Abstract
Objective:
Fatigued cancer patients often have high peripheral inflammation; however, the biological mechanisms of this association remain unclear. We examined whether decreased sensitivity of immune cells to the anti-inflammatory effects of glucocorticoids may contribute to inflammation and fatigue in head and neck cancer (HNC) patients during treatment.
Methods:
HNC patients without distant metastasis and with curative intent (n = 77) were studied 1 week before intensity-modulated radiotherapy (IMRT) and 1 month after IMRT. At each time point, fatigue was measured by the Multidimensional Fatigue Inventory-20 along with plasma inflammation markers and glucocorticoid receptor (GR) sensitivity as determined by in vitro dexamethasone suppression of lipopolysaccharide-induced interleukin 6. Linear regression models were used.
Results:
In contrast to our hypothesis, GR sensitivity increased during treatment; however, increased fatigue was associated with a lesser increase in GR sensitivity from baseline to 1 month after IMRT (unstandardized estimate = 4.07, p = .02). This effect was more prominent in human papillomavirus–unrelated HNCs (unstandardized estimate = 8.22, p = .002). Lower increases in GR sensitivity were also associated with increased inflammation at 1 month after IMRT as represented by C-reactive protein, interleukin 6, and tumor necrosis factor α. Addition of inflammation markers to models of GR sensitivity predicting fatigue indicated that these inflammation markers were stronger predictors of fatigue than GR sensitivity.
Conclusions:
Lower increases in GR sensitivity during HNC treatment were significantly predictive of increased fatigue and inflammation markers. Inflammation markers in turn predicted fatigue above and beyond levels of GR sensitivity. Our findings indicate that HNC patients with cancer-related fatigue may exhibit a decreased capacity for glucocorticoids to regulate inflammatory processes, as evidenced by a lower increase in GR sensitivity. Larger studies are necessary to verify the findings.
Keywords: fatigue, glucocorticoid receptor sensitivity, inflammation, head and neck cancer
INTRODUCTION
Fatigue is the most common and distressing symptom related to cancer and its treatment (1), including in patients with head and neck cancer (HNC), a significantly increasing cancer population due to human papillomavirus (HPV) infection (2). Most HNC patients are treated with definitive radiotherapy (RT) without surgery because of the structural complexity and functional importance of cancer sites. During RT, patients have high rates of fatigue, even compared with other cancer patients (3,4). HNC patients receiving intensity-modulated radiotherapy (IMRT), the most commonly used RT that targets tumors with higher doses while avoiding normal structures, experience even higher fatigue than conventional RT (4). In addition, combined chemotherapy with IMRT is used frequently in treating HNC, and the combination produces even worse fatigue, likely from synergistic effects among modalities (5). Like other cancer populations, fatigue affects negatively not only HNC patients’ quality of life but also their survival (6). However, no Food and Drug Administration–approved agent can effectively manage fatigue (7). Understanding the biological mechanisms is critical to the successful management of this debilitating symptom.
Cancer and its treatment such as RT and chemotherapy can activate nuclear factor κB (NF-κB) (8), a key mediator of the pro-inflammatory response (9). In addition, psychological stress associated with cancer and its treatment can trigger inflammatory responses through activating inflammatory cytokines and their signaling pathways (10). The increase of inflammation may place cancer patients at high risk of developing relevant behavioral alterations (10). Evidence from our previous studies has suggested a consistent, positive association between inflammation and fatigue (9,11). Increased peripheral inflammation markers, such as C-reactive protein (CRP), interleukin (IL) 6, and soluble tumor necrosis factor receptor 2 (sTNFR2) are predictive of fatigue in HNC patients from before IMRT until up to 3 months after IMRT (9,11). In addition, our data analysis of upregulated gene transcripts as a function of IMRT and fatigue revealed overrepresentation of transcripts related to NF-κB (12). We also found that HPV status is an important marker of vulnerability to the behavioral and immune consequences of HNC and its treatment, with patients with HPV-unrelated tumors exhibiting greater inflammation and fatigue than those with HPV-related tumors (11,12). In the current study, we examined whether anti-inflammatory responses may play a role in the association between fatigue and inflammation in HNC patients.
Glucocorticoids play a primary role in the negative regulation of inflammation; a process that is dependent on the glucocorticoid receptor (GR) (10). GR is normally inactivated in the cytoplasm. Once bound to glucocorticoid, GR undergoes a conformational change and translocates to the nucleus, where it regulates gene transcription by either binding to DNA or interacting with other transcription factors including NF-κB, with the end result being decreased production of inflammatory cytokines (13), However, evidence has demonstrated that people with chronic stress experience reduced GR sensitivity (14). This reduced GR sensitivity may lead to downregulation of anti-inflammatory responses (13,15,16). Most studies on GR sensitivity have focused on chronic stress in healthy populations, not in patients with cancer (16,17). Thus, we proposed this longitudinal study to fill this gap in patients with HNC during the acute phase of IMRT ± chemotherapy. We hypothesized that a) reduced GR sensitivity would be associated with increased fatigue over cancer treatment, b) reduced GR sensitivity would be associated with increased inflammation markers, c) inflammation markers would predict fatigue with GR sensitivity in the model, and d) HPV status would play a role in the association between GR sensitivity and fatigue.
METHODS
This was a prospective, observational study of HNC patients followed 1 week before IMRT and then at 1 month after the completion of a 6-week IMRT. The overall length of the time between the two measurement points was approximately 3 months. Surgery occurred approximately 1 month before IMRT and therefore before the baseline assessment. The selection of the two measurement times was to control baseline differences and capture high fatigue and inflammation as suggested by our previous data (9,11). The study was approved by Emory institutional review board, and all participants provided informed consent.
Participants and Procedures
The inclusion criteria and study procedures were similar to that presented in our previous publications (9,11,12). Briefly, eligible patients were enrolled at the Radiation Oncology Clinics at Emory University Hospital and Emory University Hospital at Midtown from 2013 to 2016. Inclusion criteria were as follows: histological proof of squamous cell carcinoma of the head and neck; no distant metastasis; ≥21 years of age; no evidence of uncontrolled metabolic, hematologic, cardiovascular, renal, hepatic, or neurologic disease; and being scheduled to receive IMRT with or without chemotherapy. Exclusion criteria included the following: simultaneous primaries, pregnant women, and patients with major psychiatric disorders or who cannot understand English. Other exclusion criteria that might confound the relationship between fatigue and inflammation were as follows: chronic medical conditions involving the immune system or regular use of immunosuppressive medications. Over-the-counter anti-inflammatory medications and antidepressants were allowed.
Patients’ electronic medical records were used to determine eligibility. Eligible, consented patients were enrolled into the study before the start of IMRT. Demographic and clinical variables were collected at baseline and/ or follow-up as appropriate through chart review and standardized questionnaires. All other data, including patients-reported questionnaires and blood samples, were collected at both before IMRT and 1 month after IMRT. Blood samples were collected by a phlebotomist or a certificated nurse on the same day as the questionnaires.
Social Behavioral and Clinical Measures
Demographic characteristics collected were as follows: age, sex, race (white versus nonwhite), marital status (married versus single), smoking status (never smoker versus ever smoker), and alcohol status (never drinker versus ever drinker). Clinical variables included HPV status, body mass index, primary cancer site, cancer stage (TNM) (18), radiation dose, treatment (radiation only versus radiation + surgery versus radiation + chemotherapy versus radiation + chemotherapy + surgery), and chemotherapy regimen (cisplatin versus carboplatin/paclitaxel versus other). These variables were chosen for their potential influence on fatigue, based on literature reviews and our previous studies (19–22). Patient HPV status was determined based on pathology reports of the tumor tissue before treatment. According to current practice, p16 or HPV positive were counted as HPV related; otherwise, they were counted as HPV unrelated (23). In addition, patients’ white blood cell count and neutrophil-to-lymphocyte ratio (NLR) were collected for further analysis of blood cell composition and inflammatory status.
Fatigue was measured by using the Multidimensional Fatigue Inventory (MFI)-20. The MFI is a self-report instrument with 20 items that represents five dimensions: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity (24). Each dimension consists of four items on a 1- to 5-point scale. The total score, ranging from 20 to 100 (higher score indicates more fatigue), is calculated as the sum of the 20 items or the five dimensions. The MFI-20 has well-established validity and reliability (α = .84) in cancer populations (24,25) and has been used in our published studies on HNC patients (9,11).
Depressive symptoms, given the connections between fatigue and depressive symptoms, were measured as a covariate to control in our models by using the Patient Health Questionnaire depression scale (PHQ)-8. The PHQ-8 is a well-established valid self-administered diagnostic and severity measure for depressive disorders and has been used in many large clinical studies (α = .82) (26). The PHQ-8 asks the number of days in the past 2 weeks the respondent has experienced a variety of depressive symptoms. Each item is scored from 0 (not at all) to 3 (nearly every day); the sum of each item is the total score (0 to 24). Higher scores indicate more severe depressive symptoms.
Laboratory Measures
Sensitivity of GR Function
Whole blood drawn into heparinized tubes was subjected to procedures similar to those described by DeRijk et al. (27) and adapted by Miller et al. (28). Equal portions of whole blood diluted 10:1 with sterile saline weres incubated with lipopolysaccharide (derived from Escherichia coli 055:B5; Difco, Augsburg, Germany; final concentration 30 ng/ml) along with increasing concentrations of dexamethasone (Sigma, Deisenhofen, Germany; 10−9 – 10−5 M) for 6 hours at 37°C in 5% CO2. Supernatants were separated by centrifugation at 2000g for 10 minutes at 4°C, and then aliquoted into siliconized polypropylene tubes and stored at −80°C until assay for IL6 using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). GR sensitivity of peripheral blood immune cells was calculated as described in statistical analysis.
Inflammation Markers
Whole blood was collected into chilled EDTA tubes for the isolation of plasma. Plasma were separated by centrifugation at 1000g for 10 minutes at 4°C, and then aliquoted into siliconized polypropylene tubes and stored at −80°C until batched assay for inflammation biomarkers. Plasma concentrations of IL1 receptor antagonist (IL1ra), IL1β, IL6, IL6 soluble receptor (IL6sr), IL10, tumor necrosis factor α (TNFα), sTNFR2, and monocyte chemoattractant protein 1 (MCP1) were determined in duplicate using Magnetic Luminex Screening Assay (R&D Systems) (29,30). CRP was measured using a standard turbidimetric assay (9). Mean intra-assay and interassay coefficients of variation were reliably less than 10%, and all samples were run as duplicates. These inflammation markers were selected because of their associations with cancer-related fatigue in our studies and others (9,10,20,31–33).
Statistical Analyses
Area under the curve (AUC) was computed for GR sensitivity (14,34,35). For this analysis, each participant’s AUC was calculated using the trapezoidal method from the geometric shape created by the five IL6 data points from the five dexamethasone concentrations (10−9–10−5). We standardized the AUCs by calculating individual percentages: each participant’s AUC was divided by the total possible area of that participant’s geometric shape from the five dexamethasone concentrations using the highest and lowest IL6 as the boundary. This standardization process reduces variability and random error by using absolute values of AUC and can make each AUC calculation specific to the patients’ individual response to LPS over the different concentrations of dexamethasone (see supplemental methods, Supplemental Digital Content http://links.lww.com/PSYMED/A627, for additional information). The end result was individual GR AUC percentages, in which a lower percentage means a participant’s GR is more sensitive to dexamethasone and a higher AUC percentage means decreased dexamethasone sensitivity.
Descriptive statistics (mean, standard deviation, N, percentage) were used to describe the sample population. We compared the average fatigue and GR sensitivity at baseline to the patients’ levels at 1 month after IMRT using paired t tests.
For the primary analysis, we used linear regression to model the relationship between patient-reported fatigue and change in GR sensitivity across the two time points. Fatigue at 1 month after RT was regressed onto the change in GR sensitivity while adjusting for fatigue at baseline. The resulting estimate is residualized change in a patient’s reported fatigue score, interpreted as the ability of GR sensitivity to predict a patient’s post-treatment fatigue score independent of that patient’s pretreatment fatigue score. The defined units for GR sensitivity are a 10% increase in the AUC percentage. In addition to baseline fatigue, the model adjusted for age, sex, race, body mass index, HPV status, smoking status, alcohol consumption status, marital status, and chemotherapy regimen.
Our recent publication demonstrated a significant impact of HPV on the association between inflammation and fatigue: patients with HPV-positive tumors had significantly lower fatigue than those with HPV-negative tumors before IMRT, whereas their fatigue levels increased significantly to the similar levels as those with HPV-negative tumors at 1 month after IMRT (11). Thus, we additionally examined GR sensitivity with fatigue stratified on HPV status using the similar regression model as in our primary analysis.
Regression analyses were also used to examine the association between the change in GR sensitivity over time and inflammation markers at 1 month after treatment controlling for baseline values as well as the covariates indicated above.
We further considered if inflammation markers predicted fatigue with GR sensitivity in the model. In these analyses, we added log-transformed inflammation markers separately (CRP, IL1β, IL6, IL10, IL1ra, IL6sr, MCP1, TNFR2, and TNFα) along with GR sensitivity to the primary model and report the association of these variables with fatigue controlling for the covariates indicated previously.
All statistical tests had a significance threshold of <0.05, and all analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Selected demographics and clinical characteristics are shown in Table 1. The sampled population was approximately 59 years old, most were male (71%), and most were white (83%). Sixty-one percent of the sample population had previously smoked, and half had previously consumed alcohol. Seventy-eight percent of the diagnosed cancers were stage IV. Overall, 56% were in the oropharynx and 52% were HPV-related tumors. Eighty-four percent received concurrent chemoradiotherapy with (19%) or without surgery (65%). Among the patients receiving chemotherapy, 66% had cisplatin. Chemotherapy drug was the only variable that had significant effects on different fatigue levels: patients receiving cisplatin reported significantly lower fatigue levels at 1 month after IMRT compared with those who had carboplatin/paclitaxel. In addition, we examined whether patients with different HPV status were different by demographic and clinical variables. Patients with HPV-related tumors were significantly more likely to be male (p = .001), have no history of tobacco use (p < .001), be diagnosed with oropharyngeal cancer (p < .001), receive concurrent chemoradiotherapy (p = .008), and receive higher dose of RT (p = .009). Table S1 (Supplemental Digital Content http://links.lww.com/PSYMED/A627) describes correlation coefficients among fatigue, GR sensitivity, and inflammation markers in the sample as a whole at baseline and 1 month after IMRT.
TABLE 1.
Demographic and Clinical Characteristics of the Participants (N = 77) and Their Associations With Post-RT Fatigue
| Mean (SD) or N (%) | Mean Post-IMRT Fatigue | p | |
|---|---|---|---|
| Age, y | 58.8 (10.1) | 53.6 | .99 |
| Sex | |||
| Male | 55 (71) | 51.6 | |
| Female | 22 (29) | 58.6 | .089 |
| Race | |||
| White | 63 (83) | 54.7 | |
| Nonwhite | 13 (17) | 48.3 | .22 |
| Marital statusa | |||
| Married | 53 (70) | 51.1 | |
| Not married | 23 (30) | 59.8 | .035 |
| Smoking status | |||
| Never smoker | 30 (39) | 51.6 | |
| Ever smoker | 46 (61) | 55.0 | .37 |
| Alcohol status | |||
| Never drinker | 38 (51) | 55.6 | |
| Ever drinker | 37 (49) | 52.3 | .39 |
| Body mass index, kg/m2 | 24.7 (4.4) | 53.6 | .44 |
| HPV status | |||
| Unrelated | 36 (47) | 54.5 | |
| Related | 41 (53) | 52.8 | .64 |
| Cancer site | |||
| Oropharynx | 43 (56) | 53.7 | |
| Nonoropharynx | 34 (44) | 53.5 | .95 |
| Stage | |||
| ≤III | 17 (22) | 53.4 | |
| IV | 60 (78) | 53.8 | .94 |
| Treatment | |||
| Radiotherapy only | 2 (3) | 48.5 | |
| Radiotherapy and surgery | 10 (13) | 49.5 | |
| Radiotherapy and chemotherapy | 50 (65) | 55.4 | |
| Radiotherapy, chemotherapy, and surgery | 15 (19) | 51.2 | .78 |
| Chemotherapy | |||
| Cisplatin | 43 (66) | 50.6 | |
| Carboplatin/paclitaxel | 20 (31) | 62.6 | |
| Other | 2 (3) | 51.5 | .002 |
| Radiation dose, Gy | 67.9 (4.0) | 53.6 | .28 |
| Anti-inflammatories | |||
| No | 61 (82) | 53.8 | |
| Yes | 13 (18) | 52.0 | .71 |
| Antidepressants | |||
| No | 62 (84) | 53.2 | |
| Yes | 12 (16) | 55.1 | .70 |
| Feeding tubes | |||
| No | 25 (32) | 50.7 | |
| Yes | 52 (68) | 54.6 | .35 |
| Time of blood drawn | |||
| Pre-IMRT | 10:57 AM (162 min) | ||
| 1 mo after IMRT | 11:08 AM (139 min) | 53.6 | 0.55 |
| White blood cell counts, μlb | |||
| Pre-IMRT | 7593 (2548) | ||
| 1 mo after IMRT | 5854 (2607) | 56.1 | .042 |
| NLRc | |||
| Pre-IMRT | 3.25 (2.15) | ||
| 1 mo after IMRT | 5.63 (3.56) | 56.1 | .16 |
RT = radiation therapy; SD = standard deviation; IMRT = intensive modulated radiation therapy; HPV = human papillomavirus; NLR = neutrophil-to-lymphocyte ratio. Paired t test or correlation coefficient was used for the association between demographic and clinical characteristics and their associations with post-RT fatigue.
Married includes patients married or living as married. Unmarried includes patients single, separated, divorced, or widowed.
N = 75 at pre-IMRT, N = 64 at 1 month after IMRT.
N = 67 at pre-IMRT, N = 62 at 1 month after IMRT.
At 1 month after IMRT, the patients reported statistically higher levels of fatigue compared with baseline (Table 2). In contrast to our hypothesis, between baseline and 1 month after IMRT, GR sensitivity significantly increased (Table 2). Further analyses separating patients whose fatigue increased with those whose fatigue decreased showed that the increased sensitivity of GR was more likely to occur for patients whose fatigue decreased over time (Table 2). Moreover, lesser increases in GR sensitivity from baseline to 1 month after IMRT were significantly associated with fatigue at 1 month after IMRT after controlling for baseline fatigue and other covariates (unstandardized estimate of GR sensitivity = 4.07; 95% confidence interval [CI] = 0.60–7.53; Table 3 and Figure 1). Sensitivity analyses showed that the association between lesser changes in GR sensitivity and increased fatigue remained significant after controlling for depressive symptoms, antidepressants, anti-inflammatory medicines, presence of a feeding tube, and time of blood drawn. Stratifying by HPV status, we further found that among patients with HPV-related tumors, the change in GR sensitivity was not significantly associated with the change in fatigue after controlling for covariates (unstandardized estimate = −0.56; 95% CI = −5.00–3.89). However, among patients with HPV-unrelated tumors, lower GR sensitivity changes were associated with increased fatigue (unstandardized estimate = 8.22, 95% CI = 2.91–13.53).
TABLE 2.
Fatigue and Glucocorticoid Receptor Sensitivity at Baseline and 1 Month After Radiotherapy
| N | Pre-IMRT, Mean (SD) | 1-mo After IMRT, Mean (SD) | t Test | p | |
|---|---|---|---|---|---|
| Fatigue | 76a | 48.3 (16.3) | 53.6 (16.3) | −2.99 | .004 |
| GR sensitivity as measured by the AUC | 77 | 0.37 (0.06) | 0.34 (0.06) | 2.44 | .017 |
| Fatigue increased group | 48 | 0.36 (0.06) | 0.34 (0.07) | 1.19 | .24 |
| Fatigue decreased group | 28 | 0.38 (0.05) | 0.34 (0.04) | 3.46 | .002 |
IMRT = intensive modulated radiation therapy; SD = standard deviation; GR = glucocorticoid receptor; AUC = area under the curve. Paired t test was used for comparison.
Missing one participant at 1 month after treatment.
TABLE 3.
Results From Multivariate Regression Models of GR Sensitivity Over Time on Fatigue at 1 Month After IMRT
| N | Change in Fatigue | 95% CI | p | |
|---|---|---|---|---|
| Delta GR AUC estimate | 74 | 4.07 | (0.60 to 7.53) | .022 |
| Baseline fatigue | 0.55 | (0.35 to 0.76) | <.001 | |
| Age | −0.26 | (−0.57 to 0.04) | .092 | |
| Sex | 5.94 | (−1.19 to 13.07) | .10 | |
| HPV status | 3.50 | (−3.09 to 10.08) | .30 | |
| Marital status | 3.53 | (−3.00 to 10.06) | .29 | |
| Smoking history | −4.53 | (−11.30 to 2.23) | .19 | |
| Alcohol history | −2.45 | (−7.87 to 2.97) | .38 | |
| Race | −10.23 | (−18.43 to −2.03) | .014 | |
| Treatment | ||||
| Radiation or + surgerya | 3.89 | (−5.13 to 12.93) | .40 | |
| Radiation + carboplatin/paclitaxela | 12.40 | (3.09 to 21.73) | .009 | |
| BMI | −0.10 | (−0.72 to 0.53) | .76 | |
| Stratified by HPV status adjusted | ||||
| Delta GR AUC among HPV positive | 39 | −0.56 | (−5.00 to 3.89) | .81 |
| Delta GR AUC among HPV negative | 35 | 8.22 | (2.916 to 13.53) | .002 |
GR = glucocorticoid receptor; IMRT = intensive modulated radiation therapy; CI = confidence interval; AUC = area under the curve; HPV = human papillomavirus; BMI = body mass index.
Adjusted models controlled covariates of baseline fatigue, age, sex, marital status, smoking status, alcohol status, race, treatment, body mass index, and/or human papillomavirus status.
Compared with radiation + cisplatin.
FIGURE 1.
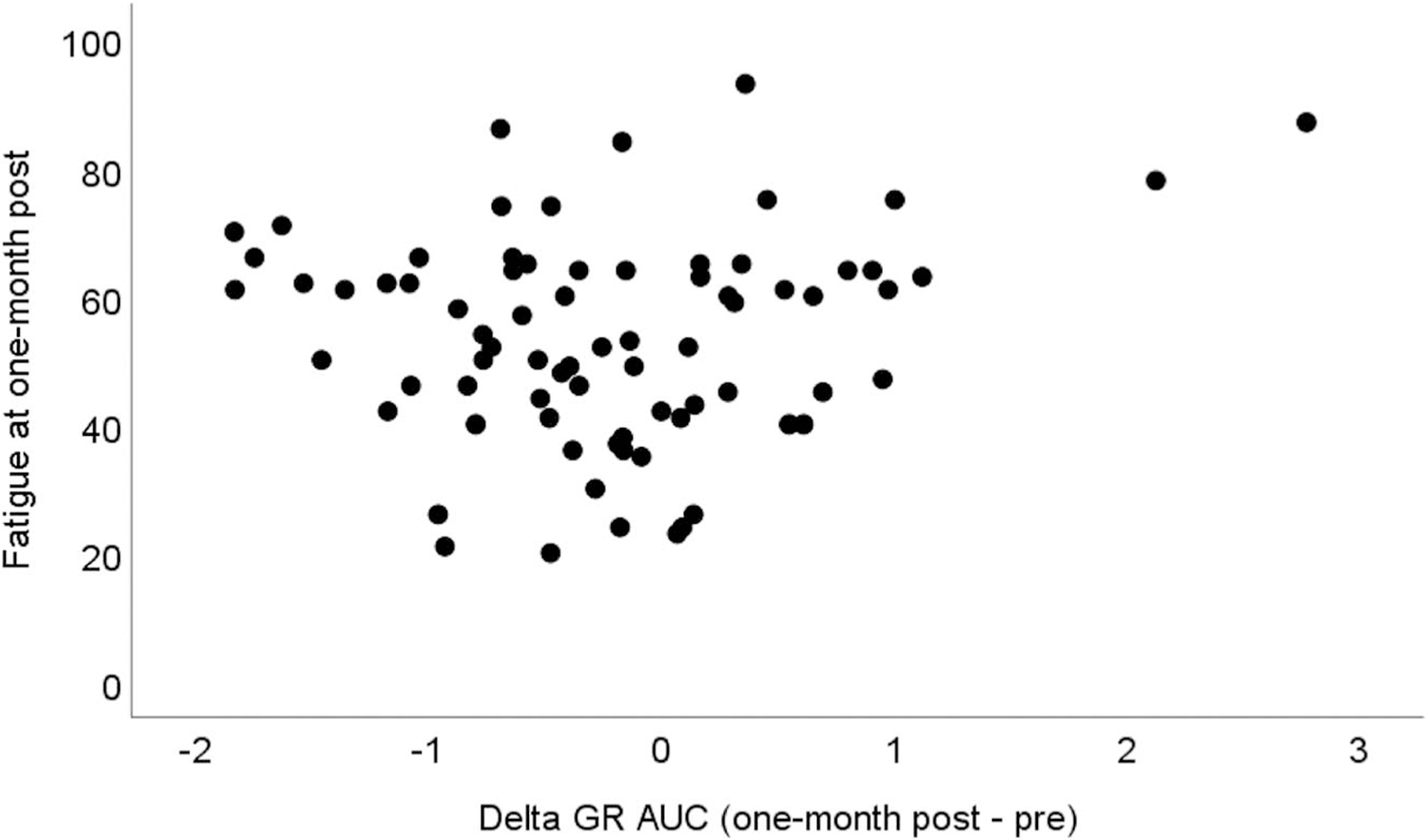
The association between GR sensitivity changes over time and fatigue at 1 month after IMRT. Decreased GR sensitivity over time as indicated by increased delta GR AUC is associated with increased fatigue. GR = glucocorticoid receptor; IMRT = intensive modulated radiation therapy; AUC = area under the curve.
Given the potential impact of NLR as another marker of inflammation (8), we did sensitivity analyses by adding NLR into our primary model using available data and found that NLR was not a significant predictor of fatigue and GR sensitivity remained significant in the same model. Similarly, because of the potential impact of cellular composition of the whole blood for the GR sensitivity assay (36), we controlled cellular composition in our model. Increased white blood cell count (unstandardized estimate = 0.0016; p = .003) and neutrophil count (unstandardized estimate = 1.8799; p = .004) over time were significantly predictive of increased fatigue, and the association of GR sensitivity and fatigue was not significant. However, further subgroup analyses of HPV-unrelated patients still showed significantly predictive effects of GR sensitivity on fatigue even with white blood cell count or neutrophil count in the model. Sensitivity analyses of adding other cell types including lymphocytes, monocytes, eosinophils, and basophils did not show significant impact on the results.
To examine whether the change in GR sensitivity was associated with increased inflammation at 1 month, we further conducted regression analyses of the change in GR sensitivity and inflammation markers at 1 month controlling for baseline values. The results showed that lesser increases in GR sensitivity over time were significantly associated with increased inflammation at 1 month after IMRT as representative by inflammation markers of CRP, IL6, IL10, and TNFα adjusting for covariates (Table 4). Similarly, sensitivity analyses of adding NLR or white blood cell count were conducted, and the associations between GR sensitivity and the four inflammation markers remained significant.
TABLE 4.
Associations Between GR Sensitivity Change Over Time and Inflammation Markers at One-Month Post-IMRT
| CRP | IL1β | IL6 | IL10 | IL1ra | IL6sr | MCP1 | TNFR2 | TNFα | |
|---|---|---|---|---|---|---|---|---|---|
| N | 74 | 63 | 73 | 72 | 74 | 74 | 74 | 74 | 74 |
| Delta GR AUC estimate | 0.59 | 0.12 | 0.41 | 0.19 | 0.16 | −0.02 | −0.01 | −0.02 | 0.1 |
| p | <.001 | .056 | .001 | .010 | .078 | .40 | .81 | .67 | .023 |
GR = glucocorticoid receptor; IMRT = intensive modulated radiation therapy; CRP = C-reactive protein; IL1β = interleukin β, IL1ra = interleukin 1 receptor antagonist, IL6sr = interleukin 1 soluble receptor, MCP1 = monocyte chemoattractant protein 1, sTNFR2 = soluble tumor necrosis factor receptor 2, TNFα = tumor necrosis factor α; AUC = area under the curve.
All models were controlled for covariates of baseline inflammation markers, age, sex, marital status, smoking status, alcohol status, race, treatment, body mass index, and human papillomavirus status.
We then explored whether inflammation markers predicted fatigue with GR sensitivity in the model. IL6 (unstandardized estimate = 2.93; p = .031) and sTNFR2 (unstandardized estimate = 6.81; p = .022) were statistically significantly associated with fatigue along with a statistically significant GR sensitivity estimate in the models (model with IL6: GR unstandardized estimate = 3.71; p = .036; model with sTNFR2: GR unstandardized estimate = 4.27; p = .012). In the models with IL1ra, CRP, TNFα, and IL10, the estimates of GR to fatigue reduced more than 20% and were no longer significant. Sensitivity analyses using mediation models showed a similar proportion of reduction in the estimate of GR to fatigue; however, the mediating effects of inflammation markers were not statistically significant.
DISCUSSION
This study examined the longitudinal association between GR sensitivity and cancer-related fatigue. In contrast to our hypothesis that GR sensitivity would decrease during IMRT, GR sensitivity increased in the sample as a whole during treatment. Moreover, the increased GR sensitivity was greater in patients with decreased fatigue over time. Furthermore, lesser increases in GR sensitivity over the treatment were significantly predictive of increased fatigue and were associated with increased inflammation markers at 1 month after IMRT. Interestingly, this effect was more prominent in patients with HPV-unrelated HNCs. In addition, inflammation markers were more strongly predictive of fatigue than GR sensitivity, suggesting a mediating effect of inflammation on the relationship between GR sensitivity and fatigue.
In this study, we observed that treatment of HNC was associated with a significant increase in GR sensitivity; however, this increase was more likely to occur in patients with decreased fatigue over time. The increase in GR sensitivity in the sample as a whole across cancer treatment is consistent with other instances of increased GR sensitivity found after acute stress and traumatic exposures such as during PTSD (37,38) and may represent an adaptive response to the trauma of treatment. After we separated patients into an increased fatigue group and a decreased fatigue group over time, we noticed that the GR sensitivity in patients with decreased fatigue was significantly greater, whereas the change in the GR sensitivity in patients with increased fatigue did not significantly change. The findings from this subgroup analyses echoed our major finding of this study: those individuals who exhibited lesser increases in GR sensitivity experienced higher levels of fatigue and plasma concentrations of inflammation markers after treatment. Glucocorticoids are key regulators of the anti-inflammatory response, and the function of glucocorticoids relies on the sensitivity of their receptors. Previous studies have shown that decreased GR sensitivity is often seen in conditions with chronic stress, such as caregivers (16,17,39). In cancer populations, we are aware of no studies that have examined the association between GR sensitivity and fatigue over time during treatment. However, decreased GR signaling was found in gene expression profile in a cross-sectional study of fatigued versus non-fatigued breast cancer survivor (19). Nevertheless, without longitudinal data, it cannot be determined whether changes in GR sensitivity were present in these populations, and the effects of cancer treatment on GR sensitivity warrant further investigation.
Our data indicate that the predictive effect of GR sensitivity on fatigue is more driven by the group of patients with HPV-unrelated tumors. Our published work suggests that patients with HPV-unrelated HNCs seem to have consistently higher levels of fatigue and inflammation from before IMRT up to 3 months after IMRT, whereas patients with HPV-related HNCs tend to have lower fatigue and inflammation before and 3 month after IMRT (11). Given the association between chronic stress and glucocorticoid resistance (17,39), it is possible that the consistently higher level of inflammation may be linked to lower relative GR sensitivity in the HPV-unrelated group. Our subsequent analyses also indicated that patients with HPV-unrelated tumors tended to have decreased GR sensitivity at both before the 1 month after IMRT compared with those with HPV-related tumors, but the differences were not statistically significant. A larger study may reveal significant results for this comparison.
Our sensitivity analyses adding cell composition to our models did not show an impact of NLR on the association between GR sensitivity and fatigue but did show an impact of white blood cell count and neutrophil count on the association. Adding white blood cell or neutrophil changes over time into the model significantly reduced the predictive effect of GR sensitivity on fatigue. These findings are consistent with published evidence that white blood cells are an important index for inflammation (40) and neutrophils are the most abundant type of white blood cell. However, this effect was attenuated in patients with HPV-unrelated tumors. In patients with HPV-unrelated tumors, although increased white blood cells and neutrophils over time were still significantly predictive of high fatigue, the association between GR sensitivity and fatigue remained. These findings further suggest a stronger drive for the association between GR sensitivity and fatigue in the HPV-unrelated patients than in the HPV-related patients, supporting our previous findings of a stronger predicative effect of inflammation on fatigue for the HPV-unrelated group than the HPV-related group (11). Nevertheless, larger studies are needed to verify the findings.
Our findings also suggest that this lesser change in glucocorticoid sensitivity was associated with increased inflammation. The etiology of this lesser change in glucocorticoid sensitivity is not well understood and is likely linked to multiple factors. However, increasing evidence has shown that inflammation itself may also contribute to reduced GR function. For instance, IL1, IL2, IL6, and TNFα have been shown to inhibit GR function at multiple levels, including GR translocation from cytoplasm to nucleus and GR-DNA binding (41–46). In addition, NF-κB, as mentioned previously, a key mediator for proinflammatory response, can directly interact with GR in the nucleus through protein-protein interactions, which lead to the repression of GR function (47). NF-κB may also suppress GR indirectly by competing for mutually required transcriptional cofactors for both NF-κB and GR in a limiting cellular pool (48). Our published study also indicated upregulated NF-κB signaling pathways in our gene expression data (9). Taken together, the findings from our study support the hypothesis that increased inflammation markers, including CRP, IL6, and TNFα, may contribute to the lesser increase in GR sensitivity in patients with fatigue.
Our previous work has demonstrated a consistent association between increased inflammation and fatigue (9,12). Given the significant association between GR sensitivity and fatigue, and between GR sensitivity and inflammation markers in this study, we would like to know whether inflammation markers would be still predictive of fatigue with GR sensitivity in the model. Our results showed that in the models with IL6 or sTNFR2, both the inflammation markers and GR sensitivity were significant predictors of fatigue. However, other inflammation markers decreased the estimates of GR sensitivity to fatigue with a more than 20% reduction. Although our sensitivity analyses of testing whether inflammation markers mediated the association between GR sensitivity and fatigue did not show statistically significant effects, adding that inflammation markers into the models did reduce GR sensitivity’s predictive effect on fatigue. These findings indicate that inflammation markers might be a stronger predictor of fatigue than GR sensitivity, but larger studies are needed for a formal test of mediation effects.
Limitations of the study include relatively small sample sizes in both HPV-related and HPV-unrelated groups and predominantly white male that may bias the results. In addition, blood samples were not drawn at the same time of day for all participants, which may add the potential of circadian influences on the data. However, we included the time of blood drawn in the regression model for sensitivity analysis, and the time of blood drawn was not a significant predictor in the models, and results did not change significantly. Larger studies with longer follow-up are warranted to further verify the results.
CONCLUSIONS
This study used a longitudinal design to explore the association among GR sensitivity, inflammation, and fatigue in a cancer population. In contrast to our hypothesis, we observed increased GR sensitivity during treatment. However, this increase was more likely to occur for patients with decreased fatigue over time. Furthermore, fatigue was associated with a lower increase in GR sensitivity; these associations were more prominent in patients with HPV-unrelated HNCs. Our findings also suggest that this lesser increase in GR sensitivity, possibly representing some level of relative reduced GR sensitivity, was also associated with higher inflammation. The findings extend and explain our previous understanding of the association between cancer-related fatigue and inflammation. The information may also help to elucidate that inflammation could be a risk factor for cancer-related fatigue and HPV status may also play a role in predicting inflammation and fatigue. Future studies may benefit from investigating whether different chemotherapeutic drugs may have different influences on the association between fatigue and GR sensitivity. Given the direct connection between GR and glucocorticoids, studies examining the change in patterns of glucocorticoid secretion and GR sensitivity during cancer treatment and its association with fatigue and inflammation are also necessary.
Supplementary Material
Acknowledgments
Source of Funding: The study was supported by National Institutes of Health (NIH)/National Institute of Nursing Research K99/R00NR014587, NIH/National Institute of Nursing Research R01NR015783, National Institutes of Health/National Cancer Institute P30CA138292, and Oncology Nursing Society Foundation.
Glossary
- AUC
area under the curve
- CI
confidence interval
- CRP
C-reactive protein
- GR
glucocorticoid receptor
- HNC
head and neck cancer
- HPV
human papillomavirus
- IMRT
intensity-modulated radiotherapy
- IL1ra
interleukin 1 receptor antagonist
- IL1β
interleukin 1β
- IL6
interleukin 6
- IL6sr
IL6 soluble receptor
- MCP1
monocyte chemoattractant protein 1
- MFI
Multidimensional Fatigue Inventory
- NF-κB
nuclear factor κB
- PHQ
Patient Health Questionnaire depression scale
- RT
radiotherapy
- sTNFR2
soluble tumor necrosis factor receptor 2
Footnotes
Conflicts of Interest
No conflicts of interest are reported.
Contributor Information
Canhua Xiao, School of Nursing, Yale University, Orange, Connecticut.
Ronald C. Eldridge, School of Nursing, Emory University, Atlanta, Gerogia..
Jonathan J. Beitler, School of Medicine, Emory University, Atlanta, Gerogia..
Kristin A. Higgins, School of Medicine, Emory University, Atlanta, Gerogia..
Cynthia E. Chico, School of Nursing, Emory University, Atlanta, Gerogia..
Jennifer C. Felger, Department of Psychiatry and Behavioral Sciences, School of Medicine, Emory University, Atlanta, Gerogia..
Evanthia C. Wommack, Department of Psychiatry and Behavioral Sciences, School of Medicine, Emory University, Atlanta, Gerogia..
Tish Knobf, School of Nursing, Yale University, Orange, Connecticut.
Nabil F. Saba, School of Medicine, Emory University, Atlanta, Gerogia..
Dong M. Shin, School of Medicine, Emory University, Atlanta, Gerogia..
Deborah W. Bruner, School of Nursing, Emory University, Atlanta, Gerogia..
Andrew H. Miller, Department of Psychiatry and Behavioral Sciences, School of Medicine, Emory University, Atlanta, Gerogia..
REFERENCES
- 1.Solberg Nes L, Ehlers SL, Patten CA, Gastineau DA. Self-regulatory fatigue in hematologic malignancies: impact on quality of life, coping, and adherence to medical recommendations. Int J Behav Med 2013;20:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage 2005;30:433–42. [DOI] [PubMed] [Google Scholar]
- 4.Gulliford SL, Miah AB, Brennan S, McQuaid D, Clark CH, Partridge M, Harrington KJ, Morden JP, Hall E, Nutting CM. Dosimetric explanations of fatigue in head and neck radiotherapy: an analysis from the PARSPORT phase III trial. Radiother Oncol 2012;104:205–12. [DOI] [PubMed] [Google Scholar]
- 5.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 2000;18:743–53. [DOI] [PubMed] [Google Scholar]
- 6.Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 2004;100:425–32. [DOI] [PubMed] [Google Scholar]
- 7.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010; CD006704. [DOI] [PubMed]
- 8.Tampa M, Mitran MI, Mitran CI, Sarbu MI, Matei C, Nicolae I, Caruntu A, Tocut SM, Popa MI, Caruntu C, Georgescu SR. Mediators of inflammation—a potential source of biomarkers in oral squamous cell carcinoma. J Immunol Res 2018;2018:1061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao C, Beitler JJ, Higgins KA, Conneely K, Dwivedi B, Felger J, Wommack EC, Shin DM, Saba NF, Ong LY, Kowalski J, Bruner DW, Miller AH. Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav Immun 2016;52: 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 2008;26:971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao C, Beitler JJ, Higgins KA, Glazer T, Huynh LK, Paul S, Felger JC, Wommack EC, Saba NF, Shin DM, Bruner DW, Miller AH. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer 2018;124:3163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao C, Beitler JJ, Higgins KA, Wommack EC, Saba NF, Shin DM, Bruner DW, Miller AH, Cole S. Differential regulation of NF-kB and IRF target genes as they relate to fatigue in patients with head and neck cancer. Brain Behav Immun 2018; 74:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003;160:1554–65. [DOI] [PubMed] [Google Scholar]
- 14.Walsh CP, Ewing LJ, Cleary JL, Vaisleib AD, Farrell CH, Wright AGC, Gray K, Marsland AL. Development of glucocorticoid resistance over one year among mothers of children newly diagnosed with cancer. Brain Behav Immun 2018; 69:364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AH. Inflammation versus glucocorticoids as purveyors of pathology during stress: have we reached the tipping point? Biol Psychiatry 2008;64: 263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 2008;64:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol 2002;21:531–41. [DOI] [PubMed] [Google Scholar]
- 18.AJCC Executive Office. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. [Google Scholar]
- 19.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun 2011;25:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun 2010;24:968–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang FM, Tsai WL, Chien CY, Chiu HC, Wang CJ, Chen HC, Hsiung CY. Changing quality of life in patients with advanced head and neck cancer after primary radiotherapy or chemoradiation. Oncology 2005;68:405–13. [DOI] [PubMed] [Google Scholar]
- 22.Xiao C, Hanlon A, Zhang Q, Movsas B, Ang K, Rosenthal DI, Nguyen-Tan PF, Kim H, Le Q, Bruner DW. Risk factors for clinician-reported symptom clusters in patients with advanced head and neck cancer in a phase 3 randomized clinical trial: RTOG 0129. Cancer 2014;120:848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol 2013;2:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995;39:315–25. [DOI] [PubMed] [Google Scholar]
- 25.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun 2007;21:413–27. [DOI] [PubMed] [Google Scholar]
- 26.Pressler SJ, Subramanian U, Perkins SM, Gradus-Pizlo I, Kareken D, Kim J, Ding Y, Sauve MJ, Sloan R. Measuring depressive symptoms in heart failure: validity and reliability of the Patient Health Questionnaire-8. Am J Crit Care 2011; 20:146–52. [DOI] [PubMed] [Google Scholar]
- 27.DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, Paciotti G, Gold PW, Sternberg EM. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. J Clin Endocrinol Metab 1997;82:2182–91. [DOI] [PubMed] [Google Scholar]
- 28.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med 2005;67: 679–87. [DOI] [PubMed] [Google Scholar]
- 29.Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med 2017;1–11. [DOI] [PubMed]
- 30.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 2016;21:1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon DE, McCain NL, Pickler RH, Munro C, Elswick RK Jr. Advancing the bio-behavioral research of fatigue with genetics and genomics. J Nurs Scholarsh 2011;43:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes-Gibby CC, Spitz MR, Yennurajalingam S, Swartz M, Gu J, Wu X, Bruera E, Shete S. Role of inflammation gene polymorphisms on pain severity in lung cancer patients. Cancer Epidemiol Biomarkers Prev 2009;18:2636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fossa SD, Ueland T, Murison R. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res 2011;71:136–41. [DOI] [PubMed] [Google Scholar]
- 34.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003;28:916–31. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A 2012;109:5995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu KD, Radom-Aizik S, Haddad F, Zaldivar F, Kraft M, Cooper DM. Glucocorticoid receptor expression on circulating leukocytes differs between healthy male and female adults. J Clin Transl Sci 2017;1:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson NS, Diolombi M, Scott-Sutherland J, Yang H, Bhatt V, Gautam S, Mullington J, Haack M. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav Immun 2016;58:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry 2004;55:745–51. [DOI] [PubMed] [Google Scholar]
- 39.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007;133:25–45. [DOI] [PubMed] [Google Scholar]
- 40.Wirth MD, Sevoyan M, Hofseth L, Shivappa N, Hurley TG, Hébert JR. The Dietary Inflammatory Index is associated with elevated white blood cell counts in the National Health and Nutrition Examination Survey. Brain Behav Immun 2018;69:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav Immun 2009;23:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biola A, Andreau K, David M, Sturm M, Haake M, Bertoglio J, Pallardy M. The glucocorticoid receptor and STAT6 physically and functionally interact in T-lymphocytes. FEBS Lett 2000;487:229–33. [DOI] [PubMed] [Google Scholar]
- 43.Biola A, Lefebvre P, Perrin-Wolff M, Sturm M, Bertoglio J, Pallardy M. Interleukin-2 inhibits glucocorticoid receptor transcriptional activity through a mechanism involving STAT5 (signal transducer and activator of transcription 5) but not AP-1. Mol Endocrinol 2001;15:1062–76. [DOI] [PubMed] [Google Scholar]
- 44.Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Adv Exp Med Biol 1999;461:107–16. [DOI] [PubMed] [Google Scholar]
- 45.Pace TW, Hu F, Miller AH. Activation of cAMP-protein kinase A abrogates STAT5-mediated inhibition of glucocorticoid receptor signaling by interferon-alpha. Brain Behav Immun 2011;25:1716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology 1999;140: 4359–66. [DOI] [PubMed] [Google Scholar]
- 47.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev 1999;20:435–59. [DOI] [PubMed] [Google Scholar]
- 48.Sheppard KA, Phelps KM, Williams AJ, Thanos D, Glass CK, Rosenfeld MG, Gerritsen ME, Collins T. Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-bindinsg protein and steroid receptor coactivator-1. J Biol Chem 1998;273:29291–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


