Abstract
Purpose:
Familial dysautonomia (FD) is a rare hereditary sensory and autonomic neuropathy (HSAN-3) with impaired pain and temperature perception and abnormal autonomic function. Patients with FD have gastrointestinal dysmotility and report a range of gastrointestinal symptoms that have yet to be systematically evaluated. The aim of this study was to establish the frequency and severity of gastrointestinal symptoms in FD.
Methods:
We distributed the validated NIH PROMIS questionnaire, and additional FD-specific questions, to 202 living patients with genetically-confirmed FD identified with the New York University FD Patient Registry or, when relevant, their caretaker. As a comparison group, we obtained PROMIS scores from a United States-based general adult population (n=71,812).
Results:
Seventy-seven (38%) questionnaires were returned, of which 53% were completed by the patient. Median age of subjects was 25 years old, and 44% were male. A quarter of patients received nutrition by gastrostomy tube solely, while 53% were reliant on the gastrostomy tube only for liquid intake. The prevalence of gastrointestinal symptoms in patients with FD was significantly higher in each of the eight domains compared to controls. Gastrointestinal symptoms as measured by raw scores on the PROMIS scale were significantly less severe in FD compared to the control population in all domains except for abdominal pain. Surveys completed by caregivers reported the same burden of symptoms as those completed by patients.
Conclusion:
Gastrointestinal symptoms affect nearly all patients with FD. In adult patients with FD, gastrointestinal symptoms are more prevalent but are reported at lesser severity than in the average U.S. adult population.
Keywords: autonomic, motility, neurogastroenterology, patient reported outcomes
INTRODUCTION
Familial dysautonomia (FD), also known as Riley-Day syndrome, is a rare hereditary sensory and autonomic neuropathy (HSAN-3) first described in 1949 in children of Jewish-Ashkenazi ancestry [22]. FD is caused by a founder mutation in the elongator complex protein 1 gene (known as ELP1 or IKBKAP), resulting in impaired development of sensory and afferent autonomic nerves [23]. This mutation originated in Eastern Europe and has a carrier rate as high as 1 in 18 individuals of Ashkenazi ancestry [18]. The key feature of FD is profound blood pressure instability that results from failure of afferent baroreflex signals [15, 17]. Patients also have widespread sensory deficits and varying degrees of cognitive dysfunction [18].
Gastrointestinal complaints are relatively frequent in FD and can have a significant impact on quality of life. Neurogenic dysphagia and gastroesophageal reflux are among the first symptoms of the disease [3, 14, 19] and can result in significant morbidity due to recurrent aspiration pneumonia [4]. In an attempt to decrease the risk of aspiration, it was previously common for patients with FD to undergo early surgical fundoplication and gastrostomy, which can interfere with the later assessment of foregut physiology [4, 14, 19]. Despite being a common clinical complaint, the prevalence of gastrointestinal symptoms in patients with FD has not been systematically studied. There are several possible reasons for this; many patients struggle to accurately locate the source of discomfort and many cannot describe their symptoms with sufficient detail to aid diagnosis. It is not clear if this is because of their sensory deficits or, in some cases, due to their limited cognitive abilities. A few small studies suggest dysmotility as an underlying mechanism of gastrointestinal complications of FD, but modern motility testing, such as esophageal manometry and ambulatory pH testing, has not been assessed, as they are often challenging to perform in this population [11, 14].
In this study, we aimed to establish the frequency and severity of gastrointestinal symptoms in patients with FD. Because there are no available validated clinical assessment tools for patient-reported gastrointestinal (GI) symptoms in patients with cognitive disabilities, we used the National Institute of Health (NIH) Gastrointestinal Patient Reported Outcomes Measurement Information System (PROMIS), a standardized, reliable tool, which has been extensively used in adult patients with gastrointestinal disorders [1, 10, 24]. Given the profound sensory neuropathy of FD and the defective afferent feedback from the visceral organs, the mechanisms driving the perceptions of gastrointestinal pain and discomfort in these patients are of particular interest. As the disorder involves both vagal and potentially enteric neurons, understanding the burden of gastrointestinal symptoms in FD may offer unique insight into the autonomic control of the gut.
METHODS
Study population
We identified participants with FD in the New York University (NYU) FD Patient Registry, a database of demographic and medical information collected from genetically-confirmed cases who have received care at NYU Langone Medical Center since 1980. Basic demographics, such as age and sex, were obtained from the NYU FD Patient Registry. The Montreal Cognitive assessment (MoCA) score from the most recent clinical visit (typically within the last 12-months) was used to screen for cognitive impairment. Cognitive impairment was defined as a MoCA score below 26 for participants without visual impairment, or 18 for visually-impaired participants [16]. Assent was obtained following local IRB guidelines for recruiting research study participants when their capacity to consent is questionable. The protocol was approved by the NYU School of Medicine Institutional Review Board who received a copy of all questions.
Survey administration and instructions
We invited by email and then by mail all living English-speaking patients with FD or their listed primary caregiver to complete the NIH Gastrointestinal PROMIS, as well as additional questions. We used the NIH Gastrointestinal PROMIS survey, which has been extensively used in adult patients with gastrointestinal disorders, and is designed for a 6th-grade reading level or lower [1]. Because of the limited number of pediatric cases, we opted to use only the adult NIH Gastrointestinal PROMIS survey tool. Questions in the adult NIH Gastrointestinal PROMIS survey fall within one of 8 assessed gastrointestinal domains: swallowing, nausea, constipation, fecal incontinence, diarrhea, belly pain, reflux, and bloating. The survey evaluates symptoms over the last 7 days and employs a 5-point categorical response scale for all questions (score 0 to 4), with higher scores reflecting more severe or frequent symptoms. The domain score is the sum of individual questions; total domain scores range from 16 to 42, as the number of questions differ in each domain. Domain scores were designed to be used either individually or summed together, to quantify severity and prevalence of gastrointestinal symptoms in a disease-agnostic manner for a wide range of populations [24]. Published tables are available that allow conversion of raw scores to normalized percentiles (ranging from 1st to 99th) using a reference population derived from online surveying of 71,812 adult U.S. individuals [1]. Age and sex distribution are shown in Table 1.
Table 1. Demographic characteristics of responders, the complete population of the FD patient registry, and the control population.
The responders and the population of the FD registry had similar age and sex distribution. The control group was significantly different than the responders, due to the lack of pediatric patients in the control group. Pediatric FD patients were excluded from further analyses. The sex distribution between responders and controls was similar P-value obtained with Chi-squared. Asterisks denote statistical significance vs. responders.
| Responders N=77 | All NYU FD Registry patients N=682 | P-value | Controls N=71,812 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Age group | P=0.021 | P<0.001* | ||||||
| 0–17 | 16 | 21% | 207 | 30% | 0 | 0% | ||
| 18–24 | 24 | 31% | 131 | 19% | 12,419 | 17% | ||
| 25–44 | 34 | 44% | 268 | 39% | 37,055 | 52% | ||
| 45–64 | 3 | 4% | 73 | 11% | 20,468 | 28% | ||
| ≥65 | 0 | 0% | 3 | < 1% | 1,870 | 3% | ||
| Sex | P=0.714 | P=0.381 | ||||||
| Women | 42 | 55% | 357 | 52% | 42,696 | 60% | ||
| Men | 35 | 45% | 325 | 48% | 29,116 | 40% | ||
In addition to the NIH Gastrointestinal PROMIS we included additional questions pertaining to the use of gastrostomy (G)-tubes for feeding including: 1) Do you have a gastrostomy tube? 2) Do you have the ability to swallow liquids? and 3) Do you have the ability to swallow solids? Parents or legal guardians were requested to assist with survey completion when needed (e.g. patients who were of young age or had cognitive or visual impairment that prevented independent survey completion). In addition, the responders were asked to identify which body system was associated with the most bothersome symptoms among the respiratory, gastrointestinal, visual, orthopedic, and psychiatric systems. Surveys were first sent to the email address listed in the NYU FD Patient Registry. Those who did not respond or did not use email or internet were mailed paper versions of the survey to complete. Duplicate responses for the same patient who previously completed online questionnaires were omitted.
Unlike the NIH Gastrointestinal PROMIS survey, which instructs participants to only complete relevant domains [1], we asked survey participants to answer all sections (e.g., marking 0/none for any symptoms they did not experience). Study data were collected and managed using a custom-designed research electronic data capture (REDCap) instrument [9]. For internet-based responses, each participant received a unique link to access the survey. They could save their answers to take a break. In rare cases, there were missing or omitted survey questions, which were analyzed as “0 or none.” Patients who ingested fluids and nutrition by G-tube only were instructed to omit questions in the swallowing domain, and any responses in this domain were not analyzed. Therefore, G-tube-dependent patients were not included in any analyses of swallowing domain sub-scores and total PROMIS scores, and were removed from the denominator.
Statistical analysis
We could not retrieve sufficient public data on a well-matched control population with a chronic, congenital neurologic disease requiring gastrostomy and fundoplication surgery. We found published data from the general population that reported normalized values using a ranked percentile score for each PROMIS domain [1], and compared only mean raw scores of the FD and control populations between these two groups. Corresponding raw data for the previously published control subjects were kindly provided by the study authors (Chris Almario MD, personal communication).
Data were assessed for normality using the Shapiro-Wilk test. Chi-square tests or Fisher’s exact test were used to compare categorical variables, such as symptom prevalence. Two-sample t-tests were used to compare continuous variables, such as symptom severity.
To correct for multiple comparisons between the control and FD population groups, we adjusted the significance level to a lower value using the Bonferroni correction method [5]. The significance level was set at P<0.01. Statistical analyses were conducted using the R software package. Data are presented as mean±standard deviation, unless otherwise stated.
RESULTS
Survey responders
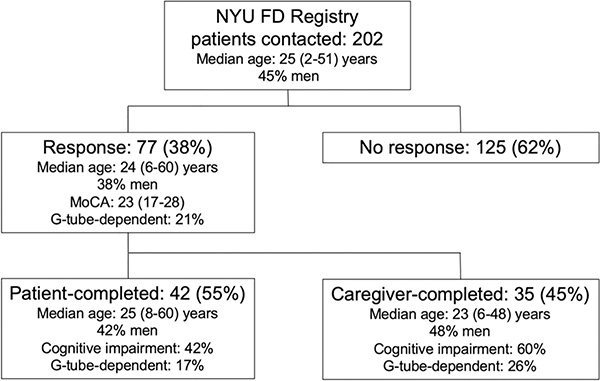
Of the 202 patients contacted to participate in the survey, 77 (38%) responded. Sixteen (21%) responders were younger than 18 years old. A total of 16 (20.8%) were G-tube dependent. The responders’ sample was representative of the cohort followed in the NYU FD Patient Registry in terms of disease duration, age at diagnosis, genotype, presence of autonomic dysfunction, and thus thought to reflect the phenotypic spectrum of the entire cohort (Table 1). The responders’ sample was significantly different from the sample of adult patients included in the control reference population derived from online surveying of 71,812 U.S. individuals [1]. This is because the control samples only included adults. The control sample also included individuals aged 65 and older, whereas no patient with FD responding to the survey was in that age group (Table 1).
In total, 55% of surveys were completed by patients themselves (Figure 1). Patients who had caregiver-completed surveys were younger than patients who completed the survey on their own (mean 20±9.98 vs 30±10.14 years, P<0.01). Sex, MoCA scores, and G-tube-dependence were similar between surveys completed directly by subjects versus those that required caregiver assistance. Moreover, total mean GI PROMIS raw scores were not statistically different in those whose surveys were completed with the assistance of caregivers (66.3±31.18) vs. those who completed the survey themselves (48.7±30.96, P=0.16).
Figure 1.
Flowchart of study participants.
Prevalence and severity of gastrointestinal symptoms
In total 97% (n=75) of patients with FD reported GI symptoms within the 7 days prior to completing the survey. Forty-two percent of patients reported GI symptoms as their most bothersome medical problem, more burdensome than other frequent comorbidities in FD such as orthopedic problems (23%), respiratory complications (14%), visual problems (13%), or psychiatric manifestations of the disease (4%).
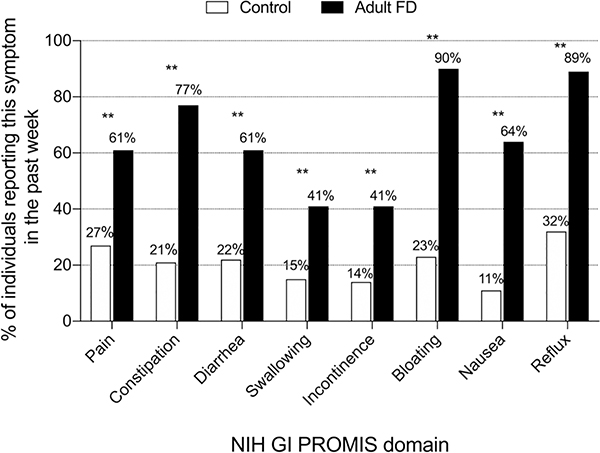
Because the available GI PROMIS data from the general U.S. populations was obtained from adults only [1], we excluded the 16 pediatric FD patients from further comparisons to this control reference sample. The prevalence of adult patients with FD reporting symptoms was significantly higher than those in the general U.S. population. This was the case in all the eight PROMIS domains, with the most striking differences in symptoms of bloating and reflux (Figure 2).
Figure 2. Percentage of patients with familial dysautonomia and controls reporting gastrointestinal symptoms in the past week.
Adult patients with familial dysautonomia (n=61, black bars) compared to a previously published general U.S. adult population (n=71,812, white bars) as measured by the National Institute of Health Gastrointestinal PROMIS score [1]. The prevalence of symptoms in all domains was significantly higher in the FD adult population. **Denotes p<0.01.
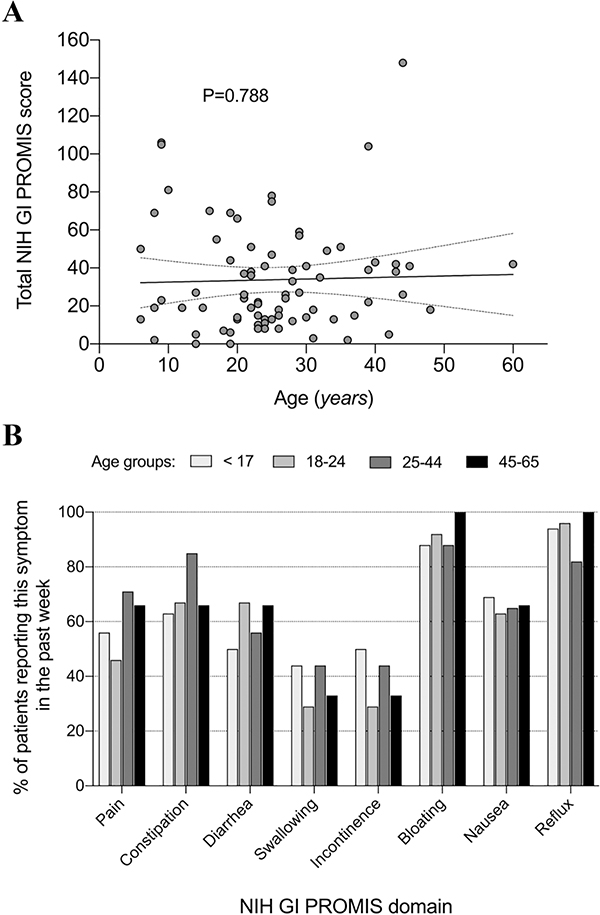
A simple regression showed lack of association between the GI PROMIS total score and the FD patients’ age (simple regression R2=0.0009; P=0.788, Figure 3A). To further understand if the prevalence of symptoms differed across age groups in patients with FD, we sub-divided our sample into 4 age groups: aged <17 (n=16), aged 18–24 (n=24), aged 25–44 (n=34), and aged 45–64 (n=3). There were no significant differences among age groups, although constipation tended to be more frequently reported in the 25–44 age group (Figure 3B).
Figure 3. Relationship between gastrointestinal symptoms and age.
A. Association between GI PROMIS symptom score and age. A regression model showed no association between the GI PROMIS total score and the age of patients with familial dysautonomia (simple regression R2=0.0009; P=0.788). B. Percentage of patients with familial dysautonomia reporting gastrointestinal symptoms in the past week in different age groups. There are no differences among age groups in any of the PROMIS domains (P=n.s.). Collectively, these data suggest that neither the severity nor the prevalence of GI symptoms are related to age in patients with familial dysautonomia.
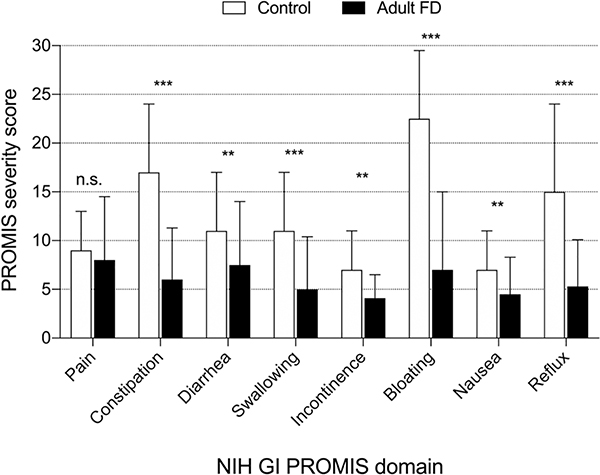
Despite having a higher prevalence of GI symptoms, adult patients with FD reported significantly lower symptomatic burden compared to adult controls in all the domains of the PROMIS, except for pain, which was not different in FD and controls (Figure 4).
Figure 4. Severity of gastrointestinal symptoms in adult patients with familial dysautonomia and controls.
Adult patients with familial dysautonomia (n=61, black bars) compared to a published general adult U.S. population (n=71,812, white bars [1]) as measured by raw domain scores of the National Institute of Health Gastrointestinal PROMIS. As the maximum raw score can vary based on the number of survey questions in each domain, scores should not be compared across domains. Overall the severity was significantly lower in the familial dysautonomia population in all domains except for pain. **Denotes p<0.01, ***Denotes p<0.001. Error bars denote standard deviation.
Subgroup analysis
To identify possible factors influencing the PROMIS survey outcomes we conducted sub-group analyses. Of the 77 responders, 16 patients (20.8%) were fully G-tube-dependent for nutrition and liquid intake, while 53% used G-tubes only for liquids, still using the oral route for solid food. Patients who were G-tube dependent did not score differently than patients who could tolerate oral intake for any of the GI PROMIS symptom domains (swallowing domain not assessed). Furthermore, there were no differences in GI PROMIS raw scores between surveys that were directly completed by subjects compared to those completed with caregiver assistance. Nausea tended to be more frequently reported by caregivers than by patients although the difference did not reach statistical significance (6.8 vs. 4.1; P=0.02). Of the 77 responders, 32 patients screened positive for cognitive impairment according to their MoCA score, with half having a score below 26. Although the symptomatic burden was numerically higher in patients without cognitive impairment, this was not statistically significant and there was considerable overlap between the raw GI PROMIS scores in patients with and without cognitive impairment (21.19±18.38 vs 34.65±21.87, P=0.066). Bloating tended to being lower in patients with cognitive impairment, although the difference was not statistically significant (2.9 vs 6.6, P=0.045).
DISCUSSION
Our study is the first to explore the usefulness of the PROMIS GI questionnaire in a population of chronically-ill patients of variable ages, cognitive abilities, and a high prevalence of surgical GI interventions. We found that gastrointestinal symptoms are almost universal in patients with FD, with 97% of patients reporting symptoms. Although the survey captured a diverse range of GI symptoms among the adult FD population, including bloating, upper GI dysfunction, dysphagia, dyspepsia and gastroesophageal reflux, patients and their caregivers ranked the severity of the symptoms lower compared to the general U.S. adult population.
While systematic studies of gastrointestinal function in FD are lacking, there is solid evidence that patients do indeed have a severe gastrointestinal phenotype. Indeed, gastrointestinal symptoms and death following a gastrointestinal bleed was reported in the original FD case series in 1949 [22]. Subsequent case series of patients with FD reported dysphagia and reflux accompanied by prolonged esophageal transit, absent or abnormal esophageal peristalsis [11], or consistent pylorospasm [12]. Such studies were based on noninvasive barium studies or scintigraphy, both of which are limited as they cannot measure pH, nor strength or muscle coordination. Neuropathology studies explaining the functional gastrointestinal abnormalities in FD are limited to very small and early studies showing degeneration and loss of ganglion cells in the celiac plexus [6] and abnormalities in the esophageal and gastric myenteric (Auerbach’s) plexuses [2], along with marked reduction in the number of spinal sensory neurons [20].
The impact of fundoplication and gastrostomy on gastrointestinal symptoms is hard to ascertain, given the high rate of these procedures in the cohort and the insufficient number of patients who have not undergone these procedures to enable robust statistical comparisons [4]. While it is tempting to speculate that those that did not undergo surgery had a milder gastrointestinal phenotype, this may not be the case as the decision to undergo surgery at that time was made empirically and not based on clinical criteria.
Despite ranking their gastrointestinal symptoms as more bothersome than other manifestations of the disease, adult patients with FD had lower symptoms scores on the PROMIS GI survey than the ones reported by a general U.S. adult population. This discordance between our clinical observations on the impact of gastrointestinal symptoms on quality of life suggests a possible inability to quantify symptom severity. We hypothesize that this is related to difficulty perceiving and interpreting symptoms in disorders, like FD and other hereditary sensory neuropathies [4], in which the development of sensory neurons and ganglia is impaired, and afferent feedback from most organs to the CNS is limited or aberrant from birth [8, 20]. Whether sensory perceptions from the gastrointestinal system are altered in congenital disorders with impaired innervation of the gut has not been thoroughly assessed, although our clinical experience with this population suggests it. Specifically, some patients with FD have suffered from severe gastrointestinal events, including Mallory-Weiss syndrome, gastric ulceration and perforation [21], pancreatitis, or cholecystitis, with minimal or no complaints. When inquired about their abdominal symptoms, these patients only reported a mild, diffuse, poorly-localized discomfort, and they were unable to describe it in terms that could be useful for a differential diagnosis. It is also likely that the sensory neuropathy in FD has a lasting impact on the subjective emotional quality placed on afferent inputs arising from the gut, which could affect the ability to self-report symptom severity in a meaningful context that would be comparable to a control population [7, 25].
An alternative explanation for the discordance between symptom prevalence and severity is that 45% of our surveys were completed by caregivers, who may quantify the symptomatic burden differently than patients. Against this hypothesis is that we found considerable overlap between patient-completed and caregiver-completed answers. Finally, it is also conceivable that, independently of the impaired afferent innervation in FD, the decade-long occurrence of chronic symptoms may lead to sensory desensitization and reduced self-perceived severity of symptoms, although, if this were true, we would expect a higher burden of symptoms in pediatric patients compared to adults. Comparison of PROMIS GI scores in pediatric and adult patients with chronic congenital disorders affecting the gastrointestinal system (e.g., Hirschsprung disease) should be helpful to address these questions.
Our study has limitations. We used published normative PROMIS GI data obtained from an older U.S. adult population. Rather than our own controls, we opted to use the available data, as this was representative of a large sample size of the general U.S. adult population. We were unable to identify an appropriate comparison group with a congenital neurologic illness requiring G-tube, of pediatric and adult age, with a similar variability in cognitive capacity. The GI PROMIS questionnaire was developed for adults and our sample size included 16 pediatric FD patients. To ensure that our sample was as similar as possible to the one studied during the normative GI PROMIS study, we performed our comparisons to the normative adult sample excluding our 16 pediatric patients. The response rate to the questionnaire was 38% and may not represent the entire FD patient cohort. Nevertheless, the responders’ characteristics were representative of the full spectrum of the disease phenotype as survey responders had the same range of age, disability, cognitive capacity, rate of surgical gastrointestinal interventions, and neurological features of disease expression compared to the full cohort followed in the natural history study. The GI PROMIS data used for comparison was generated by an internet-based tool, and it is theoretically possible that in the patients that answered the questionnaire on paper, their responses may have differed. Furthermore, the GI PROMIS tool has not been validated in specific populations like FD patients that have undergone extensive gastrointestinal surgery and because of the small number of patients that did not undergo surgery, subgroup analysis was not possible. We used a t-test to look for differences between FD patients and control, which is a valid statistical approach [13]. We could not perform the Mann Whitney U test because we only had average data from the normative GI PROMIS control group, and not individual results. We used the MoCA score, a test developed and validated in elderly populations, as a cognitive screen, and patients did not undergo formal neurocognitive testing. Finally, due to the small sample size, there is a possibility that the subgroup analysis might be underpowered.
In conclusion, gastrointestinal symptoms affect almost all patients with FD. Gastrointestinal symptoms are more prevalent in FD but are reported at lesser severity than in the average adult U.S. population. Our findings have relevance for both medical care and research study design. Although gastrointestinal symptoms are very prevalent in patients with FD, their actual severity may be underreported. In the setting of severe gastrointestinal issues, patients with FD may complain of only minor symptoms or diffuse poorly-localized discomfort. Further studies with objective assessments should clarify the phenotype of gastrointestinal abnormalities in patients with FD.
Acknowledgements:
We thank Chris Almario, MD for providing data for our reference study population.
Funding sources: NINDS (U54NS065736) and Familial Dysautonomia Foundation.
Conflicts of interests:
Dr. Palma has received fees as consultant or advisory board member for Lundbeck, Takeda, Biogen, Dr. Reddy’s and PTC; is the principal investigator in clinical studies sponsored by Theravance, Biohaven and Biogen; receives research support from the National Institutes of Health, the Food and Drug Administration, the Familial Dysautonomia Foundation, Inc. the MSA Coalition, and the Michael J. Fox Foundation; and is managing editor of Clinical Autonomic Research.
Dr. Norcliffe-Kaufmann has received fees as consultant or advisory board member for PTC and Theravance. She receives research support from the National Institutes of Health, the Food and Drug Administration, the Familial Dysautonomia Foundation, and the Michael J. Fox Foundation
Dr. Kaufmann serves on a scientific advisory board for Lundbeck, Biogen, Theravance, Biohaven, and PTC; serves as Editor-in-Chief of Clinical Autonomic Research; receives research support from the National Institutes of Health, the Food and Drug Administration, the Familial Dysautonomia Foundation, Inc. the MSA Coalition, and the Michael J. Fox Foundation.
Dr. Khan is a consultant for Medtronic.
No other authors have conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Almario CV, Ballal ML, Chey WD, Nordstrom C, Khanna D, Spiegel BMR (2018) Burden of Gastrointestinal Symptoms in the United States: Results of a Nationally Representative Survey of Over 71,000 Americans. Am J Gastroenterol 113:1701–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariel I, Wells TR (1985) Structural abnormalities of the myenteric (Auerbach’s) plexus in familial dysautonomia (Riley-Day syndrome) as demonstrated by flat-mount preparation of the esophagus and stomach. Pediatr Pathol 4:89–98 [DOI] [PubMed] [Google Scholar]
- 3.Axelrod FB (2004) Familial dysautonomia. Muscle Nerve 29:352–363 [DOI] [PubMed] [Google Scholar]
- 4.Axelrod FB, Schneider KM, Ament ME, Kutin ND, Fonkalsrud EW (1982) Gastroesophageal fundoplication and gastrostomy in familial dysautonomia. Ann Surg 195:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. BMJ 310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown WJ, Beauchemin JA, Linde LM (1964) A Neuropathological Study of Familial Dysautonomia (Riley-Day Syndrome) in Siblings . J Neurol Neurosurg Psychiatry 27:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig AD, Craig A (2009) How do you feel--now? The anterior insula and human awareness. Nature reviews neuroscience 10. [DOI] [PubMed] [Google Scholar]
- 8.George L, Chaverra M, Wolfe L, Thorne J, Close-Davis M, Eibs A, Riojas V, Grindeland A, Orr M, Carlson GA, Lefcort F (2013) Familial dysautonomia model reveals Ikbkap deletion causes apoptosis of Pax3+ progenitors and peripheral neurons. Proc Natl Acad Sci U S A 110:18698–18703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE (2019) The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochar B, Martin CF, Kappelman MD, Spiegel BM, Chen W, Sandler RS, Long MD (2018) Evaluation of Gastrointestinal Patient Reported Outcomes Measurement Information System (GI-PROMIS) Symptom Scales in Subjects With Inflammatory Bowel Diseases. Am J Gastroenterol 113:72–79 [DOI] [PubMed] [Google Scholar]
- 11.Krausz Y, Maayan C, Faber J, Marciano R, Mogle P, Wynchank S (1994) Scintigraphic evaluation of esophageal transit and gastric emptying in familial dysautonomia. Eur J Radiol 18:52–56 [DOI] [PubMed] [Google Scholar]
- 12.Linde LM, Westover JL (1962) Esophageal and gastric abnormalities in dysautonomia. Pediatrics 29:303–306 [PubMed] [Google Scholar]
- 13.Lumley T, Diehr P, Emerson S, Chen L (2002) The importance of the normality assumption in large public health data sets. Annual review of public health 23:151–169 [DOI] [PubMed] [Google Scholar]
- 14.Margulies SI, Brunt PW, Donner MW, Silbiger ML (1968) Familial dysautonomia. A cineradiographic study of the swallowing mechanism. Radiology 90:107–112 [DOI] [PubMed] [Google Scholar]
- 15.Mora CF, Norcliffe-Kaufmann L, Palma J-A, Kaufmann H (2015) Chewing-induced hypertension in afferent baroreflex failure: a sympathetic response? Experimental physiology 100:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 53:695–699 [DOI] [PubMed] [Google Scholar]
- 17.Norcliffe-Kaufmann L, Axelrod F, Kaufmann H (2010) Afferent baroreflex failure in familial dysautonomia. Neurology 75:1904–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norcliffe-Kaufmann L, Slaugenhaupt SA, Kaufmann H (2017) Familial dysautonomia: History, genotype, phenotype and translational research. Progress in neurobiology 152:131–148 [DOI] [PubMed] [Google Scholar]
- 19.Palma JA, Spalink C, Barnes EP, Norcliffe-Kaufmann L, Kaufmann H (2018) Neurogenic dysphagia with undigested macaroni and megaesophagus in familial dysautonomia. Clin Auton Res 28:125–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson J, Pytel BA, Grover-Johnson N, Axelrod F, Dancis J (1978) Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci 35:77–92 [DOI] [PubMed] [Google Scholar]
- 21.Ramprasad C, Spalink C, Vanegas I, Norcliffe-Kaufmann L, Palma JA, Levy J, Kaufmann H, Chen LA (2018) Gastrointestinal Bleeding Is Common in Children With Familial Dysautonomia: A Case-Control Study (1980–2017): Presidential Poster Award: 1054. American Journal of Gastroenterology 113:S603 [Google Scholar]
- 22.Riley CM, Day RL, et al. (1949) Central autonomic dysfunction with defective lacrimation; report of five cases. Pediatrics 3:468–478 [PubMed] [Google Scholar]
- 23.Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, Cuajungco MP, Liebert CB, Chadwick B, Idelson M, Reznik L, Robbins C, Makalowska I, Brownstein M, Krappmann D, Scheidereit C, Maayan C, Axelrod FB, Gusella JF (2001) Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 68:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiegel BM, Hays RD, Bolus R, Melmed GY, Chang L, Whitman C, Khanna PP, Paz SH, Hays T, Reise S, Khanna D (2014) Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol 109:1804–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strigo IA, Craig AD (2016) Interoception, homeostatic emotions and sympathovagal balance. Philos Trans R Soc Lond B Biol Sci 371:20160010. [DOI] [PMC free article] [PubMed] [Google Scholar]