Abstract
Magnetic CoFe2O4–gC3N4 nanocomposites were successfully synthesized, and their photocatalytic activities toward the decomposition of model synthetic dyes (e.g., methylene blue, methyl orange, and Congo red) in the presence of H2O2 were evaluated under simulated solar light irradiation. The 50CoFe2O4–50gC3N4 nanocomposite exhibited the highest catalytic activity. The catalytic activity of 50CoFe2O4–50gC3N4 toward the photodegradation of some industrially used dyes (such as Drimaren Turquoise CL-B p, Drimaren Yellow CL-2R p, and Drimaren Red CL-5B p) was also examined, and the catalyst exhibited its capability to decompose the industrial dyes completely. An aqueous mixture of these dyes was prepared to mimic the dye-containing wastewater, which was fully photodegraded within 30 min. 50CoFe2O4–50gC3N4 also exhibited facile magnetic separability from the reaction mixture after the accomplishment of photocatalysis reaction and stable performance after five cycles. The high photocatalytic efficiency to degrade several dyes, including dyes used in textile industries, under solar light irradiation makes 50CoFe2O4–50gC3N4 a promising photocatalyst for the treatment of dye-containing wastewater discharged from industries.
1. Introduction
A variety of dyes are quite often used by several industries (e.g., textile, paper, plastic, rubber, printing, cosmetics, leather, pharmaceuticals, food processing, etc.) to color their products.1−4 These industries are causing environmental pollution by discharging dye-containing effluents into the soil and aquatic systems and posing a great threat to the environment. The strong color of the dyes and pigments poses serious esthetic and ecological problems to the acquiring aquatic ecosystem, such as inhibition of benthic photosynthesis.5 Moreover, some of these dyes are toxic and carcinogenic in nature.6 To address this issue, scientists and technologists have developed a variety of physical, chemical, and biological techniques to treat the dye-containing effluents.7−10 Adsorption method, coagulation–flocculation technique, membrane filtration, ion-exchange technique, and so forth are some of the examples of physical processes. Though physical methods are quite often used for wastewater treatment, they are also associated with some limitations. For example, the adsorption process is a slow process and is not very effective for highly colored wastewater. In the case of the membrane separation process, the slow separation rate, the special requirement of filtration, ultrahigh vacuum conditions, and frequent clogging of membrane pores by organic pollutants make this process limited for use in the dye effluent treatment. The generation of a huge amount of sludge is the main disadvantage of coagulation–flocculation-based methods. Another drawback of the physical treatment techniques is that most of the time complete degradation of dyes is not possible. In these processes, although the separation of a pollutant (such as dyes) from water occurs to a large extent, the pollutant is not destroyed/decomposed. As the recovery of dyes from the adsorbents or sludge is not at all cost-effective, the dye molecules are again discharged in the environment in a more concentrated form.7,11 Hence, biological and chemical treatments of dye-containing wastewater become attractive alternatives. Aerobic treatment, anaerobic treatment, and combined anaerobic–aerobic treatment are the three major types of biological treatment techniques. In these methods, microorganisms play critical roles.7 The major limitation of the biodegradation processes is that they are not efficient for the dyes having complex aromatic structures. The large-scale application of pure cultures (algal, fungal, and bacterial) is practically limited for wastewater containing various types of dyes because of the dye-specific nature of most of the isolated cultures.5,12
For the last few years, oxidation process, particularly advanced oxidation process (AOP), to treat wastewater has gained immense attention from scientists.9,13,14 AOP involves the formation of highly reactive free radicals that oxidize and destroy organic contaminants in a short period. The use of Fenton reagent (H2O2–Fe(II)) is a widely used method to treat wastewater that contains dyes exhibiting resistance toward biological treatment or are poisonous to live biomass.15,16 However, the generation of excessive sludge and short life of some of the oxidizing agents (e.g., O3), which generate radicals, are the disadvantages of this process.13
Recently, the UV light-assisted oxidation process has become an attractive approach. Here, UV radiation activates chemicals (e.g., H2O2) and produces free radicals. These free radicals degrade the dye molecules to CO2 and H2O. Several wide band gap semiconductors (such as TiO2, ZnO, etc.) have been examined for the photocatalytic degradation of dyes.17,18 In this aspect, TiO2 is one of the most widely explored semiconductors by several researchers.17,19−22 However, the photocatalytic activity of TiO2 is limited to UV or near-UV radiation (≤380 nm) because of its wide band gap (3.2 eV).23−26 The large-scale application of usage of UV radiation is limited because it makes the process costly and non-ecofriendly. Therefore, the current research is concentrated on the development of such photocatalysts for wastewater treatment, which can efficiently degrade dyes under solar light irradiation.27−31 Tuning the band gap of the photocatalyst, so that it can be activated under visible or solar light irradiation, is a viable solution. To achieve such photocatalysts, synthesizing nanocomposites by combining two semiconducting nanoparticles having matching band gaps is an interesting strategy adopted by several researchers.32−39 Our previous study showed that reduced graphene oxide (rGO)-CoFe2O4 nanocomposites can exhibit photocatalytic activity toward the degradation of some dyes under visible light irradiation. However, the synthesis of rGO via preparation of graphene oxide by the Hummers method, followed by its reduction to rGO, is a cumbersome, environment-unfavorable, and time-consuming method. Therefore, we have aimed to develop a photocatalyst that is capable of photodegrading different types of dyes (model dyes as well as industrially used dyes) under solar light and at the same time can be synthesized by a simple but environment-friendly method as well as can be separated from the reaction mixture easily.
In the present study, we have designed a photocatalyst composed of graphitic carbon nitride (gC3N4) and CoFe2O4 (CF) nanoparticles to degrade several dyes under visible light irradiation. Here, we have chosen gC3N4 as one of the components of the nanocomposite because the advantages it offers are low cost, easy preparation protocol, nontoxicity, ability to adsorb organic molecules via π–π interaction, and so forth. However, gC3N4 suffers from a high recombination rate of the photogenerated electron–hole,37,40 low conductivity, low specific surface area,41 insignificant absorption above 460 nm in solar spectra, and so forth.42 These factors limit the wide application of gC3N4 as the photocatalyst. Designing a nanocomposite composed of gC3N4 with another semiconductor having a matching band gap is an attractive solution to tune the band gap and to address the limitation associated with gC3N4. Examples of such nanocomposites are ZnO/gC3N4,43 TiO2/gC3N4,44 SnO2/gC3N4,45 WO3/gC3N4,46 and so forth.
We have chosen CoFe2O4, which is a low band gap semiconductor (band gap ∼ 1.08 eV),47,48 as another component of the photocatalyst. Though CoFe2O4 does not exhibit an appreciable photocatalytic activity toward dye degradation,49 it can suppress the electron–hole recombination of gC3N4 by forming a heterojunction when coupled with gC3N4. By varying the composition of gC3N4 and CF, the band gap energy of the nanocomposite can be tuned in such a way that it can act as a photocatalyst in the visible light region. Moreover, as CF is magnetic in nature (saturation magnetization = 75 emu/g),50 its presence in the nanocomposite makes the photocatalyst a magnetically separable catalyst. This feature of this catalyst solves the separation-related problem of the general nanosized catalysts.
A very few reports are available on the synthesis of the gC3N4–CoFe2O4 composite and the study of its photocatalytic activity toward the degradation of dyes. Recently, Hassani et al. have reported the synthesis of mesoporous gC3N4–CoFe2O4 composite and its ability to photodegrade Malachite green dye under UV radiation.41 This catalyst exhibited the maximum degradation efficiency of 93.41% in a reaction time of 120 min. They have also studied the photodegradation of methylene blue, acid orange 7, and rhodamine B. They have also reported the sonocatalytic activity of mesoporous gC3N4–CoFe2O4 toward the removal of methylene blue.51 Huang et al. have synthesized the CoFe2O4–gC3N4 nanocomposite and showed that this photocatalyst can degrade 97.3% of methylene blue dye under visible light irradiation within 3 h.49 Inbaraj et al. have synthesized the CoFe2O4–gC3N4 nanocomposite by employing the sol–gel autocombustion method and hydrothermal method and reported that the synthesized nanocomposite exhibited 98% of photodecomposition of methylene blue under solar light irradiation within 150 min.52 Yao et al. have reported the synthesis of the CoFe2O4–gC3N4 catalyst and demonstrated its photo-Fenton-like photocatalysis for organic dyes.53 To the best of our knowledge, till date, the application of the CF–gC3N4 nanocomposite as a photocatalyst to degrade various types of dyes, particularly industrially used dyes, has not yet been well explored.
We have synthesized nanocomposites composed of varying amounts of CoFe2O4 and gC3N4. The synthesized materials (i.e., pure CF, pure gC3N4, and CF–gC3N4 nanocomposites) were characterized by using X-ray diffraction (XRD), thermogravimetric analysis (TGA), FT-IR spectroscopy, Raman spectroscopy, UV–vis diffuse reflectance spectroscopy (UV-DRS), field emission scanning electron spectroscopy (FESEM), and energy-dispersive X-ray spectrometry (EDXS). We have determined the change of band gap energy of the synthesized nanocomposites with the variation of their compositions. To evaluate the photocatalytic activity of the synthesized nanocomposites, we have performed the photodegradation reaction of different dye solutions in the presence of H2O2 under simulated solar light irradiation with a light intensity of 10000 lux (measured by using a light meter (LX-101A). Initially, the photocatalytic degradation of different model dyes [such as methylene blue (MB), methyl orange (MO), Congo red (CR), and a mixture of these dyes] was investigated, and the optimum composition of the nanocomposite which can exhibit the highest photocatalytic efficiency was determined. Then, the photocatalytic activity of this catalyst was also tested for the industrially used dyes [such as Drimaren Turquoise CL-B p (Turq CL-B), Drimaren Yellow CL-2R p (Yell CL-2R), and Drimaren Red CL-5B p (Red CL-5B)], which are widely used in textile industries. To mimic the dye-containing wastewater, we have prepared an aqueous mixture of these industrial dyes and performed the photocatalysis reaction. The synthesized catalyst exhibited excellent photocatalytic activity toward the degradation of model dyes as well as industrial dyes. After the first cycle of catalysis reaction, the catalyst was magnetically recovered, and the efficiency of the recovered catalyst was tested for a couple of cycles.
2. Results and Discussion
2.1. Structure and Morphology of the CF–gC3N4 Nanocomposites
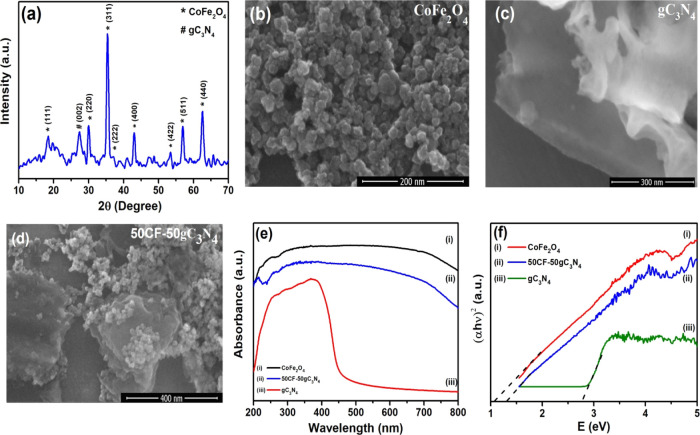
The XRD patterns of the synthesized materials (e.g., pure gC3N4, CF, and CF–gC3N4 nanocomposites) are presented in Figures S1–S3. In the XRD pattern of the pure CF sample, the diffraction peaks at 2θ = 18.2, 30.1, 35.5, 37.1, 43.1, 53.4, 56.9, and 62.6°, corresponding to the (111), (220), (311), (222), (400), (422), (511), and (440) diffraction planes [JCPDS card no 22-1086], are observed.48,54 The XRD pattern of pure gC3N4 displays two peaks at 2θ = 13.1 and 27.3°, which can be indexed as (100) and (002) planes, respectively. These peaks indicate the in-plane structural packing motifs of tri-s-triazine units and the interlayer stacking of the conjugated aromatic system of gC3N4.55,56 In the XRD pattern of CF–gC3N4 nanocomposites, all the diffraction peaks of CF and gC3N4 are observed, but in some cases (where the gC3N4 amount is less) the diffraction peaks characteristic of gC3N4 are not present. This could be because of the too thin (nanometer scale) nature of gC3N4 layers.33,57−59 As a representative, the XRD pattern of 50CoFe2O4–50gC3N4 is shown in Figure 1a, which displays both the characteristic peaks of CF and gC3N4.
Figure 1.
(a) XRD patterns of the 50CF–50gC3N4 nanocomposite, (b–d) FESEM micrographs, (e) UV–vis absorption spectra, (f) (αhν)2 versus photon energy plots of pure CoFe2O4, pure gC3N4, and 50CF–50gC3N4 nanocomposite.
We have investigated the microstructures of the synthesized pure gC3N4, pure CF, and CF–gC3N4 nanocomposites by FESEM. Figure 1b shows the agglomeration of almost spherical CF nanoparticles (with an average particle size of ∼20–25 nm). Figure 1c displays the nanometer-thin sheets of gC3N4. Figure 1d displays the FESEM image of the 50CF–50gC3N4 nanocomposite, depicting the dispersion of CF nanoparticles on the surface of the gC3N4 sheet. The EDS spectra (Figure S4) shows the presence of peaks for C, N, Co, Fe, and O in this nanocomposite.
Figure S5 presents the FT-IR spectra of pure gC3N4, CF, and CF–gC3N4 nanocomposites. In the FT-IR spectrum of gC3N4, the prominent peaks are present at (i) 1636 cm–1 for C=N stretching vibration, (ii) 1563 and 1411 cm–1 characteristic to the s-triazine ring vibrations, (iii) 1326 and 1246 cm–1 for C–N stretching, and (iv) 809 cm–1 due to the s-triazine ring vibration.60−62 The FT-IR spectrum of CF shows a peak at 591 cm–1, which corresponds to the lattice absorption of M–O (where M = Fe3+, Co2+).63 In the FT-IR spectra of CF–gC3N4 nanocomposites, all the characteristic peaks of gC3N4 and CF are observed.
Figure S6 shows the Raman spectra of pure CF, pure gC3N4, and 50CF–50gC3N4. In the Raman spectra of pure gC3N4, the Raman peaks are present at 461, 570, 688, 745, 962, 1253, 1406, 1464, 1559, and 1616 cm–1, which can be attributed to the layer–layer deformation vibrations or the correlation vibrations, out-of-plane bending mode of the graphitic domain, breathing mode of the s-triazine ring, lattice vibration of gC3N4, D band, G band, and vibration modes of CN heterocycles, respectively.64−68 The Raman spectra of pure CF displays the T2g and A1g modes at 460 and 672 cm–1, corresponding to the vibrational modes of octahedral iron and tetrahedral cobalt, respectively, present in CF.69 The presence of Raman bands corresponding to both CF and gC3N4 in the Raman spectra of the synthesized nanocomposite also confirms their presence in the 50CF–50gC3N4 nanocomposite.
XRD, FESEM, IR spectroscopy, and Raman spectral analysis clearly indicate the formation of pure CF nanoparticles on the surface of gC3N4 sheets. The chemical reactions involved in the synthesis of CoFe2O4 can be presented by the following equations48
| 1 |
| 2 |
| 3 |
To estimate the amount of gC3N4 present in the CF–gC3N4 nanocomposites, we have performed TGA of pure CF, pure gC3N4, and CF–gC3N4 nanocomposites in the temperature range of 30–800 °C (Figure S7). Pure CF shows its thermal stability in this temperature range. For pure gC3N4, the decomposition starts at ∼400 °C, and 100% decomposition completes at 700 °C. TGA of CF–gC3N4 nanocomposites was performed to calculate the amount of undecomposed sample after 700 °C. This remaining amount of solid indicates the amount of CF present in the composite. TGA thermograms of the nanocomposites show that the weight % of CF and gC3N4 matches well with the composition, which is expected as per the synthesis of the samples. For example, in the case of the 50CF–50gC3N4 composite, ∼50% solid (i.e., CF) remains undecomposed above 600 °C, indicating the presence of 50 wt % CF and 50 wt % gC3N4 in this nanocomposite.
2.2. Optical Properties of the CF–gC3N4 Nanocomposites
We have studied the optical absorption properties of the as-synthesized materials by using UV–vis diffuse reflectance spectroscopy. Figure 1e represents the UV–vis DRS spectra of pure CF, pure gC3N4, and 50CF–50gC3N4 nanocomposite. Pure gC3N4 shows an absorption edge at ∼460 nm, and pure CF displays a broad absorption range from 200 to 800 nm. In the case of CF–gC3N4 nanocomposites, the red shift of the absorption edge position was observed compared to pristine gC3N4, and the absorption of visible light enhances to a great extent with the increasing CF weight % in the nanocomposite (Figure S8). This observation is similar to the results reported by several researchers.35,36,42,49,53,70,71 This property of the nanocomposites can be exploited for their application as photocatalysts, which can be activated upon visible light irradiation. The band gap energy (Eg) of the synthesized materials was calculated from the (αhν)2 versus photon energy plot (Figure 1f) by using the Kubelka–Munk equation72
| 4 |
where h, ν, and Eg are Planck’s constant, frequency of light, and band gap, respectively. The band gap values of the synthesized pristine gC3N4 and pure CF nanoparticles are 2.76 and 1.04 eV, respectively. For the nanocomposites, the band gap value is increased with the increasing gC3N4 content, and the band gap values increase from 1.11 to 1.31 eV when the weight % of gC3N4 is increased from 5 to 50 wt %. This is mainly owing to the synergistic effect arising from the formation of heterojunctions between the CF nanoparticles and gC3N4 in the nanocomposite, which causes an easy electron transfer between these two components. This observation is in agreement with the other substantive findings in the literature.36,42,49,70,71
2.3. Photocatalytic Activity of CF–gC3N4 Nanocomposites
To evaluate the photocatalytic activity of the synthesized nanocomposites, we have performed the photodegradation reaction of different dye solutions under visible light irradiation (emitted from the solar light simulator) in the presence of H2O2. Initially, we used several model synthetic dyes (e.g., MB, MO, and CR) to determine the optimum composition of the nanocomposites which can exhibit the highest catalytic activity. Then, we conducted the photocatalytic reaction toward the degradation of some of the industrially used dyes (e.g., Turq CL-B, Yell CL-2R, and Red CL-5B) to demonstrate the potential use of the synthesized nanocomposite as an effective photocatalyst to treat the dye-containing wastewater discharged from industries.
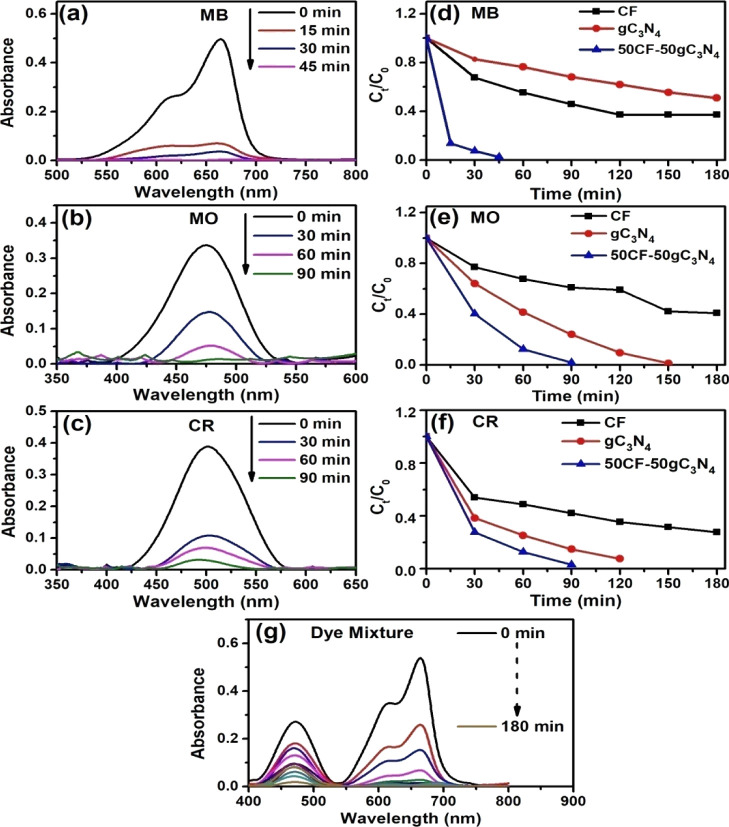
Of all the dyes, pure CF showed a poor photocatalytic activity. The highest efficiency of the photocatalyst is observed when the catalyst contained 50 wt % CF and 50 wt % gC3N4 (50CF–50gC3N4). For MB, after 3 h of exposure to light, pure CF- and pure gC3N4-catalyzed reactions showed ∼49 and ∼63% of dye degradation, respectively. With the increasing weight % of gC3N4 in the nanocomposite, the performance of the nanocomposite as photocatalyst has increased. For example, in the case of 95CF–5gC3N4, 83% photodegradation of MB has occurred after 3 h of reaction, whereas 50CF–50gC3N4 showed ∼100% MB degradation in 45 min. A similar trend has been observed for the photocatalysis reaction of other dyes. The photocatalyst that composed of 50 wt % CF and 50 wt % gC3N4 (50CF–50gC3N4) displayed the highest efficiency toward the degradation of dyes. The change of photocatalytic efficiency (i.e., % of dye degradation at different reaction times) with varying compositions of the photocatalyst is tabulated in Table 1 and depicted in Figures 2 and S9–S11. Figure 2a–c shows the UV–vis spectra displaying the decrease of the intensity of λmax with increasing reaction times for MB, MO, and CR, respectively, for the 50CF–50gC3N4-catalyzed photodegradation reaction. Figure 2d–f displays the variation of Ct/C0 ratio for MB, MO, and CR with the progress of reaction time when photocatalytic reactions are performed in the presence of a catalyst (pure CF, gC3N4, and 50CF–50gC3N4). Upon photocatalysis, the total organic carbon (TOC) removal ratio for MB, MO, and CR solutions is 69, 36, and 78.11%, respectively. We have also performed the 50CF–50gC3N4-catalyzed photocatalysis reaction with an aqueous solution containing a mixture of MB, MO, and CR dyes (60 ppm). The complete photocatalytic decomposition of this dye mixture occurred in 3 h (Figure 2g). We have observed that the photocatalytic activity of 50CF–50gC3N4 is better than that of the rGO-CoFe2O4 nanocomposites, which we have investigated in our previous study.48
Table 1. Photocatalytic Activity of CF–gC3N4 Nanocomposites toward the Decomposition of Different Dyes.
| % of degradation
(time) |
|||
|---|---|---|---|
| catalyst | MB | MO | CR |
| pure CF | 49% (3 h) | 59% (3 h) | 69% (2.5 h) |
| pure gC3N4 | 63% (3 h) | 100% (2.5 h) | 93% (2 h) |
| 95CF–5gC3N4 | 83% (3 h) | 76% (3 h) | 75% (2.5 h) |
| 90CF–10gC3N4 | 87% (3 h) | 81% (3 h) | 86% (2.5 h) |
| 85CF–15gC3N4 | 98% (3 h) | 87.7% (3 h) | 89% (2.5 h) |
| 75CF–25gC3N4 | ∼100% (1.5 h) | 93% (2 h) | 95% (2 h) |
| 50CF–50gC3N4 | ∼100% (45 min) | ∼100% (1.5 h) | ∼100% (1.5 h) |
Figure 2.
(a–c) Time-dependent UV–vis spectral changes of the 50CF–50gC3N4-catalyzed photodecomposition reaction of different dyes (MB, MO, and CR). (d–f) Photodegradation rates of different dyes catalyzed by gC3N4, CF, and 50CF–50gC3N4. (g) Time-dependent UV–vis spectral changes of the 50CF–50gC3N4-catalyzed photodecomposition reaction of the model dye mixture.
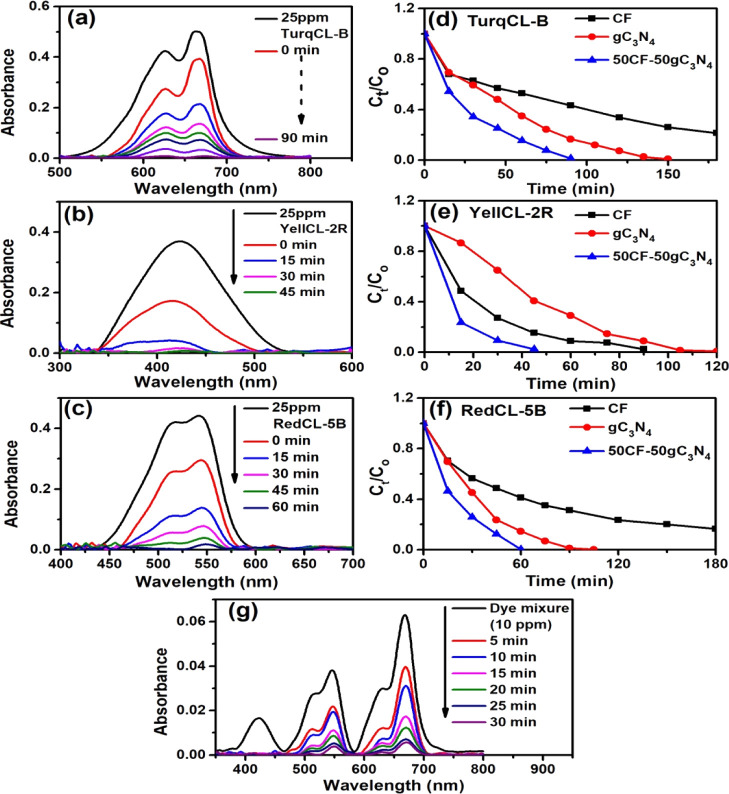
Now, to examine whether 50CF–50gC3N4 can be used to treat dye-containing industrial wastewater, we performed the photocatalysis reaction using three different dyes (e.g., Turq CL-B, Yell CL-2R, and Red CL-5B), which are widely used in textile industries and present in the wastewater discharged from these industries. Here also, it is observed that 50CF–50gC3N4 exhibited a better photocatalytic activity than pure CF and pure gC3N4 (Figure 3d–f). In the presence of 50CF–50gC3N4, the time required for 100% decomposition of Turq CL-B, Yell CL-2R, and Red CL-5B is 90, 45, and 60 min, respectively. To mimic the industrial wastewater, we have prepared an aqueous mixture of these three dyes, and the complete photodegradation of this dye mixture occurred within 30 min when catalyzed by 50CF–50gC3N4 (Figure 3g). In this case, the TOC removal ratio is 66.74%.
Figure 3.
(a–c) Time-dependent UV–vis spectral changes of the 50CF–50gC3N4-catalyzed photodecomposition reaction of industrial dyes (Turq CL-B, Yell CL-2R, and Red CL-5B). (d–f) Photodegradation rates of the dyes catalyzed by gC3N4, CF, and 50CF–50gC3N4. (g) Time-dependent UV–vis spectral changes of the 50CF–50gC3N4-catalyzed photodecomposition reaction of the industrial dye mixture.
2.4. Photocatalytic Reaction Mechanism
To understand the role of H2O2 and the 50CF–50gC3N4 catalyst in the photodecomposition reaction of MB, we have performed the experiment in the presence of only H2O2 (but no catalyst) and only with the catalyst (but no H2O2). In both cases, the reaction is found to be significantly slower. After 45 min of reaction time, only 17% decomposition occurred when only H2O2 is present. Similarly, when only the catalyst (50CF–50gC3N4) is present, 35% degradation of MB occurred after 45 min. However, when both H2O2 and 50CF–50gC3N4 are present in the reaction mixture, 100% degradation of MB is achieved in 45 min (Figure 4). These experiments confirmed that the activation of H2O2 was promoted by the catalyst when excited by visible light. As discussed before, the % of degradation of dyes is appreciably higher when the photocatalysis reaction is catalyzed by the 50CF–50gC3N4 nanocomposite in comparison with pure CF- or pure gC3N4-catalyzed reaction (Figure 2d). This enhancement of the catalytic activity of the 50CF–50gC3N4 nanocomposite can be attributed to the synergistic effect arising from the coupling of CF and gC3N4 in the nanocomposite.
Figure 4.
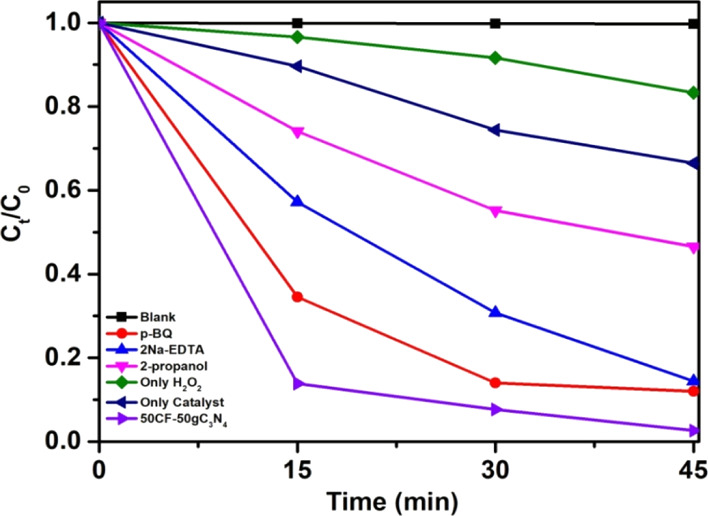
Effect of different radical scavengers on the photodegradation reaction of MB.
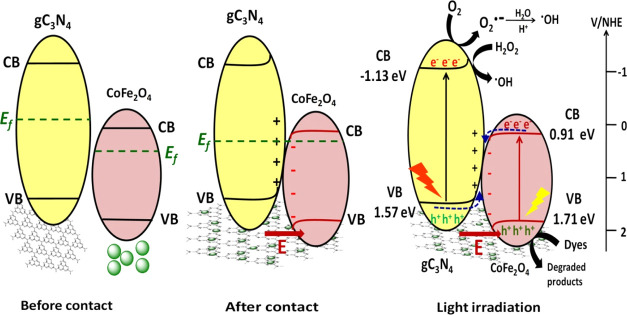
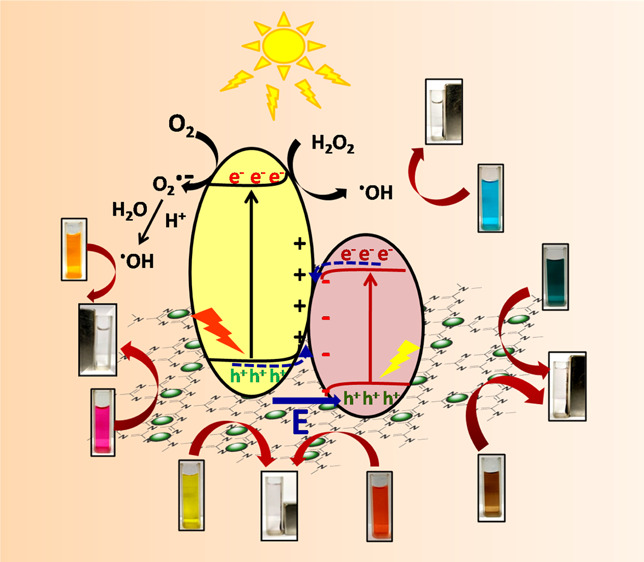
The CF–gC3N4-catalyzed photocatalytic reaction proceeds via the step-scheme (S-scheme) heterojunction mechanism, as described by Xu et al., and is presented in Scheme 1.73 In the S-scheme heterojunction mechanism, heterojunctions are formed at the interface between two photocatalysts having a staggered band structure. These heterojunctions can increase the usage of photoinduced charge carriers to generate an enormous amount of active species.74−77 The conduction (CB) and valence band (VB) values of CF are +0.91 and +1.71 eV, whereas those for gC3N4 are −1.13 and +1.57 eV, respectively.49 Under visible light irradiation, both CF and gC3N4 became excited, which leads to the formation of photogenerated holes and electrons in their VB and CB, respectively. The powerful photogenerated electrons and holes are reserved in the CB of gC3N4 and VB of CF, respectively, and the pointless charge carriers recombine and introduce a redox potential. When CF and gC3N4 come in contact, the electrons of gC3N4 diffuse into CF, and in the contact interface, an electron depletion layer and electron accumulation layer form. This electric field helps in the transfer of photogenerated electrons from CF to gC3N4. In the contact interface between CF and gC3N4, their individual Fermi energies (Ef) align to the same level, which causes band bending and results in the recombination of electrons in the CB of CF and holes in the VB of gC3N4. Moreover, because of the Coulombic attraction, the photogenerated electrons in the CB of CF and holes in the VB of gC3N4 also recombine. Thus, the useless electrons and holes recombine, whereas the powerful photogenerated electrons in the CB of gC3N4 and holes in the VB of CF are retained to proceed the photocatalysis reaction. During the photocatalysis reaction, these powerful photogenerated electrons and holes produce superoxide radicals (O2•–) and hydroxyl radicals (•OH), and these radicals degrade the dye molecules. The photocatalytic reactions which are involved in this dye degradation process can be presented in eqs 5–9.
Scheme 1. Proposed S-Scheme Mechanism of the Degradation of Dyes by CF–gC3N4 Nanocomposites.
To understand the role of these reactive species, we have performed a series of radical tapping experiments by using p-benzoquinone (BQ), EDTA-2Na, and isopropanol (IPA) which act as scavengers for O2•–, holes, and •OH respectively.33,37,49,78Figure 4 shows that the % decomposition of MB was slightly decreased with the addition of BQ and EDTA-2Na. It is observed that after 45 min of reaction, ∼88 and ∼86% decomposition of MB occurred in the presence of BQ and EDTA-2Na, respectively. However, ∼100% photodegradation of MB occurred when no scavenger was present. The rate of MB degradation became significantly slow in the presence of IPA, and only 55% of MB is decomposed within 45 min. These results indicate that •OH plays a more critical role than holes and O2.
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
3. Reusability
After the photocatalysis reaction, the catalyst was magnetically separated from the reaction mixture and used for the next cycle. Figure 5a,b shows the complete decolorization of the dyes because of photodegradation and the separation of the 50CF–50gC3N4 catalyst by a magnet. We have observed that the catalyst retains its efficiency for the first three cycles, after which it slightly decreases (Figure 6). The XRD and FESEM investigations of the recycled catalyst reveal that no significant change in the crystal structure and morphology of the catalyst occurred during the photocatalysis reaction (Figure S12).
Figure 5.
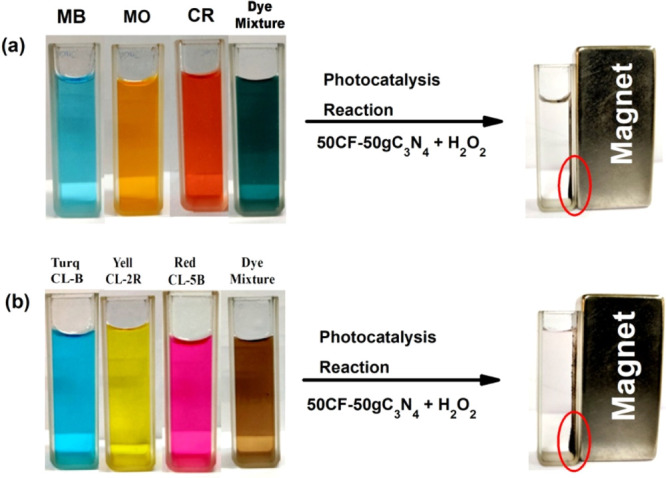
(a) Decolorization of model dyes by the photocatalysis reaction; (b) decolorization of industrial dyes by the photocatalysis reaction, and separation of the catalyst by using a magnet.
Figure 6.
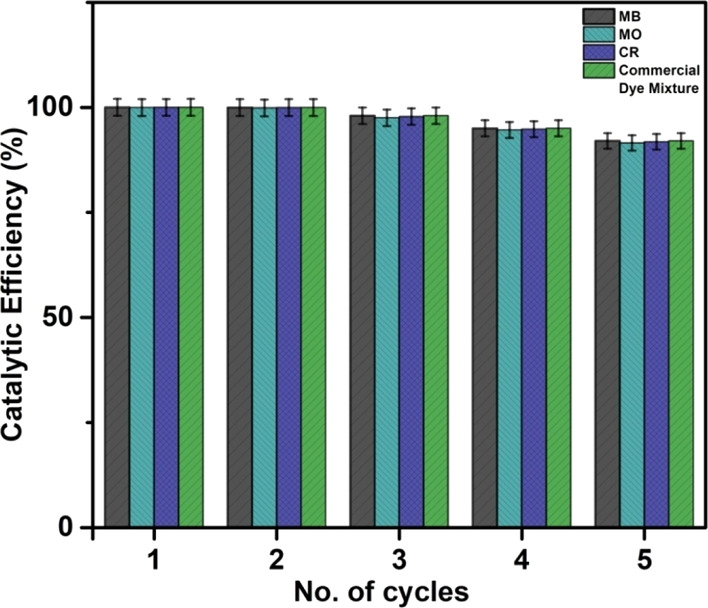
Reusability of the catalyst 50CF–50gC3N4 toward the photodegradation of MB, MO, CR, and the industrial dye mixture.
4. Conclusions
We have successfully synthesized CoFe2O4–gC3N4 nanocomposites. These nanocomposites exhibit strong absorption in the visible light region, and their band gap can be tuned simply by varying the amount of CoFe2O4 and gC3N4 in the nanocomposite. Among these nanocomposites, 50CoFe2O4–50gC3N4 shows the highest photocatalytic activity for the degradation of several model dyes (e.g., MB, MO, and CR) as well as industrially used dyes (such as Turq CL-B, Yell CL-2R, and Red CL-5B) and a mixture of dyes. The photocatalytic activities of 50CF–50gC3N4 for the degradation of dyes are significantly higher than those of individual CoFe2O4 and gC3N4. This enhancement could be due to the synergistic effect arising from the intimate coexistence of CoFe2O4 and gC3N4 in the catalysts and their staggered band structure. The photocatalytic efficiency of 50CoFe2O4–50gC3N4 is comparable and in some cases superior to that of many reported photocatalysts (Table S1).33,40,71,79−87 Moreover, the capability of this catalyst to degrade a variety of dyes, particularly industrially used dyes under solar light irradiation, makes it an attractive photocatalyst. This photocatalyst also offers easy magnetic separation and shows a stable catalytic efficiency even after five cycles. 50CoFe2O4–50gC3N4 demonstrates its potential as an efficient photocatalyst for the wastewater treatment of dye-containing effluents discharged from industries.
5. Experimental Section
5.1. Synthesis of CoFe2O4–gC3N4 Nanocomposites
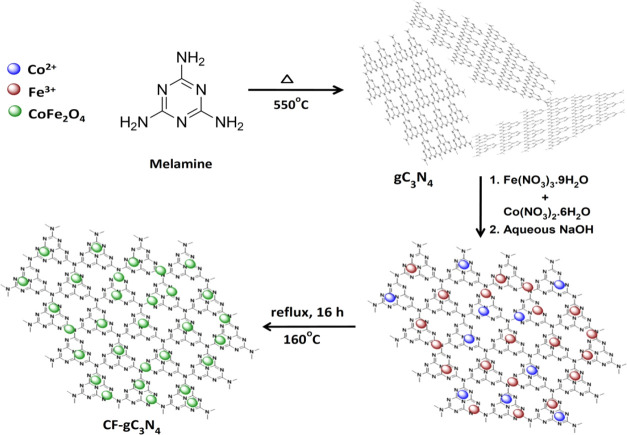
The nanocomposites composed of CoFe2O4 and gC3N4 were synthesized by employing a two-step process. The schematic presentation of this synthetic methodology is depicted in Scheme 2. In the first step, gC3N4 powder was prepared by the thermal treatment of melamine at 550 °C for 2 h at a heating rate of 5 °C/min.34 In a beaker, a dispersion of gC3N4 in an aqueous medium was prepared by adding a calculated amount of gC3N4 in water, followed by ultrasonication. In the next step, in this aqueous dispersion of gC3N4, a mixture of Co(NO3)2·6H2O and Fe(NO3)3·9H2O (molar ratio 1:2) in the polyethylene glycol and water medium (weight ratio 1: 5) was added. In this mixture, an aqueous solution of NaOH (2 M) was added dropwise till the pH reached ∼11. This mixture was refluxed at 160 °C for 16 h. After reflux, the reaction mixture was allowed to cool down to room temperature. The black-colored product thus formed was magnetically separated from the reaction mixture by applying a permanent magnet externally. After separation, this solid powder was washed with water, ethanol, and finally with acetone and then dried at 60 °C for 10 h. Using this protocol, nanocomposites having various weight % of gC3N4 and CF nanoparticles were synthesized by varying the amount of gC3N4, Co(NO3)2·6H2O, and Fe(NO3)3·9H2O. The nanocomposites having 5, 10, 15, 25, and 50 wt % gC3N4 content are referred as 95CF–5gC3N4, 90CF–10gC3N4, 85CF–15gC3N4, 75CF–25gC3N4, and 50CF–50gC3N4, respectively. Pure CF nanoparticles were also synthesized by employing the same protocol, except that gC3N4 was not added to the reaction mixture.
Scheme 2. Synthetic Route of CF–gC3N4 Nanocomposites.
5.2. Photocatalytic Activity Measurement
To evaluate the photocatalytic activity of the synthesized materials, photodegradation reactions of different dye solutions in the presence of H2O2 under simulated solar light irradiation was performed. Initially, the photocatalytic degradation of different model dyes (such as MB, MO, CR) was investigated.
First, aqueous solutions of MB (25 ppm), CR (25 ppm), and MO (10 ppm) were prepared. In a typical photocatalysis reaction, 50 mL of dye solution was taken in a beaker and mixed with 25 mg of catalyst (50 mg in the case of MO). This mixture was stirred in the dark for 30 min to reach the absorption–desorption equilibrium between the catalyst and dye solution. The UV–vis spectra of the mixture were recorded before and after the stirring of the reaction mixture in the dark. It was observed that some amount of dye was adsorbed on the surface of the catalyst during this mixing process, and the extent of dye adsorption varied with the composition and nature of the catalyst. To this mixture, 2 mL of 30% H2O2 was added, and the reaction mixture was irradiated by simulated solar light emitted from a solar light simulator, which is equipped with a 150 W Xenon lamp. A 3 mL aliquot of the reaction mixture was collected just before the exposure of light, and this point was considered as the starting point (t = 0). The color of the dye solution started to fade because of the exposure to light. The change of the concentration of dye with the progress of the reaction time was monitored spectrophotometrically by using a UV–vis spectrophotometer and following the decrease of intensity of the λmax peak with increasing reaction time (the λmax values of MB, MO, and CR are 664, 463, and 500 nm, respectively).
In the UV–vis spectra, the absorbance of the dye is proportional to its concentration. The ratio of absorbance of dye At (measured at time t) to A0 (measured at t = 0) is equal to Ct/C0 (where Ct and C0 are the concentrations of the dye at time t and t = 0, respectively). The % decomposition of the dye because of the photocatalysis reaction was determined from Ct/C0. The optimal composition of the CF–gC3N4 nanocomposite which exhibited the highest catalytic activity was determined by studying the photocatalysis reaction of these model dye solutions. Then, photodegradation reaction was performed for a mixture of dye solution containing 25 ppm MB, 25 ppm CR, and 10 ppm MO, using this catalyst.
The photocatalytic efficiency of the catalyst having optimal composition was also tested for the industrially used dyes (such as Turq CL-B, Yell CL-2R, and Red CL-5B). First, the photodegradation of individual dye solution was performed, followed by a mixture of dye solution (the total concentration of Turq CL-B, Yell CL-2R, and Red CL-5B in the mixture was 10 ppm).
After the completion of the photocatalysis reaction, the catalyst was recovered from the reaction mixture by a magnetic separation method, where a permanent magnet (N35-grade NdFeB magnet having an energy product BHmax = 33–36 MGOe) was used externally. After recovery, the catalyst was washed with ethanol. The absence of any dye in ethanol after washing indicated that all dye molecules present in the reaction mixture along with the dye molecules which were adsorbed on the surface of the catalyst were completely photodegraded. After washing the catalyst, it was dried, and the next cycle of the reaction was performed. The TOC removal ratio of the dye solutions after photocatalysis was calculated by using the following equation88
| 10 |
TOCi and TOCf are the TOC contents of the dye solution before and after the photocatalysis reaction.
The details of the chemicals and instruments used in this study are provided in the Supporting Information.
Acknowledgments
The authors are grateful to the Central Sophisticated Instrumentation Facility (CSIF) of BITS Pilani, K K Birla Goa campus, for providing the FESEM and Raman facilities and also thankful to ABSTCPL, Mumbai and BITS Pilani, for sponsoring the solar light simulator instrument.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05809.
Details of chemicals, details of instruments, comparison of photocatalytic efficiency of 50CF–50gC3N4 with other gC3N4-based photocatalysts; XRD patterns of pure CoFe2O4, pure gC3N4, 95CF–5gC3N4, 90CF–10gC3N4, 85CF–15gC3N4, and 75CF–25gC3N4; EDS of 50CF–50gC3N4; FT-IR spectra of CF, 50CF–50gC3N4, and gC3N4; Raman spectra of CF, 50CF–50gC3N4, and gC3N4; TGA curves of pure gC3N4, 50CF–50gC3N4, 75CF–25gC3N4, 85CF–15gC3N4, 90CF–10gC3N4, 95CF–5gC3N4, and pure CF in the temperature range 35–800 °C; UV–vis diffuse absorption spectra of pure gC3N4, 50CF–50gC3N4, 75CF–25gC3N4, 85CF–15gC3N4, 90CF–10gC3N4, and 95CF–5gC3N4; time-dependent UV–vis spectral changes of the photodecomposition reaction and the photodegradation rates of MB, MO, and CR catalyzed by different CF–gC3N4 nanocomposites; and XRD plot and FESEM image of the used 50CF–50gC3N4 catalyst (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hu H.; Xin J. H.; Hu H.; Wang X.; Miao D.; Liu Y. Synthesis and stabilization of metal nanocatalysts for reduction reactions–a review. J. Mater. Chem. A 2015, 3, 11157–11182. 10.1039/c5ta00753d. [DOI] [Google Scholar]
- Buthelezi S.; Olaniran A.; Pillay B. Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules 2012, 17, 14260–14274. 10.3390/molecules171214260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu Z. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 2005, 40, 997–1026. 10.1016/j.procbio.2004.04.008. [DOI] [Google Scholar]
- Ghosh B. K.; Ghosh N. N. Applications of metal nanoparticles as catalysts in cleaning dyes containing industrial effluents: a review. J. Nanosci. Nanotechnol. 2018, 18, 3735–3758. 10.1166/jnn.2018.15345. [DOI] [PubMed] [Google Scholar]
- Patel R.; Suresh S. Decolourization of azo dyes using magnesium-palladium system. J. Hazard. Mater. 2006, 137, 1729–1741. 10.1016/j.jhazmat.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Manu B.; Chaudhari S. Anaerobic decolorisation of simulated textile wastewater containing azo dyes. Bioresour. Technol. 2002, 82, 225–231. 10.1016/s0960-8524(01)00190-0. [DOI] [PubMed] [Google Scholar]
- Bafana A.; Devi S. S.; Chakrabarti T. Azo dyes: past, present and the future. Environ. Rev. 2011, 19, 350–371. 10.1139/a11-018. [DOI] [Google Scholar]
- Ahmad A.; Mohd-Setapar S. H.; Chuong C. S.; Khatoon A.; Wani W. A.; Kumar R.; Rafatullah M. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. 10.1039/c4ra16959j. [DOI] [Google Scholar]
- Divyapriya G.; Nambi I.; Senthilnathan J. Nanocatalysts in Fenton based advanced oxidation process for water and wastewater treatment. J. Bionanoscience 2016, 10, 356–368. 10.1166/jbns.2016.1387. [DOI] [Google Scholar]
- Suhas V. K. G. Application of low-cost adsorbents for dye removal–a review. J. Environ. Manage. 2009, 90, 2313–2342. 10.1016/j.jenvman.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Verma A. K.; Dash R. R.; Bhunia P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. 2012, 93, 154–168. 10.1016/j.jenvman.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Coughlin M. F.; Kinkle B. K.; Tepper A.; Bishop P. L. Characterization of aerobic azo dye-degrading bacteria and their activity in biofilms. Water Sci. Technol. 1997, 36, 215–220. 10.2166/wst.1997.0051. [DOI] [Google Scholar]
- Oturan M. A.; Aaron J.-J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. 10.1080/10643389.2013.829765. [DOI] [Google Scholar]
- Asghar A.; Raman A. A. A.; Daud W. M. A. W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J. Clean. Prod. 2015, 87, 826–838. 10.1016/j.jclepro.2014.09.010. [DOI] [Google Scholar]
- Robinson T.; McMullan G.; Marchant R.; Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Slokar Y. M.; Majcen Le Marechal A. Methods of decoloration of textile wastewaters. Dyes Pigm. 1998, 37, 335–356. 10.1016/s0143-7208(97)00075-2. [DOI] [Google Scholar]
- Schneider J.; Matsuoka M.; Takeuchi M.; Zhang J.; Horiuchi Y.; Anpo M.; Bahnemann D. W. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- Lee K. M.; Lai C. W.; Ngai K. S.; Juan J. C. Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res. 2016, 88, 428–448. 10.1016/j.watres.2015.09.045. [DOI] [PubMed] [Google Scholar]
- Kumar S. G.; Devi L. G. Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. 10.1021/jp204364a. [DOI] [PubMed] [Google Scholar]
- Nakata K.; Fujishima A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol., C 2012, 13, 169–189. 10.1016/j.jphotochemrev.2012.06.001. [DOI] [Google Scholar]
- Fujishima A.; Rao T. N.; Tryk D. A. Titanium dioxide photocatalysis. J. Photochem. Photobiol., C 2000, 1, 1–21. 10.1016/s1389-5567(00)00002-2. [DOI] [Google Scholar]
- Fujishima A.; Zhang X. Titanium dioxide photocatalysis: present situation and future approaches. C. R. Chim. 2006, 9, 750–760. 10.1016/j.crci.2005.02.055. [DOI] [Google Scholar]
- Yang X.; Chen W.; Huang J.; Zhou Y.; Zhu Y.; Li C. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4@rGO@TiO2-catalyzed photo-Fenton system. Sci. Rep. 2015, 5, 10632. 10.1038/srep10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Hydrothermal synthesis of TiO2–RGO composites and their improved photocatalytic activity in visible light. RSC Adv. 2014, 4, 36040–36045. 10.1039/c4ra06342b. [DOI] [Google Scholar]
- Fu Y.; Wang X. Magnetically separable ZnFe2O4–graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind. Eng. Chem. Res. 2011, 50, 7210–7218. 10.1021/ie200162a. [DOI] [Google Scholar]
- Leary R.; Westwood A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. 10.1016/j.carbon.2010.10.010. [DOI] [Google Scholar]
- Zhang F.; Li Y.-H.; Li J.-Y.; Tang Z.-R.; Xu Y.-J. 3D graphene-based gel photocatalysts for environmental pollutants degradation. Environ. Pollut. 2019, 253, 365–376. 10.1016/j.envpol.2019.06.089. [DOI] [PubMed] [Google Scholar]
- Weng B.; Lu K.-Q.; Tang Z.; Chen H. M.; Xu Y.-J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. 10.1038/s41467-018-04020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-H.; Li J.-Y.; Xu Y.-J. Bimetallic Nanoparticles as Cocatalysts for Versatile Photoredox Catalysis. Energy Chem. 2020, 3, 100047. 10.1016/j.enchem.2020.100047. [DOI] [Google Scholar]
- Zhang N.; Yang M.-Q.; Liu S.; Sun Y.; Xu Y.-J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. 10.1021/acs.chemrev.5b00267. [DOI] [PubMed] [Google Scholar]
- Han C.; Li Y.-H.; Qi M.-Y.; Zhang F.; Tang Z.-R.; Xu Y.-J. Surface/Interface Engineering of Carbon-Based Materials for Constructing Multidimensional Functional Hybrids. Sol. RRL 2020, 4, 1900577. 10.1002/solr.201900577. [DOI] [Google Scholar]
- Kumar S.; T S.; Kumar B.; Baruah A.; Shanker V. Synthesis of magnetically separable and recyclable g-C3N4–Fe3O4 hybrid nanocomposites with enhanced photocatalytic performance under visible-light irradiation. J. Phys. Chem. C 2013, 117, 26135–26143. 10.1021/jp409651g. [DOI] [Google Scholar]
- Tian Y.; Chang B.; Lu J.; Fu J.; Xi F.; Dong X. Hydrothermal synthesis of graphitic carbon nitride–Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. ACS Appl. Mater. Interfaces 2013, 5, 7079–7085. 10.1021/am4013819. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Bai X.; Pan C.; He J.; Zhu Y. Enhancement of photocatalytic activity of Bi2WO6hybridized with graphite-like C3N4. J. Mater. Chem. 2012, 22, 11568–11573. 10.1039/c2jm16873a. [DOI] [Google Scholar]
- Chen L.; Ma W.; Dai J.; Zhao J.; Li C.; Yan Y. Facile synthesis of highly efficient graphitic-C3N4/ZnFe2O4 heterostructures enhanced visible-light photocatalysis for spiramycin degradation. J. Photochem. Photobiol., A 2016, 328, 24–32. 10.1016/j.jphotochem.2016.04.026. [DOI] [Google Scholar]
- Ji H.; Jing X.; Xu Y.; Yan J.; Li H.; Li Y.; Huang L.; Zhang Q.; Xu H.; Li H. Magnetic g-C3N4/NiFe2O4 hybrids with enhanced photocatalytic activity. RSC Adv. 2015, 5, 57960–57967. 10.1039/c5ra07148h. [DOI] [Google Scholar]
- Yao Y.; Cai Y.; Lu F.; Qin J.; Wei F.; Xu C.; Wang S. Magnetic ZnFe2O4-C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light. Ind. Eng. Chem. Res. 2014, 53, 17294–17302. 10.1021/ie503437z. [DOI] [Google Scholar]
- Sarkar S.; Basak D. One-step nano-engineering of dispersed Ag–ZnO nanoparticles’ hybrid in reduced graphene oxide matrix and its superior photocatalytic property. CrystEngComm 2013, 15, 7606–7614. 10.1039/c3ce41043a. [DOI] [Google Scholar]
- Sarkar S.; Basak D. The reduction of graphene oxide by zinc powder to produce a zinc oxide-reduced graphene oxide hybrid and its superior photocatalytic activity. Chem. Phys. Lett. 2013, 561–562, 125–130. 10.1016/j.cplett.2013.01.050. [DOI] [Google Scholar]
- Ge L.; Han C.; Liu J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI–gC3N4 composite photocatalysts. J. Mater. Chem. 2012, 22, 11843–11850. 10.1039/c2jm16241e. [DOI] [Google Scholar]
- Hassani A.; Eghbali P.; Ekicibil A.; Metin Ö. Monodisperse cobalt ferrite nanoparticles assembled on mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4): a magnetically recoverable nanocomposite for the photocatalytic degradation of organic dyes. J. Magn. Magn Mater. 2018, 456, 400–412. 10.1016/j.jmmm.2018.02.067. [DOI] [Google Scholar]
- Zhang S.; Li J.; Zeng M.; Zhao G.; Xu J.; Hu W.; Wang X. In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. ACS Appl. Mater. Interfaces 2013, 5, 12735–12743. 10.1021/am404123z. [DOI] [PubMed] [Google Scholar]
- Sun J.-X.; Yuan Y.-P.; Qiu L.-G.; Jiang X.; Xie A.-J.; Shen Y.-H.; Zhu J.-F. Fabrication of composite photocatalyst gC3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. 10.1039/c2dt12474b. [DOI] [PubMed] [Google Scholar]
- Alcudia-Ramos M. A.; Fuentez-Torres M. O.; Ortiz-Chi F.; Espinosa-González C. G.; Hernández-Como N.; García-Zaleta D. S.; Kesarla M. K.; Torres-Torres J. G.; Collins-Martínez V.; Godavarthi S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020, 46, 38–45. 10.1016/j.ceramint.2019.08.228. [DOI] [Google Scholar]
- Shen H.; Zhao X.; Duan L.; Liu R.; Li H. Enhanced visible light photocatalytic activity in SnO2@g-C3N4 core-shell structures. Mater. Sci. Eng., B 2017, 218, 23–30. 10.1016/j.mseb.2017.01.006. [DOI] [Google Scholar]
- Huang L.; Xu H.; Li Y.; Li H.; Cheng X.; Xia J.; Xu Y.; Cai G. Visible-light-induced WO3/gC3N4 composites with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 8606–8616. 10.1039/c3dt00115f. [DOI] [PubMed] [Google Scholar]
- Gan L.; Shang S.; Yuen C. W. M.; Jiang S.-x.; Hu E. Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl. Surf. Sci. 2015, 351, 140–147. 10.1016/j.apsusc.2015.05.130. [DOI] [Google Scholar]
- Moitra D.; Chandel M.; Ghosh B. K.; Jani R. K.; Patra M. K.; Vadera S. R.; Ghosh N. N. A simple ‘in situ’co-precipitation method for the preparation of multifunctional CoFe2O4–reduced graphene oxide nanocomposites: excellent microwave absorber and highly efficient magnetically separable recyclable photocatalyst for dye degradation. RSC Adv. 2016, 6, 76759–76772. 10.1039/c6ra17384e. [DOI] [Google Scholar]
- Huang S.; Xu Y.; Xie M.; Xu H.; He M.; Xia J.; Huang L.; Li H. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf., A 2015, 478, 71–80. 10.1016/j.colsurfa.2015.03.035. [DOI] [Google Scholar]
- Lu H. M.; Zheng W. T.; Jiang Q. Saturation magnetization of ferromagnetic and ferrimagnetic nanocrystals at room temperature. J. Phys. D: Appl. Phys. 2007, 40, 320. 10.1088/0022-3727/40/2/006. [DOI] [Google Scholar]
- Hassani A.; Eghbali P.; Metin Ö. Sonocatalytic removal of methylene blue from water solution by cobalt ferrite/mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4) nanocomposites: response surface methodology approach. Environ. Sci. Pollut. Res. 2018, 25, 32140–32155. 10.1007/s11356-018-3151-3. [DOI] [PubMed] [Google Scholar]
- Inbaraj D. J.; Chandran B.; Mangalaraj C. Synthesis of CoFe2O4 and CoFe2O4/g-C3N4 nanocomposite via honey mediated sol-gel auto combustion method and hydrothermal method with enhanced photocatalytic and efficient Pb+2 adsorption property. Mater. Res. Express 2019, 6, 055501. 10.1088/2053-1591/aafd5d. [DOI] [Google Scholar]
- Yao Y.; Wu G.; Lu F.; Wang S.; Hu Y.; Zhang J.; Huang W.; Wei F. Enhanced photo-Fenton-like process over Z-scheme CoFe2O4/gC3N4 heterostructures under natural indoor light. Environ. Sci. Pollut. Res. 2016, 23, 21833–21845. 10.1007/s11356-016-7329-2. [DOI] [PubMed] [Google Scholar]
- Deng J.; Shao Y.; Gao N.; Tan C.; Zhou S.; Hu X. CoFe2O4 magnetic nanoparticles as a highly active heterogeneous catalyst of oxone for the degradation of diclofenac in water. J. Hazard. Mater. 2013, 262, 836–844. 10.1016/j.jhazmat.2013.09.049. [DOI] [PubMed] [Google Scholar]
- Dong F.; Sun Y.; Wu L.; Fu M.; Wu Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. 10.1039/c2cy20049j. [DOI] [Google Scholar]
- Xu J.; Wang Y.; Zhu Y. Nanoporous graphitic carbon nitride with enhanced photocatalytic performance. Langmuir 2013, 29, 10566–10572. 10.1021/la402268u. [DOI] [PubMed] [Google Scholar]
- Xu H.; Yan J.; Xu Y.; Song Y.; Li H.; Xia J.; Huang C.; Wan H. Novel visible-light-driven AgX/graphite-like C3N4 (X=Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal., B 2013, 129, 182–193. 10.1016/j.apcatb.2012.08.015. [DOI] [Google Scholar]
- Di J.; Xia J.; Yin S.; Xu H.; He M.; Li H.; Xu L.; Jiang Y. A gC3N4/BiOBr visible-light-driven composite: synthesis via a reactable ionic liquid and improved photocatalytic activity. RSC Adv. 2013, 3, 19624–19631. 10.1039/c3ra42269k. [DOI] [Google Scholar]
- Xu Y.; Xu H.; Yan J.; Li H.; Huang L.; Xia J.; Yin S.; Shu H. A plasmonic photocatalyst of Ag/AgBr nanoparticles coupled with g-C3N4 with enhanced visible-light photocatalytic ability. Colloids Surf., A 2013, 436, 474–483. 10.1016/j.colsurfa.2013.06.005. [DOI] [Google Scholar]
- Khabashesku V. N.; Zimmerman J. L.; Margrave J. L. Powder synthesis and characterization of amorphous carbon nitride. Chem. Mater. 2000, 12, 3264–3270. 10.1021/cm000328r. [DOI] [Google Scholar]
- Wu S.; Wen S.; Xu X.; Huang G.; Cui Y.; Li J.; Qu A. Facile synthesis of porous graphene-like carbon nitride nanosheets with high surface area and enhanced photocatalytic activity via one-step catalyst-free solution self-polymerization. Appl. Surf. Sci. 2018, 436, 424–432. 10.1016/j.apsusc.2017.11.254. [DOI] [Google Scholar]
- Niu P.; Zhang L.; Liu G.; Cheng H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. 10.1002/adfm.201200922. [DOI] [Google Scholar]
- Fu M.; Jiao Q.; Zhao Y.; Li H. Vapor diffusion synthesis of CoFe2O4 hollow sphere/graphene composites as absorbing materials. J. Mater. Chem. A 2014, 2, 735–744. 10.1039/c3ta14050d. [DOI] [Google Scholar]
- Wang H.; Zhang X.; Xie J.; Zhang J.; Ma P.; Pan B.; Xie Y. Structural distortion in graphitic-C3N4 realizing an efficient photoreactivity. Nanoscale 2015, 7, 5152–5156. 10.1039/c4nr07645a. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yang W.; Chen X.; Wang J.; Zhu Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal., B 2018, 220, 337–347. 10.1016/j.apcatb.2017.08.004. [DOI] [Google Scholar]
- Shiraishi Y.; Kanazawa S.; Sugano Y.; Tsukamoto D.; Sakamoto H.; Ichikawa S.; Hirai T. Highly selective production of hydrogen peroxide on graphitic carbon nitride (g-C3N4) photocatalyst activated by visible light. ACS Catal. 2014, 4, 774–780. 10.1021/cs401208c. [DOI] [Google Scholar]
- Wen J.; Xie J.; Chen X.; Li X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. 10.1016/j.apsusc.2016.07.030. [DOI] [Google Scholar]
- Xiang Q.; Yu J.; Jaroniec M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. 10.1021/jp200953k. [DOI] [Google Scholar]
- Mariosi F. R.; Venturini J.; da Cas Viegas A.; Bergmann C. P. Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 2020, 46, 2772–2779. 10.1016/j.ceramint.2019.09.266. [DOI] [Google Scholar]
- Wang X.; Wang A.; Ma J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92. 10.1016/j.jhazmat.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Vignesh K.; Suganthi A.; Min B.-K.; Kang M. Photocatalytic activity of magnetically recoverable MnFe2O4/g-C3N4/TiO2 nanocomposite under simulated solar light irradiation. J. Mol. Catal. A: Chem. 2014, 395, 373–383. 10.1016/j.molcata.2014.08.040. [DOI] [Google Scholar]
- Kubelka P.; Munk F. An article on optics of paint layers. Z. Tech. Phys. 1931, 12, 259–274. [Google Scholar]
- Xu Q.; Zhang L.; Cheng B.; Fan J.; Yu J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. 10.1016/j.chempr.2020.06.010. [DOI] [Google Scholar]
- Xia P.; Cao S.; Zhu B.; Liu M.; Shi M.; Yu J.; Zhang Y. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chem., Int. Ed. 2020, 59, 5218–5225. 10.1002/anie.201916012. [DOI] [PubMed] [Google Scholar]
- Xie Q.; He W.; Liu S.; Li C.; Zhang J.; Wong P. K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. 10.1016/s1872-2067(19)63481-9. [DOI] [Google Scholar]
- Xu F.; Meng K.; Cheng B.; Wang S.; Xu J.; Yu J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 1–9. 10.1038/s41467-020-18350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D.; Xia Y.; Li Q.; Yang C.; Qin Y.; Lv K. One-pot calcination synthesis of Cd0.5Zn0.5S/g-C3N4 photocatalyst with a step-scheme heterojunction structure. J. Mater. Sci. Technol. 2020, 56, 206–215. 10.1016/j.jmst.2020.03.034. [DOI] [Google Scholar]
- Guo X.; Ai S.; Yang D.; Zhao L.; Ding H. Synergistic photocatalytic and Fenton-like degradation of organic contaminants using peroxymonosulfate activated by CoFe2O4@g-C3N4 composite. Environ. Technol. 2019, 1–14. 10.1080/09593330.2019.1697378. [DOI] [PubMed] [Google Scholar]
- Xian T.; Yang H.; Di L. J.; Dai J. F. Enhanced photocatalytic activity of BaTiO3@g-C3N4 for the degradation of methyl orange under simulated sunlight irradiation. J. Alloys Compd. 2015, 622, 1098–1104. 10.1016/j.jallcom.2014.11.051. [DOI] [Google Scholar]
- Tian Y.; Cheng F.; Zhang X.; Yan F.; Zhou B.; Chen Z.; Liu J.; Xi F.; Dong X. Solvothermal synthesis and enhanced visible light photocatalytic activity of novel graphitic carbon nitride–Bi2MoO6 heterojunctions. Powder Technol. 2014, 267, 126–133. 10.1016/j.powtec.2014.07.021. [DOI] [Google Scholar]
- Zhu Z.; Wang Z.; Di J.; Long Y.; Li W. Enhanced visible-light photocatalytic properties of g-C3N4 by coupling with ZnAl2O4. Catal. Commun. 2016, 86, 86–90. 10.1016/j.catcom.2016.08.017. [DOI] [Google Scholar]
- Tian Y.; Chang B.; Yang Z.; Zhou B.; Xi F.; Dong X. Graphitic carbon nitride–BiVO4 heterojunctions: simple hydrothermal synthesis and high photocatalytic performances. RSC Adv. 2014, 4, 4187–4193. 10.1039/c3ra46079g. [DOI] [Google Scholar]
- Wang Y.; Wang Z.; Muhammad S.; He J. Graphite-like C3N4 hybridized ZnWO4 nanorods: Synthesis and its enhanced photocatalysis in visible light. CrystEngComm 2012, 14, 5065–5070. 10.1039/c2ce25517k. [DOI] [Google Scholar]
- Yu Q.; Guo S.; Li X.; Zhang M. Template free fabrication of porous g-C3N4/graphene hybrid with enhanced photocatalytic capability under visible light. Mater. Technol. 2014, 29, 172–178. 10.1179/1753555714y.0000000126. [DOI] [Google Scholar]
- Alenizi M. A.; Kumar R.; Aslam M.; Alseroury F.; Barakat M. Construction of a ternary gC3N4/TiO2@polyaniline nanocomposite for the enhanced photocatalytic activity under solar light. Sci. Rep. 2019, 9, 12091. 10.1038/s41598-019-48516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Sun Z.; Li X.; Liu L.; Zheng S. Facile fabrication of g-C3N4/precipitated silica composite with enhanced visible-light photoactivity for the degradation of rhodamine B and Congo red. Adv. Powder Technol. 2016, 27, 2051–2060. 10.1016/j.apt.2016.07.014. [DOI] [Google Scholar]
- Tu D.; Liao H.; Deng Q. Synthesis of BN/g-C3N4 as Visible-light-driven Photocatalysts for Degradation of Different Organic Pollutants. ChemistrySelect 2018, 3, 7170–7177. 10.1002/slct.201800921. [DOI] [Google Scholar]
- Hu C.; Wang M.-S.; Chen C.-H.; Chen Y.-R.; Huang P.-H.; Tung K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. 10.1016/j.memsci.2019.03.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.