Abstract
Background:
Prevention of recurrent Clostridioides difficile infection (CDI) is a challenge in clinical practice, particularly in patients who need systemic antimicrobial therapy. We aimed to evaluate the role of oral vancomycin prophylaxis (OVP) in prevention of primary or future CDI in patients on systemic antimicrobial therapy.
Methods:
A systematic search of MEDLINE, Embase, and Web of Science was performed from 2000 to January 2020. We included case-control or cohort studies that included patients on systemic antimicrobial therapy who did or did not receive oral vancomycin prophylaxis (OVP) and were evaluated for development of CDI. Odds ratio (OR) estimates with 95% confidence intervals (CI) were calculated.
Results:
Four studies including 1352 patients evaluated OVP for primary CDI prevention, with CDI occurring in 29/402 patients on OVP (7.4%) compared with 10.4% (99/950) without OVP. Meta-analysis revealed no significant decrease in risk of CDI in patients who received OVP (OR, 0.18; 95% CI, 0.03–1.03; p = 0.06). There was significant heterogeneity with I2 = 76%. Ten studies including 9258 patients evaluated OVP for secondary CDI prevention. Future CDI occurred in 91/713 patients on OVP (13.3%) compared with 21.9% (1875/8545) who did not receive OVP. Meta-analysis revealed a statistically significant decreased risk of future CDI (OR, 0.34; 95% CI, 0.20–0.59; p < 0.00001). Significant heterogeneity was seen with I2 = 59%.
Discussion:
Based on observational data, OVP appears to decrease the risk of future CDI in patients with prior CDI who require systemic antimicrobial therapy. However, OVP was not effective for primary prevention of CDI.
Keywords: antibiotics, C. difficile, diarrhea, oral vancomycin, prophylaxis
Introduction
Clostridioides difficile infection (CDI) is the most common nosocomial infection in the United States, with almost half a million cases annually. Recurrent infection is common, with antibiotic exposure being a primary risk factor.1–3 Recent data have suggested that the incidence of recurrent CDI has increased disproportionately compared with primary CDI, indicating a rising demand for strategies to prevent both primary and recurrent CDI.4
The optimal approach to reduce the risk of CDI in patients who require systemic antimicrobial therapy remains unclear. Oral vancomycin is the first line therapy for active CDI; however, the risk of recurrence after primary CDI is approximately 20–25% and is further increased with the use of additional systemic antibiotics.3 Treatment with fidaxomicin or bezlotoxumab in addition to antibiotic therapy is associated with a decreased risk of recurrence compared with vancomycin alone.5,6 A meta-analysis compared therapies for prevention of recurrent CDI in patients with active CDI and demonstrated fidaxomicin, fecal microbiota transplantation (FMT), monoclonal antibodies, and various prebiotics and probiotics demonstrated a reduction in risk of recurrent CDI, with the greatest risk reduction observed with FMT and monoclonal antibody with bezlotoxumab. This study did not address prophylaxis with oral vancomycin to prevent CDI.7
In patients who require systemic antibiotics, a primary or secondary prophylactic strategy to prevent CDI is appealing. Studies evaluating the role of probiotics for mitigating primary or recurrent CDI are inconsistent.8,9 Preventing CDI with oral vancomycin, termed oral vancomycin prophylaxis (OVP), has been reported in small studies. One study evaluated the efficacy of OVP for recurrent CDI within 90 days in adult inpatients who received systemic antibiotics and found reduced risk of CDI with OVP [odds ratio (OR), 0.63; 95% confidence intervals (CI), 0.35–1.14)].10 Another study evaluated the efficacy of OVP for hospitalized patients on systemic antibiotics and found no difference in rates of primary CDI in patients who received OVP versus no OVP.11 One recent meta-analysis of nine studies concluded that OVP was associated with decreased risk of CDI (OR, 0.263; 95% CI, 0.13–0.52) but the analysis was limited by the exclusion of key studies and inclusion of both primary and secondary prophylaxis studies together.12 In addition, studies that have controlled for confounders may be better able to identify a true association.
Due to inconsistent results from prior studies, we performed a comprehensive systematic review and meta-analysis to evaluate the role of OVP for primary and secondary CDI prevention in patients receiving systemic antimicrobial therapy.
Methods
All procedures used in this meta-analysis were consistent with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) and MOOSE (Meta-analysis Of Observational Studies in Epidemiology) criteria for observational studies.13,14
Selection criteria
The studies considered in this meta-analysis were case-control and cohort studies that evaluated patients on systemic antimicrobial therapy who did or did not receive OVP and that measured the incidence of subsequent primary or future CDI. We excluded studies that assessed patients with active CDI. Primary prevention was defined as patients with no prior history of CDI receiving OVP and secondary prevention was defined as patients with prior history of CDI receiving OVP for prophylaxis of a future episode of CDI. We used the term future CDI instead of recurrent CDI as definitions and time for future CDI recurrence were variable and there was no distinction between recurrence (subsequent infection within 8 weeks) and reinfection (subsequent infection beyond 8 weeks) in the available studies. We excluded studies that did not evaluate CDI as an outcome or if there were insufficient data to determine an estimate of an OR and 95% CI. Studies with both published full text or studies available as abstracts were included.
Data sources and search strategy
We conducted a comprehensive search of Ovid MEDLINE in-process and other non-indexed citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus from 1 January 2000 to 31 January 2020. The search strategy was designed and conducted by study investigators (SK and RT) and the Mayo Clinic library staff, independently. The search was limited to studies in the English language. Controlled vocabulary supplemented with keywords was used to search for studies of vancomycin use and CDI. Main keywords used in the search were the following: Clostridium difficile, C diff, C difficile, Clostridium difficile infection, Clostridioides difficile, CDI, Clostridium difficile-associated diarrhea or CDAD, or pseudomembranous colitis AND vancomycin OR prophylaxis OR prevention OR pre-exposure prophylaxis AND outcomes or infection. A detailed search strategy is included as an Appendix.
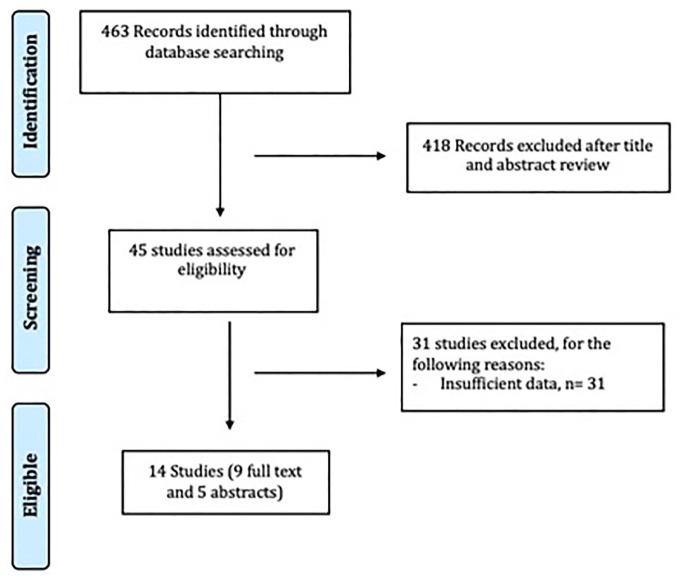
Two authors (SK and RT) independently reviewed the titles and abstracts of the identified studies, and those that did not answer the research question of interest were excluded. The full texts of the remaining articles were reviewed to determine inclusion criteria fulfillment. The reference lists of articles with information on the topic were also reviewed for additional pertinent studies. We also searched the abstracts from major gastroenterology and infectious diseases conferences from 2000 to 2019. The conferences searched were Digestive Diseases Week, American College of Gastroenterology Annual Scientific Meeting, American Society of Microbiology Microbe and Infectious Diseases Week. A flow diagram of included studies is shown in Figure 1.
Figure 1.
Flow diagram of study selection process.
The Newcastle-Ottawa scale was used to assess the methodologic quality of case-control and cohort studies by two investigators (SK and RT) independently.15 In this scale, observational studies were scored across three categories using the following parameters: selection (four questions), comparability (two questions), and ascertainment of the outcome of interest (three questions). For each question, 1 point was given if the study met the criterion, except for comparability of study groups, in which 1 point was awarded if the study controlled for age, sex, or both, and 2 points if the study controlled other confounding factors (Table 1). Studies with a cumulative score of 7 or more were considered high quality, studies with score between 4 and 6 were considered moderate-quality and low-quality studies if score was less than 4. Any discrepancies were addressed by a joint re-evaluation of the original article.
Table 1.
Characteristics of included studies.
| Study name | Study period | Was prophylaxis for recurrent versus primary CDI | Pt population | Abstract versus manuscript | Case control versus cohort | Dosing | Mean duration of vancomycin | Most recent CDI before initiation of OVP | Definition of CDI | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|
| Bajrovic and Sims16 | 2010–2015 | Rec | All adult inpatients | A | Retro cohort | NA | NA | 6 months | NA | 6 months |
| Carignan et al.17 | 2003–2011 | Rec | All adult inpatients | M | Retro cohort | 125 mg QID | 7 days | 3 months | Standard definition | 90 days |
| Carignan et al.17 | 2003–2011 | Primary | All adult inpatients | M | Retro cohort | 125 mg QID | 7 days | 3 months | NA | 90 days |
| Gantesky et al.18 | 2015–2016 | Primary | Allogenic HSCT | M | Retro cohort | 125 mg BID | 29 days | NA | Standard definition | 30 days |
| O’Connell et al.19 | 2013–2016 | Rec | All adult inpatients | A | Retro cohort | NA | NA | NA | NA | 90 days |
| Bajrovic and Brizendine20 | 2007–2013 | Primary | Lung transplant recipients | A | Retro cohort | NA | NA | NA | NA | 1 year |
| Papic et al.11 | 2015–2017 | Primary | Pts > 65 inpatient | M | Retro cohort | NA | 9 days | NA | NA | 3 months |
| Pereiras et al.21 | 2013–2014 | Rec | HSCT pts | A | Retro cohort | NA | NA | NA | NA | 1 year |
| Splinter et al.22 | 2012–2015 | Rec | Renal transplant pts | M | Retro cohort | 125 mg BID | 19 days | NA | Standard definition | 30 days |
| Van Hise et al.23 | 2010–2014 | Rec | All adult inpatients | M | Retro cohort | 125 mg BID and 250 mg BID | 13.7 days | 3 years | Standard definition | 30 days |
| Wong and Riska24 | 2011–2014 | Rec | All adult inpatients | A | Retro cohort | NA | NA | 3 months | NA | 30 days |
| Knight et al.25 | 2013–2015 | Rec | All adult inpatients | M | Retro cohort | 250 mg and 125 mg QID |
8.5 days | 12 months | Standard definition | 12 months |
| Caroff et al.10 | 2009–2015 | Rec | All adult inpatients | M | Retro cohort | NA | 2.5 days | 5 months | Standard definition | 90 days |
| Morrisette et al.26 | 2014–2018 | Rec | HSCT and hematological malignancy pts | M | Retro cohort | 125 mg BID | NA | NA | Standard definition | 60 days |
| Johnson et al.27 | 2018–2019 | Primary | All adult inpatients | M | Randomized open label prospective | 125 mg daily | NA | NA | Standard definition | 3 months |
A, abstract; BID, two times daily; CDI, Clostridioides difficile infection; CI, confidence interval; HSCT, hematopoietic stem cell transplant; M, manuscript; NA, not available; OPV, oral vancomycin prophylaxis; Pt, patient; Rec, recurrent; QID, four times daily standard definition, diarrhea with + stool test for C. difficile toxin.
The GRADE framework was used to interpret the findings of the study. The principles of the GRADE system have been adopted by the Cochrane Collaboration for evaluating the quality of evidence for the outcomes reported in systematic reviews. For systematic reviews, the GRADE approach defines the quality of the body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Quality of a body of evidence involves consideration of the study design of included studies, methodological quality, directness of evidence, heterogeneity, inconsistency of results, and risk of publication bias.28 Given the studies included in our meta-analysis were only observational in nature, the certainty of evidence was low to start with, we considered the factors including large magnitude of effect, dose response gradient and effect of adjustment of confounding factors to access the quality of outcomes.
Data abstraction
Data were abstracted independently to a predetermined collection form by two investigators (SK and RT). Data were collected for each study, including study setting and design, year of publication, location, and primary outcome reported. Conflicts in data abstraction were resolved by consensus, referring to the original article.
Outcomes assessed
Our primary analysis focused on assessing the risk of primary CDI and the risk of future CDI in patients with history of CDI with or without OVP. Recurrent CDI is defined as an episode of CDI that happens within 8 weeks of stopping treatment for CDI. An episode that occurs beyond 8 weeks is considered as a reinfection.29 Studies evaluating OVP have variable follow up and do not differentiate recurrence from reinfection. Hence, we use the term future CDI. We also assessed the risk of vancomycin-resistant Enterococcus (VRE) infections with OVP if data were available in the studies.
Statistical analyses
We used the random effects model described by DerSimonian and Laird to calculate meta-analytic OR and 95% CI for each study.30 We assessed heterogeneity within groups with the I2 statistic, which estimates the proportion of total variation across studies that is due to heterogeneity in study patients, design, or interventions rather than chance. I2 values greater than 50% suggest significant heterogeneity.31 The presence of publication bias was assessed by visual inspection of funnel plots if >10 studies were present in the analysis.31 Publication bias was also assessed using Egger’s test of intercept to quantify asymmetry of funnel plot. All p values were two-tailed and for all tests (except heterogeneity), a probability level less than 0.05 was considered statistically significant. Calculations were performed and graphs constructed using RevMan (Review Manager, version 5.3; Cochrane Inc.). Egger’s test was performed using the dmetar package in R programming version 4.0.2.32
A priori defined sensitivity analyses included subgroup analyses based on full-text and high-quality studies. We also performed subgroup analyses of studies in abstract form only; of all hospitalized patients; solid organ transplant patients; studies that performed multivariate analysis; and based on duration of follow up.
Results
Search results
The described search strategy revealed 463 potentially relevant studies; titles and abstracts were screened and full papers were obtained for relevant articles (Figure 1). In all, 45 full-text articles were reviewed, of which 31 were excluded for various reasons (Figure 1). A total of 14 studies were included in this meta-analysis, of which 9 were full-text and 5 were in abstract form.
Quality of included studies
The median New Castle Ottawa scale was 6 (range 4–9); three studies were considered high-quality and the remaining 11 studies were of moderate quality (Table 1).
Characteristics of included studies
Of the 14 studies included,10,11,16–27 9 assessed secondary prophylaxis only, 4 assessed primary prophylaxis only, and 1 evaluated both primary and secondary prophylaxis; 8 studies included any adult hospitalized patient receiving antibiotics, 3 included hospitalized hematopoietic stem cell transplant recipients, 2 included hospitalized solid organ transplant recipients, and 1 included hospitalized patients >65 years of age. A total of 13 studies were retrospective observational studies and 1 was an open-label prospective trial. Study recruitment periods ranged from 2007–2019. All patients received systemic antibiotics. Eight studies mentioned the time of most recent CDI before the initiation of OVP, which ranged from 3 months to 3 years. The dose of vancomycin was variable and the duration of OVP ranged from 7 days to 29 days in various studies. Follow-up time to assess for CDI recurrence was variable and ranged from 30 days to 1 year (Table 1).
OVP for primary prophylaxis
Five observational studies comprising a total of 1352 patients evaluated OVP for hospitalized patients with no prior CDI episode who were on systemic antibiotics (primary prevention).11,17,18,20,27 The rate of CDI in patients on OVP was 7.2% (29/402) compared with 10.4% (99/950) without OVP. Meta-analysis revealed a non-significant trend towards decrease in risk of CDI in patients who received OVP to prevent primary CDI (OR 0.18; 95% CI, 0.03–1.09; p = 0.06). There was significant heterogeneity among the studies, with an I2 of 76% (Figure 2).
Figure 2.
Analysis of studies that evaluated oral vancomycin for primary CDI prophylaxis, showing no prevention benefit.
CDI, Clostridioides difficile infection; CI, confidence interval; OPV, oral vancomycin prophylaxis.
OVP for secondary prophylaxis
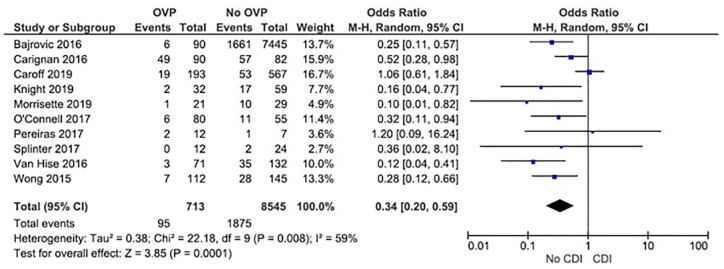
Ten observational studies comprising a total of 9258 CDI patients evaluated OVP for secondary prevention.10,16,17,19,21–26 Among these, the rate of future CDI in patients on OVP was 13.3% (95/713) compared with 21.9% (1875/8545) in patients that did not receive OVP. Meta-analysis using a random effects model revealed a statistically significant decreased risk of recurrent CDI (OR, 0.34; 95% CI, 0.20–0.59; p = 0.00001). There was significant heterogeneity among the studies, with an I2 value of 59% (Figure 3a). No publication bias was seen on visual inspection of a funnel plot (Supplemental Figure S1). Egger’s test for accessing publication bias was significant (intercept = −0.649, p = 0.02) suggesting possible publication bias.
Figure 3.
Analysis of studies that evaluated oral vancomycin for recurrent CDI prophylaxis, showing statistically significant decreased risk of CDI.
CDI, Clostridioides difficile infection; CI, confidence interval; OPV, oral vancomycin prophylaxis.
Studies that control for potential confounders for secondary prophylaxis
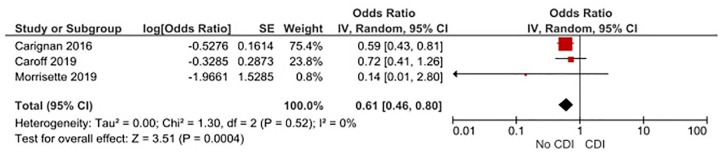
Of 10 studies, 3 included multivariable analysis after adjusting for potential confounders, including age and comorbid conditions.10,17,26 We calculated the pooled effect size of these studies by combining reported adjusted ORs and 95% CIs. Meta-analysis of these studies revealed statistically significant benefit of OVP for prevention of recurrent CDI (OR, 0.61; 95% CI, 0.46–0.80; p = 0.0004, I2 = 0%) (Figure 4)
Figure 4.
Three studies that controlled for potential confounders plot demonstrates decreased risk of recurrent CDI with OVP by the random-effects mode.
CDI, Clostridioides difficile infection; CI, confidence interval; OPV, oral vancomycin prophylaxis; SE, standard error.
Subgroup analyses of OVP for secondary prophylaxis
All hospitalized patients
A total of seven studies, including all hospitalized patients, evaluated the efficacy of OVP for secondary prophylaxis.10,16,17,19,21,23–25 Among these, the rate of future CDI in patients on OVP was 13.7% (92/668) compared with 21.9% (1862/8485) in patients that did not receive OVP. Meta-analysis revealed a statistically significant decreased risk of future CDI with OVP (OR, 0.35; 95% CI, 0.19–0.63; p = 0.0005, I2 = 69%) (Supplemental Figure S2a)
Transplant patients
A total of three studies included hospitalized solid organ transplant patients only.21,22,26 Among those, the rate of future CDI in patients on OVP was 6.7% (3/45) compared with 21.6% (13/60) in patients that did not receive OVP. Meta-analysis revealed no statistically significant decreased risk of future CDI with OVP (OR, 0.29; 95% CI, 0.06–1.38; p = 0.12, I2 = 11%) (Supplemental Figure S2b)
Follow up duration
A total of seven studies had follow up of more than 60 days.10,16,17,19,21,25,26 Among those studies, the rate of future CDI in patients on OVP was 16.4% (85/518 compared with 21.9% (1810/8244). Meta-analysis revealed a significant decreased risk of future CDI with OVP (OR, 0.41; 95% CI, 0.22–0.77; p = 0.0005, I2 = 61%) (Supplemental Figure S3a).
High quality studies
A total of three high quality studies evaluated efficacy of OVP for secondary prophylaxis.10,17,26 Among these, the rate of future CDI in patients on OVP was 22.6% (69/304) compared with 17.6% (120/678) in patients that did not receive OVP. Meta-analysis revealed no decreased risk of future CDI with OVP (OR, 0.58; 95% CI, 0.24–1.38; p = 0.22, I2 = 68%) (Supplemental Figure S3b).
Full-text studies
A total of six full-text studies evaluated the efficacy of OVP for secondary prophylaxis.10,17,22,23,25,26 Among these, the rate of future CDI in patients on OVP was 17.6% (74/419) compared with 19.4% (174/893) in patients that did not receive OVP. Meta-analysis revealed a significant decreased risk of future CDI with OVP (OR, 0.33; 95% CI, 0.14–0.78; p = 0.01, I2 = 70%) (Supplemental Figure S4a)
Abstracts only
A total of four studies in abstract form evaluated efficacy of OVP for secondary prophylaxis.16,19,21,24 Among these, the rate of future CDI in patients on OVP was 7.1% (21/294) compared with 22.2% (1701/7652) in patients that did not receive OVP. Meta-analysis revealed a significant decreased risk of recurrence with OVP (OR, 0.29; 95% CI, 0.18–0.49; p < 0.0001, I2 = 0%) (Supplemental Figure S4b).
Risk of VRE infections
Three studies assessed the risk of VRE infections after OVP.18,25,26 Among these, two included transplant patients only and one included all hospitalized patients. One study included only blood stream VRE infections, while two included all VRE infections. The rate of VRE infections was not significantly different in patients on OVP 4% (6/143) compared with 4.7% (7/143) among patients not on OVP (OR, 1.11; 95% CI, 0.35–3.49; p = 0.86, I2 = 0%) (Supplemental Figure S5).
Quality of outcomes
Per the GRADE framework, the quality of evidence for the outcomes was low because of study design (observational studies only), lack of consistency of methodology, and significant heterogeneity in all effect estimates (Supplemental Table S2).
Discussion
In our meta-analysis, we found that OVP was associated with a decreased risk of future CDI in patients with a prior history of CDI who required systemic antimicrobial therapy. However, there was no benefit of oral vancomycin as primary prophylaxis.
Oral vancomycin is well tolerated, with minimal systemic absorption from the gastrointestinal mucosa to result in toxicity or other systemic antimicrobial effects. It can be postulated that oral vancomycin might inhibit the growth of the vegetative form (but not spores) of C. difficile in patients with history of CDI who are on antibiotics, and, thus, prevent recurrence. At the same time, it should be considered that vancomycin leads to gut microbial dysbiosis. One study demonstrated that, in patients with CDI, the stool concentration of vancomycin remained adequately high for 3–5 days after completion of therapy to inhibit the in vitro growth of C. difficile.33 Therefore, it could be proposed that, in high-risk patients, vancomycin could be continued for 1–2 weeks after completion of the systemic antimicrobial therapy to continue inhibiting C. difficile growth. However, the risk of CDI continues to be high even 90 days after antibiotics, while continuing the use of vancomycin prophylaxis this long is practically difficult.
The principal concern while considering OVP would be that oral vancomycin will have a damaging effect on the microbiota of the colon and on whether OVP increases the risk of recurrent CDI following completion of therapy due to disruption of gut microbiota. A clinical trial comparing the efficacy of vancomycin, metronidazole, and placebo for eradication of asymptomatic C. difficile fecal excretion showed that vancomycin was temporarily effective, but, after 2 months, vancomycin was associated with a significantly higher rate of subsequent recolonization with new CDI strains compared with those on placebo or metronidazole.34 One study found that patients with CDI treated with oval vancomycin 125 mg four times per day had fecal concentrations of the drug 500–1000 times greater than the minimum inhibitory concentrations against C. difficile, suggesting lower doses of once or twice daily could be sufficient as a prophylactic dose.35
An additional consideration would be whether oral vancomycin could potentially increase the rate of VRE, carbapenem-resistant Klebsiella pneumoniae and Escherichia coli, although prospective data are not available.36 Three of the included studies accessed the risk of VRE infection after OVP compared with no OVP; these three studies showed no difference in VRE infection risk among the two groups. However, the sample size was very small and with a short duration of follow up. None of the studies included in our meta-analysis had included microbiome analyses.
Our meta-analysis results remained consistent on subgroup analysis of full-text studies and studies that included multivariate analysis after controlling for potential confounders. Additionally, OVP was associated with decreased risk of CDI among studies that included all hospital patients. However, on subgroup analysis of only high quality studies, no preventive effect of OVP to prevent future CDI was seen, although the number of studies was low (n = 3). Additionally, subgroup analysis of OVP prophylaxis for future CDI in transplant patients also showed no difference in risk of CDI with use of OVP, limiting the ability of the results of our meta-analysis to be applied to high-risk transplant patients; however, the number of studies was low (n = 3).
In studies evaluating secondary prophylaxis, the interval duration between the prior CDI episode and initiation of OVP for future antibiotic exposure was extremely variable among studies, ranging from 3 months to 3 years. Most guidelines define recurrent CDI as a future CDI episode within 8 weeks. Future CDI episodes beyond 8 weeks are considered reinfection. None of the studies in our meta-analysis considered the recurrent CDI episode up to 8 weeks. It remains unclear if there is any effect on OVP for secondary prophylaxis within 8 weeks only. Additionally, none of the studies in our meta-analysis included the number of prior CDI episodes before the initiation of OVP, given the risk of recurrent increases with the number of prior episodes.37 Future studies evaluating the effect of OVP on recurrent CDI within 8 weeks should be considered.
Strengths of our study include the comprehensive literature search, strict inclusion criteria and multiple subgroup analyses. There are several limitations of our findings. These include the retrospective nature of the included studies, heterogeneous patient population, inconsistent follow-up period and lack of microbiome data among the included studies. Additionally, the dosing of OVP was variable or unavailable in the included studies, only one study included the duration of OVP after discontinuation of antibiotics; hence, the relation of dosing and duration for prophylaxis could not be evaluated. Lastly, there was significant heterogeneity in our results, which was likely expected due to heterogeneous population of patients, different methodology of included studies, none of the studies controlled for any potential confounders.
In conclusion, based on observational data with low quality, OVP appears to decrease the risk of future CDI in patients with history of CDI who require systemic antimicrobial therapy. However, OVP for primary prevention was not associated with a statistically significant decreased risk. It may be reasonable to consider the use of OVP for secondary prevention in patients with history of multiple CDI episodes. However, the use for primary prevention might lead to increased vancomycin resistance with no prevention benefit. A prospective well-designed randomized controlled trial is needed to better define the optimal dosing, cost effectiveness, and risks and benefits of OVP in this vulnerable population.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-4-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sahil Khanna  https://orcid.org/0000-0002-7619-8338
https://orcid.org/0000-0002-7619-8338
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Raseen Tariq, Divison of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA; Department of Internal Medicine, Rochester General Hospital, Rochester, NY, USA.
Maryrose Laguio-Vila, Department of Infectious Diseases, Rochester General Hospital, NY, USA.
Muhammad Waqas Tahir, Department of Internal Medicine, Rochester General Hospital, Rochester, NY, USA.
Robert Orenstein, Division of Infectious Diseases, Mayo Clinic, AZ, USA.
Darrell S. Pardi, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
Sahil Khanna, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA.
References
- 1. Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. Difficile Surveillance Team. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 2369–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(Suppl. 2): S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med 2008; 359: 1932–1940. [DOI] [PubMed] [Google Scholar]
- 4. Ma GK, Brensinger CM, Wu Q, et al. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167: 152–158. [DOI] [PubMed] [Google Scholar]
- 5. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376: 305–317. [DOI] [PubMed] [Google Scholar]
- 6. Cornely OA, Vehreschild M, Adomakoh N, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection: EXTEND study subgroup analyses. Eur J Clin Microbiol Infect Dis. Epub ahead of print 25 March 2019. DOI: 10.1007/s10096-019-03525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madoff SE, Urquiaga M, Alonso CD, et al. Prevention of recurrent Clostridioides difficile infection: a systematic review of randomized controlled trials. Anaerobe 2020; 61: 102098. [DOI] [PubMed] [Google Scholar]
- 8. Sinclair A, Xie X, Saab L, et al. Lactobacillus probiotics in the prevention of diarrhea associated with Clostridium difficile: a systematic review and Bayesian hierarchical meta-analysis. CMAJ Open 2016; 4: E706–E718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 878–888. [DOI] [PubMed] [Google Scholar]
- 10. Caroff DA, Menchaca JT, Zhang Z, et al. Oral vancomycin prophylaxis during systemic antibiotic exposure to prevent Clostridiodes difficile infection relapses. Infect Control Hosp Epidemiol 2019; 40: 662–667. [DOI] [PubMed] [Google Scholar]
- 11. Papic N, Maric LS, Vince A. Efficacy of oral vancomycin in primary prevention of Clostridium difficile infection in elderly patients treated with systemic antibiotic therapy. Infect Dis (Lond) 2018; 50: 483–486. [DOI] [PubMed] [Google Scholar]
- 12. Babar S, El Kurdi B, El Iskandarani M, et al. Oral vancomycin prophylaxis for the prevention of Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. Epub ahead of print 29 June 2020. DOI: 10.1017/ice.2020.277. [DOI] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 5 May 2012).
- 16. Bajrovic V, Sims M. Vancomycin as prophylaxis of relapsing Clostridium difficile infection. Open Forum Infect Dis 2016; 3(Suppl. 1). [Google Scholar]
- 17. Carignan A, Poulin S, Martin P, et al. Efficacy of secondary prophylaxis with vancomycin for preventing recurrent Clostridium difficile infections. Am J Gastroenterol 2016; 111: 1834–1840. [DOI] [PubMed] [Google Scholar]
- 18. Ganetsky A, Han JH, Hughes ME, et al. Oral vancomycin prophylaxis is highly effective in preventing Clostridium difficile infection in allogeneic hematopoietic cell transplant recipients. Clin Infect Dis 2019; 68: 2003–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Connell M, Slish J, Shelly M. Efficacy of oral vancomycin, oral metronidazole, or IV metronidazole prophylaxis at reducing the risk of Clostridium difficile recurrence. Open Forum Infect Dis 2017; 4(Suppl. 1): S384. [Google Scholar]
- 20. Bajrovic V, Brizendine K. Primary oral vancomycin prophylaxis reduces the incidence of Clostridium difficile infection among lung transplant recipients. Am J Transplant 2017; 17(Suppl. 3): abstract c258. [DOI] [PubMed] [Google Scholar]
- 21. Pereiras MA, Urnoski E, Wynd M, et al. Does oral vancomycin prophylaxis for Clostridium difficile infection improve allogeneic hematopoietic stem cell transplant outcomes? Biol Blood Marrow Transplant 2017; 23: S395. [Google Scholar]
- 22. Splinter LE, Kerstenetzky L, Jorgenson MR, et al. Vancomycin prophylaxis for prevention of Clostridium difficile infection recurrence in renal transplant patients. Ann Pharmacother 2018; 52: 113–119. [DOI] [PubMed] [Google Scholar]
- 23. Van Hise NW, Bryant AM, Hennessey EK, et al. Efficacy of oral vancomycin in preventing recurrent Clostridium difficile infection in patients treated with systemic antimicrobial agents. Clin Infect Dis 2016; 63: 651–653. [DOI] [PubMed] [Google Scholar]
- 24. Wong D, Riska P. Secondary prophylaxis in high risk patients reduces the risk of Clostridium difficile recurrence. In: Interscience conference of antimicrobial agents and chemotherapy, San Diego, California, 17–21 September 2015. [Google Scholar]
- 25. Knight EM, Schiller DS, Fulman MK, et al. Long-term efficacy of oral vancomycin prophylaxis for the prevention of Clostridium difficile recurrence. J Pharm Pract. Epub ahead of print 11 February 2019. DOI: 10.1177/0897190019825994. [DOI] [PubMed] [Google Scholar]
- 26. Morrisette T, Van Matre AG, Miller MA, et al. Oral vancomycin prophylaxis as secondary prevention against Clostridioides difficile infection in the hematopoietic stem cell transplantation and hematologic malignancy population. Biol Blood Marrow Transplant 2019; 25: 2091–2097. [DOI] [PubMed] [Google Scholar]
- 27. Johnson SW, Brown SV, Priest DH. Effectiveness of oral vancomycin for prevention of healthcare facility-onset Clostridioides difficile infection in targeted patients during systemic antibiotic exposure. Clin Infect Dis 2020; 71: 1133–1139. [DOI] [PubMed] [Google Scholar]
- 28. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 31. Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet 1991; 337: 867–872. [DOI] [PubMed] [Google Scholar]
- 32. Harrer M, Cuijpers P, Furukawa T, et al. dmetar: companion R package for the guide ‘doing meta-analysis in R’. R package version 0.0.9000, http://dmetar.protectlab.org/. (2019, accessed 10 January 2021).
- 33. Abujamel T, Cadnum JL, Jury LA, et al. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One 2013; 8: e76269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson S, Homann SR, Bettin KM, et al. Treatment of asymptomatic Clostridium-difficile carriers (fecal excretors) with vancomycin or metronidazole - a randomized, placebo-controlled trial. Ann Intern Med 1992; 117: 297–302. [DOI] [PubMed] [Google Scholar]
- 35. Gonzales M, Pepin J, Frost EH, et al. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis 2010; 10: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Nassir WN, Sethi AK, Li Y, et al. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother 2008; 52: 2403–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McFarland LV, Surawicz CM, Rubin M, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999; 20: 43–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-4-tag-10.1177_1756284821994046 for Efficacy of oral vancomycin prophylaxis for prevention of Clostridioides difficile infection: a systematic review and meta-analysis by Raseen Tariq, Maryrose Laguio-Vila, Muhammad Waqas Tahir, Robert Orenstein, Darrell S. Pardi and Sahil Khanna in Therapeutic Advances in Gastroenterology