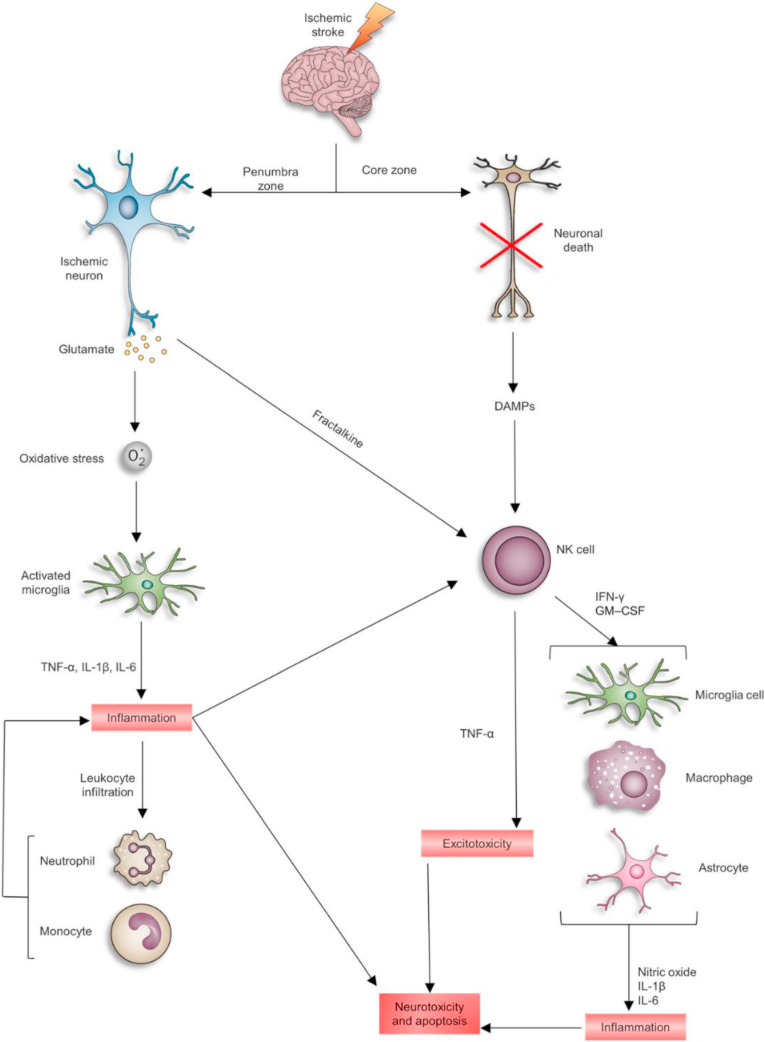
One of the molecular mechanisms responsible for ischemic damage is excitotoxicity [9]. Energy failure induces rapid release of excitatory amino acids, as well as inhibited reuptake of the excitatory amino acids. The high concentration of glutamate in synapses activates metabotropic glutamate receptors such as AMPA and NMDA receptors [10,11], resulting in disruption of calcium homeostasis. The initial calcium influx following excitotoxic glutamate stimulation is known to cause a secondary intracellular calcium overload, and this secondary response leads to a deleterious cascade of metabolic events through oxidative stress-mediated mechanisms such as apoptosis and inflammation (Fig. 2).
Fig. 2.
Schematic of neurotoxicity induced by changes in inflammatory cytokines and immune cells after cerebral ischemia. Reprinted with permission from Ref. [12]. Copyright 2017 SPRINGER.
2.2. Oxidative and nitrative stress
High oxygen consumption, high oxidized lipids levels as well as low endogenous antioxidant capacity in brain can cause the antioxidant defense system to be insufficient to scavenge free radicals (reactive oxygen and nitrogen species), resulting in oxidative and nitrosative stress-induced injury [13]. Free radical formation has been shown to be increased following a stroke and is thought to play a role in stroke damage. Reactive oxygen (ROS) and nitrogen species (RNS) may be important mediators of tissue injury in ischemic stroke [14]. ROS have many adverse effects at the cellular level, including the destruction of cellular macromolecules, induction of apoptotic cell death via lipid peroxidation; they cause oxidative DNA damage, protein annihilation, chemotaxis, and cytoskeletal structural injury [15]. Additionally, ROS and subsequent oxidative stress affect the cerebral vasculature by disrupting the functional integrity of BBB due to changes in the molecular organization and expression of critical tight junction proteins, such as claudin-5 and occludin at the BBB [16].
The NO molecule, produced by NOS, which is activated by ischemia, can combine with superoxide to produce peroxynitrite, which is an effective oxidant [17]. RNS also have prominent cellular effects, such as inhibition of mitochondrial enzymes, improving the opening of the mitochondrial permeability transition pore, and causing DNA damage. They also accelerate the influx of Ca2+ ions by activation of the cation channel subfamily M member 7 channels, which are permeable to the Ca2+ ions. Additionally, NO can affect cells due to its regulatory effect on important proteins, including metalloproteases, caspases, and glycolytic enzymes [18].
2.3. Inflammatory
After cerebral ischemia, the inflammatory cascade is activated and mediated by a wide variety of molecules such as cytokines and lymphokines released from injured tissues and ROS species. Ischemia also induces microglial (resident macrophages of the brain) transformation into phagocytic cells that generate various cytotoxic and/or cytoprotective substances. Microglia cells exert neuroprotective effects by releasing neurotrophic factors, including brain derived neurotrophic factor and insulin-like growth factor I. Also, in response to ischemia, activated microglia generate proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) [19]. While the initial purpose of activated microglial is to protect neuron cells, the over-activation of microglia leads to deleterious inflammation and neuronal death [20].
In the brain, toll-like receptors (TLRs) are expressed on microglia, astrocytes, endothelial cells, and neurons [21]. Under hypoxic conditions, TLR4 is upregulated on the surface of microglial [22]. TLRs can activate transcriptional mediators that induce nuclear gene expression of pro-inflammatory factors, cytokines, and adhesion molecules. Moreover, endogenous ligands, such as Hsp70 are upregulated after ischemia, and could potentially cause ischemic damage via TLR activation.
3. Treatment strategies
For stroke treatment, time is a key factor. The first stroke therapeutic strategy aims to increase brain reperfusion by removing clots by pharmacological or mechanical means. tPA-induced thrombolysis and intravascular recanalization techniques are now both standard treatments for acute ischemic stroke, but both have a narrow time frame when they can be applied, and there is the risk of cerebral hemorrhage.
3.1. Tissue-type plasminogen activator
Recombinant rt-PA for thrombolysis is the only commercially-available pharmaceutical agent approved by the US FDA. After rt-PA treatment, brain re-oxygenation occurs quickly. However, this treatment has a narrow therapeutic window in order to be effective (no more than 4.5 h after symptom onset). Because of this, it is estimated that only 7% of patients can benefit from rt-PA administration [23]. In addition, in patients with large infarcts, rt-PA has a relatively low success rate, and can also increase the risk of bleeding [24].
In 2015, several randomized controlled trials showed that intravascular thrombectomy was superior to intravenous rt-PA to improve the prognosis of stroke in patients with proximal occlusion of the large arteries, and three of four patients had complete reperfusion. In addition, a comprehensive meta-analysis of eight RCTs revealed that mechanical thrombectomy was correlated with an increased probability of positive outcome when compared with standard rt-PA treatment [25]. Moreover, mechanical thrombectomy, after treatment with rt-PA is now regarded as a standard guideline-recommended therapy for patients with large vessel occlusion.
3.2. Neuroprotective agents
As mentioned above, during ischemic cell death, several mechanisms play an important role, including excitotoxicity, oxidative and nitrosative stress, and inflammation. Neuroprotection is thought to be a potential method to modulate one or more of these mechanisms. These interventions include drug-induced neurotransmitter receptor blockade, anti-oxidants, anti-inflammatory drugs, and inhibition of cell death pathways [26].
In the past two decades, more than 1000 neuroprotective agents have been developed and studied to some extent, and more than 100 have progressed to clinical trials, most of which were poorly conceived and designed. It is not surprising that none of these drugs showed efficacy in randomized controlled trials.
Neuroprotection in the laboratory is mainly designed in the rodent model of transient cerebral ischemia [27], while the majority of patients in neuroprotection trials had permanent ischemia. About one-third (37%) of patients included in neuroprotection trials could receive thrombolytic treatment. The failure of most clinical trials could be attribute to lack of methodological rigor in preclinical studies, and the low BBB permeability of neuroprotectants is considered an obstacle.
3.3. Photothermal therapy
Photothermal therapy(PTT) has been widely used in the treatment of cancer [[28], [29], [30], [31], [32]]. In PTT, photothermal agents generate a localized increase in temperature to, achieve tumor ablation. Graphene and black phosphorus have also been employed in phototherapy of neurodegenerative diseases, offering advantages, including high therapeutic efficiency and minimal invasiveness [[33], [34], [35], [36], [37]]. In addition, improved BBB permeability of black phosphorus nanosheets could be achieved under NIR irradiation [38]. black phosphorus may also have additional therapeutic benefits, such as decreasing ROS and increased mitochondrial membrane potential (MMP).
In addition to the direct cytotoxic effect of photothermal therapy, lasers may also be used to promote smart drug release [[39], [40], [41], [42]]. Qiu et al. [43] designed a fully biodegradable black phosphorus hydrogel as a smart drug release platform. After loading the drug by electrostatic adsorption, black phosphorus nanosheets were mixed with a low–melting-point agarose aqueous solution to obtain the black phosphorus hydrogel. The hydrogel is capable of reversible hydrolysis and softening with an increase in temperature induced by the laser, thereby achieving photo-controlled release of the drug. The drug release rate of black phosphorus hydrogel is significantly improved under the irradiation compared with the dark condition. Light-controlled drug release may have clinical application for the treatment of stroke.
4. Passage of nanomedicine across the BBB
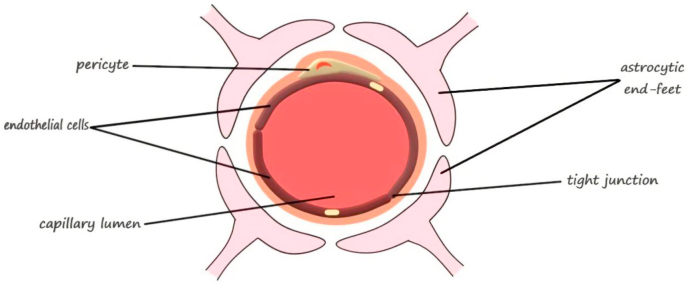
The BBB is an important barrier between the nervous system and other parts of the body. The BBB protects the brain from harmful stimuli. BBB permeability is controlled by tight junction protein complexes that limiting paracellular diffusion between capillary endothelial cells. Tight junction proteins complexes include occludin, claudins, junctional adhesion molecules, membrane-associated guanylate kinase-like proteins, and membrane associated degrading enzymes. There are specific mechanisms involved in the BBB transport of molecules that may be utilized for therapeutic drug delivery (Fig. 3).
Fig. 3.
Schematic illustration of the main structural components of the BBB. Reprinted with permission from Ref. [44]. Copyright 2018 MDPI.
4.1. Altered permeability of the BBB
After brain ischemia, oxidative stress induced by ROS production changes the grouping of tight junction proteins at the BBB, leading to heightened paracellular leakage [45]. Astrocytes also have a marked impact on the BBB destruction after ischemic injury by mechanisms that cause physical disruption of astrocyte–endothelial junctions, opening of paracellular channels, and digestion of BBB matrix proteins [46].
Ischemic stroke initiates the opening of BBB for a short period of time, about minutes to hours. The second long-term (hours to days) reopening is initiated by reperfusion of the ischemic zone, by restoring blood supply. BBB dysfunction (primary and secondary opening) allows for a variety of normally restricted substances, including proteins and red blood cells, to enter the damaged brain parenchyma. The leaky BBB provides opportunities for nanomedicines because of their small size and good fluidity.
4.2. Active BBB transport mechanisms
Another attractive strategy to promote transport of nanomedicines across the BBB is transcytosis. Active transport, including adsorptive-mediated and receptor-mediated transcytosis have been studies in order to achieve intercellular spacing and improve selective targeting. Positively charged nanoparticles interact with the negatively charged BBB luminal surface, leading to adsorption to endothelial membranes where they may be transcytosed. Coating nanomedicines with cationic biomolecules, for example, cell-penetrating peptides and cationic protein, is another promising way to increase BBB transport. Cell-penetrating peptides such as TAT improve BBB transit both in vitro and in vivo [47,48].
An alternative strategy for nanomedicine delivery across the BBB during the first few hours after injury is receptor-mediated transcytosis. Antibodies, ligand-mimicking peptides or whole ligands can be functionalized to nanoparticles targeting receptors on the surface of cells. These molecules include insulin receptors, transferrin receptors, lactoferrin receptors, and low-density lipoprotein receptor-related protein-1 [49].
5. Nanomedicine for ischemic stroke
In this section we summarize relevant nanomaterials and their corresponding drug-loading strategies or modification methods as tools to develop new therapies. Similarly, we summarize their advantages and existing shortcomings. Please see Table 1, Table 2.
Table 1.
List of nanomaterials/strategies for ischemic stroke therapy.
| Nanomaterials | Strategies | Major findings |
|---|---|---|
| Liposome | Encapsulation of superoxide dismutase | Animal models using liposome agents showed augmented levels of superoxide dismutase (SOD) and decreased infarct volume [50] |
| Changing Surface Charge | The absorption rate in ischemic area is higher [51] | |
| PEGylation | The circulation time of liposomes can be prolonged and can accumulate in ischemic brain area [52] | |
| Bind specific ligands to the surface of liposomes | Liposomes could effectively reach the brain injury area, and significantly decrease infarct volume and neurological deficit following middle cerebral artery occlusion [53] | |
| Polymeric nanoparticles | Loaded with Z-DEVD-FMK | Showed significant decrease in nerve injury, caspase-3 activity and reduced infarct volume [54] |
| Cationic polymer micelles | High efficiency, safe and reliable for tracing stem cells in vivo using magnetic resonance imaging [55] | |
| Metal nanoparticles | BBB permeation mediated by external magnetic field | Under the external magnetic field, metal nanoparticles showed accumulation a perivascular zone of the brain parenchyma and on-demand drug release [56] |
| MRI-monitored magnetic targeting | Magnetic targeting induced a 5-fold increase in the total glioma exposure to magnetic nanoparticles over non-targeted tumors [57] | |
| Free radical scavenging by autocatalytic | Cerium oxide nanoparticles show a significant neuroprotective effect on adult rat spinal cord neurons [57] | |
| Free radical scavenging | Cerium and yttrium oxide nanoparticles may be used as effective agents in prevention and possibly treatment of diabetic neuropathy [58] | |
| Carbon nanotubes | chemical bonding of amine groups on the surface | Aminemodified single-walled carbon nanotubes protected the brains of treated rats from ischaemic injury [59] |
| Graphene | Adsorption of ruthenium carbonyl clusters | Commodified GO can be used for CO-mediated vasodilatory treatment [60] |
| Modification with poly(amidoamine) dendrimer-grafted gadolinium | The modified GO be used as a contrast agent for magnetic resonance imaging to identify the location and extent of blood-brain barrier opening and quantitate drug [61] | |
| Modification with PEG | The PEGylation of rGO did not improve interaction with components of the BBB. In contrast, the attachment of PEG to rGO induced deleterious effects [62] | |
| matrix-assisted laser desorption/ionization mass spectrometry imaging | rGO systemically-injected was found mainly located in the thalamus and hippocampus of rats [63] | |
| Black phosphorus | BBB permeability increased by NIR irradiation | BP nanosheets can selectively capture Cu2+ and enhance the BBB permeability [64] |
| Hydrogels | Local injection of hydrogel | Effectively cross the blood-brain barrier, thereby promoting the infiltration of parenchyma cells around the scaffold and promoting local regeneration. |
| Gelatin microspheres(GMS) loaded with osteopondin | Duration of osteopondin release was significantly extended [65] | |
| Dendrimers | PEGylated poly(amido amine) (PAMAM) | Reduced blood clotting. |
| Dexamethasone-conjugated polyamidoamine generation 2 (PAMAM G2-Dexa) | Efficiently delivered heme oxygenase-1 (HO-1) gene into the ischemic brain [66] |
Table 2.
List of the advantage and disadvantage for nanomaterials.
| Nanomaterials | Advantages | Disadvantages |
|---|---|---|
| Liposome | High efficiency, low toxicity, long-term efficacy, ability to deliver both hydrophilic and lipophilic compounds [262] | Rapid systemic elimination, rapid metabolic degradation of phospholipids. Stability problems associated with long-term storage. Inability to provide sustained drug release. They are only moderately efficient for the encapsulation of lipophilic compounds [[263], [264], [265], [266], [267], [268]] |
| Polymeric nanoparticles | the ability to modify drug release; increase the stability of volatile drug; incorporate into other activities related to drug delivery [269] | high cost; the preparation process is complex and the reproducibility is low |
| Metal nanoparticles | MRI and magnetic targeting performance; free radical scavenging | Potential toxicity associated with complex ingredients [270] |
| Carbon nanotubes | High penetration power and surface area; more than one molecule can be conjugated to their surface | Low biodegradability and dispersivity, possible induced oxidative stress and Lung disease [170,171] |
| Graphene | Polyaromatic structure and higher surface area | The lack of standardization; Difficult to biodegrade; Damage to the lungs [215,271] |
| Black phosphorus | Biodegradable; selectively capture Cu2+64 | High cost; difficult to control shape and size |
| Hydrogels | Similar to the flexibility of natural tissue; PH or temperature sensitive; Biocompatible and biodegradable [272] | Thermosensitive hydrogel may cause excessive or insufficient drug release due to temperature stimulation [273]. |
| Dendrimers | Easy surface modification; ability to interact with charged functional groups [274]. | The specific toxicology, biocompatibility and in vivo distribution of various dendrimers need further in-depth study [274]. |
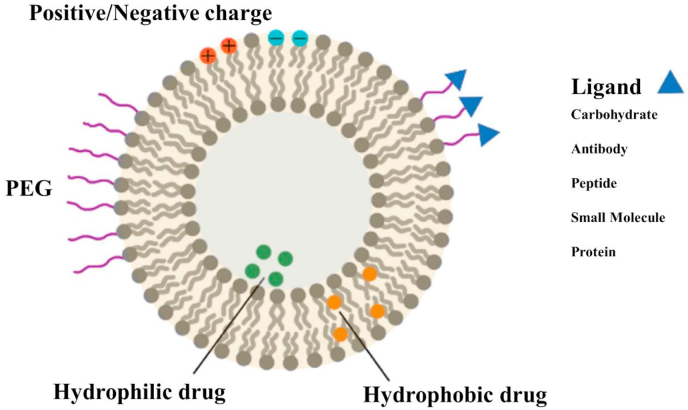
5.1. Liposome
Liposomes are synthetic spherical cells composed of single amphiphilic lipid bilayers (Fig. 4) [[67], [68], [69], [70], [71]] that have been widely used as drug delivery systems to improve the safety and effectiveness of therapeutic molecules, such as drugs, nucleic acids, vaccines, and proteins [[72], [73], [74], [75], [76]].
Fig. 4.
Schematic diagram of different liposome membrane functionalization strategies. Reprinted with permission from Ref. [83]. Copyright 2019 PERGAMON-ELSEVIER.
The application of liposomes in the treatment of stroke has been widely studied because they have the ability to cross the BBB and stay in the blood stream for a relatively long time. As a result, they can deliver enough therapeutic drugs to brain tissue [77]. Imaizumi et al. studied the role of liposomes as precursors in the treatment of focal ischemic stroke. With short half-life and inability to pass the blood-brain barrier, superoxide dismutase (SOD), a free radical scavenger, is administered through the jugular vein in the form of an encapsulated liposome. An animal model study showed augmented levels of SOD and decreased infarct volume [50]. In addition, the circulation time of liposomes can be prolonged through modification of their surface by polyethylene glycol (PEG) [52]. The accumulation of PEGylated liposomes in ischemic brain has been measured [78], attributed to disruption of the BBB, heightened vesicle infiltration and long accumulation at the ischemic site. Evidence shows that in middle cerebral artery occlusion (MCAO) rat model, a single injection of polyethylene glycol liposomes containing antiepileptic drug F506 at a low dose can significantly reduce infarct volume and improve motor function [79]. Other studies showed that nonpolyethylene glycol liposomes with different surface charges achieved early brain deposition following intratracheal cervical injection. The accumulation of these liposomes in the brain after intra-arterial injection is greater than that of cationic or neutral cationic vesicles, which may be due to the electrostatic interaction between cationic liposomes and negatively charged cell surface, and the absorption of nanoparticles is enhanced through the absorption of intercellular removal [80].
Another method to further improve drug transport across the BBB is to bind specific ligands to the surface of liposomes and target the surface proteins expressed on BBB. This strategy can increase the uptake of liposomes by BEC through receptor-mediated cell transport, thus leading to greater concentrations of the drug reaching the ischemic areas. It is reported that liposomes containing the neuroprotectant (ZL006) may be a double target therapeutic strategy for ischemic stroke [81]. HSP72 (heat shock protein 72) is used as a target protein specifically expressed in the ischemic area around the infarct. PEGylated liposome with cytosine as carrier combined with HSP72 antibody can selectively aggregate in the ischemic area following a middle cerebral artery occlusion and increase the beneficial effect of drugs [82]. When other neuroprotective drugs such as VEGF (vascular endothelial growth factor) were loaded into liposomes and immune-targeted at the ischemic region, the same promising results were observed. The liposomes could effectively reach the brain injury area, and significantly reduce infarct volume and neurological deficits [53]. The surface of liposomes was modified with a transferrin receptor derived peptide ligand (T7) that improving the permeability of the BBB and a stroke-homing peptide (SHp) that targeting the ischemic areas. As a result, T7&SHp-P-L/ZL006 liposomes reduced the neurological deficit, infarct size, and histopathological changes in the MCAO rat model [81].
In general, liposomes have good drug loading capacity, drug protection ability, and high biocompatibility. However, its use in the treatment of stroke has the disadvantage of low targeting. Studies have demonstrated that the brain targeting ability of liposomes can be improved by conjugating PEG, changing the surface charge, or by conjugating specific targeting ligands, thus enhancing the therapeutic effect.
5.2. Nanoparticles
5.2.1. Polymeric nanoparticles
Biodegradable polymeric nanoparticles have been studied as drug delivery platforms because of their high biocompatibility and good sustained-release profiles [[84], [85], [86], [87], [88]]. There are clinical trials of polymer nanoparticles for anticancer and oral antimicrobial adjuvants [89,90].Poly(D, l-lactide-co-glycolide acid) (PLGA) was observed to be one of the most extensively used to create nanoparticles for stroke. Mdzinarishvili et al. used glutathione-coated PLGA-b-PEG NPs to delivery thyroid hormones (T3) to promote neuroprotection to ischemic brain [91]. The neuroprotective effect of T3 was markedly lower compared to T3 in the PLGA-PEG formulations [91]. Although polymeric nanoparticles are promising options for targeted drug delivery, there are limitations that include high cost and complex manufacturing [92].
Polymeric nanoparticles can carry hydrophilic reagents and they can internalize drugs into cells via endocytosis. In one study, PLGA nanoparticles were encapsulated in balloon-expandable stents by a hydrophilic dye, fluorescein isothiocyanate (FITC). When coronary smooth muscle was co-incubated with FITC, the effective transmission of Polymeric NPs encapsulated by FITC was observed [93].Cationic polymer micelles with neural stem cell labeling capabilities were designed recently. It has been shown that the micelles have high efficiency, safety and reliability in tracing stem cells in vivo using magnetic resonance imaging [55]. In other studies, polymersomes have been developed to form a polymeric NP for the application of therapeutic stem cell MRI image analysis in stroke patients. In addition to polymersome nanospheres are also a key component of NP [94] because of their large surface area and neuroprotective properties. Nanospheres loaded with Z-DEVD-FMK showed significant alleviation of nerve injury, caspase-3 activity and reduced infarct volume, which may help treat stroke [54].
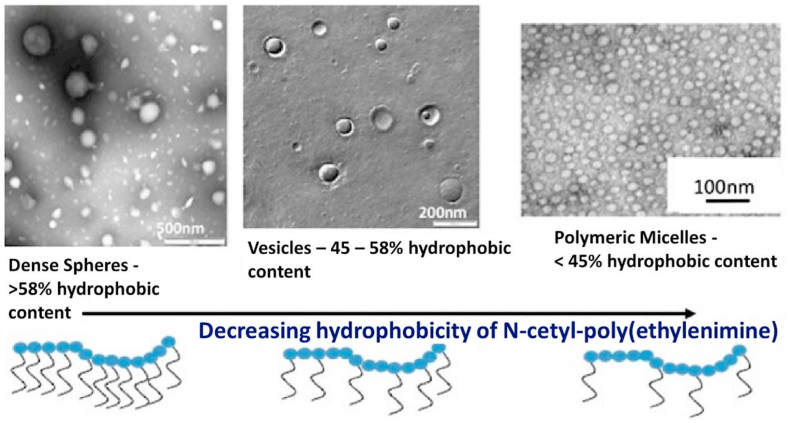
Nanoparticles possess excellent drug delivery properties, which can improve drug targeting efficiency and may be useful for controlled drug release. A number types of nanoparticles have been used as drug delivery carriers to cross the BBB since they can avoid phagocytosis by the reticuloendothelial system (Fig. 5), and increase the concentration of drugs in brain [95].
Fig. 5.
Polymeric nanoparticles, polymeric vesicles and polymeric micelles all formed from various cetyl poly(ethylenimine) amphiphiles. Reprinted with permission from Ref. [96]. Copyright 2013 Springer Science+Business Media New York.
5.2.2. Metal nanoparticles
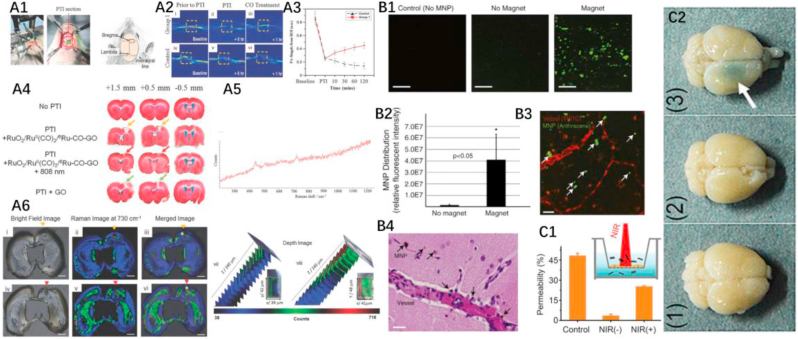
Metal nanoparticles also show great potential for the treatment and detection of brain diseases [[97], [98], [99], [100], [101]]. The shape and size of metal nanoparticles are uniform and easy to control during synthesis, which would allow them to meet quality control requirements for clinical application [28,[102], [103], [104], [105]]. With unique surface plasmon resonance characteristics [[106], [107], [108], [109], [110]], metal nanoparticles not only have enhanced photothermal capability, but they also have excellent imaging and detection performance. Since some metal nanoparticles are magnetic [[111], [112], [113], [114]], they can be used for magnetic hyperthermia, magnetic targeting, and magnetic resonance imaging (MRI) [[115], [116], [117], [118]]. The surface of metal nanoparticles are also easy to modify, which is a prerequisite for the application of drug delivery platform. Chertok et al. [57] successfully used magnetic fields to target iron oxide nanoparticles with a hydrodynamic diameter of 110 ± 22 nm to brain lesions. Iron oxide nanoparticles have superparamagnetic behavior, which can protect materials from self-aggregation when there is no external magnetic field. In vivo experiments have demonstrated that effective magnetic targeting can be achieved by placing the head of the rat between the poles of an electromagnet. For this, magnetic field densities of 0.4 T were used to demonstrate enhanced (by 500%) iron oxide nanoparticles accumulation in. Kong et al. [56] utilized a modified emulsion process to obtain iron oxide particles with significantly improved magnetization and uniform particle size (~100 nm). Experimental evidence of BBB transport is shown in Fig. 8 B1–B5. After implantation of the Nd-Fe-B magnet, the right hemisphere of the mouse brain exhibited a much stronger nanoparticle enrichment than the untreated sample (left hemisphere), indicating effective magnetic targeting. Further non-invasive experiments have shown that magnets placed outside the head of the mouse can also effectively guide the enrichment of nanoparticles into the brain (25-fold improvement compared to the control group). After exposure to the magnetic field, the nanoparticles entered the peripheral space and parenchyma from capillaries. It is worth noting that the nanoparticles can penetrate the BBB under a magnetic field without damaging BBB integrity. Atomic force microscopy images show that nanomaterials can efficiently accumulate in human endothelial cells (>800 nm), indicating that endothelial cell membrane-mediated translocation may be the mechanism involved in the process. After targeting brain, an external magnetic field can effectively control the release of the nanoparticles-based drug delivery platform.
Fig. 8.
A1-A6. The therapeutic performance of GO-based drugs on stroke. (A1) Experimental setup. (A2) Photoacoustic vessel cross-section images of vasodilatation under irradiation with an 808 nm laser in a PTI rat treated with (i–iii) Ru–CO–GO (group 1) and (iv–vi) GO (control). The enhanced photoacoustic signal of the experimental group indicates that the released CO can effectively promote vasodilation. (A3)The intensity of photoacoustic signals of group 1 and control at different time points indicated the rapid therapeutic. (A4)The infarct volume of 1 mm coronal brain sections of rats at +1.5 to −0.5 mm to the Bregma, by TTC staining. (A5) Raman spectroscopy of brain slides. (A6) Raman imaging of brain slides (+0.5 mm) (i–iii and vii) Ru–CO–GO and (iv–vi and viii) GO. Scale bar: 0.4 cm. Reprinted with permission from Ref. [244]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. *Region of depth scan. (B1–B4): BBB crossing of magnetic nanoparticles in the magnetic field. (B1) Images of mouse brain sections treated without magnetic nanoparticles (left); with magnetic field, but without magnetic nanoparticles (middle); with magnetic fields and magnetic nanoparticles (right). Scale bar, 20 μm (B2) Relative fluorescence intensity of brain sections in B1. The enrichment of magnetic nanoparticles increased 26-fold after the treatment with the magnetic field. (B3) Confocal image of extravasation of magnetic nanoparticles in the vessels. Scale bar, 50 μm (B4) Image of brain sections showing aggregation of magnetic nanoparticles around blood vessels. Scale bar, 20 μm. Reprinted with permission from ref [56]. Copyright 2012 PERGAMON-ELSEVIER. (C1–C2): In vitro and in vivo BBB penetration of BP. (C1) bEnd.3 cell monolayer was seeded in transwells to obtain an in vitro BBB model, as shown in the inset. 48% of BP was transported from the upper chamber to the lower chamber in the control group (no bEnd.3 cell). With bEnd.3 cell monolayer, only 3.5% of BP spontaneously traversed to the lower chamber, while the delivery of BP was increased by 6-fold under the illumination. (C2) The images of mouse brains with Evans blue staining show obvious BBB crossing ability of BP under the irradiation. (1) BP (2) NIR, (3) BP + NIR. Reprinted with permission from ref [64]. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The use of nanoparticles in the treatment of stroke is not limited to drug delivery system. Studies have indicated that some specific metallic nanoparticles can act as scavengers of ROS. Platinum nanoparticles displayed antioxidant effect by scavenging superoxide anions and hydrogen peroxide in an in vivo test system. Cerium oxide nanoparticles were also used as ROS scavengers to reduce oxidation and provide neuroprotection to rat spinal cord neurons [119]. A recent study indicated that cerium oxide nanoparticles increased the survival of undifferentiated PC12 cells under oxidative stress. Cerium oxide nanoparticle pre-treatment decreased ROS production, proteins expression of LPO, Bax and caspase-3 [120]. These findings suggest that Cerium oxide nanoparticles can be used as a therapeutic agent against oxidative stress and apoptosis. After years of development, some metal nanoparticles have achieved clinical application. For example, iron oxide-based MRI contrast agents have been clinically approved in Europe and the United States, and human trials of photothermal therapy based upon gold nanoparticles have also been reported [57,[121], [122], [123], [124], [125]].
5.2.3. Carbon nanotubes
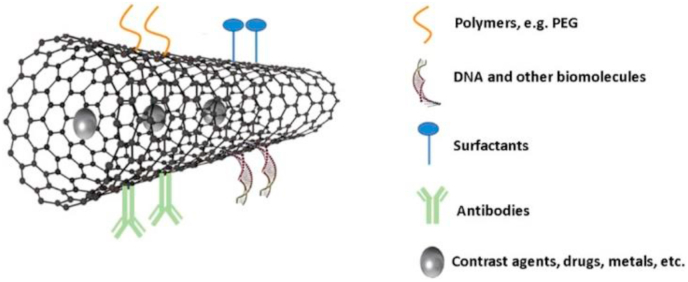
Carbon nanotubes are a class of nanomaterials, composed of graphite sheets tubes with nanometer diameters [[126], [127], [128], [129], [130], [131]]. They exhibit single- or multi-walled structures, characterized by open ends or closed with fullerene caps (Fig. 6) [[132], [133], [134], [135], [136], [137]]. With unique structures, excellent electrical, mechanical, optical and thermal properties and high specific surface area, carbon nanotubes (CNTs) have great application in various fields [[138], [139], [140], [141], [142], [143], [144], [145]]. Their main medical applications include drugs, tissue engineering, biosensor, gene therapy, hormone and enzyme delivery [[146], [147], [148], [149], [150]]. CNTs have been used in non-carrier systems due to the possibility of functionalization of their physical and biological properties using specific chemical components [151]. As the BBB cannot be penetrated through passive diffusion, the conjugation of compounds that promote the active transport to the brain is essential for emerging applications in nanomedicine. In the last two decades, they have gained a great attention as nanocarrier systems considering the unique property of carbon nanotubes [152]. With high specific surface area, they can effectively bind different molecules [[153], [154], [155], [156], [157], [158]], and their properties can be manipulated by functionalization with chemical compounds to further improve their physical and biological properties [[159], [160], [161], [162], [163]].
Fig. 6.
Schematic diagram of preparation of PEG-MWCNTs (multiwalled carbon nanotubes) Reprinted with permission from ref[171]. Copyright 2018 MDPI.
Due to the numerous advantages mentioned above, the application of carbon nanotubes in brain diseases has received some attention. As a prerequisite for clinical application, fluorescently labeled multi-walled carbon nanotubes have been found to penetrate microvascular cerebral endothelial monolayers without significant toxicity to cerebral endothelial cells [164]. Another research group also obtained similar results by 111In labeling method, Raman microscope and multiphoton luminescence microscope image [165]. Excellent BBB penetration distinguishes carbon nanotubes from common drug carriers. You et al. [166] designed dual-targeted carbon nanotubes as a drug delivery platform against orthotopic glioma. Compared with free oxaliplatin, the oxaliplatin drug-loading platform based upon carbon nanotubes shows significantly improved BBB permeability. In addition, a recent study showed that carbon nanotubes can be used in nanofiber scaffolds, which can significantly improve neuronal growth and differentiation, indicating potential application in the field of peripheral nerve restoration [167]. After modification, multi-walled carbon nanotubes (f-MWCNTs) were co-incubated with an in vitro BBB model. Because of energy-dependent transcytosis, f-MWCNTs effectively penetrated cell monolayers. Amine-modified single-walled carbon nanotubes have also been shown to provide neuroprotection to rats following MCAO, and benefit behavioral functions [59]. Although carbon nanotubes have many benefits, there are limitations to their use, including poor solubility in water, low biodegradability and dispersivity, and deleterious drug-induced oxidative stress and lung disease [[168], [169], [170]].
5.2.4. Graphene
Graphene is another recently discovered nanomaterial with interesting properties. With the quantum confinement effect caused by ultrathin thickness, graphene exhibits novel performance in electrons [[172], [173], [174], [175], [176]], thermal [42,[177], [178], [179], [180]] and optical properties [[181], [182], [183], [184], [185], [186]], and is widely used in laser [184,[187], [188], [189], [190]], sensing [[191], [192], [193], [194], [195]], optoelectronic devices [[196], [197], [198]], biomedicine [31,[199], [200], [201], [202], [203]] and other fields. The atomic thickness determines the extremely large specific surface area of graphene [188,[204], [205], [206], [207]], which makes it suitable as a drug carrier. It is also very sensitive to the action of photons [[208], [209], [210], [211]], so it has a good performance profile in the field of light-controlled drug release and photothermal therapy, which can improve the permeability of BBB. The delocalized π-electrons of graphene are advantageous for binding various aromatic ring containing drug molecules through π-π stacking. As an important member of the graphene family, graphene oxide (GO) and reduced graphene oxide(r-GO) have a rich oxygen-containing group on the surface, which ensures water dispersibility and easy modification. Considering the above advantages, researchers have used graphene as a drug carrier to cross the BBB. Yang et al. [61] synthesized graphene oxide with a single layer thickness and size range of 100–300 nm via the modified Hummers' method. It can be suspended in water for several months due to the oxygen-containing groups enriched on the surface. Transcription activator peptide as well as methoxy polyethylene glycol were used to modify the surface of GO to further improve blood circulation stability and BBB penetration. GO exhibits significant broadband absorption in the near-infrared region, which is beneficial for photoacoustic imaging. In vivo experiments confirmed that drug-loaded functionalized GO was able to efficiently aggregate in mouse brain, while the control group (pure GO) was almost incapable of penetrating the blood-brain barrier. The functionalized GO drug delivery platform enables a safe combination of treatment and rapid detection compared to simple drugs.
A study of PEG-modified rGO showed significant toxicity to primary rat astrocytes, while unmodified rGO showed negligible toxicity [62]. Astrocytes treated with PEG-rGO and rGO resulted in completely different immunofluorescence images. The former shows a complete lack of normal cellular structure, loss of contact between cells, and a marked decrease in the number of cells while the latter exhibits only moderate levels of cell body and process retraction. Similar results were obtained from in vitro experiments with rat brain endothelial cells as the study model.
In vivo experiments have shown that PEG-rGO triggers a significant and sustained down-regulation of BBB-associated proteins compared to rGO. Further experiments confirmed that PEG-rGO can induce greater levels of ROS in cells than rGO, which may be a source of PEG-rGO toxicity. It is worth noting that the sizes of rGO and PEG-rGO used in this work are 342 ± 23.5 nm and 910 ± 32.7 nm, respectively, which are much larger than those commonly used in biological applications, and may be the reason of significant differences.
Few studies have suggested that GO can be applied to the treatment of stroke.
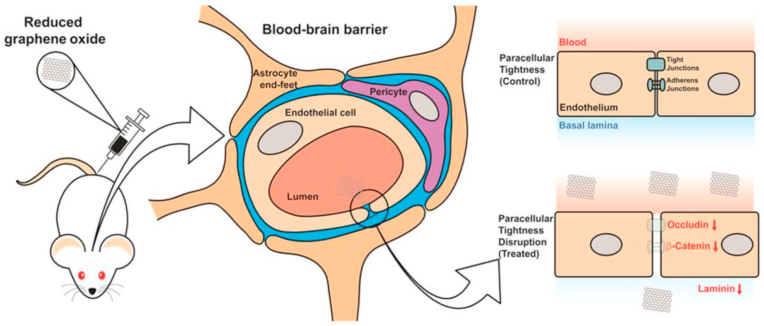
Because of the transient decrease of the BBB's paracellular tightness, rGO can reach the hippocampus area through blood circulation following ischemia [212]. rGO-induced transient opening of BBB did not show significant deleterious effects. Due to the ability of rGO to temporarily increase permeability of BBB, drug delivery to the lesion site can be measured (Fig. 7) [213]. Improved BBB permeability after using rGO indicates the great potential of graphene group materials in brain diseases.
Fig. 7.
Schematic diagram of rGO opening BBB. Reprinted with permission from ref. [63] Copyright 2015 BMC.
A recent study developed a facile CO-release platform based on the size-dependent adsorption properties of ruthenium carbonyl clusters (Ru–carbon monoxide (CO)) onto graphene oxide (GO) for treatment of stroke, as shown in Fig. 8 A1-A6. Photothermal therapy induced oxidation of RuII(CO)2 to RuO2 on the GO surface, leading to the release of CO. A cortical photothrombotic ischemia (PTI) rat model was used to demonstrate vasodilation and stroke protective effect of the RuO2/RuII(CO)2/6Ru–CO–GO composite. The results suggested that infarct volume was decreased in the group treated with RuO2/RuII(CO)2/6Ru–CO–GO composite [33]. Advances are promising, but there are still many important problems to be resolved before clinical application. First, the graphene product family includes GO, rGO and graphene nanosheets, which have very different characteristics. A standardization protocol to distinguish and characterize different molecules has not been developed [214]. In addition, poor degradation of the graphene family in vivo also needs to be improved. Although some work has shown that most of the graphene can be metabolized out of the body through urine, a small but significant amount of graphene will still remain in organs for more than 270 days [215].
5.2.5. Black phosphorus
With unique structure and properties, black phosphorus (BP), namely phosphorene, is becoming an important molecule [[216], [217], [218], [219], [220]]. BP has a broadband-adjustable direct band gap, which ensures that it has a broad spectrum of intense light absorption [5,218,[221], [222], [223], [224],[224], [224], [225], [226], [227], [228]]. BP exhibits a high extinction coefficient in the near-infrared region, optimal photothermal conversion efficiency and attractive fluorescence quantum yield, thus has application in the field of photothermal therapy, photoacoustic imaging and fluorescence imaging, etc. [34,[229], [230], [231]].
Sun et al. [232] used a simple liquid phase exfoliation method to prepare BP quantum dots (BPQDs) with an average size of 2.6 ± 1.8 nm, which can be quickly cleared by the kidneys to avoid possible long-term toxicity. Cell experiments have shown that ultra-small BPQDs have effective photothermal killing effects and great biocompatibility. PEG modification on the surface of the BPQDs enhances the stability and dispersion under physiological conditions. BPQDs coated with PEG were prepared via a one-pot method using red phosphorus as a raw material. The photoacoustic signal intensity of the BPQDs increases linearly with increasing concentration (in the range of 0–250 mg/mL). In vivo experiments have also confirmed the excellent photoacoustic imaging capabilities of BPQDs. BP also shows intense blue light emission, indicating the potential for bioluminescent imaging. Lee et al. [233] obtained BPQDs with great biocompatibility and water dispersibility via liquid phase exfoliation in chloroform. BPQDs exhibits the maximum fluorescence at 437 nm under the excitation of 377 nm, which may be derived from the band gap transitions in the cases of chloroform and DMAc or the radiative recombination of the surface-confined electrons and holes in the case of pyridine. Further experiments have shown that BPQDs have a high quantum yield (~5%) and significant fluorescence characteristics as well as negligible toxicity in cells. The ultrathin thickness and unique puckered lattice configuration [207,223,234,235], allow BP nanosheets to be used as a drug platform. Tao et al. [35] found that BP has drug-loading ratios tested to be 108%, higher than traditional materials. After loading of drugs, targeting molecules and fluorescent molecules, BP nanosheets achieved targeted detection-therapeutic combined performance and light-controlled drug release capabilities. In addition to its excellent therapeutic and detection capabilities, the most important feature of BP is its complete biodegradation under physiological conditions [236]. BP can degrade into phosphate [[237], [238], [239], [240], [241]], which is a ubiquitous metabolite in body. Illumination and modification can effectively control the degradation rate from 1 h to one month, indicating the potential of BP as the next generation of an intelligent drug-loading platform.
2D BP nanosheets can be used as a robust nanocaptor for Cu2+ and protect neuronal cells against Cu2+ induced neurotoxicity, as shown in Fig. 8C1 and C2. BP nanosheets displayed good BBB permeability under near-infrared irradiation because of their photothermal effect. Moreover, BP nanosheets decreased intracellular ROS content and increased mitochondrial membrane potential (MMP). BP nanosheets have excellent biocompatibility and stability in vitro and in vivo. Therefore, BP could be regarded as a promising neuroprotective drug delivery system [38]. The biodegradability of BP distinguishes it from traditional inorganic nanomaterials. However, balancing the biodegradability and blood circulation time is still a key factor to achieve effective treatment. In addition, the 2D BP discovered in 2014 is so young that some of the consequences of its exposure to biological environments are still unclear [242]. For example, studies have shown that phosphate anions produced by the rapid degradation of BP can kill cancer cells, which also arouses concerns about the side effects of BP degradation products to normal cells [243].
5.3. Hydrogels
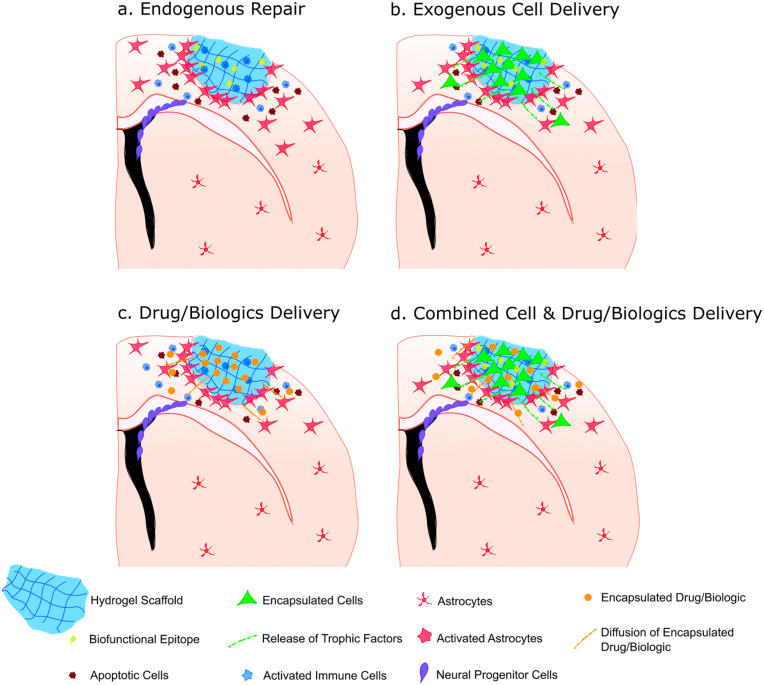
Recent efforts have focused on engineered injectable hydrogels that can be directly transplanted in the stroke cavity. In situ injectable hydrogel materials provide a unique platform to bioengineer repair environments at the stroke site. The hydrogels can be produced to match the mechanical properties of the normal brain by modulating the crosslinking density. Hydrogels promote repair by offering structural support to the injured tissue, leading to minimized secondary cell death (Fig. 9).
Fig. 9.
The potential application of injectable hydrogels in stroke treatment. (a) injecting bio functional hydrogels into infarcted areas can induce endogenous repair mechanisms, such as angiogenesis and neurogenesis. (b) injectable hydrogel can be used as a cell delivery carrier to provide a three-dimensional environment in the infarcted area. Then, coated exogenous cells can release therapeutic nutrients to the surrounding environment to help regeneration. (c) injectable hydrogel can be used as a reservoir for drug/biological agents in the infarct area for controlled and sustained administration. The goal of promoting regeneration includes increasing neural precursor cell migration from the SVZ, reducing inflammation and attenuating the immune response. (d) It may also be a dual function method to combine the delivery of exogenous cells and drug/biological agents. Reprinted with permission from Ref. [254]. Copyright 2019 ROYAL SOC CHEMISTRY.
Local injection of hydrogels can effectively bypass the blood-brain barrier, thereby promoting the infiltration of parenchyma cells around the scaffold and promoting local regeneration. Injectable hydrogels can also be used as cell transplantation carriers to transport neural progenitor cells. Since invasive delivery is required to enter the brain and skull, direct delivery of cells to the brain after stroke along with hydrogel biomaterials must be controlled so that adjacent tissues are not damaged [245]. Ideally, the injection should be guided by non-invasive imaging [246]. Modo et al. have successfully used magnetic resonance imaging to guide hydrogel injection and drainage of the brain at the same time to prevent the accumulation of intracranial pressure [247]. Currently, hydrogels are used for manufacturing contact lenses, hygiene products, tissue engineering scaffolds, drug delivery systems, wound dressings [248], and as antimicrobial agents [249].
Hydrogels can be used as drug carriers loaded with anti-inflammatory drugs to improve bioavailability. Gelatin microspheres (GMS) loaded with osteopondin were injected into a mouse following a stroke. After encapsulation with GMS, the duration of osteopondin release was significantly extended [65]. Delivery from the microspheres showed increased neuroprotection, and decreased inflammation after stroke. Hydrogel can be applied to achieve sustained and sequential delivery of epidermal growth factor (EGF) [250], erythopoietin [251,252], and cyclosporine A [253]. These results indicated improved tissue repair and reduced stroke-induced damage when compared to the delivery of the same drugs via traditional bolus delivery in the brain.
5.4. Dendrimers
Dendrimers are a class of synthetic macromolecule that present a tree-like topology and specific encapsulation properties. Dendrimers have interesting structural features, including globular and layers of branched nanostructure, and several terminal functional groups on the outside layer. They have the ability to form complexes and encapsulate a variety of molecular [255]. These polymer-based nanostructures are widely used as nanocarriers to transport various therapeutic and imaging agents because polyamidoamine, polypropylenimine, and polyaryl ether are used in dendrimer formulations and they can encapsulate both hydrophilic and hydrophobic molecules [256,257]. Dendritic macromolecules are widely used in the treatment of central nervous system diseases as their ability to transverse the blood-brain barrier [258]. In addition, they have the ability to cross various membrane or biological barriers through endocytosis-mediated cellular internalization.
In a recent study, the biocompatibility of cationic poly(amido amine) (PAMAM) dendrimers was significantly increased by PEGylation as a degree of functionalization [259]. The PEGylated PAMAM dendrimers did not influence the integrity of the BBB in an in vitro model, nor did it show cytotoxicity in an in vitro hypoxia model induced by oxygen-glucose deprivation. Interestingly, the PEGylated dendrimers reduced blood clotting, which provided an additional beneficial function in the treatment of stroke. Further, optimized PAMAM formulation could be detected in the brain 24 h after administration in a permanent focal brain ischemia mouse model. The PEGylated PAMAM dendrimers prolong blood circulation half-life and have the potential for application in drug delivery system. In another study, dexamethasone-conjugated polyamidoamine generation 2 (PAMAM G2-Dexa) efficiently delivered the heme oxygenase-1 (HO-1) gene into the ischemic brain [66]. e-PAM-R, a biodegradable arginine ester of PAMAM dendrimer, provided an efficient means of transfecting High mobility group box-1 (HMGB1) siRNA into primary neuronal cells and in the post-ischemic brain [260]. Starpharma has developed a dendrimer based product ‘vivagel’, which is designed to be used as an antiviral agent in the vagina and has successfully completed phase I clinical trials [261].
6. Conclusion and perspectives
Traditional drug therapy for ischemic stroke has limitations, and drugs need to penetrate the BBB to reach the ischemic region to achieve therapeutic effect. BBB is a barrier between the nervous system and other parts of the body, which can protect the brain from harmful stimulation. However, nanomedicines, such as liposomes, nanoparticles and hydrogels display great versatility regarding morphology, and surface physicochemical properties. They can effectively cross the BBB and deliver drugs to the lesion, reaching areas that are difficult to reach by conventional drugs. Nanomedicines are important molecules to be applied to treating stroke, based upon their ability to cross the BBB, transiently decrease the BBB paracellular tightness, and act as antioxidants. Nanomedicines can be actively transported by transcytosis, thereby improving BBB transport and improving drug permeability.
Considering that nanomedicines are not naturally existing in the human body, studies are mandatory to evaluate the bio-distribution and toxicological effects of these nanomedicines after systemic administration. Currently, there is little information regarding the pharmacological effects and interaction of nanomedicines with the human brain. According to the relevant literature, only 3 positive phase III randomized clinical trials (RCTs) in acute ischemic stroke were all reperfusion therapy. They are (National Institutes of Neurological Disorders and Stroke) (NINDS) validates the clinical effect of intravenous (IV) tPA for acute ischemic stroke within 3 h; Prolyse in Acute Cerebral Thromboembolism (PROACT) II demonstrated efficacy of IV tPA in selected patients out to 4.5 h; European Cooperative Acute Stroke Study (ECASS III) demonstrated the clinical and recanalization effect of intra-arterial (IA) prourokinase in the treatment of M1 or M2 middle cerebral artery occlusion within 6 h after stroke. Meanwhile, as a result of recruitment of RCTs for acute stroke is often difficult, it is hard to have sufficient samples to support the conduct of clinical trials. At present, because of such great challenges, such as the extremely short therapeutic window and the issue of stroke heterogeneity, there are no mature nanomaterials for acute cerebral ischemic stroke reported [275]. Animals and clinical studies investigating the toxic effects of nanomedicines on brain are limited. According to recent studies, carbon nanotubes have related toxicity in animal models [276], while liposomes, and hydrogels have limited negative effects [277]. Neurotoxicity of nanomedicines can originate not only from the core structure, but from their surface functionalization for active delivery. Although surface functionalization, such as surfactants and peptides can promote drug delivery via BBB permeability, it may also increase the risk of promoting toxic substance transport into the brain.
Nanomedicines are at an early stage of development and more in-depth research studies are needed to successfully translate these drugs to treat cerebral ischemia. Further investigations should be pursued to reveal the fate of nanomedicines after brain delivery and the potential side effects to offer more insight on translation from preclinical to clinical applications.
Declaration of competing interest
NO Conflict of Interest.
Acknowledgements
The research was partially supported by the National Natural Science Foundation of China (NSFC) (81960334, U1803128, 81960648); Science and Technology Innovation Commission of Shenzhen (KQTD2015032416270385); Science and Technology Development Fund (STDF) (007/2017/A1); Macao SAR; China and the Postgraduate Innovation Development Fund Project of Shenzhen University (PIDFPZR2018004).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Wen Chen, Email: chen-wen2000@126.com.
Bing Wang, Email: wangbing@szu.edu.cn.
Zhongjian Xie, Email: zjxie2011@163.com.
References
- 1.S H., S S., L L., Stroke Y.C.J. Vol. 48. 2017. pp. 271–275. (Age-period-cohort analysis of stroke mortality in China: data from the global burden of disease study 2013). %A Wang Z. 2. [DOI] [PubMed] [Google Scholar]
- 2.Shichita T. [Molecular and cellular mechanisms underlying the sterile inflammation after ischemic stroke] Nihon yakurigaku zasshi. Folia pharmacologica Japonica. 2018;151(1):9–14. doi: 10.1254/fpj.151.9. [DOI] [PubMed] [Google Scholar]
- 3.Lai T.W., Zhang S., Wang Y.T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo R., Fernandez-Gajardo R., Gutierrez R., Matamala J.M., Carrasco R., Miranda-Merchak A., Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol. Disord. - Drug Targets. 2013;12(5):698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 5.Tang S., He Z., Liang G., Chen S., Ge Y., Sang D.K., Lu J., Lu S., Wen Q., Zhang H. Pulse duration dependent nonlinear optical response in black phosphorus dispersions. Optic Commun. 2018;406:244–248. [Google Scholar]
- 6.Röther J., Ford G.A., Thijs V.N.S. Vol. 35. 2013. p. 313. (Thrombolytics in acute ischaemic stroke: historical perspective and future opportunities). 4. [DOI] [PubMed] [Google Scholar]
- 7.Kelly P., Kavanagh E., Murphy S.J. S.i.N. Stroke: new developments and their application in clinical practice. 2016;36(4):317–323. doi: 10.1055/s-0036-1586261. [DOI] [PubMed] [Google Scholar]
- 8.Han L., Cai Q., Tian D., Kong D.K., Gou X., Chen Z., Strittmatter S.M., Wang Z., Sheth K.N., Zhou J. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016;12(7):1833–1842. doi: 10.1016/j.nano.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Á C., U D., X U., Neurology P.A.J.T.L. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 10.Gascon S., Sobrado M., Roda J.M., Rodriguez-Pena A., Diaz-Guerra M. Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Mol. Psychiatr. 2008;13(1):99–114. doi: 10.1038/sj.mp.4002017. [DOI] [PubMed] [Google Scholar]
- 11.Besancon E., Guo S., Lok J., Tymianski M., Lo E.H. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 2008;29(5):268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Khoshnam S.E., Winlow W., Farzaneh M., Farbood Y., Moghaddam H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017;38(7):1167–1186. doi: 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang A., Zhang X., Li S., Zhao X., Liu L., Johnston S.C., Meng X., Lin J., Zuo Y., Li H., Wang Y., Wang Y. Oxidative lipoprotein markers predict poor functional outcome in patients with minor stroke or transient ischemic attack. Eur. J. Neurol. 2019;26(8):1082–1090. doi: 10.1111/ene.13943. [DOI] [PubMed] [Google Scholar]
- 14.Liu P.K. Ischemia-reperfusion-related repair deficit after oxidative stress: implications of faulty transcripts in neuronal sensitivity after brain injury. J. Biomed. Sci. 2003;10(1):4–13. doi: 10.1159/000068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P.K., Robertson C.S., Valadka A. The association between neuronal nitric oxide synthase and neuronal sensitivity in the brain after brain injury. Ann. N. Y. Acad. Sci. 2002;962:226–241. doi: 10.1111/j.1749-6632.2002.tb04071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao H., Wang Z., Liu Y., Wang P., Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. : MN. 2011;44(2):130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- 17.Bayir H., Kagan V.E., Clark R.S., Janesko-Feldman K., Rafikov R., Huang Z., Zhang X., Vagni V., Billiar T.R., Kochanek P.M. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J. Neurochem. 2007;101(1):168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima H., Kubo T., Ihara H., Hikida T., Danjo T., Nakatsuji M., Shahani N., Itakura M., Ono Y., Azuma Y.T., Inui T., Kamiya A., Sawa A., Takeuchi T. Nuclear-translocated glyceraldehyde-3-phosphate dehydrogenase promotes poly(ADP-ribose) polymerase-1 activation during oxidative/nitrosative stress in stroke. J. Biol. Chem. 2015;290(23):14493–14503. doi: 10.1074/jbc.M114.635607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S.C., Ma L.S., Chu Z.H., Xu H., Wu W.Q., Liu F. Regulation of microglial activation in stroke. Acta Pharmacol. Sin. 2017;38(4):445–458. doi: 10.1038/aps.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guruswamy R., ElAli A. Complex roles of microglial cells in ischemic stroke pathobiology: new insights and future directions. Int. J. Mol. Sci. 2017;18(3) doi: 10.3390/ijms18030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun M., Deng B., Zhao X., Gao C., Yang L., Zhao H., Yu D., Zhang F., Xu L., Chen L., Sun X. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci. Rep. 2015;5:11445. doi: 10.1038/srep11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao X., Liu S., Ding W., Yue P., Jiang Q., Zhao M., Hu F., Zhang H. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J. Neuroimmunol. 2017;310:38–45. doi: 10.1016/j.jneuroim.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Majidi S., Leon Guerrero C.R., Burger K.M., Sigounas D., Olan W.J., Qureshi A.I. Fixed dose IV rt-PA and clinical outcome in ischemic stroke patients with body weight >100 kg: pooled data from 3 randomized clinical trials. J. Stroke Cerebrovasc. Dis. 2018;27(10):2843–2848. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Ueno T., Nishijima H., Hikichi H., Haga R., Arai A., Suzuki C., Nunomura J.I., Tomiyama M. Association of survival and hyperthermia after rt-PA for ischemic stroke. Acta Neurol. Scand. 2018;138(6):574–578. doi: 10.1111/ane.13011. [DOI] [PubMed] [Google Scholar]
- 25.Saver J.L., Goyal M., van der Lugt A., Menon B.K., Majoie C.B., Dippel D.W., Campbell B.C., Nogueira R.G., Demchuk A.M., Tomasello A., Cardona P., Devlin T.G., Frei D.F., du Mesnil de Rochemont R., Berkhemer O.A., Jovin T.G., Siddiqui A.H., van Zwam W.H., Davis S.M., Castano C., Sapkota B.L., Fransen P.S., Molina C., van Oostenbrugge R.J., Chamorro A., Lingsma H., Silver F.L., Donnan G.A., Shuaib A., Brown S., Stouch B., Mitchell P.J., Davalos A., Roos Y.B., Hill M.D. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. J. Am. Med. Assoc. 2016;316(12):1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto S., Murozono M., Kanazawa M., Nara T., Ozawa T., Watanabe Y. Edaravone and cyclosporine A as neuroprotective agents for acute ischemic stroke. Acute Med Surg. 2018;5(3):213–221. doi: 10.1002/ams2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Y., Tang H., Li H., Zhao R., Huang Q., Liu J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur. J. Med. Chem. 2019;162:132–146. doi: 10.1016/j.ejmech.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Huang X.H., El-Sayed I.H., Qian W., El-Sayed M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 29.Jain P.K., Huang X., El-Sayed I.H., El-Sayed M.A. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008;41(12):1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 30.Link S., El-Sayed M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000;19(3):409–453. [Google Scholar]
- 31.Yang K., Zhang S., Zhang G., Sun X., Lee S.-T., Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 32.Loo C., Lowery A., Halas N.J., West J., Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5(4):709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 33.MJ T., HC P., HR T., JW C., QF L., TI W., X Z., ZY H., LD L., materials K.K. J.A.h. Flexible modulation of CO-release using various nuclearity of metal carbonyl clusters on graphene oxide for stroke remediation. 2018;7(5):undefined. doi: 10.1002/adhm.201701113. [DOI] [PubMed] [Google Scholar]
- 34.Xie Z., Wang D., Fan T., Xing C., Li Z., Tao W., Liu L., Bao S., Fan D., Zhang H. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B. 2018;6(29):4747–4755. doi: 10.1039/c8tb00729b. [DOI] [PubMed] [Google Scholar]
- 35.Tao W., Zhu X., Yu X., Zeng X., Xiao Q., Zhang X., Ji X., Wang X., Shi J., Zhang H., Mei L. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv. Mater. 2017;29(1) doi: 10.1002/adma.201603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Duo Y., Bao S., He L., Ling K., Luo J., Zhang Y., Huang H., Zhang H., Yu X. EpCAM aptamer-functionalized polydopamine-coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int. J. Nanomed. 2017;12:6239–6257. doi: 10.2147/IJN.S143293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X., Ye X., Wang C., Xing C., Miao Q., Xie Z., Chen X., Zhang X., Zhang H., Mei L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Contr. Release. 2019;296:150–161. doi: 10.1016/j.jconrel.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 38.J O., X Y., Y X., C N., W Z., L W., J S., L D., YN L., materials G.S.J.A. Vol. 30. 2018. p. undefined. (Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy). %A Chen W. 3. [DOI] [PubMed] [Google Scholar]
- 39.Kim J., Kim H., Kim W.J. Single-layered MoS2-PEI-PEG nanocomposite-mediated gene delivery controlled by photo and redox stimuli. Small. 2016;12(9):1184–1192. doi: 10.1002/smll.201501655. [DOI] [PubMed] [Google Scholar]
- 40.Liu G., Zou J., Tang Q., Yang X., Zhang Y., Zhang Q., Huang W., Chen P., Shao J., Dong X. Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl. Mater. Interfaces. 2017;9(46):40077–40086. doi: 10.1021/acsami.7b13421. [DOI] [PubMed] [Google Scholar]
- 41.Li Z., Zhang H., Han J., Chen Y., Lin H., Yang T. Surface nanopore engineering of 2D MXenes for targeted and synergistic multitherapies of hepatocellular carcinoma. Adv. Mater. 2018;30(25) doi: 10.1002/adma.201706981. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W., Guo Z., Huang D., Liu Z., Guo X., Zhong H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32(33):8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 43.Qiu M., Wang D., Liang W., Liu L., Zhang Y., Chen X., Sang D.K., Xing C., Li Z., Dong B., Xing F., Fan D., Bao S., Zhang H., Cao Y. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 2018;115(3):501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teleanu D.M., Chircov C., Grumezescu A.M., Volceanov A., Teleanu R.I. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10(4) doi: 10.3390/pharmaceutics10040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takemori K., Murakami T., Kometani T., Ito H. Possible involvement of oxidative stress as a causative factor in blood-brain barrier dysfunction in stroke-prone spontaneously hypertensive rats. Microvasc. Res. 2013;90:169–172. doi: 10.1016/j.mvr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Shindo A., Maki T., Mandeville E.T., Liang A.C., Egawa N., Itoh K., Itoh N., Borlongan M., Holder J.C., Chuang T.T., McNeish J.D., Tomimoto H., Lok J., Lo E.H., Arai K. Astrocyte-derived pentraxin 3 supports blood-brain barrier integrity under acute phase of stroke. Stroke. 2016;47(4):1094–1100. doi: 10.1161/STROKEAHA.115.012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meloni B.P., Milani D., Edwards A.B., Anderton R.S., O'Hare Doig R.L., Fitzgerald M., Palmer T.N., Knuckey N.W. Neuroprotective peptides fused to arginine-rich cell penetrating peptides: neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol. Therapeut. 2015;153:36–54. doi: 10.1016/j.pharmthera.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Meloni B.P., Craig A.J., Milech N., Hopkins R.M., Watt P.M., Knuckey N.W. The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cell. Mol. Neurobiol. 2014;34(2):173–181. doi: 10.1007/s10571-013-9999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhaskar S., Tian F., Stoeger T., Kreyling W., de la Fuente J.M., Grazu V., Borm P., Estrada G., Ntziachristos V., Razansky D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010;7:3. doi: 10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imaizumi S., Woolworth V., Fishman R.A., Chan P.H. J Stroke; circulation, a. j. o. c., Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. 1990;21(9):1312–1317. doi: 10.1161/01.str.21.9.1312. [DOI] [PubMed] [Google Scholar]
- 51.Campos-Martorell M., Cano-Sarabia M., Simats A., Hernández-Guillamon M., Rosell A., Maspoch D., Montaner J.J. Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats. Int. J. Nanomed. 2016;11:3035–3048. doi: 10.2147/IJN.S107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rip J., Chen L., Hartman R., van den Heuvel A., Reijerkerk A., van Kregten J., van der Boom B., Appeldoorn C., de Boer M., Maussang D., de Lange E.C., Gaillard P.J. Glutathione PEGylated liposomes: pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target. 2014;22(5):460–467. doi: 10.3109/1061186X.2014.888070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying-Mei L., Ji-Yun H., Huan W., Xue-Fang L., Mei-Hua L., Ling-Juan H., Rong-Rong T., Ahmed M.M., Chun-Lei S., Xiao-Liang W.J.B. Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. 2014;35(1):530–537. doi: 10.1016/j.biomaterials.2013.09.093. [DOI] [PubMed] [Google Scholar]
- 54.Costas P., Regent L., Marios M., Charalambos A.J.M. Vol. 8. 2012. pp. S59–S68. (Nanomedicine for the prevention, treatment and imaging of atherosclerosis). 1. [DOI] [PubMed] [Google Scholar]
- 55.Lu L., Wang Y., Cao M., Chen M., Lin B., Duan X., Zhang F., Mao J., Shuai X., Shen J. A novel polymeric micelle used for in vivo MR imaging tracking of neural stem cells in acute ischemic stroke. RSC Adv. 2017;7(25):15041–15052. [Google Scholar]
- 56.Kong S.D., Lee J., Ramachandran S., Eliceiri B.P., Shubayev V.I., Lal R., Jin S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Contr. Release. 2012;164(1):49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chertok B., Moffat B.A., David A.E., Yu F., Bergemann C., Ross B.D., Yang V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29(4):487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghaznavi H., Najafi R., Mehrzadi S., Hosseini A., Tekyemaroof N., Shakeri-Zadeh A., Rezayat M., Sharifi A.M.J.N.R. Neuro-protective effects of cerium and yttrium oxide nanoparticles on high glucose-induced oxidative stress and apoptosis in undifferentiated PC12 cells. 2015;37(7):624–632. doi: 10.1179/1743132815Y.0000000037. [DOI] [PubMed] [Google Scholar]
- 59.Lee H.J., Park J., Yoon O.J., Kim H.W., Lee D.Y., Kim D.H., Lee W.B., Lee N.E., Bonventre J.V., Kim S.S. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat. Nanotechnol. 2011;6(2):121–125. doi: 10.1038/nnano.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan M.J., Pan H.C., Tan H.R., Chai J.W., Lim Q.F., Wong T.I., Zhou X., Hong Z.Y., Liao L.D., Kong K.V. Flexible modulation of CO-release using various nuclearity of metal carbonyl clusters on graphene oxide for stroke remediation. Adv. Healthcare Mater. 2018;7(5) doi: 10.1002/adhm.201701113. [DOI] [PubMed] [Google Scholar]
- 61.Yang H.W., Huang C.Y., Lin C.W., Liu H.L., Huang C.W., Liao S.S., Chen P.Y., Lu Y.J., Wei K.C., Ma C.C. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials. 2014;35(24):6534–6542. doi: 10.1016/j.biomaterials.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 62.Mendonca M.C., Soares E.S., de Jesus M.B., Ceragioli H.J., Batista A.G., Nyul-Toth A., Molnar J., Wilhelm I., Marostica M.R., Jr., Krizbai I., da Cruz-Hofling M.A. PEGylation of reduced graphene oxide induces toxicity in cells of the blood-brain barrier: an in vitro and in vivo study. Mol. Pharm. 2016;13(11):3913–3924. doi: 10.1021/acs.molpharmaceut.6b00696. [DOI] [PubMed] [Google Scholar]
- 63.Mendonca M.C., Soares E.S., de Jesus M.B., Ceragioli H.J., Ferreira M.S., Catharino R.R., da Cruz-Hofling M.A. Reduced graphene oxide induces transient blood-brain barrier opening: an in vivo study. J. Nanobiotechnol. 2015;13:78. doi: 10.1186/s12951-015-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W., Ouyang J., Yi X., Xu Y., Niu C., Zhang W., Wang L., Sheng J., Deng L., Liu Y.N., Guo S. Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv. Mater. 2018;30(3) doi: 10.1002/adma.201703458. [DOI] [PubMed] [Google Scholar]
- 65.Y J., IY K., ID K., HK L., JY P., PL H., KK K., H C., biomaterialia L.J.J.A. Biodegradable gelatin microspheres enhance the neuroprotective potency of osteopontin via quick and sustained release in the post-ischemic brain. 2014;10(7):3126–3135. doi: 10.1016/j.actbio.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 66.P J., M C., J O., bioscience L.M.J.M. Dexamethasone-conjugated polyamidoamine dendrimer for delivery of the heme oxygenase-1 gene into the ischemic brain. 2015;15(7):1021–1028. doi: 10.1002/mabi.201500058. [DOI] [PubMed] [Google Scholar]
- 67.Chang H.-I., Yeh M.-K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koltover I., Salditt T., Radler J.O., Safinya C.R. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281(5373):78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 69.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., Samiei M., Kouhi M., Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8 doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanrooijen N., Sanders A. Liposome-mediated depletion of macrophages - mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 71.Lian T., Ho R.J.Y. Trends and developments in liposome drug delivery systems. J. Pharmaceut. Sci. 2001;90(6):667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 72.Kraft J.C., Freeling J.P., Wang Z., Ho R.J.Y. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharmaceut. Sci. 2014;103(1):29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi M., Uechi S., Takara K., Asikin Y., Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009;57(19):9141–9146. doi: 10.1021/jf9013923. [DOI] [PubMed] [Google Scholar]
- 74.Chen C., Han D., Cai C., Tang X. An overview of liposome lyophilization and its future potential. J. Contr. Release. 2010;142(3):299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 75.Hong K.L., Zheng W.W., Baker A., Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400(2):233–237. doi: 10.1016/s0014-5793(96)01397-x. [DOI] [PubMed] [Google Scholar]
- 76.Lasic D.D., Needham D. The ''Stealth'' liposome: a prototypical biomaterial. Chem. Rev. 1995;95(8):2601–2628. [Google Scholar]
- 77.Kawaguchi A.T., Haida M., Ohba H., Yamano M., Fukumoto D., Tsukada H. Liposome-encapsulated hemoglobin ameliorates ischemic stroke in nonhuman primates: longitudinal observation. Artif. Organs. 2013;37(10):904–912. doi: 10.1111/aor.12091. [DOI] [PubMed] [Google Scholar]
- 78.Fukuta T., Ishii T., Asai T., Nakamura G., Takeuchi Y., Sato A., Agato Y., Shimizu K., Akai S., Fukumoto D., Harada N., Tsukada H., Kawaguchi A.T., Oku N. Real-time trafficking of PEGylated liposomes in the rodent focal brain ischemia analyzed by positron emission tomography. Artif. Organs. 2014;38(8):662–666. doi: 10.1111/aor.12350. [DOI] [PubMed] [Google Scholar]
- 79.Ishii T., Asai T., Dai O., Agato Y., Yasuda N., Fukuta T., Shimizu K., Minamino T., Oku N.J.F.J. Treatment of cerebral ischemia-reperfusion injury with PEGylated liposomes encapsulating FK506. 2013;27(4):1362–1370. doi: 10.1096/fj.12-221325. [DOI] [PubMed] [Google Scholar]
- 80.Shailendra J., Rajinder S.M., Mei W., Chaudhuri D.B., Ellis J.A., Bruce J.N., Bigio I.J., Straubinger R.M. Cationic surface charge enhances early regional deposition of liposomes after intracarotid injection. J. Neuro-Oncol. 2014;120(3):489–497. doi: 10.1007/s11060-014-1584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Y., Jiang Y., Lv W., Wang Z., Lv L., Wang B., Liu X., Liu Y., Hu Q., Sun W., Xu Q., Xin H., Gu Z. Dual targeted nanocarrier for brain ischemic stroke treatment. J. Contr. Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 82.Jesús A., David B., Francisco C., Tomás S., Bárbara A., Wajih A.S., Miguel B., José C., Pedro R.C.J.T. In vivo theranostics at the peri-infarct region in cerebral ischemia. 2014;4(1):90–105. doi: 10.7150/thno.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruch G.E., Fernandes L.F., Bassi B.L.T., Alves M.T.R., Pereira I.O., Frézard F., Massensini A.R. Liposomes for drug delivery in stroke. Brain Res. Bull. 2019;152:246–256. doi: 10.1016/j.brainresbull.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Fournier E., Passirani C., Montero-Menei C.N., Benoit J.P. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24(19):3311–3331. doi: 10.1016/s0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 85.Miura Y., Takenaka T., Toh K., Wu S., Nishihara H., Kano M.R., Ino Y., Nomoto T., Matsumoto Y., Koyama H., Cabral H., Nishiyama N., Kataoka K. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7(10):8583–8592. doi: 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- 86.Swamy H., Smith T.K., MacDonald E.J., Boermans H.J., Squires E.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2002;80(12):3257–3267. doi: 10.2527/2002.80123257x. [DOI] [PubMed] [Google Scholar]
- 87.Lim K.J., Bisht S., Bar E.E., Maitra A., Eberhart C.G. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Canc. Biol. Ther. 2011;11(5):464–473. doi: 10.4161/cbt.11.5.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z., Wang Y., Wang Z., Zhao J., Gutkind J.S., Srivatsan A., Zhang G., Liao H.-S., Fu X., Jin A., Tong X., Niu G., Chen X. Polymeric nanovehicle regulated spatiotemporal real-time imaging of the differentiation dynamics of transplanted neural stem cells after traumatic brain injury. ACS Nano. 2015;9(7):6683–6695. doi: 10.1021/acsnano.5b00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase 1a/1b clinical data with CALAA-01, a targeted polymer-based nanoparticle containing siRNA. 2014;111(31):11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beyth N., Yudovin-Farber I., Perez-Davidi M., Domb A.J., Weiss E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. U.S.A. 2010;107(51):22038–22043. doi: 10.1073/pnas.1010341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.A M., V S., PK T., WJ G., delivery S.P.J.D., research t. Engineering triiodothyronine (T3) nanoparticle for use in ischemic brain stroke. 2013;3(4):309–317. doi: 10.1007/s13346-012-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.TH M., MM I., A N., C R., G D., A Z., S M. Nanotheragnostic applications for ischemic and hemorrhagic strokes: improved delivery for a better prognosis. Neurology. 2015;15(1):505. doi: 10.1007/s11910-014-0505-1. K. F. J. C.; reports, n. [DOI] [PubMed] [Google Scholar]
- 93.Nakano K., Egashira K., Masuda S., Funakoshi K., Zhao G., Kimura S., Matoba T., Sueishi K., Endo Y., Kawashima Y.J.J.C.I. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology : efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. 2009;2(4):277–283. doi: 10.1016/j.jcin.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 94.Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Contr. Release. 2001;70(1):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 95.Zhang T.-T., Li W., Meng G., Wang P., Liao W. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater. Sci. 2016;4(2):219–229. doi: 10.1039/c5bm00383k. [DOI] [PubMed] [Google Scholar]
- 96.Uchegbu I.F., Lalatsa A., Wong D. Polymeric nanoparticles. In: Uchegbu I.F., Schätzlein A.G., Cheng W.P., Lalatsa A., editors. Fundamentals of Pharmaceutical Nanoscience. Springer New York; New York, NY: 2013. pp. 211–234. [Google Scholar]
- 97.Geppert M., Hohnholt M., Gaetjen L., Grunwald I., Baeumer M., Dringen R. Accumulation of iron oxide nanoparticles by cultured brain astrocytes. J. Biomed. Nanotechnol. 2009;5(3):285–293. doi: 10.1166/jbn.2009.1033. [DOI] [PubMed] [Google Scholar]
- 98.Muldoon L.L., Sandor M., Pinkston K.E., Neuwelt E.A. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–796. doi: 10.1093/neurosurgery/57.4.785. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Huang Y., David A.E., Chertok B., Zhang L., Yu F., Yang V.C. Magnetic nanoparticles for MRI of brain tumors. Curr. Pharmaceut. Biotechnol. 2012;13(12):2403–2416. doi: 10.2174/138920112803341824. [DOI] [PubMed] [Google Scholar]
- 100.Nadeem M., Ahmad M., Saeed M.A., Shaari A., Riaz S., Naseem S., Rashid K. Uptake and clearance analysis of Technetium(99m) labelled iron oxide nanoparticles in a rabbit brain. IET Nanobiotechnol. 2015;9(3):136–141. doi: 10.1049/iet-nbt.2014.0012. [DOI] [PubMed] [Google Scholar]
- 101.Hohnholt M.C., Dringen R. Uptake and metabolism of iron and iron oxide nanoparticles in brain astrocytes. Biochem. Soc. Trans. 2013;41:1588–1592. doi: 10.1042/BST20130114. [DOI] [PubMed] [Google Scholar]
- 102.Skrabalak S.E., Chen J., Sun Y., Lu X., Au L., Cobley C.M., Xia Y. Gold nanocages: synthesis, properties, and applications. Acc. Chem. Res. 2008;41(12):1587–1595. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dykman L., Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev. 2012;41(6):2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 104.Boisselier E., Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38(6):1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 105.Huang X., Jain P.K., El-Sayed I.H., El-Sayed M.A. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostic and therapy. Nanomedicine. 2007;2(5):681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 106.Lee K.-S., El-Sayed M.A. Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B. 2006;110(39):19220–19225. doi: 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh S.K., Pal T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem. Rev. 2007;107(11):4797–4862. doi: 10.1021/cr0680282. [DOI] [PubMed] [Google Scholar]
- 108.Link S., El-Sayed M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B. 1999;103(40):8410–8426. [Google Scholar]
- 109.El-Sayed I.H., Huang X.H., El-Sayed M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5(5):829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 110.Eustis S., El-Sayed M.A. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006;35(3):209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 111.Pathak A.K., Khan M., Gschneidner K.A., Jr., McCallum R.W., Zhou L., Sun K., Dennis K.W., Zhou C., Pinkerton F.E., Kramer M.J., Pecharsky V.K. Cerium: an unlikely replacement of dysprosium in high performance Nd-Fe-B permanent magnets. Adv. Mater. 2015;27(16):2663–2667. doi: 10.1002/adma.201404892. [DOI] [PubMed] [Google Scholar]
- 112.Li W.F., Ohkubo T., Hono K. Effect of post-sinter annealing on the coercivity and microstructure of Nd-Fe-B permanent magnets. Acta Mater. 2009;57(5):1337–1346. [Google Scholar]
- 113.Sepehri-Amin H., Ohkubo T., Shima T., Hono K. Grain boundary and interface chemistry of an Nd-Fe-B-based sintered magnet. Acta Mater. 2012;60(3):819–830. [Google Scholar]
- 114.Sepehri-Amin H., Ohkubo T., Nagashima S., Yano M., Shoji T., Kato A., Schrefl T., Hono K. High-coercivity ultrafine-grained anisotropic Nd-Fe-B magnets processed by hot deformation and the Nd-Cu grain boundary diffusion process. Acta Mater. 2013;61(17):6622–6634. [Google Scholar]
- 115.Corot C., Robert P., Idee J.-M., Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58(14):1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 116.Gao J., Gu H., Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc. Chem. Res. 2009;42(8):1097–1107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 117.Hao R., Xing R., Xu Z., Hou Y., Gao S., Sun S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010;22(25):2729–2742. doi: 10.1002/adma.201000260. [DOI] [PubMed] [Google Scholar]
- 118.Wu W., He Q., Jiang C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008;3(11):397–415. doi: 10.1007/s11671-008-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.M D., S P., N B., JF K., LM R., S S., Biomaterials H.J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. 2007;28(10):1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.H G., R N., S M., A H., N T., A S.Z., M R., research S.A.J.N. Vol. 37. 2015. pp. 624–632. (Neuro-protective effects of cerium and yttrium oxide nanoparticles on high glucose-induced oxidative stress and apoptosis in undifferentiated PC12 cells). 7. [DOI] [PubMed] [Google Scholar]
- 121.Faa G., Gerosa C., Fanni D., Lachowicz J.I., Nurchi V.M. Gold - old drug with new potentials. Curr. Med. Chem. 2018;25(1):75–84. doi: 10.2174/0929867324666170330091438. [DOI] [PubMed] [Google Scholar]
- 122.Penninckx S., Heuskin A.C., Michiels C., Lucas S. Gold nanoparticles as a potent radiosensitizer: a transdisciplinary approach from physics to patient. Cancers. 2020;12(8) doi: 10.3390/cancers12082021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerosa C., Crisponi G., Nurchi V.M., Saba L., Cappai R., Cau F., Faa G., Van Eyken P., Scartozzi M., Floris G., Fanni D. Gold nanoparticles: a new golden era in oncology? Pharmaceuticals. 2020;13(8) doi: 10.3390/ph13080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu Y., Xu Y.J., Zhang G.B., Ling D., Wang M.Q., Zhou Y., Wu Y.D., Wu T., Hackett M.J., Hyo Kim B., Chang H., Kim J., Hu X.T., Dong L., Lee N., Li F., He J.C., Zhang L., Wen H.Q., Yang B., Hong Choi S., Hyeon T., Zou D.H. Iron oxide nanoclusters for T 1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017;1(8):637–643. doi: 10.1038/s41551-017-0116-7. [DOI] [PubMed] [Google Scholar]
- 125.Yilmaz A., Rosch S., Yildiz H., Klumpp S., Sechtem U. First multiparametric cardiovascular magnetic resonance study using ultrasmall superparamagnetic iron oxide nanoparticles in a patient with acute myocardial infarction: new vistas for the clinical application of ultrasmall superparamagnetic iron oxide. Circulation. 2012;126(15):1932–1934. doi: 10.1161/CIRCULATIONAHA.112.108167. [DOI] [PubMed] [Google Scholar]
- 126.Baughman R.H., Zakhidov A.A., de Heer W.A. Carbon nanotubes - the route toward applications. Science. 2002;297(5582):787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 127.Thess A., Lee R., Nikolaev P., Dai H.J., Petit P., Robert J., Xu C.H., Lee Y.H., Kim S.G., Rinzler A.G., Colbert D.T., Scuseria G.E., Tomanek D., Fischer J.E., Smalley R.E. Crystalline ropes of metallic carbon nanotubes. Science. 1996;273(5274):483–487. doi: 10.1126/science.273.5274.483. [DOI] [PubMed] [Google Scholar]
- 128.Treacy M.M.J., Ebbesen T.W., Gibson J.M. Exceptionally high Young's modulus observed for individual carbon nanotubes. Nature. 1996;381(6584):678–680. [Google Scholar]
- 129.Gong K., Du F., Xia Z., Durstock M., Dai L. Nitrogen-Doped carbon nanotube Arrays with high electrocatalytic activity for oxygen reduction. Science. 2009;323(5915):760–764. doi: 10.1126/science.1168049. [DOI] [PubMed] [Google Scholar]
- 130.Tans S.J., Verschueren A.R.M., Dekker C. Room-temperature transistor based on a single carbon nanotube. Nature. 1998;393(6680):49–52. [Google Scholar]
- 131.Iijima S., Ichihashi T. SINGLE-SHELL carbon nanotubes OF 1-NM diameter. Nature. 1993;363(6430):603–605. [Google Scholar]
- 132.Thostenson E.T., Ren Z.F., Chou T.W. Advances in the science and technology of carbon nanotubes and their composites: a review. Compos. Sci. Technol. 2001;61(13):1899–1912. [Google Scholar]
- 133.O'Connell M.J., Bachilo S.M., Huffman C.B., Moore V.C., Strano M.S., Haroz E.H., Rialon K.L., Boul P.J., Noon W.H., Kittrell C., Ma J.P., Hauge R.H., Weisman R.B., Smalley R.E. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297(5581):593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 134.Tasis D., Tagmatarchis N., Bianco A., Prato M. Chemistry of carbon nanotubes. Chem. Rev. 2006;106(3):1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- 135.Fan S.S., Chapline M.G., Franklin N.R., Tombler T.W., Cassell A.M., Dai H.J. Self-oriented regular arrays of carbon nanotubes and their field emission properties. Science. 1999;283(5401):512–514. doi: 10.1126/science.283.5401.512. [DOI] [PubMed] [Google Scholar]
- 136.Dillon A.C., Jones K.M., Bekkedahl T.A., Kiang C.H., Bethune D.S., Heben M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature. 1997;386(6623):377–379. [Google Scholar]
- 137.Balandin A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011;10(8):569–581. doi: 10.1038/nmat3064. [DOI] [PubMed] [Google Scholar]
- 138.Sinha N., Member S., Yeow T.W. Carbon nanotubes for biomedical applications. IEEE Trans. NanoBiosci. 2005;4(2):180–195. doi: 10.1109/tnb.2005.850478. [DOI] [PubMed] [Google Scholar]
- 139.Odom T.W., Huang J.L., Kim P., Lieber C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature. 1998;391(6662):62–64. [Google Scholar]
- 140.Baughman R.H., Cui C.X., Zakhidov A.A., Iqbal Z., Barisci J.N., Spinks G.M., Wallace G.G., Mazzoldi A., De Rossi D., Rinzler A.G., Jaschinski O., Roth S., Kertesz M. Carbon nanotube actuators. Science. 1999;284(5418):1340–1344. doi: 10.1126/science.284.5418.1340. [DOI] [PubMed] [Google Scholar]
- 141.Spitalsky Z., Tasis D., Papagelis K., Galiotis C. Carbon nanotube-polymer composites: chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010;35(3):357–401. [Google Scholar]
- 142.Zheng M., Jagota A., Semke E.D., Diner B.A., McLean R.S., Lustig S.R., Richardson R.E., Tassi N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003;2(5):338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 143.Qian D., Dickey E.C., Andrews R., Rantell T. Load transfer and deformation mechanisms in carbon nanotube-polystyrene composites. Appl. Phys. Lett. 2000;76(20):2868–2870. [Google Scholar]
- 144.Chen R.J., Zhang Y.G., Wang D.W., Dai H.J. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001;123(16):3838–3839. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- 145.Hata K., Futaba D.N., Mizuno K., Namai T., Yumura M., Iijima S. Water-Assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science. 2004;306(5700):1362–1364. doi: 10.1126/science.1104962. [DOI] [PubMed] [Google Scholar]
- 146.Alshehri R., Ilyas A., Hasan A., Arnaout A., Ahmed F., Memic A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity: Miniperspective. J. Med. Chem. 2016;59(18):8149–8167. doi: 10.1021/acs.jmedchem.5b01770. [DOI] [PubMed] [Google Scholar]
- 147.Bianco A., Kostarelos K., Prato M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005;9(6):674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Liu Z., Tabakman S., Welsher K., Dai H. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Research. 2009;2(2):85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Z., Chen K., Davis C., Sherlock S., Cao Q., Chen X., Dai H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Canc. Res. 2008;68(16):6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Z., Sun X., Nakayama-Ratchford N., Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1(1):50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 151.Ahmed W., Elhissi A., Dhanak V., Subramani K. Carbon nanotubes Chapter 18-Carbon Nanotubes : Applications in Cancer Therapy and Drug Delivery Research. Emerging Nanotechnologies in Dentistry; 2018. pp. 371–389. [Google Scholar]
- 152.Hassanzadeh P., Arbabi E., Atyabi F., Dinarvand R. Nerve growth factor-carbon nanotube complex exerts prolonged protective effects in an in vitro model of ischemic stroke. Life Sci. 2017;179:15–22. doi: 10.1016/j.lfs.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 153.Komane P.P., Kumar P., Marimuthu T., Toit L.C.D., Kondiah P.P.D., Choonara Y.E., Pillay V. Dexamethasone-loaded, PEGylated, vertically aligned, multiwalled carbon nanotubes for potential ischemic stroke intervention. Molecules. 2018;23(6) doi: 10.3390/molecules23061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pastorin G., Wu W., Wieckowski S., Briand J.P., Kostarelos K., Prato M., Bianco A. Double functionalisation of carbon nanotubes for multimodal drug delivery. Chem. Commun. 2006;11:1182–1184. doi: 10.1039/b516309a. [DOI] [PubMed] [Google Scholar]
- 155.Prato M., Kostarelos K., Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008;41(1):60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 156.Chen J., Chen S., Zhao X., Kuznetsova L.V., Wong S.S., Ojima I. Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J. Am. Chem. Soc. 2008;130(49):16778–16785. doi: 10.1021/ja805570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feazell R.P., Nakayama-Ratchford N., Dai H., Lippard S.J. Soluble single-walled carbon nanotubes as longboat delivery systems for Platinum(IV) anticancer drug design. J. Am. Chem. Soc. 2007;129(27):8438–+. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhirde A.A., Patel V., Gavard J., Zhang G., Sousa A.A., Masedunskas A., Leapman R.D., Weigert R., Gutkind J.S., Rusling J.F. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3(2):307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maiti U.N., Lee W.J., Lee J.M., Oh Y., Kim J.Y., Kim J.E., Shim J., Han T.H., Kim S.O. 25th anniversary article: chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices. Adv. Mater. 2014;26(1):40–67. doi: 10.1002/adma.201303265. [DOI] [PubMed] [Google Scholar]
- 160.Banks C.E., Davies T.J., Wildgoose G.G., Compton R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005;(7):829–841. doi: 10.1039/b413177k. [DOI] [PubMed] [Google Scholar]
- 161.Jiang L.-C., Zhang W.-D. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles-modified carbon nanotube electrode. Biosens. Bioelectron. 2010;25(6):1402–1407. doi: 10.1016/j.bios.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 162.Sljukic B., Banks C.E., Compton R.G. Iron oxide particles are the active sites for hydrogen peroxide sensing at multiwalled carbon nanotube modified electrodes. Nano Lett. 2006;6(7):1556–1558. doi: 10.1021/nl060366v. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X., Meng L., Lu Q., Fei Z., Dyson P.J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 2009;30(30):6041–6047. doi: 10.1016/j.biomaterials.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 164.Shityakov S., Salvador E., Pastorin G., Forster C. Blood-brain barrier transport studies, aggregation, and molecular dynamics simulation of multiwalled carbon nanotube functionalized with fluorescein isothiocyanate. Int. J. Nanomed. 2015;10:1703–1713. doi: 10.2147/IJN.S68429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J.T., Rubio N., Kafa H., Venturelli E., Fabbro C., Menard-Moyon C., Da Ros T., Sosabowski J.K., Lawson A.D., Robinson M.K., Prato M., Bianco A., Festy F., Preston J.E., Kostarelos K., Al-Jamal K.T. Kinetics of functionalised carbon nanotube distribution in mouse brain after systemic injection: spatial to ultra-structural analyses. J. Contr. Release. 2016;224:22–32. doi: 10.1016/j.jconrel.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.You Y., Wang N., He L., Shi C., Zhang D., Liu Y., Luo L., Chen T. Designing dual-functionalized carbon nanotubes with high blood-brain-barrier permeability for precise orthotopic glioma therapy. Dalton Trans. 2019;48(5):1569–1573. doi: 10.1039/c8dt03948h. [DOI] [PubMed] [Google Scholar]
- 167.Shrestha S., Shrestha B.K., Lee J., Joong O.K., Kim B.S., Park C.H., Kim C.S. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:511–523. doi: 10.1016/j.msec.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 168.Henna T.K., Raphey V.R., Sankar R., Ameena Shirin V.K., Gangadharappa H.V., Pramod K. Carbon nanostructures: the drug and the delivery system for brain disorders. Int. J. Pharm. 2020;587:119701. doi: 10.1016/j.ijpharm.2020.119701. [DOI] [PubMed] [Google Scholar]
- 169.Negri V., Pacheco-Torres J., Calle D., Lopez-Larrubia P. Carbon nanotubes in biomedicine. Top. Curr. Chem. 2020;378(1):15. doi: 10.1007/s41061-019-0278-8. [DOI] [PubMed] [Google Scholar]
- 170.Loh K.P., Ho D., Chiu G.N.C., Leong D.T., Pastorin G., Chow E.K. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv. Mater. 2018;30(47) doi: 10.1002/adma.201802368. [DOI] [PubMed] [Google Scholar]
- 171.Fernandes L.F., Bruch G.E., Massensini A.R., Frézard F. Recent advances in the therapeutic and diagnostic use of liposomes and carbon nanomaterials in ischemic stroke. 2018;12(453) doi: 10.3389/fnins.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hess L.H., Seifert M., Garrido J.A. Graphene transistors for bioelectronics. Proc. IEEE. 2013;101:1780. [Google Scholar]
- 173.Ponraj J.S., Xu Z.Q., Dhanabalan S.C., Mu H., Wang Y., Yuan J., Li P., Thakur S., Ashrafi M., McCoubrey K., Zhang Y., Li S., Zhang H., Bao Q. Photonics and optoelectronics of two-dimensional materials beyond graphene. Nanotechnology. 2016;27(46):462001. doi: 10.1088/0957-4484/27/46/462001. [DOI] [PubMed] [Google Scholar]
- 174.Ray S.J. First-principles study of MoS2, phosphorene and graphene based single electron transistor for gas sensing applications. Sensor. Actuator. B Chem. 2016;222:492–498. [Google Scholar]
- 175.Yang Y., Yang X., Tan Y., Yuan Q. Recent progress in flexible and wearable bio-electronics based on nanomaterials. Nano Research. 2017;10(5):1560–1583. [Google Scholar]
- 176.Huang H., Ren X., Li Z., Wang H., Huang Z., Qiao H., Tang P., Zhao J., Liang W., Ge Y., Liu J., Li J., Qi X., Zhang H. Two-dimensional bismuth nanosheets as prospective photo-detector with tunable optoelectronic performance. Nanotechnology. 2018;29(23):235201. doi: 10.1088/1361-6528/aab6ee. [DOI] [PubMed] [Google Scholar]
- 177.Yang K., Zhang S., Zhang G., Sun X., Lee S.T., Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 178.Robinson J.T., Tabakman S.M., Yongye L., Hailiang W., Hernan Sanchez C., Daniel V., Hongjie D. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133(17):6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 179.Sun H., Cao L., Lu L. Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Research. 2011;4(6):550–562. [Google Scholar]
- 180.Akhavan O., Ghaderi E., Aghayee S., Fereydooni Y., Talebi A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 2012;22(27):13773. [Google Scholar]
- 181.Jiang Y., Miao L., Jiang G., Chen Y., Qi X., Jiang X.F., Zhang H., Wen S. Broadband and enhanced nonlinear optical response of MoS2/graphene nanocomposites for ultrafast photonics applications. Sci. Rep. 2015;5:16372. doi: 10.1038/srep16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miao L., Jiang Y., Lu S., Shi B., Zhao C., Zhang H., Wen S. Broadband ultrafast nonlinear optical response of few-layers graphene: toward the mid-infrared regime. Photon. Res. 2015;3(5):214. [Google Scholar]
- 183.Wang Z., Mu H., Zhao C., Bao Q., Zhang H. Harmonic mode-locking and wavelength-tunable Q-switching operation in the graphene–Bi2Te3heterostructure saturable absorber-based fiber laser. Opt. Eng. 2016;55(8) [Google Scholar]
- 184.Zhang H., Tang D., Zhao L., Bao Q., Loh K.P. Vector dissipative solitons in graphene mode locked fiber lasers. Optic Commun. 2010;283(17):3334–3338. [Google Scholar]
- 185.Zhang H., Tang D.Y., Zhao L.M., Bao Q.L., Loh K.P., Lin B., Tjin S.C. Compact graphene mode-locked wavelength-tunable erbium-doped fiber lasers: from all anomalous dispersion to all normal dispersion. Laser Phys. Lett. 2010;7(8):591–596. [Google Scholar]
- 186.Zhao J., Wang Y., Ruan S., Yan P., Zhang H., Tsang Y.H., Yang J., Huang G. Three operation regimes with an L-band ultrafast fiber laser passively mode-locked by graphene oxide saturable absorber. J. Opt. Soc. Am. B. 2014;31(4):716. [Google Scholar]
- 187.Bao Q., Zhang H., Wang Y., Ni Z., Yan Y., Shen Z.X., Loh K.P., Tang D.Y. Atomic-layer graphene as a saturable absorber for ultrafast pulsed lasers. Adv. Funct. Mater. 2009;19(19):3077–3083. [Google Scholar]
- 188.Zhang H., Bao Q., Tang D., Zhao L., Loh K. Large energy soliton erbium-doped fiber laser with a graphene-polymer composite mode locker. Appl. Phys. Lett. 2009;95(14):141103. [Google Scholar]
- 189.Bao Q., Zhang H., Ni Z., Wang Y., Polavarapu L., Shen Z., Xu Q.-H., Tang D., Loh K.P. Monolayer graphene as a saturable absorber in a mode-locked laser. Nano Research. 2010;4(3):297–307. [Google Scholar]
- 190.Zhang H., Tang D., Knize R.J., Zhao L., Bao Q., Loh K.P. Graphene mode locked, wavelength-tunable, dissipative soliton fiber laser. Appl. Phys. Lett. 2010;96(11):111112. [Google Scholar]
- 191.Huang X., Yin Z., Wu S., Qi X., He Q., Zhang Q., Yan Q., Boey F., Zhang H. Graphene-based materials: synthesis, characterization, properties, and applications. Small. 2011;7(14):1876–1902. doi: 10.1002/smll.201002009. [DOI] [PubMed] [Google Scholar]
- 192.Lopez-Sanchez O., Lembke D., Kayci M., Radenovic A., Kis A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013;8(7):497–501. doi: 10.1038/nnano.2013.100. [DOI] [PubMed] [Google Scholar]
- 193.Shao Y., Wang J., Wu H., Liu J., Aksay I.A., Lin Y. Graphene based electrochemical sensors and biosensors: a review. Electroanalysis. 2010;22(10):1027–1036. [Google Scholar]
- 194.Chen D., Feng H., Li J. Graphene oxide: preparation, functionalization, and electrochemical applications. Chem. Rev. 2012;112(11):6027–6053. doi: 10.1021/cr300115g. [DOI] [PubMed] [Google Scholar]
- 195.Lu C.-H., Yang H.-H., Zhu C.-L., Chen X., Chen G.-N. A graphene platform for sensing biomolecules. Angew. Chem. Int. Ed. 2009;48(26):4785–4787. doi: 10.1002/anie.200901479. [DOI] [PubMed] [Google Scholar]
- 196.Avouris P. Graphene: electronic and photonic properties and devices. Nano Lett. 2010;10(11):4285–4294. doi: 10.1021/nl102824h. [DOI] [PubMed] [Google Scholar]
- 197.Bonaccorso F., Sun Z., Hasan T., Ferrari A.C. Graphene photonics and optoelectronics. Nat. Photon. 2010;4(9):611–622. [Google Scholar]
- 198.Loh K.P., Bao Q., Eda G., Chhowalla M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010;2(12):1015–1024. doi: 10.1038/nchem.907. [DOI] [PubMed] [Google Scholar]
- 199.He S., Song B., Li D., Zhu C., Qi W., Wen Y., Wang L., Song S., Fang H., Fan C. A graphene nanoprobe for rapid, sensitive, and multicolor fluorescent DNA analysis. Adv. Funct. Mater. 2010;20(3):453–459. [Google Scholar]
- 200.Mohanty N., Berry V. Graphene-based single-bacterium resolution biodevice and DNA transistor: interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 2008;8(12):4469–4476. doi: 10.1021/nl802412n. [DOI] [PubMed] [Google Scholar]
- 201.Yang K., Feng L., Shi X., Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 2013;42(2):530–547. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 202.Yang W., Ratinac K.R., Ringer S.P., Thordarson P., Gooding J.J., Braet F. Carbon nanomaterials in biosensors: should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010;49(12):2114–2138. doi: 10.1002/anie.200903463. [DOI] [PubMed] [Google Scholar]
- 203.Zhang L., Xia J., Zhao Q., Liu L., Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6(4):537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 204.Song Y., Liang Z., Zhang H., Zhang Q., Zhao L., Shen D., Tang D. Period-doubling and quadrupling bifurcation of vector soliton bunches in a graphene mode locked fiber laser. IEEE Photon. J. 2017;9(5):1–8. [Google Scholar]
- 205.Huang Z., Han W., Tang H., Ren L., Chander D.S., Qi X., Zhang H. Photoelectrochemical-type sunlight photodetector based on MoS2/graphene heterostructure. 2D Mater. 2015;2(3) [Google Scholar]
- 206.Wang Z.T., Chen Y., Zhao C.J., Zhang H., Wen S.C. Switchable dual-wavelength synchronously Q-switched erbium-doped fiber laser based on graphene saturable absorber. IEEE Photon. J. 2012;4(3):869–876. [Google Scholar]
- 207.Shi J., Li Z., Sang D.K., Xiang Y., Li J., Zhang S., Zhang H. THz photonics in two dimensional materials and metamaterials: properties, devices and prospects. J. Mater. Chem. C. 2018;6(6):1291–1306. [Google Scholar]
- 208.Song Y.F., Zhang H., Zhao L.M., Shen D.Y., Tang D.Y. Coexistence and interaction of vector and bound vector solitons in a dispersion-managed fiber laser mode locked by graphene. Optic Express. 2016;24(2):1814–1822. doi: 10.1364/OE.24.001814. [DOI] [PubMed] [Google Scholar]
- 209.Wang H., Miao L., Jiang Y., Lu S., Li Z., Li P., Zhao C., Zhang H., Wen S. Enhancing the saturable absorption and carrier dynamics of graphene with plasmonic nanowires. Phys. Status Solidi. 2015;252(10):2159–2166. [Google Scholar]
- 210.Feng Q., Chen Y., Zhao C., Li Y., Wen J., Zhang H. Experimental study on the multisoliton pattern formation in an erbium-doped fiber laser passively mode-locked by graphene saturable absorber. Opt. Eng. 2013;52(4) [Google Scholar]
- 211.Mu H., Wang Z., Yuan J., Xiao S., Chen C., Chen Y., Chen Y., Song J., Wang Y., Xue Y., Zhang H., Bao Q. Graphene–Bi2Te3 heterostructure as saturable absorber for short pulse generation. ACS Photonics. 2015;2(7):832–841. [Google Scholar]
- 212.Mendonça M.C.P., Soares E.S., de Jesus M.B., Ceragioli H.J., Ferreira M.S., Catharino R.R., da Cruz-Höfling M.A. Reduced graphene oxide induces transient blood–brain barrier opening: an in vivo study. J. Nanobiotechnol. 2015;13(1):78. doi: 10.1186/s12951-015-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fernandes L.F., Bruch G.E., Massensini A.R., Frézard F.J. F.i.N. Recent advances in the therapeutic and diagnostic use of liposomes and carbon nanomaterials in ischemic stroke. 2018;12:453. doi: 10.3389/fnins.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Reina G., Gonzalez-Dominguez J.M., Criado A., Vazquez E., Bianco A., Prato M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017;46(15):4400–4416. doi: 10.1039/c7cs00363c. [DOI] [PubMed] [Google Scholar]
- 215.Newman L., Jasim D.A., Prestat E., Lozano N., de Lazaro I., Nam Y., Assas B.M., Pennock J., Haigh S.J., Bussy C., Kostarelos K. Splenic capture and in vivo intracellular biodegradation of biological-grade graphene oxide sheets. ACS Nano. 2020;14(8):10168–10186. doi: 10.1021/acsnano.0c03438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yan Z.-Q., Zhang W. The development of graphene-based devices for cell biology research. Front. Mater. Sci. 2014;8(2):107–122. [Google Scholar]
- 217.Qi X., Zhang Y., Ou Q., Ha S.T., Qiu C.W., Zhang H., Cheng Y.B., Xiong Q., Bao Q. Photonics and optoelectronics of 2D metal-halide perovskites. Small. 2018 doi: 10.1002/smll.201800682. [DOI] [PubMed] [Google Scholar]
- 218.Li J., Luo H., Zhai B., Lu R., Guo Z., Zhang H., Liu Y. Black phosphorus: a two-dimension saturable absorption material for mid-infrared Q-switched and mode-locked fiber lasers. Sci. Rep. 2016;6:30361. doi: 10.1038/srep30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lu S., Ge Y., Sun Z., Huang Z., Cao R., Zhao C., Wen S., Fan D., Li J., Zhang H. Ultrafast nonlinear absorption and nonlinear refraction in few-layer oxidized black phosphorus. Photon. Res. 2016;4(6):286. [Google Scholar]
- 220.Liu J., Chen Y., Li Y., Zhang H., Zheng S., Xu S. Switchable dual-wavelength Q-switched fiber laser using multilayer black phosphorus as a saturable absorber. Photon. Res. 2018;6(3):198. [Google Scholar]
- 221.Guo Z., Zhang H., Lu S., Wang Z., Tang S., Shao J., Sun Z., Xie H., Wang H., Yu X.-F., Chu P.K. From black phosphorus to phosphorene: basic solvent exfoliation, evolution of Raman scattering, and applications to ultrafast photonics. Adv. Funct. Mater. 2015;25(45):6996–7002. [Google Scholar]
- 222.Jiang Q., Xu L., Chen N., Zhang H., Dai L., Wang S. Facile synthesis of black phosphorus: an efficient electrocatalyst for the oxygen evolving reaction. Angew. Chem. 2016;55(44):13849–13853. doi: 10.1002/anie.201607393. [DOI] [PubMed] [Google Scholar]
- 223.Kong L., Qin Z., Xie G., Guo Z., Zhang H., Yuan P., Qian L. Black phosphorus as broadband saturable absorber for pulsed lasers from 1μm to 2.7μm wavelength. Laser Phys. Lett. 2016;13(4) [Google Scholar]
- 224.Li C., Liu J., Guo Z., Zhang H., Ma W., Wang J., Xu X., Su L. Black phosphorus saturable absorber for a diode-pumped passively Q-switched Er:CaF2 mid-infrared laser. Optic Commun. 2018;406:158–162. [Google Scholar]
- 225.Chu Z., Liu J., Guo Z., Zhang H. 2 μm passively Q-switched laser based on black phosphorus. Opt. Mater. Express. 2016;6(7):2374. [Google Scholar]
- 226.Song Y., Chen S., Zhang Q., Li L., Zhao L., Zhang H., Tang D. Vector soliton fiber laser passively mode locked by few layer black phosphorus-based optical saturable absorber. Optic Express. 2016;24(23):25933–25942. doi: 10.1364/OE.24.025933. [DOI] [PubMed] [Google Scholar]
- 227.Qin Z., Xie G., Zhang H., Zhao C., Yuan P., Wen S., Qian L. Black phosphorus as saturable absorber for the Q-switched Er:ZBLAN fiber laser at 2.8 mum. Optic Express. 2015;23(19):24713–24718. doi: 10.1364/OE.23.024713. [DOI] [PubMed] [Google Scholar]
- 228.Lu S.B., Miao L.L., Guo Z.N., Qi X., Zhao C.J., Zhang H., Wen S.C., Tang D.Y., Fan D.Y. Broadband nonlinear optical response in multi-layer black phosphorus: an emerging infrared and mid-infrared optical material. Optic Express. 2015;23(9):11183–11194. doi: 10.1364/OE.23.011183. [DOI] [PubMed] [Google Scholar]
- 229.Fan T., Zhou Y., Qiu M., Zhang H. Black phosphorus: a novel nanoplatform with potential in the field of bio-photonic nanomedicine. J. Innov. Optic. Health Sci. 2018;11(6):1830003. [Google Scholar]
- 230.Qiu M., Ren W.X., Jeong T., Won M., Park G.Y., Sang D.K., Liu L.P., Zhang H., Kim J.S. Omnipotent phosphorene: a next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018;47(15):5588–5601. doi: 10.1039/c8cs00342d. [DOI] [PubMed] [Google Scholar]
- 231.Luo M., Fan T., Zhou Y., Zhang H., Mei L. 2D black phosphorus–based biomedical applications. Adv. Funct. Mater. 2019;29(13):1808306. [Google Scholar]
- 232.Sun Z., Xie H., Tang S., Yu X.F., Guo Z., Shao J., Zhang H., Huang H., Wang H., Chu P.K. Ultrasmall black phosphorus quantum dots: synthesis and use as photothermal agents. Angew. Chem. 2015;54(39):11526–11530. doi: 10.1002/anie.201506154. [DOI] [PubMed] [Google Scholar]
- 233.Lee M., Park Y.H., Kang E.B., Chae A., Choi Y., Jo S., Kim Y.J., Park S.-J., Min B., An T.K., Lee J., S I., Kim S.Y., Park S.Y., In I. Highly efficient visible blue-emitting black phosphorus quantum dot: mussel-inspired surface functionalization for bioapplications. ACS Omega. 2017;2(10):7096–7105. doi: 10.1021/acsomega.7b01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Qiu M., Sun Z.T., Sang D.K., Han X.G., Zhang H., Niu C.M. Current progress in black phosphorus materials and their applications in electrochemical energy storage. Nanoscale. 2017;9(36):13384–13403. doi: 10.1039/c7nr03318d. [DOI] [PubMed] [Google Scholar]
- 235.Huang W., Xie Z., Fan T., Li J., Wang Y., Wu L., Ma D., Li Z., Ge Y., Huang Z.N., Dai X., Xiang Y., Li J., Zhu X., Zhang H. Black-phosphorus-analogue tin monosulfide: an emerging optoelectronic two-dimensional material for high-performance photodetection with improved stability under ambient/harsh conditions. J. Mater. Chem. C. 2018;6(36):9582–9593. [Google Scholar]
- 236.Shao J., Xie H., Huang H., Li Z., Sun Z., Xu Y., Xiao Q., Yu X.F., Zhao Y., Zhang H., Wang H., Chu P.K. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016;7:12967. doi: 10.1038/ncomms12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ren X., Li Z., Huang Z., Sang D., Qiao H., Qi X., Li J., Zhong J., Zhang H. Environmentally robust black phosphorus nanosheets in solution: application for self-powered photodetector. Adv. Funct. Mater. 2017;27(18):1606834. [Google Scholar]
- 238.Tang X., Chen H., Ponraj J.S., Dhanabalan S.C., Xiao Q., Fan D., Zhang H. Fluorination-enhanced ambient stability and electronic tolerance of black phosphorus quantum dots. Adv. Sci. 2018;5(9):1800420. doi: 10.1002/advs.201800420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xing C., Jing G., Liang X., Qiu M., Li Z., Cao R., Li X., Fan D., Zhang H. Graphene oxide/black phosphorus nanoflake aerogels with robust thermo-stability and significantly enhanced photothermal properties in air. Nanoscale. 2017;9(24):8096–8101. doi: 10.1039/c7nr00663b. [DOI] [PubMed] [Google Scholar]
- 240.Xing C., Chen S., Qiu M., Liang X., Liu Q., Zou Q., Li Z., Xie Z., Wang D., Dong B., Liu L., Fan D., Zhang H. Conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy. Adv. Healthcare Mater. 2018;7(7) doi: 10.1002/adhm.201701510. [DOI] [PubMed] [Google Scholar]
- 241.Tang X., Liang W., Zhao J., Li Z., Qiu M., Fan T., Luo C.S., Zhou Y., Li Y., Guo Z., Fan D., Zhang H. Fluorinated phosphorene: electrochemical synthesis, atomistic fluorination, and enhanced stability. Small. 2017;13(47) doi: 10.1002/smll.201702739. [DOI] [PubMed] [Google Scholar]
- 242.Li L., Yu Y., Ye G.J., Ge Q., Ou X., Wu H., Feng D., Chen X.H., Zhang Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014;9(5):372–377. doi: 10.1038/nnano.2014.35. [DOI] [PubMed] [Google Scholar]
- 243.Zhou W., Pan T., Cui H., Zhao Z., Chu P.K., Yu X.-F. Black phosphorus: bioactive nanomaterials with inherent and selective chemotherapeutic effects. Angew. Chem. Int. Ed. 2019;58(3):769–774. doi: 10.1002/anie.201810878. [DOI] [PubMed] [Google Scholar]
- 244.Tan M.J., Pan H.-C., Tan H.R., Chai J.W., Lim Q.F., Wong T.I., Zhou X., Hong Z.-Y., Liao L.-D., Kong K.V. Flexible Modulation of CO-Release Using Various Nuclearity of Metal Carbonyl Clusters on Graphene Oxide for Stroke Remediation. Adv. Healthcare Mater. 2018;7(5):1701113. doi: 10.1002/adhm.201701113. [DOI] [PubMed] [Google Scholar]
- 245.Robin L., Steinberg G.K. Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? J. Curr. Opin. Neurol. 2013;26(6):617–625. doi: 10.1097/WCO.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Massensini A.R., Ghuman H., Saldin L.T., Medberry C.J., Keane T.J., Nicholls F.J., Velankar S.S., Badylak S.F., Modo M.J.A.B. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. 2015;27:116–130. doi: 10.1016/j.actbio.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nicholls F.J., Rotz M.W., Ghuman H., Macrenaris K.W., Meade T.J., Modo M.J.B. DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells. 2016;77:291–306. doi: 10.1016/j.biomaterials.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Caló E., Khutoryanskiy V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Europ. Polym. J. 2015;65:252–267. [Google Scholar]
- 249.Yong-Li D., Shu-Jun W., Meng C.J. Clinical Application of Hydrogel Dressing Aquacel-Ag in Treatment of Superficial Ⅱ Burns. J. Southeast Univ. 2012;31(6):729–731. [Google Scholar]
- 250.Wang Y., Cooke M.J., Sachewsky N., Morshead C.M., Shoichet M.S. Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J. Contr. Release. 2013;172(1):1–11. doi: 10.1016/j.jconrel.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 251.Y W., MJ C., CM M., Biomaterials S.M.J. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. 2012;33(9):2681–2692. doi: 10.1016/j.biomaterials.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 252.Pitaksuteepong T., Somsiri A., Waranuch N. Targeted transfollicular delivery of artocarpin extract from artocarpus incisus by means of microparticles. Eur. J. Pharmaceut. Biopharmaceut. 2007;67(3):639–645. doi: 10.1016/j.ejpb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 253.Tuladhar A., Morshead C.M., Shoichet M.S. Circumventing the blood–brain barrier: Local delivery of cyclosporin A stimulates stem cells in stroke-injured rat brain. J. Contr. Release. 2015;215:1–11. doi: 10.1016/j.jconrel.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 254.Hong A., Aguilar M.-I., Del Borgo M.P., Sobey C.G., Broughton B.R.S., Forsythe J.S. Self-assembling injectable peptide hydrogels for emerging treatment of ischemic stroke. J. Mater. Chem. B. 2019;7(25):3927–3943. [Google Scholar]
- 255.RS N., YE K., B W., S K., R R., chemistry K.R.J.B. Dendrimer-drug conjugates for tailored intracellular drug release based on glutathione levels. 2008;19(12):2446–2455. doi: 10.1021/bc800342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Verma G., Rajagopalan M.D., Valluru R., Sridhar K.A.J.N.-., Systems M.D.D. 2017. Chapter 7 – Nanoparticles: A Novel Approach to Target Tumors; pp. 113–129. [Google Scholar]
- 257.Gumustas M., Sengel-Turk C.T., Gumustas A., Ozkan S.A., Uslu B. Chapter 5 - Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. Multifunct. Syst. Comb. Deliv. 2017:67–108. [Google Scholar]
- 258.Srinageshwar B., Peruzzaro S., Andrews M., Johnson K., Hietpas A., Clark B., Mcguire C., Petersen E., Kippe J., Stewart A., Lossia O., Al-Gharaibeh A., Antcliff A., Culver R., Swanson D., Dunbar G., Sharma A., Rossignol J. PAMAM Dendrimers Cross the Blood–Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Molecul. Sci. 2017;18(3):628. doi: 10.3390/ijms18030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.SD S., M X., DM L., DA M., B C., M T., R C., V L., J R., H T., Society, P. A. J. J. o. c. r. o. j. o. t. C. R PAMAM dendrimers: blood-brain barrier transport and neuronal uptake after focal brain ischemia. 2018;291(undefined):65–79. doi: 10.1016/j.jconrel.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 260.Kim I.-D., Lim C.-M., Kim J.-B., Nam H.Y., Nam K., Kim S.-W., Park J.-S., Lee J.-K. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J. Contr. Release. 2010;142(3):422–430. doi: 10.1016/j.jconrel.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 261.Jiang Y.H., Emau P., Cairns J.S., Flanary L., Morton W.R., Mccarthy T.D., Tsai C.C.J.A.R., Retroviruses H. Vol. 21. 2005. pp. 207–213. (SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques). 3. [DOI] [PubMed] [Google Scholar]
- 262.Costantino L., Tosi G., Ruozi B., Bondioli, Vandelli M.A., Research F.J. P.i.B. 2009. Colloidal Systems for CNS Drug Delivery. [DOI] [PubMed] [Google Scholar]
- 263.Alving C.R. Macrophages as targets for delivery of liposome-encapsulated antimicrobial agents. 1988;2(1):107–128. [Google Scholar]
- 264.Allison A.G., Gregoriadis G.J.N. Liposomes as immunological adjuvants. 1974;252(5480):252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 265.Baserga; Renato, Introduction of Macromolecules into Viable Mammalian Cells. A.R. Liss.
- 266.De Marie S., Janknegt R., Bakker-Woudenberg I.A. Clinical use of liposomal and lipid-complexed amphotericin B. J. Antimicrob. Chemother. 1994;33(5):907–916. doi: 10.1093/jac/33.5.907. [DOI] [PubMed] [Google Scholar]
- 267.Lasic D.D., Papahadjopoulos D.J.e. Vol. 267. 1995. pp. 1275–1276. (Liposomes revisited). 5202. [DOI] [PubMed] [Google Scholar]
- 268.Emanuel N., Kedar E., Bolotin E.M., Smorodinsky N.I., Barenholz Y.J.P.R. Preparation and characterization of doxorubicin-loaded sterically stabilized immunoliposomes. 1996;13(3):352–359. doi: 10.1023/a:1016028106337. [DOI] [PubMed] [Google Scholar]
- 269.Kayser O., A L., Hernandez-Trejo N. The impact of nanobiotechnology on the development of new drug delivery systems. J. Curr. Pharm. Biotechnol. 2005;6(1) doi: 10.2174/1389201053167158. [DOI] [PubMed] [Google Scholar]
- 270.Kumar C.S., Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011;63(9):789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kostarelos K., Novoselov K.S. Exploring the interface of graphene and biology. Science. 2014;344(6181):261–263. doi: 10.1126/science.1246736. [DOI] [PubMed] [Google Scholar]
- 272.Zaman M. HYDROGELS, their applications and polymers used for hydrogels: a review. Int. J. Biol. Pharm. Allied Sci. 2015;4:6581–6603. [Google Scholar]
- 273.SA, T.; Harde, S.; Sherkar, M.; Raka, K.; Honde, B., HYDROGEL-A SMART POLYMER.
- 274.Gajbhiye V., Palanirajan V.K., Tekade R.K., Jain N.K. Dendrimers as therapeutic agents: a systematic review. J. Pharm. Pharmacol. 2009;61(8):989–1003. doi: 10.1211/jpp/61.08.0002. [DOI] [PubMed] [Google Scholar]
- 275.Furlan A.J. Challenges in Acute Ischemic Stroke Clinical Trials. Curr. Cardiol. Rep. 2012;14(6):761–766. doi: 10.1007/s11886-012-0311-9. [DOI] [PubMed] [Google Scholar]
- 276.Harik V.M. Geometry of carbon nanotubes and mechanisms of phagocytosis and toxic effects. Toxicol. Lett. 2017;273:69–85. doi: 10.1016/j.toxlet.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 277.Cereda C.M., Guilherme V.A., Alkschbirs M.I., de Brito Junior R.B., Tofoli G.R., Franz-Montan M., de Araujo D.R., de Paula E. Liposomal butamben gel formulations: toxicity assays and topical anesthesia in an animal model. J. Liposome Res. 2017;27(1):74–82. doi: 10.3109/08982104.2016.1160924. [DOI] [PubMed] [Google Scholar]